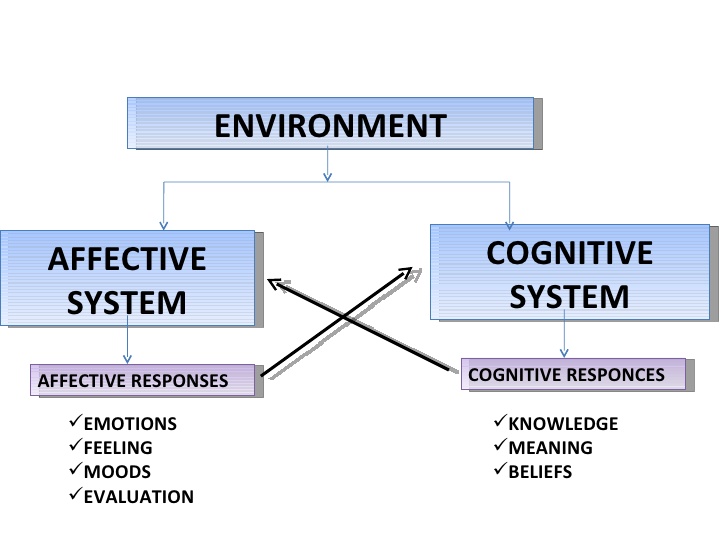
Dark chocolate and serotonin levels
The neuroprotective effects of cocoa flavanol and its influence on cognitive performance
1. Gu LW, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL USDA ARS. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–617. [PubMed] [Google Scholar]
2. Whiting D. Natural phenolic compounds 1900‐2000: a bird's eye view of a centuries chemistry. Nat Prod Rep. 2001;18:583–606. [PubMed] [Google Scholar]
3. Clapperton J, Hammerstone JF, Romanczyk R, Yow S, Chau J, Lin D, Lookwood R. 1992. pp. 112–115. Genetic Variation in Cocoa Flavour. In 16th International Conference Groupe Polyphenols;
4. Clapperton J. Contribution of genotype to cocoa (Theobroma cacao L.) Tropic Agric (Trinidad) 1994;71:303–308. [Google Scholar]
5. Rusconi M, Conti A. Theobroma cacao L., the food of the gods: a scientific approach beyond myths and claims. Pharmacol Res. 2010;61:5–13. [PubMed] [Google Scholar]
6. Kim H, Keeney PG. (−)‐Epicatechin content in fermented and unfermented cocoa beans. J Food Sci. 1984;49:1090–1092. [Google Scholar]
7. Lorist MM, Tops M. Caffeine, fatigue, and cognition. Brain Cogn. 2003;53:82–94. [PubMed] [Google Scholar]
8. Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20:S85–94. [PubMed] [Google Scholar]
9. Costa J, Lunet N, Santos C, Santos J, Vaz‐Carneiro A. Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta‐analysis of observational studies. J Alzheimers Dis. 2010;20:S221–238. [PubMed] [Google Scholar]
10. Santos C, Costa J, Santos J, Vaz‐Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta‐analysis. J Alzheimers Dis. 2010;20:S187–204. [PubMed] [Google Scholar]
11. Smit HJ. Theobromine and the pharmacology of cocoa. Handb Exp Pharmacol. 2011;200:201–234. [PubMed] [Google Scholar]
12. McCarty MF. Toward prevention of Alzheimers disease–potential nutraceutical strategies for suppressing the production of amyloid beta peptides. Med Hypotheses. 2006;67:682–697. [PubMed] [Google Scholar]
Med Hypotheses. 2006;67:682–697. [PubMed] [Google Scholar]
13. Patel AK, Rogers JT, Huang X. Flavanols, mild cognitive impairment, and Alzheimer's dementia. Int J Clin Exp Med. 2008;1:181–191. [PMC free article] [PubMed] [Google Scholar]
14. Spencer JPE. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–1161. [PubMed] [Google Scholar]
15. Vauzour D, Vafeiadou K, Rodriguez‐Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3:115–126. [PMC free article] [PubMed] [Google Scholar]
16. Cooper KA, Donovan JL, Waterhouse AL, Williamson G. Cocoa and health: a decade of research. Br J Nutr. 2008;99:1–11. [PubMed] [Google Scholar]
17. Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, Sies H. Sustained increase in flow‐mediated dilation after daily intake of high‐flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol. 2007;49:74–80. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
18. Faria A, Pestana D, Teixeira D, Couraud PO, Romero I, de Weksler B, Freitas V, Mateus N, Calhau C. Insights into the putative catechin and epicatechin transport across blood‐brain barrier. Food Funct. 2011;2:39–44. [PubMed] [Google Scholar]
19. Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice‐Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. [PubMed] [Google Scholar]
20. van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant‐derived flavanol (‐)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. [PMC free article] [PubMed] [Google Scholar]
21. Ferruzzi MG, Lobo JK, Janle EM, Cooper B, Simon JE, Wu QL, Welch C, Ho L, Weaver C, Pasinetti GM. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer's disease. J Alzheimers Dis. 2009;18:113–124. [PMC free article] [PubMed] [Google Scholar]
J Alzheimers Dis. 2009;18:113–124. [PMC free article] [PubMed] [Google Scholar]
22. Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson's disease. Neuroreport. 2001;12:3871–3875. [PubMed] [Google Scholar]
23. Andres‐Lacueva C, Shukitt‐Hale B, Galli RL, Jauregui O, Lamuela‐Raventos RM, Joseph JA. Anthocyanins in aged blueberry‐fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. [PubMed] [Google Scholar]
24. Ghosh D, Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res. 2009;53:322–331. [PubMed] [Google Scholar]
25. Fisher ND, Hughes M, Gerhard‐Herman M, Hollenberg NK. Flavanol‐rich cocoa induces nitric‐oxide‐dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. [PubMed] [Google Scholar]
26. Hollenberg NK, Fisher ND, McCullough ML. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J Am Soc Hypertens. 2009;3:105–112. [PMC free article] [PubMed] [Google Scholar]
J Am Soc Hypertens. 2009;3:105–112. [PMC free article] [PubMed] [Google Scholar]
27. Fisher ND, Sorond FA, Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharmacol. 2006;47:S210–214. [PubMed] [Google Scholar]
28. Heiss C, Kleinbongard P, Dejam A, Perré S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol‐rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46:1276–1283. [PubMed] [Google Scholar]
29. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow‐dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. [PubMed] [Google Scholar]
30. Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan‐3‐ols. JAMA. 2003;290:1030–1031. [PubMed] [Google Scholar]
31. Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus‐Snyder ML. Flavonoid‐rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. [PubMed] [Google Scholar]
Flavonoid‐rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. [PubMed] [Google Scholar]
32. Schroeter HC, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik‐Uribe C, Schmitz HH, Kelm M. (‐)‐Epicatechin mediates beneficial effects of flavanol‐rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. [PMC free article] [PubMed] [Google Scholar]
33. Fisher ND, Sorond FA, Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharmacol. 2006;47:S210–S214. [PubMed] [Google Scholar]
34. Francis ST, Head K, Morris PG, Macdonald IA. The effect of flavanol‐rich cocoa on the fMRI response to a cognitive task in healthy young people. J Cardiovasc Pharmacol. 2006;47:S215–220. [PubMed] [Google Scholar]
35. Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–26. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
36. Sorond FA, Lipsitz LA, Hollenberg NK, Fisher ND. Cerebral blood flow response to flavanol‐rich cocoa in healthy elderly humans. Neuropsychiatr Dis Treat. 2008;4:433–440. [PMC free article] [PubMed] [Google Scholar]
37. Sorond FA, Hollenberg NK, Panych LP, Fisher ND. Brain blood flow and velocity: correlations between magnetic resonance imaging and transcranial Doppler sonography. J Ultrasound Med. 2010;29:1017–1022. [PMC free article] [PubMed] [Google Scholar]
38. Ancelin ML, Christen Y, Ritchie K. Is antioxidant therapy a viable alternative for mild cognitive impairment? Examination of the evidence. Dement Geriatr Cogn Disord. 2007;24:1–19. [PubMed] [Google Scholar]
39. Joseph J, Cole G, Head E, Ingram D. Nutrition, brain aging, and neurodegeneration. J Neurosci. 2009;29:12795–12801. [PMC free article] [PubMed] [Google Scholar]
40. Macready AL, Kennedy OB, Ellis JA, Williams CM, Spencer JP, Butler LT. Flavonoids and cognitive function: a review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009;4:227–242. [PMC free article] [PubMed] [Google Scholar]
Genes Nutr. 2009;4:227–242. [PMC free article] [PubMed] [Google Scholar]
41. Field DT, Williams CM, Butler LT. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol Behav. 2011;103:255–260. [PubMed] [Google Scholar]
42. Huber KK, Adams H, Remky A, Arend KO. Retrobulbar haemodynamics and contrast sensitivity improvements after CO2 breathing. Acta Ophthalmol Scand. 2006;84:481–487. [PubMed] [Google Scholar]
43. Kalt W, Blumberg JB, McDonald JE, Vinqvist‐Tymchuk MR, Fillmore SA, Graf BA, O'Leary JM, Milbury PE. Identification of anthocyanins in the liver, eye, and brain of blueberry‐fed pigs. J Agric Food Chem. 2008;56:705–712. [PubMed] [Google Scholar]
44. Kalt W, Hanneken A, Milbury P, Tremblay F. Recent research on polyphenolics in vision and eye health. J Agric Food Chem. 2010;58:4001–4007. [PubMed] [Google Scholar]
45. Scholey AB, French SJ, Morris PJ, Kennedy DO, Milne AL, Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort.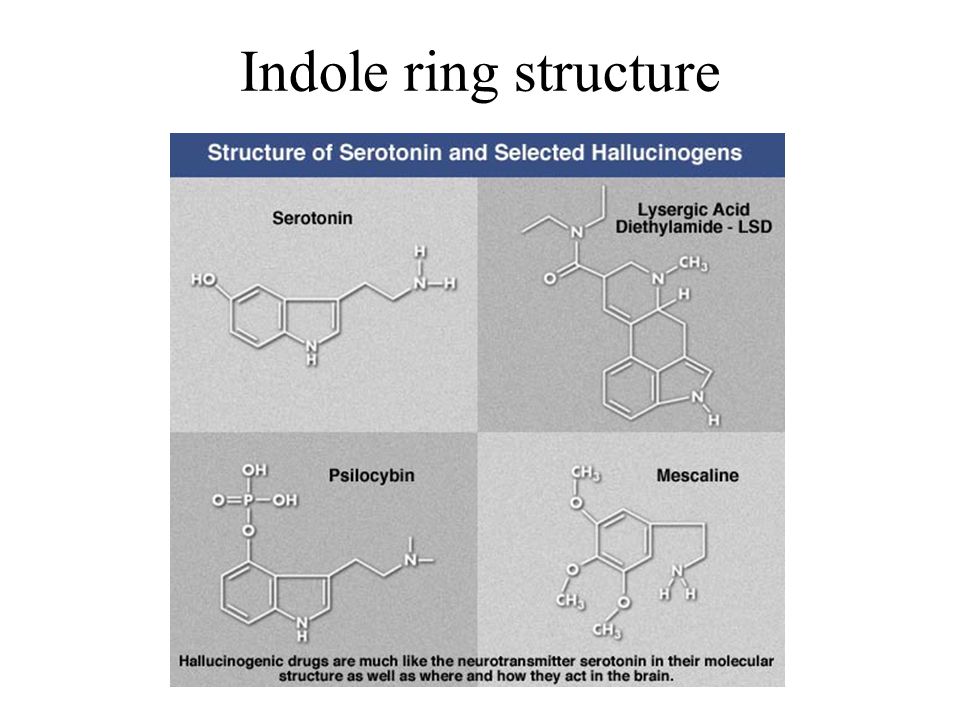 J Psychopharmacol. 2010;24:1505–1514. [PubMed] [Google Scholar]
J Psychopharmacol. 2010;24:1505–1514. [PubMed] [Google Scholar]
46. Ruitenberg A, den Heijer T, van Bakker SL, Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. [PubMed] [Google Scholar]
47. Camfield DA, Scholey A, Pipingas A, Silberstein R, Kras M, Nolidin K, Wesnes K, Pase M, Stough C. Steady state visually evoked potential (SSVEP) topography changes associated with cocoa flavanol consumption. Physiol Behav. 2012;105:948–957. [PubMed] [Google Scholar]
48. Crews WD, Jr, Harrison DW, Wright JW. A double‐blind, placebo‐controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am J Clin Nutr. 2008;87:872–880. [PubMed] [Google Scholar]
49. Spencer JPE. Flavonoids: modulators of brain function? Br J Nutr. 2008;99(E Suppl. 1):ES60–77. [PubMed] [Google Scholar]
2008;99(E Suppl. 1):ES60–77. [PubMed] [Google Scholar]
50. Spencer JPE. Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro‐cognitive performance. Proc Nutr Soc. 2008;67:238–252. [PubMed] [Google Scholar]
51. Spencer JPE. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–273. [PMC free article] [PubMed] [Google Scholar]
52. Yamada T, Yamada Y, Okano Y, Terashima T, Yokogoshi H. Anxiolytic effects of short‐ and long‐term administration of cacao mass on rat elevated T‐maze test. J Nutr Biochem. 2009;20:948–955. [PubMed] [Google Scholar]
53. Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. [PubMed] [Google Scholar]
54. Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52:35–45. [PubMed] [Google Scholar]
55. Crowley TJ, Hoehn MM, Rutledge CO, Stallings MA, Heaton RK, Sundell S, Stilson D. Dopamine excretion and vulnerability to drug‐induced Parkinsonism in schizophrenic patients. Arch Gen Psychiatry. 1978;35:97–104. [PubMed] [Google Scholar]
Dopamine excretion and vulnerability to drug‐induced Parkinsonism in schizophrenic patients. Arch Gen Psychiatry. 1978;35:97–104. [PubMed] [Google Scholar]
56. Hoehn MM, Crowley TJ, Rutledge CO. The Parkinsonian syndrome and its dopamine correlates. Adv Exp Med Biol. 1977;90:243–254. [PubMed] [Google Scholar]
57. Bisson JF, Nejdi A, Rozan P, Hidalgo S, Lalonde R, Messaoudi M. Effects of long‐term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats. Br J Nutr. 2008;100:94–101. [PubMed] [Google Scholar]
58. Rozan P, Hidalgo S, Nejdi A, Bisson JF, Lalonde R, Messaoudi M. Preventive antioxidant effects of cocoa polyphenolic extract on free radical production and cognitive performances after heat exposure in Wistar rats. J Food Sci. 2007;72:S203–206. [PubMed] [Google Scholar]
59. Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145:33–41. [PubMed] [Google Scholar]
1997;145:33–41. [PubMed] [Google Scholar]
60. Letenneur L, Proust‐Lima C, Le Gouge A, Dartigues JF, Barberger‐Gateau P. Flavonoid intake and cognitive decline over a 10‐year period. Am J Epidemiol. 2007;165:1364–1371. [PubMed] [Google Scholar]
61. Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Intake of flavonoid‐rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009;139:120–127. [PubMed] [Google Scholar]
62. Pak T, Cadet P, Mantione KJ, Stefano GB. Morphine via nitric oxide modulates beta‐amyloid metabolism: a novel protective mechanism for Alzheimer's disease. Med Sci Monit. 2005;11:BR357–366. [PubMed] [Google Scholar]
63. Luchsinger J, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol. 2004;3:579–587. [PubMed] [Google Scholar]
64. Luchsinger JA, Tang M, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–208. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
65. Nagahama Y, Nabatame H, Okina T, Yamauchi H, Narita M, Fujimoto N, Murakami M, Fukuyama H, Matsuda M. Cerebral correlates of the progression rate of the cognitive decline in probable Alzheimer's disease. Eur Neurol. 2003;50:1–9. [PubMed] [Google Scholar]
66. Commenges D, Scotet V, Renaud S, Jacqmin‐Gadda H, Barberger‐Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–363. [PubMed] [Google Scholar]
67. Valente T, Hidalgo J, Bolea I, Ramirez B, Anglés N, Reguant J, Morelló JR, Gutiérrez C, Boada M, Unzeta M. A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain. J Alzheimers Dis. 2009;18:849–865. [PubMed] [Google Scholar]
68. Fernández‐Fernández L, Comes G, Bolea I, Valente T, Ruiz J, Murtra P, Ramirez B, Anglés N, Reguant J, Morelló JR, Boada M, Hidalgo J, Escorihuela RM, Unzeta M. LMN diet, rich in polyphenols and polyunsaturated fatty acids, improves mouse cognitive decline associated with aging and Alzheimer's disease. Behav Brain Res. 2012;228:261–271. [PubMed] [Google Scholar]
Behav Brain Res. 2012;228:261–271. [PubMed] [Google Scholar]
69. Buitrago‐Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, Franco OH. Chocolate consumption and cardiometabolic disorders: systematic review and meta‐analysis. BMJ. 2011;343:d4488. [PMC free article] [PubMed] [Google Scholar]
70. Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31:1616–1623. [PubMed] [Google Scholar]
71. Rautiainen S, Larsson S, Virtamo J, Wolk A. Total antioxidant capacity of diet and risk of stroke: a population‐based prospective cohort of women. Stroke. 2012;43:335–340. [PubMed] [Google Scholar]
72. Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Doré S. The flavanol (‐)‐epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. [PMC free article] [PubMed] [Google Scholar]
73. Villarreal‐Calderon R, Torres‐Jardón R, Palacios‐Moreno J, Osnaya N, Pérez‐Guillé B, Maronpot RR, Reed W, Zhu H, Calderón‐Garcidueñas L. Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int J Toxicol. 2010;29:604–615. [PubMed] [Google Scholar]
Villarreal‐Calderon R, Torres‐Jardón R, Palacios‐Moreno J, Osnaya N, Pérez‐Guillé B, Maronpot RR, Reed W, Zhu H, Calderón‐Garcidueñas L. Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int J Toxicol. 2010;29:604–615. [PubMed] [Google Scholar]
74. Williams RJ, Spencer JP, Rice‐Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. [PubMed] [Google Scholar]
75. Rendeiro C, Spencer JP, Vauzour D, Butler LT, Ellis JA, Williams CM. The impact of flavonoids on spatial memory in rodents: from behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009;4:251–270. [PMC free article] [PubMed] [Google Scholar]
76. Noé V, Peñuelas S, Lamuela‐Raventós RM, Permanyer J, Ciudad CJ, Izquierdo‐Pulido M. Epicatechin and a cocoa polyphenolic extract modulate gene expression in human Caco‐2 cells. J Nutr. 2004;134:2509–2516. [PubMed] [Google Scholar]
77. Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases.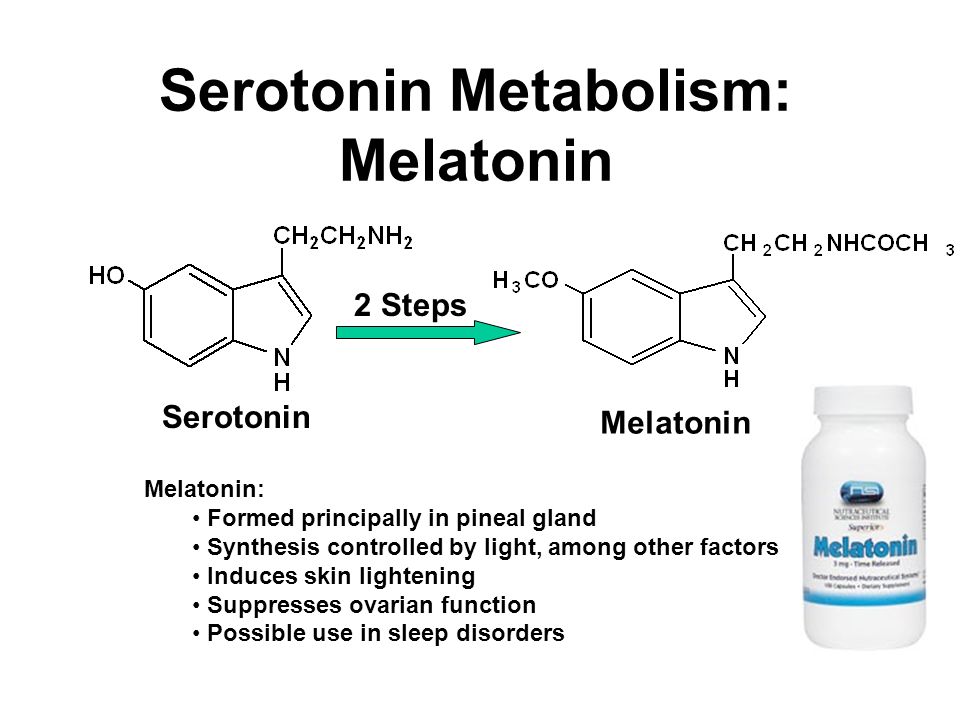 Free Radic Biol Med. 2004;37:304–317. [PubMed] [Google Scholar]
Free Radic Biol Med. 2004;37:304–317. [PubMed] [Google Scholar]
78. Jellinger KA. Cell death mechanisms in neurodegeneration. J Cell Mol Med. 2001;5:1–17. [PMC free article] [PubMed] [Google Scholar]
79. Spires TL, Hannan AJ. Nature, nurture and neurology: gene‐environment interactions in neurodegenerative disease. FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005;272:2347–2361. [PubMed] [Google Scholar]
80. Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat Disord. 2005;11:S9–15. [PubMed] [Google Scholar]
81. McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. [PubMed] [Google Scholar]
82. Zheng Z, Lee JE, Yenari MA. Stroke: molecular mechanisms and potential targets for treatment. Curr Mol Med. 2003;3:361–372. [PubMed] [Google Scholar]
83. Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243–250. [PMC free article] [PubMed] [Google Scholar]
Genes Nutr. 2009;4:243–250. [PMC free article] [PubMed] [Google Scholar]
84. Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. [PubMed] [Google Scholar]
85. McCarty MF. Vascular nitric oxide may lessen Alzheimer's risk. Med Hypotheses. 1998;51:465–476. [PubMed] [Google Scholar]
86. Parker G, Roy K, Mitchell P, Wilhelm K, Malhi G, Hadzi‐Pavlovic D. Atypical depression: a reappraisal. Am J Psychiatry. 2002;159:1470–1479. [PubMed] [Google Scholar]
87. di Tomaso E, Beltramo M, Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382:677–678. [PubMed] [Google Scholar]
88. Tytgat J, Van Boven M, Daenens P. Cannabinoid mimics in chocolate utilized as an argument in court. Int J Legal Med. 2000;113:137–139. [PubMed] [Google Scholar]
89. Messaoudi M, Bisson JF, Nejdi A, Rozan P, Javelot H. Antidepressant‐like effects of a cocoa polyphenolic extract in Wistar‐Unilever rats. Nutr Neurosci. 2008;11:269–276. [PubMed] [Google Scholar]
Nutr Neurosci. 2008;11:269–276. [PubMed] [Google Scholar]
90. Benton D, Donohoe RT. The effects of nutrients on mood. Public Health Nutr. 1999;2:403–409. [PubMed] [Google Scholar]
91. Reid LD. Endogenous opioid peptides and regulation of drinking and feeding. Am J Clin Nutr. 1985;42(5 Suppl):1099–1132. [PubMed] [Google Scholar]
92. Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS. Naloxone's anorectic effect is dependent upon the relative palatability of food. Pharmacol Biochem Behav. 1993;46:917–921. [PubMed] [Google Scholar]
93. Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87:173–177. [PubMed] [Google Scholar]
94. Si EC, Bryant HU, Yim GK. Opioid and non‐opioid components of insulin‐induced feeding. Pharmacol Biochem Behav. 1986;24:899–903. [PubMed] [Google Scholar]
95. Ottley C. Food and mood. Nurs Stand. 2000;15:46–52. [PubMed] [Google Scholar]
96. Parker G, Parker I, Brotchie H. Mood state effects of chocolate. J Affect Disord. 2006;92:149–159. [PubMed] [Google Scholar]
Parker G, Parker I, Brotchie H. Mood state effects of chocolate. J Affect Disord. 2006;92:149–159. [PubMed] [Google Scholar]
97. Fullerton DT, Getto CJ, Swift WJ, Carlson IH. Sugar, opioids and binge eating. Brain Res Bull. 1985;14:673–680. [PubMed] [Google Scholar]
98. Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. ‘Depression’ increases ‘craving’ for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl) 1998;136:272–283. [PubMed] [Google Scholar]
99. Hetherington MM, MacDiarmid JI. ‘Chocolate addiction’: a preliminary study of its description and its relationship to problem eating. Appetite. 1993;21:233–246. [PubMed] [Google Scholar]
100. Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991;17:199–212. [PubMed] [Google Scholar]
101. Smeets PA, de Graaf C, van Stafleu A, Osch MJ, van der Nievelstein RA, Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–1305. [PubMed] [Google Scholar]
Am J Clin Nutr. 2006;83:1297–1305. [PubMed] [Google Scholar]
102. Michener W, Rozin P. Pharmacological versus sensory factors in the satiation of chocolate craving. Physiol Behav. 1994;56:419–422. [PubMed] [Google Scholar]
103. Small DM, Zatorre RJ, Dagher A, Evans AC, Jones‐Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. [PubMed] [Google Scholar]
104. Smits M, van Peeters RR, Hecke P, Sunaert S. A 3 T event‐related functional magnetic resonance imaging (fMRI) study of primary and secondary gustatory cortex localization using natural tastants. Neuroradiology. 2007;49:61–71. [PubMed] [Google Scholar]
105. Beaver JD, van Lawrence AD, Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. [PMC free article] [PubMed] [Google Scholar]
106. Martin GN. Human electroencephalographic (EEG) response to olfactory stimulation: two experiments using the aroma of food.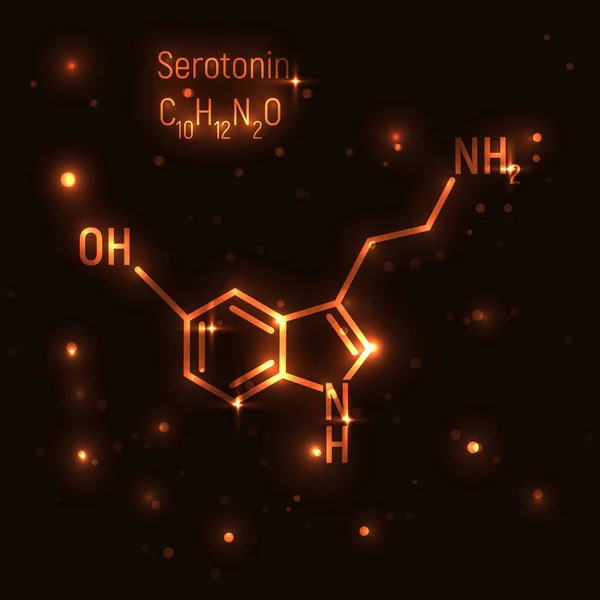 Int J Psychophysiol. 1998;30:287–302. [PubMed] [Google Scholar]
Int J Psychophysiol. 1998;30:287–302. [PubMed] [Google Scholar]
107. Rolls ET, McCabe C. Enhanced affective brain representations of chocolate in cravers vs. non‐cravers. Eur J Neurosci. 2007;26:1067–1076. [PubMed] [Google Scholar]
108. Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine‐ or chocolate‐associated contextual cues. Neuroscience. 2001;105:535–545. [PubMed] [Google Scholar]
109. Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev. 1999;23:563–576. [PubMed] [Google Scholar]
110. Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. [PubMed] [Google Scholar]
111. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779–2811. [PMC free article] [PubMed] [Google Scholar]
112. Golomb BA, Koperski S, White HL. Association between more frequent chocolate consumption and lower body mass index. Arch Intern Med. 2012;172:519–521. [PMC free article] [PubMed] [Google Scholar]
Association between more frequent chocolate consumption and lower body mass index. Arch Intern Med. 2012;172:519–521. [PMC free article] [PubMed] [Google Scholar]
113. Nogueira L, Ramirez‐Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. (‐)‐Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol. 2011;589:4615–4631. [PMC free article] [PubMed] [Google Scholar]
The Chia Co UK | Can dark chocolate help with anxiety?
Anxiety disorders are the most common mental disorders, affecting more than 8 million people in the UK. Anxiety can come in many different forms and can have a significant impact on day-to-day activities for those suffering from it, affecting concentration, sleep and the ability carry out ordinary tasks at work, home or school.
Those who suffer from severe anxiety will usually require professional medical help, and we would never intend to replace the advice of your doctor or psychologist*. But, for mild-to-moderate levels of anxiety, making changes to diet and lifestyle can make a real difference. Just like there are foods that harm or help our bodies, there are foods that harm or help our mental health.
But, for mild-to-moderate levels of anxiety, making changes to diet and lifestyle can make a real difference. Just like there are foods that harm or help our bodies, there are foods that harm or help our mental health.
Dark Chocolate:
The mood-boosting effects of chocolate have been touted for quite some time, but more recent research has shown a particular link between dark chocolate and anxiety. And here’s why:
- Polyphenols
A 2013 study in the Journal of Pharmacology showed that a special compound found in cocoa called polyphenols can positively affect anxiety and enhance calmness. - Serotonin
Low serotonin is one of the leading causes (and outcomes) of anxiety. Dark chocolate provides large amounts of Tryptophan, an amino acid that also works as a precursor to serotonin. So, it’s possible that by ingesting more dark chocolate, you’re improving your serotonin levels. - Mood Elevation
Another ingredient in chocolate is theobromine — an ingredient that studies have shown can have a positive, mood elevating effect on those that ingest it.
- Magnesium
Dark chocolate also contains high amounts of magnesium. Studies are starting to show that magnesium may be one of the few nutrients that has a noticeable effect on anxiety. People often take magnesium supplements, but getting your magnesium through food is considered a much healthier overall option.
Darker the better
It’s important to note that these benefits are only found in dark chocolate. Milk chocolate has almost no nutritional value, contains very little of those all-important nutrients mentioned above, and contains high levels of sugar that can actually lead to worse feelings of anxiety!
The closer you get to pure dark cocoa (preferably sugar-free) the more benefit you will see. We know that dark chocolate can be bitter, so try starting with a percentage of around 75% cocoa while your tastebuds adjust. We love Norwegian small-batch organic chocolate makers, Fjak — start with their 70% Mork Brasil, before moving up to the more intense award-winning Chocolat Madagascar 100%.
Other diet tips to improve anxiety:
- Drink more water. Many studies have found that dehydration affects up to 25% of those with persistent stress, and dehydration is known to cause more anxiety.
- Look for foods rich in magnesium, vitamin B12 (and other B vitamins), zinc, and antioxidants
- Avoid eating foods that contribute to anxiety, such as fried foods, high glycemic carbs, refined sugars, and alcoholic beverages.
Sweet life
In early August, we wrote that British scientists on a sample of 13,000 people showed a relationship between the consumption of dark chocolate (namely dark - with a cocoa content of more than 45 percent) and a decrease in symptoms of depression. According to the results of the work, the conditions characteristic of depression were 70 percent less common among those who ate dark chocolate, compared with those who did not eat it at all. Of course, this is only a correlation, and based on these data, it cannot be argued that chocolate can save a person from depression.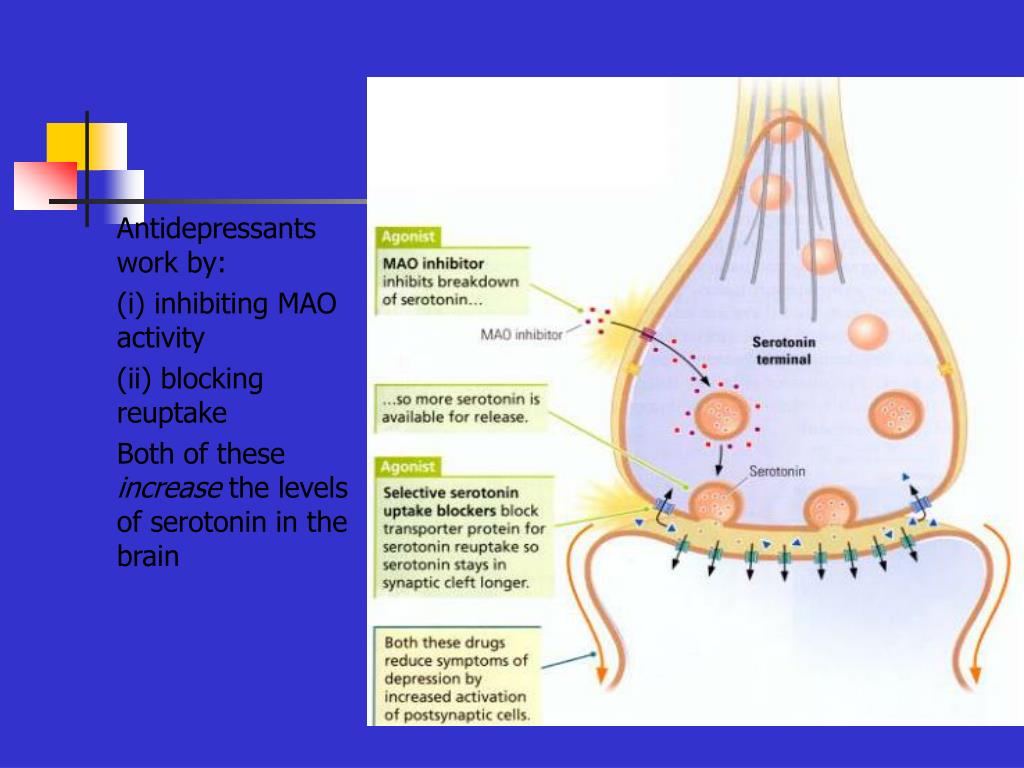 Edition N + 1 talks about the possible mechanisms that allow dark chocolate to influence our emotional state and finds out if it can be called an antidepressant.
Edition N + 1 talks about the possible mechanisms that allow dark chocolate to influence our emotional state and finds out if it can be called an antidepressant.
Chocolate is one of the most popular treats of people all over the world: thanks to the Mayans and Aztecs, who fell in love with the bitter aromatic drink made from cocoa beans, the conquistadors, who were also attracted by it, and especially thanks to the Dutch chemist Konrad van Guten, who in At the beginning of the 19th century, he patented a cheap method for extracting cocoa butter from beans.
I would like to say special thanks to those who added sugar and milk to the drink, which was probably quite unpleasant for the taste buds.
We have repeatedly written about the fact that chocolate is quite good for health: scientists have proven its benefits for the cardiovascular system, and for the brain and immunity.
The positive effect on the body is explained by the presence of natural compounds of flavonoids (or rather, flavonols) in cocoa butter and other derivative products of cocoa beans. They act on the human body primarily as an antioxidant, that is, they neutralize reactive oxygen species that can damage macromolecules such as proteins and DNA.
They act on the human body primarily as an antioxidant, that is, they neutralize reactive oxygen species that can damage macromolecules such as proteins and DNA.
The vast majority of studies, of course, do not conclude that chocolate is a panacea. What’s more, even studies in major peer-reviewed journals suggest that the benefits found from eating chocolate, both short-term and long-term—often statistically significant—remain rather small.
The same goes for mental health. Chocolate, with its sweetness and pleasant texture and aroma, can actually lift your mood, but it is not always clear how this works - whether we are dealing exclusively with a psychological effect, or some physiological mechanism is triggered here, triggered by those properties of chocolate, which are similar to the properties of antidepressants.
Both need to be dealt with separately, but first we need to explain why what we eat can be important for mental health at all.
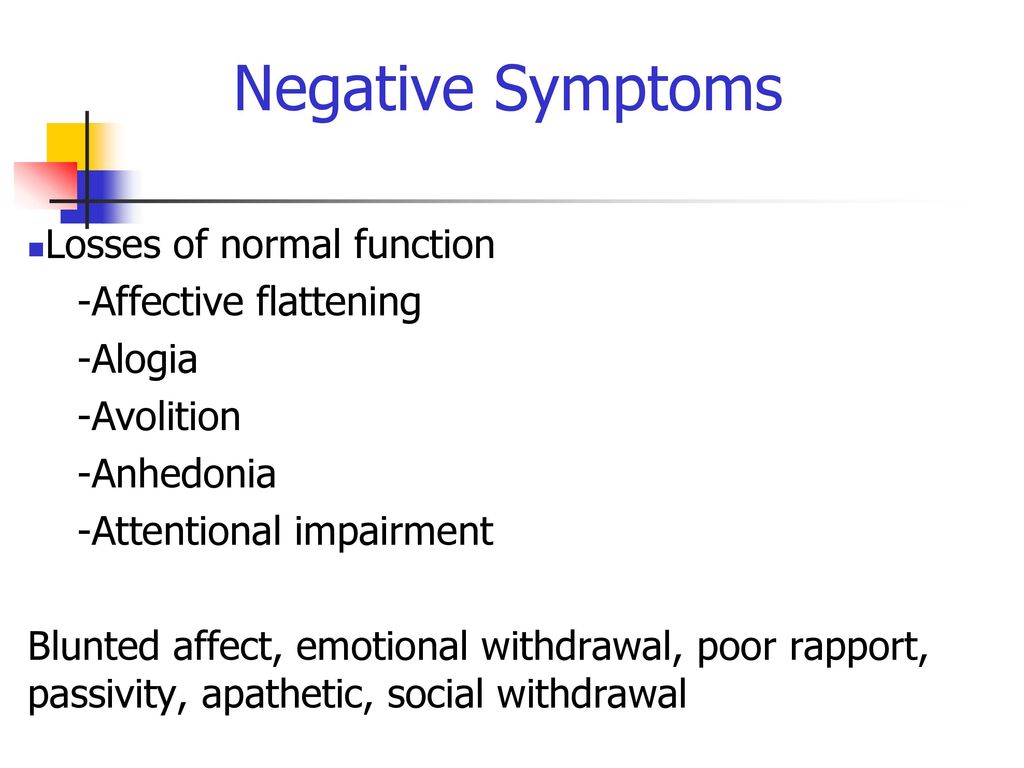
Serotonin from food
Depression is known to be endogenous: sometimes it does not require any external triggers, such as the loss of a job or the death of a loved one.
At a certain point, the brain ceases to synthesize and use the neurotransmitters important for it (serotonin, dopamine and noradrenaline) that are involved in the reward system, all together or only some of them. What used to work well and make you get up in the morning, enjoy meeting friends, feel the pleasure of your favorite food, breaks down.
It is difficult to predict this breakdown, especially considering that it can appear quite suddenly, therefore even those people who do not experience daily stresses that affect the state of the central nervous system are not protected from depression.
Depression, however, can be prevented by a correct - or relatively correct - lifestyle. So, sleep hygiene, physical activity, alcohol and, of course, nutrition affect the risk of developing depression.
Chocolate is one of the foods with a high content of tryptophan, one of the aromatic alpha-amino acids. True, 200 milligrams of tryptophan per 100 grams of chocolate is actually not the highest figure, for red or black caviar it is much higher (960 and 910 milligrams per 100 grams, respectively).
The proteinogenic enantiomer (optical isomer) of tryptophan, L-tryptophan, is part of the proteins of all living organisms, and its metabolite is serotonin, the same neurotransmitter, which, among other things, is very important for the brain reward system.
Synthesizes serotonin from tryptophan through another precursor step, 5-hydroxytryptophan. This substance, also known as 5-HTP, is sold as a dietary supplement that, according to some reports, is more effective than placebo in the treatment of depression, although there has not yet been enough high-quality medical research on this subject.
An impenetrable barrier
Unlike tryptophan or 5-HTP, which enter the body in its pure form (in the form of supplements) and can really increase the level of serotonin, with the same alpha-amino acid, which enters the body with food, everything is not so simple. The fact is that tryptophan, before being synthesized into a neurotransmitter, must overcome the blood-brain barrier.
Other amino acids must overcome the same barrier, and tryptophan is far from being the main one among them: the transport system that controls the movement across the blood-brain barrier prefers other amino acids that enter the body with proteins.
Therefore, simply adding foods rich in tryptophan to food will not raise the level of serotonin in the brain. Research shows that in order for the body to have enough tryptophan for further efficient synthesis of 5-HTP and serotonin, the amount of calories coming from protein should not exceed 2-4 percent of the total energy value of the food.
Chocolate does not belong to such products, since it contains about five percent protein: this is enough for tryptophan to give way to other amino acids, and serotonin is not synthesized from it.
The same can be said about dopamine. This neurotransmitter is synthesized from the alpha amino acid phenylalanine via tyrosine synthesis or from tyrosine itself.
Both amino acids are found in food products (including chocolate), but they can cross the blood-brain barrier only in their pure form (strictly speaking, dopamine is also present in pure form in food, for example, a banana peel, but it just does not pass the barrier between the circulatory system and the brain at all).
Studies on the synthesis of dopamine (as well as its metabolite norepinephrine) from food are rather limited, but it is known, for example, that tyrosine in its pure form in a sufficiently large dosage (100 milligrams per kilogram of body weight) does not affect a person's mood in any way.
That is why it is absolutely impossible to consider chocolate as a food source of serotonin and dopamine (therefore, do not believe it if they write about it somewhere). The good news, however, is that foods do not have to directly synthesize neurotransmitters in order to have an impact on how the human brain works.
Life in chocolate
Food is one of the most important incentives for the brain's reward system, and pleasant food (that is, of course, chocolate) is also a strong incentive. In studies of eating behavior or reinforcement learning, both in humans and laboratory animals, chocolate as a stimulus is considered a so-called “appetizing food” (palatable food), which can significantly increase the activity of dopaminergic neurons.
So, in an experiment on mice, it was shown that chocolate consumption increases the release of dopamine in the nucleus accumbens - the brain structure that receives signals from dopaminergic neurons and is an important part of the reward system.
The nucleus accumbens is sometimes called the "pleasure center": the activity of this zone increases when exposed to certain drugs, during sex. In addition, this area is also subject to the influence of social approval.
An experiment on young people showed that the activity of the nucleus accumbens increases when people like their own photos. By the way, it’s about the same as when eating chocolate: from this we can conclude that the lack of attention can be compensated for by chocolate - and the nucleus accumbens will not notice any difference.
The question arises: can't chocolate be considered a drug and isn't chocolate lovers threatened by "chocoholism"?
Some studies, for example, show that former heroin addicts in compulsory treatment or behind bars experience increased cravings for sugary foods. It is therefore possible that the physiological mechanisms that cause people to experience sugar cravings and experience withdrawal symptoms may be similar.
It is therefore possible that the physiological mechanisms that cause people to experience sugar cravings and experience withdrawal symptoms may be similar.
At the same time, the opioid system is really involved in obtaining pleasure from eating: in response to sweet food, opioid peptides are released in the body, which, in turn, increase the concentration of beta-endorphins in the hypothalamus.
Curiously, this effect is reversible with naltrexone, an opioid receptor antagonist prescribed, for example, after withdrawal of opiate painkillers.
However, this effect is not exclusively associated with chocolate. Rather, it occurs with the consumption of any high-carbohydrate food, even not necessarily sweet, so the work of the body's opioid system during the consumption of chocolate is not specifically explained by its unique properties.
Therefore, it is definitely impossible to consider chocolate as a drug, even in a simplified way, just as it is impossible to assert that its effect on the human body and consciousness is comparable, for example, with the effect of taking opiates. Otherwise, the production and sale of chocolate would be regulated by law. It's good that it isn't.
Otherwise, the production and sale of chocolate would be regulated by law. It's good that it isn't.
Hedonists approve
Of course, the "appetizing" of any sweet food, including chocolate, depends not only on the sweet taste as such - any person would prefer to eat a piece of chocolate cake, rather than a couple of tablespoons of sugar. Moreover, with chocolate, because of its physical properties and the aromatic substances contained in it, the situation is special.
The melting point of chocolate is about 30-38 degrees Celsius, which allows it to remain solid at room temperature and melt in your mouth. The melting process is almost the main thing in the consumption of chocolate.
At this stage, alkaloids, which are part of the product, are released in large quantities, which are responsible for the bitter taste and aroma familiar to cocoa, and the resulting liquidish, slightly viscous and moderately fatty consistency gives additional orosensory stimulation.
Thanks to the combination of these factors, chocolate achieves a high hedonic rating.
By the way, this concerns more milk chocolate than dark chocolate, since milk chocolate has a little more fat and carbohydrates - namely, the combination of these nutrients in the product makes it extremely attractive.
In general, it can be concluded that chocolate, despite the fact that it can improve mood, should not be considered an antidepressant. With the consumption of chocolate, any effect similar to taking real drugs is rather short-lived and certainly not as deep.
Therefore, it is not worth relying on it if you start to feel depressed, and if you have any cause for concern, you should consult a doctor.
And be sure to remember that, despite its wonderful health benefits, pleasant texture and flavor, chocolate is just chocolate.
Elizaveta Ivtushok
How chocolate cakes help us stock up on the hormone of happiness
10/16/2018
Share on social networks:
In a cold northern country it is difficult to rejoice at the approach of autumn. The fleeting Indian summer will be followed by gloomy leaden clouds, prolonged rains, cold and impenetrable longing, when everything falls out of hand, and life turns into a meaningless fuss. On such days, a chocolate dessert becomes a lifesaver - intuition correctly tells you how to deal with spleen and breakdown. Chocolate contains the essential amino acid tryptophan, from which the neurotransmitter serotonin is formed, nicknamed the "happiness hormone". Serotonin helps us to live in harmony with an imperfect world - to enjoy life with all its quirks, to find pleasure in simple things, to appreciate our uniqueness and not to succumb to illness and despondency.
The fleeting Indian summer will be followed by gloomy leaden clouds, prolonged rains, cold and impenetrable longing, when everything falls out of hand, and life turns into a meaningless fuss. On such days, a chocolate dessert becomes a lifesaver - intuition correctly tells you how to deal with spleen and breakdown. Chocolate contains the essential amino acid tryptophan, from which the neurotransmitter serotonin is formed, nicknamed the "happiness hormone". Serotonin helps us to live in harmony with an imperfect world - to enjoy life with all its quirks, to find pleasure in simple things, to appreciate our uniqueness and not to succumb to illness and despondency.
Why does the body need serotonin?
Serotonin is formed in the cells of the epiphysis, small intestine and pancreas under the influence of sunlight. In inclement weather, mediator production slows down, which immediately affects the speed and accuracy of transmission of nerve impulses. For this reason, many Russians suffer from seasonal depression. Constant serotonin starvation in regions where the sun is not visible for half a year leads to high anxiety, apathy, impaired memory and concentration, increased fatigue, sleep disorders and emotional instability. A high stress load also limits the production of serotonin: the stress hormones adrenaline and cortisol act as antagonists of the hormone of happiness.
Constant serotonin starvation in regions where the sun is not visible for half a year leads to high anxiety, apathy, impaired memory and concentration, increased fatigue, sleep disorders and emotional instability. A high stress load also limits the production of serotonin: the stress hormones adrenaline and cortisol act as antagonists of the hormone of happiness.
Severe serotonin deficiency is accompanied by a sharp increase in sensitivity to stress and pain. The close connection between chronic pain syndromes and depression of the psyche is confirmed by scientific research: according to the expert group of the University of Michigan, the same areas of the cerebral cortex are responsible for physical and mental suffering.
Currently, Russian scientists are actively studying the prospects for the use of serotonin preparations in order to prevent age-related changes in blood vessels. The expected effect is to prolong life by 20–30 years with the preservation of physical and intellectual capacity for work in old age.
Happiness factory inside oneself: how to adjust serotonin production?
Lack of serotonin is not a condition to be put up with. If depression has not yet gone far, it is enough to carve out a few hours a day for a hobby. Serotonin is actively produced at the moment when we are doing what we love and enjoy the creative process without regard to the result and external evaluation. Also, the production of serotonin is positively influenced by sports and dancing, listening to your favorite music, walking and outdoor games in the fresh air, regular sex life, meeting friends and communicating with pets.
What do they eat happiness with?
If your own resources to fight seasonal depression are not enough, you can make up for the deficiency of the hormone of happiness through diet. To the delight of the sweet tooth, dark chocolate and chocolate cakes are one of the most valuable sources of tryptophan.
Snickers cake will hit seasonal depression with peanut shot and caramel shrapnel. Chocolate products have a great advantage - due to the high content of fast carbohydrates, the delivery of tryptophan to the sites of serotonin synthesis is noticeably accelerated. The positive effect is felt already after the first piece of cake. In addition, cocoa butter contains a natural stimulant, theobromine, which acts similarly to caffeine. A little motivating kick will help you get out of hibernation, and serotonin will make awakening easy and joyful.
Chocolate products have a great advantage - due to the high content of fast carbohydrates, the delivery of tryptophan to the sites of serotonin synthesis is noticeably accelerated. The positive effect is felt already after the first piece of cake. In addition, cocoa butter contains a natural stimulant, theobromine, which acts similarly to caffeine. A little motivating kick will help you get out of hibernation, and serotonin will make awakening easy and joyful.
Of course, not all types of chocolate are equally healthy. Capricious nerve cells agree only on natural dark chocolate without admixture of vegetable fats and flavorings. Therefore, the Sweet Express confectionery orders chocolate in Belgium from a regular supplier.
Do you want to experience the difference between real chocolate and consumer chocolate? Try our Snickers cake - and you will no longer want to be interrupted by store-bought bars, in which there is only one name for real chocolate. A light chocolate biscuit with small pieces of peanuts gently envelops streams of thick caramel-peanut cream with white and milk chocolate, and the provocative crunch of airy biscuits will amuse even the unsmeian princess.