The side effects of abilify
Common and Rare Side Effects for Abilify
COMMON side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
INFREQUENT side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
RARE side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
Full Drug Information
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Related Links
Drug Survey
Are you currently using Abilify?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
ABILIFY® (aripiprazole) | Official Site
IMPORTANT SAFETY INFORMATION:
Elderly people with psychosis related to dementia (for example, an inability to perform daily activities as a result of increased memory loss), treated with antipsychotic medicines including ABILIFY, are at an increased risk of death compared to placebo. ABILIFY is not approved for the treatment of people with dementia-related psychosis (see BOXED WARNING).
Antidepressant medicines may increase suicidal thoughts or behaviors in some children, teenagers, and young adults, especially within the first few months of treatment or when the dose is changed. Depression and other serious mental illnesses are themselves associated with an increase in the risk of suicide. Patients on antidepressants and their families or caregivers should watch for new or worsening depression symptoms, unusual changes in behavior, or thoughts of suicide. Such symptoms should be reported to the patient’s healthcare provider right away, especially if they are severe or occur suddenly. ABILIFY is not approved for use in pediatric patients with depression (see BOXED WARNING).
ABILIFY is not approved for use in pediatric patients with depression (see BOXED WARNING).
Do not take ABILIFY if you are allergic to aripiprazole or any of the ingredients in ABILIFY. Allergic reactions have ranged from rash, hives, and itching to anaphylaxis, which may include difficulty breathing, tightness in the chest, and swelling of the mouth, face, lips, or tongue.
ABILIFY may cause serious side effects, including:
- Unusual urges. Some people taking ABILIFY have had unusual urges, such as gambling, binge eating or eating that you cannot control (compulsive), compulsive shopping and sexual urges. If you or your family members notice that you are having unusual urges or behaviors, talk to your healthcare provider.
- Orthostatic hypotension (decreased blood pressure). Lightheadedness or fainting may happen when rising too quickly from a sitting or lying position.
- Falls.
 Taking ABILIFY may make you sleepy or dizzy, cause a decrease in your blood pressure when changing position, or slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries.
Taking ABILIFY may make you sleepy or dizzy, cause a decrease in your blood pressure when changing position, or slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries. - Low white blood cell count
- Seizures (convulsions)
- Problems with control of your body temperature especially when you exercise a lot or are in an area that is very hot. It is important for you to drink water to avoid dehydration. Avoid getting over-heated or dehydrated. Do not over-exercise. In hot weather, stay inside in a cool place if possible. Stay out of the sun. Do not wear too much or heavy clothing. Drink plenty of water.
- Difficulty swallowing that can cause food or liquid to get into your lungs.
Do not drive, operate heavy machinery, or do other dangerous activities until you know how ABILIFY affects you. ABILIFY may make you drowsy.
Before taking ABILIFY, tell your healthcare provider about all your medical conditions, including if you have or had:
- diabetes or high blood sugar in you or your family; your healthcare provider should check your blood sugar before you start ABILIFY and also during therapy.

- seizures (convulsions).
- low or high blood pressure.
- heart problems or stroke.
- pregnancy or plans to become pregnant. It is not known if ABILIFY will harm your unborn baby. If you become pregnant while taking ABILIFY, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/
- breast-feeding or plans to breast-feed. ABILIFY can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you receive ABILIFY.
- low white blood cell count.
- phenylketonuria. ABILIFY DISCMELT® Orally Disintegrating Tablets contain phenylalanine.
For patients who must limit their sugar intake, ABILIFY Oral Solution contains sugar.
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.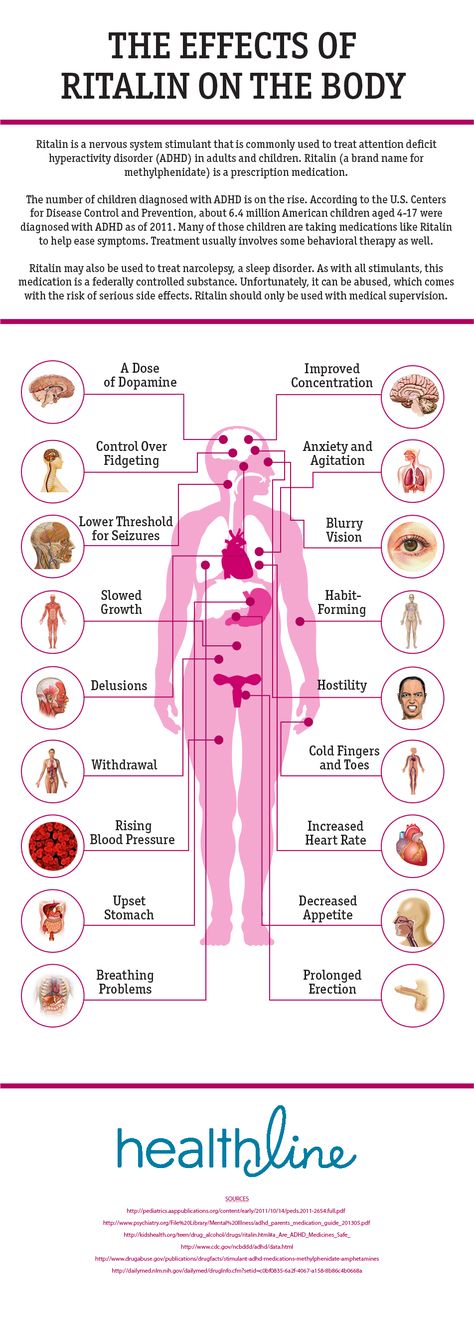
ABILIFY and other medicines may affect each other causing possible serious side effects. ABILIFY may affect the way other medicines work, and other medicines may affect how ABILIFY works. Do not start or stop any medicines while taking ABILIFY without talking to your healthcare provider first.
The most common side effects of ABILIFY in adults include: nausea, vomiting, constipation, headache, blurred vision, upper respiratory illness, dizziness, anxiety, insomnia, restlessness, and inner sense of restlessness or need to move (akathisia).
The most common side effects of ABILIFY in children include: feeling sleepy, headache, vomiting, fatigue, increased or decreased appetite, increased saliva or drooling, insomnia, nausea, stuffy nose, weight gain, uncontrolled movement such as restlessness or tremor, and muscle stiffness.
It is important to contact your healthcare provider if you experience prolonged, abnormal muscle spasms or contractions, which may be signs of a condition called dystonia.
These are not all the possible side effects of ABILIFY.
Call your doctor for medical advice about side effects.
If you have any questions about your health or medicines, talk to your healthcare provider.
You are encouraged to report side effects of ABILIFY. Please contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATIONS:
ABILIFY is indicated for:
- Treatment of Schizophrenia in adults and in adolescents 13 to 17 years of age
- Treatment of manic or mixed episodes associated with Bipolar I Disorder in adults and in pediatric patients 10 to 17 years of age
- Use as an add-on treatment to an antidepressant for adults with Major Depressive Disorder who have had an inadequate response to antidepressant therapy
- Treatment of irritability associated with Autistic Disorder in pediatric patients 6 to 17 years of age
- Treatment of Tourette’s Disorder in pediatric patients 6 to 18 years of age
Special Considerations for Pediatric Uses:
- Discuss the risks and benefits of treatment with your child’s healthcare provider.
 Treatment should be started only after a thorough diagnostic evaluation and as part of a total treatment program
Treatment should be started only after a thorough diagnostic evaluation and as part of a total treatment program
Please see U.S. FULL PRESCRIBING INFORMATION, including BOXED WARNING, and MEDICATION GUIDE.
Commonly prescribed autism drug linked to serious side effects
06/08/16
A new study shows that the antipsychotic risperidone (Rispolept) often leads to overweight and metabolic disorders in children with autism. Scientists call for careful prescribing and nutritional counseling for parents when taking it So far, only two drugs (the neuroleptics risperidone and aripiprazole) have been approved in the United States for the treatment of behavioral problems in children with autism. However, both drugs are associated with a risk of side effects. The most common unwanted side effect is being overweight.
The recommended approach for tantrums, aggression and self-injury in children with autism is behavioral therapy. However, in some cases it may not be enough to correct behavior. In such cases, doctors may recommend risperidone (Rispolept) to reduce these problems that interfere with the child's learning and social life.
However, in some cases it may not be enough to correct behavior. In such cases, doctors may recommend risperidone (Rispolept) to reduce these problems that interfere with the child's learning and social life.
However, risperidone can lead to weight gain, and a new study published in the Journal of the American Academy of Child and Adolescent Psychiatry confirms that weight gain with the drug may have undesirable effects on overall health. The results of the study showed that weight gain while taking risperidone is associated with a significant increase in insulin resistance and metabolic syndrome, which increases the risk of developing diabetes, heart disease and liver disease. (The same may be true for aripiprazole, which is also associated with a risk of weight gain, but was not studied in this study.)
We asked the lead author of the study, Lawrence Scahill of Emory University School of Medicine and Marcus Autism Center, a few questions to understand how the results of his study might influence the decisions of parents and doctors, and whether safer and more effective treatments can be expected in the future. . Dr. Scahill is a leading expert in behavioral and pharmacological treatments for autism.
. Dr. Scahill is a leading expert in behavioral and pharmacological treatments for autism.
Are you suggesting that the metabolic disturbances while taking risperidone are directly caused by weight gain? Is the increase in appetite the cause of weight gain? And why is it important to answer these questions?
It seems that the sequence of events here is as follows. Soon after the start of the drug, the child's appetite increases, which leads to an increase in daily food intake. Many, though not all, children begin to eat a lot of high-carbohydrate foods at this time. On average, after 24 weeks of clinical trials, children's weight increased by about 15% compared to baseline.
Faster weight gain was observed in those children whose parents reported an increase in appetite during the first eight weeks of the study. An increase in appetite was observed in three quarters of the sample. Importantly, we found a link between an increase in appetite and significant weight gain.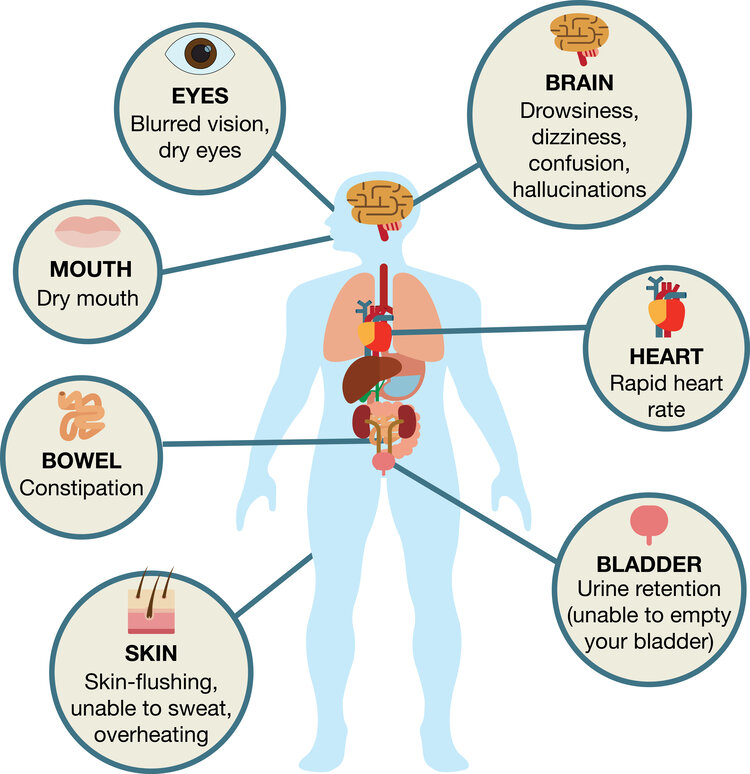
In your conclusions from the study, you suggest giving parents clear instructions on nutrition and weight control when prescribing risperidone. Who can provide such advice, and what should it ideally be?
The results of the study suggest that the prescribing physician or other specialist should talk to parents about the child's nutrition and food choices on the first day of prescribing. Parents should be advised that risperidone is very likely to cause increased appetite, which may lead to weight gain, and that this depends largely on what the child eats.
There should be no soda and other sugary drinks, sweets and the like at home. I know it sounds too harsh. However, rapid weight gain must be prevented. More importantly, drugs should only be used as a last resort if the child has serious behavioral problems.
During the 24 weeks of the study, each child taking risperidone was followed up by a physician who assessed behavioral changes and a physician who was in charge of taking the drug.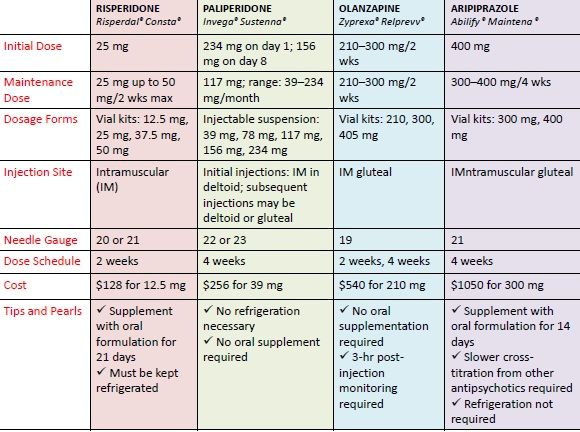 Why?
Why?
We wanted one specialist to focus entirely on whether there was improvement in the child's behavior, regardless of which group the child was in (in the risperidone-only group, or taking the drug at the same time as the parent education course). And of course, we had to watch the dosage and side effects of risperidone. This was done by a therapist as part of the study. During each examination, children were weighed and parents were asked questions about the child's appetite, sleep, and physical activity level to assess possible side effects.
In your study, how did you choose the dosage of the drug for each child?
In our study, the dose of the drug remained almost unchanged from the fourth week. Thereafter, changes were minimal, usually a reduction in dosage due to side effects.
Do you or other studies indicate that the negative effect on metabolism is related to the duration of treatment? What about other side effects such as breast enlargement or obsessive-compulsive behavior?
It is probably more accurate to say that metabolic disorders are associated with rapid weight gain. As already mentioned, risperidone seems to be able to lead to a dramatic increase in appetite, which leads to overweight. The metabolic effects were similar to those seen in obese children. However, the consequences of obesity are well known to worsen if a person remains overweight.
As already mentioned, risperidone seems to be able to lead to a dramatic increase in appetite, which leads to overweight. The metabolic effects were similar to those seen in obese children. However, the consequences of obesity are well known to worsen if a person remains overweight.
Many physicians and researchers have reported that risperidone can cause breast enlargement in boys and menstrual irregularities in girls. These side effects are likely related to increased levels of the hormone prolactin. The attending physician should discuss with parents their concerns about these side effects. In our study, we did not observe such phenomena, nor did we record an increase in compulsive behavior.
Your study compared risperidone dosage and side effects in a group of children whose parents participated in a parent education program and a group of children whose parents did not. What exactly did this training include? And did parental education help to reduce the required dosage of the drug? Did it prevent weight gain and other unwanted side effects?
The parent education program was based on the principles of applied behavior analysis. During the training, parents were taught specific strategies for dealing with temper tantrums, non-cooperation, and aggression. The program included 12-13 specialist home visits over 24 weeks. The study compared a group of children who were simply prescribed risperidone and a group of children who took risperidone and whose parents participated in the education program. Thus, children from both groups took the drug. In the group where parents were trained, the effective dose of the drug was slightly lower. Side effects in both groups did not differ.
During the training, parents were taught specific strategies for dealing with temper tantrums, non-cooperation, and aggression. The program included 12-13 specialist home visits over 24 weeks. The study compared a group of children who were simply prescribed risperidone and a group of children who took risperidone and whose parents participated in the education program. Thus, children from both groups took the drug. In the group where parents were trained, the effective dose of the drug was slightly lower. Side effects in both groups did not differ.
In your study, some children (22 out of 124 participants) did not experience an increase in appetite or weight when taking risperidone. How can this be explained?
It is not clear why about 20% of the sample did not have an increase in appetite. We know that children whose parents did not report increased appetite gained less weight. So far, we can conclude that changes in appetite and weight should be monitored from the start of taking the drug.
During your study, 12 children switched from risperidone to aripiprazole because they did not benefit from risperidone. Is there evidence that aripiprazole has fewer side effects?
The drug change was considered as part of the study. Existing evidence suggests that aripiprazole also leads to weight gain. We focused on risperidone because we had a large enough sample to collect clinically relevant information. In addition, children who switched to aripiprazole took risperidone for an average of eight weeks. So either or both drugs could affect weight in children who switched to aripiprazole.
If both drugs can lead to weight gain, why did your team only study risperidone?
We have been studying risperidone for a long time. We started studying it in the late 1990s with a grant from the US National Institutes of Health. In those days, risperidone was still considered a relatively new drug. We were interested in it because we thought it would be much better than the old antipsychotics in treating serious behavioral problems. Older antipsychotics caused serious neurological side effects in some children. We were looking for an alternative. Then our studies showed that risperidone is much less likely to cause neurological problems.
Older antipsychotics caused serious neurological side effects in some children. We were looking for an alternative. Then our studies showed that risperidone is much less likely to cause neurological problems.
In the first study, children with ASD and severe behavioral problems were randomly assigned to risperidone or an identical-looking placebo. Researchers, parents, and children did not know until the end of the study who was taking the active drug and who was taking the placebo. After eight weeks of treatment, behavioral problems were significantly reduced in the risperidone group of children. The placebo group showed little to no improvement.
After we had shown that risperidone could reduce problems such as tantrums, aggression and self-injury, we planned a second study. In this study, children with ASD and the same behavioral problems either simply took risperidone or took the drug, and their parents learned behavioral strategies for dealing with the problem behavior for six months. We did not include in the sample children who had previously taken risperidone or whose parents had already received similar training.
We did not include in the sample children who had previously taken risperidone or whose parents had already received similar training.
In the conclusion of your study, you say that more research is needed on safer treatments for risky behaviors associated with autism. Which treatment approaches seem more promising to you?
As I have already said, old generation antipsychotics have shown certain results in correcting serious behavioral problems in children with ASD. We hoped that new generation antipsychotics such as risperidone and aripiprazole would be better than older drugs. And so it turned out. But we have to go beyond just antipsychotics. We must also carefully plan large-scale randomized clinical trials of new drugs. For example, before investing in a multimillion-dollar study, we must conduct small trials to identify the most promising drugs. Biological measurements such as EEG, pulse changes, eye-tracking technologies, and direct behavioral assessments in the laboratory may be useful in such studies. Although this approach is not possible in large-scale trials, it is suitable for small-scale studies.
Although this approach is not possible in large-scale trials, it is suitable for small-scale studies.
What can you tell parents about drugs such as metformin, which can reduce the side effects of risperidone in some patients?
I don't particularly like prescribing another drug to treat the side effects of the first one, especially if it's a preventable problem. The best approach is to prescribe such drugs only to children with severe behavioral problems, and to advise parents on nutrition at the beginning of treatment.
Do you agree that lack of access to quality behavioral therapy leads to drug overuse?
The availability of effective behavioral therapy is a huge problem. A recent study by the US National Institutes of Health found that behavioral problems in children with ASD between the ages of 3 and 7 can only be reduced with parental education. So, at least in the case of young children, we can safely recommend parental education as the first line of intervention.
How can scientists, doctors, teachers and government officials solve this problem?
The Parental Education Study included 180 children. This is the largest randomized clinical trial of a behavioral intervention for children with ASD. Now we are looking for ways to introduce such training everywhere.
We hope that the information on our website will be useful or interesting for you. You can support people with autism in Russia and contribute to the work of the Foundation by clicking on the "Help" button.
Methods and treatments, Research, Psychiatry
Smart pills as an ethical issue - expert material, Lahta Clinic
in the focus of attention of the world medical community and became the subject of the widest discussion. The FDA, the most authoritative organization, whose opinion is heeded all over the world, "only" approved the drug Abilify MySite for use in clinical practice. The seemingly ordinary procedure for certification of a medicinal product gave rise to heated discussions and again raised a wave of public interest in such a painful at all times, urgent and acute problem as medical ethics.
The terms “smart tablet” (mostly dietary supplements) and “smart case for tablets”, as well as several pharmaceutical trademarks registered in the post-Soviet territory using the word “smart”, have nothing to do with that “smart pill "(eng. "smart pill"), which is devoted to this review.
Let's start with the trade name "Abilify MyCite". It is very difficult to translate this game of words-neologisms into Russian: something like “Give capacity ...” or “Bring back opportunities in my case (precedent)”. Previously, the drug "Abilify Maintena" (approx. translation "Source of Opportunities Supporting") has already been approved for use, certified in the Russian Federation under the current registration number LS-001812 dated 11/16/09and sold in pharmacies under the brand name Abilify.
The drug was developed and manufactured by one of the largest Japanese pharmaceutical corporations - Otsuka Pharmaceutical Co., Ltd. In America, Abilify is distributed jointly with Bristol-Myers Squibb (New York), a company in whose name, by the way, a Russian registration certificate has been issued. However, it was Otsuka who submitted the recently approved application for certification to the FDA. This time, she is performing in a somewhat unusual collaboration for a pharmaceutical giant with Proteus Digital Health, a Silicon Valley digital technology developer.
However, it was Otsuka who submitted the recently approved application for certification to the FDA. This time, she is performing in a somewhat unusual collaboration for a pharmaceutical giant with Proteus Digital Health, a Silicon Valley digital technology developer.
Abilify is the trade name for aripiprazole, one of the newer atypical antipsychotics. In turn, antipsychotics are a group of antipsychotic drugs that, since the second half of the 1990s, have become one of the breakthroughs in psychopharmacology, having a number of undeniable advantages over the "traditional" or "typical" first antipsychotics - chlorpromazine and haloperidol. Like other drugs in this group, aripiprazole is used primarily in the treatment of endogenous "major psychoses" - schizophrenia, bipolar disorder and psychotic depression; clinical trials are also underway for the efficacy of aripiprazole in the treatment of alcoholism. This drug has already proven itself quite well in psychiatric practice and, according to the results of comparative studies, surpasses clozapine, olanzapine, risperidone, etc. , released a little earlier, in a number of indicators. The FDA approved the use of aripiprazole in the United States in 2002.
, released a little earlier, in a number of indicators. The FDA approved the use of aripiprazole in the United States in 2002.
And today, after 15 years, Abilify with a new clarifying name is again in the spotlight. For the first time, a mass-produced dosage form containing the so-called. oral or "swallowed" tracer - a microscopic sealed radio frequency emitter that is located inside a standard capsule with the active substance. Once in the intestine, the emitter is activated and sends a signal to a miniature sensor-transmitter, which is attached, like a regular adhesive plaster, to the patient's stomach. This transmitter, in turn, transmits information about the medication to the smartphone. Thus, the patient can keep track of the time of admission and the number of doses he has already taken; in addition, he can provide the doctor and caregivers with access to this information through a dedicated web portal.
Mitchell Mathis, director of psychiatric drugs at the FDA's Center for Drug Research and Evaluation, believes that the ability to monitor prescription medications can be beneficial for some patients with mental disorders. According to M. Mathis - which, apparently, should be considered a reflection of the official position, since they are quoted in a press release - “FDA supports the development and application of new technology, therefore it intends to work with companies in studying all its benefits for patients and doctors."
According to M. Mathis - which, apparently, should be considered a reflection of the official position, since they are quoted in a press release - “FDA supports the development and application of new technology, therefore it intends to work with companies in studying all its benefits for patients and doctors."
So Abilify as such was certified 15 years ago; the oral sensor used in the new dosage form received FDA approval back in 2012. However, the official press release says nothing about the fact that a year and a half ago, an application for certification of a hybrid drug-and-device form was already submitted by Otsuka - and was rejected. The FDA then required companies to provide additional information about the intended use of the product and associated human risk factors, as well as to confirm that the hybrid drug can be safely and effectively used by patients.
In response, Otsuka and Proteus expressed “disappointment with the regulator’s decision,” which did not surprise commentators: a year and a half ago, a lot was written about the fact that the rejection of an application causes serious financial damage to companies, given the funds spent on the upgraded Abilify and fierce competition in the market (formerly only from authorized generic manufacturers of this drug).
Discussions today are dominated by restrained joy at the advancement of new technologies, but there is also some bewilderment. So, commentator Rebecca Robbins notes: forgetfulness and twilight consciousness can accompany those mental disorders for which Abilify is approved for treatment, and patients have to make a lot of effort to take the required medications on time. However, the new drug will come with a warning on the packaging: "Technology has not been proven to help patients take their medications as prescribed."
Immediately following the FDA decision, the journal Anesthesia & Analgesia published the results of a mini-study conducted in Boston on a sample of 15 patients who had suffered fractures and were prescribed opioid analgesics. The study used smart tablets similar to Abilify. The paper's lead author, Dr. Edward Boyer, says the new technology is highly promising, saying it provides a "direct measure" of how many opioids are being taken, which in some cases could be a warning sign of developing tolerance or dependence.
Professor of Psychiatry Tia P. Powell (Albert Einstein College of Medicine, New York) looks at the news from a completely different perspective.
“Unfortunately,” Dr. Powell's article begins, “unfortunately, the first FDA-approved smart pill was a drug used in the treatment of schizophrenia and bipolar disorder. This again raises confusing ethical questions."
Clinical experience and decades of research, it goes on to say, confirm that off-label drug use is a major problem in all branches of medicine. Smart pills can help forgetful people with diabetes, heart problems, and other drug-dependent patients who would like to achieve more and feel better with a prescribed treatment regimen. There is no doubt that, says Prof. Powell that mental illness drastically reduces the quality of life and its duration, and in this regard, drugs can really be useful. It is the WHO who defines mental disorders as the “major contributor” to the global damage from diseases in general. Psychotic episodes are typical of patients who are not taking psychiatrist-prescribed drugs, and such episodes not only further damage the brain, but often endanger both the patients themselves and others. Compliance with prescriptions for patients with mental illness is just as important as for somatic patients. What makes the high-tech version of aripiprazole problematic, however, is that this hybrid drug can easily be used in coercive treatment that ignores the values and preferences of the mentally ill themselves. In general, forced therapy is a long and painful problem in the history of psychiatry. In certain situations, a person with a mental disorder may be subjected, regardless of consent or disagreement, to inpatient or outpatient treatment, sometimes with compulsory medication. Of course, the best medicine in the world won't help, notes Tia P. Powell, if you don't take it. But only at 19In 1970, the US Supreme Court for the first time recognized a legal deficit in people hospitalized against their will.
Psychotic episodes are typical of patients who are not taking psychiatrist-prescribed drugs, and such episodes not only further damage the brain, but often endanger both the patients themselves and others. Compliance with prescriptions for patients with mental illness is just as important as for somatic patients. What makes the high-tech version of aripiprazole problematic, however, is that this hybrid drug can easily be used in coercive treatment that ignores the values and preferences of the mentally ill themselves. In general, forced therapy is a long and painful problem in the history of psychiatry. In certain situations, a person with a mental disorder may be subjected, regardless of consent or disagreement, to inpatient or outpatient treatment, sometimes with compulsory medication. Of course, the best medicine in the world won't help, notes Tia P. Powell, if you don't take it. But only at 19In 1970, the US Supreme Court for the first time recognized a legal deficit in people hospitalized against their will. Both clinicians and patients around the world struggle to strike the right balance between safety and individual rights.
Both clinicians and patients around the world struggle to strike the right balance between safety and individual rights.
A new dosage form is at the very center of this struggle. Manufacturers assure us that patients will consent to such treatment and the use of tracking technology. However, this smart tablet is very attractive for judicial applications. For those patients who are ordered to take drugs by the court, "consent" takes on a very different meaning if it is exchanged for freedom, custody of a child, or a reduced sentence.
I'll bet, says T. P. Powell, that people will look for ways to outsmart the tracker, while the manufacturers of this expensive drug have gone to great lengths to make it harder to disable the tracker. But I'd rather see an innovation that would serve to build trust and collaboration between doctor and patient.
We need to take a closer look not only at the benefits of Abilify MySite's hybrid drug, the article says, but also at the risks associated with it. It will help some people take their medication as prescribed, but it can also be a high-tech form of psychiatric coercion. If the new drug improves the treatment of "major psychoses", then it is necessary to carefully consider who, in fact, will benefit and who may suffer.
It will help some people take their medication as prescribed, but it can also be a high-tech form of psychiatric coercion. If the new drug improves the treatment of "major psychoses", then it is necessary to carefully consider who, in fact, will benefit and who may suffer.
Abilify MySite has been approved without any indication of its ethical use. “I am convinced,” Professor Powell concludes, “that both clinicians and consumers should be involved in the development of such ethical guidelines. True progress in psychiatry involves a genuine respect for those who struggle with mental illness, and not just the development of new ways to apply treatment to these people.
The publication evoked heated and sometimes diametrically opposed comments from specialists, patients, their relatives and people who are simply not indifferent to the problem.
Robert P.N. Shirin: “Professor Powell seems to be inclined to think of the new drug as something like a Trojan horse in the mouth. I wonder if she sees an ethical problem in DOT (the so-called "Treatment under direct supervision" - approx. Lakhta Clinic), which is a common practice in the fight against tuberculosis?
I wonder if she sees an ethical problem in DOT (the so-called "Treatment under direct supervision" - approx. Lakhta Clinic), which is a common practice in the fight against tuberculosis?
Steven J. Leitner: "The article ignores the fact that, say, long-acting drugs have been around in psychiatry for decades and perform essentially the same function."
Marie Lutz: “There is absolutely no evidence that psychotic episodes “destroy the brain”… Another question, and it is not raised in the article, is who and how will justify compulsory treatment with an antipsychotic that has such extensive and serious side effects. effects such as weight gain, tardive dyskinesia, akathisia and reduced life expectancy. In addition, it is doubtful that if atypical antipsychotics can help some people (usually for a short time), then they should necessarily be taken by all other patients. Personally, I believe that the new drug is a "bad faith development" and should be recalled.
Karen D'Angelo: “While I wholeheartedly support a patient's right to choose, as a neighbor of a schizophrenic patient, I also see the consequences of patients not taking their prescribed medications when the judicial system is overly concerned with ensuring compliance (compliance is therapeutic union of a doctor and a patient - approx.