Does cymbalta cause nausea
What They Are and How to Manage Them
If you have depression, an anxiety disorder, or pain caused by certain conditions, your doctor might suggest Cymbalta (duloxetine) as a treatment option. Along with other questions you may have about the drug, you could be wondering about its side effects.
Cymbalta is a prescription drug that’s used as a long-term treatment for several different conditions.
Cymbalta is approved to treat the following conditions in adults:
- major depressive disorder (MDD)
- generalized anxiety disorder (GAD)
- pain caused by diabetic neuropathy (nerve damage caused by diabetes)
- fibromyalgia (a condition that causes pain throughout the body)
- long-term musculoskeletal pain (pain in the bones, muscles, tendons, ligaments, and nerves)
Cymbalta is also approved to treat these conditions in children:
- GAD in children ages 7 years and older
- fibromyalgia in children ages 13 years and older
For more information about Cymbalta, including details about its uses, see this in-depth article.
Like other drugs, Cymbalta can cause mild or serious side effects. Keep reading to learn more.
Some people may experience mild or serious side effects during Cymbalta treatment. Some side effects are more common than others.
Cymbalta’s more common side effects include:
- fatigue (lack of energy)
- nausea
- constipation
- reduced appetite
- sweating more than usual*
- dizziness
* To learn more about this side effect, see “Side effects explained” below.
Mild side effects have been reported with Cymbalta, many of which are also more common side effects of the drug. Cymbalta’s mild side effects include:
- sleepiness
- fatigue (lack of energy)
- nausea
- dry mouth
- constipation
- reduced appetite
- dizziness
- headache
- sexual side effects in females and males*
- sweating more than usual†
- trouble sleeping†
- headache
- abdominal (belly) pain
* In this article, we use the terms “female” and “male” to refer to someone’s sex assigned at birth. For information about the difference between sex and gender, see this article. For details on sexual side effects Cymbalta may cause, see “Sexual side effects in women and men” below.
For information about the difference between sex and gender, see this article. For details on sexual side effects Cymbalta may cause, see “Sexual side effects in women and men” below.
† To learn more about this side effect, see “Side effects explained” below.
In most cases, these side effects should be temporary. Some may be easily managed, too. But if you have any symptoms that are ongoing or that bother you, talk with your doctor or pharmacist. And don’t stop using Cymbalta unless your doctor recommends it.
Cymbalta may cause mild side effects other than the ones listed above. See the Cymbalta medication guide for details.
Note: After the Food and Drug Administration (FDA) approves a drug, it tracks side effects of the medication. If you’d like to notify the FDA about a side effect you’ve had with Cymbalta, visit MedWatch.
Serious side effects from Cymbalta aren’t common, but they can happen. Serious side effects that have been reported with Cymbalta include:
- suicidal behaviors and thoughts*
- liver damage†
- eye problems†
- allergic reaction†‡
- fainting or dizziness when standing up
- blood pressure changes
- serotonin syndrome, a rare side effect of drugs that affect serotonin, a brain chemical
- low sodium levels
- urination problems
- severe skin reaction, such as Stevens-Johnson syndrome
- unusual bleeding or bruising
* Cymbalta has a boxed warning for this side effect. This is the most serious warning from the Food and Drug Administration (FDA). To learn more, see “Side effects explained” below.
This is the most serious warning from the Food and Drug Administration (FDA). To learn more, see “Side effects explained” below.
† To learn more about this side effect, see “Side effects explained” below.
‡ An allergic reaction is possible after using Cymbalta. But this side effect wasn’t reported in studies.
If you develop serious side effects while taking Cymbalta, call your doctor right away. If the side effects seem life threatening or if you think you’re having a medical emergency, immediately call 911 or your local emergency number.
The most common side effects of Cymbalta in children may include:
- weight loss
- reduced appetite
- fatigue (lack of energy)
- nausea or vomiting
- diarrhea
Sexual side effects from taking Cymbalta are possible and may be more common in males than females.* In studies, sexual side effects were reported in a small percentage of males and females during Cymbalta treatment. Some of these side effects, such as erectile dysfunction, may be more likely to occur when taking a higher dosage of Cymbalta.
Males who took Cymbalta reported significantly more sexual side effects compared with those who took a placebo (a treatment that contains no active drug). The sexual side effects reported in males included:
- decrease in or loss of libido (sex drive)
- trouble becoming aroused
- erectile dysfunction
- difficulty reaching orgasm
- delayed ejaculation or being unable to ejaculate
Females who took Cymbalta also reported sexual side effects. But these side effects were similar to those experienced by females who received a placebo. Sexual side effects included:
- decreased libido (sex drive)
- trouble becoming aroused
- reduced vaginal lubrication
- difficulty reaching orgasm
Note that some males and females in this study reported improvements in sexual desire, performance, and satisfaction with Cymbalta treatment. This may be because the medication helped to reduce the symptoms of their condition. As a result, their sexual health may have also improved.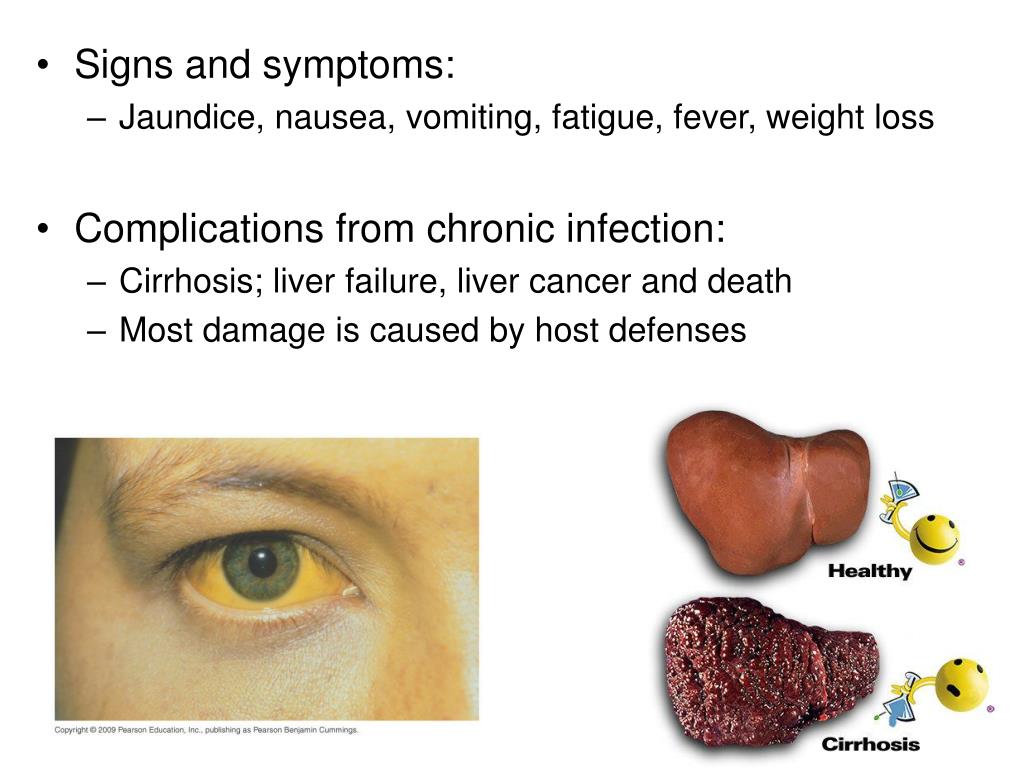
* In this article, we use the terms “female” and “male” to refer to someone’s sex assigned at birth. For information about the difference between sex and gender, see this article.
Some side effects of Cymbalta can affect your long-term health, but this isn’t common.
For example, liver failure is a rare but serious side effect of Cymbalta. Heavy alcohol use may increase the risk of liver failure. The liver damage that develops from this side effect doesn’t go away once a person stops taking Cymbalta.
You may be wondering if it’s safe to take Cymbalta long term. Studies have tested the drug’s safety for up to 6 months. A 2009 study has shown Cymbalta to be safe to use when taken for 12 months. Your doctor can tell you about their understanding of long-term Cymbalta use.
It’s a good idea to go over all of your medications with your doctor every so often. Together, you can discuss your condition and consider whether you should continue Cymbalta long term.
Keep reading to get answers to some frequently asked questions about Cymbalta’s side effects.
How long do Cymbalta’s side effects last?
How long side effects from Cymbalta last can vary. Some of the more common side effects are usually temporary, such as sleepiness, dizziness, and reduced appetite. These side effects typically ease within a few days or weeks after starting treatment.
Common side effects may get worse after your doctor increases your dosage, but this is usually temporary.
Other side effects are more likely to continue for as long as you’re taking the drug. Examples include sweating more than usual and sexual side effects. These side effects usually aren’t severe.
Cymbalta side effects can affect each person differently. For example, nausea may be a mild, temporary side effect for some people. For others it can be bothersome. In studies, a small percentage of people had to stop taking the drug due to nausea.
If you’re experiencing troublesome side effects, you shouldn’t suddenly stop taking Cymbalta. It’s best to talk with your doctor first. If you and your doctor decide that you should stop the drug, they’ll guide you on how best to do so.
It’s best to talk with your doctor first. If you and your doctor decide that you should stop the drug, they’ll guide you on how best to do so.
Do seniors have a higher risk for side effects from Cymbalta?
No, this doesn’t seem to be the case. In general, older adults (ages 65 years and older) have a higher risk for medication side effects compared with younger adults. But in studies of Cymbalta, older adults had similar side effects to those of younger adults.
Can Cymbalta cause weight gain?
Cymbalta doesn’t typically cause weight gain. In studies, weight gain wasn’t reported as a side effect.
In fact, weight loss is more likely than weight gain with Cymbalta. This is because the drug commonly causes reduced appetite and nausea.
These side effects may lead to weight loss, especially in children. Because of this, if your child is taking Cymbalta, their doctor will monitor your child’s weight and height during Cymbalta treatment.
If you have questions about weight changes with Cymbalta, talk with your doctor.
Will Cymbalta side effects differ depending on the strength I use (20 mg, 30 mg, or 60 mg)?
Some side effects of Cymbalta may be dependent on dose. Cymbalta comes in the following strengths: 20 milligrams (mg), 30 mg, and 60 mg. A higher strength of the drug might come with a higher risk of certain side effects.
Common dose-dependent side effects of Cymbalta include nausea, fatigue (lack of energy), constipation, dizziness, reduced appetite, and sweating more than usual.
Learn more about some of the side effects Cymbalta may cause.
Sweating more than usual
Sweating more than usual is a common side effect of Cymbalta. This side effect may be worse with higher doses of the drug. In addition, hot flashes (also called hot flushes) are a possible side effect of this medication.
Some people may notice increased sweating only in certain situations, such as when they’re active or during humid weather. Others may have increased sweating more often, including while trying to sleep.
What might help
Increased sweating isn’t a harmful side effect, but it may be uncomfortable. Here are a few tips that may help ease this side effect:
- Use a strong deodorant.
- Shower more often.
- Wear light fabrics
- Use a fan at night.
If this side effect continues to bother you, your doctor may suggest adjusting your dosage or switching to a different drug.
But if Cymbalta is particularly effective for your condition, you may not want to switch to a different drug. In this case, your doctor may suggest treatments for your sweating. Examples of drugs sometimes used to treat this side effect include:
- benztropine (Cogentin)
- cyproheptadine
- terazosin
If you have concerns about sweating more than usual with Cymbalta, talk with your doctor.
Eye problems
Eye problems aren’t a common side effect of Cymbalta. But this drug may increase the risk of serious eye problems, such as glaucoma (a buildup of pressure within the eye).
Cymbalta can cause a person’s pupils to dilate. This can trigger a serious eye problem, including vision loss, especially for someone who has closed-angle glaucoma. Symptoms can include:
- sudden vision changes
- eye pain
- eye redness
- swelling in or around your eye
What might help
If you have closed-angle glaucoma, you shouldn’t take Cymbalta. If you’re not sure whether you have this condition, consider visiting an eye doctor. The results of an eye exam can help you and your doctor decide if it’s safe for you to take Cymbalta.
If you develop any of the above symptoms while taking Cymbalta, you should seek medical attention. Urgent treatment is needed to help prevent permanent vision loss.
If you have questions about eye problems that Cymbalta may cause, talk with your doctor.
Liver damage
Although rare, Cymbalta can cause serious liver damage that could be fatal. The risk of this side effect may be higher with alcohol use. It could also be higher in people who already had liver problems before starting Cymbalta.
It could also be higher in people who already had liver problems before starting Cymbalta.
The following may indicate that there’s a problem with your liver:
- pain in the upper right part of your abdomen (belly)
- itching
- dark urine
- yellowing of your skin or whites of your eyes
- increased liver enzyme levels
What might help
To help prevent this side effect, talk with your doctor about any liver problems you’ve had. It’s also important to be honest about your alcohol use. Talk with your doctor about whether you’ve had problems with your liver or alcohol use in the past.
If you develop any of the above symptoms, get emergency medical care right away.
Trouble sleeping
Insomnia (trouble falling asleep or staying asleep) can occur with Cymbalta. In studies, this side effect was more commonly reported in people taking the drug for long-term musculoskeletal pain. (This is pain in the bones, muscles, tendons, ligaments, and nerves. ) Waking up earlier than desired was also reported with Cymbalta.
) Waking up earlier than desired was also reported with Cymbalta.
Insomnia is also a common side effect reported in children taking Cymbalta.
What might help
Here are a few tips that may help to improve your sleep:
- Try to exercise regularly.
- Avoid caffeine after lunch.
- Practice good sleep hygiene.
If you’re having insomnia since starting Cymbalta, talk with your doctor. They may suggest the temporary use of a sleep aid, such as melatonin. Or they may adjust your dosage or discuss other treatment options with you.
Suicidal behaviors and thoughts
Cymbalta has a boxed warning for suicidal behaviors and thoughts. A boxed warning is the most serious warning from the Food and Drug Administration (FDA). It alerts doctors and patients about drug effects that may be dangerous.
Antidepressants such as Cymbalta may increase the risk of suicidal behaviors and thoughts in children and young adults ages 24 years or younger. This is a rare side effect. Studies show that the risk is higher after a person first starts treatment or increases their dose.
This is a rare side effect. Studies show that the risk is higher after a person first starts treatment or increases their dose.
What might help
While taking Cymbalta, you should watch for any new behaviors, feelings, or thoughts. This is especially important in the first few weeks after starting Cymbalta or after your dose is adjusted.
Consider using a journal or app to make notes about your mood. You may want to ask your loved ones to let you know if they notice that you’re acting differently. Tell your doctor right away if you or someone else notices any changes in your behavior or moods.
Suicide preventionIf you think someone is at immediate risk of self-harm or hurting another person:
- Call 911 or your local emergency number.
- Stay with the person until help arrives.
- Remove any guns, knives, medications, or other things that may cause harm.
- Listen, but don’t judge, argue, threaten, or yell.
If you or someone you know is considering suicide, get help from a crisis or suicide prevention hotline.
Try the National Suicide Prevention Lifeline at 800-273-8255.
Allergic reaction
Like most drugs, Cymbalta can cause an allergic reaction in some people. But this side effect wasn’t reported in studies.
Symptoms can be mild or serious and can include:
- skin rash
- itchiness
- flushing (temporary warmth, redness, or deepening of skin color)
- swelling under your skin, typically in your eyelids, lips, hands, or feet
- swelling of your mouth, tongue, or throat, which can make it hard to breathe
What might help
If you have mild symptoms of an allergic reaction, such as a mild rash, call your doctor right away. To manage symptoms, they may suggest an over-the-counter antihistamine you take by mouth, such as Benadryl (diphenhydramine). Or they may recommend a product you apply to your skin, such as hydrocortisone cream.
If your doctor confirms you had a mild allergic reaction to Cymbalta, they’ll decide if you should continue using it.
If you have symptoms of a severe allergic reaction, such as swelling or trouble breathing, call 911 or your local emergency number right away. These symptoms could be life threatening and require immediate medical care.
If your doctor confirms you had a serious allergic reaction to Cymbalta, they may have you switch to a different treatment.
Keeping track of side effectsDuring Cymbalta treatment, consider keeping notes on any side effects you’re having. You can then share this information with your doctor. This is especially helpful to do when you first start taking new drugs or using a combination of treatments.
Your side effect notes can include things such as:
- what dose of drug you were taking when you had the side effect
- how soon after starting that dose you had the side effect
- what your symptoms were from the side effect
- how it affected your daily activities
- what other medications you were also taking
- any other information you feel is important
Keeping notes and sharing them with your doctor will help your doctor learn more about how Cymbalta affects you.
Your doctor can use this information to adjust your treatment plan if needed.
Cymbalta has several warnings that may affect whether you can safely use this drug to treat your condition.
Boxed warning: Suicidal behaviors and thoughts
Cymbalta has a boxed warning for suicidal behaviors and thoughts. A boxed warning is the most serious warning from the Food and Drug Administration (FDA).
Antidepressants such as Cymbalta may increase the risk of suicidal behaviors and thoughts in children and young adults ages 24 years or younger. After starting Cymbalta, you should watch for any new behaviors, feelings, or thoughts. Tell your doctor right away if you or your loved ones notice any changes in your behavior or moods.
To learn more, see “Side effects explained” above.
Other warnings
Cymbalta may not be right for you if you have certain medical conditions or other factors that affect your health. Talk with your doctor about your health history before you take Cymbalta. The list below includes factors to consider.
The list below includes factors to consider.
Liver or kidney problems. The liver and kidneys help clear Cymbalta from the body. In a person who has liver or kidney problems, Cymbalta levels could become too high in their body. This can worsen the drug’s side effects. In rare cases, Cymbalta may cause liver failure. People who already have liver problems may be at higher risk for this side effect. Before taking Cymbalta, tell your doctor about any liver or kidney problems you have.
Closed-angle glaucoma. Cymbalta can cause the pupils to dilate, which may worsen certain eye problems. If you have closed-angle glaucoma, vision loss could occur with Cymbalta. Talk with your doctor about other treatment options.
Heart or blood pressure conditions. Cymbalta may increase your blood pressure. If you already have high blood pressure or heart problems, taking Cymbalta could worsen your condition. Before starting this drug, tell your doctor about any heart or blood pressure problems you may have.
Slow stomach emptying. Cymbalta capsules are delayed-release. As such, they have a special coating that helps protect the drug against the acid in your stomach. If you have a condition that can slow stomach emptying, such as diabetes, the special coating may get destroyed. This could make Cymbalta less effective for treating your condition. Before taking Cymbalta, talk with your doctor about any medical conditions that you have.
Diabetes. If you have diabetes, Cymbalta may make it more difficult to manage your blood sugar levels. Before taking Cymbalta, talk with your doctor about a plan for managing your blood sugar levels.
Seizures. Cymbalta may increase the risk of seizures. But the drug hasn’t been studied in people with epilepsy (a seizure disorder). If you have a seizure disorder, your doctor may suggest another treatment option for your condition.
Bipolar disorder or mania. Cymbalta may bring on or worsen certain symptoms of bipolar disorder or mania. If you have bipolar disorder or mania, talk with your doctor about the risks involved in taking Cymbalta. If you aren’t sure whether you have either condition, your doctor may screen you for them before you take Cymbalta.
If you have bipolar disorder or mania, talk with your doctor about the risks involved in taking Cymbalta. If you aren’t sure whether you have either condition, your doctor may screen you for them before you take Cymbalta.
Low sodium levels. Cymbalta can cause low sodium levels. If you have problems with your sodium levels, talk with your doctor before you take Cymbalta.
Bleeding problems. Cymbalta may raise your risk for bruising or bleeding problems. If you have a condition that causes bleeding problems, taking this drug may worsen your condition. Before starting Cymbalta, tell your doctor about any current or past bleeding problems.
Allergic reaction. If you’ve had an allergic reaction to Cymbalta or any of its ingredients, you shouldn’t take Cymbalta. Ask your doctor what other medications are better options for you.
Alcohol use and Cymbalta
Drinking alcohol isn’t recommended with Cymbalta, especially heavy alcohol use.
Alcohol may worsen some of Cymbalta’s common side effects, such as:
- nausea
- sleepiness
- dizziness
Heavy alcohol use while taking Cymbalta can increase your risk for serious liver problems and liver failure. This can be life threatening.
If you drink alcohol, talk with your doctor about whether it’s safe for you to do so while taking Cymbalta. You can also ask them how much alcohol is safe for you to drink.
Pregnancy and breastfeeding while taking Cymbalta
Cymbalta use isn’t recommended during pregnancy because its effects aren’t fully known. The drug may cause harm to a developing fetus.
If you’re pregnant or you’re considering a pregnancy, talk with your doctor. They’ll tell you about treatment options that may be safer during this time.
Cymbalta passes into breast milk, and the drug may affect a child who is breastfed. There have been reports of drowsiness and feeding problems in children breastfed by people taking Cymbalta.
If you’re breastfeeding or have plans to breastfeed, talk with your doctor. They can help you weigh the pros and cons of breastfeeding while taking this drug.
Many people find that Cymbalta is an effective treatment for their condition. When you’re considering Cymbalta as a treatment option, it’s a good idea to talk with your doctor about your risk for side effects. Here are some questions that you may want to ask:
- Do my medical conditions increase my risk for side effects with Cymbalta?
- Are there other ways to help me manage side effects from Cymbalta?
- Are there any lifestyle changes I can make that may help to reduce my need to take Cymbalta in the future?
For tips on managing your mental health and personal stories, you can sign up for Healthline’s newsletter on anxiety and depression.
Q:
My child has generalized anxiety disorder, and their doctor suggested Cymbalta treatment. I’m concerned about the drug’s suicide warning. How common or rare is this side effect, and how can it be avoided?
How common or rare is this side effect, and how can it be avoided?
Anonymous patient
A:
The risk of suicidal behaviors and thoughts with Cymbalta use is rare. But Cymbalta does have a boxed warning for this side effect. A boxed warning is the most serious warning from the Food and Drug Administration (FDA).
When compared with a placebo (a treatment with no active drug), antidepressants such as Cymbalta were associated with increased suicidal behaviors and thoughts. This risk affected children and young adults ages 24 years and younger.
For more about this FDA boxed warning, see “Side effects explained” above.
If your child’s doctor prescribes Cymbalta, make sure to monitor your child for any changes in their behavior or mood. This is especially important when treatment with Cymbalta begins or the dosage is changed. Contact your child’s doctor right away if you notice any changes in your child’s behavior or mood.
Melissa Badowski, PharmD, MPH, FCCPAnswers represent the opinions of our medical experts. All content is strictly informational and should not be considered medical advice.
Disclaimer: Healthline has made every effort to make certain that all information is factually correct, comprehensive, and up to date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or another healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
Cymbalta Side Effects | Warnings & Withdrawal Symptoms
Common Cymbalta side effects include nausea, headache and insomnia. While rare, some serious documented side effects are bleeding of the gastrointestinal tract, liver damage and suicidal ideation. Long lasting withdrawal symptoms have been reported when patients stopped taking Cymbalta.
Cymbalta, a serotonin-norepinephrine reuptake inhibitor (SNRI), is used to treat depression, anxiety, fibromyalgia, chronic musculoskeletal pain and pain related to nerve damage from diabetes.
The most common side effects of the drug include nausea, headache, dry mouth and sleepiness — but more serious side effects may occur.
Like most antidepressants, Cymbalta (duloxetine) may trigger suicidal thoughts and behavior in children, adolescents and adults under the age of 24. As a result, patients should be closely monitored for changes in mood and behavior and the emergence of such symptoms.
People who stop using the antidepressant have also reported debilitating withdrawal symptoms, including electric shock sensations, extreme mood swings, and physical and neurological problems. Other serious side effects — ranging from birth defects to liver toxicity to a dangerous rise in serotonin levels — have also been reported.
Other serious side effects — ranging from birth defects to liver toxicity to a dangerous rise in serotonin levels — have also been reported.
Common Side Effects of Cymbalta
A review and analysis published in July 2021 looked at more than 300 studies of antidepressants. Cymbalta showed significantly higher rates of gastrointestinal side effects than the placebo.
Another review, published in April 2021, analyzed clinical trials including those submitted in the FDA Approval Package for duloxetine. The review found trial participants who took Cymbalta were more likely to experience common side effects than those given a placebo.
The most common side effects associated with Cymbalta were:
- Dry mouth
- Nausea
- Insomnia
- Sexual Dysfunction
- Constipation
- Diarrhea
- Dizziness
- Decreased appetite
- Sweating
- Intestinal gas
Related symptoms reported with duloxetine also include heartburn, vomiting, urinary frequency and stomach pains. As a result of several of these symptoms, patients also reported fatigue and weight loss.
As a result of several of these symptoms, patients also reported fatigue and weight loss.
Cymbalta, other types of SNRIs and selective serotonin reuptake inhibitors (SSRIs) remain the most widely prescribed antidepressants for major depression. But the common side effects of Cymbalta and other antidepressants can make them poorly tolerated, often leading patients to want to stop taking them.
Subscribe to Our Newsletter
Stay up to date on dangerous drugs and devices, keep up on lawsuit and settlement news, learn about FDA recalls and more.
Sign Up Now
Long Term Side Effects of Cymbalta
Most Cymbalta side effects, including severe adverse reactions like drug-induced liver injury (DILI), are reversible with proper treatment. There are examples of long term side effects of Cymbalta, however.
Weight gain can occur for some patients, especially people prescribed Cymbalta in combination with other medications. Research published in May 2021 found that veterans taking duloxetine with pregabalin to treat neuropathy had increased rates of weight gain.
Research published in May 2021 found that veterans taking duloxetine with pregabalin to treat neuropathy had increased rates of weight gain.
Neuropathy patients experience muscle weakness as a result of nerve damage or dysfunction, making exercising to lose weight challenging. Significant weight gain can increase risk of long-term health conditions such as coronary artery disease and cancer.
For adults 65 years or older, dizziness increased rates of falls, according to a May 2019 study. Injuries from falls can have long-term health consequences for geriatric patients.
Some potential long term effects require more study. A May 2020 report in The Psychiatric Journal notes, “Well-designed pharmaco-epidemiological studies evaluating the causal relationship between long-term use of duloxetine and cardiovascular disease is still necessary.”
Typically side effects diminish as patients adjust to the medication. Many patients can successfully take Cymbalta long term without complications. But protracted withdrawal syndrome from Cymbalta, as with other antidepressants, can be long-lasting, especially after long-term use.
But protracted withdrawal syndrome from Cymbalta, as with other antidepressants, can be long-lasting, especially after long-term use.
Cymbalta Withdrawal
Cymbalta addiction is a common problem, according to a research paper on duloxetine and its potential damage to the nervous system published in May 2020. The paper explained addiction is common with most psychoactive antidepressants.
Withdrawal symptoms can occur both when patients taper or completely stop taking it. Withdrawal symptoms can be managed with help from a doctor.
The most common withdrawal symptom reported for Cymbalta is nausea. Some people experience more severe and longer lasting protracted withdrawal symptoms.
Reported Cymbalta withdrawal symptoms include:
- Anxiety
- Depression
- Emerging suicidality
- Agitation
- Headache
- Fatigue
- Dizziness
- Brain Zaps (electrical shock sensations in the brain)
- Visual changes
- Muscle aches
- Tremor
- Diarrhea
- Nausea
- Sleep problems
- Cognitive impairment
A qualitative analysis published December 2020 noted, “It is now suspected that antidepressant withdrawal syndrome is more common and severe than earlier presumed, affecting roughly 30–50% of those who attempt to stop their treatment. ”
”
The authors also noted protracted withdrawal syndrome can be debilitating for some patients and pointed to the link between withdrawal and suicide.
Alexander Bingham, MA, PhD | 1:29 Can you explain the withdrawal symptoms associated with antidepressants?
Somatic Clinical Psychologist Andrew Bingham explains the withdrawal symptoms associated with antidepressants.
Cymbalta Tapering Timeline
Those looking to stop taking Cymbalta should follow a tapered approach under the guidance of a doctor. An individual’s personal Cymbalta tapering timeline should be customized for their specific situation.
Medical history, current dosage, amount of time taking duloxetine and any reactions they’ve had to Cymbalta should be discussed with their doctor.
Broad guidelines suggest tapering in increments of 30 mgs, progressing from a 120 mg dose to 90 mgs to 60 mgs, etc. Depending on a patient’s reaction to each dose reduction, the amount and time between changes can be adjusted.
Depending on a patient’s reaction to each dose reduction, the amount and time between changes can be adjusted.
Cymbalta’s label states: “If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that discontinuation can be associated with certain symptoms.”
Serious Side Effects and Warnings
Most serious side effects are rare and reversible. Some of the most serious side effects are related to drug interactions, alcohol use and co-existing medical conditions.
Cymbalta may cause enlarged liver and increased liver enzymes.
A March 2021 case report of a 43-year-old woman who was admitted to the hospital for acute liver failure demonstrates that while drug induced liver injury (DILI) is rare, it has been linked to use of Cymbalta. The patient was negative for viral and bacterial causes of liver failure and had no prior history of hepatic or liver diseases.
Between 2005 and 2017, the U.S. Food and Drug Administration issued several warnings regarding serious side effects, including the particular dangers of liver injury for heavy drinkers. The FDA advises against prescribing Cymbalta for patients who are heavy drinkers or have chronic liver disease.
Serious side effects include:
- Gastrointestinal bleeding: Also referred to as “abdominal bleeding,” this symptom was prevalent enough among patients taking antidepressants like Cymbalta to prompt the FDA to require it be listed on product labeling.
- Liver injury: As noted above, drug induced liver injury, liver damage and failure and hepatotoxicity (toxic liver disease) are rare, but serious side effects.
-
Serotonin Syndrome: The FDA required warnings about duloxetine’s link to increased levels of accumulated serotonin in the body that can result in agitation, palpitations and changes in blood pressure.
 There have even been reports of hallucinations, coma and suicidal ideation.
There have even been reports of hallucinations, coma and suicidal ideation. - Skin reactions: Skin reactions can include rash and even hair loss. Hair loss and other skin conditions typically were reversible.
- Glaucoma: While exceptionally rare, there have been reported cases of duloxetine-induced bilateral acute angle-closure glaucoma. One patient in a report published in 2017 developed glaucoma within 15 days of beginning Cymbalta.
The FDA required Cymbalta to add a black box warning regarding the increased risk of suicidal ideation while taking Cymbalta. All SNRI and SSRI antidepressants are required to carry this warning in their labeling information. A black box warning is the FDA’s strongest warning.
Suicidal ideation risk increases with interactions with other medications and the use of alcohol or drugs. For anyone experiencing thoughts of suicide, please contact your doctor or a crisis line immediately.
Feeling Hopeless? Get Help Now.
- National Suicide Hotline
- 1-800-273-8255
suicidepreventionlifeline.org - Crisis Textline
- Text “HELLO” to 741741
crisistextline.org
Black Box Warning for Suicide Risk
Cymbalta carries a black box warning stating that antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents and young adults in short-term studies. A black box warning is the FDA’s strongest warning, and the agency requires manufacturers to include this warning in the labeling information for all selective serotonin reuptake inhibitor (SSRI) and SNRI medications.
“In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors.”
Some patients also reported that discontinuing the drug has led to developing suicidal thoughts. During clinical trials, a 19-year-old patient who was being weaned off the drug killed herself in an Eli Lilly laboratory, The Associated Press reported. Nearly 20 percent of the remaining participants dropped out of the trial within a month.
During clinical trials, a 19-year-old patient who was being weaned off the drug killed herself in an Eli Lilly laboratory, The Associated Press reported. Nearly 20 percent of the remaining participants dropped out of the trial within a month.
New users should be aware of these dangers when they receive prescriptions for Cymbalta.
“In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors,” the drug’s label reads.
Pregnancy and Breastfeeding Risks
Women taking Cymbalta for depression, stress urinary incontinence (SUI) or other conditions should be aware of potential dangers of taking the drug while pregnant. Adverse effects have occurred in testing of pregnant animals, and the risk cannot be ruled out in humans.
A 2013 analysis of data from the Lilly Safety System database identified 90 abnormal outcomes in 233 pregnancies with “known pregnancy outcomes.”
These outcomes included 41 spontaneous abortions, 25 post/perinatal conditions and 19 premature births. But Dr. Sharon L. Hoog and other authors of the study concluded that these rates were consistent with abnormalities in the general population. It’s important to note that Eli Lilly conducted the study, and the authors recognized limitations of the data.
But Dr. Sharon L. Hoog and other authors of the study concluded that these rates were consistent with abnormalities in the general population. It’s important to note that Eli Lilly conducted the study, and the authors recognized limitations of the data.
Regardless, you should to talk to your doctor if you are pregnant or may become pregnant during Cymbalta use. Breastfeeding while using the drug is not recommended.
Medical advice for doctors | Remedium.ru
21.10.2022
Some aspects of adherence to combined hormonal contraception in young women
I.G. Zhukovskaya, L.F. Khuzin; IGMA
Introduction. In addition to protecting against unwanted pregnancies, combined hormonal contraceptives (CHCs) can improve women's health and quality of life. The awareness of Russian women about CHC is 85%, however, only 13% of women in Russia have experience of a long. ..
..
More
20.10.2022
Efficacy of probiotics in the treatment of irritable bowel syndrome
V.V. Tsukanov, A.V. Vasyutin, Yu.L. Thin ; Krasnoyarsk Scientific Center SB RAS
A review of current literature data was made, substantiating the high prevalence and social significance of irritable bowel syndrome (IBS). In different regions of the world, the prevalence of IBS ranges from ...
More
10/19/2022
Anticoagulant therapy with direct oral anticoagulants in polypharmacy: a course towards safety
I.N. Sychev 1.2 , L.V. Fedina 1.2 , D.A. Gabrielyan 1 Etc. Rastvorova 1 , E.V. Strigunkova 1 , K.B. Mirzaev 1 , D.A. Sychev 1 ; 1 Russian Medical Academy of Continuous Professional Education; 2 City Clinical Hospital named after S.S. Yudina
Rastvorova 1 , E.V. Strigunkova 1 , K.B. Mirzaev 1 , D.A. Sychev 1 ; 1 Russian Medical Academy of Continuous Professional Education; 2 City Clinical Hospital named after S.S. Yudina
Cardiovascular diseases...
More
10/18/2022
Inosine pranobex in the treatment of mild cervical intraepithelial neoplasia (clinical experience)
I.O. Borovikov, I.I. Kutsenko, V.P. Bulgakov, A.A. Gorbulin; KubGMU
Introduction. The article reflects the experience of treating patients with papillomavirus-associated lesions of the cervix - mild cervical intraepithelial neoplasia (CIN I) using an immunostimulant with antiviral activity...
More
10/17/2022
Efficacy of an esophagoprotector in the treatment of patients with gastroesophageal reflux disease: a systematic review
I. V. Maev 1 , D.N. Andreev 1 , Yu.A. Curly 2 , E.G. Lobanova 1 , D.I. Schaefer 1 ; 1 Moscow State University of Medicine and Dentistry named after A.I. Evdokimova, 2 Ilyinsky hospital
V. Maev 1 , D.N. Andreev 1 , Yu.A. Curly 2 , E.G. Lobanova 1 , D.I. Schaefer 1 ; 1 Moscow State University of Medicine and Dentistry named after A.I. Evdokimova, 2 Ilyinsky hospital
Gastroesophageal reflux disease (GERD) is one of the most...
More
10/14/2022
Dental anomalies: classification, causes and treatment
The close relationship between oral health, systemic and psychological health requires careful assessment of oral health as part of health maintenance surveillance. Understanding the normal sequence and patterns of teething is the basis for identifying and treating children...
More
10/13/2022
Painful sexual disorder
N. N. Stenyaev; NMITs AGP them. IN AND. Kulakova
N. Stenyaev; NMITs AGP them. IN AND. Kulakova
Painful sexual disorder, which combines the concepts of dyspareunia, vaginismus, pelvic pain, penetration disorder, is widespread in women of reproductive and postmenopausal age in the world (up to 34-45%) and more often manifests itself as coming ...
More
10/11/2022
Diagnosis of disorders in the coagulation system, assessment of the risk of hemorrhagic complications in severe cirrhosis/liver diseases according to global screening tests of the hemostasis system and principles for their correction: guidelines
M.V. Mayevskaya 1 * , M.S. Zharkova 1 , V.T. Ivashkin 1 , E.N. Bessonova 2 , N.I. Geyvandova 3 , E. A. Kitsenko 4 , N.V. Korochanskaya 5.6 , I.A. Kurkina 1 , A.L. Melikyan 7 , VG Morozov 8 , Yu.V. Horonko 9 ; 1 First Moscow State Medical University named after I.M. Sechenov (Sechenov University), 2...
A. Kitsenko 4 , N.V. Korochanskaya 5.6 , I.A. Kurkina 1 , A.L. Melikyan 7 , VG Morozov 8 , Yu.V. Horonko 9 ; 1 First Moscow State Medical University named after I.M. Sechenov (Sechenov University), 2...
More
10.10.2022
Features of eating behavior in women: risk assessment of complications
T.P. Shevlyukova, E.A. Mateikovich, P.A. Ermakova, A.A. Ermakova, Tyumen State Medical University
Introduction. Studies show that up to 8% of pregnant women suffer from eating disorders. Such problems are found everywhere, most often women do not even realize that. ..
..
More
09/29/2022
How to treat sinusitis in adults?
Sinusitis, like rhinosinusitis, refers to inflammation in the nasal cavity and paranasal sinuses. Acute sinusitis lasts less than four weeks. The most common etiology is a viral infection associated with the common cold. Distinguish acute viral sinusitis associated with colds and flu-like...
More
Load more
Simbalta (duloxetine) in the treatment of depressive disorders P.B. Gannushkin №01 2007
Subscribe to new numbers
Author: S.V. Ivanov
NTsPZ RAMS, Moscow
Page numbers in the issue: 36-39
Major depressive disorder (MDD) is currently estimated to affect up to 340 million people worldwide (Greden, 2001). It is assumed that by 2020 MDD will take the second place among the main causes of population maladaptation.
It is assumed that by 2020 MDD will take the second place among the main causes of population maladaptation.
In modern clinical psychiatry, antidepressants remain the first choice for the treatment of MDD. However, the effectiveness of currently available drugs of this class in the treatment of MDD remains unsatisfactory. The use of antidepressants achieves remission in only 30-40% of patients with MDD
It is currently estimated that up to 340 million people worldwide suffer from major depressive disorder (MDD) (Greden, 2001). By 2020, MDD is expected to be the second leading cause of population maladaptation (Murray and Lopez, 1996).
In modern clinical psychiatry, antidepressants remain the first choice for the treatment of MDD. However, the effectiveness of currently available drugs of this class in the treatment of MDD remains unsatisfactory. The use of antidepressants achieves remission in only 30-40% of patients with MDD (Richelson, 1993; Greden, 2001).
The introduction of new antidepressants with a selective mechanism of action into clinical practice has made it possible to solve many problems in the treatment of MDD associated with unsatisfactory tolerability and safety profiles of drugs of past generations - tricyclic derivatives (TCAs) and irreversible monoamine oxidase inhibitors (MAOIs). At the same time, we have to state that the new antidepressants did not significantly improve the effectiveness of treatment. For example, antidepressants of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants do not show superior efficacy over TCAs (Anderson and Tomenson, 1994; Steffens et al., 1997) and achieve asymptomatic remission in only about 35% of patients with MDD (Thase et al., 2001).
Dual action antidepressants (serotonin and norepinephrine reuptake inhibitors)
Currently, in the context of improving the effectiveness of depression therapy, special attention is paid to a new generation of antidepressants with the so-called dual mechanism of action - serotonin and norepinephrine reuptake inhibitors (SNRIs). It is assumed that the double blockade of the reuptake of serotonin and noradrenaline allows to achieve a more pronounced reduction in depressive symptoms than a selective effect on the metabolism of only one of these neurotransmitters. The feasibility of this approach has been confirmed in a number of studies. Thus, the combined use of fluoxetine (SSRI) and desipramine (relative to a selective inhibitor of norepinephrine) has been shown to be superior in efficacy to desipramine monotherapy (Nelson et al., 1991). There is also evidence that the tricyclic antidepressant clomipramine, whose mechanism of action includes simultaneous blockade of serotonin and norepinephrine reuptake, provides a higher therapeutic effect in patients with MDD compared with the SSRIs citalopram (Anderson et al., 1986) and paroxetine (Danish Univeristy). Antidepressant Group, 1990). Of note are the results of a meta-analysis of data from several trials of venlafaxine, an SNRI class of antidepressant, which results in significantly higher MDD remission rates compared to SSRIs and placebo (Thase et al.
It is assumed that the double blockade of the reuptake of serotonin and noradrenaline allows to achieve a more pronounced reduction in depressive symptoms than a selective effect on the metabolism of only one of these neurotransmitters. The feasibility of this approach has been confirmed in a number of studies. Thus, the combined use of fluoxetine (SSRI) and desipramine (relative to a selective inhibitor of norepinephrine) has been shown to be superior in efficacy to desipramine monotherapy (Nelson et al., 1991). There is also evidence that the tricyclic antidepressant clomipramine, whose mechanism of action includes simultaneous blockade of serotonin and norepinephrine reuptake, provides a higher therapeutic effect in patients with MDD compared with the SSRIs citalopram (Anderson et al., 1986) and paroxetine (Danish Univeristy). Antidepressant Group, 1990). Of note are the results of a meta-analysis of data from several trials of venlafaxine, an SNRI class of antidepressant, which results in significantly higher MDD remission rates compared to SSRIs and placebo (Thase et al. , 2001).
, 2001).
Additional advantages of dual-acting antidepressants include their ability to affect the somatic symptoms of depression (persistent pain, early awakening, morning sickness, loss/loss of appetite and aversion to food associated with weight loss, etc.). The relevance of this aspect of the treatment of depressive disorders becomes even more important in the light of recent publications concerning the clinical characteristics of incomplete remissions in patients with depression. It was found that at 9In 4% of cases, residual symptoms are represented mainly by somatic symptoms, which show a tendency to persistent persistence even with a minimal (subclinical) level or complete reduction of hypothymic manifestations proper.
In this regard, it is also necessary to note the high prevalence of depressive disorders, comorbid somatic pathology among patients in the general medical network, reaching 45.9%, of which MDD accounts for 23.8% (A.B. Smulevich et al., 2006). In these cases, as a rule, synergistic forms of comorbidity are revealed, which are realized in a bidirectional negative interaction between affective and somatic pathology at the symptomatic / syndromic level: aggravation of severity and expansion of algic and functional disorders due to depression along with retention of hypothymia under the influence of nosogenic factors of somatic disease ( A.B. Smulevich et al., 2006).
In these cases, as a rule, synergistic forms of comorbidity are revealed, which are realized in a bidirectional negative interaction between affective and somatic pathology at the symptomatic / syndromic level: aggravation of severity and expansion of algic and functional disorders due to depression along with retention of hypothymia under the influence of nosogenic factors of somatic disease ( A.B. Smulevich et al., 2006).
Numerous studies have shown that depression is one of the main factors that reduce the quality of life in patients with somatic diseases (F.Creed et al., 2002; C.Li et al., 2002). The proportion of patients in whom depression significantly disrupts their usual way of life varies depending on the severity of the affective disorder and increases from 18.1% with erased to 52.3% with distinct forms of depression (M. Gostynski et al., 2002).
Obviously, successful treatment of depression in such difficult clinical situations requires the use of antidepressants that can not only effectively affect the nuclear symptoms of depression, but also stop algic and functional disorders associated with hypothymia without adversely affecting the course of somatic pathology.
The data obtained suggest that dual-acting antidepressants may take the place of first-choice drugs in the treatment of such depressive disorders due to the peculiarities of their mechanism of action. Along with being involved in the pathogenesis of depression, serotonin and norepinephrine are involved in the transmission of pain impulses in descending pathways (Richardson, 1990; Jones, 1991; Zhou and Gebhart, 1992). Dual action antidepressants are expected to be highly effective against both hypothymia and associated pain disorders (Ansari, 2000).
The publication presents an overview of data from clinical trials of the new dual-acting antidepressant duloxetine.
Duloxetine (Cymbalta)
Duloxetine hydrochloride (Cymbalta) is a selective, potent and balanced serotonin and norepinephrine reuptake inhibitor, which, unlike TCAs, does not show significant affinity for muscarinic, histamine H 1 -, a 1 -adrenergic, dopaminergic, serotonergic 5-HT 1A -, 5-HT 1B -, 5-HT 1D -, 5-HT 2A -, 5-HT 2C -, and opioid receptors (Bymaster et al. , 2001).
, 2001).
Antidepressant effect
The clinical efficacy of duloxetine in the treatment of MDD has been confirmed by the results of many large-scale studies. The advantages of a dual mechanism of action over a selective effect on one of the neurotransmitter systems (serotonergic, noradrenergic) have been confirmed. It has been shown that duloxetine not only has a significant antidepressant effect compared to placebo, but also significantly outperforms selective serotonergic antidepressants in terms of effectiveness. This is evidenced by the data of a meta-analysis of 6 placebo-controlled studies of duloxetine (doses from 40 to 120 mg / day), in which antidepressants of the SSRI class fluoxetine (20 mg / day) and paroxetine (20 mg) are widely used in modern practice. /day) (M.Thase et al., 2003).
Moreover, if in the total sample of all studies duloxetine is comparable to SSRIs in terms of the effectiveness of relieving depressive symptoms, as evidenced by approximately equal and significant differences between drugs of both classes and placebo, then when analyzing the results of treatment in a subgroup of patients with more severe depressive disorders, a clear advantage of duloxetine is found. In patients with a baseline Hamilton Depression Inventory (HAMD) total score of 19, duloxetine was associated with a significantly higher chance of achieving complete remission than SSRIs (38% versus 29%).% respectively).
In patients with a baseline Hamilton Depression Inventory (HAMD) total score of 19, duloxetine was associated with a significantly higher chance of achieving complete remission than SSRIs (38% versus 29%).% respectively).
Another supposed advantage of dual action - the speed of onset of the antidepressant effect - is also supported by the results of relevant studies. Thus, when comparing duloxetine with the SSRI escitalopram, which has the fastest therapeutic effect in its class, it was shown that the antidepressant effect of duloxetine unfolds at the same time (A. Nierenberg et al., 2005). In the first 2 weeks of therapy, the use of both escitalopram and duloxetine provides more than 20% reduction in the nuclear symptoms of depression (Mayer factor of the HAMD scale), which corresponds to a significant and relatively rapid antidepressant effect of both antidepressants with significant differences from placebo. At the same time, a clinically significant improvement according to the subjective assessments of patients and the results of objective observation occurs as early as the 1st week of treatment.
Along with the relatively rapid deployment of duloxetine, the therapeutic effect of duloxetine is characterized by persistence and continuously increases against the background of long-term use of the drug, which is confirmed by a steady reduction in depressive symptoms throughout
9 weeks of treatment. Differences from placebo, fixed from the 1st week of therapy, not only retain the level of statistical significance, but also increase in accordance with the absolute indicators of the dynamics of the initial total HAMD scores (M. Detke et al., 2002). Moreover, as a result of a detailed assessment of individual HAMD items, over the same period, there is a pronounced and continuous reduction in both hypothymia itself and concomitant anxiety symptoms (D. Dunner et al., 2003; Data on file, Lilly Research Laboratories).
Number (absolute and %) of patients with persistent* arterial hypertension treated with different doses of duloxetine compared with placebo (data from 8 placebo-controlled studies)
| Drug dose | abs. (n) (n) | % of sample |
| Duloxetine 40 mg/day (n=174) | 0 | 0 |
| 0359 6 | 0.8 | |
| * Criteria for sustained hypertension: three consecutive measurements or systolic blood pressure is 140 mm Hg. Art. and more, while exceeding the initial level by at least 10 mm Hg. Art., or diastolic blood pressure is 90 mm Hg. Art. and more, while exceeding the initial level by at least 10 mm Hg. Art. | ||
Efficacy on somatic symptoms in patients with depressive disorders
As already noted, an important aspect of the treatment of depression is the impact on its accompanying somatic symptoms, contributing to the achievement of faster and better remission. In this context, of particular interest are the data of a number of studies evaluating the clinical efficacy of duloxetine in relation to pain disorders of various origins, acting both as part of the psychopathological manifestations of depression (different variants of the so-called somatic depressions) and as manifestations of somatic pathology. At the same time, it is important to take into account that of the antidepressants used in modern practice, only TCAs, primarily amitriptyline, have a significant analgesic effect, but are characterized by a wide range of adverse side effects.
At the same time, it is important to take into account that of the antidepressants used in modern practice, only TCAs, primarily amitriptyline, have a significant analgesic effect, but are characterized by a wide range of adverse side effects.
As shown in a special study, duloxetine exhibits a wide range of analgesic activity in patients with depressive disorders, and therefore indicates the comparability of duloxetine with TCAs in this characteristic of clinical efficacy. In a study by C. Nemeroff et al. (2002), involved more than 130 patients with depression occurring with severe and polymorphic algic symptoms. Of the patients available for final evaluation, 120 received duloxetine at a daily dose of 60 mg (single daily dose), another 113 received placebo.
The study showed that duloxetine was significantly more effective than placebo in all types of pain disorders observed in the studied sample. At the same time, the weak placebo effect in relation to algia should be emphasized (in contrast to the traditionally high level of placebo effectiveness in assessing affective symptoms in patients with moderate depression). This fact emphasizes the significance of the clinical effect of duloxetine and suggests that duloxetine, like drugs of the TCA class, has a true analgesic effect, to a certain extent independent of the thymoanaleptic effect itself.
This fact emphasizes the significance of the clinical effect of duloxetine and suggests that duloxetine, like drugs of the TCA class, has a true analgesic effect, to a certain extent independent of the thymoanaleptic effect itself.
It should also be noted the stability and speed (1-2 weeks of treatment) of the onset of the analgesic effect, noted in the above study and confirmed by M. Detke et al. (2002).
As a result of the analysis of the dynamics of the severity of back pain in patients with MDD, the authors showed that the analgesic effect of duloxetine reaches statistical and clinical significance already at the 1st week of treatment and then steadily increases within at least 9 weeks of therapy (the term of the final assessment in this study) .
Additional confirmation of the independent thymoanaleptic effect and significant analgesic effect of duloxetine are the results of a number of studies using duloxetine for pain relief in patients with diabetic neuropathy (J. Wernicke et al., 2004; Data on File, Lilly Research Laboratories; D.Goldstein et al. , 2005). These studies established a significant superiority of duloxetine over placebo in the reduction of pain syndrome with signs of a dose-dependent effect. Duloxetine in daily doses of 60 and 120 mg was significantly superior to placebo and duloxetine (20 mg/day) starting from the 1st week of therapy. Differences in efficacy between placebo and duloxetine per dose
Wernicke et al., 2004; Data on File, Lilly Research Laboratories; D.Goldstein et al. , 2005). These studies established a significant superiority of duloxetine over placebo in the reduction of pain syndrome with signs of a dose-dependent effect. Duloxetine in daily doses of 60 and 120 mg was significantly superior to placebo and duloxetine (20 mg/day) starting from the 1st week of therapy. Differences in efficacy between placebo and duloxetine per dose
20 mg/day not established. In turn, duloxetine at a dose of 60 mg/day was only slightly inferior in efficacy to duloxetine at a dose of 120 mg/day (without statistically significant differences during 12 weeks of therapy) (J.Wernicke et al., 2004; D.Goldstein et al., 2005).
Tolerability and safety
While comparable to TCAs in terms of severity and spectrum of analgesic action, duloxetine is characterized by a significantly more favorable tolerability and safety profile, comparable to the profiles of selective monoaminergic antidepressants.
One of the serious aspects of the safety of dual-acting drugs, mainly associated with the noradrenergic component, is the risk of developing arterial hypertension, as was found in the case of the use of another dual-acting antidepressant venlafaxine (M.Yu. Drobizhev, 2005). According to the results of a special meta-analysis, it was found that duloxetine therapy is not associated with a serious risk of a persistent increase in blood pressure (BP), although a certain trend towards a dose-dependence of this undesirable phenomenon was revealed (see table).
It was shown that, in accordance with the data of 8 placebo-controlled studies, the rates of development of stable arterial hypertension in the treatment of duloxetine do not have statistically significant differences from those observed against the background of placebo, with a small (non-statistically significant) increase in the proportion of patients with hypertension in the subgroup who received maximum dose of duloxetine (120 mg/day) (Data on file, Lilly Research Laboratories).
Unlike SSRIs, duloxetine does not adversely affect sexual function, which was confirmed by the results of the analysis of data from 4 clinical studies using the Arizona Sexual Functionality Scale (ASEX) [S.K.Brannan et al., 2003]. The authors report that during duloxetine therapy in women who do not initially suffer from sexual dysfunction, no changes were found compared with placebo. In turn, in men who do not initially suffer from sexual dysfunction, there is a deterioration (compared to placebo) in only two indicators - ease of achievement and satisfaction with orgasm (S. Brannan et al., 2003).
Despite a clear efficacy in the treatment of urinary incontinence syndrome, duloxetine, unlike TCAs, does not cause urinary retention, which, apparently, is explained by the significantly more limited spectrum of duloxetine receptor activity compared to TCAs (Thor et al., 1995).
Duloxetine does not cause adverse metabolic effects, as evidenced by the results of the analysis of body weight indicators recorded in the pooled database of placebo-controlled studies (Data on file, Lilly Research Laboratories). Within short-term (8-9weeks) courses of cupping therapy in all patients treated with duloxetine (at doses from 40 to 120 mg / day; n = 1115), no significant change in body weight was found: -0.5 kg in the duloxetine group versus +0.2 kg in the group placebo (n=757). Similar favorable indicators were also established with long-term maintenance therapy (average 6.5 months): the average changes in body weight in the duloxetine 80 mg/day (n=141) and 120 mg/day (n=156) groups were +0.9 and +1.2 kg, and in the placebo group this figure was -0.05 kg (n=129) (Data on file, Lilly Research Laboratories).
Within short-term (8-9weeks) courses of cupping therapy in all patients treated with duloxetine (at doses from 40 to 120 mg / day; n = 1115), no significant change in body weight was found: -0.5 kg in the duloxetine group versus +0.2 kg in the group placebo (n=757). Similar favorable indicators were also established with long-term maintenance therapy (average 6.5 months): the average changes in body weight in the duloxetine 80 mg/day (n=141) and 120 mg/day (n=156) groups were +0.9 and +1.2 kg, and in the placebo group this figure was -0.05 kg (n=129) (Data on file, Lilly Research Laboratories).
The only side effect of duloxetine is nausea. When using duloxetine at a dose
60 mg / day nausea develops relatively often (in about 40% of patients). A feature of this undesirable effect is its early onset (usually in the first days of therapy) and subsequent spontaneous reduction to the placebo level by the end of the 1st week of treatment, which, apparently, is associated with the adaptation of receptors to the action of the drug. According to the results of a differentiated assessment in the framework of placebo-controlled studies, 9Nausea remained mild/moderate in 4% of patients who developed this side effect (M. Detke et al., 2002; Data on file, Lilly Research Laboratories).
According to the results of a differentiated assessment in the framework of placebo-controlled studies, 9Nausea remained mild/moderate in 4% of patients who developed this side effect (M. Detke et al., 2002; Data on file, Lilly Research Laboratories).
Thus, dual-acting antidepressants, serotonin and norepinephrine reuptake inhibitors, such as duloxetine, expand the possibilities of effective and safe treatment of depressive disorders, including patients with the most difficult conditions to treat, determined by the comorbidity of hypothymic disorders with pain and other somatic symptoms. The dual mechanism of action of duloxetine provides a wide spectrum of clinical activity with a pronounced effect on the nuclear symptoms of depression, which is complemented by distinct analgesic properties. As a result, the use of duloxetine achieves symptomatic remission in a larger number of patients and at an earlier time than most currently available antidepressants with a selective monoaminergic mechanism of action.