Venlafaxine adverse effects
Side effects of venlafaxine - NHS
Like all medicines, venlafaxine can cause side effects in some people, but many people have no side effects, or only minor ones.
Some of the common side effects of venlafaxine will gradually improve as your body gets used to it.
Common side effects
These common side effects of venlafaxine happen in more than 1 in 100 people. There are things you can do to help cope with them:
Feeling sick (nausea)Try taking venlafaxine with or after food. It may also help if you avoid rich or spicy food. If it carries on, tell your doctor.
Sweating and hot flushesTry wearing loose clothing and using or a fan, where possible. If there is no improvement after a week, speak to your doctor.
Make sure you rest, and drink plenty of fluids. It’s also best to avoid drinking too much alcohol. Ask your pharmacist to recommend a painkiller.
Headaches usually go away after the first week of taking venlafaxine. Talk to your doctor if they last longer than a week or are severe.
A dry mouthChew sugar-free gum or sugar-free sweets.
Feeling dizzyIf venlafaxine makes you feel dizzy when you stand up, try getting up very slowly or stay sitting down until you feel better. If you begin to feel dizzy, lie down so that you do not faint, then sit until you feel better.
Do not drive, ride a bike or use tools or machinery if you feel dizzy.
Feeling sleepyDo not drive, ride a bike or use tools or machinery if you're feeling sleepy. Cut down the amount of alcohol you drink as this will make you feel more tired. If this symptom does not go away after a week or two, ask your doctor for advice.
If this symptom does not go away after a week or two, ask your doctor for advice.
Take venlafaxine first thing in the morning.
ConstipationGet more fibre into your diet such as fresh fruit and vegetables and cereals, and drink plenty of water. Try to exercise, for example, by going for a daily walk or run. If this does not help, talk to your pharmacist or doctor.
If this advice does not help and any of these side effects bother you, talk to your doctor or pharmacist.
Serious side effects
It's not common, but some people (less than 1 in 100) may have serious side effects when taking venlafaxine.
Book an appointment with your doctor if:
- you gain weight or lose weight without trying
- you have changes in your periods such as heavy bleeding, spotting, or bleeding between periods
Call your doctor or contact 111 now if:
- you get constant headaches, long-lasting confusion, weakness, or frequent muscle cramps – these can all be signs of low sodium levels in your blood you have feelings of overwhelming happiness (euphoria), excessive enthusiasm or excitement, or a feeling of restlessness that means you cannot sit or stand still
- you have unexplained muscle pain or weakness
- the whites of your eyes or skin turn yellow, although this may be less obvious on black or brown skin – this can be a sign of liver problems
- you have any changes in your eyesight, like blurred vision or dilated pupils
- you cough up blood or have blood in your pee
- you have black or red poo, or blood in your vomit – these can be signs of bleeding in the stomach
- you are bleeding from the gums, or have bruises that appear without a reason or that get bigger
- you get shortness of breath, or a fast or irregular heart beat
- you have thoughts about harming yourself or ending your life
Go to 111. nhs.uk or call 111.
nhs.uk or call 111.
Immediate action required: Call 999 now if:
- you get chest pain or pressure in your chest, shortness of breath, or a fast or irregular heart beat
Serious allergic reaction
In rare cases, it's possible to have a serious allergic reaction (anaphylaxis) to venlafaxine.
Immediate action required: Call 999 or go to A&E now if:
- you get a skin rash that may include itchy, red, swollen, blistered or peeling skin
- you're wheezing
- you get tightness in the chest or throat
- you have trouble breathing or talking
- your mouth, face, lips, tongue or throat start swelling
You could be having a serious allergic reaction and may need immediate treatment in hospital.
Long-term side effects
For most people, venlafaxine is safe to take for a long time and there are no lasting effects.
A few people may get sexual side effects, such as problems getting an erection or a lower sex drive. In some cases these can continue even after stopping the medicine. Speak to your doctor if you are worried.
Other side effects
These are not all the side effects of venlafaxine. For a full list, see the leaflet inside your medicine packet.
Information:
You can report any suspected side effect using the Yellow Card safety scheme.
Visit Yellow Card for further information.
Page last reviewed: 10 February 2022
Next review due: 10 February 2025
Venlafaxine Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
. .. Show More
.. Show More
Uses
Venlafaxine is used to treat depression. It may improve your mood and energy level, and may help restore your interest in daily living. Venlafaxine is known as a serotonin-norepinephrine reuptake inhibitor (SNRI). It works by helping to restore the balance of certain natural substances (serotonin and norepinephrine) in the brain.
How to use venlafaxine oral
Read the Medication Guide provided by your pharmacist before you start using venlafaxine and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth as directed by your doctor, usually 2 to 3 times daily with food.
The dosage is based on your medical condition and response to treatment. To reduce your risk of side effects, your doctor may direct you to start this medication at a low dose and gradually increase your dose. Follow your doctor's instructions carefully. Take this medication regularly to get the most benefit from it. To help you remember, take it at the same times each day.
To help you remember, take it at the same times each day.
Keep taking this medication even if you feel well. Do not stop taking this medication without consulting your doctor. Some conditions may become worse when this drug is suddenly stopped. Also, you may experience symptoms such as confusion, mood swings, blurred vision, headache, tiredness, sleep changes, and brief feelings similar to electric shock. Your dose may need to be gradually decreased to reduce side effects. Report any new or worsening symptoms right away.
It may take several weeks to feel the benefit of this medication. Tell your doctor if your condition lasts or gets worse.
Side Effects
See also Warning section.
Nausea, drowsiness, dizziness, dry mouth, constipation, loss of appetite, blurred vision, nervousness, trouble sleeping, unusual sweating, or yawning may occur. If any of these effects last or get worse, tell your doctor promptly.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Many people using this medication do not have serious side effects.
This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high.
Tell your doctor right away if you have any serious side effects, including: easy bruising/bleeding, decreased interest in sex, changes in sexual ability, muscle cramps/weakness, shaking (tremor).
Get medical help right away if you have any very serious side effects, including: cough that doesn't go away, shortness of breath, chest pain, severe/pounding headache, black/bloody stools, vomit that looks like coffee grounds, eye pain/swelling/redness, widened pupils, vision changes (such as seeing rainbows around lights at night), seizure.
This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before taking venlafaxine, tell your doctor or pharmacist if you are allergic to it; or to desvenlafaxine; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: bleeding problems, personal or family history of glaucoma (angle-closure type), high blood pressure, heart problems (such as heart failure, previous heart attack), high cholesterol, kidney disease, liver disease, seizure disorder, thyroid disease.
This drug may make you dizzy or drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Older adults may be more sensitive to the side effects of this drug, especially dizziness when standing. Older adults may also be more likely to develop a type of salt imbalance (hyponatremia), especially if they are taking "water pills" (diuretics). Dizziness and salt imbalance can increase the risk of falling. Older adults may also be at greater risk for bleeding while using this drug.
Older adults may also be more likely to develop a type of salt imbalance (hyponatremia), especially if they are taking "water pills" (diuretics). Dizziness and salt imbalance can increase the risk of falling. Older adults may also be at greater risk for bleeding while using this drug.
Children may be more sensitive to the side effects of the drug, especially loss of appetite and weight loss. Monitor weight and height in children who are taking this drug.
During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. Also, babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop withdrawal symptoms such as feeding/breathing difficulties, seizures, muscle stiffness, or constant crying. If you notice any of these symptoms in your newborn, tell the doctor promptly.
Since untreated mental/mood problems (such as depression, anxiety, panic attacks) can be a serious condition, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss the benefits and risks of using this medication during pregnancy with your doctor.
If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss the benefits and risks of using this medication during pregnancy with your doctor.
This drug passes into breast milk and may have undesirable effects on a nursing infant. Consult your doctor before breast-feeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: other drugs that can cause bleeding/bruising (including antiplatelet drugs such as clopidogrel, NSAIDs such as ibuprofen/naproxen, "blood thinners" such as dabigatran/warfarin).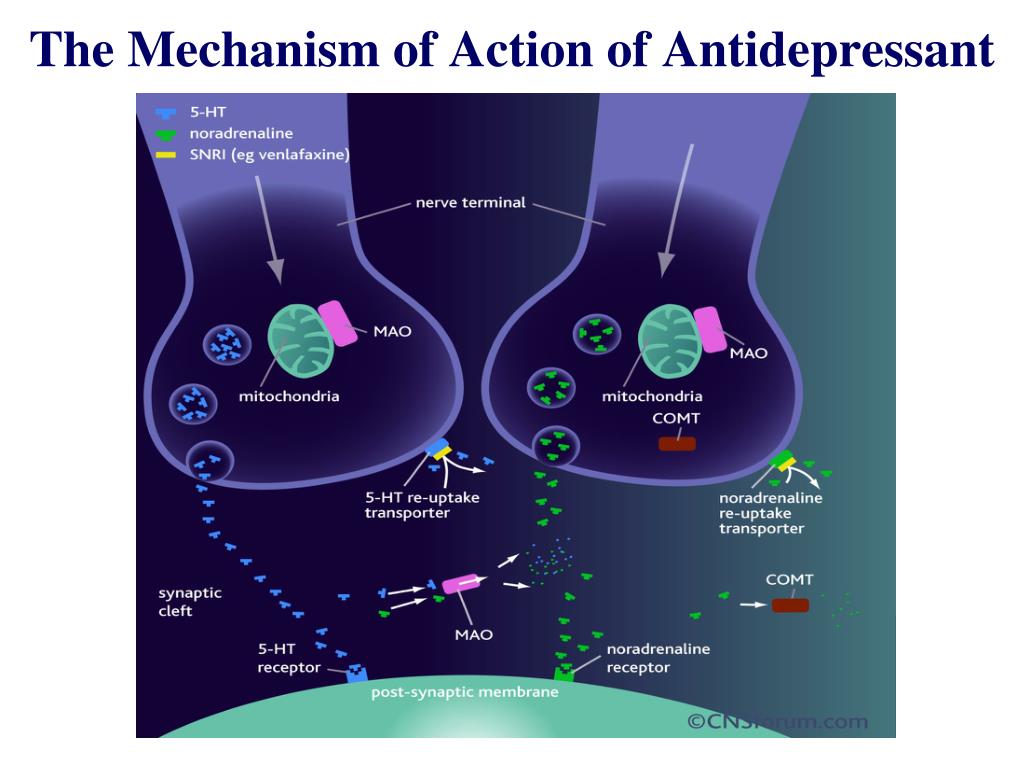
Aspirin can increase the risk of bleeding when used with this medication. However, if your doctor has directed you to take low-dose aspirin for heart attack or stroke prevention (usually 81-162 milligrams a day), you should continue taking it unless your doctor instructs you otherwise. Ask your doctor or pharmacist for more details.
Taking MAO inhibitors with this medication may cause a serious (possibly fatal) drug interaction. Avoid taking MAO inhibitors (isocarboxazid, linezolid, metaxalone, methylene blue, moclobemide, phenelzine, procarbazine, rasagiline, safinamide, selegiline, tranylcypromine) during treatment with this medication. Most MAO inhibitors should also not be taken for two weeks before and at least 7 days after treatment with this medication. Ask your doctor when to start or stop taking this medication.
The risk of serotonin syndrome/toxicity increases if you are also taking other drugs that increase serotonin. Examples include street drugs such as MDMA/"ecstasy," St.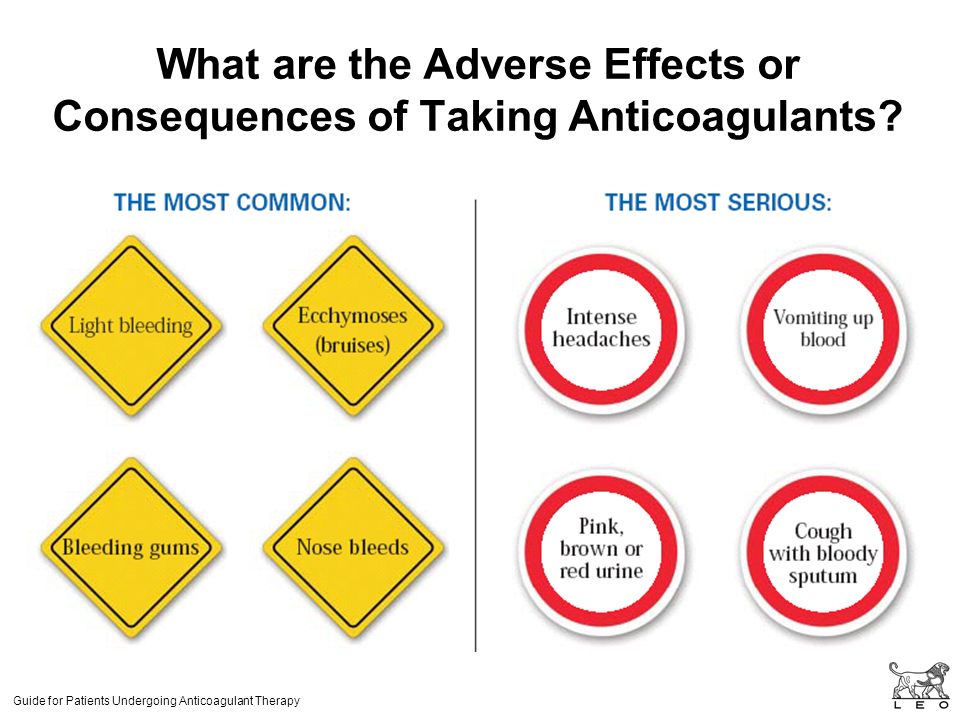 John's wort, certain antidepressants (including SSRIs such as fluoxetine/paroxetine, other SNRIs such as duloxetine/milnacipran), tryptophan, among others. The risk of serotonin syndrome/toxicity may be more likely when you start or increase the dose of these drugs.
John's wort, certain antidepressants (including SSRIs such as fluoxetine/paroxetine, other SNRIs such as duloxetine/milnacipran), tryptophan, among others. The risk of serotonin syndrome/toxicity may be more likely when you start or increase the dose of these drugs.
Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine).
Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
Venlafaxine is very similar to desvenlafaxine. Do not take medications containing desvenlafaxine while using venlafaxine.
This medication may interfere with certain lab tests (including urine tests for amphetamines), possibly causing false test results. Make sure lab personnel and all your doctors know you use this drug.
Make sure lab personnel and all your doctors know you use this drug.
Does venlafaxine oral interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: severe drowsiness, seizures, fast/irregular heartbeat.
Do not share this medication with others.
Keep all regular medical and psychiatric appointments. Laboratory and/or medical tests (such as blood pressure, cholesterol) should be performed periodically to monitor your progress or check for side effects. Consult your doctor for more details.
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
Next
Related Links
Drug Survey
Are you currently using venlafaxine oral?
This survey is being conducted by the WebMD marketing sciences department.
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.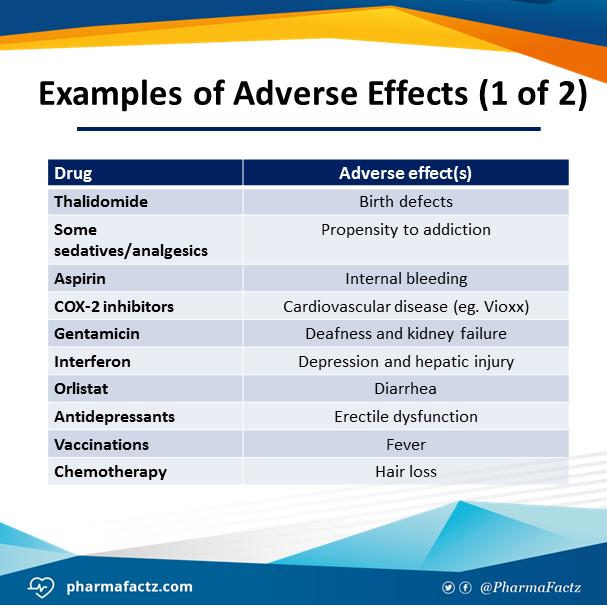
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
clinical effect, tolerability and personalized indications for prescription
At the present stage of development and improvement of methods for the treatment of endogenous depression, more and more attention is paid to the participation of monoamine neurotransmitters in the pathogenesis of the disease and the peculiarities of the impact on these links of newly created psychotropic antidepressant drugs [1, 2]. The pharmacological action of different classes of antidepressants introduced into psychiatric practice is due to different mechanisms of their interaction with certain brain receptors and participation in various metabolic processes. The search for thymoanaleptics that interact with both serotonergic and noradrenergic receptors, i.e. with a dual mechanism of action, led to the creation of a new class of antidepressants - selective serotonin and norepinephrine reuptake inhibitors (SNRIs). The appearance of antidepressants with a dual mechanism of action was accompanied by an increase in the effectiveness of the treatment of depressive conditions and made it possible to achieve remission in 45% of patients [2, 3].
The search for thymoanaleptics that interact with both serotonergic and noradrenergic receptors, i.e. with a dual mechanism of action, led to the creation of a new class of antidepressants - selective serotonin and norepinephrine reuptake inhibitors (SNRIs). The appearance of antidepressants with a dual mechanism of action was accompanied by an increase in the effectiveness of the treatment of depressive conditions and made it possible to achieve remission in 45% of patients [2, 3].
One of the SNRIs is venlafaxine, the first thymoanaleptic of the third generation. In many studies [4-6], it is evaluated as a "reference" in the group of these compounds.
Venlafaxine and its main metabolite, O-desmethylvenlafaxine, are potent inhibitors of serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake. Both the main drug and its specified metabolite reduce β-adrenergic reactivity. Venlafaxine has no affinity for muscarinic, cholinergic, histamine, and α1-adrenergic, opioid, benzodiazepine, orencyclidine and N-methyl-α-aspartate receptors in the brain; while the ratio of monoamine transfer (serotonin/norepinephrine) with venlafaxine is 30 (compared to 9,4 for another SNRI, duloxetine). This indicates a significant predominance of the serotonergic effect in the action of venlafaxine, which is similar in strength to selective serotonin reuptake inhibitors (SSRIs). A group of authoritative experts in the field of psychopharmacotherapy showed [7] that venlafaxine, along with escitalopram and clomipramine, can be considered as one of the most effective antidepressants.
This indicates a significant predominance of the serotonergic effect in the action of venlafaxine, which is similar in strength to selective serotonin reuptake inhibitors (SSRIs). A group of authoritative experts in the field of psychopharmacotherapy showed [7] that venlafaxine, along with escitalopram and clomipramine, can be considered as one of the most effective antidepressants.
Venlafaxine is a racemic mixture of two enantiomers: the S-enantiomer, which is a more potent inhibitor of serotonin reuptake, and the R-enantiomer, a potent norepinephrine reuptake inhibitor. Due to this structure, the drug in neuronal synapses eliminates the deficiency of both serotonin and norepinephrine, which provides a more balanced effect on neurotransmission and synergism of psychopharmacological effects. The features of venlafaxine should also include the high selectivity of its pharmacological action, which leads to a favorable profile of its tolerability and safety [8-10].
To date, data have been accumulated on the dose-dependence of the therapeutic effect of venlafaxine. Possessing a wide range of therapeutic action, venlafaxine consistently includes serotonergic, noradrenergic and dopaminergic receptors in the spectrum of its neurochemical activity [11]. When comparing the effectiveness of venlafaxine with SSRI antidepressants in a meta-analysis of 34 randomized double-blind trials [12], which included 8000 patients with depressive disorders of varying severity, statistically significant advantages of venlafaxine as a means to achieve remission were established.
Possessing a wide range of therapeutic action, venlafaxine consistently includes serotonergic, noradrenergic and dopaminergic receptors in the spectrum of its neurochemical activity [11]. When comparing the effectiveness of venlafaxine with SSRI antidepressants in a meta-analysis of 34 randomized double-blind trials [12], which included 8000 patients with depressive disorders of varying severity, statistically significant advantages of venlafaxine as a means to achieve remission were established.
The relatively short half-life necessitates strict adherence to the daily regimen of the drug [13, 14]. Many years of successful world experience in the use of venlafaxine indicates a dose-dependent mechanism of its action, which makes it possible to use the drug as an alternative to SSRIs and tricyclic antidepressants in the treatment of both therapeutically resistant depression and acute primary depression, both in inpatient and outpatient practice [7, 14— 19]. A favorable tolerability profile and a low incidence of side effects during venlafaxine therapy are emphasized [14].
Venlafaxine has been shown to be highly effective in a wide range of depressive conditions within recurrent and bipolar affective disorders [14], as well as in generalized anxiety, somatogenic and post-stroke depression [4, 20].
A number of authors [5, 21, 22] note the early onset of the antidepressant action of venlafaxine and its positive effect on the manifestations of the thymic component of depression, anxiety, agitation, motor retardation, and cognitive functions. However, the issues of the effect of venlafaxine on depressive states of various structures remain insufficiently developed [23].
The aim of the study was to evaluate the clinical effect of venlafaxine during course treatment of patients with various depressive states of endogenous nature.
Material and methods
We examined 32 patients, 15 women and 17 men, aged 18 to 57 years (mean age 31.75 years) with endogenous depression, who underwent inpatient treatment in the clinical departments of the Department for the Study of Endogenous Mental Disorders and Affective States of the Federal State Budgetary Institution Scientific Center for Mental health" RAS.
The study was open naturalistic in design. The average duration of the disease from its first manifestation was 7.9 years, the average number of depressive episodes transferred before inclusion in the study was 3.3. The duration of the current depressive episode prior to Velaxin was on average 4.5 months.
According to the nosological affiliation, the patients were distributed as follows: 22 (68.6%) patients were diagnosed with endogenous affective diseases - manic-depressive psychosis (MDP), cyclothymia; in 5 (15.6%) depression developed in the dynamics of low-progressive schizophrenia, and in 5 (15.6%) there was post-psychotic depression in the framework of paroxysmal schizophrenia. According to ICD-10, the depressive state of patients was diagnosed according to the following headings: F31.3—F31.4; F32.0-F32.2; F33.0-F33.2; F21; F20.4. Typologically, depressive states were defined as melancholy (3 patients, 9.4%), anxious (11 cases; 34.4%) and apatho-adynamic (18 patients; 56. 2%). Thus, in accordance with the two-level psychopathological model of depression [24], its first 2 types were defined as typical, representing positive affectivity (dreary and anxious depressions), apatho-adynamic depressions as atypical and related to negative affectivity.
2%). Thus, in accordance with the two-level psychopathological model of depression [24], its first 2 types were defined as typical, representing positive affectivity (dreary and anxious depressions), apatho-adynamic depressions as atypical and related to negative affectivity.
According to the sum of scores on the Hamilton Depression Scale (HAM-D) before treatment: mild depressive disorders were registered only in 2 (6.25%) people, moderate - in 13 (40.6%), severe depression was in 17 (53.15%), i.e., the vast majority of patients had moderate to severe depression.
The study used venlafaxine, sold under the name Velaxin in 75 mg tablets by Egis (Hungary). The drug was administered orally, starting with 37.5-75 mg per day. In the future, based on the condition of the patients, over the next days the daily dose was increased, bringing it to the maximum for this patient, but not more than 300 mg. On average, the highest mean dose of Velaxin used was 182.1-198.2 mg per day. The drug was taken twice, in the morning and in the evening. According to the study protocol, Velaxin therapy was 56 days (8 weeks).
The drug was taken twice, in the morning and in the evening. According to the study protocol, Velaxin therapy was 56 days (8 weeks).
To assess the severity of depressive symptoms and determine the therapeutic effect, in addition to the method of clinical observation, the HAM-D-24 scale containing 24 signs was used. The severity of depressive symptoms was assessed before the start of the course of treatment (day 0), then on days 1, 3, 5 of therapy, and then at the end of each of the 8 weeks of course treatment. The effectiveness of the antidepressant action of Velaxin was assessed by the degree of reduction of HAM-D scores in % in relation to the assessment before treatment in the following gradations: reduction of scores to 20% - as "insignificant", by 21-50% - as "moderate", by 51- 80% - as "good" and 81-100% - as a "significant" effect (including "practical recovery"). A decrease in the total score for HAM-D to 6 or less meant a complete "exit" to remission. Maintaining the HAM-D depression severity score at the same level or increasing the total score was regarded as no effect or “worsening” of the condition. To assess the spectrum of the antidepressant action of Velaxin, the degree of reduction in the average total score of individual signs identified in the HAM-D, conditionally characterizing melancholic or melancholy (points 1-3, 22-24), apatho-adynamic (points 7 and 8) and anxious (points 9and 10) manifestations in the structure of a depressive state.
To assess the spectrum of the antidepressant action of Velaxin, the degree of reduction in the average total score of individual signs identified in the HAM-D, conditionally characterizing melancholic or melancholy (points 1-3, 22-24), apatho-adynamic (points 7 and 8) and anxious (points 9and 10) manifestations in the structure of a depressive state.
In addition, the severity of the depressive state and the degree of its improvement over time were assessed within the above timeframes using the CGI clinical impression scale and its CGI-S and CGI-I subscales. To register side effects, the UKU interview scale was used, which consists of 4 subscales that make it possible to distinguish mental, neurological, vegetative and so-called other side effects.
Results
During the prescribed 56-day course of treatment with Velaxin, 31 (96.9%) people were recognized as responders, of which "insignificant" effect from the treatment was observed in 1 (3. 1%) patients, "moderate" - in 3 (9, 4%), "good" - in 2 (6.25%), and 25 (78.1%) patients showed a "significant" therapeutic effect. Thus, a decrease in the intensity of depressive disorders by 50% or more during treatment with Velaxin was found in the vast majority of cases - in 27 (84.4%) patients, which is shown in Fig. 1
1%) patients, "moderate" - in 3 (9, 4%), "good" - in 2 (6.25%), and 25 (78.1%) patients showed a "significant" therapeutic effect. Thus, a decrease in the intensity of depressive disorders by 50% or more during treatment with Velaxin was found in the vast majority of cases - in 27 (84.4%) patients, which is shown in Fig. 1
Rice. 1. The severity of the therapeutic effect of Velaksin in endogenous depression. On the abscissa - the degree of reduction according to HAM-D,%; along the y-axis - % of patients.
In general, the effectiveness of Velaxin, assessed on the 56th day of therapy, was quite high: the average total depression score according to HAM-D was reduced by 85.9%. Already by the end of the 1st week of therapy with Velaxin, a positive therapeutic response to the drug was observed: the reduction in depression was at the level of a “slight” effect, but approached a 20% level of reduction in disorders bordering on a “moderate” improvement. A distinct "moderate" improvement (reduction of the HAM-D score by 33. 2%) was observed by the 14th day of treatment; between the 21st and 28th days of treatment, the therapeutic effect reached the “good” range with a decrease in HAM-D scores by 50% or more, and by the 6th week, the effect of Velaxin treatment was already approaching the “significant” border (up to 76.7% reduction in the severity of symptoms of depression). In the next 7-8 weeks of therapy, an indisputable "significant" improvement in the condition of patients was found (reduction of disorders by more than 80%), up to "recovery" (Fig. 2). In 20 out of 31 patients (in 64.5% of cases) who completed the course of treatment with Velaxin, depression was completely reduced and there was an "exit" to remission (their total HAM-D score became 6 or lower).
2%) was observed by the 14th day of treatment; between the 21st and 28th days of treatment, the therapeutic effect reached the “good” range with a decrease in HAM-D scores by 50% or more, and by the 6th week, the effect of Velaxin treatment was already approaching the “significant” border (up to 76.7% reduction in the severity of symptoms of depression). In the next 7-8 weeks of therapy, an indisputable "significant" improvement in the condition of patients was found (reduction of disorders by more than 80%), up to "recovery" (Fig. 2). In 20 out of 31 patients (in 64.5% of cases) who completed the course of treatment with Velaxin, depression was completely reduced and there was an "exit" to remission (their total HAM-D score became 6 or lower).
Rice. 2. Spectrum of therapeutic action of Velaxin in endogenous depression (HAM-D score). Here and in Figures 3, 4 and 5 (the last one shows the right axis): along the y-axis - the average score in % of the score before the start of therapy, along the abscissa - the day of therapy.
The severity of the condition, assessed by the CGI-S subscale, decreased on average during treatment with Velaxin from 4.7 to 1.6 points by the end of the study (from “significantly pronounced” to less than “mildly expressed”, i.e. to almost no disorders). Moreover, a noticeable decrease in the severity of symptoms was noted between the 7th and 21st days of treatment, when the intensity of depression manifestations consistently decreased on average to "moderately" and "weakly" pronounced. From the 5th week of therapy, the severity of depressive disorders became "very weak", and on the 7th-8th week of the course therapy there was a practical "exit" from depression with the "absence" of depressive symptoms (the average level of their severity was 1.6 points). During the 56-day course of therapy, an assessment of the degree of improvement in the depressive state on the CGI-I subscale showed a “deterioration” in the mental state (5th level of assessment) only in 1 (3.1%) person, remained “unchanged” (4th level). assessment level) also in 1 (3.1%) patient, "slight improvement" (3rd level) - also in 1 (3.1%), "pronounced" improvement (2nd level) was observed in 4 (12 .6%), the remaining 25 (78.1%) patients showed "significant" improvement (grade 1).
assessment level) also in 1 (3.1%) patient, "slight improvement" (3rd level) - also in 1 (3.1%), "pronounced" improvement (2nd level) was observed in 4 (12 .6%), the remaining 25 (78.1%) patients showed "significant" improvement (grade 1).
It was found that in the spectrum of the antidepressant action of Velaxin, all 3 components of its psychotropic activity, in accordance with the final results of treatment, are represented almost equally with a slight advantage in terms of antidepressant action (see Fig. 2). Thus, the degree of reduction in the average total score of disorders on points 1-3 and 22-24 of HAM-D (reflecting the actual thymoleptic effect of the drug) was 88.2%, on points 7 and 8 (stimulating effect) - 83.7%, and on paragraphs 9and 10 (anti-anxiety action) - 85.1%. However, the implementation of all 3 components of the action of Velaxin was different in time and had its own characteristics. Thus, thymoleptic and especially anxiolytic effects were detected most quickly during treatment.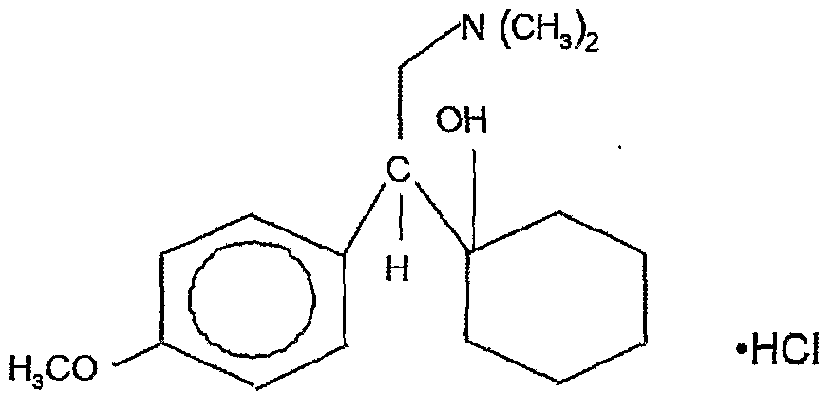 They developed almost in parallel, reaching the level of reduction in the severity of disorders according to HAM-D by 32.8 and 37.1%, respectively, already by the 14th day of therapy (“moderate” effect). At the same time, the stimulating effect of Velaxin was somewhat "late" in comparison with the anti-anxiety and anti-sadness effects, reaching similar values ("moderate" effect - reduction of disorders by 36.9%) only by the 21st day of the study.
They developed almost in parallel, reaching the level of reduction in the severity of disorders according to HAM-D by 32.8 and 37.1%, respectively, already by the 14th day of therapy (“moderate” effect). At the same time, the stimulating effect of Velaxin was somewhat "late" in comparison with the anti-anxiety and anti-sadness effects, reaching similar values ("moderate" effect - reduction of disorders by 36.9%) only by the 21st day of the study.
Later, in the period from the 21st to the 28th days of treatment, the thymoleptic and anti-anxiety components of action in the spectrum of antidepressant activity of Velaxin leveled off in terms of the rate of development and increased to the range of a distinct "good" effect (reduction of the score by 60.2 and 59 .2%). But already from the 5th week of treatment, the thymoleptic effect proper began to surpass the anxiolytic effect in terms of the depth of reduction of disorders according to the corresponding HAM-D items: the thymoleptic effect was manifested at the “significant” level already at the 6th week, and the anti-anxiety effect only after the 7th week course therapy. The formation of the stimulating effect was “delayed” in terms of the degree of manifestations compared with the other two components of the action of Velaxin at week 1, and its “significant” severity was determined only at the 8th week of treatment. And only at this stage of treatment, as already mentioned, the depth of the stimulating effect of Velaxin approximately coincided with the severity of the thymoleptic and anxiolytic effects.
The formation of the stimulating effect was “delayed” in terms of the degree of manifestations compared with the other two components of the action of Velaxin at week 1, and its “significant” severity was determined only at the 8th week of treatment. And only at this stage of treatment, as already mentioned, the depth of the stimulating effect of Velaxin approximately coincided with the severity of the thymoleptic and anxiolytic effects.
In accordance with the established data on the features of the implementation of individual components of the antidepressant action in the spectrum of psychotropic activity of Velaxin, its therapeutic efficacy was analyzed depending on the syndromic type of depression, i.e. on the dominant affect that determines the picture of the depressive state (Fig. 3). It was found that with the leading anxiety affect, the reduction in the average total score for HAM-D was 85.5% by the 56th day of treatment; with the predominance of apato-adynamic affect, the effectiveness of Velaxin was similar - the average total score for HAM-D decreased by 85. 9%. The highest efficacy of Velaxin was observed in the treatment of patients with the leading melancholy affect in the picture of depression - the reduction in the average total score according to HAM-D was 91.1%. But with a relatively similar degree of reduction in depressive symptoms, differences were also noted in the dynamics of the implementation of the antidepressant effect of the drug. So, with leading anxious and melancholy affects, i.e. in patients with positive affectivity and the most typical manifestations of depression, the reduction of depressive symptoms proceeded at an equal rate, reaching 21.1% (with anxiety) and 22.5% (with melancholy depression) already by the 7th day of therapy. Subsequently, the decrease in the average total score for HAM-D was more intense and reached more than 50% reduction in symptoms of depression by the 28th day of treatment. Treatment with Velaxin in patients with negative affectivity (atypical apatho-adynamic depression) improved more slowly.
9%. The highest efficacy of Velaxin was observed in the treatment of patients with the leading melancholy affect in the picture of depression - the reduction in the average total score according to HAM-D was 91.1%. But with a relatively similar degree of reduction in depressive symptoms, differences were also noted in the dynamics of the implementation of the antidepressant effect of the drug. So, with leading anxious and melancholy affects, i.e. in patients with positive affectivity and the most typical manifestations of depression, the reduction of depressive symptoms proceeded at an equal rate, reaching 21.1% (with anxiety) and 22.5% (with melancholy depression) already by the 7th day of therapy. Subsequently, the decrease in the average total score for HAM-D was more intense and reached more than 50% reduction in symptoms of depression by the 28th day of treatment. Treatment with Velaxin in patients with negative affectivity (atypical apatho-adynamic depression) improved more slowly. The reduction of the average total score on HAM-D by 20% or more ("moderate" effect) occurred in them only at the 2nd-3rd week of therapy; and only by the 4th week a “good” effect of Velaxin was recorded (reduction of disorders by 61.3%). By the 7th-8th week of treatment, the improvement in the condition was already “significant” (more than 80% reduction in disorders), it coincided in severity with the therapeutic effect of Velaxin in anxious depressions and somewhat “lagged behind” the effect in melancholy depression.
The reduction of the average total score on HAM-D by 20% or more ("moderate" effect) occurred in them only at the 2nd-3rd week of therapy; and only by the 4th week a “good” effect of Velaxin was recorded (reduction of disorders by 61.3%). By the 7th-8th week of treatment, the improvement in the condition was already “significant” (more than 80% reduction in disorders), it coincided in severity with the therapeutic effect of Velaxin in anxious depressions and somewhat “lagged behind” the effect in melancholy depression.
Rice. Fig. 3. Dynamics of Velaxin efficacy in different types of endogenous depression (HAM-D score).
An analysis of the effectiveness of Velaxin depending on the nosological affiliation of depression also revealed some features (Fig. 4): the smallest decrease in the average total score for HAM-D by the end of the course of treatment was in patients with low-progressive schizophrenia, it was equal to 82.3%. In these same patients, the slowest rate of reduction of depressive disorders was also noted - by the 7th day it was only 15. 2%, and a reduction of 50% or more (the border of the "good" effect) was achieved only by the 28th day; A "significant" effect was found in patients with low-progressive schizophrenia only after the 7th week of Velaxin treatment. In patients with an affective disease (MDP, cyclothymia), the reduction in the mean total HAM-D score at the end of course therapy was close in value (86.6%), and the rate of decrease in depressive symptoms was similar: by the 7th day, this indicator reached 18 .2%, and by the 4th week of therapy it was already 59.4% ("good" effect). The best result of therapy was achieved in patients with postpsychotic depression with paroxysmal schizophrenia - 90.4% reduction of disorders on the 56th day of treatment. Despite the fact that by the end of the 1st week of the study, the rate of reduction of depressive manifestations reached gradations of "insignificant" improvement (19.2%), further reduction in symptoms of depression was more intense than with MDP, demonstrating a "good" effect already by 14 Day 1 of treatment (symptom reduction score -53.
2%, and a reduction of 50% or more (the border of the "good" effect) was achieved only by the 28th day; A "significant" effect was found in patients with low-progressive schizophrenia only after the 7th week of Velaxin treatment. In patients with an affective disease (MDP, cyclothymia), the reduction in the mean total HAM-D score at the end of course therapy was close in value (86.6%), and the rate of decrease in depressive symptoms was similar: by the 7th day, this indicator reached 18 .2%, and by the 4th week of therapy it was already 59.4% ("good" effect). The best result of therapy was achieved in patients with postpsychotic depression with paroxysmal schizophrenia - 90.4% reduction of disorders on the 56th day of treatment. Despite the fact that by the end of the 1st week of the study, the rate of reduction of depressive manifestations reached gradations of "insignificant" improvement (19.2%), further reduction in symptoms of depression was more intense than with MDP, demonstrating a "good" effect already by 14 Day 1 of treatment (symptom reduction score -53. 9%), and by the 28th day it was already 76.5%, that is, it was almost on the border of "significant". However, it should be noted that in these patients, the severity of depressive disorders was initially significantly lower, at the level of "moderate" severity, than in other, more "severe" depressions (23 points according to HAM-D versus 31.3 points for affective illness and 31.6 for low-progressive schizophrenia).
9%), and by the 28th day it was already 76.5%, that is, it was almost on the border of "significant". However, it should be noted that in these patients, the severity of depressive disorders was initially significantly lower, at the level of "moderate" severity, than in other, more "severe" depressions (23 points according to HAM-D versus 31.3 points for affective illness and 31.6 for low-progressive schizophrenia).
Rice. 4. Antidepressant effect of Velaxin in different nosological groups of patients (HAM-D score).
All the indicated patterns of development of the therapeutic effect of Velaxin indicate the absence of a direct dependence on the value of its daily dose. As can be seen from Fig. 5, the therapeutic effect began to appear and increase from the 3rd–4th week of treatment (reduction of depression symptoms by 50% or more according to HAM-D), with an increase in the average daily dose to the maximum (182.1–198.2 mg per day) . But in the future, despite the stabilization and even a slight decrease in the achieved maximum average daily dose of Velaxin (up to 190. 3 mg per day), response to therapy continued to increase at a faster rate, to a level of "significant" improvement,
3 mg per day), response to therapy continued to increase at a faster rate, to a level of "significant" improvement,
Rice. 5. The ratio of the therapeutic effect (HAM-D score) and the daily dose of Velaxin. On the left y-axis - the average daily dose of the drug (mg).
During course therapy with Velaxin, 45 adverse events of various types were recorded in 21 (65.6%) patients; in 5 they were single, and in 16 they were combined. The most frequent were mental and vegetative adverse events noted on the UKU scale, they occurred in 40% of patients. 1 patient dropped out of the study ahead of schedule (after 2 weeks of treatment) due to the development of acute affective-delusional psychosis, requiring a change in therapy. Neurological and other side effects occurred in only 8.9and 11.1% of patients. The most common among the individual side effects were such as "agitation/anxiety" (in 15.5% of patients), decreased sleep duration and nausea (in 11.1%), tachycardia and hyperhidrosis (in 6. 6%), sedation and constipation (in 4.4%). All other side effects occurred in isolated cases. The frequency of side effects during course treatment with Velaxin was highest on the 2nd week of treatment, when the average daily dose of the drug was insignificant (157.7 mg per day). The most frequent during this period were mental and autonomous adverse events. But in the future, despite the increase in the average daily dose, the incidence of side effects gradually decreased. The exception was "other" side effects, the frequency of which practically remained at the same level until the end of treatment. These were mainly violations of sexual functions, which developed in accordance with an increase in the daily dose of Velaxin.
6%), sedation and constipation (in 4.4%). All other side effects occurred in isolated cases. The frequency of side effects during course treatment with Velaxin was highest on the 2nd week of treatment, when the average daily dose of the drug was insignificant (157.7 mg per day). The most frequent during this period were mental and autonomous adverse events. But in the future, despite the increase in the average daily dose, the incidence of side effects gradually decreased. The exception was "other" side effects, the frequency of which practically remained at the same level until the end of treatment. These were mainly violations of sexual functions, which developed in accordance with an increase in the daily dose of Velaxin.
It should be noted that, in general, all adverse events were mild or approached moderate in the first 2 weeks of treatment, and then the average degree of their severity, as assessed by UKU, decreased to a “mild” level, despite a gradual increase in the average daily doses of Velaxin. At the same time, mental side effects were initially the most severe: the average UKU score for their severity at the 1st–2nd week of treatment was 1.9–2.0. However, in the course of further course treatment, the severity of adverse mental symptoms decreased from the 3rd week. The severity of some other adverse events slightly increased compared to the initial one: if on the 2nd week of treatment their severity corresponded on average to the UKU of a “mild” degree (1 point), then in the future they reached 1.5 points, and at the same time their degree severity increased correspondingly with an increase in the mean dose of Velaxin.
At the same time, mental side effects were initially the most severe: the average UKU score for their severity at the 1st–2nd week of treatment was 1.9–2.0. However, in the course of further course treatment, the severity of adverse mental symptoms decreased from the 3rd week. The severity of some other adverse events slightly increased compared to the initial one: if on the 2nd week of treatment their severity corresponded on average to the UKU of a “mild” degree (1 point), then in the future they reached 1.5 points, and at the same time their degree severity increased correspondingly with an increase in the mean dose of Velaxin.
Discussion
The results of the clinical use of Velaxin in endogenous depression showed its high antidepressant activity. In 84.4% of patients, there was a clear positive therapeutic effect with a decrease in the severity of disorders (according to HAM-D) by 50% or more. Of these, the majority (78.1%) achieved a "significant" therapeutic effect with a reduction in the HAM-D score by more than 80%, and in 62. 5% of patients, at the end of the 8-week course treatment with Velaxin, a good quality remission occurred with complete "out" of depression. The distinct antidepressant properties of Velaxin are also evidenced by the inversion of depressive affect into hypomanic affect in 2 patients by the end of the course of treatment. It is very important that the therapeutic effect of Velaxin manifests itself early, already at the 2nd week of treatment it is determined at the level of "moderate" (up to 50% reduction in the severity of symptoms according to HAM-D), and by the 3rd-4th week of therapy, the reduction of the HAM score -D reached 50% or more ("good" effect).
5% of patients, at the end of the 8-week course treatment with Velaxin, a good quality remission occurred with complete "out" of depression. The distinct antidepressant properties of Velaxin are also evidenced by the inversion of depressive affect into hypomanic affect in 2 patients by the end of the course of treatment. It is very important that the therapeutic effect of Velaxin manifests itself early, already at the 2nd week of treatment it is determined at the level of "moderate" (up to 50% reduction in the severity of symptoms according to HAM-D), and by the 3rd-4th week of therapy, the reduction of the HAM score -D reached 50% or more ("good" effect).
According to the clinical characteristics, Velaxin can be legitimately classified as a balanced antidepressant. In the spectrum of its antidepressant activity, all 3 components of the antidepressant effect are approximately equally represented with a certain predominance (in terms of depth of effect) of the thymoleptic effect, an earlier anxiolytic effect and a “lag” in the timing of the manifestation of the stimulating effect. These properties of the antidepressant activity of Velaxin ensured its high therapeutic efficacy in the treatment of syndromic different types of depressive states - anxiety, apatho-adynamic and, primarily, melancholic. By the end of the course of treatment, the effectiveness of Velaxin in anxiety and apatho-adynamic depression "stopped" at the level of 85.5 and 85.9, respectively.% reduction of disorders according to HAM-D, and with melancholy depressions was deeper at this stage of treatment and reached 91.1% reduction of the corresponding symptoms.
These properties of the antidepressant activity of Velaxin ensured its high therapeutic efficacy in the treatment of syndromic different types of depressive states - anxiety, apatho-adynamic and, primarily, melancholic. By the end of the course of treatment, the effectiveness of Velaxin in anxiety and apatho-adynamic depression "stopped" at the level of 85.5 and 85.9, respectively.% reduction of disorders according to HAM-D, and with melancholy depressions was deeper at this stage of treatment and reached 91.1% reduction of the corresponding symptoms.
Features of the spectrum of therapeutic action of Velaxin depend on the nosological affiliation of the disease and the form of its course. In cases where the basis of the clinical essence of an endogenous disease was a distinct property of phase- or paroxysmal formation (MDP, cyclothymia, paroxysmal schizophrenia), the effectiveness of Velaxin in the treatment of depression was higher than in cases of diseases in which depression developed as part of exacerbation against the background of continuous (sluggish) ) course (low-progressive schizophrenia). But in general, the depth of therapeutic improvement in all these diseases was quite high, respectively, at the level of 86.6; 90.4% and 82.3% reduction in HAM-D depressive symptom severity score.
But in general, the depth of therapeutic improvement in all these diseases was quite high, respectively, at the level of 86.6; 90.4% and 82.3% reduction in HAM-D depressive symptom severity score.
The results of the study substantiate the expediency of prescribing long-term courses of treatment with Velaxin, since the formation of its therapeutic effect at the level of "good" begins only from the 3rd-4th week of the course of therapy, and the "significant" effect is achieved only at the 2nd month of treatment. The data obtained allow us to recommend moderate daily doses of the drug to achieve a "significant" therapeutic effect and "exit" into remission: doses of 180-19 can be considered optimal.8 mg, i.e. 2-3 tablets per day (75 mg each), since a further increase in the daily dose does not lead to a clear increase in the clinical effect.
In general, Velaxin is well tolerated, rare side effects associated with its administration are mild or moderate and develop mainly in the first 2 weeks of treatment; they do not require discontinuation of the drug and do not affect the regimen of therapy. Despite ongoing treatment and an increase in daily doses of Velaxin, the severity and frequency of adverse events are decreasing, which may justify their dose-independence. It is important to note that such severe side effects as urinary retention, lowering blood pressure, dry mouth, vomiting, convulsions, orthostatism, which are usually characteristic of tricyclic antidepressants, did not occur during treatment with Velaxin.
Despite ongoing treatment and an increase in daily doses of Velaxin, the severity and frequency of adverse events are decreasing, which may justify their dose-independence. It is important to note that such severe side effects as urinary retention, lowering blood pressure, dry mouth, vomiting, convulsions, orthostatism, which are usually characteristic of tricyclic antidepressants, did not occur during treatment with Velaxin.
Possessing a wide range and depth of antidepressant action, as well as good tolerance, Velaxin can be recommended as a drug of choice in the treatment of nosologically various endogenous diseases that occur with a picture of depression, both moderate and severe. Velaxin, a drug with a distinct antidepressant activity of balanced action, at the present stage of the development of psychopharmacotherapy can rightly be classified as an active antidepressant as a highly effective representative of the third generation of this class of psychotropic compounds.
Clinical efficacy and tolerability of venlafaxine (Velaxin) in the treatment of moderate and severe depression :: DIFFICULT PATIENT
S.N. Mosolov, E.G. Kostyukova, A.V. Gorodnichev, I.V. Timofeev, M.Ya. Ladyzhensky, O.V. Serditov
Moscow Research Institute of Psychiatry, Roszdrav
The treatment of depressive spectrum disorders, from the 1960s to the present, remains a complex and urgent problem. Despite the variety of antidepressants available in the arsenal of psychiatrists, there is a large group of patients in whom known drugs are ineffective or cause undesirable effects. In this regard, studies of the pathogenesis of depression and the mechanisms of action of antidepressants are actively continuing, which makes it possible to synthesize new effective drugs with an improved tolerability profile and a more powerful mechanism of action.
Venlafaxine is the first third-generation thymoanaleptic (selective serotonin and norepinephrine reuptake inhibitor - SNRI), followed by a whole group of such drugs (milnacipran, duloxetine). It should be noted that venlafaxine is the most studied and most commonly prescribed drug from the SNRI group and is used in many studies (in order to prove the effectiveness of new antidepressants) as a reference.
It should be noted that venlafaxine is the most studied and most commonly prescribed drug from the SNRI group and is used in many studies (in order to prove the effectiveness of new antidepressants) as a reference.
In the last decade, convincing data have accumulated on the high effectiveness of tricyclic antidepressants (TCA) compared with SSRIs in the treatment of severe, including melancholic depression. The results of two independent studies are known [1, 2], which compared the effectiveness of clomipramine, citalopram and paroxetine. The therapeutic response in treatment with clomipramine was 2 times higher than that of citalopram (60 and 30%). Similar results were noted when comparing clomipramine and paroxetine. The superiority of TCAs in the treatment of severe depressive states was also revealed in two meta-analyses conducted by Anderson [3, 4]. They confirmed that TCAs with a broad, non-selective mechanism of action (amitriptyline and clomipramine) are more effective than SSRIs. In our country, similar data were obtained by A.B. Smulevich and S.N. Mosolov on the material of open studies [5, 6].
In our country, similar data were obtained by A.B. Smulevich and S.N. Mosolov on the material of open studies [5, 6].
The results of these studies, as well as clinical practice, led to the idea to enhance the effectiveness of antidepressants by expanding the neurochemical application, which became the basis for the synthesis of a new generation of antidepressants, namely: a wide range of clinical action; 2) compared to TCAs, dual action antidepressants are safer; 3) "dual" action antidepressants have a number of features and specific effects. These provisions, as shown by clinical practice, were fully realized in the process of using the "double" action antidepressant venlafaxine.
Venlafaxine, having a wide therapeutic range of doses, consistently includes serotonergic, noradrenergic and dopaminergic effects in the spectrum of its neurochemical activity. So, at a dose of 75-125 mg, venlafaxine exhibits a serotonergic effect, when the dose is increased to 225 mg, the noradrenergic effect is turned on, and a further increase in the dose to 375 mg leads to the appearance of a dopaminergic effect. Such a dose-dependent pharmacology of venlafaxine fundamentally distinguishes this drug from SSRIs, a limited range of therapeutic doses, which narrows the possibility of other neurochemical effects other than serotonergic ones [7].
Such a dose-dependent pharmacology of venlafaxine fundamentally distinguishes this drug from SSRIs, a limited range of therapeutic doses, which narrows the possibility of other neurochemical effects other than serotonergic ones [7].
The high efficacy of venlafaxine has been demonstrated in comparative studies with TCAs. Thus, a comparison of venlafaxine and clomipramine did not reveal differences in the antidepressant effect, with slightly better tolerability of venlafaxine due to a lower frequency of anticholinergic adverse events (p ≤ 0.05) [8]. Venlafaxine is as effective as amitriptyline [9] and imipramine, although the doses used in the study were close to the maximum (imipramine 179 mg/day versus venlafaxine 183 mg/day) [10].
A meta-analysis of 19 studies including 2181 patients showed that the side effects of venlafaxine, the frequency of which exceeds 10%, include: nausea, headache, insomnia, drowsiness, dry mouth, dizziness, constipation, asthenia, sweating and nervousness [ eleven]. Of these, nausea is the most common adverse event and leads to drug withdrawal in 6% of cases. At the same time, the frequency of occurrence of this undesirable effect decreases by 50% already after the 1st week of therapy and further - during the entire course of treatment. Anticholinergic side effects occur with venlafaxine 2 times less frequently than with TCAs.
Of these, nausea is the most common adverse event and leads to drug withdrawal in 6% of cases. At the same time, the frequency of occurrence of this undesirable effect decreases by 50% already after the 1st week of therapy and further - during the entire course of treatment. Anticholinergic side effects occur with venlafaxine 2 times less frequently than with TCAs.
However, some side effects of venlafaxine are noted to be dose-dependent (which is a direct reflection of the drug's dose-dependent pharmacology). These include with high certainty such serotonergic adverse events as nausea, insomnia and sexual dysfunctions [12].
The aim of this work was to study the efficacy, features of thymoanaleptic action and tolerability of the drug venlafaxine in the treatment of moderate and severe depression.
The study was open, non-comparative. The duration of the study was 6 weeks.
The study included patients aged 18 to 65 years with an ICD-10 diagnosis of recurrent depressive disorder (F32. 1), moderate or severe depressive episode (F33.1 or F33.2), bipolar affective disorder (F31): episode moderate or severe depression (F31.3 and F31.4). The minimum sum of points when assessed on the 17-point Hamilton Scale (HH) was at least 17, and on the Global Clinical Impression Scale (GCI) - at least 4 points. The study included patients who gave written informed consent to participate in it. Quality of life was also assessed using the WHO Brief Quality of Life Scale at baseline and end of the study.
1), moderate or severe depressive episode (F33.1 or F33.2), bipolar affective disorder (F31): episode moderate or severe depression (F31.3 and F31.4). The minimum sum of points when assessed on the 17-point Hamilton Scale (HH) was at least 17, and on the Global Clinical Impression Scale (GCI) - at least 4 points. The study included patients who gave written informed consent to participate in it. Quality of life was also assessed using the WHO Brief Quality of Life Scale at baseline and end of the study.
The exclusion criteria were the period of pregnancy and lactation in women; high suicidal risk for outpatients; any clinically significant uncompensated diseases of the kidneys, liver, cardiovascular, respiratory system, cerebrovascular disorders or other serious progressive somatic diseases; organic diseases of the central nervous system, any of the listed methods of treatment at the indicated time intervals before the start of the study: MAO inhibitors (including reversible ones) - 2 weeks; electroconvulsive therapy - 3 months; depot forms of neuroleptics - 4 weeks.
Patients who were taking any psychotropic medication prior to study entry were subject to a 7-day "wash out" period.
In the first two weeks of the study, including the "withdrawal" period, hypnotics were allowed to correct sleep disorders and anxiolytics (with the exception of alprazolam) for anxiety symptoms that could lead to premature termination of the study. Subsequently, this concomitant therapy was discontinued in all patients, with the exception of those who took benzodiazepine tranquilizers for a long time before the start of the study. No specific psychotherapy was used in the study. Patients were given explanations about their condition, including the expected effect of the drug.
During follow-up, the severity of depressive symptoms was recorded using the Hamilton Scale for Depression (17 items) (HAM-D), the Global Clinical Impression (CGI) Scale and the WHO Concise Quality of Life Scale. The condition of patients was assessed immediately before the start of the drug (day 0 of therapy), and then on days 4, 7, 14, 21, 28, 35, and 42 of treatment. On the same days, throughout the study, all emerging adverse events (AEs) were recorded with an indication of their severity, time of occurrence and duration. An AE was any adverse (medically) event that occurred in a patient receiving study drug, or regardless of its causal relationship to it. Worsening of the patient's pre-study symptoms was also recorded as an adverse event.
On the same days, throughout the study, all emerging adverse events (AEs) were recorded with an indication of their severity, time of occurrence and duration. An AE was any adverse (medically) event that occurred in a patient receiving study drug, or regardless of its causal relationship to it. Worsening of the patient's pre-study symptoms was also recorded as an adverse event.
The study included 30 patients, of which 26 (86.6%) women and 4 (13.4% men). The mean age of the patients was 43.6 ± 2.7 years.
The duration of the disease averaged 13.0 ± 1.9 years, the number of previous depressive episodes was 6.6 ± 1.2. The duration of the present episode of depression averaged 3.6 ± 1.22 months. Drug treatment of the current depressive episode before the start of the study was carried out in 13 (50%) patients.
Baseline mean group score for the first 17 HAM-D items was 21.96 ± 2.4. According to CGI, the average disease severity score for the group was 4.34 ± 0.48 points.
Venlafaxine (Velaxin) was given as a single daily dose of 75 mg/day followed by an increase to 150 mg/day in 2 divided doses during the first week. With insufficient effectiveness of the drug, the dose was increased to 225 mg / day. The dose increase was carried out 3 weeks after the start of therapy. The mean daily dose of venlafaxine was 137.5 mg/day.
With insufficient effectiveness of the drug, the dose was increased to 225 mg / day. The dose increase was carried out 3 weeks after the start of therapy. The mean daily dose of venlafaxine was 137.5 mg/day.
Completed the study 26 patients. One patient withdrew due to refusal to continue the study, 2 patients withdrew due to deterioration between days 14 and 21 of therapy, and 1 patient withdrew due to adverse events on day 3 of therapy.
Results of the study
When analyzing the data, the following results were obtained. The overall effectiveness of therapy by the end of the study for SH (the percentage of patients with a reduction in the total score by at least 50%) was 77% (20 out of 26 patients). Qualitative remission (≤ 7 points) by the end of the study was established in 61%. According to the CGI scale, a positive effect (pronounced and significant improvement) was observed in 18 (69%) patients.
Already at the second week of therapy, 40% of patients were assessed as responders to ongoing therapy, by the third week of therapy 50% were responders, and by the 6th week of treatment their number exceeded two thirds of the total number (20 out of 27-74. 7%) . A significant increase in the number of responders by the 3rd week of therapy indicates a fairly rapid effect, somewhat ahead of the time characteristic of most antidepressants. From fig. Table 1 shows that the increase in the number of patients with a positive effect of therapy was observed until the end of the study, although it was most pronounced between the 3rd and 5th weeks of therapy, which suggests a fairly rapid onset of the effect in comparison with other new generation antidepressants, as well as Extend recommendations to continue therapy for at least 6 weeks for venlafaxine.
7%) . A significant increase in the number of responders by the 3rd week of therapy indicates a fairly rapid effect, somewhat ahead of the time characteristic of most antidepressants. From fig. Table 1 shows that the increase in the number of patients with a positive effect of therapy was observed until the end of the study, although it was most pronounced between the 3rd and 5th weeks of therapy, which suggests a fairly rapid onset of the effect in comparison with other new generation antidepressants, as well as Extend recommendations to continue therapy for at least 6 weeks for venlafaxine.
These data are also confirmed by the analysis of the dynamics of depression severity indicators according to CGI. A change in the severity of the disease was observed already on the 7th day of therapy (p The effect of therapy occurred quite quickly. The value of the total SH score for the group as a whole decreased by 12% already on the 4th day and by 23% on the 7th day of treatment ( the changes are statistically significant, p ≤ 0. 01) (Fig. 2). Subsequently, there was a consistent decrease in the severity of depressive symptoms. By the end of the study, the total score of SH was 6.9± 0.9 points, which practically corresponds to the generally accepted indicator of therapeutic remission for SH and indicates a distinct antidepressant effect of the study drug.
01) (Fig. 2). Subsequently, there was a consistent decrease in the severity of depressive symptoms. By the end of the study, the total score of SH was 6.9± 0.9 points, which practically corresponds to the generally accepted indicator of therapeutic remission for SH and indicates a distinct antidepressant effect of the study drug.
The main indicators that determine the spectrum of the psychotropic action of the antidepressant (low mood, mental anxiety and lethargy) decreased fairly evenly (Fig. 3), the statistical significance of changes for each indicator was observed already on the 7th day of therapy (p Despite a fairly harmonious reduction in anxiety and lethargy, in the first days the reduction of anxiety symptoms prevailed, but by the end of the first week of therapy, the reduction in indicators leveled off.0096 The nature of the thymoanaleptic effect is clearly confirmed by the analysis of the coefficient (K) of the ratio of anxiety reduction and lethargy (Fig. 4). The value of K, equal to 1, reflects a uniform reduction in the SH indicators "mental anxiety" and "lethargy" and indicates a balanced thymoanaleptic effect of the drug. As can be seen from fig. 4, at the beginning of therapy, the K value was significantly greater than 1, especially from the 4th day until the 21st day of therapy. This means that at the beginning of therapy, anxiety was reduced much faster than inhibition. In the study, this led to a rapid reduction in anxiety symptoms, the absence of the need for additional use of tranquilizers. However, by the 4th week of therapy, the effect of venlafaxine becomes balanced until the end of the course of therapy.
As can be seen from fig. 4, at the beginning of therapy, the K value was significantly greater than 1, especially from the 4th day until the 21st day of therapy. This means that at the beginning of therapy, anxiety was reduced much faster than inhibition. In the study, this led to a rapid reduction in anxiety symptoms, the absence of the need for additional use of tranquilizers. However, by the 4th week of therapy, the effect of venlafaxine becomes balanced until the end of the course of therapy.
It has already been mentioned above that venlafaxine, used in different doses, can have different effects on the reduction of depressive symptoms. Thus, venlafaxine at a dose of 75-150 mg / day affects mainly serotonergic transmission and is similar to SSRIs. The reduction in the anxiety/lethargy ratio for the group of patients who took venlafaxine at doses up to 150 mg/day is shown in Figure 5.
influence on lethargy, however, by the end of the first week, the action of venlafaxine became more balanced with an extremely insignificant activating effect after a month of therapy.
The effect of venlafaxine at a dose of more than 150 mg/day on the coefficient of reduction of anxiety/lethargy is somewhat different (Fig. 6).
Noteworthy is the long-term, up to the 3rd week of therapy, the predominance of the reduction of anxiety symptoms in relation to the reduction of lethargy, which was assessed by patients as the absence of anxiety and did not require anxiolytics. Subsequently, until the end of the study, the drug prescribed at this dose was characterized as a balanced antidepressant with a mild sedative effect.
Clinically, at the end of the first week of treatment, most patients felt better. Along with a rapid decrease in the severity of anxiety, there was an improvement in sleep. Patients noted an improvement in the quality of sleep, it became deeper, a feeling of cheerfulness returned when waking up in the morning. The results are shown in Figure 7.
A rapid decrease in the severity of anxiety allowed most patients to stop taking anxiolytics prescribed during the "wash out" period or at previous stages of treatment. Objectively, patients became more contact, more active in conversation, noted that the interest lost during the period of depression in the usual range of activities and hobbies was beginning to be restored. These changes were reflected in the analysis of the WHO Brief Quality of Life Scale (Fig. 8).
Objectively, patients became more contact, more active in conversation, noted that the interest lost during the period of depression in the usual range of activities and hobbies was beginning to be restored. These changes were reflected in the analysis of the WHO Brief Quality of Life Scale (Fig. 8).
The results show a high reliability of improvement in this indicator (p ≤ 0.01) in patients after the course of therapy compared with the moment of inclusion in the study.
In the present study, venlafaxine appeared to be well tolerated. In one case, a patient was withdrawn from the study due to mydriasis. The adverse event was not severe, but the patient refused to continue therapy and dose adjustments. In 14 (43.3%) of 29 patients, 20 cases of AE were registered, however, the relationship of their development with venlafaxine was assessed as "probable" only in 6 cases, and in other cases as "supposed". Assessment of the intensity of AE showed that 12 (60%) of 20 cases of AE were of low intensity, 8 (40%) were moderate. In most cases, no measures were taken regarding therapy, but in 5 of 20 (40%) cases, the dose of the drug was reduced. All AEs that occurred during the study period and occurred with a frequency of more than 1 case are presented in the table.
In most cases, no measures were taken regarding therapy, but in 5 of 20 (40%) cases, the dose of the drug was reduced. All AEs that occurred during the study period and occurred with a frequency of more than 1 case are presented in the table.
As can be seen from the table, nausea and mydriasis occupy the largest share among AEs. In most cases, they appeared in the first two weeks of therapy. Drowsiness, as a rule, was observed on the 1st week of treatment, which, along with indicators of the dynamics of the ratio of anxiety reduction and lethargy, probably reflects some sedative effect of the drug in the first days of therapy. Analysis of the outcomes of AEs showed that in the vast majority of cases (80%) AEs were transient. In most cases, they developed in the first week of therapy when using a dose of 150 mg / day and, in the future, as therapy continued, they were reduced. With a further increase in the dose of the drug in most patients, an increase in the frequency of AE, as a rule, did not occur. In addition, after the first week of therapy, adverse events were noted only when using doses of 150 mg / day.
In addition, after the first week of therapy, adverse events were noted only when using doses of 150 mg / day.
Discussion
Summing up the results of the study, it is necessary to note the high efficacy of venlafaxine, and, most importantly, a 50% reduction in symptoms occurred fairly quickly, on the 2-3rd week of therapy. There are data in the literature on an earlier clinical response (within the 1st, rarely 2nd week of therapy) when using high doses of venlafaxine [13, 14, 15]. However, the design of these studies was based on the rapid buildup of venlafaxine. The most revealing study compared 3 groups of patients taking different doses of venlafaxine (75 mg - the first group; 150-225 mg - the second group; 300-375 mg - the third group). The results of this work showed that in the 1st and 2nd groups of patients, the onset of the primary response was observed in the first 2 weeks of therapy, while in the 3rd group of patients taking the highest dose of the drug, a statistically significant effect was noted already in the 1st week of treatment [15].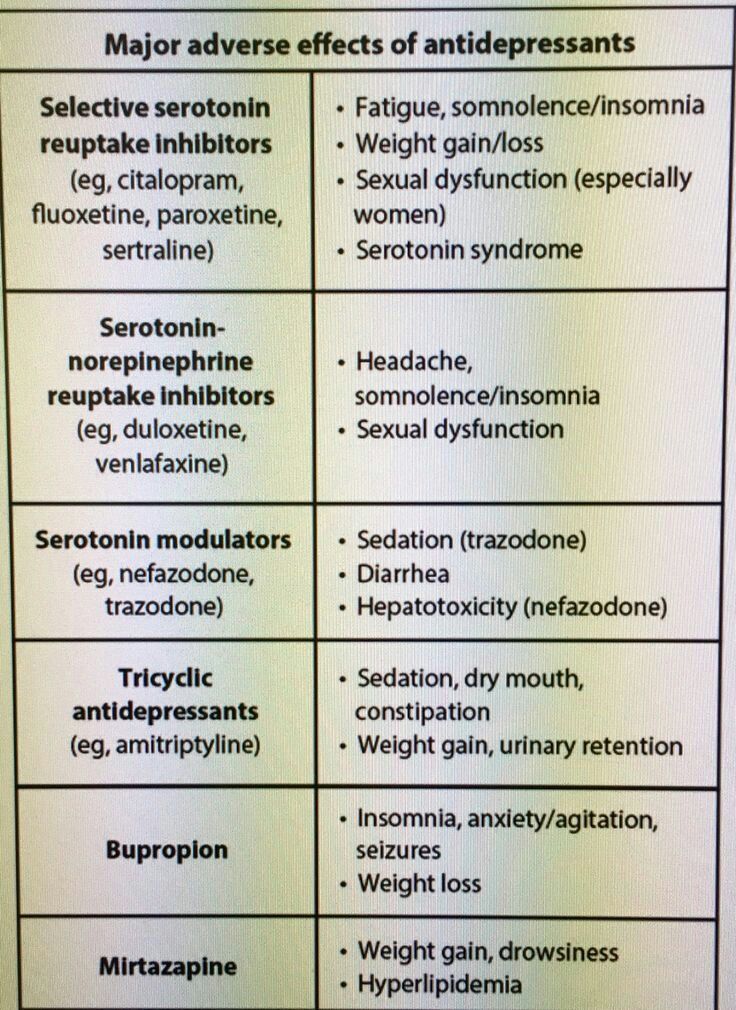 The dose escalation regimen in the present study differed from those described above in greater flexibility, which allowed the safe and effective use of venlafaxine for the treatment of depressive disorders in outpatients. It is clear that a rapid dose escalation strategy that allows full use of the dose-dependent effect of venlafaxine is especially appropriate in the treatment of major depressive disorders in an inpatient setting.
The dose escalation regimen in the present study differed from those described above in greater flexibility, which allowed the safe and effective use of venlafaxine for the treatment of depressive disorders in outpatients. It is clear that a rapid dose escalation strategy that allows full use of the dose-dependent effect of venlafaxine is especially appropriate in the treatment of major depressive disorders in an inpatient setting.
The dose-dependent effect of venlafaxine, associated with the gradual inclusion of various mediator structures (serotonin, norepinephrine, dopamine) into the orbit of the drug's influence, indirectly leads to a difference in the features of the clinical action. Thus, in the study, the drug used in high doses (more than 150 mg/day) proved to be predominantly sedative, while in smaller doses it had a more balanced effect.
The drug was quite well tolerated. None of the patients had severe adverse events, the vast majority of side effects were dose-dependent and were observed mainly in the first 2 weeks of therapy.
Given the rather pronounced sedative effect of the drug in the first weeks of therapy and the ability to control it depending on the dynamics of the dose, as well as the rapid reduction of sleep disturbances and the balanced nature of the action after 3 weeks of therapy, it can be noted that venlafaxine is one of the optimal drugs of choice for depressive patients on an outpatient basis.
Thus, summarizing the results of the study, we can conclude that venlafaxine has a distinct thymoanaleptic effect and has a balanced effect on the symptoms of anxiety and lethargy, with a moderate sedative effect in the first week of therapy. Of particular importance is the ability of the drug to quickly reduce depressive disorders of any severity, as well as a wide range of comorbid depression and anxiety disorders.
Literature.
1. Danish University Antidepressant Group. Citalopram: clinical effect profile in comparison with clomipramine. A controlled multicenter study // Psychopharmacology 1986; 90:131-138.
2. Danish University Antidepressant Group. Paroxetine: a selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study // J Affective Disorders 1990; 18:289-299.
3. Anderson I.M., Tomenson B.M. The efficacy of selective serotonin re-uptake inhibitors in depression: a meta-analysis of studies against tricyclic antidepressants. J Psychopharmacol 1994; 8:238-49.
4. Anderson I.M. SSRIs versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability // Depression Anxiety 1998; 7: Suppl. 1:11-17.
5. Mosolov S.N. Clinical use of modern antidepressants. St. Petersburg: 1995.
6. Smulevich A.B. Depression in general medicine. M.: 2001.
7. Preskorn S.H. Applied Clinical Psychopharmacology // J Practical Psychiatry and Behavioral Health, 1999, July; 224-8.
8. Samuelian J.C., Hackett D. A randomized, double-blind comparison of venlafaxine and clomipramine in outpatients with major depression // J Psychopharmacol 1998; 12:3:273-278.
9. Benedictis E. Double-blind comparison of venlafaxine and amitriptyline in outpatients with or without melancholia // J Psychopharmacol 2000; 14:1:61-66.
10. Schweizer E., Feigner J., Mandos et al. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients // J Clin Psychiatry 1994; 55:3:104-108.
11. Danjou P, Hackett D. Safety and tolerance profile of venlafaxine // Internat Clin Psychopharmacol 1995; 10:15-20.
12. Preskorn S.H. Comparison of the tolerability of buproprion, fluoxetine, imipramine, nefazodone, paroxetine, sertraline and venlafaxine // J Clin Psychiat 1995; 56: 1221.
13. Wang C.P., Howell S.R., Scatina J., Sisenwine S.F. The disposition of venlafaxine enantomers in dogs, rats, and humans receiving venlafaxine// Chirality 1992; 4:84-90.
14. Khan A., Fabre L., Rudolph R. Venlafaxine in depressed outpatients // Psychopharmacol Bull 1991; 27:141-4.
15. Schweizer E., Weise C., Clary C. et al.