Ssri pregnancy category
ACOG Guidelines on Psychiatric Medication Use During Pregnancy and Lactation
CARRIE ARMSTRONG
Am Fam Physician. 2008;78(6):772-778
Guideline source: American College of Obstetricians and Gynecologists
Literature search described? Yes
Evidence rating system used? Yes
Published source: Obstetrics & Gynecology, November 2007
Available at: http://www. greenjournal.org/content/vol110/issue5
An estimated 500,000 pregnancies in the United States each year involve women who have or who will develop psychiatric illness during the pregnancy. The use of psychotropic medications in these women is a concern because of the risks of adverse perinatal and postnatal outcomes. However, advising these women to discontinue medication presents new risks associated with untreated or inadequately treated mental illness, such as poor adherence to prenatal care, inadequate nutrition, and increased alcohol and tobacco use.
Ideally, decisions about psychiatric medication use during and after pregnancy should be made before conception. The use of a single medication at a higher dosage is preferred over multiple medications, and those with fewer metabolites, higher protein binding, and fewer interactions with other medications are also preferred. All psychotropic medications cross the placenta, are present in amniotic fluid, and can enter breast milk. The U.S. Food and Drug Administration has categorized medications according to risk during pregnancy (Table 1).
The U.S. Food and Drug Administration has categorized medications according to risk during pregnancy (Table 1).
| Drug | FDA pregnancy category* | AAP rating | Lactation risk category† | |
|---|---|---|---|---|
| Anxiolytics and hypnotics | ||||
| Benzodiazepines | ||||
| Alprazolam (Xanax) | D | Unknown, of concern | L3 | |
| Chlordiazepoxide (Librium) | D | NA | L3 | |
| Clonazepam (Klonopin) | D | NA | L3 | |
| Clorazepate (Tranxene) | D | NA | L3 | |
| Diazepam (Valium) | D | Unknown, of concern | L3; L4 if used chronically | |
| Estazolam (Prosom)‡ | X | NA | L3 | |
| Flurazepam (Dalmane) | X | NA | L3 | |
| Lorazepam (Ativan) | D | Unknown, of concern | L3 | |
| Oxazepam (Serax)‡ | D | NA | L3 | |
| Quazepam (Doral) | X | Unknown, of concern | L2 | |
| Temazepam (Restoril) | X | Unknown, of concern | L3 | |
| Triazolam (Halcion) | X | NA | L3 | |
| Nonbenzodiazepine anxiolytics and hypnotics | ||||
| Buspirone (Buspar) | B | NA | L3 | |
| Chloral hydrate | C | Compatible | L3 | |
| Eszopiclone (Lunesta) | C | NA | NA | |
| Zaleplon (Sonata) | C | Unknown, of concern | L2 | |
| Zolpidem (Ambien) | B | NA | L3 | |
| Antiepileptics and mood stabilizers | ||||
| Carbamazepine (Tegretol) | D | Compatible | L2 | |
| Lamotrigine (Lamictal) | C | Unknown | L3 | |
| Lithium | D | Contraindicated | L4 | |
| Valproic acid (Depakene) | D | Compatible | L2 | |
| Antidepressants | ||||
| Tricyclics and heterocyclics | ||||
| Amitriptyline | C | Unknown, of concern | L2 | |
| Amoxapine (Asendin)‡ | C | Unknown, of concern | L2 | |
| Clomipramine (Anafranil) | C | Unknown, of concern | L2 | |
| Desipramine (Norpramin) | C | Unknown, of concern | L2 | |
| Doxepin (Sinequan)‡ | C | Unknown, of concern | L5 | |
| Imipramine (Tofranil) | C | Unknown, of concern | L2 | |
| Maprotiline (Ludiomil)‡ | B | NA | L3 | |
| Nortriptyline (Pamelor) | C | Unknown, of concern | L2 | |
| Protriptyline (Vivactil) | C | NA | NA | |
| Selective serotonin reuptake inhibitors | ||||
| Citalopram (Celexa) | C | NA | L3 | |
| Escitalopram (Lexapro) | C | NA | L3 in older infants | |
| Fluoxetine (Prozac) | C | Unknown, of concern | L2 in older infants; L3 in neonates | |
| Fluvoxamine (Luvox)‡ | C | Unknown, of concern | L2 | |
| Paroxetine (Paxil) | D | Unknown, of concern | L2 | |
| Sertraline (Zoloft) | C | Unknown, of concern | L2 | |
| Other antidepressants | ||||
| Bupropion (Wellbutrin) | B | Unknown, of concern | L3 | |
| Duloxetine (Cymbalta) | C | NA | NA | |
| Mirtazapine (Remeron) | C | NA | L3 | |
| Nefazodone (Serzone)‡ | C | NA | L4 | |
| Trazodone (Desyrel)‡ | C | Unknown, of concern | L2 | |
| Venlafaxine (Effexor) | C | NA | L3 | |
| Antipsychotics | ||||
| Aripiprazole (Abilify) | C | NA | L3 | |
| Chlorpromazine (Thorazine)‡ | C | Unknown, of concern | L3 | |
| Clozapine (Clozaril) | B | Unknown, of concern | L3 | |
| Fluphenazine (Prolixin)‡ | C | NA | L3 | |
| Haloperidol (Haldol) | C | Unknown, of concern | L2 | |
| Loxapine (Loxitane) | C | NA | L4 | |
| Olanzapine (Zyprexa) | C | NA | L2 | |
| Perphenazine (Trilafon)‡ | C | Unknown, of concern | NA | |
| Pimozide (Orap) | C | NA | L4 | |
| Quetiapine (Seroquel) | C | Unknown, of concern | L4 | |
| Risperidone (Risperdal) | C | NA | L3 | |
| Thioridazine (Mellaril)‡ | C | NA | L4 | |
| Thiothixene (Navane) | C | NA | L4 | |
| Trifluoperazine (Stelazine)‡ | C | Unknown, of concern | NA | |
| Ziprasidone (Geodon) | C | Unknown, of concern | L4 | |
Major Depression
Ten to 16 percent of pregnant women meet diagnostic criteria for depression, and up to 70 percent of pregnant women have symptoms of depression. Studies have shown a relapse rate of 68 percent in women who discontinue antidepressant therapy during pregnancy. Untreated maternal depression is associated with increased rates of adverse outcomes (e.g., premature birth, low birth weight, fetal growth restriction, postnatal complications), especially when depression occurs in the late second to early third trimesters.
Studies have shown a relapse rate of 68 percent in women who discontinue antidepressant therapy during pregnancy. Untreated maternal depression is associated with increased rates of adverse outcomes (e.g., premature birth, low birth weight, fetal growth restriction, postnatal complications), especially when depression occurs in the late second to early third trimesters.
There is limited evidence of teratogenic effects from the use of antidepressants in pregnancy and adverse effects from exposure during breastfeeding. Exposure to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy has been associated with transient neonatal complications; however, the potential risks associated with SSRI use must be weighed against the risk of relapse if treatment is discontinued. Treatment with SSRIs or selective norepinephrine reuptake inhibitors during pregnancy should be individualized.
Paroxetine (Paxil) should be avoided by pregnant women and women who plan to become pregnant, and fetal echocardiography should be considered for women exposed to paroxetine during early pregnancy. Because abrupt discontinuation of this drug is associated with withdrawal symptoms and a high rate of relapse, prescribing information about discontinuation of therapy should be followed carefully.
Because abrupt discontinuation of this drug is associated with withdrawal symptoms and a high rate of relapse, prescribing information about discontinuation of therapy should be followed carefully.
The combination of breastfeeding and SSRI use has not been studied extensively; however, medication exposure from breastfeeding is less than the exposure that occurs transplacentally. Isolated adverse effects have been reported, the most notable of which was an infant who had transient apnea after being exposed to citalopram (Celexa). Generally, no long-term neurobehavioral studies have been done in infants exposed to SSRIs through breast milk. Most tricyclic antidepressants seem to be safe during lactation except for doxepin (Sinequan), which reportedly led to an incident of infant respiratory depression.
Bipolar Disorder
Rates of postpartum relapse in women with bipolar disorder range from 32 to 67 percent. Perinatal episodes of the disorder tend to be depressive and are more likely to recur in subsequent pregnancies. The risk of postpartum psychosis is increased by as much as 46 percent in women with this disorder.
The risk of postpartum psychosis is increased by as much as 46 percent in women with this disorder.
LITHIUM THERAPY
The use of lithium during pregnancy has been associated with congenital cardiac malformations, fetal and neonatal cardiac arrhythmias, hypoglycemia, premature delivery, and other adverse outcomes. However, neurobehavioral sequelae were not found in a five-year follow-up of 60 school-age children exposed to lithium during gestation. The decision to discontinue lithium therapy during pregnancy because of fetal risks should be weighed against the maternal risks of illness exacerbation.
The physiologic changes of pregnancy may affect the absorption, distribution, metabolism, and elimination of lithium, and close monitoring of lithium levels during pregnancy and the postpartum period is recommended. The following guidelines have been suggested for women with bipolar disorder who are taking lithium and plan to conceive:
Lithium therapy should be gradually tapered before conception in women who have mild, infrequent episodes.
Lithium therapy should be tapered before conception, but gradually restarted after organogenesis in women who have more severe episodes and are at moderate risk of short-term relapse.
Lithium therapy should be continued throughout the pregnancy in women who have severe, frequent episodes, and these patients should be counseled about the reproductive risks associated with therapy.
Fetal echocardiography should be considered in women exposed to lithium in the first trimester.
The use of lithium during breastfeeding has been associated with a number of adverse effects; however, only 10 maternal-infant dyads have been studied. Effects included lethargy, hypotonia, hypothermia, cyanosis, and electrocardiography changes. No long-term studies have examined the neurobehavioral consequences of lithium therapy during breastfeeding.
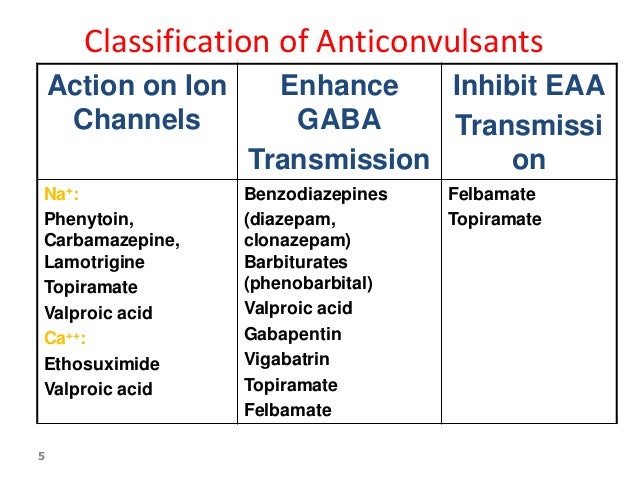
ANTIEPILEPTIC THERAPY FOR BIPOLAR DISORDER
Several antiepileptic drugs are used in the treatment of bipolar disorder, including valproic acid (Depakene), carbamazepine (Tegretol), and lamotrigine (Lamictal). However, data on the fetal effects of these drugs come primarily from studies of women with seizures. It is not clear whether the underlying pathology of epilepsy contributes to the teratogenic effect of these drugs on the fetus.
However, data on the fetal effects of these drugs come primarily from studies of women with seizures. It is not clear whether the underlying pathology of epilepsy contributes to the teratogenic effect of these drugs on the fetus.
Exposure to valproic acid during pregnancy is associated with an increased risk of neural tube defects, craniofacial and cardiovascular anomalies, fetal growth restriction, and cognitive impairment. Carbamazepine exposure during pregnancy is associated with facial dysmorphism and fingernail hypoplasia. It is unclear whether carbamazepine use increases the risk of neural tube defects or developmental delay. Although these drugs are superior to lithium in the treatment of patients with mixed episodes or rapid cycling, they should be avoided during pregnancy.
The use of lamotrigine during pregnancy has not been associated with any major fetal anomalies and is an option for maintenance therapy in women with bipolar disorder.
Valproic acid use during lactation has been studied in 41 maternal-infant dyads; only one infant was adversely affected with thrombocytopenia and anemia. The American Academy of Pediatrics and the World Health Organization consider valproic acid safe in breastfeeding women. Carbamazepine is ruled “probably safe”; rare side effects include transient cholestatic hepatitis and hyperbilirubinemia.
The American Academy of Pediatrics and the World Health Organization consider valproic acid safe in breastfeeding women. Carbamazepine is ruled “probably safe”; rare side effects include transient cholestatic hepatitis and hyperbilirubinemia.
Anxiety Disorders
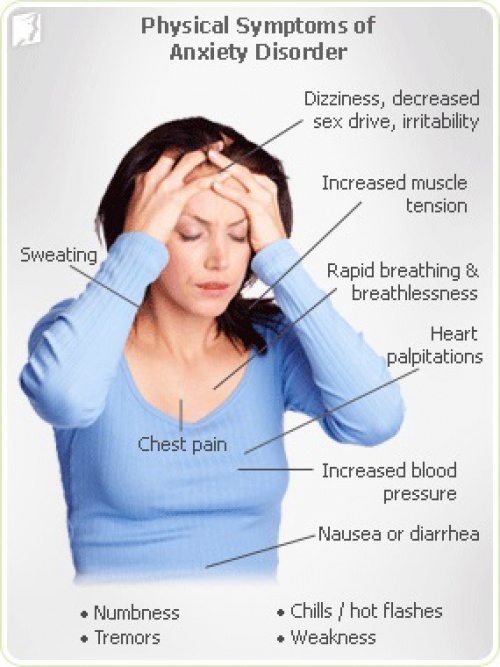
Anxiety disorders are the most common psychiatric disorders, and some (e.g., panic disorder, generalized anxiety disorder, posttraumatic stress disorder, agoraphobia) are twice as likely to be diagnosed in women than in men. Anxiety and stress during pregnancy are associated with spontaneous abortion, preterm delivery, and delivery complications, although a direct causal relationship has not been established.
The use of benzodiazepines in women with anxiety disorders does not carry a significant teratogenic risk. Prenatal exposure to diazepam (Valium) increases the risk of oral cleft, but the absolute risk increases by only 0.01 percent (from six to seven in 10,000 infants). Maternal use of benzodiazepines shortly before delivery is associated with floppy infant syndrome (i. e., hypothermia, lethargy, poor respiratory effort, and feeding difficulties), and withdrawal syndromes may persist for several months after delivery in infants whose mothers took alprazolam (Xanax), chlordiazepoxide (Librium), or diazepam.
e., hypothermia, lethargy, poor respiratory effort, and feeding difficulties), and withdrawal syndromes may persist for several months after delivery in infants whose mothers took alprazolam (Xanax), chlordiazepoxide (Librium), or diazepam.
In general, use of benzodiazepines during breastfeeding affects the infant only if he or she has an impaired ability to metabolize the drug. In this situation, the infant may demonstrate sedation and poor feeding.
Schizophrenia
Adverse outcomes have been reported in women with schizophrenia, including preterm delivery, low birth weight, placental abnormalities, increased rates of congenital malformation, and a higher incidence of postnatal death. If left untreated during pregnancy, schizophrenia can have devastating effects on the mother and child.
Atypical antipsychotics have replaced typical agents as first-line therapy for psychotic disorders because these drugs are better tolerated and may be more effective in managing the negative symptoms of schizophrenia. The reproductive safety data on atypical antipsychotics are limited, but the use of olanzapine (Zyprexa), risperidone (Risperdal), quetiapine (Seroquel), and clozapine (Clozaril) has been associated with increased rates of low birth weight and therapeutic abortion. No long-term studies of children exposed to atypical antipsychotics during gestation have been conducted. Therefore, the routine use of these drugs during pregnancy and lactation is not recommended.
The reproductive safety data on atypical antipsychotics are limited, but the use of olanzapine (Zyprexa), risperidone (Risperdal), quetiapine (Seroquel), and clozapine (Clozaril) has been associated with increased rates of low birth weight and therapeutic abortion. No long-term studies of children exposed to atypical antipsychotics during gestation have been conducted. Therefore, the routine use of these drugs during pregnancy and lactation is not recommended.
Typical antipsychotics have a larger reproductive safety profile; no significant teratogenic effect has been documented with chlorpromazine (Thorazine), haloperidol (Haldol), or perphenazine (Trilafon). Doses of typical antipsychotics should be minimized during the peri-partum period to limit the necessity of using additional medications to manage extrapyramidal side effects.
Data on antipsychotic use in breastfeeding women are limited. A small study of chlorpromazine use during breastfeeding showed no developmental deficits in children up to five years of age; however, a study of both chlorpromazine and haloperidol revealed developmental deficits in children 12 to 18 months of age.
Coverage of guidelines from other organizations does not imply endorsement by AFP or the AAFP.
This series is coordinated by Michael J. Arnold, MD, contributing editor.
A collection of Practice Guidelines published in AFP is available at https://www.aafp.org/afp/practguide.
FDA Antidepressant Drug Labels for Pregnant and Postpartum Women - Screening for Depression in Adults
On October 7, 2014, we searched for the current drug label information of brand name antidepressants on the Drugs@FDA website (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/). We also examined drug approval and labeling revision documents for any medical or statistical reviews associated with labeling considerations for pregnant or postpartum women. Discontinued drugs were not evaluated.
| Generic (Brand Name) | FDA Pregnancy Category* | Drug Label: Fetal/Neonate Complications | Drug Label: Nursing Considerations | Other Nursing Considerations82 |
|---|---|---|---|---|
| SSRIs | ||||
| Sertraline (Zoloft) | C | Nonteratogenic effects include complications requiring prolonged hospitalization, respiratory support, and tube feeding upon delivery. Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN | It is not known whether, and in what amount, sertraline or its metabolites are excreted in human milk. Caution should be exercised when administered to nursing women | Studies generally confirm that the transfer of sertraline and its metabolite to the infant is minimal and attaining clinically relevant plasma levels in infants is remote |
| Paroxetine (Pereva, Paxil) | D | Epidemiological studies have shown that infants exposed to paroxetine in the first trimester have an increased risk of congenital malformations, particularly cardiovascular malformations; nonteratogenic effects include complications requiring prolonged hospitalization, respiratory support, and tube feeding upon delivery. Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN | Paroxetine is secreted in human milk and caution should be exercised when administering to nursing women | Studies suggest minimal to no effect on breastfed infants. Most studies show minimal to no plasma levels in breastfed infants |
| Fluvoxamine (Luvox) | C | Increased embryofetal death, increased incidences of fetal eye abnormalities, decreased fetal body weight; nonteratogenic effects include complications requiring prolonged hospitalization, respiratory support, and tube feeding upon delivery. Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN | Fluvoxamine is secreted in human breast milk, potential for serious adverse effects from exposure in the nursing infant should be taken into consideration when the decision to continue or discontinue use is made | Data from studies suggests only minuscule amounts of fluvoxamine are transferred to infants, plasma levels in infants are too low to be detected, and no adverse effects have been noted |
| Fluoxetine (Prozac) | C | Fetal cardiovascular malformations; nonteratogenic effects include complications requiring prolonged hospitalization, respiratory support, and tube feeding upon delivery. Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN | Because Prozac is excreted in human milk, nursing while on Prozac is not recommended. Studies show mixed results in nursing infants; some show no adverse effects and others reporting increased crying, sleep disturbance, vomiting, and watery stools in exposed infants. | Women taking fluoxetine should be advised to continue breastfeeding and observe the infant for side effects. Severe colic, fussiness, and crying have been reported. |
| Escitalopram (Lexapro) | C | Nonteratogenic effects include complications requiring prolonged hospitalization, respiratory support, and tube feeding upon delivery. Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN | Escitalopram is excreted in human breast milk, so caution should be exercised and breastfeeding infants should be observed for adverse reactions when administering to nursing women. Some reports of infants experiencing excessive somnolence, decreased feedings, and weight loss | Recent data concerning use in breastfeeding mothers suggests the relative infant dose is low and plasma levels in breastfed infants are largely undetectable. No adverse events in infants were reported No adverse events in infants were reported |
| Citalopram (Celexa) | C | Nonteratogenic effects include complications requiring prolonged hospitalization, respiratory support, and tube feeding upon delivery. Other clinical findings include respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying; infants exposed to SSRIs in pregnancy may have an increased risk for PPHN and is associated with substantial neonatal morbidity and mortality. Several recent studies suggest a positive statistical association between SSRI use in pregnancy and PPHN-Serotonin syndrome | Citalopram is excreted in human breast milk, caution should be exercised and breastfeeding infants should be observed for adverse reactions when administering to nursing women. Some reports of infants experiencing excessive somnolence, decreased feedings, and weight loss | Reports of excessive somnolence, decreased feeding, and weight loss in breastfed infants. However, majority of studies show no or limited side effects in breastfed infants. Risks of this product are quite low |
| SNRIs | ||||
| Venlafaxine* | C | No teratogenic effects reported; non-teratogenic effects included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia , tremor, jitteriness, irritability, and constant crying | Venlafaxine has been reported to be excreted in milk, potential for serious adverse reactions in nursing infants. A decision should be made to discontinue nursing or to discontinue the drug | Venlafaxine does enter the milk in moderate amounts, however no side-effects have been reported following its lactational exposure |
| Duloxetine (Cymbalta) | C | Non-teratogenic effects included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia , tremor, jitteriness, irritability, and constant crying | The safety of duloxetine in infants is not known, nursing while on Cymbalta is not recommended | Milk levels in one study (6 mothers) are low and the relative infant dose is low. Subsequent study suggests weight-adjusted infant dose of 0.14% of the maternal dose |
| Desvenlafaxine (Pristiq) | C | Neonates exposed to SNRIs or SSRIs late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Non-teratogenic effects included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia , tremor, jitteriness, irritability, and constant crying Non-teratogenic effects included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia , tremor, jitteriness, irritability, and constant crying | Potential for serious adverse reactions in nursing infants from PRISTIQ | Desvenlafaxine does enter the milk in moderate amounts, however no side-effects have been reported following its lactational exposure |
| DRIs | ||||
| Bupropion (Wellbutrin) | C | No increased risk of congenital malformations overall | Bupropion and its metabolites are present in human milk, exercise caution when administering to nursing women | Plasma levels in breastfed infants are undetectable, one case of seizure in 6-month old infant |
| 5-HT2A Receptor Antagonists | ||||
| Nefazodone * | C | Premature birth, infants drowsiness and lethargy, infant failure to thrive, and poor temperature control | It is not known whether Nefazodone or its metabolites are excreted in human milk, caution should be exercised when administered to nursing women | Medication should not be used in breastfeeding mothers with young infants, premature infants, infants subject to apnea, or other weakened infants |
| SRIs | ||||
| Trazodone (Oleptro) | C | Increased fetal resorption, increase in congenital anomalies, may cause fetal harm | Oleptro use in pregnant and nursing women is not recommended | Milk levels are probably too low to be clinically relevant in the breastfed infant, did not report any pediatric concerns in breastfeeding infants |
| TeCAs | ||||
| Miratazapine (Remeron) | C | No evidence of teratogenic effects | Remeron may be excreted into breast milk, caution should be exercised in administering to nursing women | Two studies found no adverse effects among infants of nursing mothers and suggest breastfeeding is safe during Miratazapine therapy |
Note: No Black Box Warnings for Pregnant.
- *
FDA Pregnancy Categories: Category C = Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of drug in pregnant women despite potential risks; Category D = There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
Abbreviations: DRI = dopamine reuptake inhibitors; FDA = U.S. Food and Drug Administration; PPHN = persistent pulmonary hypertension of the newborn; SNRI = serotonin-norepinephrine reuptake inhibitors; SRI = serotonin reuptake inhibitors; SSRI = selective serotonin reuptake inhibitors; TeCA = tricyclic antidepressants.

✚ Teratogenic effect of psychotropic drugs. Neuropsychiatric clinic of Professor Minutko. Article No. 3618
The teratogenic effect and fetotoxic effect of psychotropic drugs is manifested when they are taken by a pregnant woman or during her lactation. Below are the main groups of drugs that show their negative effect on the body of a woman, her fetus (newborn)
Antidepressants
suggest that SSRIs are associated with a slight increase in the risk of cardiovascular malformations, mainly manifested in ventricular and atrial septal defects.
SSRIs cross the placenta and thus have both short-term and chronic teratogenic effects on fetal development during prenatal exposure to this class of drugs. Epidemiological studies document that 10-30% of newborns whose mothers took SSRI had mild neonatal adaptation syndrome (PNAS) at birth. Some authors have also reported persistent pulmonary hypertension of the newborn (PPHN) weakly associated with uterine penetration of antidepressants, but other researchers have not confirmed these data. Similarly, the effect of antidepressants taken by a pregnant woman on the later stages of the development of the nervous system of a newborn has been controversial in terms of the formation of autism. However, symptoms of depression during pregnancy are also associated with an increased risk of fetal growth retardation, preterm birth, and postpartum depression. 9All antipsychotics cross the placenta (Newport et. first trimester of pregnancy. In general, we note that the rate of malformations in the general population is 1%-3%.
Similarly, the effect of antidepressants taken by a pregnant woman on the later stages of the development of the nervous system of a newborn has been controversial in terms of the formation of autism. However, symptoms of depression during pregnancy are also associated with an increased risk of fetal growth retardation, preterm birth, and postpartum depression. 9All antipsychotics cross the placenta (Newport et. first trimester of pregnancy. In general, we note that the rate of malformations in the general population is 1%-3%.
Literature reviews on the effect of first-generation antipsychotics (promethazine, chlorpromazine, prochlorperazine, haloperidol, perphenazine, triofluoperazine, loxapine, thioridazine) on the fetus in terms of teratogenic risk did not show a significant effect on their level of teratogenic risk to the fetus. (Einarson and Boskovic, 2009).
Similarly, second-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole) have also been shown to be teratogenic to the fetus (Einarson and Boskovic, 2009). trimester of pregnancy, both first and second generation antipsychotics are statistically associated with a small but increased risk of major malformations, mainly the atrial-ventricular septum (Reis and Kallen, 2008)
trimester of pregnancy, both first and second generation antipsychotics are statistically associated with a small but increased risk of major malformations, mainly the atrial-ventricular septum (Reis and Kallen, 2008)
Antiepileptic drugs
A systematic review published in 2010 on the teratogenic risk of psychotropic drugs [Galbally et.al.2010] showed that antiepileptic drugs can cause various fetal complications. There is an obvious association between certain drugs in this class, such as valproate, and an increased risk of malformations, especially when sodium valproate is used in doses above 1000 mg. children show signs of cognitive decline. [Galbally et al. et al . 2010) . The literature on lithium carbonate is more limited, but it is clear that this drug can cause Ebstein's anomaly. The teratogenic effect is characteristic of a large number of psychotropic drugs, so their use should be justified and carefully monitored.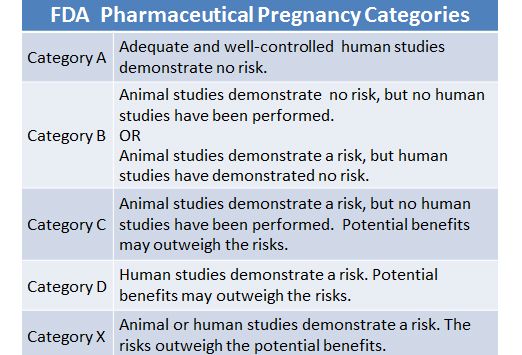
Blog Post Category:
Biological Psychiatry
You must enable JavaScript to use this form.
Your name *
Your phone number (privacy guaranteed) *
Main complaint *
Presumptive diagnosis
Taking medication
Consent to the processing of personal data *
I agree to the processing of personal data, I have read the privacy policy
The study confirmed the absence of the effect of antidepressants taken during pregnancy on the growth of the baby
svetazo, with 1 comment
According to a new study by scientists at Northwestern Medicine, serotonin reuptake inhibitors (SSRIs), antidepressants that women take during pregnancy, do not affect the growth of an infant in the first year of life.
Doctors feared that antidepressant treatment during pregnancy could negatively affect the growth of the unborn baby in the first year of his life. Previously, studies have shown that depression during pregnancy can reduce a child's growth rate.
However, experimental data show that infants born to women who took inhibitors during pregnancy had similar weight, length and head circumference at the end of the first year of life with infants born to women who did not take any antidepressants during pregnancy. Children whose mothers took antidepressants were slightly smaller at birth, but after two weeks this difference disappeared.
In addition, the height of infants whose mothers did not take antidepressants was similar to the average height of infants in general.
"Most women want to know about the impact of their depression and the medications they use during pregnancy, not only on the baby at birth, but also on their physical and mental development in the future," said lead author of the study Northwestern Medicine and Dr. Medical Sciences Katherine L. Wisner. "This information may help women analyze the balance of risks and benefits when continuing antidepressant treatment during pregnancy," the study author continued.
Medical Sciences Katherine L. Wisner. "This information may help women analyze the balance of risks and benefits when continuing antidepressant treatment during pregnancy," the study author continued.
Dr. Wisner is Director of the Northwestern's Asher Center for the Study and Treatment of Depressive Disorders and Professor of Psychiatry and Behavioral Sciences at the Norman and Helen Asher Center. Asher, professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine and physician at Northwestern Memorial Hospital.
The results of this study will be published March 20, 2013 in the American Journal of Psychiatry. The online information on the website was exposed prior to publication.
“Depression has a negative impact on the health of both mother and child,” Dr. Wisner noted, and warned women against not taking antidepressant medication during pregnancy, as it could lead to relapses.
Maternal prenatal stress and depression can precipitate preterm labor and cause low birth weight, which increases the risk of cardiovascular disease.