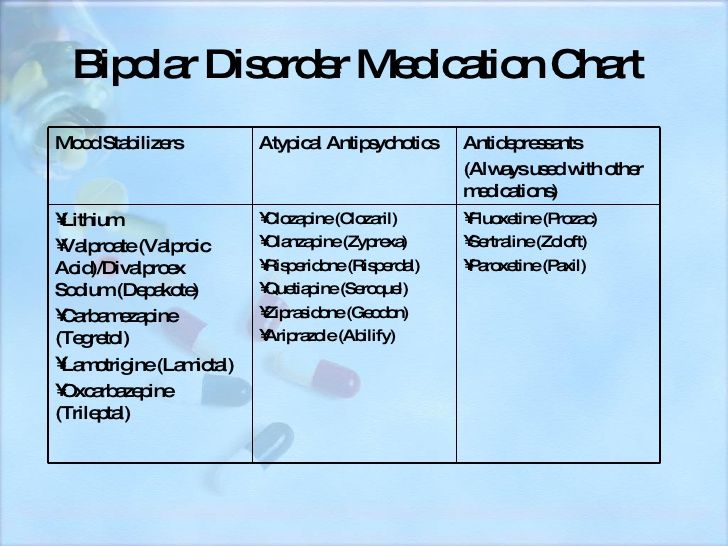
The pain i feel
The Neuroscience of Empathy – Association for Psychological Science – APS
Whether it’s watching a friend get a paper cut or staring at a photo of a child refugee, observing someone else’s suffering can evoke a deep sense of distress and sadness — almost as if it’s happening to us. In the past, this might have been explained simply as empathy, the ability to experience the feelings of others, but over the last 20 years, neuroscientists have been able to pinpoint some of the specific regions of the brain responsible for this sense of interconnectedness. Five scientists discussed the neuroscience behind how we process the feelings of others during an Integrative Science Symposium chaired by APS Fellow Piotr Winkielman (University of California, San Diego) at the 2017 International Convention of Psychological Science in Vienna.
Mirroring the Mind
Cultural emphasis on ingroups and outgroups may create an “empathy gap” between people of different races and nationalities, says Ying-yi Hong.
“When we witness what happens to others, we don’t just activate the visual cortex like we thought some decades ago,” said Christian Keysers of the Netherlands Institute for Neuroscience in Amsterdam. “We also activate our own actions as if we’d be acting in similar ways. We activate our own emotions and sensations as if we felt the same.”
Through his work at the Social Brain Lab, Keysers, together with Valeria Gazzola, has found that observing another person’s action, pain, or affect can trigger parts of the same neural networks responsible for executing those actions and experiencing those feelings firsthand. Keysers’ presentation, however, focused on exploring how this system contributes to our psychology. Does this mirror system help us understand what goes on in others? Does it help us read their minds? Can we “catch” the emotions of others?
To explore whether the motor mirror system helps us understand the inner states behind the actions of others, Keysers in one study asked participants to watch a video of a person grasping toy balls hidden within a large bin.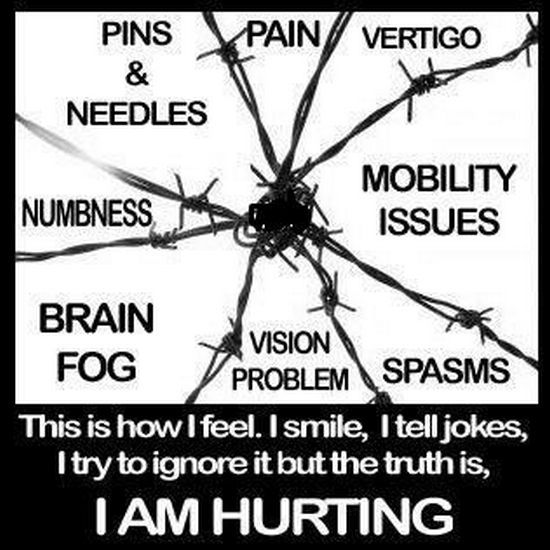 In one condition, participants determined whether or not the person in the video hesitated before selecting a ball (a theory-of-mind task). Using transcranial magnetic stimulation (TMS) in combination with fMRI, Keysers showed that interfering with the mirror system impaired people’s ability to detect the level of confidence of others, providing evidence that this system indeed contributes to perceiving the inner states of others. Performing fMRI and TMS on other brain regions such as the temporoparietal junction (TPJ) further suggests that this motor simulation in the mirror system is then sent onward to more cognitive regions in the TPJ.
In one condition, participants determined whether or not the person in the video hesitated before selecting a ball (a theory-of-mind task). Using transcranial magnetic stimulation (TMS) in combination with fMRI, Keysers showed that interfering with the mirror system impaired people’s ability to detect the level of confidence of others, providing evidence that this system indeed contributes to perceiving the inner states of others. Performing fMRI and TMS on other brain regions such as the temporoparietal junction (TPJ) further suggests that this motor simulation in the mirror system is then sent onward to more cognitive regions in the TPJ.
“Very rapidly, we got this unifying notion that when you witness the states of others you replicate these states in yourself as if you were in their shoes, which is why we call these activities ‘vicarious states,’” Keysers said.
Studies have suggested that this ability to mentalize the experiences of others so vividly can lead us to take prosocial steps to reduce their pain, but Keysers also wanted to investigate the depth of this emotional contagion — how and to what extent we experience other people’s suffering. To do this, Keysers’ lab studied two very different populations: human psychopaths and rats.
To do this, Keysers’ lab studied two very different populations: human psychopaths and rats.
While witnessing the pain of others is correlated with activity in the insula, which is thought to contribute to self-awareness by integrating sensory information, and the anterior cingulate cortex (ACC), which is associated with decision making and impulse control, the researchers found that psychopaths who passively observed an aggressor twisting someone’s hand exhibited significantly less brain activity than their neurotypical peers. When the psychopathic individuals were asked to attempt to empathize with the person in the video, however, their brain activity increased to baseline levels.
This suggests that the current model of empathy as a one-dimensional scale with empathic individuals at one end and psychopaths at the other may be overly simplistic, Keysers said.
“Psychopaths are probably equally high on ability, it’s just that they don’t recruit this spontaneously, so their propensity is modified,” he explained.
These findings could lead to more effective interventions for psychopathic individuals, as well as to future research into where people with autism spectrum disorders may fall on these axes.
Shared Pain
Studies of emotional contagion in animal models have allowed researchers to further examine the role of deep brain activity, which can be difficult to neurostimulate in humans. Keysers’ work with rats has found that these animals are more likely to freeze after watching another rat receive an electric shock if they themselves had been shocked in the past.
Inhibiting a region analogous to the ACC in the rats’ brains reduced their response to another rat’s distress, but not their fear of being shocked themselves, suggesting that the area deals specifically with socially triggered fear, Keysers said.
Claus Lamm, University of Vienna, investigates the processes that regulate firsthand pain and those that cause empathy for pain through numerous studies on the influence of painkillers.
In these experiments, participants who took a placebo “painkiller” reported lower pain ratings after receiving a shock than did those in the control group. When those same participants watched a confederate get shocked, they reported a similar drop in their perception of the actor’s pain.
“If you reduce people’s self-experienced pain, if you induce analgesia, that not only helps people to deal with their own pain, but it also reduces empathy for the pain of another person,” Lamm said.
On the neural level, Lamm said, fMRI scans showed that people in the placebo group displayed lower levels of brain activity in the anterior insula and mid cingulate cortex in both cases. These results were further confirmed in another study that compared participants who received only the painkiller placebo with those who received both the placebo and naltrexone, an opioid antagonist that prevents the brain from regulating pain.
This resulted in a “complete reversal” of the placebo effect, causing participants to report both their own pain and the pain of others at near baseline rates, supporting Lamm’s previous claims about the pain system’s role in empathy.
“This suggests that empathy for pain is grounded in representing others’ pain within one’s own pain systems,” Lamm said.
The Self/Other Divide
Empathy may not give us a full sense of someone else’s experiences, however. When observers in one of Keysers’ studies were given the opportunity to pay to reduce the severity of the electric shocks a confederate was about to receive, on average participants paid only enough to reduce her pain by 50%.
Lamm studied this self/other distinction through a series of experiments that measured people’s emotional egocentricity bias. To do so, participants were presented with visuo-tactile stimulation that was either congruent or incongruent with that of a partner under fMRI. In an incongruent pair, for example, one participant might be presented with an image of a rose and be touched with something that felt like a rose, while the other was shown a slug and touched with a slimy substance.
Participants’ own emotions were found to color their perception of other people’s affect at a relatively low rate — however, when researchers inhibited the right supramarginal gyrus (rSMG), a region of the brain previous associated mainly with language processing, this egocentricity bias increased, suggesting that the rSMG may be responsible for maintaining a self/other divide, Lamm said.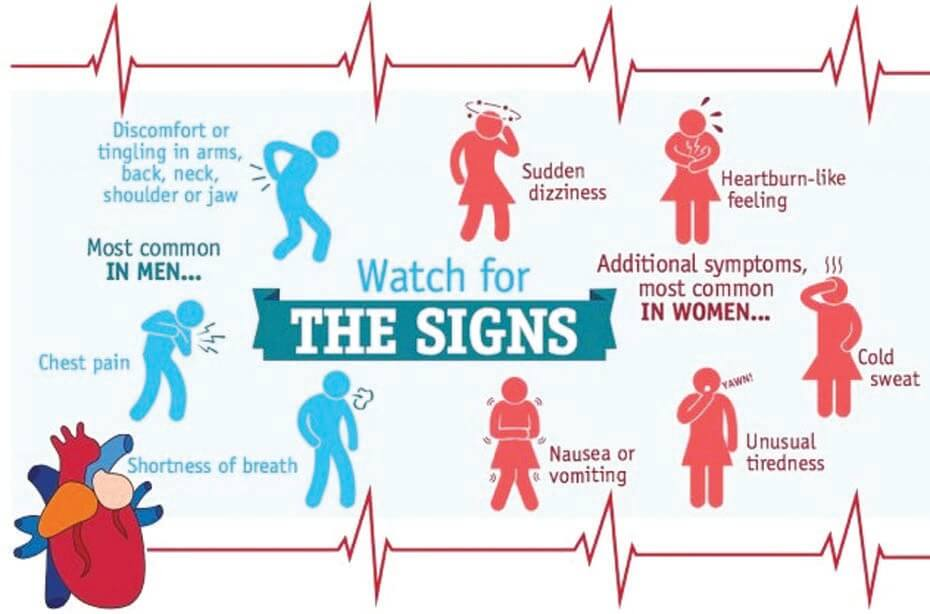
“Empathy not only requires a mechanism for sharing emotions, but also for keeping them separate. Otherwise we are getting ‘contaged,’ emotionally distressed and so on,” he said.
The rate of rSMG activation also changes significantly across a lifetime, Lamm added, with the area’s developmental trajectory causing emotional egocentricity to be more common in adolescents and the elderly.
Developing Division
Researchers are working to unite neuroscientific and psychological perspectives on feelings, empathy, and identity, says Piotr Winkielman.
Rebecca Saxe (Massachusetts Institute of Technology) said her work with developmental psychology confirms this trend. In one series of experiments, Saxe monitored the brain networks that 3- to 5-year-old children used to consider a character’s mind (the temporoparietal junction, posterior cingulate, and prefrontal cortex) and body (the secondary somatosensory cortex, insula, middle frontal gyrus, and ACC) throughout a short film.
Saxe found that while these brain regions may interact with each other, there were no points of overlap between the mind and body networks’ activities.
“When we’re getting information from the same source and about the same people, we still nevertheless impose a kind of dualism where we alternate between considering what their bodies feel like and the causes of their minds,” Saxe said.
Furthermore, Saxe and her colleagues found that while these networks were more distinct in children who were able to pass an explicit-false-belief task (e.g., if Sally puts her sandwich on a shelf and her friend moves it to the desk, where will she look for it?), the division was present in participants of all ages.
“Most people have treated explicit false belief as if it were the milestone,” Saxe said. “Actually, the false-belief task is just one measure of a much more continuous developmental change as children become increasingly sophisticated in their thinking about other people’s minds. ”
”
Next, Saxe scaled this experiment down to test the theory of mind of infants as young as 6 months, this time measuring their response to children’s facial expressions, outdoor scenes, and visual static. This time period may be key to understanding the neuropsychology of empathy because most of the brain’s cognitive development happens within the first year of life, she explained.
“A baby’s brain is more different from a 3-year-old’s brain than a 3-year-old’s brain is from a 33-year-old’s brain,” Saxe said.
Under fMRI, the infants’ brains were found to have many of the same regional responses that allow adults to distinguish between faces and scenes. Their brains didn’t show any regional preferences for objects and bodies, however.
This level of regional specificity suggests that the Kennard Principle, the theory that infants’ brains possess such resilience and plasticity because the cortex hasn’t specialized yet, may be only partially true. There does appear to be some functional organization of social process, Saxe said, with gradually increasing specialization as the child ages.
Empathy in Action
Brian D. Knutson says analysis of individuals’ brain activity when considering a purchase may be predictive of aggregate market choices.
On the surface, neuroforecasting sounds like a concept that would be right at home in the world of Philip K. Dick’s Minority Report — a science fiction thriller about a society that stops crime before it happens based on the brainwaves of three mutant “precogs” — said APS Fellow Brian D. Knutson (Stanford University), but someday it could play a very real role in the future of economics.
Knutson’s research on the brain mechanisms that influence choice homes in on three functional targets: the nucleus accumbens (NAcc) for gain anticipation, the anterior insula for loss anticipation, and the medial prefrontal cortex (mPFC) for value integration.
Using fMRI, Knutson was able to predict participants’ purchases in a simulated online shopping environment on the basis of brain activations in these areas. Before participants chose to buy a product, increased activity in the NAcc and mPFC was paired with a decrease in the insula, while the reverse was true of trials in which participants chose not to make a purchase.
Before participants chose to buy a product, increased activity in the NAcc and mPFC was paired with a decrease in the insula, while the reverse was true of trials in which participants chose not to make a purchase.
“This was very exciting to me as a psychologist to be able to say, ‘Wow, we can take activity out of the brain and, not knowing anything else about who it is and what product they’re seeing, we can predict choice,’” Knutson said.
His economist colleagues weren’t as impressed: They were interested in market activity, not individual choice. Knutson said he accepted this challenge by applying his neuroanaylsis to large-scale online markets such as Kiva and Kickstarter.
Knutson asked 30 participants to rate the appeal and neediness of loan requests on Kiva and found that posts with photos of people displaying a positive affect were most likely to trigger the increased NAcc activity that caused them to make a purchase — or in this case, a loan. More importantly, the averaged choices of those participants forecasted the loan appeal’s success on the internet. Two similar studies involving Kickstarter campaigns also suggested a link between NAcc activity and aggregate market activity.
Two similar studies involving Kickstarter campaigns also suggested a link between NAcc activity and aggregate market activity.
While brain activity doesn’t scale perfectly to aggregate choice, Knutson said, some components of decision making, such as affective responses, may be more generalizable than others.
“The paradox may be that the things that make you most consistent as an individual, that best predict your choices, may not be the things that make your choices conform to those of others. We may be able to deconstruct and decouple those components in the brain,” Knutson said.
Global Empathy
The neuroanatomy of our brains may allow us to feel empathy for another’s experiences, but it can also stop us from making cross-cultural connections, said APS Fellow Ying-yi Hong (Chinese University of Hong Kong).
“Despite all these neurobiological capabilities enabling us to empathize with others, we still see cases in which individuals chose to harm others, for example during intergroup conflicts or wars,” Hong said.
This may be due in part to the brain’s distinction between in-group and out-group members, she explained. People have been found to show greater activation in the amygdala when viewing fearful faces of their own race, for example, and less activation in the ACC when watching a needle prick the face of someone of a different race.
The cultural mixing that accompanies globalization can heighten these responses, Hong added. In one study, she and her colleagues found that melding cultural symbols (e.g., combining the American and Chinese flags, putting Chairman Mao’s head on the Lincoln Memorial, or even presenting images of “fusion” foods) can elicit a pattern of disgust in the anterior insula of White Americans similar to that elicited by physical contaminant objects such as insects.
These responses can also be modulated by cultural practices, Hong said. One study comparing the in-group/out-group bias in Korea, a more collectivist society, and the United States, a more individualistic society, found that more interdependent societies may foster a greater sense of in-group favoritism in the brain.
Further research into this empathy gap should consider not just the causal relationship between neural activation and behavior, she said, but the societal context in which they take place.
“What I want to propose,” Hong said, “is that maybe there is another area that we can also think about, which is the culture, the shared lay theories, values, and norms.”
Why the sexes don’t feel pain the same way
Robert Sorge was studying pain in mice in 2009, but he was the one who ended up with a headache.
At McGill University in Montreal, Canada, Sorge was investigating how animals develop an extreme sensitivity to touch. To test for this response, Sorge poked the paws of mice using fine hairs, ones that wouldn’t ordinarily bother them. The males behaved as the scientific literature said they would: they yanked their paws back from even the finest of threads.
But females remained stoic to Sorge’s gentle pokes and prods1. “It just didn’t work in the females,” recalls Sorge, now a behaviourist at the University of Alabama at Birmingham. “We couldn’t figure out why.” Sorge and his adviser at McGill University, pain researcher Jeffrey Mogil, would go on to determine that this kind of pain hypersensitivity results from remarkably different pathways in male and female mice, with distinct immune-cell types contributing to discomfort2.
“We couldn’t figure out why.” Sorge and his adviser at McGill University, pain researcher Jeffrey Mogil, would go on to determine that this kind of pain hypersensitivity results from remarkably different pathways in male and female mice, with distinct immune-cell types contributing to discomfort2.
Sorge and Mogil would never have made their discovery if they had followed the conventions of most pain researchers. By including male and female mice, they were going against the crowd. At the time, many pain scientists worried that females’ hormone cycles would complicate results. Others stuck with males because, well, that’s how things were done.
Today, inspired in part by Sorge and Mogil’s work and spurred on by funders, pain researchers are opening their eyes to the spectrum of responses across sexes. Results are starting to trickle out, and it’s clear that certain pain pathways vary considerably, with immune cells and hormones having key roles in differing responses.
This push is part of a broader movement to consider sex as an important variable in biomedical research, to ensure that studies cover the range of possibilities rather than gleaning results from a single population. A major change came in 2016, when the US National Institutes of Health (NIH) made it a requirement for grant applicants to justify their choice of the sex of animals used in experiments. The discoveries in pain research are among the most exciting to emerge, says Cara Tannenbaum, scientific director of the Institute of Gender and Health in Montreal, part of the Canadian Institutes of Health Research. And of Sorge and Mogil’s work, she adds, “To my knowledge, no other field of science has identified this type of sex difference.”
Microglia, the nervous system’s immune cells, are behind forms of pain in male mice.Steve Gschmeissner/SPL
The research could open the door for new medical advances, adds Tannenbaum. These are sorely needed: some 20% of people worldwide experience chronic pain — and the majority are women. Today, the pharmaceutical market offers the same pain drugs to everyone. But if the roots of pain are different, some drugs might work better in some people than in others.
Today, the pharmaceutical market offers the same pain drugs to everyone. But if the roots of pain are different, some drugs might work better in some people than in others.
Moreover, people might require different pain medications when hormone levels fluctuate through life. And a person’s sex doesn’t always fit clearly into the categories of male and female: it is determined by a spectrum of characteristics, including genetics, anatomical development and hormone levels, each of which might affect a person’s needs in pain therapy. The picture is a long way from complete, and studies — most in rodents — have so far focused on biological sex, as opposed to gender, a psychosocial concept that doesn’t necessarily match sex.
Iain Chessel, vice-president and head of neuroscience at AstraZeneca in Cambridge, UK, predicts that future pain medications will be tailored to individuals — and that sex will be a key factor in those personalized prescriptions. “But we don’t understand it yet,” he adds.
Hear reporter Amber Dance talk more about pain processing in different sexes.
Your browser does not support the audio element.Download MP3
Immune to the pain
Pain happens when neural sensors in the skin, muscles, joints or organs register a potentially harmful sensation, such as heat or tissue damage. They send signals through peripheral nerves to the spinal cord, activating other nerves that send signals to the brainstem and on to the cerebral cortex, which interprets those signals as ‘ouch!’. But pain happens in many ways, and diverse chemical pathways contribute. Some pain types are distinguished by timing. There’s the acute response to something hot, sharp or otherwise noxious, and there’s long-term, chronic pain that might persist even after the initial injury has healed.
Chronic pain can manifest as hypersensitivity to otherwise non-painful stimuli, as in the case of Sorge’s male mice. Back in 2009, he and Mogil were studying a model of chronic pain triggered by inflammation.
Injecting a bacterial molecule called lipopolysaccharide into the spines of mice drew the attention of microglia, the nervous system’s resident immune cells. But in Sorge’s studies, this led to inflammation only in the males, explaining why they were so sensitive to the hair-prick test, Sorge and Mogil reported in 2011 (ref. 1). The microglia remained quiet in females, which seemed to account for their indifference to Sorge poking their paws with fine hairs.
To better understand why male and female mice dealt with pain so differently, Sorge and Mogil turned to a pain source that affects all mice. They injured the animals’ sciatic nerves, which run from the lower back down each leg. This led to a form of chronic pain that happens when the body’s pain-detecting system is damaged or malfunctioning. It caused both male and female mice to become extra sensitive to touch.
Yet even in this case, there were differences. Microglia seemed to have a prominent role in the pain of males, but not in that of female mice2. Sorge and a team of collaborators from three institutions found that, no matter how they blocked microglia, this eliminated the pain hypersensitivity in males alone.
Sorge and a team of collaborators from three institutions found that, no matter how they blocked microglia, this eliminated the pain hypersensitivity in males alone.
It’s not that females were immune to pain. They were just as bothered by nerve injury as the males were, but they weren’t using microglia to become hypersensitive to touch. Mogil and Sorge wondered whether another immune component, called a T cell, was behind the chronic pain in females. These cells have a known role in pain sensitization in mice.
Sorge tried the same nerve injury in female mice lacking T cells. They still became hypersensitive to the fine hairs, but the mechanism now seemed to occur through microglia. In females lacking T cells, blocking the activity of microglia prevented this pain response, just as it did in males. And when the researchers transferred T cells back to female mice that were lacking them, the animals stopped using microglia in nerve-injury pain (see ‘Two routes to pain’).
The team’s findings2, reported in 2015, had a big influence on the pain field, says Greg Dussor, a neuropharmacologist at the University of Texas at Dallas. The results showed that even though everybody’s pain might look similar from the outside, scientists can’t assume it’s the same on the inside.
The results showed that even though everybody’s pain might look similar from the outside, scientists can’t assume it’s the same on the inside.
Pain points
If animals can switch between pain pathways, what controls the switch? Researchers have long attributed sex differences in pain perception to oestrogen, a hormone that controls the development of the uterus, ovaries and breasts, and which regulates the menstrual cycle. Oestrogen can either exacerbate or dull pain, depending on its concentration and location. Testosterone, the hormone involved in development of the penis, testes and prostate, as well as of secondary characteristics such as body hair, has received much less attention from pain researchers, although studies suggest it can reduce pain3, and some people with chronic pain take testosterone treatments4.
In the case of microglia and pain hypersensitivity, Mogil’s research points squarely at testosterone as the control switch for pain pathways. In the 2011 and 2015 studies1,2, when Sorge tested castrated male mice, which have low testosterone levels, the animals exhibited a response similar to females. And when the researchers provided testosterone to castrated males, or to females, the pain pathway switched to one dependent on microglia.
In the 2011 and 2015 studies1,2, when Sorge tested castrated male mice, which have low testosterone levels, the animals exhibited a response similar to females. And when the researchers provided testosterone to castrated males, or to females, the pain pathway switched to one dependent on microglia.
Since then, researchers have continued to find evidence shoring up the importance of microglia — and the cells’ enzymes and receptors — in male mice experiencing pain. And the phenomenon isn’t restricted to mice: one of Mogil’s collaborators, neuroscientist Michael Salter, also found microglial receptors at work in male rats that had hypersensitivity from nerve injury5. Salter, who is chief of research at the Hospital for Sick Children in Toronto, Canada, is now investigating the question in macaques, which are likely to process pain in a more similar way to humans.
It’s much harder to investigate these pain pathways in people, but clues are emerging. Neuropharmacologist Ted Price, at the University of Texas at Dallas, and his collaborators have found preliminary evidence, published this month6, of differences in how immune cells contribute to pain in people.
Neuropharmacologist Ted Price, at the University of Texas at Dallas, and his collaborators have found preliminary evidence, published this month6, of differences in how immune cells contribute to pain in people.
They’re working with nerve tissue removed from individuals with cancer, whose tumours had invaded their spines. In nerves excised from men experiencing pain, Price’s team found signs of inflammation caused by an immune cell called a macrophage. These cells serve a similar function to microglia. In women who were in pain, however, the more important players seemed to be nerve cells themselves and a short stretch of protein building blocks (called a peptide) that stimulates nerve growth. The results suggest parallels between human and rodent sex differences, says Price.
But immune cells and hormones don’t fully explain pain differences. For instance, Sarah Linnstaedt, a translational biologist at the University of North Carolina Medical Center in Chapel Hill, has found hints that some women might have a genetic predisposition to chronic pain. Her team has identified a suite of RNA molecules in the bloodstream that are more likely to be elevated in women who develop chronic neck, shoulder or back pain after a motor-vehicle accident. Many of these RNA molecules are encoded by genes on the X chromosome, of which there are two copies in most women7.
Her team has identified a suite of RNA molecules in the bloodstream that are more likely to be elevated in women who develop chronic neck, shoulder or back pain after a motor-vehicle accident. Many of these RNA molecules are encoded by genes on the X chromosome, of which there are two copies in most women7.
That’s useful information, says Linnstaedt. “It will enable us to develop new therapeutics that can either be used specifically in women, or at higher doses in women.”
Drug differential
Others are thinking about sex-specific pain treatments, too. In a study published online in November 2018, Price and his team reported that a diabetes drug called metformin reduces microglial populations surrounding sensory neurons in the spinal cord. They also showed that the drug blocks pain hypersensitivity from nerve damage only in male mice8. “It didn’t do anything in the females; in fact, it got a little bit worse,” says Price, who has a theory as to why: to enter the nervous system, metformin relies on a protein that’s expressed at higher levels in cells from males. Higher doses didn’t make a difference in females, however, presumably because the medication was trapped outside the nerves.
Higher doses didn’t make a difference in females, however, presumably because the medication was trapped outside the nerves.
Higher doses do help females receiving one of the oldest pain drugs in the pharmacy: morphine. Women and female rodents both usually require higher doses of morphine to achieve the same pain relief as men and male rodents, says Anne Murphy, a neuroscientist at Georgia State University in Atlanta. She’s one of a handful of researchers who was studying sex differences well before the NIH changed its guidelines.
Microglia are also behind morphine’s differing effects, Murphy’s team reported9 in 2017. The drug dulls pain by blocking neurons in a brain region called the periaqueductal grey, or PAG. But the drug can also activate microglia there, counteracting morphine’s pain-relieving effects. This is exactly what happens in female rats, which have more active microglia in the PAG than males have. When rats were treated with morphine before the scientists applied a hot light beam to their paws, the female animals had more inflammation in the PAG and pulled back their legs more quickly than did males given the same dose. When Murphy’s team blocked morphine’s effects on microglia, males and females responded to the pain in a similar way9.
When Murphy’s team blocked morphine’s effects on microglia, males and females responded to the pain in a similar way9.
There’s at least one drug already on the market that scientists have reason to think might work differently across sexes. In 2018, the US Food and Drug Administration approved migraine treatments based on antibodies against CGRP, a peptide found in the nervous system that is involved in these kinds of headache. Migraines affect three times as many women as men.
In an as-yet-unpublished study of mice and rats, a team led by Price and Dussor applied CGRP to the thick membrane surrounding the brain. In females, the peptide created a response that looked like a migraine: the animals grimaced and their faces were hypersensitive to touch. In males: “Nothing,” says Dussor. Modern anti-CGRP medicines might work better in women than in men, he adds — but the drug’s clinical trials didn’t check for such effects.
That’s typical of many drug trials. They usually include men and women, but the numbers of each often aren’t high enough to suss out differences. There’s a real possibility that pain drugs that failed clinical trials in the past might have succeeded if they had been tested separately by sex, says Price. “It seems really obvious,” he adds, “but nobody was really doing it.”
There’s a real possibility that pain drugs that failed clinical trials in the past might have succeeded if they had been tested separately by sex, says Price. “It seems really obvious,” he adds, “but nobody was really doing it.”
Personalized pills
Chessel, at AstraZeneca, would be happy to develop a pain drug that works only in people of a certain sex. But the sex of study participants and animal subjects is driven by practicality, ethical concerns and government regulations, he says. AstraZeneca uses female rodents in most of its preclinical pain research because they’re less aggressive and easier to house and handle than males. In early clinical trials, safety is the focus, so companies often exclude people who could become pregnant. As a result, drugs are mostly trialled on men and on women who are past menopause.
Even if scientists develop drugs that are targeted to male- or female-specific pain pathways, these might not be enough. It might be best to customize drugs more closely, to take into account the spectrum of genetics, hormone levels and anatomical development.
Little research has been done on pain mechanisms in people who don’t fit into a binary definition of sex and gender. In one study, researchers in Italy surveyed transgender people undergoing hormone treatment. They found that 11 out of 47 people who transitioned from male to female reported pain issues that arose after the transition. Six out of 26 people transitioning from female to male reported that their pain problems lessened after taking testosterone10.
On the basis of his team’s experiments with castration and testosterone treatments in mice, Mogil thinks that pain pathways will be determined by hormone levels. He predicts that people with more than a certain threshold of testosterone will have pain mechanisms associated with males, and those whose testosterone falls below that level will experience pain through mechanisms common in females.
Pain responses also seem to change throughout life, around the time hormone levels rise or fall. Studies looking only at biological sex have found that, at puberty, the rates of pain conditions rise more in girls than in boys. And as people age, and some hit menopause, hormonal levels change again, and sex differences in chronic pain rates begin to disappear. Pregnancy changes pain responses, too. Mogil’s group reported in 2017 that, early in pregnancy, mice switch from a typically female, microglia-independent mechanism of pain sensitization to a more male-associated one that involves microglia. By late pregnancy, the animals don’t seem to feel chronic pain at all11.
And as people age, and some hit menopause, hormonal levels change again, and sex differences in chronic pain rates begin to disappear. Pregnancy changes pain responses, too. Mogil’s group reported in 2017 that, early in pregnancy, mice switch from a typically female, microglia-independent mechanism of pain sensitization to a more male-associated one that involves microglia. By late pregnancy, the animals don’t seem to feel chronic pain at all11.
But he’s no longer one of a few scientists looking for such sex differences. “People are finding this left, right and centre now,” says Mogil. “I don’t think we know the half of it at this point.”
George Gregory Plitt Jr. quote: The pain you feel today will be the strength you feel ...
- George Gregory Plitt Jr.
.
Last updated October 27, 2022
Topics
pain, strength, tomorrow
George Gregory Plitt Jr.
46 1977 - 2017 0023 “Today feel what you want to be tomorrow. ”
”
— Alexander Grigorievich Sviyash 1953
“Today is the tomorrow that we think about yesterday.”
— Thomas La Mans
“The impossible today will become possible tomorrow .“
— Konstantin Eduardovich Tsiolkovsky scientist, cosmist philosopher, science fiction writer 1857 - 1935
“Decisions that are not taken today will not be taken tomorrow.”
— Alexei Khristinin
"The people who are left without honor today will be left without bread tomorrow."
- Eliza Orzeszko 1841 - 1910
"Anxiety tomorrow does not relieve sorrow, it loses strength today."
- Corrie ten Boom Dutch Christian 1892 - 1983
“I ... can juggle with masks that I change from day to day: today I am a professor, it’s very nice, and tomorrow I’m a laureate, today I’m a decorator, and tomorrow a dresser.” fashion collector, interior decorator, theater artist, author of books and articles, lecturer… 1958
“Don't try to be perfect tomorrow. Try to be a little better today.”
Try to be a little better today.”
- Joe Rogan 1967
“It was good today. Today was fun. Tomorrow another one.”
- Dr. Seuss American children's writer and cartoonist 1904 - 1991
philosopher 1844 - 1900
„It is not possible for everyone to feel the taste of tomorrow, if today they are mired in despair and pain, if they are imprisoned in a dark room, they have never seen the sunlight, if they have never tasted happiness and have no desire for life ...... Today will have no value
— Khalaroshi
“The more insecure I am, the more powerful I feel because I am sure that there are no emotions to be ashamed of.”
nine0002 - Alanis Morissette Canadian singer, songwriter, actress and producer 1974“Do today what you are going to do tomorrow; say tomorrow what you want to say today.”
— Kazimierz Psherva-Tetmajer Polish poet, prose writer, playwright 1865 - 1940
“What is the difference between an artist and an amateur? Only the pain that he feels, the amateur seeks only pleasure in art. ”
”
— Odilon Redon French painter, graphic artist, decorator 1840 - 1916
“Today, precisely today, there is no alternative to Yeltsin. Tomorrow. But today it is not.”
— Boris Nikolayevich Yeltsin, the first President of the Russian Federation, Russian politician and statesman, reformer; in the Soviet period ... 1931 - 2007
“about Kiriyenko: Today he will say, and tomorrow he will be hanged. He is clean, young, full of strength and energy. We chose a virgin!”
— Vladimir Volfovich Zhirinovsky Russian politician 1946
“Better to work tomorrow than today!”
- Mikhail Sergeevich Gorbachev Soviet, Russian state, political and public figure 1931
“As long as you feel pain, you are alive. As long as you feel someone else's pain, you are human.”
— Demi Lovato American actress and singer 1992
“It's just fashion, and as you know, it is changeable: today one thing is fashionable, tomorrow another. But there are things that seem to be above fashion. “
“
— Renars Kaupers leader and vocalist of the Latvian rock band Brainstorm 1974
“I have no plans for the future. It's stupid to have plans. No one can know what will happen to him tomorrow. Today you are married, tomorrow you are divorced. Today you rustle with money, tomorrow you rustle with leaves. Today you look out the window, tomorrow you look at the grave. “
- Peter Jackson New Zealand film director, screenwriter, actor and producer 1961
Related topics
- Strength
- Tomorrow 9013
12 taps and parades of Bols0001
- Yana Litvinova
- BBC, London
Image copyright, Getty Images
Image caption,We all know that pain is an objective reality, but its perception is deeply subjective. Pain can be a symptom, a disease, mental or physical. How close are we to understanding what it is?
Acute, dull, sudden, chronic, aching, throbbing, blinding.
 .. This is not a complete list of epithets that we use without hesitation when talking about the sensation that we all experienced and continue to experience: about pain.
.. This is not a complete list of epithets that we use without hesitation when talking about the sensation that we all experienced and continue to experience: about pain. - Why is pain so difficult to measure and relieve?
- Medicine of the future: how bioglass will revolutionize surgery
- Why paper cuts are so painful
She doesn't care about skin color, eye shape, or social status. It doesn't care what level of evolution this or that creature is at. People, dogs, cats, dolphins, whales, birds, frogs, and even, according to scientists, earthworms experience pain. nine0003
At the same time, if scientists say that the mechanism of pain is more or less clear to them, then what is it: a signal system of malfunctions, an obligatory part of being, without which it is impossible to understand physical and mental well-being, a purely physiological process or a result complex chemical processes in the brain, neither doctors nor even the clergy came to a unanimous agreement.

Image copyright Getty Images
Image captionWe know how the signaling system works from neurons to the brain and back, but many questions remain unanswered
In addition, there is a group of people who, due to a genetic anomaly, do not experience pain at all.
In fact, they do not need to envy, because they can easily miss the onset of some disease, and die, although painlessly, but completely in vain.
All our knowledge of pain is based on paradoxes.
1. Our brain registers pain signals, but does not feel pain itself
Photo author, Getty Images
Photo caption,The brain registers and processes pain signals from all other parts of the body, but does not feel pain itself
Let's say you twisted your ankle or burned your finger. The nerve fibers immediately send a signal to your brain that interprets the sensation as pain.
No wonder modern surgery became possible only after the discovery of anesthesia.

However, if the brain itself is the object of the operation, then pain medication is useless.
Nerve cells in the brain send themselves the same signals as in a broken limb, but there is no data center for them. nine0003
The brain, accustomed to being responsible for the whole organism, does not understand at all when it should hurt itself.
It's kind of creepy, but patients are often fully awake during brain surgery, which lets surgeons know if they've dug too deep into our body's main processing unit.
2. We all feel pain differently
Photo by DanielVilleneuve
Photo caption,Pain is subjective: for some it's agony, but for others it's a slight inconvenience. nine0003
The fact that, after, for example, natural childbirth, one woman says that she was a little uncomfortable, but that's okay, and the other needs pain relief at the very beginning of the contractions, does not mean at all that one of them is stoic, and the other is weak slur.

How we feel pain is influenced by many factors: what chemical reactions are taking place in your brain at that time, whether there is an inflammatory process somewhere in your body, and how much you "remember" pain sensations that you experienced before . nine0003
As Kenneth Hansraj, head of the New York Spinal Surgery Center, once said: “Someone can drill into the tibia without anesthesia, and he will calmly tell you, they say, buddy, pull that thing out! the skin of a thin needle."
3. Pain can be distracted
The author of the photo, Portra
Photo caption,Pain can be deceived: if you start shaking a bruised finger, it becomes easier
Our brain, of course, is the most complex computer ever or created by nature, but at the same time he is a little blunt. nine0003
The fact is that it is difficult for him to analyze several sensations at the same time.
Let's say you've been bitten by a mosquito and the bite is itchy.
 Attach an ice cube to it, and suddenly you will realize that you still feel the cold, but the itching is gone.
Attach an ice cube to it, and suddenly you will realize that you still feel the cold, but the itching is gone. That's why we instinctively rub a bruised area or frantically shake a finger that is accidentally pinched by a door.
4. Redheads do worse
Image copyright, Getty Images
Photo caption,Redheads have a hard time: fiery hair color is accompanied by a non-standard attitude to painkillers
It's hard to believe, but in 2009 an article appeared in the Journal of the American Dental Association, according to which redheads really do not like visiting dentists.
The fact is that the same genetic combination that gives them a fiery hair color makes them less susceptible to certain painkillers.
And sometimes they need a dose that is twice as high as what would be enough for some brunette. nine0003
It is also possible that their body reacts to anesthesia in a way that is not entirely trivial.
 Some doctors, by the way, make adjustments for the patient's hair color.
Some doctors, by the way, make adjustments for the patient's hair color. 5. Sex relieves pain
Image copyright, Getty Images
Image captionHaving sex can reduce migraine pain... if you have the energy to do it, of course
Well, objectively speaking, if you have a migraine attack, then sex in such a situation seems to be somewhat doubtful. nine0003
However, there are some statistics that say that 60% of migraine sufferers feel much better if they do it during an attack.
Sexual arousal releases endorphins in the brain, which are natural pain relievers.
By the way, things are not so simple with migraine patients. It is suspected that the same gene variant that bestows migraine sufferers at the same time significantly increases their libido. nine0003
6. We are mercilessly divided into women and men
The author of the photo, Getty Images
Image caption,We all feel the same way, only men think that we should endure
In fact, there is no scientific evidence that that men and women experience pain differently.

Although doctors note that, in general, women are more likely to admit that they are in pain.
Perhaps this is due to a social stereotype that requires "real" men to endure with clenched teeth. nine0003
7. Those who don't feel pain
Image copyright, Getty Images
Image captionThose who don't feel pain don't do so well: just touching a hot stove can result in a third-degree burn
This is a very rare genetic anomaly. So rare that in the entire history of medicine it has met only a few dozen times.
Those who are very unlucky to be born with it can, for example, feel whether an object is hot or cold, but they do not feel pain. nine0003
And that, by the way, is really bad. For example, accidentally touching a hot stove might result in a third-degree burn, instead of the small blister that would result if they quickly figured out what was happening and pulled their hand away.

According to available statistics (which, for quite obvious reasons, are extremely small), the average life expectancy of such insensitive people is significantly below the average.
8. The most common pain
Image copyright Getty Images
Image caption,The most common pain in developed countries is pain in the lower back.
This is back pain. Approximately 27% of people in developed countries claim to suffer from low back pain.
Whereas from constant headaches or migraines - only 15%. Experts advise not to disdain physical exercises and not to gain excess weight.
However, this is a consequence of our evolutionary successes. Bipedalism is not at all conducive to spinal health. Quadrupeds, in which the weight is distributed much more evenly, do not face back pain. nine0003
9. What hurt kings and dinosaurs
Photo copyright, Getty Images
Photo caption,Both kings and dinosaurs suffered from gout.
 True, there is a dragon here, but it is probably a close relative of the Tyrannosaurus rex
True, there is a dragon here, but it is probably a close relative of the Tyrannosaurus rex Gout, also known as arthritis, used to be called the disease of kings, since it was allegedly the result of excessive consumption of fatty foods and alcohol.
It is clear that in the distant Middle Ages only very wealthy people could afford it. We now know that gout pain is caused by the formation of sharp uric acid crystals inside the joints. nine0003
Examination of the skeletal upper limb of a female Tyrannosaurus rex (which palaeontologists named Sue) showed that this particular Jurassic predator also suffered from gout, and in a very advanced form. It is likely that Sue suffered from chronic pain throughout the last years of her life.
10. The nature of pain is not at all unambiguous
The author of the photo, Getty Images
Photo caption,Sometimes pain turns from a symptom into a disease.
 It hurts everywhere, and why is not clear
It hurts everywhere, and why is not clear Pain is a symptom, which, however, gives only a general idea that something is wrong, but does not give any specifics.
And in patients suffering from central pain syndrome, the pain itself becomes the disease, not its symptom.
Such patients complain of pain throughout the body, with sensations ranging from "pins and needles" to "strong pressure". In this case, the brain is not just a registrar and processor of pain sensations, but also their main generator. nine0003
11. Don't underestimate your brain
The author of the photo, Getty Images
Image caption evaluates the signals it receives, deciding how serious the danger is and whether immediate action should be taken.Having received an alarm signal, the brain immediately tries to answer the main question: "How dangerous is all this?" nine0003
In assessing the situation, our central processor uses all the information available to it: from subjective, coming from our past experience, to objective, obtained from the whole complex of physical and chemical parameters of the body.

And having received a signal, it sends "instructions" to the nerve endings on how to behave. Canadian physician Paul Ingram described the process in the following imaginary dialogue:
Image copyright, Getty Images
Image caption,The brain commands neurons as it wants, and they have to obey
Nerves: Problem! Problem! Huge! Big! Red alert! Turn on immediately!
Brain: Mmmmm, right? Okay, I took note. But guys, I have a database here, sorry, it's strictly secret, so take my word for it: it's not all that scary. Relax.
Nerves : No, no, listen, this is all very serious!
Brain: Nope, I don't believe it.
Nerves: Look, maybe we don't have access to this "information" that you talk about all the time, but we know very well what tissue damage is! And we're not playing games here. We won't shut up until you take action!
Brain (hypnotist voice): You don't remember what it was.
 There is absolutely no need to send me signals. Everything is absolutely fine, breathe deeply...
There is absolutely no need to send me signals. Everything is absolutely fine, breathe deeply... Nerves: Ah, yes... What are we talking about? Damn, it looks like they just wanted to report something important... Well, okay, we'll be back later. nine0003
12. The ultimate boss
Photo credit, Getty Images
Image caption,The brain decides how to regulate the pain button in our body, and why sometimes it stops at six and sometimes at ten, we are up to we still don't know for sure
The brain can really twirl its peripheral nerve endings as it pleases.
If he doesn't like something, he can ask for more information. Or maybe order his subordinates not to fuss. nine0003
In recent years, a lot of information has appeared, according to which nerves in the periphery can actually change both physically and chemically, possibly following a command coming from the brain.