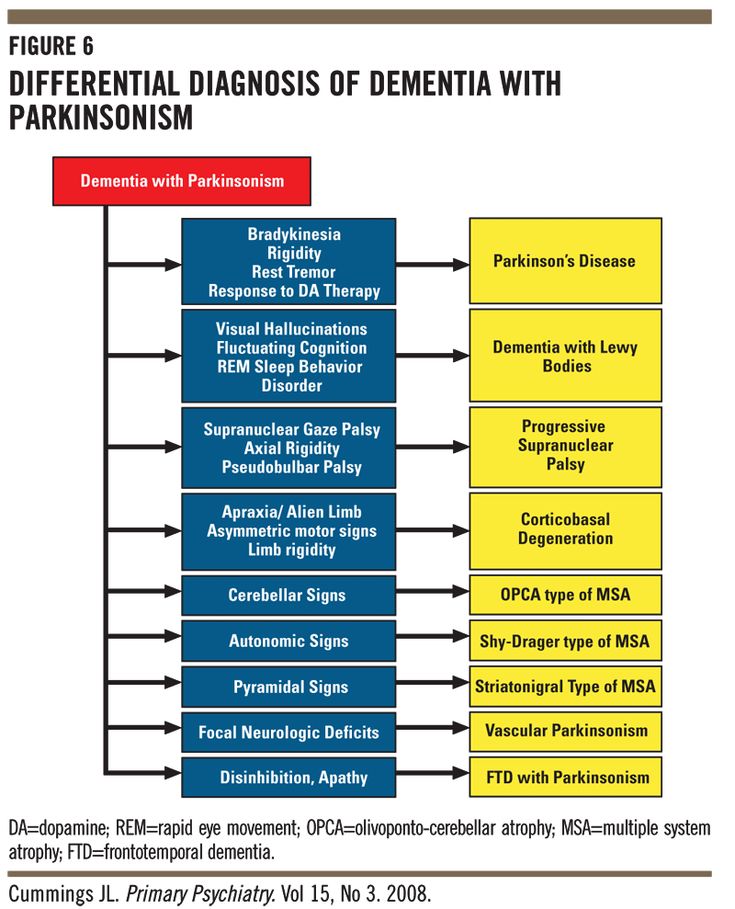
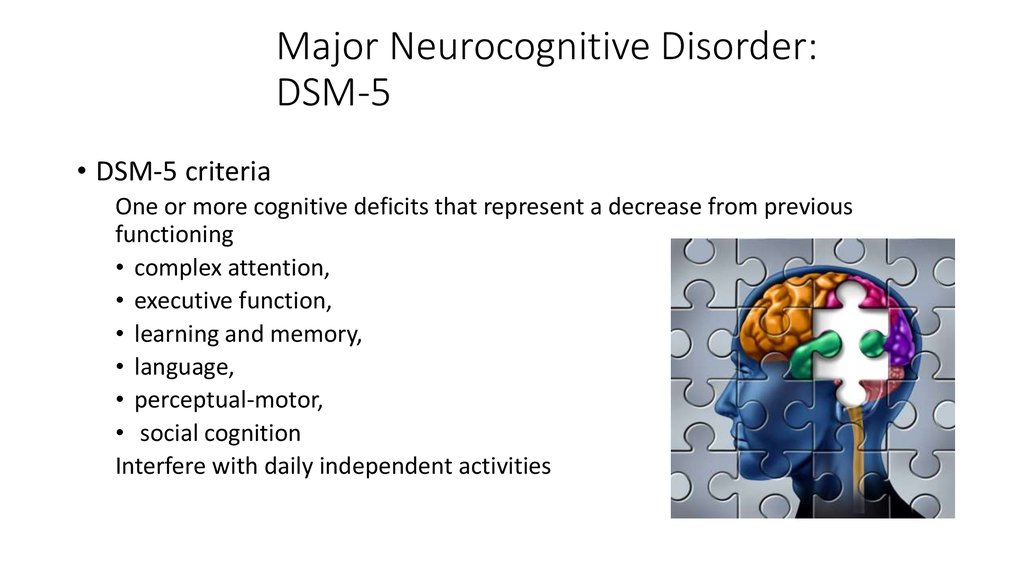
Diagnostic features of neurocognitive disorders
Dementia and Cognitive Impairment: Epidemiology, Diagnosis, and Treatment
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
2. Murphy SL, Xu J, Kochanek KD. Division of Vital Statistics. Deaths: final data for 2010. [Accessed on-line December 19, 2013];National Vital Statistics Reports. 2013 61(4) Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. [Google Scholar]
3. Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. [PubMed] [Google Scholar]
4. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. [PubMed] [Google Scholar]
5. Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51(3):728–733. [PubMed] [Google Scholar]
6. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8(1):14–21. [PubMed] [Google Scholar]
7. Prince M, Prina M, Guerchet Alzheimer’s Disease International. World Alzheimer Report 2013 Journey of Caring: An analysis of long term care for dementia. [Accessed on-line December 17, 2013]; Available at: http://www.alz.co.uk/research/world-report-2013. [Google Scholar]
8. Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93. [PMC free article] [PubMed] [Google Scholar]
9. Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405–1412. [PMC free article] [PubMed] [Google Scholar]
10. Gurland BJ, Wilder DE, Lantigua RF, et al. Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
Gurland BJ, Wilder DE, Lantigua RF, et al. Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
11. Hall KS, Gao S, Baiyewu O, et al. Prevalence rates for dementia and Alzheimer's disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5(3):227–233. [PMC free article] [PubMed] [Google Scholar]
12. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics and memory study. Neuroepidemiology. 2007;29:125–132. [PMC free article] [PubMed] [Google Scholar]
13. Graves AB, Larson EB, Edland SD, et al. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. Am J Epidemiol. 1996;144(8):760–771. [PubMed] [Google Scholar]
14. Canadian Study of Health and Aging Working Group. Canadian Study of Health and Aging: study methods and prevalence of dementia. Canadian Medical Association Journal. 1994;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
1994;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
15. Aarsland D, Zaccai J, Brayne C. A symptomatic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20(10):1255–1263. [PubMed] [Google Scholar]
16. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561–566. [PubMed] [Google Scholar]
17. Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population- based study. Brain. 2003;126:2016–2022. [PubMed] [Google Scholar]
18. Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55(9):809–815. [PubMed] [Google Scholar]
19. Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. [PubMed] [Google Scholar]
20. Tsuang D, Leverenz JB, Lopez OL, et al. APO ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–228. [PMC free article] [PubMed] [Google Scholar]
Tsuang D, Leverenz JB, Lopez OL, et al. APO ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–228. [PMC free article] [PubMed] [Google Scholar]
21. Chuang Y, Hayden KM, Norton MC, et al. Association between APOE ε4 allele and vascular dementia: the Cache County study. Dement Geriatr Cogn Disord. 2010;29:248–253. [PMC free article] [PubMed] [Google Scholar]
22. Yin YW, Li JC, Wang JZ, et al. Association between apolipoprotein E gene polymorphism and the risk of vascular dementia: a meta-analysis. Neurosci Lett. 2012;514(1):6–11. [PubMed] [Google Scholar]
23. Srinivasan R, Davidson Y, Gibbons L, et al. The apolipoprotein E ε4 allele selectively increases the risk of frontotemporal lobar degeneration in males. J Neurol Neurosurg Psychiatry. 2006;77(2):154–158. [PMC free article] [PubMed] [Google Scholar]
24. Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–145. [PMC free article] [PubMed] [Google Scholar]
25. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. [PubMed] [Google Scholar]
Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. [PubMed] [Google Scholar]
26. Schnaider Beeri M, Goldbourt U, Silverman JM, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63(10):1902–1907. [PubMed] [Google Scholar]
27. Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274. [PubMed] [Google Scholar]
28. Qui C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166(9):1003–1008. [PubMed] [Google Scholar]
29. Santangeli P, Di Biase L, Bai R, et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9(11):1761–1768. [PubMed] [Google Scholar]
Heart Rhythm. 2012;9(11):1761–1768. [PubMed] [Google Scholar]
30. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry and Neurol. 2012 Article ID 367516, 15 pages. [PMC free article] [PubMed] [Google Scholar]
31. Nation DA, Edland SD, Bond MW, et al. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81(23):2024–2027. [PMC free article] [PubMed] [Google Scholar]
32. Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52(2):168–174. [PubMed] [Google Scholar]
33. Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: the Conselice Study of Brain Aging. Neurobiol Aging. 2007;28(12):1810–1820. [PubMed] [Google Scholar]
34. Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. [PMC free article] [PubMed] [Google Scholar]
Neurobiol Aging. 2000;21(3):383–421. [PMC free article] [PubMed] [Google Scholar]
35. Schott JM, Revesz T. Inflammation in Alzheimer’s disease: insights from immunotherapy. Brain. 2013;136(9):2654–2656. [PubMed] [Google Scholar]
36. Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37(2):289–305. [PubMed] [Google Scholar]
37. Budhiraja R, Budhiraja P, Quan SF. Sleep-disordered breathing and cardiovascular disorders. Respiratory Care. 2010;55(10):1322–1332. [PMC free article] [PubMed] [Google Scholar]
38. Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36(5):709–715. [PMC free article] [PubMed] [Google Scholar]
39. Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLOS One. 2013;8(10):e78655. [PMC free article] [PubMed] [Google Scholar]
40. van Kooten F, Koudstaal PJ. Epidemiology of post-stroke dementia. Haemostasis. 1998;28(3–4):124–133. [PubMed] [Google Scholar]
van Kooten F, Koudstaal PJ. Epidemiology of post-stroke dementia. Haemostasis. 1998;28(3–4):124–133. [PubMed] [Google Scholar]
41. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. [PubMed] [Google Scholar]
42. Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27–34. [PMC free article] [PubMed] [Google Scholar]
43. Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116. [PubMed] [Google Scholar]
44. Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. [PMC free article] [PubMed] [Google Scholar]
45. Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16(10):790–803. [PubMed] [Google Scholar]
Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16(10):790–803. [PubMed] [Google Scholar]
46. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608–613. [PMC free article] [PubMed] [Google Scholar]
47. Wilson RS, Boyle PA, Buchman AS, Yu L, Arnold SE, Bennett DA. Harm avoidance and risk of Alzheimer’s disease. Psychosom Med. 2011;73(8):690–696. [PMC free article] [PubMed] [Google Scholar]
48. Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74(7):857–862. [PMC free article] [PubMed] [Google Scholar]
49. Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. [PubMed] [Google Scholar]
50. Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. [PubMed] [Google Scholar]
Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. [PubMed] [Google Scholar]
51. Gavett BE, Stern RA, Cantu RD, Nowinski CJ, McKee AC. Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2(3):18–21. [PMC free article] [PubMed] [Google Scholar]
52. Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113(9):1250–1256. [PMC free article] [PubMed] [Google Scholar]
53. Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166(4):367–378. [PubMed] [Google Scholar]
54. Chang CC, Zhao Y, Lee CW, Ganguli M. Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis Assoc Disord. 2012;26(4):300–306. [PMC free article] [PubMed] [Google Scholar]
55. Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947–957. [PMC free article] [PubMed] [Google Scholar]
Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947–957. [PMC free article] [PubMed] [Google Scholar]
56. Anttila T, Helkala EL, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329(7465):539. [PMC free article] [PubMed] [Google Scholar]
57. Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289(11):1405–1413. [PubMed] [Google Scholar]
58. Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem Biol Interact. 2010;188(2):289–300. [PMC free article] [PubMed] [Google Scholar]
59. Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12(1):11–22. [PubMed] [Google Scholar]
2007;12(1):11–22. [PubMed] [Google Scholar]
60. Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. [PubMed] [Google Scholar]
61. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
62. Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PloS One. 2012;7(6):e38268. [PMC free article] [PubMed] [Google Scholar]
63. Alladi S, Bak T, Duggirala V, et al. Bilingualism delays age at onset of dementia, independent of education and immigrations status. Neurology. 2013;81(2):1938–1944. [PubMed] [Google Scholar]
64. Craik FI, Bialystok E, Freedman M. Delaying the onset of Alzheimer disease: bilingualism as a form a cognitive reserve.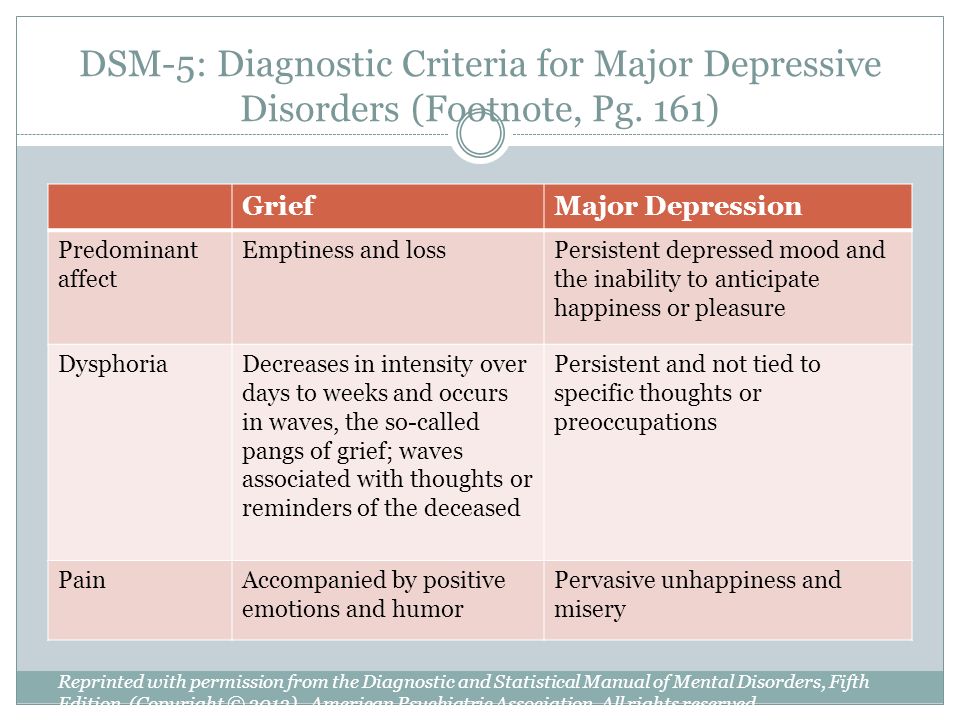 Neurology. 2010;75(19):1726–1729. [PMC free article] [PubMed] [Google Scholar]
Neurology. 2010;75(19):1726–1729. [PMC free article] [PubMed] [Google Scholar]
65. Bonaiuto S, Rocca WA, Lippi A, et al. Education and occupation as risk factors for dementia: a population-based case control study. Neuroepidemiology. 1995;14(3):101–109. [PubMed] [Google Scholar]
66. Bickel H, Kurz A. Education, occupation, and dementia: the Bavarian school sisters study. Dement Geriatr Cogn Disord. 2009;27(6):548–556. [PubMed] [Google Scholar]
67. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. [PubMed] [Google Scholar]
68. Hughes TF. Promotion of cognitive health through activity in the aging population. Aging Health. 2010;6(1):111–121. [PMC free article] [PubMed] [Google Scholar]
69. Yip AG, Green RC, Huyck M, Cupples LA, Farrer LA MIRAGE Study Group. Nonsteroidal anti-inflammatory drug use and Alzheimer’s disease risk: the MIRAGE study. BMC Geriatrics. 2005;5:2. [PMC free article] [PubMed] [Google Scholar]
70. Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70(1):17–24. [PMC free article] [PubMed] [Google Scholar]
Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70(1):17–24. [PMC free article] [PubMed] [Google Scholar]
71. in‘t Veld BA, Launer LJ, Hoes AW, et al. NSAIDs and incident Alzheimer’s disease. The Rotterdam Study. Neurobiol Aging. 1998;19(6):607–611. [PubMed] [Google Scholar]
72. de Craen AJ, Gussekloo J, Vrijsen B, Westendorp RG. Meta-analysis of nonsteroidal anti-inflammatory drug use and risk of dementia. Am J Epidemiol. 2005;161(2):114–120. [PubMed] [Google Scholar]
73. Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88(11):1213–1221. [PubMed] [Google Scholar]
74. Wong WB, Lin VW, Boudreau D, Devine EB. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiology Drug Saf. 2013;22(4):345–358. [PubMed] [Google Scholar]
Pharmacoepidemiology Drug Saf. 2013;22(4):345–358. [PubMed] [Google Scholar]
75. Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. [PubMed] [Google Scholar]
76. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. [PubMed] [Google Scholar]
77. O’Brien J, Jackson JW, Grodstein F, Blacker D, Weuve J. Postmenopausal hormone therapy is not associated with risk of all-cause dementia and Alzheimer’s Disease. Epidemiol Rev. first published online September 15, 2013. [Google Scholar]
78. Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852. [PMC free article] [PubMed] [Google Scholar]
Neurology. 2012;79(18):1846–1852. [PMC free article] [PubMed] [Google Scholar]
79. Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69(1):163–169. [PMC free article] [PubMed] [Google Scholar]
80. Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288(17):2123–2129. [PubMed] [Google Scholar]
81. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam study. Lancet. 2002;359(9303):281–286. [PubMed] [Google Scholar]
82. Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65(8):1210–1217. [PubMed] [Google Scholar]
83. Lourida I, Soni M, Thompson-Coon J, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24(4):479–489. [PubMed] [Google Scholar]
Epidemiology. 2013;24(4):479–489. [PubMed] [Google Scholar]
84. Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11. [PubMed] [Google Scholar]
85. Crooks VC, Lubben J, Petitti DB, Little D, Chiu V. Social network, cognitive function, and dementia incidence among elderly women. Am J Public Health. 2008;98(7):1221–1227. [PMC free article] [PubMed] [Google Scholar]
86. Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am J Epidemiol. 2002;155(12):1081–1087. [PubMed] [Google Scholar]
87. Fratiglioni L, Paillard-Bog S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. [PubMed] [Google Scholar]
88. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state. ” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [PubMed] [Google Scholar]
” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [PubMed] [Google Scholar]
89. Nasreddine ZS, Phillips NA, Bédirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [PubMed] [Google Scholar]
90. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027. [PubMed] [Google Scholar]
91. Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59(11):1764–1767. [PubMed] [Google Scholar]
92. Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71(19):1489–1495. [PMC free article] [PubMed] [Google Scholar]
93. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [PMC free article] [PubMed] [Google Scholar]
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [PMC free article] [PubMed] [Google Scholar]
94. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. [PMC free article] [PubMed] [Google Scholar]
95. Jellinger KA. Morphological diagnosis of “vascular dementia”- a critical update. J Neurol Sci. 2008;270(1–2):1–12. [PubMed] [Google Scholar]
96. Sneed JR, Culang-Reinlieb ME. The vascular depression hypothesis: an update. Am J Geriatr Psychiatry. 2011;19(2):99–103. [PMC free article] [PubMed] [Google Scholar]
97. Rabinovici GD, Miller BL.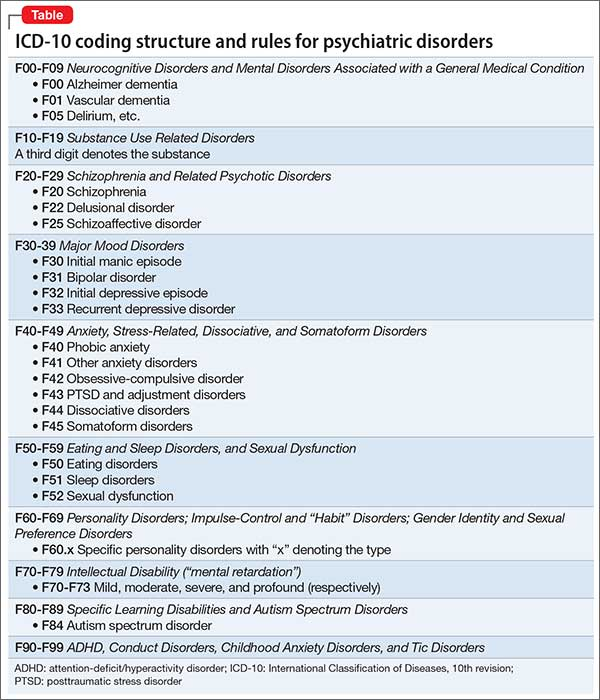 Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375–398. [PMC free article] [PubMed] [Google Scholar]
Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375–398. [PMC free article] [PubMed] [Google Scholar]
98. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65(12):1863–1872. [PubMed] [Google Scholar]
99. Brown K, Mastrianni JA. The prion diseases. J Geriatr Psychiatry Neurol. 2010;23(4):277–298. [PubMed] [Google Scholar]
100. Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database of Systematic Reviews. 2006;(1):CD005593. [PMC free article] [PubMed] [Google Scholar]
101. Press D, Alexander M. Cholinesterase inhibitors in the treatment of dementia. In: Basow DS, editor. UpToDate. Waltham, MA: UpToDate; [Accessed on December 17, 2013]. [Google Scholar]
102. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509–2518. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
103. Maidment I, Fox C, Boustani M. Cholinesterase inhibitors for Parkinson's disease dementia. Cochrane Database of Systematic Reviews. 2006;(1):CD004747. [PubMed] [Google Scholar]
104. McKeith I, Mintzer J, Aarsland D, et al. Dementia with Lewy Bodies. Lancet Neurology. 2004;3(1):19–28. [PubMed] [Google Scholar]
105. Wright CB. Treatment and prevention of vascular dementia. In: Basow DS, editor. UpToDate. Waltham, MA: UpToDate; [Accessed on December 17, 2013]. [Google Scholar]
106. Miller BL, Lee SE. Frontotemporal dementia: treatment. In: Basow DS, editor. UpToDate. Waltham, MA: UpToDate; [Accessed on December 17, 2013]. [Google Scholar]
107. Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84–87. [PubMed] [Google Scholar]
108. Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database of Systematic Reviews. 2012;9:CD009132. [PMC free article] [PubMed] [Google Scholar]
Cochrane Database of Systematic Reviews. 2012;9:CD009132. [PMC free article] [PubMed] [Google Scholar]
109. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database of Systematic Reviews. 2006;2:CD003154. [PubMed] [Google Scholar]
110. Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66(1):17–22. [PMC free article] [PubMed] [Google Scholar]
111. Gareri P, DeFazio P, Manfredi VG, DeSarro G. Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. [2013 October 23];J Clin Psychopharmacol. Published Ahead-of-Print. [PubMed] [Google Scholar]
Classifying neurocognitive disorders: the DSM-5 approach
Review
. 2014 Nov;10(11):634-42.
doi: 10.1038/nrneurol.2014.181. Epub 2014 Sep 30.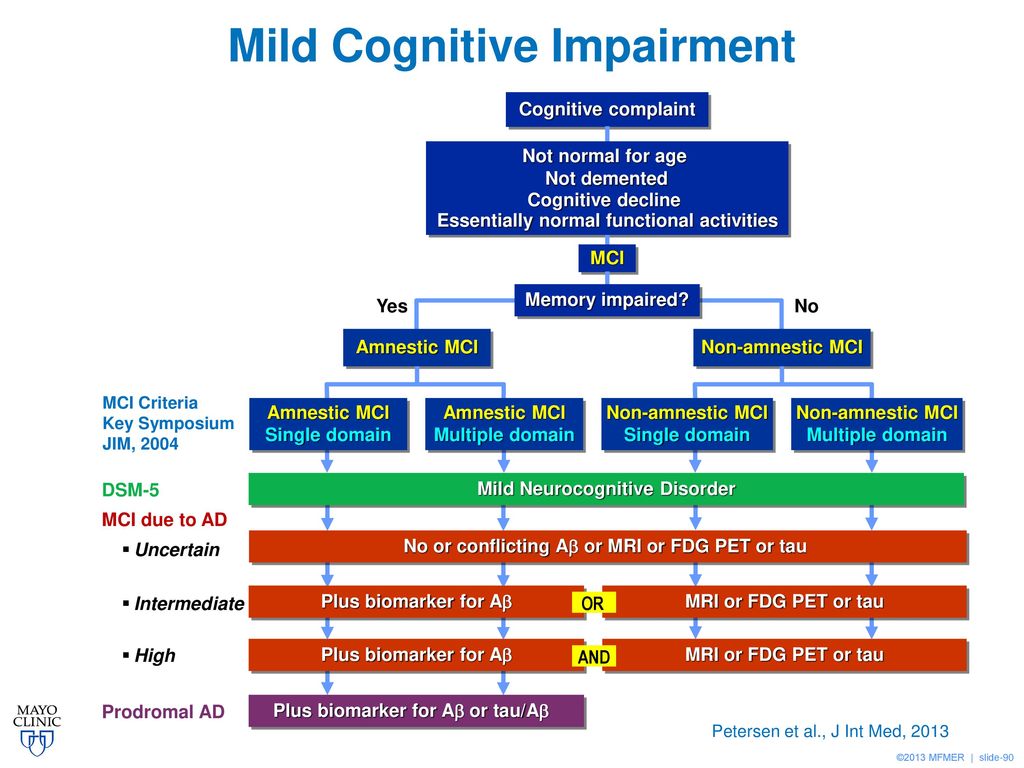
Perminder S Sachdev 1 , Deborah Blacker 2 , Dan G Blazer 3 , Mary Ganguli 4 , Dilip V Jeste 5 , Jane S Paulsen 6 , Ronald C Petersen 7
Affiliations
Affiliations
- 1 Centre for Healthy Brain Ageing, School of Psychiatry, University of New South Wales, Prince of Wales Hospital, Barker Street, Randwick, NSW 2031, Australia.
- 2 Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, 401 Park Drive, Boston, MA 02215, USA.
- 3 Duke Institute for Brain Sciences, Duke University, 3521 Hospital South, Durham, NC 27710, USA.

- 4 Department of Psychiatry, University of Pittsburgh, 3811 O'Hara Street, Pittsburgh, PA 15213, USA.
- 5 Department of Psychiatry, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA.
- 6 Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
- 7 Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA.
- PMID: 25266297
- DOI: 10.1038/nrneurol.2014.181
Free article
Review
Perminder S Sachdev et al. Nat Rev Neurol. 2014 Nov.
Nat Rev Neurol. 2014 Nov.
Free article
. 2014 Nov;10(11):634-42.
doi: 10.1038/nrneurol.2014.181. Epub 2014 Sep 30.
Authors
Perminder S Sachdev 1 , Deborah Blacker 2 , Dan G Blazer 3 , Mary Ganguli 4 , Dilip V Jeste 5 , Jane S Paulsen 6 , Ronald C Petersen 7
Affiliations
- 1 Centre for Healthy Brain Ageing, School of Psychiatry, University of New South Wales, Prince of Wales Hospital, Barker Street, Randwick, NSW 2031, Australia.

- 2 Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, 401 Park Drive, Boston, MA 02215, USA.
- 3 Duke Institute for Brain Sciences, Duke University, 3521 Hospital South, Durham, NC 27710, USA.
- 4 Department of Psychiatry, University of Pittsburgh, 3811 O'Hara Street, Pittsburgh, PA 15213, USA.
- 5 Department of Psychiatry, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA.
- 6 Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
- 7 Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA.
- PMID: 25266297
- DOI: 10.1038/nrneurol.2014.181
Abstract
Neurocognitive disorders--including delirium, mild cognitive impairment and dementia--are characterized by decline from a previously attained level of cognitive functioning. These disorders have diverse clinical characteristics and aetiologies, with Alzheimer disease, cerebrovascular disease, Lewy body disease, frontotemporal degeneration, traumatic brain injury, infections, and alcohol abuse representing common causes. This diversity is reflected by the variety of approaches to classifying these disorders, with separate groups determining criteria for each disorder on the basis of aetiology. As a result, there is now an array of terms to describe cognitive syndromes, various definitions for the same syndrome, and often multiple criteria to determine a specific aetiology.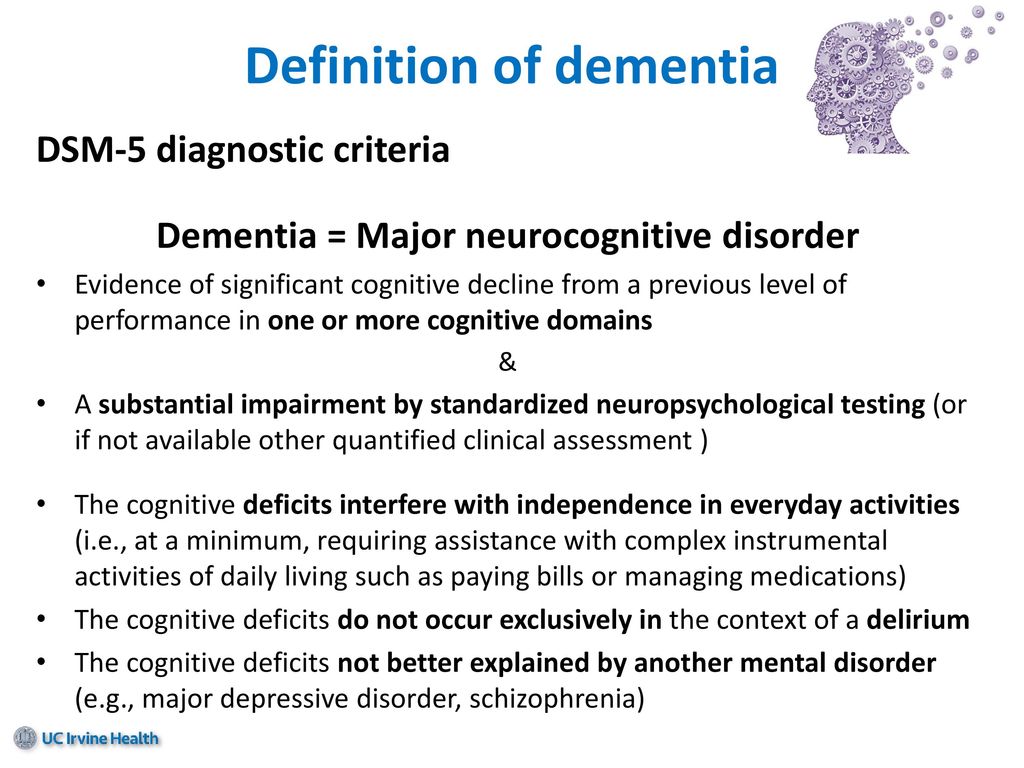 The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) provides a common framework for the diagnosis of neurocognitive disorders, first by describing the main cognitive syndromes, and then defining criteria to delineate specific aetiological subtypes of mild and major neurocognitive disorders. The DSM-5 approach builds on the expectation that clinicians and research groups will welcome a common language to deal with the neurocognitive disorders. As the use of these criteria becomes more widespread, a common international classification for these disorders could emerge for the first time, thus promoting efficient communication among clinicians and researchers.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) provides a common framework for the diagnosis of neurocognitive disorders, first by describing the main cognitive syndromes, and then defining criteria to delineate specific aetiological subtypes of mild and major neurocognitive disorders. The DSM-5 approach builds on the expectation that clinicians and research groups will welcome a common language to deal with the neurocognitive disorders. As the use of these criteria becomes more widespread, a common international classification for these disorders could emerge for the first time, thus promoting efficient communication among clinicians and researchers.
Similar articles
-
Mild Neurocognitive Disorder: An Old Wine in a New Bottle.
Stokin GB, Krell-Roesch J, Petersen RC, Geda YE. Stokin GB, et al. Harv Rev Psychiatry.
 2015 Sep-Oct;23(5):368-76. doi: 10.1097/HRP.0000000000000084. Harv Rev Psychiatry. 2015. PMID: 26332219 Free PMC article. Review.
2015 Sep-Oct;23(5):368-76. doi: 10.1097/HRP.0000000000000084. Harv Rev Psychiatry. 2015. PMID: 26332219 Free PMC article. Review. -
The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment.
Sachs-Ericsson N, Blazer DG. Sachs-Ericsson N, et al. Aging Ment Health. 2015 Jan;19(1):2-12. doi: 10.1080/13607863.2014.920303. Epub 2014 Jun 10. Aging Ment Health. 2015. PMID: 24914889
-
Neurocognitive disorders: cluster 1 of the proposed meta-structure for DSM-V and ICD-11.
Sachdev P, Andrews G, Hobbs MJ, Sunderland M, Anderson TM. Sachdev P, et al. Psychol Med. 2009 Dec;39(12):2001-12. doi: 10.1017/S0033291709990262. Epub 2009 Oct 1. Psychol Med. 2009.
 PMID: 19796426
PMID: 19796426 -
New DSM-V neurocognitive disorders criteria and their impact on diagnostic classifications of mild cognitive impairment and dementia in a memory clinic setting.
Tay L, Lim WS, Chan M, Ali N, Mahanum S, Chew P, Lim J, Chong MS. Tay L, et al. Am J Geriatr Psychiatry. 2015 Aug;23(8):768-79. doi: 10.1016/j.jagp.2015.01.004. Epub 2015 Jan 29. Am J Geriatr Psychiatry. 2015. PMID: 25728011
-
Dementia and cognitive impairment: epidemiology, diagnosis, and treatment.
Hugo J, Ganguli M. Hugo J, et al. Clin Geriatr Med. 2014 Aug;30(3):421-42. doi: 10.1016/j.cger.2014.04.001. Epub 2014 Jun 12. Clin Geriatr Med. 2014. PMID: 25037289 Free PMC article. Review.
See all similar articles
Cited by
-
Neurocognitive Impairment in Cardiovascular Disease Patients Taking Statins Versus Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors: A Systematic Review.
Shahid R, Naik SS, Ramphall S, Rijal S, Prakash V, Ekladios H, Mulayamkuzhiyil Saju J, Mandal N, Kham NI, Hamid P. Shahid R, et al. Cureus. 2022 Oct 31;14(10):e30942. doi: 10.7759/cureus.30942. eCollection 2022 Oct. Cureus. 2022. PMID: 36465767 Free PMC article. Review.
-
A new approach to diagnosing and researching developmental prosopagnosia: Excluded cases are impaired too.
Burns EJ, Gaunt E, Kidane B, Hunter L, Pulford J. Burns EJ, et al. Behav Res Methods. 2022 Dec 2:1-24. doi: 10.3758/s13428-022-02017-w. Online ahead of print. Behav Res Methods. 2022. PMID: 36459376 Free PMC article.
-
Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review.
Thangaleela S, Sivamaruthi BS, Kesika P, Chaiyasut C.
 Thangaleela S, et al. Microorganisms. 2022 Nov 15;10(11):2268. doi: 10.3390/microorganisms10112268. Microorganisms. 2022. PMID: 36422338 Free PMC article. Review.
Thangaleela S, et al. Microorganisms. 2022 Nov 15;10(11):2268. doi: 10.3390/microorganisms10112268. Microorganisms. 2022. PMID: 36422338 Free PMC article. Review. -
The neurocognitive disorder cohort RIFADE: Aims, methods, first results showing cognitive improvement in a subgroup.
Baumann B, Lipka T, Jänner M, Kujovic M. Baumann B, et al. Eur Arch Psychiatry Clin Neurosci. 2022 Nov 21. doi: 10.1007/s00406-022-01516-3. Online ahead of print. Eur Arch Psychiatry Clin Neurosci. 2022. PMID: 36416960
-
Target engagement of ginsenosides in mild cognitive impairment using mass spectrometry-based drug affinity responsive target stability.
Zhu Z, Li R, Qin W, Zhang H, Cheng Y, Chen F, Chen C, Chen L, Zhao Y.
 Zhu Z, et al. J Ginseng Res. 2022 Nov;46(6):750-758. doi: 10.1016/j.jgr.2021.12.003. Epub 2021 Dec 17. J Ginseng Res. 2022. PMID: 36312734 Free PMC article.
Zhu Z, et al. J Ginseng Res. 2022 Nov;46(6):750-758. doi: 10.1016/j.jgr.2021.12.003. Epub 2021 Dec 17. J Ginseng Res. 2022. PMID: 36312734 Free PMC article.
See all "Cited by" articles
References
-
- Stroke. 2011 Sep;42(9):2672-713 - PubMed
-
- Psychol Med. 2009 Dec;39(12):2001-12 - PubMed
-
- Brain. 2011 Sep;134(Pt 9):2456-77 - PubMed
-
- J Intern Med.
 2004 Sep;256(3):183-94 - PubMed
2004 Sep;256(3):183-94 - PubMed
- J Intern Med.
-
- Neurology. 2007 Oct 30;69(18):1789-99 - PubMed
Publication types
MeSH terms
Grant support
- P50 AG005134/AG/NIA NIH HHS/United States
- R01 MH099987/MH/NIMH NIH HHS/United States
- K07 AG044395/AG/NIA NIH HHS/United States
classification, main causes and treatment uMEDp
The article provides a definition, classification, diagnostic criteria, principles of pathogenetic and symptomatic therapy of non-dementia cognitive impairment. The possibilities of using the dopaminergic and noradrenergic drug piribedil (Pronoran) for the treatment of mild and moderate cognitive impairments that do not reach the severity of dementia are considered in detail.
The possibilities of using the dopaminergic and noradrenergic drug piribedil (Pronoran) for the treatment of mild and moderate cognitive impairments that do not reach the severity of dementia are considered in detail.
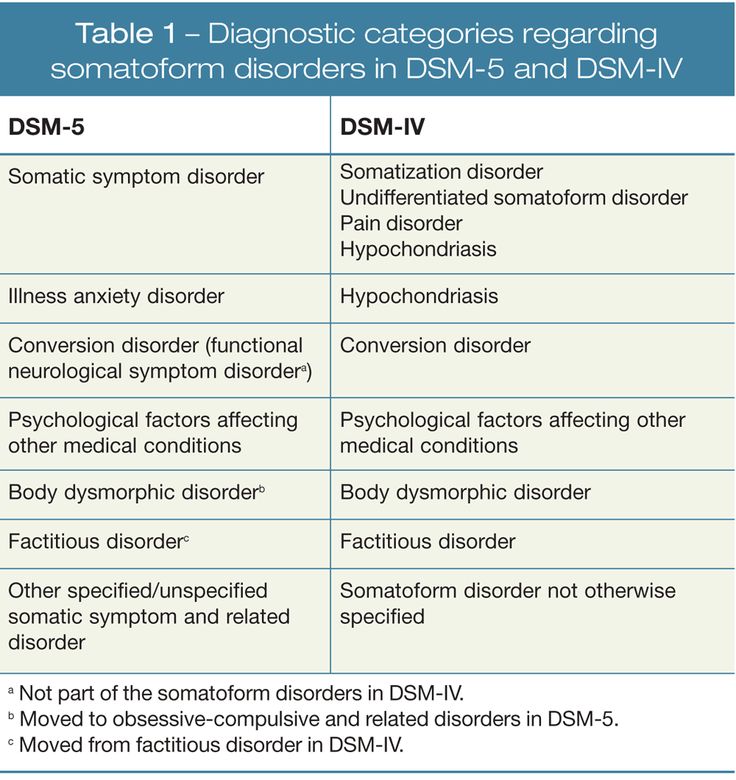
Table 1. Cognitive Functions (according to DSM-V)
Table 2. DSM-V
Diagnostic Criteria for Moderate and Severe Neurocognitive ImpairmentTable 3. Classification of cognitive impairment by severity [5]
Table 4. Diagnostic criteria for depression according to the International Classification of Diseases, 10th Revision
Fig. 1. The increase in the sum of points on the Montreal scale for assessing cognitive functions during therapy with Pronoran (group A), piracetam (group B), ginkgo biloba (group C) and vinpocetine (group D)
Fig. 2. Dynamics of subjective neurological symptoms during therapy with Pronoran (p < 0.05)
About 90% of the area of the human cerebral cortex is involved in cognitive activity. Therefore, most neurological diseases with an interest in the brain are accompanied by certain cognitive disorders. Usually they are combined with changes in the emotional-behavioral sphere, being united by a common pathomorphological and pathophysiological substrate. A practicing neurologist needs to assess the presence and characteristics of cognitive and other neuropsychiatric disorders and take this information into account when diagnosing syndromic, topical and nosological diseases of the nervous system.
Usually they are combined with changes in the emotional-behavioral sphere, being united by a common pathomorphological and pathophysiological substrate. A practicing neurologist needs to assess the presence and characteristics of cognitive and other neuropsychiatric disorders and take this information into account when diagnosing syndromic, topical and nosological diseases of the nervous system.
Cognitive impairment is of equal importance to clinicians in other medical specialties. The target organ of many somatic diseases, in particular, diseases of the cardiovascular system that are widespread in old age, is the brain. In this case, the assessment of the state of the brain is extremely important for assessing the effectiveness of controlling the underlying disease and choosing a therapeutic tactic.
The presence of cognitive impairments has an extremely negative impact on the quality of life of the patient and his immediate family, makes it difficult to treat concomitant diseases and carry out rehabilitation measures. Therefore, timely diagnosis and the earliest possible start of therapy for existing cognitive disorders are very important.
Therefore, timely diagnosis and the earliest possible start of therapy for existing cognitive disorders are very important.
Definition and classification of cognitive impairments
According to the latest revision of the international recommendations for the diagnosis of mental disorders (Diagnostic and statistical manual of mental diseases - DSM-V), cognitive disorders include a decrease in comparison with the premorbid level of one or more higher brain functions that provide the processes of perception, storage, transformation and transmission of information (Table 1) [1].
It is important not only to establish cognitive decline and conduct its qualitative analysis, but also to quantify the severity of existing disorders. It is known that some drugs that are effective in severe cognitive impairment (dementia) have a much lesser effect on cognitive impairment that does not reach the degree of dementia. This is probably due to various neurochemical changes that are noted at the early and later stages of the pathological process [2-4].
Dementia (or, according to DSM-V, severe neurocognitive impairment) is characterized by significant impairment of higher brain functions that interfere with the normal functioning of the patient. With dementia, due to severe cognitive impairment, the patient is at least partially deprived of independence and needs outside help in the most common life situations (for example, when orienting in the area, shopping in a store) (Table 2) [1].
In the treatment of patients with severe cognitive disorders, priority should be given to drugs with a symptomatic effect, which can reduce the severity of disorders and thereby improve the quality of life of patients and their relatives.
The diagnosis of non-dementia cognitive impairment is established in cases where, despite the existing intellectual defect, the patient retains independence in everyday life. At the same time, the patient may feel some difficulties in mental work, which is reflected in complaints. However, the patient overcomes these difficulties without resorting to outside help (Table 2) [1].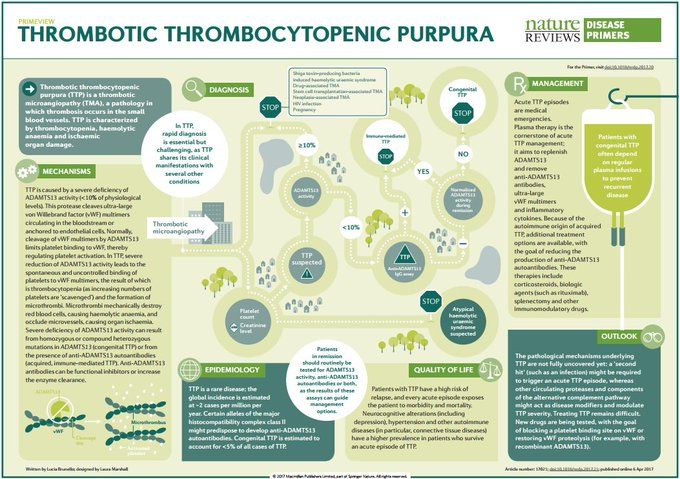 In the treatment of patients with non-dementia cognitive disorders, one should not only use symptomatic therapy, but also take measures to prevent dementia.
In the treatment of patients with non-dementia cognitive disorders, one should not only use symptomatic therapy, but also take measures to prevent dementia.
According to the classification of Academician N.N. Yakhno, non-dementia cognitive disorders are divided into mild and moderate (Table 3) [5]. At the same time, patients with moderate impairments may experience difficulties in the most complex and unusual activities for the patient. At the same time, patients with mild disorders are completely independent and independent in all types of activity, including the most difficult one.
In recent years, the attention of neurologists, psychiatrists and representatives of other neurosciences has increased to an even earlier stage of cognitive deficiency - the so-called subjective cognitive impairment. The wording "subjective cognitive impairment" (subjective memory impairment, cognitive complaints) is currently widely used both in the scientific literature and in everyday clinical practice as an independent diagnosis. This diagnosis is made if there are cognitive complaints, while the results of objective cognitive tests remain within the age norm.
This diagnosis is made if there are cognitive complaints, while the results of objective cognitive tests remain within the age norm.
Patients may complain of increased forgetfulness, decreased concentration of attention, increased fatigue during mental work, and sometimes difficulty in finding the right word in a conversation. These complaints are a very urgent problem for the patient, which can serve as an independent or main reason for contacting a doctor. At the same time, the use of standard cognitive tests does not reveal any significant deviations from the accepted standards. Patients with subjective cognitive disorders fully maintain independence in everyday life. Cognitive difficulties are also invisible from the outside: relatives, colleagues and other persons always assess the patient's cognitive abilities as quite intact.
Currently, the following international diagnostic criteria (2014) for the syndrome of subjective cognitive impairment are known [6]:
-
patient complaints about a persistent deterioration in mental performance compared to the past, which arose for no apparent reason;
-
the absence of any deviations from the age norm according to the cognitive tests used to diagnose Alzheimer's disease and other dementing diseases;
-
cognitive complaints are not associated with any established diagnosis of a neurological, psychiatric disease or intoxication.
The dissociation between patient complaints, test results, and patients' day-to-day functioning raises legitimate questions about the true nature of the complaints. These issues are still far from being resolved and are being actively studied. At the present stage of scientific knowledge, it seems that patients with subjective cognitive impairments represent a very heterogeneous group, which includes both patients with the earliest stages of the dementing process, and patients with disorders of the anxiety-depressive and hypochondria spectrum.
In some cases, the predominantly subjective nature of disorders is explained by methodological difficulties in objectifying cognitive status. Currently, there are no generally accepted recommendations on the use of specific methods for the diagnosis of dementia or non-dementia cognitive impairment. Therefore, in practice, tests of varying degrees of sensitivity, specificity and reproducibility are used. The use of tests with low sensitivity will lead to underdiagnosis of mild and moderate cognitive impairments and to overdiagnosis of so-called subjective impairments.
The diagnosis of "subjective cognitive impairment" is often received by patients with a high premorbid intellectual level. The cognitive functions reduced as a result of a cerebral disease in comparison with the individual norm will formally be within the average statistical standard for a long time. Consequently, cognitive decline can remain formally unconfirmed for a long time, in other words, “subjective”.
Complaints of a cognitive nature may be due to anxiety-depressive disorders in the absence of an organic cerebral disease. Thus, patients with a high level of anxiety will be overly concerned about minor situational forgetfulness. In this case, the reason for going to the doctor is such widespread complaints, including among healthy people, such as “I don’t remember why I came to the room”, “I don’t remember what I put where”, “I didn’t recognize a familiar person or didn’t remember him surname", etc.
However, the greatest research interest in a heterogeneous group of patients with subjective cognitive impairments is caused by patients with reduced tolerance to mental stress, since this pathological phenomenon may indeed be the earliest clinical manifestation of the dementing process. As is known, at the very initial stages of a neurodegenerative or cerebrovascular disease, clinical symptoms may be absent, despite the presence of organic brain damage, sometimes significant. This is due to the so-called cerebral reserve, that is, the compensatory capabilities of the brain. The presence of such opportunities will lead to a false negative test result. At the same time, in everyday life, the patient may experience difficulties in special conditions when the cerebral reserve is depleted and cannot overcome the difficulties that arise, for example, in a state of fatigue or emotional stress. At present, the development of the "intellectual treadmill" methodology is being carried out very actively in the world. It will allow assessing the degree of tolerance to increased mental stress, which may decrease to the development of clinically defined cognitive disorders.
As is known, at the very initial stages of a neurodegenerative or cerebrovascular disease, clinical symptoms may be absent, despite the presence of organic brain damage, sometimes significant. This is due to the so-called cerebral reserve, that is, the compensatory capabilities of the brain. The presence of such opportunities will lead to a false negative test result. At the same time, in everyday life, the patient may experience difficulties in special conditions when the cerebral reserve is depleted and cannot overcome the difficulties that arise, for example, in a state of fatigue or emotional stress. At present, the development of the "intellectual treadmill" methodology is being carried out very actively in the world. It will allow assessing the degree of tolerance to increased mental stress, which may decrease to the development of clinically defined cognitive disorders.
International studies show that the risk of developing dementia among patients with subjective cognitive impairment is significantly higher than the average in the population [6]. Therefore, even isolated complaints that are not confirmed by cognitive tests should not be ignored by the attending physicians. They cannot serve as a basis for any specific clinical diagnosis, but their presence is an indication for active prevention, primarily non-pharmacological (mental and physical activity, optimization of nutrition and lifestyle).
Therefore, even isolated complaints that are not confirmed by cognitive tests should not be ignored by the attending physicians. They cannot serve as a basis for any specific clinical diagnosis, but their presence is an indication for active prevention, primarily non-pharmacological (mental and physical activity, optimization of nutrition and lifestyle).
Diagnosis of moderate cognitive impairment
As follows from the above criteria (Table 2), the diagnosis of the syndrome of moderate neurocognitive impairment is based, firstly, on the complaints of patients and / or their relatives, and secondly, on objective test results. At the same time, it should be borne in mind that complaints of a cognitive nature are far from always straightforward. Usually, patients with the so-called amnestic type of the syndrome of moderate neurocognitive impairment complain of a decrease in memory or increased forgetfulness, in which progressive memory disorders predominate in their cognitive status. In such patients, Alzheimer's disease is most often established in the future. However, according to the analysis of specialized outpatient admission of patients with cognitive impairment, the most common cause of the syndrome of moderate cognitive impairment is cerebrovascular pathology. Thus, the experience of the first Russian clinic of memory disorders shows that dyscirculatory encephalopathy or the consequences of acute cerebrovascular accidents cause 68% of moderate cognitive impairments [7].
In such patients, Alzheimer's disease is most often established in the future. However, according to the analysis of specialized outpatient admission of patients with cognitive impairment, the most common cause of the syndrome of moderate cognitive impairment is cerebrovascular pathology. Thus, the experience of the first Russian clinic of memory disorders shows that dyscirculatory encephalopathy or the consequences of acute cerebrovascular accidents cause 68% of moderate cognitive impairments [7].
Vascular cognitive impairments in most cases are of the so-called subcortical-frontal type. At the same time, the memory for current events and life events practically does not suffer, and the cognitive status is dominated by a decrease in the concentration of attention and the pace of cognitive activity (bradyphrenia), a violation of frontal control functions (planning and control). A characteristic feature is also the frequent combination of cognitive and emotional-behavioral disorders: depression, apathy or affective lability. It should be emphasized that emotional and behavioral disorders in chronic cerebrovascular insufficiency are of an organic nature and are caused by the same brain damage (dysfunction of fronto-striatal connections) as cognitive impairment. The comorbidity of vascular depression and vascular cognitive impairment is at least 80% [8–11].
It should be emphasized that emotional and behavioral disorders in chronic cerebrovascular insufficiency are of an organic nature and are caused by the same brain damage (dysfunction of fronto-striatal connections) as cognitive impairment. The comorbidity of vascular depression and vascular cognitive impairment is at least 80% [8–11].
Patients with vascular cognitive disorders rarely complain of forgetfulness, since their memory is relatively intact. The structure of complaints is dominated by the so-called subjective neurological symptoms: headache, non-systemic dizziness, noise and heaviness in the head, increased fatigue, sleep disturbances. These symptoms are quite typical for the initial stages of dyscirculatory encephalopathy and in the recent past were considered as an important sign of chronic ischemic brain damage. It is now obvious that headache, dizziness and other unpleasant sensations in the head cannot be a direct result of cerebral ischemia. The pathogenesis of subjective neurological symptoms is more complex and is associated primarily with existing cognitive, emotional and motor disorders. So, headache most often has the character of a tension headache, which, as you know, is almost always caused by anxiety and / or depression. Sleep disturbances also have an emotional cause. Increased fatigue can either be a sign of depression (Table 4) or reflect a decrease in mental performance. In the latter case, this complaint is the subjective equivalent of cognitive disorders. Dizziness in chronic cerebrovascular insufficiency is usually non-systemic and is described as a feeling of unsteadiness when walking. Behind this sensation, as a rule, there are real imbalances due to damage to the fronto-striatal and fronto-cerebellar connections.
So, headache most often has the character of a tension headache, which, as you know, is almost always caused by anxiety and / or depression. Sleep disturbances also have an emotional cause. Increased fatigue can either be a sign of depression (Table 4) or reflect a decrease in mental performance. In the latter case, this complaint is the subjective equivalent of cognitive disorders. Dizziness in chronic cerebrovascular insufficiency is usually non-systemic and is described as a feeling of unsteadiness when walking. Behind this sensation, as a rule, there are real imbalances due to damage to the fronto-striatal and fronto-cerebellar connections.
Subjective neurological symptoms are almost always present in the initial stages of chronic cerebrovascular insufficiency. They cannot be the basis for a diagnosis, but should lead the doctor to suspect a chronic cerebrovascular disease. To confirm the diagnosis, a thorough assessment of the cognitive and emotional status using objective methods is required. At the stage of moderate (non-dementic) cognitive disorders, the most sensitive methods should be used, for example, the Montreal Cognitive Assessment Scale [12].
At the stage of moderate (non-dementic) cognitive disorders, the most sensitive methods should be used, for example, the Montreal Cognitive Assessment Scale [12].
Pathogenetic and symptomatic therapy of non-dementia cognitive impairments
To date, a unified generally accepted protocol for the management of patients with cognitive impairments that do not reach the severity of dementia has not been finally developed. Many international studies have failed to demonstrate that pharmacotherapy with drugs such as acetylcholinesterase inhibitors, piracetam, non-steroidal anti-inflammatory drugs prevents or reduces the risk of dementia [2-4]. At the same time, the same studies showed the ability of some of the above drugs to reduce the severity of symptoms in patients with mild cognitive impairment syndrome.
Currently, vasotropic and neurometabolic drugs, the dopaminergic and noradrenergic drug piribedil (Pronoran), and NMDA receptor blockers are widely used empirically in everyday clinical practice.
The results of a number of large studies and practical experience indicate the clinical effectiveness of the drug piribedil (Pronoran). Pronoran has a complex mechanism of action: it stimulates postsynaptic D 2 / D 3 - dopamine receptors and blocks presynaptic alpha-adrenergic receptors. At the same time, the blockade of presynaptic adrenergic receptors leads to an increase in cerebral noradrenergic activity. Thus, against the background of the use of this drug, the activity of two cerebral neurotransmitter systems increases: dopaminergic and noradrenergic. Both of these systems are directly involved in cognitive activity. At the same time, it is believed that dopaminergic stimulation of the prefrontal cortex, indirectly through the mesocortical dopaminergic pathway, plays an important role in attention processes and provides intellectual flexibility, that is, the ability to change the behavioral paradigm. Noradrenergic activation is important for the processes of memorization and reproduction of information, since it provides the optimal level of concentration and motivation for mnestic activity. With age, the synthesis and activity of both dopamine and norepinephrine decrease. Therefore, the correction of these neurotransmitter disorders against the background of the use of Pronoran helps to reduce the severity of age-associated disorders of attention and memory. In addition, due to the adrenoblocking and dopaminergic action, Pronoran also has a favorable vasotropic effect, which creates additional benefits in case of cognitive impairment of vascular etiology [13-16].
With age, the synthesis and activity of both dopamine and norepinephrine decrease. Therefore, the correction of these neurotransmitter disorders against the background of the use of Pronoran helps to reduce the severity of age-associated disorders of attention and memory. In addition, due to the adrenoblocking and dopaminergic action, Pronoran also has a favorable vasotropic effect, which creates additional benefits in case of cognitive impairment of vascular etiology [13-16].
In clinical practice, Pronoran is used to treat mild and moderate cognitive impairment that does not reach the severity of dementia in patients over 50 years of age. The drug can be prescribed both for vascular cognitive impairment and at the initial stages of the neurodegenerative process. A large number of clinical studies have been performed for this indication, including those using a double-blind method. For example, in France in the 1980s. 14 clinical studies were conducted, in which more than 7 thousand patients with non-dementia cognitive impairments took part. It has been shown that Pronoran contributes to a significant improvement in memory, concentration and intellectual flexibility, that is, the ability to change the paradigm of behavior depending on external conditions [17, 18]. In 2001, the clinical efficacy of Pronoran was again demonstrated by D. Nagaradja and S. Jayashree. The authors used Pronoran in the syndrome of moderate cognitive impairment in accordance with modern diagnostic criteria. It was shown that against the background of the study drug, there was a more than two-fold increase in the frequency of cognitive improvement on the short mental status assessment scale compared with placebo, which was statistically and clinically significant [19].
It has been shown that Pronoran contributes to a significant improvement in memory, concentration and intellectual flexibility, that is, the ability to change the paradigm of behavior depending on external conditions [17, 18]. In 2001, the clinical efficacy of Pronoran was again demonstrated by D. Nagaradja and S. Jayashree. The authors used Pronoran in the syndrome of moderate cognitive impairment in accordance with modern diagnostic criteria. It was shown that against the background of the study drug, there was a more than two-fold increase in the frequency of cognitive improvement on the short mental status assessment scale compared with placebo, which was statistically and clinically significant [19].
Currently, Russian specialists also have significant experience in using Pronoran in patients with cognitive impairments that do not reach the severity of dementia. Thus, as part of the PROMETHEUS study, 574 patients from 33 cities in 30 regions of Russia, including 336 women and 207 men, aged 60 to 89 years (mean age 69. 5 ± 5.5 years) received Pronoran with mild or moderate cognitive impairment. . Patients with cognitive complaints who scored 25–27 points on the Mini Mental Status Scale or who performed the clock drawing test with errors but did not meet the diagnostic criteria for dementia were selected for treatment. During therapy, a statistically significant improvement in cognitive functions was recorded, which was noted as early as the sixth week of treatment and further increased until the end of the 12-week follow-up. At the same time, one part of the patients received Pronoran monotherapy, and the other part received Pronoran in combination with vasotropic and / or neurometabolic drugs. No significant difference was shown between these groups of patients, that is, the combination of Pronoran with vasotropic and neurometabolic therapy had no advantages over monotherapy with the study drug [20, 21].
5 ± 5.5 years) received Pronoran with mild or moderate cognitive impairment. . Patients with cognitive complaints who scored 25–27 points on the Mini Mental Status Scale or who performed the clock drawing test with errors but did not meet the diagnostic criteria for dementia were selected for treatment. During therapy, a statistically significant improvement in cognitive functions was recorded, which was noted as early as the sixth week of treatment and further increased until the end of the 12-week follow-up. At the same time, one part of the patients received Pronoran monotherapy, and the other part received Pronoran in combination with vasotropic and / or neurometabolic drugs. No significant difference was shown between these groups of patients, that is, the combination of Pronoran with vasotropic and neurometabolic therapy had no advantages over monotherapy with the study drug [20, 21].
In the largest Russian non-comparative study, Pronoran was treated by more than 2,000 patients aged 50 to 94 years (mean age 64. 9 ± 8.3 years) with a diagnosis of stage 1 or 2 dyscirculatory encephalopathy and with mild or moderate cognitive impairment. violations. All patients took Pronoran for three months. According to the attending physicians, in 2/3 of cases there was a significant or moderate improvement in cognitive and other neurological functions [22].
9 ± 8.3 years) with a diagnosis of stage 1 or 2 dyscirculatory encephalopathy and with mild or moderate cognitive impairment. violations. All patients took Pronoran for three months. According to the attending physicians, in 2/3 of cases there was a significant or moderate improvement in cognitive and other neurological functions [22].
According to some data, the magnitude of the therapeutic effect of dopamine and noradrenergic therapy in relation to non-dementia cognitive disorders may be greater than that of other vasotropic and neurometabolic drugs actively used in clinical practice. In the FUETE study, 189 patients were observed, including 139 women and 57 men, aged 42 to 82 years (mean age 63.6 ± 8.5 years) with cognitive disorders that did not reach the severity of dementia, against the background of arterial hypertension and cerebral atherosclerosis . The patients were treated with various drugs, while the representatives of the therapeutic groups did not differ in age, level of education and clinical features of the underlying disease. Against the background of the therapy, there was a regression of both subjective and objective cognitive disorders in all compared therapeutic groups. At the same time, the severity of subjective improvement and objective dynamics of cognitive tests against the background of the use of Pronoran after two months of therapy were significantly greater compared with vasotropic and neurometabolic therapy (Fig. 1) [23].
Against the background of the therapy, there was a regression of both subjective and objective cognitive disorders in all compared therapeutic groups. At the same time, the severity of subjective improvement and objective dynamics of cognitive tests against the background of the use of Pronoran after two months of therapy were significantly greater compared with vasotropic and neurometabolic therapy (Fig. 1) [23].
Regression of cognitive disorders, according to special tests, is the main criterion for the effectiveness of the therapy. However, as noted above, many patients with moderate cognitive impairment, primarily of a vascular nature, also complain of headache, non-systemic dizziness, noise, heaviness or other unpleasant sensations in the head, increased fatigue and sleep disturbances. These complaints are of the same nature and are associated with both cognitive impairment and changes in the emotional status of patients at the initial stage of chronic cerebrovascular insufficiency. They significantly reduce the quality of life of patients and are often the main reason for visiting a neurologist. Therefore, the dynamics of subjective neurological symptoms in patients with a syndrome of moderate neurocognitive disorders of vascular etiology during therapy is extremely important for assessing the significance of the clinical effect and the degree of influence of therapy on the daily life of patients. Regression of subjective neurological symptoms contributes most to adherence to therapy.
They significantly reduce the quality of life of patients and are often the main reason for visiting a neurologist. Therefore, the dynamics of subjective neurological symptoms in patients with a syndrome of moderate neurocognitive disorders of vascular etiology during therapy is extremely important for assessing the significance of the clinical effect and the degree of influence of therapy on the daily life of patients. Regression of subjective neurological symptoms contributes most to adherence to therapy.
In the study by N.N. Yakhno et al. (2006) 29 patients with a diagnosis of "moderate cognitive impairment" against the background of dyscirculatory encephalopathy of the first or second stage received Pronoran for three months [24]. At the same time, no other vasotropic or neurometabolic drugs were used. During treatment with Pronoran, the frequency and severity of headache, dizziness, fatigue and subjective feeling of forgetfulness significantly decreased (Fig. 2). Other authors also reported on the weakening of subjective neurological symptoms during the use of Pronoran [17, 18]. Thus, dopamine and noradrenergic therapy contributes to a significant improvement in the well-being of patients, and, consequently, improves the quality of life and adherence to ongoing therapeutic measures.
Thus, dopamine and noradrenergic therapy contributes to a significant improvement in the well-being of patients, and, consequently, improves the quality of life and adherence to ongoing therapeutic measures.
It can be summarized that to date, Pronoran has established itself as an effective drug that improves cognitive abilities and well-being in patients with the initial stages of organic cerebral diseases without dementia. In contrast to Parkinson's disease, in which significantly higher doses are used, for non-demented cognitive impairment, Pronoran is prescribed at a dose of 50 mg / day once a day. The recommended duration of therapy is at least three months.
Diagnosis of mental disorders in the elderly: modern classifications | Savina
1. American Psychiatric Association. Diagnostic and manual statistical of mental disorders. DSM IV. 1994.
2. Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. American Journal of Psychiatry. 1970;126(7):983–987. DOI:10.1176/ajp.126.7.983.
American Journal of Psychiatry. 1970;126(7):983–987. DOI:10.1176/ajp.126.7.983.
3. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. International Schizophrenia Consortium. Common polygenic variation contributes to the risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. DOI:10.1038/nature08185.
4. American Psychiatric Association. Diagnostic and manual statistical of mental disorders. 5th ed. Arlington, VA: APA, 2013.
5. Krasnov VN. Modern principles of diagnosis and classification of mental disorders. Social and clinical psychiatry. 2018;28(1): 58–61.
6. Sachdev PS. Is DSM-5 defensible? Aust NZ J. Psychiatry. 2013;47(1):10–11. DOI:10.1177/0004867412468164.
7. Ghaemi SN. DSM-5 and the miracle that never happened. Acta Psychiatr. Scand. 2014;129(6):410–412. DOI:10.1111/acps.12263.
8. Parker G. The DSM-5 classification of mood disorders: some fallacies and fault lines. Acta Psychiatr. Scand. 2014;129(6):404–409. DOI:10.1111/acps.12253.
Scand. 2014;129(6):404–409. DOI:10.1111/acps.12253.
9. Maglione, JE, Thomas, SE, Jeste DV. Lateonset schizophrenia: do recent studies support categorizing LOS as a subtype of schizophrenia? Current opinion in psychiatry. 2014;27(3):173. DOI:10.1097/YCO.0000000000000049.
10. Howard R, Rabins PV, Seeman MV, Jeste DV. Lateonset schizophrenia and verylateonset schizophrenia-like psychosis: an international consensus. Am. J. Psychiatry. 2000;157(2):172–178. DOI:10.1176/appi.ajp.157.2.172.
11. Uher R, Payne JL, Pavlova B, Perlis RH. Major depressive disorder in DSM-5: implications for clinical practice and research changes. Depress Anxiety. 2014;31(6):459–471. DOI:10.1002/da.22217.
12. Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. 2013;12(2):92–98. DOI:10.1002/wps.20050.
13. Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, Kupfer DJ. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry. 2013;170(1):59-70. https://doi.org/10.1176/appi.ajp.2012.12070999.
Am. J. Psychiatry. 2013;170(1):59-70. https://doi.org/10.1176/appi.ajp.2012.12070999.
14. Gallo J, Rabins P, Anthony J. Sadness in older per-sons: 13-year follow-up of a community sample in Baltimore, Maryland. Psychol. Med 1999;29(2):341–350. DOI:10.1017/s0033291798008083.
15. American Psychiatric Association. Diagnostic and manual statistical of mental disorders. 4th ed., text rev. Washington, DC: APA, 2000.
16. Kendler KS, Myers J, Zisook S. Does bereavement-related major depression differ from major depression associated with other stressful life events? Am. J. Psychiatry. 2008;165(11):1449–1455. DOI:10.1176/appi.ajp.2008.07111757.
17. Kessing LV, Bukh JD, Bock C, Vinberg M, Gether U. Does bereavement-related episode first depression differ from other kinds of first depressions? soc. Psychiatry epidemiol. 2010;45(8):801–808. DOI:10.1007/s00127-009-0121-6.
18. Mojtabai R. Bereavement-related depressive episodes: characteristics, 3-year course, and implications for the DSM-5. Arch. Gen. Psychiatry. 2011;68(9):920–928. DOI:10.1001/archgenpsychiatry.2011.95
Arch. Gen. Psychiatry. 2011;68(9):920–928. DOI:10.1001/archgenpsychiatry.2011.95
19. Karam EG, Tabet CG, Alam D, Shamseddeen W, Chatila Y, Mneimneh Z, Salamoun MM, Hamalian M. Bereavement related and non-bereavement related depressions: a comparative field study. J. Affect. Discord. 2009;112(1–3):102–110. https://doi.org/10.1016/j.jad.2008.03.016.
20. Wakefield JC, Schmitz MF. Recurrence of depression after bereavement-related depression: evidence for the validity of DSM–IV bereavement exclusion from the Epidemiologic Catchment Area Study. J. Nerv. Ment. Dis. 2012;200(6):480–485. DOI:10.1097/NMD.0b013e318248213f.
21. Kornilov VV. The role of pathological grief reaction in the development of dementia of late age. Psychiatry. 2018;1(77):5–15. https://doi.org/10.30629/2618-6667-2018-77-5-15.
22. Wakefield JC, First MB. Validity of the bereavement exclusion to major depression: does the empirical evidence support the proposal to eliminate the exclusion in DSM-5? World Psychiatry. 2012;11(3):3–10. DOI:10.1016/j.wpsyc.2012.01.002.
2012;11(3):3–10. DOI:10.1016/j.wpsyc.2012.01.002.
23. Reed GM, MendonçaCorreia J, Esparza P, Saxena S, Maj M. The WPA-WHO global survey of psychiatrists’ attitudes towards mental disorders classification. World Psychiatry. 2011;10(2):118–131. DOI:10.1002/j.2051-5545.2011.tb00034.x.
24. Maj M. Clinical judgment and the DSM-5 diagnosis of major depression. World Psychiatry. 2013;12(2):89–91. DOI:10.1002/wps.20049.
25. Sachdev PS, Mohan A, Taylor L, Jeste DV. DSM-5 and mental disorders in older individuals: an overview. Harvard Review of Psychiatry. 2015;23(5):320–328. DOI:10.1097/HRP.0000000000000090.
26. End VA. Depression in old age. In: Guide to Psychiatry. Edited by AS Tiganov. 1999:668–673.
27. Judd L, Schettler P, Akiskal H, Coryell W, Fawcett J, Fiedorowicz JG, Solomon DA, Keller MB. Prevalence and clinical significance of subsyndromal manic symptoms, including irritability and psychomotor agitation, during bipolar major depressive episodes. J. Affect. Discord. 2012;138(3):440–448. https://doi.org/10.1016/j.jad.2011.12.046.
J. Affect. Discord. 2012;138(3):440–448. https://doi.org/10.1016/j.jad.2011.12.046.
28. Siranchiev MA. Dysthymia: background, age aspects. Psychiatry. 2003;2(2):51–60.
29. Byers AL, Vittinghoff E, Lui LY, Hoang T, Blazer DG, Covinsky KE, Ensrud KE, Cauley JA, Hillier TA, Fredman L, Yaffe K. Twenty-year depressive trajectories among older women. Arch. Gen. Psychiatry. 2012;69(10):1073–1079. https://doi:10.1001/archgenpsychiatry.2012.43.
30. Fawcett J. An overview of mood disorders in the DSM-5. Curr. Psychiatry Rep. 2010;12(6):531–538. DOI:10.1007/s11920-010-0154-2.
31. Dumlu K, Orhon Z, Ozerdem A, Tural U, Ulas H, Tunca Z. Treatment-induced manic switch in the course of unipolar depression can predict bipolarity: cluster analysis based evidence. J. Affect. Discord. 2011;(1,3):91–134. https://doi.org/10.1016/j.jad.2011.06.019.
32. Oquendo MA, Baca-Garcia E. Suicidal behavior disorder as a diagnostic entity in the DSM–5 classification system: advantages outweigh limitations. World Psychiatry. 2014;13(2):128–130. DOI:10.1002/wps.20116.
World Psychiatry. 2014;13(2):128–130. DOI:10.1002/wps.20116.
33. Shah A. The relationship between suicide rates and age: an analysis of multinational data from the World Health Organization Int. Psychogeriatr. 2007;19(6):1141–1152. DOI:10.1017/S1041610207005285.
34. Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am. J. Psychiatry. 1996;153(8):1001–1008. DOI:10.1176/ajp.153.8.1001.
35. Van Ameringen M, Patterson B, Simpson W. DSM-5 obsessive compulsive and related disorders: clinical implications of new criteria. Depress Anxiety. 2014;31(6):487–489. DOI:10.1002/da.22259.
36. Stein DJ, Craske MG, Friedman MJ, Phillips KA. Meta-structure issues for the DSM–5: how do anxiety disorders, obsessive-compulsive disorder and related disorders, posttraumatic disorders, and dissociative disorders fit together? Curr. Psychiatry Rep. 2011;13(4):248–250. DOI:10.1007/s11920-011-0207-1.
DOI:10.1007/s11920-011-0207-1.
37. Lenze EJ, Wetherell JL. A lifespan view of anxiety disorders. Dialogue Clin. neurosci. 2011;13(4):381–399. PMCID: PMC3263387.
38. Boegels SM, Knappe S, Clark LA. Adult separation anxiety disorder in DSM-5. Clin. Psychol. Rev. 2013;33(5):663–674. DOI:10.1016/j.cpr.2013.03.006.
39. Manicavasagar V, Marnane C, Pini S, Abelli M, Rees S, Eapen V, Silove D. Adult separation anxiety disorder: a disorder comes of age. Curr. Psychiatry Rep. 2010;12(4):290–297. https://doi.org/10.1007/s11920-010-0131-9.
40. Shear K, Jin R, Meron Ruscio A, Walters E, Kessler R. Prevalence and correlates ofestimated DSM-IV child and adult separation anxiety disorder in the National Comorbidity Survey Replication. Am. J. Psychiatry. 2006;163(6):1074–1083. DOI:10.1176/ajp.2006.163.6.1074.
41. Strain JJ, Freidman MS. Considering adjustment disorders as stress response syndromes. Depress Anxiety. 2011;28(9):818–823. DOI:10.1002/da.20782.
42. Casey P, Doherty A. Adjustment disorder: implications for ICD-11 and DSM-5. Br. J. Psychiatry. 2012;(201):90–92. DOI:10.1192/bjp.bp.112.110494.
Casey P, Doherty A. Adjustment disorder: implications for ICD-11 and DSM-5. Br. J. Psychiatry. 2012;(201):90–92. DOI:10.1192/bjp.bp.112.110494.
43. Savina MA. Clinic of post-stroke depression. Mental disorders in general medicine. 2006;(2):31–35.
44. Savina MA, Serpukhovitina IA. Clinical picture of generalized anxiety disorder. Mental disorders in general medicine. 2009;2:4–10.
45. Sachdev P, Andrews G, Hobbs MJ, Sunderland M, Anderson TM. Neurocognitive disorders: cluster 1 of the proposed metastructure for DSM-V and ICD-11. Psychol Med. 2009;39(12):2001–2012. DOI:10.1017/S0033291709990262.
46. Rodriguez-Bailón M, Montoro-Membila N., Garcia-Morán T, Arnedo-Montoro ML, Funes Molina MJ. Preliminary cognitive scale of basic and instrumental activities of daily living for dementia and mild cognitive impairment. J.Clin. Exp. Neuropsychol. 2015;37(4):339– 53. DOI:10.1080/13803395.2015.1013022.
47. Petersen R, Roberts RO, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Jack CR Jr. Mild cognitive impairment: ten years later. Arch. Neurol 2009;66(12):1447–1455. https://doi.org/10.1001/archneurol.2009.266.
Mild cognitive impairment: ten years later. Arch. Neurol 2009;66(12):1447–1455. https://doi.org/10.1001/archneurol.2009.266.
48. Gavrilova SI. Predemental neurocognitive disorders: diagnostic and therapeutic aspects. Review of Psychiatry and Medical Psychology named after V.M. Bekhterev. 2018;(1):89–98.
49. Blazer D. Neurocognitive disorders in DSM-5. Am. J. Psychiatry. 2013;170(6):585–587. DOI:10.1176/appi.ajp.2013.13020179.
50. Mikhailova NM, Roshchina IF. Ethical aspects of late age dementia: problems and solutions. Clinical and special psychology. 2013;(2):91–109.
51. Roshchina IF, Gavrilova SI, Zharikov GA., Kalyn YaB, Kolykhalov IV, Mikhailova NM, Selezneva ND, Sokolova ON. Neuropsychological prospective study of patients with so-called questionable dementia. Psychiatry. 2003;4(4):54–57.
52. Ganguli M. Can the DSM-5 framework enhance the diagnosis of MCI? Neurology. 2013;81(23):2045–2050. DOI:10.1212/01.wnl.0000436944.01023.e5.
53. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256(3):240–246. DOI:10.1111/j.1365–2796.2004.01380.x.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256(3):240–246. DOI:10.1111/j.1365–2796.2004.01380.x.
54. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. DOI:10.1016/j.jalz.2011.03.008.
55. Mikhailova NM. Depression at a later age. Russian medical journal.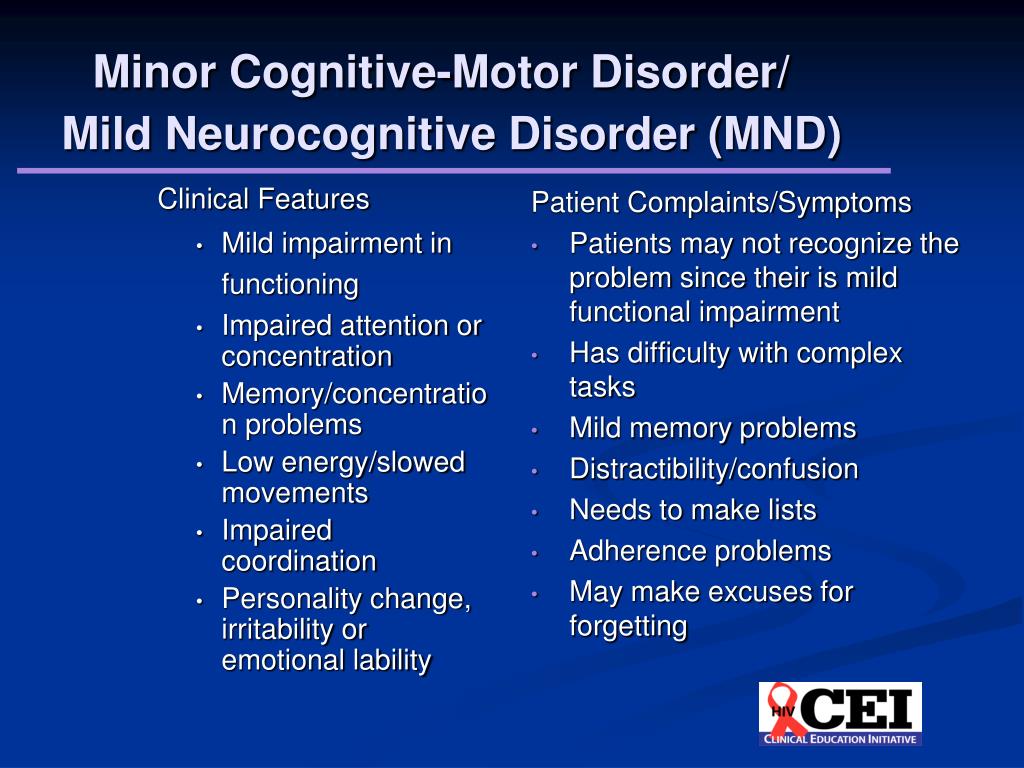 2007;12(14):835.
2007;12(14):835.
56. Kolykhalov IV, Selezneva ND. Non-cognitive mental disorders in Alzheimer's disease: symptomatology and therapy. Psychiatry and psycho-pharmacotherapy, 2001;3(2):48–53.
57. Fischer CE, Agüera-Ortiz L. Psychosis and dementia: risk factor, prodrome, or cause? International psychogeriatrics. 2018;30(2):209–219. DOI:10.1017/S1041610217000874.
58. Nagao S, Yokota O, Ikeda C, Takeda N, Ishizu H, Kuroda S, Terada S, Murayama S, Uchitomi Y. Argyrophilic grain disease as a neurodegenerative substrate in late-onset schizophrenia and delusional disorders. European archives of psychiatry and clinical neuroscience. 2014;264(4):317–331. DOI:10.1007/s00406-013-0472-6.
59. Alexoupolos GS. The Vascular Depression Hypothesis: 10 Years Later. Biol. Psychiatry. 2006;60(12):1304–1305. DOI:10.1016/j.biopsych.2006.09.006.
60. Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. DOI:10.1016/S1474-4422(07)70178-3.
Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. DOI:10.1016/S1474-4422(07)70178-3.
61. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. DOI:10.1016/j.jalz.2011.03.005
62. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. DOI:10.1212/WNL. 0b013e31821103e6.
0b013e31821103e6.
63. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP , Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL . Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. brain. 2011;134(9):2456–2477. DOI:10.1093/brain/awr179.
64. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ , Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. DOI:10.1212/01.wnl.0000187889.17253.b1.
Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. DOI:10.1212/01.wnl.0000187889.17253.b1.
65. Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, Jellinger K, Jeste DV, Pasquier F, Paulsen J , Prins N, Rockwood K, Roman G, Scheltens P; International Society for Vascular Behavioral and Cognitive Disorders. Diagnostic criteria for vascular cognitive disorder—a VASCOG statement. Alzheimers Dis. Assoc. Discord. 2014;28(3):206–218. DOI:10.1097/WAD.0000000000000034.
66. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson's disease. mov discord. 2007;22(12):1689– 1707. DOI:10.1002/mds.21507.
67.