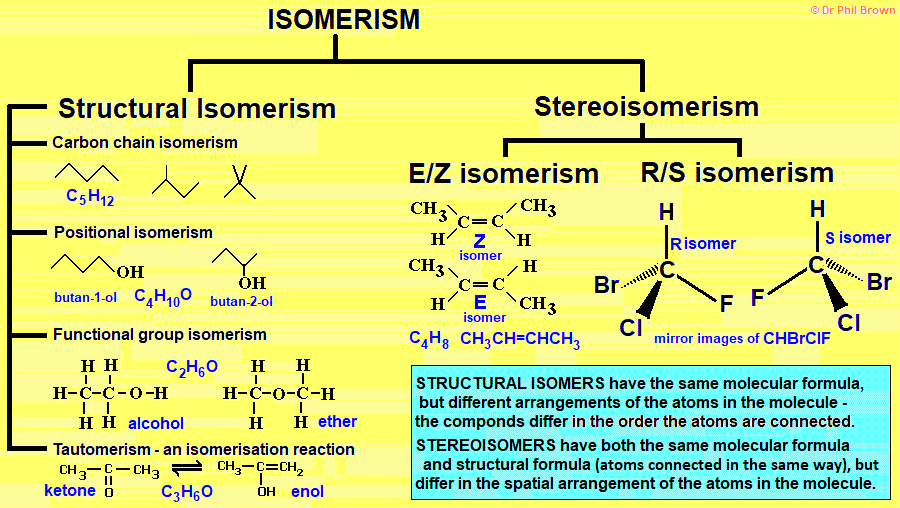
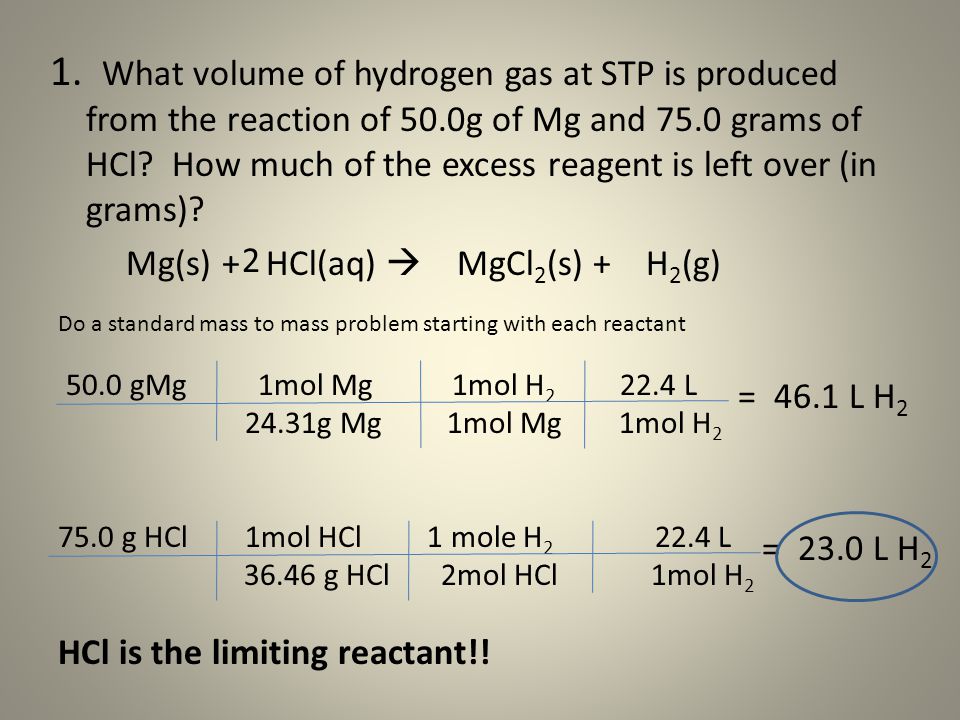
What is the highest mg of klonopin
Klonopin (clonazepam) dosing, indications, interactions, adverse effects, and more
clonazepam increases and albuterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and alfentanil both increase sedation. Use Caution/Monitor.
alprazolam and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and amitriptyline both increase sedation. Use Caution/Monitor.
amobarbital and clonazepam both increase sedation. Use Caution/Monitor.
amobarbital will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and amoxapine both increase sedation. Use Caution/Monitor.
clonazepam and apomorphine both increase sedation. Use Caution/Monitor.
clonazepam increases and arformoterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and aripiprazole both increase sedation. Use Caution/Monitor.
clonazepam increases and armodafinil decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
atazanavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Potential for increased toxicity. Use alternatives if available. Consider lowering benzodiazepine dose.
azelastine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and baclofen both increase sedation. Use Caution/Monitor.
clonazepam and belladonna and opium both increase sedation. Use Caution/Monitor.
belzutifan will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. If unable to avoid coadministration of belzutifan with sensitive CYP3A4 substrates, consider increasing the sensitive CYP3A4 substrate dose in accordance with its prescribing information.
Modify Therapy/Monitor Closely. If unable to avoid coadministration of belzutifan with sensitive CYP3A4 substrates, consider increasing the sensitive CYP3A4 substrate dose in accordance with its prescribing information.
clonazepam and benperidol both increase sedation. Use Caution/Monitor.
clonazepam increases and benzphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
bosentan will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
brexanolone, clonazepam. Either increases toxicity of the other by sedation. Use Caution/Monitor.
brompheniramine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and buprenorphine both increase sedation. Use Caution/Monitor.
clonazepam and buprenorphine buccal both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
clonazepam increases toxicity of buprenorphine subdermal implant by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Studies have shown that the combination of benzodiazepines and buprenorphine altered the usual ceiling effect on buprenorphine-induced respiratory depression, making the respiratory effects of buprenorphine appear similar to those of full opioid agonists. There have been postmarketing reports of coma and death with coadministration of buprenorphine and benzodiazepines. In many, but not all of these cases, buprenorphine was misused by self-injection. If a benzodiazepine must be used for an indication other than seizures, lower the benzodiazepine initial dose and cautiously titrate to clinical response.
clonazepam increases toxicity of buprenorphine, long-acting injection by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of buprenorphine and benzodiazepines or other CNS depressants increases risk of adverse reactions including overdose, respiratory depression, and death.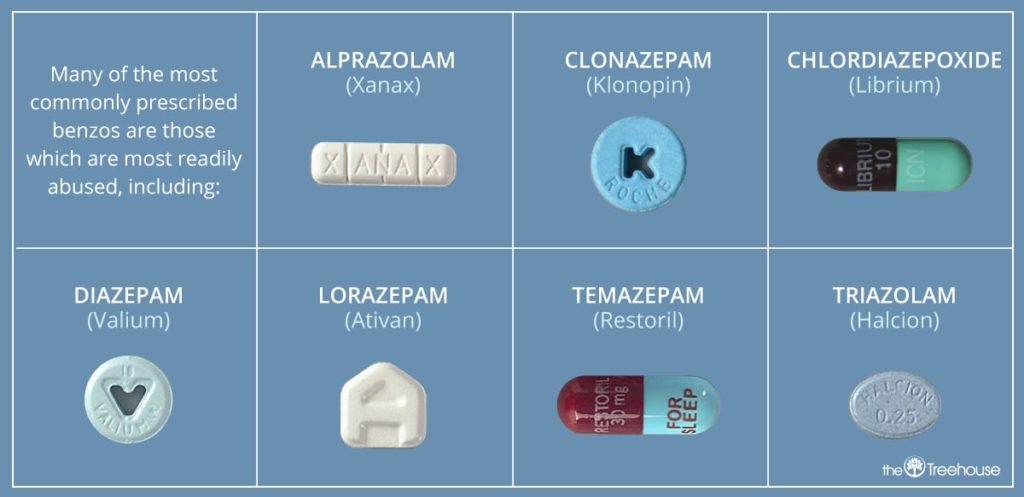 Cessation of benzodiazepines or other CNS depressants is preferred in most cases. In some cases, monitoring at a higher level of care for tapering CNS depressants may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
Cessation of benzodiazepines or other CNS depressants is preferred in most cases. In some cases, monitoring at a higher level of care for tapering CNS depressants may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
butabarbital and clonazepam both increase sedation. Use Caution/Monitor.
butalbital and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and butorphanol both increase sedation. Use Caution/Monitor.
clonazepam increases and caffeine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
carbinoxamine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and carisoprodol both increase sedation. Use Caution/Monitor.
cenobamate will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Increase dose of CYP3A4 substrate, as needed, when coadministered with cenobamate.
Modify Therapy/Monitor Closely. Increase dose of CYP3A4 substrate, as needed, when coadministered with cenobamate.
cenobamate, clonazepam. Either increases effects of the other by sedation. Use Caution/Monitor.
ceritinib will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and chloral hydrate both increase sedation. Use Caution/Monitor.
chlordiazepoxide and clonazepam both increase sedation. Use Caution/Monitor.
chlorpheniramine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and chlorpromazine both increase sedation. Use Caution/Monitor.
clonazepam and chlorzoxazone both increase sedation. Use Caution/Monitor.
cimetidine increases levels of clonazepam by decreasing metabolism. Use Caution/Monitor.
Use Caution/Monitor.
cinnarizine and clonazepam both increase sedation. Use Caution/Monitor.
clarithromycin will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clemastine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam, clobazam. Other (see comment). Use Caution/Monitor. Comment: Concomitant administration can increase the potential for CNS effects (e.g., increased sedation or respiratory depression).
clonazepam and clomipramine both increase sedation. Use Caution/Monitor.
clonidine, clonazepam. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Enhanced CNS depressant effects.
clonazepam and clorazepate both increase sedation. Use Caution/Monitor.
clonazepam and clozapine both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
cobicistat will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Clinical monitoring is recommended upon coadministration with anticonvulsants.
clonazepam and codeine both increase sedation. Use Caution/Monitor.
crizotinib increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Dose reduction may be needed for coadministered drugs that are predominantly metabolized by CYP3A.
crofelemer increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Crofelemer has the potential to inhibit CYP3A4 at concentrations expected in the gut; unlikely to inhibit systemically because minimally absorbed.
cyclizine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and cyclobenzaprine both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
cyproheptadine and clonazepam both increase sedation. Use Caution/Monitor.
dabrafenib will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely.
clonazepam and dantrolene both increase sedation. Use Caution/Monitor.
clonazepam and daridorexant both increase sedation. Modify Therapy/Monitor Closely. Coadministration increases risk of CNS depression, which can lead to additive impairment of psychomotor performance and cause daytime impairment.
darunavir will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
desflurane and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and desipramine both increase sedation. Use Caution/Monitor.
clonazepam and deutetrabenazine both increase sedation. Use Caution/Monitor.
dexchlorpheniramine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam increases and dexfenfluramine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and dexmedetomidine both increase sedation. Use Caution/Monitor.
clonazepam increases and dexmethylphenidate decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and dextroamphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and dextromoramide both increase sedation. Use Caution/Monitor.
clonazepam and diamorphine both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
clonazepam and diazepam both increase sedation. Use Caution/Monitor.
diazepam intranasal, clonazepam. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Coadministration may potentiate the CNS-depressant effects of each drug.
clonazepam increases and diethylpropion decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
difelikefalin and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and difenoxin hcl both increase sedation. Use Caution/Monitor.
dimenhydrinate and clonazepam both increase sedation. Use Caution/Monitor.
diphenhydramine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and diphenoxylate hcl both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
clonazepam and dipipanone both increase sedation. Use Caution/Monitor.
disulfiram increases levels of clonazepam by decreasing metabolism. Use Caution/Monitor.
clonazepam increases and dobutamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and dopamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and dopexamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and dosulepin both increase sedation. Use Caution/Monitor.
clonazepam and doxepin both increase sedation. Use Caution/Monitor.
clonazepam and doxylamine both increase sedation. Use Caution/Monitor.
clonazepam and droperidol both increase sedation.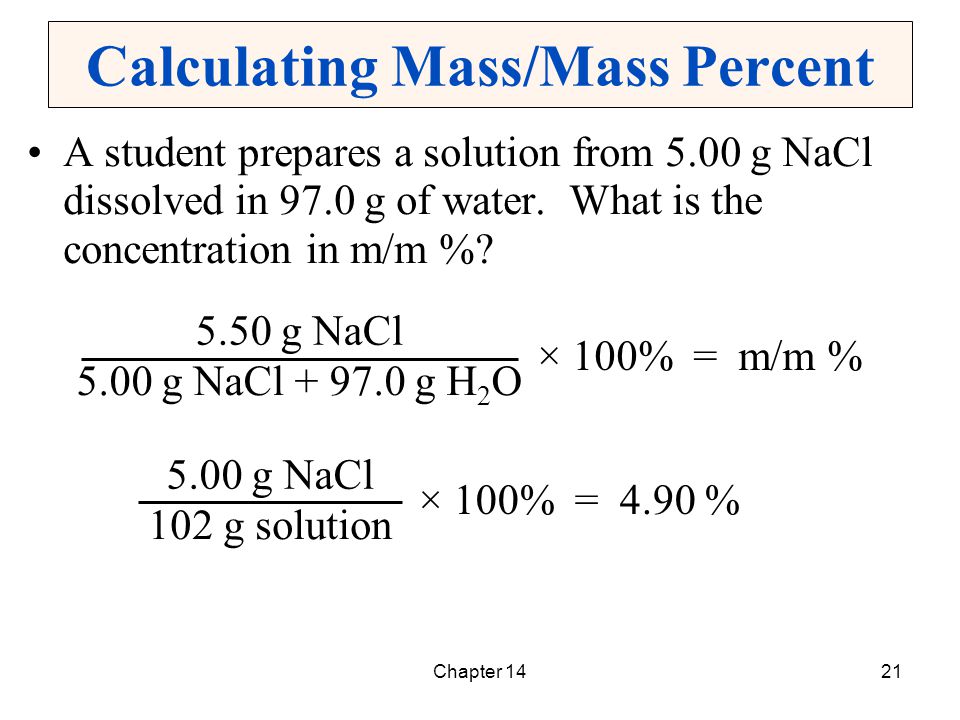 Use Caution/Monitor.
Use Caution/Monitor.
duvelisib will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. will increase the level or effect of
efavirenz will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
elagolix will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Elagolix is a weak-to-moderate CYP3A4 inducer. Monitor CYP3A substrates if coadministered. Consider increasing CYP3A substrate dose if needed.
elvitegravir/cobicistat/emtricitabine/tenofovir DF increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Cobicistat is a CYP3A4 inhibitor; consider benzodiazepine dose reduction.
encorafenib, clonazepam. affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Encorafenib both inhibits and induces CYP3A4 at clinically relevant plasma concentrations. Coadministration of encorafenib with sensitive CYP3A4 substrates may result in increased toxicity or decreased efficacy of these agents.
enzalutamide will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam increases and ephedrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and epinephrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and epinephrine racemic decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
esketamine intranasal, clonazepam. Either increases toxicity of the other by sedation. Modify Therapy/Monitor Closely.
Either increases toxicity of the other by sedation. Modify Therapy/Monitor Closely.
clonazepam and estazolam both increase sedation. Use Caution/Monitor.
clonazepam and ethanol both increase sedation. Use Caution/Monitor.
ethinylestradiol will increase the level or effect of clonazepam by Mechanism: decreasing metabolism. Use Caution/Monitor. Ethinyl estradiol may inhibit the clearance of benzodiazepines that undergo oxidation, thereby increasing serum concentrations of concomitantly administered benzodiazepines.
etomidate and clonazepam both increase sedation. Use Caution/Monitor.
etravirine will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam increases and fenfluramine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.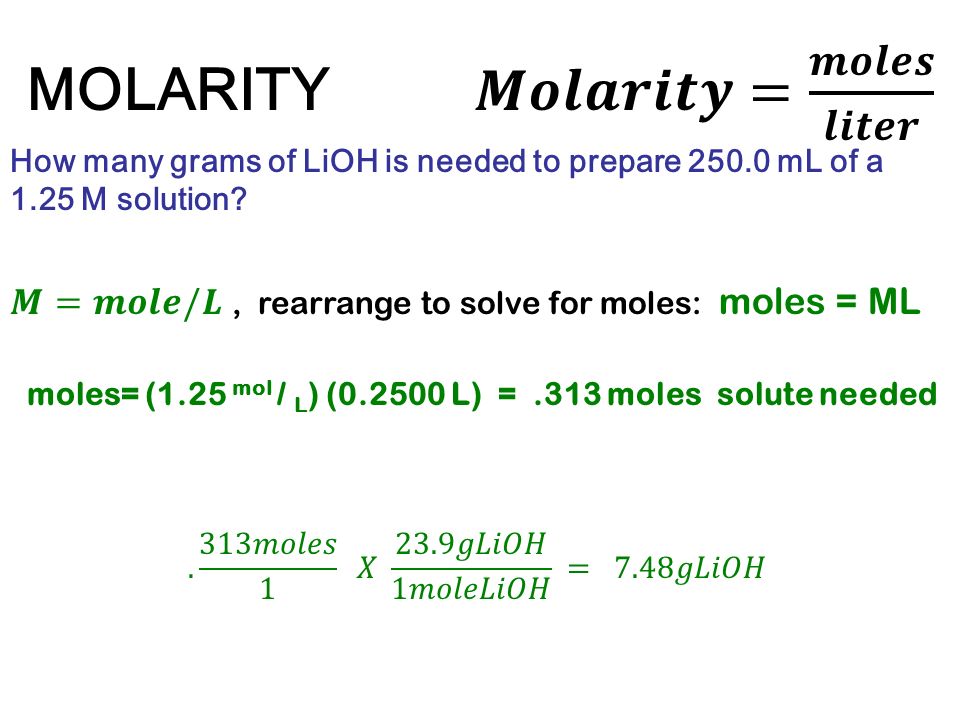
clonazepam and flibanserin both increase sedation. Modify Therapy/Monitor Closely. Risk for sedation increased if flibanserin is coadministration with other CNS depressants.
clonazepam and fluphenazine both increase sedation. Use Caution/Monitor.
clonazepam and flurazepam both increase sedation. Use Caution/Monitor.
clonazepam increases and formoterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
fosamprenavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Potential for increased toxicity. Use alternatives if available. Consider lowering benzodiazepine dose.
fosphenytoin will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
gabapentin, clonazepam. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
gabapentin enacarbil, clonazepam. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
clonazepam and ganaxolone both increase sedation. Use Caution/Monitor.
clonazepam and haloperidol both increase sedation. Use Caution/Monitor.
hyaluronidase, clonazepam. Other (see comment). Use Caution/Monitor.
Comment: Drug combination has been found to be incompatible.
clonazepam and hydromorphone both increase sedation. Use Caution/Monitor.
hydroxyzine and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and iloperidone both increase sedation. Use Caution/Monitor.
iloperidone increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Iloperidone is a time-dependent CYP3A inhibitor and may lead to increased plasma levels of drugs predominantly eliminated by CYP3A4.
clonazepam and imipramine both increase sedation. Use Caution/Monitor.
indinavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Potential for increased toxicity. Use alternatives if available. Consider lowering benzodiazepine dose.
clonazepam increases and isoproterenol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
Effect of interaction is not clear, use caution. Use Caution/Monitor.
istradefylline will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Istradefylline 40 mg/day increased peak levels and AUC of CYP3A4 substrates in clinical trials. This effect was not observed with istradefylline 20 mg/day. Consider dose reduction of sensitive CYP3A4 substrates.
itraconazole will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely.
kava and clonazepam both increase sedation. Use Caution/Monitor.
ketamine and clonazepam both increase sedation. Use Caution/Monitor.
ketoconazole will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely.
clonazepam and ketotifen, ophthalmic both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
lasmiditan, clonazepam. Either increases effects of the other by sedation. Use Caution/Monitor. Coadministration of lasmiditan and other CNS depressant drugs, including alcohol have not been evaluated in clinical studies. Lasmiditan may cause sedation, as well as other cognitive and/or neuropsychiatric adverse reactions.
lemborexant, clonazepam. Either increases effects of the other by sedation. Modify Therapy/Monitor Closely. Dosage adjustment may be necessary if lemborexant is coadministered with other CNS depressants because of potentially additive effects.
lenacapavir will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Lencapavir (a moderate CYP3A4 inhibitor) may increase CYP3A4 substrates initiated within 9 months after last SC dose of lenacapavir, which may increase potential risk of adverse reactions of CYP3A4 substrates.
letermovir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam increases and levalbuterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
levoketoconazole will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely.
levonorgestrel oral/ethinylestradiol/ferrous bisglycinate will increase the level or effect of clonazepam by decreasing metabolism. Use Caution/Monitor. Ethinyl estradiol may inhibit the clearance of benzodiazepines that undergo oxidation, thereby increasing serum concentrations of concomitantly administered benzodiazepines.
clonazepam and levorphanol both increase sedation. Use Caution/Monitor.
clonazepam increases and lisdexamfetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and lofepramine both increase sedation. Use Caution/Monitor.
clonazepam and lofexidine both increase sedation. Use Caution/Monitor.
lopinavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Potential for increased toxicity. Use alternatives if available. Use alternatives if available. Consider lowering benzodiazepine dose.
clonazepam and loprazolam both increase sedation. Use Caution/Monitor.
clonazepam and lorazepam both increase sedation. Use Caution/Monitor.
lorlatinib will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and lormetazepam both increase sedation. Use Caution/Monitor.
clonazepam and loxapine both increase sedation. Use Caution/Monitor.
clonazepam and loxapine inhaled both increase sedation. Use Caution/Monitor.
lurasidone, clonazepam. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: Potential for increased CNS depressant effects when used concurrently; monitor for increased adverse effects and toxicity.
clonazepam and maprotiline both increase sedation. Use Caution/Monitor.
clonazepam and marijuana both increase sedation. Use Caution/Monitor.
clonazepam and melatonin both increase sedation. Use Caution/Monitor.
clonazepam and meperidine both increase sedation. Use Caution/Monitor.
clonazepam and meprobamate both increase sedation. Use Caution/Monitor.
clonazepam increases and metaproterenol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and metaxalone both increase sedation. Use Caution/Monitor.
clonazepam and methadone both increase sedation. Use Caution/Monitor.
clonazepam increases and methamphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and methocarbamol both increase sedation. Use Caution/Monitor.
clonazepam increases and methylenedioxymethamphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
methylphenidate transdermal will increase the level or effect of clonazepam by decreasing metabolism. Modify Therapy/Monitor Closely. Consider decreasing the dose of these drugs when given coadministered with methylphenidate. Monitor for drug toxiticities when initiating or discontinuing methylphenidate.
clonazepam and midazolam both increase sedation. Use Caution/Monitor.
midazolam intranasal, clonazepam. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Concomitant use of barbiturates, alcohol, or other CNS depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect.
clonazepam increases and midodrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
mifepristone will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and mirtazapine both increase sedation. Use Caution/Monitor.
mitotane decreases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Mitotane is a strong inducer of cytochrome P-4503A4; monitor when coadministered with CYP3A4 substrates for possible dosage adjustments.
Use Caution/Monitor. Mitotane is a strong inducer of cytochrome P-4503A4; monitor when coadministered with CYP3A4 substrates for possible dosage adjustments.
clonazepam increases and modafinil decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and morphine both increase sedation. Use Caution/Monitor.
clonazepam and motherwort both increase sedation. Use Caution/Monitor.
clonazepam and moxonidine both increase sedation. Use Caution/Monitor.
clonazepam and nabilone both increase sedation. Use Caution/Monitor.
nafcillin will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and nalbuphine both increase sedation. Use Caution/Monitor.
nefazodone will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
Use Caution/Monitor.
nelfinavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Potential for increased toxicity. Use alternatives if available. Consider lowering benzodiazepine dose.
clonazepam increases and norepinephrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and nortriptyline both increase sedation. Use Caution/Monitor.
clonazepam and olanzapine both increase sedation. Use Caution/Monitor.
oliceridine, clonazepam.
Either increases toxicity of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
Monitor closely for signs of respiratory depression and sedation.
clonazepam and opium tincture both increase sedation. Use Caution/Monitor.
orlistat decreases levels of clonazepam by inhibition of GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Risk of convulsions.
clonazepam and orphenadrine both increase sedation. Use Caution/Monitor.
clonazepam and oxazepam both increase sedation. Use Caution/Monitor.
clonazepam and oxycodone both increase sedation. Use Caution/Monitor.
clonazepam and oxymorphone both increase sedation. Use Caution/Monitor.
clonazepam and paliperidone both increase sedation. Use Caution/Monitor.
clonazepam and papaveretum both increase sedation. Use Caution/Monitor.
clonazepam and papaverine both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
clonazepam and pentazocine both increase sedation. Use Caution/Monitor.
pentobarbital and clonazepam both increase sedation. Use Caution/Monitor.
perampanel and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and perphenazine both increase sedation. Use Caution/Monitor.
clonazepam increases and phendimetrazine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
phenobarbital and clonazepam both increase sedation. Use Caution/Monitor.
phenobarbital will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam increases and phentermine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and phenylephrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and phenylephrine PO decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor. .
phenytoin will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and pholcodine both increase sedation. Use Caution/Monitor.
clonazepam and pimozide both increase sedation. Use Caution/Monitor.
clonazepam increases and pirbuterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
posaconazole will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
pregabalin, clonazepam.
Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
primidone and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and prochlorperazine both increase sedation. Use Caution/Monitor.
promethazine and clonazepam both increase sedation. Use Caution/Monitor.
propofol and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam increases and propylhexedrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and protriptyline both increase sedation. Use Caution/Monitor.
clonazepam and quazepam both increase sedation. Use Caution/Monitor.
clonazepam and quetiapine both increase sedation. Use Caution/Monitor.
Use Caution/Monitor.
clonazepam and ramelteon both increase sedation. Use Caution/Monitor.
remimazolam, clonazepam. Either increases toxicity of the other by sedation. Modify Therapy/Monitor Closely. Coadministration may result in profound sedation, respiratory depression, coma, and/or death. Continuously monitor vital signs during sedation and recovery period if coadministered. Carefully titrate remimazolam dose if administered with opioid analgesics and/or sedative/hypnotics.
ribociclib will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
rifabutin will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
rifampin will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
Use Caution/Monitor.
rifapentine will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
clonazepam and risperidone both increase sedation. Use Caution/Monitor.
ritonavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Potential for increased toxicity. Use alternatives if available. Consider lowering benzodiazepine dose.
rucaparib will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Adjust dosage of CYP3A4 substrates, if clinically indicated.
clonazepam increases and salmeterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
saquinavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Use alternatives if available. Consider lowering benzodiazepine dose.
Modify Therapy/Monitor Closely. Use alternatives if available. Consider lowering benzodiazepine dose.
clonazepam and scullcap both increase sedation. Use Caution/Monitor.
secobarbital and clonazepam both increase sedation. Use Caution/Monitor.
secobarbital will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. May also enhance CNS depressant effect of clonazepam
sevelamer decreases levels of clonazepam by increasing elimination. Use Caution/Monitor.
sevoflurane and clonazepam both increase sedation. Use Caution/Monitor.
clonazepam and shepherd's purse both increase sedation. Use Caution/Monitor.
stiripentol, clonazepam. affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Stiripentol is a CYP3A4 inhibitor and inducer. Monitor CYP3A4 substrates coadministered with stiripentol for increased or decreased effects. CYP3A4 substrates may require dosage adjustment.
Monitor CYP3A4 substrates coadministered with stiripentol for increased or decreased effects. CYP3A4 substrates may require dosage adjustment.
stiripentol, clonazepam. Either increases effects of the other by sedation. Use Caution/Monitor. Concomitant use stiripentol with other CNS depressants, including alcohol, may increase the risk of sedation and somnolence.
clonazepam and sufentanil both increase sedation. Use Caution/Monitor.
suvorexant and clonazepam both increase sedation. Modify Therapy/Monitor Closely. Dosage adjustments of suvorexant and concomitant CNS depressants may be necessary
clonazepam and tapentadol both increase sedation. Use Caution/Monitor.
tazemetostat will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
tecovirimat will decrease the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Tecovirimat is a weak CYP3A4 inducer. Monitor sensitive CYP3A4 substrates for effectiveness if coadministered.
Use Caution/Monitor. Tecovirimat is a weak CYP3A4 inducer. Monitor sensitive CYP3A4 substrates for effectiveness if coadministered.
teduglutide increases levels of clonazepam by Other (see comment). Use Caution/Monitor. Comment: Teduglutide may increase absorption of concomitant PO medications; caution with with drugs requiring titration or those with a narrow therapeutic index; dose adjustment may be necessary.
clonazepam and temazepam both increase sedation. Use Caution/Monitor.
clonazepam increases and terbutaline decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and thioridazine both increase sedation. Use Caution/Monitor.
clonazepam and thiothixene both increase sedation. Use Caution/Monitor.
tipranavir increases levels of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Use alternatives if available. Consider lowering benzodiazepine dose.
Modify Therapy/Monitor Closely. Use alternatives if available. Consider lowering benzodiazepine dose.
clonazepam and topiramate both increase sedation. Modify Therapy/Monitor Closely.
clonazepam and tramadol both increase sedation. Use Caution/Monitor.
clonazepam and trazodone both increase sedation. Use Caution/Monitor.
clonazepam and triazolam both increase sedation. Use Caution/Monitor.
clonazepam and triclofos both increase sedation. Use Caution/Monitor.
clonazepam and trifluoperazine both increase sedation. Use Caution/Monitor.
clonazepam and trimipramine both increase sedation. Use Caution/Monitor.
triprolidine and clonazepam both increase sedation. Use Caution/Monitor.
voriconazole will increase the level or effect of clonazepam by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
Use Caution/Monitor.
clonazepam increases and xylometazoline decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam increases and yohimbine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
clonazepam and ziconotide both increase sedation. Use Caution/Monitor.
clonazepam and ziprasidone both increase sedation. Use Caution/Monitor.
clonazepam and zotepine both increase sedation. Use Caution/Monitor.
Form, strengths, how to take, and more
Klonopin (clonazepam) is a brand-name prescription medication. The Food and Drug Administration (FDA) has approved it to treat the following conditions:
- certain seizure disorders, including absence seizures, akinetic seizures (also called atonic seizures), and myoclonic seizures, in adults and children of all ages
- panic disorder with or without agoraphobia (fear of leaving home or going places that may be difficult to escape) in adults
Klonopin is available as an oral tablet.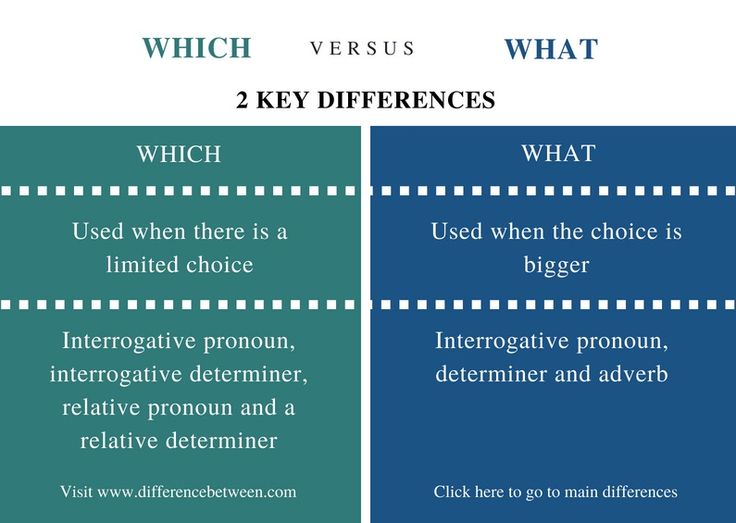 It belongs to a class of drugs called benzodiazepines. And it’s available in a generic form called clonazepam.
It belongs to a class of drugs called benzodiazepines. And it’s available in a generic form called clonazepam.
Dosage summary
Below is a dosage chart that summarizes Klonopin dosages. Your doctor will determine the dosage that’s best for you.
| Klonopin form | Strengths | Typical starting dosage |
| oral tablet | • 0.5 milligrams (mg) • 1 mg • 2 mg | • for seizures in adults: 0.5 mg three times per day • for panic disorder in adults: 0.25 mg twice per day |
For information about the dosage of Klonopin, including its strengths and how to take the drug, keep reading. For a comprehensive look at Klonopin, see this article.
This article describes typical dosages for Klonopin provided by the drug’s manufacturer. When taking Klonopin, always follow the dosage prescribed by your doctor.
Before you start treatment with Klonopin, your doctor will recommend the best dosage for you.
Klonopin form
Klonopin is available as a tablet that is taken by mouth.
Klonopin strengths (0.5 mg, 1 mg, 2 mg)
Klonopin comes in three strengths:
- 0.5 milligrams (mg)
- 1 mg
- 2 mg
Typical dosages
Typically, your doctor will start you on a low dosage. Then they’ll adjust it over time to reach the amount that’s right for you. Your doctor will ultimately prescribe the smallest dosage that provides the desired effect.
The following information describes dosages that are commonly used or recommended. However, be sure to take the dosage your doctor prescribes for you. Your doctor will determine the best dosage to fit your needs.
Dosage for panic disorder
The recommended starting dosage of Klonopin for panic disorder in adults is 0.25 mg twice per day. This is the lowest dose of Klonopin that your doctor will recommend. Starting with the smallest dose helps your body adjust to the medication. It also helps your doctor see how well the drug is working for you.
It also helps your doctor see how well the drug is working for you.
Your doctor may monitor you for the first 3 days after you start treatment with Klonopin to see if the smallest dose is working for you. In some cases, they may recommend a dose increase every 3 days until the drug is working to manage your symptoms.
Many people taking Klonopin for panic disorder take a dose of 0.5 mg twice per day. However, in some cases, it’s possible a maximum daily dose of 4 mg of Klonopin (2 mg twice daily) is needed to treat panic disorder. This is the highest dose of Klonopin that’s recommended for panic disorder.
Dosage for seizure disorders
The recommended starting dosage of Klonopin for seizure disorders in adults is 0.5 mg three times per day. This is likely the lowest dose of Klonopin that your doctor will recommend for seizure disorders.
Your doctor may recommend increasing your dose of Klonopin every 3 days as needed to better manage your seizures. So, the best dose for you will be determined by how well Klonopin is working to treat your condition.
The maximum recommended dosage of Klonopin to treat seizure disorders is 20 mg per day. This may be split up into multiple doses per day. Be sure to follow your doctor’s instructions about how long to go between doses.
Children’s dosage
The recommended dosage of Klonopin to treat children with seizure disorders is based on your child’s age and weight. The starting dose for children up to 10 years of age and younger or children who weigh less than 30 kg* (about 66 lb) is between 0.01 and 0.03 milligrams per kilogram (mg/kg) of body weight per day.
So, if your child weighs 20 kg (about 44 lb), their starting dosage would be from 0.2 mg to 0.6 mg per day. This dosage would likely be split into two or three doses per day. For example, your child may get a dose of 0.2 mg three times daily. The starting daily dose of Klonopin in children should not be more than 0.05 mg/kg. For the example above, the maximum starting dosage of Klonopin should be 1 mg per day.
After your child starts treatment with Klonopin, their doctor may increase their dose every 3 days until their symptoms are managed. The recommended maintenance dosage of Klonopin in children is 0.1 to 0.2 mg/kg of body weight per day. So, if your child weighs 20 kg (about 44 lb), their maintenance dosage would be from 2 to 4 mg per day. This may be split into three daily doses, taken as 1 mg three times per day.
The recommended maintenance dosage of Klonopin in children is 0.1 to 0.2 mg/kg of body weight per day. So, if your child weighs 20 kg (about 44 lb), their maintenance dosage would be from 2 to 4 mg per day. This may be split into three daily doses, taken as 1 mg three times per day.
For children ages 10 years and older who weigh at least 30 kg (about 66 lb), the recommended dosage is the same as for adults. For more information on the dosage recommended in children ages 10 years and older, see the “Dosage for seizure disorders” section above.
Klonopin is not approved for use in children with panic disorder. So there’s no recommended dosage for this use.
* One kilogram (kg) is about 2.2 pounds (lb).
Long-term treatment
It’s possible that Klonopin may be a long-term treatment. How long you take this medication may depend on many factors, including what condition you’re taking the drug to treat. If Klonopin works to manage your condition, your doctor may recommend that you take it long term.
However, it’s important to note that Klonopin hasn’t been studied in people with panic disorder for longer than 9 weeks. So, it’s not known what long-term effects the drug may have. Taking Klonopin long term may also increase your risk of dependence.
Due to these risks, your doctor will recommend taking the lowest dosage of Klonopin for the shortest amount of time possible. It’s possible that your doctor may recommend taking Klonopin short term for your condition. Then, they may recommend switching to a different treatment option.
For more information about how long you should take Klonopin for your condition, talk with your doctor. They can discuss the best treatment plan for you.
Klonopin isn’t approved to treat anxiety. In fact, this medication is only approved to treat panic disorder and seizures in certain people.
However, panic disorder is a type of anxiety disorder. So, it’s possible that your doctor may recommend treating panic disorder related to your anxiety with Klonopin.
Because the drug isn’t approved to treat anxiety specifically, there aren’t “normal” or maximum doses. However, if your doctor recommends taking Klonopin for your anxiety, they will recommend the best dosage for you.
Klonopin is not approved to treat sleep disorders, such as insomnia. However, this medication may cause sleepiness or drowsiness as side effects.
Due to this, your doctor may recommend taking Klonopin off-label for sleep problems. Off-label use of a drug is when your doctor prescribes a medication for a different use than what it was approved for.
There isn’t a recommended dosage of Klonopin to treat sleep problems. However, if your doctor recommends taking the drug off-label for this condition, they will prescribe the best dosage for you.
Below are some frequently asked questions about Klonopin.
What are the maximum and lowest doses of Klonopin?
The maximum and lowest doses of Klonopin will depend on which condition you’re using the drug to treat. To treat panic disorder in adults, the lowest recommend dosage is 0.25 milligrams (mg) twice per day. The maximum dosage for this use is 2 mg twice per day.
To treat panic disorder in adults, the lowest recommend dosage is 0.25 milligrams (mg) twice per day. The maximum dosage for this use is 2 mg twice per day.
For people with seizure disorders, the lowest recommended dosage of Klonopin is typically 0.5 mg three times per day. And the maximum recommended dosage for this use is up to 20 mg per day, divided into multiple doses throughout the day.
For more information about the specific dosing of Klonopin, see “Typical dosages” in the “Klonopin dosage” section above.
Is there a dosage for Klonopin’s use as a muscle relaxer?
Klonopin isn’t approved for use as a muscle relaxer. So, there isn’t a dosage that’s recommended for this use.
However, other benzodiazepines, such as Valium (diazepam), may be used along with other medications to treat muscle spasms.
If you’re experiencing muscle spasms and think you may need a muscle relaxer, talk with your doctor about the best treatment option for you.
The Klonopin dosage your doctor prescribes will depend on several factors. These include:
These include:
- the type and severity of the condition you’re using Klonopin to treat
- your age
- your weight (for children)
Other medical conditions you have can also affect your Klonopin dosage.
Dosage adjustments
Your doctor may recommend a lower dosage for you if you take medications that may interact with Klonopin, including opioid medications, such as Roxicodone (oxycodone). They may also recommend a lower dosage if you have other medical conditions, such as liver problems.
Klonopin comes as an oral tablet. The tablet should be swallowed whole with water.
If you have trouble swallowing tablets, see this article for tips on how to take this form of medication. You can also talk with your doctor or pharmacist.
There isn’t a sublingual dosage form (a dose that dissolves under your tongue) or a liquid form of Klonopin available. However, if you’re having trouble swallowing your dose of Klonopin, talk with your doctor about switching to clonazepam (the generic form of Klonopin). This version is available as an orally disintegrating tablet.
This version is available as an orally disintegrating tablet.
If you only need to take Klonopin once per day, take your dose at bedtime. However, in most cases, you will take Klonopin two to three times per day. It may be helpful to take Klonopin around the same times each day. This helps maintain a steady level of the drug in your body so Klonopin can work effectively.
It’s important to note that if you need to stop treatment with Klonopin for any reason, you should talk with your doctor. They will recommend slowly decreasing your dosage of Klonopin to prevent withdrawal symptoms. This is very important since Klonopin withdrawal can cause serious risks and can be life threatening. For more information about withdrawal symptoms, see the “Klonopin and withdrawal and dependence” section below.
ACCESSIBLE DRUG LABELS AND CONTAINERSIf you’re having trouble reading your prescription label, talk with your doctor or pharmacist. Some pharmacies offer labels with large print, braille, or a code you scan with a smartphone to convert text to speech.
If your local pharmacy doesn’t have these options, your doctor or pharmacist might be able to recommend a pharmacy that does.
If you’re having trouble opening medication bottles, ask your pharmacist about putting Klonopin in an easy-open container. They also may recommend tools that can make it easier to open bottles.
If you miss your dose of Klonopin, talk with your doctor or pharmacist about when to take your next dose. In some cases, they may recommend taking your dose as soon as you remember. However, if it’s almost time for your next scheduled dose, they may recommend skipping your missed dose and continuing with your regular dosing schedule.
You should not take an extra dose to make up for a missed dose. Taking extra doses can increase your risk of serious side effects, including overdose.
To help make sure that you don’t miss a dose, try using a medication reminder. This can include setting an alarm or using a timer. You could also download a reminder app on your phone.
Klonopin has a boxed warning for the risk of misuse and addiction occurring during treatment. In fact, all benzodiazepines, including Klonopin, may increase your risk of misuse and addiction. A boxed warning is the most serious warning from the FDA. The purpose of a boxed warning is to inform doctors and patients about the risks of taking a medication.
Misuse means using a medication differently than how it was prescribed. And addiction refers to the inability to stop taking a medication or substance, even if you know it can be harmful to your health. It’s possible for addiction to occur even if you’re taking Klonopin exactly as your doctor prescribed.
Misuse and addiction can increase your risk of serious side effects, including overdose, which can be life threatening. Symptoms of misuse may include:
- abdominal pain
- anxiety or depression
- aggression
- blurry vision
- confusion
- slurred speech
- paranoia
- suicidal thoughts or actions
- seizures
- coma
- difficulty breathing
Due to this risk, your doctor will monitor you throughout your treatment for signs of misuse or addiction. In some cases, they may recommend a different treatment option for you.
In some cases, they may recommend a different treatment option for you.
It’s important to tell your doctor if you have a history of drug misuse or addiction before taking Klonopin. They can recommend the best treatment option for you.
If you take more Klonopin than your doctor prescribes, you may develop serious side effects.
It’s important that you do not use more Klonopin than your doctor advises.
Symptoms of an overdose
Overdose symptoms of Klonopin can include:
- sleepiness
- confusion
- low blood pressure
- slower reflexes
- coma
If you take more than the recommended amount of Klonopin
Call your doctor right away if you believe you’ve taken too much Klonopin. Another option is to call the American Association of Poison Control Centers at 800-222-1222 or use its online tool. If you have severe symptoms, immediately call 911 or your local emergency number, or go to the nearest emergency room.
It’s possible for benzodiazepines, such as Klonopin, to cause symptoms of withdrawal or dependence. These symptoms may occur even if you take Klonopin exactly as your doctor prescribed. In fact, Klonopin has a boxed warning for the risk of withdrawal and dependence. A boxed warning is the most serious warning from the FDA. The purpose of a boxed warning is to inform doctors and patients about the risks of taking a medication.
Withdrawal symptoms can occur if your body becomes used to taking a medication and then you stop treatment. Dependence can occur if your body is used to a drug and you need it to feel well. Klonopin can cause both withdrawal and dependence.
You may be at an increased risk of withdrawal or dependence if you’re taking a higher dose of Klonopin or you’ve taken the drug for a long period of time. However, it’s possible for withdrawal to occur even if you’re using Klonopin for a short period of time.
Klonopin withdrawal symptoms
Withdrawal symptoms vary from person to person, but most people experience symptoms in two phases. The first phase is called acute withdrawal and occurs right after you stop Klonopin. How long acute withdrawal lasts can vary based on how much Klonopin you were taking and for how long. However, it’s possible for acute withdrawal to last for a few weeks. Symptoms of acute withdrawal from Klonopin may include:
The first phase is called acute withdrawal and occurs right after you stop Klonopin. How long acute withdrawal lasts can vary based on how much Klonopin you were taking and for how long. However, it’s possible for acute withdrawal to last for a few weeks. Symptoms of acute withdrawal from Klonopin may include:
- anxiety
- depression
- nausea or vomiting
- diarrhea
- high blood pressure
- panic attacks
- seizures
- suicidal thoughts or actions
- insomnia
- hallucinations
After acute withdrawal, you may experience the second phase of withdrawal, called protracted withdrawal. This can occur for weeks after your acute withdrawal. In some cases, symptoms may even last 12 months or longer. Symptoms of protracted withdrawal may include:
- anxiety
- depression
- muscle weakness or twitching
- ringing in the ears
- numbness or tingling in your arms or legs
- difficulty thinking
- memory problems
- insomnia
If you need to stop treatment with Klonopin for any reason, be sure to talk with your doctor first. They will recommend slowly decreasing your dose of Klonopin to prevent withdrawal symptoms. This is very important, since Klonopin withdrawal can cause serious risks and can be life threatening. Your doctor will be able to recommend a dosage taper, or slow decrease in your Klonopin dose, to avoid these side effects.
They will recommend slowly decreasing your dose of Klonopin to prevent withdrawal symptoms. This is very important, since Klonopin withdrawal can cause serious risks and can be life threatening. Your doctor will be able to recommend a dosage taper, or slow decrease in your Klonopin dose, to avoid these side effects.
The dosages in this article are typical dosages provided by the drug’s manufacturer. If your doctor recommends Klonopin for you, they will prescribe the dosage that’s right for you. Always follow the dosage that your doctor prescribes for you.
As with any drug, never change your dosage of Klonopin without your doctor’s recommendation. If you have questions about the dosage of Klonopin that’s best for you, talk with your doctor.
Besides learning about dosage, you may want other information about Klonopin. These additional articles might be helpful to you:
- More about Klonopin. For information about other aspects of Klonopin, refer to this article.

- Drug comparison. The active drug in Klonopin is clonazepam. To find out how clonazepam compares with Xanax, read this article.
- Interactions. If you’d like to learn about Klonopin’s interactions, you can see this article.
- Details about your condition. For details about seizure disorders, see our epilepsy and seizures hub and list of related articles. And to learn more about panic disorder, visit our mental health hub.
Disclaimer: Medical News Today has made every effort to make certain that all information is factually correct, comprehensive, and up to date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or another healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
Arpimed
What Azaleptin is and what it is used for
Clozapine is the active ingredient in Azaleptin, which belongs to the group of antipsychotics (drugs used to treat certain mental disorders such as psychosis).
Azaleptin is used to treat schizophrenia in people who have not responded well to other drugs.
Schizophrenia is a mental illness that affects the way a person thinks, feels, and behaves. You should only use this drug if you have already used at least two other antipsychotics, one of which was a new-generation atypical antipsychotic, for schizophrenia that did not work or caused serious untreated side effects.
Azaleptin is also used to treat severe thinking, emotional and behavioral disorders in people with Parkinson's disease for whom other drugs have failed.
What you need to know before you use Azaleptin
Do not take Azaleptin if you are :
- you are allergic (hypersensitive) to any of the ingredients of clozapinus 902
- You do not have periodic blood tests,
- have had a history of having a low white blood cell count (such as leukopenia or agranulocytosis), especially if it was caused by drugs. The drug is also not used if you have a low level of leukocytes in the blood, due to previous chemotherapy,
- You suffer or have ever suffered from diseases of the bone marrow,
- You are taking a drug that interferes with the proper functioning of the bone marrow,
- You are taking a drug that reduces the number of white blood cells in your blood,
- You have previously stopped Azaleptin due to serious side effects (eg, agranulocytosis or heart problems),
- You suffer from uncontrolled epilepsy (seizures or convulsions),
- you have an acute mental disorder caused by the use of alcohol or drugs (for example, narcotic drugs),
- You suffer from myocarditis (inflammation of the heart muscle),
- You suffer from other severe heart conditions,
- You suffer from severe kidney disease,
- you have symptoms of active liver disease such as jaundice (yellowing of the skin and whites of the eyes, nausea and loss of appetite),
- You suffer from severe liver disease,
- You have impaired consciousness and severe drowsiness,
- You suffer from circulatory collapse, which can develop as a result of acute shock,
- You suffer from paralytic ileus (the bowel does not work properly and you are severely constipated),
- You are or have been treated with long-acting depot injections of antipsychotics.

If any of the above apply to you, contact your doctor and stop taking Azaleptin.
Azaleptin should not be used in people who are unconscious or in a coma.
Special instructions and precautions
The safety precautions mentioned in this section are very important. You must follow them to minimize your risk of developing serious, life-threatening side effects.
Before starting treatment with Azaleptin, tell your doctor if you have or have ever had:0026 Tell your doctor right away before taking Azaleptin tablets if: Medical examinations and blood tests Before you start taking Azaleptin, your doctor will need to review your medical history and do blood tests to make sure your white blood cell count is normal. It is important to be aware of this, as the body needs white blood cells to fight infections. It is necessary to conduct periodic blood tests before starting treatment, during treatment and after discontinuation of Azaleptin. Your doctor can also do physical examinations before starting treatment. To check the condition of the heart, he may do an electrocardiogram (ECG), but only if necessary or if you have an indication for this. If you have impaired liver function, you should have regular tests to evaluate liver function throughout the course of taking Azaleptin. If you have high blood sugar (diabetes mellitus), your doctor will need to monitor your blood sugar regularly. Azaleptin may cause changes in blood lipid levels. Azaleptin may cause weight gain. Your healthcare provider should monitor your weight and blood lipid levels. If you experience dizziness or weakness while taking Azaleptin, gently get up from a sitting or lying position. If you are scheduled for surgery or if for some reason you are unable to walk for a long period of time, talk to your doctor about taking Azaleptin as there is a risk of thrombosis (venous thrombosis). Children and adolescents under 16 If you are under 16 years old, you should not take Azaleptin because there is not enough data on its use in this age group. Elderly (aged 60 years and over) In the elderly (aged 60 years and over), the following side effects may be more likely to occur during treatment with Azaleptin: weakness or dizziness after changing body posture, fast heartbeat, difficulty urinating and constipation. Tell your doctor or pharmacist if you have dementia. Other medicines and Azaleptin Tell your doctor or pharmacist if you are taking, have recently taken or should take any other medicines. Do not take Azaleptin at the same time as drugs that depress the bone marrow and/or reduce the number of blood cells, such as: These drugs increase the risk of agranulocytosis (low white blood cell count). Use of Azaleptin concomitantly with other medicinal products that may affect the effects of Azaleptin and/or other medicinal products. Tell your doctor if you plan to take, are taking (even if your treatment is ending), or if you have recently had to stop taking any of the following medicines: This is not an exhaustive list. Your doctor and pharmacist may have more information about medicines that you should be careful about or avoid taking Azaleptin. They can also tell you if the drugs you are taking are in the above groups or not. Consult with them. Azaleptin with food and drink Avoid alcohol while using Azaleptin. Tell your doctor if you smoke and how often you drink caffeinated drinks (coffee, tea, cola). Sudden changes in smoking habits or intake of caffeinated beverages may also affect the effects of Azaleptin. How to take Azaleptin In order to minimize the risk of falling blood pressure, seizures and drowsiness, it is necessary that your doctor gradually increase the dose of the drug. You should always take Azaleptin exactly as prescribed by your doctor. If you have any doubts, you should consult your doctor or pharmacist. In the first place, it is important not to change the dosage or stop taking Azaleptin without notifying your doctor. If the dose you have been prescribed cannot be reached with this dose, there are other doses of this drug that must be used to reach the desired dose of the drug. Azaleptin tablets can be divided into equal doses. Treatment of schizophrenia The recommended starting dose is 12.5 mg (half a 25 mg tablet) once or twice on the first day followed by 25 mg once or twice on the second day. Swallow the tablet with water. If this dose is well tolerated, your doctor may gradually increase the dose in steps of 25 to 50 mg over the next 2-3 weeks until the dose reaches 300 mg per day. Thereafter, if necessary, the daily dose may be increased further in steps of 50 to 100 mg at half a week, or preferably at weekly intervals. The recommended daily dose is 200 to 450 mg divided into several single doses per day. Some people may need a higher dose. The permissible daily dose can be up to 900 mg. Frequent side effects (in particular, convulsions) are possible at daily doses of more than 450 mg. Always take the individually determined minimum effective dose of the drug for you. Most people take part of their dose in the morning and another part in the evening. Your healthcare professional will advise you on how to properly divide your daily dose. If your daily dose is only 200 mg, then you can take it once in the evening. During a certain period of time when you are taking Azaleptin and you have positive results, your doctor may reduce the dose. It is necessary to take Azaleptin for at least 6 months. Treatment of severe mental disorders in patients with Parkinson's disease The recommended starting dose is 12.5 mg (half a 25 mg tablet) in the evening. The recommended daily dose is 25 to 37.5 mg taken once in the evening. The dose of 50 mg per day should only be exceeded in exceptional cases. The maximum daily dose is 100 mg. Always take the minimum effective dose of the drug individually selected for you. If you have taken more Azaleptin than recommended . Symptoms of overdose include: salivation, pupil dilation, blurred vision, low blood pressure, collapse, fast or irregular heartbeat, shallow or labored breathing. If you forget to take Azaleptin If you forget to take your next dose, take it as soon as you remember. If you stop taking Azaleptin Do not stop taking Azaleptin without your doctor's advice as you may develop withdrawal symptoms. It includes sweating, headache, nausea, vomiting, and diarrhea. If you experience any of the above symptoms, tell your doctor immediately. These symptoms may be followed by more serious side effects if not treated immediately. Symptoms of the underlying disease may reappear. A gradual dose reduction in 12.5 mg increments over one to two weeks is recommended if treatment is to be discontinued. Your doctor will tell you how to reduce the daily dose of the drug. If you have to suddenly stop treatment with Azaleptin, you will need to consult your doctor for this. If your doctor decides to resume treatment with Azaleptin and the last dose of Azaleptin was taken more than two days ago, then the recommended initial dose should be 12.5 mg. If you have any further questions on the use of this medicine, ask your doctor or pharmacist. Pregnancy and breastfeeding If you are pregnant or breastfeeding, think you may be pregnant or are planning to become pregnant, ask your doctor or pharmacist before taking this medicine. Your doctor can discuss with you the benefits and possible risks of taking the drug during pregnancy. Contact your doctor immediately if you become pregnant while taking Azaleptin. Pregnancy Newborns whose mothers took Azaleptin during the last trimester (last three months) of pregnancy may experience the following symptoms: trembling, muscle rigidity and/or weakness, drowsiness, excitability, breathing problems and difficulty when feeding. If your child has any of the above symptoms, you should contact your doctor. Breastfeeding Do not breastfeed while taking Azaleptin. Clozapine, the active ingredient in Azaleptin, is excreted in breast milk and may harm your baby. Women of childbearing potential Some women who take certain medications for mental illness have irregular or no periods. If you have this observed, you can restore the regularity of menstruation if you replace the medication you are taking with Azaleptin. This means that you must use effective contraception. Effects on the ability to drive and use machines Azaleptin may cause fatigue, drowsiness and convulsions, especially at the beginning of treatment. You should not drive or operate machinery while you have these symptoms. Azaleptin contains lactose If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine. Possible side effects Like all medicines, Azaleptin can cause side effects, although not everyone gets them. Some side effects can be serious and require medical attention: Call your doctor right away before taking Azaleptin tablets if you experience any of the following side effects: Very common ( may affect more than 1 in 10 people ) : Common (may affect up to 1 in 10 people): Uncommon (may affect up to 1 in 100 people): Rare (may affect up to 1 in 1,000 people) Your doctor will need to check your heart and refer you to a cardiologist immediately if necessary. Very rare (may affect up to 1 in 10,000 people): Unknown (frequency of adverse reactions not estimated according to available data): neck and upper abdomen), shortness of breath, sweating, weakness, dizziness, nausea, vomiting, and palpitations (symptoms of a heart attack). You must seek emergency medical attention immediately. Other side effects0074 ) : Drowsiness, dizziness, increased salivation. Common (may affect up to 1 in 10 people): weight gain, blurred vision, headache, trembling, stiffness, restlessness, convulsions, impaired movement, inability to start moving, inability to control movement of different parts of the body, ECG changes, high blood pressure, weakness or dizziness after changing body posture, nausea, vomiting , loss of appetite, dry mouth, minor liver function test abnormalities, loss of urinary control, difficulty urinating, fatigue, fever, increased sweating, fever, speech disturbances (eg, slurred speech). Uncommon (may affect up to 1 in 100 people): Decreased levels of white blood cells (agranulocytosis), speech problems (eg, stuttering). Rare (may affect up to 1 in 1,000 people): surrounding the heart muscle (pericarditis), accumulation of fluid in the pericardial cavity (pericardial exudate), high blood sugar, diabetes mellitus, increased levels of the enzyme creatine phosphokinase in the blood. Very rare (may affect up to 1 in 10,000 people) behavior (obsessive-compulsive symptoms), skin reactions, salivary gland enlargement, difficulty breathing, complications from uncontrolled blood sugar (eg, coma or ketoacidosis), elevated blood triglycerides or cholesterol, heart muscle disease (cardiomyopathy), cardiac arrest , sudden unexplained death. Unknown (frequency of adverse reactions not estimated to the available data): Liver disorders including hepatic steatosis (fatty liver), liver cell necrosis, liver toxicity, liver disorders that include replacement of normal liver tissue coarse fibrous (scar) tissue leading to loss of liver function, including those that lead to life-threatening consequences such as liver failure (which can lead to death), liver damage (damage to liver cells, liver bile ducts, or both) together) and liver transplantation, electroencephalogram (EEG) changes, diarrhea, stomach discomfort, heartburn, stomach discomfort after eating, muscle weakness, muscle spasms, muscle pain, nasal congestion, bedwetting, sudden, uncontrolled increase in blood pressure ( pseudopheochromocytoma), uncontrolled bending of the body to one side (pleura totonus) if you are a man; ejaculation disorders, accompanied by an abnormal passage of seminal fluid, as a result of which the ejaculate enters the cavity of the bladder instead of ejaculating through the penis (orgasm without semen or retrograde ejaculation), rash, purplish-red spots, fever or itching due to inflammation of the blood vessels, inflammation large intestine leading to diarrhea, abdominal pain, fever, discoloration of the skin, butterfly rash on the face, joint pain, muscle pain, fever and fatigue (lupus erythematosus). Older people with dementia have been reported to experience a slight increase in the number of deaths among those taking antipsychotics compared with patients not taking antipsychotics. Reporting side effects: Tell your doctor, pharmacist or nurse if you notice any side effects. This includes any possible side effects not listed in this package insert. You can also report side effects to Arpimed LLC by going to the website www.arpimed.com and filling out the appropriate form “Report a side effect or ineffectiveness of a drug” and to the Scientific Center for Expertise of Drugs and Medical Technologies named after. Academician E.Gabrielyan, by going to the website www.pharm.am in the section “Report a side effect of a drug” and fill out the form “Map of reporting a side effect of a drug”. Scientific center hotline: +37410237665; +37498773368 How to store Azaleptin Keep out of the reach of children, dry, dark place at a temperature of 15 0 C-25 0 C. Shelf life - 3 years. Do not take Azaleptin after the expiry date which is stated on the package and on each blister. When specifying the expiration date, the last day of the specified month is meant. Do not dispose of medicines in wastewater or sewers. Ask your pharmacist how to dispose of a medicine you no longer need. These measures are aimed at protecting the environment. Contents of the pack and additional information What Azaleptin contains One tablet contains: active substance: 1 mg clozapine - excipients: microcrystalline cellulose, lactose monohydrate, corn starch, povidone, sodium starch glycolate, magnesium stearate. What Azaleptin looks like and contents of the pack Yellow, round, biconvex tablets, notched on one side, odorless. Packaging description 10 tablets in a blister pack (PVC/aluminium). 10 tablets in blisters (PVC/aluminium). 4 blister packs (40 tablets), together with the leaflet, are placed in a carton box. Dispensing conditions Available by prescription. Severe effects of constipation caused by clozapine can be fatal. Clozapine is an atypical antipsychotic used in the treatment of schizophrenia resistant to or intolerant to other antipsychotics; for the relief of suicidal behavior in schizophrenia or schizoaffective disorder; in the treatment of psychosis in Parkinson's disease. Currently, clozapine is reliably one of the most effective drugs for the treatment of schizophrenia. The antipsychotic effect of clozapine is probably mediated by a combination of dopamine D 9 receptor antagonist effects0773 2 in the mesolimbic pathway and 5-HT serotonin receptors 2A in the frontal cortex. The antagonism of D 2 alleviates the positive (psycho-productive) symptoms of schizophrenia, while the antagonism of 5-HT 2A alleviates its negative (psycho-deficiency) symptoms. Since clozapine simultaneously affects other receptors, its use is accompanied by adverse reactions. Thus, antagonism of serotonin receptors 5-HT 2C leads to a violation of lipid metabolism and an increase in body weight, muscarinic receptors M 1-5 - anticholinergic manifestations, histamine receptors H 1 - drowsiness, adrenergic receptors α 1 - orthostatic hypotensive effect. The regulator took care of the fact that for the 10-year period 2006-2016. The FDA Adverse Event Reporting System (FAERS) and the medical literature identified 10 cases of clozapine-induced constipation that progressed to serious complications and resulted in hospitalization, surgery, or death. Adverse events included necrotizing colitis (n=4), intestinal ischemia or necrosis (n=5), volvulus (n=1). The cumulative daily dose of clozapine ranged from 200 to 600 mg, with a median of 400 mg. The time to onset of serious bowel events ranged from 3 days to 6 months, with a median of 46 days. Preliminary review of additional FAERS data up to the end of 2019year confirmed the above. Clozapine has been found to cause serious bowel problems. Other antipsychotics have also been associated with severe complications of constipation, but only when used in conjunction with anticholinergics. A New Zealand study evaluating colonic transit time (CTT) using radiopaque markers found a median CTT of 104. Monitoring of bowel function and proactive use of laxatives in patients on clozapine therapy has been shown to improve colonic transit time and reduce the risk of serious sequelae. Clozapine, which marked the beginning of atypical antipsychotics as a class of drugs, appeared in 1972 in Switzerland and Austria under the name Leponex, and entered the US market only in February 1990 under the brand name Clozaril. Important information Mosmedpreparaty.ru is a specialized research and reference and analytical service of the Mosmedpreparaty group of companies, targeted at key events in the global pharmaceutical, biotechnology, medicine and healthcare industries. 
 Later tests should be done at least once a month.
Later tests should be done at least once a month. 
 This includes over-the-counter or herbal medicines. You may need to change the dosage of the drug or take other drugs.
This includes over-the-counter or herbal medicines. You may need to change the dosage of the drug or take other drugs.

 Continue taking the drug for as long as your doctor tells you to. If you are 60 years of age or older, your doctor may start treatment at a lower dosage and increase it gradually, as patients in this age group are more likely to develop some unwanted side effects (See "What you need to know before using Azaleptin").
Continue taking the drug for as long as your doctor tells you to. If you are 60 years of age or older, your doctor may start treatment at a lower dosage and increase it gradually, as patients in this age group are more likely to develop some unwanted side effects (See "What you need to know before using Azaleptin"). 
 Swallow the tablet with water. Your healthcare provider may then gradually increase your dose in 12.5 mg steps, no faster than two steps per week, up to a maximum dose of 50 mg by the end of the second week. Increasing the dosage should be stopped or postponed if you feel weak, dizzy or confused. In order to avoid these symptoms, blood pressure should be measured during the first weeks of treatment.
Swallow the tablet with water. Your healthcare provider may then gradually increase your dose in 12.5 mg steps, no faster than two steps per week, up to a maximum dose of 50 mg by the end of the second week. Increasing the dosage should be stopped or postponed if you feel weak, dizzy or confused. In order to avoid these symptoms, blood pressure should be measured during the first weeks of treatment.  If it is time for your next dose, skip the missed dose and take your next dose at the usual recommended time. Do not take a double dose to make up for a missed dose. Contact your doctor as soon as possible if you have not taken Azaleptin at all for more than 48 hours.
If it is time for your next dose, skip the missed dose and take your next dose at the usual recommended time. Do not take a double dose to make up for a missed dose. Contact your doctor as soon as possible if you have not taken Azaleptin at all for more than 48 hours. 

severe constipation Your doctor must treat this side effect so that you can avoid further complications.


 These symptoms may be signs that you are developing a liver disorder that can lead to fulminant liver necrosis.
These symptoms may be signs that you are developing a liver disorder that can lead to fulminant liver necrosis. 



 5 blister packs (50 tablets), together with the leaflet, are placed in a carton box.
5 blister packs (50 tablets), together with the leaflet, are placed in a carton box. Clozapine: the most effective but far from safe drug against schizophrenia
Image: Wikimedia.NEWS, REGULATORS At a Glance


Details
 5 hours in clozapine-treated patients versus 23 hours in other antipsychotics such as olanzapine , risperidone (risperidone), paliperidone (paliperidone), aripiprazole (aripiprazole), zuclopenthixol (zuclopenthixol), haloperidol (haloperidol). Clozapine resulted in a more than four-fold slowdown in CTT (p<0.0001), regardless of gender, age, race, duration of therapy, or total drug dose, but with a positive correlation consistent with plasma levels of clozapine. In 80% of subjects in the clozapine group, an abnormally long CTT was noted in the ascending and descending colon and its rectosigmoid region, which indicates total intestinal hypokinesia. Worse, patients often did not show any subjective symptoms associated with constipation.
5 hours in clozapine-treated patients versus 23 hours in other antipsychotics such as olanzapine , risperidone (risperidone), paliperidone (paliperidone), aripiprazole (aripiprazole), zuclopenthixol (zuclopenthixol), haloperidol (haloperidol). Clozapine resulted in a more than four-fold slowdown in CTT (p<0.0001), regardless of gender, age, race, duration of therapy, or total drug dose, but with a positive correlation consistent with plasma levels of clozapine. In 80% of subjects in the clozapine group, an abnormally long CTT was noted in the ascending and descending colon and its rectosigmoid region, which indicates total intestinal hypokinesia. Worse, patients often did not show any subjective symptoms associated with constipation.  Clozapine has long passed into the category of generic drugs and is known under the following trade names:
Clozapine has long passed into the category of generic drugs and is known under the following trade names:
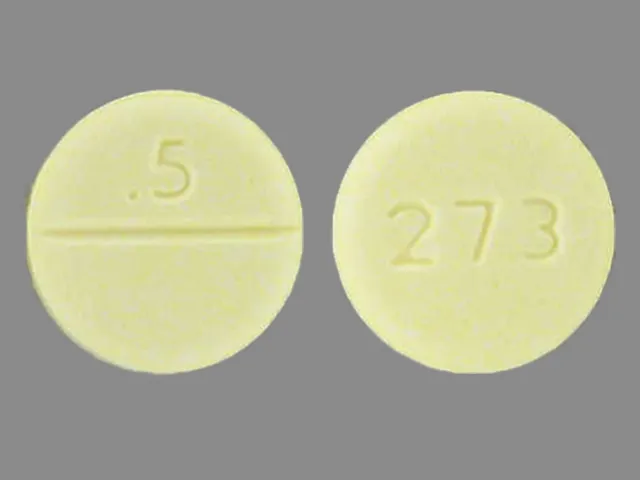

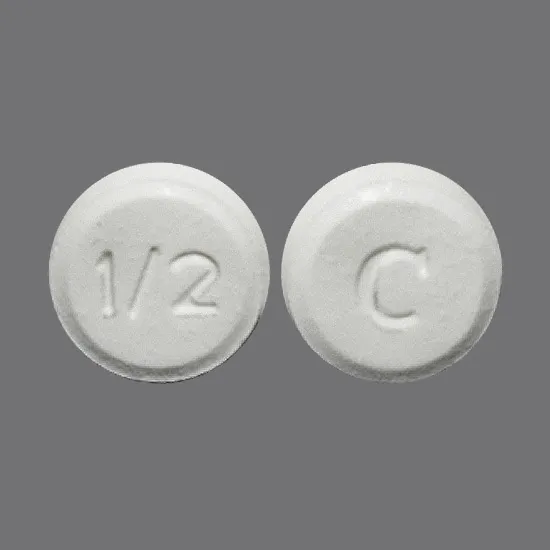
Learn more