Gut bacteria and ocd
The Association Between OCD And Gut Health
Recent years have seen innovative studies that examine newly uncovered connections between different biological and mental systems. Among them is the association between obsessive-compulsive disorder (OCD), gut health and the microorganisms that influence both. Read on to learn more on the subject.
Understanding OCD
OCD is a mental health disorder defined by obsessive thoughts on a destabilizing subject, compulsive actions that repeat themselves, or both. Between 1.1%-1.8% of the world’s population are believed to contend with OCD.
An individual experiencing OCD may initially be overwhelmed by thoughts or imagery about a subject they find concerning. The four most common OCD obsessions are:
- Cleanliness.
- Order/checking.
- Catastrophes.
- Immoral/taboo actions.
To distract themselves from these thoughts, the individual might begin to carry out repetitive actions of a ritualistic nature. While such behavior may start off by providing some relief, eventually the individual begins to feel compelled to perform it, as the behavior itself is incorporated into their condition. Feeding into each other, the loop of OCD-related obsessions and compulsions is set in place.
A number of possible root causes have been linked to OCD. Among them are:
- Abnormal brain activity.
- Genetics.
- Experiencing physical or sexual abuse as a child.
- Exposure to traumatic events.
- Higher negative emotionality.
- Gender, with males more likely to develop early-onset OCD.
The Gut-Brain Axis
The gut-brain axis refers to the connection between the central nervous system, the gastrointestinal system, and the trillions of microbiomes that exist within the gut. Research from the past years has uncovered a direct link between the gut and the brain, thereby drawing a relationship between the thoughts and feelings that stem from the brain, and the intestinal functions of the digestive system.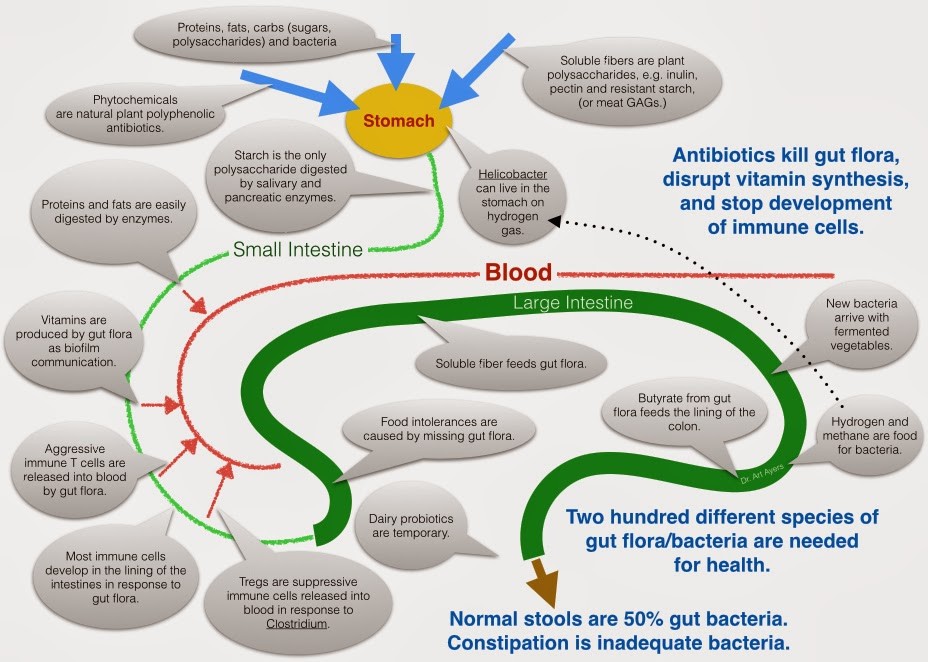
How OCD and Gut Health can Influence Each Other
The correlation between gut health and OCD appears to present itself through the intestinal microflora. A study from 2016 examined the stool samples of patients with OCD, and found they were significantly less diverse and contained a lower abundance of microorganisms, when compared to the general population. It should also be noted that in addition to scoring higher for symptoms of OCD, these patients were found to have a higher prevalence of depression, anxiety and stress than the general population.
The results of a more recent study from 2022 highlighted that patients with OCD usually have an imbalanced microbial biosystem, when compared to the general population. As a result, not only was there a trend toward lower levels of certain bacteria types and an influx in others, but the digestive system as a whole was more likely to suffer from inflammation.
Additional studies have similarly replicated the above finding that correlates OCD with increased inflammation of the gut. This stands to reason, since gut inflammation has been shown to facilitate greater permeation of the blood-brain barrier, allowing harmful cytokines to cross into the brain’s neural system and influence its functioning.
This stands to reason, since gut inflammation has been shown to facilitate greater permeation of the blood-brain barrier, allowing harmful cytokines to cross into the brain’s neural system and influence its functioning.
Exposure to both stressful events and excessive antibiotics have been suggested as possible root causes that originally altered the gut’s microbiota system, thereby setting the stage for an eventual onset of OCD symptoms. In terms of stressful life events, pregnancy in particular is being examined, as an experience that can bring about both OCD and changes in the mother’s gut health.
Treating OCD and Gut Health
Due to the bidirectionality of the gut-brain axis, treating one aspect of this connection can lead to improvement overall.
As mentioned earlier, OCD has been linked to irregular brain activity. This is particularly true for specific brain structures, such as the anterior cingulate gyrus. A number of treatments have been recognized by the FDA for their ability to help regulate this activity. Among them are:
Among them are:
- Selective serotonin reuptake inhibitors, or SSRIs. This family of medications has been shown to significantly alleviate OCD symptoms, by extending the activation period of the mood-regulating neurotransmitter serotonin. Considered to be relatively tolerable, the two most common SSRI side effects are weight gain and sexual dysfunction.
- Cognitive-behavioral therapy, or CBT. This goal-focused form of psychotherapy has also been approved to treat OCD for its ability to offer effective relief, without physical side effects.
- Deep Transcranial Magnetic Stimulation, or Deep TMS™. FDA-cleared to treat OCD, depression/anxious depression, and smoking addiction, Deep TMS is a noninvasive medical device treatment that utilizes electromagnetic fields to safely and effectively regulate brain activity in structures linked to the targeted condition. It does not use anesthesia, does not necessitate a lengthy recovery period, and does not cause any long-term or significant side effects.
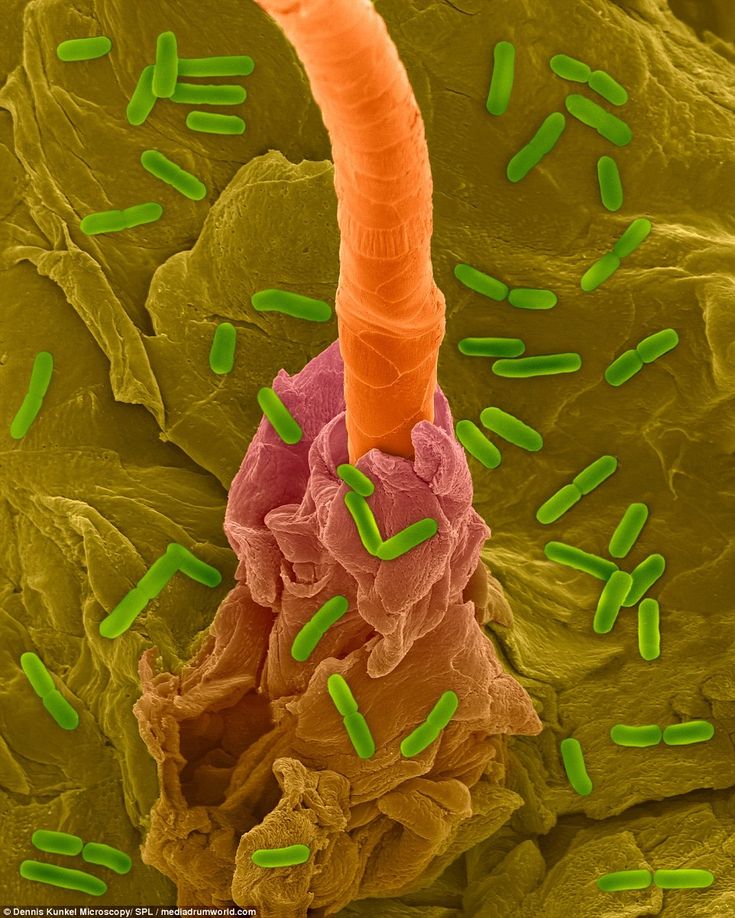
Similarly, improving one’s gut health can lead to improvement in their OCD symptoms. Three ways shown to achieve this are:
- Improved Diet. Opting for a Mediterranean diet over a Western one can improve gut health by reducing inflammation and promoting consistent digestive functioning. Preferring fresh fruit and vegetables, whole grains and olive oil, and steering clear of red or processed meat, high-fat dairy products, white flour and candy, can all improve one’s gut health, and as a result, reduce their OCD symptoms.
- Probiotics. Incorporating probiotics—microorganisms believed to improve the diversity of the gut’s microflora—can also improve one’s mental health. A study from 2015, for example, found that probiotics help lower the production of the stress-inducing hormone cortisol that has been found in higher levels among patients with OCD.
- Sleep. A good night’s sleep has been shown to improve gut health, while a lack of sleep has been implicated in gut inflammation, immune dysfunction, and a leaky gut.
 OCD, as a disorder found in correlation to such digestive conditions, can similarly be influenced by improved sleep.
OCD, as a disorder found in correlation to such digestive conditions, can similarly be influenced by improved sleep.
You may also be interested in...
Obsessive-compulsive disorder and gut microbiota dysregulation
Save citation to file
Format: Summary (text)PubMedPMIDAbstract (text)CSV
Add to Collections
- Create a new collection
- Add to an existing collection
Name your collection:
Name must be less than 100 characters
Choose a collection:
Unable to load your collection due to an error
Please try again
Add to My Bibliography
- My Bibliography
Unable to load your delegates due to an error
Please try again
Your saved search
Name of saved search:
Search terms:
Test search terms
Email: (change)
Which day? The first SundayThe first MondayThe first TuesdayThe first WednesdayThe first ThursdayThe first FridayThe first SaturdayThe first dayThe first weekday
Which day? SundayMondayTuesdayWednesdayThursdayFridaySaturday
Report format: SummarySummary (text)AbstractAbstract (text)PubMed
Send at most: 1 item5 items10 items20 items50 items100 items200 items
Send even when there aren't any new results
Optional text in email:
Create a file for external citation management software
Full text links
Elsevier Science
Full text links
. 2014 Feb;82(2):163-6.
2014 Feb;82(2):163-6.
doi: 10.1016/j.mehy.2013.11.026. Epub 2013 Dec 1.
Jon C Rees 1
Affiliations
Affiliation
- 1 3075 Blackshear Court, Lawrenceville, GA 30044, United States. Electronic address: [email protected].
- PMID: 24332563
- DOI: 10.1016/j.mehy.2013.11.026
Jon C Rees. Med Hypotheses. 2014 Feb.
. 2014 Feb;82(2):163-6.
doi: 10. 1016/j.mehy.2013.11.026. Epub 2013 Dec 1.
1016/j.mehy.2013.11.026. Epub 2013 Dec 1.
Author
Jon C Rees 1
Affiliation
- 1 3075 Blackshear Court, Lawrenceville, GA 30044, United States. Electronic address: [email protected].
- PMID: 24332563
- DOI: 10.1016/j.mehy.2013.11.026
Abstract
Obsessive-compulsive disorder (OCD) is a debilitating disorder for which the cause is not known and treatment options are modestly beneficial. A hypothesis is presented wherein the root cause of OCD is proposed to be a dysfunction of the gut microbiome constituency resulting in a susceptibility to obsessional thinking. Both stress and antibiotics are proposed as mechanisms by which gut microbiota are altered preceding the onset of OCD symptomology. In this light, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) leading to episodic OCD is explained not by group A beta-hemolytic streptococcal infections, but rather by prophylactic antibiotics that are administered as treatment. Further, stressful life events known to trigger OCD, such as pregnancy, are recast to show the possibility of altering gut microbiota prior to onset of OCD symptoms. Suggested treatment for OCD would be the directed, specie-specific (re)introduction of beneficial bacteria modifying the gut microbiome, thereby ameliorating OCD symptoms. Special considerations should be contemplated when considering efficacy of treatment, particularly the unhealthy coping strategies often observed in patients with chronic OCD that may need addressing in conjunction with microbiome remediation.
Both stress and antibiotics are proposed as mechanisms by which gut microbiota are altered preceding the onset of OCD symptomology. In this light, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) leading to episodic OCD is explained not by group A beta-hemolytic streptococcal infections, but rather by prophylactic antibiotics that are administered as treatment. Further, stressful life events known to trigger OCD, such as pregnancy, are recast to show the possibility of altering gut microbiota prior to onset of OCD symptoms. Suggested treatment for OCD would be the directed, specie-specific (re)introduction of beneficial bacteria modifying the gut microbiome, thereby ameliorating OCD symptoms. Special considerations should be contemplated when considering efficacy of treatment, particularly the unhealthy coping strategies often observed in patients with chronic OCD that may need addressing in conjunction with microbiome remediation.
Published by Elsevier Ltd.
Similar articles
-
Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder.
Domènech L, Willis J, Alemany-Navarro M, Morell M, Real E, Escaramís G, Bertolín S, Sánchez Chinchilla D, Balcells S, Segalàs C, Estivill X, Menchón JM, Gabaldón T, Alonso P, Rabionet R. Domènech L, et al. Sci Rep. 2022 Jan 27;12(1):1448. doi: 10.1038/s41598-022-05480-9. Sci Rep. 2022. PMID: 35087123 Free PMC article.
-
Is obsessive-compulsive disorder an autoimmune disease?
Arnold PD, Richter MA. Arnold PD, et al. CMAJ. 2001 Nov 13;165(10):1353-8. CMAJ. 2001. PMID: 11760984 Free PMC article. Review.
-
"WHAT'S BUGGING THE GUT IN OCD?" A REVIEW OF THE GUT MICROBIOME IN OBSESSIVE-COMPULSIVE DISORDER.
Turna J, Grosman Kaplan K, Anglin R, Van Ameringen M. Turna J, et al. Depress Anxiety. 2016 Mar;33(3):171-8. doi: 10.1002/da.22454. Epub 2015 Dec 2. Depress Anxiety. 2016. PMID: 26629974 Review.
-
Obsessive-compulsive disorder and immunology: a review.
da Rocha FF, Correa H, Teixeira AL. da Rocha FF, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Jul 1;32(5):1139-46. doi: 10.1016/j.pnpbp.2007.12.026. Epub 2008 Jan 11. Prog Neuropsychopharmacol Biol Psychiatry. 2008. PMID: 18262706 Review.
-
Searching for host immune-microbiome mechanisms in obsessive-compulsive disorder: A narrative literature review and future directions.
Troyer EA, Kohn JN, Ecklu-Mensah G, Aleti G, Rosenberg DR, Hong S.
 Troyer EA, et al. Neurosci Biobehav Rev. 2021 Jun;125:517-534. doi: 10.1016/j.neubiorev.2021.02.034. Epub 2021 Feb 24. Neurosci Biobehav Rev. 2021. PMID: 33639178 Free PMC article. Review.
Troyer EA, et al. Neurosci Biobehav Rev. 2021 Jun;125:517-534. doi: 10.1016/j.neubiorev.2021.02.034. Epub 2021 Feb 24. Neurosci Biobehav Rev. 2021. PMID: 33639178 Free PMC article. Review.
See all similar articles
Cited by
-
Gut Barrier Dysfunction and Type 2 Immunity: Implications for Compulsive Behavior.
Fields CT, Chassaing B, de Vries GJ. Fields CT, et al. Med Hypotheses. 2022 Apr;161:110799. doi: 10.1016/j.mehy.2022.110799. Epub 2022 Feb 14. Med Hypotheses. 2022. PMID: 36060122
-
Regulation of oxytocin receptor gene expression in obsessive-compulsive disorder: a possible role for the microbiota-host epigenetic axis.
D'Addario C, Pucci M, Bellia F, Girella A, Sabatucci A, Fanti F, Vismara M, Benatti B, Ferrara L, Fasciana F, Celebre L, Viganò C, Elli L, Sergi M, Maccarrone M, Buzzelli V, Trezza V, Dell'Osso B.
 D'Addario C, et al. Clin Epigenetics. 2022 Mar 31;14(1):47. doi: 10.1186/s13148-022-01264-0. Clin Epigenetics. 2022. PMID: 35361281 Free PMC article.
D'Addario C, et al. Clin Epigenetics. 2022 Mar 31;14(1):47. doi: 10.1186/s13148-022-01264-0. Clin Epigenetics. 2022. PMID: 35361281 Free PMC article. -
The Slowest Shared Resonance: A Review of Electromagnetic Field Oscillations Between Central and Peripheral Nervous Systems.
Young A, Hunt T, Ericson M. Young A, et al. Front Hum Neurosci. 2022 Feb 16;15:796455. doi: 10.3389/fnhum.2021.796455. eCollection 2021. Front Hum Neurosci. 2022. PMID: 35250508 Free PMC article. Review.
-
Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder.
Domènech L, Willis J, Alemany-Navarro M, Morell M, Real E, Escaramís G, Bertolín S, Sánchez Chinchilla D, Balcells S, Segalàs C, Estivill X, Menchón JM, Gabaldón T, Alonso P, Rabionet R.
 Domènech L, et al. Sci Rep. 2022 Jan 27;12(1):1448. doi: 10.1038/s41598-022-05480-9. Sci Rep. 2022. PMID: 35087123 Free PMC article.
Domènech L, et al. Sci Rep. 2022 Jan 27;12(1):1448. doi: 10.1038/s41598-022-05480-9. Sci Rep. 2022. PMID: 35087123 Free PMC article. -
The Microbiota/Microbiome and the Gut-Brain Axis: How Much Do They Matter in Psychiatry?
Marazziti D, Buccianelli B, Palermo S, Parra E, Arone A, Beatino MF, Massa L, Carpita B, Barberi FM, Mucci F, Dell’Osso L. Marazziti D, et al. Life (Basel). 2021 Jul 28;11(8):760. doi: 10.3390/life11080760. Life (Basel). 2021. PMID: 34440503 Free PMC article. Review.
See all "Cited by" articles
MeSH terms
Substances
Full text links
Elsevier Science
Cite
Format: AMA APA MLA NLM
Send To
Impact of gut microbiota on brain function
The intestines are a kind of microbial "metropolis", inhabited by thousands of species of microorganisms.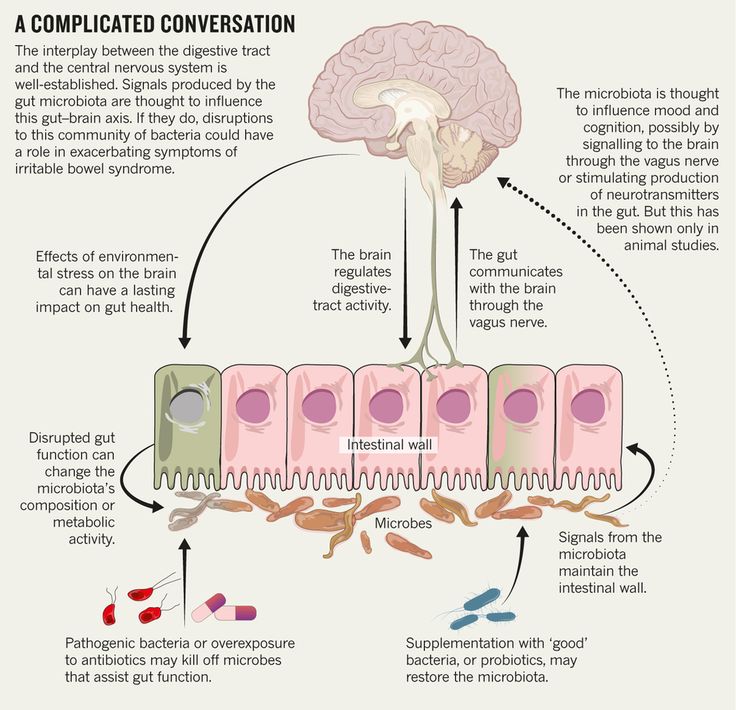 Their community has a huge impact on what is happening in the body, including the functioning of the brain.
Their community has a huge impact on what is happening in the body, including the functioning of the brain.
What interferes with the normal functioning of the “microbiota-gut-brain” axis and what consequences can its failure lead to?
Free webinars on anti-aging medicineLearn about the features of the International School of Anti-Age Expert, as well as opportunities to improve your medical practice day by day. Also in the webinar program are fascinating reviews of innovations in anti-aging medicine and analyzes of the most difficult clinical cases with recommendations that really work.
Learn moreBrain-gut connection
Scientists have long known that our gut and brain communicate with each other. And relatively recently it became known that a third party is involved in this “conversation” - the intestinal microbiota. This trio makes up the microbiota-gut-brain axis.
The intestine contains various groups of microorganisms. These are mainly bacteria, but also fungi and viruses. The community of microbes is called the microbiota. The gene set of these microbes is the microbiome.
These are mainly bacteria, but also fungi and viruses. The community of microbes is called the microbiota. The gene set of these microbes is the microbiome.
We start collecting our own “collection” of microbes during childbirth or even in the womb. After we are born, we “hoard” microbes from food and the environment.
The microbiota makes up a significant part of our body. There is approximately one microbe for every human cell. The vast majority of bacteria are found in the large intestine. But they also live on the skin, eyes and other areas of the body.
We and our gut microbiota can live in a mutually beneficial relationship. While in the colon, bacteria eat fiber and other digestive residues. In turn, they can keep our mind and body healthy. After all, the gut microbiota interacts with the brain and the intestinal (enteral) nervous system.
How exactly does the brain “talk” to the intestines?
Scientists have identified several different ways in which this communication works.
When microbes release neurotransmitters, they relay messages to the enteric nervous system, which travel to the brain through the bloodstream.
Another important communication pathway is through the vagus nerve. It starts at the base of the neck and extends to the abdomen, connecting with the intestines and other organs.
We technically have two vagus nerves - one on each side of the body. They carry messages from the gut to the brain and vice versa.
For example, messages from the brain to the gut via the vagus nerve control the release of stomach acid and digestive enzymes. They also help regulate inflammation levels, appetite, and the body's response to stress.
The messages carried by the vagus nerve depend largely on the microbiota. When microbes release neurotransmitters and short-chain fatty acids, they can activate the vagus nerve.
If the microbiota is diverse and includes more “good” microbes, there will be better communication between the gut and the brain. But when gut bacteria get out of balance, that relationship doesn't change for the better. And with a high probability, mental health problems, dysfunction of the brain and intestines can develop.
But when gut bacteria get out of balance, that relationship doesn't change for the better. And with a high probability, mental health problems, dysfunction of the brain and intestines can develop.
Importance of intestinal microflora
The intestinal nervous system, in fact, can be called the second brain - its role is so great.
There are 100-500 million neurons in the walls of the digestive tract that make up the intestinal nervous system. This is the largest group of neurons in your body. This network of nerves extends from the esophagus to the anus and produces over 30 different neurotransmitters (chemical messengers).
The gut microbiota influences the development of the enteric nervous system during childhood. Its well-coordinated work is very important, because it helps to control many functions of the small and large intestines.
Specifically, the enteric nervous system regulates:
-
Blood flow to the intestines;
-
Intestinal secretion of fluids, electrolytes and hormones;
-
Movement of intestinal contents;
-
The function of the intestinal barrier, which ensures the absorption of nutrients;
-
Immune activity in the intestine;
The enteric nervous system can do all this without the direct involvement of the brain.
Another constant source of information for the gut is the microbiota. It produces neurotransmitters such as serotonin and dopamine that provide communication between the gut and the brain.
Microbes in the gut also produce short-chain fatty acids such as propionate and butyrate. Some bacteria produce them when they eat dietary fiber in the gut. These fatty acids are involved in communication between the gut and the brain, and help control inflammation, for example.
Consequences of microbiota imbalance
An imbalance in the microbiota occurs when the number of harmful bacteria outweighs the number of beneficial ones, and this is already called dysbacteriosis.
In such a situation, inflammation and oxidative stress can increase significantly. As a result, chronic diseases can remind me of myself.
Here are some of the health problems that can occur when communication between the gut and the brain is disrupted.
-
Anxiety, depression and other mental health problems.

Experts used to look at mental health from the top down. They believed that the symptoms of anxiety, depression, and other mental health issues were caused by an imbalance in your brain. But this idea is evolving along with new research.
Scientists now believe that gut dysbiosis may be one of the main causes of imbalances in the brain. In other words, gut health can go hand in hand with mental health.
For example, when your microbiota is out of balance, it produces less butyric acid (butyrate). This short-chain fatty acid helps reduce inflammation. And if there is not enough butyrate, it can increase. And when nerves in the brain are inflamed, a person is at a higher risk of depression.
Insufficient amounts of butyric acid can also lead to increased intestinal permeability, also known as leaky gut syndrome. It is also responsible for increased inflammation and a higher risk of mental health problems.
For example, intestinal dysbiosis is common in people with autism.
 In one study, 43% of children with autism had leaky gut. And children without autism didn't have what's called a leaky gut.
In one study, 43% of children with autism had leaky gut. And children without autism didn't have what's called a leaky gut. -
Neurodegenerative diseases. Gut dysbiosis can impair memory and other brain functions. This is a factor that increases the risk of Alzheimer's disease and other types of dementia. Gut dysbiosis is also associated with cognitive problems in Parkinson's disease.
According to animal studies, certain gut microbes are associated with improved memory and brain function. But an excess of other gut microbes has been linked to brain inflammation, oxidative stress, and plaque formation on the brain.
Some research shows that "bad" gut microbes cause disordered proteins to accumulate in the brain, forming plaque. This can interfere with the normal functioning of your brain cells.
This accumulation of proteins can contribute to the development of Parkinson's and Alzheimer's diseases. And the symptoms of the disease worsen depending on the growth of these protein "lumps".

Disruption of the microbiota-gut-brain axis is also associated with insomnia. And poor sleep increases the risk of emotional problems, including anxiety and depression.
Animal studies show that restoring certain microbes to the gut microbiota can help fight anxiety and depression. However, more research is needed.
Anti-Age Medicine
Learn the intricacies of anti-aging medicine from anywhere in the world. For the convenience of doctors, we have created an online training platform Anti-Age Expert: Lectures from our educational programs are consistently laid out here, to which access is open 24/7. Doctors can study the materials as many times as necessary, ask questions and discuss interesting clinical cases with colleagues in special chats
Learn moreWhat breaks the connection between the microbiota and the brain
The microbiota-gut-brain axis can be compromised by many factors. Let's highlight the main ones:
Let's highlight the main ones:
-
Heavy metals. Toxic heavy metals can disrupt cellular processes and damage mitochondria. Some of these are found in air pollutants, water and amalgam fillings.
-
Glyphosate and other pesticides. Glyphosate, commonly sold under the name Roundup, is a pesticide commonly used to control weeds in crops. Laboratory studies show that glyphosate can kill beneficial microbes in the gut. Another pesticide, chlorpyrifos, has the same effect.
-
Parasites. Parasitic infections are very common and can disrupt the microbiota-gut-brain axis. Animal and human studies show that protozoan parasites and helminths can cause long-term changes in the gut microbiota. This can contribute to problems such as intestinal inflammation and increased permeability.
-
Viruses. They are able to interact with our genes, including those involved in inflammatory bowel disease.
 Animal studies show that certain viruses promote the expression of genes associated with Crohn's disease and ulcerative colitis. These are the two main types of inflammatory bowel disease.
Animal studies show that certain viruses promote the expression of genes associated with Crohn's disease and ulcerative colitis. These are the two main types of inflammatory bowel disease. In addition, the inflammatory products of viruses can contribute to the development of brain diseases such as Alzheimer's disease. Scientists have found viruses in areas of the brain affected by dementia or memory loss. For example, the herpes simplex virus has been linked to Alzheimer's disease.
-
A diet low in fiber and high in sugar.
The good bacteria eat the fiber in the colon. And if we don't "feed" them, the good bacteria won't be able to make protective short-chain fatty acids like butyrate. As a result, we become more vulnerable to dysbiosis and increased intestinal permeability.
A diet high in sugar can also lead to dysbiosis and increased intestinal permeability. However, artificial sweeteners (sucralose, aspartame, and saccharin) can also increase gut inflammation.
-
Antibiotics and other drugs. Antibiotics are prescribed to kill germs that cause infections. But drugs can destroy most of the beneficial bacteria. This allows harmful microbes, including Candida albicans and Clostridium difficile, to multiply rapidly.
The more antibiotics you take, the stronger the harmful microbes. They can become drug resistant. Meanwhile, the good microbes are getting weaker. This further increases the microbial imbalance in the gut.
In addition to antibiotics, the following medications harm the gut microbiota:
-
non-steroidal anti-inflammatory drugs;
-
proton pump inhibitors for acid reflux;
-
Some psychiatric medications, such as those for bipolar disorder.
-
-
Chemicals. Food and environment contain trace amounts of chemicals, such as those from plastics and flame retardants.
 They accumulate in foods. And some harmful chemicals are produced when meat is cooked. The gut microbiota is exposed to these toxins during meals.
They accumulate in foods. And some harmful chemicals are produced when meat is cooked. The gut microbiota is exposed to these toxins during meals.
Our gut bacteria can break down some of these chemicals. For example, one laboratory study showed that beneficial gut microbes are able to convert harmful chemicals from fried meat into less toxic compounds.
But the impact of the gut microbiota on toxic chemicals is not always in our favor.
In some cases, bacteria produce more harmful toxins when they break down chemicals. This can trigger the release of inflammatory molecules that can damage the intestinal wall.
-
Stress. This includes factors such as fear, lack of sleep, passing exams, strenuous exercise, extreme temperatures and excessive noise. Stress can disrupt the composition and activity of the gut microbiota. Gut microbes can recognize and respond to your hormones and neurotransmitters. In particular, those involved in your body's response to stress.
Both mental and physical stress can disrupt the microbiota. For example, scientists have found that stress causes a decrease in the number of beneficial lactobacilli, bacteria that help protect the intestinal wall.
In addition, when we are under stress, the intestines, brain, and vagus nerve signal the digestive tract to decrease its activity. Slower digestion reduces the absorption of nutrients.
When the connection between the intestines and the brain is disrupted, this affects not only the work of our “command center”, but also the functioning of many organs and systems.
Brief conclusions
-
The gut, gut microbiota, and brain communicate with each other, representing the microbiota-gut-brain axis.
-
There are several different ways in which this communication works.
-
The intestinal nervous system, due to its importance, can be called the second brain.
-
Microbiota imbalances may be one of the key factors that undermine mental health.
-
The normal functioning of the microbiota-gut-brain axis is hindered by many factors from heavy metals to viruses.
References
-
Wong CGT, Bottiglieri T, Snead OC. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54 Suppl 6:S3–12.
-
D'Argenio V, Sarnataro D. Microbiome Influence in the Pathogenesis of Prion and Alzheimer's Diseases. Int JMol Sci. 2019 Sep 23;20(19).
-
Kundu P, Blacher E, Elinav E, Pettersson S. Our Gut Microbiome: The Evolving Inner Self. cell. 2017 Dec 14;171(7):1481–93.
symptoms, diet, treatment in Sochi, clinic "ARMED"
The mass of the microflora of the gastrointestinal tract is 2.5 kg. The normal intestinal microflora performs a number of functions in the human body. It is antagonistic in relation to pathogenic and conditionally pathogenic, which prevents the development of acute intestinal infection, synthesizes vitamins, and activates the immune system.
It is antagonistic in relation to pathogenic and conditionally pathogenic, which prevents the development of acute intestinal infection, synthesizes vitamins, and activates the immune system.
The term "gut dysbiosis" includes:
- change in the quantitative and qualitative composition of the microflora in the small and large intestine;
- the appearance of conditionally pathogenic strains that are not part of the resident microflora: Proteus, morganella, Klebsiella, Enterobacter, E. coli (with enzymatic deficiency or hemolytic properties).
It should be noted that dysbacteriosis (synonyms for excessive bacterial growth in the intestine, dysbiosis) is not an independent disease and occurs in patients against the background of various conditions and diseases, which should be taken into account in the process of developing treatment tactics.
According to statistics, dysbacteriosis most often affects young children or women.
Causes of occurrence
- Nutrition: imbalance, diet change, water features.
- Iatrogenic: taking antibiotics and other drugs, vaccination.
- Occupational hazards: chemical production, night schedule.
- Environment: pollution, climate change, radiation.
- Genetics: heredity, gastrointestinal pathology, NOD2 mutations.
- Lifestyle: stress, jet lag.
- Body weight: obesity, underweight.
- Diseases: infectious, nervous, endocrine, immune.
A huge number of able-bodied active population admits violations in the diet (in the modern rhythm of life, a person sometimes replaces a full breakfast, which should take place in a leisurely atmosphere, with snacks on the run), experiences frequent and chronic stress, and takes medications.
Incorrectly planned nutrition, as a rule, is combined with nervous stress during the working day and adverse factors: traffic jams on the way to work, queues, stress.
As a result, one of these factors, or their combination, becomes the cause of many diseases of the gastrointestinal tract, including dysbacteriosis.
Clinical manifestations of dysbacteriosis include local (intestinal) symptoms, as well as systemic disorders caused by the translocation of the intestinal microflora into the internal environment of the body (lymph nodes-mesadenitis, urinary system-pyelonephritis, liver-steatosis, cirrhosis, cholestasis).
Symptoms of intestinal dysbiosis
- Loose stools or constipation. With dysbacteriosis, the intestinal walls absorb fluid. There is a deficiency of trace elements;
- Bloating and pain in the abdomen due to strong gas;
- The appearance of unpleasant sensations such as colic, nausea, heartburn, pain;
- Halitosis, allergies and sweating;
- In a severe form of dysbacteriosis, anemia, insomnia, shortness of breath, pale skin, and hair loss occur.
Dysbacteriosis is divided into 4 degrees:
- In the first degree there are minor changes in the intestines, which are manifested by a slight disorder and over time it goes away on its own;
- Due to the small amount of enzymes for food processing, diarrhea with a green tint with a sour smell, as well as additional symptoms such as nausea and rumbling in the stomach;
- High fever, apathy and weakness appear, undigested food particles appear in the feces;
- Due to the large number of pathogenic microorganisms, an intestinal infection occurs in the form of salmonella or dysentery; this causes beriberi, lack of appetite.
Diagnosis
This disease can only be definitively diagnosed by bacteriological culture or biochemical analysis. In the first case, feces are sown on a nutrient medium and within a week, specialists receive the result of the ratio of bacteria. In the second case, the amount of fatty acids is revealed, as well as the department in which the violation occurred and its stage.
One of the directions in the diagnosis of dysbacteriosis is the study of the content of various metabolites in the exhaled air, which are produced with the participation of intestinal bacteria. In addition, chemical methods are currently being introduced into practice, which make it possible to determine the types of bacteria and fungi in different media using gas chromatography.
ARMED Medical Center has its own certified laboratory. To detect dysbacteriosis, the patient will need to pass a series of tests, a list of which will be given by your doctor.
Treatment of dysbacteriosis
Therapy of patients with dysbacteriosis includes treatment of the underlying disease (against which dysbacteriosis developed) and restoration of the normal composition of intestinal bacteria.
In general, the treatment consists of:
- getting rid of the increased bacterial growth in the gastrointestinal tract, as a rule, these are antibacterial drugs for 10-14 days;
- restoration of normal and beneficial microflora in the gastrointestinal tract: probiotics, prebiotics, synbiotics, metabiotics;
- improve absorption of beneficial bacteria and intestinal digestion;
- stimulating the body to resist external infections.
A diet is prescribed taking into account the type of dyspepsia, motor disorders and the underlying disease.
In order for the treatment to be more effective, it is necessary to follow the rules: eat in small portions, but every 3 hours, exclude white bread, salty and fatty foods. Drinks should be consumed after meals after half an hour.
Thus, the problem of intestinal dysbacteriosis remains relevant. The phenomenon of dysbacteriosis should be considered as a secondary condition that requires clarification of the causes of its development.