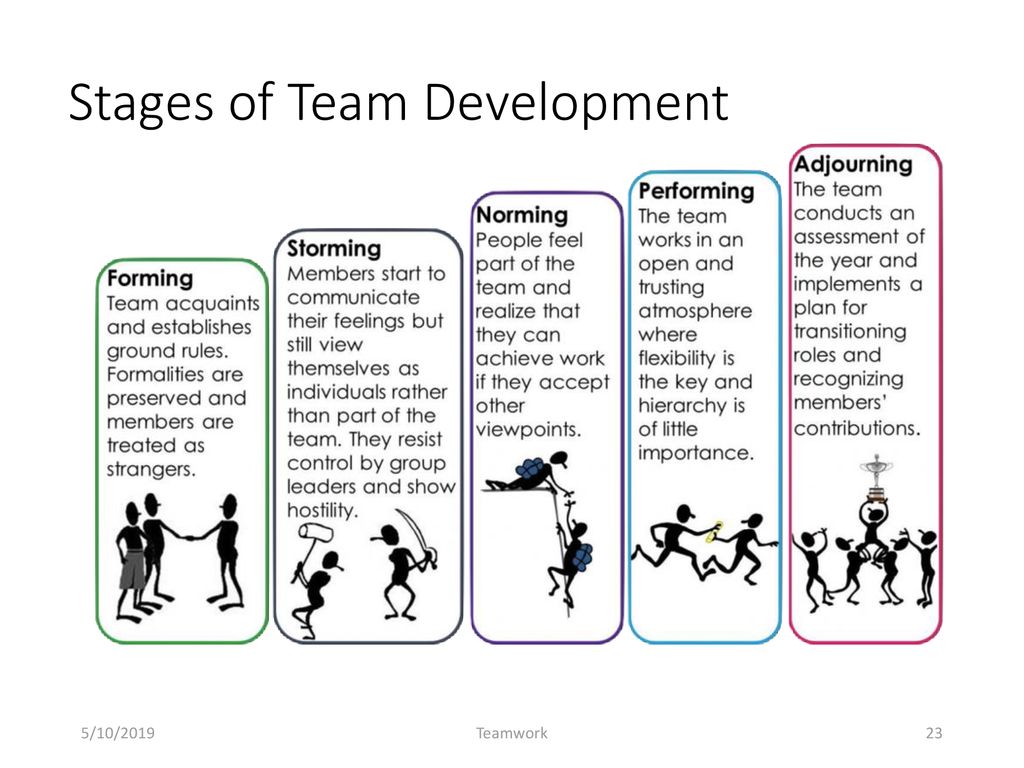
Types of control groups
Control Group: Definition, Examples and Types
Design of Experiments > Control Group
What is a Control Group?
Red pill or blue pill? If Neo in The Matrix takes the blue pill (the placebo), nothing happens. Image: W.Carter|Wikimedia Commons
The control group (sometimes called a comparison group) is used in an experiment as a way to ensure that your experiment actually works. It’s a way to make sure that the treatment you are giving is causing the experimental results, and not something outside the experiment.
An experiment is split into two groups: the experimental group and the control group. The experimental group is given the experimental treatment and the control group is given either a standard treatment or nothing. For example, let’s say you wanted to know if Gatorade increased athletic performance. Your experimental group would be given the Gatorade and your control group would be given regular water.
The conditions must be exactly the same for all members in the experiment. The only difference between members must be the item or thing you are conducting the experiment to look at. Let’s say you wanted to know if a new fertilizer makes plants grow taller. You must ensure that the lighting, water supply, size of container and other important factors are held constant for every member in every group. The only thing that differs in this case is the type of fertilizer given to the plants.
Types of Control Groups in Medical Experiments
Control groups can be subdivided into the following types (see: FDA):
- Placebo concurrent control: one group is given the treatment, the other a placebo (“sugar pill”).
- Dose-comparison concurrent control: two different doses are administered, a different one to each group.
- No treatment concurrent control: one group is given the treatment, the other group is given nothing.
- Active treatment concurrent control: one group is given the treatment, the other group is given an existing therapy that is known to be effective.
- Historical control: only one physical group exists experimentally (the experimental group). the control group is compiled from historical data.
Which type of control group you use depends largely on what type of patients you are administering a treatment too. In many cases, it would be unethical to withhold treatment from a control group or provide a placebo.
Next: The Placebo Effect.
References
Beyer, W. H. CRC Standard Mathematical Tables, 31st ed. Boca Raton, FL: CRC Press, pp. 536 and 571, 2002.
Agresti A. (1990) Categorical Data Analysis. John Wiley and Sons, New York.
Dodge, Y. (2008). The Concise Encyclopedia of Statistics. Springer.
Gonick, L. (1993). The Cartoon Guide to Statistics. HarperPerennial.
CITE THIS AS:
Stephanie Glen. "Control Group: Definition, Examples and Types" From StatisticsHowTo.com: Elementary Statistics for the rest of us! https://www. statisticshowto.com/control-group/
statisticshowto.com/control-group/
---------------------------------------------------------------------------
Need help with a homework or test question? With Chegg Study, you can get step-by-step solutions to your questions from an expert in the field. Your first 30 minutes with a Chegg tutor is free!
Comments? Need to post a correction? Please Contact Us.
Control Groups and Treatment Groups
Published on July 3, 2020 by Lauren Thomas. Revised on November 11, 2022.
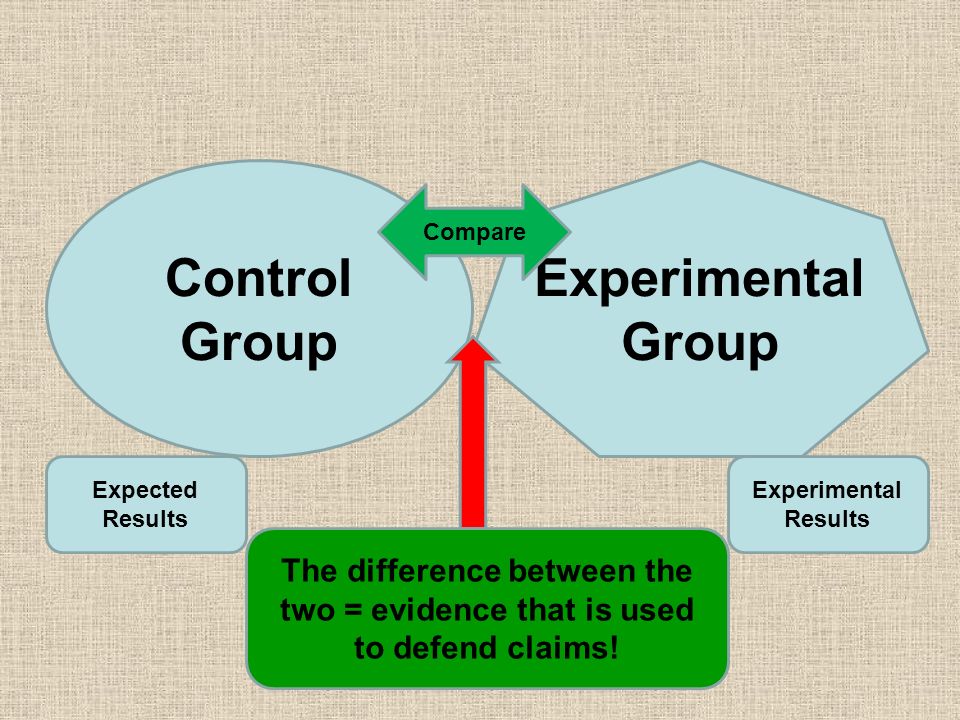
In a scientific study, a control group is used to establish a cause-and-effect relationship by isolating the effect of an independent variable.
Researchers change the independent variable in the treatment group and keep it constant in the control group. Then they compare the results of these groups.
Then they compare the results of these groups.
Using a control group means that any change in the dependent variable can be attributed to the independent variable.
Table of contents
- Control groups in experiments
- Control groups in non-experimental research
- Importance of control groups
- Frequently asked questions about control groups
Control groups in experiments
Control groups are essential to experimental design. When researchers are interested in the impact of a new treatment, they randomly divide their study participants into at least two groups:
- The treatment group (also called the experimental group) receives the treatment whose effect the researcher is interested in.
- The control group receives either no treatment, a standard treatment whose effect is already known, or a placebo (a fake treatment).
The treatment is any independent variable manipulated by the experimenters, and its exact form depends on the type of research being performed. In a medical trial, it might be a new drug or therapy. In public policy studies, it could be a new social policy that some receive and not others.
In a medical trial, it might be a new drug or therapy. In public policy studies, it could be a new social policy that some receive and not others.
In a well-designed experiment, all variables apart from the treatment should be kept constant between the two groups. This means researchers can correctly measure the entire effect of the treatment without interference from confounding variables.
Example of a control groupYou are interested in whether college students perform better in school if they are paid for their performance. To test this, you divide several students into control and treatment groups.- You pay the students in the treatment group for achieving high grades.
- Students in the control group do not receive any money.
By comparing the average change in their grades over the year, you can find out whether monetary incentives improve school performance.
Studies can also include more than one treatment or control group. Researchers might want to examine the impact of multiple treatments at once, or compare a new treatment to several alternatives currently available.
- The treatment group gets the new pill.
- Control group 1 gets an identical-looking sugar pill (a placebo)
- Control group 2 gets a pill already approved to treat high blood pressure
Since the only variable that differs between the three groups is the type of pill, any differences in average blood pressure between the three groups can be credited to the type of pill they received.
- The difference between the treatment group and control group 1 demonstrates the effectiveness of the pill as compared to no treatment.
- The difference between the treatment group and control group 2 shows whether the new pill improves on treatments already available on the market.
Control groups in non-experimental research
Although control groups are more common in experimental research, they can be used in other types of research too. Researchers generally rely on non-experimental control groups in two cases: quasi-experimental or matching design.
Researchers generally rely on non-experimental control groups in two cases: quasi-experimental or matching design.
Control groups in quasi-experimental design
While true experiments rely on random assignment to the treatment or control groups, quasi-experimental design uses some criterion other than randomization to assign people.
Often, these assignments are not controlled by researchers, but are pre-existing groups that have received different treatments. For example, researchers could study the effects of a new teaching method that was applied in some classes in a school but not others, or study the impact of a new policy that is implemented in one state but not in the neighboring state.
In these cases, the classes that did not use the new teaching method, or the state that did not implement the new policy, is the control group.
Control groups in matching design
In correlational research, matching represents a potential alternate option when you cannot use either true or quasi-experimental designs.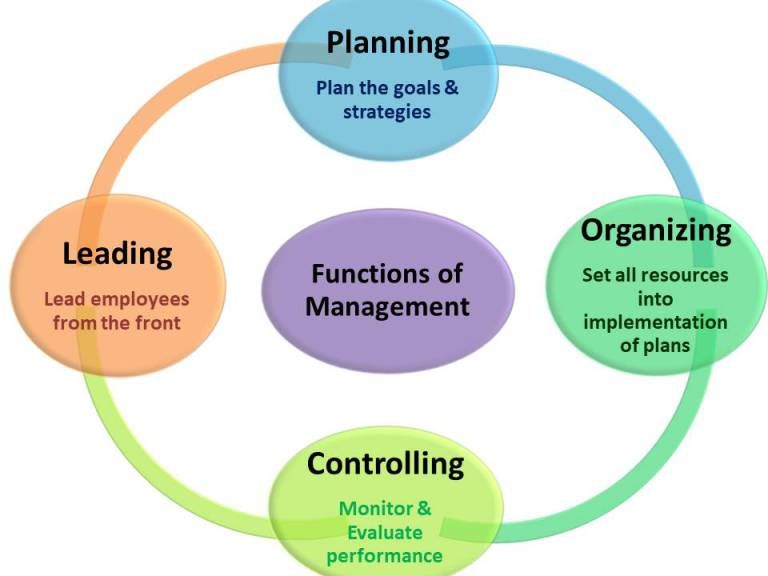
In matching designs, the researcher matches individuals who received the “treatment”, or independent variable under study, to others who did not–the control group.
Each member of the treatment group thus has a counterpart in the control group identical in every way possible outside of the treatment. This ensures that the treatment is the only source of potential differences in outcomes between the two groups.
Example of a matched control groupYou are interested in whether smoking electronic cigarettes can cause lung cancer. Here, the “treatment” is whether or not someone has smoked e-cigarettes. You cannot simply compare the cancer rates of those who smoked e-cigarettes against those who have not–the two groups most likely differ in ways that might affect their rates of cancer.Instead, you can create a control group by matching individuals who do not smoke with those who do (the treatment group) on age, gender, diet, level of exercise, and so on, ensuring that the only difference between the two groups–and thus the only variable that could cause differences in their rates of lung cancer–is their use of e-cigarettes.
Importance of control groups
Control groups help ensure the internal validity of your research. You might see a difference over time in your dependent variable in your treatment group. However, without a control group, it is difficult to know whether the change has arisen from the treatment. It is possible that the change is due to some other variables.
If you use a control group that is identical in every other way to the treatment group, you know that the treatment–the only difference between the two groups–must be what has caused the change.
For example, people often recover from illnesses or injuries over time regardless of whether they’ve received effective treatment or not. Thus, without a control group, it’s difficult to determine whether improvements in medical conditions come from a treatment or just the natural progression of time.
Risks from invalid control groups
If your control group differs from the treatment group in ways that you haven’t accounted for, your results may reflect the interference of confounding variables instead of your independent variable.
Since those who come from a family of smokers are more likely to be exposed to secondhand smoke, a known cause of cancer, higher rates may occur among individuals in your treatment group, but you can’t know for sure if this difference is due to the use of e-cigarettes.
Minimizing this risk
A few methods can aid you in minimizing the risk from invalid control groups.
- Ensure that all potential confounding variables are accounted for, preferably through an experimental design if possible, since it is difficult to control for all the possible confounders outside of an experimental environment.
- Use double-blinding. This will prevent the members of each group from modifying their behavior based on whether they were placed in the treatment or control group, which could then lead to biased outcomes.
- Randomly assign your subjects into control and treatment groups. This method will allow you to not only minimize the differences between the two groups on confounding variables that you can directly observe, but also those you cannot.
Frequently asked questions about control groups
- Do experiments always need a control group?
-
A true experiment (a.k.a. a controlled experiment) always includes at least one control group that doesn’t receive the experimental treatment.
However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group’s outcomes before and after a treatment (instead of comparing outcomes between different groups).
For strong internal validity, it’s usually best to include a control group if possible. Without a control group, it’s harder to be certain that the outcome was caused by the experimental treatment and not by other variables.
- What is a confounding variable?
-
A confounding variable, also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design, it’s important to identify potential confounding variables and plan how you will reduce their impact.

- How do I prevent confounding variables from interfering with my research?
-
There are several methods you can use to decrease the impact of confounding variables on your research: restriction, matching, statistical control and randomization.
In restriction, you restrict your sample by only including certain subjects that have the same values of potential confounding variables.
In matching, you match each of the subjects in your treatment group with a counterpart in the comparison group. The matched subjects have the same values on any potential confounding variables, and only differ in the independent variable.
In statistical control, you include potential confounders as variables in your regression.
In randomization, you randomly assign the treatment (or independent variable) in your study to a sufficiently large number of subjects, which allows you to control for all potential confounding variables.
- What is experimental design?
-
Experimental design means planning a set of procedures to investigate a relationship between variables. To design a controlled experiment, you need:
- A testable hypothesis
- At least one independent variable that can be precisely manipulated
- At least one dependent variable that can be precisely measured
When designing the experiment, you decide:
- How you will manipulate the variable(s)
- How you will control for any potential confounding variables
- How many subjects or samples will be included in the study
- How subjects will be assigned to treatment levels
Experimental design is essential to the internal and external validity of your experiment.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Thomas, L. (2022, November 11). Control Groups and Treatment Groups | Uses & Examples. Scribbr. Retrieved December 1, 2022, from https://www.scribbr.com/methodology/control-group/
Cite this article
Is this article helpful?
You have already voted. Thanks :-) Your vote is saved :-) Processing your vote...
Lauren has a bachelor's degree in Economics and Political Science and is currently finishing up a master's in Economics. She is always on the move, having lived in five cities in both the US and France, and is happy to have a job that will follow her wherever she goes.
She is always on the move, having lived in five cities in both the US and France, and is happy to have a job that will follow her wherever she goes.
Types and design of clinical trials
Clinical trial of a drug - a systematic study of a drug through its use in humans to assess its safety and / or efficacy, as well as to identify and / or confirm its clinical, pharmacological, pharmacodynamic properties, assessment of absorption, distribution , metabolism, excretion and / or interaction with other drugs.
Such research is conducted in accordance with the founding ethical principles of the Declaration of Helsinki, GCP (Good Clinical Practice) and applicable regulatory requirements.
Risk-benefit assessment and review and approval of the study protocol and other documentation related to the conduct of clinical trials are the responsibility of the Institutional Review Board/Independent Ethics Committee (IRB/IEC). Once approved by the IRB/IEC, the clinical trial can proceed.
There are several types of clinical trials:
1. Pilot study
2. Randomized clinical trial
3. Controlled and uncontrolled
4. Parallel and crossover studies
5. Open and blind studies
6. Prospective
7. Single center and multicenter
8. Cohort
9. Case-control study
All clinical trials are classified according to certain characteristics: goals, duration of time, presence of interference in the usual tactics of patient management, etc. patients;
- screening studies - the search for the best ways to detect certain diseases or conditions;
- diagnostic studies - the search for ways to diagnose a particular disease or condition;
- therapeutic studies are conducted to study the efficacy and safety of experimental drugs, new combinations of drugs, or new techniques in surgery or radiotherapy;
- quality of life studies are conducted to explore ways to improve the quality of life of patients suffering from chronic diseases;
- Expanded access programs (in exceptional circumstances, involve the use of an experimental drug in patients with serious or life-threatening diseases who cannot be included in a clinical trial because they do not meet the inclusion criteria.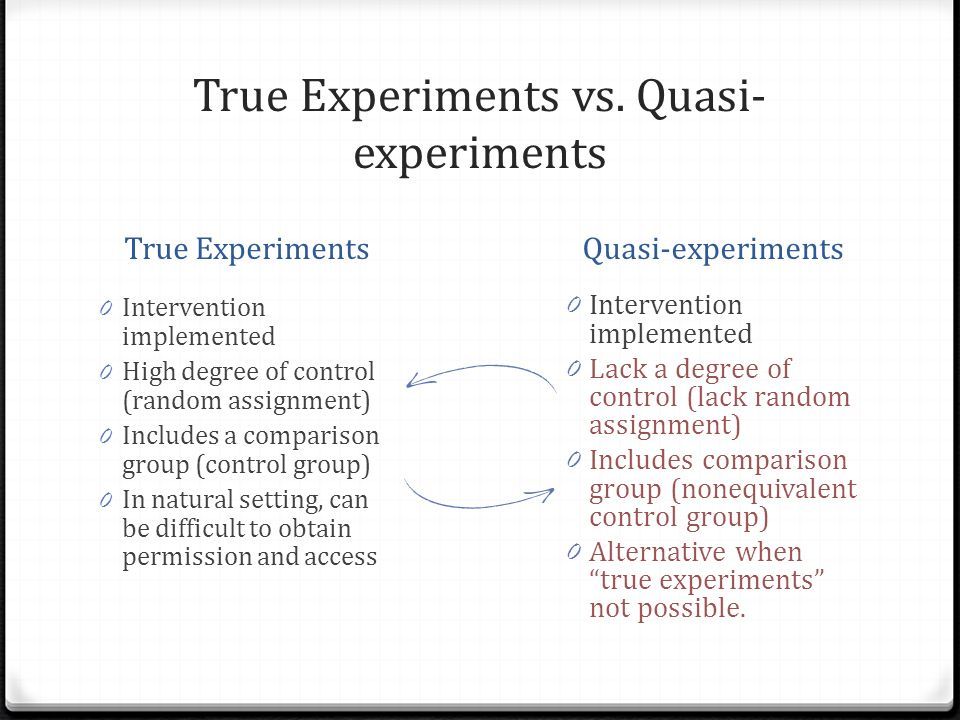
Studies on the presence of interference with usual patient management ( standard procedures for the examination and treatment of the patient):
- observational (observational) study - a clinical study in which the researcher collects data by simply observing events in their natural course, without actively interfering in them;
- non-interventional study ("non-interventional study") - a study in which a medicinal product is prescribed in the usual way in accordance with the conditions set out in the marketing authorization.
- intervention study - a study of new, unregistered drugs, immunobiological agents, medical equipment, or a study in which drugs, immunobiological agents, medical equipment are prescribed or used in a way different from the conditions set forth in the registered instruction (whether it is a new indication, a new dosage of a drug, a new route of administration, a new route of administration, or a new category of patients).
Follow-up studies:
- retrospective (historical) study - a study in which the outcomes of previous clinical trials or studies are studied, that is, the outcomes have already occurred before the study was started. The researcher reviews medical records and selects patients according to certain criteria in order to study the results of treatment.
The researcher reviews medical records and selects patients according to certain criteria in order to study the results of treatment.
is a prospective study - a study in which patients are recruited according to the criteria set out in the study protocol.
Patients receive an investigational drug and are followed up for some time. The formation of groups receiving or not receiving the investigational medicinal product occurs before the results are recorded. Most clinical studies are prospective.
Studies by duration:
- cross-sectional study - examines the impact of risk factors on a population and / or the prevalence of a disease (condition) in it at a certain point in time.
- continuous (longitudinal) - collecting data several times over a long period.
- longitudinal study - a long-term clinical study in which long-term periodic observation of the same individuals is carried out.
Site studies:
- international study - a study conducted in several countries;
- multicenter study - a study conducted in accordance with a single protocol in several research centers;
- meta-analysis - data from different studies on the same topic are summarized.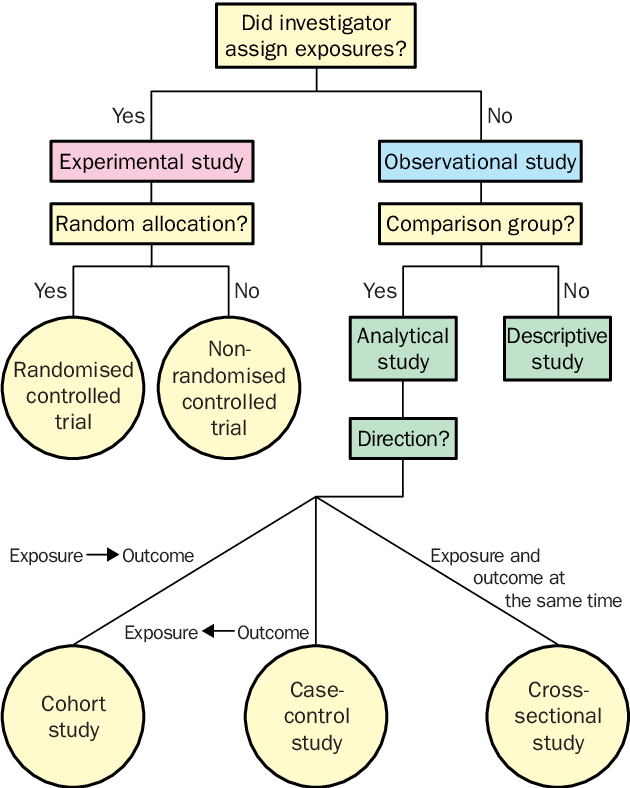
Studies on the degree of randomness of the experiment:
- randomized - when, after signing the consent to the experiment, the participants (scientists and subjects) draw lots - who should conduct which type or part of the experiment.
- not randomized. At the moment, they are not taken seriously in the scientific world. Participants may consciously or unconsciously collude and sabotage the results.
Study on the awareness of participants about the course of the study process:
Blind or masked study - there is no information on which group - experimental or control group.
Masked studies are used to eliminate bias in clinical studies.
Research on the effectiveness of the results obtained:
- direct - definitely leads to an improvement in the patient's life. Direct criteria for effectiveness include recovery, reduction in mortality and complications, reduction in hospital stay, improvement in quality of life;
- indirect (surrogate) - lead to the normalization of some medical indicator (for example, blood pressure), which, theoretically, should improve the patient's life.
All studies are divided into three classes.
Class I studies include the "Gold Standard" - randomized controlled (prospective) trials with double or triple "blind" control. The materials of these trials and the meta-analysis based on them should be used in medical practice as a source of the most reliable information.
Class II includes well-designed open experimental studies, observational prospective and retrospective, which, with a certain degree of criticality, the results of these tests can be applied in practice.
Class III - studies in which significant errors were made, description of cases and series of cases. They, as well as individual medical experience, the opinion of experts or "authorities" are considered as having no sufficient scientific basis.
Clinical trials are also classified according to their design. They can be classified as follows:
* Depending on the method used to allocate participants to treatment and control groups (non-randomized and randomized controlled trials).
* Depending on the knowledge of participants or investigators (or both) as to which group the participants are assigned to (single-blind or double-blind studies).
* Depending on the perceived degree of difference between treatment and control groups (tests to confirm greater or lesser effectiveness).
In non-randomized controlled clinical trials, the investigator assigns participants to treatment groups and control groups. In these trials, the control groups may be concurrent or historical. If historical controls are used, all patients in the trial receive investigational medicinal product; the results are compared with the patient's previous condition (for example, in a patient with a chronic disease) or with a control group from a previous study.
In randomized controlled trials, trial participants are randomly assigned to either treatment or control groups. The process of randomly assigning trial participants to treatment or control groups is called "randomization". Different methods are used for randomization (closed envelopes, computer generated sequence, random numbers). Randomization requires two components: the creation of a random sequence and the application of a random sequence, preferably in such a way that the participants do not know this sequence. Randomization eliminates potential systematic errors.
Different methods are used for randomization (closed envelopes, computer generated sequence, random numbers). Randomization requires two components: the creation of a random sequence and the application of a random sequence, preferably in such a way that the participants do not know this sequence. Randomization eliminates potential systematic errors.
Comparative Trial Designs
There are several different types of comparative trials:
* Higher performance - to confirm that an investigational drug is better than a control.
* Equivalences - to confirm that the endpoint score is no different (neither better nor worse) than the control.
* Not less effective - to confirm that the investigational drug is not worse than the control.
* Dose-effect tests to determine rates for different doses, including starting dose and maximum dose. are a necessary step in the development of any new drug, or expansion of indications for the use of a drug already known to doctors.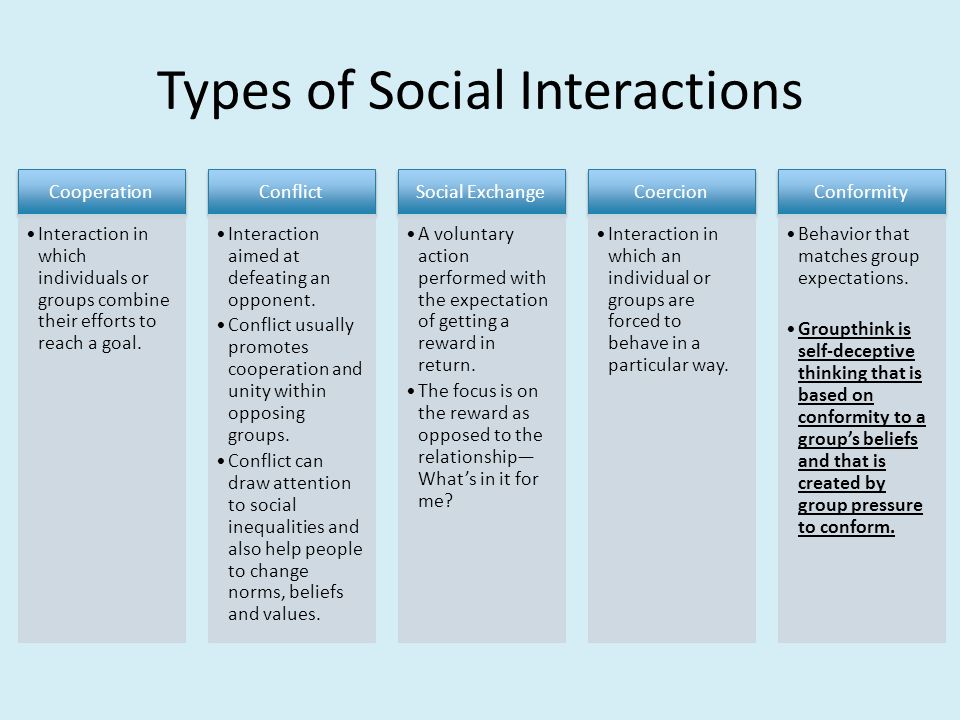 At the initial stages of drug development, chemical, physical, biological, microbiological, pharmacological, toxicological and other studies are carried out on tissues (in vitro) or on laboratory animals. These are the so-called pre-clinical studies , the purpose of which is to obtain, by scientific methods, evaluations and evidence of the effectiveness and safety of medicines. However, these studies cannot provide reliable information about how the studied drugs will act in humans, since the body of laboratory animals differs from the human body both in terms of pharmacokinetic characteristics and in the response of organs and systems to drugs. Therefore, it is necessary to conduct clinical trials of drugs in humans.
At the initial stages of drug development, chemical, physical, biological, microbiological, pharmacological, toxicological and other studies are carried out on tissues (in vitro) or on laboratory animals. These are the so-called pre-clinical studies , the purpose of which is to obtain, by scientific methods, evaluations and evidence of the effectiveness and safety of medicines. However, these studies cannot provide reliable information about how the studied drugs will act in humans, since the body of laboratory animals differs from the human body both in terms of pharmacokinetic characteristics and in the response of organs and systems to drugs. Therefore, it is necessary to conduct clinical trials of drugs in humans.
So, what is the clinical study (trial) of the drug ? This is a systematic study of a medicinal product through its use in a person (patient or healthy volunteer) in order to assess its safety and / or efficacy, as well as to identify and / or confirm its clinical, pharmacological, pharmacodynamic properties, assessment of absorption, distribution, metabolism, excretion and /or interactions with other drugs.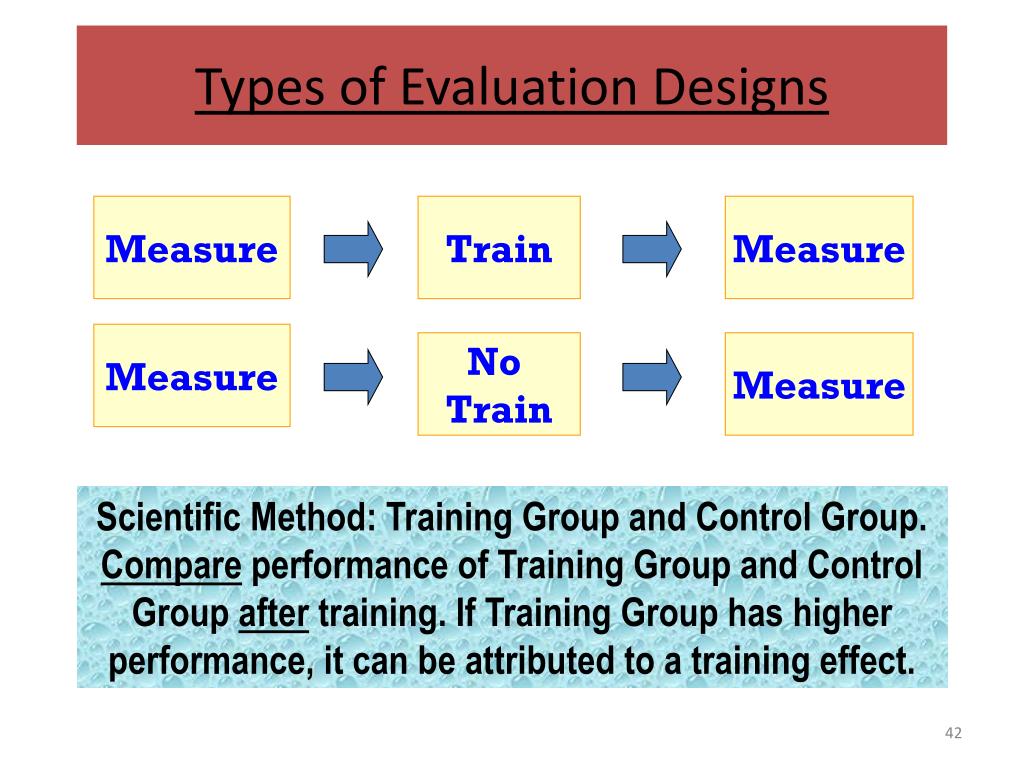 The decision to initiate a clinical trial is made by the Sponsor/Customer who is responsible for organizing, supervising and/or funding the study. Responsibility for the practical conduct of the study rests with the Investigator (individual or group of individuals). As a rule, the sponsors are pharmaceutical companies - drug developers, however, the researcher can also act as a sponsor if the study was initiated on his initiative and he bears full responsibility for its conduct.
The decision to initiate a clinical trial is made by the Sponsor/Customer who is responsible for organizing, supervising and/or funding the study. Responsibility for the practical conduct of the study rests with the Investigator (individual or group of individuals). As a rule, the sponsors are pharmaceutical companies - drug developers, however, the researcher can also act as a sponsor if the study was initiated on his initiative and he bears full responsibility for its conduct.
Clinical research must be conducted in accordance with the founding ethical principles of the Declaration of Helsinki, GCP Rules ( Good Clinical Practice , Good Clinical Practice) and applicable regulatory requirements. Prior to the start of a clinical trial, an assessment should be made of the relationship between the foreseeable risk and the expected benefit for the subject and society. At the forefront is the principle of priority of the rights, safety and health of the subject over the interests of science and society.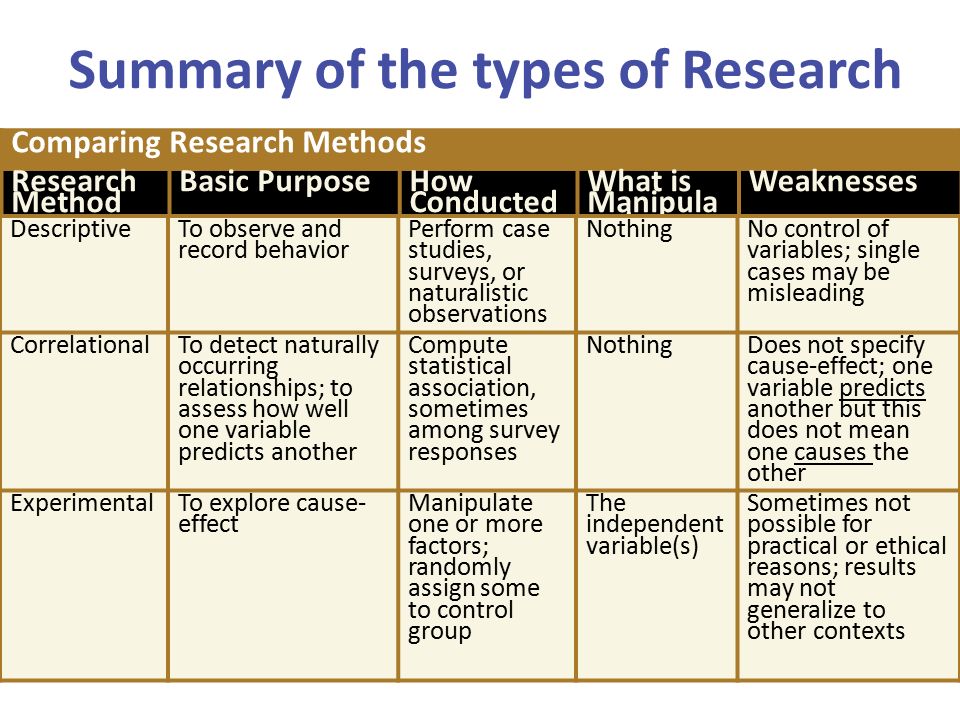 A subject may be included in the study only on the basis of voluntary informed consent (IC), obtained after a detailed acquaintance with the study materials.
A subject may be included in the study only on the basis of voluntary informed consent (IC), obtained after a detailed acquaintance with the study materials.
The clinical investigation must be scientifically justified, detailed and clearly described in the study protocol . Evaluation of the balance of risks and benefits, as well as review and approval of the protocol of the study and other documentation related to the conduct of clinical trials, are the responsibility of the Expert Council of the Organization / Independent Ethics Committee (ESO / NEC). Once approved by the IRB/IEC, the clinical trial can proceed.
Pilot study is intended to provide preliminary data important for planning further stages of the study (determining the possibility of conducting a study in a larger number of subjects, the sample size in a future study, the required study power, etc.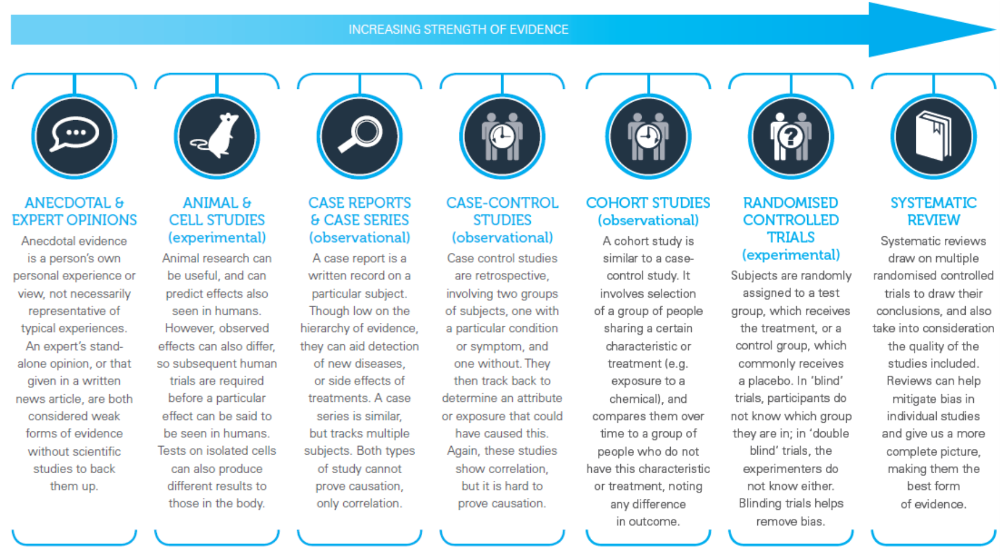 ).
).
Randomized clinical trial in which patients are assigned to treatment groups at random (randomization procedure) and have the same chance of receiving an investigational or control drug (comparator or placebo). In a non-randomized study, there is no randomization procedure.
A controlled (sometimes used synonymous with "comparative") clinical trial in which an investigational drug whose efficacy and safety has not yet been fully established is compared with a drug whose efficacy and safety is well known (comparator drug). This may be placebo, standard therapy, or no treatment at all. AT uncontrolled (non-comparative) study control / comparison group (group of subjects taking the comparator drug) is not used. In a broader sense, controlled research refers to any research in which potential sources of bias are controlled (if possible, minimized or eliminated) (i.e., it is carried out in strict accordance with the protocol, monitored, etc. ).
).
When conducting parallel studies Subjects in different groups receive either the study drug alone or the comparator/placebo alone. In crossover studies, each patient receives both comparator drugs, usually in random order.
The study can be open when all participants in the study know which drug the patient is receiving, and blind ( masked ) when one (single-blind study) or several parties participating in the study (double-blind, triple-blind or full blind study) are kept in the dark about the allocation of patients to treatment groups.
The prospective study divides participants into groups who will or will not receive study drug before outcomes occur. In contrast, the retrospective (historical) study examines the outcomes of previous clinical trials, i.e. outcomes occur before the study is started.
Depending on the number of research centers where the study is carried out in accordance with a single protocol, studies are single center and multi center .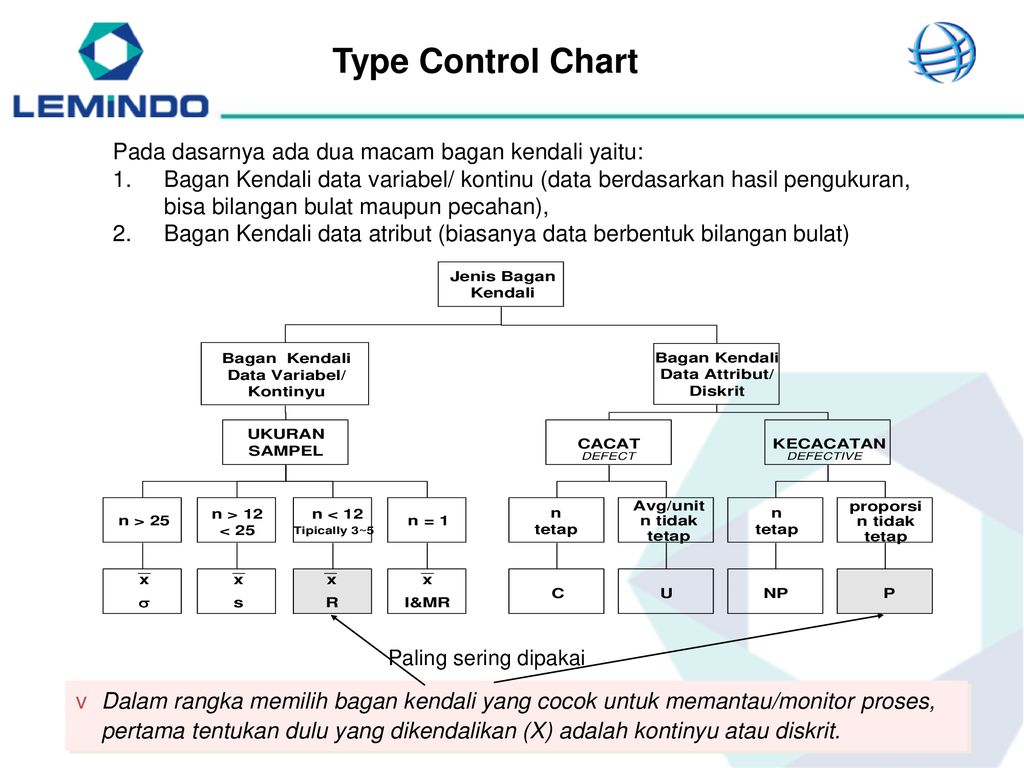 If the study is conducted in several countries, it is called international.
If the study is conducted in several countries, it is called international.
The parallel study compares two or more groups of subjects, one or more of whom receive an investigational drug and one group is a control. Some parallel studies compare different treatments without including a control group. (This design is called independent group design.)
The cohort study is an observational study in which a selected group of people (cohort) is followed over time. The outcomes of subjects in different subgroups of this cohort, those who were or were not treated (or were treated to varying degrees) with the study drug are compared. In the prospective cohort study, cohorts are formed in the present and followed up in the future. In retrospective (or historical ) cohort study The cohort is selected from archival records and traced from then to the present.
The case-control study (synonym: similar case study ) compares people with a particular disease or outcome (“case”) with people in the same population who do not have that disease or who do not experience that outcome (“ control") to identify associations between outcome and prior exposure to certain risk factors. In Study case series follow several individuals, usually receiving the same treatment, without the use of a control group. The case report (synonyms: case report, case history, single case report ) is a study of treatment and outcome in one person.
The current preference for clinical drug trial design is to provide the most reliable data, such as prospective, controlled, comparative, randomized, and preferably double-blind trials.
Recently, the role of clinical drug trials has increased due to the introduction of the principles of evidence-based medicine into practical healthcare.