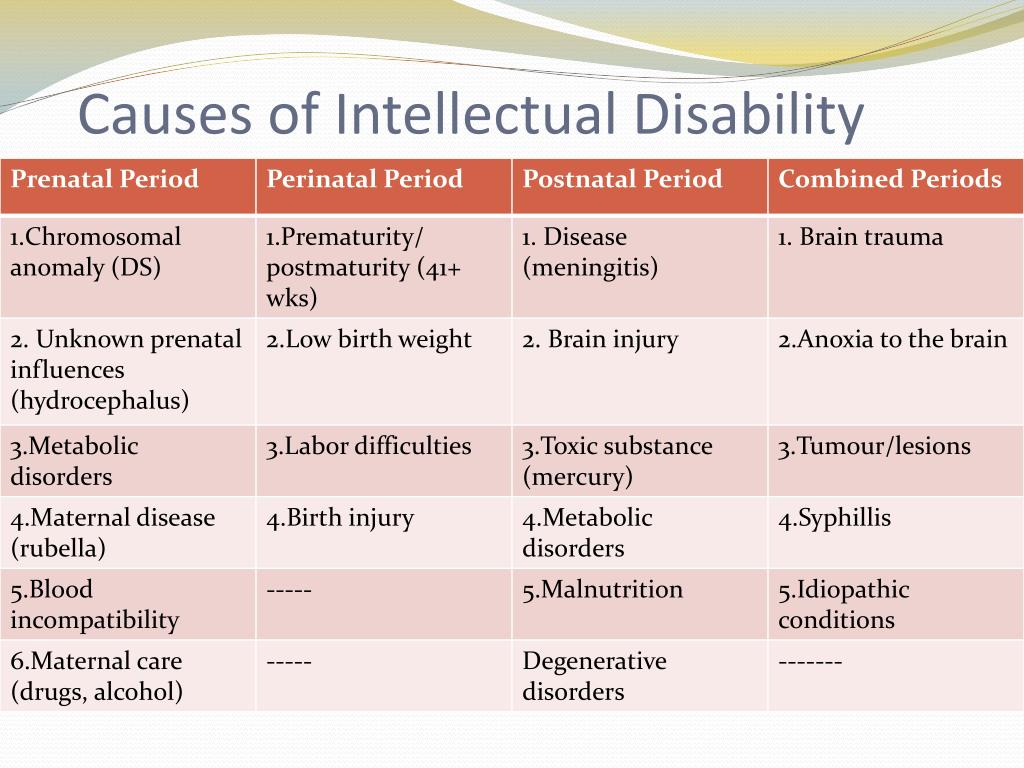
List of cognitive disorders in childhood
Childhood Cognitive Disorders | Principles of Drug Therapy in Neurology
Navbar Search Filter Oxford AcademicPrinciples of Drug Therapy in Neurology (2 edn)Contemporary Neurology SeriesNeurologyPain MedicineBooksJournals Mobile Microsite Search Term
Close
Navbar Search Filter Oxford AcademicPrinciples of Drug Therapy in Neurology (2 edn)Contemporary Neurology SeriesNeurologyPain MedicineBooksJournals Microsite Search Term
Advanced Search
-
Cite Icon Cite
-
Permissions
-
Share
- More
Cite
Lipkin, Paul H. ,
'Childhood Cognitive Disorders'
,
in Michael V. Johnston, and Robert Gross (eds)
,
Principles of Drug Therapy in Neurology
, 2 edn
, Contemporary Neurology Series
(
New York,
2010;
online edn,
Oxford Academic
, 1 Apr. 2013
), https://doi.org/10.1093/med/9780195146837.003.0768,
accessed 20 Oct. 2022.
Select Format Select format.ris (Mendeley, Papers, Zotero).enw (EndNote).bibtex (BibTex).txt (Medlars, RefWorks)
Close
Navbar Search Filter Oxford AcademicPrinciples of Drug Therapy in Neurology (2 edn)Contemporary Neurology SeriesNeurologyPain MedicineBooksJournals Mobile Microsite Search Term
Close
Navbar Search Filter Oxford AcademicPrinciples of Drug Therapy in Neurology (2 edn)Contemporary Neurology SeriesNeurologyPain MedicineBooksJournals Microsite Search Term
Advanced Search
Abstract
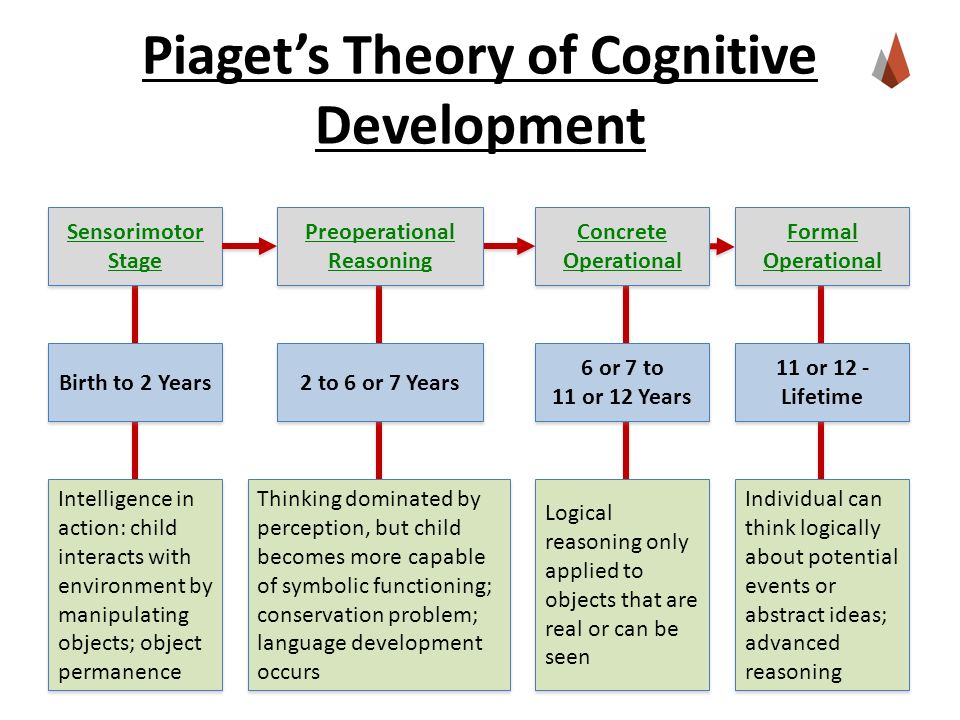
Cognitive disorders are common causes of disability in children, with medications such as stimulants widely used to improve attention, behavior, and learning. The spectrum of childhood cognitive disorders ranges from the lower-prevalence, higher-severity disorder of intellectual disability (ID; also commonly referred to as mental retardation, global developmental delay, or learning disability [United Kingdom]) to the high-prevalence, lower severity learning disabilities (LD) including reading disorder (RD; developmental dyslexia) and attention-deficit hyperactivity disorder (ADHD).
The spectrum of childhood cognitive disorders ranges from the lower-prevalence, higher-severity disorder of intellectual disability (ID; also commonly referred to as mental retardation, global developmental delay, or learning disability [United Kingdom]) to the high-prevalence, lower severity learning disabilities (LD) including reading disorder (RD; developmental dyslexia) and attention-deficit hyperactivity disorder (ADHD).
Subject
NeurologyPain Medicine
Series
Contemporary Neurology Series
Disclaimer
Oxford University Press makes no representation, express or implied, that the drug dosages in this book are correct.
Readers must therefore always …
More
Oxford University Press makes no representation, express or implied, that the drug dosages in this book are correct.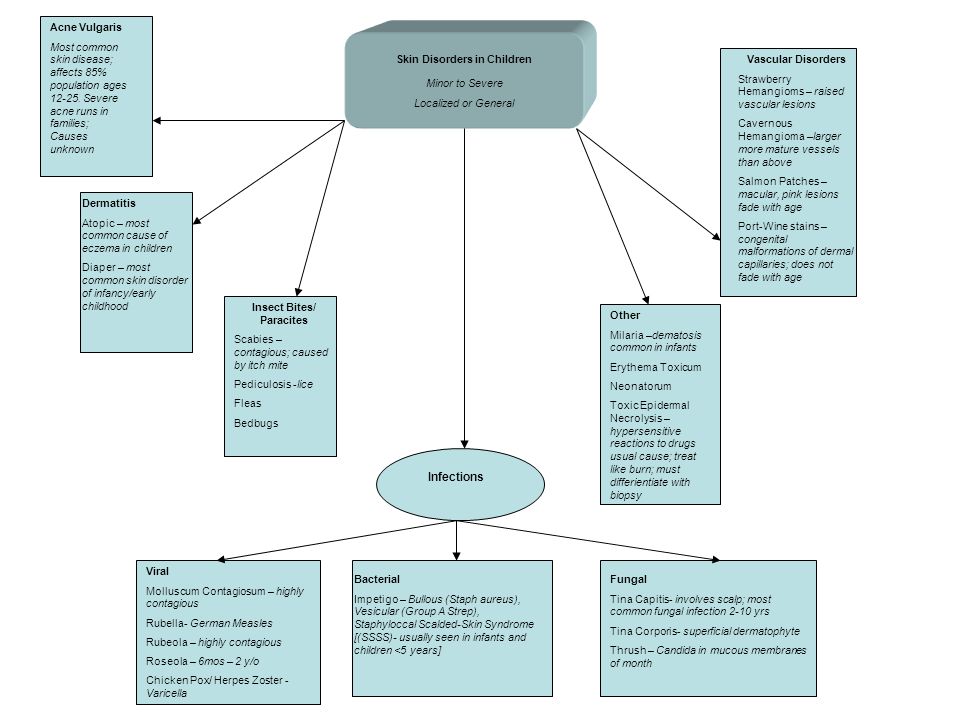 Readers must therefore always check the product information and clinical procedures with the most up to date published product information and data sheets
provided by the manufacturers and the most recent codes of conduct and safety regulations. The authors and the publishers do not accept responsibility or
legal liability for any errors in the text or for the misuse or misapplication of material in this work. Except where otherwise stated, drug dosages
and recommendations are for the non-pregnant adult who is not breastfeeding.
Readers must therefore always check the product information and clinical procedures with the most up to date published product information and data sheets
provided by the manufacturers and the most recent codes of conduct and safety regulations. The authors and the publishers do not accept responsibility or
legal liability for any errors in the text or for the misuse or misapplication of material in this work. Except where otherwise stated, drug dosages
and recommendations are for the non-pregnant adult who is not breastfeeding.
You do not currently have access to this chapter.
Sign in
Get help with access
Get help with access
Institutional access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth / Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.

- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.

- Following successful sign in, you will be returned to Oxford Academic.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
Personal account
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
Institutional account management
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Purchase
Our books are available by subscription or purchase to libraries and institutions.
Purchasing information
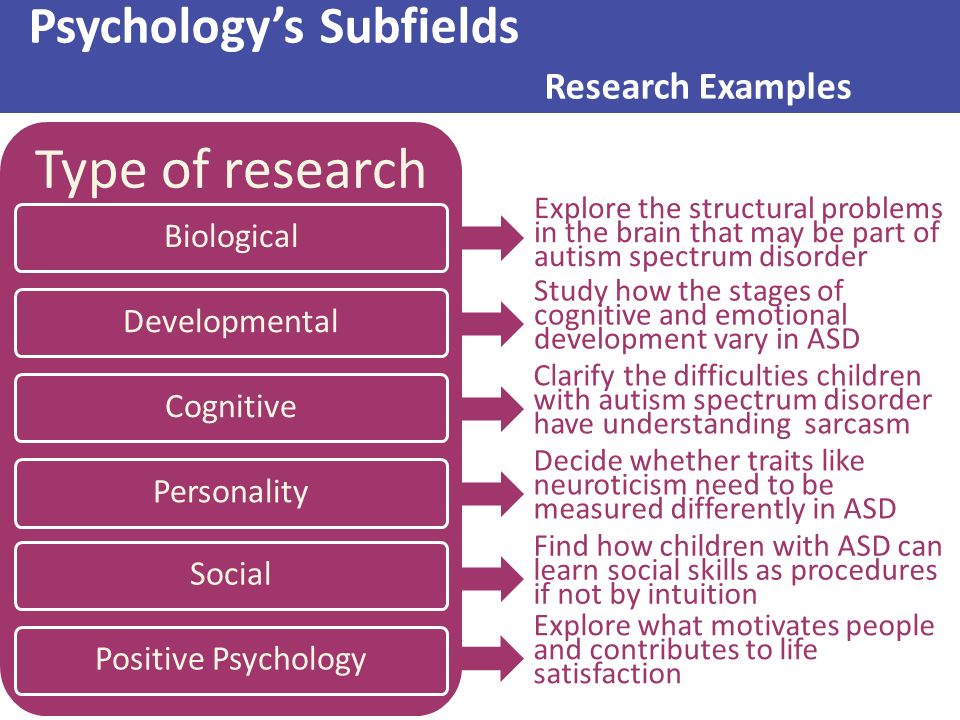
Cognitive Disorders
The term cognitive disorders is a very broad one that means any type of problem with cognition (i. e., thinking skills). There are many types of cognitive skills, including attention and concentration, memory, reasoning, problem solving, visual-spatial skills, and a group of skills called executive functions. Executive functions are high level thinking skills that allow a person to plan and carry out activities independently. Initiation, planning, goal setting, organization, time management, self-monitoring, and evaluation are all executive function skills.
e., thinking skills). There are many types of cognitive skills, including attention and concentration, memory, reasoning, problem solving, visual-spatial skills, and a group of skills called executive functions. Executive functions are high level thinking skills that allow a person to plan and carry out activities independently. Initiation, planning, goal setting, organization, time management, self-monitoring, and evaluation are all executive function skills.
Cognitive disorders may start at any point in life when brain damage occurs. Traumatic brain injury, stroke, tumor, dementia, Parkinson's disease, substance abuse, or other neurological injuries and diseases can cause this damage. Other cognitive disorders are evident from birth or early childhood, such as those associated with developmental conditions like attention deficit/hyperactivity disorder (ADHD) or autism. Executive function problems, in particular, often emerge as a child moves into later elementary grades and school demands increase. Psychiatric disorders (e.g., depression) may also negatively affect cognitive functioning.
Psychiatric disorders (e.g., depression) may also negatively affect cognitive functioning.
Symptoms of Cognitive Disorders
The type(s) and severity of cognitive disorders depend on how much and where damage has occurred. Symptoms might be barely noticeable and only affect ability to do very challenging mental tasks. Some people with cognitive disorders can do basic daily tasks (e.g., dressing, bathing, fixing simple meals) but cannot hold a job or go to school. And some people's thinking skills are so impaired that they need a lot of help with even simple tasks.
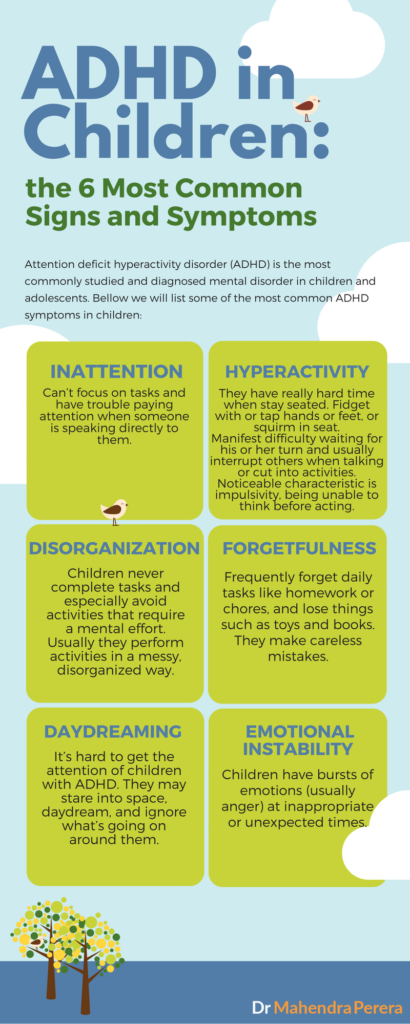
Symptoms of cognitive disorders may include:
- Difficulty paying attention to a task, or switching attention from one task to another
- Confusion about date or time of day, or where they are
- Problems recalling recent events, learning new information, or remembering to do things in the future
- Rarely starts conversations, or needs cuing to get started with tasks
- Needs longer than normal to process what he hears or reads
- Misses appointments, loses items, or seems disorganized in general
- Cannot think of more than one solution to a problem
- Problems reading maps, charts and graphs
- Difficulty completing multi-step tasks
- Often underestimates how long it will take to complete a task; often late to appointments
- Poor judgment or impulsive behavior (i.
 e., acting without thinking)
e., acting without thinking) - Not aware of his weaknesses/impairments
Assessing and Treating Cognitive Disorders
A team of professionals will often assess cognitive skills. The team includes a Speech-Language Pathologist (SLP) in addition to psychologists, teachers and/or occupational therapists. They may give formal and informal tests to check many different cognitive skills. Such tests may not fully measure how well a person might function in real-life situations, so the SLP and other team members will also observe the client during normal daily activities.
Therapy activities will vary widely depending on what cognitive problems are present. The main goal will be to improve the client's ability to complete daily activities (e.g., self-care, school work, job skills) as independently as possible. The SLP will teach family and possibly friends, teachers or bosses about how much help the client needs with different tasks, and how best to assist him.
The SLP will teach the client compensatory strategies to help work around cognitive problems. These are systematic ways of doing things that use a person's strengths to overcome his weaknesses. Some examples include: decreasing visual and auditory distractions in the environment; using aids such as paper or electronic organizers, calendars, alarms, and checklists; and breaking tasks down into separate steps. SLPs may also use paper/pencil or verbal activities or computer programs to improve impaired thinking skills by practicing memory drills, attentional focus, visual spatial skills, reasoning tasks, etc.
These are systematic ways of doing things that use a person's strengths to overcome his weaknesses. Some examples include: decreasing visual and auditory distractions in the environment; using aids such as paper or electronic organizers, calendars, alarms, and checklists; and breaking tasks down into separate steps. SLPs may also use paper/pencil or verbal activities or computer programs to improve impaired thinking skills by practicing memory drills, attentional focus, visual spatial skills, reasoning tasks, etc.
COGNITIVE DISORDERS IN CHILDREN WITH CEREBRAL PALSY (STRUCTURE, DIAGNOSIS, TREATMENT) | Nemkova
1. Baranov A. A., Kuchma V. R., Namazova-Baranova L. S. et al. Strategy “Health and development of adolescents in Russia” (harmonization of European and Russian approaches to the theory and practice of protecting and promoting the health of adolescents ). M., 2010. 108 p.
2. Karkashadze GA, Maslova OI, Namazova-Baranova LS Actual problems of diagnosis and treatment of mild cognitive impairment in children. Pediatric pharmacology. 2011; 8(5):36–41.
Pediatric pharmacology. 2011; 8(5):36–41.
3. Nemkova S. A., Namazova-Baranova L. S., Maslova O. I. et al. Cerebral palsy: diagnosis and correction of cognitive impairment: Educational and methodological manual. M.: Union of Pediatricians of Russia. 2012. 45 p.
4. Garfinkle J., Shevell M. I. Cerebral palsy, developmental delay, and epilepsy after neonatal seizures. Pediatr. Neurol. 2011; 44(2): 88–96.
5. Himmelmann K., Uvebrant P. Function and neuroimaging in cerebral palsy: a population-based study. Dev Med Child Neurol. 2011; 53(6): 516–521.
6. Badalyan L. O., Zhurba L. T., Timonina O. V. Cerebral palsy. Kyiv: Health. 1988. 327 p.
7. Savina MV Problems of mental development of children and adolescents with cerebral palsy. International medical journal. 2010; 3:12–16.
8. Nemkova SA Psychological aspects of the rehabilitation of disabled children with cerebral palsy. Materials of the scientific-practical conference "New opportunities for providing comprehensive assistance to children with disabilities in the conditions of the center of psychological, medical and social support. " M., 2008. 46 p.
" M., 2008. 46 p.
9. Semenova KA Restorative treatment of children with perinatal lesions of the nervous system and cerebral palsy. M.: Code. 2007. 616 p.
10. International Classification of Diseases (10th revision). Classification of mental and behavioral disorders. Research diagnostic criteria. St. Petersburg: Addis. 1994. 300 p.
11. Ermolenko NA, Skvortsov IA, Neretina AF Clinical and psychological analysis of the development of motor, perceptual, intellectual and speech functions in children with cerebral palsy. Journal of Neurology and Psychiatry. S. S. Korsakov. 2000; 3:19-23.
12. Kozyavkin VI, Shestopalova LF Psychological examination of children with organic lesions of the central nervous system, including cerebral palsy: Guidelines. Kharkiv. 1995. 21 p.
13. Sadovskaya Yu.E. Sensory processing disorder and dyspraxia in preschool children. Abstract dis. … doc. honey. Sciences. M., 2011. 44 p.
14. Nemkova SA The study of the individual profile of functional asymmetries, vertical stability and intellectual functions in patients with cerebral palsy with somatosensory stimulation. Abstract dis. … cand. honey. Sciences. M., 2000. 27 p.
Abstract dis. … cand. honey. Sciences. M., 2000. 27 p.
15. Kalizhnuk ES Mental disorders in children with cerebral palsy. Kyiv: Vishcha school. 1987. 269 p.
16. Mastyukova EM Children with cerebral palsy. Special psychology / ed. V. I. Lubovsky. M., 2003.
17. Salkov VN Vision disorders in children with cerebral palsy. Journal of Neurology and Psychiatry. S. S. Korsakov. 2011; 111(4): 8–11.
18. Mamaychuk II Psychology of dysontogenesis and basics of psychocorrection. St. Petersburg: Publishing House of St. Petersburg State University. 2001. 158 p.
19. Kornev AN Neuropsychological research methods. Psychodiagnostic methods in pediatrics and child psychoneurology. SPb., 1991. 95 p.
20. Arkhipova EF Correctional work with children with cerebral palsy. M.: Enlightenment. 1989. 77 p.
21. Maslova OI Neurorehabilitation in pediatrics. Bulletin of RAMN. 2011; 6:41–44.
22. Nemkova SA, Kobrin VI, Sologubov EG et al. Influence of the method of dynamic proprioceptive correction on vertical stability and intellectual functions in patients with cerebral palsy. Neurological journal. 2000; 2:21–24.
Neurological journal. 2000; 2:21–24.
23. Nemkova SA, Maslova OI, Zavadenko NN New technologies in the complex rehabilitation of cognitive impairment in children with cerebral palsy. Materials of the scientific-practical conference "Topical issues of child neurology". Kyiv. 2011, pp. 137–139.
24. Nemkova S. A., Maslova O. I., Zavadenko N. N. The use of space technologies for the rehabilitation of the cognitive disorders in children with cerebral palsy. Europaediatrics-2011. Vienna. 2011.
25. Platonova T. N., Skoromets A. P., Shabalova N. P. Cortexin — long-term use in pediatric practice. Collection of scientific articles “Cortexin. Five years of experience in domestic neurology. St. Petersburg: Science. 2008. 160 p.
26. Ukhanova TA, Gorbunov FE, Ivanova VV Treatment of speech disorders in children with cerebral palsy by combination of reflexotherapy with Cortexin. Journal of Neurology and Psychiatry. S. S. Korsakov. 2011; 8:19–22.
27. Pak LA, Smirnov IE, Goryunova AV et al. Efficacy of Cortexin in the treatment of cerebral palsy. Materials of the 9th All-Russian Congress of Neurologists. Yaroslavl. 2006. P. 206.
Efficacy of Cortexin in the treatment of cerebral palsy. Materials of the 9th All-Russian Congress of Neurologists. Yaroslavl. 2006. P. 206.
28. Isanova VA, Ismagilov MF Korteksin in the complex rehabilitation of patients with cerebral palsy. Neurological Bulletin. 2008; 15(4): 125–127.
29. Ivannikova NV, Esaulova IV, Avdonina V. Yu. et al. Cortexin in complex habilitation and rehabilitation of children with disabilities. Terra Medica. Special issue. 2004, pp. 7–8.
30. Smirnova IA Special education for preschool children with cerebral palsy. St. Petersburg: Detstvo-Press. 2003. 160 p.
Pre-dementia cognitive disorders debuting at a young age: diagnostic and treatment options | Kolokolov O.V., Maleina A.Yu., Kolokolova A.M.
Pre-dementia cognitive disorders debuting at a young age: diagnostic and treatment options | Kolokolov O.V., Maleina A.Yu., Kolokolova A.M. | "RMJ" No. 14 dated 08/30/2017
August 30, 2017
Share material print Add to favorites- Kolokolov O.
 V. 1 ,
V. 1 , - Maleina A.Yu. 2 ,
- Kolokolova A.M. 1
one
Saratov State Medical University im. IN AND. Razumovsky Ministry of Health of Russia, Saratov, Russia
2
Saratov State Medical University im. IN AND. Razumovsky" of the Ministry of Health of Russia
The article discusses the topical problem of timely diagnosis of pre-dementia cognitive disorders (CD), debuting at a young age. Criteria for clinical diagnosis, neuropsychological approaches, neuroimaging methods, and biomarkers that allow determining the type of CR and the etiology of the disease are listed. The most appropriate approach in difficult situations is the referral of patients to clinics that specialize in the diagnosis of CR.
Criteria for clinical diagnosis, neuropsychological approaches, neuroimaging methods, and biomarkers that allow determining the type of CR and the etiology of the disease are listed. The most appropriate approach in difficult situations is the referral of patients to clinics that specialize in the diagnosis of CR.
Information about the relationship between sleep disorders, depression, systemic inflammation and CR is presented. Attention is focused on the fact that the correction of sleep disorders and depression in some cases makes it possible to achieve the restoration of cognitive functions.
It is stated that the treatment of predemental CR, debuting at a young age, largely depends on the etiology of the disease. In some cases, the timely appointment of etiotropic therapy allows for the restoration of impaired functions, in others it may be ineffective.
We present our own data on the use of Divaza in patients under the age of 65 suffering from chronic cerebrovascular disease (CVD). The use of Divaza, which has a nootropic, vasoactive and antioxidant effect, in combination with basic therapy, makes it possible to restore the quality of night sleep and reduce the severity of moderate CR.
The use of Divaza, which has a nootropic, vasoactive and antioxidant effect, in combination with basic therapy, makes it possible to restore the quality of night sleep and reduce the severity of moderate CR.
Keywords: cognitive function, dementia, mild cognitive impairment, dementia with onset at a young age, neuroimaging, biomarkers, S100 protein, Divaza.
Early dement cognitive impairments with debut at a young age: the possibilities of diagnosis and treatment
Kolokolov O.V., Maleina A.Yu., Kolokolova A.M.
Saratov State Medical University named after V.I. Razumovsky
The article discusses the urgent problem of timely diagnosis of early dement cognitive impairments (CIs), debuting at a young age. The criteria for clinical diagnosis, neuropsychological approaches, methods of neuroimaging and biomarkers that allow determining the type of CI and the etiology of the disease are listed. The most appropriate approach in complex situations is the referral of patients to clinics that specialize in CI diagnosis.
Data are presented on the relationship between sleep disorders, depression, systemic inflammation and CI. Attention is focused on the fact that correction of sleep disorders and depression in some cases allows to achieve restoration of cognitive functions.
It is stated that the treatment of early dement cognitive impairments, debuting at a young age, largely depends on the etiology of the disease. In some cases, the timely administration of etiotropic therapy allows recovery of impaired functions, in others it may not be effective.
The authors provide their own data on the use of the drug Divaza in patients younger than 65 years with chronic cerebrovascular disease (CVB). The use of the drug Divaza, which has the nootropic, vasoactive and antioxidant action, in combination with basic therapy, allows to restore the quality of night sleep and reduce the severity of moderate cognitive impairments.
Key words: cognitive functions, dementia, mild cognitive impairments, dementia with debut at a young age, neuroimaging, biomarkers, S100 protein, Divaza.
For citation: Kolokolov O.V., Maleina A.Yu., Kolokolova A.M. Early dement cognitive impairments with debut at a young age: the possibilities of diagnosis and treatment // RMJ. 2017. No. 14. P.1014–1020.
Keywords:
For citation: Predemental cognitive disorders that debut at a young age: diagnostic and treatment options. breast cancer. Medical review. 2017;25(14):1014-1020.
The article is devoted to the possibilities of diagnosing and treating pre-dementia cognitive disorders that debut at a young age
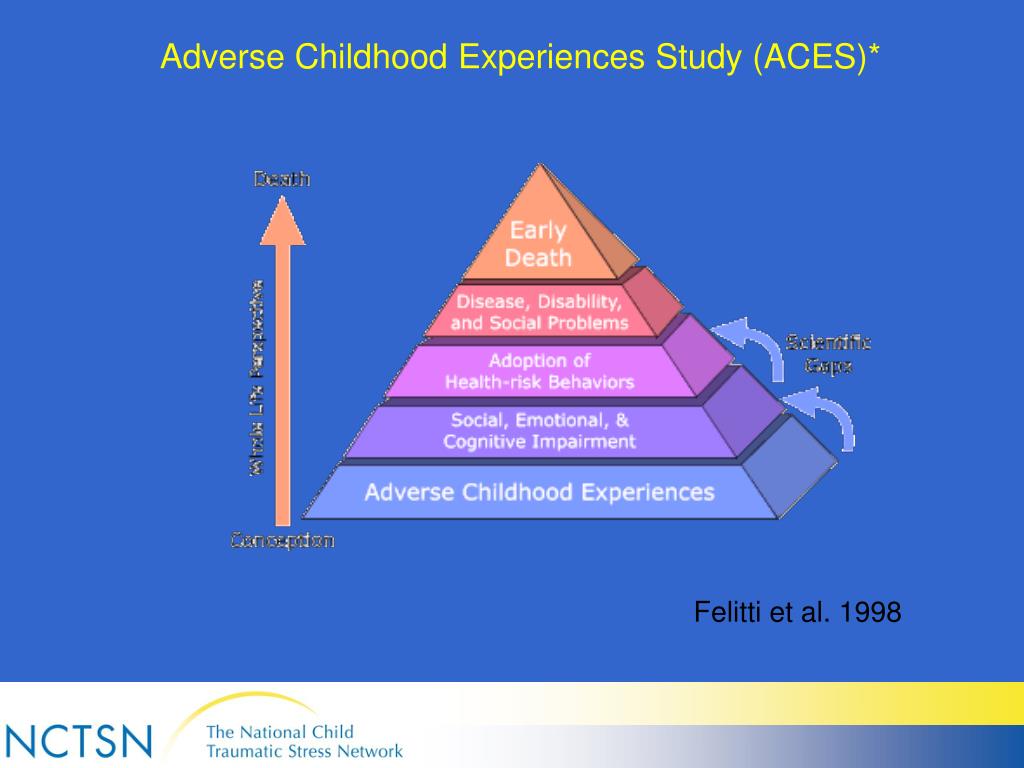
According to the WHO, about 47.5 million people with dementia currently live in the world. The annual number of new cases of the disease is 7.7 million. The term "dementia" is used to denote a syndrome in which degradation of cognitive functions occurs, which is accompanied by a deterioration in control over the emotional state, as well as a violation of the behavior and motivation of patients. Dementia is most common among the elderly, but it is not a sign of physiological aging. Obviously, dementia, being one of the main causes of disability in the elderly, will in the future become of increasing medical and socio-economic importance throughout the world, especially in aging populations, since it has a negative impact not only on the patients themselves, but also on their environment. , especially for caregivers [1].
Obviously, dementia, being one of the main causes of disability in the elderly, will in the future become of increasing medical and socio-economic importance throughout the world, especially in aging populations, since it has a negative impact not only on the patients themselves, but also on their environment. , especially for caregivers [1].
Achieving a lasting effect of therapy prescribed for severe polyfunctional cognitive disorders (CD) remains an unsolved problem. For this reason, the development of algorithms for the early diagnosis of CR at the initial stages of diseases and at a younger age, when CR has not yet reached the degree of dementia, and attempts to correct them may be the most successful, is of particular relevance.
Normally, cognitive functions provide the process of rational cognition of the world and purposeful interaction with it [2]. The main components of the cognition process are memory (remembering and storing information), gnosis (information perception), executive (information processing and analysis) and expressive (speech and praxis) functions.
Cognitive impairment can be said to be in the case of a deterioration in cognitive abilities compared to an individual norm or baseline. One of the first definitions of pre-dementia (not reaching the degree of dementia) CR was formulated by V.A. Kral, who, after observing patients with sleep apnea and finding distracted attention, decreased memory ability, irritability and impaired social interactions, called this disorder “benign senile forgetfulness” [3]. Later, Mayo Clinic experts proposed the term "Mild Cognitive Impairment" (MCI), which is currently included in the ICD-10 (F06.7) [4]. Current RBM criteria were formulated by J. Touchon and R. Retersen and include:
- Complaints of cognitive decline on the part of the patient himself and (or) people from his environment;
– decrease in cognitive functions within 1 year;
– lack of influence of a cognitive defect on daily activities;
– moderate cognitive deficit according to neuropsychological research;
– a preserved general level of intelligence and the absence of clinical signs of dementia.
In accordance with the recommendations of the ICD-10, MCD (F06.7) is diagnosed in the presence of:
- decrease in memory;
- learning difficulties;
- decreased ability to concentrate on a task for a long time;
- pronounced mental fatigue when trying to solve a mental problem.
It must be borne in mind that none of the listed symptoms is so pronounced in MCD that it is possible to diagnose dementia.
According to modern data of foreign authors, the frequency of MCI is from 11 to 20% among people over 65 years of age [5–7]. The results of epidemiological studies regarding the prevalence of MCI vary in different regions, depend on the nature of the sample, research methods and the qualifications of specialists. In the epidemiological study "PROMETHEUS" (RF, 2005–2007), it was found that the incidence of MCI and so-called "mild" CI among elderly patients who consulted a neurologist is 43% [8].
To denote cognitive impairment that develops in persons under 65 years of age, in modern literature it is customary to use the term "Young Onset Dementia" (YOD) - "dementia with a debut at a young age" (DDM). The frequency of DDM, as well as dementia in older age groups, increases with age. According to A. Withall et al., 1 out of 1500 people aged 30–44 years old suffers from dementia, and 1 out of 750 people aged 45–64 years old [9].
The frequency of DDM, as well as dementia in older age groups, increases with age. According to A. Withall et al., 1 out of 1500 people aged 30–44 years old suffers from dementia, and 1 out of 750 people aged 45–64 years old [9].
Etiology and pathogenesis
DDM is characterized by a lower proportion of cases due to degenerative diseases of the nervous system than late-onset dementia. Undoubtedly, Alzheimer's disease (AD) is the most common cause of cognitive impairment at a younger age, but its proportion in DDM is significantly lower (15–40%) compared to that in late-onset dementia (50–70%) [10] . Most cases of AD-associated dementia in people under the age of 45 are genetically determined and inherited in an autosomal dominant manner [11-13]. Degenerative diseases such as frontotemporal dementia (FTD) and Huntington's chorea are more common among younger people, while the prevalence of Lewy body disease and Parkinson's disease is lower among them. The proportion of cases of dementia that developed as a result of cerebrovascular pathology in the age groups mentioned above, according to the estimates of most authors, is approximately the same [10–12].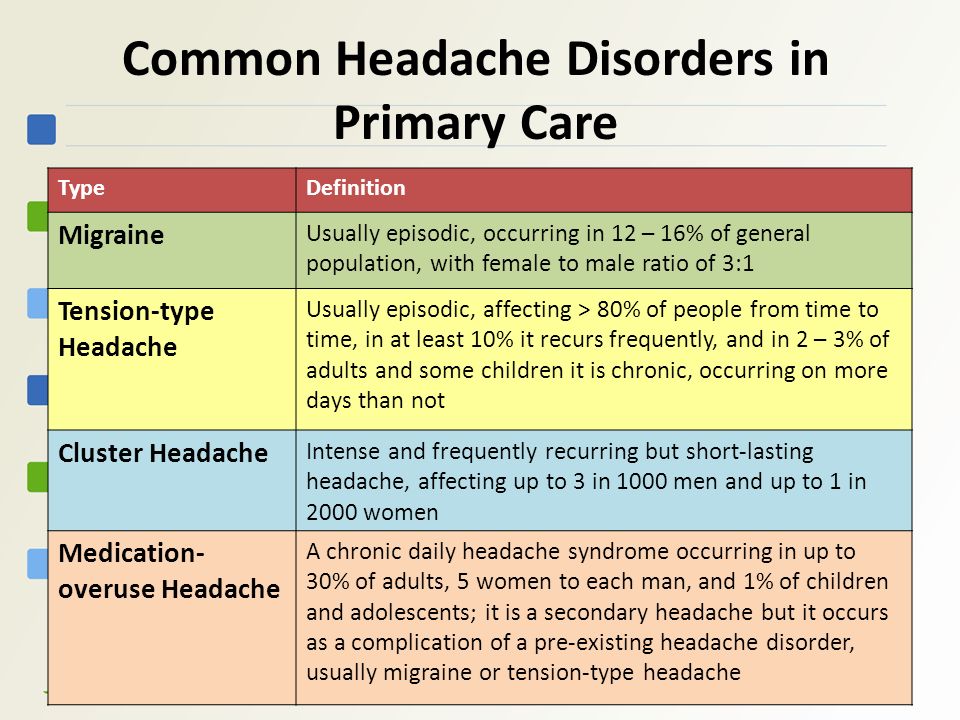
Secondary dementias are more common (about 20% of cases) at younger ages, and causes of DDM include alcohol abuse (5–10% of cases), HIV infection, multiple sclerosis, traumatic brain injury, and a wide range of metabolic, infectious, neoplastic, and autoimmune disorders, many of which are extremely rare, some of them are genetically determined [9–12]. Importantly, some cases of secondary dementia occurring in people younger than 65 years of age may be potentially curable, but adequate therapy should be provided early in the course of the disease (eg, penicillin treatment of neurosyphilis, corticosteroid treatment of exacerbations of multiple sclerosis or cerebral vasculitis, avoidance of alcohol). together with the appointment of thiamine in alcoholism).
Clinical picture
Partly because of the broader etiological spectrum, the clinical manifestations of DDM are more varied than those of late-onset dementia. The subjective and objective manifestations of cognitive impairments, which are the core of the clinical picture of the disease, are quite often accompanied by such disorders as depression, behavioral disorders, and focal neurological symptoms (gait disorder, epileptic seizures, signs of damage to the peripheral nervous system, decreased vision, etc.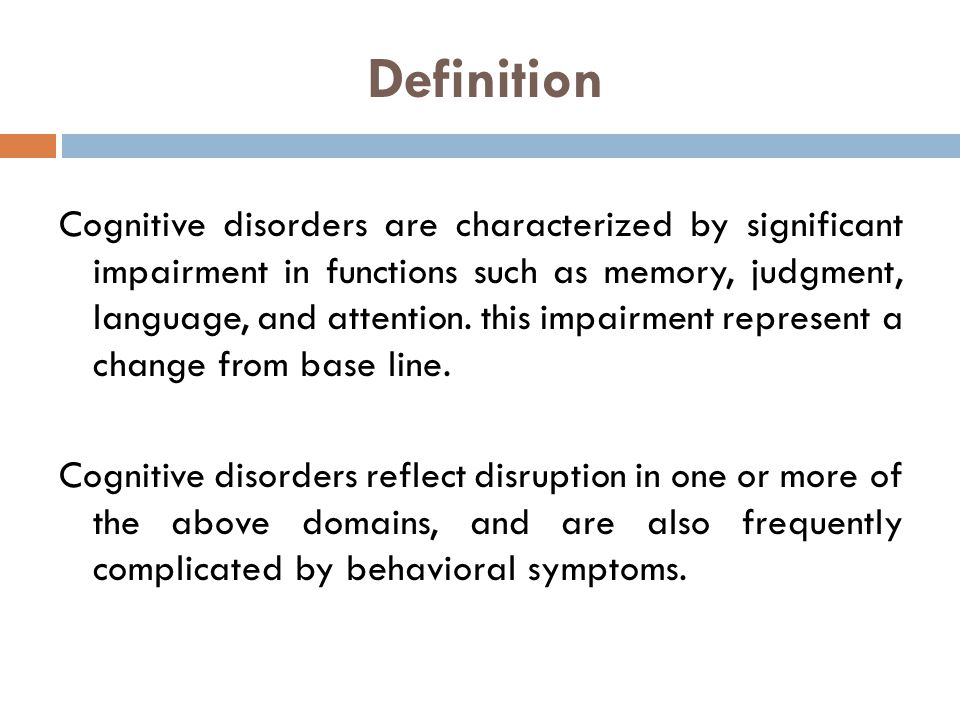 ) [11 , 12]. It is often these signs, not associated with cognitive deficits, that neurologists detect at the onset of the disease.
) [11 , 12]. It is often these signs, not associated with cognitive deficits, that neurologists detect at the onset of the disease. Clinical manifestations of BA differ little in different (under and over 65) age groups. In some cases, patients actively complain about memory impairment. In other cases, work colleagues are the first to notice cognitive decline, which is important because younger people with DDM tend to remain professional and socially active, at least at the onset of the disease. At the workplace, they experience increasing organizational difficulties, their relationships with colleagues worsen, problems arise in solving tasks that previously did not cause any difficulties.
In most cases, DDM has symptoms of depression [14]. On the other hand, it is not uncommon for disorders that were initially treated as depressive disorders to later turn out to be manifestations of frontal dementia. D. Souery et al. draw attention to the relevance of revising the diagnosis of depression in cases of formation of resistance to treatment with antidepressants [15].
In FTD and alcohol-associated CR, behavioral disturbances and personality changes are common and early signs of the disease. Characterized by apathy, decreased social activity, sexual disinhibition, compulsive behavior, perversion of appetite. Personality changes include irritability, selfishness, and loss of empathy. The changes described above usually develop gradually over several years [16].
Among the focal neurological disorders characteristic of a group of diseases called "dementia-plus" (FTD, vascular dementia, Lewy body disease, parkinsonism, progressive supranuclear palsy, corticobasal degeneration, Huntington's chorea, alcoholism, multiple sclerosis, HIV infection, neurosyphilis, post-traumatic dementia, normotensive hydrocephalus, cerebral vasculitis, etc.), the most common are gait disorders, hyperkinesis, epileptic seizures, parkinsonism, pyramidal insufficiency, ataxia. Often, systemic manifestations of the disease include skin lesions, metabolic disorders, decreased vision, and anemia.
Diagnostic
Thus, among the clinical situations where manifestations of the disease in persons under the age of 65 years should alert the clinician and motivate him to conduct a more thorough assessment of cognitive functions using standardized cognitive screening tools, as well as obtaining additional information about the history of the disease from family members patient and (or) his friends, should be attributed [17]:- treatment-resistant depression and (or) anxiety, especially if there are complaints of cognitive decline;
- problems with memory and (or) thinking that arose at a young age, especially in the presence of an aggravating family history;
- a situation where a close family member expresses concern about the patient's cognitive impairment;
- a change in behavior that is inconsistent with the patient's premorbid personality;
- alcoholism and/or substance abuse for 5 years or more;
– HIV infection;
- progressive neurological deficit;
- an indication in the anamnesis of the development of dementia at a young age among family members;
- chronic systemic diseases.

Of particular interest and create significant diagnostic difficulties are such clinical situations when mild and moderate CR develop in people under 65 years of age. Diagnostic search can take a long period. A reliable diagnosis of dementia, the type of dementia, and the exact etiological diagnosis may remain uncertain for 1–2 years or more. In such cases, it is obvious that something is wrong with the patient, but the information necessary to meet the criteria for diagnosing dementia, determining its type and establishing the etiology of CR, may not be enough. Some clinicians are tempted to “diagnose” AD and prescribe treatment with cholinesterase inhibitors. However, this should be avoided because, in addition to the lack of reliable evidence of the long-term effectiveness of cholinesterase inhibitors in the treatment of moderate CR, there is also the risk of misdiagnosis of CR at all, with all the consequences of this error. It is expected that biomarkers developed for diagnosing AD will eventually allow reliable identification of this disease even at the stage of MC in people younger than 65 years, but at present the most promising of them (for example, imaging of the distribution of amyloid) are not available in routine practice.
The difficulties described above are difficult to overcome in the conditions of outpatient medical organizations. In a number of countries, in such situations, it is recommended to refer patients to clinics specializing in the diagnosis of CR, which significantly speeds up the diagnostic process. In cases where such clinics are not available, it is recommended to contact doctors who have their own experience in the diagnosis and treatment of dementia. Investigations such as positron emission tomography, single photon emission tomography, advanced neuropsychological testing, and imaging of amyloid distribution are not universally available and take time. The latter can negatively affect the emotional state and contribute to a decrease in the quality of life not only of the patient himself, but also of his environment.
Satisfactory results obtained when testing using screening scales focused on the detection of memory disorders, such as MMSE, do not always allow adequate and timely assessment of the current clinical situation, which can lead to late diagnosis of dementia.
 This dictates the need to use a set of different tests and develop new ones, for example, Addenbrookes Cognitive Examination (ACE), which has demonstrated high sensitivity and specificity in the differential diagnosis of BA and FTD [18].
This dictates the need to use a set of different tests and develop new ones, for example, Addenbrookes Cognitive Examination (ACE), which has demonstrated high sensitivity and specificity in the differential diagnosis of BA and FTD [18]. The examination plan required for suspected DDM is somewhat different from that required for late-onset dementia, and includes a detailed analysis of the medical history (using information coming not only from the patient himself, but also from his environment), a thorough examination of the patient with a special focus on neurological status, the use of a wider range of tests to assess cognitive functions, the assessment of behavior and psychological status, the wider use of functional diagnostics and neuroimaging methods [19]. If necessary, the list of studies can be expanded. In the presence of symptoms and signs of neurological pathology, regular monitoring by a neurologist and the exclusion of rare, including genetically determined, neurological disorders that can manifest themselves as DDM are necessary, including: CADASIL, Fabry disease, FXTAS syndrome, Gaucher disease, Kufs disease, neuroacanthocytosis, Nimmann-Pick disease (type C), spinocerebellar ataxia, Wilson-Konovalov disease; as well as infections (Whipple disease, syphilis), Creutzfeldt-Jakob disease, limbic encephalitis, etc.
Indeed, there is a higher risk in DDM that CR is genetic, with familial dementia most likely if two or more family members under 65 years of age have dementia. Autosomal dominant inheritance occurs in 5% of cases of AD and in 10–15% of cases of FTD; it is characteristic of the CADASIL syndrome and Huntington's chorea [12]. In these situations, the patient's family members are understandably concerned about their high risk of developing dementia. To avoid suicide and other problems, genetic testing should always be accompanied by medical genetic counseling by qualified geneticists.
Other rare, non-genetic causes of dementia are also most likely to occur before the age of 45. Many of them are associated with metabolic disorders and infections (see above). Most of these present with symptoms consistent with somatic or psychiatric illnesses, often many years before cognitive decline becomes noticeable. In such cases, it is very important to study the medical history in detail in order to find the connection between somatic, mental and cognitive problems, trace their evolution over time and establish the correct diagnosis [17].

Currently, enough evidence has been accumulated to judge the relationship between sleep disorders and CR, namely, the association of early and progressive circadian rhythm disturbances, changes in the quality and architecture of sleep with an increased risk of developing dementia [20–22]. Sleep disturbances entail impaired memory consolidation and consequently cognitive decline. On the other hand, the correction of sleep disorders leads to the restoration of cognitive functions and an improvement in the quality of life [23].
According to the National Institutes of Health (NIH), the incidence of sleep disorders in the US population is 6–10%, and among patients with neurological pathology it reaches 40–83%, depending on the form of the disease [24]. The most common sleep disorders in patients with MCI include insomnia, increased daytime sleepiness, circadian sleep-wake rhythm disturbance with evening confusion, sleep breathing disorders including obstructive sleep apnea (OSA), primary central sleep apnea syndrome, central sleep apnea syndrome with Cheyne-Stokes breathing, hypoventilation / hypoxemia syndrome during sleep with pathology of the parenchyma or pulmonary vessels, parasomnias, in particular: behavior disorder during REM sleep, restless legs syndrome.
When deciding on the diagnosis and treatment of sleep disorders in patients with MCI, it is necessary to carefully conduct an objective examination. In particular, it is important to assess the risk of obstructive sleep apnea, taking into account these diseases, structural features of the ENT organs, the neck, chronic pulmonary diseases, and neurological status. Identification of somatic and mental disorders, including anxiety and depression, requires specialized examination and treatment [25].
A significant range of possibilities for neuropsychological testing, as well as a high frequency of sleep disorders in patients with MCD, dictate the need to create an algorithm for diagnosing these disorders and methods for their correction, aimed at a wide range of physicians. To a certain extent, this was implemented by the staff of the Italian Dementia Research Association (SINDem), who developed recommendations for the correction of sleep disorders in patients with MCD [26].
Thus, on the one hand, for persons complaining of a decrease in cognitive functions, it is advisable not only to use scales focused on the detection of CR, but also to conduct an additional examination to identify sleep disorders. When prescribing treatment, it is important to take into account that the correction of insomnia helps to slow down the rate of increase in CR. On the other hand, there is an obvious need to conduct screening for CR and a more detailed examination (primarily aimed at identifying somatic and mental diseases) of patients suffering from sleep disorders, regardless of the effectiveness/ineffectiveness of hypnotics. Timely recognition of CR in the early stages allows one to start their adequate treatment and avoid the progression of CR and maladjustment of patients.
Numerous studies indicate the relationship between cognitive impairment and sleep breathing disorders. It has been established that most often (40%) in patients with AD and other dementias OSA is registered, which contributes to neurodegeneration due to sleep fragmentation and transient hypoxia.
 Along with this, OSA is associated with the development of cardiovascular diseases (CVD), including hypertension, coronary pathology, and cerebral infarction.
Along with this, OSA is associated with the development of cardiovascular diseases (CVD), including hypertension, coronary pathology, and cerebral infarction. In patients with MCI, along with sleep disturbance, neurosis-like, asthenic, anxiety, and depressive disorders are often diagnosed [22, 27, 28]. This is due to dysregulation of the hypothalamic-pituitary-adrenal and immune systems of the body [29–31]. The risk of anxiety and depressive disorders, as well as changes in sleep duration, correlate with the activity of systemic inflammation [32]. The general neurochemical processes underlying sleep disorders and the development of depression are mediated by increased levels of C-reactive protein (hs-CRP), S100 protein, and monocytic chemoattractant protein-1 (MCP-1) [33, 34]. Inflammation is considered to be the most significant risk factor for CR [23, 35], the most significant relationship between markers of inflammation and CR was established for hs-CRP [36]. In some cases, inflammatory changes can be mediated by the presence of CVD [37, 38].
 An additional confirmation of the above-described relationship between insomnia, depression, systemic inflammation, and CR can be considered the results of a study by M. Heinzelmann et al., which showed that with normalization of sleep, the level of hs-CRP in the blood decreases, while the level of depression also decreases [39].
An additional confirmation of the above-described relationship between insomnia, depression, systemic inflammation, and CR can be considered the results of a study by M. Heinzelmann et al., which showed that with normalization of sleep, the level of hs-CRP in the blood decreases, while the level of depression also decreases [39]. As mentioned above, the diagnosis of MCI is traditionally based on the analysis of patient complaints, subjective assessment by a neurologist and psychiatrist of the clinical manifestations of the disease, the results of instrumental studies and neuropsychological testing. To improve the objectivity of diagnosing CR in assessing cognitive functions, it is recommended to use special scales, one of which is MoCA. In subsequent years, various testing methods were developed and implemented, including ACE, CAMCOG, CAMDEX, CDT, IQCODE, VFT, etc. [40]. However, in everyday clinical practice, the use of detailed neuropsychological testing may be limited due to the lack of qualified specialists, high cost, and long duration of the examination.
 A wide range of neuropsychological testing methods, their lack of sensitivity and specificity, along with a high incidence of CI, dictate the need to develop algorithms and methods for diagnosing CI that would be focused on application in practical healthcare, highly sensitive and specific, accessible and cost-effective, easily interpretable and effective, applicable to all clinical forms of RBM. In this regard, it seems promising to use special computer programs for diagnosing and evaluating MCI, for example, CANS-MCI, CANTAB, which are adapted and validated in the regions of their development [41]. However, these methods are still of limited use. From the practitioner's point of view, the identification of patients with CM, as well as the assessment of the risk of CM progression to dementia, still largely depend on clinical judgment and the results of the assessment of cognitive status by available methods.
A wide range of neuropsychological testing methods, their lack of sensitivity and specificity, along with a high incidence of CI, dictate the need to develop algorithms and methods for diagnosing CI that would be focused on application in practical healthcare, highly sensitive and specific, accessible and cost-effective, easily interpretable and effective, applicable to all clinical forms of RBM. In this regard, it seems promising to use special computer programs for diagnosing and evaluating MCI, for example, CANS-MCI, CANTAB, which are adapted and validated in the regions of their development [41]. However, these methods are still of limited use. From the practitioner's point of view, the identification of patients with CM, as well as the assessment of the risk of CM progression to dementia, still largely depend on clinical judgment and the results of the assessment of cognitive status by available methods. The complexity of the situation is exacerbated by the fact that at present there are no highly specific clinical criteria for assessing the early manifestations of CR [42].
 The mechanisms of the occurrence of CR, which at first glance appear to be different, may turn out to be links in a single pathological process [5]. Many believe that the most adequate and promising method for early diagnosis of CR is still the determination of biological markers [35, 43–47], while the quantitative assessment of biomarkers makes it possible to determine the prognosis of the disease [47]. It has been established that one of the universal risk factors for the occurrence of CR is the inflammatory process [23, 35]. Research results P.B. Gorelick et al. indicate the relationship of inflammation markers, in particular, in particular, hs-CRP and CR [36]. Along with this, it is known that inflammatory mediators may indicate a high risk of CVD [30] and type 2 diabetes mellitus [28, 31, 48–50].
The mechanisms of the occurrence of CR, which at first glance appear to be different, may turn out to be links in a single pathological process [5]. Many believe that the most adequate and promising method for early diagnosis of CR is still the determination of biological markers [35, 43–47], while the quantitative assessment of biomarkers makes it possible to determine the prognosis of the disease [47]. It has been established that one of the universal risk factors for the occurrence of CR is the inflammatory process [23, 35]. Research results P.B. Gorelick et al. indicate the relationship of inflammation markers, in particular, in particular, hs-CRP and CR [36]. Along with this, it is known that inflammatory mediators may indicate a high risk of CVD [30] and type 2 diabetes mellitus [28, 31, 48–50]. One of the biomarkers of damage to the nervous system is the S100 proteins, which are a family of low molecular weight Ca2+-binding proteins, among which S100β occupies a leading position.
 The S100β protein is synthesized in glial cells and subsequently transported to neurons, where it can be in free form or be associated with intracellular components. It is involved in many intra- and extracellular processes, in particular, it stimulates the differentiation and proliferation of neurons, the growth of dendrites, increases the survival of neurons, and also regulates the cell's energy metabolism [51, 52]. The indicated effects contribute to the stabilization of the impulse activity of the neuron and a more differentiated response to stimulation. S100β was first detected in the cerebrospinal fluid (CSF) of a patient with multiple sclerosis [53]. Then it was detected in biological fluids (peripheral blood, CSF, amniotic fluid, saliva) of patients with brain damage of various etiologies (acute encephalomyelitis, amyotrophic lateral sclerosis, intracranial tumors, etc.). The S100β protein is used as a diagnostic marker of various diseases: hypoxic-ischemic brain damage (in particular, ischemia in stroke) and AD [54].
The S100β protein is synthesized in glial cells and subsequently transported to neurons, where it can be in free form or be associated with intracellular components. It is involved in many intra- and extracellular processes, in particular, it stimulates the differentiation and proliferation of neurons, the growth of dendrites, increases the survival of neurons, and also regulates the cell's energy metabolism [51, 52]. The indicated effects contribute to the stabilization of the impulse activity of the neuron and a more differentiated response to stimulation. S100β was first detected in the cerebrospinal fluid (CSF) of a patient with multiple sclerosis [53]. Then it was detected in biological fluids (peripheral blood, CSF, amniotic fluid, saliva) of patients with brain damage of various etiologies (acute encephalomyelitis, amyotrophic lateral sclerosis, intracranial tumors, etc.). The S100β protein is used as a diagnostic marker of various diseases: hypoxic-ischemic brain damage (in particular, ischemia in stroke) and AD [54]. It has been shown that the level of S100β in blood plasma is increased in depression [55]. This fact testifies in favor of the hypothesis of a significant role of neurodegenerative processes in the pathogenesis of anxiety and depressive disorder. Enough information has also been accumulated on the involvement of S100 proteins in the pathogenesis of anxiety. For example, transgenic mice with increased expression of the S100 protein demonstrated a high level of anxiety in open field tests [56], while S100 knockout mice, on the contrary, had reduced anxiety [57]. Interestingly, an increase in the amount of S100β occurs in the blood and urine of healthy people as a result of prolonged mental and physical activity [58]. It follows from the above that S100 proteins are a promising pharmacological target for the treatment of neurological and psychoemotional disorders, the correction of neurodegenerative processes, and the regulation of the body's work under stress.
It has been shown that the level of S100β in blood plasma is increased in depression [55]. This fact testifies in favor of the hypothesis of a significant role of neurodegenerative processes in the pathogenesis of anxiety and depressive disorder. Enough information has also been accumulated on the involvement of S100 proteins in the pathogenesis of anxiety. For example, transgenic mice with increased expression of the S100 protein demonstrated a high level of anxiety in open field tests [56], while S100 knockout mice, on the contrary, had reduced anxiety [57]. Interestingly, an increase in the amount of S100β occurs in the blood and urine of healthy people as a result of prolonged mental and physical activity [58]. It follows from the above that S100 proteins are a promising pharmacological target for the treatment of neurological and psychoemotional disorders, the correction of neurodegenerative processes, and the regulation of the body's work under stress. Treatment
In connection with the above diagnostic difficulties in the treatment of MCI, especially among people under 65 years of age, a special place is occupied by etiotropic (if it is possible - for example, antibacterial therapy for neuroinfections caused by bacteria; antihypertensive therapy for CVD against the background of arterial hypertension) and universal pathogenetic therapy.
One of the promising nootropic and vasoactive drugs with antioxidant action, the use of which is advisable in patients with CR, is Divaza. The active components of Divaza are represented by release-active forms of antibodies to the S100 protein (RA AT S100) and release-active forms of antibodies to endothelial NO synthase (RA AT eNOS). Obtaining RA AT is carried out using a fundamentally new technology (US Patent 8,535,664 B2, 2013), which ensures that such forms have a common distinctive property - the ability to have a modifying effect on the original substance (or structurally similar biological molecules) by changing its spatial structure, which entails a change physical, chemical and biological properties [59].
Thus, the molecular target of RA AT S100 is the S100 protein involved in the conjugation of synaptic and metabolic processes in the CNS. The influence of RA AT S100 contributes to a more stable binding of the S100 protein to Ca2+, which triggers the influx of Na+ into the cell and the outflow of Ca2+ into the extracellular space.
 A change in the transmembrane potential generates an action potential, as a result of which the nerve impulse propagates towards neighboring neurons. The modulating effect of RA AT S100 on the synaptic transmission of various receptors, including GABA, serotonin, sigma1-, NMDA, has been shown. RA AT S100, having a GABA-mimetic and neurotrophic effect, increases the activity of stress-limiting systems, contributes to the restoration of neuronal plasticity processes. Thus, a wide range of pharmacological activity of RA AT S100 provides nootropic, neuroprotective, membrane stabilizing and antioxidant effects. The mechanism of action of the second component of Divaza, RA AT eNOS, is aimed at restoring endothelial function (endothelioprotective effect) and at regulating the work of the cascade: NO-synthase → NO-guanylate cyclase → cGMP (vasoactive effect). Divaza increases the activity of endothelial NO-synthase, and also increases the production of cGMP, which activates intracellular protein kinase-mediated reactions in response to the binding of peptide hormones to the cell membrane.
A change in the transmembrane potential generates an action potential, as a result of which the nerve impulse propagates towards neighboring neurons. The modulating effect of RA AT S100 on the synaptic transmission of various receptors, including GABA, serotonin, sigma1-, NMDA, has been shown. RA AT S100, having a GABA-mimetic and neurotrophic effect, increases the activity of stress-limiting systems, contributes to the restoration of neuronal plasticity processes. Thus, a wide range of pharmacological activity of RA AT S100 provides nootropic, neuroprotective, membrane stabilizing and antioxidant effects. The mechanism of action of the second component of Divaza, RA AT eNOS, is aimed at restoring endothelial function (endothelioprotective effect) and at regulating the work of the cascade: NO-synthase → NO-guanylate cyclase → cGMP (vasoactive effect). Divaza increases the activity of endothelial NO-synthase, and also increases the production of cGMP, which activates intracellular protein kinase-mediated reactions in response to the binding of peptide hormones to the cell membrane.
Previously [17], we conducted a study to assess the clinical and laboratory efficacy and safety of using Divaza (taking 2 tablets 3 times a day for 12 weeks in addition to basic therapy) in comparison with basic therapy in patients (n= 80) under the age of 65 suffering from chronic cerebral ischemia (CCI), the main clinical manifestations of which were CR. Initially, the majority of patients (77%) showed signs of moderate cognitive decline (23–25 points according to the results of the MoCA test). The vast majority (93%) of patients had sleep disorders (total score on the LSEQ questionnaire < 300).
While taking Divaza, there was a significant improvement in cognitive functions, as evidenced by a decrease in the proportion of patients with moderate cognitive impairment. At the beginning of the study, these were 77%, after 12 weeks. treatment - only 3%. In the vast majority (97%) of patients, the MoCA test result was 26–28 points. The improvement in cognitive functions, according to the assessment of the means, was statistically significant (p < 0.
 05) - the mean score of the MoCA test result increased from 24.67±0.25 to 26.30±0.15.
05) - the mean score of the MoCA test result increased from 24.67±0.25 to 26.30±0.15. At the same time, during therapy with Divaza, restoration of night sleep was noted in most patients. According to the analysis of the scoring of the subjective characteristics of sleep using the LSEQ questionnaire, after 12 weeks. after the start of treatment, significant sleep disturbances (total LSEQ score < 300) persisted only in a small (17%) part of patients. At the same time, the proportion of patients with moderate sleep disorders significantly decreased only in the group treated with Divaza: when assessing the GTS parameter (difficulty falling asleep), from 67 to 10%; QOS (quality of sleep) - from 57 to 3%; AFS (difficulty waking up) from 63% to 17% and BFW (behavior integrity after waking up) from 57% to 0%. There was a correlation (r=0.60) between the relief of anxiety and the restoration of nocturnal sleep.
Conclusion
Thus, it should be recognized that the problem of timely diagnosis of pre-dementia (not reaching the degree of dementia) CR, debuting at a young age, has not yet been resolved. The most appropriate approach around the world is to refer patients to clinics that specialize in the diagnosis of CR, or to refer to doctors who have their own experience in the diagnosis and treatment of dementia.
The most appropriate approach around the world is to refer patients to clinics that specialize in the diagnosis of CR, or to refer to doctors who have their own experience in the diagnosis and treatment of dementia. Many studies have proven the relationship between sleep disturbance, depression, systemic inflammation and CR. Moreover, the correction of sleep disorders and depression in some cases allows to achieve the restoration of cognitive functions and improve the quality of life.
Treatment of predemental CR, debuting at a young age, largely depends on the etiology of the disease. In some cases (neuroinfections), the timely appointment of etiotropic therapy makes it possible to achieve a complete restoration of impaired functions, in others (genetically determined diseases), the treatment may be completely ineffective.
The use of the drug Divaza, which has a nootropic, vasoactive and antioxidant effect, in patients under the age of 65 with chronic CVD, in combination with basic therapy, makes it possible to restore the quality of night sleep and reduce the severity of the main clinical manifestations of the disease - predemental CR.
Share material print Add to favorites
1. World Health Organization. Dementia. Internet resource: http://www.who.int/mediacentre/factsheets/fs362/ru [Vsemirnaja Organizacija Zdravoohranenija. Demencija Internet-resource: http://www.who.int/mediacentre/factsheets/fs362/ru (in Russian)].
2. Zakharov V.V., Yakhno N.N. Cognitive disorders in the elderly and senile age: A guide for doctors. M., 2005. 71 p. [Zaharov V.V., Jahno N.N. Kognitivnye rasstrojstva v pozhilom i starcheskom vozraste: Metodicheskoe posobie dlja vrachej. M., 2005. 71 s. (in Russian)].
3. Kales A., Caldwell A., Cadieux R et al. Severe obstructive sleep apnea - II: Associated psychopathology and psychosocial consequences // Journal of Chronic Diseases. 1985 Vol. 38(5). P. 427–434. doi:10.1016/0021-9681(85)
-9.
4. Zakharov V.V., Voznesenskaya T.G. Neuropsychiatric disorders: diagnostic tests. 3rd ed. M., 2014. 320 p. [Zaharov V.V., Voznesenskaja T.G. Nervno-psihicheskie narushenija: diagnosticheskie testy. 3rd ed. M., 2014. 320 s. (in Russian)].
3rd ed. M., 2014. 320 p. [Zaharov V.V., Voznesenskaja T.G. Nervno-psihicheskie narushenija: diagnosticheskie testy. 3rd ed. M., 2014. 320 s. (in Russian)].
5. Forlenza O., Diniz B., Stella F. et al. Mild cognitive impairment (part 1): clinical characteristics and predictors of dementia // Rev Bras Psiquiatr. 2013. Vol. 35(2). P. 178–185. doi:10.1590/1516-4446-2012-3503.
6. Ravaglia G., Forti P., Montesi F. et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population // J Am Geriatr Soc. 2008 Vol. 56. P. 51–58.
7. Luck T., Riedel-Heller S.G., Kaduszkiewicz H. et al. Mild cognitive impairment in general practice: agespecific prevalence and correlated results from the German study on aging, cognition and dementia in primary care patients (AgeCoDe) // Dement Geriatr Cogn Disord. 2007 Vol. 24. P. 307–316.
8. Zakharov V.V. Prevalence and treatment of cognitive impairment in the neurological clinic (results of the All-Russian study "PROMETHEUS") // Сonsilium Medicum.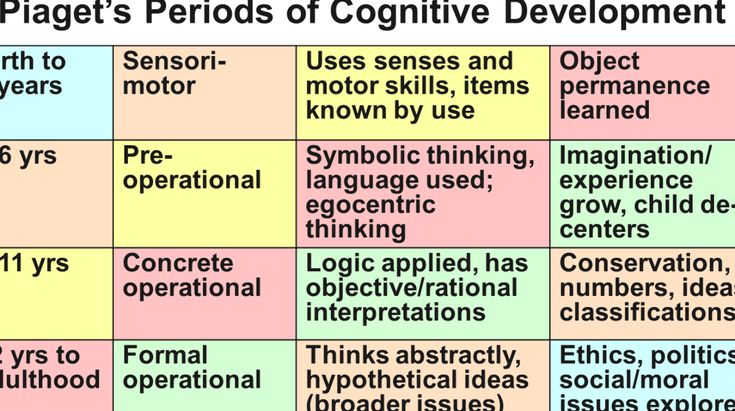 2012. Vol. No. 10. No. 2. P. 25–29 [Zaharov V.V. Rasprostranennost' i lechenie kognitivnyh narushenij v nevrologicheskoj klinike (Rezul'taty Vserossijskogo issledovanija "PROMETEJ") // Сonsilium Medicum. 2012. T. No. 10. No. 2. S. 25–29 (in Russian)].
2012. Vol. No. 10. No. 2. P. 25–29 [Zaharov V.V. Rasprostranennost' i lechenie kognitivnyh narushenij v nevrologicheskoj klinike (Rezul'taty Vserossijskogo issledovanija "PROMETEJ") // Сonsilium Medicum. 2012. T. No. 10. No. 2. S. 25–29 (in Russian)].
9. Withall A., Draper B., Seeher K., Brodaty H. The prevalence and causes of younger onset dementia in Eastern Sydney, Australia // Int Psychogeriatrics. 2014. Vol. 26.1955–1965.
10. Vieira R.T., Caixeta L., Machado S. et al. Epidemiology of early-onset dementia: a review of the literature // Clin Pract Epidemiol Ment Health. 2013. Vol. 9. P. 88–95.
11. Rossor M.N., Fox N.C., Mummery C.J. et al. The diagnosis of young-onset dementia // Lancet Neurol. 2010 Vol. 9. P. 793–806.
12. Masellis M., Sherborn K., Neto P.R. et al. Early-onset dementias: diagnostic and aetiological considerations // Alzheimers Res Ther. 2013. Vol. 5. P. 7.
13. Kelley B.J., Boeve B.F., Josephs K.A. Young-onset dementia. Demographic and etiologic characteristics of 235 patients // Arch Neurol. 2008 Vol. 65. P. 1502–1508.
2008 Vol. 65. P. 1502–1508.
14. Rosness T.A., Barca M.L., Engedal K. Occurrence of depression and its correlates in early onset dementia patients // Int J Geriatr Psychiatry. 2010 Vol. 25. P. 704–711.
15. Souery D., Papakostas G.I., Trivedi M.H. Treatment-resistant depression // J Clin Psychiatry. 2006 Vol. 67. P. 16–22.
16. Ridley N., Draper B., Withall A. Alcohol-related dementia: an update of the evidence // Alzheimers Res Ther. 2013. Vol. 5. P. 3.
17. Fateeva V.V., Kolokolov O.V., Zakharova N.B. and other Disturbances of sleep and cognitive functions as a manifestation of chronic cerebral ischemia and the pathogenetic basis of their correction // Attending physician. 2016. No. 5. P. 16 [Fateeva V.V., Kolokolov O.V., Zaharova N.B. i dr. Narushenie sna i kognitivnyh funkcij kak projavlenie hronicheskoj ishemii golovnogo mozga i patogeneticheskie osnovy ih korrekcii // Lechashhij vrach. 2016. No. 5. S. 16 (in Russian)].
18. Mioshi E., Dawson K., Mitchell J. et al. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening // Int J Geriatr Psychiatry. 2006 Vol. 21. P. 1078–1085.
et al. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening // Int J Geriatr Psychiatry. 2006 Vol. 21. P. 1078–1085.
19. Brodaty H., Connors M., Pond D. et al. Dementia: 14 essentials of assessment and care planning // Medicine Today. 2013. Vol. 14. P. 18–27.
20. Tranah G. J., Blackwell T., Stone K. L et al.; SOF Research Group: Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women // Ann Neurol. 2011 Vol. 70. P. 722–732.
21. Chen P.L., Lee W.J., Sun W.Z. et al. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study // PLoS One. 2012. Vol. 7. P. e49113.
22. Virta J.J., Heikkilä K., Perola M. et al. Midlife sleep characteristics associated with late life cognitive function // Sleep. 2013. Vol. 36. P. 1533-1541.
23. Eikelenboom P., van Exel E., Hoozemans J.J.M. et al. Neuroinflammation – An Early Event in Both the History and Pathogenesis of Alzheimer’s Disease // Neurodegenerative Dis. 2010 Vol. 7(1-3). P. 38–41. doi:10.1159/000283480.
2010 Vol. 7(1-3). P. 38–41. doi:10.1159/000283480.
24. Poluektov M.G. Sleep disorders in the practice of a neurologist // Neurology, neuropsychiatry, psychosomatics. 2012. No. 4. P. 18–24 [Polujektov M.G. Narushenija sna v praktike nevrologa // Nevrologija, nejropsihiatrija, psihosomatika. 2012. No. 4. S. 18–24 (in Russian)].
25. Yakhno N.N., Damulin I.V. Dyscirculatory encephalopathy and vascular dementia in the elderly // BC. 1997. No. 5(20). P. 45–56 [Jahno N.N., Damulin I.V. Discirkuljatornaja jencefalopatija i sosudistaja demencija u pozhilyh // RMZh. 1997. No. 5(20). S. 45–56 (in Russian)].
26. Schutte-Rodin S., Broch L., Buysse D. et al. Clinical guideline for the evaluation and management of chronic insomnia in adults // J Clin Sleep Med. 2008 Vol. 4(5). P. 487–504.
27. Eremenko S.I. Dyscirculatory encephalopathy (etiology, pathogenesis, pathomorphology, clinic, disability examination, principles of treatment). Blagoveshchensk: Amur State Medical Academy, 2008. Internet resource: http://dis.konflib.ru/metodichki-meditsina/101267-3-si-eremenko-discirkulyatornaya-encefalopatiya-etiologiya-patogenez-patomorfologiya-klinika-ekspertiza-trudosposobnosti-princi .php [Eremenko S.I. Discirkuljatornaja jencefalopatija (jetiologija, patogenez, patomorfologija, clinic, jekspertiza trudosposobnosti, principy lechenija). Blagoveshhensk: Amurskaja GMA, 2008. Internet-resurs: http://dis.konflib.ru/metodichki-meditsina/101267-3-si-eremenko-discirkulyatornaya-encefalopatiya-etiologiya-patogenez-patomorfologiya-klinika-ekspertiza-trudosposobnosti-princi .php (in Russian)].
Internet resource: http://dis.konflib.ru/metodichki-meditsina/101267-3-si-eremenko-discirkulyatornaya-encefalopatiya-etiologiya-patogenez-patomorfologiya-klinika-ekspertiza-trudosposobnosti-princi .php [Eremenko S.I. Discirkuljatornaja jencefalopatija (jetiologija, patogenez, patomorfologija, clinic, jekspertiza trudosposobnosti, principy lechenija). Blagoveshhensk: Amurskaja GMA, 2008. Internet-resurs: http://dis.konflib.ru/metodichki-meditsina/101267-3-si-eremenko-discirkulyatornaya-encefalopatiya-etiologiya-patogenez-patomorfologiya-klinika-ekspertiza-trudosposobnosti-princi .php (in Russian)].
28. Levin Ya.I. Depression and sleep // Attending physician. 2008. No. 8. P. 29–32 [Levin Ja.I. Depressija i son // Lechashhij vrach. 2008. No. 8. S. 29–32 (in Russian)].
29. Suarez E.C., Sundy J.S., Erkanli A. Depressogenic vulnerability and gender-specific patterns of neuro-immune dysregulation: What the ratio of cortisol to C-reactive protein can tell us about loss of normal regulatory control // Brain, Behavior, and Immunity. 2015. Vol. 44. P. 137–147. doi:10.1016/j.bbi.2014.09.008.
2015. Vol. 44. P. 137–147. doi:10.1016/j.bbi.2014.09.008.
30. Matsuzaka H., Maeshima H., Kida S. et al. Gender Differences in Serum Testosterone and Cortisol in Patients with Major Depressive Disorder Compared with Controls // The International Journal of Psychiatry in Medicine. 2013. Vol. 46(2). P. 203–221. doi:10.2190/pm.46.2.g.
31. Pace T.W.W., Raison C.L., Miller A.H. Neuroendocrine–Immune Interactions: Implications for Health and Behavior // Hormones, Brain and Behavior. 2009. P. 2597–634. doi:10.1016/b978-008088783-8.00083-8.
32. Prather A.A., Vogelzangs N., Penninx B.W.J.H. Sleep duration, insomnia, and markers of systemic inflammation: Results from the Netherlands Study of Depression and Anxiety (NESDA) // Journal of Psychiatric Research. 2015. Vol. 60. P. 95–102. doi:10.1016/j.jpsychires.2014.09.018.
33. Iskesen I., Kurdal A.T., Yilmaz H. et al. Sleep disturbances after cardiac surgery with or without elevated S100B levels // Acta Cardiologica. 2009 Vol.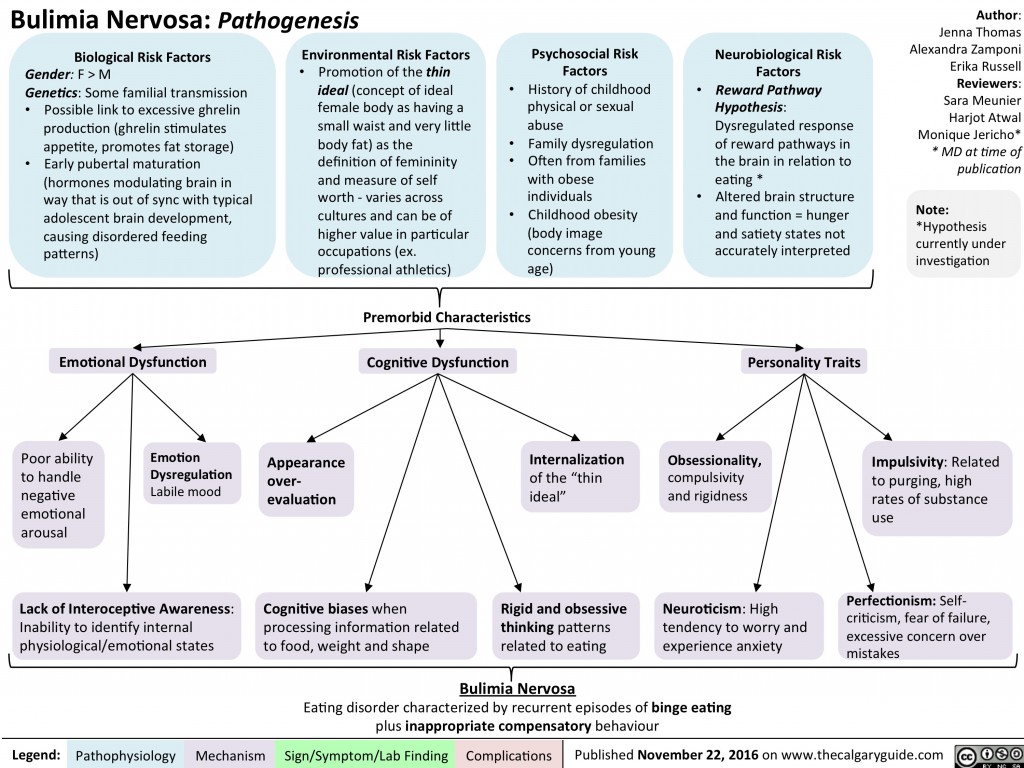 64(6). P. 741–746. doi:10.2143/AC.64.6.2044737
64(6). P. 741–746. doi:10.2143/AC.64.6.2044737
34. Choa H.J., Seeman T.E., Kiefe C.I. et al. Sleep disturbance and longitudinal risk of inflammation: Moderatinginfluences of social integration and social isolation in the CoronaryArtery Risk Development in Young Adults (CARDIA) study // Brain Behav. Immun. 2015. P. 1–8. doi:10.1016/j.bbi.2015.02.023.
35. Saleem M., Herrmann N., Swardfager W. et al. Inflammatory Markers in Mild Cognitive Impairment: A Meta-Analysis // Arosio B, editor. JAD. 2015. Vol. 47(3). P. 669–679. doi:10.3233/jad-150042.
36. Gorelick P.B., Scuteri A., Black S.E. et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association // Stroke. 2011 Vol. 42(9). P. 2672–2713. doi:10.1161/str.0b013e3182299496.
37. Pradhan A.D. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus // JAMA. 2001 Vol. 286(3). P. 327. doi:10. 1001/jama.286.3.327.
1001/jama.286.3.327.
38. Grandner M.A., Buxton O.M., Jackson N. et al. Extreme Sleep Durations and Increased C-Reactive Protein: Effects of Sex and Ethnoracial Group // SLEEP. 2013. Vol. 36(5). P. 769–779. doi:10.5665/sleep.2646.
39. Heinzelmann M., Lee H., Rak H. et al. Sleep restoration is associated with reduced plasma C-reactive protein and depression symptoms in military personnel with sleep disturbance after deployment // Sleep Medicine. 2014. Vol. 15(12). P. 1565–1570. doi:10.1016/j.sleep.2014.08.004.
40. Ladeira R.B., Diniz B.S., Nunes P.V., Forlenza O.V. Combining cognitive screening tests for the evaluation of mild cognitive impairment in the elderly // Clinics (Sao Paulo). 2009 Vol. 64. P. 967–973.
41. Duara R., Loewenstein D., Greig M. et al. Reliability and Validity of an Algorithm for the Diagnosis of Normal Cognition, Mild Cognitive Impairment, and Dementia: Implications for Multicenter Research Studies // The American Journal of Geriatric Psychiatry. 2010 Vol. 18(4). P. 363–370. doi:10.1097/jgp.0b013e3181c534a0.
2010 Vol. 18(4). P. 363–370. doi:10.1097/jgp.0b013e3181c534a0.
42. Van Rossum I.A., Vos S., Handels R., Visser P.J. Biomarkers as Predictors for Conversion fromMild Cognitive Impairment toAlzheimer-Type Dementia: Implications forTrial Design // Journal of Alzheimer's Disease. 2010 Vol. 20. P. 881–891. doi 10.3233/JAD-2010-091606.
43. Horvath I., Jia X., Johansson P. et al. Pro-inflammatory S100A9 Protein as a Robust Biomarker Differentiating Early Stages of Cognitive Impairment in Alzheimer’s Disease // ACS Chemical Neuroscience. 2016. Vol. 7(1). P. 34–39. doi:10.1021/acschemneuro.5b00265.
44. Karim S., Hopkins S., Purandare N. et al. Peripheral inflammatory markers in amnestic mild cognitive impairment // International Journal of Geriatric Psychiatry. 2013. Vol. 29(3). P. 221–226. doi:10.1002/gps.3988.
45. Koyama A., O'Brien J., Weuve J. et al. The Role of Peripheral Inflammatory Markers in Dementia and Alzheimer's Disease: A Meta-Analysis // The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013. Vol. 68(4). P. 433–440. doi: 10.1093/gerona/gls187.
2013. Vol. 68(4). P. 433–440. doi: 10.1093/gerona/gls187.
46. Cunningham C., Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research // Alzheimer’s Research and Therapy. 2015. Vol. 7(1). P. 33. doi: 10.1186/s13195-015-0117-2.
47. Rosa-Neto P., Hsiung G.Y., Masellis M. Fluid biomarkers for diagnosing dementia: rationale and the Canadian Consensus on Diagnosis and Treatment of Dementia recommendations for Canadian physicians // Alzheimer’s Research and Therapy. 2013. Vol. 5(Suppl 1). P. 8. doi: 10.1186/alzrt223.
48. Besedovsky H.O. Immune-neuro-endocrine interactions: facts and hypotheses // Endocrine Reviews. 1996 Vol. 17(1). P. 64–102. Available from: doi:10.1210/er.17.1.64.
49. Setiawan E., Wilson A.A., Mizrahi R. et al. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain During Major Depressive Episodes // JAMA Psychiatry. 2015. Vol. 72(3). P. 268. doi:10.1001/jamapsychiatry.2014.2427.
72(3). P. 268. doi:10.1001/jamapsychiatry.2014.2427.
50. Irwin M.R., Cole J.C. Depression and psychoneuroimmunology // Human Psychoneuroimmunology. 2005. P. 243–264. doi:10.1093/med:psych/9780198568841.003.0010.
51. Michetti F., Corvino V., Geloso M.C. et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress // Journal of Neurochemistry. 2012. Vol. 120(5). P. 644–659. doi:10.1111/j.1471-4159.2011.07612.x.
52. Donato R., Sorci G., Riuzzi F. et al. S100B's double life: Intracellular regulator and extracellular signal // Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009 Vol. 1793(6). P. 1008–1022. doi:10.1016/j.bbamcr.2008.11.009.
53. Michetti F., Massaro A., Murazio M. The nervous system-specific S-100 antigen in cerebrospinal fluid of multiple sclerosis patients // Neuroscience Letters. 1979 Vol. 11(2). P. 171–175. doi:10.1016/0304-3940(79)-8.
54. Korfias S., Stranjalis G., Papadimitriou A. et al. Serum S-100B Protein as A Biochemical Marker of Brain Injury: A Review of Current Concepts // CMC. 2006 Vol. 13(30). P. 3719–3731. doi:10.2174/09298670677
Serum S-100B Protein as A Biochemical Marker of Brain Injury: A Review of Current Concepts // CMC. 2006 Vol. 13(30). P. 3719–3731. doi:10.2174/09298670677
29.
55. Rothermundt M., Arolt V., Wiesmann M. et al. S-100B is increased in melancholic but not in non-melancholic major depression // Journal of Affective Disorders. 2001 Vol. 66(1). P. 89-93. doi:10.1016/s0165-0327(00)00321-9.
56. Bell K., Shokrian D., Potenzieri C., Whitaker-Azmitia P. Harm Avoidance, Anxiety, and Response to Novelty in the Adolescent S-100β Transgenic Mouse: Role of Serotonin and Relevance to Down Syndrome // Neuropsychopharmacology. 2003 Vol. 28(10). P. 1840–1845. doi:10.1038/sj.npp.1300242.
57. Ackermann G.E., Marenholz I., Wolfer D.P. et al. S100A1-deficient male mice exhibit increased exploratory activity and reduced anxiety-related responses // Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2006 Vol. 1763(11). P. 1307–1319. doi:10.1016/j.bbamcr.