Alternative to lamictal for bipolar
Top lamotrigine alternatives and how to switch your Rx
Lamotrigine doesn't work for everyone. Lithium, Depakote, Tegretol, Seroquel, and Keppra are some lamotrigine alternatives. Get the full list here.
Compare lamotrigine alternatives | Lithium | Depakote | Tegretol | Seroquel | Keppra | Natural alternatives | How to switch meds
Anyone can feel reluctant to change medication for their bipolar disorder or seizures. Those two conditions are frightening to experience, and altering the treatment plan could make you susceptible to a relapse of seizures or mental health problems. Lamotrigine, the generic form of brand name Lamictal, has Food and Drug Administration (FDA) approval for maintenance treatment of both bipolar I disorder and prevention of seizures. Although many find Lamictal helpful, you may not and could rightfully be considering a switch to a different drug. Lamictal may simply be ineffective, prompting you to want something better, or it could be causing you unacceptable side effects.
But even if your reason for looking into an alternative is justified, it is crucial to understand the options in detail and take your ideas to a healthcare provider before making any changes. Those details are exactly what we will cover.
What can I take in place of lamotrigine?
Lamotrigine is categorized as an anticonvulsant, a drug used to treat epilepsy. Anticonvulsants or antiepileptic drugs are a broad category with various mechanisms for blocking the repetitive firing of neurons (brain cells) that causes seizures. If Lamictal is not the right choice for you, there are a number of alternatives to look into. Most are not overly expensive, but a SingleCare discount card may still be advisable to help reduce the cost.
Treatment of bipolar disorder often incorporates a mood stabilizer as a core element. Certain anticonvulsants, including Lamictal, can function as mood stabilizers. Changing to a different anticonvulsant or a different type of mood stabilizer could be options for you.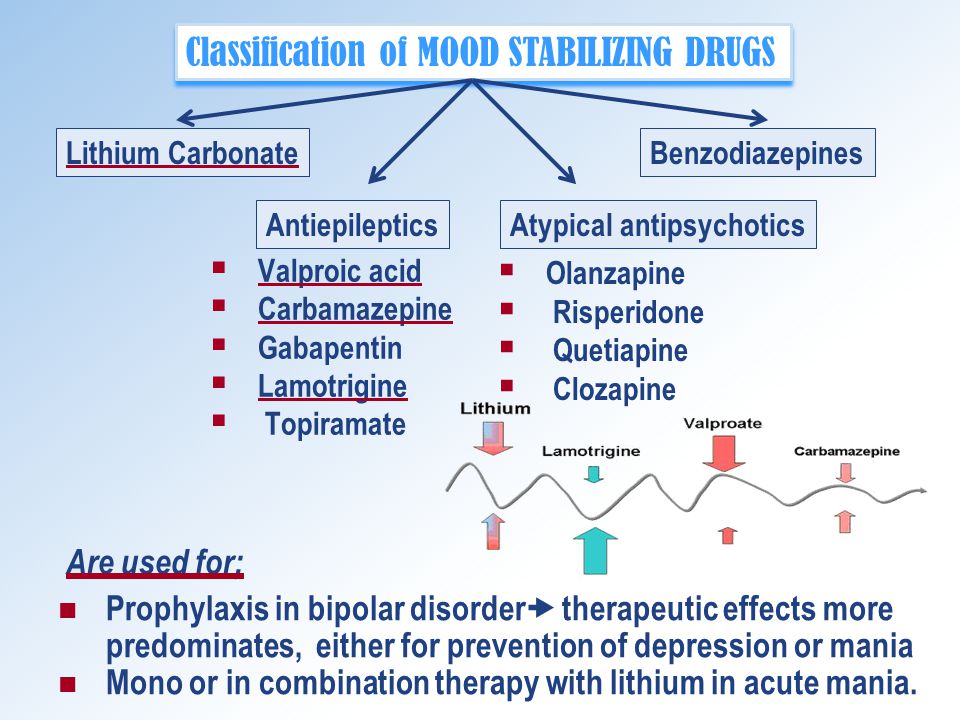 Aside from mood stabilizers, antipsychotic drugs are another category that is effective for bipolar disorder and may be a worthy substitute for Lamictal. Comparing your options will hopefully help you find the right one.
Aside from mood stabilizers, antipsychotic drugs are another category that is effective for bipolar disorder and may be a worthy substitute for Lamictal. Comparing your options will hopefully help you find the right one.
RELATED: Bipolar disorder statistics 2022
Other alternatives to lamotrigine
- Haloperidol
- Geodon (ziprasidone)
- Clozaril (clozapine)
- Fycompa (perampanel)
- Mysoline (primidone)
- Phenobarbital
- Klonopin (clonazepam)
- Onfi (clobazam)
- Felbatol (felbamate)
- Xcompri (cenobamate)
- Trileptal (oxcarbazepine)
- Valproic acid
- Valproate
- Neurontin (gabapentin)
- Dilantin (phenytoin)
- Keppra XR (levetiracetam extended release)
- Briviact (brivaracetam)
Top 5 lamotrigine alternatives
The following are some of the most common alternatives to lamotrigine.
1. Lithium
Lithium is a long-standing, well-studied mood stabilizer for bipolar disorder. How exactly it works remains a mystery, but its efficacy is undeniable. Clinical trials have shown that lithium and Lamictal are equally good at reducing the overall risk of relapse, although lithium seems a bit better at lowering risk of mania recurrence. If Lamictal is not working for you, lithium is certainly an alternative to be aware of.
Unfortunately, lithium has a multitude of side effects which make it less tolerable than the effects of Lamictal in studies. Potential adverse effects of lithium on kidney, thyroid, and parathyroid function can be problematic, as can untoward symptoms like diarrhea, nausea, tremor, and weight gain. Toxic effects of lithium are experienced at drug levels not much higher than those required for effectiveness. Accordingly, lithium drug levels are monitored with bloodwork to help avoid toxicity. When done right, lithium therapy can be a good choice, but the prospect of serious side effects and drug monitoring may keep you looking for other options.
RELATED: Lithium side effects and how to avoid them
2. Depakote
Divalproex sodium is the generic form of Depakote and is chemically similar to valproate and valproic acid. Depakote shares both seizure and bipolar treatment indications with lamotrigine. They both are anticonvulsants that are effective in preventing seizures in epilepsy and mood stabilizers that are effective for preventing mood swings in bipolar disorder. Extended-release formulations are available for both, if the convenience of once-daily dosing is important. Depakote and Lamictal can each match lithium for efficacy and surpass it for tolerability.
Given all the similarities, the reason to consider changing from Lamictal to Depakote is the difference in side effects and potential allergic reactions. Either can make you nauseous, dizzy, tremulous, or cause blurred vision, but there are serious toxicities that each is notorious for. Lamotrigine has a black box warning from the FDA for the potential to cause the life-threatening skin rash condition Stevens Johnson syndrome and others like it. It can also provoke substantial adverse effects on the heart. In comparison, the black box warning on Divalproex is regarding the potential for hepatotoxicity, including fatal liver failure, and pancreatitis, an attack of pancreas inflammation. It can trigger dangerous drops in blood cell counts as well. A pre-existing medical condition or bad initial experience with Lamictal could sway you toward Depakote.
It can also provoke substantial adverse effects on the heart. In comparison, the black box warning on Divalproex is regarding the potential for hepatotoxicity, including fatal liver failure, and pancreatitis, an attack of pancreas inflammation. It can trigger dangerous drops in blood cell counts as well. A pre-existing medical condition or bad initial experience with Lamictal could sway you toward Depakote.
RELATED: Depakote side effects and how to avoid them
3. Tegretol
Another anticonvulsant/mood stabilizer with a potential for life threatening rashes, blood cell count disturbances, and liver effects, Tegretol does not immediately distinguish itself from Lamictal and Depakote. Like them, it has a generic form and extended-release product too. Sometimes the reason to change may simply be to try to find something that works better. While carbamazepine, the generic form of Tegretol, is a common first-line option for epilepsy, it is not usually an early choice for bipolar disorder. A reason to hesitate regarding carbamazepine is the large list of meds that interact with it. The search for the right medication must take into account your list of prescription drugs and over-the-counter medications to evaluate for drug interactions.
A reason to hesitate regarding carbamazepine is the large list of meds that interact with it. The search for the right medication must take into account your list of prescription drugs and over-the-counter medications to evaluate for drug interactions.
4. Seroquel
Seroquel (quetiapine) is solely an alternative to Lamictal for those with bipolar mood disorder. It is not an anticonvulsant. Quetiapine is a second generation or atypical antipsychotic, but it is a popular mood stabilizer for those with bipolar. The popularity likely stems from its ability to treat both manic episodes and depressive symptoms of bipolar. Limitations that you should be aware of are its propensity to cause tiredness and weight gain.
RELATED: Seroquel side effects and how to avoid them
5. Keppra
If you are searching for an alternative to Lamictal for epilepsy control rather than bipolar, Keppra (levetiracetam) is worth a look. Levetiracetam’s efficacy and tolerability leads many who start it to stick with it. Other attractive features are the lack of a FDA black box warning and a relative paucity of contraindications and drug interactions compared to lamotrigine. Before choosing Keppra, you should review the common side effects, including sedation and mood disturbance. Caution when starting any of these drugs is advisable, because excessive tiredness could cause impairment with driving and other tasks.
Other attractive features are the lack of a FDA black box warning and a relative paucity of contraindications and drug interactions compared to lamotrigine. Before choosing Keppra, you should review the common side effects, including sedation and mood disturbance. Caution when starting any of these drugs is advisable, because excessive tiredness could cause impairment with driving and other tasks.
Natural alternatives to lamotrigine
Facing warning labels, serious side effects, drug interactions, and contraindications could have you fearful of just about any medication. For that reason, many folks turn to natural alternatives. However, just because something is considered natural does not mean it is safe. Many supplements have not been adequately tested either. A review of supplements for bipolar depression reached the conclusion that there is not enough evidence to support any particular one, but it also pointed out that natural options can have potential, as evidenced by the mood stabilizer lithium being a natural mineral.
Cannabidiol (CBD) garners a lot of attention as a potential, natural remedy for bipolar and seizure disorders. Only weak evidence exists for use in bipolar treatment, according to a 2020 review. Likewise, a review indicated that the use of CBD for certain seizure disorders is backed by clinical trials, but numerous questions remain about its safety and effectiveness.
RELATED: The best diet for mental health | Physical activity and mental health
How to switch to a lamotrigine alternative
The gravity of a decision about seizure or bipolar treatment can seem overwhelming. Hopefully, gathering information has helped to put you on the right track in the decision-making process. The next step is to discuss the matter with the prescribing healthcare professional. If the effects of lamotrigine have you considering discontinuation, be prepared to explain why you are dissatisfied and what you are looking for in a substitute, including any of the options noted above. Your pharmacist can be another great resource for medical advice. When a decision is made, your entire healthcare team should be on the same page.
Your pharmacist can be another great resource for medical advice. When a decision is made, your entire healthcare team should be on the same page.
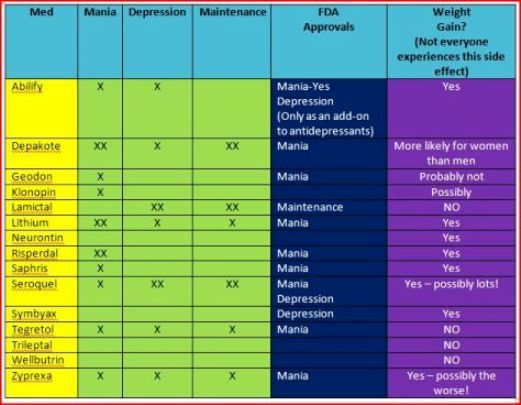
Top Mood Stabilizers for Bipolar Disorder
Chris Aiken, MD
None of them are perfect, but a few rise to the top, according to recent guidelines.
RESEARCH UPDATE
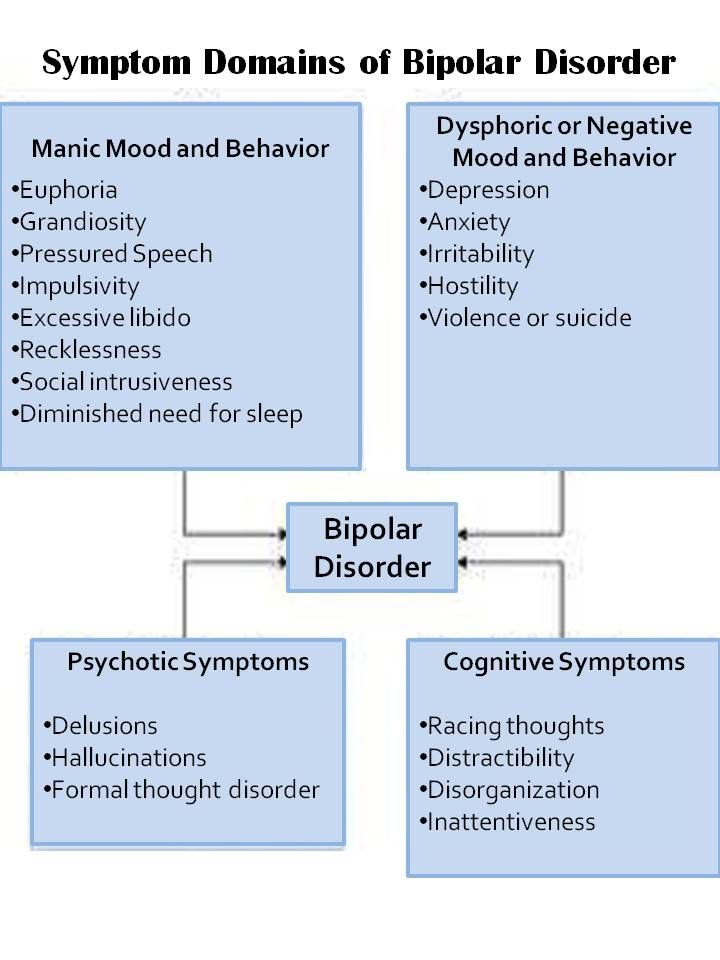
I’ve been pouring over textbooks and treatment guidelines, and these four mood stabilizers keep rising to the top.1-6 None of them are perfect, but each has a unique role in bipolar disorder:
- Lithium
- Quetiapine (Seroquel)
- Lurasidone (Latuda)
- Lamotrigine (Lamictal)
Lithium and quetiapine top the lists for all three phases of the illness: mania, depression, and the maintenance phase. Lurasidone and lamotrigine are either untested (lurasidone) or ineffective (lamotrigine) in mania, but they are essential tools for bipolar depression.
Lithium
Lithium stands out for its preventative effects in bipolar disorder, but it also has important benefits outside of the manic-depressive symptom lists. It is the only mood stabilizer that significantly reduces the risk of suicide, and it reduces mortality in other ways as well. Although lithium is often avoided out of concerns of toxicity, it actually lowers the risk of cancer, heart disease, stroke, and viral illnesses. 7 All of those occur at higher frequency in bipolar disorder. Vascular disease is the leading cause of death in bipolar disorder,8 and viral illnesses are both a consequence (ie, STDs) and cause (ie. inflammatory effects) of bipolar symptoms.9
Patients with classic, “textbook,” bipolar disorder tend to respond best to lithium. They are characterized by full remission between episodes, predominance of manic over depressive symptoms, illness onset in late teens, and a family history of bipolar disorder. They also tend to lack significant mixed states, rapid cycling, and psychiatric comorbidities, although a recent study found that comorbid panic disorder predicted lithium response.10
They also tend to lack significant mixed states, rapid cycling, and psychiatric comorbidities, although a recent study found that comorbid panic disorder predicted lithium response.10
Quetiapine
Quetiapine covers ground that lithium does not. It is more effective against mixed manias, while lithium is preferred for the purer, euphoric highs. It also works better in acute depressive episodes. Quetiapine cannot claim the medical benefits that lithium does, but it does assist with two symptoms that patients find particularly troubling: anxiety and insomnia.11,12 Although its long-term adverse effects give me pause, it did provide the best protection against both poles of the illness in Terrence Ketter’s analysis of the numbers needed to treat (NNT) for long-term maintenance.6
Lurasidone (Latuda) and Lamotrigine (Lamictal)
Both of these occupy a unique niche in bipolar depression. Lurasidone is the more effective of the two, with a number needed to treat (NNT) of 5 compared to lamotrigine’s 12. 6 But lamotrigine is the better tolerated option, with few of the adverse effects that matter most to patients: weight gain, fatigue, sexual dysfunction, and long-term medical risks. Lamotrigine is better at preventing depression than it is at treating it.
6 But lamotrigine is the better tolerated option, with few of the adverse effects that matter most to patients: weight gain, fatigue, sexual dysfunction, and long-term medical risks. Lamotrigine is better at preventing depression than it is at treating it.
There is some evidence that lamotrigine works better in bipolar II disorder, where frequent cycles of depression predominate, than bipolar I.13 Some experts favor it for the rapid mood fluctuations of cyclothymic disorder, and I’ve found it useful there as well.14 The main risk of lamotrigine is during the first three months of treatment, when Stevens-Johnson syndrome and other life threatening allergic reactions can occur at a rate of about 1:3000.
Long-term tolerability is not the strong suit of atypical antipsychotics like lurasidone, but these medicines can work quickly to get patients out of severe episodes. The ones that treat bipolar depression are cariprazine (Vraylar), lurasidone (Latuda), olanzapine-fluoxetine combo (Symbyax), and quetiapine (Seroquel).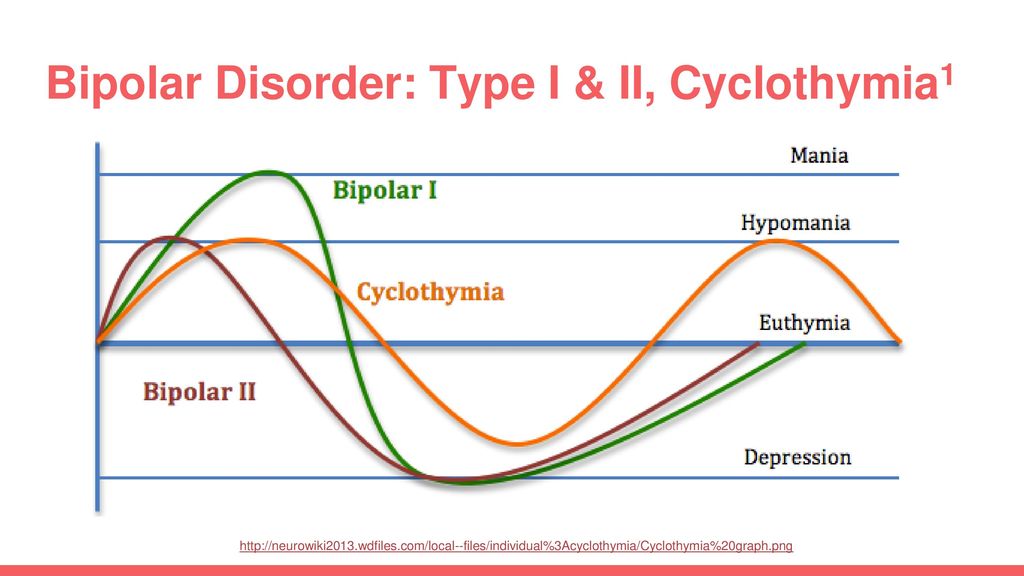 Among them, lurasidone offers a good balance of efficacy and tolerability. Lurasidone’s main problems â nausea, akathisia, cost, and lack of evidence in mania â are less likely to occur with quetiapine, so patients who cannot tolerate one will often tolerate the other.
Among them, lurasidone offers a good balance of efficacy and tolerability. Lurasidone’s main problems â nausea, akathisia, cost, and lack of evidence in mania â are less likely to occur with quetiapine, so patients who cannot tolerate one will often tolerate the other.
The Bottom Line
Matching the mood stabilizer to the patient is a matter of balancing competing goals. Each stands out in its own way, whether for tolerability, treatment of comorbidities, or benefits in depression, mania, or the maintenance phase. Together, these four treatments cover lot of ground, but you also need to know how to use them. That is covered in the next installment.
For related content, see Dosing Secrets for the Top Mood Stabilizers.
Disclosures:
Dr Aiken is the Mood Disorders Section Editor for Psychiatric Times, the Editor in Chief of The Carlat Psychiatry Report, and the Director of the Mood Treatment Center. His written several books on mood disorders, most recently The Depression and Bipolar Workbook. He can be heard in the weekly Carlat Psychiatry Podcast with his co-host Kellie Newsome, PMH-NP. The author does not accept honoraria from pharmaceutical companies but receives royalties from PESI for The Depression and Bipolar Workbook and from W.W. Norton & Co. for Bipolar, Not So Much
His written several books on mood disorders, most recently The Depression and Bipolar Workbook. He can be heard in the weekly Carlat Psychiatry Podcast with his co-host Kellie Newsome, PMH-NP. The author does not accept honoraria from pharmaceutical companies but receives royalties from PESI for The Depression and Bipolar Workbook and from W.W. Norton & Co. for Bipolar, Not So Much
References:
1. Parker GB, Graham RK, Tavella G. Is there consensus across international evidence-based guidelines for the management of bipolar disorder? Acta Psychiatr Scand. 2017;135(6):515â526.
2. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97â170.
3. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: The Clinical Guidelines. Int J Neuropsychopharmacol. 2017;20(2):180â195.
Int J Neuropsychopharmacol. 2017;20(2):180â195.
4. Wang D, Osser DN. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: An update on bipolar depression. Bipolar Disord. 2019;10.1111/bdi.12860.
5. Mohammad O, Osser DN. The psychopharmacology algorithm project at the Harvard South Shore Program: an algorithm for acute mania. Harv Rev Psychiatry. 2014;22(5):274â294.
6. Ketter T (2015). Advances in Treatment of Bipolar Disorders, 1st Edition. American Psychiatric Publishing, Washington, DC.
7. Post RM. The new news about lithium: An underutilized treatment in the united states. Neuropsychopharmacology. 2018;43(5):1174â1179.
8. Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23(1):40â47.
9. Tucker JD, Bertke AS. Assessment of cognitive impairment in HSV-1 positive schizophrenia and bipolar patients: Systematic review and meta-analysis. Schizophr Res. 2019;209:40â47.
Schizophr Res. 2019;209:40â47.
10. Scott J, Bellivier F, Manchia M, et al. Can network analysis shed light on predictors of lithium response in bipolar I disorder? Acta Psychiatr Scand. 2020;10.1111/acps.
11. Pompili M, Rihmer Z, Gonda X, Serafini G, Sher L, Girardi P. Early onset of action and sleep-improving effect are crucial in decreasing suicide risk: the role of quetiapine XR in the treatment of unipolar and bipolar depression. Riv Psichiatr. 2012;47(6):489â497.
12. Wang Z, Kemp DE, Chan PK, et al. Comparisons of the tolerability and sensitivity of quetiapine-XR in the acute treatment of schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and generalized anxiety disorder. Int J Neuropsychopharmacol. 2011;14(1):131â142.
13. Terao T, Ishida A, Kimura T, Yarita M, Hara T. Preventive effects of lamotrigine in bipolar II versus bipolar I disorder. J Clin Psychiatry. 2017;78(8):e1000âe1005.
14. Manning JS, Haykal RF, Connor PD, Cunningham PD, Jackson WC, Long S. Sustained remission with lamotrigine augmentation or monotherapy in female resistant depressives with mixed cyclothymic-dysthymic temperament. J Affect Disord. 2005;84(2-3):259â266.
Related Article >>>
Advertisement
Advertisement
updated recommendations of the British Association for Psychopharmacology
01/08/2017
Treatment outcomes for bipolar disorder (BD) have been, and still are, modest. However, British experts believe that there is significant potential for improvement if therapy is carried out in accordance with modern recommendations based on evidence-based medicine. This summer, the British Association for Psychopharmacology presented the third update of its evidence-based BD treatment guidelines, with the key provisions of which we would like to acquaint our readers with.
The quality of the evidence on which recommendations were made was rated from I to IV (descending), and the strength of the recommendations, respectively, by the number of asterisks, from very low (*) to high (****). The provisions, the observance of which, according to the authors, should be the most stringent, they marked with the letter S.
Acute manic episode
Most patients with manic states require short-term medical treatment in an adequate clinical setting (I). A network meta-analysis of randomized controlled trials (RCTs) demonstrated the efficacy of a range of drugs (I). When choosing a drug, it is necessary to take into account its effectiveness and the risk of developing adverse events.
There is currently no alternative psychotherapeutic treatment for acute mania.
For patients not receiving long-term treatment with BR . In the case of severe manic episodes, oral dopamine receptor antagonists, which provide a rapid anti-manic effect, should be considered (****). Thus, a systematic analysis of clinical trial data indicates that haloperidol, olanzapine, risperidone, and quetiapine are highly effective for the short-term relief of symptoms of acute mania. An alternative is valproate, which has a lower risk of developing adverse motor reactions, but they should not be prescribed to women of childbearing age due to the unacceptable risk of a teratogenic effect and a slowdown in the child's intellectual development in the future. It is also possible to use aripiprazole, other antagonists and partial agonists of dopamine receptors, carbamazepine and lithium preparations.
Thus, a systematic analysis of clinical trial data indicates that haloperidol, olanzapine, risperidone, and quetiapine are highly effective for the short-term relief of symptoms of acute mania. An alternative is valproate, which has a lower risk of developing adverse motor reactions, but they should not be prescribed to women of childbearing age due to the unacceptable risk of a teratogenic effect and a slowdown in the child's intellectual development in the future. It is also possible to use aripiprazole, other antagonists and partial agonists of dopamine receptors, carbamazepine and lithium preparations.
If an agitated patient requires parenteral therapy to correct behavior without full consent, dopamine antagonists/partial agonists and GABAergics (benzodiazepines) should be used (S).
It is recommended to prescribe the minimum effective dose of drugs (S). Do not increase the dose of dopamine receptor antagonists just to achieve a sedative effect (S).
For less severe, non-psychotic patients with mania and hypomania, treatment can be extrapolated from mania practice (IV).
In order to quickly induce sleep in agitated hyperactive patients, it is worth considering the additional prescription of GABA-ergic drugs (***).
Whenever possible, the choice of therapy should be based on the patient's previous experience and preferences.
Antidepressants (i.e. drugs approved for the treatment of unipolar depression) during a manic episode are usually discontinued by tapering the dose (**).
Long-term therapy with BR should be considered if treatment of mania has been successful (S).
For patients with a manic episode that developed during long-term therapy. If the current condition is caused by insufficient effectiveness of basic therapy, it is recommended to increase the dose of the drug used to the maximum well tolerated (S). Increasing the dose of dopamine receptor antagonists and partial agonists or valproate may be sufficient to relieve manic symptoms (IV).
In the case of treatment with lithium preparations, it should be ensured that its concentration in the blood serum is in the target range. Try increasing the dose until a concentration of 0.6-0.8 mmol/L (mEq/L) is reached. A concentration of 0.8-1.0 mmol/L may be more effective, but long-term maintenance is associated with an increased risk of side effects (I).
Try increasing the dose until a concentration of 0.6-0.8 mmol/L (mEq/L) is reached. A concentration of 0.8-1.0 mmol/L may be more effective, but long-term maintenance is associated with an increased risk of side effects (I).
If the patient is on lithium, consider adding a dopamine receptor antagonist/partial agonist or valproate to therapy (****).
If the manic episode was the result of the patient's failure to comply with medical recommendations, the cause should be determined and adequate measures taken (S). For example, if insufficient adherence to therapy is due to adverse events, it is necessary to reduce the dose of the drug (if the side effect is dose-dependent) or transfer the patient to an alternative treatment regimen with better tolerance. If non-compliance is deliberate and not related to tolerability of therapy, long-term use of lithium preparations is not indicated due to the risk of developing mania or depression if they are discontinued (I).
If symptoms cannot be controlled with optimal doses of first-line drugs and/or the manic state is extremely severe, another treatment should be added. Consider a combination of lithium or valproate with a dopamine antagonist/partial agonist (****).
Consider a combination of lithium or valproate with a dopamine antagonist/partial agonist (****).
Clozapine is considered in more refractory cases (**).
Electroconvulsive therapy may be considered in patients with very severe or treatment-resistant mania, in cases where the patient prefers this type of treatment, in severe mania during pregnancy (***).
Manic or hypomanic episode with mixed features. DSM-5 proposes the term "mixed traits" instead of the definition of "mixed episode" used in DSM-IV. A secondary analysis of the results of studies in relation to the effectiveness of the treatment of mixed episodes indicates that the treatment of episodes such as manic is adequate.
Use of psychoactive substances. If substance use may have contributed to a manic or hypomanic episode, consider medical support for drug withdrawal (S).
Termination of therapy for an acute episode. Drug withdrawal should be planned based on the need for further long-term maintenance treatment (S).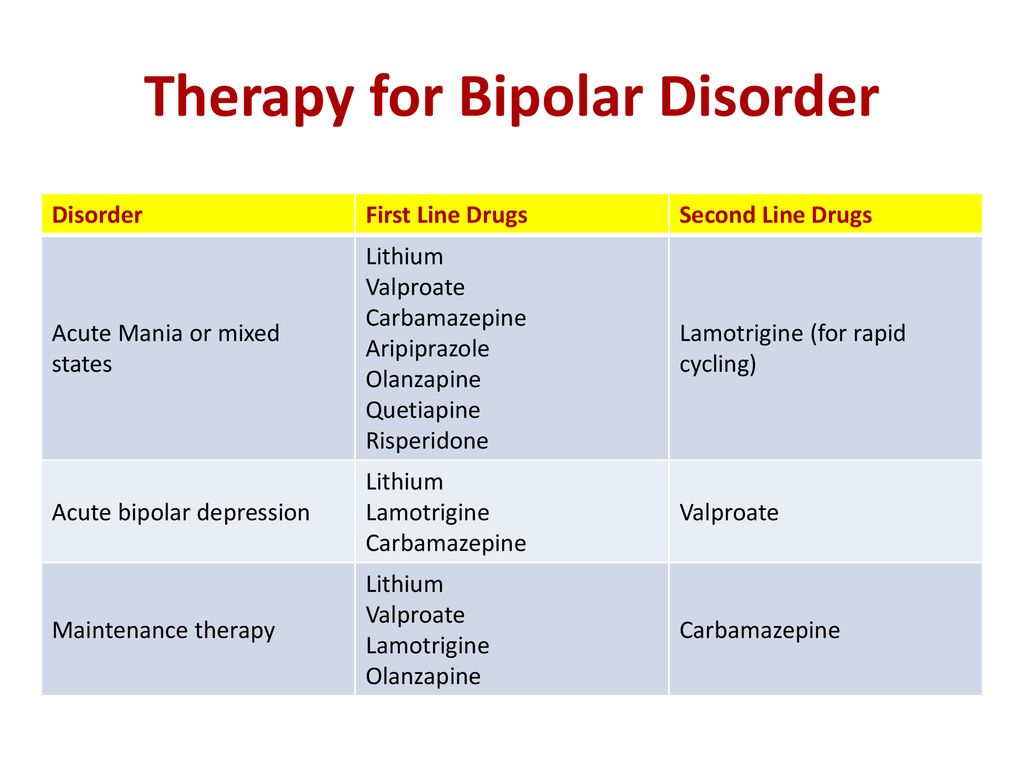 Many drugs are effective not only for the treatment of manic states, but also for the prevention of their relapse (I).
Many drugs are effective not only for the treatment of manic states, but also for the prevention of their relapse (I).
The dose of drugs used only for the intensive treatment of mania, upon reaching a complete remission, is gradually reduced (for 4 weeks or more) until complete withdrawal (IV). Remission often occurs after 3 months, but mood stabilization may take 6 months or more.
Any drugs used additionally for symptomatic treatment (sedation, sleep induction) should be discontinued immediately after improvement (S).
Acute depressive episode
Network meta-analysis of RCTs showed the effectiveness of a limited range of drugs with different pharmacodynamics and different evidence bases.
There is no consensus on the appropriateness of the use of antidepressants, that is, drugs that are effective in unipolar depression (IV).
Most of the data come from studies involving patients with type I BD, but it seems logical to extrapolate them to people with type II BD.
For patients not receiving long-term BR treatment. Consider quetiapine, lurasidone, or olanzapine (***). An important benefit of dopamine receptor antagonists is their antimanic properties (I).
The use of antidepressants in BD is currently not well understood. Only the combination of fluoxetine with olanzapine has the necessary evidence base for BD (***). The widespread use of other antidepressants in patients with BD is the result of an extrapolation of data obtained in the treatment of unipolar depression. However, when prescribing antidepressants to patients with a history of mania, anti-manic agents (dopamine receptor antagonists, lithium preparations, valproates) should be added to them (S).
Initial therapy with lamotrigine (with gradual dose escalation) should be considered, usually as an adjunct to drugs to prevent relapse of mania (****).
Electroconvulsive therapy should be considered in patients with increased risk of suicide, treatment resistance, psychosis, severe depression during pregnancy, or life-threatening exhaustion (***). If possible, reduce previous polypharmacy, which can cause an increase in the seizure threshold.
If possible, reduce previous polypharmacy, which can cause an increase in the seizure threshold.
If symptoms of depression are mild, despite a limited evidence base, lithium may be used, especially as a preparation for long-term treatment (**).
Family CBT or Interpersonal Rhythm Therapy should be considered as adjunctive treatment if possible, as this may help shorten the duration of the acute episode (**).
For patients with a depressive episode that developed during long-term therapy. Check that the current long-term treatment regimen is able to prevent relapse of mania (lithium drugs, valproates, dopamine antagonists / partial agonists), assess the adequacy of drug doses and / or serum lithium concentration (S). If possible, current stressors should be eliminated.
If the patient does not respond to the optimization of long-term treatment, and the symptoms of depression are sufficiently pronounced, it is necessary to start the treatment indicated in the paragraph above. The following are recommendations for the treatment of resistant depression.
The following are recommendations for the treatment of resistant depression.
Drug choice for a depressive episode. Preferable treatment options cannot be determined based on the available studies (IV).
In the treatment of depression, there is a risk of transition to a manic state or unstable mood (I). And although such a phase change is characteristic of the disease itself, with monotherapy with antidepressants, the risk increases. Non-selective monoamine reuptake inhibitors (venlafaxine, duloxetine, amitriptyline, and imipramine) are more likely to induce mania than selective drugs, especially selective serotonin (II) reuptake inhibitors. The risk of developing a manic state while taking antidepressants in combination with antimanic drugs is minimal (I). If an antidepressant is prescribed as monotherapy for type II BD, dose escalation should be gradual and closely monitored by the clinician for early management of adverse reactions such as hypomania, comorbidity, or agitation (IV).
In contrast to the widespread use of antidepressants, lamotrigine is rarely used outside of specialized centers, despite its effectiveness in type I BD and the possibility of use in type II BD.
Long-term therapy should be considered after successful treatment of a depressive episode (S).
Withdrawal of antidepressants. The possibility of gradual withdrawal of antidepressants is considered after achieving complete remission (IV). Depressive episodes in BD are usually shorter than in unipolar depression (I). In the absence of indications for prolongation of therapy, it is worth canceling the antidepressant no earlier than 12 weeks after the start of remission (*). Longer treatment with antidepressants is justified if their withdrawal causes a relapse in the patient.
Treatment of resistant depression. BD patients with depression may show relative or even marked resistance to treatment (I). This means that the patient does not respond to treatment not only with antidepressants, but also with quetiapine, olanzapine, lurasidone and lamotrigine both as monotherapy and in combinations. The evidence base for the treatment of such patients with BD is extremely scarce. Electroconvulsive therapy may be a treatment option (***). It is possible to extrapolate the experience of treating unipolar depression using augmentation strategies. Coverage with anti-manic drugs such as lithium, valproate, dopamine antagonists/partial agonists (S) is needed.
The evidence base for the treatment of such patients with BD is extremely scarce. Electroconvulsive therapy may be a treatment option (***). It is possible to extrapolate the experience of treating unipolar depression using augmentation strategies. Coverage with anti-manic drugs such as lithium, valproate, dopamine antagonists/partial agonists (S) is needed.
Psychosocial therapy as initial treatment. There is very little evidence of the effectiveness of psychotherapy alone (without medication) in the treatment of acute bipolar depression. More evidence is needed that this approach is effective (IV).
Long-term treatment
Long-term treatment should be considered even after one severe manic episode, i.e. type I BD (***).
Without active consent to long-term treatment, treatment adherence may be low (I). Extended treatment should be considered, including psychoeducation, motivational and family support, especially in the initial stages of the disease, to model the patient's behavior and help him adhere to the regimen (***).
If the patient has been successfully treated for several years, continued therapy is recommended due to the high risk of relapse (***).
Recommendations for the treatment of type I BD can be extrapolated to type II BD as clinical studies show similar efficacy (**).
Long-term treatment strategies. Currently, continuous rather than intermittent oral therapy is the preferred relapse prevention strategy.
A network meta-analysis of studies evaluating long-term therapy showed the effectiveness of a limited range of drugs with different pharmacodynamics and different evidence bases in preventing relapses of mania: lithium preparations, olanzapine, quetiapine, long-acting injectable risperidone and valproates. Lamotrigine, lithium, quetiapine, and lurasidone have been shown to be effective in preventing recurrence of depressive episodes.
Although relatively few patients remain in such studies for 6 months or more, there is strong evidence of a high efficacy of lithium in preventing relapse in patients who have not experienced an early response to therapy with these drugs (I).
Short-term addition of other drugs (GABA receptor modulators or dopamine receptor antagonists/partial agonists) is necessary if a stressor is likely or present, early symptoms of relapse (especially insomnia) or severe anxiety (IV). It is worth considering the possibility of providing the patient with these drugs in advance, instructing him on the cases in which they should be used (*). Increasing the doses of long-term therapy drugs instead of additional drugs may also be effective (*).
Due to the fact that the optimal long-term treatment strategy has not yet been determined, clinicians and patients are involved in clinical trials designed to answer key therapeutic questions (S).
Choice of drug for long-term therapy. In addition to studies on relapse prevention, analysis of treatment results in real clinical practice indicates the effectiveness of such drugs: lithium > valproate > olanzapine > quetiapine > carbamazepine (I).
It is recommended to consider lithium preparations as monotherapy (****). Lithium monotherapy is effective in preventing relapses of manic, depressive and mixed states (I), has a greater evidence base for the prevention of new episodes compared to other agents (I) and a better studied long-term safety profile (I). According to RCTs and observational studies, patients with BD who use lithium preparations are less at risk of suicide and self-harmful behavior (I).
Lithium monotherapy is effective in preventing relapses of manic, depressive and mixed states (I), has a greater evidence base for the prevention of new episodes compared to other agents (I) and a better studied long-term safety profile (I). According to RCTs and observational studies, patients with BD who use lithium preparations are less at risk of suicide and self-harmful behavior (I).
In the treatment of lithium, biochemical monitoring, including the determination of its concentration in blood serum, is mandatory (S). The target range is 0.6-0.8 mmol/L. Lithium concentrations above 0.8 mmol/L are associated with an increased risk of impaired renal function, especially in women (I). Monitoring of lithium concentration in somatically healthy patients should be carried out at intervals of 3 months in the first year of treatment and 6 months thereafter (S).
If lithium is ineffective, poorly tolerated, or non-compliant, other treatment options should be considered: valproate, dopamine antagonists/partial agonists (****).
Valproates are often put on the same level with lithium as "mood stabilizers". The evidence base based on the results of controlled clinical trials is weaker, however, data from real practice indicate a greater effectiveness of valproates compared to other drugs (I). Risk factors in women have been discussed above.
Additional data obtained from studies on the treatment of acute episodes. If a patient experiences a sustained remission of a manic or depressive episode while taking any drug, this may be a reason to use this drug as long-term monotherapy (IV). At the same time, preference will probably be given to dopamine receptor antagonists due to their effectiveness in short-term use, however, the possibility of using lithium preparations as a better alternative should also be considered (IV).
Carbamazepine as a maintenance treatment is less effective than lithium preparations, but can sometimes be used as monotherapy when lithium is ineffective, especially in patients without classic episodes of euphoric mania (II). The drug seems to be effective mainly only for the prevention of relapses of manic states (I). Do not forget about drug interactions, which are a big problem in the treatment of carbamazepine. One might consider oxcarbazepine, which is less prone to such interactions (I).
The drug seems to be effective mainly only for the prevention of relapses of manic states (I). Do not forget about drug interactions, which are a big problem in the treatment of carbamazepine. One might consider oxcarbazepine, which is less prone to such interactions (I).
If mania relapse prevention is needed, but the patient is not following oral medication recommendations, or parenteral route of administration is preferred, long-acting drugs should be considered (**). There are a large number of long-acting dopamine receptor antagonists/partial agonists such as fluphenazine decanoate, haloperidol decanoate, olanzapine pamoate, risperidone microspheres, paliperidone palmitate, and aripiprazole monohydrate. In RCTs, only risperidone (II) has been shown to be effective. The use of other drugs is based on the class efficacy of dopamine antagonists/partial agonists, extrapolation of the results of their use in oral form and clinical experience (IV).
Lamotrigine and quetiapine can be considered as monotherapy for type II BD (***). Type I BD usually requires a combination of lamotrigine with a long-term antimanic drug (IV).
Type I BD usually requires a combination of lamotrigine with a long-term antimanic drug (IV).
Insufficient efficacy of monotherapy. If a patient fails to respond to monotherapy and subthreshold symptoms or relapses persist, long-term combination therapy is considered. When it comes to symptoms or relapses of mania, a combination of two predominantly anti-manic drugs (i.e., lithium, valproate, dopamine antagonists/partial agonists) would be logical (IV). In case of persisting symptoms or recurrence of depression, a combination of lithium, lamotrigine, quetiapine, lurasidone, or olanzapine (IV) may be appropriate.
The role of antidepressants in the long-term treatment of BD has not been studied in RCTs, but their long-term use appears to be effective in some patients (**).
If clozapine has been shown to be effective in the treatment of resistant mania, continued use is considered (**).
Supportive electroconvulsive therapy may be considered in patients who respond well to it during an acute episode but respond poorly to oral medications (*).
Consider adding psychotherapy if subthreshold symptoms are present (**).
Fast cycling. Conditions that contribute to rapid cycling, such as hypothyroidism or substance use, should be identified and addressed (**).
Gradual withdrawal of antidepressants should also be considered as they may cause cycling (**).
There is no specific treatment for rapid cycling. Many patients with this often debilitating form of BD require a combination of drugs. The countercyclical effect of therapy is assessed in dynamics over a period of six months or more, monitoring the patient's mood. Ineffective drugs are canceled to avoid unnecessary polypharmacy (S).
Cancellation of long-term treatment . After discontinuation of drugs, there is a risk of relapse even after years of stable remission (II). Thus, the decision to discontinue treatment should be made after assessing the potential risks (S).
The gradual withdrawal of any drug should take at least 4 weeks, and longer if possible (S). Abrupt withdrawal of lithium preparations is fraught with early relapse of mania (I).
Abrupt withdrawal of lithium preparations is fraught with early relapse of mania (I).
Discontinuation of drugs should not lead to termination of patient follow-up. It is necessary to continue monitoring and develop a plan to recognize and eliminate early signs of a relapse of a manic or depressive state (S).
Specific psychosocial interventions. Psychosocial interventions may improve treatment outcomes, reduce subthreshold symptoms, and reduce the risk of relapse (II). Psychoeducation is an important component of good clinical practice, as without it clinical interaction cannot be effective (S).
Various forms of psychotherapy (family therapy, cognitive behavioral therapy, interpersonal rhythm therapy) have been studied to prevent relapse. Psychological interactions are more effective in the initial stages of the disease (I).
Functional impairment in BD patients may require the use of cognitive and functional remediation strategies (II).
Patient groups provide the patient with support and additional information about BD and its treatment (IV).
Treatment of alcoholism in patients with BD
In alcoholics, even a modest reduction in alcohol consumption can improve health (I).
To reduce alcohol consumption, the patient should be offered naltrexone or nalmefene in addition to a behavioral therapy program (**).
If taking naltrexone has not helped the patient stay sober, acamprosate is recommended (**).
If a patient wants to remain sober, but acamprosate and naltrexone do not help, disulfiram is considered. However, the patient should be aware of the risks associated with the use of disulfiram and the need to monitor his mood (*).
Treatment of comorbid borderline personality disorder in patients with BD
Patients with comorbidity require treatment for both disorders. In this regard, one should not give preference only to medical treatment (carried out in BD) or only psychotherapy (indicated in borderline conditions) (S). In the absence of appropriate indications, there is no need to cancel or suspend adequate therapy for BD or borderline personality disorder.
Although there are few data supporting the effectiveness of medical treatment of borderline conditions, mood instability common to both pathological conditions is successfully treated with drugs (lamotrigine, lithium preparations, olanzapine, risperidone, aripiprazole and quetiapine), which improves the course of borderline disorder .
Treatment of anxiety and other comorbid disorders in patients with BD
Treat comorbid anxiety disorders, attention deficit hyperactivity disorder, and substance abuse according to relevant clinical guidelines (*).
Antidepressants should be used with caution (S).
Treatment of BR in selected groups of patients
Manic states in children and adolescents. Aripiprazole is preferred because it is approved for the treatment of type I BD in adolescents (over 13 years of age) (***). In other cases, it is worth adhering to the recommendations for the treatment of BD in adults. There is evidence that olanzapine and risperidone are effective in adolescents (**).![]()
Children should be given adequate doses of drugs (S). It is necessary to be aware of the increased risk of side effects, in particular the possible weight gain (S).
Bipolar depression in children and adolescents. Considers medication and psychotherapy based on adult treatment data (*).
Antidepressants are more likely to cause mania in children and adolescents than in adults (II).
Long-term treatment is recommended in young patients, as relapses and mood instability can have a detrimental effect on the formation of cognitive and emotional functions (S).
Elderly patients . If obvious side effects are observed when using normal doses of psychotropic drugs, a dose reduction is considered (*).
Women who can become pregnant. Valproate and carbamazepine may be teratogenic (I). Given the risk/benefit ratio, valproate is contraindicated in women who may become pregnant (I).
Concerns about the association of lithium intake and the development of heart defects appear to be unfounded (II).
Since at least 50% of pregnancies today are unplanned, family planning counseling should be provided to patients whenever possible (S).
Pregnancy and postpartum period. Dopamine antagonists/partial agonists, antidepressants, lamotrigine, and lithium are not or very low risk of teratogenicity. However, it should be remembered that the risk of new drugs is always unknown, so they should be used with caution.
Pregnant women are not immune from relapse. Discontinuation of medications can lead to mood destabilization (IV). Thus, the potential negative impact on the fetus must be weighed against the risk of a mental disorder in the mother and how this will affect the child's condition (S).
Many psychotropic drugs used to treat BD can cause symptoms in newborns. Babies need to be watched hours and even days after birth (S).
After childbirth, women have an increased risk of recurrence of mania or depression (I). In this regard, they need constant monitoring and preventive treatment (S).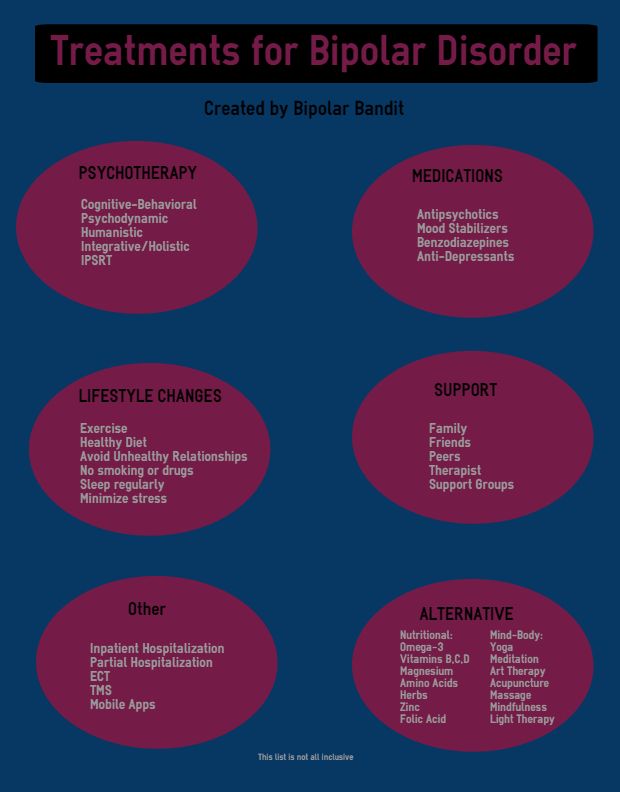
There are anecdotal reports of adverse reactions in breastfed infants when mothers take psychotropic drugs, but the frequency of such events is not yet clear (III).
Women who continue to receive psychotropic medication after childbirth make their own decision to breastfeed or formula-feed after a detailed explanation of the benefits and risks (S). If the mother decides to continue breastfeeding while on therapy, the baby should be monitored (S).
There are regular reports about the adverse effects of taking antidepressants and other psychotropic drugs during pregnancy on the further development of children. Very often, such studies are of inadequate design and low quality. Thus, claims that the use of psychotropic drugs during pregnancy may cause behavioral disorders in children in the future should be treated with caution.
The main recommendations for the treatment of BD are summarized in the table.
Prepared by Natalia Mishchenko
Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. Goodwin G.M., Haddad P.M., FerrierI.N. et al. J Psychopharmacol. Jun 2016; 30(6): 495-553.
Goodwin G.M., Haddad P.M., FerrierI.N. et al. J Psychopharmacol. Jun 2016; 30(6): 495-553.
- Number:
- Thematic issue "Neurology, Psychiatry, Psychotherapy" No. 3 (38), spring 2016
11/25/2022 NeurologyTherapy and family medicine
The problem of acute and chronic stress on the body (in front of the brain) is one of the most common in medicine; in modern medical practice in modern minds it has a special meaning, both in the medical, and in the medical and social sense, scho without intermediary occupies the sphere of professional activity of various physicians - neurologists, cardiologists, gastroenterologists, pulmonologists, endocrinologists, psychiatrists, and also doctors-internists wide profile and family doctors. A special place is added to the influx of chronic stress in the pathogenesis of t. sickness of civilization - psychosomatic and cerebrovascular pathology, as well as neuroses [6, 9, 16]. Podnuє guessing the form of pathology, the primary lesion of the central nervous system (CNS) on all levels of the structural and functional organization of the brain - in terms of molecular to systemic with a further development of dysregulation of internal organs, central and peripheral blood circulation, the disease of civilization also belongs to the category of dysregulation pathology. ...
A special place is added to the influx of chronic stress in the pathogenesis of t. sickness of civilization - psychosomatic and cerebrovascular pathology, as well as neuroses [6, 9, 16]. Podnuє guessing the form of pathology, the primary lesion of the central nervous system (CNS) on all levels of the structural and functional organization of the brain - in terms of molecular to systemic with a further development of dysregulation of internal organs, central and peripheral blood circulation, the disease of civilization also belongs to the category of dysregulation pathology. ...
11/25/2022 PsychiatryTherapy and family medicine Treating depressive disorder
In today's world, depression is one of the most widespread mental disorders. Show illness and negative consequences both on the psychological and on the somatic level, and severe depression can become a cause of suicide. In the absence of a decrease in the quality of life of ailments, and a stronger focus on the economic and medical systems of the country, the urgency of this illness is mindful. About modern standards of diagnostics and methods of treating depressive disorder in its dopovіdі at the international neurological conference "XIV Neurosymposium" by Prof. Allan H. Young....
In the absence of a decrease in the quality of life of ailments, and a stronger focus on the economic and medical systems of the country, the urgency of this illness is mindful. About modern standards of diagnostics and methods of treating depressive disorder in its dopovіdі at the international neurological conference "XIV Neurosymposium" by Prof. Allan H. Young....
11/20/2022 Psychiatry Somatically unreasonable syndromes and functional disorders from the look of a psychiatrist
The conference “Internal medicine in the minds of modern people” was organized by the Department of Internal Diseases of the Faculty of Dentistry of the National Medical University. O.O. Bogomolets (m. Kyiv), community organizations "Academy of Internal Medicine" (m. Kiev) and "Polish Medical Association in Ukraine named after. A.S. Svіncіtskogo” (m. Kiev). Tsya zustrіch bula was dedicated to the nutrition of diagnostics, exaltation, as well as the prevention of illness in the ambush of evidence-based medicine ....
A.S. Svіncіtskogo” (m. Kiev). Tsya zustrіch bula was dedicated to the nutrition of diagnostics, exaltation, as well as the prevention of illness in the ambush of evidence-based medicine ....
04.11.2022 NeurologyCiticoline for improving memory in older people
Antiquity is characterized by the development of a wide range of pathologies, traditionally viewed as a natural process, and then, not like a disease. If old age is not aggravated by neurological or psychiatric ailments, cognitive functions do not break down, but rather calm down. Zokrema, in the middle of that frail age, the memory of those warehouses that are respected by physiological ones is commemorated. Mayzhe skin is subjectively aware of the memory of the memory of old times, which manifests itself, in the middle, like an hour later, necessary for memorizing new information and guessing information, as it was guessed earlier.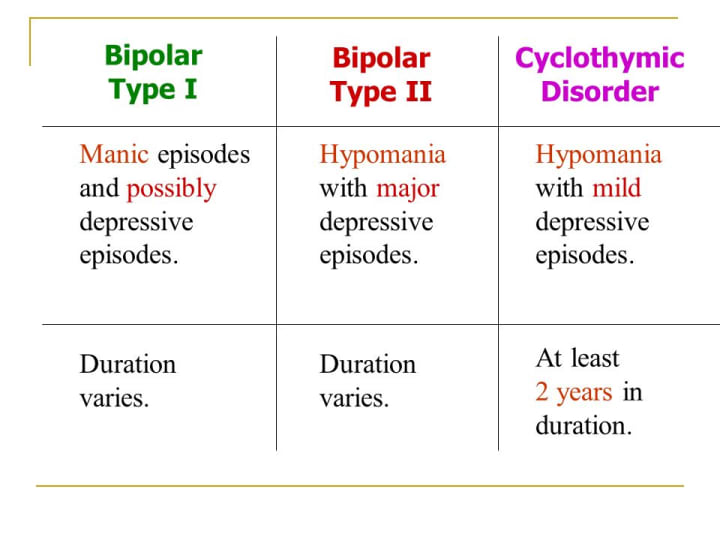 ...
...
Antidepressant therapy with antidepressants. Lamictal and the problem of drug resistance | Yastrebov D.V.
Introduction The initial prescription of antidepressants, the effectiveness of which has been proven in clinical studies, involves achieving a drug response rate of 50-75% within 3-4 weeks of therapy. From this it follows that from 25 to 50% of patients fall into a group of heterogeneous conditions, defined as "treatment-resistant depression" [I.M. Anderson et al., 2000; Schatzberg A.F., 2003]. Today there is no generally accepted definition of the phenomenon of drug resistance. For the most part, the use of this term implies the absence of a satisfactory response to treatment. However, the criteria used in clinical trials, which involve a rating system-based assessment of symptom dynamics as a result of therapy (eg, a 50% reduction in the total score of the HAMD1 scale), are of little use in everyday practice. As one of the reasons for this, one can mention that an improvement of this level is often not recognized by the patient as a positive outcome, which is confirmed by the discrepancy between subjective and objective ratings that is often found in clinical studies [A.J. Rush et al., 2006].
As one of the reasons for this, one can mention that an improvement of this level is often not recognized by the patient as a positive outcome, which is confirmed by the discrepancy between subjective and objective ratings that is often found in clinical studies [A.J. Rush et al., 2006].
The initial prescription of antidepressants, the effectiveness of which has been proven in clinical studies, involves achieving a drug response rate of 50-75% within 3-4 weeks of therapy. From this it follows that from 25 to 50% of patients fall into a group of heterogeneous conditions, defined as "treatment-resistant depression" [I.M. Anderson et al., 2000; Schatzberg A.F., 2003]. Today there is no generally accepted definition of the phenomenon of drug resistance. For the most part, the use of this term implies the absence of a satisfactory response to treatment. However, the criteria used in clinical trials, which involve a rating system-based assessment of symptom dynamics as a result of therapy (eg, a 50% reduction in the total score of the HAMD1 scale), are of little use in everyday practice. As one of the reasons for this, one can mention that an improvement of this level is often not recognized by the patient as a positive outcome, which is confirmed by the discrepancy between subjective and objective ratings that is often found in clinical studies [A.J. Rush et al., 2006].
As one of the reasons for this, one can mention that an improvement of this level is often not recognized by the patient as a positive outcome, which is confirmed by the discrepancy between subjective and objective ratings that is often found in clinical studies [A.J. Rush et al., 2006].
Other definitions based on the clinical assessment of the condition suggest an estimate of the number of unsuccessful treatments (2 or more). And finally, the third version of the definitions involves the persistence of depressive symptoms in the absence of success in achieving a state of remission as a result of the therapy (it should be noted that in this case the concepts of remission and drug response are combined) - M.R. Nelsen, D.L. Dunner, 1993; M.E. Thase, P.T. Ninan, 2002.
Any of the listed approaches for determining drug resistance is justified only for patients for whom there were no diagnostic inconsistencies and for whom adequate therapy was initially prescribed. In these cases, one speaks of the so-called "true" resistance. However, there are other options in which the achieved drug response against the doctor's expectations can be assessed as insufficient. Among them can be named: an error in the choice of an antidepressant or its dosage; poor tolerance, which makes it impossible to use a specific drug in the appropriate dose, violations of compliance, and a number of others (Fig. 1). It is obvious that in these cases, sometimes defined as "pseudo-resistance" or "relative resistance", medical tactics are fundamentally different and should be aimed at overcoming or eliminating factors that interfere with the achievement of a drug response [H.E. Lehmann, 1974].
However, there are other options in which the achieved drug response against the doctor's expectations can be assessed as insufficient. Among them can be named: an error in the choice of an antidepressant or its dosage; poor tolerance, which makes it impossible to use a specific drug in the appropriate dose, violations of compliance, and a number of others (Fig. 1). It is obvious that in these cases, sometimes defined as "pseudo-resistance" or "relative resistance", medical tactics are fundamentally different and should be aimed at overcoming or eliminating factors that interfere with the achievement of a drug response [H.E. Lehmann, 1974].
After eliminating the signs that allow attributing the lack of a drug response to the account of "pseudo-resistance", the question arises of determining the "true" (absolute) resistance [D. Bird et al., 2002]. In contrast to the previously existing ideas about drug resistance, as a phenomenon of the atomic order, M. Thase and A. Rush (1997) proposed a dynamic multi-stage system for assessing therapy-resistant depression, which is currently widely used. This typology reflects the authors' idea of drug resistance as a stage concept that requires comparison of the entire set of previous therapeutic measures that did not lead to an improvement in the condition (Table 1).
This typology reflects the authors' idea of drug resistance as a stage concept that requires comparison of the entire set of previous therapeutic measures that did not lead to an improvement in the condition (Table 1).
More complex is the taxonomically organized European staged method for assessing drug resistance, partially echoing the approach of M. Thase and A. Rush. Each of the stages of resistance, instead of a serial number, has its own definition with the designation of groups of drugs and the terms of their therapy that did not lead to an improvement in the condition (Table 2).
Depression variants,
therapy resistant
The issue of determining the clinical subtype of depression (psychotic, atypical, seasonal) is an important component in the management of this group of patients, since such differences may determine sensitivity to a particular therapy option. In addition, resistance to therapy may be due to an erroneous diagnosis with the definition of a unipolar variant of a depressive disorder in a patient with an undiagnosed bipolar course. Due to the fact that depressive phases in patients with bipolar disorder are clinically detected more often (including due to the greater attendance of patients with depressive symptoms), it is expected that up to half of patients with bipolar disorder can receive treatment according to therapeutic algorithms developed for unipolar depression. [S.N. Ghaemi et al., 1999]. As will be shown below, the question of the correct diagnosis of bipolar disorder is key in the appointment of therapy.
Due to the fact that depressive phases in patients with bipolar disorder are clinically detected more often (including due to the greater attendance of patients with depressive symptoms), it is expected that up to half of patients with bipolar disorder can receive treatment according to therapeutic algorithms developed for unipolar depression. [S.N. Ghaemi et al., 1999]. As will be shown below, the question of the correct diagnosis of bipolar disorder is key in the appointment of therapy.
Coping methods
drug resistance
Identification of a case of drug resistance dictates to the doctor the need to change the tactics of managing the patient. At the first stage, the choice is made between two main options: changing the antidepressant or prescribing an additional drug to "enhance" the antidepressant effect of the main drug. Let's take a closer look at each of them.
Changing Therapy
Changing the drug is preferable in the event of a complete lack of response to therapy or in the presence of side effects requiring the withdrawal of the originally prescribed antidepressant. Despite the apparent obviousness of the fact that the change of an antidepressant that turned out to be ineffective should imply the choice of a “second-line” drug with a fundamentally different mechanism of action, there are separate data arguing the expediency of switching to a more potent drug of the same group. In particular, for the most commonly prescribed antidepressants - selective serotonin reuptake inhibitors (SSRIs), it has been shown that the change of less potent drugs (fluoxetine, fluvoxamine, citalopram) that did not improve the symptoms of depression to more potent ones (paroxetine, sertraline) gives a percentage of improvements similar to selective ones. serotonin and norepinephrine reuptake inhibitors (venlafaxine) and selective norepinephrine and dopamine reuptake inhibitors (bupropion) up to 30% of cases. At the same time, the number of cases of early discontinuation of therapy due to developed side effects for "double-acting" drugs is 25% higher than for drugs of the SSRI group.
Despite the apparent obviousness of the fact that the change of an antidepressant that turned out to be ineffective should imply the choice of a “second-line” drug with a fundamentally different mechanism of action, there are separate data arguing the expediency of switching to a more potent drug of the same group. In particular, for the most commonly prescribed antidepressants - selective serotonin reuptake inhibitors (SSRIs), it has been shown that the change of less potent drugs (fluoxetine, fluvoxamine, citalopram) that did not improve the symptoms of depression to more potent ones (paroxetine, sertraline) gives a percentage of improvements similar to selective ones. serotonin and norepinephrine reuptake inhibitors (venlafaxine) and selective norepinephrine and dopamine reuptake inhibitors (bupropion) up to 30% of cases. At the same time, the number of cases of early discontinuation of therapy due to developed side effects for "double-acting" drugs is 25% higher than for drugs of the SSRI group.:strip_icc():format(jpeg)/kly-media-production/medias/3018655/original/008121600_1578662842-Infografis_Penderita_Skizofrenia_Meningkat_di_Indonesia.jpg) Thus, the assumption that the lack of sensitivity of depressive symptoms to the initially prescribed antidepressant indicates the ineffectiveness of the entire class of drugs with a similar mechanism of action has not been confirmed to date [A.J. Rush et al., 2006]. These data, presented in the form of practical recommendations, show that when changing therapy, it is justified to choose a drug not with the largest number of cumulative mechanisms of action, but with the maximum potential in relation to the leading neurotransmitter system (for SSRIs, this is paroxetine (Paxil) and sertraline). At the same time, certain arguments can be given that do not allow defining this tactic as the main one. In particular, an increase in drug potential automatically means a decrease in tolerability. Thus, in patients who notice the occurrence of certain side effects even during first-line therapy, tactics aimed at enhancing the potential of the drug can lead to worse tolerability and a decrease in the level of drug compliance [Thase M.
Thus, the assumption that the lack of sensitivity of depressive symptoms to the initially prescribed antidepressant indicates the ineffectiveness of the entire class of drugs with a similar mechanism of action has not been confirmed to date [A.J. Rush et al., 2006]. These data, presented in the form of practical recommendations, show that when changing therapy, it is justified to choose a drug not with the largest number of cumulative mechanisms of action, but with the maximum potential in relation to the leading neurotransmitter system (for SSRIs, this is paroxetine (Paxil) and sertraline). At the same time, certain arguments can be given that do not allow defining this tactic as the main one. In particular, an increase in drug potential automatically means a decrease in tolerability. Thus, in patients who notice the occurrence of certain side effects even during first-line therapy, tactics aimed at enhancing the potential of the drug can lead to worse tolerability and a decrease in the level of drug compliance [Thase M.![]() E., Rush A.J., 1997].
E., Rush A.J., 1997].
Antidepressant withdrawal policy
Discontinuation of drug antidepressant therapy (AD-therapy) may be accompanied by the occurrence of certain disorders, which are currently accepted to be classified as part of the "anti-adhesion discontinuation syndrome" (AD-AD cessation syndrome).
In earlier studies, the terms “AD withdrawal reaction” and “AD withdrawal syndrome” were more often used to denote pathological symptoms associated with antidepressant (AD) withdrawal [Rotschild, 1995; Dilsaver, 1984; Garner, 1993], which were later changed to emphasize the difference between these disorders and classical withdrawal, which already “reserved” these concepts for itself. However, until now, uncertainty remains in the terminological attribution of the same clinical manifestations, reflecting the opinion of a particular author about the advisability of considering them as withdrawal symptoms [Haddad, 2005].
Early descriptions of SPP AD appeared already in the course of clinical trials of the first ADs (imipramine) [Mann, 1959].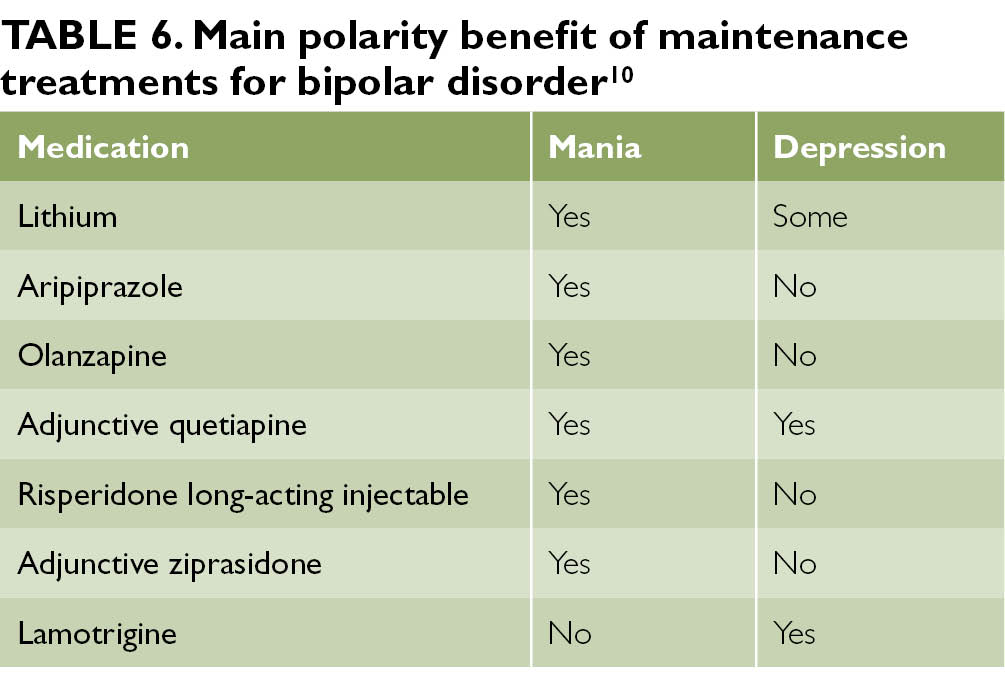 Subsequently, reports of similar disorders were given for almost all groups of AD: other tricyclic antidepressants (TCAs), MAO inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), selective serotonin and norepinephrine reuptake inhibitors (SNRIs) and other modern antidepressants [ Hadad, 2004]. It was revealed that the features and severity of SPP BP vary both in relation to different groups of drugs, and for representatives within the same class.
Subsequently, reports of similar disorders were given for almost all groups of AD: other tricyclic antidepressants (TCAs), MAO inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), selective serotonin and norepinephrine reuptake inhibitors (SNRIs) and other modern antidepressants [ Hadad, 2004]. It was revealed that the features and severity of SPP BP vary both in relation to different groups of drugs, and for representatives within the same class.
Despite the fact that usually SPP AD manifests itself within a few days after stopping the use of AD or reducing the dosage of the drug and is characterized by a short duration and relative mildness of manifestations, correct recognition if they occur is important, because. these conditions are associated with certain disturbances in daily activities, causing a marked decrease in performance, and their occurrence in a patient unaware of this possibility can lead to serious violations of drug compliance. Also, in some cases, SPP BP can take on a rather pronounced character, requiring hospitalization of the patient [Warner, 2006].
As the main measure to prevent the occurrence of SPP AD or minimize its manifestations, most authors declare the need to follow the prescribed regimen of taking the drug with the elimination of possible interruptions. If it is necessary to cancel blood pressure, it is advisable to follow the method of gradual dose reduction, taking into account the pharmacokinetic properties of a particular drug. The therapeutic value of the last recommendation, in our opinion, however, needs additional verification. According to the data of comparative studies, the gradual abolition of blood pressure allows to achieve an approximately two-fold decrease in the severity of SPP AD compared with one-stage [Van Geffen, 2005]. However, there are other data that show that the gradual withdrawal of the drug rather "blurs" the "rebound" symptoms throughout the entire period of withdrawal. If the manifestations of SPP BP are mild, this technique really makes it possible to additionally “smooth out” them, however, with a greater severity of violations, such tactics of “delaying” may be inappropriate [Yastrebov, 2007]. In addition, the result of gradual withdrawal does not guarantee an asymptomatic outcome of AD withdrawal.
In addition, the result of gradual withdrawal does not guarantee an asymptomatic outcome of AD withdrawal.
According to various sources, up to 50% of patients diagnosed with SPP AD discontinued the drug gradually [Barr, 1994; Pacheco, 169]. Among the factors associated with an increased risk of developing SPP AD, we can name episodes of reactions to the abolition of AD that have already been noted in the anamnesis. In this case, the risk of developing SPP BP increases to 75% [Black, 2000]. For such patients, when canceling AD, gradual withdrawal over 4–8 weeks is desirable, possibly under the “cover” of a short course of replacement therapy, which can be tranquilizers or AD with a different mechanism of action than the drug being withdrawn. These drugs, on the one hand, stop SPP BP, and on the other hand, allow the corresponding receptor subsystems (but not the concentration of the corresponding neurotransmitter) to return to the “pre-therapeutic” state (for example, when paroxetine is canceled - mirtazapine), which in most cases allows you to “cover up” the manifestations reactive symptomatology throughout its course up to complete regression [Warner, 2006].
Combination therapy
In the event that a certain positive shift in the clinical picture of depression is recorded, which, however, indicates only a partial response to therapy, and at the same time the tolerability of the initially prescribed antidepressant is assessed as satisfactory, it is proposed to use combination therapy with the addition of either another antidepressant ("dual therapy"). ”), or a drug of another class that enhances the effect of the main antidepressant (enhanced therapy). There are some differences between these two combination therapies.
"Dual" therapy involves the addition of a second antidepressant to an existing monotherapy. At the same time, unlike the procedure for changing an antidepressant, its mechanism of action, if possible, should not overlap with that of the “primary” drug. An example is the combination of a selective serotonin reuptake inhibitor (paroxetine, sertraline, citalopram) or a selective serotonin and norepinephrine reuptake inhibitor with a drug such as mirtazapine. However, this tactic has not been widely adopted for a variety of reasons. The main one is that this approach, despite the simplicity of its definition, is largely based on empirical experience and is difficult to rationally evaluate the effectiveness and formalize the assignment algorithm. To date, there are no comparable data on the effectiveness of various combinations of antidepressants; at the same time, the number of side effects, due to which patients stop treatment, increases significantly. Separately, it is necessary to specify the special care required in these cases in the choice of drugs to exclude undesirable drug interactions.
However, this tactic has not been widely adopted for a variety of reasons. The main one is that this approach, despite the simplicity of its definition, is largely based on empirical experience and is difficult to rationally evaluate the effectiveness and formalize the assignment algorithm. To date, there are no comparable data on the effectiveness of various combinations of antidepressants; at the same time, the number of side effects, due to which patients stop treatment, increases significantly. Separately, it is necessary to specify the special care required in these cases in the choice of drugs to exclude undesirable drug interactions.
An enhanced version of combination therapy involves the appointment of an antidepressant, coupled with one of the antipsychotics, or with drugs from the group of mood stabilizers. The latter include lithium salts, carbamazepine, salts of valproic acid, and lamotrigine (Table 3).
Limitations in the use of antidepressants
The choice of drugs for antidepressant therapy requires mandatory consideration of the characteristics of the clinical picture of an affective disease and its dynamics in general. So, R. El-Mallakh and A. Karippot (2006) indicate that the presence of a history of signs of a bipolar course makes the appointment of antidepressants alone undesirable because of the possibility of affect inversion, cycle acceleration and the appearance of prolonged irritable dysphoria.
So, R. El-Mallakh and A. Karippot (2006) indicate that the presence of a history of signs of a bipolar course makes the appointment of antidepressants alone undesirable because of the possibility of affect inversion, cycle acceleration and the appearance of prolonged irritable dysphoria.
Thus, it is suggested that antidepressant monotherapy is undesirable in patients with depression as part of bipolar disorder due to the possible worsening of the course of bipolar disorder [C.B. Nemeroff, 2001]. Official guidelines published by the American Psychiatric Association [APA Practice Guidelines, 2002] also recommend that antidepressants should not be used as monotherapy in these patients at all initially. Instead, a one-time appointment of at least combination therapy is proposed; it is recommended to use lithium salts or Lamictal (lamotrigine) along with antidepressants as first-line agents at the stage of active therapy. These recommendations are based on recent studies of depression in bipolar disorder defined as resistant to prior therapy (stage II resistance). It has been shown that the appointment of such patients with mood stabilizers (Lamictal (lamotrigine) at doses up to 150-250 mg per day) for combination with antidepressants can significantly increase the level of drug response in comparison with atypical antipsychotics (risperidone) and reduce the likelihood of relapse within a year (with 5% for risperidone to 24% for Lamictal (lamotrigine)) [A. A. Nierenberg et al., 2006].
It has been shown that the appointment of such patients with mood stabilizers (Lamictal (lamotrigine) at doses up to 150-250 mg per day) for combination with antidepressants can significantly increase the level of drug response in comparison with atypical antipsychotics (risperidone) and reduce the likelihood of relapse within a year (with 5% for risperidone to 24% for Lamictal (lamotrigine)) [A. A. Nierenberg et al., 2006].
Separate attention should be paid to the possibility of using drugs that do not belong to the class of antidepressants for monotherapy of depression in bipolar disorder. Existing data confirm the possibility of using some mood stabilizers and atypical antipsychotics in this capacity (Table 4) [K. Fountoulakis et al., 2007].
It must be noted that the appointment of some of the above drugs for long-term therapy (atypical antipsychotics, especially olanzapine), despite proven effectiveness, may be undesirable due to their inherent undesirable side effects, mainly manifested with long-term use (weight gain, metabolic disorders) .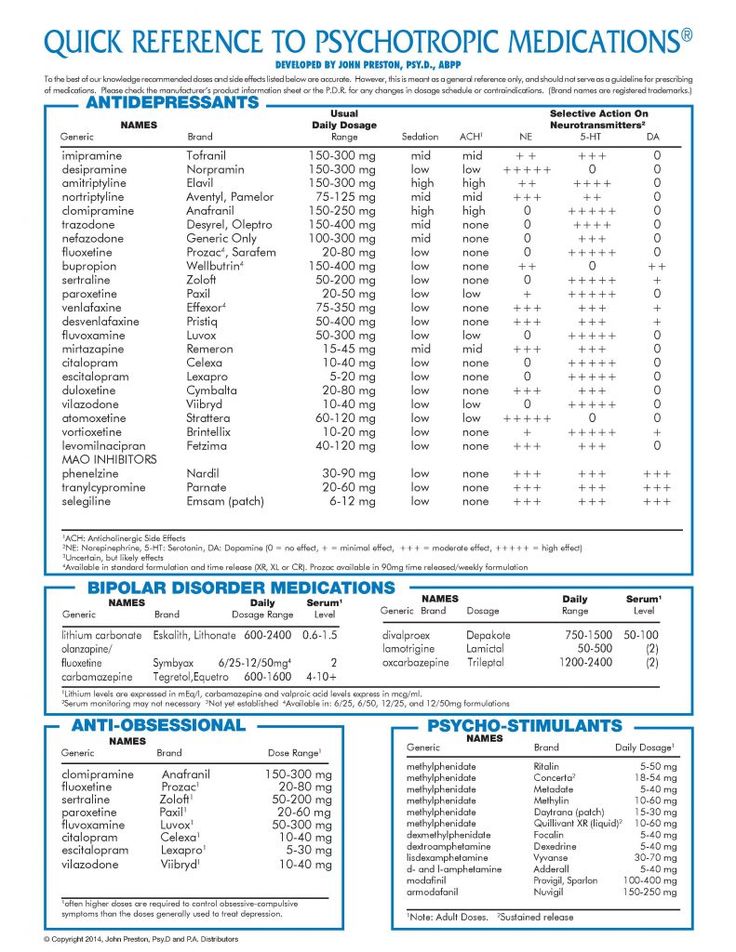
Long-term prophylactic monotherapy with lamotrigine for bipolar depression
Experience with long-term use of Lamictal has shown that this drug is equally effective in bipolar I and II disorders [Geddes, 2009]. The advantages of Lamictal, which justify its use at the stage of long-term preventive therapy, are: the effect on the residual manifestations of depression, the absence of rebound symptoms upon withdrawal, and the minimal presence of side effects [Calabrese, 2008]. Of particular value to the drug is the absence of the ability to cause weight gain with long-term use, which may justify the need to transfer patients with a tendency to obesity from other drugs (including lithium) to it [Bowden, 2006].
Supplement
Article sponsored by GlaxoSmithKline
1 HAMD: Hamilton Depression Rating Scale
Literature
1. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder (Revision).
 Am J Psychiatry 2002; 159 (April suppl).
Am J Psychiatry 2002; 159 (April suppl). 2. Anderson I. M., Nutt D. J., Deakin J. F. W. Evidence–based guidelines for treating depressive illness with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. J Psychopharmacol (2000), 14, 3–20.
3. Barr L. C., Goodman, W. K., Price, L. H. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry (1994), 151, 289.
3. Bird D., Haddad P. M., Dursun S. M. An overview of the definition and management of treatment–resistant depression. Klinik Psikofarmakoloji Bulteni 2002; 12:92–101.
4. Black K., Shea, C., Dursun, S., Kutcher, S. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry NeuroSci (2000), 25, 255–261.
5. Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials.
 Bipolar Disord 2008; 10:323–33.
Bipolar Disord 2008; 10:323–33. 6. Charles L. Bowden, M.D. Joseph R. Calabrese, M.D. Terence A. Ketter, M.D. Gary S. Sachs, M.D. Robin L. White, M.S. Thomas R. Thompson, M.D. Impact of Lamotrigine and Lithium on Weight in Obese and Nonobese Patients With Bipolar I Disorder. Am J Psychiatry 2006; 163:1199–1201.
7. Dilsaver S. C., Greden, F. J. Antidepressant withdrawal phenomena. Biol Psychiatry (1984), 19, 237–256.
8. Ghaemi S. N., Sachs G. S., Chiou A. M., Pandurangi A. K., Goodwin K. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52:135–44.
9. El–Mallakh R. S., Karippot A. Chronic depression in bipolar disorder. Am J Psychiatry. 2006 Aug;163(8):1337–1341; quiz 1478.
10. Fountoulakis K. N., Vieta E., Siamouli M. et al. Treatment of bipolar disorder: a complex treatment for a multi-facet disorder. Annals of General Psychiatry 2007, 6:27 (http://www.annals-general-psychiatry.com/content/6/1/27).
11.
 Garner E.M., Kelly, M.W., Thompson, D.F. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother (1993), 27, 1068–1072.
Garner E.M., Kelly, M.W., Thompson, D.F. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother (1993), 27, 1068–1072. 12. Haddad P.M. Do antidepressants cause dependence? Epidemiologie e Psychiatria Sociale (2005), 14, 58–62.
13. Haddad P. M., Anderson, I., Rosenbaum, J. (2004). Antidepressant discontinuation syndromes. In Haddad P., Dursun, P., Deakin, B. (Ed.), Adverse syndromes and psychiatric drugs: a clinical guide. (pp. 183–205). Oxford: Oxford University Press.
14. John R. Geddes, Joseph R. Calabrese and Guy M. Goodwin. Lamotrigine for treatment of bipolar depression. The British Journal of Psychiatry (2009) 194, 4–9.
15. Lehmann H. E. Therapy–resistant depressions–a clinical classification. Pharmakopsychiatr Neuropsychopharmakol 1974;7:156–63.
16. Mann A. M., MacPherson, A. Clinical experience with imipramine (G22355) in the treatment of depression. Canadian Psychiatric Association Journal (1959), 4, 38–47.
17. Messenheimer J., Mullens E.
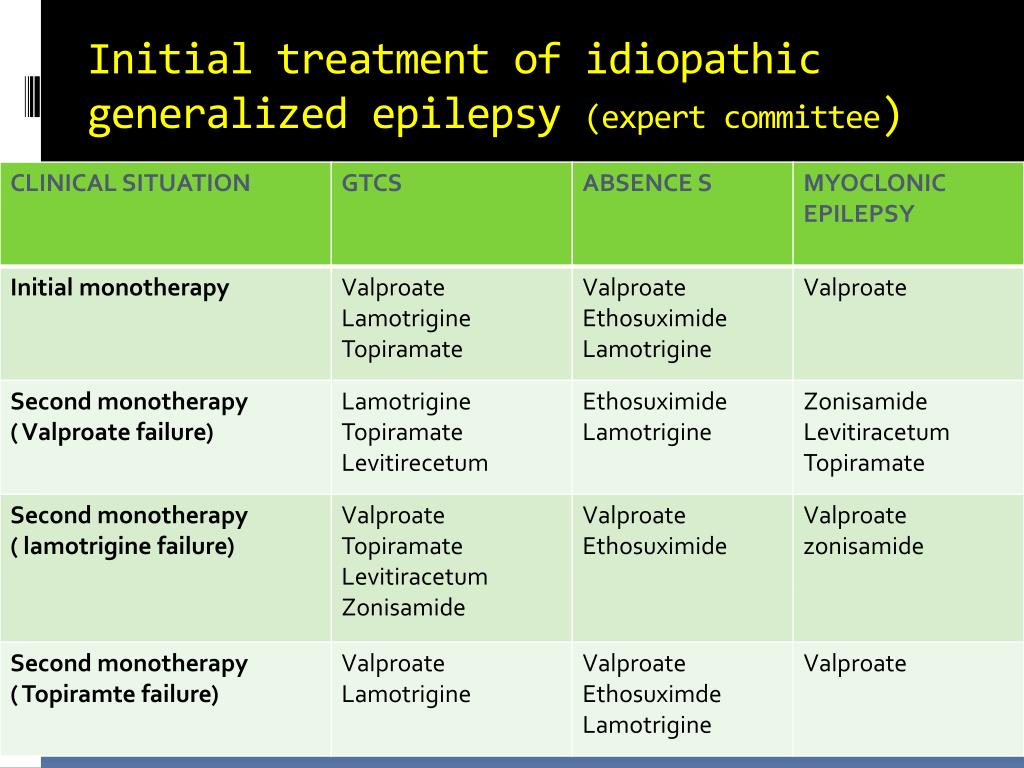 L., Giorgi L., Young F. Safety of adult clinical trial experience with lamotrigine. Drug Safety 1998, 18: 281–296.
L., Giorgi L., Young F. Safety of adult clinical trial experience with lamotrigine. Drug Safety 1998, 18: 281–296. 18. Nelsen M. R., Dunner D. L. Clinical and differential diagnostic aspects of treatment-resistant depression. Journal of Psychiatric Research. Vol. 29, 1, January–February 1995, 43–50.
19. Nemeroff C. B., Evans D. L., Gyulai L., Sachs G. S., Bowden C. L., Gergel I. P., Oakes R., Pitts C. D.: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912
20. Nierenberg A. A., Ostacher M. J., Calabrese J. R., Ketter T. A., Marangell L. B., Miklowitz D. J., Miyahara S., Bauer M. S., Thase M. E., Wisniewski S. R., Sachs G. S. Treatment–resistant bipolar depression: a STEP–BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006 Feb;163(2):210–6.
21. Pacheco L., Malo, P., Aragues, E., Etxebeste, M.
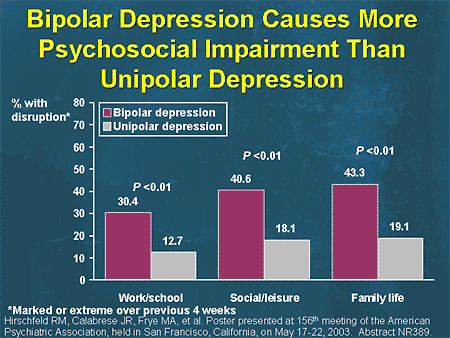 More cases of paroxetine withdrawal symptoms. Brit J Psychiatry (169), 169, 384.
More cases of paroxetine withdrawal symptoms. Brit J Psychiatry (169), 169, 384. 22. Rotschild A. J. Selective serotonin reuptake inhibitor–induced sexual dysfunction: efficacy of a drug holiday. Am J Psychiatry (1995), 152, 1514–1516.
23. Rush A. J., Kraemer H. C., Sackeim H. A., et al. Report by the ACNP Task Force on response and remission in major depressive disorder. neuropsychopharmacology. 2006;31:1841–1853.
24. Rush A. J., Trivedi M. H., Wisniewski S. R., Stewart J. W., Nierenberg A. A., Thase M. E., Ritz L., Biggs M. M., Warden D., Luther J. F., Shores–Wilson K., Niederehe G., Fava M. Bupropion–SR , sertraline, or venlafaxine–XR after failure of SSRIs for depression. N Engl J Med. 2006 Mar 23;354(12):1231–1242.
25. Santos M. A., Rocha F. L., Hara C. Efficacy and Safety of Antidepressant Augmentation With Lamotrigine in Patients With Treatment–Resistant Depression: A Randomized, Placebo–Controlled, Double–Blind Study. Prim Care Companion J Clin Psychiatry.
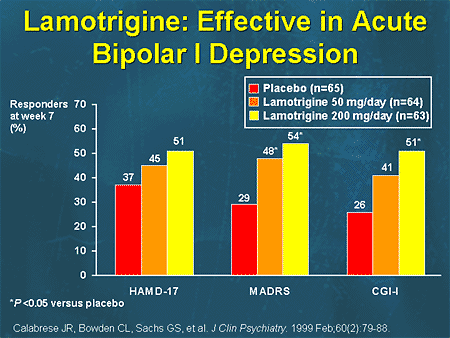 2008; 10(3): 187–190.
2008; 10(3): 187–190.26. Schatzberg A. F., Cole J. O., DeBattista C. Manual of Clinical Psychopharmacology. 4th ed. Washington DC: American Psychiatric Publishing, Inc.; 2003:42.
27. Souery D., Amsterdam J., de Montigny C., Lecrubier Y., Montgomery S., Lipp O., Racagni G., Zohar J., Mendlewicz J. Treatment resistant depression: methodological overview and operational criteria. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 1999;9(1–2):83–91.
28. Thase M. E., Rush A. J. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58:23–29.
29. Thase M. E., Ninan P. T. New goals in the treatment of depression: moving towards recovery. Psychopharma Bull. 2002;36(Suppl 2):24–35.
30. Van Geffen E.C., Hugtenburg, J.G., Heerdink, E.R., Van Hulten R.P., Egberts, A.C.G. Discontinuation symptoms in users of selective serotonin reuptake inhibitors in clinical practice: tapering versus abrupt discontinuation.