Max dosage of ritalin
Ritalin, Concerta (methylphenidate) dosing, indications, interactions, adverse effects, and more
albuterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
aluminum hydroxide decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
amitriptyline, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate will decrease the level or effect of amlodipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
amoxapine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
apomorphine, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
arformoterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
aripiprazole increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.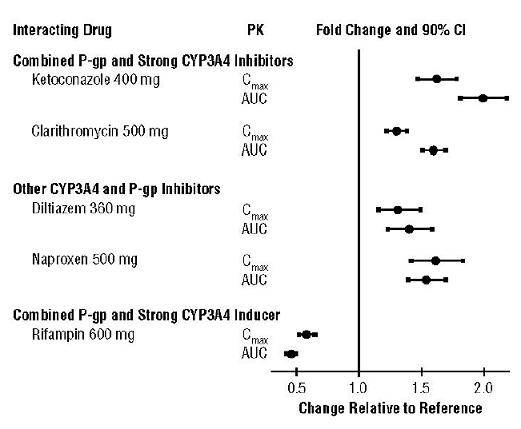
armodafinil increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
asenapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
aspirin/citric acid/sodium bicarbonate decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will increase the level or effect of atomoxetine by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of azilsartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of benazepril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
benzhydrocodone/acetaminophen, methylphenidate. Either increases effects of the other by serotonin levels. Use Caution/Monitor. Coadministration of drugs that affect the serotonergic neurotransmitter system may result in serotonin syndrome. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment.
bromocriptine, methylphenidate.
Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
Use Caution/Monitor. Potential for additive CNS stimulation.
caffeine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
calcium carbonate decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of candesartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of captopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
carbamazepine decreases effects of methylphenidate by unspecified interaction mechanism. Use Caution/Monitor. Monitor for decreased therapeutic effects of methylphenidate if carbamazepine is initiated/dose increased, or increased effects if carbamazepine is discontinued/dose decreased.
cariprazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
chlorpromazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
cimetidine decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of clevidipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
clomipramine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
clozapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
cocaine topical increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
desipramine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
dexfenfluramine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
dexlansoprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
dexmethylphenidate increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
dextroamphetamine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
didanosine will decrease the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Use Caution/Monitor. Interaction specifically associated with Ritalin LA.
methylphenidate will decrease the level or effect of diltiazem by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
dobutamine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
Use Caution/Monitor.
dopamine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
dopexamine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
doxepin, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate will increase the level or effect of dronabinol by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of enalapril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Monitor BP.
ephedrine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
epinephrine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate, epinephrine inhaled. Either increases effects of the other by sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
epinephrine racemic and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of eprosartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
esketamine intranasal, methylphenidate. Either increases toxicity of the other by sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor. Closely monitor blood pressure with concomitant use of esketamine nasal with stimulants. .
Either increases toxicity of the other by sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor. Closely monitor blood pressure with concomitant use of esketamine nasal with stimulants. .
esomeprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
famotidine will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Applies only to extended release formulation
famotidine decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of felodipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
fenfluramine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
fluphenazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
only.
fluphenazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
formoterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of fosinopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will increase the level or effect of fosphenytoin by unknown mechanism. Use Caution/Monitor. Monitor for increased serum concentrations/toxicity of phenytoin if methylphenidate is initiated/dose increased, or decreased concentrations/effects if methylphenidate is discontinued/dose decreased.
green tea, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Green tea may include caffeine. Caffeine is a CNS-stimulant and additive effects may be seen when coadministered with other CNS stimulants. Caffeine should be avoided or used cautiously.
Other (see comment). Use Caution/Monitor.
Comment: Green tea may include caffeine. Caffeine is a CNS-stimulant and additive effects may be seen when coadministered with other CNS stimulants. Caffeine should be avoided or used cautiously.
haloperidol increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
hydralazine, methylphenidate. Mechanism: pharmacodynamic antagonism. Use Caution/Monitor. Sympathomimetics can antagonize the activity of some antihypertensive agents.
hydrocodone, methylphenidate.
Either increases effects of the other by serotonin levels. Use Caution/Monitor. Coadministration of drugs that affect the serotonergic neurotransmitter system may result in serotonin syndrome. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment.
ibuprofen/famotidine will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Applies only to extended release formulation
iloperidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
imipramine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate decreases effects of iohexol by unspecified interaction mechanism. Modify Therapy/Monitor Closely. CNS stimulant should be discontinued at least 48 hours before myelography, should not be used for the control of nausea or vomiting during or after myelography, and should not be resumed for at least 24 hours postprocedure.
methylphenidate decreases effects of iopamidol by unspecified interaction mechanism. Modify Therapy/Monitor Closely. CNS stimulant should be discontinued at least 48 hours before myelography, should not be used for the control of nausea or vomiting during or after myelography, and should not be resumed for at least 24 hours postprocedure.
methylphenidate will decrease the level or effect of irbesartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
isoproterenol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of isradipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
lansoprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
levalbuterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
levodopa, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
lisdexamfetamine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of lisinopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of losartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
loxapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
loxapine inhaled increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
lurasidone, methylphenidate.
Either increases toxicity of the other by Other (see comment). Use Caution/Monitor.
Comment: Potential for additive CNS effects.
lurasidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
magnesium oxide decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
metaproterenol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methamphetamine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
Use Caution/Monitor. Risk of acute hypertensive episode.
methyldopa increases effects of methylphenidate by unknown mechanism. Use Caution/Monitor.
modafinil increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of moexipril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
molindone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of nadolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nicardipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nifedipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nimodipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nisoldipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
nizatidine will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Use Caution/Monitor. Applies only to extended release formulation
Applies only to extended release formulation
nizatidine decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
norepinephrine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
nortriptyline, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
olanzapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of olmesartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
omeprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
oxytocin increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor.
paliperidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
pantoprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of penbutolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of perindopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Methylphenidate may diminish antihypertensive effects. Monitor BP.
perphenazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
perphenazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will increase the level or effect of phenobarbital by unknown mechanism. Use Caution/Monitor. Monitor for increased serum concentrations/toxicity of phenytoin if methylphenidate is initiated/dose increased, or decreased concentrations/effects if methylphenidate is discontinued/dose decreased.
methylphenidate will decrease the level or effect of phenoxybenzamine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of phentolamine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will increase the level or effect of phenytoin by unknown mechanism. Use Caution/Monitor. Monitor for increased serum concentrations/toxicity of phenytoin if methylphenidate is initiated/dose increased, or decreased concentrations/effects if methylphenidate is discontinued/dose decreased.
pimavanserin increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
pimozide increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
pirbuterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
pramipexole, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
methylphenidate will decrease the level or effect of prazosin by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
procarbazine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
prochlorperazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
promazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
promethazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
methylphenidate will decrease the level or effect of propranolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
protriptyline, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
quetiapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of quinapril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
rabeprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of ramipril by pharmacodynamic antagonism.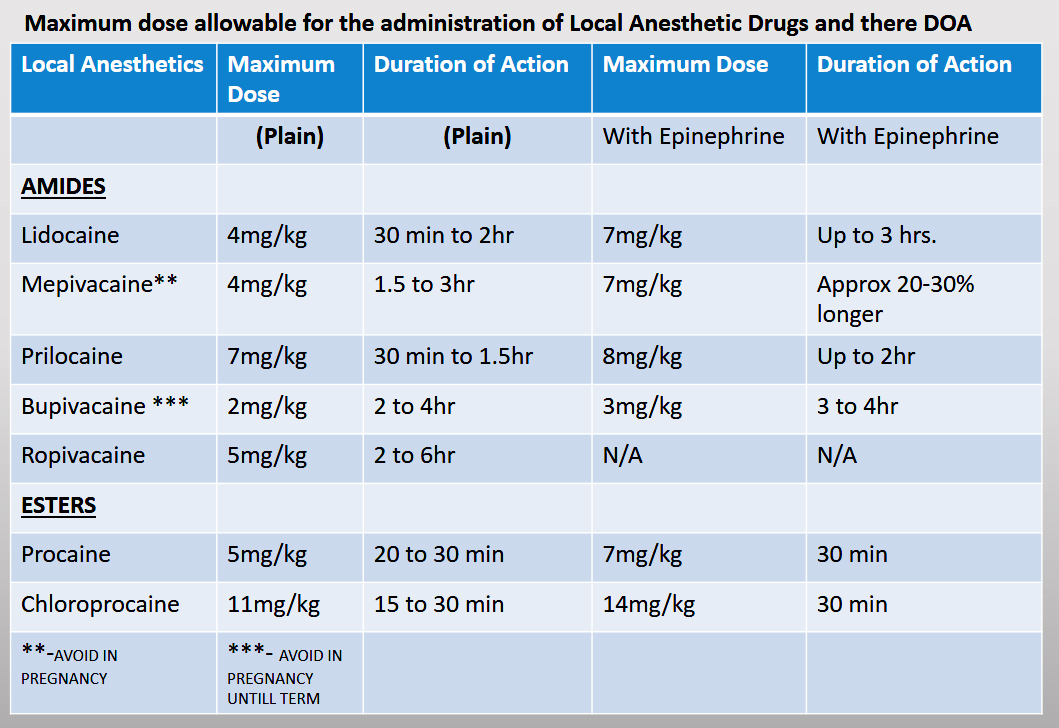 Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
risperidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
ropinirole, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
rotigotine, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
methylphenidate will decrease the level or effect of sacubitril/valsartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
salmeterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
Use Caution/Monitor.
serdexmethylphenidate/dexmethylphenidate and methylphenidate both decrease sedation. Use Caution/Monitor.
serdexmethylphenidate/dexmethylphenidate and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
serdexmethylphenidate/dexmethylphenidate increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
sodium zirconium cyclosilicate will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Check specific recommendations for drugs that exhibit pH-dependent solubility that may affect their systemic exposure and efficacy. In general, administer drugs at least 2 hr before or after sodium zirconium cyclosilicate. Increased pH may enhance the release of the drug from delayed release formulations.
methylphenidate and solriamfetol both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of sotalol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
sufentanil SL, methylphenidate. Either increases effects of the other by serotonin levels. Use Caution/Monitor. Coadministration of drugs that affect the serotonergic neurotransmitter system may result in serotonin syndrome. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment.
methylphenidate will decrease the level or effect of telmisartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of terazosin by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
terbutaline and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
thioridazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
thiothixene increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of timolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of trandolapril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate increases toxicity of trazodone by Other (see comment). Modify Therapy/Monitor Closely. Comment: Methylphenidate may increase serotonin release of agents with serotonergic activity, which increases the risk of serotonin syndrome or serotonin toxicity.
trifluoperazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
trifluoperazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
trimipramine, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate will decrease the level or effect of valsartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of verapamil by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate increases effects of warfarin by unspecified interaction mechanism. Use Caution/Monitor.
ziprasidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Evaluation of Methylphenidate Safety and Maximum-Dose Titration Rationale in Attention-Deficit/Hyperactivity Disorder
1. de Zwaan M, Gruss B, Müller A, et al.. The estimated prevalence and correlates of adult ADHD in a German community sample.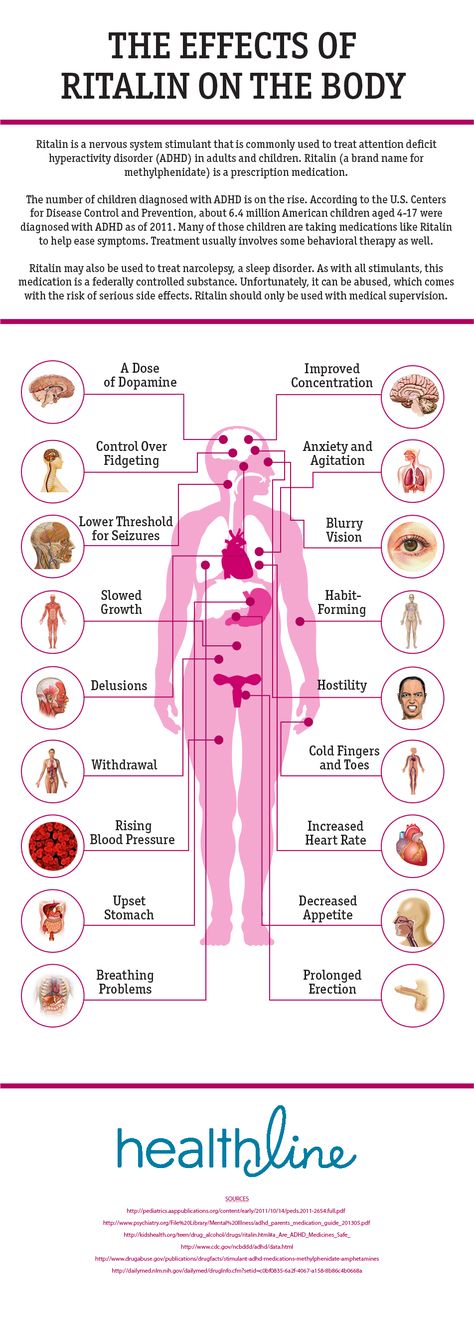 Eur Arch Psychiatry Clin Neurosci. 2012;262(1):79-86. doi: 10.1007/s00406-011-0211-9 [PubMed] [CrossRef] [Google Scholar]
Eur Arch Psychiatry Clin Neurosci. 2012;262(1):79-86. doi: 10.1007/s00406-011-0211-9 [PubMed] [CrossRef] [Google Scholar]
2. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490-499. doi: 10.1007/s13311-012-0135-8 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Sawyer MG, Reece CE, Sawyer ACP, Johnson SE, Lawrence D. Has the prevalence of child and adolescent mental disorders in Australia changed between 1998 and 2013 to 2014? J Am Acad Child Adolesc Psychiatry. 2018;57(5):343-350.e5. doi: 10.1016/j.jaac.2018.02.012 [PubMed] [CrossRef] [Google Scholar]
4. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
5. Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73(7):941-950. doi: 10.4088/JCP.11m07529 [PubMed] [CrossRef] [Google Scholar]
J Clin Psychiatry. 2012;73(7):941-950. doi: 10.4088/JCP.11m07529 [PubMed] [CrossRef] [Google Scholar]
6. Biederman J, Quinn D, Weiss M, et al.. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs. 2003;5(12):833-841. doi: 10.2165/00148581-200305120-00006 [PubMed] [CrossRef] [Google Scholar]
7. Arnold LE, Hodgkins P, Caci H, Kahle J, Young S. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS One. 2015;10(2):e0116407. doi: 10.1371/journal.pone.0116407 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Subcommittee on Attention-Deficit/Hyperactivity Disorder Steering Committee on Quality Improvement and Management ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1023-1029. doi: 10.1542/peds.2011-2654 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2011;128(5):1023-1029. doi: 10.1542/peds.2011-2654 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. J Am Acad Child Adolesc Psychiatry. 1997;36(6):754-763. doi: 10.1097/00004583-199706000-00011 [PubMed] [CrossRef] [Google Scholar]
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666. doi: 10.1542/peds.100.4.662 [PubMed] [CrossRef] [Google Scholar]
11. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237. doi: 10.2165/00023210-200822030-00003 [PubMed] [CrossRef] [Google Scholar]
12. Taylor E, Döpfner M, Sergeant J, et al.. European Clinical Guidelines for Hyperkinetic Disorder–first upgrade. Eur Child Adolesc Psychiatry. 2004;13(suppl 1):I7-I30. doi: 10.1007/s00787-004-1002-x [PubMed] [CrossRef] [Google Scholar]
Eur Child Adolesc Psychiatry. 2004;13(suppl 1):I7-I30. doi: 10.1007/s00787-004-1002-x [PubMed] [CrossRef] [Google Scholar]
13. CONCERTA (methylphenidate HCl) extended-release tablets CII. Food and Drug Administration. 2007. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021121s014lbl.pdf. Accessed November 8, 2016.
14. Ritalin hydrochloride methylphenidate hydrochloride tablets USP. Food and Drug Administration. 2007. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/010187s069,018029s040,021284s011lbl.pdf. Accessed November 14, 2016.
15. eTG Complete. Attention deficit hyperactivity disorder. Therapeutic Guidelines Limited. http://online.tg.org.au. Accessed November 14, 2016.
16. The Royal Australian College of General Practitioners; The Pharmaceutical Society of Australia . Australasian Society for Clinical and Experimental Pharmacologists and Toxicologists. Methylphenidate. Australian Medicines Handbook. Rundle Mall, SA: Australian Medicines Handbook Pty Ltd; 2016. [Google Scholar]
[Google Scholar]
17. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [PubMed] [CrossRef] [Google Scholar]
18. Storebø OJ, Ramstad E, Krogh HB, et al.. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev. 2015;(11):CD009885. doi: 10.1002/14651858.CD009885.pub2 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
20. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
21. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
22. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
23. World Health Organization International Classification of Diseases, Ninth Revision (ICD-9). Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
24. World Health Organization International Statistical Classification of Diseases, Tenth Revision (ICD-10). Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [PubMed] [CrossRef] [Google Scholar]
26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8(2):157-159. doi: 10.2307/1164923 [CrossRef] [Google Scholar]
29. Arnold LE, Lindsay RL, Conners CK, et al.. A double-blind, placebo-controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2004;14(4):542-554. doi: 10.1089/cap.2004.14.542 [PubMed] [CrossRef] [Google Scholar]
30. Findling RL, Bukstein OG, Melmed RD, et al.. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(1):149-159. doi: 10.4088/JCP.v69n0120 [PubMed] [CrossRef] [Google Scholar]
31. Findling RL, Quinn D, Hatch SJ, Cameron SJ, DeCory HH, McDowell M. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2006;15(8):450-459. doi: 10.1007/s00787-006-0565-0 [PubMed] [CrossRef] [Google Scholar]
Findling RL, Quinn D, Hatch SJ, Cameron SJ, DeCory HH, McDowell M. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2006;15(8):450-459. doi: 10.1007/s00787-006-0565-0 [PubMed] [CrossRef] [Google Scholar]
32. Greenhill LL, Findling RL, Swanson JM; ADHD Study Group . A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002;109(3):E39. doi: 10.1542/peds.109.3.e39 [PubMed] [CrossRef] [Google Scholar]
33. Law SF, Schachar RJ. Do typical clinical doses of methylphenidate cause tics in children treated for attention-deficit hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 1999;38(8):944-951. doi: 10.1097/00004583-199908000-00009 [PubMed] [CrossRef] [Google Scholar]
34. Riggs PD, Winhusen T, Davies RD, et al.. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914. doi: 10.1016/j.jaac.2011.06.010 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914. doi: 10.1016/j.jaac.2011.06.010 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Simonoff E, Taylor E, Baird G, et al.. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry. 2013;54(5):527-535. doi: 10.1111/j.1469-7610.2012.02569.x [PubMed] [CrossRef] [Google Scholar]
36. Wilens TE, McBurnett K, Bukstein O, et al.. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2006;160(1):82-90. doi: 10.1001/archpedi.160.1.82 [PubMed] [CrossRef] [Google Scholar]
37. Wolraich ML, Greenhill LL, Pelham W, et al.. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):883-892. doi: 10.1542/peds.108.4.883 [PubMed] [CrossRef] [Google Scholar]
doi: 10.1542/peds.108.4.883 [PubMed] [CrossRef] [Google Scholar]
38. Alsen S, Resmi H, Ozek H, Tufan AE, Bulbul M, Pekcanlar AA. Plasma norepinephrine and dopamine levels in prepubertal male children with attention-deficit hyperactivity disorder do not change with 8 weeks of methylphenidate treatment. Klinik Psikofarmakol Bülteni. 2015;25(3):259-266. doi: 10.5455/bcp.20150802050349 [CrossRef] [Google Scholar]
39. Arnold LE, Bozzolo DR, Hodgkins P, et al.. Switching from oral extended-release methylphenidate to the methylphenidate transdermal system: continued attention-deficit/hyperactivity disorder symptom control and tolerability after abrupt conversion. Curr Med Res Opin. 2010;26(1):129-137. doi: 10.1185/03007990903437412 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
40. Blader JC, Pliszka SR, Jensen PS, Schooler NR, Kafantaris V. Stimulant-responsive and stimulant-refractory aggressive behavior among children with ADHD. Pediatrics. 2010;126(4):e796-e806. doi: 10.1542/peds.2010-0086 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
doi: 10.1542/peds.2010-0086 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
41. Charles L, Schain R, Zelniker T. Optimal dosages of methylphenidate for improving the learning and behavior of hyperactive children. J Dev Behav Pediatr. 1981;2(3):78-81. doi: 10.1097/00004703-198109000-00003 [PubMed] [CrossRef] [Google Scholar]
42. Childress AC, Kollins SH, Cutler AJ, Marraffino A, Sikes CR. Efficacy, safety, and tolerability of an extended-release orally disintegrating methylphenidate tablet in children 6-12 years of age with attention-deficit/hyperactivity disorder in the laboratory classroom setting. J Child Adolesc Psychopharmacol. 2017;27(1):66-74. doi: 10.1089/cap.2016.0002 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
43. Cho SC, Kim BN, Cummins TD, Kim JW, Bellgrove MA. Norepinephrine transporter -3081(A/T) and alpha-2A-adrenergic receptor MspI polymorphisms are associated with cardiovascular side effects of OROS-methylphenidate treatment. J Psychopharmacol. 2012;26(3):380-389. doi: 10.1177/0269881111405356 [PubMed] [CrossRef] [Google Scholar]
2012;26(3):380-389. doi: 10.1177/0269881111405356 [PubMed] [CrossRef] [Google Scholar]
44. Chou WJ, Chen SJ, Chen YS, et al.. Remission in children and adolescents diagnosed with attention-deficit/hyperactivity disorder via an effective and tolerable titration scheme for osmotic release oral system methylphenidate. J Child Adolesc Psychopharmacol. 2012;22(3):215-225. doi: 10.1089/cap.2011.0006 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Connor DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr (Phila). 2000;39(1):15-25. doi: 10.1177/000992280003900102 [PubMed] [CrossRef] [Google Scholar]
46. Correia Filho AG, Bodanese R, Silva TL, Alvares JP, Aman M, Rohde LA. Comparison of risperidone and methylphenidate for reducing ADHD symptoms in children and adolescents with moderate mental retardation. J Am Acad Child Adolesc Psychiatry. 2005;44(8):748-755. doi: 10.1097/01.chi.0000166986.30592.67 [PubMed] [CrossRef] [Google Scholar]
2005;44(8):748-755. doi: 10.1097/01.chi.0000166986.30592.67 [PubMed] [CrossRef] [Google Scholar]
47. Findling RL, Katic A, Rubin R, Moon E, Civil R, Li Y. A 6-month, open-label, extension study of the tolerability and effectiveness of the methylphenidate transdermal system in adolescents diagnosed with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010;20(5):365-375. doi: 10.1089/cap.2009.0122 [PubMed] [CrossRef] [Google Scholar]
48. Findling RL, Wigal SB, Bukstein OG, et al.. Long-term tolerability of the methylphenidate transdermal system in pediatric attention-deficit/hyperactivity disorder: a multicenter, prospective, 12-month, open-label, uncontrolled, phase III extension of four clinical trials. Clin Ther. 2009;31(8):1844-1855. doi: 10.1016/j.clinthera.2009.08.002 [PubMed] [CrossRef] [Google Scholar]
49. Firestone P, Kelly MJ, Goodman JT, Davey J. Differential effects of parent training and stimulant medication with hyperactives: a progress report. J Am Acad Child Psychiatry. 1981;20(1):135-147. doi: 10.1016/S0002-7138(09)60723-8 [PubMed] [CrossRef] [Google Scholar]
J Am Acad Child Psychiatry. 1981;20(1):135-147. doi: 10.1016/S0002-7138(09)60723-8 [PubMed] [CrossRef] [Google Scholar]
50. Hoare P, Remschmidt H, Medori R, et al.. 12-Month efficacy and safety of OROS MPH in children and adolescents with attention-deficit/hyperactivity disorder switched from MPH. Eur Child Adolesc Psychiatry. 2005;14(6):305-309. doi: 10.1007/s00787-005-0486-3 [PubMed] [CrossRef] [Google Scholar]
51. Kemner JE, Starr HL, Ciccone PE, Hooper-Wood CG, Crockett RS. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther. 2005;22(5):498-512. doi: 10.1007/BF02849870 [PubMed] [CrossRef] [Google Scholar]
52. Kim SW, Lee JH, Lee SH, Hong HJ, Lee MG, Yook K-H. ABCB1 c.2677G>T variation is associated with adverse reactions of OROS-methylphenidate in children and adolescents with ADHD. J Clin Psychopharmacol. 2013;33(4):491-498. doi: 10.1097/JCP.0b013e3182905a8d [PubMed] [CrossRef] [Google Scholar]
53. Kral MC, Lally MD, Boan AD. Effectiveness and side effect profile of stimulant medication for the treatment of attention-deficit/hyperactivity disorder in youth with epilepsy. J Child Adolesc Psychopharmacol. 2017;27(8):735-740. doi: 10.1089/cap.2016.0186 [PubMed] [CrossRef] [Google Scholar]
Kral MC, Lally MD, Boan AD. Effectiveness and side effect profile of stimulant medication for the treatment of attention-deficit/hyperactivity disorder in youth with epilepsy. J Child Adolesc Psychopharmacol. 2017;27(8):735-740. doi: 10.1089/cap.2016.0186 [PubMed] [CrossRef] [Google Scholar]
54. Kronenberger WG, Giauque AL, Lafata DE, Bohnstedt BN, Maxey LE, Dunn DW. Quetiapine addition in methylphenidate treatment-resistant adolescents with comorbid ADHD, conduct/oppositional-defiant disorder, and aggression: a prospective, open-label study. J Child Adolesc Psychopharmacol. 2007;17(3):334-347. doi: 10.1089/cap.2006.0012 [PubMed] [CrossRef] [Google Scholar]
55. Kurlan R, Goetz CG, McDermott MP, et al.; Tourette’s Syndrome Study Group . Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527-536. doi: 10.1212/WNL.58.4.527 [PubMed] [CrossRef] [Google Scholar]
56. Loo SK, Bilder RM, Cho AL, et al.. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1. doi: 10.1016/j.jaac.2016.04.020 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1. doi: 10.1016/j.jaac.2016.04.020 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
57. Maayan L, Paykina N, Fried J, Strauss T, Gugga SS, Greenhill L. The open-label treatment of attention-deficit/hyperactivity disorder in 4- and 5-year-old children with beaded methylphenidate. J Child Adolesc Psychopharmacol. 2009;19(2):147-153. doi: 10.1089/cap.2008.053 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
58. McGough JJ, McBurnett K, Bukstein O, et al.. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16(3):351-356. doi: 10.1089/cap.2006.16.351 [PubMed] [CrossRef] [Google Scholar]
59. Na K-S, Lee SI, Hong SD, et al.. Effect of osmotic-release oral system methylphenidate on learning skills in adolescents with attention-deficit/hyperactivity disorder: an open-label study. Int Clin Psychopharmacol. 2013;28(4):184-192. doi: 10.1097/YIC.0b013e3283612509 [PubMed] [CrossRef] [Google Scholar]
2013;28(4):184-192. doi: 10.1097/YIC.0b013e3283612509 [PubMed] [CrossRef] [Google Scholar]
60. Newcorn JH, Kratochvil CJ, Allen AJ, et al.; Atomoxetine/Methylphenidate Comparative Study Group . Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721-730. doi: 10.1176/appi.ajp.2007.05091676 [PubMed] [CrossRef] [Google Scholar]
61. Newcorn JH, Stein MA, Cooper KM. Dose-response characteristics in adolescents with attention-deficit/hyperactivity disorder treated with OROS methylphenidate in a 4-week, open-label, dose-titration study. J Child Adolesc Psychopharmacol. 2010;20(3):187-196. doi: 10.1089/cap.2009.0102 [PubMed] [CrossRef] [Google Scholar]
62. Palumbo DR, Sallee FR, Pelham WE Jr, Bukstein OG, Daviss WB, McDermott MP. Clonidine for attention-deficit/hyperactivity disorder: I. efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(2):180-188. doi: 10.1097/chi.0b013e31815d9af7 [PubMed] [CrossRef] [Google Scholar]
2008;47(2):180-188. doi: 10.1097/chi.0b013e31815d9af7 [PubMed] [CrossRef] [Google Scholar]
63. Purper-Ouakil D, Cortese S, Wohl M, et al.. Temperament and character dimensions associated with clinical characteristics and treatment outcome in attention-deficit/hyperactivity disorder boys. Compr Psychiatry. 2010;51(3):286-292. doi: 10.1016/j.comppsych.2009.08.004 [PubMed] [CrossRef] [Google Scholar]
64. Radziuk AL, Kieling RR, Santos K, Rotert R, Bastos F, Palmini AL. Methylphenidate improves the quality of life of children and adolescents with ADHD and difficult-to-treat epilepsies. Epilepsy Behav. 2015;46:215-220. doi: 10.1016/j.yebeh.2015.02.019 [PubMed] [CrossRef] [Google Scholar]
65. Sayer GR, McGough JJ, Levitt J, et al.. Acute and long-term cardiovascular effects of stimulant, guanfacine, and combination therapy for attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(10):882-888. doi: 10.1089/cap.2015.0264 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
66. Song DH, Choi S, Joung YS, et al.. Titrating optimal dose of osmotic-controlled release oral delivery (OROS)-methylphenidate and its efficacy and safety in Korean children with ADHD: a multisite open labeled study. Psychiatry Investig. 2012;9(3):257-262. doi: 10.4306/pi.2012.9.3.257 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Song DH, Choi S, Joung YS, et al.. Titrating optimal dose of osmotic-controlled release oral delivery (OROS)-methylphenidate and its efficacy and safety in Korean children with ADHD: a multisite open labeled study. Psychiatry Investig. 2012;9(3):257-262. doi: 10.4306/pi.2012.9.3.257 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
67. Soutullo C, Banaschewski T, Lecendreux M, et al.. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743-751. doi: 10.1007/s40263-013-0086-6 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
68. Su Y, Li H, Chen Y, et al.. Remission rate and functional outcomes during a 6-month treatment with osmotic-release oral-system methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2015;35(5):525-534. doi: 10.1097/JCP.0000000000000389 [PubMed] [CrossRef] [Google Scholar]
69. Su Y, Yang L, Stein MA, Cao Q, Wang Y. Osmotic release oral system methylphenidate versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in Chinese youth: 8-week comparative efficacy and 1-year follow-up. J Child Adolesc Psychopharmacol. 2016;26(4):362-371. doi: 10.1089/cap.2015.0031 [PubMed] [CrossRef] [Google Scholar]
Su Y, Yang L, Stein MA, Cao Q, Wang Y. Osmotic release oral system methylphenidate versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in Chinese youth: 8-week comparative efficacy and 1-year follow-up. J Child Adolesc Psychopharmacol. 2016;26(4):362-371. doi: 10.1089/cap.2015.0031 [PubMed] [CrossRef] [Google Scholar]
70. Tucker JD, Suter W, Petibone DM, et al.. Cytogenetic assessment of methylphenidate treatment in pediatric patients treated for attention deficit hyperactivity disorder. Mutat Res. 2009;677(1-2):53-58. doi: 10.1016/j.mrgentox.2009.05.005 [PubMed] [CrossRef] [Google Scholar]
71. Wang Y, Zheng Y, Du Y, et al.. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry. 2007;41(3):222-230. doi: 10.1080/00048670601057767 [PubMed] [CrossRef] [Google Scholar]
72. Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L. Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR™) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized, double-blind study. CNS Drugs. 2015;29(4):331-340. doi: 10.1007/s40263-015-0241-3 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR™) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized, double-blind study. CNS Drugs. 2015;29(4):331-340. doi: 10.1007/s40263-015-0241-3 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
73. Wilens T, Pelham W, Stein M, et al.. ADHD treatment with once-daily OROS methylphenidate: interim 12-month results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry. 2003;42(4):424-433. doi: 10.1097/01.CHI.0000046814.95464.7D [PubMed] [CrossRef] [Google Scholar]
74. Wilens TE, Boellner SW, López FA, et al.. Varying the wear time of the methylphenidate transdermal system in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(6):700-708. doi: 10.1097/CHI.0b013e31816bffdf [PubMed] [CrossRef] [Google Scholar]
75. Geladé K, Bink M, Janssen TW, van Mourik R, Maras A, Oosterlaan J. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur Child Adolesc Psychiatry. 2017;26(4):457-468. doi: 10.1007/s00787-016-0902-x [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Eur Child Adolesc Psychiatry. 2017;26(4):457-468. doi: 10.1007/s00787-016-0902-x [PMC free article] [PubMed] [CrossRef] [Google Scholar]
76. Tannock R, Schachar RJ, Carr RP, Logan GD. Dose-response effects of methylphenidate on academic performance and overt behavior in hyperactive children. Pediatrics. 1989;84(4):648-657. [PubMed] [Google Scholar]
77. Horn WF, Ialongo NS, Pascoe JM, et al.. Additive effects of psychostimulants, parent training, and self-control therapy with ADHD children. J Am Acad Child Adolesc Psychiatry. 1991;30(2):233-240. doi: 10.1097/00004583-199103000-00011 [PubMed] [CrossRef] [Google Scholar]
78. Schachar R, Tannock R. Childhood hyperactivity and psychostimulants: a review of extended treatment studies. J Child Adolesc Psychopharmacol. 1993;3(2):81-97. doi: 10.1089/cap.1993.3.81 [PubMed] [CrossRef] [Google Scholar]
79. Stein MA, Sarampote CS, Waldman ID, et al.. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003;112(5):e404. doi: 10.1542/peds.112.5.e404 [PubMed] [CrossRef] [Google Scholar]
Pediatrics. 2003;112(5):e404. doi: 10.1542/peds.112.5.e404 [PubMed] [CrossRef] [Google Scholar]
Ritalin 10 mg tablets; Virgo: 10 mg; package: ;
lv|en|RU
With this diagnostic code, the condition compensates % of the price
- General information
- Pharmacist
Instructions for use
Before starting treatment, your doctor will perform various tests at each dose change and every six months thereafter, or at each visit, to make sure methylphenidate is still reasonably safe and helpful. These checks will include:
- measure blood pressure and heart rate and record the results in a table each time the dose is changed, and then every six months or at each visit;
- Determining height, weight and appetite and tabulating the results each time the dose is changed and then every six months or at each visit.
- assessment of mental symptoms each time the dose is changed and every six months thereafter or at each visit.
Dose titration
Careful dose titration is required at the start of methylphenidate therapy. Dose titration should begin with the lowest possible dose.
Always take this medicine exactly as your doctor has told you. If you are not sure, talk to your doctor or pharmacist. The usual dose is 20 to 30 mg in 2 to 3 divided doses, but some patients may require a higher or lower dose. The highest recommended daily dose is 60 mg.
If you or your child do not feel better after taking this medicine, your doctor may decide that other treatment is needed. Tell your doctor if your child's condition does not improve after one month of treatment. nine0021
Ritalin 10 mg tablets
Ritalin is used to treat Attention Deficit Hyperactivity Disorder (ADHD) in adolescents and children 6 years of age and older who have not responded well to other non-pharmaceutical drugs.
Ritalin should be used in combination with other treatments as part of an extensive treatment program. An extensive treatment program usually includes psychological, educational and social measures, as well as medication, and aims to stabilize children with ADHD with symptoms that may include chronically short attention span, difficulty concentrating, emotional lability, impulsivity, moderate to severe hyperactivity, medical history. minor neurological signs and abnormal electroencephalography (EEG). Learning may or may not be disrupted. The diagnosis cannot be made on the basis of one or more symptoms. To make an adequate diagnosis, it is necessary to use medical and specialized psychology, education and social resources. nine0003
An extensive treatment program usually includes psychological, educational and social measures, as well as medication, and aims to stabilize children with ADHD with symptoms that may include chronically short attention span, difficulty concentrating, emotional lability, impulsivity, moderate to severe hyperactivity, medical history. minor neurological signs and abnormal electroencephalography (EEG). Learning may or may not be disrupted. The diagnosis cannot be made on the basis of one or more symptoms. To make an adequate diagnosis, it is necessary to use medical and specialized psychology, education and social resources. nine0003
Ritalin should only be started and used under the supervision of a specialist in behavioral disorders in children and/or adolescents.
Ritalin therapy is not intended for all children with ADHD and the decision to use this medication should be based on a careful assessment of the severity and chronicity of the child's symptoms according to age. Use of Ritalin should always be done as directed, according to licensed indications, and in accordance with prescribing/diagnosis instructions. nine0003
Use of Ritalin should always be done as directed, according to licensed indications, and in accordance with prescribing/diagnosis instructions. nine0003
Additional text
Ritalin with food
Taking Ritalin with food can help relieve stomach pain, nausea, or vomiting.
Ritalin with alcohol
You or your child should not drink alcohol while taking this medicine because alcohol can make the side effects of this medicine worse. Please note that some foods and medicines also contain alcohol.
Pregnancy and lactation
Talk to your doctor or pharmacist before taking Ritalin if you or your child has:
- sexually active. Your doctor will discuss contraception with you;
- pregnant or think you are pregnant. Your doctor will decide if you or your daughter should take methylphenidate;
- breastfeeding or planning to breastfeed. You or your daughter should not breastfeed during treatment with Ritalin. The active ingredient in Ritalin can be excreted in breast milk.
You or your daughter should not breastfeed during treatment with Ritalin. The active ingredient in Ritalin can be excreted in breast milk.
Driving and using machines
Dizziness, drowsiness and visual disturbances may occur when taking methylphenidate. If these side effects occur, it may be dangerous to do any dangerous activity, such as driving a car, operating machinery, cycling, or climbing trees, until you are sure that it will not affect you or your child.
Ritalin contains lactose and wheat starch.
Ritalin contains lactose - each tablet contains 40 mg lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine. nine0015 Ritalin also contains wheat starch - each tablet contains 48 mg of wheat starch. This medicine is suitable for people with celiac disease. Should not be used in patients with wheat allergy (other than celiac disease).
- Reimbursable medicines
| Drugs | packaging | Price | There is a surcharge |
The price may vary depending on the prescription and the diagnosis code.
Price may be higher than max. pharmacy price, non-residents or ind. orders.
If the state reimburses 100%, the pharmacy must pay 0.71 euros per prescription.
- Other products
| Drugs | packaging | nine0086 PriceThere is a surcharge |
- Similar drugs
| 222 Drugs | packaging | Price | Compensation |
- Other products nine0011
- Methylphenidate oral tablets are available as generic and branded products. Trade names: Ritalin, Ritalin-SR, Concerta, Metadata ER, QuilliChew ER, Cotempla XR-ODT.
- Methylphenidate is available in the following forms: immediate-release oral tablet, extended-release oral tablet, oral chewable tablet, extended-release chewable tablet, and extended-release orally disintegrating tablet.
 Methylphenidate is also available as an extended-release oral capsule, transdermal patch, oral suspension, and oral solution. nine0008
Methylphenidate is also available as an extended-release oral capsule, transdermal patch, oral suspension, and oral solution. nine0008 - Methylphenidate oral tablets are used to treat narcolepsy and attention deficit hyperactivity disorder (ADHD).
- This drug has a black box warning. This is the most serious warning from the Food and Drug Administration (FDA). A black box warning alerts doctors and patients to exposure to drugs that may be dangerous. nine0008
- Taking methylphenidate over a long period of time can lead to addiction and dependence. Use it with caution if you have a history of alcohol or drug abuse. Your doctor will gradually stop this medication to prevent withdrawal symptoms.
- Heart warning: Methylphenidate may cause stroke, heart attack, or sudden death in people with heart problems. People with severe heart problems should not take this medicine. This medicine may increase blood pressure and heart rate.
 If you have high blood pressure, heart failure, a history of heart attack, or an abnormal heart rate, ask your doctor if this medicine is safe for you. nine0008
If you have high blood pressure, heart failure, a history of heart attack, or an abnormal heart rate, ask your doctor if this medicine is safe for you. nine0008 - Mental health warning: If you have a mental health problem, this medicine may make your symptoms worse. It can also cause psychotic or manic symptoms in children and adolescents without a history of such problems. They may have symptoms such as hallucinations (seeing, hearing, or believing a lie) or paranoia (suspicion).
- Digestive Warning: This warning only applies to the Concerta brand. Concerts can cause a blockage in the esophagus, stomach, or intestines in people who already have a constriction in any of these organs. Concerta tablets should only be used if you can swallow the tablet whole. Cutting or breaking tablets can increase the amount of the drug in the body. This increases the risk of side effects. nine0008
- Headache
- LEARD INFORMATION
- Disorders of the stomach 9000 these effects are mild and may disappear within a few days or a couple of weeks. If they are more severe or do not go away, talk to your doctor or pharmacist. nine0003
- Heart problems. Symptoms may include:
- pain in the chest, left arm, jaw, or between the shoulders
- high blood pressure
- increased heart rate
- shortness of breath
- Stroke. Symptoms may include:
- weakness in one part or side of your body
- Slurred speech
- Impaired liver function, which may be mild or lead to severe liver damage
- Mental health problems.
 Symptoms may include:
Symptoms may include: - symptoms of mania such as racing thoughts, feelings of power and excessive energy
- Aggression or hostility
- hallucinations (observation or listening to unrealistic things)
- paranoia (sensation of suspicion)
- Sensity 9,0008
- Ceremons
- Slow growth (height) in children ,
- visual vision or fifty vision
- Circulation problems. Symptoms on the toes or toes may include:
- numbness
- feeling cold (sensitivity to temperature)
- bowl
- skin color changes from pale to blue and red
- new unexplained wounds
- Priapism (painful and prolonged erections)
- antacids
- h3 blockers
- proton pump inhibitors
- selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline
- serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine and venlafaxine
- tricyclic antidepressants (TCAs) such as amitriptyline and clomipramine monooxidase inhibitors MAOIs) such as selegiline and phenelzine
- opioids fentanyl and tramadol
- anxiolytic buspirone
- triptans
- lithium
- tryptophan
- St. Ivana
- Angiotensin II receptor blockers, such as Lozartan, Valsartan and Irbesartan
- Angiotensin -Run -Riding enzyme (APF), such as analapril and lisinopril
- diuretics (diuretic tablets), such as hydrochlorotiazide and fusemide
Methylphenidate: side effects, dosage, uses and more
contents
FDA Warning: Abuse and Dependence
Other warnings
Methylphenidate oral tablet is a prescription drug. It is available in the following forms: oral tablet, extended-release tablet, extended-release capsule, chewable tablet, extended-release chewable tablet, and extended-release tablet for oral disintegration.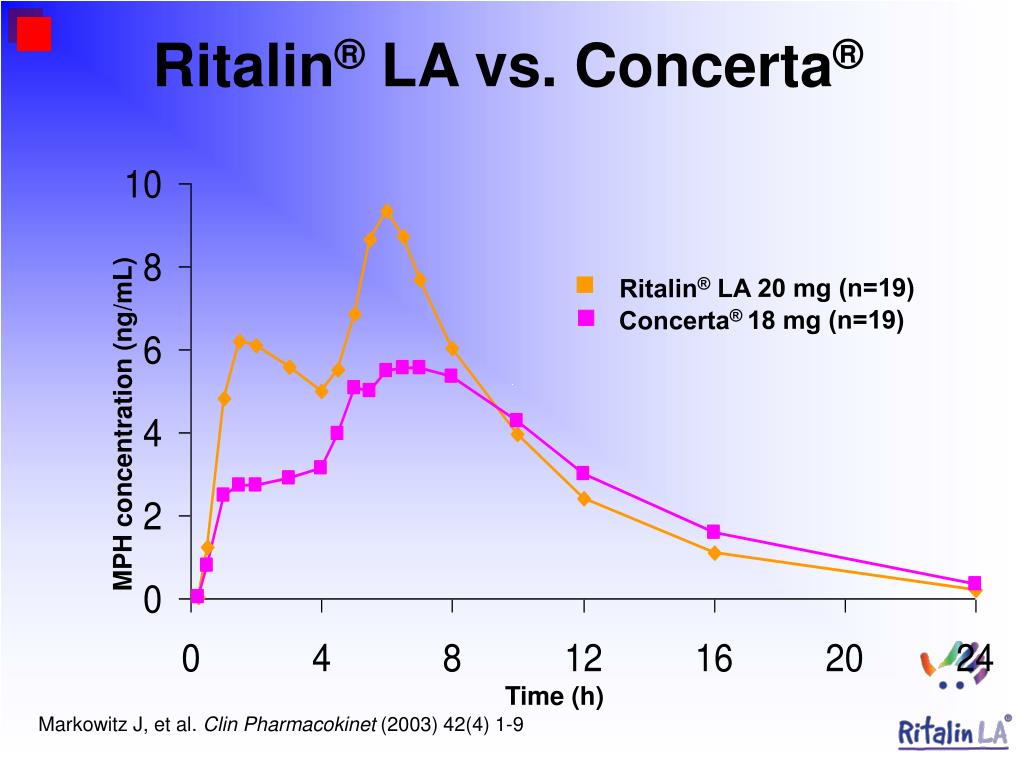 Methylphenidate is also available as a transdermal patch, oral suspension, and oral solution. nine0003
Methylphenidate is also available as a transdermal patch, oral suspension, and oral solution. nine0003
Methylphenidate is a controlled substance, which means your doctor will monitor your use closely.
Methylphenidate oral tablets are available as Ritalin, Ritalin SR, Concerta, Metadate ER, QuilliChew ER, and Cotempla XR-ODT protected formulations. It is also available as a generic drug. Generics usually cost less than branded versions. In some cases, they may not be available in all strengths or forms as brand name medicines. nine0003
Methylphenidate oral tablets can be used as part of combination therapy. This means that you may need to take it with other medicines. Why is it used?
How it works
Methylphenidate belongs to a class of drugs called central nervous system (CNS) stimulants. It works by increasing the amount of norepinephrine and dopamine chemicals in your brain. These chemicals send signals to other parts of your body that help relieve your symptoms. nine0003
nine0003
Methylphenidate oral tablets do not cause drowsiness but may cause other side effects.
more common side effects
are more common side effects that may occur when taking methylphenidate include:
Serious side effects
Call your doctor if you have serious side effects. Call 911 if your symptoms seem life-threatening or if you think you need emergency medical attention. Serious side effects and their symptoms may include the following:
Disclaimer: Our goal is to provide you with the most current and up-to-date information. But because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information does not replace medical advice. Always discuss possible side effects with a doctor who knows your medical history. nine0003
Always discuss possible side effects with a doctor who knows your medical history. nine0003
Methylphenidate oral tablets may interact with other medicines, vitamins or herbs you are taking. An interaction is when a substance changes the way a drug works. It can be harmful or prevent the medicine from working well.
To avoid interactions, your doctor must carefully administer all of your medicines. Tell your doctor about any medications, vitamins, or herbs you are taking. To find out how this medicine may interact with other medicines you are taking, talk to your doctor or pharmacist. nine0003
Examples of drugs that can interact with methylphenidate are listed below.
Acid reflux drugs
Taking these medicines with methylphenidate may increase the levels of methylphenidate in the body and lead to additional side effects. These drugs may also interfere with the mechanism of action of the long-acting forms of methylphenidate. Examples of such drugs include:
Serotonergic drugs
Taking these medicines with methylphenidate may increase the risk of serotonin syndrome, which can be fatal.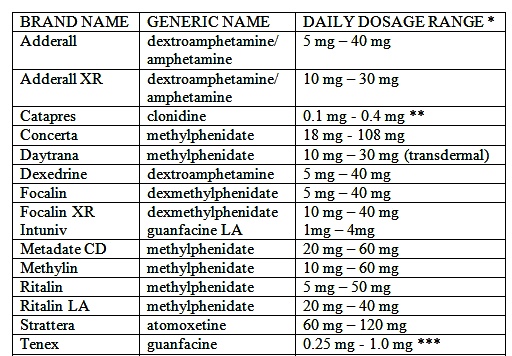 If you are taking any of these medicines, your doctor will prescribe a reduced dose of methylphenidate for you and monitor you for signs of serotonin syndrome. Symptoms may include restlessness, sweating, muscle twitching, and confusion.
If you are taking any of these medicines, your doctor will prescribe a reduced dose of methylphenidate for you and monitor you for signs of serotonin syndrome. Symptoms may include restlessness, sweating, muscle twitching, and confusion.
Examples of such preparations include:
Methylphenidate should not be used during MAOI treatment. You cannot take it within 14 days of stopping your MAOI treatment. Taking these drugs together can lead to a dangerous increase in blood pressure.
Blood pressure medicines
Taking these drugs with methylphenidate may reduce the expected effects of these drugs. This means they will be less effective. Examples of such drugs include:
- chlorpromazine
- haloperidol
Anticonvulsants
Using these medicines with methylphenidate may increase the amount of medicine in your throat in your body. This can lead to additional side effects from anticonvulsants. Examples of such drugs include:
This can lead to additional side effects from anticonvulsants. Examples of such drugs include:
- phenytoin
- phenobarbital
warfarin
Using warfarin, a blood thinner, with methylphenidate may increase the effect of warfarin in the body. This may increase the risk of bleeding. nine0003
Disclaimer: Our goal is to provide you with the most current and up-to-date information. However, because drugs affect each person differently, we cannot guarantee that these data include all possible interactions. This information does not replace medical advice. Always talk to your doctor about potential interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter medications you are taking. nine0003
This medicine comes with several caveats.
Allergy Alert
Methylphenidate may cause a severe allergic reaction. Symptoms may include:
- trouble breathing
- swelling of the throat or tongue
- Osip
- hives (itching)
If you have these symptoms, call 911 or go to the nearest emergency room.
Do not take this medicine again if you have ever had an allergic reaction to it. Repeated use may be lethal (lead to death). nine0003
Alcohol Interaction Warning
Alcohol may increase the effects of methylphenidate. You must not drink alcohol while taking this medicine.
Alcohol may release Metadate CD and Ritalin LA into your body faster. This can cause more side effects and reduce the effect of the drug.
Warnings for people with certain medical conditions
For people with heart problems: methylphenidate may increase the risk of sudden death, stroke, and heart attack. If you have heart problems, a history of heart attack, high blood pressure, or an abnormal heart rate, ask your doctor if this medicine is safe for you. nine0003
For people with mental disorders: methylphenidate may make your symptoms worse. It can also cause new psychotic symptoms, especially in children and adolescents. You may need to stop taking this medicine if this happens.
For people with circulation problems: This medicine may make circulation problems in the toes worse.
For people with seizures: If you or your child has seizures, do not take methylphenidate. This may increase the risk of seizures. nine0003
For people with glaucoma: methylphenidate may impair your vision.
For people with growth problems: Methylphenidate has been shown to slow the growth of children. Your child's doctor will monitor your child's height and weight while taking this medicine. If your child is not gaining height or weight, methylphenidate may need to be stopped.
For people with digestive problems: Do not use Concerta's name medicine if you have a blockage in your esophagus, stomach, or small or large intestine. Concerts can exacerbate this problem. nine0003
Warnings for other groups
For pregnant women: Methylphenidate is a category C pregnancy drug. This means two things:
Tell your doctor if you are pregnant or plan to become pregnant. Methylphenidate should be used during pregnancy only if the potential benefit justifies the potential risk. nine0003
If you become pregnant while taking this medicine, call your doctor right away.
For lactating women: it is not known whether methylphenidate passes into breast milk. You may need to decide whether to take methylphenidate or breastfeed.
For the elderly: This medicine has not been found to be safe and effective for use in people over 65 years of age.
For children: This medicine has not been found to be safe and effective for use in children under 6 years of age. nine0003
Children should be under medical supervision when taking methylphenidate.
Not all possible dosages and schedules are included here. Your dose, form and frequency of administration will depend on:
- your age
- the condition being treated
- how serious your condition is
- other health conditions you have
- how you respond to the first dose
Dosage with attention deficit hyperactivity disorder (ADHD)
Generic: Methylphenidate
- Presentation: immediate release oral tablets.
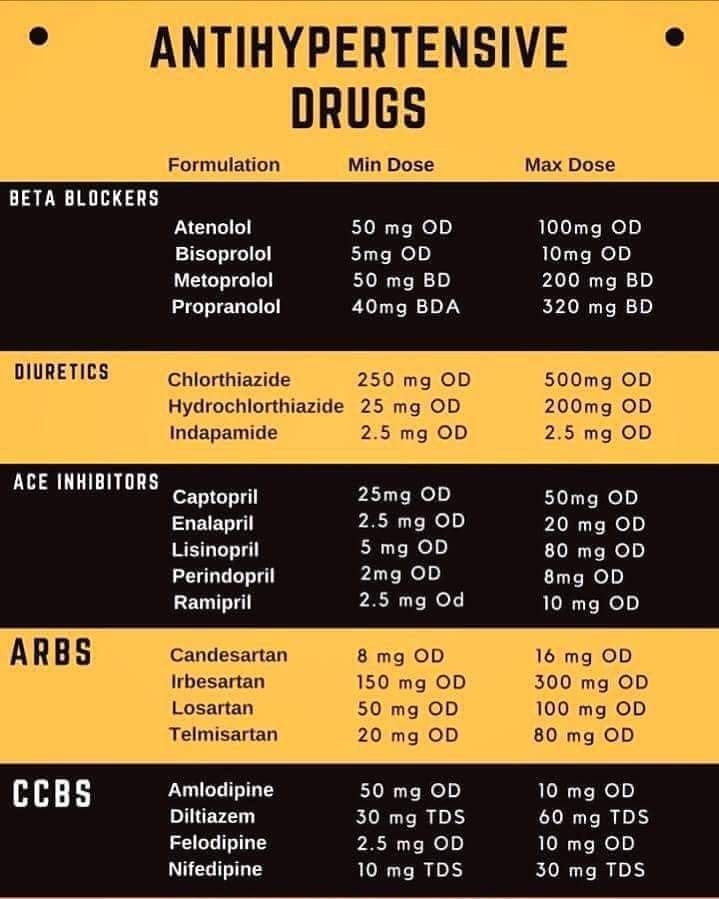
- Strength: 5mg, 10mg, 20mg
- Form: chewable tablet
- Strength: 2.5mg, 5mg, 10mg
- Form: extended release oral tablet.
- Strengths: 10 mg, 18 mg, 20 mg, 27 mg, 36 mg, 54 mg, 72 mg.
Trademark: Ritalin
- Presentation: tablets for oral administration with immediate release.
- Strengths: 5mg, 10mg, 20mg
Trademark: Ritalin CP
- Presentation: prolonged release tablet for oral administration.
- Strength: 20 mg
Trademark: Concerta
- Presentation: prolonged release tablet for oral administration.
- Strengths: 18 mg, 27 mg, 36 mg, 54 mg
Trademark: Cotempla XR-ODT
- Presentation: long-acting tablet for oral disintegration.
- Strengths: 8.6 mg, 17.3 mg. 25.9 mg
Brand: Metadate ER
- Presentation: extended release tablet for oral administration.
- Strengths: 20 mg
Trademark: QuilliChew ER
- Presentation: sustained release chewable tablets.
 nine0008
nine0008 - Strengths: 20mg, 30mg, 40mg
Concert:
Adult Dose (18 years and older)
- Typical Dose: 18mg or 36mg once a day.
- Dose increases: Your doctor may increase your dose by 18 mg each week.
- Maximum dose: 72 mg per day.
Pediatric dose (ages 13-17)
- Typical dosage: 18 mg per day.
- Dose increases: Your doctor may increase your child's dose by 18 mg each week. nine0008
- Maximum dose: 72 mg per day.
Pediatric (ages 6-12)
- Typical dosage: 18 mg per day.
- Dose increases: Your doctor may increase your dose by 18 mg each week.
- Maximum dose: 54 mg per day.
Pediatric dose (ages 0-5 years)
Doses for persons under 6 years of age have not been established.
RITALIN, GENERAL AND AVAILABLE TABLETS:
Adult dose (18-64 years)
- Typical dose: 20-30 mg per day divided into 2-3 doses.
Pediatric dose (ages 6-17)
- Typical dosage: 5 mg twice a day before breakfast and lunch.
- Dose increases: Your doctor may increase your dose by 5-10 mg each week.
Pediatric dose (ages 0-5 years)
Doses for persons under 6 years of age have not been established.
RITALIN SR, METADATE ER, GENERAL TABLETS AVAILABLE:
Adult Dose (18 years and older)
- Typical dosage: These extended release tablets last approximately 8 hours. These tablets can be used instead of immediate release tablets when the 8 hour dose of the extended release tablet corresponds to the titrated 8 hour dose of the immediate release tablet. Your doctor can tell you more.
Pediatric Dose (ages 6-17)
- Typical Dosage: These extended release tablets last about 8 hours. These tablets can be used instead of immediate release tablets when the 8 hour dose of the extended release tablet corresponds to the titrated 8 hour dose of the immediate release tablet. Your child's doctor can tell you more. nine0008
Pediatric dose (ages 0-5 years)
Doses for persons under 6 years of age have not been established.
COTEMPLA XR-ODT
Adult dose (18 years and over)
This medicine is not prescribed for this age range.
Pediatric dose (ages 6-17 years)
- Typical dose: 17.3 mg once a day in the morning.
- Dose increases: Your doctor may increase your child's dose by 8.6 mg to 17.3 mg each week until an appropriate dose is determined. If it is prescribed for long-term use, your doctor may adjust your child's dose from time to time. nine0008
- Maximum dose: 51.8 mg per day.
Pediatric (age 0-5 years)
This medicine has not been found to be safe or effective in children under 6 years of age.
KILISHJU E.R.
Adult Dose (18 years and older)
- Typical dosage: 20 mg once daily in the morning.
- Dose increase: Your doctor may increase or decrease your dosage by 10, 15, or 20 mg each week until you determine the appropriate dosage. nine0008
- Maximum dose: 60 mg per day.
Pediatric dose (ages 6-17)
- Typical dosage: 20 mg once a day in the morning.
- Dose increase: Your doctor may increase or decrease your dosage by 10, 15, or 20 mg each week until you determine the appropriate dosage.
- Maximum dose: 60 mg per day.
Pediatric (age 0-5 years)
This medicine has not been found to be safe or effective in children under 6 years of age. nine0003
Dosage for narcolepsy
General: Methylphenidate
- Formulation: immediate release oral tablets.
- Strengths: 5mg, 10mg, 20mg
- Presentation: extended release oral tablet.
- Strengths: 10 mg, 20 mg
- Form: chewable tablet
- Strengths: 2.5 mg, 5 mg, 10 mg
Trademark: Ritalin
- Release form: tablets for oral administration with instant release.
- Strengths: 5mg, 10mg, 20mg
Trademark: Ritalin CP
- Presentation: prolonged release tablet for oral administration.
- Strength: 20mg
Brand: Metadate ER
- Presentation: prolonged release tablet for oral use.
- Strengths: 20 mg
RITALIN, GENERAL TABLETS AND AVAILABLE TABLETS:
Adult Dose (18-64 years old)
- Typical Dose: 20-30 mg per day, divided into 2-3 doses.
Pediatric dose (ages 6-17)
- Typical dosage: 5 mg twice a day before breakfast and lunch.
- Dose increases: Your doctor may increase your dose by 5-10 mg each week.
Pediatric dose (ages 0-5 years)
Doses for persons under 6 years of age have not been established. nine0003
RITALIN SR, METADATE ER, GENERAL TABLETS AVAILABLE:
Adult Dose (18-64 years old)
- Typical Dose: These extended release tablets last about 8 hours. These tablets can be used instead of immediate release tablets when the 8 hour dose of the extended release tablet corresponds to the titrated 8 hour dose of the immediate release tablet. Your doctor can tell you more.
Pediatric dose (ages 6-17)
- Typical dosage: These extended release tablets last approximately 8 hours.
 These tablets can be used instead of immediate release tablets when the 8 hour dose of the extended release tablet corresponds to the titrated 8 hour dose of the immediate release tablet. Your child's doctor can tell you more.
These tablets can be used instead of immediate release tablets when the 8 hour dose of the extended release tablet corresponds to the titrated 8 hour dose of the immediate release tablet. Your child's doctor can tell you more.
Pediatric dose (ages 0-5 years)
Doses for persons under 6 years of age have not been established.
Dosage warning
Do not take methylphenidate late at night. This can cause sleep problems.
Disclaimer: Our goal is to provide you with the most current and up-to-date information. But because drugs affect each person differently, we cannot guarantee that this list includes all possible doses. This information does not replace medical advice. Always talk to your doctor or pharmacist about the doses that are right for you.
Methylphenidate oral tablet is used for short or long term treatment. This medicine is usually stopped after puberty. Your doctor may occasionally try to stop your methylphenidate treatment to see if you need to take it. If symptoms return, you may need to continue taking it. nine0003
If symptoms return, you may need to continue taking it. nine0003
Methylphenidate poses a serious risk if you do not take it as prescribed.
If you stop taking it: symptoms will not be controlled. If you take large doses of this medication for a long time and stop abruptly, you may experience extreme tiredness, tiredness, or severe depression.
If you don't take it as scheduled: If you take methylphenidate later in the day, you may have trouble sleeping. nine0003
If you take too much: if you take too many methylphenidate, the following can occur:
- Anxiety
- Muscle pain and weakness
- Fatherer breathing
- Confusion
- High or low blood pressure
- vomit
- diarrhea
- seizures
- eating
If you think you have taken too much of this medicine, call your doctor or local poison control center. If you have severe symptoms, call 911 or go to the nearest emergency room immediately.
What to do if you miss a dose: If you miss a dose, take it as soon as possible. If it's almost time for the next dose, wait until then and take one dose.
Do not double your dose to try and catch up. This can lead to dangerous side effects.
How to tell if a drug is working: For ADHD: You need to concentrate and pay attention better, be less impulsive and hyperactive. nine0003
For narcolepsy: You should feel less sleepy and less awake.
Important considerations for taking methylphenidate
Keep this in mind if your doctor prescribes methylphenidate.
Basic information
- Some forms should not be taken with meals. If you are taking immediate-release tablets or chewable tablets, take methylphenidate 30 to 45 minutes before meals.
- You can take extended-release oral disintegration tablets with or without food. However, you must take them the same way every time. nine0008
- Take the extended release tablets when you wake up in the morning.
 This form releases the drug into your body throughout the day. Do not take it late in the day or at night as it may cause sleep problems.
This form releases the drug into your body throughout the day. Do not take it late in the day or at night as it may cause sleep problems.
Self-administered
For instant release and chewable tablets:
- You can cut these tablets.
For extended release tablets (not disintegrating in the mouth):
- Do not cut, chew, crush or split these tablets. nine0008
- Swallow them whole with water or other liquids.
For extended release oral disintegrating tablets:
- Use each tablet immediately after removing from the blister pack.
- Remove foil from blister pack with dry hands. Do not push the tablet through the foil.
- Place the tablet on the tongue immediately. Let dissolve without chewing. Liquid is not required.
Warehouse
- Each form must be stored at the appropriate temperature:
- o All generic tablets: Store between 68°C to 77°C and 20°F to 25°F.

- Concerta, Ritalin: Store these tablets at room temperature (77°C) from 25°F. You can store them briefly at 59°C to 86°F (15°C to 30°C).
- Metadate ER: Store at 68°C to 77°F (20°C to 25°C). You can store it for a short time at a temperature of 59°C to 86°F (15°C to 30°C).
- Cotempla XR-ODT: Store at 59°C to 86°F (15°C to 30°C). After removing the blisters from the cardboard, store them in a reusable travel box.
- Do not freeze methylphenidate. Keep it away from high temperatures.
- Keep this medicine away from light.
- Do not store this medicine in damp or damp places such as bathrooms.
tapping
The prescription for this drug is not completed. You or your pharmacy will need to talk to your doctor about a new prescription if you need a different medicine.
Travel
When traveling with medication:
- Always carry your medication with you. Never put it in your checked bag while flying.
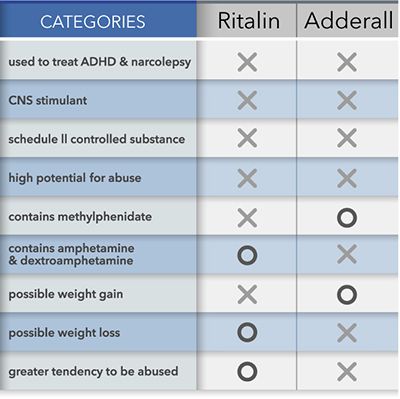 Keep it in your bag.
Keep it in your bag. - Don't worry about air devices at the airport. I can't hurt drugs.
- You may need to show the pharmacy sticker for your medicines to the airport staff. Always carry the original prescription container with you. nine0008
- Do not put this medicine in the glove compartment of your car or leave it in your car. Be sure to avoid this when the weather is very hot or very cold.
Clinical monitoring
While you are taking this medicine, your doctor will examine you for the following:
- blood pressure and heart rate
- signs of aggressive behavior or mental health changes
- height and weight in children 8
- height and weight in children 8
- nine0161
Not every pharmacy has this medicine. When filling out a prescription, be sure to call in advance and make sure that it is in the pharmacy.
Prior approval
Many insurance companies require prior approval for this drug.
 This means that your doctor will need to get insurance company approval before your insurance company will pay for the prescription.
This means that your doctor will need to get insurance company approval before your insurance company will pay for the prescription. There are other medicines available to treat your condition. Some may suit you better than others. Talk to your doctor about other treatment options that may help you. nine0003
Disclaimer: Healthline has made every effort to ensure that all information is correct, complete, and up-to-date. However, this article should not be used as a substitute for the knowledge and experience of a licensed healthcare professional. Always check with your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, instructions, precautions, warnings, drug interactions, allergic reactions, or side effects. The absence of warnings or other information for a particular drug does not mean that the drug or combination of drugs is safe, effective, or suitable for all patients or for all special uses.