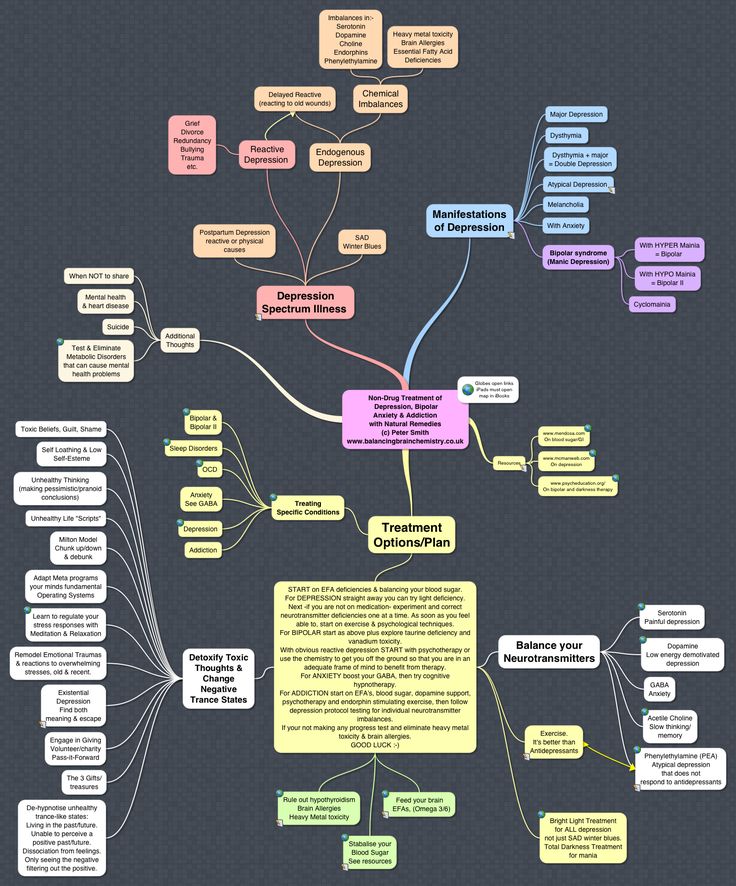
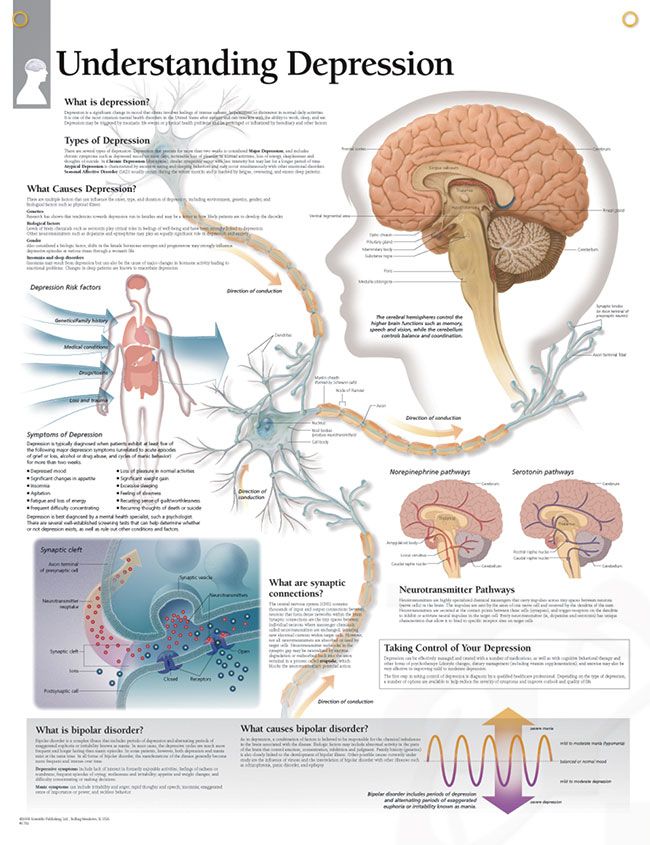
What neurotransmitters cause depression
How Depression Affects the Brain > News > Yale Medicine
When we think about depression, what comes to mind are feelings and emotions – or, for some, the absence of feelings and emotions. In order to really understand depression, however, it’s important to be aware that the condition has physical aspects as well. Most people understand what depression looks like on the outside, in terms of a person’s behavior, but our medical understanding of the actual progression of the disease and its treatments continues to evolve.
What we know right now is that, on a chemical level, depression involves neurotransmitters, which can be thought of as the messengers that carry signals between brain cells, or neurons.
“The current standard of care for the treatment of depression is based on what we call the ‘monoamine deficiency hypothesis,’ essentially presuming that one of three neurotransmitters in the brain is deficient or underactive,” says Rachel Katz, MD, a Yale Assistant Professor of Clinical Psychiatry.
But according to Dr. Katz, this is only part of the story. There are about 100 types of neurotransmitters overall, and billions of connections between neurons in each person’s brain.
There remains much to learn.
For years and years, doctors and researchers assumed that depression stemmed from an abnormality within these neurotransmitters, particularly serotonin or norepinephrine. But over time, these two neurotransmitters did not seem to account for the symptoms associated with major depression. As a result, doctors began to look elsewhere.
The search proved fruitful. “There are chemical messengers, which include glutamate and GABA, between the nerve cells in the higher centers of the brain involved in regulating mood and emotion,” says John Krystal, MD, chair of Yale’s Department of Psychiatry, noting that these may be alternative causes for the symptoms of depression.
These two are the brain’s most common neurotransmitters.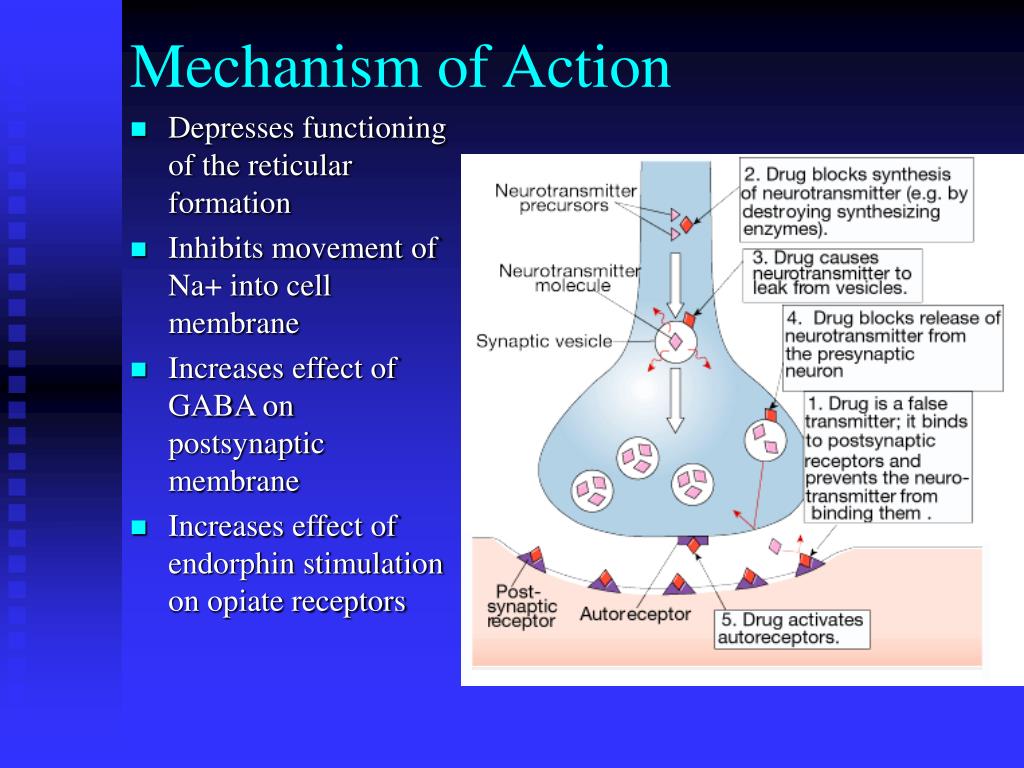 They regulate how the brain changes and develops over a lifetime. When a person experiences chronic stress and anxiety, some of these connections between nerve cells break apart. As a result, communication between the affected cells becomes “noisy,” according to Dr. Krystal. And it’s this noise, along with the overall loss of connections, that many believe contribute to the biology of depression.
They regulate how the brain changes and develops over a lifetime. When a person experiences chronic stress and anxiety, some of these connections between nerve cells break apart. As a result, communication between the affected cells becomes “noisy,” according to Dr. Krystal. And it’s this noise, along with the overall loss of connections, that many believe contribute to the biology of depression.
This “neurobiology of depression” is important to understand. First, it helps doctors understand how the disease develops and evolves. Also important, though, is that those same doctors can then use their new understanding of depression’s mechanisms to build targeted treatment plans. And, since depression is often a long-term disease, people needs long-term treatments for it.
“There are clear differences between a healthy brain and a depressed brain,” Dr. Katz says. “And the exciting thing is, when you treat that depression effectively, the brain goes back to looking like a healthy brain. ”
”
We have entered a new era of psychiatry, Dr. Katz adds. As we shift away from a single hypothesis about what causes depression, we are also learning more about the brain as a whole, in all of its complexities.
In this video, Drs. Katz and Krystal explain how depression affects the brain.
Read more Yale Medicine newsRelated Specialists
Should You Be Screened for Anxiety?
Relationship of neurotransmitters to the symptoms of major depressive disorder
Review
. 2008;69 Suppl E1:4-7.
David J Nutt 1
Affiliations
Affiliation
- 1 Psychopharmacology Unit, University of Bristol, United Kingdom. [email protected]
- PMID: 18494537
Review
David J Nutt. J Clin Psychiatry. 2008.
J Clin Psychiatry. 2008.
. 2008;69 Suppl E1:4-7.
Author
David J Nutt 1
Affiliation
- 1 Psychopharmacology Unit, University of Bristol, United Kingdom. [email protected]
- PMID: 18494537
Abstract
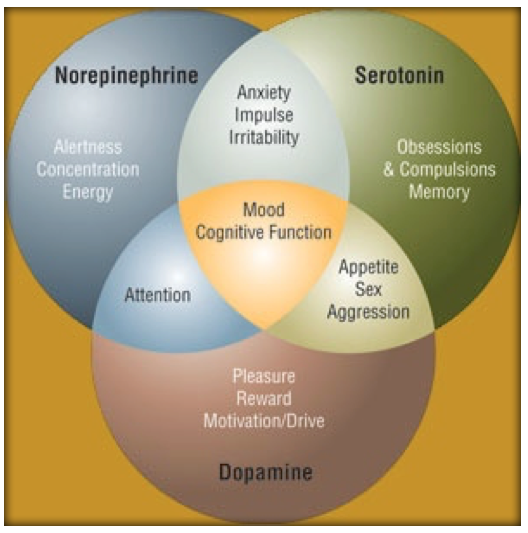
A relationship appears to exist between the 3 main monoamine neurotransmitters in the brain (i.e., dopamine, norepinephrine, and serotonin) and specific symptoms of major depressive disorder. Specific symptoms are associated with the increase or decrease of specific neurotransmitters, which suggests that specific symptoms of depression could be assigned to specific neurochemical mechanisms, and subsequently specific antidepressant drugs could target symptom-specific neurotransmitters. Research on electroconvulsive therapy has supported a correlation between neurotransmitters and depression symptoms. A 2-dimensional model of neurotransmitter functions is discussed that describes depression as a mixture of 2 separate components--negative affect and the loss of positive affect--that can be considered in relation to the 3 amine neurotransmitters. Owing to the different methods of action of available antidepressant agents and the depression symptoms thought to be associated with dopamine, serotonin, and norepinephrine, current treatments can be targeted toward patients' specific symptoms.
Research on electroconvulsive therapy has supported a correlation between neurotransmitters and depression symptoms. A 2-dimensional model of neurotransmitter functions is discussed that describes depression as a mixture of 2 separate components--negative affect and the loss of positive affect--that can be considered in relation to the 3 amine neurotransmitters. Owing to the different methods of action of available antidepressant agents and the depression symptoms thought to be associated with dopamine, serotonin, and norepinephrine, current treatments can be targeted toward patients' specific symptoms.
Similar articles
-
The other face of depression, reduced positive affect: the role of catecholamines in causation and cure.
Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S. Nutt D, et al. J Psychopharmacol.
 2007 Jul;21(5):461-71. doi: 10.1177/0269881106069938. Epub 2006 Oct 18. J Psychopharmacol. 2007. PMID: 17050654 Review.
2007 Jul;21(5):461-71. doi: 10.1177/0269881106069938. Epub 2006 Oct 18. J Psychopharmacol. 2007. PMID: 17050654 Review. -
[A new concept of antidepressant drugs action: clinical and preclinical data].
Płaźnik A, Kostowski W. Płaźnik A, et al. Psychiatr Pol. 1994 Jan-Feb;28(1):39-50. Psychiatr Pol. 1994. PMID: 7910691 Review. Polish.
-
Emerging drugs for major depressive disorder.
Kennedy SH, Rizvi SJ. Kennedy SH, et al. Expert Opin Emerg Drugs. 2009 Sep;14(3):439-53. doi: 10.1517/14728210903107751. Expert Opin Emerg Drugs. 2009. PMID: 19637989 Review.
-
[Monoamine's role in depression and possible consequences for the clinical management of the core symptoms of major depression].
La Pia S. La Pia S. Riv Psichiatr. 2009 Jan-Feb;44(1):1-14. Riv Psichiatr. 2009. PMID: 20066933 Review. Italian.
-
Biological basis of depression and therapeutic relevance.
Richelson E. Richelson E. J Clin Psychiatry. 1991 Jun;52 Suppl:4-10. J Clin Psychiatry. 1991. PMID: 1646793 Review.
See all similar articles
Cited by
-
The Role of Beta-Adrenergic Receptors in Depression and Resilience.
Zhang H, Cui M, Cao JL, Han MH. Zhang H, et al. Biomedicines. 2022 Sep 23;10(10):2378. doi: 10.3390/biomedicines10102378. Biomedicines. 2022. PMID: 36289638 Free PMC article. Review.
-
Gabrb2 knock-out mice exhibit double-directed PMDD-like symptoms: GABAAR subunits, neurotransmitter metabolism disruption, and allopregnanolone binding.
Gao M, Zhang H, Sun Y, Gao Z, Sun C, Wei F, Gao D. Gao M, et al. Aging (Albany NY). 2022 Oct 24;14(20):8437-8447. doi: 10.18632/aging.204351. Epub 2022 Oct 24. Aging (Albany NY). 2022. PMID: 36287173 Free PMC article.
-
Dopamine Receptors: Is It Possible to Become a Therapeutic Target for Depression?
Zhao F, Cheng Z, Piao J, Cui R, Li B. Zhao F, et al. Front Pharmacol. 2022 Aug 17;13:947785. doi: 10.3389/fphar.2022.947785. eCollection 2022. Front Pharmacol. 2022. PMID: 36059987 Free PMC article. Review.
-
Pathophysiology of Depression: Stingless Bee Honey Promising as an Antidepressant.
Zakaria FH, Samhani I, Mustafa MZ, Shafin N. Zakaria FH, et al. Molecules. 2022 Aug 10;27(16):5091.
 doi: 10.3390/molecules27165091. Molecules. 2022. PMID: 36014336 Free PMC article. Review.
doi: 10.3390/molecules27165091. Molecules. 2022. PMID: 36014336 Free PMC article. Review. -
Factors related to the length of stay for major depressive disorder patients in China: A real-world retrospective study.
Cheng P, Wang L, Xu L, Zhou Y, Zhao G, Zhang L, Li W. Cheng P, et al. Front Public Health. 2022 Jul 29;10:892133. doi: 10.3389/fpubh.2022.892133. eCollection 2022. Front Public Health. 2022. PMID: 35968457 Free PMC article.
See all "Cited by" articles
Publication types
MeSH terms
Substances
The role of the brain - iFightDepression [EN]
The role of the brain - iFightDepression [EN] Zur optimalen Präsentation der Website muss JavaScript in den Einstellungen ihres Browsers aktiviert sein.
During depression, the functioning of the brain changes. The balance of neurotransmitters, the neurotrophic factor of the brain is disturbed, the state of synapses changes.
In people with chronic depression beginning in childhood or adolescence, some brain structures may even be smaller.
(The prognosis of antidepressant treatment in combination with psychotherapy is generally better in terms of relapse prevention than antidepressant treatment alone).
However, the mechanisms of depression are still not entirely clear. It is likely that the concentration of neurotransmitters in the synaptic cleft and brain-derived neurotrophic factor is too low, which leads to a decrease in the number of synapses and an unbranched dendritic tree.
People with depression have different parts of the brain that function differently.
In this process, neurotransmitters such as norepinephrine and serotonin play an important role.
We looked at the various causes of depression, how they interact, and what happens at the brain level. Here is a list of the most common causes:
Here is a list of the most common causes:
- Life difficulties - we all need time to sort out and cope with some stressful situations, such as a bereavement or relationship breakdown. When these events occur, the risk of developing depression increases, especially if you withdraw into yourself, stop seeing friends and family, and try to cope with the situation yourself.
- Long-term difficulties - financial problems, poverty or caring for a disabled relative increase the risk of depression
- Illness - If a person has a chronic or life-threatening illness such as heart disease, immune disease, or cancer, there is a high risk of developing depression.
- Traits - Some people with low self-esteem may be more vulnerable to depression. Character traits are associated with heredity, as well as education and social environment.
- Family history - if one of your first-order relatives has already suffered from depression, the likelihood that you will also be much higher.
- Personal history - if a person has had a previous episode of depression, the risk of relapse is increased. The more episodes a person has in the past, the greater the risk of developing further depressive episodes in the future.
- Pregnancy and menopause - women are more vulnerable to depression than men due to differences in hormonal environments. Hormonal imbalance and variability in different phases of a woman's life is the reason for the high risk of developing depression. A well-known example is postpartum depression.
- Alcohol and drugs - People tend to use drugs to achieve altered states of consciousness and also as a form of self-medication. In some cultures, depressed people are particularly prone to alcohol abuse. The toxic effects of alcohol can lead to worsening depression. In addition, cannabis use can lead to depression, especially in teenagers.
- Sleep and wake disorders - depression causes sleep disorders such as insomnia and hypersomnia.
 However, sleep disturbances and rest can themselves lead to depression. Healthy sleep is necessary for the normal functioning of the body.
However, sleep disturbances and rest can themselves lead to depression. Healthy sleep is necessary for the normal functioning of the body.
Neurobiology of depression: serotonin system of the brain
Major depression is a common mental disorder that is one of the most common causes of disability [1, 2]. This disease occurs in all age groups and affects people of both sexes in every region of the world. The experience of the last decades has shown that the prospects for studying depression are related to its neurobiology. The molecular hypothesis is widely used to explain the pathogenetic mechanisms of depression. According to the latter, adverse environmental factors such as stress affect genetic vulnerability, which causes maladaptive changes in the chain of neurotransmitters, among which monoamines play a major role. In most of the existing achievements in the treatment of the disease, the effects on the deciphered mediator mechanisms of pathogenesis are also realized [3].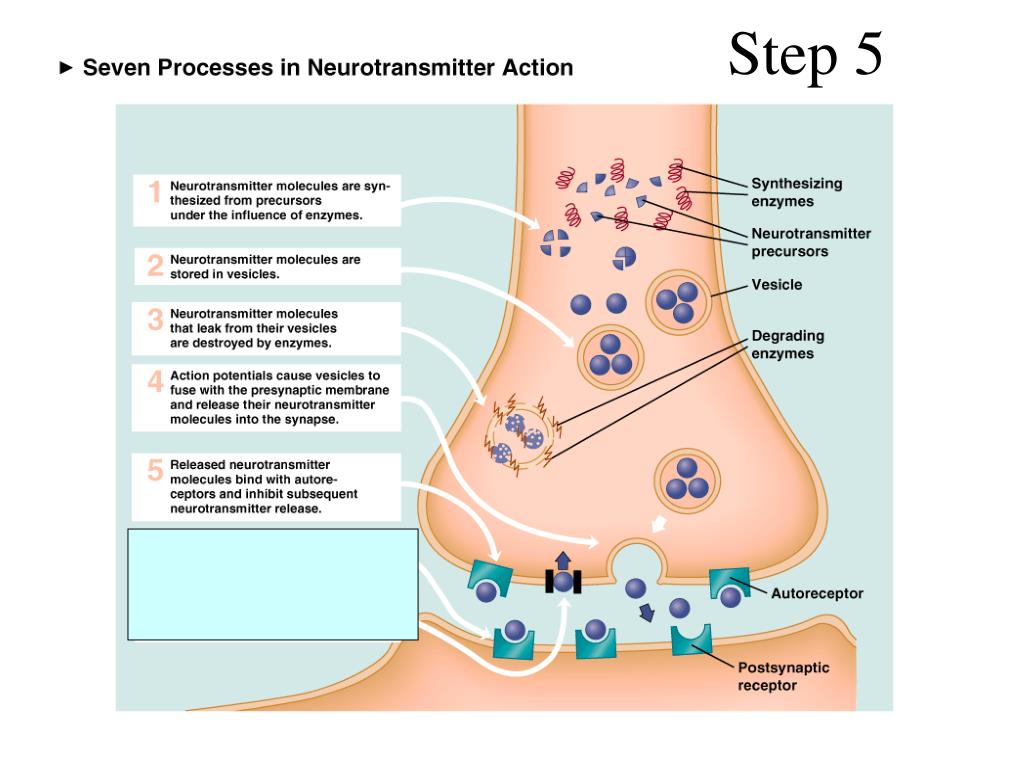
One of the most important systems of cerebral neurotransmission involved in the pathogenesis of depression is the serotonin system. This neurotransmitter system has a long evolutionary history and is involved in a number of behavioral acts and emotional manifestations [4]. It is the subject of a significant number of studies, a review of which is presented in this publication.
To better understand the integration of the serotonin system into the brain's mood regulation processes, one should first consider the available data on the influence of different cerebral regions on affective manifestations. Thus, executive functions, including the modulation of emotional behavior, which may be related to the formation of cognitive symptoms of depression (depressive vision of the future), are associated with hypoactivation of the left frontal cortex [5].
The emotional memory system, including the amygdala and hippocampus, is also involved in the realization of manifestations of depression. Depressed patients demonstrate a predominant focus on negative past events [6]. Dysfunction of striatal circles that perform psychomotor functions can explain motor symptoms depression. Eating Disorders and violations of a number of other somatic functions indicate the involvement of the hypothalamus in the process and the hypothalamic-pituitary-adrenal axis.
Depressed patients demonstrate a predominant focus on negative past events [6]. Dysfunction of striatal circles that perform psychomotor functions can explain motor symptoms depression. Eating Disorders and violations of a number of other somatic functions indicate the involvement of the hypothalamus in the process and the hypothalamic-pituitary-adrenal axis.
These brain formations are anatomically and functionally interconnected with the help of neuronal circles [4].
Numerous experimental literature indicate the importance of pathways that unite the frontal, paralimbic (ventral frontal cortex, cingulate gyrus, insula, anterior temporal pole), striatal and brainstem regions into a single network in the implementation of affective and motivational processes [7–9]. In turn, with the help of functional neuroimaging methods, disturbances in the activity of the above brain regions in depressive patients were detected [10]. The development of a neuroanatomical model of depression was facilitated by data on the occurrence of depressive disorders in organic lesions of various brain structures.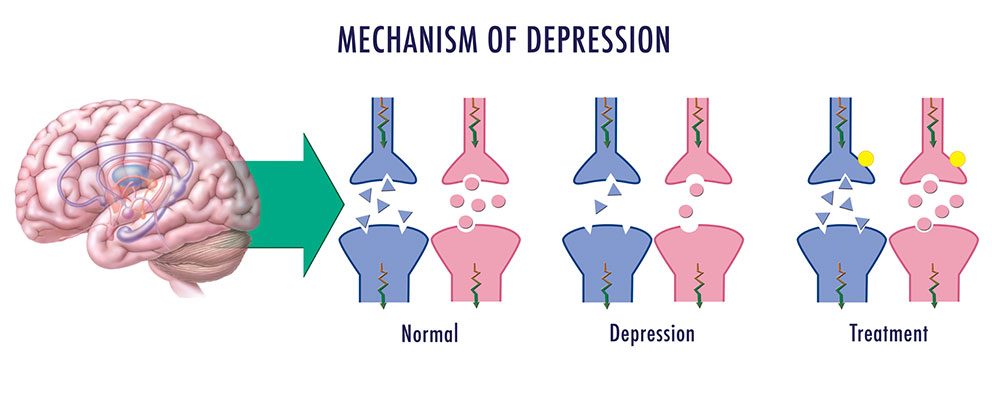 An example is ischemic lesions of the left frontal lobe in post-stroke depression [11, 12], as well as damage to the frontostriatal pathways in patients with vascular depression and Parkinson's disease [13-15].
An example is ischemic lesions of the left frontal lobe in post-stroke depression [11, 12], as well as damage to the frontostriatal pathways in patients with vascular depression and Parkinson's disease [13-15].
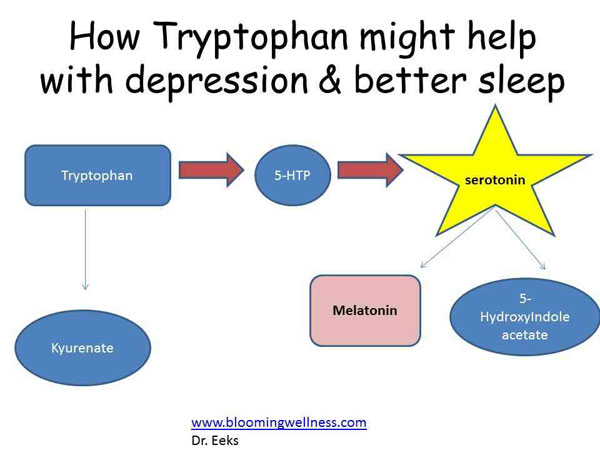
The serotonin system of the brain is an integral part of the described neuronal networks of mood regulation. Serotonergic neurons are grouped in 9 nuclei of the brainstem. Most of them coincide with the medially located raphe nucleus [16]. Serotonin (5-hydroxytryptamine [5-HT]) is synthesized in these nuclei from tryptophan.
The ascending terminals of serotonergic nuclei take part in the regulation of affective processes, which end in a large number of brain structures: subcortical formations (caudate nucleus, putamen, anterior and medial nuclei of the thalamus), diencephalon, olfactory brain and a number of formations associated with the reticular formation, cortex of large hemispheres, amygdala and hypothalamus. At the same time, there is much more serotonin in the cortex of the limbic system than in neocortical regions [17-19].
The importance of impaired serotonin synthesis for the onset of depression has been shown in studies investigating the effects of dietary restriction of tryptophan intake. The hypotryptophan diet resulted in depressive symptoms in healthy individuals and in depressed patients in remission. According to positron emission tomography, the examined patients showed a decrease in the activity of the pre- and orbitofrontal cortex, as well as the thalamus [20]. There is convincing evidence of the genetic determinism of serotonin synthesis in the brain. It is known that the human genome contains the 5-HTT gene, whose activity regulates the level of serotonin produced by the brain [21].
Serotonin fulfills its physiological role by acting on 5-HT receptors.
Currently, more than 15 types of serotonin receptors are known [22–26], but not all of them have been identified in the human brain.
In the central nervous system (CNS) of mammals, serotonin 5-HT 1 receptors and five of their subtypes - A, B, D, E, F, are found, which are proteins containing 365-422 amino acid residues. Through inhibitory G-proteins, these receptors are coupled to adenylate cyclase, the activity of which is suppressed upon their activation.
Through inhibitory G-proteins, these receptors are coupled to adenylate cyclase, the activity of which is suppressed upon their activation.
5-HT 1A receptors are predominantly localized in the hippocampus, tonsils, septum pellucidum - structures involved in mood formation. These CNS receptors are located on the pre- and postsynaptic membranes [27]. Presynaptic 5-HT 1A receptors regulate the intensity of serotonin release from presynaptic neuronal terminals by the feedback principle. Through stimulation of postsynaptic 5-HT 1A receptors, a number of important physiological functions of serotonin are realized: mood regulation, obsessive-compulsive reactions, sexual behavior, appetite control, thermoregulation, cardiovascular regulation. It is this type of receptor that is involved in the implementation of the antidepressant effect of selective serotonin reuptake inhibitors, the antidepressant and anti-anxiety effect of buspirone.
The subtype of human 5-HT 1D receptors (functional analog of rat 5-HT 1B receptors) is localized in the frontal cortex, striatum, and basal ganglia [22, 28]. Presynaptic 5-HT 1D receptors play the role of autoreceptors through which a negative feedback between the level of extra- and intraneuronal serotonin is carried out. Perhaps they also play the role of heteroreceptors, through which the release of other neurotransmitters, such as dopamine, acetylcholine, glutamate, is controlled. Stimulation of postsynaptic receptors of this subtype in experimental models caused prolonged hyperactivity, antidepressant effect, decreased pain sensitivity and appetite, and hypothermia.
Presynaptic 5-HT 1D receptors play the role of autoreceptors through which a negative feedback between the level of extra- and intraneuronal serotonin is carried out. Perhaps they also play the role of heteroreceptors, through which the release of other neurotransmitters, such as dopamine, acetylcholine, glutamate, is controlled. Stimulation of postsynaptic receptors of this subtype in experimental models caused prolonged hyperactivity, antidepressant effect, decreased pain sensitivity and appetite, and hypothermia.
It has recently been shown that the functioning of the 5-HT 1B/D receptor depends on the P11 peptide belonging to the S100 protein group. The concentration of P11 peptide in the brain of patients with depression was low. Long-term antidepressant treatment increases the level of this peptide in brain tissue [3]. The function of other subtypes of 5-HT 1 receptors has not yet been established.
5-HT 2 receptors have been found in the human CNS. Their family consists of three subtypes: 5-HT 2A , 5-HT 2B , 5-HT 2C [22, 29, 30]. To a greater extent, such receptors are present in the pyramidal neurons of the frontal cortex, the putamen, and to a lesser extent, in the hippocampus and caudate nucleus. They are part of the brain reinforcement system, the low activity of which causes the occurrence of anhedonia, one of the key symptoms of depression [22]. 5-HT 2A receptors mediate the anxiogenic effect, participate in the formation of sexual behavior, and are involved in the regulation of sleep. A decrease in their number was noted in post-mortem studies in people who suffered from depression and committed suicide. 5-HT 9 activation0067 2A receptor causes an increase in concentration dopamine in the striatum. Modern atypical Antipsychotics are highly active in relation to this subtype, which is associated with the antidepressant effect of these drugs [31]. Antagonists of 5-HT 2A receptors increase the duration of slow-wave sleep, improving its quality, while agonists reduce the phase of fast-wave sleep.
Their family consists of three subtypes: 5-HT 2A , 5-HT 2B , 5-HT 2C [22, 29, 30]. To a greater extent, such receptors are present in the pyramidal neurons of the frontal cortex, the putamen, and to a lesser extent, in the hippocampus and caudate nucleus. They are part of the brain reinforcement system, the low activity of which causes the occurrence of anhedonia, one of the key symptoms of depression [22]. 5-HT 2A receptors mediate the anxiogenic effect, participate in the formation of sexual behavior, and are involved in the regulation of sleep. A decrease in their number was noted in post-mortem studies in people who suffered from depression and committed suicide. 5-HT 9 activation0067 2A receptor causes an increase in concentration dopamine in the striatum. Modern atypical Antipsychotics are highly active in relation to this subtype, which is associated with the antidepressant effect of these drugs [31]. Antagonists of 5-HT 2A receptors increase the duration of slow-wave sleep, improving its quality, while agonists reduce the phase of fast-wave sleep.
5-HT 2C CNS receptors are found in the greatest amount in the hippocampus, cerebral cortex, striatum, substantia nigra. Agonists of these receptors cause anxiogenic and panic effects, disturb sleep. Blockade 5-HT 2C receptor is one of the mechanisms of depression treatment.
Associated with this is the effectiveness of antidepressants that are antagonists of these receptors (mianserin, imipramine, maprotiline, amitriptyline, desipramine, agomelatine) [32, 33]. Antagonists 5-HT 2C receptors improve sleep [22, 30] and have anxiolytic properties. The latter partly explains the anti-anxiety effect of selective serotonin reuptake inhibitors.
5-HT 3 receptors are located in the solitary tract, gelatinous substance, nuclei of the trigeminal and vagus nerves, hippocampus. Their central antagonists have an anxiolytic effect, increase cognitive abilities, change the sensitivity of nociceptive neurons, and have an antiemetic effect.
5-HT 4 receptors are maximally represented in areas saturated with dopaminergic neurons (basal nuclei, accumbens). They are localized on GABAergic and cholinergic interneurons and GABAergic projections into the substantia nigra. Agonists of these receptors can increase the activity of dopaminergic systems, while antagonists can block this effect. There are data on the anxiolytic effect of antagonists of 5-HT 4 receptors [22, 34].
5-HT 6 receptors are located in the striatum, amygdala, hippocampus, cortex, olfactory bulb. Various antidepressants (clomipramine, amitriptyline, nortriptyline, doxepin) have a high affinity for them and are their antagonists.
5-HT 7 receptors are present in the hypothalamus, thalamus, and brain stem. They can participate in the organization of circadian rhythms by influencing the suprachiasmatic nuclei. In the future, 5-HT 6 - and 5-HT 7 receptors may become a target for modeling depression [3].
The next level of disorders of the serotonin system in depression is reuptake 5-HT from the synaptic cleft to the presynaptic neuron, which is carried by the serotonin transporter protein. The density of this protein in the brain of depressed patients decreased, which was detected using functional neuroimaging methods, and in those who died due to suicide, according to postmortem histochemical studies [35].
Individual features of serotonin turnover in the CNS, among other hereditary factors, depend on the effects of the
serotonin carrier gene (5-HTT). This gene is located on the 17th chromosome. It describes several polymorphic regions, including an insertion-deletion polymorphism (5-HTTLPR) found in the promoter region and represented by two allelic variants - l (long) and s (short - with a deletion). This polymorphism is functional [36-38].
A number of authors found an association between 5-HTTLPR polymorphism and the development of depressive states in response to various stressors [39].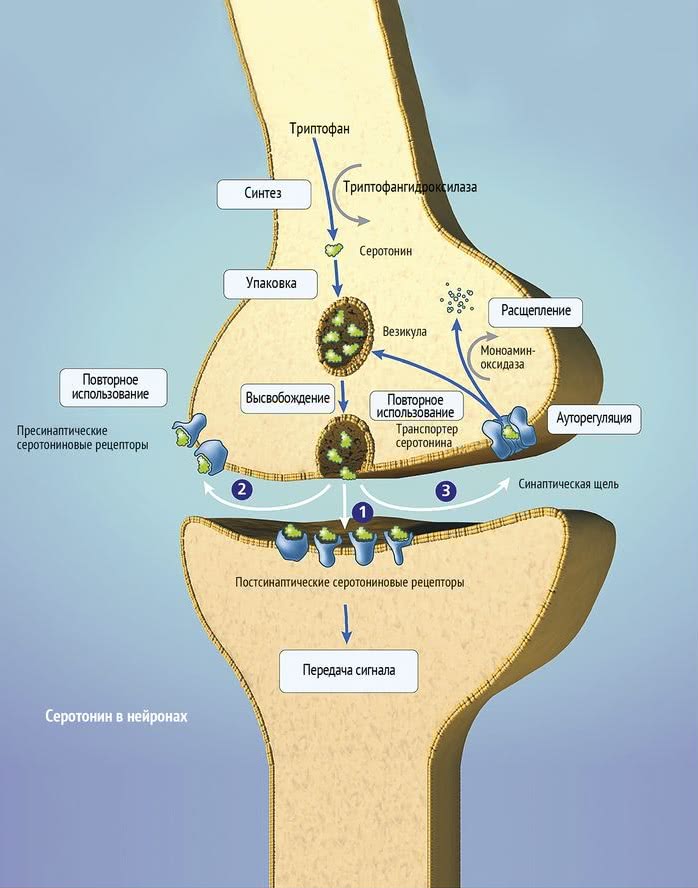 Individuals with at least one short allele in the
Individuals with at least one short allele in the
genotype exhibited more severe depressive symptoms, were more likely to be diagnosed with a DSM-IV depressive episode, and reported more suicidal thoughts and attempts during depressive episodes compared with homozygotes for the long allele. The role of the serotonin transporter gene in mediating the relationship between stressful life events and the subsequent development of depressive symptoms and physical distress was later confirmed by other authors [40–42]. In addition, it was found that healthy people - carriers of the short allele - are more characterized by increased emotional reactivity and anxiety, that is, personality traits that are considered as predispositional in relation to affective disorders [43, 44].
The facts described above testify to the great importance of the serotonin system for the functioning of brain areas that are directly related to the regulation of affective processes: frontal regions that modulate emotional behavior; the limbic region, which is related to emotional and cognitive impairment in depression; fronto-striatal structures that determine the occurrence of anhedonia; psychomotor disorders. Separately, it is necessary to single out the role of the serotonin system in the functioning of the hypothalamic region - the most important link in neuro-endocrine, autonomic, circadian regulation.
Separately, it is necessary to single out the role of the serotonin system in the functioning of the hypothalamic region - the most important link in neuro-endocrine, autonomic, circadian regulation.
Serotonin dysfunction directly affects limbic-hypothalamic-pituitary-adrenal regulation in patients with depression [45]. Depression is associated with increased daily production of adrenocorticotropic hormone. His hyperproduction can be explained by an increase in the production of corticotropin-releasing factor, the synthesis of which is normally limited by the feedback mechanism by the level of cortisol in the blood plasma.
Violation of the inhibitory effects of cortisol on the production of corticotropin-releasing factor in depression is associated with impaired function of glucocorticoid and 5-HT 1A receptors. The result of hyperactivity hypothalamic-pituitary-adrenal axis in patients with depression is an increase in plasma cortisol levels. Hypercortisolemia, in turn, leads to a decrease in the activity of postsynaptic 5-HT 1A receptors, one of the main manifestations of serotonin dysfunction. Thus, a vicious circle is closed.
Cortisol also potentiates an increase in adrenaline production. This is associated with an increase in the activity of the sympathetic link of the segmental division of the autonomic nervous system. These mechanisms are responsible for many autonomic symptoms of depression.
The serotonergic system is involved in the regulation of the sleep-wake cycle. Not surprisingly, one of the most common symptoms of depression is sleep disturbance. It is believed that the main generator of circadian rhythms, localized in the suprachiasmatic nucleus of the anterior hypothalamus [46], receives information about the level of body activity from the raphe nuclei along with stimuli from the intergeniculate nuclei of the lateral geniculate body [47, 48]. Blockade 5-HT 2C receptors in the hypothalamic region, which become hypersensitive in depression, according to Krauchi et al. (1997) and Leproult et al. (2005), can resynchronize the circadian rhythm and induce antidepressant effects [3].
Effects on serotonin neurotransmission are implemented in the mechanisms of action of many modern antidepressants and other psychotropic drugs. For some drugs, these mechanisms are the main pharmacodynamic effect, for others they are of additional importance.
Inhibition of serotonin reuptake underlies the pharmacodynamics of a large number of antidepressants: selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs).
SSRIs (citalopram, sertraline, fluoxetine, fluvoxamine, paroxetine) act on the main site of the serotonin transporter protein. Escitalopram blocks both the basic and allosteric sites of this protein. Blockade of the serotonin transporter protein causes an initial increase in the concentration of 5-HT in the somatodendritic zone (but not in the zone of the axonal terminal). This, in turn, causes a decrease in the activity of 5-HT 1A autoreceptors. Since their role is to suppress impulses coming to serotonergic neurons, as well as to suppress the synthesis and release of serotonin, the blockade of receptors causes the release of neurons from inhibitory influences and increases the release of serotonin from the axonal terminal into the synaptic cleft. An increase in the concentration of serotonin in the synaptic cleft allows it to exercise its effects on postsynaptic receptors, what is the antidepressant effect of this group of drugs. Time required to decrease the activity of somatodendritic 5-HT autoreceptors 1A and the resulting release of serotonin from the axonal terminal explains the 2–3 week delay in the onset of the effect of SSRIs [49–51]. The main advantages of this group of drugs include their selective effect on the serotonin system, and the absence or minimal effect on other mediator systems of the brain, which minimizes side effects [3]. The selectivity of drugs in the SSRI group is not the same. As selectivity decreases, SSRIs can be ranked as follows: escitalopram, citalopram, sertraline, fluoxetine, paroxetine.
An increase in the concentration of serotonin in the synaptic cleft allows it to exercise its effects on postsynaptic receptors, what is the antidepressant effect of this group of drugs. Time required to decrease the activity of somatodendritic 5-HT autoreceptors 1A and the resulting release of serotonin from the axonal terminal explains the 2–3 week delay in the onset of the effect of SSRIs [49–51]. The main advantages of this group of drugs include their selective effect on the serotonin system, and the absence or minimal effect on other mediator systems of the brain, which minimizes side effects [3]. The selectivity of drugs in the SSRI group is not the same. As selectivity decreases, SSRIs can be ranked as follows: escitalopram, citalopram, sertraline, fluoxetine, paroxetine.
SNRIs (venlafaxine, milnacipran, duloxetine) inhibit serotonin reuptake along with inhibition of norepinephrine reuptake. The significance of norepinephrine disorders in depression will be discussed in future publications. Blockade of serotonin reuptake is one of the main mechanisms of action of most TCAs (clomipramine, amitriptyline, doxepin, imipramine, protriptyline).
Blockade of serotonin reuptake is one of the main mechanisms of action of most TCAs (clomipramine, amitriptyline, doxepin, imipramine, protriptyline).
Unfortunately, the interaction of these drugs with other receptor systems (especially with cholinergic and histamine ones) leads to a large number of side effects and the refusal to use TCAs as first-line antidepressants [3].
Several drugs are active against 5-HT 1A receptors. Pindolol blocks presynaptic 5-HT 1A receptors and, therefore, should prevent an undesirable feedback effect, which is expressed in an increase in the concentration of somatodendritic serotonin. He showed the possibility of accelerating the onset of action of antidepressants [3]. Buspirone, gepirone, azaperone, partial antagonists of presynaptic 5-HT 1A receptors and activators of postsynaptic have an antidepressant effect [3].
Antidepressants of various chemical groups have a blocking effect on 5-HT 2C receptors: tetracyclic (mianserin), noradrenergic and specific serotonergic (mirtazapine), serotonin modulators (nefazodone, trazodone), an agonist of M 1 - and M 2 melatonin receptors and an antagonist of 5-HT 2C receptors (agomelatine). The antidepressant activity of modern atypical antipsychotics is also associated with the blockade of 5-HT 2C - and 5-HT 2A receptors [3]. In addition to their antidepressant action, these 5-HT 2 receptor antagonists synchronize biological rhythms disturbed in depression. In addition to the inhibition of 5-HT 2C receptors, mirtazapine, by blocking a2 receptors, stimulates the synthesis of serotonin [3].
The antidepressant activity of modern atypical antipsychotics is also associated with the blockade of 5-HT 2C - and 5-HT 2A receptors [3]. In addition to their antidepressant action, these 5-HT 2 receptor antagonists synchronize biological rhythms disturbed in depression. In addition to the inhibition of 5-HT 2C receptors, mirtazapine, by blocking a2 receptors, stimulates the synthesis of serotonin [3].
Potentially interesting possibilities in the treatment of depression may be related to exposure to on 5-HT 1B/D -, 5-HT 6 - and 5-HT 7 receptors. The emerging experimental data on the pharmacological efficacy of these targets require clinical validation [3].
Summarizing the presented data, we are fully aware that only an attempt was made to integrate modern information about the neurobiology of the serotonin system of the brain and the pharmacotherapy of depression based on the correction of serotonin metabolism disorders. The results of many studies are beyond the scope of this review. The prism through which the selection of data for inclusion in the work was carried out was the possibility of practical refraction of the knowledge gained. After all, "there is nothing more practical than a good theory." Isolation of isolated serotonin dysfunction in depression is also very conditional. It is obvious that the activity of this neurotransmitter system must be considered in the structure of the complex of interrelationships of disorders of the noradrenergic, dopamine, GABA, peptidergic, and other mediator systems. The presented information, which is part of the modern molecular hypothesis of depression, must be supplemented with data on other biological disorders that occur in this disease. They will be reflected in our subsequent publications. We very much hope that the proposed information on the neurobiological mechanisms of depressive disorders will be useful to practitioners.
The results of many studies are beyond the scope of this review. The prism through which the selection of data for inclusion in the work was carried out was the possibility of practical refraction of the knowledge gained. After all, "there is nothing more practical than a good theory." Isolation of isolated serotonin dysfunction in depression is also very conditional. It is obvious that the activity of this neurotransmitter system must be considered in the structure of the complex of interrelationships of disorders of the noradrenergic, dopamine, GABA, peptidergic, and other mediator systems. The presented information, which is part of the modern molecular hypothesis of depression, must be supplemented with data on other biological disorders that occur in this disease. They will be reflected in our subsequent publications. We very much hope that the proposed information on the neurobiological mechanisms of depressive disorders will be useful to practitioners.
Literature
1. Kessler R.S. et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey // Arch Gen Psychiatry. - 1994. - Vol. 51. – P. 8-19.
Kessler R.S. et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey // Arch Gen Psychiatry. - 1994. - Vol. 51. – P. 8-19.
2. Murray C.J.L., Lopez A.D. Global burden of disease: a comprehensive assessment of mortality and morbidity from diseases, injuries and risk factors in 1990 and projects to 2020, Vol. I. - Harvard: World Health Organization, 1996.
3. Reasonable use of antidepressants: a technical review of the data prepared by the CINP Working Group / Ed. T. Bagai, H. Grunze, N. Sartorius: per. from English. - St. Petersburg, 2006. - 174 p.
4. Stein D.J. Serotonergic neurocircuitry in mood and anxiety disorders // Martin Dunitz Ltd. - 2003. - 82 p.
5. Mineka S., Watson D., Clark L.A. Comorbidity of anxiety and unipolar mood disorders // Annu Rev Psychol. - 1998. - Vol. 49.- P. 377-412.
6. MacLeod A.K., Byrne A. Anxiety, depression, and the anticipation of future positive and negative experience // J Abnorm Psychol.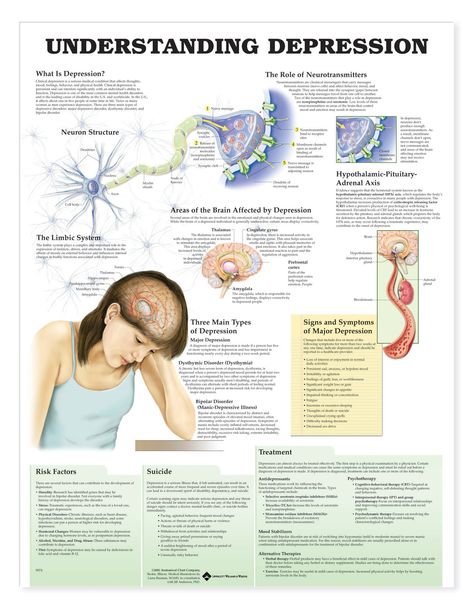 - 1993. - Vol. 102. - P. 238-247.
- 1993. - Vol. 102. - P. 238-247.
7. Damasio A.R. The somatic marker hypothesis and the possible function of the prefrontal cortex // Philos Trans R Sos. - 1996.- Vol. 54S. - P. 1413-1420.
8. MacLean P.D. Psychosomatic disease and the visceral brain: recent developments bearing on the Papez theory of emotion // Psychosom Med. - 1949. - Vol. 11. - P. 338-353.
9. Rolls E.T. A theory of emotion, and its application to understanding the neural basis of emotions // Cognition Emotion. - 1990. - Vol. 4. - P.161-190.
10. Videbach P. PET measurements of brain glucose metabolism and blood flow in major depression: a critical review // Acta Psychiatr Scand. - 2000. - Vol. 101. - P. 11-20.
11. Narushima K., Kosier J.T., Robinson R.G. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis // J Neuropsychiatry Clin Neurosci. - 2003. - Vol. 15. - P. 422-430.
12. Shimoda K., Robinson R.G. The relationship between poststroke depression and lesion location in long-term follow-up // Biol Psychiatry. - 1999. - Vol. 45. – P. 187-192.
- 1999. - Vol. 45. – P. 187-192.
13. Camus V., Kraehenbuhl H., Preisig M. et al. Geriatric depression and vascular diseases: what are the links? // J Affect Discord. - 2004. - Vol. 81, No. 1. - P. 1-16.
14. Firbank M.J., Lloyd A.J., Ferrier N., O'Brien J.T. A volumetric study of MRI signal hyperintensities in late-life depression // Am J Geriatr Psychiatry. - 2004. - Vol. 12, No. 6. - P. 606-612.
15. Seki T., Awata S., Koizumi Y. et al. Association between depressive symptoms and cerebrovascular lesions on MRI in community-dwelling elderly individuals // Nippon Ronen Igakkai Zasshi. - 2006. - Vol. 43, N 1. - P. 102-107.
16. Dahlstrom A., Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system // Acta Physiol Scand. - 1965.-Vol. 64. – P. 1-85.
17. Barkhatova V.P. Neurotransmitters and extrapyramidal pathology. - M.: Medicine, 1988.
18. Gromova E.A. Serotonin and its role in the body. - M.: Medicine, 1966.
19. Lutsenko N.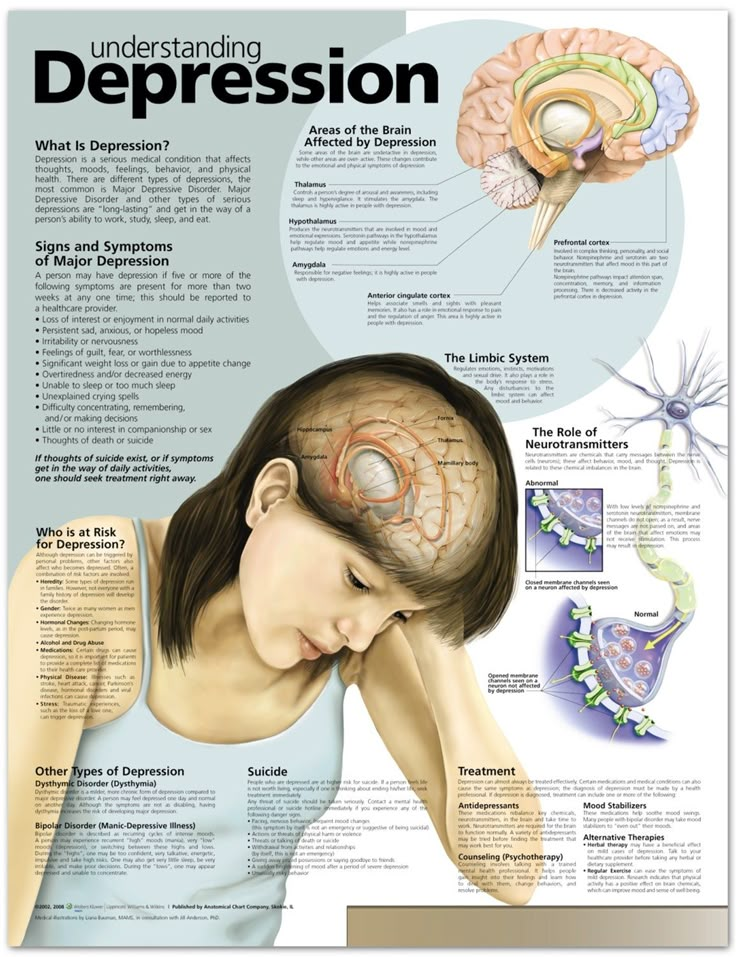 G., Suvorov N.N. Regulation of serotonin biosynthesis in the central nervous system // Uspekhi sovrem. biol. - 1982. - T. 94. - S. 243-251.
G., Suvorov N.N. Regulation of serotonin biosynthesis in the central nervous system // Uspekhi sovrem. biol. - 1982. - T. 94. - S. 243-251.
20. Bremmer J.D., Innis R.B., Salomon R.M. et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse // Arch Gen Psychiatry. - 1997.-Vol. 54. – P. 364-374.
21. Konysova A.Zh. Serotonin metabolism in multiple sclerosis and retrobulbar neuritis (clinical and biochemical study): Diss. …cand. honey. Sciences. M., 1995.
22. Sergeev P.V. Receptors. – Volgograd, 1999.
23. Cox C., Cohen J. 5-HT2B receptor signaling in the rat stomach fundus: dependence on calcium influx, calcium release and protein kinase C // Behav. Brain Res. - 1996. - Vol. 73. – P. 289.
24. Fox S.H., Brotchie J.M. Anti-parkinsonian action of 5-HT2C receptor antagonism in the substantia nigra pars reticulata // Mov. Discord. - 1997. - Vol. 12, Suppl. 1. – P. 116.
25. Hanssen E., Nilsson A.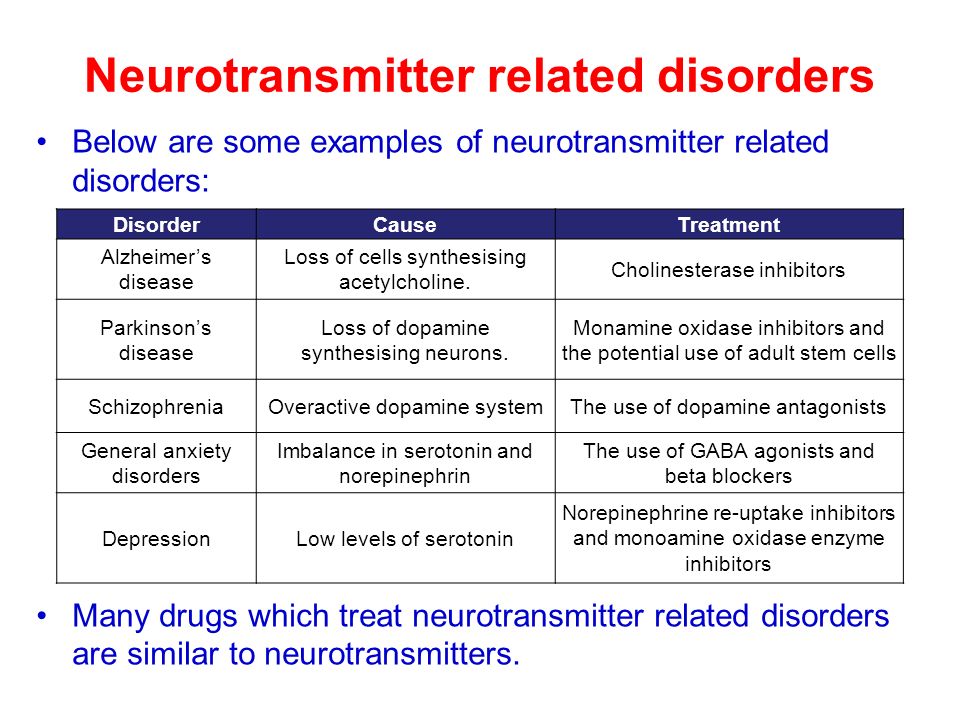 , Ericsson P. Heterogeneity among astrocytes evaluated biochemical parameters // Adv. biosci. - 1986. -
, Ericsson P. Heterogeneity among astrocytes evaluated biochemical parameters // Adv. biosci. - 1986. -
Vol. 61. - P. 235-241.
26. Holstege J.S., Knypers H.G. Brainstem projections to spinal motoneurons: an update commentary // Neuro. sci. - 1987. - Vol. 23. – P. 809-821.
27. Blier P., Ward N.M. Is the role for 5HT-1A-agonists in the treatment of depression // Biol. Psychiatry. - 2003. - Vol. 53. – P. 193-203.
28. Connor J.D. et al. Use of GR 55562, a selective 5-HT1D antagonist, to investigate 5-HT1D receptor subtypes mediating cerebral vasoconstriction // Cephalgia. - 1995. - Vol. 15, Suppl. 14. – P. 99.
29. Choi C, Maroteaux J. Immunohistochemical localization of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain // FEBS Lett. - 1996. - Vol. 391. – P. 45.
30. Martin G.R. et al. 5-HT2C receptor agonists and antagonists in animal models of anxiety // Eur. Neuropharmacol. - 1995.-Vol. 5. – P. 209.
31. Misyuk N.S. et al.