Tegretol for bipolar
Carbamazepine (Tegretol) | NAMI: National Alliance on Mental Illness
Download PDF
Generic name: carbamazepine (kar ba MAZ e peen)
- Tablets: 200 mg
- Chewable tablets: 100 mg
- Oral suspension: 100 mg/5 mL
- Extended release tablets: 100 mg, 200 mg, 400 mg
- Extended release capsules: 100 mg, 200 mg, 300 mg
Brand names:
- Tegretol®
- Tablets: 200 mg
- Oral suspension: 100 mg/5 mL
- Tegretol XR®
- Extended release tablets: 100 mg, 200 mg, 400 mg
- Carbatrol®, Equetro®
- Extended release capsules: 100 mg, 200 mg, 300 mg
- Epitol®
- Tablets: 200 mg
All FDA black box warnings are at the end of this fact sheet. Please review before taking this medication.
What Is Carbamazepine And What Does It Treat?
Carbamazepine is a mood stabilizer medication that works in the brain. It is approved for the treatment of bipolar 1 disorder (also known as manic depression) as well as for epilepsy and trigeminal neuralgia. Bipolar disorder involves episodes of depression and/or mania.
Symptoms of depression include:
- Depressed mood — feeling sad, empty, or tearful
- Feeling worthless, guilty, hopeless, or helpless
- Loss of interest or pleasure in normal activities
- Sleep and eat more or less than usual (for most people it is less)
- Low energy, trouble concentrating, or thoughts of death (suicidal thinking)
- Psychomotor agitation (‘nervous energy’)
- Psychomotor retardation (feeling like you are moving in slow motion)
Symptoms of mania include:
- Feeling irritable or “high”
- Having increased self esteem
- Feeling like you don’t need to sleep
- Feeling the need to continue to talk
- Feeling like your thoughts are too quick (racing thoughts)
- Feeling distracted
- Getting involved in activities that are risky or could have bad consequences (e.
 g., excessive spending)
g., excessive spending)
Carbamazepine may also be helpful when prescribed “off-label” for behavioral or psychological symptoms of dementia. “Off-label” means that it hasn’t been approved by the Food and Drug Administration for this condition. Your mental health provider should justify his or her thinking in recommending an “off-label” treatment. They should be clear about the limits of the research around that medication and if there are any other options.
What Is The Most Important Information I Should Know About Carbamazepine?
Bipolar disorder requires long-term treatment. Do not stop taking carbamazepine, even when you feel better. With input from you, your health care provider will assess how long you will need to take the medicine. Missing doses of carbamazepine may increase your risk for a relapse in your mood symptoms.
Do not stop taking carbamazepine or change your dose without talking to with your healthcare provider first.
In order for carbamazepine to work properly, it should be taken every day as ordered by your healthcare provider.
Periodically, your healthcare provider may ask you to provide a blood sample to make sure the appropriate level of medication is in your body and to assess for side effects, such as changes in blood cell counts.
Are There Specific Concerns About Carbamazepine And Pregnancy?
If you are planning on becoming pregnant, notify your healthcare provider so that he/she can best manage your medications. People living with bipolar disorder who wish to become pregnant face important decisions. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
Carbamazepine has been associated with and increased risk of defects of the head and face, fingernails, and developmental delay. There may be precautions to decrease the risk of this effect. Do not stop taking carbamazepine without first speaking to your healthcare provider. Discontinuing mood stabilizer medications during pregnancy has been associated with a significant increase in symptom relapse.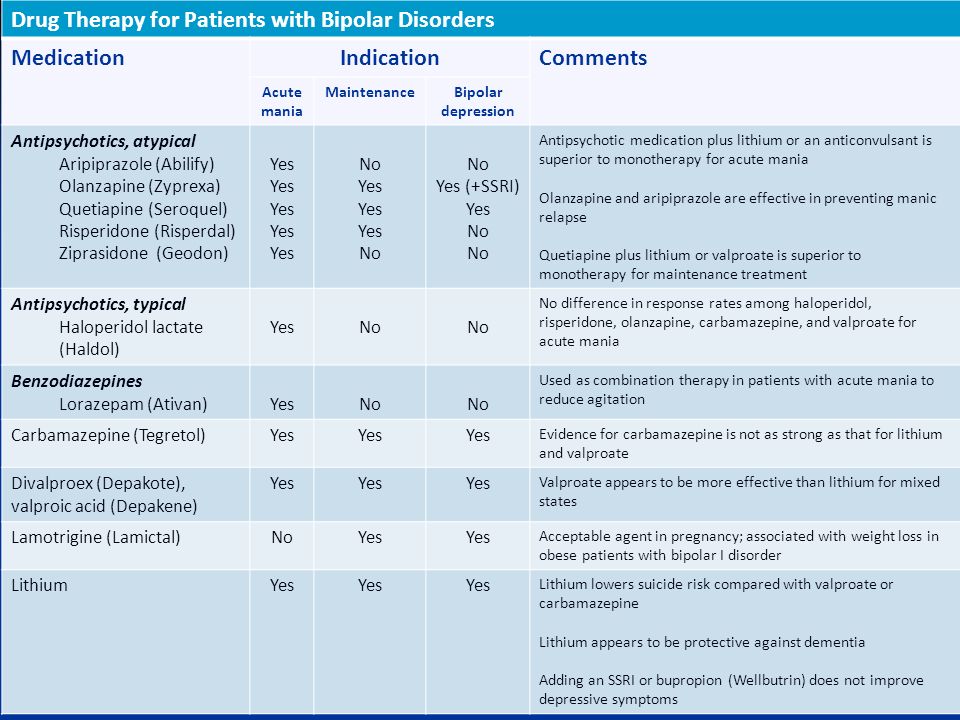
Regarding breast-feeding, caution is advised since carbamazepine does pass into breast milk. Talk with your doctor about whether or not it is safe to breastfeed while taking carbamazepine.
What Should I Discuss With My Health Care Provider Before Taking Carbamazepine?
- Symptoms of your condition that bother you the most
- If you have thoughts of suicide or harming yourself
- Medications you have taken in the past for your condition, whether they were effective or caused any adverse effects
- If you experience side effects from your medications, discuss them with your provider. Some side effects may pass with time, but others may require changes in the medication.
- Any other psychiatric or medical problems you have
- All other medications you are currently taking (including over the counter products, herbal and nutritional supplements) and any medication allergies you have
- Other non-medication treatment you are receiving, such as talk therapy or substance abuse treatment.
 Your provider can explain how these different treatments work with the medication.
Your provider can explain how these different treatments work with the medication. - If you are pregnant, plan to become pregnant, or are breast-feeding
- If you drink alcohol or use illegal drugs
How Should I Take Carbamazepine?
Carbamazepine is usually taken 2-4 times per day with or without food.
Typically patients begin at a low dose of medicine and the dose is increased slowly over several weeks.
The dose usually ranges from 200 mg to 1600 mg each day, but some patients may require more based on symptoms. Only your healthcare provider can determine the correct dose for you.
Carbamazepine suspension: Measure with a dosing spoon or oral syringe, which you can get from your pharmacy.
Extended-release capsules: Swallow whole or sprinkle onto food, such as applesauce or pudding and eat immediately. Do not chew the sprinkle capsule or contents.
Use a calendar, pillbox, alarm clock, or cell phone alert to help you remember to take your medication. You may also ask a family member a friend to remind you or check in with you to be sure you are taking your medication.
You may also ask a family member a friend to remind you or check in with you to be sure you are taking your medication.
What Happens If I Miss A Dose Of Carbamazepine?
If you miss a dose of carbamazepine, take it as soon as you remember, unless it is closer to the time of your next dose. Discuss this with your health care provider. Do not double your dose or take more than what is prescribed.
What Should I Avoid While Taking Carbamazepine?
Avoid drinking alcohol or using illegal drugs while you are taking carbamazepine. They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g., sedation) of the medication.
Avoid consuming large quantities (8 ounces or more) of fresh grapefruit juice, as this can increase levels of carbamazepine and increase your risk of side effects or rash.
What Happens If I Overdose With Carbamazepine?
If an overdose occurs call your doctor or 911. You may need urgent medical care. You may also contact the poison control center at 1-800-222-1222.
A specific treatment to reverse the effects of carbamazepine does not exist.
What Are The Possible Side Effects Of Carbamazepine?
Common side effects
- Dizziness or drowsiness
- Unsteadiness when standing or walking
- Nausea or vomiting
- Dry mouth
- Constipation
- Blurry or double vision
Rare/serious side effects
Carbamazepine can cause a decrease in the body’s sodium level. Some signs of low sodium include nausea, tiredness, lack of energy, headache, confusion, or more frequent or more severe seizures.
Carbamazepine may cause rare but serious blood problems including low white blood cell counts. Symptoms may include: fever, sore throat, or other infections that come and go or do not go away, easy bruising, red or purple spots on your body, bleeding gums or nose bleeds, or severe fatigue or weakness. Your doctor will occasionally order blood work to monitor for this.
In rare cases (<1%) a severe, spreading rash with blistering of the skin in patches over the entire body along with fever, headache and cough can occur (Stevens-Johnson syndrome). Although this is rare with carbamazepine, discontinuation of this medication is necessary. Rare cases of severe allergic reactions have been reported. Symptoms include swelling of the face, eyes, lips, or tongue, difficulty swallowing or breathing. If you experience any of these side effects, it is important to seek medical care immediately.
Studies have found that individuals who take antiepileptic medications including carbamazepine have suicidal thoughts or behaviors up to twice as often than individuals who take placebo (inactive medication). These thoughts or behaviors occurred in approximately 1 in 500 patients taking the antiepileptic class of medications. If you experience any thoughts or impulses to hurt yourself, you should contact your doctor immediately.
Are There Any Risks For Taking Carbamazepine For Long Periods Of Time?
To date, there are no known problems associated with long term use of carbamazepine. It is a safe and effective medication when used as directed.
It is a safe and effective medication when used as directed.
It is important to note that some of the side effects listed above (particularly changes in blood sodium, rash, and suicidal thoughts) may continue to occur or worsen if you continue taking the medication. It is important to follow up with your doctor for blood work and to contact your doctor immediately if you notice any skin rash or changes in mood or behavior.
What Other Medications May Interact With Carbamazepine?
The following medications may increase the level and effects of carbamazepine:
- The mood stabilizer and antiseizure medication valproic acid/divalproex (Depakote®)
- Certain antibiotics, such as ciprofloxacin (Cipro®), erythromycin (Ery-tab®), clarithrymycin (Biaxin®)
- Medications for heartburn or reflux, such as cimetidine (Tagamet®) and omeprazole (Prilosec®)
- Antifungal medications, such as ketoconazole (Nizoral®), fluconazole (Diflucan®), itraconazole (Sporanox®), voriconazole (Vfend®)
- Certain heart medications, such as diltiazem (Cardizem®) or verapamil (Calan®, Isoptin®)
- Certain medications used for mood or sleep, such as trazodone (Desyrel®) or nefazodone (Serzone®)
The following medications may decrease the level and effect of carbamazepine:
- Phenytoin (Dilantin®), phenobarbital, primidone (Mysoline®)
- Rifampin (Rifadin®)
Carbamazepine may decrease the level and effects of:
- Oral contraceptives
- Certain medications used for psychiatric disorders, such as lurasidone (Latuda®), aripiprazole (Abilify®), alprazolam (Xanax®), escitalopram (Lexapro®), trazodone (Desyrel®), and nefazodone (Serzone®)
- Certain heart medications, such as diltiazem (Cardizem®) or verapamil (Calan®, Isoptin®)
- Anti-rejection medications used in organ transplants, like tacrolimus (Prograf®) and cyclosporine (Neoral®, Sandimmune®)
- Certain cholesterol medications, like simvastatin (Zocor®), atorvastatin (Lipitor®)
- The blood thinner warfarin (Coumadin®)
- Antiseizure medications like phenytoin (Dilantin®), phenobarbital, and valproic acid/divalproex (Depakote®), lamotrigine (Lamictal®)
How Long Does It Take For Carbamazepine To Work?
It is very important to tell your doctor how you feel things are going during the first few weeks after you start taking carbamazepine. It will probably take several weeks to see big enough changes in your symptoms to decide if carbamazepine is the right medication for you.
It will probably take several weeks to see big enough changes in your symptoms to decide if carbamazepine is the right medication for you.
Mood stabilizer treatment is generally needed lifelong for persons with bipolar disorder. Your doctor can best discuss the duration of treatment you need based on your symptoms and illness.
Summary of FDA Black Box Warnings
Serious Skin Reactions and HLA-B*1502 Allele
Serious and sometimes fatal skin reactions have been reported with carbamazepine use. These reactions may be accompanied by mucous membrane ulcers, fever, or painful rash. Seek medical care immediately at the first sign of rash, as treatment must be stopped to avoid progression of the rash. These reactions are estimated to occur in 1 to 6 per 10,000 new users in countries with mainly Caucasian populations, but the risk in some Asian countries is estimated to be about 10 times higher. HLA-B*1502 is found almost exclusively in patients with ancestry across broad areas of Asia. Patients with ancestry in genetically at-risk populations should be screened for the presence of HLA-B*1502 prior to initiating treatment with carbamazepine.
Patients with ancestry in genetically at-risk populations should be screened for the presence of HLA-B*1502 prior to initiating treatment with carbamazepine.
Aplastic Anemia and Agranulocytosis
Carbamazepine has been associated with a condition where the body does not make enough new blood cells and also with a decrease in white blood cells. People taking carbamazepine can be at an increased risk of infection if white blood cell counts drop too low.
Provided by
(January 2023)
©2022 The American Association of Psychiatric Pharmacists (AAPP) and the National Alliance on Mental Illness (NAMI). AAPP and NAMI make this document available under the Creative Commons Attribution-No Derivatives 4.0 International License. Last Updated: January 2016.
This information is being provided as a community outreach effort of the American Association of Psychiatric Pharmacists. This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The American Association of Psychiatric Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The American Association of Psychiatric Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
Tegretol (Carbamazepine) for Bipolar Disorder
What is Tegretol?
Tegretol is an antiseizure medication used to treat manic, hypomanic, or mixed episode symptoms of bipolar disorder. It is also used to treat seizures and nerve pain.
When did the U.S. Food and Drug Administration (FDA) approve the medication?
Tegretol was first approved by the FDA in 1968, originally for treatment of trigeminal neuralgia, a nerve pain disorder.
Is there a generic version of Tegretol?
Yes, the generic version is known as carbamazepine and is sold in the U.S.
Are there any major differences between Tegretol and other medications used to treat bipolar disorder?
Tegretol belongs to the class of medications known as antiseizure drugs, or anticonvulsants. These are sometimes taken to treat or prevent the manic, hypomanic, or mixed episodes associated with bipolar disorder. Tegretol may be prescribed alone or in combination with other medications to treat symptoms.
What warning information do I need to know about Tegretol?
Tegretol may cause a life-threatening allergic reaction known as Stevens-Johnson syndrome or toxic epidermal necrolysis. This can cause damage to your internal organs or skin. The primary risk factor is genetic, with people of Asian ancestry being at higher risk. Talk to your doctor about getting genetic testing to evaluate the risk for this rare but severe allergic reaction to taking Tegretol. Tegretol can also decrease the number of blood cells in the body, so talk to your doctor if you already have a low number or are taking other medications that increase this risk.
Tegretol can also decrease the number of blood cells in the body, so talk to your doctor if you already have a low number or are taking other medications that increase this risk.
Can children take Tegretol?
Children over the age of 6 can be prescribed Tegretol for seizures. Talk to your doctor about the risks and benefits and potential side effects to monitor.
Are there potential interaction issues for people taking Tegretol and any other drugs?
There are hundreds of drugs which are known to interact with Tegretol in major, moderate, or mild ways, so let your doctor know what other medications, including any vitamins or over the counter prescriptions, you are taking before you begin taking the medication. Some of these might include medication for mental illness such as depression or anxiety, antifungals, seizure medications, HIV protease inhibitors, malaria medications, or sleeping medications. Tegretol may also decrease the effectiveness of hormonal contraceptives.
Are there any other medical conditions that would make someone ineligible for Tegretol therapy?
Talk to your doctor about other medical conditions before you take Tegretol, such as glaucoma, heart disease, history of low sodium, thyroid or liver disease, or a history of psychosis. Also tell your doctor if you have a history of low blood cell counts.
What is the typical dose that would be prescribed to someone taking Tegretol?
Dosage will vary depending on the age of the patient and the condition being treated.
What do I do if I miss a dose?
Take the dose of Tegretol when you remember, but skip the missed dose if it is almost time for your next dose. You should never take extra doses of the medication to make up for missed doses.
What side effects can Tegretol cause?
Common side effects can include:
nausea
dry mouth
constipation
dizziness
drowsiness
It also is recommended that you wait to drive or operate machinery until you know how the medication affects you and to avoid alcohol and illegal drugs while on the medication, as they can worsen adverse effects. Report major side effects to your doctor immediately, which can include swelling, shortness of breath, skin reaction, and fever. Report side effects to the FDA at 1-800-FDA-1088 or online.
Report major side effects to your doctor immediately, which can include swelling, shortness of breath, skin reaction, and fever. Report side effects to the FDA at 1-800-FDA-1088 or online.
What are the potential psychological side effects of taking Tegretol?
Antiseizure medications sometimes cause suicidal thoughts and behaviors in a small percentage of people who take the medication. Seek medical help if you experience these thoughts or other changes in your behavior or mood.
What are the potential long-term effects of taking Tegretol?
Tegretol can cause lower blood counts and sodium levels, and increase your risks for vitamin D deficiency and osteoporosis. Your doctor may want to monitor these periodically while you’re taking the medication.
Is it safe for a woman who is pregnant, about to become pregnant, or nursing to take Tegretol?
Tegretol can cause birth defects and fetal harm when taken during pregnancy. The drug can be transferred via breast milk and potentially harm a baby. Therefore, talk to your doctor if you are pregnant, planning to become pregnant, or are nursing before you take Tegretol.
Therefore, talk to your doctor if you are pregnant, planning to become pregnant, or are nursing before you take Tegretol.
Can symptoms occur if Tegretol is discontinued?
It’s important not to discontinue use of the drug before talking with your doctor. Withdrawal symptoms of Tegretol can include sleep problems, increased anxiety, numbness in limbs, joint pain, shaking, and the return of manic or depressive symptoms.
What should I do if I overdose on Tegretol?
An overdose of Tegretol could be fatal, so seek immediate help or call the Poison Help Line at 1-800-222-1222 if you overdose. Overdose symptoms can include abnormal or uncontrollable movements, restlessness, seizures, loss of balance, dizziness, drowsiness, blurred vision, slower or irregular breathing, rapid heartbeat, nausea, vomiting, trouble urinating, and unconsciousness.
Is Tegretol habit-forming?
Tegretol has no habit-forming potential, but it is not recommended that you discontinue use of the drug before talking with your doctor, as withdrawal symptoms can occur.
How much does Tegretol cost?
According to goodrx.com, 60 tables of 200 mg generic carbamazepine cost approximately $65. 60 tablets of 200 mg Tegretol cost approximately $150.
Are there any disadvantages to Tegretol?
The biggest disadvantages of Tegretol are potential side effects, which include severe allergic reaction and decreased blood cell counts, and increased risk for drug interactions. Pregnant women are also typically advised not to take the medication due to the risk of birth defects.
DISCLAIMER: The information contained herein should NOT be used as a substitute for the advice of an appropriately qualified and licensed physician or other healthcare providers. This article mentions drugs that were FDA-approved and available at the time of publication and may not include all possible drug interactions or all FDA warnings or alerts. The author of this page explicitly does not endorse this drug or any specific treatment method. If you have health questions or concerns about interactions, please check with your physician or go to the FDA site for a comprehensive list of warnings.
- FDA – Tegretol
- NIH – Carbamazepine
Notes: This article was originally published January 2, 2017 and most recently updated August 26, 2022.
Co-administration of carbamazepine and lithium in the treatment of outpatients with fast cycling bipolar disorder without psychiatric comorbidity: a randomized, double-blind, placebo-controlled trial | Aliev
1. Blanco C, Compton WM, Saha TD, Goldstein BI, Ruan WJ, Huang B, Grant BF. Epidemiologic Survey on Alcohol and Related Conditions-III. J. Psychiatr. Res. 2017;84:310–317. PMID: 27814503 PMCID: PMC7416534 DOI: 10.1016/j.jpsychires.2016.10.003
2. Cloutier M, Greene M, Guerin A, Touya M, Wu E. The economic burden of bipolar I disorder in the United States in 2015. J. Affect. Disorder. 2018;226:45–51. PMID: 28961441 DOI: 10.1016/j.jad.2017.09.011
3. Maj M, Pirozzi R, Magliano L, Bartoli L. The long-term result of lithium proaxis in bipolar disorder: a 5-year prospective study of 402 patients in a lithium clinic. Am. J. Psychiatry. 1998;155:30–35. PMID: 9433335 DOI: 10.1176/ajp.155.1.30
Am. J. Psychiatry. 1998;155:30–35. PMID: 9433335 DOI: 10.1176/ajp.155.1.30
4. Fountoulakis KN, Kontis D, Gonda X, Yatham LN. A systematic review of the evidence on the treatment of rapid cycling bipolar disorder. 2013;15:115–137. PMID: 23437958 DOI: 10.1111/bdi.12045
5. Carvalho AF, Dimellis D, Gonda X, Vieta E, Mclntyre RS, Fountoulakis KN. Rapid cycling in bipolar disorder: a systematic review. J.Clin. Psychiatry. 2014;75:e578–586. PMID: 25004199 DOI: 10.4088/JCP.13r08905
6. Goldberg JF, Bowden CL, Calabrese JR, Ketter TA, Dann RS, Frye MA, Suppes T, Post RM. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in rapid cycling bipolar disorder. Biol. Psychiatry. 2008;63:125–130. DOI: 10.1016/j.biopsych.2006.12.031
7. Aditya H, Aditi S, Malvika R, Janardhanan CN, Suresh BM. Pramipexole in the treatment of refractory depression in a patient with rapid cycling bipolar disorder. Indian J. Psychol. Med. 2015;37:473–474. PMCID: PMC4676223 PMID: 26702189 DOI: 10.4103/0253-7176.168614
PMCID: PMC4676223 PMID: 26702189 DOI: 10.4103/0253-7176.168614
8. Chen P, Huang Y. Remission of classic rapid cycling bipolar disorder with levothyroxine augmentation therapy in a male patient having clinical hypothyroidism. Neuropsychiatr Dis. treat. 2015;11:339–342. DOI: 102147/NDT.S76973
9. Viguera AC, Baldessarini RJ, Tondo L. Response to lithium maintenance treatment in bipolar disorders: comparison of women and men. Bipolar Discord. 2001;3:245–252. PMID: 11903207 DOI: 10.1034/j.1399-5618.2001.30503.x
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatric Association. 2013.
11. Glantz SA. Primer of Biostatistics. Seventh Edition. 2012:321. ISBN-13: 978-0071781503; ISBN-10: 0071781501
12. Huber J, Burke D. ECT and lithium in old age depression—cause or treatment of rapid cycling? Australas Psychiatry. 2015;23:500–502. PubMed DOI: 10.1177/1039856215591328
13. Maj M, Pirozzi R, Formacola AMR, Tortrella A. Reliability and validity of four alternative definitions of bipolar disorder with fast cycling. Am. J. Psychiatry. 1999;158:303–305. PMID: 10484955. DOI: 10.1176/ajp.156.9.1421
Maj M, Pirozzi R, Formacola AMR, Tortrella A. Reliability and validity of four alternative definitions of bipolar disorder with fast cycling. Am. J. Psychiatry. 1999;158:303–305. PMID: 10484955. DOI: 10.1176/ajp.156.9.1421
14. Sampath H, Sharma I, Dutta S. Treatment of suicidal depression with ketamine in rapid cycling bipolar disorder. Asia Pack. Psychiatry. 2016;8:98–101. PMID: 26871425 DOI: 10.1111/appy.12220
15. Goldberg JF, Bowden CL, Calabrese JR, Ketter TA, Dann RS, Frye MA, Suppes T, Post RM. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in rapid cycling bipolar disorder. Biol. Psychiatry. 2008;63:125–130. DOI: 10.1016/j.biopsych.2006.12.031
16. Bobo WV, Vande Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA. Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety. 2016;33(8):698–710. DOI: 10.1002/da.22505. Epub 2016 Apr 6. PMID: 27062450
PMID: 27062450
17. Calabrese JR, Fatemi SH, Kujawa M, Woyshville MJ. Predictors of response to mood stabilizers. J.Clin. Psychopharmacol. 1996;16:24–31. PMID: 8707997 DOI: 10.1097/00004714-199604001-00004
18. Baldessarini RJ, Tondo L, Hennen J, Viguera AC. Is Lithium Still Worth Using? An Update of Selected Recent Research. Harvard Review of Psychiatry. 2002; 10(2):59–75. DOI: 10.1080/hrp.10.2.59.75
19. Calabrese JR, Shelton MD, Rapport DJ, Youngstrom EA, Jackson K, Bilali S, Ganocy SJ, Findling RL. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am. J. Psychiatry. 2005;162:2152–2161. PMID: 16263857. DOI: 10.1176/appi.ajp.162.11.2152
20. Koukopoulos A, Reginalsi D, Laddomada P, Floris G, Serra G, Tondo L. Course of the manic-depressive cycle and changes caused by treatments. Pharmacopsychiatry. 1980;13:156–167. PMID: 6108577. DOI: 10.1055/s-2007-1019628
21. Health at a Glance: Europe 2018. State of Health in the EU Cycle. OECD Publishing. 2018:216.
State of Health in the EU Cycle. OECD Publishing. 2018:216.
22. Calabrese JR, Rapport DJ, Youngstrom EA, Jackson K, Bilali S, Findling RL. New data on the use of lithium, divalproate, and lamotrigine in rapid cycling bipolar disorder. Eur. Psychiatry. 2005;20(2):92–95. PMID: 15797691 DOI: 10.1016/j.eurpsy.2004.12.003
23. Kostyukova EG, Shafarenko AA, Ladyzhensky MY. Problems and new possibilities of differentiated therapy in patients with bipolar disorder. Modern therapy of mental disorders. 2014;4:8–14.
Carbamazepine (tegretol) in the prevention of affective disorders (literature review) - Psychiatry and psychopharmacotherapy named after. P.B. Gannushkin №03 2004
Subscribe to new numbers
Author: E.G. Kostyukova Federal Scientific and Methodological Center for Therapy of Mental Illnesses of the Ministry of Health and Social Development of the Russian Federation, Moscow
The diagnosis and treatment of affective disorders has been one of the most widely discussed medical problems in recent years. This is due both to the significant prevalence of these diseases in the population, and to the improvement of methods for their diagnosis and therapy.
This is due both to the significant prevalence of these diseases in the population, and to the improvement of methods for their diagnosis and therapy.
D Diagnostics and therapy of affective disorders in recent years is one of the most widely discussed medical problems. This is due both to the significant prevalence of these diseases in the population, and to the improvement of methods for their diagnosis and therapy.
Until the late 1960s, psychiatry was dominated by Kraepelin's unitary concept of manic-depressive psychosis, which had very wide diagnostic boundaries and included not only those conditions that modern scientists understand as bipolar disorder-I, bipolar disorder-II and cyclothymia, but also a wide range of recurrent depression. In 1957, Leonhard proposed a division into bipolar and unipolar depressive types of the course of the disease. Subsequently, this dichotomy was enshrined in official diagnostic manuals: the American Diagnostic and Statistical Manual, 4th revision (DSM-IV) and the International Classification of Diseases, 10th revision (ICD-10). The validity of the diagnostic distinction between bipolar disorder and recurrent depression has been supported by numerous clinical and biological studies.
The validity of the diagnostic distinction between bipolar disorder and recurrent depression has been supported by numerous clinical and biological studies.
From a clinical point of view, such a division is of great importance for the choice of therapeutic tactics, taking into account the existing risk of developing affect inversion, which is considered an unfavorable factor that aggravates the overall course of the disease. According to modern concepts, "an attack generates an attack" and a greater number of previous episodes determines a greater risk of subsequent exacerbations (R. Post, 1992; R. Post et al., 2002; A. Ehnvall, H. Agren, 2002). At the same time, we are talking about the most intact contingent of patients in whom the disease begins at a young, working age, and the degree of social maladjustment is almost completely determined by the frequency and severity of developing depressive and manic phases.
At the moment, there is no universal drug that, under monotherapy, could provide all the necessary psychotropic effects in bipolar disorder - antimanic, antidepressant and prophylactic - in relation to both manic and depressive phases. At the same time, properly selected pharmacotherapy can significantly reduce the severity of symptoms characteristic of this disease (R. Hirshfield et al., 2002).
At the same time, properly selected pharmacotherapy can significantly reduce the severity of symptoms characteristic of this disease (R. Hirshfield et al., 2002).
Up to now, the drugs of first choice for the treatment of a wide range of diseases characterized by phasic affective disorders remain normotimics. The term "normothymic", ie. leveling mood (unlike neuroleptics and antidepressants), proposed by the Danish psychiatrist M.Schou in the early 60s, implies the bimodal action of the drug - the ability to suppress the development of symptoms of both poles without causing an inversion of affect, and fix the patient's condition on an even, i.e. e. close to euthymic level (S.N. Mosolov, 1996). This group of drugs includes lithium carbonate, sodium valproate and carbamazepine (CRB), as well as a new drug with a proven preventive effect on depressive phases - lamotrigine.
One of the most widely used drugs in this group is CRB (tegretol). The effectiveness of CRP in stopping manic states is inferior to that of lithium. However, according to most studies of the anti-manic effect of CRP, the number of responders to therapy is 50–70% (L. Strömgren, S. Boller, 1985; H. Kravitz, J. Fawcett, 1987). The antidepressant effect of CRP is less pronounced than the antimanic effect (J. Ballenger, R. Post, 1980; J. Neumann et al., 1984), but more than that of lithium and valproate.
However, according to most studies of the anti-manic effect of CRP, the number of responders to therapy is 50–70% (L. Strömgren, S. Boller, 1985; H. Kravitz, J. Fawcett, 1987). The antidepressant effect of CRP is less pronounced than the antimanic effect (J. Ballenger, R. Post, 1980; J. Neumann et al., 1984), but more than that of lithium and valproate.
The first observations indicating a possible normothymic effect of CRP in manic-depressive psychosis are contained in the works of Japanese authors devoted to the thymoleptic properties of the drug (H. Takezaki et al., 1971; T. Okuma et al., 1973). Subsequently, a significant number of specially planned and methodically sustained studies were carried out, as a result of which convincing evidence of a clear normothymic effect of CRP was obtained (R. Ya. Vovin et al., 1984; S.N. Mosolov, 1985; A.I. Skorik et al., 1986; E.G. Kostyukova, 1989; M.V. Kuzavkova et al., 2001; Okuma et al., 1983, 1984; Lipinski and Pope 1982; Nolen, 1983; Gaspar and Kielholz 1984; Greil et al. , 1984; Kishimoto, 1984; Post et al., 1984; Elphic, 1985; Emrich et al., 1985; Fawcett and Kravitz 1985; Watkins et al., 1987; R. Post et al., 1996; V. Sharma et al., 1997, etc.). Summarizing the results of these studies shows that the effectiveness of CRP is not inferior to other mood stabilizers and with long-term continuous use in 70% of patients, it is possible to completely or partially prevent recurrent attacks (M.V. Kuzavkova et al., 2002).
, 1984; Kishimoto, 1984; Post et al., 1984; Elphic, 1985; Emrich et al., 1985; Fawcett and Kravitz 1985; Watkins et al., 1987; R. Post et al., 1996; V. Sharma et al., 1997, etc.). Summarizing the results of these studies shows that the effectiveness of CRP is not inferior to other mood stabilizers and with long-term continuous use in 70% of patients, it is possible to completely or partially prevent recurrent attacks (M.V. Kuzavkova et al., 2002).
The manifestation of the preventive properties of CRP occurs quite quickly. According to A. Kishimoto et al. (1983), if the CRP is effective, then its effect is detected in a short time after administration. This idea is concretized in the works of domestic researchers (R.Ya. Vovin, 1987; S.N. Mosolov et al., 1994). They found that a stable effect with the subsequent formation of remission in CRP can be noted already in the first 2-3 months of treatment. Such efficiency, i.e. termination of the phase or interruption of the continuum at the beginning of the course of prophylactic therapy significantly correlates with the positive effect of therapy over the next year. At the same time, the rate of development of the clinical action of CRP is higher than that of lithium carbonate, the preventive effect of which can be judged not earlier than the 6th month of treatment.
At the same time, the rate of development of the clinical action of CRP is higher than that of lithium carbonate, the preventive effect of which can be judged not earlier than the 6th month of treatment.
In the studies of the last 10 years, in the absence of any doubts about the presence of a clearly pronounced normothymic effect in CRP, various aspects of its clinical use are specified, such as efficacy and tolerability compared with other normotimics, differentiated indications for prescribing, features of the clinical picture of the disease in the process of prophylactic therapy, criteria for predicting effectiveness, the necessary and sufficient duration of preventive therapy, etc.
The authors agree that CRP is effective in the presence of circular affective disorders in the disease picture, regardless of the polarity of the course of the disease and its nosological affiliation. At the same time, its influence is more fully manifested in relation to the reduction of depressions in comparison with manias.
In the process of long-term CRP therapy, not only the frequency, severity and duration of seizures are reduced, but their syndromic structure is also transformed. The most sensitive components of the depressive syndrome are anxiety, restlessness, sleep disorders (A.G. Digilov, 1986). Unlike lithium carbonate, the treatment of CRP does not reveal the development of the phenomenon designated by O. Arnold (1974) as "automatic existence syndrome". In patients with a positive effect of CRP prophylactic therapy, the development of depressive states is either completely suppressed or their duration is reduced with a simultaneous decrease in the severity of symptoms, i.e. there is a uniform reduction of the entire symptom complex. Vital experiences of melancholy, anxiety lose their dominant position in the complaints of patients and do not have the same painful character that previously prompted patients to be hospitalized. Subdepressions that change during CRP therapy most often begin to take on the character of asthenic conditions.![]() At the same time, astheno-hypochondriacal disorders may come to the fore.
At the same time, astheno-hypochondriacal disorders may come to the fore.
Manic states during CRP therapy regress primarily due to the affective and ideomotor components. Persistent manic states, as a rule, lose the severity of symptoms. First of all, the severity of psychopathic manifestations decreases, especially conflict, anger, while in the treatment of lithium, manic syndromes are often replaced by dysphoric and mixed states (P. Baastrup et al., 1967, etc.).
Most authors note that CRP not only has a reducing effect on attacks of affective and schizoaffective psychosis, but also reveals its effect during periods of remission in the form of a distinct tranquilizing effect.
Comparative studies of the features of the clinical action of drugs from the group of mood stabilizers show that in terms of the severity of the preventive action in relation to depressive phases, CRP is superior to lithium carbonate and salts of valproic acid, but is somewhat inferior to them in terms of the effect on manic seizures (S. N. Mosolov, 1985; 1994, and etc.). In a number of other drugs of normothymic action, it is most effective in the continual course of psychosis, especially in cases with a rapid change of phases (S.N. Mosolov, 1985; R.Ya.Vovin et al., 1987; E.G. Kostyukova, 1989; M.V. Kuzavkova, 2001; M. Gastpar et al., 1984, etc.). CRP has also been found to be more effective than lithium in atypical and schizoaffective psychoses (Placidi et al., 1986,
N. Mosolov, 1985; 1994, and etc.). In a number of other drugs of normothymic action, it is most effective in the continual course of psychosis, especially in cases with a rapid change of phases (S.N. Mosolov, 1985; R.Ya.Vovin et al., 1987; E.G. Kostyukova, 1989; M.V. Kuzavkova, 2001; M. Gastpar et al., 1984, etc.). CRP has also been found to be more effective than lithium in atypical and schizoaffective psychoses (Placidi et al., 1986,
and etc.).
The data of scientific research, as well as many years of experience in clinical use, make it possible to determine CRP as the drug of first choice with the predominance of depressive disorders in the picture of the disease, as well as with a continual course with a rapid change of phases.
In the last decade, more and more attention of researchers has been attracted by the possibility of an early individual prediction of the effectiveness of drugs prescribed for the purpose of prevention. The importance of such a search is undeniable, since the evaluation of the effectiveness of any mood stabilizer is possible no earlier than a year after the start of therapy. In this regard, a differentiated approach to the choice of a drug for preventive treatment is an important factor in improving the quality of therapy.
In this regard, a differentiated approach to the choice of a drug for preventive treatment is an important factor in improving the quality of therapy.
The possibility of identifying clinical predictive factors for the effectiveness of CRP is discussed in a significant number of studies (S.N. Mosolov, 1985; O.P. Vertogradova, V.N. Krasnov, 1986; R.Ya.Vovin et al., 1988; M.V. Kuzavkova, 2001; S.N. Mosolov et al., 1994, 2001; A.Finzen, 1991; V. Sharma, Persad, 1992; S. Dilsaver et al., 1993, and others), the results of which generally agree and complement each other. Of the most significant clinical and anamnestic signs that correlate with the effectiveness of CRP therapy, the most informative for a differentiated approach to choosing a drug are those that distinguish it from lithium carbonate and valproic acid salts, namely: an earlier manifestation of the disease, a higher frequency of episodes, a lower average duration of seizures. In other words, when it comes to patients with a relatively long duration of the disease (early ill) and frequent exacerbations, the prognosis of CRP therapy is better compared to other mood stabilizers. A more favorable prognosis for CRP preventive therapy is also evidenced by the predominance of anxiety in depressive states, the absence of a circadian-vital syndrome in the structure of exacerbations, an inverted daily rhythm, and the absence of early awakenings (S.N. Mosolov et al., 1994). In addition, the effect of the drug is higher in patients resistant to lithium therapy, and in case of termination of the phase or interruption of the continuum during the first 2 months of prophylactic therapy.
A more favorable prognosis for CRP preventive therapy is also evidenced by the predominance of anxiety in depressive states, the absence of a circadian-vital syndrome in the structure of exacerbations, an inverted daily rhythm, and the absence of early awakenings (S.N. Mosolov et al., 1994). In addition, the effect of the drug is higher in patients resistant to lithium therapy, and in case of termination of the phase or interruption of the continuum during the first 2 months of prophylactic therapy.
Already from the first steps of studying the clinical action of CRP in affective disorders, the authors made attempts to find pharmacokinetic predictors of a positive response to therapy. First of all, they concerned the search for the relationship between the dose of the drug, its concentration in blood plasma and the clinical effect. To date, most researchers agree that there is no direct relationship between the dose of the drug and its concentration in the blood, as well as between the concentration and the effect of prophylactic therapy (T. B. Kudryakova et al., 1987, 1989; A. Kishimoto et al., 1983; Post et al., 1983; Greil et al., 1984; T. Okuma, 1984; T. Uhde et al., 1984; Ballenger, 1988, etc.).
B. Kudryakova et al., 1987, 1989; A. Kishimoto et al., 1983; Post et al., 1983; Greil et al., 1984; T. Okuma, 1984; T. Uhde et al., 1984; Ballenger, 1988, etc.).
In the works of domestic authors (E.G. Kostyukova, 1989; T.B. Kudryakova, 1990), for the first time, an opinion was expressed about the possible relationship between the effect of preventive therapy for CRP and the characteristics of the patient's individual metabolism. This hypothesis was confirmed and further developed in subsequent years. It has been established that the ratios of the concentrations of CRP and its metabolites (epoxide/diol) characterize different rates of individual metabolism, and if complete responders have the slowest metabolism, nonresponders have the fastest metabolism (M.V. Kuzavkova et al., 2001). Thus, the determination of pharmacokinetic parameters allows for an early biological prediction of the effectiveness of CRP as early as the 4th week of prophylactic therapy after the completion of the process of autoinduction of liver enzymes.
CRP is generally well tolerated in long-term prophylactic use. Its side effects are most pronounced, as a rule, in the early stages of therapy (A.I. Skorik et al. 1986; S.N. Mosolov, 1988; M.V. Kuzavkova, 2001; Placidi et al., 1986; K. Stoll et al., 1986, and others), which is associated with the peculiarities of the pharmacokinetics of the drug, namely with the phenomenon of autoinduction of hepatic enzymes responsible for its metabolism. Side effects that develop at the first stage of treatment are a guideline in selecting an adequate dose for further preventive therapy (A.I. Skorik et al., 1986). The most common are drowsiness, slurred speech, dizziness, mild ataxia and disturbances of accommodation (diplopia), which can be observed in 30% of patients in the first weeks after the appointment of CRP (Lerer, 1985; Fawcett et al., 1985, etc.). These side effects are easily corrected at an individual rate of dosage increase for each patient and do not require discontinuation of the drug. Nausea, vomiting, appetite disorders in most cases disappear spontaneously even without dose reduction (H. Wunderlich, GrЯnes et al., 1983, etc.).
Nausea, vomiting, appetite disorders in most cases disappear spontaneously even without dose reduction (H. Wunderlich, GrЯnes et al., 1983, etc.).
According to various studies, the development of these early side effects is associated not so much with the level of CRP concentration in the blood serum, but with its sharp fluctuations (T.B. Kudryakova, 1987, 1990; Tomson, 1984, etc.). This pattern determines the practical recommendations for dividing the daily dose of the drug into 3-4 doses with more or less equal intervals of time. The appearance in the arsenal of prophylactic agents of a prolonged form of CRP - tegretol CR, which can significantly reduce the range of fluctuations in the concentration of the drug in the blood and at the same time cause fewer side effects (Aldenkamp et al., 1987; Persson et al., 1990, and others), proves this pattern and provides a more convenient two-time daily dosing regimen.
Allergic skin reactions in the treatment of CRP occur in 5-15% of patients (M. SillanpKa, 1981; B. Lerer, 1985; A. I. Skorik et al., 1986, etc.). Their frequency is minimal if the drug is started with small doses, followed by a gradual increase. There is an opinion that the frequency of skin allergic reactions in the treatment of CRP is higher in psychiatric patients compared with patients with epilepsy, which is associated with the already existing sensitization of psychiatric patients to previously taken other psychotropic drugs (Elphick et al., 1988). In most cases, they are mild, manifest maculopapillary erythematous rash, occur mainly at the beginning of therapy and disappear after discontinuation of the drug. Only single observations of more severe allergic reactions, such as exfoliative dermatitis, erythroderma, Lyle's symptom or Stevens-Johnson syndrome, have been described (M. SillanpKa, 1981; Pellock, 1987). Extremely rarely, allergic skin reactions may be accompanied by other disorders characteristic of general hypersensitivity (Mullick et al., 1979; Stewart et al.
SillanpKa, 1981; B. Lerer, 1985; A. I. Skorik et al., 1986, etc.). Their frequency is minimal if the drug is started with small doses, followed by a gradual increase. There is an opinion that the frequency of skin allergic reactions in the treatment of CRP is higher in psychiatric patients compared with patients with epilepsy, which is associated with the already existing sensitization of psychiatric patients to previously taken other psychotropic drugs (Elphick et al., 1988). In most cases, they are mild, manifest maculopapillary erythematous rash, occur mainly at the beginning of therapy and disappear after discontinuation of the drug. Only single observations of more severe allergic reactions, such as exfoliative dermatitis, erythroderma, Lyle's symptom or Stevens-Johnson syndrome, have been described (M. SillanpKa, 1981; Pellock, 1987). Extremely rarely, allergic skin reactions may be accompanied by other disorders characteristic of general hypersensitivity (Mullick et al., 1979; Stewart et al. , 1980; Hogg et al., 1981; Lewis, Rosenbloom, 1982), which disappear after drug withdrawal.
, 1980; Hogg et al., 1981; Lewis, Rosenbloom, 1982), which disappear after drug withdrawal.
It is known that approximately 10% of patients taking CRP develop a relatively short leukopenia at the first stage of therapy (G. Blijham et al., 1984; T. KerKnen et al., 1983). It is not related to the level of drug concentration in the blood serum, changes, as a rule, occur within clinically acceptable limits, are reversible and do not require discontinuation of the drug. In 2% of patients, prolonged but non-progressive stable leukopenia is possible (T. Brewerton, 1986; I. Fawcett et al., 1985; G. Placidi et al., 1984; R. Joffe et al., 1985; M. Post et al., 1982). There are separate reports of the development of agranulocytosis, aplastic anemia (Pisciotta, 1982; Hart, Easton, 1982; Pellock, 1987) and thrombocytopenia (Pearce, Ron, 1968; Rutman, 1978; Baciewicz, Yerevanian, 1984) as complications of CRP therapy. Such cases, however, are rare - approximately 1 in 20,000–50,000 patients. The described observations, as a rule, concerned patients with epilepsy who had previously taken other antiepileptic drugs, which can also cause changes in the blood picture. In this regard, it is often difficult to determine whether changes in the blood picture developed as a result of taking CRP or whether they were associated with previous or simultaneous use of another antiepileptic drug (M. Brodie, F. Johnson, 1997). However, given the existing risk of developing hematological complications, it is recommended to conduct regular clinical blood tests once every 3 months during CRP therapy (D.Scheffner, 1972; Shiefer, 1972; F.Silverstein et al., 1982, etc.) . According to Fawcett et al. (1985), the drug must be discontinued when the number of leukocytes decreases to more than 3000 in 1mm 3 .
The described observations, as a rule, concerned patients with epilepsy who had previously taken other antiepileptic drugs, which can also cause changes in the blood picture. In this regard, it is often difficult to determine whether changes in the blood picture developed as a result of taking CRP or whether they were associated with previous or simultaneous use of another antiepileptic drug (M. Brodie, F. Johnson, 1997). However, given the existing risk of developing hematological complications, it is recommended to conduct regular clinical blood tests once every 3 months during CRP therapy (D.Scheffner, 1972; Shiefer, 1972; F.Silverstein et al., 1982, etc.) . According to Fawcett et al. (1985), the drug must be discontinued when the number of leukocytes decreases to more than 3000 in 1mm 3 .
Cardiovascular complications and liver dysfunction associated with the use of CRP are uncommon. Albany et al. (1995 g.) described the development of a dose-dependent bradyarrhythmia, which disappeared after a decrease in the dosage of the drug. Single observations of the toxic effect of CRP on the liver were associated with the use of high doses of the drug (Soffer et al., 1983; Luke et al., 1986).
Single observations of the toxic effect of CRP on the liver were associated with the use of high doses of the drug (Soffer et al., 1983; Luke et al., 1986).
In general, the authors positively assess the tolerability of CRP with long-term use, noting that taking the drug rarely leads to the development of life-threatening conditions. Even with overdose, deaths are rare (Berry et al., 1983; Fisher and Cysyk 1988).
To date, there are no strict guidelines for the selection of a prophylactic dose of CRP, but there are general principles that are supported by almost all researchers working in this field. Treatment begins with a small dose (100-200 mg / day), which is prescribed in the evening. The dose is increased gradually (100 mg every 2-3 days or 200 mg once a week) to the maximum tolerated. The daily dose is distributed evenly over a 3-time intake. If the dose is not a multiple of "3", a larger dosage is prescribed at night. Tegretol CR is prescribed 2 times a day - in the morning and in the evening. When side effects occur, the dose is reduced, returning to the previous one, which is the most tolerated for this patient. This dose is maintained for the entire period of further therapy. For most patients, it is 400-1000 mg / day (R.Ya. Vovin et al., 1984; S.N. Mosolov, 1991, etc.). If a clear prophylactic effect is not observed in patients, then in the course of therapy, CRP dosages are adjusted (E.G. Kostyukova, 1989). The indication for correction of dosages is the appearance in patients in remission of affective fluctuations of the subclinical level, in the form of hypomania or subdepression. Increasing the dose is carried out at the same slow pace as at the beginning of therapy. The doses of CRP newly selected in this way in different patients are 600–1600 mg/day, i.e. they are higher than those that were established as maximally tolerated at the very beginning of therapy. A flexible, dynamic approach to the choice of dosages, which underlies this technique, in 80% of cases allows to increase the effectiveness of preventive therapy.
When side effects occur, the dose is reduced, returning to the previous one, which is the most tolerated for this patient. This dose is maintained for the entire period of further therapy. For most patients, it is 400-1000 mg / day (R.Ya. Vovin et al., 1984; S.N. Mosolov, 1991, etc.). If a clear prophylactic effect is not observed in patients, then in the course of therapy, CRP dosages are adjusted (E.G. Kostyukova, 1989). The indication for correction of dosages is the appearance in patients in remission of affective fluctuations of the subclinical level, in the form of hypomania or subdepression. Increasing the dose is carried out at the same slow pace as at the beginning of therapy. The doses of CRP newly selected in this way in different patients are 600–1600 mg/day, i.e. they are higher than those that were established as maximally tolerated at the very beginning of therapy. A flexible, dynamic approach to the choice of dosages, which underlies this technique, in 80% of cases allows to increase the effectiveness of preventive therapy.
The main feature of the use of drugs for the secondary prevention of relapses in phase-producing endogenous psychoses and at the same time a condition for success is their continuous (indefinitely long) long-term use. Cancellation of therapy, even with complete well-being, significantly increases the risk of relapse and can contribute to the strengthening of adverse trends in the course of the disease (G.Ya. Avrutsky, A.A. Neduva, 1988; S.N. Mosolov, 1996, etc.). Although according to K. Macritchie, N. Hunt (2000), the abolition of CRP, unlike lithium carbonate, does not cause the so-called rebound effect (the development of mania in bipolar patients), the results of a 4-year follow-up study conducted by R. Post and et al., 1990 showed a significant worsening of the course of the disease in patients who refused CRP prophylactic therapy, compared with those who continued the drug.
With such a long-term long-term use of CRP, the issues of its interactions with other drugs, which can be prescribed with the development of concomitant somatic diseases or exacerbations of a mental state, are of particular importance. If necessary, other psychotropic drugs (neuroleptics, antidepressants, tranquilizers) can be prescribed against the background of prophylactic therapy for CRP. However, for the correct choice of dosages, it should be taken into account that CRP, having a powerful inducing effect on the P-450 cytochrome system (Albani et al., 1995), enhances the metabolism of drugs, the exchange of which affects the same system of cytochromes, in particular sodium valproate, lamotrigine and antipsychotics (M. Freeman, A. Stoll, 1998). CRP taken together with haloperidol can lead to a decrease in the serum concentration of haloperidol (Kahn et al., 1990). CRP also enhances the metabolism of imipramine, desipramine, nortriptyline, and buproprion. The combination of CRP and clozapine is contraindicated due to an increased risk of hematological side effects (M. Freeman, A. Stoll, 1998), and with monoamine oxidase inhibitors because of the risk of toxic reactions. A pronounced interaction with the development of toxic reactions has been established between CRP and clarithromycin (O'Connor and Fris, 1994).
If necessary, other psychotropic drugs (neuroleptics, antidepressants, tranquilizers) can be prescribed against the background of prophylactic therapy for CRP. However, for the correct choice of dosages, it should be taken into account that CRP, having a powerful inducing effect on the P-450 cytochrome system (Albani et al., 1995), enhances the metabolism of drugs, the exchange of which affects the same system of cytochromes, in particular sodium valproate, lamotrigine and antipsychotics (M. Freeman, A. Stoll, 1998). CRP taken together with haloperidol can lead to a decrease in the serum concentration of haloperidol (Kahn et al., 1990). CRP also enhances the metabolism of imipramine, desipramine, nortriptyline, and buproprion. The combination of CRP and clozapine is contraindicated due to an increased risk of hematological side effects (M. Freeman, A. Stoll, 1998), and with monoamine oxidase inhibitors because of the risk of toxic reactions. A pronounced interaction with the development of toxic reactions has been established between CRP and clarithromycin (O'Connor and Fris, 1994).
CRP reduces the effectiveness of oral contraceptives and makes it difficult to interpret the dexamethasone test, reduces the blood levels of theophylline and indirect anticoagulants.
It is known that a number of drugs increase the level of CRP in blood plasma by suppressing its metabolism. These include, in particular, cimetidine (Macphee et al., 1984), dextropropoxyphene (Hansen et al., 1980), triacetyloleandromycin (Wong et al., 1983), isoniazid (Wright et al., 1982), danazol (Kramer et al., 1986), diltiazem (Brodie and Macphee, 1986 ) and veloxazine (Pisani et al., 1984).
In this review, it seems important to dwell separately on the interaction of CRB and lithium carbonate. Combination therapy with these drugs in recent years is traditional in cases of monotherapy failure. The results of numerous studies have shown that there is a group of patients who respond to combination therapy, but are resistant to each of the drugs in monotherapy (F.Rose, F. Johnson, 1997). These studies concern both topical relief of affective disorders and preventive therapy (Inoue et al. 1981; Lipinski and Pope 1982; Keisling 1983; Moss and James 1983; Nolen 1983; Kwamie et al. 1984; Fawcett and Kravitz, 1985; Shukla et al., 1985; Cabrera et al., 1987; Arana et al., 1989; Kraminger, Post, 1989; Stromgren, 1990; Stuppaeck et al., 1990; Chou, 1991; Sousek, 1991, etc.).
Johnson, 1997). These studies concern both topical relief of affective disorders and preventive therapy (Inoue et al. 1981; Lipinski and Pope 1982; Keisling 1983; Moss and James 1983; Nolen 1983; Kwamie et al. 1984; Fawcett and Kravitz, 1985; Shukla et al., 1985; Cabrera et al., 1987; Arana et al., 1989; Kraminger, Post, 1989; Stromgren, 1990; Stuppaeck et al., 1990; Chou, 1991; Sousek, 1991, etc.).
The known caution in the use of this combination therapy is probably dictated by the literature on the increased risk of side effects and toxic reactions associated with drug interactions of lithium and CRP (Chaudry, Waters, 1983; Shukla et al., 1984, etc.). In this case, the risk factor is called signs of residual organic insufficiency of the central nervous system or concomitant metabolic diseases. Other reports (I. Fawcett, H. Kravitz, 1985; Cabrera et al., 1987, and others) do not contain information about any specific side effects or toxic reactions during combination therapy. However, combination therapy of CRP and lithium is recommended to be prescribed with caution, to use lower dosages of drugs and a slower rate of increasing the dose of CRP when it is added to lithium therapy and to maintain the concentration of lithium in the blood at a lower level (S.N. Mosolov et al., 1991; Post et al., 1987).
However, combination therapy of CRP and lithium is recommended to be prescribed with caution, to use lower dosages of drugs and a slower rate of increasing the dose of CRP when it is added to lithium therapy and to maintain the concentration of lithium in the blood at a lower level (S.N. Mosolov et al., 1991; Post et al., 1987).
Many years of clinical experience with the use of CRP as a mood stabilizer confirms the results of numerous scientific studies. To date, it is widely used throughout the world and, along with lithium carbonate, and in some cases as an alternative, is a traditional means of preventive therapy for phase-producing endogenous psychoses. This can be confirmed by the results of a survey in which more than 9,000 members of the American Psychiatric Association participated. The survey showed that the frequency of prescribing CRP as a means of prevention is constantly and significantly increasing. If at 1988, normothymic CRP monotherapy was carried out only in 3.