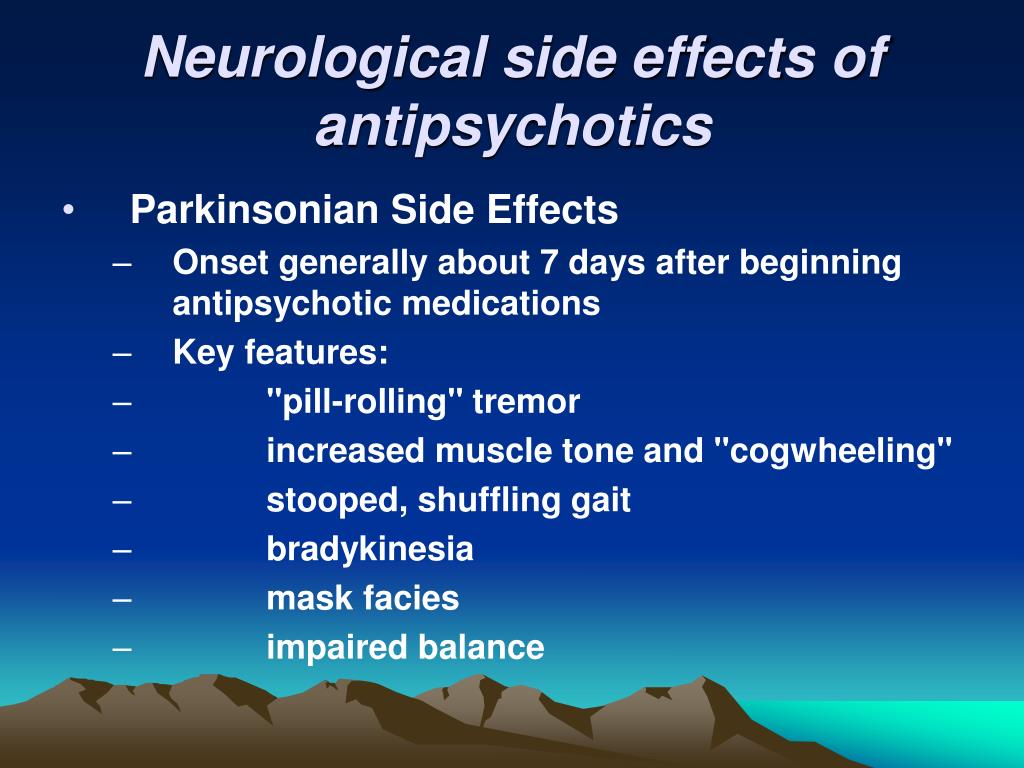
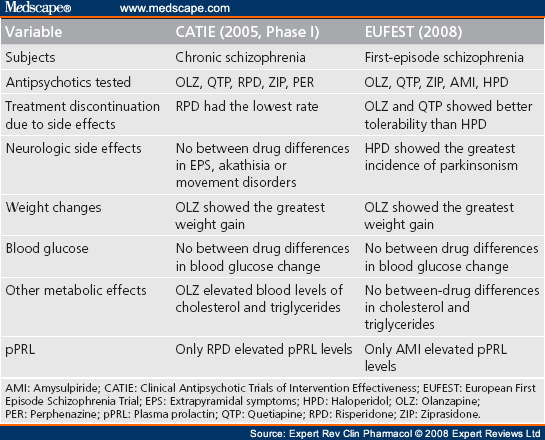
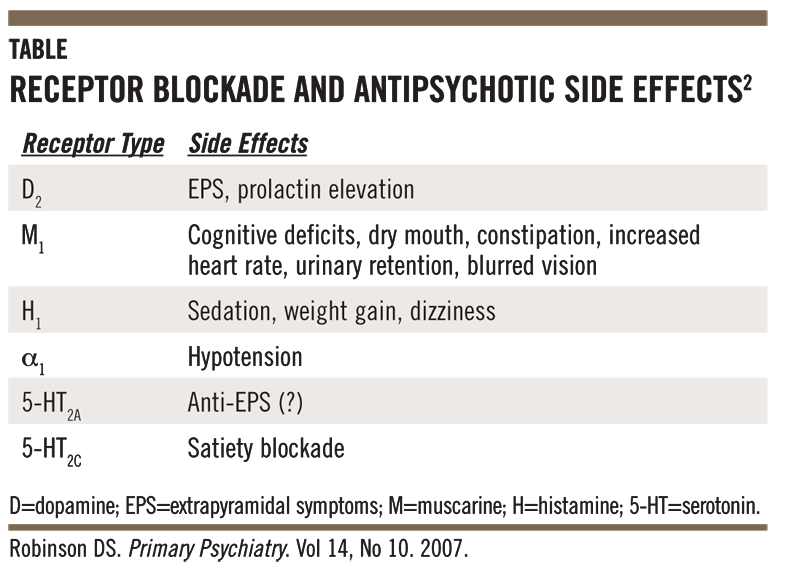
Long term side effects of antipsychotics
What is the risk‐benefit ratio of long‐term antipsychotic treatment in people with schizophrenia?
1. Kahn RS, Sommer IE, Murray RM et al. Schizophrenia. Nat Rev Dis Primer 2015;1:15067. [PubMed] [Google Scholar]
2. Lehman AF, Lieberman JA, Dixon LB et al. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(Suppl. 2):1‐56. [PubMed] [Google Scholar]
3. Buchanan RW, Kreyenbuhl J, Kelly DL et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010;36:71‐93. [PMC free article] [PubMed] [Google Scholar]
4. Crockford D, Addington D. Canadian schizophrenia guidelines: schizophrenia and other psychotic disorders with coexisting substance use disorders. Can J Psychiatry 2017;62:624‐34. [PMC free article] [PubMed] [Google Scholar]
5. Galletly C, Castle D, Dark F et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry 2016;50:410‐72. [PubMed] [Google Scholar]
6. Davis JM, Matalon L, Watanabe MD et al. Depot antipsychotic drugs. Place in therapy. Drugs 1994;47:741‐73. [PubMed] [Google Scholar]
7. Kissling W. The current unsatisfactory state of relapse prevention in schizophrenic psychoses – suggestions for improvement. Clin Neuropharmacol 1991;14(Suppl. 2):S33‐44. [PubMed] [Google Scholar]
8. Leucht S, Barnes TRE, Kissling W et al. Relapse prevention in schizophrenia with new‐generation antipsychotics: a systematic review and exploratory meta‐analysis of randomized, controlled trials. Am J Psychiatry 2003;160:1209‐22. [PubMed] [Google Scholar]
9. Leucht S, Tardy M, Komossa K et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta‐analysis. Lancet 2012;379:2063‐71. [PubMed] [Google Scholar]
10. Leucht S, Cipriani A, Spineli L et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple‐treatments meta‐analysis. Lancet 2013;382:951‐62. [PubMed] [Google Scholar]
Lancet 2013;382:951‐62. [PubMed] [Google Scholar]
11. Takeuchi H, Suzuki T, Uchida H et al. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr Res 2012;134:219‐25. [PubMed] [Google Scholar]
12. Murray RM, Quattrone D, Natesan S et al. Should psychiatrists be more cautious about the long‐term prophylactic use of antipsychotics? Br J Psychiatry 2016;209:361‐5. [PubMed] [Google Scholar]
13. Wunderink L, Nieboer RM, Wiersma D et al. Recovery in remitted first‐episode psychosis at 7 years of follow‐up of an early dose reduction/discontinuation or maintenance treatment strategy: long‐term follow‐up of a 2‐year randomized clinical trial. JAMA Psychiatry 2013;70:913‐20. [PubMed] [Google Scholar]
14. Harrow M, Jobe TH, Faull RN. Does treatment of schizophrenia with antipsychotic medications eliminate or reduce psychosis? A 20‐year multi‐follow‐up study. Psychol Med 2014;44:3007‐16. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
15. Harrow M, Jobe TH, Faull RN et al. A 20‐year multi‐followup longitudinal study assessing whether antipsychotic medications contribute to work functioning in schizophrenia. Psychiatry Res 2017;256:267‐74. [PMC free article] [PubMed] [Google Scholar]
16. Samaha A‐N, Seeman P, Stewart J et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci 2007;27:2979‐86. [PMC free article] [PubMed] [Google Scholar]
17. Moncrieff J. Antipsychotic maintenance treatment: time to rethink? PLoS Med 2015;12:e1001861. [PMC free article] [PubMed] [Google Scholar]
18. Goff DC, Falkai P, Fleischhacker WW et al. The long‐term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry 2017;174:840‐9. [PubMed] [Google Scholar]
19. Gøtzsche PC, Young AH, Crace J. Does long term use of psychiatric drugs cause more harm than good? BMJ 2015;350:h3435.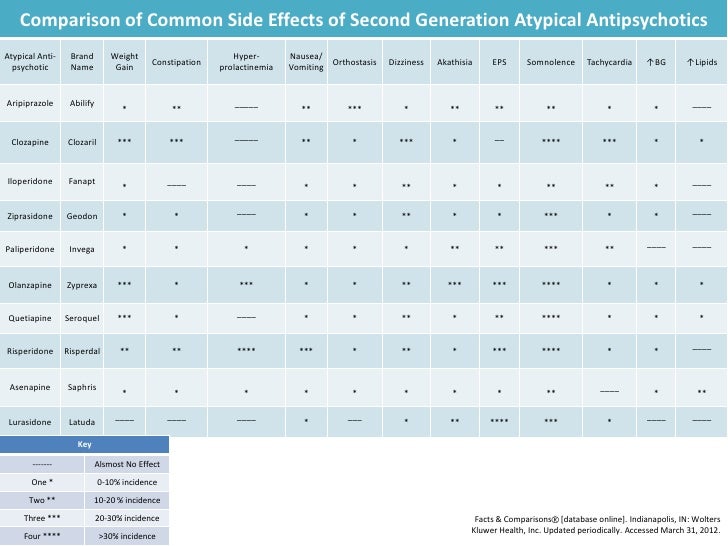 [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
20. Correll CU, Kishimoto T, Nielsen J et al. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther 2011;33:B16‐39. [PMC free article] [PubMed] [Google Scholar]
21. Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long‐acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol 2013;66(Suppl. 8):S37‐41. [PMC free article] [PubMed] [Google Scholar]
22. Correll CU, Kishimoto T, Kane JM. Randomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternatives. Dialogues Clin Neurosci 2011;13:155‐72. [PMC free article] [PubMed] [Google Scholar]
23. Kane JM, Kishimoto T, Correll CU. Non‐adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 2013;12:216‐26. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
24. Valenstein M, Ganoczy D, McCarthy JF et al. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry 2006;67:1542‐50. [PubMed] [Google Scholar]
25. Rajagopalan K, Wade S, Meyer N et al. Real‐world adherence assessment of lurasidone and other oral atypical antipsychotics among patients with schizophrenia: an administrative claims analysis. Curr Med Res Opin 2017;33:813‐20. [PubMed] [Google Scholar]
26. Lopez LV, Shaikh A, Merson J et al. Accuracy of clinician assessments of medication status in the emergency setting: a comparison of clinician assessment of antipsychotic usage and plasma level determination. J Clin Psychopharmacol 2017;37:310‐4. [PubMed] [Google Scholar]
27. Alvarez‐Jimenez M, Priede A, Hetrick SE et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta‐analysis of longitudinal studies. Schizophr Res 2012;139:116‐28. [PubMed] [Google Scholar]
Schizophr Res 2012;139:116‐28. [PubMed] [Google Scholar]
28. Winton‐Brown TT, Elanjithara T, Power P et al. Five‐fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res 2017;179:50‐6. [PubMed] [Google Scholar]
29. Schoeler T, Petros N, Di Forti M et al. Poor medication adherence and risk of relapse associated with continued cannabis use in patients with first‐episode psychosis: a prospective analysis. Lancet Psychiatry 2017;4:627‐33. [PMC free article] [PubMed] [Google Scholar]
30. Novick D, Haro JM, Suarez D et al. Predictors and clinical consequences of non‐adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 2010;176:109‐13. [PubMed] [Google Scholar]
31. Kishimoto T, Robenzadeh A, Leucht C et al. Long‐acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta‐analysis of randomized trials. Schizophr Bull 2014;40:192‐213. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
32. Kishimoto T, Nitta M, Borenstein M et al. Long‐acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta‐analysis of mirror‐image studies. J Clin Psychiatry 2013;74:957‐65. [PubMed] [Google Scholar]
33. Barnes TRE, Drake RJ, Dunn G et al. Effect of prior treatment with antipsychotic long‐acting injection on randomised clinical trial treatment outcomes. Br J Psychiatry 2013;203:215‐20. [PubMed] [Google Scholar]
34. Voss EA, Ryan PB, Stang PE et al. Switching from risperidone long‐acting injectable to paliperidone long‐acting injectable or oral antipsychotics: analysis of a Medicaid claims database. Int Clin Psychopharmacol 2015;30:151‐7. [PMC free article] [PubMed] [Google Scholar]
35. Kishimoto T, Hagi K, Nitta M et al. Effectiveness of long‐acting injectable vs oral antipsychotics in patients with schizophrenia: a meta‐analysis of prospective and retrospective cohort studies.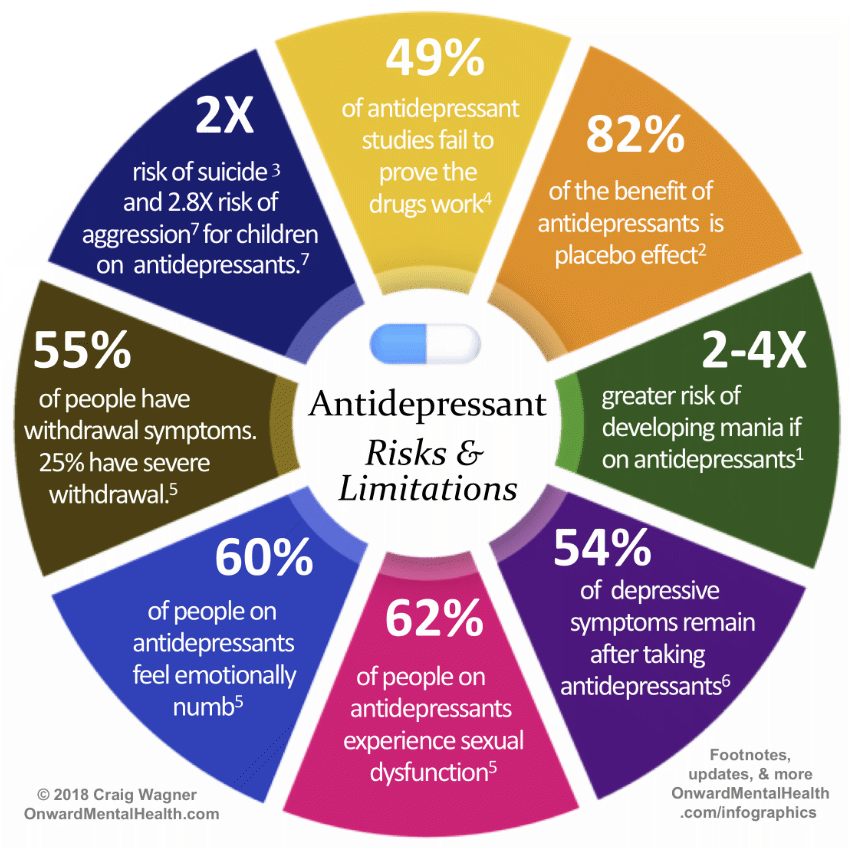 Schizophr Bull (in press). [Google Scholar]
Schizophr Bull (in press). [Google Scholar]
36. Tiihonen J, Haukka J, Taylor M et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011;168:603‐9. [PubMed] [Google Scholar]
37. Tiihonen J, Mittendorfer‐Rutz E, Majak M et al. Real‐world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiatry 2017;74:686‐93. [PMC free article] [PubMed] [Google Scholar]
38. Misawa F, Kishimoto T, Hagi K et al. Safety and tolerability of long‐acting injectable versus oral antipsychotics: a meta‐analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res 2016;176:220‐30. [PubMed] [Google Scholar]
39. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci 2014;16:505‐24. [PMC free article] [PubMed] [Google Scholar]
40. Kotov R, Fochtmann L, Li K et al.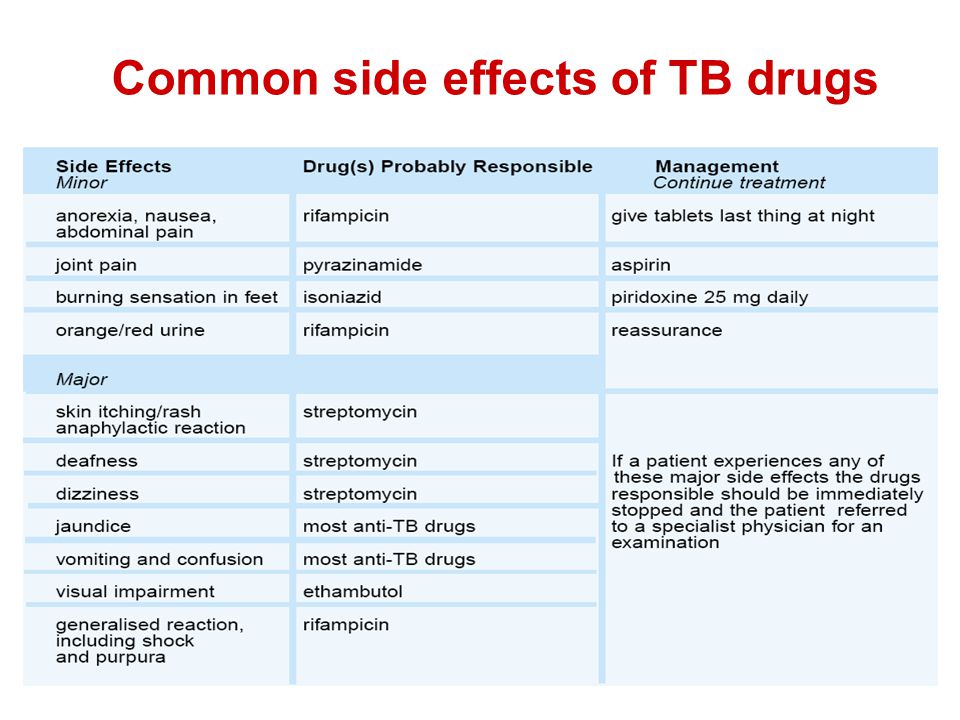 Declining clinical course of psychotic disorders over the two decades following first hospitalization: evidence from the Suffolk County Mental Health Project. Am J Psychiatry 2017;174:1064‐74. [PMC free article] [PubMed] [Google Scholar]
Declining clinical course of psychotic disorders over the two decades following first hospitalization: evidence from the Suffolk County Mental Health Project. Am J Psychiatry 2017;174:1064‐74. [PMC free article] [PubMed] [Google Scholar]
41. Husa AP, Rannikko I, Moilanen J et al. Lifetime use of antipsychotic medication and its relation to change of verbal learning and memory in midlife schizophrenia – an observational 9‐year follow‐up study. Schizophr Res 2014;158:134‐41. [PubMed] [Google Scholar]
42. Moilanen J, Haapea M, Miettunen J et al. Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication – A 10‐year follow‐up of the Northern Finland 1966 Birth Cohort study. Eur Psychiatry 2013;28:53‐8. [PubMed] [Google Scholar]
43. Gotfredsen DR, Wils RS, Hjorthøj C et al. Stability and development of psychotic symptoms and the use of antipsychotic medication – long‐term follow‐up. Psychol Med 2017;47:2118‐29. [PubMed] [Google Scholar]
44. Wils RS, Gotfredsen DR, Hjorthøj C et al. Antipsychotic medication and remission of psychotic symptoms 10 years after a first‐episode psychosis. Schizophr Res 2017;182:42‐8. [PubMed] [Google Scholar]
Wils RS, Gotfredsen DR, Hjorthøj C et al. Antipsychotic medication and remission of psychotic symptoms 10 years after a first‐episode psychosis. Schizophr Res 2017;182:42‐8. [PubMed] [Google Scholar]
45. Ran M‐S, Weng X, Chan CL‐W et al. Different outcomes of never‐treated and treated patients with schizophrenia: 14‐year follow‐up study in rural China. Br J Psychiatry 2015;207:495‐500. [PMC free article] [PubMed] [Google Scholar]
46. Tiihonen J, Mittendorfer‐Rutz E, Torniainen M et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow‐up study. Am J Psychiatry 2016;173:600‐6. [PubMed] [Google Scholar]
47. Tiihonen J, Lönnqvist J, Wahlbeck K et al. 11‐year follow‐up of mortality in patients with schizophrenia: a population‐based cohort study (FIN11 study). Lancet 2009;374:620‐7. [PubMed] [Google Scholar]
48. Wunderink L, Nienhuis FJ, Sytema S et al. Guided discontinuation versus maintenance treatment in remitted first‐episode psychosis: relapse rates and functional outcome. J Clin Psychiatry 2007;68:654‐61. [PubMed] [Google Scholar]
J Clin Psychiatry 2007;68:654‐61. [PubMed] [Google Scholar]
49. Uchida H, Suzuki T, Takeuchi H et al. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta‐analysis. Schizophr Bull 2011;37:788‐99. [PMC free article] [PubMed] [Google Scholar]
50. World Health Organization . Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2017. http://www.whocc.no/atcddd/.
51. Takeuchi H, Suzuki T, Remington G et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open‐label, randomized, controlled, pilot study. Schizophr Bull 2013;39:993‐8. [PMC free article] [PubMed] [Google Scholar]
52. Mayoral‐van Son J, de la Foz VO, Martinez‐Garcia O et al. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first‐episode nonaffective psychosis individuals: a 3‐year naturalistic follow‐up study. J Clin Psychiatry 2016;77:492‐500. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
53. De Hert M, Detraux J, van Winkel R et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011;8:114‐26. [PubMed] [Google Scholar]
54. De Hert M, Correll CU, Bobes J et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52‐77. [PMC free article] [PubMed] [Google Scholar]
55. Correll CU, Solmi M, Veronese N et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163‐80. [PMC free article] [PubMed] [Google Scholar]
56. Stubbs B, Koyanagi A, Veronese N et al. Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low‐ and middle‐income countries.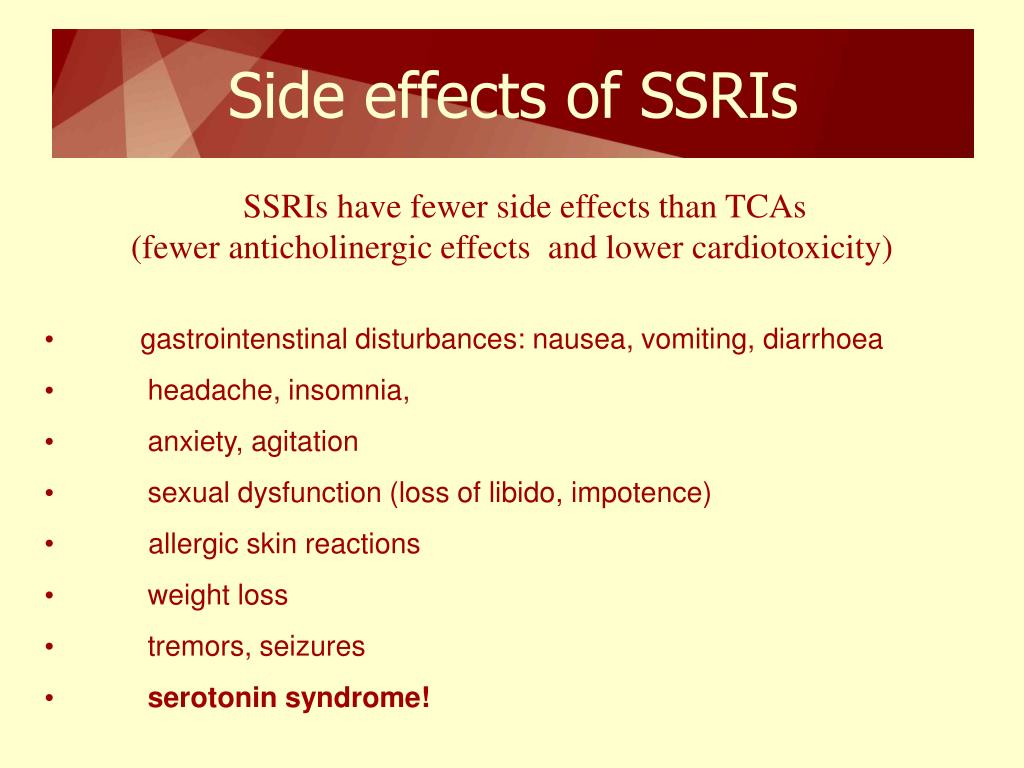 BMC Med 2016;14:189. [PMC free article] [PubMed] [Google Scholar]
BMC Med 2016;14:189. [PMC free article] [PubMed] [Google Scholar]
57. Vancampfort D, Correll CU, Galling B et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta‐analysis. World Psychiatry 2016;15:166‐74. [PMC free article] [PubMed] [Google Scholar]
58. Vancampfort D, Stubbs B, Mitchell AJ et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339‐47. [PMC free article] [PubMed] [Google Scholar]
59. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [PMC free article] [PubMed] [Google Scholar]
60. Vancampfort D, Wampers M, Mitchell AJ et al.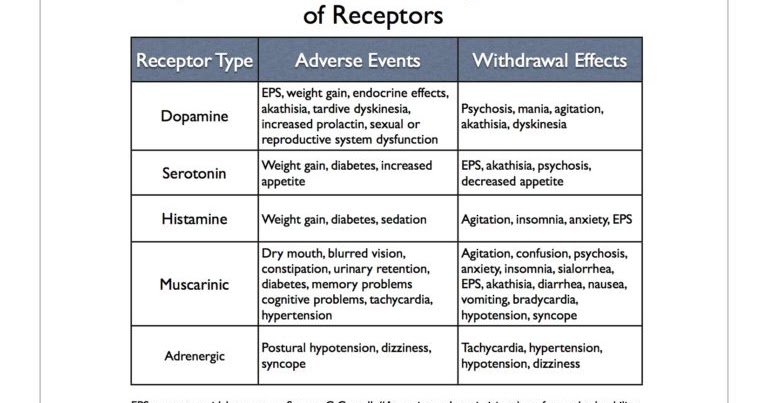 A meta‐analysis of cardio‐metabolic abnormalities in drug naïve, first‐episode and multi‐episode patients with schizophrenia versus general population controls. World Psychiatry 2013;12:240‐50. [PMC free article] [PubMed] [Google Scholar]
A meta‐analysis of cardio‐metabolic abnormalities in drug naïve, first‐episode and multi‐episode patients with schizophrenia versus general population controls. World Psychiatry 2013;12:240‐50. [PMC free article] [PubMed] [Google Scholar]
61. Vancampfort D, Firth J, Schuch FB et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta‐analysis. World Psychiatry 2017;16:308‐15. [PMC free article] [PubMed] [Google Scholar]
62. Leucht S, Burkard T, Henderson J et al. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand 2007;116:317‐33. [PubMed] [Google Scholar]
63. Liu NH, Daumit GL, Dua T et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017;16:30‐40. [PMC free article] [PubMed] [Google Scholar]
64. Hjorthøj C, Stürup AE, McGrath JJ. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta‐analysis. Lancet Psychiatry 2017;4:295‐301. [PubMed] [Google Scholar]
Hjorthøj C, Stürup AE, McGrath JJ. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta‐analysis. Lancet Psychiatry 2017;4:295‐301. [PubMed] [Google Scholar]
65. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123‐31. [PubMed] [Google Scholar]
66. Olfson M, Gerhard T, Huang C et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172‐81. [PubMed] [Google Scholar]
67. Rubio JM, Correll CU. Duration and relevance of untreated psychiatric disorders, 1: Psychotic disorders. J Clin Psychiatry 2017;78:358‐9. [PubMed] [Google Scholar]
68. Fekadu A, Medhin G, Kebede D et al. Excess mortality in severe mental illness: 10‐year population‐based cohort study in rural Ethiopia. Br J Psychiatry 2015;206:289‐96. [PubMed] [Google Scholar]
69. Ran MS, Chan CL, Chen EY et al.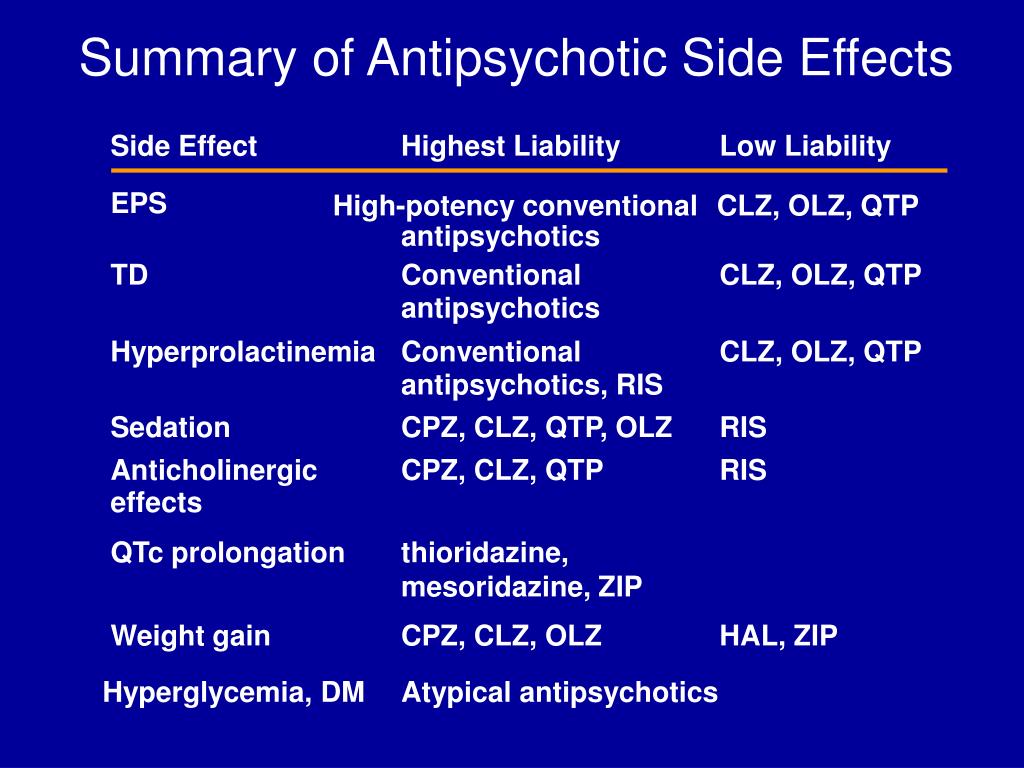 Differences in mortality and suicidal behaviour between treated and never‐treated people with schizophrenia in rural China. Br J Psychiatry 2009;195:126‐31. [PMC free article] [PubMed] [Google Scholar]
Differences in mortality and suicidal behaviour between treated and never‐treated people with schizophrenia in rural China. Br J Psychiatry 2009;195:126‐31. [PMC free article] [PubMed] [Google Scholar]
70. Charlson FJ, Baxter AJ, Dua T et al. Excess mortality from mental, neurological, and substance use disorders in the Global Burden of Disease Study 2010 In: Patel V, Chisholm D, Dua T. et al (eds). Mental, neurological, and substance use disorders: disease control priorities, 3rd ed Washington: International Bank for Reconstruction and Development/World Bank, 2016. [Google Scholar]
71. Ponnudurai R, Jayakar J, Sathiya Sekaran B. Assessment of mortality and marital status of schizophrenic patients over a period of 13 years. Indian J Psychiatry 2006;48:84‐7. [PMC free article] [PubMed] [Google Scholar]
72. Casey DE, Haupt DW, Newcomer JW et al. Antipsychotic‐induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia.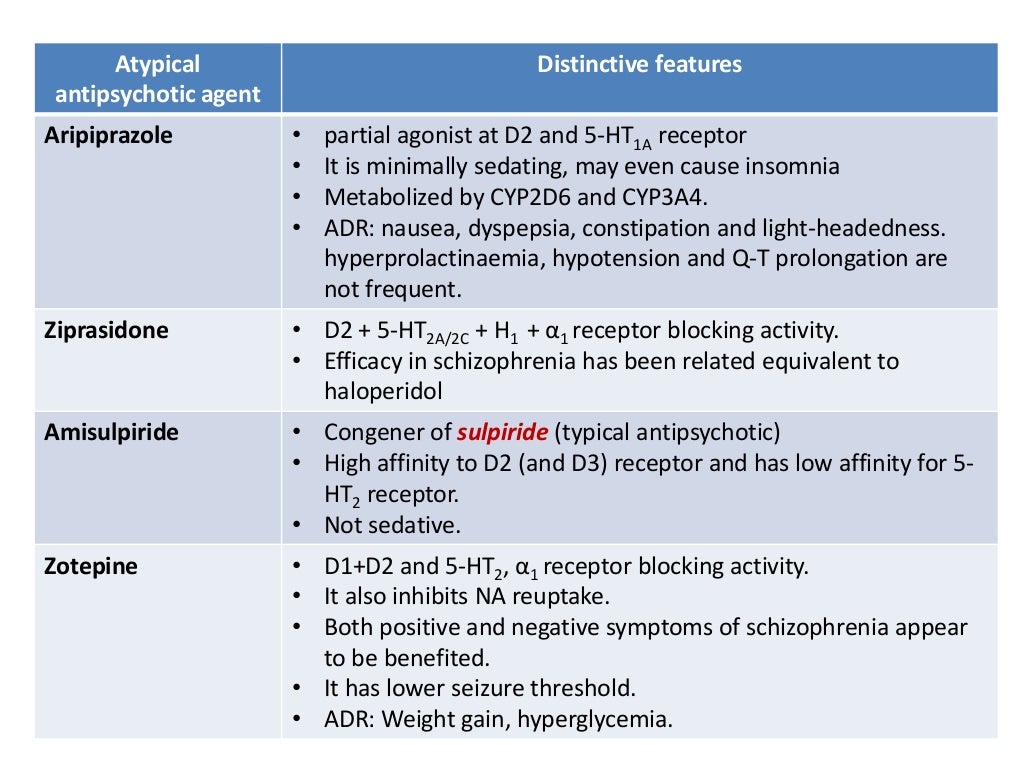 J Clin Psychiatry 2004;65(Suppl. 7):4‐18. [PubMed] [Google Scholar]
J Clin Psychiatry 2004;65(Suppl. 7):4‐18. [PubMed] [Google Scholar]
73. Crump C, Winkleby MA, Sundquist K et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013;170:324‐33. [PubMed] [Google Scholar]
74. Lahti M, Tiihonen J, Wildgust H et al. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med 2012;42:2275‐85. [PubMed] [Google Scholar]
75. Vermeulen J, van Rooijen G, Doedens P et al. Antipsychotic medication and long‐term mortality risk in patients with schizophrenia; a systematic review and meta‐analysis. Psychol Med 2017;47:2217‐28. [PubMed] [Google Scholar]
76. Torniainen M, Mittendorfer‐Rutz E, Tanskanen A et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull 2015;41:656‐63. [PMC free article] [PubMed] [Google Scholar]
77. Tiihonen J, Mittendorfer‐Rutz E, Alexanderson K et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Presented at the 30th Congress of the European College of Neuropsychopharmacology, Paris, September 2017.
Presented at the 30th Congress of the European College of Neuropsychopharmacology, Paris, September 2017.
78. Rubio JM, Correll CU. Reduced all‐cause mortality with antipsychotics and antidepressants compared to increased all‐cause mortality with benzodiazepines in patients with schizophrenia observed in naturalistic treatment settings. Evid Based Ment Health 2017;20:e6. [PubMed] [Google Scholar]
79. Johnstone EC, Crow TJ, Frith CD et al. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976;2:924‐6. [PubMed] [Google Scholar]
80. Bakhshi K, Chance SA. The neuropathology of schizophrenia: a selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience 2015;303:82‐102. [PubMed] [Google Scholar]
81. Honea R, Crow TJ, Passingham D et al. Regional deficits in brain volume in schizophrenia: a meta‐analysis of voxel‐based morphometry studies. Am J Psychiatry 2005;162:2233‐45. [PubMed] [Google Scholar]
82. Crow TJ, Chance SA, Priddle TH et al. Laterality interacts with sex across the schizophrenia/bipolarity continuum: an interpretation of meta‐analyses of structural MRI. Psychiatry Res 2013;210:1232‐44. [PubMed] [Google Scholar]
Crow TJ, Chance SA, Priddle TH et al. Laterality interacts with sex across the schizophrenia/bipolarity continuum: an interpretation of meta‐analyses of structural MRI. Psychiatry Res 2013;210:1232‐44. [PubMed] [Google Scholar]
83. Zhang W, Deng W, Yao L et al. Brain structural abnormalities in a group of never‐medicated patients with long‐term schizophrenia. Am J Psychiatry 2015;172:995‐1003. [PubMed] [Google Scholar]
84. Harrisberger F, Buechler R, Smieskova R et al. Alterations in the hippocampus and thalamus in individuals at high risk for psychosis. NPJ Schizophr 2016;2:16033. [PMC free article] [PubMed] [Google Scholar]
85. McIntosh AM, Owens DC, Moorhead WJ et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry 2011;69:953‐8. [PubMed] [Google Scholar]
86. Takayanagi Y, Kulason S, Sasabayashi D et al. Reduced thickness of the anterior cingulate cortex in individuals with an at‐risk mental state who later develop psychosis. Schizophr Bull 2017;43:907‐13. [PMC free article] [PubMed] [Google Scholar]
Schizophr Bull 2017;43:907‐13. [PMC free article] [PubMed] [Google Scholar]
87. Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry 2002;59:553‐8. [PubMed] [Google Scholar]
88. Mathalon DH, Rapoport JL, Davis KL et al. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry. Arch Gen Psychiatry 2003;60:846‐8. [PubMed] [Google Scholar]
89. Pakkenberg B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry 1993;34:768‐72. [PubMed] [Google Scholar]
90. Selemon LD, Rajkowska G, Goldman‐Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three‐dimensional, stereologic counting method. J Comp Neurol 1998;392:402‐12. [PubMed] [Google Scholar]
91. Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain J Neurol 1999;122(Pt. 4):593‐624. [PubMed] [Google Scholar]
A critical review of the data and their interpretation. Brain J Neurol 1999;122(Pt. 4):593‐624. [PubMed] [Google Scholar]
92. Haijma SV, Van Haren N, Cahn W et al. Brain volumes in schizophrenia: a meta‐analysis in over 18 000 subjects. Schizophr Bull 2013;39:1129‐38. [PMC free article] [PubMed] [Google Scholar]
93. Swayze VW, Andersen A, Arndt S et al. Reversibility of brain tissue loss in anorexia nervosa assessed with a computerized Talairach 3‐D proportional grid. Psychol Med 1996;26:381‐90. [PubMed] [Google Scholar]
94. Pfefferbaum A, Sullivan EV, Mathalon DH et al. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 1995;19:1177‐91. [PubMed] [Google Scholar]
95. Schroth G, Naegele T, Klose U et al. Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology 1988;30:385‐9. [PubMed] [Google Scholar]
96. Gordon N. Apparent cerebral atrophy in patients on treatment with steroids. Dev Med Child Neurol 1980;22:502‐6. [PubMed] [Google Scholar]
Dev Med Child Neurol 1980;22:502‐6. [PubMed] [Google Scholar]
97. Vita A, De Peri L, Deste G et al. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta‐analysis and meta‐regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry 2015;78:403‐12. [PubMed] [Google Scholar]
98. Brugger S, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia. JAMA Psychiatry 2017;74:1104‐11. [PMC free article] [PubMed] [Google Scholar]
99. Fusar‐Poli P, Smieskova R, Kempton MJ et al. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta‐analysis of longitudinal MRI studies. Neurosci Biobehav Rev 2013;37:1680‐91. [PMC free article] [PubMed] [Google Scholar]
100. van Erp TGM, Hibar DP, Rasmussen JM et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016;21:547‐53. [PMC free article] [PubMed] [Google Scholar]
Mol Psychiatry 2016;21:547‐53. [PMC free article] [PubMed] [Google Scholar]
101. Leung M, Cheung C, Yu K et al. Gray matter in first‐episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta‐analyses with sample size weighting. Schizophr Bull 2011;37:199‐211. [PMC free article] [PubMed] [Google Scholar]
102. Boonstra G, van Haren NEM, Schnack HG et al. Brain volume changes after withdrawal of atypical antipsychotics in patients with first‐episode schizophrenia. J Clin Psychopharmacol 2011;31:146‐53. [PubMed] [Google Scholar]
103. Ho BC, Andreasen NC, Ziebell S et al. Long‐term antipsychotic treatment and brain volumes: a longitudinal study of first‐episode schizophrenia. Arch Gen Psychiatry 2011;68:128‐37. [PMC free article] [PubMed] [Google Scholar]
104. Andreasen NC, Liu D, Ziebell S et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study.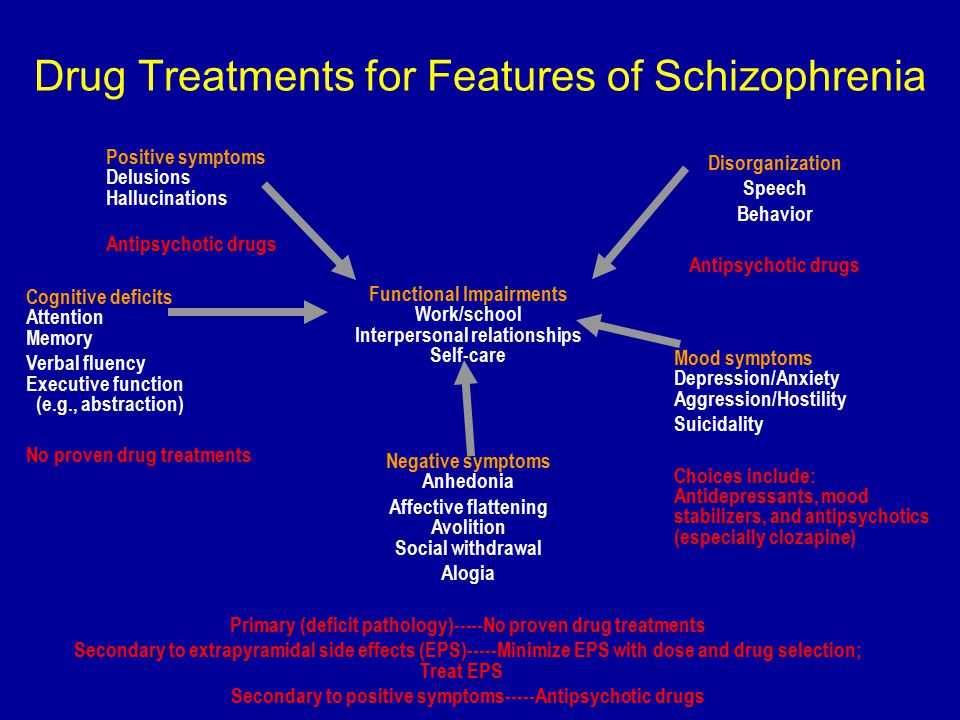 Am J Psychiatry 2013;170:609‐15. [PMC free article] [PubMed] [Google Scholar]
Am J Psychiatry 2013;170:609‐15. [PMC free article] [PubMed] [Google Scholar]
105. Ahmed M, Cannon DM, Scanlon C et al. Progressive brain atrophy and cortical thinning in schizophrenia after commencing clozapine treatment. Neuropsychopharmacology 2015;40:2409‐17. [PMC free article] [PubMed] [Google Scholar]
106. Lieberman J, Chakos M, Wu H et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001;49:487‐99. [PubMed] [Google Scholar]
107. Lesh TA, Tanase C, Geib BR et al. A multimodal analysis of antipsychotic effects on brain structure and function in first‐episode schizophrenia. JAMA Psychiatry 2015;72:226‐34. [PMC free article] [PubMed] [Google Scholar]
108. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science 1977;196:326‐8. [PubMed] [Google Scholar]
109. Silvestri S, Seeman MV, Negrete JC et al. Increased dopamine D2 receptor binding after long‐term treatment with antipsychotics in humans: a clinical PET study.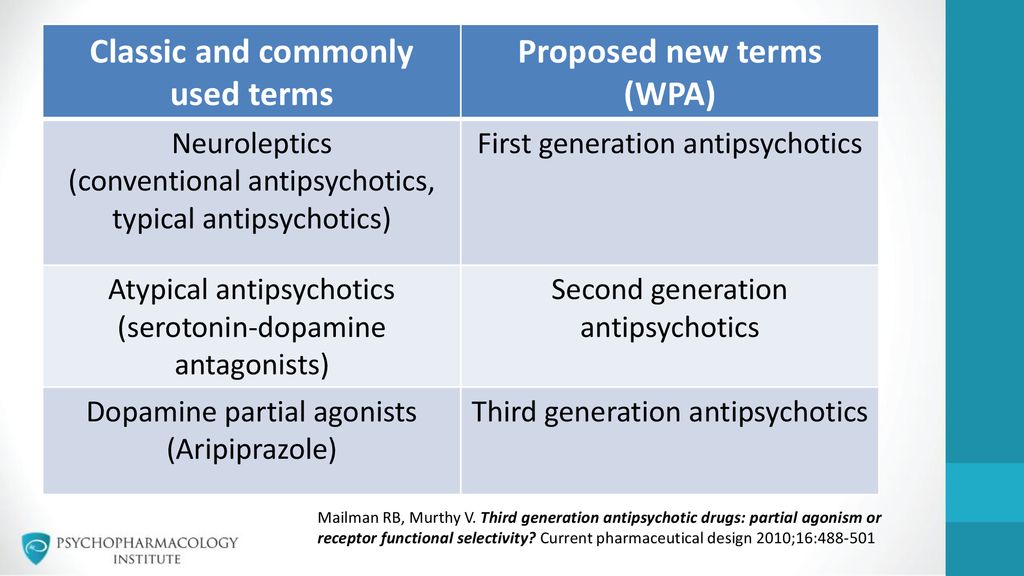 Psychopharmacology 2000;152:174‐80. [PubMed] [Google Scholar]
Psychopharmacology 2000;152:174‐80. [PubMed] [Google Scholar]
110. Klawans HL, Goetz CG, Perlik S. Tardive dyskinesia: review and update. Am J Psychiatry 1980;137:900‐8. [PubMed] [Google Scholar]
111. Lohr JB, Kuczenski R, Niculescu AB. Oxidative mechanisms and tardive dyskinesia. CNS Drugs 2003;17:47‐62. [PubMed] [Google Scholar]
112. Bakker PR, van Harten PN, van Os J. Antipsychotic‐induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: a meta‐analysis of pharmacogenetic interactions. Mol Psychiatry 2008;13:544‐56. [PubMed] [Google Scholar]
113. Kane JM, Woerner M, Weinhold P et al. A prospective study of tardive dyskinesia development: preliminary results. J Clin Psychopharmacol 1982;2:345‐9. [PubMed] [Google Scholar]
114. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second‐generation antipsychotics: a systematic review of 1‐year studies. Am J Psychiatry 2004;161:414‐25.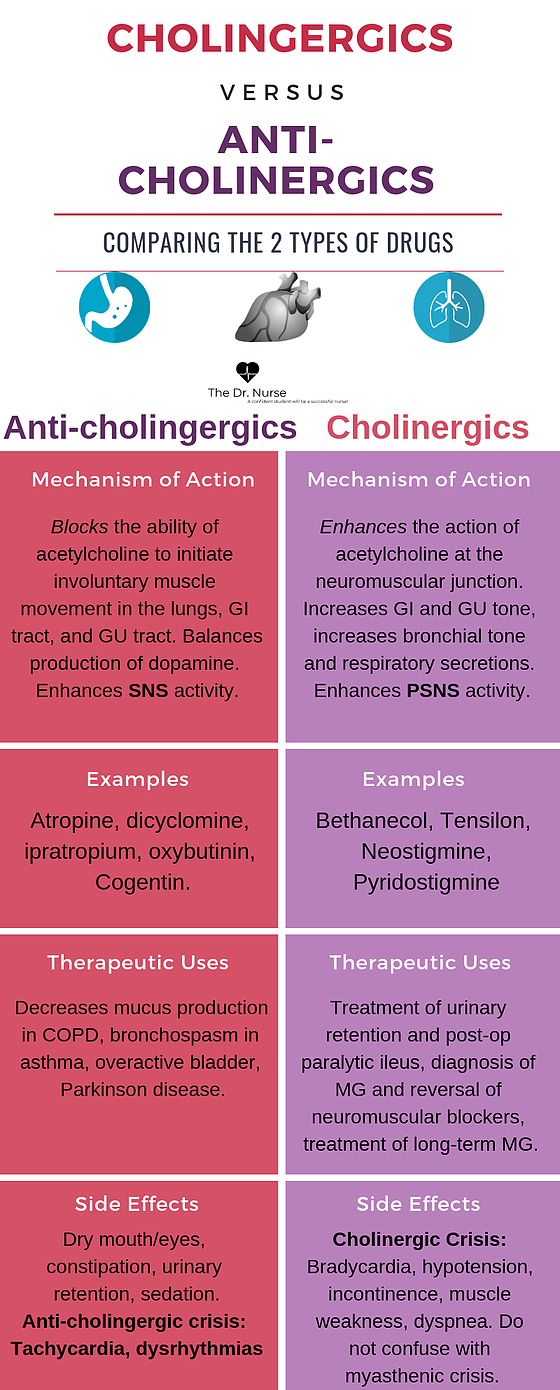 [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
115. Carbon M, Hsieh C‐H, Kane JM et al. Tardive dyskinesia prevalence in the period of second‐generation antipsychotic use: a meta‐analysis. J Clin Psychiatry 2017;78:e264‐78. [PubMed] [Google Scholar]
116. Lieberman JA, Alvir J, Geisler S et al. Methylphenidate response, psychopathology and tardive dyskinesia as predictors of relapse in schizophrenia. Neuropsychopharmacology 1994;11:107‐18. [PubMed] [Google Scholar]
117. Yamanaka H, Kanahara N, Suzuki T et al. Impact of dopamine supersensitivity psychosis in treatment‐resistant schizophrenia: an analysis of multi‐factors predicting long‐term prognosis. Schizophr Res 2016;170:252‐8. [PubMed] [Google Scholar]
118. Youssef HA, Waddington JL. Morbidity and mortality in tardive dyskinesia: associations in chronic schizophrenia. Acta Psychiatr Scand 1987;75:74‐7. [PubMed] [Google Scholar]
119. Apud JA, Egan MF, Wyatt RJ. Neuroleptic withdrawal in treatment‐resistant patients with schizophrenia: tardive dyskinesia is not associated with supersensitive psychosis. Schizophr Res 2003;63:151‐60. [PubMed] [Google Scholar]
Schizophr Res 2003;63:151‐60. [PubMed] [Google Scholar]
120. Kane JM. Tardive dyskinesia circa 2006. Am J Psychiatry 2006;163:1316‐8. [PubMed] [Google Scholar]
121. Kane JM, Correll CU, Liang GS et al. Efficacy of valbenazine (nbi‐98854) in treating subjects with tardive dyskinesia and schizophrenia or schizoaffective disorder. Psychopharmacol Bull 2017;47:69‐76. [PMC free article] [PubMed] [Google Scholar]
122. Fernandez HH, Factor SA, Hauser RA et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM‐TD study. Neurology 2017;88:2003‐10. [PMC free article] [PubMed] [Google Scholar]
123. Kimura H, Kanahara N, Komatsu N et al. A prospective comparative study of risperidone long‐acting injectable for treatment‐resistant schizophrenia with dopamine supersensitivity psychosis. Schizophr Res 2014;155:52‐8. [PubMed] [Google Scholar]
124. Chouinard G, Jones BD. Neuroleptic‐induced supersensitivity psychosis: clinical and pharmacologic characteristics.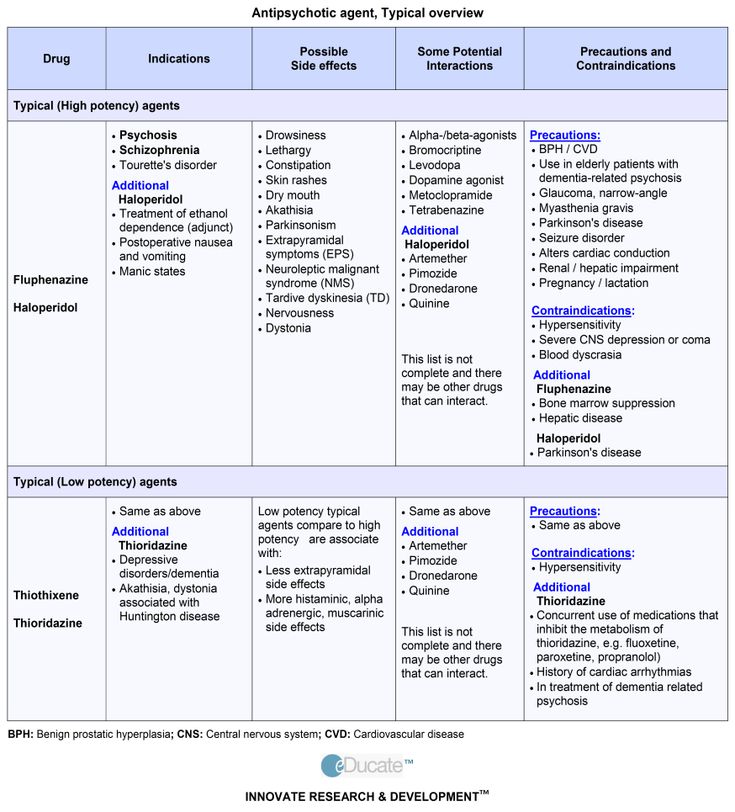 Am J Psychiatry 1980;137:16‐21. [PubMed] [Google Scholar]
Am J Psychiatry 1980;137:16‐21. [PubMed] [Google Scholar]
125. Moncrieff J. Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand 2006;114:3‐13. [PubMed] [Google Scholar]
126. Nakata Y, Kanahara N, Iyo M. Dopamine supersensitivity psychosis in schizophrenia: concepts and implications in clinical practice. J Psychopharmacol 2017;31:1511‐8. [PubMed] [Google Scholar]
127. Yin J, Barr AM, Ramos‐Miguel A et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol 2017;15:174‐83. [PMC free article] [PubMed] [Google Scholar]
128. Koener B, Goursaud S, Van De Stadt M et al. Pharmacological blockade of dopamine D2 receptors by aripiprazole is not associated with striatal sensitization. Naunyn‐Schmiedeberg's Arch Pharmacol 2011;383:65‐77. [PubMed] [Google Scholar]
129. Kane JM, Osuntokun O, Kryzhanovskaya LA et al. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry 2009;70:572‐81. [PubMed] [Google Scholar]
A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry 2009;70:572‐81. [PubMed] [Google Scholar]
130. Stauffer V, Ascher‐Svanum H, Liu L et al. Maintenance of response with atypical antipsychotics in the treatment of schizophrenia: a post‐hoc analysis of 5 double‐blind, randomized clinical trials. BMC Psychiatry 2009;9:13. [PMC free article] [PubMed] [Google Scholar]
131. Jääskeläinen E, Juola P, Hirvonen N et al. A systematic review and meta‐analysis of recovery in schizophrenia. Schizophr Bull 2013;39:1296‐306. [PMC free article] [PubMed] [Google Scholar]
132. Van Eck RM, Burger TJ, Vellinga A et al. The relationship between clinical and personal recovery in patients with schizophrenia spectrum disorders: a systematic review and meta‐analysis. Schizophr Bull (in press). [PMC free article] [PubMed] [Google Scholar]
133. Kane JM, Robinson DG, Schooler NR et al. Comprehensive versus usual community care for first‐episode psychosis: 2‐year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry 2016;173:362‐72. [PMC free article] [PubMed] [Google Scholar]
Am J Psychiatry 2016;173:362‐72. [PMC free article] [PubMed] [Google Scholar]
134. Dixon LB, Dickerson F, Bellack AS et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull 2010;36:48‐70. [PMC free article] [PubMed] [Google Scholar]
135. Wykes T, Steel C, Everitt B et al. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523‐37. [PMC free article] [PubMed] [Google Scholar]
136. Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry 2006;163:365‐73. [PubMed] [Google Scholar]
137. Morrison AP, Turkington D, Pyle M et al. Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: a single‐blind randomised controlled trial. Lancet 2014;383:1395‐403. [PubMed] [Google Scholar]
138. Sensky T, Turkington D, Kingdon D et al. A randomized controlled trial of cognitive‐behavioral therapy for persistent symptoms in schizophrenia resistant to medication. Arch Gen Psychiatry 2000;57:165‐72. [PubMed] [Google Scholar]
Arch Gen Psychiatry 2000;57:165‐72. [PubMed] [Google Scholar]
139. Burns AMN, Erickson DH, Brenner CA. Cognitive‐behavioral therapy for medication‐resistant psychosis: a meta‐analytic review. Psychiatr Serv 2014;65:874‐80. [PubMed] [Google Scholar]
140. Guo X, Zhai J, Liu Z et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early‐stage schizophrenia: a randomized, 1‐year study. Arch Gen Psychiatry 2010;67:895‐904. [PMC free article] [PubMed] [Google Scholar]
141. Pilling S, Bebbington P, Kuipers E et al. Psychological treatments in schizophrenia: I. Meta‐analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002;32:763‐82. [PubMed] [Google Scholar]
142. Schooler NR, Keith SJ, Severe JB et al. Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry 1997;54:453‐63. [PubMed] [Google Scholar]
143. Robinson DG, Schooler NR, Correll CU et al. Psychopharmacological treatment in the RAISE‐ETP study: outcomes of a manual and computer decision support system based intervention. Am J Psychiatry 2018;175:169‐79. [PMC free article] [PubMed] [Google Scholar]
Robinson DG, Schooler NR, Correll CU et al. Psychopharmacological treatment in the RAISE‐ETP study: outcomes of a manual and computer decision support system based intervention. Am J Psychiatry 2018;175:169‐79. [PMC free article] [PubMed] [Google Scholar]
144. Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non‐pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta‐analytic comparison of randomized controlled trials. Schizophr Res 2012;140:159‐68. [PubMed] [Google Scholar]
145. Speyer H, Nørgaard HCB, Birk M et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016;15:155‐65. [PMC free article] [PubMed] [Google Scholar]
146. Jakobsen AS, Speyer H, Nørgaard HCB et al. Effect of lifestyle coaching versus care coordination versus treatment as usual in people with severe mental illness and overweight: two‐years follow‐up of the randomized CHANGE trial. PLoS One 2017;12:e0185881. [PMC free article] [PubMed] [Google Scholar]
Effect of lifestyle coaching versus care coordination versus treatment as usual in people with severe mental illness and overweight: two‐years follow‐up of the randomized CHANGE trial. PLoS One 2017;12:e0185881. [PMC free article] [PubMed] [Google Scholar]
147. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med 2018;48:229‐44. [PubMed] [Google Scholar]
148. van Os J, Guloksuz S. A critique of the “ultra‐high risk” and “transition” paradigm. World Psychiatry 2017;16:200‐6. [PMC free article] [PubMed] [Google Scholar]
149. Nishikawa T, Hayashi T, Koga I et al. Neuroleptic withdrawal with remitted schizophrenics: a naturalistic follow‐up study. Psychiatry 2007;70:68‐79. [PubMed] [Google Scholar]
150. Gitlin M, Nuechterlein K, Subotnik KL et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent‐onset schizophrenia. Am J Psychiatry 2001;158:1835‐42. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
151. Zhang J‐P, Lencz T, Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta‐analysis. Am J Psychiatry 2010;167:763‐72. [PMC free article] [PubMed] [Google Scholar]
152. Sarpal DK, Robinson DG, Lencz T et al. Antipsychotic treatment and functional connectivity of the striatum in first‐episode schizophrenia. JAMA Psychiatry 2015;72:5. [PMC free article] [PubMed] [Google Scholar]
153. Sarpal DK, Argyelan M, Robinson DG et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry 2016;173:69‐77. [PMC free article] [PubMed] [Google Scholar]
154. Hadley JA, Nenert R, Kraguljac NV et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 2014;39:1020‐30. [PMC free article] [PubMed] [Google Scholar]
155. Carrión RE, Cornblatt BA, Burton CZ et al. Personalized prediction of psychosis: external validation of the NAPLS‐2 psychosis risk calculator with the EDIPPP project. Am J Psychiatry 2016;173:989‐96. [PMC free article] [PubMed] [Google Scholar]
Personalized prediction of psychosis: external validation of the NAPLS‐2 psychosis risk calculator with the EDIPPP project. Am J Psychiatry 2016;173:989‐96. [PMC free article] [PubMed] [Google Scholar]
156. Prata D, Mechelli A, Kapur S. Clinically meaningful biomarkers for psychosis: a systematic and quantitative review. Neurosci Biobehav Rev 2014;45:134‐141. [PubMed] [Google Scholar]
Long-Term Effects of Antipsychotics | Psychology Today
Donald Goff, Jeffrey Lieberman, and colleagues recently published a review article entitled “The Long-Term Effects of Antipsychotic Medication on Clinical Course in Schizophrenia” in the American Journal of Psychiatry. In this paper, the authors review the use of antipsychotics for initial treatment of psychosis associated with schizophrenia as well as the effects of long-term use, including clinical outcomes and relapse prevention. The benefits of antipsychotics in the initial treatment of psychotic symptoms are clear. Data also support long-term use of these medications to minimize the occurrence of relapse. The authors note that up to 20% of individuals with schizophrenia may “maintain remission or partial remission for extended periods off medication.” However, it is difficult to identify this subset of individuals who might do well without long-term “maintenance” treatment.
The authors note that up to 20% of individuals with schizophrenia may “maintain remission or partial remission for extended periods off medication.” However, it is difficult to identify this subset of individuals who might do well without long-term “maintenance” treatment.
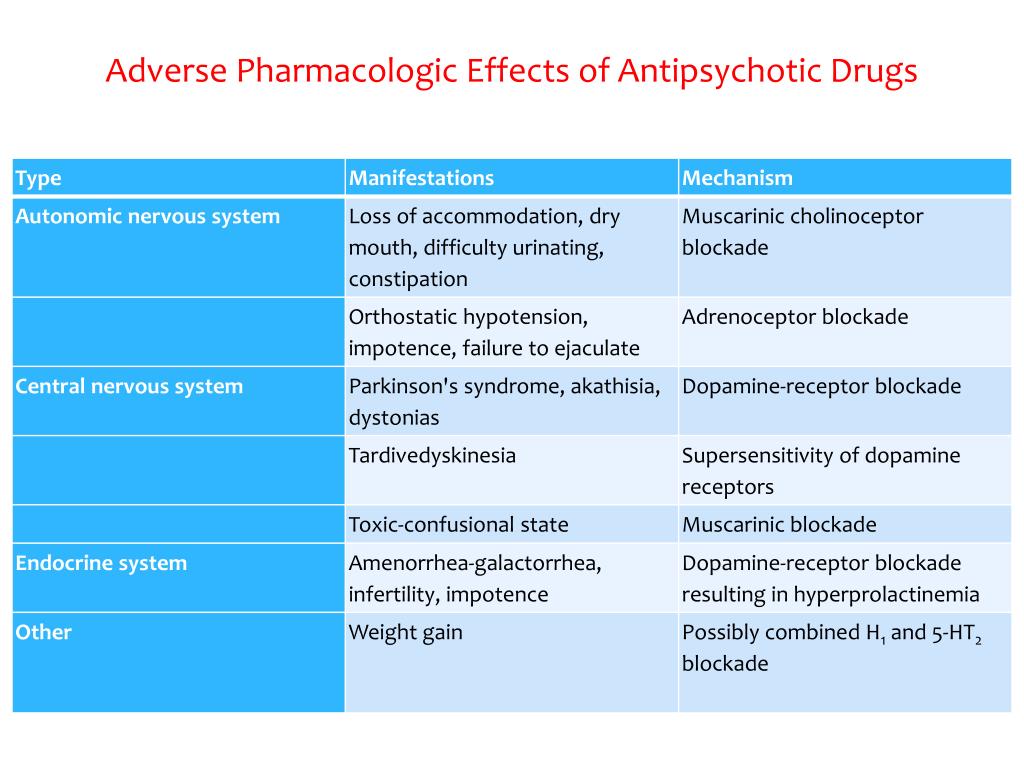
Although antipsychotic medications are effective, some have substantial side effects, including several types of movement disorders, weight gain, and effects on sugar and lipid regulation. They may increase the risk of stroke and are associated with higher rates of death in the elderly.
We agree with the authors that for many patients with schizophrenia, the long-term use of antipsychotics, together with lifestyle changes, can be very helpful. However, what about the long-term use of these medications for symptoms other than the psychotic symptoms associated with schizophrenia? These agents are increasingly being used to treat symptoms associated with a variety of conditions, including bipolar disorder, depression, dementia, borderline personality disorder, and autism. Some health care providers even prescribe these drugs to help with sleep or anxiety.
Some health care providers even prescribe these drugs to help with sleep or anxiety.
For these other indications, there are limited data demonstrating long-term benefit. Antipsychotic medications may help some patients for weeks or months, but longer-term use may lead to unwanted side effects. It is difficult to evaluate the risk-to-benefit ratio when long-term benefit hasn’t been demonstrated.
We teach our psychiatry residents to prescribe medications based on evidence that they work, and there are limited data supporting the long-term use of antipsychotic medications for conditions other than schizophrenia. Nevertheless, it is common to encounter patients who do not have schizophrenia but have been prescribed antipsychotics for long periods of time. Unfortunately, some of these patients are taking two or more such drugs simultaneously. It is often necessary for patients with schizophrenia to continue antipsychotics, but patients with other psychiatric disorders may be able to be weaned off these medications.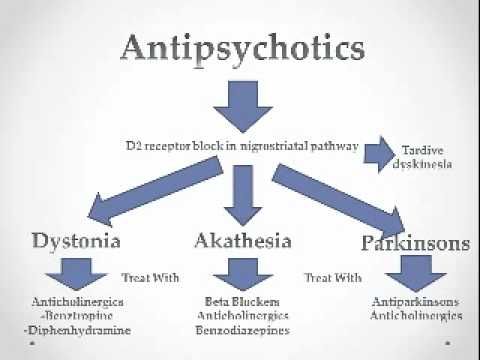
Antipsychotics are often prescribed by non-psychiatrists. It is important for non-psychiatric physicians and other prescribers such as nurse practitioners and physician assistants to review their use of antipsychotics each time they see a patient for whom these drugs are prescribed. Antipsychotics are important and powerful weapons against certain illnesses, but like any powerful treatment they need to be used carefully.
This column was written by Eugene Rubin MD, PhD and Charles Zorumski MD.
References
Goff, D.C., Falkai, P., Fleischhacker, W.W., Girgis, R.R., Kahn, R.M., Uchida, H., Zhao, J., & Lieberman, J.A. (2017). The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 174:840-849.
✚ Stop taking antipsychotics (advice from an old psychiatrist). Neuropsychiatric clinic of Professor Minutko. Article No. 8603
When are neuroleptics prescribed?
Discontinuation of neuroleptic treatment is possible and appropriate in a number of clinical situations. Patients requiring long-term treatment may need to switch from one antipsychotic to another antipsychotic if their response to treatment has been inadequate or side effects have occurred. Some psychiatrists believe that some antipsychotics are more effective than others for psychosis - Clozapine is recognized as the most effective antipsychotic, followed by a moderately effective group consisting of Amisulpride, Olanzapine, Risperidone and Paliperidone, and then the remaining new and old antipsychotics, such as Haloperidol and Chlorpromazine. on the contrary, other psychiatrists consider haloperidol to be one of the best antipsychotics. A number of psychiatrists believe that antipsychotics can be used in severe major depression, when rapid relief of agitation, insomnia, and the prevention of the risk of suicide are needed while waiting for the action of antidepressants. nine0005
Patients requiring long-term treatment may need to switch from one antipsychotic to another antipsychotic if their response to treatment has been inadequate or side effects have occurred. Some psychiatrists believe that some antipsychotics are more effective than others for psychosis - Clozapine is recognized as the most effective antipsychotic, followed by a moderately effective group consisting of Amisulpride, Olanzapine, Risperidone and Paliperidone, and then the remaining new and old antipsychotics, such as Haloperidol and Chlorpromazine. on the contrary, other psychiatrists consider haloperidol to be one of the best antipsychotics. A number of psychiatrists believe that antipsychotics can be used in severe major depression, when rapid relief of agitation, insomnia, and the prevention of the risk of suicide are needed while waiting for the action of antidepressants. nine0005
Side effects of neuroleptics
Long-term use of antipsychotics can have serious consequences, including tardive dyskinesia, weight gain, metabolic syndrome, diabetes, and cardiovascular complications.
Discontinuation of antipsychotics
When discontinuing an antipsychotic, individual circumstances must be carefully considered, including disease severity and medical history, risk of relapse and its consequences, response to treatment and prognostic factors, and the social situation of the patient. Antipsychotics should be stopped very slowly under close medical supervision. Abrupt discontinuation of treatment may lead to psychosis, which may be more severe than before treatment with antipsychotics. It is not uncommon for Clozapine to be discontinued as a result of complications such as agranulocytosis or myocarditis. Depending on the pharmacological action of the antipsychotic, several withdrawal syndromes may occur. nine0005
When evaluating the benefits and harms of taking an antipsychotic, careful consideration should be given to disease severity, treatment complications (eg, obesity), previous pattern of relapse, risk to self and others, and the psychosocial consequences of relapse. Repeated episodes of psychosis worsen the long-term prognosis. Because the risk of recurrence after a second episode is high, most physicians recommend long-term treatment.
Repeated episodes of psychosis worsen the long-term prognosis. Because the risk of recurrence after a second episode is high, most physicians recommend long-term treatment.
When can antipsychotics be safely discontinued?
Antipsychotics can be safely discontinued if antipsychotics (eg, low-dose quetiapine) have been used for anxiety or sleep disturbance and ongoing treatment is not required or undesirable, antipsychotics have been used to treat behavioral disturbances in dementia but are no longer needed because of -for the correct correction of behavioral behavior or the positive impact of the patient's environment. In addition, neuroleptics should be discontinued if antipsychotics have been tested for indications not covered by the instructions and have not been effective. nine0005
Withdrawal of antipsychotics after severe depression
Patients who have experienced psychotic depression and have responded to a combination of antidepressants and antipsychotics with or without electroconvulsive therapy can often continue to be treated with antidepressants alone. There are no clear recommendations as to when antipsychotics can be discontinued in these patients. However, after the patient has recovered for some time, it is often possible to gradually reduce the dose while continuing to monitor the patient's mental state (especially if serious risk factors such as suicidality were initially present). nine0005
There are no clear recommendations as to when antipsychotics can be discontinued in these patients. However, after the patient has recovered for some time, it is often possible to gradually reduce the dose while continuing to monitor the patient's mental state (especially if serious risk factors such as suicidality were initially present). nine0005
Discontinuation of antipsychotics under medical supervision
Under the supervision of a psychiatrist, antipsychotics are discontinued in the following cases: after the first episode of psychosis, there was a qualitative remission within a year (up to 40% of patients who survived one episode of psychosis can remain healthy after stopping antipsychotic drugs or at least need only low doses), in bipolar mood disorder when antipsychotics are no longer needed, especially if lithium monotherapy is appropriate. If there have been two episodes of psychosis, then an antipsychotic is required for at least two years. Antipsychotics can also be discontinued under medical supervision if there has been a complete recovery from drug-induced psychosis and clinical evaluation indicates that antipsychotic treatment is no longer needed (eg, drug use has been stopped).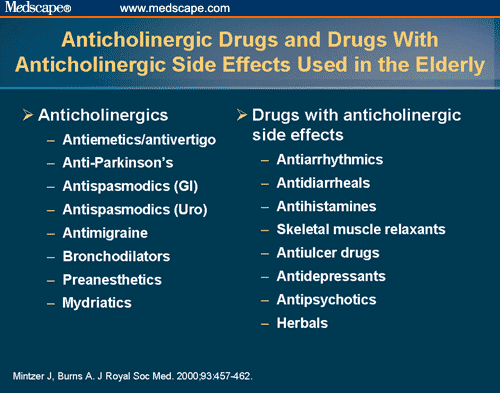 Many psychiatrists believe that if there have been several episodes of psychosis or recovery was incomplete, then permanent treatment with antipsychotics is recommended, since when the drug is stopped, the psychosis is more likely to worsen or recur. nine0005
Many psychiatrists believe that if there have been several episodes of psychosis or recovery was incomplete, then permanent treatment with antipsychotics is recommended, since when the drug is stopped, the psychosis is more likely to worsen or recur. nine0005
Withdrawal of long-acting antipsychotics
Some antipsychotics (especially depot injections) have a long half-life and are unlikely to be associated with significant withdrawal symptoms.
Switching antipsychotic
There are a number of clinical situations in which switching from one antipsychotic to another is considered. Before switching, a psychiatric examination is recommended, especially in difficult clinical situations or when an urgent switch is needed. Changing antipsychotics is recommended when there is an inadequate clinical response to acute symptoms despite dose optimization and adequate duration of treatment. Changing the neuroleptic is possible if there is poor control of chronic symptoms of psychosis and functional impairment persists during maintenance therapy, if there has been a relapse despite adequate preventive or maintenance treatment of psychotic illness. Persistence of certain symptoms of psychotic illness (eg, negative symptoms and cognitive dysfunction) despite adequate doses of a single antipsychotic that may respond better to an alternative neuroleptic is also an indication for a change of medication. Unacceptable side effects at low therapeutic doses before a clinical response in susceptible individuals (eg, extrapyramidal effects in Asian patients) also require consideration of switching to antipsychotics with a lower risk of side effects. The need to change the antipsychotic drug due to a physical complication (eg, ziprasidone is contraindicated in cardiovascular disease, antipsychotics that cause severe hyperprolactinemia are contraindicated in breast cancer) are known reasons for switching antipsychotics. Poor adherence to treatment is an indication for switching from an oral antipsychotic to a long-acting injectable depot. nine0005
Persistence of certain symptoms of psychotic illness (eg, negative symptoms and cognitive dysfunction) despite adequate doses of a single antipsychotic that may respond better to an alternative neuroleptic is also an indication for a change of medication. Unacceptable side effects at low therapeutic doses before a clinical response in susceptible individuals (eg, extrapyramidal effects in Asian patients) also require consideration of switching to antipsychotics with a lower risk of side effects. The need to change the antipsychotic drug due to a physical complication (eg, ziprasidone is contraindicated in cardiovascular disease, antipsychotics that cause severe hyperprolactinemia are contraindicated in breast cancer) are known reasons for switching antipsychotics. Poor adherence to treatment is an indication for switching from an oral antipsychotic to a long-acting injectable depot. nine0005
Switching from one antipsychotic to another is not necessarily a panacea. During the switch, an exacerbation of the disease may occur and new side effects may appear. When a switch is made due to an inadequate response, it is important to ensure that the dose of the first antipsychotic has been optimized, that the patient has been on treatment for an adequate period of time, and that the patient is adhering to the regimen and tolerating the course of treatment.
When a switch is made due to an inadequate response, it is important to ensure that the dose of the first antipsychotic has been optimized, that the patient has been on treatment for an adequate period of time, and that the patient is adhering to the regimen and tolerating the course of treatment.
These factors should be taken into account when changing antipsychotics
The choice of a new drug will be determined in part by the reasons for the switch, but will also need to take into account the likely efficacy, side effects, dosing regimen, and patient or caregiver preference.
Withdrawal syndrome
Depending on the pharmacology of the antipsychotic, switching from one antipsychotic to another can lead to withdrawal syndromes, especially withdrawal of anticholinergics with drugs such as Quetiapine, Clozapine, Chlopromazine and Olanzapine. Switching from one antipsychotic to another (for example, when looking for a drug with a lower risk of weight gain) can lead to loss of efficacy and withdrawal symptoms. nine0005
nine0005
Clozapine withdrawal
When a patient is switched from clozapine to another antipsychotic drug, relapsing psychosis and other serious withdrawal effects may occur, regardless of which clozapine drug is switched to. Clozapine should be discontinued under the supervision of a psychiatrist. The dose should be gradually reduced, and not stopped abruptly. However, sometimes this may be unavoidable if, for example, we observe agranulocytosis.
How to stop an antipsychotic
Unlike switching antidepressants, a drug-free period between stopping the first antipsychotic and starting the second is not recommended due to the risk of relapse. When switching from an oral antipsychotic to a depot, a depot to a depot, or a depot to an oral antipsychotic, special instructions must be followed.
Direct switching
Although it is possible to stop the first drug and immediately start the second the next day, this can lead to withdrawal symptoms and possible drug interactions. When the first antipsychotic is aripiprazole or brexpiprazole, one can immediately switch to them, since both of these drugs have a very long half-life and do not have anticholinergic effects. nine0005
When the first antipsychotic is aripiprazole or brexpiprazole, one can immediately switch to them, since both of these drugs have a very long half-life and do not have anticholinergic effects. nine0005
Cross-titration
Available data in the literature indicate little difference in the risk of relapse with immediate or gradual discontinuation or switching of antipsychotics. Most psychiatrists use a cross-titration strategy. (reducing the dose of the first neuroleptic with the introduction of the second). A slower approach to titration is to continue the first antipsychotic for a period at its usual dose while gradually increasing the therapeutic dose of the second antipsychotic. Then you can gradually reduce and stop taking the first antipsychotic. With this approach, the risk of relapse is minimized, but additional side effects may occur. nine0005
When it is necessary to switch from one antipsychotic to another during the treatment of psychoses, clinicians need to have some understanding of the pharmacokinetics and dynamics of antipsychotics in order to plan and carefully manage regime change. This usually involves taking both drugs at the same time. Discontinuation and switching of neuroleptics can lead to serious consequences, especially relapse of psychosis, which can carry serious risks and worsen long-term prognosis. Withdrawal syndromes associated with cholinergic and dopaminergic effects may occur depending on the characteristics of the antipsychotics being taken. nine0005
This usually involves taking both drugs at the same time. Discontinuation and switching of neuroleptics can lead to serious consequences, especially relapse of psychosis, which can carry serious risks and worsen long-term prognosis. Withdrawal syndromes associated with cholinergic and dopaminergic effects may occur depending on the characteristics of the antipsychotics being taken. nine0005
Blog Post Category:
Biological Psychiatry
You must enable JavaScript to use this form.
Your name *
Your phone (privacy guaranteed) *
Main complaint *
Presumptive diagnosis
Taking medication
Consent to the processing of personal data *
I agree to the processing of personal data, I have read the privacy policy
Long-term side effects of levodopa - Center for Extrapyramidal and Cognitive Disorders
For about 50 years, levodopa (L-DOPA, the levorotatory isomer of the amino acid deoxyphenylalanine) has been the most effective treatment for Parkinson's disease. In terms of effectiveness, it is ahead of any other anti-Parkinsonian drug and even neurosurgical intervention. It provides the most guaranteed anti-Parkinsonian effect, causing improvement in almost 100% of patients with Parkinson's disease. Not a single patient with Parkinson's disease who seeks to maximize the period of his active life can avoid its intake. Levodopa is considered the "gold standard" for the treatment of Parkinson's disease. It increases the life expectancy of patients. To date, there are no convincing data on the neurotoxic effect of levodopa. Moreover, the ability of small doses of levodopa to exert a neuroprotective effect cannot be ruled out. nine0005
In terms of effectiveness, it is ahead of any other anti-Parkinsonian drug and even neurosurgical intervention. It provides the most guaranteed anti-Parkinsonian effect, causing improvement in almost 100% of patients with Parkinson's disease. Not a single patient with Parkinson's disease who seeks to maximize the period of his active life can avoid its intake. Levodopa is considered the "gold standard" for the treatment of Parkinson's disease. It increases the life expectancy of patients. To date, there are no convincing data on the neurotoxic effect of levodopa. Moreover, the ability of small doses of levodopa to exert a neuroprotective effect cannot be ruled out. nine0005
After oral administration, levodopa enters the brain and is converted by functioning nerve cells into dopamine, compensating for its deficiency. Modern drugs (such as Nakom or Madopar) contain a combination of levodopa with an inhibitor of the enzyme DOPA-decarboxylase, which blocks the metabolism of levodopa in peripheral tissues, resulting in a large part of levodopa reaching the brain and reducing the likelihood of side effects associated with the peripheral action of the drug. In the early stages of Parkinson's disease, even small doses of levodopa often have a significant effect, almost completely eliminating the symptoms of parkinsonism. Unfortunately, a few years after the start of treatment with levodopa in a significant proportion of patients, the reaction to the drug changes: the duration of the action of a single dose decreases, violent movements (dyskinesias) appear. nine0005
In the early stages of Parkinson's disease, even small doses of levodopa often have a significant effect, almost completely eliminating the symptoms of parkinsonism. Unfortunately, a few years after the start of treatment with levodopa in a significant proportion of patients, the reaction to the drug changes: the duration of the action of a single dose decreases, violent movements (dyskinesias) appear. nine0005
The patient takes the prescribed single dose of levodopa, it begins to act after 40 minutes - the patient begins the "on" period, characterized by a decrease in the symptoms of parkinsonism, and after 3-4 hours, and subsequently even faster - after 2- 2.5 hours - the effect of the drug weakens - a period of "off" begins with a decrease in motor activity. Sometimes "on" and "off" are accompanied by involuntary (violent) movements of a different nature. During the day there are also stiffenings when walking, when for a few seconds or minutes the patient cannot move. For patients with this disorder, turning or passing through a relatively narrow doorway may be particularly difficult. Fluctuations in motor activity, which are possible in a wide range - from excessive motor activity at the peak of the dose to a sharp weakening of motor capabilities during the "off" period, are called motor fluctuations in the medical literature. nine0005
For patients with this disorder, turning or passing through a relatively narrow doorway may be particularly difficult. Fluctuations in motor activity, which are possible in a wide range - from excessive motor activity at the peak of the dose to a sharp weakening of motor capabilities during the "off" period, are called motor fluctuations in the medical literature. nine0005
There are several types of motor fluctuations. The phenomenon of "exhaustion" of the end of the dose is characterized by a gradual predictable partial return of the symptoms of parkinsonism by the end of the next dose of levodopa. Many patients, as the effect of a single dose of levodopa wears off, also note depression of mood, anxiety, sweating, palpitations, increased pain, etc. ("non-motor fluctuations").
Over time, the patient's transition from the "on" state to the "off" state becomes more and more brief and abrupt, which is referred to as the "on-off" phenomenon. nine0005
Sometimes the "off" period comes on suddenly, regardless of when the drug is taken. In addition to the above fluctuations, delayed development of “on” or complete absence of “on” is possible, when taking the next dose is not accompanied by an improvement in clinical symptoms or does not occur quickly enough.
In addition to the above fluctuations, delayed development of “on” or complete absence of “on” is possible, when taking the next dose is not accompanied by an improvement in clinical symptoms or does not occur quickly enough.
Against the background of "on" (at the peak dose of levodopa), rapid "dancing" (choreiform) movements may occur, mainly involving the upper half of the body ("peak dose dyskinesia"). This is a kind of limiter, requiring the weakening of dopaminergic therapy, since increasing the dose will lead to an increase in dyskinesias. This phenomenon is based on hypersensitivity of dopamine receptors. nine0005
Off-period dystonia is a violent, prolonged, often painful contraction of the muscles of the lower extremities, often with plantar flexion or tucking of the foot. It usually appears at night or in the morning before the next dose of the drug and decreases with longer dopaminergic stimulation, achieved by prescribing a sustained-release levodopa drug (Madopar GSS), a long-acting dopamine receptor agonist, or a combination of levodopa with a DOPA decarboxylase inhibitor and a catechol inhibitor. -Omethyltransferase entacapone (Stalevo). nine0005
-Omethyltransferase entacapone (Stalevo). nine0005
y bSince the risk of fluctuations and dyskinesias depends on the total dose of levodopa preparations, it is customary to prescribe them only with a real decrease in the patient's functional capabilities, which interferes with his professional or daily household activity.
In younger patients (up to 60 years of age), fluctuations in the effect of levodopa develop faster, so they try to delay the time of prescribing levodopa in this age group by starting treatment with other antiparkinsonian drugs: dopamine receptor agonists (ropinirole, pramipexole, piribedil, rotigotine) , monoamine oxidase inhibitors - rasagiline (Azilect) or selegiline (Yumex). Additionally, to enhance the effect, amantadine and an anticholinergic (especially with rest tremor) or a combination of all of these drugs can be prescribed. And only when they no longer give the desired effect and do not provide sufficient patient mobility, small doses of levodopa are added to them. However, some experts believe that excessive delay in the appointment of levodopa is not the best tactic in the management of patients, as it is often accompanied by insufficient control of symptoms. Meanwhile, adequate correction of motor functions is the main guarantee of the best long-term results of treatment. nine0005
However, some experts believe that excessive delay in the appointment of levodopa is not the best tactic in the management of patients, as it is often accompanied by insufficient control of symptoms. Meanwhile, adequate correction of motor functions is the main guarantee of the best long-term results of treatment. nine0005
In the elderly, who are at lower risk of fluctuations and dyskinesias, it is common to start levodopa immediately. In this case, the minimum effective dose should be adhered to, which can be achieved by adding dopamine receptor agonists, type B monoamine oxidase inhibitors or amantadine.
"Off" periods and dyskinesias can be excruciating for patients, and they may be tempted to relieve their condition by taking an emergency dose of levodopa or another antiparkinsonian drug. Often, in a vicious circle, this leads to an aggravation of the instability of the patient's condition. Because of this, any changes in the treatment regimen, especially with fluctuations, should be agreed with the attending physician. Correction of fluctuations and dyskinesias is a difficult task even for an experienced specialist, and it can be solved only with close cooperation between the doctor and the patient. nine0005
Correction of fluctuations and dyskinesias is a difficult task even for an experienced specialist, and it can be solved only with close cooperation between the doctor and the patient. nine0005
The goal of adjusting the treatment regimen is to maximize the duration of the "on" period in the absence or minimal presentation of dyskinesias. Filling in a daily activity diary by the patient at 1-hour intervals can help the clinician analyze the relationship between the “off” period and dyskinesias, as well as their occurrence relative to the timing of medications.
When the duration of action of levodopa decreases (the “end-of-dose exhaustion” phenomenon), most specialists first resort to splitting the dose of levodopa (reducing the single dose with a shortening of the interval between doses of the drug) and switching from an immediate-release drug to a sustained-release drug (Madopar GSS) . In addition, the effect of levodopa can be enhanced by improving its absorption. To do this, the drug is taken at least 60 minutes before a meal and not earlier than 2 hours after a meal. In addition, they reduce protein intake during the day (amino acids formed during the breakdown of food proteins compete in the intestines with levodopa for absorption). If this does not work, one of the dopamine receptor agonists (preferably controlled-release), the catechol-O-methyltransferase inhibitor entacapone (switching to Stalevo), or a monoamine oxidase inhibitor type B must be added sequentially.
In addition, they reduce protein intake during the day (amino acids formed during the breakdown of food proteins compete in the intestines with levodopa for absorption). If this does not work, one of the dopamine receptor agonists (preferably controlled-release), the catechol-O-methyltransferase inhibitor entacapone (switching to Stalevo), or a monoamine oxidase inhibitor type B must be added sequentially.
The sequence of prescribing drugs is determined individually, including taking into account the risk of side effects in each individual patient.
Freezes that develop during the “off” period respond to techniques that reduce the “off” phase. It is useful to teach the patient how to overcome freezing by walking in place, unusual dance movements, stepping over an imaginary line drawn on the patient's path, or using a special cane with a thin metal bar that folds down at the bottom, stepping over which the patient can start moving. nine0005
Other recommendations for freezing:
• stop trying to continue driving;
• try to shift from one foot to the other;
• slightly bend your knees, lift your foot off the floor and step forward;
• count "one, two, three" or tell yourself "left-right, left-right";
• hum a rhythmic melody;
• imagine the sound of footsteps on the pavement and raise your leg to take a step;
• try to imitate the walking of the person in front; nine0171 • if you have a problem getting through a narrow space, try to look beyond it: imagine where you will be when you pass this space.
In cases where the transition from "on" to "off" becomes unpredictable, it is possible to return to less frequent use of relatively large doses of levodopa ("dose piling"). This will lead, on the one hand, to an increase in the period of "on" in the daytime period, when the patient needs to be active; on the other hand, the “off” period will also increase, but will move to the evening and become more predictable. Treatment of on-off fluctuations becomes more difficult when the on-period is accompanied by dyskinesias. In these cases, there may not be an unambiguously satisfactory solution, and the patient himself must decide whether to put up with hyperkinesis during the "on" or suffer from a long period of "off". Usually patients choose the first. nine0005
In cases where drugs fail to achieve sufficient effect, resort to stereotaxic operations (constant stimulation of certain areas in the basal ganglia) (see below). An alternative may be subcutaneous administration of apomorphine, the introduction of a gel with levodopa / carbidopa (duodopa) into the duodenum.
But sometimes it is not possible to completely get rid of motor fluctuations. Then it remains to adapt to them: you do all the things you need during the hours when you feel better, spend the rest of the hours doing activities that do not require movement: reading, watching TV, listening to the radio. nine0005
In domestic clinical practice, patients with Parkinson's disease are often treated with drugs whose effectiveness in this disease has not been convincingly proven. Often these are the so-called "vascular drugs", often administered intravenously by drip. Their appointment is often justified by naive and archaic ideas about the connection of Parkinson's disease with "bad vessels of the brain", which in most patients, even the elderly, do not suffer significantly. The positive experience of their use, if it exists, is explained by nothing more than the placebo effect. nine0005
The placebo effect is achieved by the patient's faith in the doctor, by misrepresented or inaccurately perceived information, by the power of tradition, and sometimes by the patient's desperation in the methods of classical medicine. Parkinson's specialists are well aware that a significant proportion of patients respond well to placebo, but this effect is never sustainable. Most importantly, ineffective drugs and other treatments do not prevent adequate treatment of a disease that, if delayed, can have adverse long-term consequences. nine0005
Parkinson's specialists are well aware that a significant proportion of patients respond well to placebo, but this effect is never sustainable. Most importantly, ineffective drugs and other treatments do not prevent adequate treatment of a disease that, if delayed, can have adverse long-term consequences. nine0005
In general, patients with Parkinson's disease should be cautious when using any medication, some of which carry the risk of worsening their condition. First of all, this applies to neuroleptics, such as haloperidol, triftazin, etaperazine, clopixol, olanzapine (Zyprexa), risperidone (Rispolept). Even such relatively mild drugs as sulpiride (Eglonil), thioridazine (Sonapax), teraligen, when taken regularly, can cause significant deterioration.
The same can be said about a number of drugs that are widely used in neurological and therapeutic practice, but can block dopamine receptors and increase the symptoms of parkinsonism: cinnarizine (including combined preparations of cinnarizine and piracetam, for example, Omaron or Phezam), metoclopramide (Cerucal), pipolfene . Iron preparations prescribed for iron deficiency anemia can reduce the effectiveness of levodopa and often require an increase in its dose.
Iron preparations prescribed for iron deficiency anemia can reduce the effectiveness of levodopa and often require an increase in its dose.
Be careful with drugs that cause sedation, including sleeping pills, especially if they are long-acting (eg phenazepam). When using them, inform your doctor about the severity of the sedative effect, the possible increase in symptoms during the day. On the other hand, such "nootropic" drugs as piracetam or fenotropil can cause arousal in some patients. nine0005
Finally, it should be borne in mind that during surgical interventions for concomitant diseases, inhalation anesthetics are often used for anesthesia - drugs with a strong sedative effect that can cause an artificial coma, which is necessary for surgical intervention. In older people, they can cause confusion. Some surgeries can be performed under the influence of less dangerous drugs, in some conditions you can do without surgery. Ask your doctor about alternative treatments before opting for surgery. nine0005
nine0005
The list of drugs that can cause deterioration in patients with Parkinson's disease is very extensive, so it makes sense to consult a neurologist before starting a new drug on a regular basis, even if it is prescribed for a different disease.
Rapid progress in the study of the pathogenesis of Parkinson's disease, as well as the development of the latest medical technologies (in particular, the technique of planting stem cells that can take the place of dying cells, or genetic therapy) allow us to hope that in the next decade a way will be found to significantly slow down the progression of this disease . nine0005
To get the most out of your doctor's visit, try these simple tips:
• Most doctors welcome information about your condition. This will help you to participate in making decisions about therapy, and you will be able to articulate your complaints more precisely.
• Make a list of questions you have with your doctor ahead of time.
• Do not put off seeing your doctor for too long, especially if you feel your treatment is worsening or getting worse.