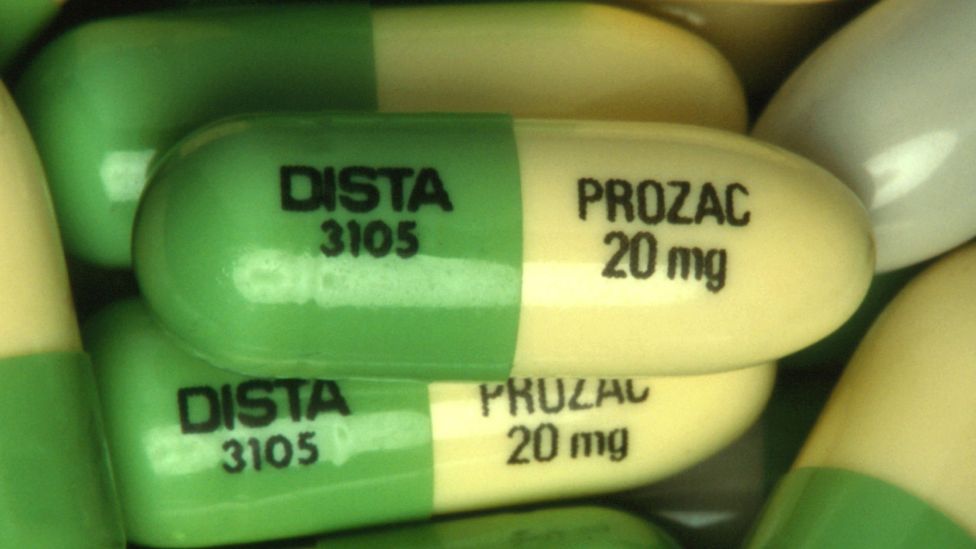
Who makes prozac
Generic Prozac: Can You Trust It?
Written by Jeanie Lerche Davis
Aug. 28, 2001 -- Since the new generic Prozac got FDA approval, the WebMD message boards have been buzzing with consumer questions and comments:
lucia18: "My health plan just sent a note that they'd like for me to switch to the new form of generic Prozac. I'm very hesitant to switch. Am I foolish? Are they truly the same thing? How can they be the same thing (did the Prozac fellows send the recipe to the generic fellows)?"
iberne: "On an emotional level, I can understand a general discomfort with 'generic' medications. Name brand does mean quality with many consumer products. Personally I like my Coca-Cola, and lots of name brand cereals taste better to me. But a better analogy is table salt. The difference between brands is virtually nil for the most part, and quality isn't so much an issue."
murray500: "My mother-in-law swears that generic ibuprofen is not as good as her Motrin. I can't tell any difference except a lot less $$$."
Is the active ingredient in generic Prozac really the same as the name-brand version? Can different inactive ingredients -- binders and fillers -- affect the drug's quality?
For answers, WebMD turned to Bob West, RPh, acting deputy director of the FDA's Office of Generic Drugs.
"We want to assure the public that through rigorous testing, generic Prozac has been proven to be as safe and effective as the Lilly product," West tells WebMD. Drugmaker Eli Lilly and Co. manufactures Prozac.
"When you swallow the capsule or tablet, it dissolves in a manner similar to the Lilly product," he says. "The active ingredient is absorbed by the body in a manner comparable to the Lilly product, so the blood levels obtained are comparable to those obtained with the Lilly product."
What sort of rigorous testing is West talking about? FDA regulations require that generic drugs:
- Contain the same active ingredients as the brand-name drug (the binders and fillers may vary, however).
- Must be the same in strength and dosage (whether pill or capsule) as brand-name drugs.
- Be manufactured under the same strict standards as brand-name products. Meet batch requirements for, among other things, strength, purity, and quality.
- Be bioequivalent to the name-brand drug.
Translation: If two drugs are "bioequivalent," it means that the active ingredient in one drug works the same as in the other drug.
As with other generic drugs, generic Prozac's bioequivalency study was conducted over a month's time, with 40-50 people taking the generic version, then switching to the name-brand drug. Each patient's blood was analyzed multiple times each day to observe the amount of drug that had been absorbed, how long it took the drug to be absorbed, and the total amount of drug that was absorbed.
Generic Prozac passed all the tests, West tells WebMD. Bottom line: "It should work in the same way as the Lilly product," he says. "We have no concern that it will not."
"We have no concern that it will not."
While in the past, there may have been differences between generics and name-brand drugs, "it's not as serious a problem as it used to be," says Stuart Schweitzer, PhD, professor of health services at UCLA School of Public Health.
"Generic drugs are being tested more carefully, and manufacturing standards are tighter than they were 10 years ago," he says. "In almost every case, the generic drugs today are therapeutically equivalent to brand-name products."
However, he adds, "it is possible that people will react differently to a different formulation."
Even though the FDA finds generic and brand-name drugs to be bioequivalent, "that doesn't mean that a few people might not notice a difference," he tells WebMD. "No two people are exactly the same. An inert ingredient -- a binder -- is different in this formulation than in another formulation. Just because your body and my body see no difference in that formulation, that's not to say that a third body might not respond differently to it. "
"
Those perceived differences are "possible, but unlikely," Schweitzer says. What may be at work is what researchers call "placebo effect."
"Because I know I'm taking a generic version, I'll be really suspicious," he says. "If I trip over the curb walking to my office, I'm going to start attributing that to the generic drug -- although it has nothing to do with it."
Generic Prozac brings "substantial" cost savings to consumers, he says. "It could eventually fall to half the original price."
Prices of the brand-name product will likely increase, as Eli Lilly recognizes.
"They have a small, loyal following," says Schweitzer of brand-name drugs. "There are some people who will purchase the original product regardless of cheaper versions available."
Is Prozac more effective than generic fluoxetine?
Commentary
Upon its introduction in 1987, fluoxetine revolutionized drug therapy for mood disorders and has become a cornerstone in depression treatment. After 14 years of being the sole manufacturer of fluoxetine (under the brand name Prozac), Eli Lilly and Company’s exclusivity patent expired.
1 Generic fluoxetine is now available through multiple manufacturers.
After 14 years of being the sole manufacturer of fluoxetine (under the brand name Prozac), Eli Lilly and Company’s exclusivity patent expired.
1 Generic fluoxetine is now available through multiple manufacturers.
While use of generic fluoxetine rather than Prozac will decrease medication costs, the question arises: Is the brand-name drug more effective than its generic equivalent? Some anecdotal reports have suggested a clinical difference, but these claims have not yet been supported in the literature.
Some clinicians have found that select patients require a higher dosage of generic fluoxetine than Prozac to control their symptoms, but several issues may contribute to these increased requirements. First, depression and depressive symptoms wax and wane; an increase in symptoms may be part of the course of illness rather than differences between brand and generic formulations.
Increased symptoms may also reflect patient bias. The patient knows he or she is taking a generic and may be more inclined to notice or report symptoms. Additionally, some patients who believe generics are less effective than brand-name equivalents experience a reverse placebo effect—their belief that a generic drug is inferior diminishes its effectiveness. Finally, subtle differences in bioavailability and bioequivalence between the brand-name and generic drugs may be seen clinically.
Additionally, some patients who believe generics are less effective than brand-name equivalents experience a reverse placebo effect—their belief that a generic drug is inferior diminishes its effectiveness. Finally, subtle differences in bioavailability and bioequivalence between the brand-name and generic drugs may be seen clinically.
To receive FDA approval, a generic drug must be proven to be therapeutically equivalent to its brand-name counterpart. This entails both pharmaceutical equivalence (identical amounts of the same ingredient in the same dosage form and route of administration) and bio-equivalence (comparable rate and extent to which the active ingredient is absorbed and becomes available at the site of action).2 Statistical analysis of pharmacokinetics includes evaluating measures such as area under the curve and peak concentration. The test drug and reference drug are compared by calculating the 90% confidence interval for their respective population geometric means. The calculated confidence interval should fall within the bioequivalence limit, typically between 80 and 125% for the population geometric mean. Other factors typically considered include the logarithmic transformation of pharmacokinetic data, methods to evaluate sequence effects, and evaluation of outlier data.3
The calculated confidence interval should fall within the bioequivalence limit, typically between 80 and 125% for the population geometric mean. Other factors typically considered include the logarithmic transformation of pharmacokinetic data, methods to evaluate sequence effects, and evaluation of outlier data.3
Comparator generic drugs must be rigorously tested before receiving FDA approval. One would hope that the variability that may exist between the brand and generic product does not significantly change patient response.
To date, more than 20 companies have received approval or tentative approval for almost 50 generic fluoxetine products. The approval package data (which includes bioequivalency data) for these agents are not yet available.4
Still, there is no evidence that generic fluoxetine is less effective than Prozac, despite increased attention from patients, clinicians, and pharmaceutical companies. The bottom line is that each patient needs individual treatment. If symptoms increase or worsen, then increase the dosage, which would be done in any case. If adverse effects increase, lower the dosage. If a true difference is suspected in a specific patient, it should be promptly reported to the FDA, which evaluates drugs after marketing by regularly assessing product quality and investigating and evaluating allegations of drug product inequivalence.5
If symptoms increase or worsen, then increase the dosage, which would be done in any case. If adverse effects increase, lower the dosage. If a true difference is suspected in a specific patient, it should be promptly reported to the FDA, which evaluates drugs after marketing by regularly assessing product quality and investigating and evaluating allegations of drug product inequivalence.5
Cynthia A. Mascarenas, PharmD
Lisa M.Mican, PharmD
University of Texas Health Science Center at San Antonio
References
- McLean B. A bitter pill. Fortune, August 13, 2001.
- Vasquez CA, Vasquez R. Therapeutic equivalence guidelines: what the codes mean. Available at: www.pharmacytimes.com/GPR2.html.
- Center for Drug Evaluation and Research (CDER). Guidance for industry: statistical approaches to establishing bioequivalence. U.S. Department of Health and Human Services, Food and Drug Administration, 2001.
- CDER.
 New and generic drug approvals: 1998-2002. Available at: www.fda.gov/cder/approval/index.htm.
New and generic drug approvals: 1998-2002. Available at: www.fda.gov/cder/approval/index.htm. - U.S. Food and Drug Administration. Therapeutic equivalence of generic drugs (letter). Available at: www.pharmweb.net/forum/0086/1998/msg00010.html.
Adolescent depression: Diagnostic skills can differentiate teen angst from psychopathology
MDedge Psychiatry
Adolescent depression: Diagnostic skills can differentiate teen angst from psychopathology
MDedge Psychiatry
Short-term cognitive therapy shows promise for dysthymia
MDedge Psychiatry
Antidepressants for fibromyalgia: Latest word on the link to depression and anxiety
MDedge Psychiatry
MAO inhibitors: An option worth trying in treatment-resistant cases
MDedge Psychiatry
- Depression
| 💊 Ingredients of Prozac ® ✅ How to use Prozac ® Save Search for analogues Interaction Description of the active ingredients of the preparation Prozac ® (Prozac ® ) The scientific information provided is general and cannot be used to make decisions. Update date: 2020.06.09 Marketing authorization holder:ELI LILLY VOSTOK, S.A. (Switzerland)
Made:PATHEON FRANCE, S.A.S. (France) ATX code: N06AB03 (Fluoxetine) Active substance: fluoxetine (fluoxetine) Rec.INN WHO registered Dosage form
Release form, packaging and composition Prozac®Capsules hard gelatin capsules, size #3, opaque, green/cream, imprinted with the LILLY logo and the identification code "3105"; the contents of the capsules are white powder.0008 Excipients : starch, dimethicone. Composition of the capsule shell: patented blue dye (patent blue V dye), iron oxide yellow dye, titanium dioxide, gelatin, food ink (for identification printing). 14 pcs. - blisters (1) - packs of cardboard. Clinical and pharmacological group: Antidepressant Pharmacotherapeutic group: Antidepressant Pharmacological action Antidepressant, propylamine derivative. Pharmacokinetics Absorbed from the gastrointestinal tract. Weakly metabolized during the "first pass" through the liver. Eating does not affect the degree of absorption, although it may slow down its rate. C max in plasma is achieved after 6-8 hours. C ss in plasma is achieved only after continuous administration for several weeks. Protein binding 94.5%. Easily penetrates through the BBB. It is metabolized in the liver by demethylation to form the main active metabolite of norfluoxetine. T 1/2 fluoxetine is 2-3 days, norfluoxetine is 7-9 days. Excreted by the kidneys 80% and through the intestines - about 15%. Indications of the active substances of the drug Prozac®Depression of various origins, obsessive-compulsive disorders, bulimic neurosis. Open list of ICD-10 codes
Dosage regimenThe method of administration and dosing regimen of a particular drug depends on its form of release and other factors. The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed. Initial dose - 20 mg 1 time / day in the morning; if necessary, the dose can be increased after 3-4 weeks. The frequency of admission is 2-3 times / day. The maximum daily oral dose of for adults is 80 mg. Side effectsFrom the side of the central nervous system: possible anxiety, tremor, nervousness, drowsiness, headache, sleep disturbances. From the digestive system: possible diarrhea, nausea. From the side of metabolism: may increase sweating, hypoglycemia, hyponatremia (especially in elderly patients and with hypovolemia). From the reproductive system: decreased libido. Allergic reactions: possible skin rash, itching. Other: joint and muscle pain, shortness of breath, fever. Contraindications for useGlaucoma, bladder atony, severe renal dysfunction, benign prostatic hyperplasia, simultaneous administration of MAO inhibitors, convulsive syndrome of various origins, epilepsy, pregnancy, lactation, hypersensitivity to fluoxetine. Use in pregnancy and lactationContraindicated in pregnancy and lactation. Use in hepatic impairmentUse with extreme caution in patients with hepatic impairment. Use in impaired renal functionContraindicated in severe renal impairment. Use with extreme caution in patients with moderate and mild renal impairment. Use in childrenThe safety of fluoxetine in children has not been established. Use in elderly patients Elderly patients require dosage adjustment. Special instructionsUse with extreme caution in patients with impaired liver and kidney function, with a history of epileptic seizures, cardiovascular diseases. In patients with diabetes mellitus, changes in blood glucose levels are possible, which requires correction of the dosing regimen of hypoglycemic drugs. When used in debilitated patients while taking fluoxetine, the likelihood of developing epileptic seizures increases. With the simultaneous use of fluoxetine and electroconvulsive therapy, the development of prolonged epileptic seizures is possible. Fluoxetine can be used no earlier than 14 days after discontinuation of MAO inhibitors. The period after the abolition of fluoxetine before the start of therapy with MAO inhibitors should be at least 5 weeks. Elderly patients require dosage adjustment. The safety of fluoxetine in children has not been established. Do not drink alcohol during treatment. Influence on the ability to drive vehicles and mechanisms During the period of treatment, one should refrain from potentially hazardous activities that require increased attention and rapid psychomotor reactions. Drug InteractionsWhen used simultaneously with drugs that have a depressant effect on the central nervous system, with ethanol, a significant increase in the inhibitory effect on the central nervous system, as well as an increase in the likelihood of convulsions, is possible. With simultaneous use with MAO inhibitors, furazolidone, procarbazine, tryptophan, serotonin syndrome may develop (confusion, hypomania, restlessness, agitation, convulsions, dysarthria, hypertensive crisis, chills, tremor, nausea, vomiting, diarrhea). With simultaneous use, fluoxetine inhibits the metabolism of tricyclic and tetracyclic antidepressants, trazodone, carbamazepine, diazepam, metoprolol, terfenadine, phenytoin, which leads to an increase in their concentration in blood serum, an increase in their therapeutic and side effects. With simultaneous use, inhibition of the biotransformation of drugs metabolized with the participation of the CYP2D6 isoenzyme is possible. When used simultaneously with hypoglycemic agents, their effect may be enhanced. There have been reports of increased effects of warfarin when co-administered with fluoxetine. When used simultaneously with haloperidol, fluphenazine, maprotiline, metoclopramide, perphenazine, periciazine, pimozide, risperidone, sulpiride, trifluoperazine, cases of extrapyramidal symptoms and dystonia have been described; with dextromethorphan - a case of the development of hallucinations is described; with digoxin - a case of increasing the concentration of digoxin in the blood plasma. When used simultaneously with lithium salts, an increase or decrease in the concentration of lithium in the blood plasma is possible. With simultaneous use, it is possible to increase the concentration of imipramine or desipramine in the blood plasma by 2-10 times (may persist for 3 weeks after fluoxetine is discontinued). When used simultaneously with propofol, a case is described in which spontaneous movements were observed; with phenylpropanolamine - a case is described in which dizziness, weight loss, hyperactivity were observed. Co-administration may enhance the effects of flecainide, mexiletine, propafenone, thioridazine, zuclopenthixol. Keep |
How Prozac Made the World Love Depression — Bird In Flight
At the end of the twentieth century, Prozac helped humanity cope with one of the most difficult diseases of our time - depression. But at the same time, he created a cult out of the disease. Bird in Flight tells the story of the rise and fall of the happiness pill.
The diagnosis of "clinical depression" began a century and a half ago, today it is already the second most common in the world. This disorder affects about 300 million people. One in six people with severe depression is at risk of committing suicide. Despite the complexity of the disease, it is now not considered incurable, and for this we should be grateful to Prozac, a drug that has changed the idea of depression.
An incurable disease
For a long time, medicine did not know how to help people with depression: in the Middle Ages, it was treated with prayers, in the Renaissance, with vegetarianism. Only in the 19th century did doctors realize that the main cause of depression was a malfunction in the nervous system. Patients were prescribed stimulant psychoactive substances such as alcohol, opium, cocaine, and eventually sedative barbiturates. They did not cure the disease, but only temporarily relieved the symptoms, while causing addiction.
Accidental discovery
A breakthrough in the fight against depression occurred in the early 1950s, when a drug for tuberculosis, iprovizid, was being studied in the United States. The medicine coped with its task poorly, but it was found to have an unusual side effect: after taking it, appetite returned to tuberculosis patients, their mood improved. As the doctors recalled, "the patients danced despite the holes in their lungs. " Psychiatrists who undertook to study iprovisid confirmed its psychoactive properties. Thus, a new class of drugs appeared - antidepressants.
" Psychiatrists who undertook to study iprovisid confirmed its psychoactive properties. Thus, a new class of drugs appeared - antidepressants.
The first such drugs were very toxic and had many side effects. Patients taking them suffered from heart problems, excess weight and slow reflexes. However, the medications did not help everyone.
The first antidepressants had many side effects. Patients taking them suffered from heart problems, excess weight and slow reflexes.
In the early 1970s, a number of pharmaceutical companies began to develop a new generation of depression medication that would not have these unpleasant side effects. Its discoverer was David T. Wong, a researcher at the American pharmaceutical company Eli Lilly. For a long time, Wong studied the mechanisms of reuptake of serotonin, a neurotransmitter responsible, among other things, for the emotional background of a person. The researcher's task was to find a substance that would prevent the absorption of serotonin by brain neurons. It was opened at 1972, the substance was named fluoxetine.
It was opened at 1972, the substance was named fluoxetine.
Unlike the old-style antidepressants, which, like weapons of mass destruction, acted on all neurotransmitters at once, fluoxetine worked selectively. Patients could no longer be afraid of lethargy; the problem of side effects seemed to be solved.
"Prozac: wash away your blues!" Source: Tumblr / Adbusters
Killer marketing
Another advantage of the new drug was that, in addition to reuptake, it also blocked the uptake of noradrenaline, that is, it acted as a stimulant on the nervous system. So Eli Lilly made the right decision to sell fluoxetine not as an antidepressant but as a mood booster. The company wanted customers not to think about taking the medicine. Fluoxetine was supposed to be associated with energy and vivacity, so the first step in its promotion was a name change. This task was entrusted to Interbrand, whose clients included Sony, Microsoft and Nintendo. Thanks to her, fluoxetine became Prozac.
Eli Lilly introduced the drug to the US Food and Drug Administration in the late 1970s. Consideration of the application stretched for ten years. Department officials were embarrassed that Prozac had a stimulant rather than a sedative property. Despite this, it was still approved, and in December 1987 the drug entered the market.
The Cult of Sorrow and Joy
In its first year, Prozac sold $350 million in the US and $2.6 billion worldwide. The drug finally convinced humanity that depression is not a sentence, as previously thought, and happiness is not an abstract concept, but a state that can be achieved with the help of pills.
"Prozac" finally convinced humanity that depression is not a sentence, as previously thought, and happiness can be achieved with the help of pills.
The popularity of the drug was also supported by media attention. So, on December 18, 1989, New York Magazine came out with Prozac pills on the cover. The headline was "Buy-Buy Blues." After that, almost all the major magazines of that time wrote about the pills.
The headline was "Buy-Buy Blues." After that, almost all the major magazines of that time wrote about the pills.
Eli Lilly's marketing idea worked. The Americans perceived the drug as an energy drink, after drinking which one could become more productive. It seemed that the whole country fell in love with the medicine, including doctors. At 19In 1993, psychiatrist Peter Kramer published Listening to Prozac, which became a New York Times bestseller. In it, the author suggested that the drug not only allows you to cope with depression, but also can make a mediocre person more bright, a shy person more open and sociable, and an ambitious employee can help climb the career ladder. After the publication of the book, sales of the pills increased another 15%.
Shot from the movie Prozac Nation (2001) / Miramax / Album
Prozac's popularity peaked in the 1990s when comedians and writers started talking about the drug. “When do the holidays start in New York? When drug dealers start selling more Prozac than crack" - this joke was made on the evening show with David Letterman in 1994. In the same year, writer Elisabeth Wurzel published Prozac Nation, a book about her experience of dealing with clinical depression. Wurzel's work is considered one of the examples of the romanticization of the image not only of Prozac, but of antidepressants in general.
In the same year, writer Elisabeth Wurzel published Prozac Nation, a book about her experience of dealing with clinical depression. Wurzel's work is considered one of the examples of the romanticization of the image not only of Prozac, but of antidepressants in general.
Prozac has made its way into music as well. In 1996, the rock group Mr. T Experience released the song That Prozac Moment, in which the effect of taking the drug was compared to a moment of fleeting happiness, when you can forget about everything. Also, the drug is mentioned in the compositions of Aerosmith, Pixies, Red Hot Chili Peppers, Neil Diamond, Nick Cave and other artists.
Love no matter what
When INXS lead singer Michael Hutchence took his own life in 1997, the media wrote that Prozac killed the star. The musician's case was one of several dozen suicide cases that swept across the United States, all of which were associated with the use of fluoxetine. Eli Lilly started getting lawsuits. Later it turned out that the company knew that the drug could increase the risk of suicide even at the stage of clinical trials. It was especially dangerous for teenagers.
Eli Lilly started getting lawsuits. Later it turned out that the company knew that the drug could increase the risk of suicide even at the stage of clinical trials. It was especially dangerous for teenagers.
How could a pill designed to treat depression cause people to commit suicide? It was assumed that suicidal thoughts were in patients who committed suicide even before the start of the drug, but due to depression they lacked the strength to realize their plans. Prozac just became a source of energy. Defenders of the drug argued that it was not the drug that was responsible for the deaths, but the disease. However, according to The Guardian, the drug can cause suicidal thoughts in people who do not suffer from depression.
The fact that the drug can increase the risk of suicide, the company knew even at the stage of clinical trials.
When Eli Lilly's patent for Prozac expired in 2001, fluoxetine-based competitor products filled the market. The side effects of the new generation of antidepressants were even less. However, even today, Prozac, along with them, remains one of the most effective means to combat depression.
The side effects of the new generation of antidepressants were even less. However, even today, Prozac, along with them, remains one of the most effective means to combat depression.
The drug scandals have caused the medical community and the media to become more critical of antidepressants—they are no longer considered a panacea. In 2008, a study was published proving that drugs for depression are effective in severe manifestations of the disease, but in the lungs they are barely noticeable than placebo. The approaches of pharmaceutical companies to the promotion of antidepressants on the market were also subject to revision. The Guardian believes that manufacturers often do not publish the results of their research or, even worse, influence them. Often, companies use advertising to change the idea of the disease, convincing the public that even minor sleep disturbances and mood swings are a cause for concern.
However, the global antidepressant market is expected to surpass $18 billion by the end of 2024, with fluoxetine accounting for a significant portion of the profits.
 decisions about the use of a particular drug.
decisions about the use of a particular drug.  20 mg: 14 or 28 pcs.
20 mg: 14 or 28 pcs.  The mechanism of action is associated with selective blockade of neuronal reuptake of serotonin in the CNS. Fluoxetine is a weak antagonist of cholino-, adreno- and histamine receptors. Unlike most antidepressants, fluoxetine does not appear to cause a decrease in the functional activity of postsynaptic β-adrenergic receptors. Helps improve mood, reduces feelings of fear and tension, eliminates dysphoria. Does not cause sedation. When taken in average therapeutic doses, it practically does not affect the functions of the cardiovascular and other systems.
The mechanism of action is associated with selective blockade of neuronal reuptake of serotonin in the CNS. Fluoxetine is a weak antagonist of cholino-, adreno- and histamine receptors. Unlike most antidepressants, fluoxetine does not appear to cause a decrease in the functional activity of postsynaptic β-adrenergic receptors. Helps improve mood, reduces feelings of fear and tension, eliminates dysphoria. Does not cause sedation. When taken in average therapeutic doses, it practically does not affect the functions of the cardiovascular and other systems. 
 2
2