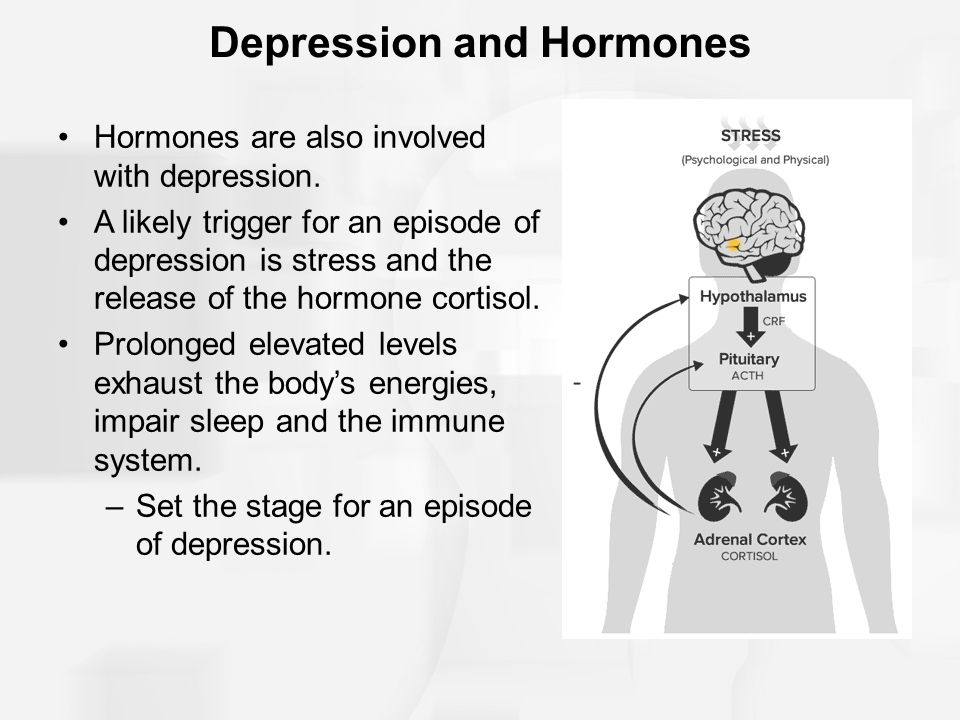
Trileptal for depression
Trileptal (Oxcarbazepine) for Bipolar Disorder
What is Trileptal? Trileptal is a medication known as an anticonvulsant that is used to treat seizures. It is also sometimes used as a mood stabilizer to treat the symptoms of bipolar disorder.
When did the U.S. Food and Drug Administration (FDA) approve the medication? Trileptal was approved by the FDA in 2000.
Is there a generic version of Trileptal? Yes, the generic version is known as oxcarbazepine and is sold in the US.
Are there any major differences between Trileptal and other medications used to treat bipolar disorder? Trileptal belongs to the class of medications known as anticonvulsants. Anticonvulsants are sometimes prescribed to treat manic and depressive episodes associated with bipolar disorder. Trileptal may be prescribed in combination with other medications to treat symptoms. Talk to your doctor about the potential risks and benefits of taking Trileptal.
Can children take Trileptal? Children are prescribed Trileptal for seizures, but the effectiveness and safety of use for bipolar disorder has not been established. Talk to your child’s doctor about the risks and benefits and potential side effects to monitor.
Are there potential interaction issues for people taking Trileptal and any other drugs? There are hundreds of drugs which are known to interact with Trileptal in major, moderate, or mild ways, so let your doctor know what other medications you are taking before you begin taking the medication. Some of these might include amiodarone, amitriptyline, calcium channel blockers, chlorpromazine, clomipramine, cyclophosphamide, desmopressin, diazepam, diuretics, hormonal contraceptives, other anticonvulsants, proton-pump inhibitors, theophylline, and selective serotonin reuptake inhibitors. The medication can also decrease the effectiveness of hormonal contraceptives.
Are there any other medical conditions that would make someone ineligible for Trileptal therapy? Talk to your doctor about other medical conditions before you take Trileptal, such as kidney or liver disease.
What is the typical dose that would be prescribed to someone taking Trileptal? Dosage will vary depending on the age of the patient and the condition being treated.
What do I do if I miss a dose? You should never take extra doses of the medication to make up for missed doses. Talk to your doctor about how long you should wait in between doses.
What side effects can Trileptal cause? Common side effects can include:
It also is recommended that you wait to drive or operate machinery until you know how the medication affects you. It is also recommended that people avoid alcohol and illegal drugs while on the medication, as they can worsen adverse effects. Report major side effects to your doctor immediately, which can include swelling, thirst, nausea, vomiting, headache, confusion, decreased alertness, rash, fever, blisters, irritated eyes, blotches on skin, yellowing of skin or eyes, bleeding or bruising, joint pain, chest pain, signs of infection, or changes in urination. You can also report side effects to the FDA at 1-800-FDA-1088 or online.
What are the potential psychological side effects of taking Trileptal? Taking anticonvulsant medications can sometimes result in increased suicidal thoughts and behaviors in a small percentage of people. Seek medical help if you experience these thoughts or other changes in your behavior or mood.
Seek medical help if you experience these thoughts or other changes in your behavior or mood.
Is it safe for a woman who is pregnant, about to become pregnant, or nursing to take Trileptal? Trileptal may cause birth defects and fetal harm when taken during pregnancy. The drug can be transferred via breast milk and potentially harm a baby. Therefore, talk to your doctor if you are pregnant, planning to become pregnant, or are nursing before you take Trileptal. The medication can also impact the effectiveness of hormonal contraceptives.
Can symptoms occur if Trileptal is discontinued? It’s important not to discontinue use of the drug before talking with your doctor. Withdrawal symptoms of Trileptal can include insomnia and return of seizures or bipolar symptoms (depending on the purpose of taking the medication).
What should I do if I overdose on Trileptal? An overdose of Trileptal could be fatal, so seek immediate help or call the Poison Help Line at 1-800-222-1222 if you overdose. Overdose symptoms can include dizziness, sedation, decreased heart rate, low blood pressure, low sodium levels, seizures, and coma.
Overdose symptoms can include dizziness, sedation, decreased heart rate, low blood pressure, low sodium levels, seizures, and coma.
Is Trileptal habit-forming? Trileptal is not habit-forming, but it is not recommended that you discontinue use of the drug before talking with your doctor, as withdrawal symptoms can occur.
How much does Trileptal cost? According to goodrx.com, 60 tablets of 300 mg generic oxcarbazepine cost approximately $86.50. Sixty tablets of 300 mg Trileptal cost approximately $160.66.
Are there any disadvantages to Trileptal? The biggest disadvantages of Trileptal are potential side effects. Pregnant women are also typically advised not to take the medication due to the risks.
DISCLAIMER: The information contained herein should NOT be used as a substitute for the advice of an appropriately qualified and licensed physician or other healthcare providers. This article mentions drugs that were FDA-approved and available at the time of publication and may not include all possible drug interactions or all FDA warnings or alerts. The author of this page explicitly does not endorse this drug or any specific treatment method. If you have health questions or concerns about interactions, please check with your physician or go to the FDA site for a comprehensive list of warnings.
The author of this page explicitly does not endorse this drug or any specific treatment method. If you have health questions or concerns about interactions, please check with your physician or go to the FDA site for a comprehensive list of warnings.
- FDA – Trileptal
- NIH – Oxcarbazepine
Notes: This article was originally published January 2, 2017 and most recently updated September 12, 2022.
Mental Health Medications | NAMI: National Alliance on Mental Illness
Brand names: Trileptal®, Oxtellar XR®
- Tablet: 150 mg, 300 mg, 600 mg
- Liquid: 300 mg/5 mL
- Extended-release tablet: 150 mg, 300 mg, 600 mg
Generic name: oxcarbazepine (ox car BAZ e peen)
All FDA black box warnings are at the end of this fact sheet. Please review before taking this medication.
What Is Oxcarbazepine And What Does It Treat?
Oxcarbazepine is an antiepileptic medication that works in the brain to prevent and control seizures. It is approved for the treatment of partial seizures.
It is approved for the treatment of partial seizures.
Oxcarbazepine may also be helpful when prescribed “off-label” for nerve pain or as a mood stabilizer for bipolar disorder. “Off-label” means that it hasn’t been approved by the Food and Drug Administration for this condition. Your mental health provider should justify his or her thinking in recommending an “off-label” treatment. They should be clear about the limits of the research around that medication and if there are any other options.
Bipolar disorder involves episodes of depression and/or mania.
Symptoms of depression include:
- Depressed mood — feeling sad, empty, or tearful
- Feeling worthless, guilty, hopeless, or helpless
- Loss of interest or pleasure in normal activities
- Sleep and eat more or less than usual (for most people it is less)
- Low energy, trouble concentrating, or thoughts of death (suicidal thinking)
- Psychomotor agitation (‘nervous energy’)
- Psychomotor retardation (feeling like you are moving in slow motion)
Symptoms of mania include:
- Feeling irritable or “high”
- Having increased self esteem
- Feeling like you don’t need to sleep
- Feeling the need to continue to talk
- Feeling like your thoughts are too quick (racing thoughts)
- Feeling distracted
- Getting involved in activities that are risky or could have bad consequences (e.
 g., excessive spending)
g., excessive spending)
What Is The Most Important Information I Should Know About Oxcarbazepine?
Bipolar disorder requires long-term treatment. Do not stop taking oxcarbazepine, even when you feel better. With input from you, your health care provider will assess how long you will need to take the medicine. Missing doses of oxcarbazepine may increase your risk for a relapse in your mood symptoms.
Do not stop taking oxcarbazepine or change your dose without talking to with your healthcare provider first.
In order for oxcarbazepine to work properly, it should be taken every day as ordered by your healthcare provider.
Periodically, your healthcare provider may ask you to provide a blood sample to make sure the appropriate level of medication is in your body and to assess for side effects, such as changes in blood cell counts and sodium levels.
Are There Specific Concerns About Oxcarbazepine And Pregnancy?
If you are planning on becoming pregnant, notify your healthcare provider so that he/she can best manage your medications. People living with bipolar disorder who wish to become pregnant face important decisions. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
People living with bipolar disorder who wish to become pregnant face important decisions. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
Oxcarbazepine has been associated with an increased risk of craniofacial defects and heart malformations. There may be precautions to decrease the risk of this effect. Do not stop taking oxcarbazepine without first speaking to your healthcare provider. Discontinuing mood stabilizer medications during pregnancy has been associated with a significant increase in symptom relapse.
Regarding breast-feeding, caution is advised since oxcarbazepine does pass into breast milk.
What Should I Discuss With My Healthcare Provider Before Taking Oxcarbazepine?
- Symptoms of your condition that bother you the most
- If you have thoughts of suicide or harming yourself
- Medications you have taken in the past for your condition, whether they were effective or caused any adverse effects
- If you experience side effects from your medications, discuss them with your provider.
 Some side effects may pass with time, but others may require changes in the medication.
Some side effects may pass with time, but others may require changes in the medication. - Any other psychiatric or medical problems you have
- All other medications you are currently taking (including over the counter products, herbal and nutritional supplements) and any medication allergies you have
- Other non-medication treatment you are receiving, such as talk therapy or substance abuse treatment. Your provider can explain how these different treatments work with the medication.
- If you are pregnant, plan to become pregnant, or are breast-feeding
- If you drink alcohol or use illegal drugs
How Should I Take Oxcarbazepine?
Oxcarbazepine is usually taken once or twice a day with or without food.
Typically patients begin at a low dose of medicine and the dose is increased slowly over several weeks.
The dose usually ranges from 900-1200 mg per day. People taking this medication for seizure control may take up to 2400 mg daily. Only your healthcare provider can determine the correct dose for you.
Only your healthcare provider can determine the correct dose for you.
Oxcarbazepine suspension: Measure your dose with a dosing spoon or oral syringe, which you can get from your pharmacy. Before using, shake the bottle well to ensure the medicine is mixed thoroughly. Your dose can be mixed in a small glass of water just prior to taking or may be swallowed directly from the dosing spoon.
Use a calendar, pillbox, alarm clock, or cell phone alert to help you remember to take your medication. You may also ask a family member or a friend to remind you or check in with you to be sure you are taking your medication.
What Happens If I Miss A Dose Of Oxcarbazepine?
If you miss a dose of oxcarbazepine, take it as soon as you remember, unless it is closer to the time of your next dose. Discuss this with your healthcare provider. Do not double your dose or take more than what is prescribed.
What Should I Avoid While Taking Oxcarbazepine?
Avoid drinking alcohol or using illegal drugs while you are taking oxcarbazepine.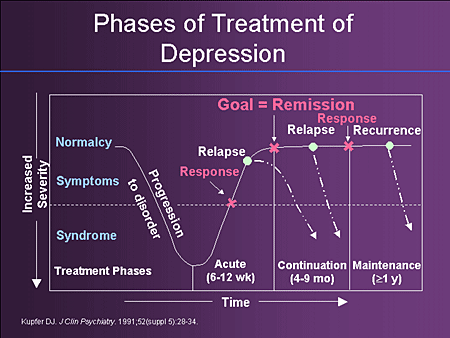 They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g., sedation, dizziness) of the medication.
They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g., sedation, dizziness) of the medication.
What Happens If I Overdose With Oxcarbazepine?
If an overdose occurs call your doctor or 911. You may need urgent medical care. You may also contact the poison control center at 1-800-222-1222.
A specific treatment to reverse the effects of oxcarbazepine does not exist.
What Are Possible Side Effects Of Oxcarbazepine?
Common side effects
- Dizziness
- Drowsiness
- Vision changes
- Nausea/vomiting
- Headache
- Fatigue
- Uncoordinated movements
- Tremor
Rare/serious side effects
Oxcarbazepine can cause a decrease in the body’s sodium level. Some signs of low sodium include nausea, tiredness, lack of energy, headache, confusion, or more frequent or more severe seizures.
Oxcarbazepine may also cause allergic reactions or serious problems which may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
You may or may not have a rash with these types of reactions.
In rare cases (<1%) a severe, spreading rash with blistering of the skin in patches over the entire body along with fever, headache and cough can occur (Stevens-Johnson syndrome). Although this is rare with oxcarbazepine, discontinuation of this medication is necessary. Rare cases of severe allergic reactions have been reported. Symptoms include swelling of the face, eyes, lips, or tongue, difficulty swallowing or breathing. If you experience any of these side effects, it is important to seek medical care immediately.
Studies have found that individuals who take antiepileptic medications including oxcarbazepine may be twice as likely to have suicidal thoughts or behaviors as individuals who take placebo (inactive medication). These thoughts or behaviors are rare and occurred in approximately 1 in 500 patients taking the antiepileptic class of medications. If you experience any thoughts or impulses to hurt yourself, you should contact your doctor immediately.
Are There Any Risks For Taking Oxcarbazepine For Long Periods Of Time?
To date, there are no known problems associated with long term use of oxcarbazepine. It is a safe and effective medication when used as directed.
It is important to note that some of the side effects listed above (particularly changes in blood sodium, rash, and suicidal thoughts) may continue to occur or worsen if you continue taking the medication. It is important to follow up with your doctor for blood work and to contact your doctor immediately if you notice any skin rash or changes in mood or behavior.
What Other Medications May Interact With Oxcarbazepine?
The seizure medications phenobarbital and phenytoin may decrease the effects of oxcarbazepine.
Oxcarbazepine may increase the effects of phenobarbital and phenytoin.
Oxcarbazepine may decrease the level and effects of:
- Oral contraceptives (birth control pills)
- Anti-rejection medications used in organ transplants, like tacrolimus (Prograf®) and cyclosporine (Neoral®, Sandimmune®)
- Certain blood pressure medications, such as amlodipine (Norvasc®) or felodipine (Plendil®)
How Long Does It Take For Oxcarbazepine To Work?
It is very important to tell your doctor how you feel things are going during the first few weeks after you start taking oxcarbazepine and whether or not you are experiencing any side effects from the medication. It will probably take several weeks to see big enough changes in your symptoms to decide if oxcarbazepine is the right medication for you.
It will probably take several weeks to see big enough changes in your symptoms to decide if oxcarbazepine is the right medication for you.
Mood stabilizer treatment is generally needed lifelong for persons with bipolar disorder. Your doctor can best discuss the duration of treatment you need based on your symptoms and illness.
Summary of FDA Black Box Warnings
There are no FDA black box warnings for oxcarbazepine.
Provided by
(June 2019)
©2019 The College of Psychiatric and Neurologic Pharmacists (CPNP) and the National Alliance on Mental Illness (NAMI). CPNP and NAMI make this document available under the Creative Commons Attribution-No Derivatives 4.0 International License. Last Updated: January 2016.
This information is being provided as a community outreach effort of the College of Psychiatric and Neurologic Pharmacists. This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
New gold standard | www.mgzt.ru
Within the framework of the VI All-Russian Congress of Neurologists held in Yaroslavl, a symposium "New possibilities for the use of antiepileptic drugs in neurology" was held, which was organized by Novartis Pharma Services Inc. The presidium of the scientific meeting included Academician of the Russian Academy of Medical Sciences Nikolai Yakhno, Chairman of the Russian Anti-Epileptic League Professor Gagik Avakyan, Secretary of the Russian Anti-Epileptic League Professor Alla Gekht, Chief Expert Pediatric Neurologist of the Ministry of Health and Social Development of Russia Professor Larisa Kalinina.
Differences and advantages
Professor Konstantin Mukhin (Russian State Medical University) opened the symposium with the report “Clinical experience with Trileptal (oxcarbazepine) in symptomatic focal epilepsy”. He spoke in detail about the history of creation and requirements for antiepileptic drugs, which include high efficiency in the treatment of epilepsy, a wide range of effects on various seizures, the absence of seizure aggravation, and good tolerance.
The speaker dwelled on the general principles of epilepsy treatment - the effectiveness of monotherapy, prescribing drugs depending on the form of the disease, the nature of the attacks and the age of the patients. It was emphasized that if a drug was not effective enough, it should be gradually withdrawn and replaced by another.
Pharmacoeconomic data were presented, according to which, per inpatient, only antiepileptic drugs in Germany were spent per month in 1986 - 28 euros, in 1992 - 33 euros, in 2001 - 70 euros (when new generation drugs entered clinical practice), in 2002 - 92 euros. The author stressed that this figure will grow due to the cost of modern drugs for the treatment of epilepsy. However, the costs to society associated with the disability of patients with epilepsy are much greater, which is why the availability of new effective drugs is important.
The author stressed that this figure will grow due to the cost of modern drugs for the treatment of epilepsy. However, the costs to society associated with the disability of patients with epilepsy are much greater, which is why the availability of new effective drugs is important.
Trileptal (oxcarbazepine) is a drug that has become firmly established in clinical practice and has been used in some countries for 10 years. It preferentially blocks voltage-gated sodium and calcium channels, peaking at 4.5 hours after oral administration of 600 mg. Trileptal has a linear pharmacokinetics, unlike, for example, otdifenin and phenytoin, where, due to non-linear pharmacokinetics, even with a small increase in dose, it is possible to quickly reach a peak concentration and get a toxic effect.
The binding of Trileptal to plasma proteins is low - 40%. This is especially important in pediatric practice, where drugs with high plasma protein binding must be used with caution.
The fundamental difference between Trileptal and drugs of the carbamazepine group is a different metabolic pathway with the formation of a completely different metabolite - monohydroxy derivative. Thus, during the transformation of Trileptal, the body does not form 10,11 epoxide, the active metabolite of carbamazepine, which is traditionally associated with its main side effects. A side effect that is possible when taking both carbamazepine and Trileptal is hyponatremia, that is, a decrease in the level of sodium in the blood plasma of less than 120 mmol / l. Hyponatremia occurs more often in adults, especially in the elderly; in pediatric practice, hyponatremia is rare.
Thus, during the transformation of Trileptal, the body does not form 10,11 epoxide, the active metabolite of carbamazepine, which is traditionally associated with its main side effects. A side effect that is possible when taking both carbamazepine and Trileptal is hyponatremia, that is, a decrease in the level of sodium in the blood plasma of less than 120 mmol / l. Hyponatremia occurs more often in adults, especially in the elderly; in pediatric practice, hyponatremia is rare.
The half-life is on average 2.5 hours for Trileptal itself (oxcarbazepine) and 9.5 hours for its metabolite, monohydroxy derivative, which allows you to take the drug 2 times a day. 95% of the drug is metabolized in the kidneys.
An important advantage of oxcarbazepine is that, unlike the drugs of the carbamazepine group, it is a weak inducer of microsomal liver enzymes and does not affect the metabolism of other drugs. Thus, it is convenient to combine oxcarbazepine with other antiepileptic drugs, knowing that their concentration will not change significantly. However, caution should be exercised when using oxcarbazepine while taking oral contraceptives.
However, caution should be exercised when using oxcarbazepine while taking oral contraceptives.
Trileptal is currently positioned as a starting monotherapy for symptomatic focal forms of epilepsy in adults and children over 2 years of age. In the near future, Trileptal suspension for very young children will be approved for use.
The second indication is the use as adjunctive therapy for symptomatic forms of focal epilepsy. Trileptal is contraindicated in patients with typical absence seizures or myoclonus within idiopathic generalized forms of epilepsy.
Dosages for children - from 8 to 46 mg / kg of body weight per day, the average dose is 20-30 mg / kg of body weight per day in two divided doses. For adults - 10-45 mg per kg of body weight per day, an average of 1000 mg / day. The starting dose ranges from 150 mg/day in young children to 300 mg/day in older children, followed by a weekly increase in dose from 150 to 200 mg. For adults, it is also possible to titrate the dose from 150 mg with a further weekly increase in the dose by 600 mg.
According to clinical studies, in 90% of patients with partial epilepsy, a decrease in the frequency of seizures by more than 50% was observed, and in 60% of patients with newly diagnosed epilepsy, complete relief of seizures was observed with Trileptal monotherapy.
The drug demonstrates the highest percentage of retention on therapy - up to 91% of patients continue to take Trileptal during the year. Interruption of therapy due to side effects - only 3%. An important point - if necessary, it is possible to replace other antiepileptic drugs with Trileptal "overnight", that is, switching drugs can be one-step.
Therapy of neuropathic pain
Professor Valery Alekseev (Moscow Medical Academy named after I.M. Sechenov) in the report "Anticonvulsants in the treatment of neuropathic pain" spoke in detail about the functioning of the nociceptive and antinociceptive systems, the mechanisms of pain formation, and gave characteristics to various types of pain.
The therapy strategy for neuropathic pain is based on a complex effect both on the peripheral components of the system that conduct pain, and on the regulation of behavior and the emotional sphere. All anticonvulsants used to treat neuropathic pain are conditionally divided into three groups: blockers of voltage-gated sodium channels; drugs that enhance GABA-ergic activity; drugs that block the release of activating amino acids (glutamate and aspartate). Trileptal blocks voltage-dependent sodium and calcium channels, opens potassium channels, that is, creates the effect of membrane hyperpolarization, as a result, the peripheral nociceptive stimulus is not perceived, according to the “busy here” principle, and finally blocks the release of activating amino acids, which complements the overall effect against the formation of neuropathic pain . It is important that with the combined use of a wide range of drugs with an effect on peripheral receptor apparatus, Trileptal has practically no interaction with other drugs, that is, it can be actively used in complex treatment.
There are data on the use of Trileptal in traumatic lesions of the spinal cord, complex regional pain syndrome type 1, postherpetic neuralgia, trigeminal neuralgia, diabetic neuropathy with pain syndrome.
Mood corrector
Prof. Olga Vorobieva (MMA named after I.M. Sechenov) made a presentation on “Normothymic action of antiepileptic drugs”.
After the introduction of the first anticonvulsants to the market, it became clear that in addition to the effect on epileptic seizures, they affect psychopathological syndromes.
Primarily, anticonvulsants can be used for mood disorders.
Mood disorders are divided into bipolar and depressive, these disorders are extremely numerous. And if neurologists do not encounter bipolar disorders of the 1st type, when manias are replaced by depression, in their practice, they constantly encounter bipolar disorder of the 2nd type, in which mania does not reach the degree of manic psychosis. These are shallow, mild depressions, mood disorders induced by drugs used in neurology or caused by somatic suffering, including neurological.
Anticonvulsants stabilize mood and are commonly referred to as mood correctors. Bipolar disorder is characterized by alternating manic and depressive episodes, with each successive episode requiring a lesser trigger. The gaps between the phases shorten, similar to the epilepsy model, where each seizure paves the way for the next.
First-generation anticonvulsants have proven to be truly mood stabilizing, highly effective for acute mania and long-term treatment of bipolar disorder. Modern anticonvulsants entering the market do not have such a stabilizing effect. Some of them are effective against PTSD, post-stress anxiety, some are effective against depression, but not enough to be called mood correctors. New generation drugs have a narrower, selective psychotropic spectrum.
Looking at the level of sales of anticonvulsants, it is clear that first-generation drugs remain very attractive. Therefore, now some new drugs are being developed on the basis of molecules of the first generation of anticonvulsants. Trileptal is very close in action to carbamazepine and retains its effects with a much more favorable tolerability spectrum. Trileptal gives fewer allergic reactions compared to carbamazepine and, which is very important for the treatment of psychopathological syndromes, interacts significantly less with psychotropic drugs, it has better performance in combined treatment, no matter what it is prescribed with - anticonvulsants or psychotropic drugs.
Trileptal is very close in action to carbamazepine and retains its effects with a much more favorable tolerability spectrum. Trileptal gives fewer allergic reactions compared to carbamazepine and, which is very important for the treatment of psychopathological syndromes, interacts significantly less with psychotropic drugs, it has better performance in combined treatment, no matter what it is prescribed with - anticonvulsants or psychotropic drugs.
Trileptal (oxcarbazepine) is similar in its mechanisms of action and clinical effects to carbamazepine and can be used as a mood corrector, for the treatment of manic-depressive psychosis, while mainly for the treatment of mania (for the correction of depression in doctors, there is already a fairly large arsenal of drugs) . As a mood corrector, the drug has been investigated in four large controlled trials, where dosages from 900 to 2300 mg per day were used. Its action was compared with antipsychotics (haloperidol), lithium and placebo. With regard to haloperidol, oxcarbazepine has a similar efficacy, and in some aspects looks more attractive, the same with lithium.
With regard to haloperidol, oxcarbazepine has a similar efficacy, and in some aspects looks more attractive, the same with lithium.
Trileptal (oxcarbazepine) is used as a mood corrector at dosages of 900-1200 mg per day. Neurologists deal with incomplete syndromes and use mainly the dosage of 900 mg per day. Psychiatrists use dosages sometimes significantly higher than 1200 mg per day.
Albert KHISAMOV , corr. "MG".
‹ Where are Piglet and I going? Up I forgot what candles are lit on New Year's Eve... ›
| 💊 Composition of Trileptal ® ✅ Use of Trileptal ® Save Search for analogues Interaction Description of the active ingredients of the preparation Trileptal ® (Trileptal ® ) The scientific information provided is general and cannot be used to make decisions. Update date: 2020.05.07 Marketing authorization holder:NOVARTIS PHARMA, AG (Switzerland)
Made:NOVARTIS PHARMA STEIN, AG (Switzerland) ATX code: N03AF02 (Oxcarbazepine) Active substance: oxcarbazepine (oxcarbazepine) Rec.INN WHO registered Dosage form
Release form, packaging and composition Trileptal® Film-coated tablets yellow, oval, slightly biconvex, scored on both sides, marked "TF/TF" on one side and "CG/CG" on the other. Clinical and pharmacological group: Anticonvulsant drug Pharmacotherapeutic group: Anticonvulsant Pharmacological action Antiepileptic. Pharmacological activity is primarily due to the action of the metabolite - monohydroxy derivative (MHP) oxcarbazepine. The implementation of the anticonvulsant action is facilitated by an increase in the conductivity of potassium ions and the modulation of calcium channels activated by a high membrane potential. There was no significant interaction with brain neurotransmitters or receptor binding. Experimental studies have shown that oxcarbazepine and its metabolite have a pronounced anticonvulsant effect. The efficacy of oxcarbazepine in epileptic seizures has been demonstrated both as monotherapy and when oxcarbazepine is used in combination therapy in children and adults. Pharmacokinetics After oral administration, oxcarbazepine is completely absorbed and largely metabolized to form the pharmacologically active metabolite, the 10-monohydroxy derivative. Binding of the metabolite to plasma proteins, mainly albumin, is about 40%. In the therapeutic range, the degree of binding does not depend on the concentration of the drug in the blood serum. Oxcarbazepine and MGP do not bind to α 1 -acid glycoprotein. Apparent V d - 49 l. With ss MHD in blood plasma is achieved on days 2-3 when taking oxcarbazepine 2 times / day. In the equilibrium state, the pharmacokinetic parameters of MGP are linear and dose-dependent in the range of daily doses of 300 mg - 2400 mg. Oxcarbazepine is rapidly metabolized by hepatic cytosolic enzymes to the pharmacologically active metabolite MHD, which undergoes further glucuronidation. Oxcarbazepine is excreted as metabolites mainly by the kidneys (95%), less than 1% is excreted unchanged. Approximately 80% of excreted metabolites are MHD, of which 49% are glucuronides and 27% are unchanged MGP. DGP is excreted unchanged (about 3%), oxcarbazepine conjugates account for 13%. About 4% of the dose is excreted in the feces. Oxcarbazepine is rapidly excreted from the blood plasma, the apparent T 1/2 is 1.3 - 2.3 hours. The apparent T 1/2 MGP averages 9.3 ± 1.8 hours. With CC less than 30 ml / min after a single dose of 300 mg of oxcarbazepine T 1/2 MHL increases to 19 hours, and AUC doubles. Weight-adjusted clearance of MHD in children decreases with age and body weight, approaching that of adults. Weight-adjusted clearance in children aged 1 month to 4 years is 93% higher than in adults. After taking oxcarbazepine at a single dose of 300 mg or multiple doses of 600 mg/day in healthy volunteers aged 60-82 years, plasma C max and AUC values for MHD were 30-60% higher compared with the same indicators in young volunteers (18-32 years old), which is associated with an age-related decrease in CC. Pregnant women experience a number of physiological changes that can lead to a gradual decrease in plasma levels of MHD during pregnancy. Indications of the active substances of the drug Trileptal®Simple and complex partial epileptic seizures with or without secondary generalization in adults and children aged 1 month and older. Generalized tonic-clonic epileptic seizures in adults and children aged 2 years and older. Open list of ICD-10 codes
Dosage regimenThe route of administration and dosing regimen of a particular drug depends on its form of release and other factors. The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed. Take orally. Initial dose - 8-10 mg / kg body weight / day. For patients with impaired renal function (CC less than 30 ml / min), adjustment of the initial dose and dosing regimen is required. Side effectsMost common (≥ 10%) : drowsiness, headache, dizziness, diplopia, nausea, vomiting, fatigue. From the side of the hematopoietic system: sometimes - leukopenia; very rarely - suppression of bone marrow hematopoiesis, agranulocytosis, aplastic anemia, neutropenia, pancytopenia, thrombocytopenia. On the part of the immune system: very rarely - hypersensitivity reactions accompanied by fever and rash (including multiple organ disorders). With the development of hypersensitivity reactions, damage to the circulatory and lymphatic systems (eosinophilia, thrombocytopenia, lymphadenopathy, splenomegaly), muscles and joints (myalgia, swelling in the joints, arthralgia), nervous system (encephalopathy), kidneys (proteinuria, interstitial nephritis, renal failure) is possible. From the side of metabolism: often - hyponatremia; very rarely - clinically significant hyponatremia (sodium concentration <125 mmol / l - usually during the first 3 months of drug therapy; in some patients - more than 1 g after the start of treatment), leading to the development of such manifestations and symptoms as seizures, confusion, decreased level of consciousness, encephalopathy, visual disturbances (including blurred vision), nausea, vomiting, folic acid deficiency; very rarely - hypothyroidism. From the side of the central nervous system: very often - drowsiness, headache, dizziness; often - ataxia, tremor, nystagmus, impaired attention, amnesia; confusion, depression, apathy, agitation, emotional lability. From the senses: very often - diplopia; often - visual impairment, blurred vision, vertigo. From the side of the cardiovascular system: very rarely - arrhythmias, AV blockade, arterial hypertension. From the digestive system: very often - nausea, vomiting; often - diarrhea, constipation, abdominal pain; sometimes - an increase in the activity of liver enzymes, an increase in the concentration of alkaline phosphatase in the blood; very rarely - pancreatitis and / or increased levels of lipase and / or amylase, hepatitis. Dermatological reactions: often - rash, alopecia, acne. Allergic reactions: sometimes - urticaria; very rarely - angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell's syndrome), erythema multiforme. Other: very often - feeling tired; often - asthenia; very rarely - systemic lupus erythematosus. In children under 4 years of age: very often (11%) - drowsiness; often (≥1%–<10%) - ataxia, irritability, vomiting, lethargy, fatigue, nystagmus, tremor, loss of appetite, increased concentration of uric acid in the blood. Contraindications for useChildren under 3 years of age; hypersensitivity to oxcarbazepine. Pregnancy and lactationLimited experience with pregnancy. Reports suggest that oxcarbazepine use during pregnancy may be associated with the development of birth defects (eg, cleft palate). In experimental studies with the use of oxcarbazepine in toxic doses, there was an increase in embryonic mortality, slowing down and impaired development and growth of the fetus. If the patient plans to become pregnant or becomes pregnant while using oxcarbazepine, and if there is a question about the use of oxcarbazepine during pregnancy, the expected benefits of therapy and the possible risk to the fetus, especially in the first trimester of pregnancy, should be carefully weighed. Use the lowest effective dose of oxcarbazepine during pregnancy. When clinically effective in women of childbearing age, oxcarbazepine should be used as monotherapy. Effective antiepileptic treatment should not be interrupted during pregnancy, as disease progression may adversely affect the mother and fetus. Folic acid deficiency is known to develop during pregnancy. Antiepileptic drugs can exacerbate this deficiency, which is one of the possible causes of fetal developmental disorders, so additional folic acid supplementation is recommended. When used during pregnancy, it must be taken into account that the physiological changes occurring in the body of a pregnant woman can lead to a gradual decrease in the concentration of the active metabolite in the blood plasma. To achieve maximum control of the symptoms of the disease, it is necessary to regularly evaluate the clinical effect of oxcarbazepine and determine the concentration of the metabolite in the blood plasma. Determination of the concentration of MHD in blood plasma is also recommended in the postpartum period, especially if the dose of oxcarbazepine was increased during pregnancy. There are reports that the use of antiepileptic drugs during pregnancy can lead to increased bleeding in newborns. As a precautionary measure, it is recommended that vitamin K 1 be administered during the last few weeks of pregnancy and to newborns whose mothers received oxcarbazepine. Oxcarbazepine and MHD cross the placental barrier and are excreted in breast milk. The concentration ratio in milk and plasma was 0.5 for both substances. Since the effect on newborns of oxcarbazepine and MHD, received with mother's milk, is not known, oxcarbazepine should not be used during breastfeeding. Precautions Use with caution in patients with known hypersensitivity to carbamazepine, as in this group of patients, approximately 25-30% of cases may develop hypersensitivity reactions to oxcarbazepine. In patients with no history of hypersensitivity to carbamazepine, it is also possible to develop hypersensitivity reactions to oxcarbazepine, including multiple organ disorders. Use with caution in patients with severe hepatic impairment. In patients with impaired renal function and low serum sodium levels, or in patients receiving concomitant treatment with drugs that promote sodium excretion from the body (diuretics, drugs that affect the secretion of ADH), the concentration should be determined before starting therapy with oxcarbazepine sodium in blood serum. In the future, the concentration of sodium in the blood serum should be monitored 2 weeks after the start of therapy and then monthly for 3 months or as needed. With particular attention to these risk factors should be treated in elderly patients. If it is necessary to prescribe diuretics and other drugs that reduce the concentration of sodium in the blood serum, patients receiving oxcarbazepine should follow the same recommendations. If clinical symptoms appear that suggest hyponatremia, serum sodium concentration should be measured. With the development of symptoms of severe bone marrow suppression, consider discontinuing oxcarbazepine. Patients receiving anticonvulsants rarely experienced episodes of suicidal behavior and thoughts. The mechanism of increased risk of suicide in this category of patients has not been established. Therefore, at all stages of treatment, careful monitoring of patients receiving oxcarbazepine is necessary. Weight should be monitored in all patients with heart failure to detect fluid retention in a timely manner. With fluid retention or with the progression of symptoms of heart failure, the concentration of sodium in the blood serum should be determined. If hyponatremia occurs, fluid intake should be limited. When using oxcarbazepine, in very rare cases, a violation of cardiac conduction is possible, therefore, during the period of treatment, careful monitoring of patients with previous conduction disorders (AV blockade, arrhythmia) is necessary. Dermatological reactions have been observed in both children and adults with oxcarbazepine and developed on average 19 days after the start of treatment. There are isolated reports of recurrence of skin reactions when oxcarbazepine is restarted. With the development of skin reactions against the background of the use of oxcarbazepine, consideration should be given to its cancellation and the appointment of another antiepileptic agent. If hepatitis is suspected, discontinuation of oxcarbazepine should be considered. As with all antiepileptic drugs, oxcarbazepine should be discontinued gradually due to the risk of increased seizure frequency. Influence on the ability to drive vehicles and mechanisms Patients who develop dizziness, drowsiness or other disorders of the central nervous system during the use of oxcarbazepine should not drive vehicles or work with mechanisms during the period of treatment. Drug interactions Oxcarbazepine and its pharmacologically active metabolite MHD are inhibitors of cytochrome CYP2C19. As inducers of CYP3A4 and CYP3A5, oxcarbazepine and MHD reduce plasma concentrations of drugs metabolized by these isoenzymes: dihydropyridine calcium antagonists, oral contraceptives, and antiepileptic drugs (eg, carbamazepine). With simultaneous use with oxcarbazepine, a decrease in plasma concentrations of other drugs that are substrates of CYP3A4 and CYP3A5 enzymes (for example, drugs of the immunosuppressant group - cyclosporine) is also possible. Because MHD is a weak inducer of UDP-glucuronyl transferase in vitro, it is therefore unlikely that it would interfere in vivo with the metabolism of drugs excreted as glucuronic acid conjugates (eg, valproic acid and lamotrigine). In vitro studies have confirmed the weak inducing ability of oxcarbazepine and MGP against isoenzymes of the CYP2B and CYP3A4 enzyme subsystems. The inducing effect of oxcarbazepine and MHD on other CYP isoenzymes is unknown. Plasma concentrations of phenytoin increase by up to 40% with concomitant use of oxcarbazepine at a dose of 1200 mg/day or more. Therefore, when using oxcarbazepine at such doses, a reduction in the dose of phenytoin may be required. The increase in serum concentrations of phenobarbital with simultaneous use with oxcarbazepine is insignificant (15%). Simultaneous administration of strong inducers of cytochrome P450 isoenzymes (i. Oxcarbazepine has been shown to interact with ethinyl estradiol and levonorgestrel. The average AUC values for them decreased by 48-52% and 35-52%, respectively. Interaction studies of oxcarbazepine with other oral or implantable contraceptives have not been conducted. Thus, the simultaneous use of oxcarbazepine and hormonal contraceptives may lead to a decrease in the effectiveness of the latter. Co-administration of oxcarbazepine and felodipine may result in a 28% decrease in felodipine AUC, although plasma concentrations remain within the therapeutic range. On the other hand, with simultaneous use with verapamil, a decrease in the concentration of MHD in the blood serum by 20% is possible. This reduction has no clinical significance. Cimetidine, erythromycin, dextropropoxyphene do not affect the pharmacokinetic parameters of MHD; viloxazine slightly affects the concentration of MHD in plasma (the concentration of MHD increases by 10% after repeated co-administration). |
 decisions about the use of a particular drug.
decisions about the use of a particular drug.  , coated film coated, 600 mg: 10, 20, 30 or 50 pcs.
, coated film coated, 600 mg: 10, 20, 30 or 50 pcs.  The mechanism of action of oxcarbazepine and its metabolite is mainly associated with the blockade of voltage-dependent sodium channels, which leads to stabilization of overexcited neuronal membranes, inhibition of the occurrence of serial neuronal discharges and a decrease in synaptic conduction of impulses.
The mechanism of action of oxcarbazepine and its metabolite is mainly associated with the blockade of voltage-dependent sodium channels, which leads to stabilization of overexcited neuronal membranes, inhibition of the occurrence of serial neuronal discharges and a decrease in synaptic conduction of impulses.  After a single dose of oxcarbazepine, depending on the dosage form used, C max metabolite in plasma is 24.9-34 μmol / l, the average time to reach it is about 4.5-6 hours. Pharmacokinetic studies have shown that 2 % oxcarbazepine and 70% MGP; the rest is accounted for by secondary metabolites, rapidly excreted from the blood plasma.
After a single dose of oxcarbazepine, depending on the dosage form used, C max metabolite in plasma is 24.9-34 μmol / l, the average time to reach it is about 4.5-6 hours. Pharmacokinetic studies have shown that 2 % oxcarbazepine and 70% MGP; the rest is accounted for by secondary metabolites, rapidly excreted from the blood plasma.  Minor amounts of MHP (about 4% of the dose) are oxidized to form an inactive metabolite - 10, 11-dihydroxy derivative (DHP).
Minor amounts of MHP (about 4% of the dose) are oxidized to form an inactive metabolite - 10, 11-dihydroxy derivative (DHP).  As a result, it is expected that the AUC of MHD in children of this age group will be 2 times less than that in adults when using the same doses (when adjusted for body weight). Weight-adjusted clearance in children aged 4 to 12 years is 43% higher than in adults. The estimated AUC of MHD in children of this age group is 2/3 of that in adults when using the same doses (when adjusted for body weight). It is assumed that in children aged 13 years and older, due to the increase in body weight, the clearance of MHD, adjusted for body weight, corresponds to the clearance of MHD in adults.
As a result, it is expected that the AUC of MHD in children of this age group will be 2 times less than that in adults when using the same doses (when adjusted for body weight). Weight-adjusted clearance in children aged 4 to 12 years is 43% higher than in adults. The estimated AUC of MHD in children of this age group is 2/3 of that in adults when using the same doses (when adjusted for body weight). It is assumed that in children aged 13 years and older, due to the increase in body weight, the clearance of MHD, adjusted for body weight, corresponds to the clearance of MHD in adults. 
 Further, the dose is adjusted depending on the treatment regimen, the age of the patient, the effectiveness of treatment, and kidney function.
Further, the dose is adjusted depending on the treatment regimen, the age of the patient, the effectiveness of treatment, and kidney function.  ), lungs (shortness of breath, pulmonary edema, bronchospasm, interstitial inflammation), abnormal liver function tests, angioedema, anaphylactic reactions.
), lungs (shortness of breath, pulmonary edema, bronchospasm, interstitial inflammation), abnormal liver function tests, angioedema, anaphylactic reactions. 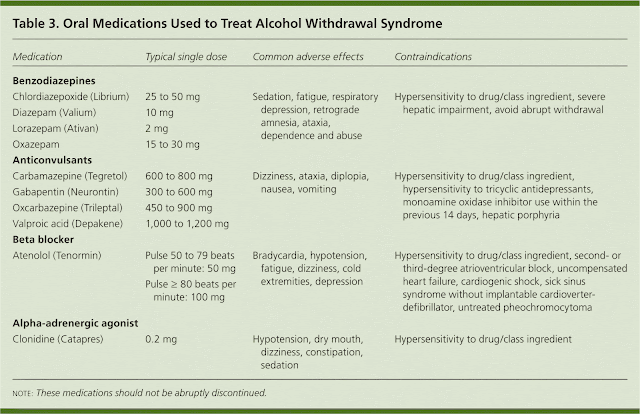



 In the event of an immediate hypersensitivity reaction, oxcarbazepine should be discontinued immediately and alternative therapy instituted.
In the event of an immediate hypersensitivity reaction, oxcarbazepine should be discontinued immediately and alternative therapy instituted.  For other patients, measurement of serum sodium concentration can be performed during routine blood tests.
For other patients, measurement of serum sodium concentration can be performed during routine blood tests. 
 Therefore, when using oxcarbazepine in high doses, drug interactions with drugs metabolized by CYP2C19 (phenobarbital, phenytoin) are possible. For some patients, dose reduction of drugs - substrates of CYP2C19 may be required. Oxcarbazepine and MHD have been shown to interact weakly or not at all with the following microsomal isoenzymes: CYP1A2, CYP2A6, CYP2C9, CYP2D9, CYP2E1, CYP4A4 and CYP4C11.
Therefore, when using oxcarbazepine in high doses, drug interactions with drugs metabolized by CYP2C19 (phenobarbital, phenytoin) are possible. For some patients, dose reduction of drugs - substrates of CYP2C19 may be required. Oxcarbazepine and MHD have been shown to interact weakly or not at all with the following microsomal isoenzymes: CYP1A2, CYP2A6, CYP2C9, CYP2D9, CYP2E1, CYP4A4 and CYP4C11.  But, taking into account even the weak inducing ability of oxcarbazepine and MHD, it may be necessary to increase the doses of concomitantly used drugs metabolized with the participation of CYP3A4 or UDP-glucuronyl transferase. If oxcarbazepine is discontinued, the dose of these drugs may need to be reduced.
But, taking into account even the weak inducing ability of oxcarbazepine and MHD, it may be necessary to increase the doses of concomitantly used drugs metabolized with the participation of CYP3A4 or UDP-glucuronyl transferase. If oxcarbazepine is discontinued, the dose of these drugs may need to be reduced.  e. carbamazepine, phenytoin and phenobarbital) reduces plasma concentrations of MHD (by 29-40%).
e. carbamazepine, phenytoin and phenobarbital) reduces plasma concentrations of MHD (by 29-40%).