Does oxcarbazepine cause weight gain
Weight gain in children on oxcarbazepine monotherapy
Save citation to file
Format: Summary (text)PubMedPMIDAbstract (text)CSV
Add to Collections
- Create a new collection
- Add to an existing collection
Name your collection:
Name must be less than 100 characters
Choose a collection:
Unable to load your collection due to an error
Please try again
Add to My Bibliography
- My Bibliography
Unable to load your delegates due to an error
Please try again
Your saved search
Name of saved search:
Search terms:
Test search terms
Email: (change)
Which day? The first SundayThe first MondayThe first TuesdayThe first WednesdayThe first ThursdayThe first FridayThe first SaturdayThe first dayThe first weekday
Which day? SundayMondayTuesdayWednesdayThursdayFridaySaturday
Report format: SummarySummary (text)AbstractAbstract (text)PubMed
Send at most: 1 item5 items10 items20 items50 items100 items200 items
Send even when there aren't any new results
Optional text in email:
Create a file for external citation management software
Full text links
Elsevier Science
Full text links
. 2016 May;122:110-3.
doi: 10.1016/j.eplepsyres.2016.03.004. Epub 2016 Mar 16.
Anastasia Garoufi 1 , George Vartzelis 2 , Charalambos Tsentidis 2 , Achilleas Attilakos 3 , Evangelia Koemtzidou 2 , Lydia Kossiva 2 , Eustathia Katsarou 4 , Alexandra Soldatou 2
Affiliations
Affiliations
- 1 Second Department of Pediatrics, National and Kapodistrian University of Athens, Medical School, 'P. & A. Kyriakou' Children's Hospital, Thivon & Levadias Str.
 , 11527 Athens, Greece. Electronic address: [email protected].
, 11527 Athens, Greece. Electronic address: [email protected]. - 2 Second Department of Pediatrics, National and Kapodistrian University of Athens, Medical School, 'P. & A. Kyriakou' Children's Hospital, Thivon & Levadias Str., 11527 Athens, Greece.
- 3 Third Department of Pediatrics, National and Kapodistrian University of Athens, Medical School, Attikon Hospital, Rimini 1, 12462, Haidari, Athens, Greece.
- 4 Department of Pediatric Neurology, 'P. & A. Kyriakou' Children's Hospital, Thivon & Levadias Str., 11527, Athens, Greece.
- PMID: 27010568
- DOI: 10.
 1016/j.eplepsyres.2016.03.004
1016/j.eplepsyres.2016.03.004
Anastasia Garoufi et al. Epilepsy Res. 2016 May.
. 2016 May;122:110-3.
doi: 10.1016/j.eplepsyres.2016.03.004. Epub 2016 Mar 16.
Authors
Anastasia Garoufi 1 , George Vartzelis 2 , Charalambos Tsentidis 2 , Achilleas Attilakos 3 , Evangelia Koemtzidou 2 , Lydia Kossiva 2 , Eustathia Katsarou 4 , Alexandra Soldatou 2
Affiliations
- 1 Second Department of Pediatrics, National and Kapodistrian University of Athens, Medical School, 'P.
 & A. Kyriakou' Children's Hospital, Thivon & Levadias Str., 11527 Athens, Greece. Electronic address: [email protected].
& A. Kyriakou' Children's Hospital, Thivon & Levadias Str., 11527 Athens, Greece. Electronic address: [email protected]. - 2 Second Department of Pediatrics, National and Kapodistrian University of Athens, Medical School, 'P. & A. Kyriakou' Children's Hospital, Thivon & Levadias Str., 11527 Athens, Greece.
- 3 Third Department of Pediatrics, National and Kapodistrian University of Athens, Medical School, Attikon Hospital, Rimini 1, 12462, Haidari, Athens, Greece.
- 4 Department of Pediatric Neurology, 'P. & A. Kyriakou' Children's Hospital, Thivon & Levadias Str., 11527, Athens, Greece.
- PMID: 27010568
- DOI: 10.
 1016/j.eplepsyres.2016.03.004
1016/j.eplepsyres.2016.03.004
Abstract
Background: Studies of the effect of oxcarbazepine (OXC) on body growth of children with epilepsy are rare and their results are controversial. To the contrary, many studies have shown significant weight gain following valproate (VPA) treatment.
Purpose: To prospectively evaluate the effect of OXC monotherapy on growth patterns of children with epilepsy and compare it with the effect of VPA monotherapy.
Method: Fifty-nine otherwise healthy children, aged 3.7-15.9 years, with primary generalized, partial or partial with secondary generalization seizure disorder, were included in the study. Twenty six children were placed on OXC and thirty three on VPA monotherapy. Body weight (BW), height and body mass index (BMI) as well as their standard deviation scores (SDS), were evaluated prior to as well as 8 months post initiation of OXC or VPA therapy.
Body weight (BW), height and body mass index (BMI) as well as their standard deviation scores (SDS), were evaluated prior to as well as 8 months post initiation of OXC or VPA therapy.
Results: Eight months post OXC-treatment, BW, SDS-BW, BMI and SDS-BMI increased significantly. The increase was similar to that observed in the VPA group. An additional 15.4% of children in the OXC group and 21.2% in the VPA group became overweight or obese. The effect of both OXC and VPA therapy on linear growth did not reach statistical significance.
Conclusion: Similarly to VPA, OXC monotherapy resulted in a significant weight gain in children with epilepsy. Careful monitoring for excess weight gain along with counseling on adapting a healthy lifestyle should be offered to children on OXC therapy.
Keywords: Antiepileptic drugs; Epilepsy; Linear growth; Obesity; Valproate.
Copyright © 2016 Elsevier B.V. All rights reserved.
Similar articles
-
Analysis of acylcarnitine levels by tandem mass spectrometry in epileptic children receiving valproate and oxcarbazepine.
Cansu A, Serdaroglu A, Biberoglu G, Tumer L, Hirfanoglu TL, Ezgu FS, Hasanoglu A. Cansu A, et al. Epileptic Disord. 2011 Dec;13(4):394-400. doi: 10.1684/epd.2011.0478. Epileptic Disord. 2011. PMID: 22258044
-
The effects of valproate, carbamazepine, and oxcarbazepine on growth and sexual maturation in girls with epilepsy.
Rättyä J, Vainionpää L, Knip M, Lanning P, Isojärvi JI. Rättyä J, et al. Pediatrics. 1999 Mar;103(3):588-93. doi: 10.1542/peds.103.3.588. Pediatrics. 1999. PMID: 10049961
-
Thyroid function in girls with epilepsy with carbamazepine, oxcarbazepine, or valproate monotherapy and after withdrawal of medication.

Vainionpää LK, Mikkonen K, Rättyä J, Knip M, Pakarinen AJ, Myllylä VV, Isojärvi JI. Vainionpää LK, et al. Epilepsia. 2004 Mar;45(3):197-203. doi: 10.1111/j.0013-9580.2004.26003.x. Epilepsia. 2004. PMID: 15009219 Clinical Trial.
-
What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs?
Schmidt D, Elger CE. Schmidt D, et al. Epilepsy Behav. 2004 Oct;5(5):627-35. doi: 10.1016/j.yebeh.2004.07.004. Epilepsy Behav. 2004. PMID: 15380112 Review.
-
Oxcarbazepine: a review of its use in children with epilepsy.
Bang L, Goa K. Bang L, et al. Paediatr Drugs. 2003;5(8):557-73. doi: 10.2165/00148581-200305080-00006. Paediatr Drugs.
 2003. PMID: 12895138 Review.
2003. PMID: 12895138 Review.
See all similar articles
Cited by
-
Levetiracetam versus Oxcarbazepine as monotherapy in newly diagnosed focal epilepsy: A systematic review and meta-analysis.
Kharel S, Ojha R, Khanal S. Kharel S, et al. Brain Behav. 2022 Nov;12(11):e2779. doi: 10.1002/brb3.2779. Epub 2022 Oct 2. Brain Behav. 2022. PMID: 36184821 Free PMC article. Review.
-
Efficacy comparison of oxcarbazepine and levetiracetam monotherapy among patients with newly diagnosed focal epilepsy in China: A multicenter, open-label, randomized study.
Zhu H, Deng X, Feng L, Lian Y, Han X, Guo Z, Gou Y, Du Y, Xie L, Yao D, Liu Y, Wu Q, Lan S, Liu K, Zhan P, Wang X, Dang J, Hou Y, Chen K, Zhu Y, Shi Y, Yu Y, Xiao B, Zhu S, Meng H.
 Zhu H, et al. CNS Neurosci Ther. 2022 Jul;28(7):1072-1080. doi: 10.1111/cns.13840. Epub 2022 Apr 15. CNS Neurosci Ther. 2022. PMID: 35429132 Free PMC article. Clinical Trial.
Zhu H, et al. CNS Neurosci Ther. 2022 Jul;28(7):1072-1080. doi: 10.1111/cns.13840. Epub 2022 Apr 15. CNS Neurosci Ther. 2022. PMID: 35429132 Free PMC article. Clinical Trial. -
Valproate-Induced Epigenetic Upregulation of Hypothalamic Fto Expression Potentially Linked with Weight Gain.
Zhang H, Lu P, Tang HL, Yan HJ, Jiang W, Shi H, Chen SY, Gao MM, Zeng XD, Long YS. Zhang H, et al. Cell Mol Neurobiol. 2021 Aug;41(6):1257-1269. doi: 10.1007/s10571-020-00895-2. Epub 2020 Jun 4. Cell Mol Neurobiol. 2021. PMID: 32500354
-
How do pediatric patients perceive adverse drug events of anticonvulsant drugs? A survey.
Neininger MP, Woltermann S, Jeschke S, Herziger B, Müller RM, Kiess W, Bertsche T, Bertsche A.
 Neininger MP, et al. Eur J Pediatr. 2020 Sep;179(9):1413-1420. doi: 10.1007/s00431-020-03571-1. Epub 2020 Mar 11. Eur J Pediatr. 2020. PMID: 32162065 Free PMC article.
Neininger MP, et al. Eur J Pediatr. 2020 Sep;179(9):1413-1420. doi: 10.1007/s00431-020-03571-1. Epub 2020 Mar 11. Eur J Pediatr. 2020. PMID: 32162065 Free PMC article. -
Carbamazepine Enhances Adipogenesis by Inhibiting Wnt/β-catenin Expression.
Im DU, Kim SC, Chau GC, Um SH. Im DU, et al. Cells. 2019 Nov 18;8(11):1460. doi: 10.3390/cells8111460. Cells. 2019. PMID: 31752244 Free PMC article.
See all "Cited by" articles
MeSH terms
Substances
Full text links
Elsevier Science
Cite
Format: AMA APA MLA NLM
Add to Collections
- Create a new collection
- Add to an existing collection
Name your collection:
Name must be less than 100 characters
Choose a collection:
Unable to load your collection due to an error
Please try again
Send To
Oxcarbazepine side effects and how to avoid them
Oxcarbazepine side effects include dizziness, double vision, sleepiness, and weight gain
Common oxcarbazepine side effects | Serious side effects | Weight gain | Side effects timeline | Contraindications | Warnings | Interactions | How to avoid side effects | How to treat side effects
Oxcarbazepine is a generic prescription anticonvulsant and the active ingredient in the brand-name prescription drugs Trileptal and Oxtellar XR (extended-release tablets). It is used to control partial-onset seizures in people diagnosed with epilepsy but is also prescribed off-label for trigeminal neuralgia and to control manic episodes in people with bipolar disorder. Related to the widely-used anti-seizure medication, carbamazepine, oxcarbazepine keeps nerves from firing quickly and repeatedly by interfering with the ability of nerves to build up an electric charge. In this way, oxcarbazepine prevents seizures from spreading throughout the brain. Many of its side effects are similar to those caused by carbamazepine and are mainly due to its effects on the nervous system.
It is used to control partial-onset seizures in people diagnosed with epilepsy but is also prescribed off-label for trigeminal neuralgia and to control manic episodes in people with bipolar disorder. Related to the widely-used anti-seizure medication, carbamazepine, oxcarbazepine keeps nerves from firing quickly and repeatedly by interfering with the ability of nerves to build up an electric charge. In this way, oxcarbazepine prevents seizures from spreading throughout the brain. Many of its side effects are similar to those caused by carbamazepine and are mainly due to its effects on the nervous system.
Common side effects of oxcarbazepine
The most common side effects of oxcarbazepine are dizziness, double vision, and sleepiness. In order, the list of the most likely side effects are:
- Dizziness
- Double vision
- Sleepiness
- Headache
- Nausea
- Vomiting
- Rapid eye movements (nystagmus)
- Unsteadiness
- Abnormal gait
- Vertigo
- Vision problems
- Fatigue
- Abdominal pain
- Tremor
- Diarrhea
- Upset stomach
- Constipation
- Tiredness
- Runny nose
- Insomnia
- Abnormal thoughts
Serious side effects of oxcarbazepine
The most serious side effects of oxcarbazepine are:
- Suicidal thoughts and behaviors
- Low sodium (hyponatremia)
- Blood disorders
- Swollen pancreas
- Withdrawal seizures
- Onset of generalized seizures (primarily in children)
- Severe allergic reactions
- Severe skin reactions
Oxcarbazepine and weight gain
At least one study has shown that children taking oxcarbazepine can experience significant increases in body weight. Weight gain in adults, however, seems to be less common. In clinical studies, people taking oxcarbazepine gained weight at just a slightly higher percentage than people taking a placebo. Adults also experienced weight loss on oxcarbazepine. Weight gain, then, seems to be uncommon among adults taking oxcarbazepine. For children, though, it is important to keep an eye on body weight when they’re on oxcarbazepine. It may be necessary to modify their diet or their activity to address weight gain.
Weight gain in adults, however, seems to be less common. In clinical studies, people taking oxcarbazepine gained weight at just a slightly higher percentage than people taking a placebo. Adults also experienced weight loss on oxcarbazepine. Weight gain, then, seems to be uncommon among adults taking oxcarbazepine. For children, though, it is important to keep an eye on body weight when they’re on oxcarbazepine. It may be necessary to modify their diet or their activity to address weight gain.
How soon do oxcarbazepine side effects start?
Many of the most common side effects of oxcarbazepine occur early in treatment. These include sleepiness, dizziness, headache, nausea, vomiting, vision problems, and problems with thinking or concentrating. Most of the serious side effects, however, are delayed side effects and require oxcarbazepine to be taken for a while.
How long do oxcarbazepine side effects last?
Many of the milder adverse effects of oxcarbazepine improve over time as the body adapts to the drug. Some of the nervous system problems, such as drowsiness, may never go away completely, but they’ll become easier to manage. Side effects that don’t go away are usually reversible when the drug is decreased or stopped.
Some of the nervous system problems, such as drowsiness, may never go away completely, but they’ll become easier to manage. Side effects that don’t go away are usually reversible when the drug is decreased or stopped.
What are the long-term side effects of oxcarbazepine?
Oxcarbazepine is intended for long-term use—years, if possible. Although it is similar to carbamazepine, it does not seem to cause bone loss when taken over the long-term in the same way as carbamazepine or other widely-used antiepileptic drugs such as phenytoin.
Oxcarbazepine contraindications
Oxcarbazepine is never prescribed to anyone who has a known hypersensitivity to the drug or to a related drug called Aptiom (eslicarbazepine acetate).
Pregnancy
Oxcarbazepine is considered a risk during pregnancy, so it is prescribed cautiously and only if the benefits outweigh the risks. There is not only a risk for birth defects, but pregnant women on oxcarbazepine may see an increase in the number of seizures when pregnant. Regular blood tests may need to be performed.
Regular blood tests may need to be performed.
Breastfeeding
Oxcarbazepine is prescribed cautiously in women who are breastfeeding. The drug is present in human breast milk, so it could cause problems in a nursing infant.
Children
Oxcarbazepine is FDA-approved for use in children as young as 2 years of age.
Seniors
Adults older than 65 may not be able to eliminate the drug as quickly as young adults. This slower clearance puts seniors at a higher risk of side effects, so healthcare professionals will usually start with a low dose of oxcarbazepine at the onset and try to keep the dose as low as possible. Seniors are also more vulnerable to falls, accidents, and injuries if they experience side effects like dizziness and unsteadiness. As a final consideration, seniors are also more likely to have problems with low sodium, a common side effect of oxcarbazepine.
Oxcarbazepine warnings
Although oxcarbazepine is related to carbamazepine, it does not have a serious black box warning from the FDA about suicidality or blood problems. It can cause those problems but is less likely to. There are, of course, other risks when taking oxcarbazepine.
It can cause those problems but is less likely to. There are, of course, other risks when taking oxcarbazepine.
Cautions
Some pre-existing medical conditions may cause problems in people taking oxcarbazepine.
The most serious caution involves people who are genetically susceptible to severe and potentially fatal drug reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis. These people are born with a variant of the gene HLA-B 1502. This variant is more common among people of Asian descent, so genetic testing may be performed before the drug is prescribed. Having this variant, however, is not a strict contraindication. The drug can still be taken, but, because of the risk, side effects will need to be carefully monitored.
People who have had an allergic reaction to carbamazepine are more likely to have an allergic reaction to oxcarbazepine. That doesn’t mean the drug is prohibited. It does mean that patients, their caregivers, and healthcare providers will need to watch more carefully for symptoms of an allergic reaction.
Some medical conditions can be worsened by oxcarbazepine. These include low sodium, depression, and suicidal tendencies. Kidney disease makes it difficult for the body to flush the drug out, so higher concentrations of the drug make side effects—including severe ones—more likely. Reduced doses are usually prescribed to people with kidney problems.
Finally, oxcarbazepine only helps with one type of seizure, partial seizures. It is not appropriate for other types of seizures.
Abuse and dependence
Drug abuse and physical dependence are not associated with oxcarbazepine.
Withdrawal
The sudden discontinuation of oxcarbazepine can provoke withdrawal seizures in people with a seizure disorder. When it’s time to stop, healthcare providers will prescribe a gradually tapering dose.
Overdose
Call a poison control center or get medical help if too much oxcarbazepine is taken. Symptoms of an overdose may include nausea, vomiting, sleepiness, low blood pressure, agitation, vision problems, confusion, dizziness, balance problems, and even coma.
Oxcarbazepine interactions
Most of the drug interactions related to oxcarbazepine involve a liver enzyme that breaks down some medications. In some cases, oxcarbazepine speeds up the breakdown of other drugs, making them less effective. These include some AIDS/HIV drugs, some antibiotics, some hepatitis C treatments, some blood thinners, some heart rhythm drugs, and hormonal contraceptives. Women taking birth control pills will be advised that they are at a higher risk for pregnancy if they also take oxcarbazepine. The list of drugs is very long, but be assured that healthcare professionals are aware of these interactions and will adjust doses accordingly. A few of these drugs are so compromised that they are never prescribed with oxcarbazepine.
Other drugs do the opposite: they speed up the body’s breakdown of oxcarbazepine, making it less effective. These include carbamazepine, the anti-seizure medication phenytoin, the tuberculosis treatment rifampin, and other drugs. This list also includes the popular herbal supplement, St. John’s wort, so it’s discouraged during oxcarbazepine therapy.
This list also includes the popular herbal supplement, St. John’s wort, so it’s discouraged during oxcarbazepine therapy.
Some drugs make seizures more likely. They are used cautiously in anyone taking drugs like oxcarbazepine to control seizures. Some are never used, such as Wellbutrin (bupropion).
How to avoid oxcarbazepine side effects
Oxcarbazepine tends to have fewer severe side effects than its sister drug, carbamazepine. However, mild to moderate side effects are slightly more likely to occur. Still, there are ways to keep these problems at bay.
1. Tell the prescriber about all medical conditions
The first rule is to make sure the prescriber knows about all medical conditions past and present. For oxcarbazepine, the most important are:
- A history of suicidal thoughts or suicide attempts
- Depression or mood problems
- Liver problems
- Kidney problems
- Any allergic reaction to carbamazepine
- Pregnancy or pregnancy plans
- Breastfeeding or breastfeeding plans
2.
 Include all the medications being taken
Include all the medications being takenYour medical history is not complete without including all prescription drugs, over-the-counter medications, and dietary supplements being taken.
3. Follow instructions
Taking too much oxcarbazepine raises the risk of adverse effects. Follow the instructions to the letter. Do not take more than the prescribed dose or take it more often than prescribed. A missed dose can be taken when remembered, but skip it if it’s almost time for the next dose. Never double up a dose to make up for a missed dose.
4. Avoid driving or other risky activities
Oxcarbazepine can cause serious impairment, so it’s wise to not drive, operate machinery, or engage in risky activities or sports for a few days. Once the effects of oxcarbazepine are understood, then it’s okay to go back to driving if impairment is not too severe. If this drug makes you too drowsy or dizzy, do not drive.
How to treat side effects of oxcarbazepine
Mild side effects can usually be handled at home. However, if they persist or get worse, get medical advice from the prescribing healthcare provider. Some serious side effects, however, will require immediate medical attention. They are not always obvious, so make sure to be familiar with the signs of possibly serious problems.
However, if they persist or get worse, get medical advice from the prescribing healthcare provider. Some serious side effects, however, will require immediate medical attention. They are not always obvious, so make sure to be familiar with the signs of possibly serious problems.
Suicidal thoughts and behaviors
Thinking about suicide or showing signs of suicidality will require immediate medical attention. Any suspicion of suicidality should be reported to the prescriber, particularly:
- Talking about suicide or death
- Making suicide plans
- Any sudden changes in mood, behaviors, or feelings
- New or worse depression
- New or worse anxiety
- New or worse irritability
- Agitation or restlessness
- Panic attacks
- Aggression, hostility, or violence
- Acting on dangerous impulses
- Unusual or extreme increase in activity or talking
- Insomnia
Low sodium levels
Low sodium is a common side effect of oxcarbazepine. A temporary and small dip in sodium levels can be resolved by eating more salt. The real problem arises if sodium gets too low. That’s dangerous, so get medical attention if any of the following signs of low sodium are noticed:
A temporary and small dip in sodium levels can be resolved by eating more salt. The real problem arises if sodium gets too low. That’s dangerous, so get medical attention if any of the following signs of low sodium are noticed:
- An increase in seizures
- Feeling more tired than usual
- Feeling weak or without energy
- Finding it hard to think
- Confusion
- Headache
- Nausea
Liver problems
Oxcarbazepine can poison the liver. The only way to tell for sure is with a blood test, but the following symptoms are clues that medical help is needed:
- Pain on the right side of the upper abdomen
- Dark urine
- Feeling less hungry than usual
- Yellowing of the skin or eyes
Blood problems
Like carbamazepine, oxcarbazepine can cause blood problems. They are all considered serious side effects including reduced white blood cell counts, red blood cell counts, and reduced numbers of blood clot-forming cells called platelets. Each of these problems will have different symptoms. The most important to watch out for are:
They are all considered serious side effects including reduced white blood cell counts, red blood cell counts, and reduced numbers of blood clot-forming cells called platelets. Each of these problems will have different symptoms. The most important to watch out for are:
- An increase in both the frequency and severity of infections
- Bleeding more than usual
- Bruising more than usual
- Nosebleeds
- Bleeding gums
- Feeling tired or fatigued more than usual
- Feeling weaker than usual
Drug reactions
Potentially life-threatening drug reactions usually affect the skin. They can develop very quickly after the first symptoms, so stop taking the drug and get immediate medical attention when noticing symptoms such as:
- Skin rash
- Hives
- Sores in the mouth and around the eyes
- Skin blisters
- Skin peeling
Allergic reactions
An allergic reaction to oxcarbazepine could swiftly evolve into a serious problem. It’s not always possible to tell if an allergic reaction is due to the drug, so for safety’s sake, get immediate medical attention at any of the following symptoms of an allergic reaction:
It’s not always possible to tell if an allergic reaction is due to the drug, so for safety’s sake, get immediate medical attention at any of the following symptoms of an allergic reaction:
- Swelling of the face, throat, or mouth
- Trouble breathing
- Trouble swallowing
- Sore throat
- Skin rash
- Hives
Sources
- Long-term anticonvulsant therapy leads to low bone mineral density — evidence for direct drug effects of phenytoin and carbamazepine on human osteoblast-like cells, Experimental and Clinical Endocrinology & Diabetes
- Oxcarbazepine, Epocrates
- Oxcarbazepine drug summary, Prescriber’s Digital Reference
- Oxcarbazepine tablet prescribing information, U.S. National Library of Medicine
- Trileptal prescribing information, U.
 S. National Library of Medicine
S. National Library of Medicine - Weight gain in children on oxcarbazepine monotherapy, Epilepsy Research
Side effects of second generation antiepileptic drugs | Belousov
Annotation
This article discusses the side effects and frequency of their occurrence in 6 modern second-generation antiepileptic drugs (PEP-II): gabapentin (GBP) - Neurontin, Pfizer and other generics; lamotrigine (Ltd) - Lamictal, GlaxoSmithKline and other generics; levetiracetam (LEV) - Keppra, Pliva; oxcarbazepine (OKS) - Trileptal, Novartis; pregabalin (PHB) - Lyrica, Pfizer; topiramate (TPM) - Topamax, Janssen-Cilag and other generics. All data on the side effects of PEP-II are from controlled clinical trials. Compared to first generation drugs (AED-I), most AED-II have much fewer side effects associated with stimulation or inhibition of enzyme activity, such as cognitive, hormonal, and others. This simplifies therapy and improves patient compliance, which is absolutely essential for the successful management of patients with partial or generalized epilepsy. AED-II expands the arsenal of treatments and is a new step in optimizing individual therapy for epilepsy. Although these drugs are safer and better tolerated, most of them still cause adverse side effects.
This simplifies therapy and improves patient compliance, which is absolutely essential for the successful management of patients with partial or generalized epilepsy. AED-II expands the arsenal of treatments and is a new step in optimizing individual therapy for epilepsy. Although these drugs are safer and better tolerated, most of them still cause adverse side effects.
Introduction
This article discusses the side effects and incidence of six current second-generation antiepileptic drugs (AED-II):
All AED-II side effect data are from controlled clinical trials.
Compared to first generation drugs (AED-I): phenobarbital (PB), primidone (PRD), phenytoin (PNT), carbamazepine (CBZ), valproic acid (VPA), most AED-II have much fewer side effects, associated with stimulation or inhibition of enzyme activity, for example, cognitive, hormonal, and others. This simplifies therapy and improves patient compliance, which is absolutely essential for the successful management of patients with partial or generalized epilepsy [25]. AED-II expands the arsenal of treatments and is a new step in optimizing individual therapy for epilepsy.
This simplifies therapy and improves patient compliance, which is absolutely essential for the successful management of patients with partial or generalized epilepsy [25]. AED-II expands the arsenal of treatments and is a new step in optimizing individual therapy for epilepsy.
Although these drugs are safer and better tolerated, most of them still cause adverse side effects. The table summarizes the frequency of occurrence of the most common side effects of some AED-II.
Table. Frequency of side effects of some new antiepileptic drugs, modified from [7]
| Side effect | GBP N = 543 (%) | LTD N = 711 (%) | (%) (%) (%) (%) | | Central Hall | Dizziness | 10 (1. | | 14 (12.2.2.2.2.2.2.2.2.2.2.2.2.2.2.2.4) 14 (12.4) 14 (12.2.2.2.ART Ataxia | 7 (1.3) | 16 (2.3) | 14 (12.4) | Violation of speech | - | 14 (12.4) | Diplopia | 4 (0.7) | 21 (3.0) | 8 (7.1) Nystagmus | 4 (0.7) | 4 (3.5) | Dyspepsia | - | 3 (0.4) | 3 (2.7) | - | 4 (0. | - 9000 | | |||
* - Low dose (200-400 mg/day) ; GBP - gabapentin ; LTD - lamotrigine ; TPM - topiramate .
Gabapentin (GBP). The most common side effects of GBP are drowsiness, weakness, dizziness, and weight gain [9]. GBP does not change the performance of the liver, kidneys and endocrine system and does not affect the cellular composition of the blood. Several cases of movement disorders have been described [20]. Data on a possible teratogenic effect in humans is insufficient.
Lamotrigine (Ltd.). The most common side effect of LTD is exanthema [17], which rarely manifests as Stevens-Johnson syndrome or Lyell's syndrome. The development of exanthema in most cases can be avoided by slowly increasing the dose. When a rash appears, the drug is immediately canceled, since in most cases the exanthema is irreversible. Other rare side effects are vetiligo, gastrointestinal symptoms, drowsiness, nausea, diplopia, idiosyncratic thrombocytopenia, leukopenia, and transaminase elevation [13].
Other rare side effects are vetiligo, gastrointestinal symptoms, drowsiness, nausea, diplopia, idiosyncratic thrombocytopenia, leukopenia, and transaminase elevation [13].
The development of blepharospasm has been described with the use of LTD [27]. As a result of the interaction of LTD with CBZ, vitiligo and nausea may occur, which are eliminated by reducing the dose of CBZ.
No teratogenic effects have been found in animal experiments, but there are insufficient data on effects in humans. To date, 707 pregnant women treated with LTD monotherapy have reported a 2.8% incidence of fetal developmental disorders; with monotherapy with other AEDs, this figure is 3.3-4.5%, according to the Register of Pregnant Women Taking Lamotrigine [14]. According to one North American registry, the incidence of cleft palate is 8.9cases per 1000 children, but this figure needs to be confirmed. LTD passes into breast milk [19].
In general, LTD is well tolerated. In some studies, the use of LTD even found mood stabilization, antidepressant effect, improvement of attention and cognitive functions [26]. In 6.4% of patients, sleep disturbances are observed, which are partially eliminated by taking the drug in the morning or in the middle of the day [22].
In 6.4% of patients, sleep disturbances are observed, which are partially eliminated by taking the drug in the morning or in the middle of the day [22].
Levetiracetam (LEV) - Studies to date suggest that LEV is well tolerated [5]. A positive effect on the quality of life of patients was also noted [6]. The most common side effects are drowsiness, asthenia and dizziness [1], with drowsiness more common at higher doses of the drug [2]. Gastrointestinal phenomena (anorexia, diarrhea) and side effects from the central nervous system (amnesia, ataxia, insomnia, nervousness, tremor, vitiligo), as well as diplopia and skin reactions, are less common. Emotional instability and aggressiveness may develop.
Oxcarbazepine (OCS). The most common side effects of ACS are headache, vitiligo, drowsiness, nausea, diplopia, vomiting, and ataxia [3]. In general, the drug is well tolerated, and side effects are less common than with CBZ [8]. Often they can be avoided by carefully increasing the initial dose (600 mg / week). It should be remembered that ACS can cause hyponatremia up to <125 mmol/l [12], which in rare cases is accompanied by confusion. OCS has less effect on free testosterone levels than CBZ due to its weak drug interaction potential [10]. This may be important given the risk of erectile dysfunction and hypogonadism. In addition to testosterone levels, after switching from CBZ to ACS, the level of total cholesterol also normalizes [4].
Often they can be avoided by carefully increasing the initial dose (600 mg / week). It should be remembered that ACS can cause hyponatremia up to <125 mmol/l [12], which in rare cases is accompanied by confusion. OCS has less effect on free testosterone levels than CBZ due to its weak drug interaction potential [10]. This may be important given the risk of erectile dysfunction and hypogonadism. In addition to testosterone levels, after switching from CBZ to ACS, the level of total cholesterol also normalizes [4].
Pregabalin (PHB). The most common side effects in controlled clinical trials were dizziness (38%), drowsiness (28%), ataxia (19.5%), and weight gain (15.9%) at the maximum dose of 600 mg [4].
Topiramate (TPM). Long-term studies have shown good tolerability and no withdrawal symptoms [21]. The most common side effect was dose-dependent weight loss [23]. In addition, side effects from the central nervous system were observed - a decrease in concentration, psychomotor retardation, speech impairment, dizziness, drowsiness, weakness, confusion and ataxia.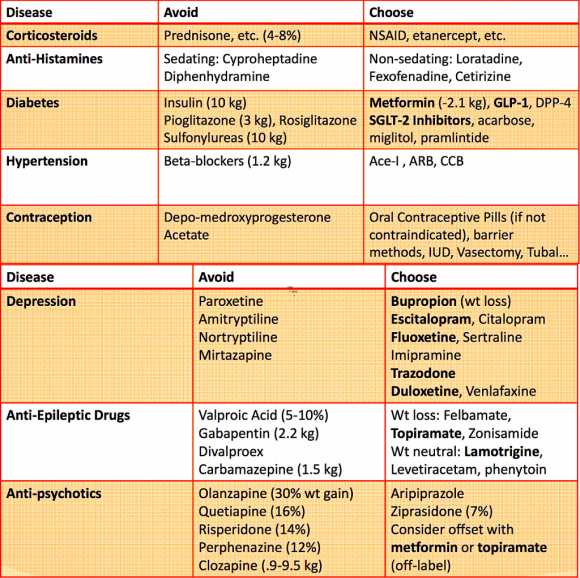 Perhaps the development of paresthesia associated with inhibition of the activity of carbonic anhydrase. Attention should be paid to the fact that TBI contributes to the formation of calcium-phosphate kidney stones due to the weakening of the excretion of citrates in the urine and, accordingly, the increase in urine pH. In some cases, the use of TPM may cause symptoms of glaucoma. There are insufficient data on the use of the drug during pregnancy, so TBI should be used during pregnancy only after a careful assessment of the risk-benefit ratio. Monotherapy at doses of 100-200 mg is generally better tolerated than combination therapy.
Perhaps the development of paresthesia associated with inhibition of the activity of carbonic anhydrase. Attention should be paid to the fact that TBI contributes to the formation of calcium-phosphate kidney stones due to the weakening of the excretion of citrates in the urine and, accordingly, the increase in urine pH. In some cases, the use of TPM may cause symptoms of glaucoma. There are insufficient data on the use of the drug during pregnancy, so TBI should be used during pregnancy only after a careful assessment of the risk-benefit ratio. Monotherapy at doses of 100-200 mg is generally better tolerated than combination therapy.
Conclusion
PEP-I (FB, PRD, FNT, CBZ, VPC) have an increased ability to develop side effects due to the activation/inhibition of enzyme activity. In addition to impaired cognitive functions and functions of the endocrine system, long-term use of these drugs may be accompanied by changes in bones (osteoporosis) and connective tissue (gingival hyperplasia, Dupuytren's contracture, hypertrichosis, etc. ). In addition, hormonal and metabolic disorders lead to sexual dysfunction [10, 11, 15, 16, 18].
). In addition, hormonal and metabolic disorders lead to sexual dysfunction [10, 11, 15, 16, 18].
These side effects are unlikely for most AED-IIs, so it seems now possible to improve treatment tolerance. In controlled trials, the rate of early discontinuation of HBP, LEV, LTD, ACS, PHB, and TPM was less than for CBA, VPC, and FNT [24].
Thus :
- the use of PEP-II improves tolerability and compliance;
- AED-II have better safety and possibly less teratogenicity than AED-I.
Currently, epileptologists expect the emergence of a new generation of AEDs: ganaloxone, BIA2-093, brivaracetam, fluorofelbamate, charcoseride, remazemide, safinamide, styripentol, talampanel, SPD 421, retigabine and others, which are still undergoing preclinical and clinical studies.
1. Ben-Menachem E. & Falter U. (2000). Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia 41, 1276-1283.
European Levetiracetam Study Group. Epilepsia 41, 1276-1283.
2. Betts T., Waegemans T. & Crawford P. (2000). A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure 9, 80-87.
3. Bill P.A., Vigonius U., Pohlmann H., Guerreiro C.A., Kochen S., Saffer D., et al. (1997). A double-blind controlled clinical trial of oxcarbazepine versus phenytoin in adults with previously untreated epilepsy. Epilepsy Res 27, 195-204.
4. Blum D.E. (1998). New drugs for persons with epilepsy. Adv Neurol 76, 57-87.
5. Cereghino J.J., Biton V., Abou K.B., Dreifuss F., Gauer L.J. & Leppik I. (2000). Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology 55, 236-242.
6. Cramer J.A., Arrigo C., Van-Hammee G., Gauer L.J. & Cereghino J.J. (2000). Effect of levetiracetam on epilepsy-related quality of life. N132 Study Group. Epilepsia 41, 868-874.
N132 Study Group. Epilepsia 41, 868-874.
7. Cramer J.A., Fisher R., Ben-Menachem E., French J. & Mattson R. (1999). New antiepileptic drugs: comparison of key trials. Epilepsia 40, 590-600.
8. Dam M., Ekberg R., Loyning Y., Waltimo O. & Jakobsen K. (1989). A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res 3, 70-76.
9. Gidal B.E., Maly M.M., Nemire R.E. & Haley K. (1995). Weight gain and gabapentin therapy [letter]. Ann Pharmacother 29, 1048-1054.
10. Herzog A., Drislane F.W. & Schomer D.I. (2003). Differential antiepileptic drug effects on sex hormones and reproductive hormones: a comparison between lamotrigne and enzyme-inducing antiepileptic drugs. Epilepsia 44(9), 107-115.
11. Isojaervi J.I.T., Pakarinen A.J., Rautio A., Pelkonen O. & Myllylae V.V. (1995). Serum sex hormone levels after replacing carbamazepine with oxcarbazepine. Eur J Clin Pharmacol 47(5), 462-464.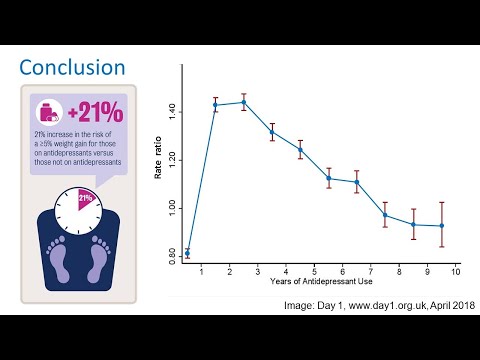
12. Johannessen A.C. & Nielsen O.A. (1987). Hyponatremia induced by oxcarbazepine. Epilepsy Res 1, 155-156.
13. Kilpatrick E.S., Forrest G. & Brodie M.J. (1996). Concentration-effect and concentration-toxicity relations with lamotrigine: a prospective study. Epilepsia 37, 534-538.
14. Lamotrigine pregnanacy registry, January 2006, GlaxoSmithKline.
15. Martin R., Kuzniecky R. & Ho S. (1999). Cognitive effects of topiramate, gabapentine and lamotrigine in healthy young adults. Neurology 52(2), 321-327.
16. Meador K.J., Loring D.W., Ray P.G., Murro, A.M., King D.W., Perrine K.R. et al. (2001). Differential cognitive and behavioral effects of carbamazepine and lamotrigine. Neurology 56, 177-1182.
17. Messenheimer J.A. & Guberman A.H. (2000). Rash with lamotrigine: dosing guidelines [letter]. Epilepsia 41, 488.
18. Pack A.M., Olarte L.S. & Morell M.J. (2003). Bone mineral density in an outpatient population receiving enzyme-inducing antiepileptic drugs. Epilepsy Behav 4, 169-174.
Epilepsy Behav 4, 169-174.
19. Rambeck B., Kurlemann G., Stodieck S.R., May T.W. & Jurgens U. (1997). Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol 51, 481-484.
20. Reeves A.L., So E.L., Sharbrough F.W. & Krahn L.E. (1996). Movement disorders associated with the use of gabapentin. Epilepsia 37, 988-990.
21. Ritter F., Glauser T.A., Elterman R.D. & Wyllie E. (2000). Effectiveness, tolerability, and safety of topiramate in children with partial-onset seizures. Topiramate YP Study Group. Epilepsia 41(1), 82-85.
22. Sadler M. (1999). Lamotrigine associated with insomnia. Epilepsia 40, 322-325.
23. Sander J.W. (1997). Practical aspects of the use of topiramate in patients with epilepsy. Epilepsia 38(1), 56-58.
24. Stefan H., Feuerstein T.J. Novel anticonvulsant drugs. Pharmacology & Therapeutics 113 (2007) 165-183
25. Steinhoff B.J., Hirsch E, Mutani R. & Nakken K. O. (2003). The ideal characteristics of antiepileptic therapy: an overview of old and new AEDs. Acta Neurol Scand 107, 87-95.
O. (2003). The ideal characteristics of antiepileptic therapy: an overview of old and new AEDs. Acta Neurol Scand 107, 87-95.
26. Uvebrant P. & Bauziene R. (1994). Intractable epilepsy in children: the efficacy of lamotrigin treatment including non-seizure related benefit. Neuropediatrics 25, 284-289.
27. Verma A., Miller P., Carwile S.T., Husain A.M. & Radtke, R.A. (1999). Lamotrigine-induced blepharospasm. Pharmacotherapy 19, 877-880.
AEDs and cosmetic adverse events
Finding the optimal treatment for epilepsy in adolescents is a particular challenge. These patients need a regimen that least disrupts their daily activities. In addition, children and adolescents are more sensitive to the perception of relationships with peers 1 . The most commonly used antiepileptic drugs in adolescents can cause cosmetic adverse events (AEs) such as weight changes, acne, skin rashes, hair loss, hirsutism, and gingival hyperplasia. Their development negatively affects the quality of life of patients 2 .
Statistics show that most often with the development of CI due to the use of AEDs, patients complain of weight gain (3.6%) 2 .
The drugs available in the doctor's arsenal affect body weight in different ways 3 :
*- drugs are not registered in the Russian Federation.
The exact mechanisms of the pathogenesis of weight change are not fully understood and differ for each drug. Obviously, in most cases, especially when it concerns adolescent girls, excessive body weight gain can cause low compliance and lead to refusal to take this drug. Studies have shown that valproate and pregabalin are the most commonly associated with overweight, and the literature describes the proposed mechanisms of pathogenesis 2 :
- increased appetite and thirst due to direct influence on the hypothalamus,
- induced hyperinsulinemia and insulin resistance,
- hyperleptinemia and leptin resistance.
Up to 1.9% of patients may complain of hair loss while taking AEDs, with women reporting this AE more often than men (2.8% versus 0.7%). Valproic acid is the most common cause of hair loss, with up to 93% of patients with these AEs having reduced their dosage or stopped taking valproate altogether. Sometimes cases of hair loss occur when taking topiramate 4 .
Gingival hyperplasia with AEDs is less common than weight gain or hair loss. Most often, this CI can be associated with phenytoin treatment (up to 2.5%). Most patients either stopped taking the drug or adjusted the dose. On average, the time of appearance of the first signs of this CI from the start of treatment was 51 days 4 .
AEs such as acne may occur in patients taking felbamate (1.9%) and lamotrigine (0.6%) 4 .
According to American researchers, different AEDs have different rates of CI development.
The table shows data on the occurrence of CI in women depending on the intake of a particular drug 4 :
 5
5 Green - the incidence of CI is below the average level of all AEDs.
Red - the incidence of CI is above the average level of all AEDs.
1. Sheth R.D.;Gidal B.E.;Optimizing epilepsy management in teenagers;J Child Neurol;2006;21;4:273-279.
2. Chen B.; Choi H.
 8)
8)  6)
6)