Lamictal for migraines
Lamotrigine in the Prevention of Migraine With Aura
Lamotrigine in the Prevention of Migraine With Aura | PracticeUpdate center of excellencealarmbookmarkcalendarcheck-clipboardclockwisecompass-navigatecompasscounterclockwisedeletedownload-bookeducation-historyemailget-helpgizmo-flaghelp-iconjournalleftlinklocklog-inlog-outnavigate-downnavigate-leftnavigate-rightnavigate-upnotifications-disabledopen-bookpersonplay-videoplayrefreshrightrightwardsearchsettingsshare-2starthumbs-upsharecheck-box3linesprinter-2insert-boxeshelp-iconlocationpluspaperclipdownloadclockwisecheck-circleexclamationPlease provide your AHPRA Number to ensure that you are given the correct level of access to our site.
Further Reading
-
featured
Safety of IV Thrombolysis in Patients With Ischemic Stroke Aged ≥90 Years
Stroke · October 20, 2022
-
story of the week
Efficacy and Safety of Thrombectomy After Stroke Due to Basilar Artery Occlusion
N.
Engl. J. Med · October 20, 2022
-
story of the week
Surgical Hematoma Evacuation of Cortical Intracerebral Hemorrhage ≥10 mL Significantly Reduces the Risk of Subsequent Epilepsy
Eur. J. Neurol. · October 20, 2022
-
featured
Differentiating MS From AQP4-Positive NMOSD and MOGAD With Imaging
Neurology · October 19, 2022
-
story of the week
Pachymeningitis in Biopsy-Proven Sarcoidosis: Clinical Course, Radiographic Findings, Response to Treatment, and Long-Term Outcomes
Neurol Neuroimmunol Neuroinflamm · October 18, 2022
updated
-
featured
International Consensus Recommendations for the Management of New-Onset Refractory Status Epilepticus/Febrile Infection–Related Epilepsy Syndrome
Epilepsia · October 18, 2022
-
featured
Detection of Atrial Fibrillation in a Large Population Using Wearable Devices
Circulation · October 16, 2022
-
featured
Association of Antidepressant Use During Pregnancy With the Risk of Neurodevelopmental Disorders in Children
JAMA Intern Med · October 13, 2022
-
featured
Frequency of New or Enlarging Lesions on MRI Outside of Clinical Attacks in Patients With MOG Antibody–Associated Disease
Neurology · October 13, 2022
-
featured
MOG and AQP4 Antibodies in Children With MS
Ann.
 Neurol
·
October 12, 2022
Neurol
·
October 12, 2022
updated
-
featured
Effect of Dimethyl Fumarate vs Interferon β-1a in Patients With Pediatric-Onset MS
JAMA Netw Open · October 12, 2022
- Efficacy and Safety of Thrombectomy After Stroke Due to Basilar Artery Occlusion
- Safety of IV Thrombolysis in Patients With Ischemic Stroke Aged ≥90 Years
- Phosphodiesterase-5 Inhibitors Not Tied to Alzheimer Disease Risk
- Differentiating MS From AQP4-Positive NMOSD and MOGAD With Imaging
- Optic Neuritis and Autoimmune Optic Neuropathies: Advances in Diagnosis and Treatment
- Safety of IV Thrombolysis in Patients With Ischemic Stroke Aged ≥90 Years
- Efficacy and Safety of Thrombectomy After Stroke Due to Basilar Artery Occlusion
- Less Sleep Tied to Higher Risk for Developing Chronic Disease
- Phosphodiesterase-5 Inhibitors Not Tied to Alzheimer Disease Risk
- Menopausal Vasomotor Symptoms Tied to White Matter Hyperintensity
NeurologyNeurology
Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura
Article Text
Article menu
- Article
Text - Article
info - Citation
Tools - Share
- Rapid Responses
- Article
metrics - Alerts
Short report
Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura
- C Lampl1,
- Z Katsarava2,
- H-C Diener2,
- V Limmroth3
- 1Department of Neurology and Psychiatry, Pain and Headache Centre, Linz General Hospital, 4020 Linz, Austria
- 2Department of Neurology, Essen University Hospital, Essen, Germany
- Correspondence to:
Dr C Lampl
Department of Neurology and Psychiatry, Pain and Headache Centre, Linz General Hospital, 4020 Linz, Austria; christian.
 lamplakh.linz.at
lamplakh.linz.at
Abstract
This study examined the efficacy of lamotrigine in the prevention of migraine aura. Fifty nine patients suffering from migraine with aura received lamotrigine in a controlled three year prospective open study. Treatment response was defined as a reduction of aura frequency each month by at least 50%. Primary endpoint was reached by three quarters of the patients. Lamotrigine significantly reduced both frequency of migraine aura (mean, 1.5 (SD, 0.6) each month before v 0.4 (0.7) after treatment; p < 0.001) and aura duration (mean, 27 (SD, 11) minutes before v 8 (14) after treatment; p < 0.001). Furthermore, more than three quarters of those patients with a reduction of aura symptoms experienced a significant reduction of frequency of migraine attacks (mean, 2.1 (SD, 1.0) each month before v 1.2 (1.1) after treatment; p < 0.001). Lamotrigine was highly effective in reducing migraine aura and migraine attacks. The strong correlation between reduction of aura symptoms and migraine attacks stresses the potential role of aura-like events and possibly cortical spreading depression as a trigger for trigeminal vascular activation, and subsequently the development of migraine headaches.
The strong correlation between reduction of aura symptoms and migraine attacks stresses the potential role of aura-like events and possibly cortical spreading depression as a trigger for trigeminal vascular activation, and subsequently the development of migraine headaches.
- CSD, cortical spreading depression
- df, degrees of freedom
- anticonvulsant
- aura without migraine
- cortical spreading depression
- lamotrigine
- migraine with aura
http://dx.doi.org/10.1136/jnnp.2005.063750
Statistics from Altmetric.com
Request Permissions
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- CSD, cortical spreading depression
- df, degrees of freedom
- anticonvulsant
- aura without migraine
- cortical spreading depression
- lamotrigine
- migraine with aura
Ten to fifteen percent of all patients with migraine suffer from migraine with aura. For most of these patients the aura phase encompasses visual or sensory deficits for 30 to 60 minutes, and in a few cases for several hours. Using high field functional magnetic resonance imaging with near continuous recording during visual aura, cortical spreading depression (CSD)-like events in the human occipital cortex could be visualised,1 confirming the concept of a transient slowly spreading excitation (depolarisation), followed by long lasting depression as the underlying mechanism of migraine aura. Despite the recent development of abortive migraine drugs such as 5-hydroxytryptamine 1B/D agonists, suitable agents for suppressing the frequency and duration of migraine aura have not been identified. Based on concepts of emphasising CSD-like events in migraine pathogenesis, anti-glutamatergic strategies have been suggested as a potential avenue for the treatment of migraine auras.2
For most of these patients the aura phase encompasses visual or sensory deficits for 30 to 60 minutes, and in a few cases for several hours. Using high field functional magnetic resonance imaging with near continuous recording during visual aura, cortical spreading depression (CSD)-like events in the human occipital cortex could be visualised,1 confirming the concept of a transient slowly spreading excitation (depolarisation), followed by long lasting depression as the underlying mechanism of migraine aura. Despite the recent development of abortive migraine drugs such as 5-hydroxytryptamine 1B/D agonists, suitable agents for suppressing the frequency and duration of migraine aura have not been identified. Based on concepts of emphasising CSD-like events in migraine pathogenesis, anti-glutamatergic strategies have been suggested as a potential avenue for the treatment of migraine auras.2
Lamotrigine is a potent sodium channel inhibitor with anti-glutamatergic actions. 3 In line with other sodium channel inhibitors, lamotrigine did not prevent migraine attacks.4 In two short term pilot studies, however, lamotrigine was shown to decrease aura frequency, duration, and symptoms.5,6 Data from one of these studies5 indicated that the successful treatment of migraine aura may also reduce migraine attack frequency in this subgroup of patients. Therefore, the aim of our study was to confirm the effects of lamotrigine on migraine aura after longterm treatment in a larger study population, and to investigate whether a reduction in migraine aura frequency is followed by a reduction of migraine attacks.
3 In line with other sodium channel inhibitors, lamotrigine did not prevent migraine attacks.4 In two short term pilot studies, however, lamotrigine was shown to decrease aura frequency, duration, and symptoms.5,6 Data from one of these studies5 indicated that the successful treatment of migraine aura may also reduce migraine attack frequency in this subgroup of patients. Therefore, the aim of our study was to confirm the effects of lamotrigine on migraine aura after longterm treatment in a larger study population, and to investigate whether a reduction in migraine aura frequency is followed by a reduction of migraine attacks.
METHODS AND PATIENTS
Our study was designed as a prospective, controlled, open, longterm dose titration study. Enrolment took place between February 1995 and December 2000. Informed consent was given by all patients. The study was approved by the local ethics committee (27/95).
Study design
Inclusion and exclusion criteria
Patients between 19 and 60 years of age were eligible if they had suffered from migraine with aura and migraine aura without headache for at least one year (diagnosis according to IHS criteria). One attack each month was the minimum attack frequency for enrolment, with an intensity described as moderate to severe on a four step scale (none, light, moderate, and severe). Exclusion criteria included patients taking prophylactic headache treatment within the three months before the beginning of the trial; previous lamotrigine treatment of epileptic seizures; cardiac, hepatic, or renal diseases; and pregnancy or the risk of pregnancy.
One attack each month was the minimum attack frequency for enrolment, with an intensity described as moderate to severe on a four step scale (none, light, moderate, and severe). Exclusion criteria included patients taking prophylactic headache treatment within the three months before the beginning of the trial; previous lamotrigine treatment of epileptic seizures; cardiac, hepatic, or renal diseases; and pregnancy or the risk of pregnancy.
Treatment and evaluation of drug effects
Aura type, frequency, and duration were determined by headache diaries for at least two months before treatment initiation. All patients had to maintain a headache diary and were obliged to document auras and migraine attacks. Control visits were performed each month with evaluation of the diaries, recording of aura symptoms, frequency and duration, and frequency of migraine attacks.
Dose regimen and titration
Lamotrigine treatment was started with a dose of 25 mg daily during the first month. If no reduction in migraine aura symptoms was seen, the dosage was increased by 25 mg monthly, not exceeding 300 mg/day. If the primary outcome criteria were reached and maintained for at least two months, the lamotrigine dosage was reduced, but not before six months of treatment. When a positive response was maintained for at least two months, lamotrigine was reduced by 25 mg, but not below a basic dose of 50 mg/day. If aura symptoms recurred for two months, dosage was increased by 25 mg monthly again until frequency or duration improved. This dosage was the final maintenance dose for the remainder of the study.
If no reduction in migraine aura symptoms was seen, the dosage was increased by 25 mg monthly, not exceeding 300 mg/day. If the primary outcome criteria were reached and maintained for at least two months, the lamotrigine dosage was reduced, but not before six months of treatment. When a positive response was maintained for at least two months, lamotrigine was reduced by 25 mg, but not below a basic dose of 50 mg/day. If aura symptoms recurred for two months, dosage was increased by 25 mg monthly again until frequency or duration improved. This dosage was the final maintenance dose for the remainder of the study.
Data analysis
The predetermined primary outcome measure was defined as percentage of patients with a reduction of migraine aura frequency by at least 50%. Secondary outcome measures were the reduction of aura frequency (mean, attacks each month) and the aura duration (mean, minutes) in addition to reduction of frequency of migraine attacks (mean, each month). Non-responders were defined as patients with continuing aura symptoms despite treatment for a minimum of three months on a minimum dosage of 100 mg for at least two months.
Statistics
A paired t test was used to compare mean frequency of migraine aura, mean duration of aura, and mean frequency of migraine attacks. The level of significance was set at 0.5. Pearson’s correlation was used to assess a possible correlation between the reduction in frequency of aura and migraine attacks. All statistics were performed using SPSS10.0.
RESULTS
Patient population
Table 1 provides details of migraine and aura presentation, patient demographics, dosages of lamotrigine, and treatment duration.
Table 1
Migraine and aura characteristics, patient demographics, dosages of lamotrigine, and treatment duration
Treatment effects
Effects on aura
A positive response to treatment was seen in 44 of the 59 patients (table 2). Lamotrigine significantly reduced the frequency of migraine aura (mean, 1.5 (SD, 0.6) each month before v 0.4 (0.7) after treatment; degrees of freedom (df) = 58; t = 10. 5; p < 0.001) and the duration of aura symptoms (mean, 26.9 (SD, 10.8) minutes before v 8.3 (13.6) after treatment; df = 58; t = 10.8; p < 0.001). Treatment effects were not different for different types of aura.
5; p < 0.001) and the duration of aura symptoms (mean, 26.9 (SD, 10.8) minutes before v 8.3 (13.6) after treatment; df = 58; t = 10.8; p < 0.001). Treatment effects were not different for different types of aura.
Table 2
Treatment response to lamotrigine
Effects on migraine attacks
The frequency of migraine attacks was significantly reduced also (mean, 2.1 (SD, 1.0) each month before v 1.2 (1.1) after treatment; df = 55; t = 7.7; p < 0.001). A significant correlation was found between the reduction in frequency of aura and migraine headache attacks (table 2).
DISCUSSION
Lamotrigine was highly effective in reducing the frequency and intensity of migraine aura. Furthermore, in more than three quarters of those patients who experienced improvement, a significant decrease in migraine frequency was also seen, whereas this effect was not seen among non-responders. These data confirm the results of pilot studies conducted with lamotrigine for the treatment of migraine aura,5,6 and suggest that the drug may also be useful in the treatment of patients with more severe types of aura (such as familiar hemiplegic migraine). For most patients, dosages in the range of 75 and 150 mg were sufficient to achieve a consistent response over the entire treatment period. Interestingly, once patients reached their “final” daily dosage, the treatment effect was sustained in almost all patients for the rest of the observation period. The fact that it took almost five months to reach the primary endpoint indicates that patients should be treated for at least six months before this treatment can be considered as ineffective. It also suggests that clinical trials for the prevention of migraine should ideally cover a treatment and evaluation phase of at least six months.
These data confirm the results of pilot studies conducted with lamotrigine for the treatment of migraine aura,5,6 and suggest that the drug may also be useful in the treatment of patients with more severe types of aura (such as familiar hemiplegic migraine). For most patients, dosages in the range of 75 and 150 mg were sufficient to achieve a consistent response over the entire treatment period. Interestingly, once patients reached their “final” daily dosage, the treatment effect was sustained in almost all patients for the rest of the observation period. The fact that it took almost five months to reach the primary endpoint indicates that patients should be treated for at least six months before this treatment can be considered as ineffective. It also suggests that clinical trials for the prevention of migraine should ideally cover a treatment and evaluation phase of at least six months.
Limitations of our study
Our study had some limitations. First, there was no placebo group comparison. However, we considered placebo treatment for six or more months not appropriate and unethical.7 Second, we did not measure serum concentrations of lamotrigine. Although in several pain studies, a significant association was found between analgesia and plasma lamotrigine concentrations, the dosage in this titration design was guided by the clinical response (reduction of aura symptoms) only. Therefore, measurements were without additional value for the dose finding procedures.
However, we considered placebo treatment for six or more months not appropriate and unethical.7 Second, we did not measure serum concentrations of lamotrigine. Although in several pain studies, a significant association was found between analgesia and plasma lamotrigine concentrations, the dosage in this titration design was guided by the clinical response (reduction of aura symptoms) only. Therefore, measurements were without additional value for the dose finding procedures.
Lessons for migraine pathophysiology and treatment
Recently, Bolay et al demonstrated elegantly that meningeal afferents can be activated by CSD in animals.8 The fact that the patients in our study not only experienced a reduction in migraine aura, but also a significant reduction in migraine frequency, suggests that the inhibition of CSD-like events may subsequently prevent migraine headache in this subgroup of patients. Our results also suggest that aura as a possible clinical entity initiates a complex neuronal pathway, resulting in typical migraine headache. In patients with migraine without aura, however, other (still unknown) mechanisms, such as a temporary impairment of the periaqueductual grey, probably play a crucial role in the activation of the trigeminal vascular system.9
In patients with migraine without aura, however, other (still unknown) mechanisms, such as a temporary impairment of the periaqueductual grey, probably play a crucial role in the activation of the trigeminal vascular system.9
Therefore, our study may provide the first clinical evidence consistent with the concept that CSD-like events activate the trigeminal vascular system under specific circumstances in the human cortex. Because there is now compelling evidence that CSD-like events may either be caused by extensive glutamate release or reduced clearance from the synaptic cleft,10 treatment by downregulating NMDA receptor activity provides an important approach to managing patients with migraine with aura.
REFERENCES
- ↵
Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 2001;98:4687–92.
- ↵
Kaube H, Herzog J, Kaufer T, et al.
 Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology 2000;55:139–41.
Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology 2000;55:139–41. - ↵
Wang SJ, Huang CC, Hsu KS, et al. Presynaptic inhibition of excitatory neurotransmission by lamotrigine in the rat amygdalar neurons. Synapse 1996;3:248–55.
- ↵
Steiner TJ, Findley LJ, Yuen AW. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia1997;17:109–12.
- ↵
Lampl C, Buzath A, Klinger D, et al. Lamotrigine in the prophylactic treatment of migraine aura—a pilot study. Cephalalgia 1999;19:58–63.
- ↵
D’Andrea G, Granella F, Cadaldini M, et al. Effectiveness of lamotrigine in the prophylaxis of migraine with aura: an open pilot study.
 Cephalalgia 1999;19:64–6.
Cephalalgia 1999;19:64–6. - ↵
Linde M, May A, Limmroth V, et al. Headache masters programme. Ethical aspects of placebo in migraine research. Cephalalgia 2003;23:491–5.
- ↵
Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 2002;8:136–42.
- ↵
Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous migraine attacks. Nat Med 1995;1:658–60.
- ↵
Moskowitz MA, Bolay H, Dalkara T. Deciphering migraine mechanisms: clues from familial hemiplegic migraine genotypes. Ann Neurol2004;55:276–80.
Footnotes
Read the full text or download the PDF:
Subscribe
Log in using your username and password
For personal accounts OR managers of institutional accounts
Username *
Password *
Forgot your log in details?Register a new account?
Forgot your user name or password?
Lamotrigine in the treatment of chronic pain syndromes
Lamotrigine in the treatment of chronic pain syndromes
E. V. Ekusheva, MD, prof., head. Department of Nervous Diseases1-2’3
V. Ekusheva, MD, prof., head. Department of Nervous Diseases1-2’3
1 Academy of Postgraduate Education Federal Research and Clinical Center for Specialized Types of Medical Care and Medical Technologies, Federal Medical and Biological Agency of Russia, Moscow
2 Academician A. Vein 3Belgorod State National Research University, Belgorod
Lamotrigine in treatment of chronic pain syndromes
E. V. Ekusheva
Academy of Postgraduate Education of the Federal Research and Clinical Center for Specialized Medical Care and Medical Technologies, Clinic for Headache and Autonomic Disorders n. a. Academician A. Wein; Moscow; Belgorod National Research University, Belgorod; Russia
Summary
Chronic pain syndromes represent a significant socio-economic problem for health care and society as a whole due to the insufficient effect of the treatment, a pronounced decrease in the quality of life and a significant degree of maladjustment of this category of patients. Therapy of chronic pain syndromes with first-line drugs from the group of anticonvulsants (gabapentin, pregabalin, carbamazepine, valproic acid and topiramate) does not always have the expected effect, and various side and undesirable effects are often observed. This involves the search for opportunities and prospects for the use of other new generation anticonvulsants, one of which is lamotrigine, as pharmacological tools. The article discusses a wide range of effective and safe use of Lamitor in a variety of diseases accompanied by chronic pain.
Therapy of chronic pain syndromes with first-line drugs from the group of anticonvulsants (gabapentin, pregabalin, carbamazepine, valproic acid and topiramate) does not always have the expected effect, and various side and undesirable effects are often observed. This involves the search for opportunities and prospects for the use of other new generation anticonvulsants, one of which is lamotrigine, as pharmacological tools. The article discusses a wide range of effective and safe use of Lamitor in a variety of diseases accompanied by chronic pain.
Key words: chronic pain, migraine with aura, neuropathic pain, trigeminal neuralgia, lamotrigine, Lamitor.
Summary
Chronic pain syndromes represent a significant socio-economic problem for health care and society as a whole due to the insufficient effect of the treatment, a pronounced decrease in the qualify of life and a significant degree of maladjustment of this category of patients. Treatment of chronic pain syndromes with first-line drugs from the group of anticonvulsants (gabapentin, pregabalin, carbamazepine, valproic acid and topiramate) does not always give the expected effect, and various side and undesirable effects are often observed. This implies the search for opportunities and prospects for the use of other new generation anticonvulsants, one of which is lamotrigine, as a means of pharmacological action. The article discusses a wide range of effective and safe use of Lamitor for various diseases accompanied by chronic pain.
This implies the search for opportunities and prospects for the use of other new generation anticonvulsants, one of which is lamotrigine, as a means of pharmacological action. The article discusses a wide range of effective and safe use of Lamitor for various diseases accompanied by chronic pain.
Key words: chronic pain, migraine with aura, neuropathic pain, trigeminal neuralgia, lamotrigine, Lamitor.
Despite the tremendous progress and achievements of modern medical science, there are still many neurological diseases in the treatment of which only a temporary effect is achieved, or it is not observed at all. This leads to the search for new technologies and therapeutic strategies to expand the capabilities of clinicians in the management of patients with diseases of the nervous system. Chronic pain syndromes represent a significant socio-economic problem for health care and society as a whole due to the insufficient effect of the treatment, a pronounced decrease in the quality of life and a significant degree of maladjustment of this category of patients. First of all, these are chronic neuropathic pain, various forms of migraine and SUNCT (Short-last ing Unilateral Neuralgiform headache attacks with Conjunctival injection and Tearing
First of all, these are chronic neuropathic pain, various forms of migraine and SUNCT (Short-last ing Unilateral Neuralgiform headache attacks with Conjunctival injection and Tearing
The use of drugs from the group of anticonvulsants for the treatment of pain syndromes of various etiologies began in the middle of the 20th century. The analgesic action of various representatives of this group is based on such mechanisms as blockade of voltage-dependent Na+ channels, voltage-dependent Ca 2 + channels of the membrane of nociceptive neurons, which limits the conduction of sodium and calcium ions through the channels, potentiation of GABAergic transmission and inhibition of glutamatergic transmission and, thus, a decrease in sensitization and excitability of central and peripheral nociceptors [1].
And in the treatment of chronic pain syndromes of various etiologies, in particular migraine, trigeminal neuralgia and other variants of chronic neuropathic pain, gabapentin, pregabalin, carbamazepine, valproic acid and topiramate are considered as first-line drugs. At the same time, these drugs do not always have the expected effect, various side and undesirable effects are often observed, which suggests the search for opportunities and prospects for the use of other representatives of the new generation of anticonvulsants, one of which is lamotrigine, as tools for the pharmacotherapy of pain syndromes.
At the same time, these drugs do not always have the expected effect, various side and undesirable effects are often observed, which suggests the search for opportunities and prospects for the use of other representatives of the new generation of anticonvulsants, one of which is lamotrigine, as tools for the pharmacotherapy of pain syndromes.
Lamotrigine has a proven effect on the leading links in the pathogenesis of chronic pain syndromes, realizing all of the above antinociceptive mechanisms [2, 3, 4], in addition, the effect on a4B2-nicotinic and transmembrane receptors is discussed as the basis of the analgesic effect of lamotrigine [5, 6]. This drug is characterized by a pronounced selectivity of the effect on the functional activity of neurons of the nociceptive and antinociceptive systems, which, along with a wide therapeutic potential, allows reasonable use of lamotrigine in the treatment of chronic pain of various etiologies. 9Ol000 (average 100 mg/day)
 et al., 2004; Lamp C. et al., 2005; Viana M. et al., 2018; Buch D. et al., 2019
et al., 2004; Lamp C. et al., 2005; Viana M. et al., 2018; Buch D. et al., 2019 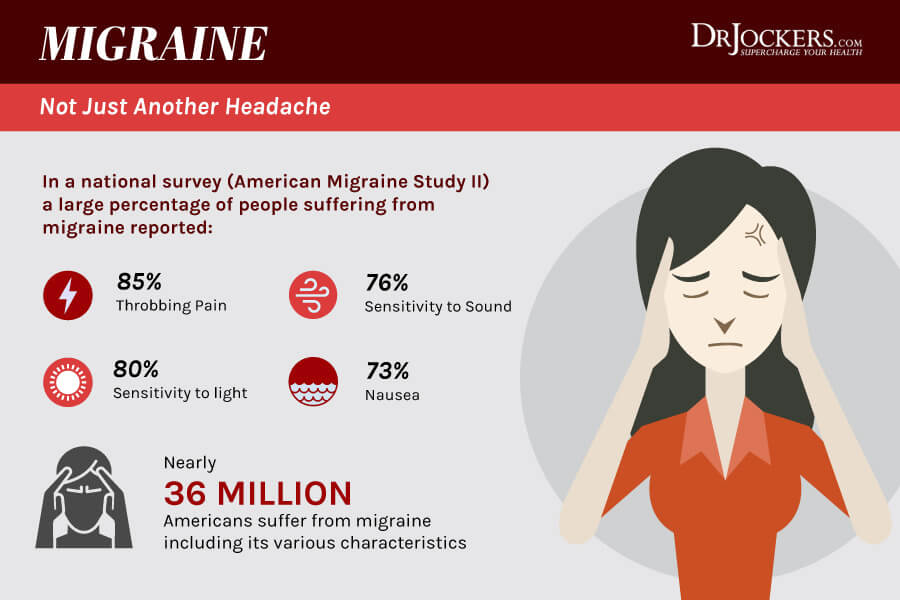 T. et al., 2001; Pascual J. et al., 2004; Lamp C. et al., 2005; Thissen S. et al., 2014; Schankin C. J. et al., 2015; Traber G. L. et al., 2019
T. et al., 2001; Pascual J. et al., 2004; Lamp C. et al., 2005; Thissen S. et al., 2014; Schankin C. J. et al., 2015; Traber G. L. et al., 2019 , 2014
, 2014 Migraine
Migraine is in second place among the causes of disability and severe maladjustment caused by a neurological disease, and in sixth place among the leading medical causes of a decrease in the quality of life of the population in the world [7]. One variant of this disease is migraine with aura, which often causes problems in therapy, in particular in patients with basilar migraine (1.2.2), hemiplegic migraine (1.2.3), typical aura without headache (1.2.1.2), vestibular migraine (A1.6.6) and migraine complications such as persistent aura without infarction (1.4.2) and visual snow (A1.4.6) [8].
Analysis of all studies of the use of lamotrigine in the treatment of migraine in the PubMed database (more than 100 works) up to February 2019 [9] demonstrated its high effect, good tolerability and safety in the preventive treatment of patients with various types of migraine with aura (Table 1) , including difficult-to-treat and resistant to other means of preventive therapy. A significant decrease in the frequency of seizures by half or more (from 4.2 to 0.7 per month) and the duration of the aura were shown after a 6-month course of therapy with lamotrigine in different types of the disease [9], in particular in patients with basilar migraine [4, 10-13], familial hemiplegic migraine types 1 and 2 [13, 14-16], migraine with prolonged aura and typical aura without headache [12, 17 ]. In some cases, a complete reduction in attacks was observed: in patients with basilar migraine after a 12-month course of lamotrigine, they were not observed over the next 5 years [10], as well as in patients resistant to therapy with other drugs [9, 17]. Thus, the high potential for efficacy and safety, the low profile of possible adverse events, and the low cost of a course of therapy make it possible to consider lamotrigine as the drug of choice in patients with frequent migraine attacks with aura, persistent aura, and severe disadaptation [12, 17].
A significant decrease in the frequency of seizures by half or more (from 4.2 to 0.7 per month) and the duration of the aura were shown after a 6-month course of therapy with lamotrigine in different types of the disease [9], in particular in patients with basilar migraine [4, 10-13], familial hemiplegic migraine types 1 and 2 [13, 14-16], migraine with prolonged aura and typical aura without headache [12, 17 ]. In some cases, a complete reduction in attacks was observed: in patients with basilar migraine after a 12-month course of lamotrigine, they were not observed over the next 5 years [10], as well as in patients resistant to therapy with other drugs [9, 17]. Thus, the high potential for efficacy and safety, the low profile of possible adverse events, and the low cost of a course of therapy make it possible to consider lamotrigine as the drug of choice in patients with frequent migraine attacks with aura, persistent aura, and severe disadaptation [12, 17].
Vestibular migraine (VM) occurs in approximately 10% of patients with migraine [18] and is characterized by excruciating attacks of headache accompanied by moderate or severe dizziness, vomiting, photo- and phonophobia. Data from randomized controlled trials of CM prophylactic therapy [18-21] have demonstrated the efficacy of lamotrigine along with first-line drugs. In particular, in a retrospective open study [20] of 19 patients with CM, seizures were more than halved in 18 of 19patients, and a quarter of them are absent while taking lamotrigine. There was also a significant decrease in the severity and frequency of dizziness (from 18.1 to 5.4 days per month) [20]. In another study [21], 65 patients with CM taking prophylactic lamotrigine showed significant improvement in 58 (85%) of them in the form of a reduction in the frequency of headache attacks and episodes of dizziness, as well as high adherence to lamotrigine therapy.
Data from randomized controlled trials of CM prophylactic therapy [18-21] have demonstrated the efficacy of lamotrigine along with first-line drugs. In particular, in a retrospective open study [20] of 19 patients with CM, seizures were more than halved in 18 of 19patients, and a quarter of them are absent while taking lamotrigine. There was also a significant decrease in the severity and frequency of dizziness (from 18.1 to 5.4 days per month) [20]. In another study [21], 65 patients with CM taking prophylactic lamotrigine showed significant improvement in 58 (85%) of them in the form of a reduction in the frequency of headache attacks and episodes of dizziness, as well as high adherence to lamotrigine therapy.
The high effect of prophylactic therapy with lamotrigine has been repeatedly shown in patients with visual snow, which in clinical practice is difficult to treat and significantly reduces the quality of life of patients [4, 13, 17, 22-24]. Longitudinal follow-up of patients with visual snow who received lamotrigine as a prophylactic therapy showed a pronounced effect in 75-84% of patients, which persisted for several subsequent years [4, 13, 23].
Chronic neuropathic pain
Chronic neuropathic pain is a complex and sometimes unpredictable task for the doctor, as it is characterized by recurrent course, the occurrence of side or adverse events during first-line therapy, as well as a decrease in their effectiveness during long-term treatment. All this leads to the search for new, more active and safe means for the treatment of pain syndrome of various origins.
In the European and American clinical guidelines for the treatment of trigeminal neuralgia [25, 26], lamotrigine is considered as a second-line drug used in the absence of effect or intolerance to carbamazepine or oxcarbazepine, as well as in complex therapy with first-line drugs in case of their insufficient effect (Table 1). one). A number of studies have shown the effect of lamotrigine in the treatment of resistant trigeminal neuralgia [27, 28]. Its high efficiency in the treatment of another variant of central post-stroke pain, which is complex from the point of view of analgesic therapy, has been demonstrated [29-32], including in the form of a decrease in the severity of the pain syndrome by half or more, according to the visual analogue scale [32]. Lamotrigine has been shown to be effective in the treatment of pain in diabetic polyneuropathy [33–36], chronic neuropathic pain in multiple sclerosis, postherpetic neuralgia, HIV-associated neuropathy [37] associated with spinal cord injury [38], or other etiologies [2] .
Lamotrigine has been shown to be effective in the treatment of pain in diabetic polyneuropathy [33–36], chronic neuropathic pain in multiple sclerosis, postherpetic neuralgia, HIV-associated neuropathy [37] associated with spinal cord injury [38], or other etiologies [2] .
Other headaches
Lamotrigine is considered as the first-line drug and the most effective treatment for painful and intractable SUNCT attacks [39, 40].
It is important to note that slow titration of the drug (addition of 25 mg per day for 1-2 weeks) will minimize adverse events during its use, and the rational use of lamotrigine (adequate daily dose and required duration of therapy) will allow its full therapeutic potential to be realized.
Thus, to date, extensive positive experience has been accumulated with the use of lamotrigine in various types of chronic pain syndromes, which makes it possible to safely and effectively help these patients. One such drug is Lamitor (Torrent Pharmaceuticals), which is therapeutically equivalent to the original drug (Class A - Orange Book, FDA). Lamitor is characterized by good tolerance, without complicating daily functioning, efficiency and breadth of the clinical and pharmacological spectrum, on the one hand, having antidepressant and anti-anxiety effects, and on the other hand, the ability to use it in children from 3 years old, in women during pregnancy, in the elderly people, including those suffering from diabetes (see the instructions for the drug). These properties are of particular clinical value, as they allow Lamitor to be effectively and safely used for the pharmacotherapy of patients with a variety of chronic pain syndromes.
Lamitor is characterized by good tolerance, without complicating daily functioning, efficiency and breadth of the clinical and pharmacological spectrum, on the one hand, having antidepressant and anti-anxiety effects, and on the other hand, the ability to use it in children from 3 years old, in women during pregnancy, in the elderly people, including those suffering from diabetes (see the instructions for the drug). These properties are of particular clinical value, as they allow Lamitor to be effectively and safely used for the pharmacotherapy of patients with a variety of chronic pain syndromes.
References
- Borouierdi A., Zeng J., Sharp K. et al. Calcium channel-2-delta-J protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. // Pain. No. 152 (3). R. 649-655.
- Titlic M., Lukir J., Tonkic A. et al. Lamotrigine in the treatment of the pain syndromes and neuropathic pain. // Bratislavske Lekarske Listy.
 2008. N 109 (9). R. 421-424.
2008. N 109 (9). R. 421-424. - Kuzniecky R. Modulation of cerebral GABA by topiramate, lamotrigine and gabapentin in healthy adults. // Neurology. 2002. No. 58. P. 368-372.
- Lampl C., Katsarava Z., Diener H.-C., Limmroth V. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. // Journal of Neurology, Neurosurgery & Psychiatry. 2005. N76 (12). R. 1730-1732.
- Zheng C., Yang K., Liu Q. et al. The anticonvulsive drug lamotrigine blocks neuronal{alpha}4{beta}2-nicotinic acetylcholine receptors. // The Journal of Pharmacology and Experimental Therapeutics. 2010. N335(2). R. 401-408.
- Yang R., Dunn J. F., Su D. et al. Lamotrigine inhibits TRESK regulated by G protein coupled receptor agonist. // The Journal of Neuroscience. 2018. N 367. R. 368-375.
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016.
 // The Lancet Neurology. 2017. N390. R. 1211-1859.
// The Lancet Neurology. 2017. N390. R. 1211-1859. - Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. // Cephalalgia. 2018. N38. P. 1-211.
- Buch D., Chabriat H. Lamotrigine in the prevention of migraine with aura: a narrative review. // header. 2019. N59(8). R. 1187-1197.
- Cologno D., d’Onofrio F., Castriota O. et al. Basilar-type migraine patients responsive to lamotrigine: a 5-year follow-up. // Neurological Sciences. 2013. N34 (Suppl 1). P. S 165-S 166.
- d'Onofrio F., Cologno D., Petretta V. et al. Basilar-type migraine responsive to lamotrigine: three case reports. // Neurological Sciences. 2007. N28 (Suppl 2). P. S239-41.
- Viana M., Afrid'iS. Migraine with prolongation aura: phenotype and treatment. // Naunyn-Schmiedeberg's Archives of Pharmacology. 2018.N39eleven). R. 1-7.
- Pascual J., Caminero A. B., Mateos V. et al. Preventing disturbing migraine aura with lamotrigine: an open study.
 // header. 2004. N44 (10). R. 1024-1028.
// header. 2004. N44 (10). R. 1024-1028. - Pelzer N., Stam A. H., Carpay J. A. et al. Familial hemiplegic migraine is treated by sodium valproate and lamotngine. // Cephalalgia. 2014. N 34. R. 708-711.
- Camia F., Pisciotta L., Morana G. et al. Combined early treatment in hemiplegic attacks related to CACNA1A encephalopathy with brain oedema: Blocking the cascade? // Cephalalgia. 2017. N 37. R. 1202-1206.
- Liguori C., Albanese M., Sancesario G. et al. May a suspicious psychiatric disorder hide sporadic hemiplegic migraine? Genetic test as prompting factor for diagnosis. // Neurological Sciences. N 34. R. 1845-1846.
- Thissen S., Vos I. G., Schreuder T. H. et al. Persistent migraine aura: New cases, a literature review, and ideas about pathophysiology. // header. N 54. R. 1290-1309.
- Bronstein A. M., Lempert T. Dizziness: a practical approach to diagnosis and management. // Cambridge University Press. 2007. P. 83-91.
- Dieterich M.
 , Obermann M., Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. // Journal of Neurology. 2016. No. 263 (Suppl. 1). S 82-89.
, Obermann M., Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. // Journal of Neurology. 2016. No. 263 (Suppl. 1). S 82-89. - Bisdorff A. R. Treatment of migraine related vertigo with lamotrigine an observational study. // Bulletin of the Medical Science Society of the Grand Duchy of Luxembourg. 2004. N2. R. 103-108.
- Zhang L., Wilson M., Yamagishi L. Lamotrigine as a prophylactic treatment for migraine-associated vertigo: A case senes. // Neurology. 2014. N82 (10 Suppl.). Poster 7.193.
- Chen W. T., Fuh J. L., Lu S. R., Wang S. J. Persistent migrainous visual phenomena might be responsive to lamotrigine. // header. 2001; 41:823-825.
- Schankin C. J., Maniyar F. H., Digre K. B., Goads-by P. J. “Visual snow’’ – A disorder distinct from persistent migraine aura. // brain. 2014. N 137. R. 1419-1428.
- Traber G. L., Piccirellic M., Michels L. Visual snow syndrome: a review on diagnosis, pathophysiology, and treatment.
 // Current Opinion Neurology. 2020. N 33 (1). P. 74-78.
// Current Opinion Neurology. 2020. N 33 (1). P. 74-78. - Bendtsen L., Zakrzewska J. M., Abbott J. et al. European Academy of Neurology guideline on trigeminal neuralgia. // European Journal of Neurology. 2019. N26(6). R. 831-849.
- Gronseth G., Cruccu G., Alksne J. et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. // Neurology. 2008. N 71 (15). R. 1183-1190.
- Alves T. C., Azevedo G. S., Carvalho E. S. [Pharmacological treatment of trigeminal neuralgia: systematic review and metanalysis] [in Portuguese]. // Brazilian Journal of Anesthesiology. 2004. N54. R. 836-849.
- Al-Quliti K. W. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. // Neurosciences. 2015. N20(2). R. 107-114.
- Kalita J.
 , Chandra S., Misra U. K. Pregabalin and lamotrigine in central poststroke pain: A pilot study. // Neurology India. 2017. N65(3). R. 506-511.
, Chandra S., Misra U. K. Pregabalin and lamotrigine in central poststroke pain: A pilot study. // Neurology India. 2017. N65(3). R. 506-511. - Finnerup N.B., Gottrup H, Jensen T.S. Anticonvulsants in central pain. // Expert Opinion Pharmacotherapy. 2002. N3 (10). R. 1411-1420.
- Vestergaard K., Andersen G., Gottrup H. et al. Lamotrigine for central poststroke pain: a randomized controlled trial. // Neurology. 2001. No. 56 (2). R. 184-190.
- Frese A., Husstedt I. W., Ringelstein E. B., Evers S. Pharmacologic treatment of central poststroke pain. // Clinical Journal of Pain. 2006. N22(3). R. 252-60.
- Eisenberg E., Lurie Y., Braker C. et al. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. // Neurology. 2001. N57(3). R. 505-509.
- Singleton J. R., Smith A. G., Russel J. et al. Polyneuropathy with impaired glucose tolerance: implication for diagnosis and therapy. // Current Treat Options in Neurology. 2005. N 7 (1).
 P. 33-42.
P. 33-42. - Vinic A.J., Tuchman M., Safirstein B. et al. Lam-otrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. // Pain. 2007. N 128 (1-2). R. 167-179.
- Chong M. S., Hester J. Diabetic painful neuropathy: current and future treatment options. //Drugs. 2007. N67(4). R. 569-585.
- Simpson D. M., McArthur J. C., Olney R. et al. Lamotrigine HIV neuropathy study team. // Neurology. 2003. N60(9). R. 1508-1514.
- Finnerup N. B., Sindrup S. H., Bach F. W. et al. Lamotrigine in spinal cord injury pain: a randomized controlled trial. // Pain. 2002. N96(3). R. 375-383.
- Cohen A. Trigeminal autonomic cephalalgias: a diagnostic and therapeutic overview. // ACNR. Headache series. 2014. No. 14 (4). R. 12-15.
- Lambru G., Matharu M. C. SUNCT and SUNA: medical and surgical treatment. // Neurological Sciences. 2013. N34 (Suppl. 1). P. S75-81.
For citation: Ekusheva E. V. Lamotrigine in the treatment of chronic pain syndromes. Medical alphabet. 2020; (22): 5-8. https://doi.org/10.33667/2078-5631-2020-22-5-8 For citation: Ekusheva E. V. Lamotrigine in treatment of chronic pain syndromes. medical alphabet. 2020; (22): 5-8. https://doi.org/10.33667/2078-5631-2020-22-
V. Lamotrigine in the treatment of chronic pain syndromes. Medical alphabet. 2020; (22): 5-8. https://doi.org/10.33667/2078-5631-2020-22-5-8 For citation: Ekusheva E. V. Lamotrigine in treatment of chronic pain syndromes. medical alphabet. 2020; (22): 5-8. https://doi.org/10.33667/2078-5631-2020-22-
Lamotrigine in the treatment of chronic pain syndromes | Ekusheva
1. Borouierdi A., Zeng J., Sharp K. et al. Calcium channel 2-delta 1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. // Pain. No. 152 (3). R. 649–655.
2. Titlic M., Lukir J., Tonkic A. et al. Lamotrigine in the treatment of the pain syndromes and neuropathic pain. // Bratislavske Lekarske Listy. 2008. N109(9). R. 421–424.
3. Kuzniecky R. Modulation of cerebral GABA by topiramate, lamotrigine and gabapentin in healthy adults. // Neurology. 2002. N58. P. 368–372.
4. Lampl C., Katsarava Z., Diener H.-C., Limmroth V. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. // Journal of Neurology, Neurosurgery & Psychiatry. 2005. N76 (12). R. 1730–1732.
// Journal of Neurology, Neurosurgery & Psychiatry. 2005. N76 (12). R. 1730–1732.
5. Zheng C., Yang K., Liu Q. et al. The anticonvulsive drug lamotrigine blocks neuronal{alpha}4{beta}2-nicotinic acetylcholine receptors. // The Journal of Pharmacology and Experimental Therapeutics. 2010. N335(2). R. 401–408.
6. Yang R., Dunn J. F., Su D. et al. Lamotrigine inhibits TRESK regulated by G protein coupled receptor agonist. // The Journal of Neuroscience. 2018. N367. R. 368–375.
7. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. // The Lancet Neurology. 2017. N390. R. 1211–1859.
8. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. // Cephalalgia. 2018. N38. P. 1–211.
9. Buch D., Chabriat H. Lamotrigine in the prevention of migraine with aura: a narrative review. // header. 2019. N59(8). R. 1187–1197.
Lamotrigine in the prevention of migraine with aura: a narrative review. // header. 2019. N59(8). R. 1187–1197.
10. Cologno D., d'Onofrio F., Castriota O. et al. Basilar-type migraine patients responsive to lamotrigine: a 5-year follow-up. // Neurological Sciences. 2013. N34 (Suppl 1). P. S 165–S 166.
11. d'Onofrio F., Cologno D., Petretta V. et al. Basilar-type migraine responsive to lamotrigine: three case reports. // Neurological Sciences. 2007. N28 (Suppl 2). P.S. 239-41.
12. Viana M., Afridi S. Migraine with proliferating aura: phenotype and treatment. // Naunyn-Schmiedeberg's Archives of Pharmacology. 2018.N39eleven). R. 1–7.
13. Pascual J., Caminero A. B., Mateos V. et al. Preventing disturbing migraine aura with lamotrigine: an open study. // header. 2004. N44 (10). R. 1024–1028.
14. Pelzer N., Stam A.H., Carpay J.A. et al. Familial hemiplegic migraine is treated by sodium valproate and lamotrigine. // Cephalalgia. 2014. N34. R. 708–711.
15. Camia F., Pisciotta L., Morana G. et al. Combined early treatment in hemiplegic attacks related to CACNA1A encephalopathy with brain oedema: Blocking the cascade? // Cephalalgia. 2017. N37. R. 1202–1206.
16. Liguori C., Albanese M., Sancesario G. et al. May a suspicious psychiatric disorder hide sporadic hemiplegic migraine? Genetic test as prompting factor for diagnosis. // Neurological Sciences. 2013. N34. R. 1845–1846.
17. Thissen S., Vos I.G., Schreuder T.H. et al. Persistent migraine aura: New cases, a literature review, and ideas about pathophysiology. // header. 2014. N54. R. 1290–1309.
18. Bronstein A.M., Lempert T. Dizziness: a practical approach to diagnosis and management. // Cambridge University Press. 2007. P. 83–91.
19. Dieterich M., Obermann M., Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. // Journal of Neurology. 2016. N263 (Suppl. 1). S 82–89.
20. Bisdorff A.R. Treatment of migraine related vertigo with lamotrigine an observational study. // Bulletin of the Medical Science Society of the Grand Duchy of Luxembourg. 2004. N2. R. 103–108.
// Bulletin of the Medical Science Society of the Grand Duchy of Luxembourg. 2004. N2. R. 103–108.
21. Zhang L., Wilson M., Yamagishi L. Lamotrigine as a prophylactic treatment for migraine-associated vertigo: A case series. // Neurology. 2014. No. 82 (10 Suppl.). Poster 7.193.
22. Chen W. T., Fuh J. L., Lu S. R., Wang S. J. Persistent migrainous visual phenomena might be responsive to lamotrigine. // header. 2001; 41:823–825.
23. Schankin C. J., Maniyar F. H., Digre K. B., Goadsby P. J. “Visual snow” – A disorder distinct from persistent migraine aura. // brain. 2014. N 137. R. 1419–1428.
24. Traber G. L., Piccirellic M., Michels L. Visual snow syndrome: a review on diagnosis, pathophysiology, and treatment. // Current Opinion Neurology. 2020. N33(1). P. 74–78.
25. Bendtsen L., Zakrzewska J. M., Abbott J. et al. European Academy of Neurology guideline on trigeminal neuralgia. // European Journal of Neurology. 2019. N26(6). R. 831–849.
26. Gronseth G., Cruccu G., Alksne J. et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. // Neurology. 2008. N71 (15). R. 1183–1190.
Gronseth G., Cruccu G., Alksne J. et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. // Neurology. 2008. N71 (15). R. 1183–1190.
27. Alves T. C., Azevedo G. S., Carvalho E. S. [Pharmacological treatment of trigeminal neuralgia: systematic review and metanalysis] [in Portuguese]. // Brazilian Journal of Anesthesiology. 2004. N54. R. 836–849.
28. Al-Quliti K.W. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. // Neurosciences. 2015. N20(2). R. 107–114.
29. Kalita J., Chandra S., Misra U.K. Pregabalin and lamotrigine in central poststroke pain: A pilot study. // Neurology India. 2017. N65(3). R. 506–511.
30. Finnerup N.B., Gottrup H, Jensen T.S. Anticonvulsants in central pain. // Expert Opinion Pharmacotherapy. 2002. N3 (10). R. 1411–1420.
2002. N3 (10). R. 1411–1420.
31. Vestergaard K., Andersen G., Gottrup H. et al. Lamotrigine for central poststroke pain: a randomized controlled trial. // Neurology. 2001. N56(2). R. 184–190.
32. Frese A., Husstedt I. W., Ringelstein E. B., Evers S. Pharmacologic treatment of central poststroke pain. // Clinical Journal of Pain. 2006. N22(3). R. 252–60.
33. Eisenberg E., Lurie Y., Braker C. et al. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. // Neurology. 2001. N 57 (3). R. 505–509.
34. Singleton J. R., Smith A. G., Russel J. et al. Polyneuropathy with impaired glucose tolerance: implication for diagnosis and therapy. // Current Treat Options in Neurology. 2005. N7 (1). P. 33–42.
35. Vinic A. J., Tuchman M., Safirstein B. et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. // Pain. 2007. N128(1–2). R. 167–179.
36.