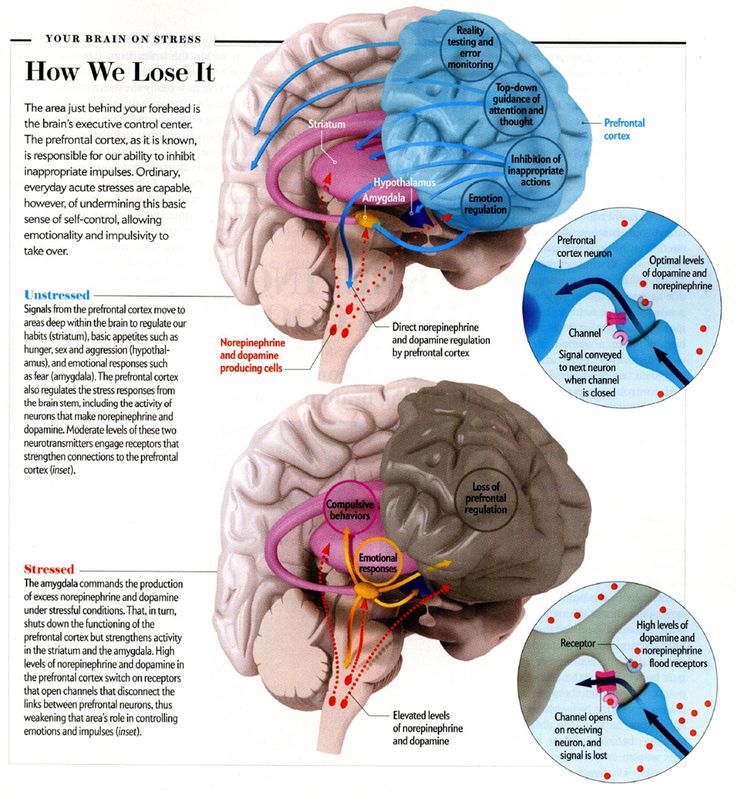
Stress damage to brain
Neurobiological and Systemic Effects of Chronic Stress
1. Lazarus RS, Folkman S. Stress, appraisal and coping, New York: Springer Verlag, 1984. [Google Scholar]
2. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010; 1186: 125–145. [PubMed] [Google Scholar]
3. Theall KP, Brett ZH, Shirtcliff EA, et al. Neighborhood disorder and telomeres: connecting children's exposure to community level stress and cellular response. Soc Sci Med 2013; 85: 50–58. [PMC free article] [PubMed] [Google Scholar]
4. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009; 16: 300–317. [PMC free article] [PubMed] [Google Scholar]
5. Dhabhar FS, Malarkey WB, Neri E, et al. Stress-induced redistribution of immune cells – from barracks to boulevards to battlefields: a tale of three hormones – Curt Richter Award winner. Psychoneuroendocrinology 2012; 37: 1345–1368.
[PMC free article] [PubMed] [Google Scholar]
6. Dhabhar FS, Saul AN, Daugherty C, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun 2010; 24: 127–137. [PMC free article] [PubMed] [Google Scholar]
7. Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 2005; 97: 1760–1767. [PMC free article] [PubMed] [Google Scholar]
8. Crum AJ, Salovey P, Achor S. Rethinking stress: the role of mindsets in determining the stress response. J Pers Soc Psychol 2013; 104: 716–733. [PubMed] [Google Scholar]
9. McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013; 79: 16–29. [PMC free article] [PubMed] [Google Scholar]
10. Gray JD, Rubin TG, Hunter RG, et al. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 2014; 19: 1171–1178. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
11. De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement 2014; 10(1 Suppl): S26–S32. [PubMed] [Google Scholar]
12. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022. [PMC free article] [PubMed] [Google Scholar]
13. McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature 1968; 220: 911–912. [PubMed] [Google Scholar]
14. Reul JM, DeKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985; 117: 2505–2511. [PubMed] [Google Scholar]
15. McEwen BS. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res 2016; 1645: 50–54. [PubMed] [Google Scholar]
16. Cameron HA, Gould E. The control of neuronal birth and survival. In: Shaw CA. (ed). Receptor dynamics in neural development. Pharmacology and toxicology: basic and clinical aspects, 1st New York: CRC Press, 1996, pp. 141–157. [Google Scholar]
In: Shaw CA. (ed). Receptor dynamics in neural development. Pharmacology and toxicology: basic and clinical aspects, 1st New York: CRC Press, 1996, pp. 141–157. [Google Scholar]
17. McEwen BS. Stress and hippocampal plasticity. Ann Rev Neurosci 1999; 22: 105–122. [PubMed] [Google Scholar]
18. Vyas A, Mitra R, Rao BSS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [PMC free article] [PubMed] [Google Scholar]
19. Bennur S, Shankaranarayana Rao BS, Pawlak R, et al. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 2007; 144: 8–16. [PubMed] [Google Scholar]
20. Lau T, Bigio B, Zelli D, et al. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Mol Psychiatry 2017; 22: 227–234. [PMC free article] [PubMed] [Google Scholar]
21. Rao RP, Anilkumar S, McEwen BS, et al. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 2012; 72: 466–475. [PMC free article] [PubMed] [Google Scholar]
Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 2012; 72: 466–475. [PMC free article] [PubMed] [Google Scholar]
22. Krishnan V, Han M-H, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404. [PubMed] [Google Scholar]
23. Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2004; 125: 1–6. [PubMed] [Google Scholar]
24. Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874. [PMC free article] [PubMed] [Google Scholar]
25. Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 2006; 27: 244–250. [PubMed] [Google Scholar]
26. Popoli M, Yan Z, McEwen BS, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012; 13: 22–37. [PMC free article] [PubMed] [Google Scholar]
Popoli M, Yan Z, McEwen BS, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012; 13: 22–37. [PMC free article] [PubMed] [Google Scholar]
27. Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci 2009; 1179: 167–178. [PubMed] [Google Scholar]
28. Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-Psychopharm Biol Psychiat 2010; 34: 791–797. [PMC free article] [PubMed] [Google Scholar]
29. Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 2009; 106: 3543–3548. [PMC free article] [PubMed] [Google Scholar]
30. Wei Q, Lu X-Y, Liu L, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856. [PMC free article] [PubMed] [Google Scholar]
31. Jacobson L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res 2014; 1583: 109–121. [PMC free article] [PubMed] [Google Scholar]
Jacobson L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res 2014; 1583: 109–121. [PMC free article] [PubMed] [Google Scholar]
32. Szyf M, Weaver ICG, Champagne FA, et al. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrin 2005; 26: 139–162. [PubMed] [Google Scholar]
33. McGowan PO, Sasaki A, D'Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neurosci 2009; 12: 241–243. [PMC free article] [PubMed] [Google Scholar]
34. Zohar J, Yahalom H, Kozlovsky N, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol 2011; 21: 796–809. [PubMed] [Google Scholar]
35. Schelling G, Roozendaal B, De Quervain DJ-F. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann NY Acad Sci 2004; 1032: 158–166.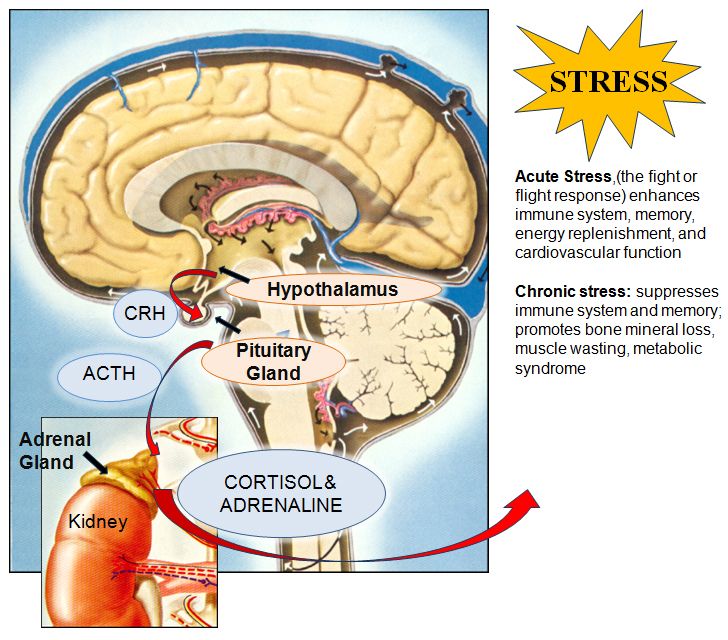 [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
36. Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 2008; 105: 5573–5578. [PMC free article] [PubMed] [Google Scholar]
37. Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA 2011; 108: 16074–16079. [PMC free article] [PubMed] [Google Scholar]
38. Liston C, Cichon JM, Jeanneteau F, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 2013; 16: 698–705. [PMC free article] [PubMed] [Google Scholar]
39. Lamont EW, Robinson B, Stewart J, et al. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 2005; 102: 4180–4184. [PMC free article] [PubMed] [Google Scholar]
40. Reddy AB, Maywood ES, Karp NA, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 2007; 45: 1478–1488. [PubMed] [Google Scholar]
Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 2007; 45: 1478–1488. [PubMed] [Google Scholar]
41. Karatsoreos IN, Bhagat SM, Bowles NP, et al. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 2010; 151: 2117–2127. [PMC free article] [PubMed] [Google Scholar]
42. Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem 1993; 61: 1957–1960. [PubMed] [Google Scholar]
43. Karst H, Berger S, Turiault M, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 2005; 102: 19204–19207. [PMC free article] [PubMed] [Google Scholar]
44. Treccani G, Musazzi L, Perego C, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex.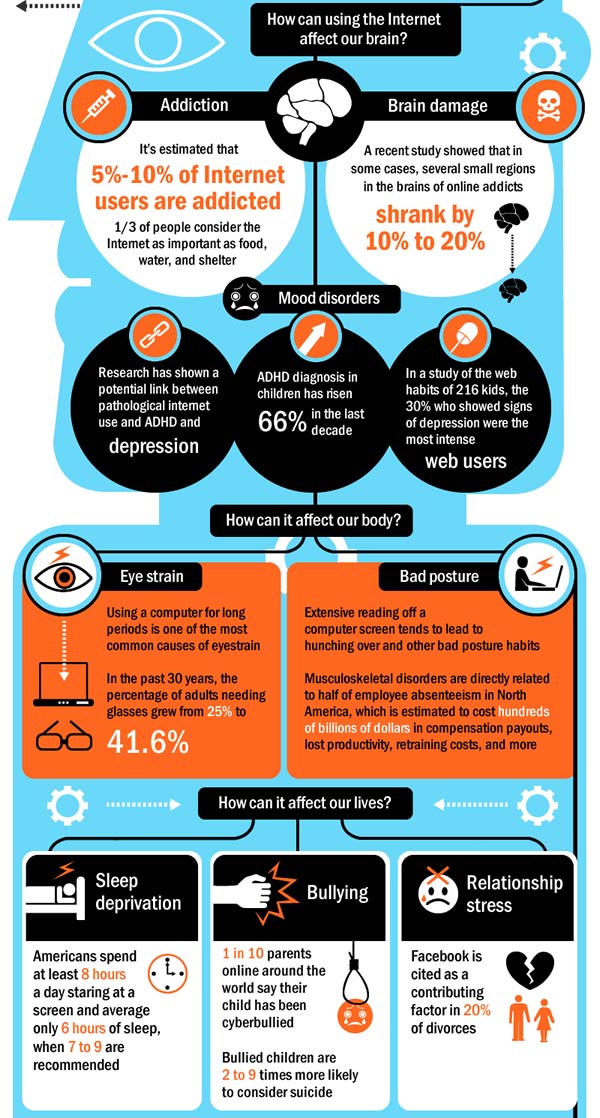 Mol Psychiatry 2014; 19: 433–443. [PubMed] [Google Scholar]
Mol Psychiatry 2014; 19: 433–443. [PubMed] [Google Scholar]
45. Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98. [PubMed] [Google Scholar]
46. Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampus CA3 pyramidal neurons. Brain Res 1992; 588: 341–344. [PubMed] [Google Scholar]
47. Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex 2011; 21: 2366–2373. [PMC free article] [PubMed] [Google Scholar]
48. Arendt T, Stieler J, Strijkstra AM, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 2003; 23: 6972–6981. [PMC free article] [PubMed] [Google Scholar]
49. Kinoshita Y, Hunter RG, Gray JD, et al. Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc Natl Acad Sci USA 2014; 111: 16130–16135. [PMC free article] [PubMed] [Google Scholar]
Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc Natl Acad Sci USA 2014; 111: 16130–16135. [PMC free article] [PubMed] [Google Scholar]
50. Sapolsky RM, Krey LC, McEwen BS. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. EndocrRev 1986; 7: 284–301. [PubMed] [Google Scholar]
51. Nasca C, Zelli D, Bigio B, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA 2015; 112: 14960–14965. [PMC free article] [PubMed] [Google Scholar]
52. Nasca C, Xenos D, Barone Y, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 2013; 110: 4804–4809. [PMC free article] [PubMed] [Google Scholar]
53. Chattarji S, Tomar A, Suvrathan A, et al. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 2015; 18: 1364–1375. [PubMed] [Google Scholar]
Nat Neurosci 2015; 18: 1364–1375. [PubMed] [Google Scholar]
54. Pereira AC, Lambert HK, Grossman YS, et al. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci USA 2014; 111: 18733–18738. [PMC free article] [PubMed] [Google Scholar]
55. Pereira AC, Gray JD, Kogan JF, et al. Age and Alzheimer's disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry 2017; 22: 296–305. [PMC free article] [PubMed] [Google Scholar]
56. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904. [PubMed] [Google Scholar]
57. Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging 2011; 32: 1942–1948. [PMC free article] [PubMed] [Google Scholar]
58. Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and medial prefrontal gyrus metabolism in women receiving hormone therapy.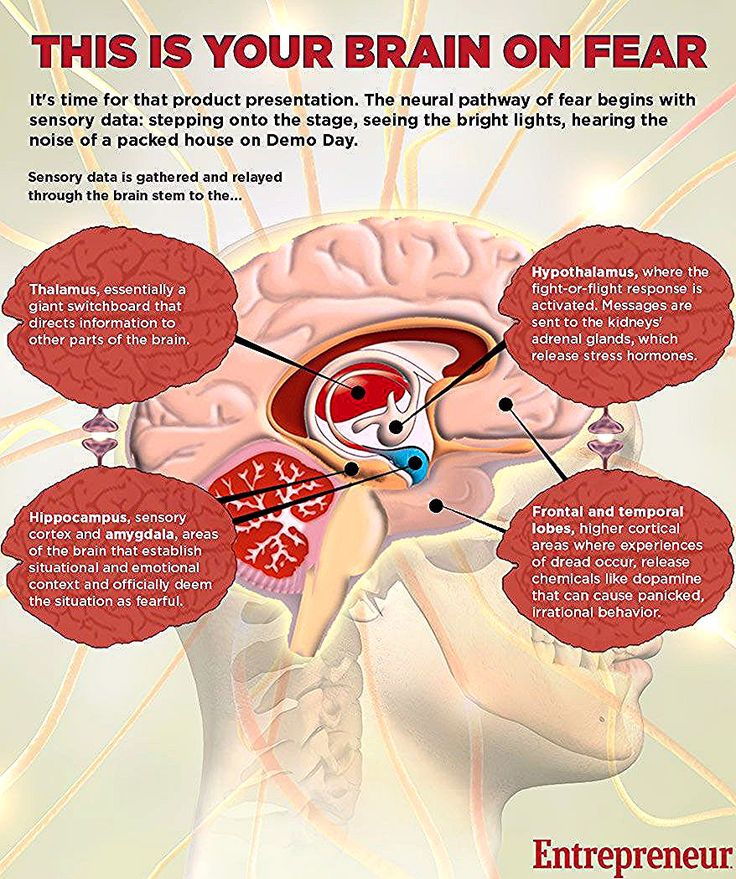 Psychiatry Res 2014; 223: 28–36. [PubMed] [Google Scholar]
Psychiatry Res 2014; 223: 28–36. [PubMed] [Google Scholar]
59. Kenna H, Hoeft F, Kelley R, et al. Fasting plasma insulin and the default mode network in women at risk for Alzheimer's disease. Neurobiol Aging 2013; 34: 641–649. [PMC free article] [PubMed] [Google Scholar]
60. Grillo CA, Piroli GG, Lawrence RC, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 2015; 64: 3927–3936. [PMC free article] [PubMed] [Google Scholar]
61. Grillo CA, Piroli GG, Kaigler KF, et al. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res 2011; 222: 230–235. [PMC free article] [PubMed] [Google Scholar]
62. Grillo CA, Piroli GG, Evans AN, et al. Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: effects of dietary restriction. Physiol Behav 2011; 104: 235–241. [PMC free article] [PubMed] [Google Scholar]
63. Grillo CA, Mulder P, Macht VA, et al. Dietary restriction reverses obesity-induced anhedonia. Physiol Behav 2014; 128: 126–132. [PMC free article] [PubMed] [Google Scholar]
Dietary restriction reverses obesity-induced anhedonia. Physiol Behav 2014; 128: 126–132. [PMC free article] [PubMed] [Google Scholar]
64. Yao J, Irwin RW, Zhao L, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 14670–14675. [PMC free article] [PubMed] [Google Scholar]
65. D'Alessio D. Is GLP-1 a hormone: whether and when?. J Diabetes Investig 2016; 7(Suppl 1): 50–55. [PMC free article] [PubMed] [Google Scholar]
66. McIntyre RS, Powell AM, Kaidanovich-Beilin O, et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res 2013; 237: 164–171. [PubMed] [Google Scholar]
67. Bigio B, Mathe AA, Sousa VC, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: implications for treatment resistance. Proc Natl Acad Sci USA 2016; 113: 7906–7911. [PMC free article] [PubMed] [Google Scholar]
68. Halfon N, Larson K, Lu M, et al. Lifecourse health development: past, present and future. Matern Child Health J 2014; 18: 344–365. [PMC free article] [PubMed] [Google Scholar]
Halfon N, Larson K, Lu M, et al. Lifecourse health development: past, present and future. Matern Child Health J 2014; 18: 344–365. [PMC free article] [PubMed] [Google Scholar]
69. Allfrey VG. Changes in chromosomal proteins at times of gene activation. Fed Proc 1970; 29: 1447–1460. [PubMed] [Google Scholar]
70. Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol 2008; 86: 305–341. [PMC free article] [PubMed] [Google Scholar]
71. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience 2014; 275: 420–435. [PubMed] [Google Scholar]
72. Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev 2007; 87: 799–823. [PubMed] [Google Scholar]
73. Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res 2012; 229: 280–288. [PubMed] [Google Scholar]
Behav Brain Res 2012; 229: 280–288. [PubMed] [Google Scholar]
74. Nasca C, Bigio B, Zelli D, et al. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 2015; 20: 755–763. [PMC free article] [PubMed] [Google Scholar]
75. Freund J, Brandmaier AM, Lewejohann L, et al. Emergence of individuality in genetically identical mice. Science 2013; 340: 756–759. [PubMed] [Google Scholar]
76. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005; 102: 10604–10609. [PMC free article] [PubMed] [Google Scholar]
77. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998; 14: 245–258. [PubMed] [Google Scholar]
78. Levine S, Haltmeyer G, Kara G, et al. Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2: 55–59. [Google Scholar]
Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2: 55–59. [Google Scholar]
79. Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 2005; 7: 103–123. [PMC free article] [PubMed] [Google Scholar]
80. Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA 2003; 100: 16131–16136. [PMC free article] [PubMed] [Google Scholar]
81. Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav 2006; 50: 454–462. [PubMed] [Google Scholar]
82. Akers KG, Yang Z, DelVecchio DP, et al. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS One 2008; 3: e2840.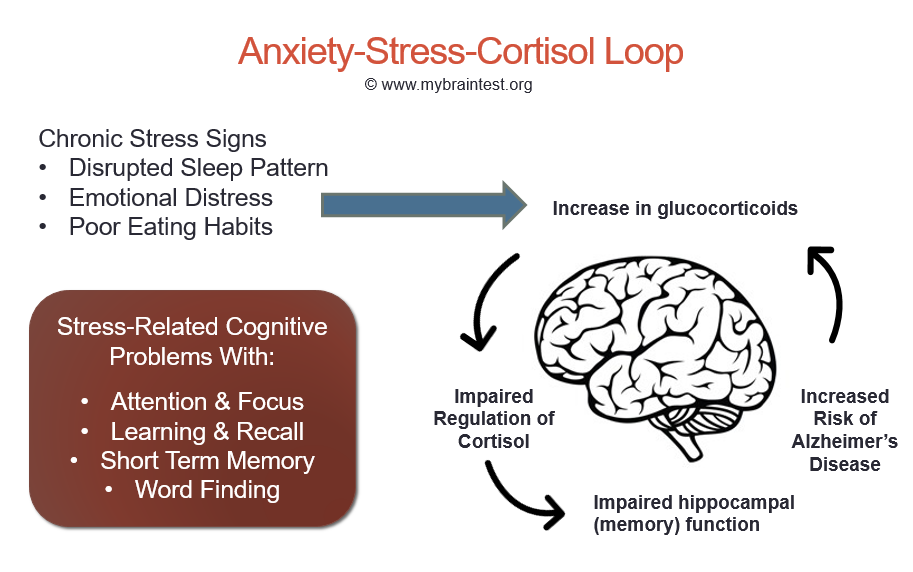 [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
83. Tang AC, Akers KG, Reeb BC, et al. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA 2006; 103: 15716–15721. [PMC free article] [PubMed] [Google Scholar]
84. Parker KJ, Buckmaster CL, Sundlass K, et al. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA 2006; 103: 3000–3005. [PMC free article] [PubMed] [Google Scholar]
85. Isgor C, Kabbaj M, Akil H, et al. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 2004; 14: 636–648. [PubMed] [Google Scholar]
86. Rice CJ, Sandman CA, Lenjavi MR, et al. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008; 149: 4892–4900. [PMC free article] [PubMed] [Google Scholar]
87. Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci 2006; 8: 1004–1006. [PMC free article] [PubMed] [Google Scholar]
Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci 2006; 8: 1004–1006. [PMC free article] [PubMed] [Google Scholar]
88. Kaufman D, Smith ELP, Gohil BC, et al. Early appearance of the metabolic syndrome in socially reared bonnet macaques. J Clin Endocrin & Metab 2005; 90: 404–408. [PubMed] [Google Scholar]
89. Coplan JD, Smith ELP, Altemus M, et al. Variable foraging demand rearing: Sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiat 2001; 50: 200–204. [PubMed] [Google Scholar]
90. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301: 386–389. [PubMed] [Google Scholar]
91. Spinelli S, Schwandt ML, Lindell SG, et al. The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Dev Psychopathol 2012; 24: 157–165. [PMC free article] [PubMed] [Google Scholar]
Dev Psychopathol 2012; 24: 157–165. [PMC free article] [PubMed] [Google Scholar]
92. Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science 2002; 297: 851–854. [PubMed] [Google Scholar]
93. Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann NY Acad Sci 2006; 1094: 52–62. [PubMed] [Google Scholar]
94. Obradovic J, Bush NR, Stamperdahl J, et al. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev 2010; 81: 270–289. [PMC free article] [PubMed] [Google Scholar]
95. Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 2005; 17: 271–301. [PubMed] [Google Scholar]
96. Galea LAM, McEwen BS, Tanapat P, et al. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress.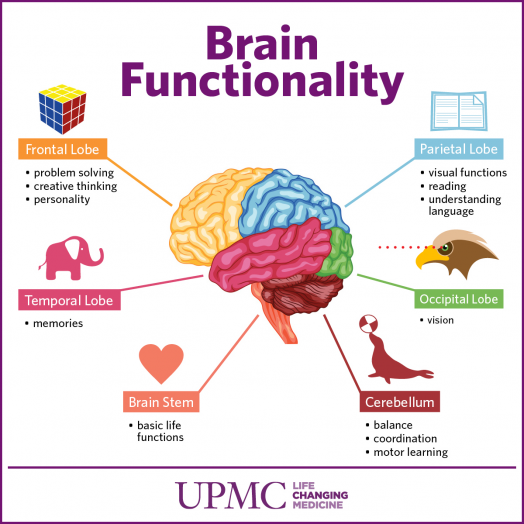 Neuroscience 1997; 81: 689–697. [PubMed] [Google Scholar]
Neuroscience 1997; 81: 689–697. [PubMed] [Google Scholar]
97. Luine V, Villegas M, Martinez C, et al. Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639: 167–170. [PubMed] [Google Scholar]
98. Luine VN, Beck KD, Bowman RE, et al. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinology 2007; 19: 743–751. [PubMed] [Google Scholar]
99. Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res 2001; 904: 279–289. [PubMed] [Google Scholar]
100. Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 1998; 95: 4066–4071. [PMC free article] [PubMed] [Google Scholar]
101. Wood GE, Shors TJ, Beylin AV. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females.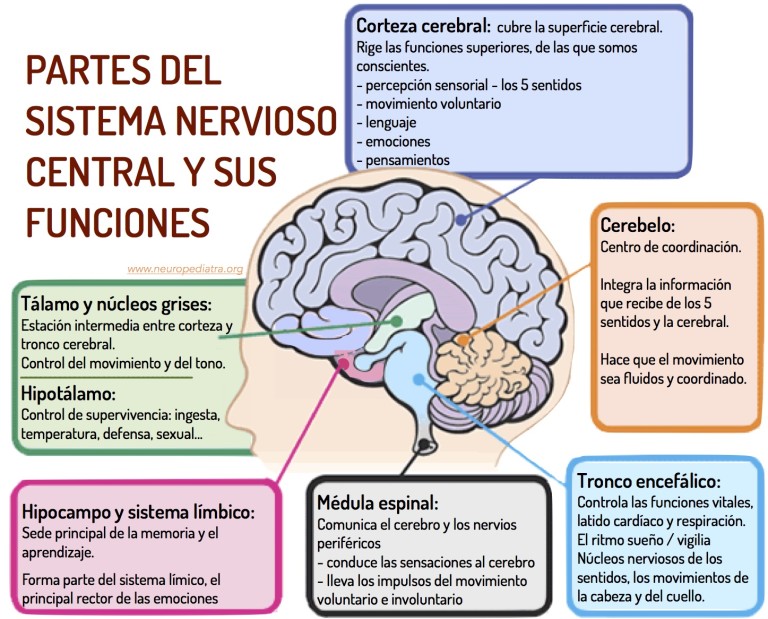 Behav Neurosci 2001; 115: 175–187. [PubMed] [Google Scholar]
Behav Neurosci 2001; 115: 175–187. [PubMed] [Google Scholar]
102. Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA 2002; 99: 13955–13960. [PMC free article] [PubMed] [Google Scholar]
103. Leuner B, Mendolia-loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiat 2004; 56: 964–970. [PMC free article] [PubMed] [Google Scholar]
104. Shansky RM, Hamo C, Hof PR, et al. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 2010; 20: 2560–2567. [PMC free article] [PubMed] [Google Scholar]
105. Bangasser DA, Curtis A, Reyes BA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 2010; 15: 877, 896–904. [PMC free article] [PubMed] [Google Scholar]
106. Bangasser DA, Zhang X, Garachh V, et al. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 2011; 103: 342–351. [PMC free article] [PubMed] [Google Scholar]
Bangasser DA, Zhang X, Garachh V, et al. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 2011; 103: 342–351. [PMC free article] [PubMed] [Google Scholar]
107. Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7: 477–484. [PubMed] [Google Scholar]
108. McEwen BS, Lasley EN. The end of sex as we know it. Cerebrum The Dana Forum on Brain Science 2005; Vol. 7, New York, NY: Dana Press. [Google Scholar]
109. McEwen BS. Introduction: the end of sex as we once knew it. Physiol Behav 2009; 97: 143–145. [PMC free article] [PubMed] [Google Scholar]
110. Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry 2007; 164: 1530–1538. [PubMed] [Google Scholar]
111. Meites J. Short history of neuroendocrinology and the international society of neuroendocrinology. Neuroendocrinology 1992; 56: 1–10.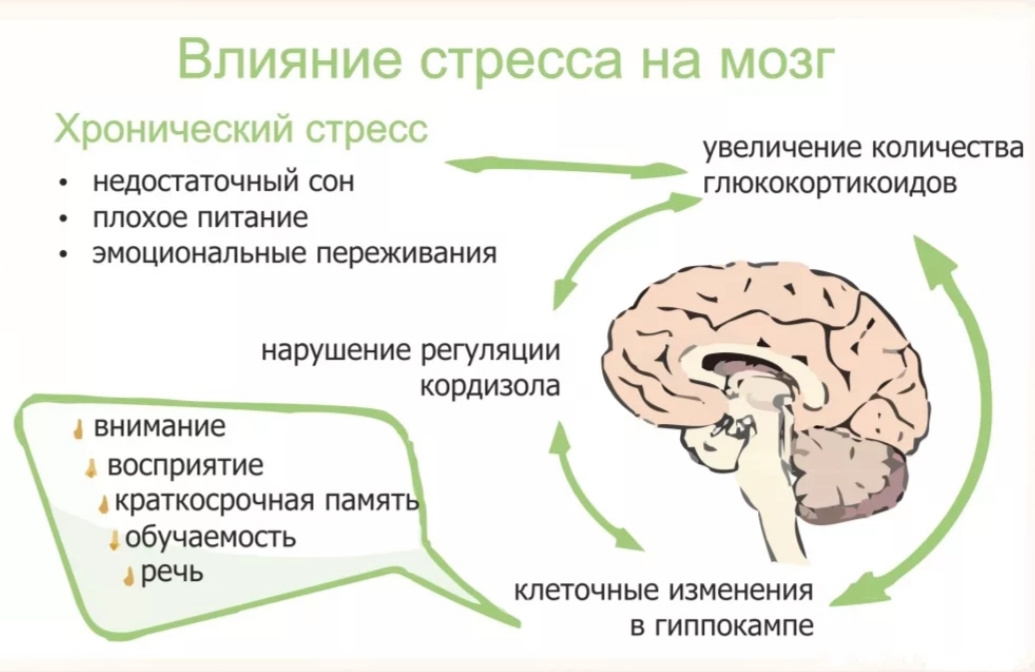 [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
112. Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nature Neurosci 2002; 5: 933–934. [PubMed] [Google Scholar]
113. Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology 2010; 35: 67–82. [PubMed] [Google Scholar]
114. McEwen BS, McEwen CA. Social, psychological, and physiological reactions to stress, New York: John Wiley & Sons, Inc, 2015. [Google Scholar]
115. Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 2009; 163: 1135–1143. [PMC free article] [PubMed] [Google Scholar]
116. Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci 2010; 21: 848–856. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
117. McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med 1998; 338: 171–179. [PubMed] [Google Scholar]
118. Anda RF, Butchart A, Felitti VJ, et al. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med 2010; 39: 93–98. [PubMed] [Google Scholar]
119. McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health 2011; 101(Suppl 1): S131–S139. [PMC free article] [PubMed] [Google Scholar]
120. Evans GW, Gonnella C, Marcynyszyn LA, et al. The role of chaos in poverty and children's socioemotional adjustment. Psychol Sci 2005; 16: 560–565. [PubMed] [Google Scholar]
121. Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res 2006; 1110: 166–174. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
122. Hart B, Risley TR. Meaningful differences in the everyday experience of young American Children, Baltimore, MD: Brookes Publishing Company, 1995, pp. 304. [Google Scholar]
123. Hanson JL, Chandra A, Wolfe BL, et al. Association between income and the hippocampus. PLoS One 2011; 6: e18712. [PMC free article] [PubMed] [Google Scholar]
124. Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci 2007; 2: 161–173. [PMC free article] [PubMed] [Google Scholar]
125. Gianaros PJ, Horenstein JA, Hariri AR, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci 2008; 3: 91–96. [PMC free article] [PubMed] [Google Scholar]
126. Adler NE, Boyce TW, Chesney MA, et al. Socioeconomic Inequalities in Health. JAMA 1993; 269: 3140–3145. [PubMed] [Google Scholar]
127. Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108: 14324–14329. [PMC free article] [PubMed] [Google Scholar]
Proc Natl Acad Sci USA 2011; 108: 14324–14329. [PMC free article] [PubMed] [Google Scholar]
128. Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev 2011; 35: 1562–1592. [PMC free article] [PubMed] [Google Scholar]
129. Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health 2010; 100: 933–939. [PMC free article] [PubMed] [Google Scholar]
130. Tomasdottir MO, Sigurdsson JA, Petursson H, et al. Self reported childhood difficulties, adult multimorbidity and allostatic load. A cross-sectional analysis of the Norwegian HUNT study. PLoS One 2015; 10: e0130591. [PMC free article] [PubMed] [Google Scholar]
131. Rasgon NL, McEwen BS. Insulin resistance-a missing link no more. Mol Psychiatry 2016; 21: 1648–1652. [PubMed] [Google Scholar]
132. Waddington CH. The epigenotype. Endeavoour 1942; 1: 18–20. [Google Scholar]
[Google Scholar]
133. Acheson SD. Independent inquiry into inequalities in health report, London: The Stationary Office, 1998. [Google Scholar]
134. McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA 2012; 109(Suppl 2): 17180–17185. [PMC free article] [PubMed] [Google Scholar]
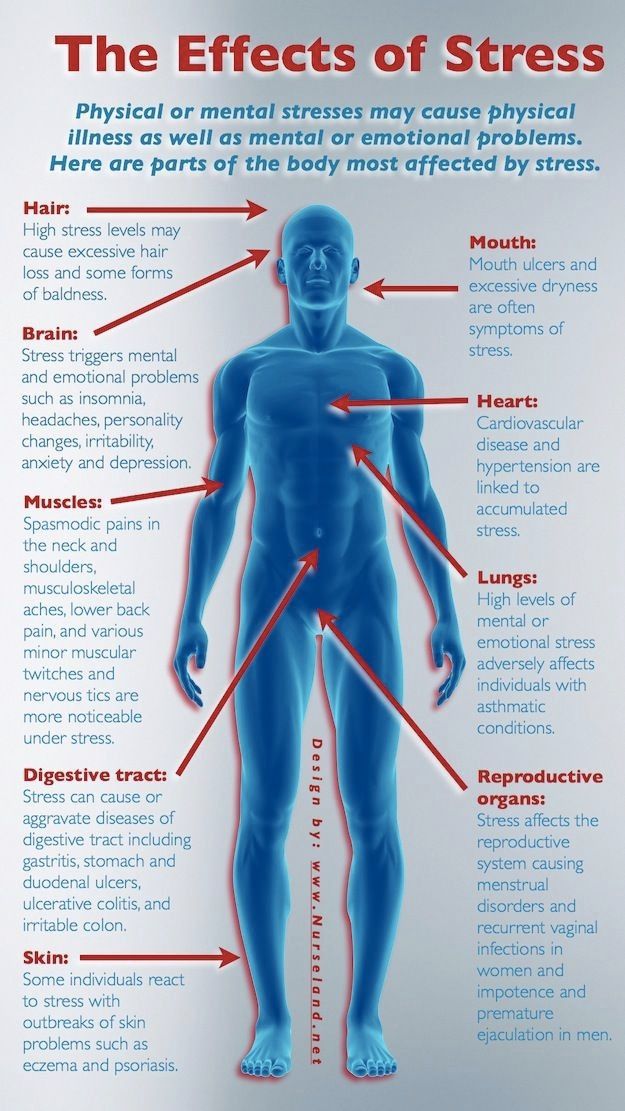
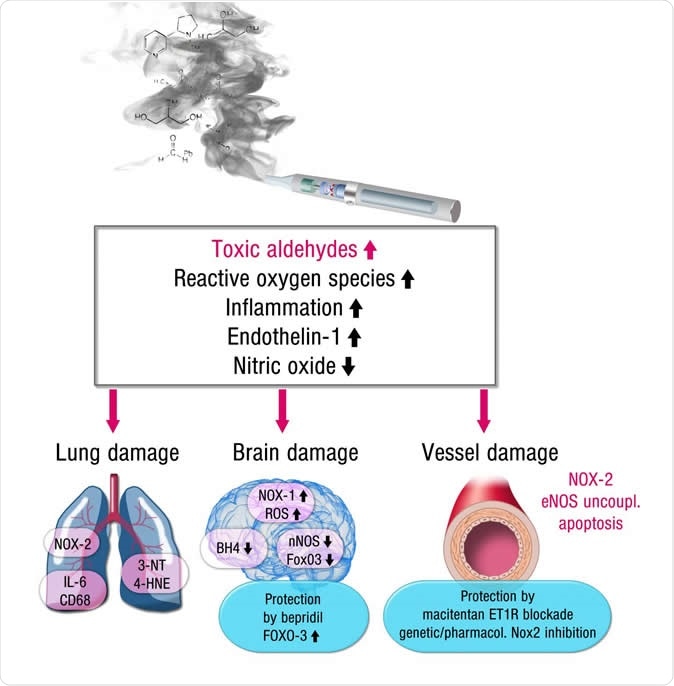
6 Ways Stress Affects Your Brain
The brain is a fascinating and complex organ. It’s the primary control center for our whole body, and it can be affected by stress in many different ways. Stress itself is an important part of life – it helps us prepare for danger or respond to emergencies. But when we’re constantly stressed out, that’s when our brain starts to pay the price. This blog post will explore how stress affects your brain, both positively and negatively, so you can develop strategies to reduce your brain’s vulnerability to its harmful effects.
For starters, it is important to understand how our body processes stress. In the simplest terms, stress is basically the “fight or flight” response to a perceived threat. This activates the amygdala, or “fear center” of the brain, and causes a cascade of events. These include the production of the stress hormone cortisol, an increase in glucose levels, increased heart rate, and an increase in blood flow to the muscles in the arms and legs. After the threat has passed, then the body will eventually return to normal.
This activates the amygdala, or “fear center” of the brain, and causes a cascade of events. These include the production of the stress hormone cortisol, an increase in glucose levels, increased heart rate, and an increase in blood flow to the muscles in the arms and legs. After the threat has passed, then the body will eventually return to normal.
In the case of chronic stress, however, the fear center of the brain is constantly activated, meaning that the body is in a constant state of stress. Cortisol levels are also constantly elevated, which can eventually start to cause problems with digestion, sleeping, and the immune system. Not only that, but when one part of the brain is constantly engaged, it is postulated that the other parts of the brain may not have enough energy to carry out their own functions properly. As a result, here are six ways that stress can affect the brain:
Impairs MemoryOne effect of chronic stress that researchers have observed is memory impairment. Specifically, it has been noted that people who are stressed tend to be more forgetful and less likely to remember specific information. Researchers believe that even minor stress, such as being late to work, can cause you to forget simple things like where your keys are. One study performed on older rats even noted that high levels of cortisol caused short-term memory declines. According to Dr. Kerry Ressler, chief scientific officer at McLean Hospital and professor of psychiatry at Harvard Medical School, “The basic idea is that the brain is shunting its resources because it’s in survival mode, not memory mode”.
Specifically, it has been noted that people who are stressed tend to be more forgetful and less likely to remember specific information. Researchers believe that even minor stress, such as being late to work, can cause you to forget simple things like where your keys are. One study performed on older rats even noted that high levels of cortisol caused short-term memory declines. According to Dr. Kerry Ressler, chief scientific officer at McLean Hospital and professor of psychiatry at Harvard Medical School, “The basic idea is that the brain is shunting its resources because it’s in survival mode, not memory mode”.
Your brain is composed of both gray matter and white matter. Gray matter is used for decision-making and problem-solving, while white matter is used to connect regions of the brain and communicate information. It has been noted that during times of chronic stress, the myelin sheaths that make up white matter become overproduced, while less gray matter is produced. When this happens, there can be an imbalance in gray and white matter. In some cases, this results in permanent changes to the brain’s structure.
When this happens, there can be an imbalance in gray and white matter. In some cases, this results in permanent changes to the brain’s structure.
An imbalance between white and gray matter can also play a role in the development of mental illness. The theory is that having excess myelin in certain areas of the brain interferes with the timing and balance of communication. It was also noted that chronic stress can negatively alter hippocampal function. The hippocampus is involved in memory, specifically spatial memory, memory consolidation, and memory transfer.
Stress Kills Brain CellsIt has been suggested by researchers that chronic stress can even kill new neurons in the brain’s hippocampus. The hippocampus is one of only two locations where neurons are produced. Despite the fact that the formation of new neurons does not seem to be affected, research shows that new neurons produced during periods of stress are more likely to die within a week.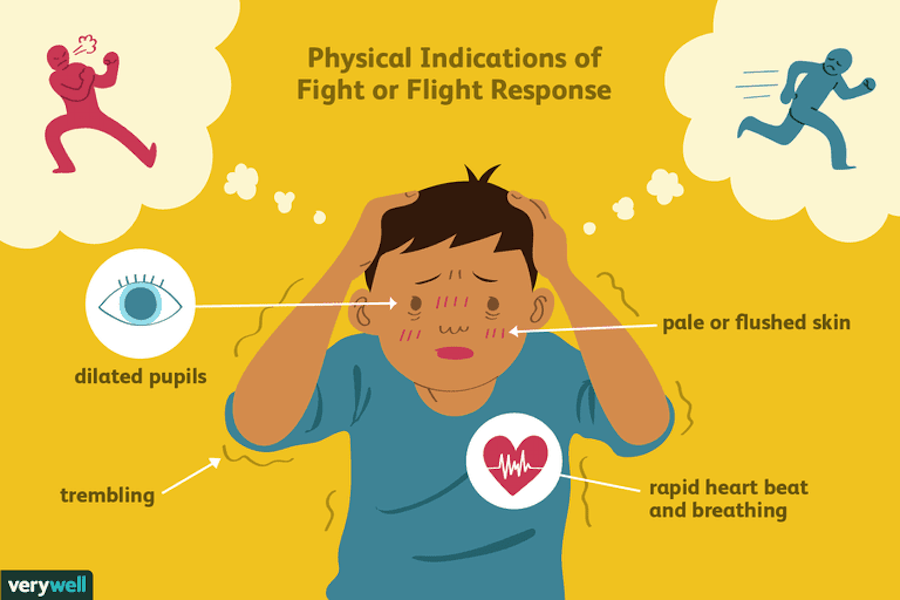
While the overall volume of the brain tends to remain about the same, it has been found that chronic stress in otherwise healthy individuals can cause areas of the brain associated with emotions, metabolism, and memory to shrink. Chronic stress also made people more likely to experience brain shrinkage when exposed to intense stressors. This means that people under constant stress may find it harder to deal with future stress.
Improves Cognitive FunctionStress is not all bad for your brain. In fact, moderate stress can actually improve brain performance by strengthening the connection between neurons in the brain. This helps to improve memory and attention span in order to make you more productive overall. This is why some people tend to perform “better under pressure”.
Dr. Kashouty, a diplomate of the American Board of Psychiatry and Neurology (ABPN), practices general neurology with fellowship trained specialization in clinical neurophysiology.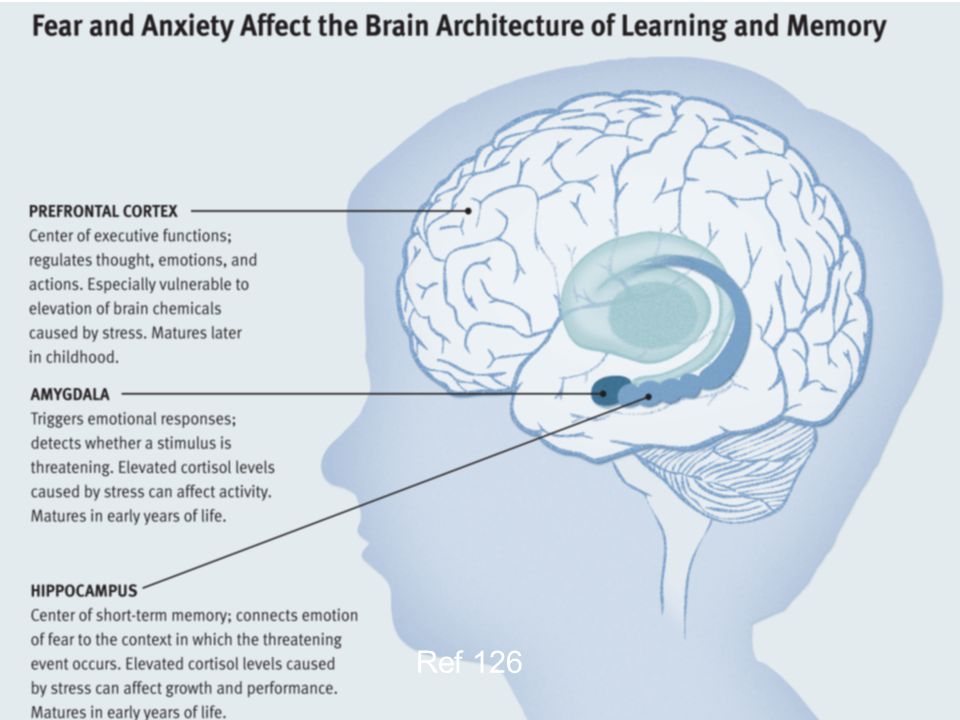 Dr. Kashouty finds the form and function of the nerves and muscles the most interesting part of neurology, which is what led him to specialize in neurophysiology with more emphasis on neuromuscular conditions. He treats all neurological diseases, but his main focus is to treat and manage headaches, movement disorders and neuromuscular diseases.
Dr. Kashouty finds the form and function of the nerves and muscles the most interesting part of neurology, which is what led him to specialize in neurophysiology with more emphasis on neuromuscular conditions. He treats all neurological diseases, but his main focus is to treat and manage headaches, movement disorders and neuromuscular diseases.
We talk about the impact of coronavirus on the brain and psyche, the likely consequences and psychological rehabilitation.
The illness may be accompanied by shortness of breath, fever, cough, and loss of smell. But the infection also affects the mental and neurological state of the patient. In addition to disorders of the central nervous system at the height of the disease, a third of those who recover have long-term complications: depression, anxiety, increased risk of stroke, parkinsonism. We talk about the impact of coronavirus on the brain and psyche, the likely consequences and psychological rehabilitation. nine0003
Why violations occur
COVID-19, like any other virus, affects the functioning of many body systems and affects not only the respiratory tract and lungs, but also the central nervous system. This is called neurotropism - the ability of an infection to infect the cells of this system. Moreover, researchers believe that the virus replicates inside the nerve cells of the brain.
This is called neurotropism - the ability of an infection to infect the cells of this system. Moreover, researchers believe that the virus replicates inside the nerve cells of the brain.
The results of post-mortem autopsies demonstrate that the coronavirus leads to inflammation of the brain tissue. And neuroimaging methods that show the structure and dysfunction of the brain detect microstrokes and leukoencephalopathy - a condition that leads to demyelination, when the coating of the processes of the nerve cell is destroyed. nine0003
These organic damages lead to the development of mental and neurological disorders in a person, which are complications.
What's going on
In a systematic review, researchers report that 20-40% of patients with coronavirus have psychiatric disorders:
- insomnia - in 42% of patients
- violation of attention and concentration - in 38%
- anxiety - 36%
- memory impairment - 34%
- depression - 33%
- disturbance of consciousness - in 21%
- post-traumatic stress disorder - 4-7%
Every fourth patient has a severe headache and dizziness, 69% have psychomotor agitation, 0. 7% have convulsions and movement disorders. Elderly people may develop delirium - a disorder of consciousness in which thinking, attention and perception of the world around are disturbed. However, most often delirium occurs in elderly patients with dementia, a syndrome of cognitive decline. Sometimes this is the only symptom of coronavirus in them without respiratory system disorders. nine0003
7% have convulsions and movement disorders. Elderly people may develop delirium - a disorder of consciousness in which thinking, attention and perception of the world around are disturbed. However, most often delirium occurs in elderly patients with dementia, a syndrome of cognitive decline. Sometimes this is the only symptom of coronavirus in them without respiratory system disorders. nine0003
“People with coronavirus infection often have negative psychological reactions: an acute reaction to stress, depression, emotional disturbances, obsessions and dysphoria — a combination of a gloomy mood and irritability,” says Olga Markina, candidate of psychological sciences, SberHealth consultant.
A large-scale outbreak of coronavirus is not the first case in history. During previous epidemics, doctors observed depressed mood and anxiety in patients - the most common symptoms in the acute phase of intoxication that accompanies the disease. In a small part of patients, psychosis occurs - an acute disturbance of mental activity, in which mental processes are out of sync, the perception of the real world is disturbed, and behavior and emotional reactions do not correspond to the real situation. nine0003
nine0003
“During illness, patients experience fear and anxiety,” Tigran Makichyan, a neurologist and rehabilitation specialist at GMS Clinic, shares his experience. “This is due to many factors: pressure from the media, stories that someone you know has died, heart palpitations, which causes anxiety and anxiety in a person.”
Another part of the patients develops catatonia, a movement disorder accompanied by either complete stupor or increased arousal. nine0003
“Work experience shows that a coronavirus infection associated with intoxication, oxygen starvation of the brain, can cause an exacerbation of diseases of the neurological and psychiatric spectrum,” warns Olga Markina. - In elderly patients, emotional and intellectual disorders may worsen. Also, COVID-19 can cause a psychotic state: confusion, hallucinations and delusions. Quite often there are episodes of anxiety-depressive disorder, panic attacks and obsessive-compulsive disorder.
nine0003
If a person with a mental disorder has a coronavirus, then the infection and its treatment can provoke a relapse and aggravate the course of the disorder. For example, medications for the treatment of COVID-19 can provoke psychosis in a patient with schizophrenia - delusions, hallucinations and inappropriate behavior, for example, the patient will think that medical personnel are trying to infect him.
“Coronavirus leads to decompensation of all chronic diseases, including schizophrenia,” says Tigran Makichyan, “Against the background of hypoxia, when there is not enough oxygen to the brain, patients become disoriented, but after recovery, as a rule, this disappears.” nine0003
Mental disorders are caused not only by the direct impact of the infection on the brain, but also by socio-psychological and economic factors associated with the coronavirus. A pandemic is associated in people with instability and unpredictability.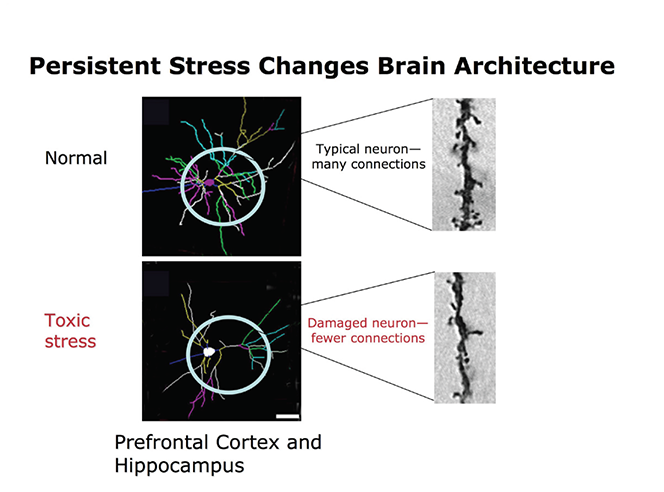 Quarantine, social restrictions, uninterrupted news flow about morbidity and mortality from COVID - all this affects the emotional state and mood of people. So, depression, anxiety, sleep disturbance, feeling of loneliness, depression and apathy can be caused by: nine0003
Quarantine, social restrictions, uninterrupted news flow about morbidity and mortality from COVID - all this affects the emotional state and mood of people. So, depression, anxiety, sleep disturbance, feeling of loneliness, depression and apathy can be caused by: nine0003
- high frequency of contact with infected people
- fear of infecting loved ones
- the need for physical distancing and wearing masks
- feelings of medical and social insecurity
- regular media reports about coronavirus
- lack of information about when the pandemic will end
A Chinese study shows that the very fact of a virus pandemic outbreak already affects the mental state of people who have not contracted the coronavirus. So, about one in six, under the influence of the epidemic, developed symptoms of moderate and severe depression, and one in three - signs of an anxiety disorder of moderate or severe degree. nine0003
In addition, some respondents have symptoms of post-traumatic stress disorder and psychological distress. The pandemic is also reported to worsen the course of mental disorders in people who do not have coronavirus.
The pandemic is also reported to worsen the course of mental disorders in people who do not have coronavirus.
Covid affects not only the patient himself. It is reported that 50% of family members where there is a coronavirus patient have symptoms of depression.
Complications after recovery
nine0002 The more severe the infectious disease, the higher the chance of complications after recovery. So, about a third of those who have recovered have complications from the central nervous system and psyche.Many people who have been ill talk about fatigue and malaise, difficulty in choosing words, memorizing, and reduced concentration. Some describe their condition as a kind of haze after COVID-19. Researchers report that one in three regularly experience headaches and dizziness, loss of smell and taste, and muscle weakness. nine0003
“Many negative psychological reactions appear after recovery,” Olga Markina shares her experience, “Sometimes symptoms do not appear immediately, but several months after recovery.
Patients develop malaise, drowsiness, and stamina decreases. Many people experience anxiety and fear of re-infection, up to hypochondria and phobia.”
A professor of psychiatry at the University of Maryland reports that, in his opinion, about 30-50% of those who recover in the future have an increased risk of mental disorders, such as depression and anxiety. Also, about half of people who have had symptoms of coronavirus suffer from insomnia and depressed mood. nine0003
Recent studies say that one in ten people who recover from coronavirus develop post-traumatic stress disorder, a third develop depression and anxiety disorder. In addition, 54% of people develop chronic fatigue syndrome within three months of infection.
A recent but not yet peer-reviewed study reports that people who recover from coronavirus are more likely to experience intracranial hemorrhage, ischemic stroke, parkinsonism, Guillain-Barré syndrome, dementia, and insomnia than those who recover from other common infections, including the flu. nine0003
nine0003
Psychological and cognitive rehabilitation
In addition to physical rehabilitation, which we have already talked about, those who have been ill need cognitive and psychological rehabilitation. The WHO reports that a recovery course is indicated for people who experience cognitive impairment during illness and after recovery, including memory and concentration decline, anxiety, depression, post-traumatic stress disorder and chronic fatigue.
There is still not enough information about which methods of rehabilitation are better. Now doctors practice cognitive-behavioral rehabilitation, which includes: nine0003
- memory and attention training
- speech therapy
- mental exercises
- psychological support
The task of psychological rehabilitation is to normalize the emotional background, relieve anxiety, tension and depression. Psychotherapy helps survivors reduce stress caused by social isolation and physical distancing. Psychotherapy can be done both online, if a person needs to observe isolation, or face-to-face, individually or in a group. nine0003
Psychotherapy can be done both online, if a person needs to observe isolation, or face-to-face, individually or in a group. nine0003
“Psychological assistance to covid patients can be provided at all stages of the disease. Medical psychologists work with patients in the "red zones". They provide psychological assistance to both patients and relatives of patients. Such help is vital for people who are in a state of great concern for their health and the health of their loved ones,” says Olga Markina.”
Psychological rehabilitation is provided to children and adults. To restore preschool children, psychologists and psychotherapists use the methods of play and fairy tale therapy. For older children - art therapy: sand therapy, isotherapy and music therapy. Adults are shown cognitive behavioral therapy, family and interpersonal psychotherapy. nine0003
Important to remember:
- Coronavirus is a neurotropic infection that multiplies in nerve cells.
 It is the cause of many neurological and mental disorders
It is the cause of many neurological and mental disorders - Patients with coronavirus may experience disorders of the central nervous system and psyche: headache, dizziness, fatigue, insomnia, anxiety, symptoms of depression. In severe patients - catatonia and disorders of consciousness
- Long-term complications may occur in recovered people: decreased memory and concentration, regular headaches, depression and anxiety, post-traumatic stress disorder, chronic fatigue syndrome
- Cognitive behavioral rehabilitation, cognitive behavioral therapy, art therapy, family and interpersonal therapy are used for mental and cognitive recovery.
Perinatal lesions of the central nervous system in Moscow - Central Clinical Hospital of the Russian Academy of Sciences
Perinatal lesions of the central nervous system (PP CNS) or hypoxic-ischemic encephalopathy is a group of pathological conditions associated with brain damage in the perinatal period. nine0003
nine0003
The main causes of PP CNS:
- Fetal hypoxia (chronic intrauterine; acute in childbirth)
- Birth injury
- Intoxication (bilirubin encephalopathy)
- Hypoglycemia
- Infectious factor
Clinical manifestations of CNS PP
- Nervous system excitability syndrome: excessive and multiple movements, tremor of the chin, tongue, limbs, regurgitation, sleep disturbance (excessive wakefulness), spontaneous Moro reflex (spreading of the arms in the supine position)
- Syndrome of depression of the nervous system: decrease in spontaneous motor activity, short-term wakefulness, excessive sleep, weakness of the sucking reflex, insufficient emotional response when interacting with the child.
- Syndrome of vegetative-visceral dysfunctions: thermoregulation disorders, transient cyanosis, disturbances in heart rate and respiratory rhythm, marbling of the skin, hypothermia of the extremities, vegetative-vascular spots on the skin, regurgitation, vomiting, unstable stool.
 nine0018
nine0018 - Intracranial hypertension syndrome, hydrocephalic syndrome: excessive increase in head circumference, fontanel bulging, head tilting back, loud monotonous crying (brain scream), head tilting back up to trunk arching (opisthotonus), persistent vomiting and regurgitation not associated with eating, increased sensitivity to sound stimuli (hyperesthesia), spontaneous bulging of the eyes (Gref's syndrome), difficulty falling asleep (wants to sleep, but cannot), short-term and superficial sleep, excitability. nine0018
- Convulsive syndrome: a variety of sudden and repetitive contractions of the eyelids, facial muscles, eye abduction, paroxysmal chewing, swallowing, sucking, sticking out of the tongue, swimming movements of the arms, pedaling, tonic tension of the trunk or limbs, single or group twitching of the muscles of the limbs, accompanied by convulsive movements eyes or "stopping" gaze, apnea.
- Dysregulation of muscle tone (muscle dystonia) increased, decreased, mixed muscle tone in the limbs, range of motion in the joints, spontaneous posture in sleep and wakefulness, position of the hands and feet, support during verticalization, head position during traction (pulling up) by the handles .
 nine0018
nine0018
Outcomes and consequences of CNS PP
The consequences of PP CNS can be determined by the age of 1 year. Below are their main manifestations:
- Impaired motor development: delayed acquisition of head holding skills, coups, sitting, crawling, standing up, independent walking relative to the physical age of the child.
- Formation of paresis and paralysis of both one and several limbs (monoplegia, diplegia, hemiparesis, tetraparesis), which are related to various forms of cerebral palsy. nine0018
- Violation of psycho-speech development: delay in acquiring the skill of cooing, babble, the first words and phrases, the quality of the sounds uttered, the timing of the formation of pincer grip and pointing gesture, understanding of addressed speech, interest in surrounding objects and their intended use, the nature of the game, memorizing a new information, concentration of attention with the formation of attention deficit hyperactivity disorder.
- Violation of behavior and emotions: the timing of the formation of the animation complex, differentiation of relatives and strangers, emotional resonance, the degree of expression of emotions, communication with peers and adults, the possibility of playing together, the formation of neatness skills, possibly leading to autism spectrum disorders. nine0018
- Hydrocephalus: excessive increase in head circumference, head deformity, pronounced saphenous veins in the temporal regions, signs of hypertension and hydrocephalus syndromes.
- Paroxysmal conditions of non-epileptic origin: affective-respiratory attacks, benign myoclonus of infancy (Fijerman's syndrome), benign neonatal sleep myoclonus, Sandiffer's syndrome, infantile torticolis, restless sleep, night terrors, rhythmic movements in sleep (rocking, shaking head, thumb sucking, gnashing of teeth). nine0018
- Age-dependent epileptic syndromes: early infantile epileptic encephalopathy (Otahara syndrome), early myoclonic encephalopathy, Dravet syndrome, West syndrome, benign neonatal epileptic syndromes, benign myoclonic epilepsy of infancy, benign partial epilepsy of infancy.
Instrumentation
In the CDC Research Institute of Pediatrics and Rehabilitation Treatment, instrumental diagnostics are carried out in order to clarify the diagnosis: nine0003
- Ultrasound of the brain (neurosonography)
- EEG of daytime sleep and wakefulness
- CT scan of the brain
- MRI of the brain and spine
Our help
Specialists from the Department of Developmental Neurobiology of the Research Institute of Pediatrics and Health Protection of the Central Clinical Hospital of the Russian Academy of Sciences work on the basis of the CDC:
- Diagnosis, monitoring and treatment are carried out by experienced neurologists, PhDs with 20 years of experience in this problem. Our specialists are the authors of the book "Modern neurobiological aspects of perinatal lesions of the CNS", published by the Russian Academy of Sciences. nine0018
- Defectologists and clinical psychologists are involved in the diagnosis to help clarify the presence of developmental disorders.
Learn more