Dosage of depakote for bipolar
Dosing for Bipolar Mania - Depakote® (divalproex sodium)
- FOR U.S. HEALTHCARE PROFESSIONALS
- Important Safety Information
- Medication Guide
- Prescribing Information
- Patient Site
- Important Safety Information
- Prescribing Information
FOR U. S. HEALTHCARE
PROFESSIONALS
Depakote Delayed-Release Tablets
125 mg
Depakote Delayed-Release Tablets
250 mg
Depakote Delayed-Release Tablets
500 mg
Depakote ER
250 mg
Depakote ER
500 mg
Depakote Delayed-Release Tablets are indicated for treatment of manic episodes associated with bipolar disorder. Depakote ER is indicated for acute treatment of manic or mixed episodes associated with bipolar disorder, with or without psychotic features.
Dosing recommendations for Depakote Delayed-Release Tablets
and Depakote ER1,2
| DEPAKOTE Delayed-Release Tablets |
DEPAKOTE ER Tablets |
|
|---|---|---|
| INITIAL DOSE | 750 mg daily divided dose | 25 mg/kg/day once daily |
| INCREASE DOSE | The dose should be increased as rapidly as possible to achieve the lowest therapeutic dose which produces the desired clinical effect or the desired range of plasma concentrations | |
| MAXIMUM RECOMMENDED DOSE |
60 mg/kg/day | |
| THERAPEUTIC BLOOD RANGE |
Clinical response was dosed at trough plasma concentrations between 50-125 μg/mL | 85 to 125 μg/mL |
Important Safety Considerations
1,2- Depakote Delayed-Release Tablets and Depakote ER are not interchangeable.

- Depakote Delayed-Release Tablets and Depakote ER tablets must be swallowed whole.
- Valproate concentration levels ≥110 mcg/mL in females or ≥135 mcg/mL in males increases the risk of thrombocytopenia.
- Use Depakote Delayed-Release Tablets if smaller dose adjustments than those available with Depakote ER are required.
- Depakote ER tablets are administered orally once a day and must be swallowed whole.
- Depakote ER should be administered once daily using a dose 8%-20% higher than the total daily dose of Depakote.
- If satisfactory clinical response has not been achieved, monitor plasma levels.
Dose conversion from Depakote Delayed-Release Tablets to Depakote ER1,2
| DEPAKOTE Delayed-Release Tablets Total Daily Dose (mg) |
DEPAKOTE ER Total Daily Dose (mg) |
|---|---|
| 500*-625 | 750 |
| 750*-875 | 1,000 |
| 1,000*-1,125 | 1,250 |
| 1,250-1,375 | 1,500 |
| 1,500-1,625 | 1,750 |
| 1,750 | 2,000 |
| 1,875-2,000 | 2,250 |
| 2,125-2,250 | 2,500 |
| 2,375 | 2,750 |
| 2,500-2,750 | 3,000 |
| 2,875 | 3,250 |
| 3,000-3,125 | 3,500 |
*These total daily doses of Depakote Delayed-Release Tablets cannot be directly converted to an 8% to 20% higher total daily dose of Depakote ER because the required dosing strengths of Depakote ER are not available. Consideration may be given at the clinician’s discretion to increase the patient’s total daily doses of Depakote Delayed-Release Tablets to the next higher dosage before converting to the appropriate total daily dose of Depakote ER.
Consideration may be given at the clinician’s discretion to increase the patient’s total daily doses of Depakote Delayed-Release Tablets to the next higher dosage before converting to the appropriate total daily dose of Depakote ER.
WRITE DAW†
Ensure patients receive the branded Depakote you prescribe.
LEARN MOREHELP YOUR PATIENTS SAVE
Eligible patients could pay as little as $5/month.‡
LEARN MOREHELP PATIENTS SAVE TRIPS
Converting to a 90-day Rx may save patients trips to the pharmacy.
†Or your state’s legal language. ‡Up to $100/month savings for eligible patients.
ELIGIBILITY
Available to patients with commercial prescription insurance coverage for Depakote who meet eligibility criteria. Copay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law or by the patient’s health insurance provider. If at any time a patient begins receiving prescription drug coverage under any such federal, state, or government-funded healthcare program, patient will no longer be able to use the Depakote Savings Card and patient must call OPUS Health at 800.364.4767 to stop participation. Patients residing in or receiving treatment in certain states may not be eligible. Patients may not seek reimbursement for value received from the Depakote Savings Card from any third-party payers. Offer subject to change or discontinuance without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. Please see full Terms and Conditions.
If at any time a patient begins receiving prescription drug coverage under any such federal, state, or government-funded healthcare program, patient will no longer be able to use the Depakote Savings Card and patient must call OPUS Health at 800.364.4767 to stop participation. Patients residing in or receiving treatment in certain states may not be eligible. Patients may not seek reimbursement for value received from the Depakote Savings Card from any third-party payers. Offer subject to change or discontinuance without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. Please see full Terms and Conditions.
TERMS AND CONDITIONS
Pharmacist Instructions
- Submit the copay card authorized for all commercially insured patients by the patient’s primary insurance as a secondary transaction to OPUS Health.
- When you use this card, you are confirming that you have not submitted and will not submit a claim for this prescription for reimbursement under any federal, state, or government-funded healthcare program, such as Medicare (including Part D), Medicare Advantage, Medicaid, Medigap, Veterans Affairs, the Department of Defense, or TRICARE.
- Pharmacists with questions please call OPUS Health at 800.364.4767.
INDICATIONS
1,2Mania
DEPAKOTE® ER (divalproex sodium) extended-release tablets, for oral use, is a valproate and is indicated for the treatment of acute manic or mixed episodes associated with bipolar disorder, with or without psychotic features.
DEPAKOTE® (divalproex sodium) delayed-release tablets, for oral use, is a valproate and is indicated for the treatment of the manic episodes associated with bipolar disorder.
A manic episode is a distinct period of abnormally and persistently elevated, expansive, or irritable mood. Typical symptoms of mania include pressure of speech, motor hyperactivity, reduced need for sleep, flight of ideas, grandiosity, poor judgment, aggressiveness, and possible hostility. A mixed episode is characterized by the criteria for a manic episode in conjunction with those for a major depressive episode (depressed mood, loss of interest or pleasure in nearly all activities).
The efficacy of DEPAKOTE ER is based in part on studies of DEPAKOTE in this indication, and was confirmed in a 3-week trial with patients meeting DSM-IV-TR criteria for bipolar I disorder, manic or mixed type, who were hospitalized for acute mania.
The efficacy of DEPAKOTE was established in 3-week trials with patients meeting DSM-III-R criteria for bipolar disorder who were hospitalized for acute mania.
The effectiveness of valproate for long-term use in mania, i.e., more than 3 weeks, has not been demonstrated in controlled clinical trials. Therefore, healthcare providers who elect to use DEPAKOTE or DEPAKOTE ER for extended periods should continually reevaluate the long-term risk-benefits of the drug for the individual patient.
Epilepsy
DEPAKOTE ER is indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures.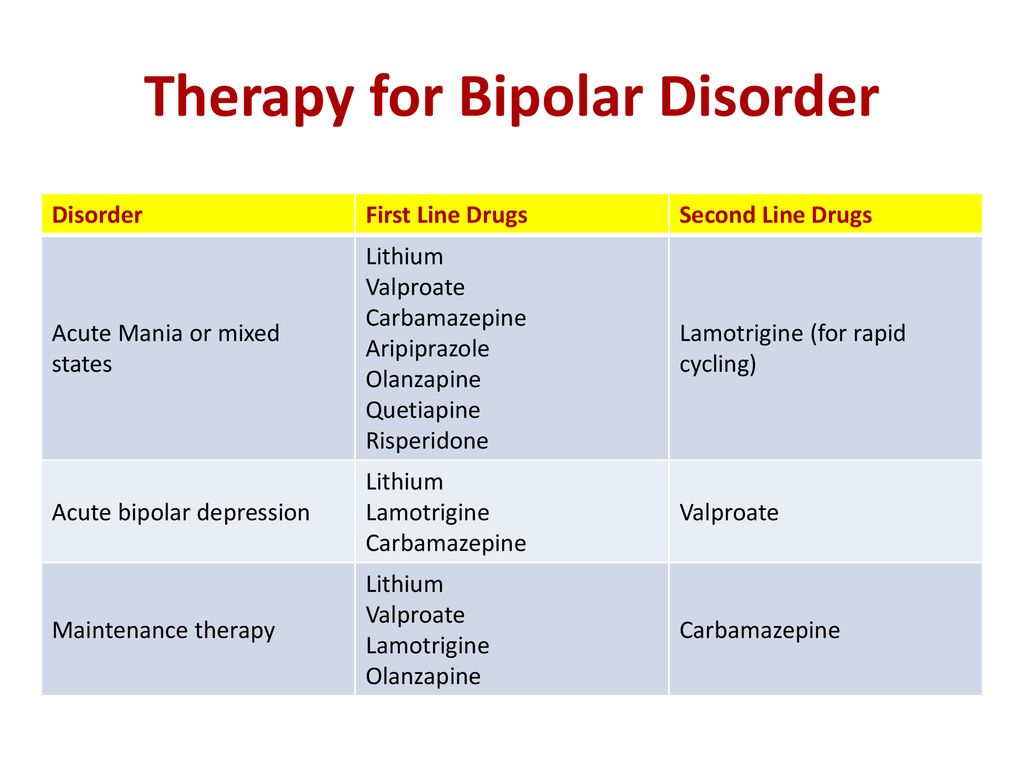 DEPAKOTE ER is also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures in adults and children 10 years of age or older, and adjunctively in adults and children 10 years of age or older with multiple seizure types that include absence seizures.
DEPAKOTE ER is also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures in adults and children 10 years of age or older, and adjunctively in adults and children 10 years of age or older with multiple seizure types that include absence seizures.
DEPAKOTE is indicated as monotherapy and adjunctive therapy in the treatment of patients with complex partial seizures that occur either in isolation or in association with other types of seizures. DEPAKOTE is also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures.
Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present.
Migraine
DEPAKOTE and DEPAKOTE ER are indicated for prophylaxis of migraine headaches. There is no evidence that DEPAKOTE ER or DEPAKOTE is useful in the acute treatment of migraine headaches.
There is no evidence that DEPAKOTE ER or DEPAKOTE is useful in the acute treatment of migraine headaches.
Important Limitations
Because of the risk to the fetus of decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations, which may occur very early in pregnancy, valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable.
For prophylaxis of migraine headaches, DEPAKOTE and DEPAKOTE ER are contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception.
IMPORTANT SAFETY INFORMATION
1,2Warning: Life-Threatening Adverse Reactions
Hepatotoxicity
General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate and its derivatives. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When DEPAKOTE (divalproex sodium) delayed-release tablets, for oral use, or DEPAKOTE ER (divalproex sodium) extended-release tablets, for oral use, is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
Patients with Mitochondrial Disease: There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e.g., Alpers Huttenlocher Syndrome). DEPAKOTE and DEPAKOTE ER are contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and in children under two years of age who are clinically suspected of having a mitochondrial disorder. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, DEPAKOTE and DEPAKOTE ER should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with DEPAKOTE or DEPAKOTE ER for the development of acute liver injury, with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice.
POLG mutation screening should be performed in accordance with current clinical practice.
Fetal Risk
Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores and neurodevelopmental disorders following in utero exposure.
Valproate is therefore contraindicated for prophylaxis of migraine headaches in pregnant women and in women of childbearing potential who are not using effective contraception [see Contraindications (4)]. Valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable.
Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable. In such situations, effective contraception should be used [see Warnings and Precautions (5.2, 5.3, 5.4)].
In such situations, effective contraception should be used [see Warnings and Precautions (5.2, 5.3, 5.4)].
A Medication Guide describing the risks of valproate is available for patients [see Patient Counseling Information (17)].
Pancreatitis
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated.
- Valproate should not be administered to patients with hepatic disease or dysfunction.
 Immediately discontinue drug if significant hepatic dysfunction is suspected or apparent. Progression of dysfunction has occurred in spite of discontinuation of valproate.
Immediately discontinue drug if significant hepatic dysfunction is suspected or apparent. Progression of dysfunction has occurred in spite of discontinuation of valproate. - Valproate is contraindicated in patients known to have mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG; e.g., Alpers Huttenlocher Syndrome) and in children under two years of age who are suspected of having a POLG-related disorder. POLG-related disorder symptoms may include unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus at presentation, developmental delays, psychomotor regression, axonal sensorimotor neuropathy, myopathy cerebellar ataxia, ophthalmoplegia, or complicated migraine with occipital aura.
- DEPAKOTE and DEPAKOTE ER are contraindicated for use in prophylaxis of migraine headaches in women who are pregnant and in women of childbearing potential who are not using effective contraception.
- Valproate is contraindicated in patients with known hypersensitivity to the drug.
- Valproate is contraindicated in patients with known urea cycle disorders (UCDs). Hyperammonemic encephalopathy, sometimes fatal, has been reported following initiation of valproate. Symptoms of unexplained hyperammonemic encephalopathy during valproate therapy require prompt treatment (including discontinuation of valproate).
- Valproate can cause decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations (e.g., craniofacial defects, hypospadias, cardiovascular and limb malformations). In epileptic mothers, the rate of congenital malformations with in utero exposure is about four times higher compared to the rate with in utero exposure to other anti-seizure monotherapies. Lower cognitive test scores, including decreased IQ scores, were associated with in utero valproate exposure compared with in utero exposure to either another or no antiepileptic drug.
 Unless other medications have failed to provide adequate symptom control or are otherwise unacceptable, women of childbearing potential should not receive valproate.
Unless other medications have failed to provide adequate symptom control or are otherwise unacceptable, women of childbearing potential should not receive valproate. - Valproate can increase the risk of suicidal thoughts or behavior. Patients treated with any AED should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or unusual changes in mood or behavior. Counsel patients and families to be alert for and to immediately report depression, any unusual changes in mood or behavior, or suicidal thoughts, behavior, or acts of self-harm.
- Thrombocytopenia is dose-related. Decreases in other cell lines and myelodysplasia have also been associated with valproate. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of ≥110 ug/mL in females and ≥135 ug/mL in males or at 50 mg/kg/d. Check complete blood counts and coagulation times before starting therapy or surgery, at periodic intervals, and during pregnancy.
 Reduce dose or discontinue if hemorrhage, bruising, or hemostasis/coagulation disorder occur.
Reduce dose or discontinue if hemorrhage, bruising, or hemostasis/coagulation disorder occur. - Hyperammonemia has been associated with valproate; it may be present despite normal liver function tests and should be considered if hypothermia occurs. Asymptomatic elevations of ammonia are more common and require close monitoring. Discontinue valproate if ammonia increases.
- Concomitant administration of topiramate and valproate has been associated with hyperammonemia (with or without encephalopathy) in patients who have tolerated either drug alone.
- Hypothermia has been associated with valproate therapy both in conjunction with, and in the absence of, hyperammonemia. It can occur after starting topiramate treatment or after increasing the daily dose of topiramate. Consider stopping valproate if hypothermia develops.
- Drug Reaction with Eosinophilia and Systemic Syndrome (DRESS) / multi-organ hypersensitivity reactions have been reported and may be fatal or life-threatening.
 Patients typically present with fever, rash, and lymphadenopathy associated with other organ system involvement, e.g., hematologic abnormalities. Early symptoms may not include rash. Regardless, immediately evaluate and discontinue therapy for any signs of hypersensitivity.
Patients typically present with fever, rash, and lymphadenopathy associated with other organ system involvement, e.g., hematologic abnormalities. Early symptoms may not include rash. Regardless, immediately evaluate and discontinue therapy for any signs of hypersensitivity. - Carbapenem antibiotics (such as ertapenem, imipenem, meropenem) may reduce serum valproate concentrations to subtherapeutic levels, resulting in loss of seizure control.
- In a clinical trial, somnolence was associated with valproate in some elderly dementia patients along with reduced nutritional intake; weight loss; and a trend to have a lower baseline albumin concentration, higher BUN, and lower valproate clearance. Discontinuation occurred in some patients.
- Valproate may interact with drugs capable of enzyme induction; check valproate and concomitant drug levels periodically in the early course of therapy.
- Rare reports of medication residue in the stool have occurred, some in patients with anatomic (ileostomy or colostomy) or functional gastrointestinal disorders with shortened GI transit times or in the context of diarrhea.
 If medication residue occurs, monitor plasma valproate levels and patient’s clinical condition; alternative treatment may be considered.
If medication residue occurs, monitor plasma valproate levels and patient’s clinical condition; alternative treatment may be considered.
ADVERSE EVENTS
1,2- Most common adverse reactions (reported >5%) are abdominal pain, abnormal thinking, alopecia, amblyopia/blurred vision, amnesia, anorexia, asthenia, ataxia, back pain, bronchitis, constipation, depression, diarrhea, diplopia, dizziness, dyspepsia, dyspnea, ecchymosis, emotional lability, fever, flu syndrome, headache, increased appetite, infection, insomnia, nausea, nervousness, nystagmus, peripheral edema, pharyngitis, rash, rhinitis, somnolence, thrombocytopenia, tinnitus, tremor, vomiting, weight gain, and weight loss.
Keep DEPAKOTE and all other medications where children cannot reach them.
REFERENCES
- Depakote Delayed-Release Tablets [package insert]. North Chicago, IL: AbbVie Inc.
- Depakote ER [package insert].
 North Chicago, IL: AbbVie Inc.
North Chicago, IL: AbbVie Inc.
If you have any questions about AbbVie's Depakote.com website that have not been answered, click here. This website and the information contained herein is intended for use by US residents only, is provided for informational purposes only, and is not intended to replace a discussion with a healthcare provider. All decisions regarding patient care must be made with a healthcare provider and consider the unique characteristics of each patient.
- Contact us
- Important Safety Information
- Medication Guide
- Prescribing Information
- Site Map
- Privacy Policy
- Terms of Use
- Cookies Settings
©2020 AbbVie Inc. North Chicago, IL 60064 US-DPKT-200006 July 2020
North Chicago, IL 60064 US-DPKT-200006 July 2020
INDICATIONS
1,2Mania
DEPAKOTE® ER (divalproex sodium) extended-release tablets, for oral use, is a valproate and is indicated for the treatment of acute manic or mixed episodes associated with bipolar disorder, with or without psychotic features.
DEPAKOTE® (divalproex sodium) delayed-release tablets, for oral use, is a valproate and is indicated for the treatment of the manic episodes associated with bipolar disorder.
IMPORTANT SAFETY INFORMATION
1,2Warning: Life-Threatening Adverse Reactions
Hepatotoxicity
General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate and its derivatives. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
Depakote (divalproex sodium) dosing, indications, interactions, adverse effects, and more
Patient Education
divalproex oral
DIVALPROEX SODIUM EXTENDED-RELEASE - ORAL
(dye-VAL-pro-ex)
COMMON BRAND NAME(S): Depakote ER
WARNING: Rarely, this medication has caused serious (sometimes fatal) liver problems, usually within the first 6 months of starting treatment. Laboratory tests should be performed before you start treatment and periodically during treatment, especially within the first 6 months, to monitor this side effect.The risk of serious liver problems is increased in children younger than 2 years, especially if they have an inherited metabolic disorder, severe seizure disorder with mental retardation, organic brain disease, or if they take more than one seizure medication.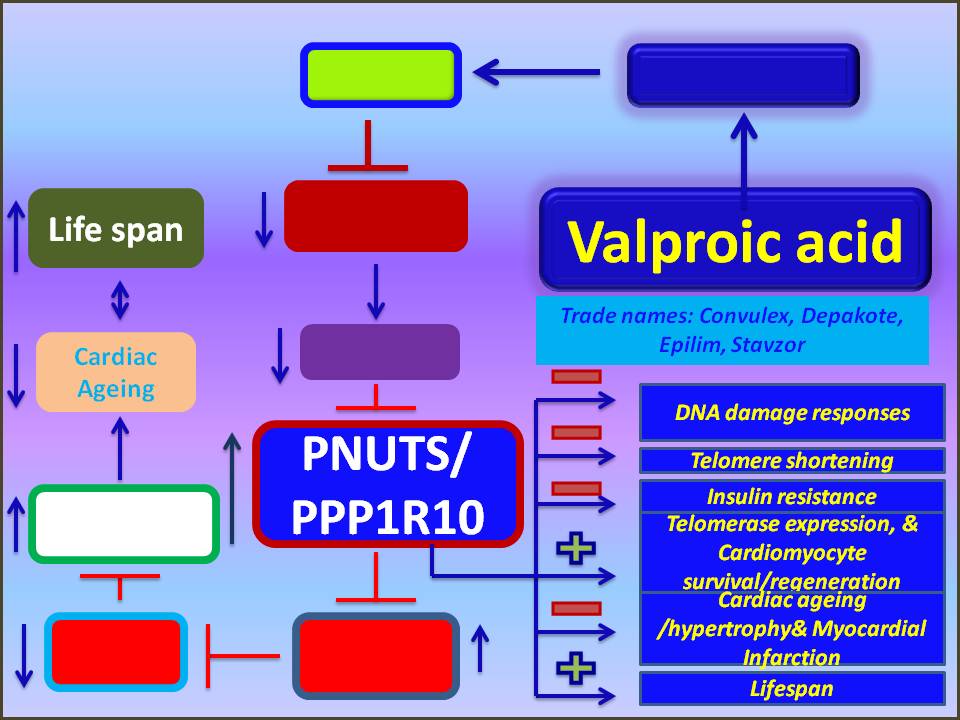 Talk with the doctor about the risks and benefits of using this medication in children younger than 2 years.Due to an increased risk for liver problems, people with certain inherited metabolic disorders (such as Alpers-Huttenlocher syndrome) should not use this medication. Children younger than 2 years who might have these disorders should not use this medication. Children older than 2 years who might have these disorders should be closely monitored during treatment with divalproex sodium. Talk to your doctor for details.This medication has rarely caused severe (sometimes fatal) disease of the pancreas (pancreatitis). This may occur at any time during treatment and can quickly worsen.Tell your doctor right away if you develop symptoms of liver problems or pancreatitis such as nausea/vomiting that doesn't stop, unusual tiredness, weakness, swelling of the face, stomach/abdominal pain, loss of appetite, dark urine, or yellowing eyes/skin.Taking this medication during pregnancy can cause birth defects, may lower your child's IQ, and may increase the risk of your child having certain brain/mental disorders (such as autism, attention deficit/hyperactivity disorder).
Talk with the doctor about the risks and benefits of using this medication in children younger than 2 years.Due to an increased risk for liver problems, people with certain inherited metabolic disorders (such as Alpers-Huttenlocher syndrome) should not use this medication. Children younger than 2 years who might have these disorders should not use this medication. Children older than 2 years who might have these disorders should be closely monitored during treatment with divalproex sodium. Talk to your doctor for details.This medication has rarely caused severe (sometimes fatal) disease of the pancreas (pancreatitis). This may occur at any time during treatment and can quickly worsen.Tell your doctor right away if you develop symptoms of liver problems or pancreatitis such as nausea/vomiting that doesn't stop, unusual tiredness, weakness, swelling of the face, stomach/abdominal pain, loss of appetite, dark urine, or yellowing eyes/skin.Taking this medication during pregnancy can cause birth defects, may lower your child's IQ, and may increase the risk of your child having certain brain/mental disorders (such as autism, attention deficit/hyperactivity disorder). Women of childbearing age should discuss the risks and benefits of this medication, other treatment options, and use of reliable forms of birth control with their doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately talk to your doctor. If you are taking divalproex sodium only to prevent migraine headaches, this medication must not be used during pregnancy. If you are taking divalproex sodium to treat seizures or mental/mood problems (such as bipolar disorder), do not stop taking this medication unless directed by your doctor. Untreated seizures and mental/mood problems (such as bipolar disorder) are serious conditions that can harm both a pregnant woman and her unborn baby.
Women of childbearing age should discuss the risks and benefits of this medication, other treatment options, and use of reliable forms of birth control with their doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately talk to your doctor. If you are taking divalproex sodium only to prevent migraine headaches, this medication must not be used during pregnancy. If you are taking divalproex sodium to treat seizures or mental/mood problems (such as bipolar disorder), do not stop taking this medication unless directed by your doctor. Untreated seizures and mental/mood problems (such as bipolar disorder) are serious conditions that can harm both a pregnant woman and her unborn baby.
USES: This medication is used to treat seizure disorders, mental/mood conditions (such as manic phase of bipolar disorder), and to prevent migraine headaches. It works by restoring the balance of certain natural substances (neurotransmitters) in the brain.
HOW TO USE: Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start taking divalproex sodium and each time you get a refill.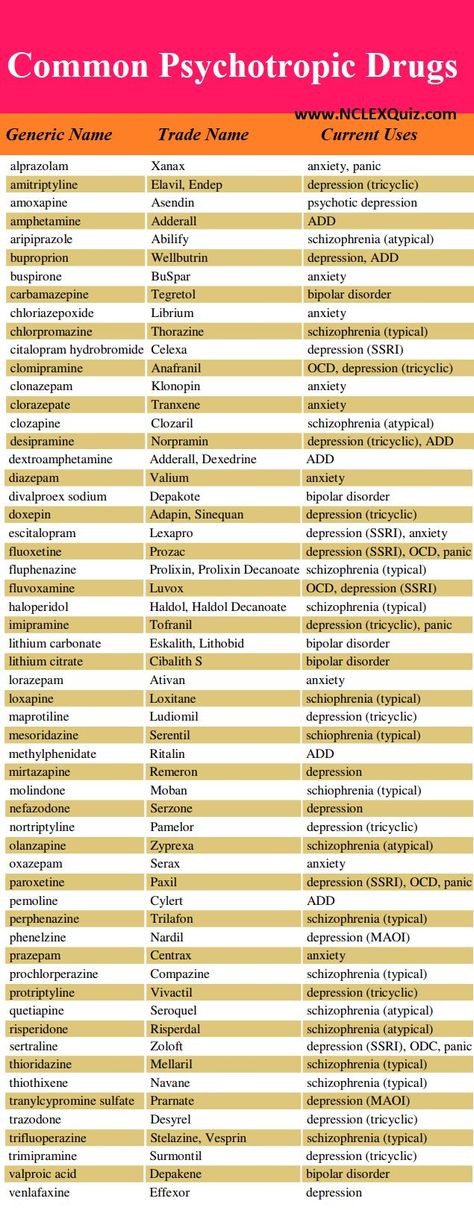 If you have any questions, ask your doctor or pharmacist.Take this medication by mouth once daily or as directed by your doctor. You may take it with food if stomach upset occurs. Swallow the tablets whole. Do not crush or chew the tablets. Doing so can release all of the drug at once, increasing the risk of side effects.The dosage is based on your age, weight, medical condition, response to treatment, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products). Use this medication regularly in order to get the most benefit from it. Remember to use it at the same time each day to keep the amount of medication in your blood constant.If this medication is used for seizures, do not stop taking it without consulting your doctor. Your condition may become worse if the drug is suddenly stopped. Your dose may need to be gradually decreased.Divalproex sodium does not relieve acute migraine headaches.
If you have any questions, ask your doctor or pharmacist.Take this medication by mouth once daily or as directed by your doctor. You may take it with food if stomach upset occurs. Swallow the tablets whole. Do not crush or chew the tablets. Doing so can release all of the drug at once, increasing the risk of side effects.The dosage is based on your age, weight, medical condition, response to treatment, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products). Use this medication regularly in order to get the most benefit from it. Remember to use it at the same time each day to keep the amount of medication in your blood constant.If this medication is used for seizures, do not stop taking it without consulting your doctor. Your condition may become worse if the drug is suddenly stopped. Your dose may need to be gradually decreased.Divalproex sodium does not relieve acute migraine headaches. Take other medications as directed by your doctor for acute attacks.Inform your doctor if your condition does not improve.
Take other medications as directed by your doctor for acute attacks.Inform your doctor if your condition does not improve.
SIDE EFFECTS: See also Warning section.Diarrhea, dizziness, drowsiness, hair loss, blurred/double vision, change in menstrual periods, ringing in the ears, shakiness (tremor), unsteadiness, weight changes may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.A small number of people who take anticonvulsants for any condition (such as seizure, bipolar disorder, pain) may experience depression, suicidal thoughts/attempts, or other mental/mood problems. Tell your doctor right away if you or your family/caregiver notice any unusual/sudden changes in your mood, thoughts, or behavior including signs of depression, suicidal thoughts/attempts, thoughts about harming yourself. Severe (sometimes fatal) brain disorder (encephalopathy) has rarely occurred, particularly in patients with certain metabolic disorders (urea cycle disorders). Tell your doctor right away if you develop unexplained weakness, vomiting, or sudden mental/mood changes (such as confusion).Get medical help right away if you have any very serious side effects, including: chest pain, easy bruising/unexplained bleeding, fast/slow/irregular heartbeat, swelling of hands/feet, uncontrolled eye movement (nystagmus), feeling cold/shivering, rapid breathing, loss of consciousness.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects.
Severe (sometimes fatal) brain disorder (encephalopathy) has rarely occurred, particularly in patients with certain metabolic disorders (urea cycle disorders). Tell your doctor right away if you develop unexplained weakness, vomiting, or sudden mental/mood changes (such as confusion).Get medical help right away if you have any very serious side effects, including: chest pain, easy bruising/unexplained bleeding, fast/slow/irregular heartbeat, swelling of hands/feet, uncontrolled eye movement (nystagmus), feeling cold/shivering, rapid breathing, loss of consciousness.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: See also Warning section.Before taking divalproex sodium, tell your doctor or pharmacist if you are allergic to it; or to valproic acid or valproate sodium; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: liver disease, pancreatitis, certain metabolic disorders (such as urea cycle disorders, Alpers-Huttenlocher syndrome), alcohol abuse, bleeding problems, brain disease (dementia), kidney disease, dehydration, poor nutrition.To lower the chance of getting cut, bruised, or injured, use caution with sharp objects like razors and nail cutters, and avoid activities such as contact sports. Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).This drug may make you dizzy or drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Limit alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).Children younger than 6 years may be at greater risk for liver problems and pancreatitis.Older adults may be more sensitive to the side effects of this drug, especially drowsiness, dizziness, unsteadiness, or tremor. Drowsiness, dizziness, unsteadiness can increase the risk of falling.Tell your doctor if you are pregnant or plan to become pregnant. You should not become pregnant while using divalproex sodium. Divalproex sodium may harm an unborn baby. If you become pregnant, talk to your doctor right away about the risks and benefits of this medication.
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).This drug may make you dizzy or drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Limit alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).Children younger than 6 years may be at greater risk for liver problems and pancreatitis.Older adults may be more sensitive to the side effects of this drug, especially drowsiness, dizziness, unsteadiness, or tremor. Drowsiness, dizziness, unsteadiness can increase the risk of falling.Tell your doctor if you are pregnant or plan to become pregnant. You should not become pregnant while using divalproex sodium. Divalproex sodium may harm an unborn baby. If you become pregnant, talk to your doctor right away about the risks and benefits of this medication.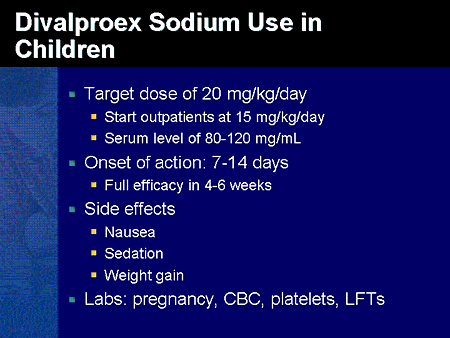 See also Warning section.This medication passes into breast milk. While there have been no reports of harm to nursing infants, consult your doctor before breast-feeding.
See also Warning section.This medication passes into breast milk. While there have been no reports of harm to nursing infants, consult your doctor before breast-feeding.
DRUG INTERACTIONS: Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some products that may interact with this drug include: certain antidepressants (such as amitriptyline, nortriptyline, phenelzine), certain antibiotics (carbapenems such as imipenem), irinotecan, mefloquine, orlistat, other medications for seizure (such as ethosuximide, lamotrigine, rufinamide, topiramate), rifampin, vorinostat, warfarin, zidovudine.Low-dose aspirin, as prescribed by your doctor for specific medical reasons such as heart attack or stroke prevention (usually 81-162 milligrams a day), should be continued. Consult your doctor or pharmacist if you are using aspirin for any reason.Tell your doctor or pharmacist if you are taking other products that cause drowsiness including alcohol, marijuana (cannabis), antihistamines (such as cetirizine, diphenhydramine), drugs for sleep or anxiety (such as alprazolam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), and opioid pain relievers (such as codeine, hydrocodone).Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.This drug may affect certain lab tests (such as urine ketones). Make sure laboratory personnel and your doctors know you use this medication.
Consult your doctor or pharmacist if you are using aspirin for any reason.Tell your doctor or pharmacist if you are taking other products that cause drowsiness including alcohol, marijuana (cannabis), antihistamines (such as cetirizine, diphenhydramine), drugs for sleep or anxiety (such as alprazolam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), and opioid pain relievers (such as codeine, hydrocodone).Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.This drug may affect certain lab tests (such as urine ketones). Make sure laboratory personnel and your doctors know you use this medication.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: excessive drowsiness, coma, irregular/slow heartbeat.
Canada residents can call a provincial poison control center. Symptoms of overdose may include: excessive drowsiness, coma, irregular/slow heartbeat.
NOTES: Do not share this medication with others.Laboratory and/or medical tests (such as drug levels, liver function tests, complete blood counts, clotting tests) should be performed before you start treatment, periodically to monitor your progress, or to check for side effects. Consult your doctor for more details.
MISSED DOSE: If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
STORAGE: Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Consult your pharmacist or local waste disposal company.
MEDICAL ALERT: Your condition can cause complications in a medical emergency. For information about enrolling in MedicAlert, call 1-888-633-4298 (US) or 1-800-668-1507 (Canada).
Information last revised July 2022. Copyright(c) 2022 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Valproate as a maintenance treatment for people with bipolar disorder after episodes of mood disorder
Bipolar disorder is a disorder that manifests itself in the form of manic and depressive states, and sometimes mixed. Depression is characterized by a decline in mood and energy, as well as an inability to experience joy, often in combination with other problems, such as sleep disturbance. Mania is the opposite - there is "too" a lot of energy, as well as problems with a good mood or irritability. In mixed states, the symptoms of depression and mania are combined. These episodes of mood disorder usually occur several times in a person's life, so long-term treatment (supportive care) can play a very important role in preventing relapses. Since valproate is a drug that may be useful in the treatment of the acute phase of bipolar disorder, in this review we wanted to answer the following question: is valproate useful as a maintenance agent in bipolar disorder?
Depression is characterized by a decline in mood and energy, as well as an inability to experience joy, often in combination with other problems, such as sleep disturbance. Mania is the opposite - there is "too" a lot of energy, as well as problems with a good mood or irritability. In mixed states, the symptoms of depression and mania are combined. These episodes of mood disorder usually occur several times in a person's life, so long-term treatment (supportive care) can play a very important role in preventing relapses. Since valproate is a drug that may be useful in the treatment of the acute phase of bipolar disorder, in this review we wanted to answer the following question: is valproate useful as a maintenance agent in bipolar disorder?
We searched for relevant studies (randomized controlled trials or RCTs) of long-term treatment of people with bipolar disorder with valproate or any other mood stabilizer, antipsychotic, or placebo. Three of us looked at RCTs to make sure the experiments were scientific. We extracted data from studies, put all the evidence together, and performed statistical analysis to find significant results.
We extracted data from studies, put all the evidence together, and performed statistical analysis to find significant results.
We searched up to 11 January 2013 and found six studies with 876 participants. The quality of the studies in terms of design was not very good, which means that the effects of some drugs may have been overestimated. The pooled studies suggest that valproate may help prevent the recurrence of bipolar disorder, especially depressive episodes. However, due to limited evidence, conclusions regarding valproate versus placebo and lithium (or other active agents) cannot be made with any reasonable degree of certainty. Lithium is an important drug to compare with valproate as it is known to be effective in preventing the recurrence of bipolar disorder. When we pooled the results of all studies that compared valproate with lithium, the evidence did not support superiority of valproate or lithium in terms of efficacy. People who took valproate for a long time were more likely to continue taking their prescribed medications than patients who were given lithium. Clinicians and patients should be aware of the side effects of valproate, including alopecia, tremor, and weight gain.
Clinicians and patients should be aware of the side effects of valproate, including alopecia, tremor, and weight gain.
We also found a study comparing valproate monotherapy with combination therapy (taking two drugs at the same time). This study compared people who took only lithium or valproate with those who took valproate and lithium at the same time. There is no evidence that the use of valproate and lithium, compared with lithium alone, provided greater patient adherence to the prescribed treatment.
Translation notes:
Translation: Lilianna Lenarovna Mullina. Editing: Kukushkin Mikhail Evgenievich. Project coordination for translation into Russian: Cochrane Russia - Cochrane Russia (branch of the Northern Cochrane Center on the basis of Kazan Federal University). For questions related to this translation, please contact us at: [email protected]; [email protected] by: Ekaterina Yudina
Valproic acid
Valproic acid is an anticonvulsant drug. It is widely used to treat various types of epilepsy in adults and children (large and small seizures, myoclonic, tonic-clonic and bipolar forms).
It is widely used to treat various types of epilepsy in adults and children (large and small seizures, myoclonic, tonic-clonic and bipolar forms).
Synonyms Russian
Apilepsin, depakine, orfiril, convulex.
Synonyms English
Acidum valproicum, Valproate, Valproic Acid, Depakote.
Test method
Immunochemiluminescent assay.
Units
μg/mL (micrograms per milliliter).
Which biomaterial can be used for research?
Venous blood.
How to properly prepare for the examination?
- Do not eat for 2-3 hours before the examination, you can drink pure non-carbonated water.
- Do not smoke for 30 minutes before the test.
General information about the study
Valproic acid inhibits the enzyme GABA transferase and, as a result, increases the content of the inhibitory neurotransmitter - gamma-aminobutyric acid (GABA) - in the nervous system. Under conditions of accumulation of GABA in the central structures of the brain, the threshold of excitability and the level of convulsive readiness decrease.
Under conditions of accumulation of GABA in the central structures of the brain, the threshold of excitability and the level of convulsive readiness decrease.
It is prescribed for patients suffering from epilepsy in all its manifestations (major and small seizures, myoclonic, tonic-clonic and bipolar form), behavioral changes inherent in epilepsy, affective disorders, as well as in the treatment of mania accompanied by bipolar disorders, with febrile convulsions in children, children's tics. Recently, it has also been used for neurological and mental disorders, for the prevention and treatment of migraine, neuropathic pain, behavioral disorders with panic episodes, aggression, etc. In addition, according to the results of studies, the antitumor activity of valproic acid, which is used in myelodysplastic syndromes, has been revealed. and acute monocytic leukemia.
Valproic acid preparations are rapidly and almost completely absorbed in the gastrointestinal tract, reaching a maximum concentration in the blood after 1.![]() 5-4 hours. Moreover, they are characterized by non-linear (dose-dependent) pharmacokinetics, when the concentration of the drug in the blood increases or decreases faster than the increased or reduced dose. If the upper limit of their average therapeutic level is exceeded, one must take into account the greater likelihood of side effects, in some cases up to intoxication.
5-4 hours. Moreover, they are characterized by non-linear (dose-dependent) pharmacokinetics, when the concentration of the drug in the blood increases or decreases faster than the increased or reduced dose. If the upper limit of their average therapeutic level is exceeded, one must take into account the greater likelihood of side effects, in some cases up to intoxication.
Valproic acid is primarily metabolized in the liver and may cause liver damage, especially during the first 6 months after starting treatment. Therefore, before therapy with valproic acid, each patient must pass a series of tests to assess liver function.
Valproic acid is characterized by low toxicity, severe side effects may occur during prolonged treatment. These include weight gain, nausea, ovarian cysts, tremors, dizziness, mental depression, manifestations of pancreatitis, and liver damage (severe abdominal pain and vomiting, lethargy, yellow discoloration of the skin or eyes). Side effects are more common in infants and young children, especially when treated in combination with other antiepileptic drugs. Poisoning with valproic acid drugs in most cases is not severe, serious overdoses, when the patient's life is in danger, are extremely rare. Most often, overdoses are associated with depression of the central nervous system. A significant excess of the dose of the drug can cause coma and respiratory failure. In this regard, in the course of treatment, it is required to determine the concentration of the drug in the blood of patients in order to assess the level of toxicity and control the excretion of drugs from the body.
Poisoning with valproic acid drugs in most cases is not severe, serious overdoses, when the patient's life is in danger, are extremely rare. Most often, overdoses are associated with depression of the central nervous system. A significant excess of the dose of the drug can cause coma and respiratory failure. In this regard, in the course of treatment, it is required to determine the concentration of the drug in the blood of patients in order to assess the level of toxicity and control the excretion of drugs from the body.
Individual dosage, based on therapeutic control of the drug concentration in the blood serum, increases the effectiveness and safety of therapy and allows you to achieve success in each case.
Blood is taken twice for analysis (this is the standard method): 1st sample - immediately before taking the drug by the patient (residual concentration after the last dose), 2nd sample - 2-3 hours after administration (reaching the maximum concentration in the blood) . At the same time, the procedure for taking blood is determined by the attending physician and may differ significantly from the standard method.
At the same time, the procedure for taking blood is determined by the attending physician and may differ significantly from the standard method.
What is research used for?
- To determine the most effective concentration of valproic acid and the mode of its administration in the treatment of certain diseases.
- To prevent the toxic effects of valproic acid preparations.
When is the test scheduled?
- In violation of the function of the liver, kidney or gastrointestinal tract, which affect the pharmacokinetics of valproic acid preparations.
- If there is doubt about the correctness of the drug intake by the patient.
- If acute poisoning with valproic acid is suspected (depression of consciousness, disorientation, increased drowsiness, tachycardia, pulmonary edema, manifestations of pancreatitis and liver damage).
- When prescribing the drug after reaching its stable concentration (minimum equal to five half-lives).
- When the patient is less than a year old - due to rapidly changing body weight (every 1-3 months).
- With positive dynamics of the disease against the background of ongoing therapy, in general, 1-2 times a year.
- When pregnancy is confirmed, at the 8-10th week of pregnancy, then 1 time in 2 months, at 34-36 weeks, after childbirth for 8 weeks twice, and with ongoing seizures - at each visit to a neurologist (epileptologist) .
- After prescribing or discontinuing other drugs in combination anticonvulsant therapy.
What do the results mean?
Reference values: 50 - 100 µg/ml.
Causes of increased valproic acid levels:
- overdosing and/or incorrect administration of valproic acid preparations;
- acute poisoning with valproic acid preparations.
Causes of a decrease in the level of valproic acid:
- excretion of valproic acid preparations from the body.

The generally accepted therapeutic concentration range for valproic acid is 50-100 µg/mL. The toxic effect develops at concentrations above 100 µg/ml.
What can influence the result?
- Decreased liver function in a number of liver diseases can cause an increase in the concentration of valproic acid in the blood serum even in the absence of an increase in the doses of the administered drug.
Important notes
- Before starting treatment with valproic acid, each patient should undergo a series of tests to evaluate liver function.
- Doses of drugs are selected by the attending physician for each patient individually, they cannot be changed independently.
- Simultaneous use of other anticonvulsants enhances the toxic effect of valproic acid and increases the risk of side effects, so it is necessary to control the concentration in the blood serum of these drugs.
Also recommended
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- Alkaline phosphatase, total
- Gamma-glutamyl transpeptidase (gamma-GT)
- Carbamazepine
- Lamotrigine
- Topiramate
Who orders the examination?
Neurologist, psychiatrist, psychotherapist, narcologist, toxicologist, general practitioner.