Zoloft dose for ocd
Sertraline in the treatment of obsessive compulsive disorder: two double-blind, placebo-controlled studies
Review
. 1992 Oct;7 Suppl 2:37-41.
doi: 10.1097/00004850-199210002-00007.
G Chouinard 1
Affiliations
Affiliation
- 1 Department of Psychiatry, University of Montreal, Hôpital Louis-H. Lafontaine, Quebec, Canada.
- PMID: 1484177
- DOI: 10.1097/00004850-199210002-00007
Review
G Chouinard. Int Clin Psychopharmacol. 1992 Oct.
. 1992 Oct;7 Suppl 2:37-41.
doi: 10.1097/00004850-199210002-00007.
Author
G Chouinard 1
Affiliation
- 1 Department of Psychiatry, University of Montreal, Hôpital Louis-H. Lafontaine, Quebec, Canada.
- PMID: 1484177
- DOI: 10.1097/00004850-199210002-00007
Abstract
Sertraline is a non-tricyclic, potent and selective serotonin reuptake inhibitor (SSRI) which is currently approved for the treatment of depression in several countries, including the UK and the USA. The role of serotonin in the aetiology of obsessive compulsive disorder (OCD) has been established through considerable indirect evidence. The strongest evidence comes from the fact that drugs known to be SSRIs have been found to be useful in the pharmacotherapy of OCD. Two double-blind, placebo-controlled studies, including a total of 412 patients, were undertaken to evaluate the efficacy and safety of sertraline in OCD. The first of these studies of a flexible dosing design showed that sertraline, given for eight weeks in daily dosages of 50-200 mg, was a safe and effective treatment for OCD, and superior to placebo. The second study of a fixed dose design and 12 weeks duration confirmed the efficacy and safety of sertraline in OCD at fixed dosages of 50, 100, or 200 mg/day and demonstrated that further improvement in OCD symptoms can be achieved through continued treatment with sertraline. A comparison between the results of these two studies and similar studies with clomipramine shows that, while both drugs have significant therapeutic efficacy, their side-effect profiles may be markedly distinct.
The role of serotonin in the aetiology of obsessive compulsive disorder (OCD) has been established through considerable indirect evidence. The strongest evidence comes from the fact that drugs known to be SSRIs have been found to be useful in the pharmacotherapy of OCD. Two double-blind, placebo-controlled studies, including a total of 412 patients, were undertaken to evaluate the efficacy and safety of sertraline in OCD. The first of these studies of a flexible dosing design showed that sertraline, given for eight weeks in daily dosages of 50-200 mg, was a safe and effective treatment for OCD, and superior to placebo. The second study of a fixed dose design and 12 weeks duration confirmed the efficacy and safety of sertraline in OCD at fixed dosages of 50, 100, or 200 mg/day and demonstrated that further improvement in OCD symptoms can be achieved through continued treatment with sertraline. A comparison between the results of these two studies and similar studies with clomipramine shows that, while both drugs have significant therapeutic efficacy, their side-effect profiles may be markedly distinct.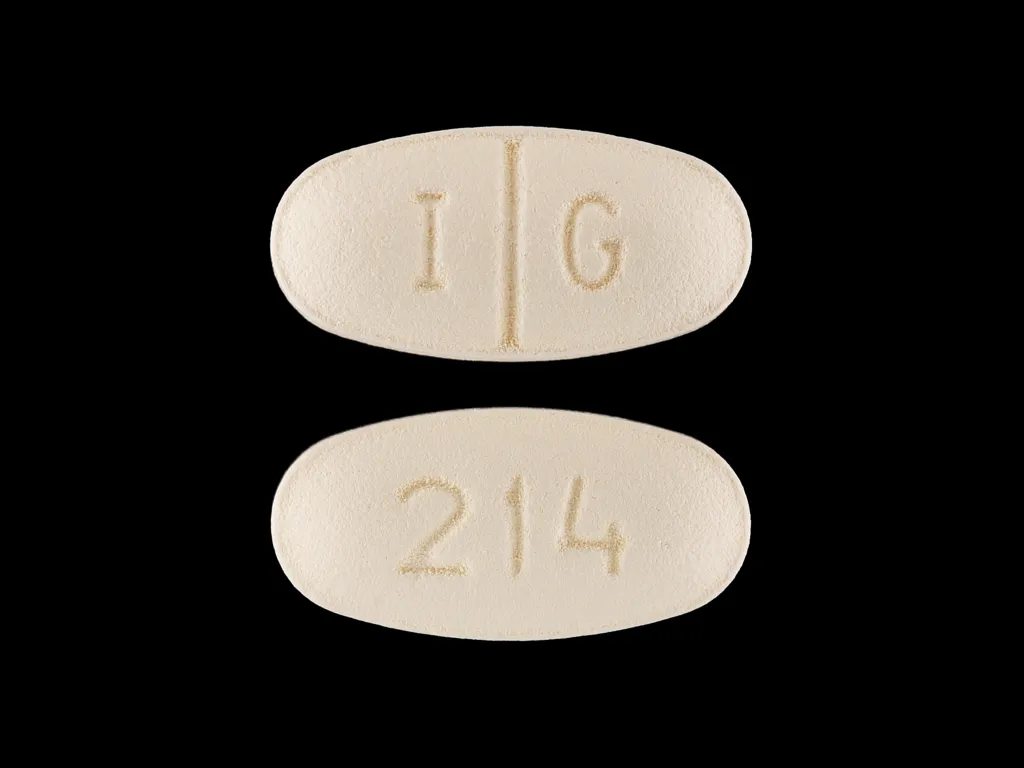
Similar articles
-
A 1 year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder.
Greist JH, Jefferson JW, Kobak KA, Chouinard G, DuBoff E, Halaris A, Kim SW, Koran L, Liebowtiz MR, Lydiard B, et al. Greist JH, et al. Int Clin Psychopharmacol. 1995 Jun;10(2):57-65. doi: 10.1097/00004850-199506000-00001. Int Clin Psychopharmacol. 1995. PMID: 7673657 Clinical Trial.
-
Double-blind parallel comparison of three dosages of sertraline and placebo in outpatients with obsessive-compulsive disorder.
Greist J, Chouinard G, DuBoff E, Halaris A, Kim SW, Koran L, Liebowitz M, Lydiard RB, Rasmussen S, White K, et al. Greist J, et al. Arch Gen Psychiatry.
 1995 Apr;52(4):289-95. doi: 10.1001/archpsyc.1995.03950160039008. Arch Gen Psychiatry. 1995. PMID: 7702445 Clinical Trial.
1995 Apr;52(4):289-95. doi: 10.1001/archpsyc.1995.03950160039008. Arch Gen Psychiatry. 1995. PMID: 7702445 Clinical Trial. -
A 2-year study of sertraline in the treatment of obsessive-compulsive disorder.
Rasmussen S, Hackett E, DuBoff E, Greist J, Halaris A, Koran LM, Liebowitz M, Lydiard RB, McElroy S, Mendels J, O'Connor K. Rasmussen S, et al. Int Clin Psychopharmacol. 1997 Nov;12(6):309-16. doi: 10.1097/00004850-199711000-00003. Int Clin Psychopharmacol. 1997. PMID: 9547132 Clinical Trial.
-
Obsessive-compulsive disorder: a new perspective in diagnosis and treatment.
Lydiard RB. Lydiard RB. Int Clin Psychopharmacol. 1994 Jun;9 Suppl 3:33-7. doi: 10.1142/9789814440912_0131. Int Clin Psychopharmacol.
 1994. PMID: 7963449 Review.
1994. PMID: 7963449 Review. -
A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder.
Pigott TA, Seay SM. Pigott TA, et al. J Clin Psychiatry. 1999 Feb;60(2):101-6. doi: 10.4088/jcp.v60n0206. J Clin Psychiatry. 1999. PMID: 10084636 Review.
See all similar articles
Cited by
-
Placebo Effect in Obsessive-Compulsive Disorder (OCD). Placebo Response and Placebo Responders in OCD: The Trend Over Time.
Kotzalidis GD, Del Casale A, Simmaco M, Pancheri L, Brugnoli R, Paolini M, Gualtieri I, Ferracuti S, Savoja V, Cuomo I, De Chiara L, Mosca A, Sani G, Girardi P, Pompili M, Rapinesi C, On Behalf Of The Sapienza Group For The Study Of The Placebo Effect In Psychiatric Disorders.
 Kotzalidis GD, et al. Curr Neuropharmacol. 2019;17(8):741-774. doi: 10.2174/1570159X16666181026163922. Curr Neuropharmacol. 2019. PMID: 30370851 Free PMC article. Review.
Kotzalidis GD, et al. Curr Neuropharmacol. 2019;17(8):741-774. doi: 10.2174/1570159X16666181026163922. Curr Neuropharmacol. 2019. PMID: 30370851 Free PMC article. Review. -
Current management of obsessive and phobic states.
Goljevscek S, Carvalho LA. Goljevscek S, et al. Neuropsychiatr Dis Treat. 2011;7:599-610. doi: 10.2147/NDT.S17032. Epub 2011 Sep 30. Neuropsychiatr Dis Treat. 2011. PMID: 22003299 Free PMC article.
-
Obsessive compulsive neurosis : clomipramine, prolactin and therapeutic response.
Ananth J, Kaur A, Poland R, Wohl M. Ananth J, et al. Indian J Psychiatry. 1997 Apr;39(2):154-9. Indian J Psychiatry. 1997. PMID: 21584063 Free PMC article.
-
The genetic studies of obsessive-compulsive disorder and its future directions.
Kim SJ, Kim CH. Kim SJ, et al. Yonsei Med J. 2006 Aug 31;47(4):443-54. doi: 10.3349/ymj.2006.47.4.443. Yonsei Med J. 2006. PMID: 16941732 Free PMC article. Review.
-
The search for new off-label indications for antidepressant, antianxiety, antipsychotic and anticonvulsant drugs.
Chouinard G. Chouinard G. J Psychiatry Neurosci. 2006 May;31(3):168-76. J Psychiatry Neurosci. 2006. PMID: 16699602 Free PMC article. Review.
See all "Cited by" articles
Publication types
MeSH terms
Substances
Zoloft Dosage Guide - Drugs.com
Print Save Generic name: SERTRALINE HYDROCHLORIDE 25mg
Dosage forms: tablet, film coated; oral concentrate
Drug class: Selective serotonin reuptake inhibitors
Medically reviewed by Drugs. com. Last updated on Oct 29, 2021.
com. Last updated on Oct 29, 2021.
Dosage in Patients with MDD, OCD, PD, PTSD, and SAD
The recommended initial dosage and maximum ZOLOFT dosage in patients with MDD, OCD, PD, PTSD, and SAD are displayed in Table 1 below. A dosage of 25 mg or 50 mg per day is the initial therapeutic dosage.
For adults and pediatric patients, subsequent dosages may be increased in case of an inadequate response in 25 to 50 mg per day increments once a week, depending on tolerability, up to a maximum of 200 mg per day. Given the 24-hour elimination half-life of ZOLOFT, the recommended interval between dose changes is one week.
| Indication | Starting Dose | Therapeutic Range |
|---|---|---|
| Adults | ||
| MDD | 50 mg | 50–200 mg |
| OCD | 50 mg | |
| PD, PTSD, SAD | 25 mg | |
| Pediatric Patients | ||
| OCD (ages 6–12 years old) | 25 mg | 50–200 mg |
| OCD (ages 13–17 years old) | 50 mg | |
Dosage in Patients with PMDD
The recommended starting ZOLOFT dosage in adult women with PMDD is 50 mg per day. ZOLOFT may be administered either continuously (every day throughout the menstrual cycle) or intermittently (only during the luteal phase of the menstrual cycle, i.e., starting the daily dosage 14 days prior to the anticipated onset of menstruation and continuing through the onset of menses). Intermittent dosing would be repeated with each new cycle.
ZOLOFT may be administered either continuously (every day throughout the menstrual cycle) or intermittently (only during the luteal phase of the menstrual cycle, i.e., starting the daily dosage 14 days prior to the anticipated onset of menstruation and continuing through the onset of menses). Intermittent dosing would be repeated with each new cycle.
- When dosing continuously, patients not responding to a 50 mg dosage may benefit from dosage increases at 50 mg increments per menstrual cycle up to 150 mg per day.
- When dosing intermittently, patients not responding to a 50 mg dosage may benefit from increasing the dosage up to a maximum of 100 mg per day during the next menstrual cycle (and subsequent cycles) as follows: 50 mg per day during the first 3 days of dosing followed by 100 mg per day during the remaining days in the dosing cycle.
Screen for Bipolar Disorder Prior to Starting ZOLOFT
Prior to initiating treatment with ZOLOFT or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [See Warnings and Precautions (5. 4)].
4)].
Dosage Modifications in Patients with Hepatic Impairment
Both the recommended starting dosage and therapeutic range in patients with mild hepatic impairment (Child Pugh scores 5 or 6) are half the recommended daily dosage [See Dosage and Administration (2.1, 2.2)]. The use of ZOLOFT in patients with moderate (Child Pugh scores 7 to 9) or severe hepatic impairment (Child Pugh scores 10–15) is not recommended [See Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant
At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of ZOLOFT. In addition, at least 14 days must elapse after stopping ZOLOFT before starting an MAOI antidepressant [See Contraindications (4), Warnings and Precautions (5.2)].
Discontinuation of Treatment with ZOLOFT
Adverse reactions may occur upon discontinuation of ZOLOFT [See Warnings and Precautions (5. 5)]. Gradually reduce the dosage rather than stopping ZOLOFT abruptly whenever possible.
5)]. Gradually reduce the dosage rather than stopping ZOLOFT abruptly whenever possible.
Preparation of ZOLOFT Oral Solution
ZOLOFT oral solution must be diluted before use.
- Use the supplied calibrated dropper to measure the amount of ZOLOFT oral solution needed
- Note: The supplied calibrated dropper has 25 mg and 50 mg graduation marks only
- Mix with 4 ounces (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. After mixing, a slight haze may appear, which is normal.
Instruct patients or caregivers to immediately take the dose after mixing.
Frequently asked questions
- SSRI’s vs SNRI’s - What's the difference between them?
- Lexapro vs Zoloft: How do they compare?
- How long for an increased dose of Zoloft to work?
- How long does Zoloft (sertraline) take to work?
- Does Zoloft (sertraline) cause weight gain?
- Prozac vs Zoloft - What are the Differences & Similarities?
- What are some common side effects of antidepressants?
- Is Zoloft (sertraline) a controlled substance?
- Can I take tramadol with sertraline?
More about Zoloft (sertraline)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1,870)
- Drug images
- Side effects
- Patient tips
- During pregnancy
- Generic availability
- Support group
- Drug class: selective serotonin reuptake inhibitors
- Breastfeeding
Patient resources
- Drug Information
- Zoloft (Sertraline Oral Liquid)
- Zoloft (Sertraline Tablets)
Professional resources
- Prescribing Information
Related treatment guides
- Obsessive Compulsive Disorder
- Major Depressive Disorder
- Panic Disorder
- Depression
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Medical Disclaimer
Treatment of comorbid obsessive-compulsive and depressive disorders in childhood
The problem of treating mental disorders in childhood is one of the most urgent and complex. This is due, on the one hand, to the complexity of their differential diagnosis in this age group and, accordingly, the choice of adequate therapy, and, on the other hand, to special care that must be taken when prescribing pharmacotherapy [5]. The latter is largely determined by the features of pharmacokinetics in an ontogenetically formed organism. The difference from adults is that in children the process of absorption of the drug, getting into the blood and its excretion is accelerated. It is also important to note a lower level of drug binding to blood proteins and, as a result, an increased amount of free fractions in the blood. This leads to a faster onset of both therapeutic effect and adverse events. In addition, drugs used in pediatric practice should have a wide “therapeutic window” so as not to pose a threat to life in case of overdose, especially in adolescents who are at risk for suicidal activity [1, 3] The incidence of side effects in drugs for pediatric practice should be minimal and they should be as mild and quickly passing as possible [3].
Given the high prevalence of depressive disorders in childhood, the issues of their treatment are especially relevant [4]. Over the past decades, there has been a steady increase in the frequency of these disorders, outstripping the general trend in the growth of mental disorders [2]. Depression leads to impaired social functioning and school performance. Adolescents suffering from depression are significantly more likely to commit suicide attempts, use psychoactive substances, are characterized by deviant behavior, difficult relationships with family and environment, and later in life have more problems in family, social and professional spheres. In the age group of 10-14 years, completed suicide is the fourth most common cause of death, and in the age group of 14-19years comes in third place [16]. All this determines the importance of early diagnosis and active treatment of depression in children and adolescents. The sooner depression is recognized, the less severe its consequences will be. The earlier treatment is started, the less effort and time it will require, and the better the result will be.
The earlier treatment is started, the less effort and time it will require, and the better the result will be.
The clinical picture of mental disorders in childhood is characterized by pronounced polymorphism, which is largely associated with the combination of several psychopathological syndromes [2], which often requires the appointment of combined therapy with antidepressants, anxiolytics and neuroleptics. Therefore, drugs used in pediatric practice need a favorable profile of drug interactions with a minimum of undesirable symptoms that occur when several drugs are co-administered [9-eleven]. In childhood, depression is most common with a predominance of somatic equivalents, as well as in combination with obsessive-compulsive disorders (OCD) and anxiety disorders, therefore, antidepressants with thymoleptic and simultaneously with anxiolytic properties that do not cause undesirable effects should be the most optimal for the treatment of childhood depression. side effects [16]. The emergence of drugs that selectively block serotonin reuptake (SSRIs) has significantly expanded the range of possibilities for treating OCD and depressive disorders in childhood. However, the existing age restrictions associated with the lack of relevant clinical trials are still an obstacle to providing the safest and most adequate therapy using these agents.
The emergence of drugs that selectively block serotonin reuptake (SSRIs) has significantly expanded the range of possibilities for treating OCD and depressive disorders in childhood. However, the existing age restrictions associated with the lack of relevant clinical trials are still an obstacle to providing the safest and most adequate therapy using these agents.
Thus, the problem of an adequate choice of antidepressant therapy in children, taking into account the age specificity of the psychopathological picture of depression, remains relevant. An important aspect of therapy in childhood should include the possibility of treatment with drugs that are sufficient to be taken once a day, which significantly improves patient compliance. These drugs include an antidepressant from the SSRI class Zoloft (sertraline) from Pfizer [5]. Due to the selective inhibition of serotonin uptake, it has a very weak effect on the reuptake of norepinephrine and dopamine, has no affinity for muscarinic cholinergic receptors, serotonin, dopamine, histamine, GABA-, benzodiazepine and adrenoreceptors and therefore does not have a stimulating, sedative or anticholinergic effect. Sertraline does not cause drug dependence and weight gain with long-term use. Data obtained in previous clinical trials on the high efficacy and safety of sertraline in the treatment of depression in children and adolescents substantiate the possibility of its use in this age group [8, 12-14, 17].
Sertraline does not cause drug dependence and weight gain with long-term use. Data obtained in previous clinical trials on the high efficacy and safety of sertraline in the treatment of depression in children and adolescents substantiate the possibility of its use in this age group [8, 12-14, 17].
One of the largest is an international multicentre, double-blind, placebo-controlled study of the effectiveness of sertraline treatment of depression in children and adolescents, conducted by S. Sharp and J. Hellings [16], who studied 376 people aged 6-17 years, whose diagnosis according to the DSM -IV met the criteria for major depressive disorder. Patients included in the main group took sertraline (zoloft) at a dose of 50 to 200 mg per day for 10 weeks. The Children Depression Rating Scale-Revised (CDRSR) and Clinical Global Impression (CGI) scales were used as assessment tools. A significant improvement in performance in children taking sertraline began to be observed already by the 7th day of admission. Side effects in the form of diarrhea, nausea and agitation were observed in only 7 people, 6 of whom belonged to the placebo group, which illustrates only the extremely high placebo reactivity characteristic of childhood.
Side effects in the form of diarrhea, nausea and agitation were observed in only 7 people, 6 of whom belonged to the placebo group, which illustrates only the extremely high placebo reactivity characteristic of childhood.
In other studies, side effects were also considered to be non-serious. So S. Donnely et al. [12], evaluating the efficacy and safety of sertraline in the treatment of 189 outpatients aged 6 to 17 years with a diagnosis of major depressive disorder, double-blind for 10 weeks, showed that sertraline is well tolerated: only 8% of patients interrupted treatment because of - for side effects (2.1% - in the placebo group). Adverse events included diarrhea, anorexia, and agitation. E. Tierney et al. [17] retrospectively assessed the therapeutic and side effects of sertraline used at doses of 25-200 mg per day in 33 patients aged 8 to 18 years with a diagnosis of major depressive disorder and identified side effects in 16 patients, while 8 patients discontinued therapy due to for these manifestations. Changes in behavior were observed in 7 patients (2 patients developed hypomania). M. Nixon et al. [14] followed 21 outpatients aged 12 to 18 years in an open 6-month study and found only minor side effects in the form of headaches.
Changes in behavior were observed in 7 patients (2 patients developed hypomania). M. Nixon et al. [14] followed 21 outpatients aged 12 to 18 years in an open 6-month study and found only minor side effects in the form of headaches.
Zoloft (sertraline) is the drug with the most extensive pharmacokinetic profile in childhood and adolescence of all SSRIs. The half-life of sertraline in children and adults is approximately the same, and the maximum concentration (Cmax) and clearance in the blood in children are slightly higher, and in adolescents somewhat lower than in adults, which is reflected in the dosing regimens of the drug in patients of different age categories [ 7] (Fig. 1) . Figure 1. Pharmacokinetic characteristics of sertraline in pediatric practice. However, in the Russian Federation, in children and adolescents aged 6 to 17 years, Zoloft is recommended for the treatment of only OCD, but not depression, despite the clinical experience obtained both in our country and abroad [6, 15], which shows a high antidepressant effect. effectiveness of Zoloft in this age group.
effectiveness of Zoloft in this age group.
Purpose of the study conducted in 2011-2012 in the Department for the Study of Problems of Child Psychiatry with the Childhood Autism Research Group of the Scientific Center for Mental Health of the Russian Academy of Medical Sciences, consisted in assessing the clinical efficacy, tolerability and safety of Zoloft in the treatment of comorbid OCD depression in pediatric patients. Zoloft was prescribed to patients on a planned basis as the drug of choice for the treatment of obsessive-compulsive symptoms. Studying the reduction of OCD symptoms was not part of the aim of the study.
Material and methods
We analyzed the condition of 26 inpatients aged 7 to 14 years (mean age 10.0±1.6 years) who had a combination of OCD and depressive disorders in the clinical picture. The picture of depression met the ICD-10 criteria for a depressive episode of moderate and mild severity (F-32). These disorders were observed in the structure of mood disorders (F3x) in 6 people, schizotypal disorder (F21x) - in 8, schizoaffective disorder (F25x) - in 5, OCD (F4x) in combination with mood disorders - in 7. Patients with schizotypal and schizoaffective disorders disorders received zoloft in combination with antipsychotics. Patients with comorbid depressive disorders and OCD received Zoloft monotherapy.
Patients with schizotypal and schizoaffective disorders disorders received zoloft in combination with antipsychotics. Patients with comorbid depressive disorders and OCD received Zoloft monotherapy.
Zoloft was administered once in the evening, starting at 25 mg. In the absence of individual intolerance, the next day the dose was increased to 50 mg per day. Subsequently, the dose was increased in steps of 25-50 mg per week until a therapeutic effect was achieved. The average effective dose of Zoloft was 100 mg per day (75 to 150 mg). The maximum dosage used mainly in children over 12 years of age was 150 mg per day. The dynamics of the condition was assessed weekly for 6 weeks of therapy using the Hamilton Depression Rating Scale (HAM-D 21), the Beck Subjective Depression Rating Scale, the Global Clinical Impression of Change (CGI-I), and the severity (CGI-S) mental state scales.
According to the structure of depressive symptoms, the patients were distributed as follows: 12 patients - depression with a predominance of anxiety; 5- melancholic depression; 9 - dysphoric depression (depression with behavioral disorders).
The severity of the depressive state of the patients included in the study before the start of Zoloft therapy corresponded to mild (4 patients), moderate (14), and severe (8) depression. The average score on the HAM-D scale was (16.4±3.2).
Results and discussion
All 26 patients included in the study completed the course of therapy, which is a high indicator of thymoanaleptic activity and tolerance to the drug. The proportion of patients receiving the maximum therapeutic dosage of the drug (150 mg per day) was 35% (9 patients), which also positively characterizes the tolerability of the drug.
The dynamics of depressive disorders during Zoloft therapy was characterized by a rapid onset (already on the 2nd week) of a positive therapeutic effect (a decrease by an average of 3.8 points on the HAM-D scale) and a further gradual reduction in depressive symptoms by the 6th week (fig. 2) .Figure 2. Dynamics of depressive symptoms according to the Hamilton scale. As the most common depressive symptoms in pediatric patients, manifestations of anxiety and sleep disturbances, as well as somatic symptoms, came to the fore. The effectiveness of Zoloft in relation to anxiety disorders was assessed by sections of the scale: mental anxiety, sleep disorders, general somatic symptoms and general depressive mood. First of all, positive changes concerned the general depressive mood and insomnia. Patients noted the disappearance of difficulties falling asleep, the restoration of the duration and depth of night sleep already from the end of the 1st - the beginning of the 2nd week of therapy, which made it possible to dispense with the additional prescription of tranquilizers with a hypnotic effect. At 2-4 weeks of therapy, anxiety, restlessness, separation anxiety, which easily occurs at the moment of separation from the mother or other significant people, decreased. The focus on anxious experiences lost its relevance, anxiety, fears of possible failures disappeared.
As the most common depressive symptoms in pediatric patients, manifestations of anxiety and sleep disturbances, as well as somatic symptoms, came to the fore. The effectiveness of Zoloft in relation to anxiety disorders was assessed by sections of the scale: mental anxiety, sleep disorders, general somatic symptoms and general depressive mood. First of all, positive changes concerned the general depressive mood and insomnia. Patients noted the disappearance of difficulties falling asleep, the restoration of the duration and depth of night sleep already from the end of the 1st - the beginning of the 2nd week of therapy, which made it possible to dispense with the additional prescription of tranquilizers with a hypnotic effect. At 2-4 weeks of therapy, anxiety, restlessness, separation anxiety, which easily occurs at the moment of separation from the mother or other significant people, decreased. The focus on anxious experiences lost its relevance, anxiety, fears of possible failures disappeared. Patients stopped complaining about the fear of the dark, being without parents, unfamiliar people or places. These changes were reflected in the reduction of scores in the mental anxiety section of the HAM-D scale. The general somatic symptoms of depression were reduced somewhat later - on the 4-6th week of therapy, which was expressed in the disappearance of complaints of abdominal pain, headache, feelings of lethargy and loss of strength.
Patients stopped complaining about the fear of the dark, being without parents, unfamiliar people or places. These changes were reflected in the reduction of scores in the mental anxiety section of the HAM-D scale. The general somatic symptoms of depression were reduced somewhat later - on the 4-6th week of therapy, which was expressed in the disappearance of complaints of abdominal pain, headache, feelings of lethargy and loss of strength.
The high overall effectiveness of treatment is reflected in the scores on the scales of the overall clinical impression - CGI-I and CGI-S. So, already from the 7th day of treatment, the value of the indicator of the severity of the disease decreased. According to the reduction of the indicator of the global assessment of the dynamics of the mental state, the significance was achieved by the 4th week of treatment. In terms of the effectiveness of therapy, there was a similar dynamics of changes in the state of patients (Fig. 3) . Figure 3. Dynamics of indicators on the CGI-I, CGI-S scales. The starting average total CGI-S score of 4.2 decreased to 2 points after 6 weeks of therapy. The mean CGI-I total score at the final visit (week 6) was 1.5 (significant improvement).
Dynamics of indicators on the CGI-I, CGI-S scales. The starting average total CGI-S score of 4.2 decreased to 2 points after 6 weeks of therapy. The mean CGI-I total score at the final visit (week 6) was 1.5 (significant improvement).
Clinically, as early as the 1st week of treatment, all children showed a significant improvement in well-being. The tearfulness disappeared rather quickly. At the 2nd week of therapy, the ability to express joy, appetite improved, and suicidal thoughts were deactivated. On the 3rd week, against the background of a gradual improvement, working capacity and social activity increased - the children willingly joined rehabilitation classes and began to attend classes.
Most of the patients included in the study did not experience adverse events while taking Zoloft. One patient noted increased morning sleepiness during the first 3 days of taking Zoloft at a dose of 50 mg per day, which disappeared on the 4th day with continued use of the drug at the same dose. After the 1st week of therapy, 2 patients with a diagnosis of schizotypal disorder developed signs of hypomania with behavioral disorders when the dose of Zoloft was increased to 100 mg per day. After reducing the dose of Zoloft to 50 mg per day and introducing a behavior-correcting drug (neuleptil - 3 mg per day), the condition returned to normal over the next week. In 1 patient, when the dose was increased to 150 mg per day, tremor of the fingers and tongue was noted, which was reduced when the dosage was reduced to 100 mg per day.
After the 1st week of therapy, 2 patients with a diagnosis of schizotypal disorder developed signs of hypomania with behavioral disorders when the dose of Zoloft was increased to 100 mg per day. After reducing the dose of Zoloft to 50 mg per day and introducing a behavior-correcting drug (neuleptil - 3 mg per day), the condition returned to normal over the next week. In 1 patient, when the dose was increased to 150 mg per day, tremor of the fingers and tongue was noted, which was reduced when the dosage was reduced to 100 mg per day.
In 2 cases, short-term episodes of nausea were noted during the 1st week, which did not recur later.
On the ECG and in the general clinical analyzes for a period of 6 weeks of therapy, no abnormalities were detected. There were no subjective complaints about side effects from the gastrointestinal tract and the autonomic nervous system (persistent nausea, vomiting, hypersalivation, diarrhea, accommodation disorders, excretion disorders). Common side effects of antidepressants, such as hypotension, tachycardia, and syncope, have also not been identified. All patients continued to take Zoloft after the end of the study - both inpatient (12 people) and outpatient. Data on the evaluation of adverse events are in good agreement with the results of previous studies and indicate a favorable tolerability and safety profile of Zoloft in pediatric patients with depression.
Common side effects of antidepressants, such as hypotension, tachycardia, and syncope, have also not been identified. All patients continued to take Zoloft after the end of the study - both inpatient (12 people) and outpatient. Data on the evaluation of adverse events are in good agreement with the results of previous studies and indicate a favorable tolerability and safety profile of Zoloft in pediatric patients with depression.
The data obtained on the efficacy and tolerability of Zoloft in the treatment of various types of depression in childhood and the possibility of its use in the combined therapy of depressive symptoms, including schizophrenic disorders, indicate the need for further study of modern antidepressants in order to expand age limits and provide children with the most modern pharmacological preparations.
Instruction Zoloft: how to take Zoloft, how long Zoloft works, why Zoloft
Composition
active substance: sertraline;
one film-coated tablet contains sertraline hydrochloride in an amount equivalent to 50 mg sertraline;
Excipients: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, hydroxypropyl cellulose, sodium starch, magnesium stearate, Opadry white (hypromellose, titanium dioxide (E 171), polyethylene glycol, polysorbate 80), Opadry transparent (hydroxypropyl methylcellulose, polyethylene glycol).
Indications.
Sertraline is indicated for the treatment of the following disorders:
Major depressive episodes. Prevention of recurrence of major depressive episodes.
Panic disorder with or without agoraphobia.
Obsessive-compulsive disorder (OCD) in adults and children 6-17 years of age.
Social anxiety disorder.
Post-traumatic stress disorder (PTSD).
Contraindications.
Hypersensitivity to the active substance or to any of the excipients listed in the Composition section.
Concomitant use of sertraline with irreversible MAOIs (MAOIs) is contraindicated due to the risk of developing serotonin syndrome with symptoms such as agitation, tremor and hyperthermia. Sertraline therapy should not be started for at least 14 days after stopping treatment with an irreversible MAO inhibitor. The use of sertraline should be discontinued at least 7 days before the start of therapy with an irreversible MAO inhibitor.
Concomitant use of sertraline and pimozide is contraindicated (see Section "Interaction with other medicinal products and other forms of interaction").
Dosage and administration.
how to use
Take sertraline once a day (morning or evening).
Sertraline tablets can be taken with or without food.
initiation of treatment
Depression and OCD
Treatment with sertraline should be initiated at a dose of 50 mg/day.
Panic disorder, PTSD and social anxiety disorder
Treatment should be initiated at a dose of 25 mg/day. After 1 week, the dose should be increased to 50 mg 1 time per day. It has been shown that this dosing regimen reduces the incidence of side effects characteristic of panic disorders at the initial stage of treatment.
dose titration
Depression, OCD, panic disorder, social anxiety disorder and PTSD
In patients not responding to the 50 mg dose, the effect may be achieved by increasing the dose. Dose adjustments should be made in increments of 50 mg at intervals of at least one week, until a maximum dose of 200 mg/day is reached. Dose adjustment should be carried out no more than 1 time per week, given the half-life of sertraline, which is 24 hours.
The first manifestations of a therapeutic effect can be observed within 7 days of treatment. However, a long period of time is usually required to achieve a therapeutic response, especially in patients with OCD.
maintenance dose
Dosage during long-term therapy should be kept at a low effective level with subsequent adjustment depending on the therapeutic response.
depression
Long-term therapy can be used to prevent recurrence of major depressive episodes (MAE). In most cases, the recommended dose to prevent recurrence of RES is the same as the dose used during the treatment of that depressive episode. Patients with depression should receive therapy for a sufficient time, for at least 6 months, to ensure the complete absence of symptoms.
Panic Disorders and OCD
Patients with panic disorders and OCD should be treated regularly during long-term therapy, as the drug has not been shown to be effective in preventing relapses for these disorders.
use in children
Children and adolescents with obsessive-compulsive disorder
Adolescents aged 13-17 years: the initial dose is 50 mg 1 time per day.
Children 6-12 years old: The initial dose is 25 mg once a day. After 1 week, the dose can be increased to 50 mg 1 time per day.
If necessary, in case of insufficient effect, further increase in the dose is possible with an increase of 50 mg 1 time over a period of several weeks. The maximum dose is 200 mg/day. However, when increasing the dose of 50 mg, the generally low body weight of children compared with adults should be taken into account. Do not change the dose more than once a week.
The efficacy of the drug in children with major depressive disorder has not been demonstrated.
Data on the use of the drug in children under 6 years of age are not available (see also section "Peculiarities of use").
Use in elderly patients
Elderly patients doses of the drug should be selected with caution, since these patients may have an increased risk of developing hyponatremia (see Section "Peculiarities of use").
Use in hepatic impairment
Caution should be exercised when using sertraline in patients with liver disease. In case of impaired liver function, it is necessary to reduce the dose or frequency of taking the drug to patients. Sertraline should not be used in patients with severe hepatic insufficiency, since there are no clinical data on the use of the drug in such a patient (see section "Peculiarities of use").
Use in renal failure
In case of impaired renal function, dose adjustment of the drug is not required (see section "Peculiarities of use").
Withdrawal symptoms observed upon discontinuation of sertraline therapy
Abrupt discontinuation of the drug should be avoided. When stopping treatment with sertraline, in order to reduce the risk of developing withdrawal reactions, the dose should be gradually reduced over at least 1-2 weeks (see Sections "Peculiarities of use" and "Adverse reactions"). If unbearable symptoms appear after discontinuation of the drug or discontinuation of its use, reinstatement of the drug at the previously prescribed dose may be considered.