New medicine for narcolepsy
Recently Approved and Upcoming Treatments for Narcolepsy
1. Kornum BR, Knudsen S, Ollila HM, Pizza F, Jennum PJ, Dauvilliers Y, et al. Narcolepsy. Nat Rev Dis Prim. 2017;3:16100. [PubMed] [Google Scholar]
2. Szabo ST, Thorpy MJ, Mayer G, Peever JH, Kilduff TS. Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med Rev. 2019;43:23–36. [PMC free article] [PubMed] [Google Scholar]
3. Ohayon MM. Epidemiology of narcolepsy. In: Bassetti C, Mignot E, Billard M, editors. Narcolepsy and hypersomnia. New York: Taylor and Francis; 2007. pp. 125–132. [Google Scholar]
4. Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25(2):197–202. [PubMed] [Google Scholar]
5. Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15(5):502–507. [PubMed] [Google Scholar]
6. Dauvilliers Y, Siegel JM, Lopez R, Torontali ZA, Peever JH. Cataplexy–clinical aspects, pathophysiology and management strategy. Nat Rev Neurol. 2014;10(7):386–395. [PMC free article] [PubMed] [Google Scholar]
7. American Academy of Sleep Medicine . International classification of sleep disorders. 3. Darien: American Academy of Sleep Medicine; 2014. [Google Scholar]
8. Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep. 2017;9:39–57. [PMC free article] [PubMed] [Google Scholar]
9. Dauvilliers Y, Arnulf I, Szakacs Z, Leu-Semenescu S, Lecomte I, Scart-Gres C, et al. Long-term use of pitolisant to treat patients with narcolepsy: Harmony III study. Sleep Biol Rhythms. 2019;42(11):1–11. [PMC free article] [PubMed] [Google Scholar]
10. Schwartz JC. The histamine h4 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163(4):713–721. [PMC free article] [PubMed] [Google Scholar]
11. Romigi A, Vitrani G, Lo Giudice T, Centonze D, Franco V. Profile of pitolisant in the management of narcolepsy: design, development, and place in therapy. Drug Des Dev Ther. 2018;12:2665–2675. [PMC free article] [PubMed] [Google Scholar]
Drug Des Dev Ther. 2018;12:2665–2675. [PMC free article] [PubMed] [Google Scholar]
12. Wakix [summary of product characteristics]. Paris: Bioprojet Pharm; 2019.
13. Wakix [package insert]. Plymouth Meeting: Harmony Biosciences; 2019.
14. Harmony Biosciences announces file acceptance of its new drug application for pitolisant [press release]. 2019. https://www.harmonybiosciences.com/newsroom/harmony-biosciences-announces-file-acceptance-of-its-new-drug-application/. Accessed 02 May 2019.
15. Ligneau X, Perrin D, Landais L, Camelin JC, Calmels TP, Berrebi-Bertrand I, et al. BF2.649 [1-{3-[3-(4-chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine h4 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365–375. [PubMed] [Google Scholar]
16. EMA. Wakix assessment report. 2015. https://www.ema.europa.eu/en/documents/assessment-report/wakix-epar-public-assessment-report_en.pdf. Accessed 29 Apr 2019
17. Robert P, Schwartz JC. Hormonal contraceptive and pitolisant CYP3A4 induction [abstract]. Neurology. 2019;92(15 suppl):P3.6-035.
Robert P, Schwartz JC. Hormonal contraceptive and pitolisant CYP3A4 induction [abstract]. Neurology. 2019;92(15 suppl):P3.6-035.
18. Doghramji Karl, Davis Craig W, Patroneva Albena, Schwartz Jean-Charles, Scart-Grès Catherine, Robert Philippe, Wanaski Stephen P, Krystal Andrew D. 0615 Pitolisant in Combination With Other Medications for the Management of Narcolepsy. Sleep. 2019;42(Supplement_1):A245–A245. [Google Scholar]
19. Dauvilliers Y, Bassetti C, Lammers GJ, Arnulf I, Mayer G, Rodenbeck A, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–1075. [PubMed] [Google Scholar]
20. Szakacs Z, Dauvilliers Y, Mikhaylov V, Poverennova I, Krylov S, Jankovic S, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200–207. [PubMed] [Google Scholar]
21. Scart-Gres C, Momah C, Roy M, Maski K, Piris S, Bogan RK.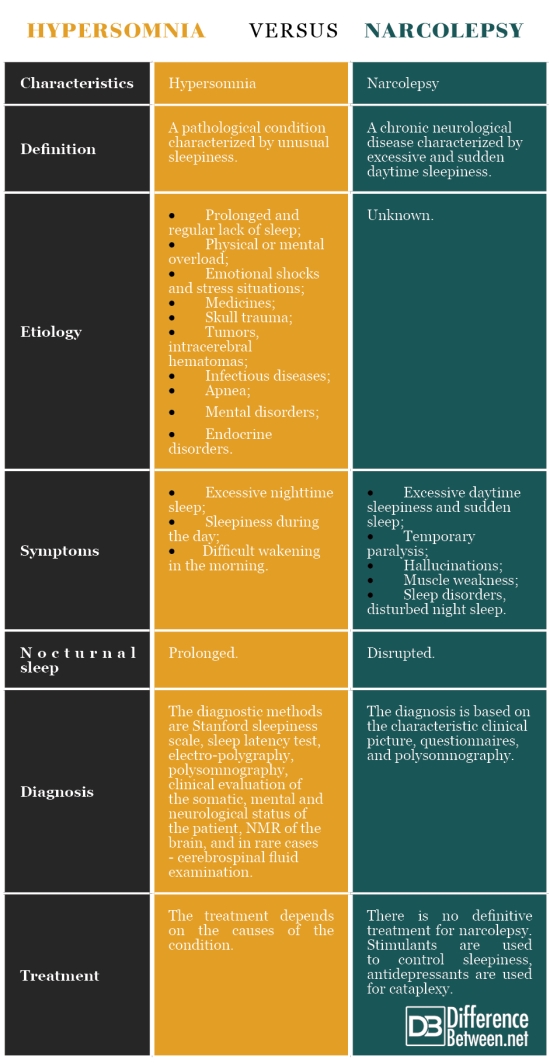 Safety and tolerability of pitolisant in the treatment of adults with narcolepsy: integrated data from clinical studies [abstract no. 0614] Sleep. 2019;42(suppl 1):A244–A5. [Google Scholar]
Safety and tolerability of pitolisant in the treatment of adults with narcolepsy: integrated data from clinical studies [abstract no. 0614] Sleep. 2019;42(suppl 1):A244–A5. [Google Scholar]
22. Bauer Eric D, Davis Craig W, Patroneva Albena, Dayno Jeffrey M, Thorpy Michael J. 0611 The Safety and Tolerability of Pitolisant in the Treatment of Excessive Daytime Sleepiness and Cataplexy in Adult Patients With Narcolepsy: An Open-Label, Expanded Access Program in the United States. Sleep. 2019;42(Supplement_1):A243–A243. [Google Scholar]
23. Kallweit Ulf, Triller Annika. 0613 Effects of Pitolisant on Nighttime Sleep. Sleep. 2019;42(Supplement_1):A244–A244. [Google Scholar]
24. Uguen M, Perrin D, Belliard S, Ligneau X, Beardsley PM, Lecomte JM, et al. Preclinical evaluation of the abuse potential of pitolisant, a histamine H(3) receptor inverse agonist/antagonist compared with modafinil. Br J Pharmacol. 2013;169(3):632–644. [PMC free article] [PubMed] [Google Scholar]
25. Setnik B, McDonnell M, Mills C, Scart-Gres C, Robert P, Dayno JM, et al. Evaluation of the abuse potential of pitolisant, a selective h4-receptor antagonist/inverse agonist, for the treatment of adult patients with narcolepsy with or without cataplexy. Sleep. 2019 doi: 10.1093/sleep/zsz252. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Setnik B, McDonnell M, Mills C, Scart-Gres C, Robert P, Dayno JM, et al. Evaluation of the abuse potential of pitolisant, a selective h4-receptor antagonist/inverse agonist, for the treatment of adult patients with narcolepsy with or without cataplexy. Sleep. 2019 doi: 10.1093/sleep/zsz252. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Sunosi® (solriamfetol) tablets [prescribing information]. Palo Alto: Jazz Pharmaceuticals, Inc.; 2019.
27. Baladi MG, Forster MJ, Gatch MB, Mailman RB, Hyman DL, Carter LP, et al. Characterization of the neurochemical and behavioral effects of solriamfetol (JZP-110), a selective dopamine and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2018;366(2):367–376. [PubMed] [Google Scholar]
28. Zomorodi K, Kankam M, Lu Y. A phase I, randomized, crossover, open-label study of the pharmacokinetics of solriamfetol (JZP-110) in healthy adult subjects with and without food. Clin Ther. 2019;41(2):196–204. [PubMed] [Google Scholar]
29. Zomorodi K, Chen D, Lee L, Lasseter K, Marbury T. Single-dose pharmacokinetics and safety of solriamfetol in participants with normal or impaired renal function and with end-stage renal disease requiring hemodialysis. J Clin Pharmacol. 2019;59(8):1120–1129. [PMC free article] [PubMed] [Google Scholar]
Zomorodi K, Chen D, Lee L, Lasseter K, Marbury T. Single-dose pharmacokinetics and safety of solriamfetol in participants with normal or impaired renal function and with end-stage renal disease requiring hemodialysis. J Clin Pharmacol. 2019;59(8):1120–1129. [PMC free article] [PubMed] [Google Scholar]
30. Ruoff C, Swick TJ, Doekel R, Emsellem HA, Feldman NT, Rosenberg R, et al. Effect of oral JZP-110 (ADX-N05) on wakefulness and sleepiness in adults with narcolepsy: a phase 2b study. Sleep. 2016;39(7):1379–1387. [PMC free article] [PubMed] [Google Scholar]
31. Thorpy MJ, Shapiro C, Mayer G, Corser BC, Emsellem H, Plazzi G, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85(3):359–370. [PMC free article] [PubMed] [Google Scholar]
32. Malhotra A, Shapiro C, Pepin JL, Hedner J, Ahmed M, Foldvary-Schaefer N, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2019 doi: 10.1093/sleep/zsz220. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Sleep. 2019 doi: 10.1093/sleep/zsz220. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
33. Zomorodi K, Chen D, Lee L, Swearingen D. A randomized, double-blind, placebo- and positive-controlled, four-period crossover study of the effects of solriamfetol (JZP-110) on QTcF intervals in healthy participants [poster]. Annual Meeting of the American College of Clinical Pharmacology; 23–25 Sep 2018; Bethesda.
34. Carter LP, Henningfield JE, Wang YG, Lu Y, Kelsh D, Vince B, et al. A randomized, double-blind, placebo-controlled, crossover study to evaluate the human abuse liability of solriamfetol, a selective dopamine and norepinephrine reuptake inhibitor. J Psychopharmacol. 2018;32(12):1351–1361. [PMC free article] [PubMed] [Google Scholar]
35. Provigil [package insert]. North Wales: Teva Pharmaceuticals; 2018.
36. Bron M, Franek J, Ronnebaum S, Menno D, Bujanover S, Stepnowsky C. Indirect treatment comparison of the efficacy of solriamfetol (JZP-110), modafinil, and armodafinil for the treatment of excessive sleepiness in obstructive sleep apnea [poster]. Annual Meeting of the Academy of Managed Care Pharmacy Nexus; 22–25 Oct 2018; Orlando.
Annual Meeting of the Academy of Managed Care Pharmacy Nexus; 22–25 Oct 2018; Orlando.
37. Avadel Pharmaceuticals receives orphan drug designation from FDA for FT 218 for the treatment of narcolepsy [press release]. 2018. http://investors.avadel.com/news-releases/news-release-details/avadel-pharmaceuticals-receives-orphan-drug-designation-fda-ft?ID=2325927&c=67519&p=irol-newsArticle. Accessed 26 Apr 2019.
38. Sodium oxybate controlled release—Avadel Pharmaceuticals [drug profile]. Adis Insight; 2018. https://adisinsight.springer.com/drugs/800041142. Accessed 27 Nov 2019.
39. Xyrem® (sodium oxybate) oral solution [prescribing information]. Palo Alto: Jazz Pharmaceuticals; 2018.
40. Monteith David, Grassot Julien, Castellan Charlotte, Roth Thomas. 0609 Pharmacokinetics And Formulation Selection Of Ft218, An Investigational Controlled-release Sodium Oxybate Formulation Designed For Once Nightly Dosing. Sleep. 2019;42(Supplement_1):A242–A243. [Google Scholar]
41. Monteith David, Grassot Julien, Castellan Charlotte, Roth Thomas. 0610 Pharmacokinetics And Dose Proportionality Of FT218, An Investigational Controlled Release Formulation Of Sodium Oxybate For Once Nightly Dosing. Sleep. 2019;42(Supplement_1):A243–A243. [Google Scholar]
Monteith David, Grassot Julien, Castellan Charlotte, Roth Thomas. 0610 Pharmacokinetics And Dose Proportionality Of FT218, An Investigational Controlled Release Formulation Of Sodium Oxybate For Once Nightly Dosing. Sleep. 2019;42(Supplement_1):A243–A243. [Google Scholar]
42. A study to compare the absorption into the body of the drug sodium oxybate in FT218 in comparison to the currently marketed product Xyrem® [EudraCT2016-004342-28]. 2017 Oct 17. https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-004342-28/NL. Accessed 29 Apr 2019.
43. Sodium oxybate for treatment of excessive daytime sleepiness and cataplexy in narcolepsy [ClinicalTrials.gov identifier NCT02720744]. National Institutes of Health, ClinicalTrials.gov. 2019.https://clinicaltrials.gov/ct2/show/{"type":"clinical-trial","attrs":{"text":"NCT02720744","term_id":"NCT02720744"}}NCT02720744. Accessed 29 Apr 2019.
44. Jazz Pharmaceuticals announces positive top-line results from phase 3 study of JZP-258 in adult narcolepsy patients with cataplexy and excessive daytime sleepiness [press release]. 2019. https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-positive-top-line-results-phase-3. Accessed 26 Apr 2019.
2019. https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-positive-top-line-results-phase-3. Accessed 26 Apr 2019.
45. JZP 258 [drug profile]: Adis Insight; 2019. https://adisinsight.springer.com/drugs/800048256. Accessed 27 Nov 2019.
46. Chen C, Skowronski R, Jenkins J, Zomorodi K. Pharmacokinetics, relative bioavailability, and food effect of JZP-258 and sodium oxybate: results of two phase 1, open-label, randomised crossover studies in healthy volunteers [abstract]. Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
47. Bogan R, Thorpy M, Dauvilliers Y, Del Rio Villegas R, Foldvary-Schaefer N, Skowronski R, et al. Efficacy and safety of JZP-258 in a phase 3 double-blind, placebo-controlled, randomised-withdrawl study in adults with narcolepsy with cataplexy [abstract]. Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
48. Dauvilliers Y, Sonka K, Bogan RK, Partinen M, Del Rio Villegas R, Foldvary-Schaefer N, et al. Changes in cataplexy frequency by prior therapy in a phase 3, double-blind, placebo-controlled, randomised withdrawal study of JZP-258 in adults with narcolepsy with cataplexy [abstract]. Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
Changes in cataplexy frequency by prior therapy in a phase 3, double-blind, placebo-controlled, randomised withdrawal study of JZP-258 in adults with narcolepsy with cataplexy [abstract]. Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
49. Drakatos P, Lykouras D, D’Ancona G, Higgins S, Gildeh N, Macavei R, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med. 2017;35:80–84. [PMC free article] [PubMed] [Google Scholar]
50. Axsome announces AXS-12 for narcolepsy [press release]. 2018. https://axsometherapeuticsinc.gcs-web.com/static-files/fa862bca-7be0-4849-9f00-68894655ab86. Accessed 26 Apr 2019.
51. Axsome Therapeutics receives FDA orphan drug designation for AXS-12 for the treatment of narcolepsy [press release]. 2018. https://axsometherapeuticsinc.gcs-web.com/news-releases/news-release-details/axsome-therapeutics-receives-fda-orphan-drug-designation-axs-12?field_nir_news_date_value%5bmin%5d=2019. Accessed 26 Apr 2019.
Accessed 26 Apr 2019.
52. Edronax [summary of product characteristics]. Kent: Pfizer Limited; 2015.
53. Schmidt C, Leibiger J, Fendt M. The norepinephrine reuptake inhibitor reboxetine is more potent in treating murine narcoleptic episodes than the serotonin reuptake inhibitor escitalopram. Behav Brain Res. 2016;308:205–210. [PubMed] [Google Scholar]
54. Fleishaker JC. Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clin Pharmacokinet. 2000;39(6):413–427. [PubMed] [Google Scholar]
55. Larrosa O, de la Llave Y, Bario S, Granizo JJ, Garcia-Borreguero D. Stimulant and anticataplectic effects of reboxetine in patients with narcolepsy: a pilot study. Sleep. 2001;24(3):282–285. [PubMed] [Google Scholar]
56. O'Gorman Cedric, Jones Amanda, Tabuteau Herriot. 0058 Scientific Rationale and Clinical Development of AXS-12 for Narcolepsy. Sleep. 2019;42(Supplement_1):A24–A25. [Google Scholar]
57. Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. [PubMed] [Google Scholar]
Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. [PubMed] [Google Scholar]
58. Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10(1):75–81. [PubMed] [Google Scholar]
59. Theranexus announces preliminary results of phase II trial of THN102 on narcolepsy patients [press release]. 2019. https://www.theranexus.com/images/pdf/Theranexus_PR_result_narcolepsy_20199227_VDEF.pdf. Accessed 26 Apr 2019
60. Liu X, Petit JM, Ezan P, Gyger J, Magistretti P, Giaume C. The psychostimulant modafinil enhances gap junctional communication in cortical astrocytes. Neuropharmacology. 2013;75:533–538. [PubMed] [Google Scholar]
61. Petit JM, Magistretti PJ. Regulation of neuron-astrocyte metabolic coupling across the sleep-wake cycle. Neuroscience. 2016;323:135–156. [PubMed] [Google Scholar]
62. Vodovar D, Duchene A, Wimberley C, Leroy C, Pottier G, Dauvilliers Y, et al. Cortico-amygdala-striatal activation by modafinil/flecainide combination. Int J Neuropsychopharmacol. 2018;21(7):687–696. [PMC free article] [PubMed] [Google Scholar]
Vodovar D, Duchene A, Wimberley C, Leroy C, Pottier G, Dauvilliers Y, et al. Cortico-amygdala-striatal activation by modafinil/flecainide combination. Int J Neuropsychopharmacol. 2018;21(7):687–696. [PMC free article] [PubMed] [Google Scholar]
63. Duchene A, Perier M, Zhao Y, Liu X, Thomasson J, Chauveau F, et al. Impact of astroglial connexins on modafinil pharmacological properties. Sleep. 2016;39(6):1283–1292. [PMC free article] [PubMed] [Google Scholar]
64. Safety and efficacy of THN102 on sleepiness in narcoleptic patients [ClinicalTrials.gov identifier NCT02821715]. National Institutes of Health, ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/{"type":"clinical-trial","attrs":{"text":"NCT02821715","term_id":"NCT02821715"}}NCT02821715. Accessed 26 Apr 2019.
65. Theranexus provides 2018 full-year results and update on activities [press release]. 2019. https://www.actusnews.com/en/THERANEXUS/pr/2019/04/17/theranexus-provides-2018-full-year-results-and-update-on-activities. Accessed 02 May 2019.
Accessed 02 May 2019.
66. Nirogi Ramakrishna, Bhyrapuneni Gopinadh, Abraham Renny, Subramanian Ramkumar, Goyal Vinod Kumar, Pandey Santosh Kumar, Badange Rajesh, Shinde Anil. 0139 SUVN-G3031, A Potent and Selective Histamine h4 Receptor Inverse Agonist - Phase-2 Investigational New Drug for the Treatment of Narcolepsy - Differentiating Factors with Competitor Clinical Candidates. Sleep. 2019;42(Supplement_1):A57–A57. [Google Scholar]
67. Benade Vijay, Daripelli Saivishal, Tirumalasetty Chaitanya, Subramanian Ramkumar, Petlu Surendra, Badange Rajesh, Nirogi Ramakrishna. 0054 SUVN-G3031, a Histamine h4 Receptor Inverse Agonist Produces Wake Promoting Effect in Orexin-2-saporin Lesioned Rats. Sleep. 2019;42(Supplement_1):A22–A23. [Google Scholar]
68. Bhayrapuneni Gopinadh, Kamuju Venkatesh, Gandipudi Sudhakar, Jayarajan Pradeep, Abraham Renny, Bojja Kumar, Pandey Santosh Kumar, Mekala Venkat Reddy, Nirogi Ramakrishna. 0120 SUVN-G3031, a Novel, Potent and Selective Histamine h4 Receptor Inverse Agonist for the Treatment of Narcolepsy: Preclinical Characterization. Sleep. 2019;42(Supplement_1):A50–A50. [Google Scholar]
Sleep. 2019;42(Supplement_1):A50–A50. [Google Scholar]
69. Nirogi Ramakrishna, Shinde Anil, Mudigonda Koteshwara, Bhyrapuneni Gopinadh, Muddana Nageswara Rao, Palacharla Raghava Chowdary, Ajjala Devender Reddy, Goyal Vinod Kumar, Ravula Jyothsna, Jetta Satish, Mohammed Abdul Rasheed. 0053 SUVN-G3031: Safety, Tolerability and Pharmacokinetics of a Potent and Selective Histamine h4 Receptor Inverse Agonist - Single and Multiple Ascending Doses in Healthy Subjects. Sleep. 2019;42(Supplement_1):A22–A22. [Google Scholar]
70. Takenoshita S, Sakai N, Chiba Y, Matsumura M, Yamaguchi M, Nishino S. An overview of hypocretin based therapy in narcolepsy. Expert Opin Investig Drugs. 2018;27(4):389–406. [PubMed] [Google Scholar]
71. Barateau L, Dauvilliers Y. Recent advances in treatment for narcolepsy. Ther Adv Neurol Disord. 2019;12:1–12. [Google Scholar]
72. Kimura Haruhide, Ishikawa Takashi, Yukitake Hiroshi, Suzuki Motohisa. 0055 An Orexin 2 Receptor-selective Agonist, TAK-925, Ameliorates Narcolepsy-like Symptoms and Obesity in Orexin/ataxin-3 Transgenic Mice. Sleep. 2019;42(Supplement_1):A23–A23. [Google Scholar]
Sleep. 2019;42(Supplement_1):A23–A23. [Google Scholar]
73. Suzuki M, Yukitake H, Ishikawa T, Kimura H. 0001 An Orexin 2 Receptor-selective Agonist TAK-925 Ameliorates Narcolepsy-like Symptoms In Orexin/ataxin-3 Mice. Sleep. 2018;41(suppl_1):A1–A1. [Google Scholar]
74. Evans R, Tanaka S, Tanaka S, Touno S, Shimizu K, Sakui S, et al. A phase 1 single ascending dose study of a novel orexin 2 receptor agonist, TAK-925, in healthy volunteers (HV) and subjects with narcolepsy type 1 (NT1) to assess safety, tolerability, pharmacokinetics, and pharmacodynamic outcomes [oral presentation]. World Sleep Congress; 20–25 Sep 2019; Vancouver.
75. Ishikawa T, Suzuki M, Kajita Y, Miyanohana Y, Koike T, Kimura H. Discovery of a novel, orally available orexin 2 receptor-selective agonist, TAK-994, as a therapeutic drug for narcolepsy [abstract]. Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
76. Kimura H, Ishikawa T, Suzuki M. A novel, orally available orexin 2 receptor-selective agonist, TAK-994, ameliorates narcolepsy-like symptoms in narcolepsy mouse models [abstract]. Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
Biennial World Sleep Congress; 20–25 Sep 2019; Vancouver.
77. Barateau L, Liblau R, Peyron C, Dauvilliers Y. Narcolepsy type 1 as an autoimmune disorder: evidence, and implications for pharmacological treatment. CNS Drugs. 2017;31(10):821–834. [PubMed] [Google Scholar]
78. Clinical outcomes in narcolepsy and cataplexy: an evaluation of reboxetine treatment (CONCERT) (CONCERT) [ClinicalTrials.gov identifier NCT03881852]. National Institutes of Health, ClinicalTrials.gov. 2019. https://clinicaltrials.gov/ct2/show/{"type":"clinical-trial","attrs":{"text":"NCT03881852","term_id":"NCT03881852"}}NCT03881852. Accessed 29 Apr 2019.
79. A study of the efficacy and safety of JZP-258 in subjects with narcolepsy with cataplexy [ClinicalTrials.gov identifier NCT03030599]. National Institutes of Health, ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/{"type":"clinical-trial","attrs":{"text":"NCT03030599","term_id":"NCT03030599"}}NCT03030599. Accessed 29 Apr 2019.
Stanford researcher shows once-nightly narcolepsy drug is safe, effective | News Center
A phase 3 study has found that an extended-release version of sodium oxybate reduces daytime sleepiness and attacks of muscle weakness in narcolepsy patients.
September 8, 2021 - By Janelle Weaver
A new version of a narcolepsy drug that patients take once at bedtime — rather than at bedtime and again in the middle of the night — safely and effectively improved symptoms in a trial led by a researcher at Stanford Medicine.
The drug the researchers were investigating, ON-SXB, is an extended-release version of sodium oxybate, which requires twice-nightly dosing. Sodium oxybate is approved by the Food and Drug Administration for the treatment of multiple narcolepsy symptoms, including excessive daytime sleepiness and cataplexy — sudden muscle weakness while awake.
In the trial, ON-SXB decreased cataplexy attacks and daytime sleepiness more effectively than a placebo, while increasing clinicians’ ratings of the overall condition of study participants. Its safety profile was favorable, with side effects that were similar to those caused by the twice-nightly version of sodium oxybate. Eliminating the need for a second dose at night, ON-SXB could help patients adhere to the medication regimen, reduce the risk of falls leading to injury, and improve nocturnal sleep and overall quality of life.
“Sodium oxybate has largely become a first-line treatment for patients with narcolepsy,” said Clete Kushida, MD, PhD, a professor of psychiatry and behavioral sciences. “Clinicians can be confident that a single bedtime dose of sodium oxybate has demonstrated efficacy for both objective and subjective symptoms of narcolepsy.”
The study was published Aug. 6 in Sleep. Kushida is the lead author.
Seeking sound sleep
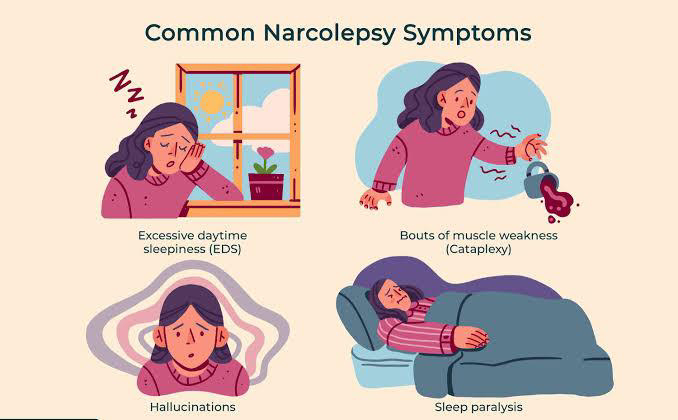
Narcolepsy is a chronic neurological disorder that affects between 135,000 and 200,000 people in the U.S. In addition to excessive daytime sleepiness and cataplexy, symptoms include disrupted nighttime sleep, sleep paralysis, and hallucinations when falling asleep or waking up. All patients experience excessive daytime sleepiness, which can manifest as sleep attacks that last several seconds or minutes and can occur while talking, eating or driving.
Cataplexy, which is another common symptom, is often triggered by strong emotions and sometimes causes falls. This collection of features can interfere with psychological and cognitive function and development; it can also severely impede daily life, including school attendance, employment and social activities.
This collection of features can interfere with psychological and cognitive function and development; it can also severely impede daily life, including school attendance, employment and social activities.
Sodium oxybate is effective at treating multiple narcolepsy symptoms, including disrupted nighttime sleep. But because its half-life is approximately 30 minutes to 1 hour, a second dose is required 2 ½ to 4 hours after the first dose. Waking in the middle of the night to take the medication can be highly disruptive, especially considering that patients typically already experience fragmented sleep and poor sleep quality.
A study in Europe showed that 27% of patients with narcolepsy did not take sodium oxybate on the recommended time schedule. When patients need to take medications more than once daily, they tend to miss doses: This problem has been seen with other conditions, including depression, schizophrenia, epilepsy, Type 2 diabetes, cardiovascular disease, chronic obstructive pulmonary disease and HIV.
“A medication that is taken twice daily — in the morning and evening — is more challenging than a once-daily medication, but it’s even more problematic for a chronic medication requiring middle-of-the-night awakening,” Kushida said. “Although the labeling advises patients to remain in bed to take the second dose, falls leading to injury and, in some cases, hospitalization have been reported when patients rise from bed.”
No-awakening alternative
The trial was conducted from November 2016 to March 2020 at 71 centers in the United States, Australia, Canada and Europe. In total, 222 narcolepsy patients, ages 16 or older, were randomly assigned to take ON-SXB, in varying amounts, or a placebo.
Compared with placebo, participants on the three highest doses of ON-SXB had significantly less daytime sleepiness, a decrease in weekly cataplexy attacks, lower self-rated sleepiness in everyday situations, and higher overall condition scores from their clinicians. For example, 72% of participants taking the highest dose of ON-SXB were considered much or very much improved, compared with 31.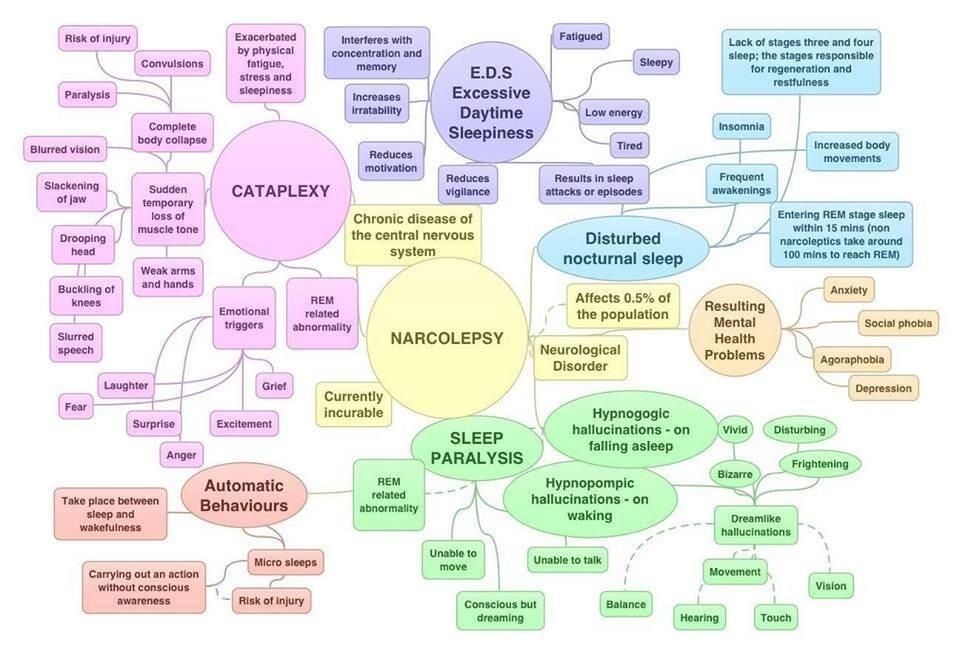 6% in the placebo group.
6% in the placebo group.
The side effects of ON-SXB are similar to those of the twice-nightly version of sodium oxybate and include nausea, headache, vomiting, dizziness, involuntary urination and decreased appetite. But with ON-SXB, rates of headaches, nausea and dizziness were lower.
ON-SXB is under review at the FDA for the treatment of excessive daytime sleepiness and cataplexy in adults with narcolepsy. It has received orphan drug designation from the FDA because it may be clinically superior to the twice-nightly formulation of sodium oxybate already approved for the same condition. Orphan status is provided to drugs for the treatment, diagnosis or prevention of rare diseases that either affect fewer than 200,000 people in the United States, or are otherwise not expected to recover the development and marketing costs. Drug companies receive financial benefits for developing orphan drugs.
“If approved, ON-SXB may be a major advance for patients experiencing the burdensome symptoms of narcolepsy and for physicians who manage their patients with this chronic, incapacitating sleep disorder,” Kushida said.
Researchers from the University of Toronto; Henry Ford Health System; Montefiore Medical Center; Sleep Management Institute; Florida Pediatric Research Institute; Neurotrials Research Inc.; Ohio Sleep Medicine and Neuroscience Institute; Avadel Pharmaceuticals; and Gui-de-Chauliac Hospital also contributed to the study.
The work was supported by Avadel Ireland.
-
Janelle Weaver
Stanford Medicine integrates research, medical education and health care at its three institutions - Stanford School of Medicine, Stanford Health Care, and Stanford Children's Health. For more information, please visit the Office of Communications website at http://mednews.stanford.edu.
Sunosi: a new drug for excessive daytime sleepiness
Main
Sunosi (Sunosi, solriamphetol) is a new drug designed to improve the wakefulness status of adult patients suffering from excessive daytime sleepiness caused by narcolepsy or obstructive sleep syndrome. sleep apnea. Sunosi was developed by Jazz Pharmaceuticals.
FDA approved. However, Sunosi is not indicated for the treatment of obstructive sleep apnea. Before prescribing Sunosi, the latter should be treated with continuous positive airway pressure (CPAP).
The European Medicines Agency (EMA) has also approved Sunosi: a drug intended to improve wakefulness status and reduce excessive daytime sleepiness in adult patients with narcolepsy (with or without cataplexy) or obstructive sleep apnea (if sleepiness is successful). not stopped by its primary therapy such as CPAP).
Sunosi: mechanism of action of solriamphetol
The precise mechanism of action by which oral solriamfetol (solriamfetol, JZP-110) promotes wakefulness in patients with excessive daytime sleepiness due to narcolepsy or obstructive sleep apnea has not been established. The therapeutic efficacy of solriamphetol is thought to be mediated by dopamine and norepinephrine reuptake inhibition (DNRI) dual: the molecule enhances the signaling of these neurotransmitters in the brainstem excitatory system.
Solriamphetol binds to dopamine and norepinephrine transporters (K and 14.2 and 3.7 µmol) and inhibits the reuptake of these neurotransmitters (IC 50 2.9 and 4.4 µmol). Solriamphetol has no significant binding affinity for the serotonin transporter (K i 81.5 µmol) and does not inhibit the reuptake of the latter (IC 50 > 100 µmol). Solriamphetol does not have a pronounced affinity for dopamine, serotonin, norepinephrine, GABA, adenosine, histamine, orexin, benzodiazepine receptors, as well as acetylcholine muscarinic or acetylcholine nicotinic receptors.
https://mosmedpreparaty.ru/wp-content/uploads/2020/09/SUNOSI-MOA.mp4
The putative dual inhibition of dopamine and norepinephrine reuptake provided by solriamphetol is similar to that provided by amphetamine or dextroamphetamine in terms of neurobehavioral effects. However, there are a number of mechanistic differences. So, solriamphetol does not affect trace amine receptor 1 (TAAR1) or vesicular monoamine transporters 1 and 2 (VMAT1 and VMAT2). This explains why solriamphetol does not trigger the release of dopamine and norepinephrine into the synaptic cleft in the same way that amphetamines do. Again, there are a number of drugs, such as bupropion, which, being in the DNRI class, are not considered amphetamines. In other words, solriamphetol is similar in its psychostimulant effect to amphetamines and modafinil, but it cannot be attributed to the amphetamine series.
Sunosi: therapeutic efficacy of solriamphetol
The TONES clinical program, which included four phase III clinical trials (randomized, double-blind, placebo-controlled, parallel groups, multicenter, international), studied the efficacy of solriamphetol in more than 900 adults patients suffering from excessive daytime sleepiness.
Treatment outcome was assessed by two primary endpoints: wakefulness maintenance test (MWT) and change in Epworth Sleepiness Scale (ESS) score. The first measures a person's ability to stay awake during the daytime in a dark, quiet environment: patients are instructed to remain awake for as long as possible in four to five 40-minute testing sessions throughout the day with a 2-hour interval between them - sleep delay is determined by the average the number of minutes of wakefulness during the first four testing sessions. The second is represented by a questionnaire of 8 questions, answering which patients assess the estimated likelihood of falling asleep during their usual daily activities.
Among the secondary endpoints: the patient's assessment of the overall impression of a change in own state (PGI-C), implemented by a 7-point scale, on which the subject assessed the severity of the symptoms of the disease.
For example, clinical trial NCT02348593 tested solriamphetol in patients (n=231) with narcolepsy. Participants, in 51% of cases of which the disease was accompanied by cataplexy, were prescribed once a day placebo or solriamphetol at a dose of 75, 150 or 300 mg.
After 12 weeks, patients with cataplexy treated with solriamphetol at the above doses showed a rms mean change (hereinafter) in MWT sleep latency of 3.4 minutes (95% CI: −0.4–7.2), 7.9 (4.1–11.7) and 10.7 (6.8–14.5) versus 1.8 minutes (−1.9 - 5.4) in the placebo group (p<0.05 for 150- and 300-mg doses of solriamphetol).
Mosmedpreparaty
Patients without cataplexy showed a change of 6.0 (2.2-9.8), 11.6 (7.9-15.4) and 13.8 (9.8-17.8) minutes versus 2 .6 minutes (−1.1 - 6.2) in the control group (p<0.05 for the 150-mg dose of solriamphetol, p<0.0001 for the 300-mg dose).
In the subgroup of patients with cataplexy who were prescribed solriamphetol, the change in ESS score was -3.1 (-5.0 - -1.2), -5.6 (-7.5 - -3.7) and -6.3 (-8.2 - -4.5) versus -1.8 (-3.7 - -0.02) change in the placebo group (p<0.05 for 150- and 300-mg doses of the drug ).
Among patients without cataplexy, the change in ESS score was -4.5 (-6.4 - -2.6), -5.2 (-7.1 - -3.4) and -6.4 (-8 .4 - -4.4) - against -1.5 (-3.3 - 0.3) in the control group (p<0.05 for all doses of solriamphetol).
For PGI-C improvement, the mean percentage difference from placebo was 10% (−15–35), 33% (9–57), and 39% (16–61) in the subgroup of patients with cataplexy (p <0.05 for 150- and 300-mg doses of solriamphetol), 48% (25-70), 44% (21-67) and 52% (30-73) in the subgroup without cataplexy (p<0. 001 for all doses ).
001 for all doses ).
The NCT02348606 clinical trial testing solriamphetol in patients (n=459) with obstructive sleep apnea similarly found Sunosi's therapeutic efficacy to be statistically significantly superior to placebo.
Comparative efficacy of solriamphetol in patients with narcolepsy or obstructive sleep apnea noted in clinical trials NCT02348593 and NCT02348606 is shown below:
Sunosi: safety of solriamphetol
Adverse reactions associated with the use of Sunosi, typical of other drugs with general pharmacodynamic properties (stimulants, bupropion): reversible anorexia, restlessness and nervousness, insomnia and irritability, dry mouth, nausea, diarrhea. However, none of these negative phenomena can cause harm.
Solriamphetol's main safety concern is an increase in blood pressure, although its increase may go unnoticed. Therefore, in the instructions for the medical use of Sunosi, it is instructed both to monitor changes in blood pressure and pulse rate during therapy with solriamphetol, and not to prescribe the drug to patients with unstable cardiovascular disease, serious cardiac arrhythmia and other severe cardiac disorders.
Sunosi: Solriamphetol's commercial future
Sunosi's market launch was ambivalent for the Jazz in terms of perceived commercial success. On the one hand, the highest 300-mg dose of the drug was not approved: despite the increased therapeutic efficacy, the regulator referred to adverse reactions. On the other hand, the instructions for medical use of "Sunoshi" turned out to be spared from the "black-framed" warning.
In addition, solriamphetol, supported by clinical evidence for the treatment of excessive daytime sleepiness due to obstructive sleep apnea, is suitable for a much wider patient population than it would be if it were only allowed for narcolepsy.
According to industry experts, demand for Sunosi will reach almost half a billion dollars by 2024.
In January 2019, Jazz managed to block the generic competition that copy drugs wanted to mount to its bestseller Zyrem (Xyrem, sodium oxybutyrate) in 2018 and 2019. earning $1.40 and $1. 64 billion, respectively. Generic Zyrema, approved by the FDA in July 2002 for the treatment of cataplexy or excessive daytime sleepiness caused by narcolepsy in patients 7 years of age and older, will not see the light of day until 2023.
64 billion, respectively. Generic Zyrema, approved by the FDA in July 2002 for the treatment of cataplexy or excessive daytime sleepiness caused by narcolepsy in patients 7 years of age and older, will not see the light of day until 2023.
Supplementary Materials
US Prescribing Information for Sunosi (Solriamphetol). [PDF]
Instructions for medical use of the drug "Sunosi" (Sunosi, solriamphetol) in Europe. [PDF]
Mosmedpreparaty
Sunosi. EMA European Public Assessment Report (EPAR). [PDF]
Sunosi. FDA Joint Supervisory Review (JSR). [PDF]
Solriamfetol for the Treatment of Excessive Daytime Sleepiness in Participants with Narcolepsy with and without Cataplexy: Subgroup Analysis of Efficacy and Safety Data by Cataplexy Status in a Randomized Controlled Trial. CNS Drugs. 2020 Jul;34(7):773-784. [PDF] [source]
Solriamfetol for Excessive Sleepiness in Obstructive Sleep Apnea (TONES 3). A Randomized Controlled Trial. Am J Respir Crit Care Med. 2019 Jun 1; 199(11): 1421–1431. [PDF] [source]
A Randomized Controlled Trial. Am J Respir Crit Care Med. 2019 Jun 1; 199(11): 1421–1431. [PDF] [source]
Measures of functional outcomes, work productivity, and quality of life from a randomized, phase 3 study of solriamfetol in participants with narcolepsy. Sleep Med. Mar 2020;67:128-136. [PDF] [source]
Effects of Solriamfetol on Quality-of-Life Measures from a 12-Week Phase 3 Randomized Controlled Trial. Ann Am Thorac Soc. 2020 Aug;17(8):998-1007. [PDF] [source]
Clinically relevant effects of solriamfetol on excessive daytime sleepiness: a post hoc analysis of the magnitude of change in clinical trials in adults with narcolepsy or obstructive sleep apnea. J Clinic Sleep Med. 2020 Nov 23. [source]
Scientists on the way to the discovery of the "drug wakefulness" | Society news | Izvestiya
Millions of sleepy city dwellers, who bring themselves to their senses with a cup of morning coffee, may soon try a new wonderful means of maintaining vigor and efficiency. Imagine: after taking a pill after a sleepless day, you lose the desire to sleep and you are again ready to work and stay awake without interrupting sleep. The tempting idea of life extension by stealing time from sleep can become a reality, and the drug that provides this is a super-effective stimulant of the future. Monkeys in the laboratories of the American city of Darpa have already tried this stimulant.
Imagine: after taking a pill after a sleepless day, you lose the desire to sleep and you are again ready to work and stay awake without interrupting sleep. The tempting idea of life extension by stealing time from sleep can become a reality, and the drug that provides this is a super-effective stimulant of the future. Monkeys in the laboratories of the American city of Darpa have already tried this stimulant.
Little monkeys, deprived of sleep by heartless scientists for 30-36 hours for scientific purposes, got rid of drowsiness after receiving a dose of a neurohormone. They almost instantly became as active and alert as their counterparts, peacefully dozing in the neighboring cage. This miraculous brain hormone that reverses sleep deprivation is called orexin-A.
Scientists suggest that on the basis of orexin-A it is possible to create a drug that can replace natural sleep for people without any serious side effects. However, this discovery will most likely find its first practical application in the treatment of severe nervous pathologies.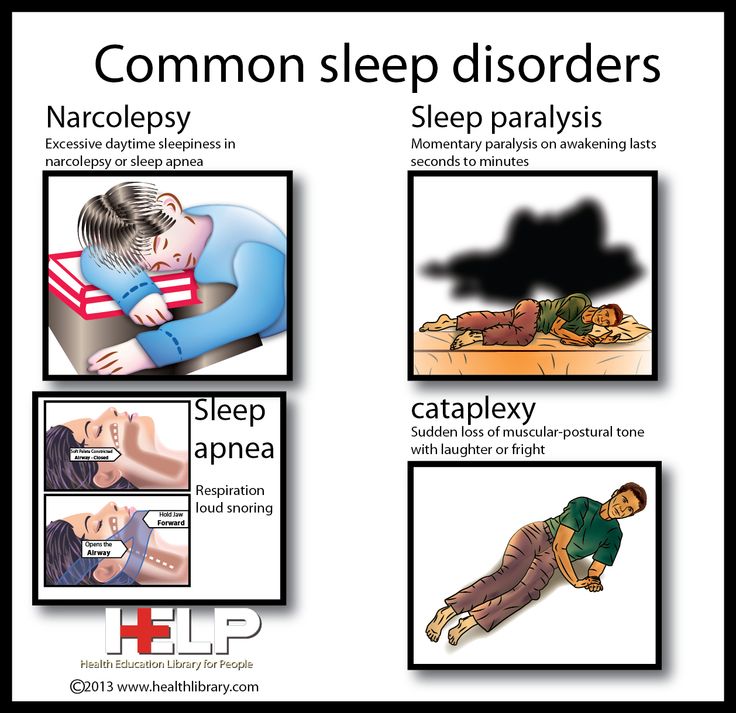 On the basis of the hormone, scientists will try to create a drug for the treatment of narcolepsy, a serious disease that manifests itself as sleep disorders. Patients with narcolepsy experience irresistible daytime sleepiness with attacks of sudden falling asleep and cataplexy, that is, a complete or partial loss of muscle tone.
On the basis of the hormone, scientists will try to create a drug for the treatment of narcolepsy, a serious disease that manifests itself as sleep disorders. Patients with narcolepsy experience irresistible daytime sleepiness with attacks of sudden falling asleep and cataplexy, that is, a complete or partial loss of muscle tone.
"This therapy represents a completely new approach to increasing the activity of the central nervous system, and the results of recent experiments indicate the relative safety of this method," - said Dr. Jerome Siegel, professor of psychiatry at the University of California at Los Angeles in an article in the Journal of Neurological Science.
Orexin-A has every chance of becoming the basis of drugs that can be sold as "sleep substitutes". For many years, people have used various stimulants to combat sleep, but they can be addictive and often have serious side effects, such as high blood pressure and mood swings. The health hazards that these drugs can cause hinder their widespread use.
A remedy that helps a person go without sleep for a long time has long been a coveted "philosopher's stone" of pharmaceuticals, along with a cure for cancer and anti-aging pills. The military would very much like to receive a "pill for vivacity". This will keep the troops in constant combat readiness. Many armies around the world already allow pilots to take amphetamines for long-haul flights. The military budget finances the development of other stimulants such as modafinil and orexin-A.
During the experiment, the scientists divided the monkeys, who had not slept for more than 30 hours in a row, into two groups, the website k2kapital.com reports. The first group was given orexin-A, and the second group was given a saline placebo. The scientists then conducted a series of standardized cognitive tests. The orexin-A treated monkeys performed as well as the sleeping monkeys, while the saline group performed less well.
The results of the study, published in the specialized Journal of Neurological Science, also indicate that orexin-A did more than just restore monkeys' cognitive abilities. The monkeys' brains looked 'awakened' on proton emission tomography images.
The monkeys' brains looked 'awakened' on proton emission tomography images.
According to Siegel, the uniqueness of orexin-A lies in the fact that the pharmacological effect of the drug was only on sleep-deprived monkeys, without affecting the condition of the wakeful animals. In this regard, scientists believe that this neurohormone acts "exclusively to reverse the effect of drowsiness", without exposing the brain to other influences. This property distinguishes this substance from the currently used stimulants.
Such a product could be in great demand all over the world. Consumers could trade their money for the ability to stay awake while staying awake and not endangering the body. The wide distribution and free sale of such a tool can have a very strong social effect, giving rise to a 24-hour society that can go without sleep and remain operational throughout the entire time of the day.
Dr. Michael Tweri, director of the National Sleep Disorders Research Center, acknowledged the great interest in developing a "peep pill" but warned of the possible very serious consequences of long-term use of this drug, if not on the central nervous system, then on other body systems . "The results of recent studies indicate that lack of sleep causes an increased risk of cardiovascular disease and metabolic disorders," - said Tver.
"The results of recent studies indicate that lack of sleep causes an increased risk of cardiovascular disease and metabolic disorders," - said Tver.
However, people are already solving the problem of lack of time for sleep with the help of various existing and freely sold stimulants. "We have to acknowledge that we already live in a society where people are already self-medicating with caffeine," said Jerome Siegel.
He also noted that the stimulant modafinil (used to treat certain conditions, including narcolepsy) is used in drugs such as Provigil, Alertec, Modiodal, which are often bought by healthy people to combat sleep instead of the traditional cup of coffee. "With these and other precedents, it's not clear why orexin-A can't be used for temporary sleep relief," Siegel said. "On the other hand, one would have to be an idiot to advocate the complete elimination of sleep by taking the drug as long as possible."
It's too early to worry about orexin-A being in pharmacies.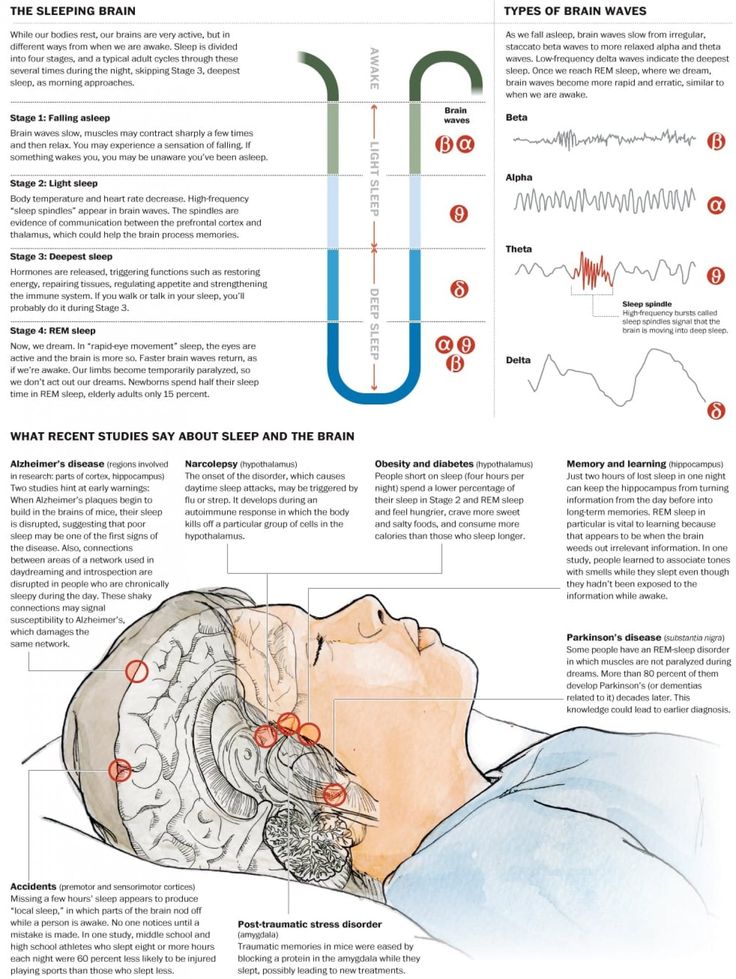 In the near future, pharmaceutical companies will not be able to produce orexin-A in industrial quantities, and its commercial use, and even more so free sale, will take more than one year of approvals, studies and consideration of the issue by regulatory authorities in various countries.
In the near future, pharmaceutical companies will not be able to produce orexin-A in industrial quantities, and its commercial use, and even more so free sale, will take more than one year of approvals, studies and consideration of the issue by regulatory authorities in various countries.
However, the market for illicit drugs, including narcotic drugs, exists without the approval of the regulatory authorities of most countries, and some countries, such as the Netherlands, have a more liberal policy on some substances that are banned in most countries of the world. It is now known that GlaxoSmithKline and Actelion are working on the development of sleeping pills based on orexin receptor antagonists that will act on brain cells and cause the opposite effect, that is, drowsiness. It is possible that in the laboratories of pharmaceutical companies, work is underway to create a drug for vigor.
However, it is very unlikely that in the coming decades orexin-A will be reproduced in clandestine laboratories in third world countries for subsequent illegal sale in developed countries, as is now happening, for example, with heroin.