Slowing of the brain
EEG in neurological conditions other than epilepsy: when does it help, what does it add?
Article Text
Article menu
- Article
Text - Article
info - Citation
Tools - Share
- Rapid Responses
- Article
metrics - Alerts
EEG in neurological conditions other than epilepsy: when does it help, what does it add?
- S J M Smith
- Correspondence to: Dr Shelagh Smith Department of Clinical Neurophysiology, The National Hospital, Queen Square, London WC1N 3BG, UK; shelaghsepilepsynse.org.uk
http://dx.doi.org/10.1136/jnnp.2005.068486
Statistics from Altmetric.com
Request Permissions
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- Creutzfeldt-Jacob disease
- dementia
- encephalopathies
- EEG
- electroencephalogram
Although the electroencephalogram (EEG) is a reliable test to assess cerebral function, its value in diagnosis and evaluation of neurological conditions apart from epilepsy has been largely superceded in recent years by other investigations with greater specificity and sensitivity. Is EEG still worthwhile, and in which cases can it provide information that affects management? Broadly speaking, EEG is most important in patients with impaired consciousness or altered mental state (table 1):
Table 1
Strengths and weaknesses of the EEG
-
Where seizures or non-convulsive status epilepticus (NCSE) may be a contributing factor
-
To demonstrate functional disturbance when cerebral dysfunction is evident and structural imaging is normal
-
To detect focal or lateralised abnormalities which could suggest a structural basis for an encephalopathy
-
To identify diagnostic EEG patterns in appropriate clinical settings, such as sporadic Creutzfeldt-Jakob disease (CJD).
ENCEPHALOPATHIES
EEG changes in encephalopathies are similar, whether the cause is septic, metabolic, toxic, or structural. There is a progressive increase in slow wave activities, the degree of which parallels the severity of brain dysfunction. In mild encephalopathic states, slowing of normal alpha (α) rhythms occurs, and with more severe encephalopathy, the appearance of theta (θ) and continuous or non-continuous delta (δ) activities. A variety of additional EEG patterns can be seen, such as frontal intermittent rhythmic delta (FIRDA), periodic lateralised or bilateral epileptiform discharges (PEDs, BIPEDs), and triphasic waves. None of these patterns is specific to a particular pathophysiological process or diagnosis, but PEDs are most likely to occur in acute or subacute focal destructive pathologies or focal epileptogenic lesions; triphasic waves are typically found in metabolic encephalopathies; and some patients with mesial fronto-parietal lesions or third ventricle tumours show FIRDA in their EEG.
Metabolic encephalopathies
A patient with acute change in awareness whose EEG shows triphasic waves and diffuse slow activity will usually have a metabolic encephalopathy. Triphasic waves (TW) consist of moderate to high amplitude complexes with three (but sometimes two or four) negative-positive-negative phases, usually occurring in runs at 1.5–3 per second. They have been considered as specific to severe hepatic encephalopathy, but TW are seen in encephalopathies associated with renal failure or electrolyte imbalance, as well as anoxia and intoxications (such as lithium, metrizamide, and levodopa). The rate of change rather than the absolute level of a given metabolite, toxin or electrolyte is more important in determining the degree of EEG abnormality. Thus, EEG changes are usually more severe in uraemic encephalopathy if there is acute deterioration of renal function, with broad correlation between degree of EEG slowing and increase in serum creatinine. Spike wave epileptiform discharges (and seizures) may occur in any severe encephalopathy, but are said to be more common than in uraemic encephalopathy and in insulin coma.
In hypoglycaemia there may be generalised slow activity or focal/lateralised δ rhythms, and these may be associated with focal neurological deficits.
EEG can be useful for early detection of dialysis dementia or encephalopathy (occurring in about 1% of dialysis patients), as abnormalities can precede clinical symptoms by several months in this disorder.
Toxic encephalopathies
Most drugs or toxins have diffuse effects on the EEG. Some agents, particularly benzodiazepines and barbiturates, induce fast or beta (β) rhythms, and the EEG may be a useful pointer to drug intoxication when this is clinically unsuspected. Generalised slow activity can alert to hyperammonaemia in a confused or obtunded patient who has epilepsy treated with sodium valproate. Clozapine is quite commonly associated with EEG change, often pronounced, and manifest by both generalised slow activity and spike wave discharge in non-epileptic patients. Induction of clinical seizures and EEG abnormalities are more likely at high dosages or with rapid dose escalation.
Endocrine disorders
EEG changes parallel the severity of hormonal disturbance, but it may be difficult to distinguish primary endocrine from secondary electrolyte effect. Hashimoto’s thyroiditis has heterogeneous clinical and electrographic manifestations; the EEG can show generalised or frontal slow activity, triphasic waves, periodic sharp waves, and lateralised temporal slowing.
Anoxic encephalopathy
Most studies have involved patients with ischaemic/anoxic brain damage following prolonged cardiorespiratory arrest, and EEG grading systems have been proposed for prognostic purposes. Some patterns—burst suppression and isoelectric EEG—are particularly associated with poor outcome (death, vegetative state), and PEDs or BIPEDs are also adverse electrographic findings. However, great caution must be exercised when judging prognosis based on a single EEG recording, and it is essential to consider other factors such as systemic complications, electrolyte imbalance, core temperature, and use of sedative medication that may reversibly affect cerebral function and the EEG. EEG variability, either spontaneous or in reaction to external stimuli, is an important feature, and reactivity to a range of stimulation procedures (auditory, pain) should be assessed during the EEG recording.
EEG variability, either spontaneous or in reaction to external stimuli, is an important feature, and reactivity to a range of stimulation procedures (auditory, pain) should be assessed during the EEG recording.
When should EEG be performed in the intensive care patient?
-
To detect non-convulsive or clinically subtle seizures. Most cases of status on the intensive care unit (ICU) are non-convulsive or difficult to identify on clinical grounds alone. NCSE should be anticipated if there is a prior history of epilepsy; in patients with CNS infection, recent neurosurgical procedures, head injury or stroke; as a sequela of convulsive status epilepticus; and following prolonged cardiorespiratory arrest. Up to 10% of patients with unexplained coma have NCSE, based on electrographic studies. Occurrence of NCSE and delay in recognition/treatment are associated with poorer outcome independent of aetiology of status and age.
-
To characterise paroxysmal clinical events that might be seizures, including grimacing, chewing, or nystagmoid eye movements; abrupt and otherwise unexplained changes in pulse, blood pressure or respiratory pattern; or abrupt deterioration in conscious level.
 Ideally, video and EEG should be recorded concurrently.
Ideally, video and EEG should be recorded concurrently. -
To distinguish coma from diminished responsiveness due to other causes (psychiatric, sedation, neuromuscular, cortical de-efferentation/locked in syndrome).
The use of EEG for detection of cerebral ischaemia at a reversible stage, such as following subarachnoid haemorrhage, is currently a research tool. Systems that utilise EEG measures to monitor the level of sedation or anaesthesia have not been widely accepted for either perioperative or intensive care use. Neurophysiological criteria—preferably a combination of EEG, somatosensory N20, and mid-latency auditory evoked potentials, central motor conduction time—have been shown to predict outcome of coma, albeit in studies mostly limited to patients with anoxic–ischaemic or traumatic brain damage. That such techniques are not in widespread use is partly a reflection of limited service availability, and perhaps the technical challenges of continuous neurophysiological monitoring in particular.
EEG no longer has a role in determination of brain death, but demonstration of an isoelectric and wholly unreactive EEG, in addition to other assessment of the patient, may sometimes help relatives accept that ongoing treatment is futile.
NEUROLOGICAL INFECTIONS
EEG is always abnormal in acute encephalitis, and demonstration of slow activity in a confused/obtunded patient with pyrexia helps to confirm the diagnosis. Electrographic abnormalities will be non-specific as to cause, although focal or lateralised changes can raise suspicion of encephalitis caused by herpes simplex infection (HSE). These include focal δ activity and PEDs, typically over the temporal lobes (fig 1), with either unilateral or bilateral involvement. PEDs were considered to be the EEG hallmark of HSE, but they are also seen in other acute encephalitides, brain tumours, and acute strokes. Moreover, it may take several days for the PEDs to appear in HSE, the discharges can be transient, and there is some evidence that prompt treatment with antiviral therapy reduces the likelihood that PEDs will occur. Thus, the role of EEG in diagnosis of HSE has been overtaken by specific immunological and viral detection tests in CSF and neuroimaging. Any patient now with suspected encephalitis will be treated with acyclovir from the time of presentation in the accident and emergency department, without delay introduced by waiting for an EEG. EEG findings in HSE may give prognostic information, as cases with bilateral epileptiform discharges tend to have poor long term neurological outcome. Unfortunately, predictive factors are difficult to identify from most studies, since these are small, retrospective, and with varying time intervals between symptomatic onset and EEG or treatment.
Thus, the role of EEG in diagnosis of HSE has been overtaken by specific immunological and viral detection tests in CSF and neuroimaging. Any patient now with suspected encephalitis will be treated with acyclovir from the time of presentation in the accident and emergency department, without delay introduced by waiting for an EEG. EEG findings in HSE may give prognostic information, as cases with bilateral epileptiform discharges tend to have poor long term neurological outcome. Unfortunately, predictive factors are difficult to identify from most studies, since these are small, retrospective, and with varying time intervals between symptomatic onset and EEG or treatment.
Figure 1
Herpes simplex encephalitis: left sided slow activity and repetitive periodic epileptiform discharges (PEDs).
As with encephalopathies, EEG is most useful in acute encephalitis to identify seizures as a complicating factor.
Subacute sclerosing pan-encephalitis (SSPE)
This neurodegenerative complication of measles virus infection in childhood is worthy of mention, being one of very few disorders in which the EEG shows relatively specific or pathognomonic changes. Although rare, UK neurologists are likely to see more cases in years to come because of the decline in measles vaccination following the MMR/autism scare. The characteristic EEG picture is of stereotyped high voltage periodic complexes, usually generalised or bilateral (fig 2). Morphology of the complexes is highly stereotyped within an individual, but differs between patients. The periodicity varies from a few to many seconds, with a gradual reduction of the interval between complexes, and eventual disappearance of complexes as disease progresses. Background cerebral activity between complexes is normal initially, with increasing slow activity and then attenuation in later stages. The complexes are usually associated with myoclonic jerks (the myoclonus may be negative). Most SSPE presents in children and adolescents. Adult onset cases are rare, but clinical and electrographic features can be atypical—prominent early visual symptoms, lack of dementia, and a slow EEG without stereotyped complexes.
Although rare, UK neurologists are likely to see more cases in years to come because of the decline in measles vaccination following the MMR/autism scare. The characteristic EEG picture is of stereotyped high voltage periodic complexes, usually generalised or bilateral (fig 2). Morphology of the complexes is highly stereotyped within an individual, but differs between patients. The periodicity varies from a few to many seconds, with a gradual reduction of the interval between complexes, and eventual disappearance of complexes as disease progresses. Background cerebral activity between complexes is normal initially, with increasing slow activity and then attenuation in later stages. The complexes are usually associated with myoclonic jerks (the myoclonus may be negative). Most SSPE presents in children and adolescents. Adult onset cases are rare, but clinical and electrographic features can be atypical—prominent early visual symptoms, lack of dementia, and a slow EEG without stereotyped complexes.
Figure 2
Stereotyped high amplitude periodic complexes in an adolescent female with subacute sclerosing pan-encephalitis.
HIV infection
Non-localised or focal EEG abnormalities can occur, depending on the nature of opportunistic infection and other central nervous system involvement. In HIV encephalopathy, the usual finding is mild EEG slowing. Epileptiform discharges occur in association with seizures (up to 10% of patients with HIV dementia complex).
DEMENTIA
EEG would be a powerful tool if it was able to distinguish reliably between the worried well and those with minimal/mild cognitive impairment, or predict which patients will go on to develop progressive cognitive decline. In practice, EEG is usually normal until there is little clinical doubt about the likelihood of dementia. It is uncertain yet whether quantitative EEG methodologies, which identify abnormalities in frequency or spatial distribution of cerebral rhythms not clearly visible to the eye, or longitudinal EEG studies in an individual patient, will be more diagnostically helpful especially in early detection of dementias.
There is limited change in the EEG in the normal aging brain. After the age of 85 years, α rhythm frequency declines slightly to around 7-8 Hz. Isolated or intermittent temporal slow waves may be seen in up to one third of healthy subjects over the age of 65 years. Their basis and whether they have any pathological significance is uncertain, but vascular factors are probable.
Interpretation of studies which have evaluated EEG in various neurodegenerative diseases is compounded by inaccuracy of purely clinical diagnosis and limited neuropathological data, inclusion of cases with varying stages of disease, inadequate control groups, and sometimes unclear description of EEG findings. Nevertheless, some general comments can be made about the range of EEG changes that are expected in more common dementias.
Alzheimer’s disease
In the early stage, with mild cognitive impairment, the EEG is usually normal. As disease progresses, α rhythm slows in frequency and then disappears, and in the moderately or severely demented patient, the EEG is dominated by slow activity. The abnormalities are usually diffuse, but may sometimes show emphasis or be most pronounced over frontal or temporal lobes. A few patients show periodic sharp waves or epileptiform discharges, which may be associated with myoclonic jerks or seizures, but if prominent and there are atypical clinical features, other diagnoses should be considered. A number of small case series have reported patients in which apparently progressive memory dysfunction presenting as dementia is due to unrecognised complex partial seizures. These patients show epileptiform discharges over temporal lobes, and introduction of antiepileptic drug treatment leads to improvement in cognitive deficit. Pointers to such cases include a previous history of epilepsy, fluctuation in memory function, and absence of progressive deterioration on repeated psychometric testing. It may be necessary in some cases to monitor interictal EEG for up to 24 hours to identify epileptiform discharges occurring during nocturnal sleep only.
The abnormalities are usually diffuse, but may sometimes show emphasis or be most pronounced over frontal or temporal lobes. A few patients show periodic sharp waves or epileptiform discharges, which may be associated with myoclonic jerks or seizures, but if prominent and there are atypical clinical features, other diagnoses should be considered. A number of small case series have reported patients in which apparently progressive memory dysfunction presenting as dementia is due to unrecognised complex partial seizures. These patients show epileptiform discharges over temporal lobes, and introduction of antiepileptic drug treatment leads to improvement in cognitive deficit. Pointers to such cases include a previous history of epilepsy, fluctuation in memory function, and absence of progressive deterioration on repeated psychometric testing. It may be necessary in some cases to monitor interictal EEG for up to 24 hours to identify epileptiform discharges occurring during nocturnal sleep only.
Vascular dementia
α rhythm may be preserved for longer than in Alzheimer’s disease, or there may be prominent intermittent temporal slow activity.
Lewy body dementia
Some cross sectional studies have reported a greater degree of EEG slowing, and more focal slow activity in temporal regions in Lewy body dementia compared with Alzheimer’s disease.
Fronto-temporal lobar dementia (FTLD)
It is usually said that the EEG is normal in FTLD. However, reappraisal of EEG findings with the benefit of pathological confirmation of diagnosis in larger numbers of cases shows that slowing occurs in up to 60%, with correlation between degree of EEG abnormality and dementia. Abnormalities are most pronounced in patients with temporal lobe variant FTLD. Hence, EEG is not a reliable discriminator between FTLD and Alzheimer’s disease either in the early or late stages of these disorders.
Pseudodementia
Due to psychiatric illness, the EEG is normal.
Creutzfeldt-Jacob disease (CJD) and other prion disorders
Periodic complexes (PC) are a sufficiently characteristic EEG finding in sporadic CJD (sCJD) for this pattern to be included by the World Health Organization as one of the criteria for diagnosis. The complexes usually have a bilateral or generalised distribution, and repetition rate of around 1 Hz (ranging from 0.5–2 Hz). Myoclonic jerks are usually present when PC develop, and may or may not be time locked to the complexes. Variations do occur—focal or lateralised complexes have been reported (such as occipital PC in the Heidenhain variant), and morphology of the complexes overlaps with that of triphasic waves and PEDs. Unsurprisingly, textbooks rarely provide a clear definition of what constitutes a periodic complex. The German CJD surveillance group has proposed objective EEG diagnostic criteria for purposes of epidemiological or multi-centre studies:
The complexes usually have a bilateral or generalised distribution, and repetition rate of around 1 Hz (ranging from 0.5–2 Hz). Myoclonic jerks are usually present when PC develop, and may or may not be time locked to the complexes. Variations do occur—focal or lateralised complexes have been reported (such as occipital PC in the Heidenhain variant), and morphology of the complexes overlaps with that of triphasic waves and PEDs. Unsurprisingly, textbooks rarely provide a clear definition of what constitutes a periodic complex. The German CJD surveillance group has proposed objective EEG diagnostic criteria for purposes of epidemiological or multi-centre studies:
-
strictly periodic cerebral potentials (most of duration 100–600 ms) with intercomplex interval of 0.5–2 seconds
-
generalised and lateralised complexes accepted
-
at least five repetitive intervals to rule out semi-periodic activity.
About 65% of patients with sCJD will show PC in the EEG. However, in early stages of disease, the EEG may be relatively normal or non-specifically slow, and serial recordings should be performed every 3–4 weeks, if there is suspicion of CJD. Absence of PC in the EEG after 12 weeks duration of illness has been said to be a strong pointer against the diagnosis of CJD, unless of the atypical long duration form. In addition to PC, the EEG shows progressive change in background cerebral rhythms, with loss of normal activity, increasing slow activity, and then decline in amplitude, eventually with a featureless appearance between complexes (fig 3). PC usually disappear in the terminal stage of disease.
Figure 3
Characteristic EEG findings in sporadic Creutzfeldt-Jacob disease (sCJD): periodic complexes at approximately 1 per second, and very low amplitude featureless background between complexes.
The reason why PC are not seen in a significant minority of patients with sCJD is unclear. The occurrence of periodicity may depend on how diffusely or extensively disease affects cortex, which in turn may be influenced by molecular and genetic factors. PC in the EEG are strongly correlated with methionine homozygosity or heterozygosity at codon 129 of the prion protein gene, and with type 2 pathological prion protein.
The occurrence of periodicity may depend on how diffusely or extensively disease affects cortex, which in turn may be influenced by molecular and genetic factors. PC in the EEG are strongly correlated with methionine homozygosity or heterozygosity at codon 129 of the prion protein gene, and with type 2 pathological prion protein.
Although diagnostic specificity of PC for sCJD is high (around 90–95%), PC are not pathognomonic of sCJD—they are described in other dementias (Alzheimer’s disease, Lewy body, vascular) and toxic encephalopathies such as those caused by lithium. However, the clinical context is paramount. In a recent necropsy based study, combination of a typical EEG and typical clinical findings gave a positive predictive value of 99% for sCJD. Hopefully, in time, clinical and EEG criteria together with other data (14-3-3 protein in CSF and MRI) will provide a secure ante-mortem diagnosis of sCJD without the need for brain biopsy.
The presence of PC helps to rule out other prion disorders or transmissible spongiform encephalopathies. PC are not found in kuru, familial fatal insomnia, Gerstmann-Staussler-Scheinker syndrome (with the exception of very rare cases) or in variant CJD.
PC are not found in kuru, familial fatal insomnia, Gerstmann-Staussler-Scheinker syndrome (with the exception of very rare cases) or in variant CJD.
The overlap between triphasic waves, periodic epileptiform discharges and periodic complexes
The morphology and other EEG characteristics of these different phenomena overlap. Fluctuation may give the appearance or impression of seizure activity, and PC can be abolished or attenuated by diazepam. Thus, even experienced electroencephalographers may have difficulty in distinguishing non-convulsive status epilepticus from an acute/subacute toxic or metabolic encephalopathy, or a rapidly progressive dementia. As always, EEG interpretation must be in clinical context, and in conjunction with other laboratory data. A few cases of sCJD presenting as complex partial status have been reported, with variable clinical and EEG response to seizure suppressing treatment. It is uncertain whether these patients really do have status epilepticus as part of the spongiform encephalopathy. More probably, misdiagnosis occurs because the clinical course, decline in conscious level, and development of PC in the EEG are particularly rapid.
More probably, misdiagnosis occurs because the clinical course, decline in conscious level, and development of PC in the EEG are particularly rapid.
Another problematic area is the significance of periodic epileptiform discharges (PEDs) with respect to clinical or subclinical seizures. PEDs occur in acute processes—strokes, tumours, and cerebral infections—that are themselves associated with epileptic seizures, impaired conscious level, and neurological dysfunction. PEDs are strongly correlated with refractory seizures (particularly focal motor, EPC, secondary generalised), and can appear during the course of convulsive and non-convulsive status epilepticus. However, not all patients with PEDs have seizures. How then to decide whether antiepileptic treatment is required in a patient with neurological dysfunction or impaired conscious level whose EEG shows PEDs? Clearly, treatment is necessary if there are overt clinical seizures, and prudent if there is a previous history of epilepsy.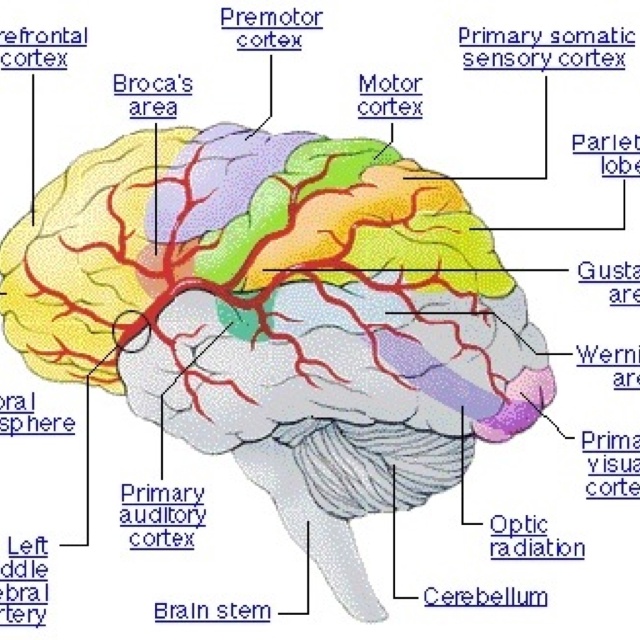 Treatment should also be considered if neurological dysfunction or impaired conscious level fluctuates or is episodic over time periods of minutes or possibly hours, suggesting an ictal basis. Whether EEG features suggesting electrographic seizure activity necessitate treatment is less certain. Identification of ictal EEG changes can be difficult, even when using specific criteria such as rhythmicity, frequency, and evolution of discharges or periodic features, and such criteria are not used consistently. Moreover, many district general hospitals in the UK do not have ready access to EEG equipment or trained personnel. Service availability of continuous EEG monitoring is even more limited. Routine EEG will not be adequate in certain patients: both coma and presence of PEDs have been shown to predict delayed time (> 24 hours) to first seizure in patients with seizures detected by continuous EEG. Some authorities in the USA advocate aggressive antiepileptic treatment in comatose patients with repetitive electrographic seizures or continuous spikes/periodic discharges, even when there are no clinical signs of seizures.
Treatment should also be considered if neurological dysfunction or impaired conscious level fluctuates or is episodic over time periods of minutes or possibly hours, suggesting an ictal basis. Whether EEG features suggesting electrographic seizure activity necessitate treatment is less certain. Identification of ictal EEG changes can be difficult, even when using specific criteria such as rhythmicity, frequency, and evolution of discharges or periodic features, and such criteria are not used consistently. Moreover, many district general hospitals in the UK do not have ready access to EEG equipment or trained personnel. Service availability of continuous EEG monitoring is even more limited. Routine EEG will not be adequate in certain patients: both coma and presence of PEDs have been shown to predict delayed time (> 24 hours) to first seizure in patients with seizures detected by continuous EEG. Some authorities in the USA advocate aggressive antiepileptic treatment in comatose patients with repetitive electrographic seizures or continuous spikes/periodic discharges, even when there are no clinical signs of seizures. There is, however, no good evidence from appropriately conducted clinical trials for improved outcome in such cases.
There is, however, no good evidence from appropriately conducted clinical trials for improved outcome in such cases.
AMNESIC STATES
Patients with transient global amnesia have normal EEGs, unless the recording is done fortuitously during the acute event, when temporal slow activity may be seen. EEG referral should be reserved for those cases in which there is high clinical suspicion or likelihood of transient epileptic amnesia—in which the amnestic episodes are recurrent and brief, or of very long duration (complex partial status epilepticus lasting days).
THE EEG IN PSYCHIATRIC DISORDERS
A wide range of EEG abnormalities, often subtle and bordering on normal variants, has been described in psychiatric conditions, including personality disorders. None appears to be of diagnostic or prognostic value. Overall, there is low EEG yield in patients presenting with purely psychiatric symptoms. This is especially so in young subjects with new onset psychotic disorders featuring auditory and visual hallucinations. Such hallucinations in isolation of any other clinical pointers are hardly ever indicative of epilepsy. Even in neurological conditions that manifest initially with psychiatric features—for example, variant CJD—EEG is usually normal in the early stages. In middle aged or elderly patients with new onset psychiatric illness, EEG can be more helpful in detection of organic brain syndromes, with diffuse abnormalities indicating a neurodegenerative or encephalopathic process, or focal slow activity alerting to a space occupying lesion.
Such hallucinations in isolation of any other clinical pointers are hardly ever indicative of epilepsy. Even in neurological conditions that manifest initially with psychiatric features—for example, variant CJD—EEG is usually normal in the early stages. In middle aged or elderly patients with new onset psychiatric illness, EEG can be more helpful in detection of organic brain syndromes, with diffuse abnormalities indicating a neurodegenerative or encephalopathic process, or focal slow activity alerting to a space occupying lesion.
EEG is essential for identification of de novo absence status of late onset. This may occur without a preceding history of epilepsy, and be triggered by abrupt withdrawal of benzodiazepine or psychotropic drugs. Some cases are associated with metabolic disturbance or alcohol abuse. A high index of suspicion is required, as clinical manifestations are very variable. They range from a mild acute confusional state with mental dullness, irritability, patchy amnesia, and slight motor retardation to profound stupor with catatonia and loss of sphincter control. Primary misdiagnosis of a psychiatric disorder is very common. The EEG confirms the organic basis of the confusional state, and typically shows continuous or repetitive generalised/diffuse spike wave discharge (1–4 Hz). A characteristic of the condition is rapid clinical and EEG resolution with intravenous benzodiazepine (diazepam or lorazepam). Patients with de novo absence status and no prior history of epilepsy do not usually require long term antiepileptic drug treatment.
Primary misdiagnosis of a psychiatric disorder is very common. The EEG confirms the organic basis of the confusional state, and typically shows continuous or repetitive generalised/diffuse spike wave discharge (1–4 Hz). A characteristic of the condition is rapid clinical and EEG resolution with intravenous benzodiazepine (diazepam or lorazepam). Patients with de novo absence status and no prior history of epilepsy do not usually require long term antiepileptic drug treatment.
REFERENCES
-
Ebersole JS, Pedley TA, eds. Current practice of clinical electroencephalography. 3rd ed. Lippincott Williams and Wilkins 2003. ▸ Authoritative multi-author reference text, covering the cellular basis of EEG, technological aspects, normal EEG across all age groups, and comprehensive description of clinical applications of EEG. Also includes chapters on evoked potentials and intra-operative monitoring.
-
Blume W, Kaibara M.
 Atlas of adult encephalography. Lippincott-Raven 1995.
Atlas of adult encephalography. Lippincott-Raven 1995. -
Goldenshohn ES, Legatt AD, Koszer S, et al.Goldenshohn’s EEG interpretation: problems of overreading and underreading. 2nd ed. Futura Publishing Co Inc 1999. ▸ Practical guides to interpretation of EEG; useful conjunctive to standard EEG texts. Both atlases include a full range of normal and abnormal EEGs (including atypical records/variants) with detailed accompanying legends and summaries of key points. The Goldenshohn’s atlas particularly stresses the pitfalls of EEG evaluation and the problems that can result from over- and under-interpretation.
-
American Clinical Neurophysiology Society.Journal of Clinical Neurophysiology: official publication of the American Clinical Neurophysiology Society with issues devoted to specific topics and including original research papers.
 ▸ Volume 21 (5) 2004 covers clinical neurophysiology in critical care and diagnosis of encephalopathy, and includes appraisal of continuous EEG monitoring in detection of seizures and ischaemia, and the role of neurophysiological assessment to evaluate prognosis in ICU patients.
▸ Volume 21 (5) 2004 covers clinical neurophysiology in critical care and diagnosis of encephalopathy, and includes appraisal of continuous EEG monitoring in detection of seizures and ischaemia, and the role of neurophysiological assessment to evaluate prognosis in ICU patients. -
Hogh P, Smith SJ, Scahill RI, et al. Epilepsy presenting as AD: electroclinical features, and response to treatment. Neurology 2000;58:298–301. ▸ A reversible/treatable pseudodementia
-
Chan D, Walters RJ, Sampson EL, et al. EEG abnormalities in fronto-temporal lobar degeneration. Neurology 2004;62:1628–30. ▸ Reappraisal of EEG findings in this increasingly recognised dementia.
-
Steinhoff BJ, Zerr I, Glatting M, et al. Diagnostic value of periodic complexes in Creutzfeld-Jakob disease.
 Ann Neurol 2004;56:702–8.
Ann Neurol 2004;56:702–8. -
Zerr I, Schulz-Schaeffer WJ, Giese A, et al. Current clinical diagnosis in Creutzfeldt-Jakob disease: identification of uncommon variants. Ann Neurol 2000;48:323–9. ▸ The German CJD group has published extensively on the value of commonly used clinical tests including EEG in diagnosis of CJD.
Read the full text or download the PDF:
Subscribe
Log in using your username and password
For personal accounts OR managers of institutional accounts
Username *
Password *
Forgot your log in details?Register a new account?
Forgot your user name or password?
Electroencephalogram (EEG) | Johns Hopkins Medicine
What is an EEG?
An EEG is a test that detects abnormalities in your brain waves, or in the electrical activity of your brain. During the procedure, electrodes consisting of small metal discs with thin wires are pasted onto your scalp. The electrodes detect tiny electrical charges that result from the activity of your brain cells. The charges are amplified and appear as a graph on a computer screen, or as a recording that may be printed out on paper. Your healthcare provider then interprets the reading.
The electrodes detect tiny electrical charges that result from the activity of your brain cells. The charges are amplified and appear as a graph on a computer screen, or as a recording that may be printed out on paper. Your healthcare provider then interprets the reading.
During an EEG, your healthcare provider typically evaluates about 100 pages, or computer screens, of activity. He or she pays special attention to the basic waveform, but also examines brief bursts of energy and responses to stimuli, such as flashing lights.
Evoked potential studies are related procedures that also may be done. These studies measure electrical activity in your brain in response to stimulation of sight, sound, or touch.
Why might I need an EEG?
The EEG is used to evaluate several types of brain disorders. When epilepsy is present, seizure activity will appear as rapid spiking waves on the EEG.
People with lesions of their brain, which can result from tumors or stroke, may have unusually slow EEG waves, depending on the size and the location of the lesion.
The test can also be used to diagnose other disorders that influence brain activity, such as Alzheimer's disease, certain psychoses, and a sleep disorder called narcolepsy.
The EEG may also be used to determine the overall electrical activity of the brain (for example, to evaluate trauma, drug intoxication, or extent of brain damage in comatose patients). The EEG may also be used to monitor blood flow in the brain during surgical procedures.
There may be other reasons for your healthcare provider to recommend an EEG.
What are the risks of an EEG?
The EEG has been used for many years and is considered a safe procedure. The test causes no discomfort. The electrodes record activity. They do not produce any sensation. In addition, there is no risk of getting an electric shock.
In rare instances, an EEG can cause seizures in a person with a seizure disorder. This is due to the flashing lights or the deep breathing that may be involved during the test. If you do get a seizure, your healthcare provider will treat it immediately.
Other risks may be present, depending on your specific medical condition. Be sure to discuss any concerns with your healthcare provider before the procedure.
Certain factors or conditions may interfere with the reading of an EEG test. These include:
- Low blood sugar (hypoglycemia) caused by fasting
- Body or eye movement during the tests (but this will rarely, if ever, significantly interfere with the interpretation of the test)
- Lights, especially bright or flashing ones
- Certain medicines, such as sedatives
- Drinks containing caffeine, such as coffee, cola, and tea (while these drinks can occasionally alter the EEG results, this almost never interferes significantly with the interpretation of the test)
- Oily hair or the presence of hair spray
How do I get ready for an EEG?
Ask your healthcare provider to tell you what you should do before your test. Below is a list of common steps that you may be asked to do.
- Your healthcare provider will explain the procedure to you and you can ask questions.
- You will be asked to sign a consent form that gives your permission to do the procedure. Read the form carefully and ask questions if something is not clear.
- Wash your hair with shampoo, but do not use a conditioner the night before the test. Do not use any hair care products, such as hairspray or gels.
- Tell your healthcare provider of all medicines (prescription and over-the-counter) and herbal supplements that you are taking.
- Discontinue using medicines that may interfere with the test if your healthcare provider has directed you to do so. Do not stop using medicines without first consulting your healthcare provider.
- Avoid consuming any food or drinks containing caffeine for 8 to 12 hours before the test.
- Follow any directions your healthcare provider gives you about reducing your sleep the night before the test.
 Some EEG tests require that you sleep through the procedure, and some do not. If the EEG is to be done during sleep, adults may not be allowed to sleep more than 4 or 5 hours the night before the test. Children may not be allowed to sleep for more than 5 to 7 hours the night before.
Some EEG tests require that you sleep through the procedure, and some do not. If the EEG is to be done during sleep, adults may not be allowed to sleep more than 4 or 5 hours the night before the test. Children may not be allowed to sleep for more than 5 to 7 hours the night before. - Avoid fasting the night before or the day of the procedure. Low blood sugar may influence the results.
- Based on your medical condition, your healthcare provider may request other specific preparations.
What happens during an EEG?
An EEG may be done on an outpatient basis, or as part of your stay in a hospital. Procedures may vary depending on your condition and your healthcare provider's practices. Talk with your healthcare provider about what you will experience during your test.
Generally, an EEG procedure follows this process:
- You will be asked to relax in a reclining chair or lie on a bed.
- Between 16 and 25 electrodes will be attached to your scalp with a special paste, or a cap containing the electrodes will be used.
- You will be asked to close your eyes, relax, and be still.
- Once the recording begins, you will need to remain still throughout the test. Your healthcare provider may monitor you through a window in an adjoining room to observe any movements that can cause an inaccurate reading, such as swallowing or blinking. The recording may be stopped periodically to let you rest or reposition yourself.
- After your healthcare provider does the initial recording while you are at rest, he or she may test you with various stimuli to produce brain wave activity that does not show up while you are resting. For example, you may be asked to breathe deeply and rapidly for 3 minutes, or you may be exposed to a bright flashing light.
- This study is generally done by an EEG technician and may take approximately 45 minutes to 2 hours.
- If you are being evaluated for a sleep disorder, the EEG may be done while you are asleep.
- If you need to be monitored for a longer period of time, you may also be admitted to the hospital for prolonged EEG (24-hour EEG) monitoring.
- In cases where prolonged inpatient monitoring is not possible, your doctor may consider doing an ambulatory EEG.
What happens after an EEG?
Once the test is completed, the electrodes will be removed and the electrode paste will be washed off with warm water, acetone, or witch hazel. In some cases, you may need to wash your hair again at home.
If you took any sedatives for the test, you may be required to rest until the sedatives have worn off. You will need to have someone drive you home.
Skin irritation or redness may be present at the locations where the electrodes were placed, but this will wear off in a few hours.
Your healthcare provider will inform you when you may resume any medicines you stopped taking before the test.
Your healthcare provider may give you additional or alternate instructions after the procedure, depending on your particular situation.
Next steps
Before you agree to the test or the procedure make sure you know:
- The name of the test or procedure
- The reason you are having the test or procedure
- What results to expect and what they mean
- The risks and benefits of the test or procedure
- What the possible side effects or complications are
- When and where you are to have the test or procedure
- Who will do the test or procedure and what that person’s qualifications are
- What would happen if you did not have the test or procedure
- Any alternative tests or procedures to think about
- When and how will you get the results
- Who to call after the test or procedure if you have questions or problems
- How much will you have to pay for the test or procedure
How to slow down age-related changes in the brain?
With the rise in the level of medicine, the average life expectancy has increased - on average, in countries, men and women live 65–70 years. With an increase in life expectancy, diseases associated with the aging of the body have arisen. One of these problems is age-related changes in the brain that reduce mental performance in older people. We tell you what it is, why they occur and how to prevent them.
With an increase in life expectancy, diseases associated with the aging of the body have arisen. One of these problems is age-related changes in the brain that reduce mental performance in older people. We tell you what it is, why they occur and how to prevent them.
What is it and when do 9 appear0005
Age-related changes in the brain are a gradual decrease in mental performance due to disturbances in the structure of nerve cells, intercellular connections, and a decrease in gray matter volume. Changes occur at all levels: from tissues to molecules and begin to form at the age of 20–25 years. In youth, this is imperceptible: they are compensated by the plasticity of the brain and its high ability to self-heal.
Over time, age-related changes are more noticeable: people remember worse, it is more difficult for them to concentrate, they learn more slowly and more often make mistakes in everyday activities. But this does not mean that cognitive functions can be put an end to. For example, research shows that in adulthood people perform better on tests of verbal ability and spatial reasoning than younger people.
For example, research shows that in adulthood people perform better on tests of verbal ability and spatial reasoning than younger people.
Age-related changes occur in everyone, regardless of gender and social status. However, education and occupation still influence.
“The lower the level of education and the intellectual load on the brain, the faster the emotional and mental degradation sets in,” says cardiologist and family doctor at GMS Clinic Mikhail Glotov.
Age-related changes, or more simply, brain aging, is the same normal process as graying of hair, a decrease in muscle volume, a decrease in visual acuity, hearing, and skin elasticity. They should not be confused with brain disorders - diseases of the nervous system that lead to a decrease in intelligence mainly in the elderly - Alzheimer's disease, Pick's disease or dementia with Lewy bodies.
Why the brain gets old
The first reason is oxidative stress. Biochemical processes take place in all cells, as a result of which waste products accumulate. Normally, they are utilized and removed from the cell. But with age, some recycling processes are disrupted, and the amount of waste in the cell increases, including the accumulation of free radicals.
Biochemical processes take place in all cells, as a result of which waste products accumulate. Normally, they are utilized and removed from the cell. But with age, some recycling processes are disrupted, and the amount of waste in the cell increases, including the accumulation of free radicals.
These are unstable atoms that damage the cell membrane, cellular organs and DNA. Their accumulation leads to oxidative stress, a process when cells and their internal structures are damaged due to excessive oxidation.
It disrupts the structure of DNA inside the cell nucleus and mitochondria, the energy center of the cell, where many structural errors accumulate. This leads to damage, death and malfunction of brain neurons.
“Toxins and combustion products in cigarette smoke increase oxidative stress,” says Dmitry Malyshev, a neurologist at the MedSwiss clinic. “Therefore, in smokers, age-related changes in the brain occur faster. The same applies to alcohol, which increases the risk of developing cerebrovascular pathologies.
”
The second reason for the decline in mental performance in old age is changes in synapses. These are the nerve connections that connect neurons to each other. As people age, the density of synaptic gaps decreases, and the fewer connections between neurons, the slower mental activity, such as memorizing or learning a new skill, proceeds. At the same time, an elderly person can perform the task just as well as a young person, but he will need more time to think and make decisions.
The third reason is demyelination. The processes of neurons are covered with myelin, thanks to which an electrical impulse travels at high speed from cell to cell. With age, myelin becomes less and less, due to which the speed of transmission of a nerve impulse slows down.
Together, these causes give the following result - with age, the brain decreases in size, the volume of gray and white matter decreases, and the transmission of the nerve impulse is disrupted. All this impairs mental faculties.
All this impairs mental faculties.
How brain aging manifests itself
In adulthood, defense mechanisms no longer have time to correct failures in nerve cells, therefore, after 30-35 years, the first signs of age-related changes appear - the amount of RAM, which is responsible for remembering actual actions and events, decreases. For example, a person may forget where he left his parked car, buy some groceries from a shopping list, or what he did this morning.
Brain aging means that cognitive processes slow down in older people. But this slowdown is not critical - healthy elderly people without nervous and mental illnesses also remember phone numbers, license plates or the place where they left the keys to the door, but they need more time to restore events from memory than young people.
With age, the amount of accumulated knowledge decreases in the elderly, events from autobiographical memory begin to disappear. At the same time, procedural memory, which is responsible for storing information about skills, is practically not disturbed. For example, if a young man once learned to ride a bicycle or drive a car, then in old age he will not forget how to drive, but he may forget which road he took to get to school, the name of the first boss at work, or the name of the capital of Germany. The same goes for muscle memory: skills, such as playing a musical instrument or the ability to dance, are fixed almost until the end of life.
For example, if a young man once learned to ride a bicycle or drive a car, then in old age he will not forget how to drive, but he may forget which road he took to get to school, the name of the first boss at work, or the name of the capital of Germany. The same goes for muscle memory: skills, such as playing a musical instrument or the ability to dance, are fixed almost until the end of life.
Elderly people have reduced concentration. With age, it becomes increasingly difficult to concentrate on a task or focus on a conversation in a noisy place. The ability to divide attention is also reduced. If in youth it is possible to cook dinner at the same time and listen attentively to the radio playing in the background, then in old age it is more difficult to do this - you need more strength to do several things at the same time.
How to slow down age-related changes
There are two ways: change your lifestyle and learn. Studies show that people with obesity and a habit of regularly eating sugar and drinking sugary sodas have 10 years earlier brain aging. To delay it, you should adjust your lifestyle - bring your body weight back to normal and reduce your daily sugar intake.
To delay it, you should adjust your lifestyle - bring your body weight back to normal and reduce your daily sugar intake.
Researchers claim that brain aging in people occurs more slowly if they:
- regularly engage in physical activity: running, playing football, swimming, exercising in the gym
- constantly load themselves with intellectual activities: read books, solve puzzles, write poetry, study foreign languages
- are socially active: visit museums, communicate regularly with friends and relatives, travel
- have stress management skills
- eat healthy
- sleep at least 7-8 hours a day.
“There are many ways to slow down age-related changes in the brain. The question is that they captivate a person,” says Dmitry Malyshev. “Any action that affects the sphere of brain activity will be useful: the development of memory, attention, and speech, counting, communication, spatial thinking.
”
Daily physical activity in the form of aerobic and strength exercises for 45 minutes increases mental capacity in people over 50 years old. At the same time, the results of other studies report that in people over 50 who do not engage in physical activity, a five-year brain aging is comparable to a ten-year one.
Age-related changes in the brain are slowed down by playing musical instruments. Studies show that playing or learning an instrument increases neuronal activity, which compensates for the physiological aging of nerve tissue. There are no restrictions on the choice of a musical instrument: it can be a violin, harmonica, banjo or electric guitar.
“Reading, learning foreign languages and developing fine motor skills slow down the development of age-related changes in the brain,” says Mikhail Glotov. “For example, residents of southern Italy who are engaged in tailoring and shoe making are less likely to develop dementia and Alzheimer's disease than in other regions.
”
The Mediterranean diet is recognized as the most healthy and beneficial for the nervous system and cardiovascular system. One of the main factors is a diet containing omega-3 and omega-6 fatty acids. We have already talked about them in detail. Studies also report that people who add kale, spinach, eggs, and avocados to their diet have slower brain aging.
Important to remember
- The first signs of brain aging appear after 30 years
- Aging is a natural physiological process, not a disease
- Causes - oxidative stress, demyelination and decreased density of synapses, lead to a decrease in the volume of the medulla
- Age-related changes are manifested by a gradual deterioration of mental processes: thinking, memorization, attention, orientation
- Brain aging can be slowed down by physical activity, proper eating habits and regular intellectual activity.
3 Simple Habits That Will Slow Down Your Brain Health
AND Help Keep Your Cognitive Performance High
Editorial
Our body is constantly aging. This is no secret to anyone, only if we throw all our efforts into anti-aging skin care and the elimination of wrinkles, then we think much less about those parts of the body that are hidden from the eyes. The brain ages almost faster than all other organs: memory deterioration and slowing down of cognitive processes can be observed already by the age of 30, and then it only becomes more serious. The good news is that brain aging can be easily delayed if you follow the basic rules. Here are three daily habits that will help keep your brain healthy and young.
This is no secret to anyone, only if we throw all our efforts into anti-aging skin care and the elimination of wrinkles, then we think much less about those parts of the body that are hidden from the eyes. The brain ages almost faster than all other organs: memory deterioration and slowing down of cognitive processes can be observed already by the age of 30, and then it only becomes more serious. The good news is that brain aging can be easily delayed if you follow the basic rules. Here are three daily habits that will help keep your brain healthy and young.
Try to get enough sleep
The most banal advice you can give a person with any problem is to improve sleep patterns. However, when it comes to maintaining brain health, one can hardly think of anything more important. Firstly, when we sleep, our brain actively builds new neural connections, strengthens existing ones, and also cuts off all “unnecessary”, which has a beneficial effect on memory.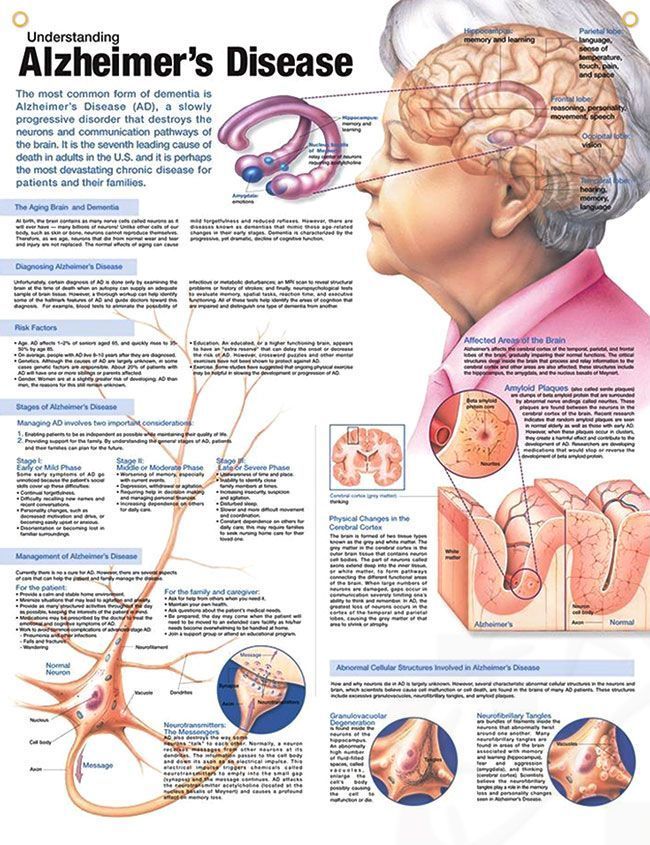 In addition, during sleep, the glial-lymphatic system is cleansed in our body. The brain gets rid of toxins (including those associated with the rapid development of Alzheimer's disease) by significantly expanding the space between brain cells. In a word, the better your sleep pattern, the longer your brain will “serve” you - and this is a rule that cannot be changed. If you are having trouble with this, be sure to read our great guide to healthy sleep to always sleep soundly and wake up easily.
In addition, during sleep, the glial-lymphatic system is cleansed in our body. The brain gets rid of toxins (including those associated with the rapid development of Alzheimer's disease) by significantly expanding the space between brain cells. In a word, the better your sleep pattern, the longer your brain will “serve” you - and this is a rule that cannot be changed. If you are having trouble with this, be sure to read our great guide to healthy sleep to always sleep soundly and wake up easily.
Never stop learning
Another trite advice, but there is no better activity for our brain than learning. In the process of recognizing information and skills, new neural connections are formed in the brain, which is also important for maintaining a good memory. It is precisely because of the lack of new information in older people, many of whom are sure that they have already learned this in this life, the brain often “loses” very quickly. You can learn anything: from the Chinese language to playing tennis. The main thing is to continuously receive and process new information. Mastering creative skills will also help improve memory. Think about what you have long wanted. Master the technique of graphic drawing? Learn to work on a potter's wheel? Or maybe dance the tango? Any activity that involves receiving and processing new information will do. So you can easily combine business with pleasure.
You can learn anything: from the Chinese language to playing tennis. The main thing is to continuously receive and process new information. Mastering creative skills will also help improve memory. Think about what you have long wanted. Master the technique of graphic drawing? Learn to work on a potter's wheel? Or maybe dance the tango? Any activity that involves receiving and processing new information will do. So you can easily combine business with pleasure.
Take dietary supplements
It is quite difficult for the brain to work in the conditions of the modern rhythm of life. According to statistics, more than 25% (and this is one in four) of working adults around the world at least once resorted to the help of dietary supplements to improve brain function. Thanks to such a high demand, a huge number of bioadditives for brain function have appeared on the market, and all of them promise an almost instantaneous and tangible effect.