Circadian rhythm sleep wake disorder
Circadian Rhythm Sleep Disorders | Sleep Foundation
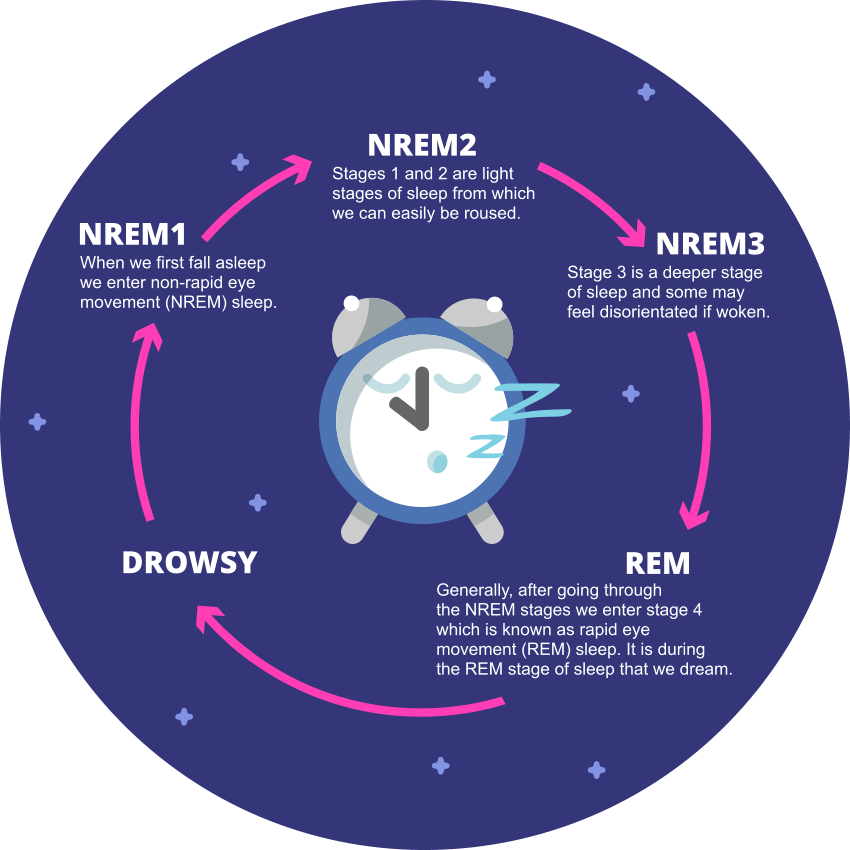
Most people operate on a 24-hour biological clock that is synchronized with bodily hormone production and natural light and darkness. These 24-hour cycles are collectively known as the circadian rhythm, and they play a major role in our sleep cycle.
Circadian rhythm sleep disorders – formally known as circadian rhythm sleep-wake disorders – are a group of conditions tied to dysfunctions or misalignments with the body’s internal clock. Examples of these disorders include mild conditions such as jet lag, as well as more debilitating conditions such as delayed and advanced sleep-wake disorder, irregular sleep-wake rhythm disorder, and shift work disorder.
What Is Circadian Rhythm?
The circadian rhythm is crucial to different physiological processes. In addition to sleep, this rhythm helps regulate body temperature, eating and digestion, and hormonal activity. The master circadian clock is found in the hypothalamus of the brain and composed of a cluster of proteins known as the suprachiasmatic nucleus (SCN). In a healthy adult, this clock resets – or “entrains” – every 24 hours based on light and darkness cycles. A healthy person who wakes up in the morning will gradually become more tired throughout the day, and feelings of sleepiness will peak in the evening when it is dark out.
A person’s sleep rhythm changes and evolves with age. This is why teenagers often go to bed later than both younger children and adults. As we get older, we tend to go to bed and wake up at earlier times of the day.
What Is a Circadian Rhythm Sleep Disorder?
According to the American Academy of Sleep Medicine (AASM) International Classification of Sleep Disorders, a circadian rhythm sleep-wake disorder occurs because of an alteration to the body’s internal timekeeping system, the clock’s inability to entrain roughly every 24 hours, or a misalignment between the clock and a person’s external environment.
What Are the Symptoms of a Circadian Rhythm Sleep Disorder?
While symptoms for these disorders can vary, most cause excessive daytime sleepiness. Insomnia – difficulty falling or staying asleep – is another common issue associated with these disorders.
Insomnia – difficulty falling or staying asleep – is another common issue associated with these disorders.
A formal diagnosis of a circadian rhythm sleep-wake disorder involves specific criteria including:
- Chronic or recurring sleep disturbances due to alterations of the individual’s internal circadian rhythm or misalignments between their circadian rhythm and their desired or required work or social schedule.
- Insomnia symptoms and/or excessive daytime sleepiness.
- Clinically significant distress or impairments to the individual’s mental, physical, social, occupational, or educational performance that can be attributed to their sleep disturbances.
As these criteria demonstrate, circadian rhythm sleep disorders can provoke significant health effects including problems in work or school as well as an elevated risk of vehicular or workplace accidents.
Types of Circadian Rhythm Sleep-Wake Disorders
Based on AASM classifications, the separate types of circadian rhythm sleep-wake disorders include the following:
Delayed and Advanced Sleep-Wake Phase Disorders
Delayed sleep-wake phase disorder occurs when a person’s sleep-wake cycle is pushed back more than two hours beyond what is considered a typical sleep schedule.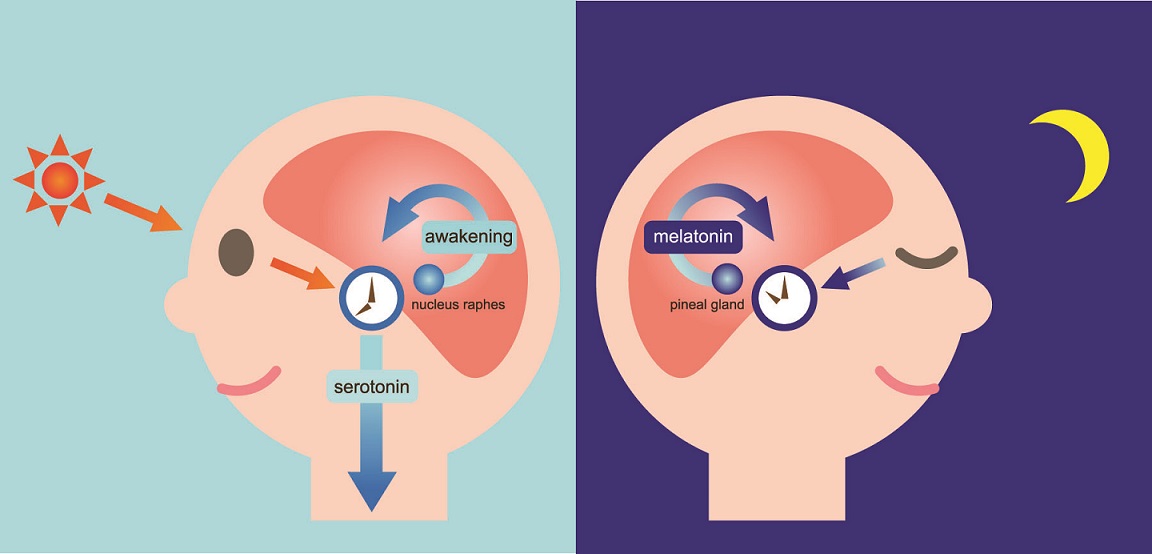 A delayed circadian rhythm can cause people to struggle with falling asleep at night and waking up earlier in the morning.
A delayed circadian rhythm can cause people to struggle with falling asleep at night and waking up earlier in the morning.
People with this condition often suffer from lack of sleep if they have school or work obligations that require an early wake-up time. Many people with this disorder are considered evening chronotypes, or night owls; its prevalence rate for young adults and adolescents is 7 to 16%.
Advanced sleep-wake phase disorder is essentially the opposite: the person tends to fall asleep and wake up more than two hours before their desired times. Advanced age is a major risk factor for this disorder.
In order to receive a diagnosis for delayed or advanced sleep-wake phase disorder, the patient must experience symptoms for at least three months. In addition, they must also report improvements to their sleep quality and duration if allowed to follow their own sleep schedule (rather than a schedule dictated by work or other obligations).
Irregular Sleep-Wake Rhythm Disorder
This disorder is characterized by inconsistent sleep patterns without a stable rhythm or entrainment to day-night cycles. Abnormal sleep periods can cause both difficulty sleeping and excessive daytime sleepiness during the course of the day. Most people with irregular sleep-wake rhythm disorder have neurodevelopmental or neurodegenerative disorder, such as Parkinson’s disease, Alzheimer’s disease, or Huntington’s Disease. The disorder has also been observed in children with developmental disabilities.
Abnormal sleep periods can cause both difficulty sleeping and excessive daytime sleepiness during the course of the day. Most people with irregular sleep-wake rhythm disorder have neurodevelopmental or neurodegenerative disorder, such as Parkinson’s disease, Alzheimer’s disease, or Huntington’s Disease. The disorder has also been observed in children with developmental disabilities.
The fragmented sleep cycle of this disorder typically yields periods of sleep that last four hours or less. As a result, people with irregular sleep-wake rhythm disorder frequently nap throughout the day. Sleep fragmentation may be more severe for Alzheimer’s patients who experience sundowning, which involves restlessness, agitation, or confusion that coincide with sunset.
Non-24-Hour Sleep-Wake Rhythm Disorder
Also known as free-running disorder, non-24-hour sleep-wake rhythm disorder occurs when the internal clock does not reset every 24 hours. As a result, a person’s normal sleep period is constantly shifting, working its way around the clock over a period of days or weeks. Severity of symptoms often depends on the person’s schedule and whether their obligations conflict with their sleep cycle.
Severity of symptoms often depends on the person’s schedule and whether their obligations conflict with their sleep cycle.
People with this condition may have insomnia symptoms and excessive daytime sleepiness when their sleep periods don’t match up with the schedule of their social and professional life. When their schedule aligns with sleep periods, a person with this condition experiences few, if any, sleep disturbances.
This disorder primarily affects people who are totally blind. The eyes of a totally blind person cannot transmit as many light signals to the brain, leading to confusion about the time of day. As a result, their internal clock is often unable to entrain on a 24-hour cycle. Between 50% and 80% of blind people report sleep disturbances, and experts estimate half of totally blind people have non-24-hour sleep-wake rhythm disorder. A diagnosis requires symptoms that persist for at least three months.
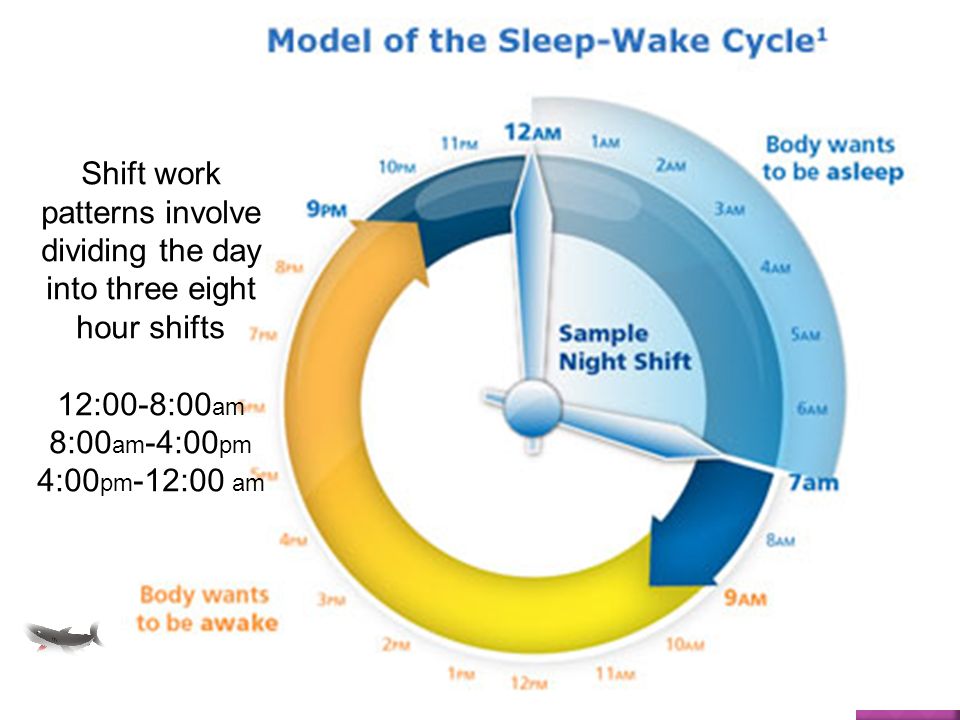
Shift Work Disorder
People whose jobs require them to work partly or completely at night often experience shiftwork disorder, which is characterized by insomnia and excessive daytime sleepiness.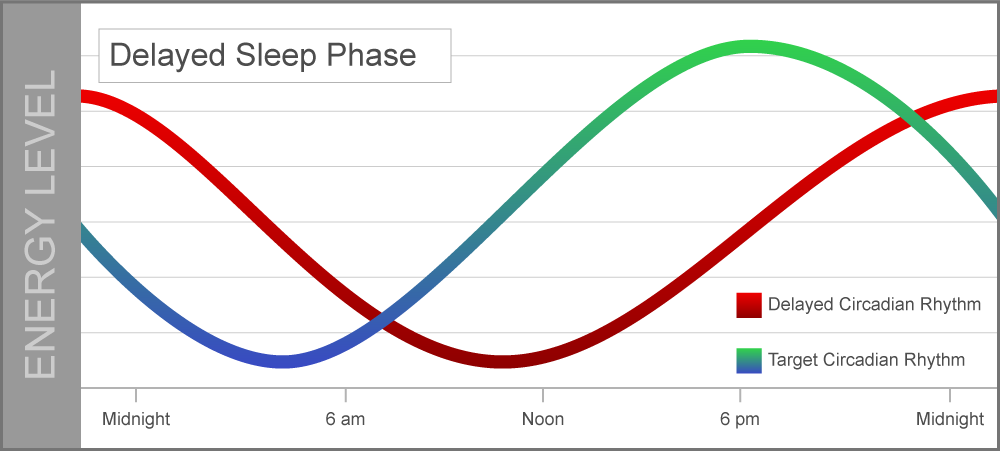 The term “shift-work” can apply to any shift that falls outside the traditional 9 a.m. to 5 p.m. schedule, but shift work disorder usually affects those who work late at night and/or early in the morning. Rotating shifts composed of daytime and nighttime hours can also lead to sleep disturbances and daytime grogginess.
The term “shift-work” can apply to any shift that falls outside the traditional 9 a.m. to 5 p.m. schedule, but shift work disorder usually affects those who work late at night and/or early in the morning. Rotating shifts composed of daytime and nighttime hours can also lead to sleep disturbances and daytime grogginess.
Most people with shift work disorder lose between one and four hours of sleep for each 24-hour period, and adjusting to work once their shift begins can become increasingly difficult over time.
This disorder can be particularly dangerous because it increases the risk of accidents either at their workplace or on the road during a late-night or early-morning commute.
People with this condition may also develop ulcers, and self-medicate with alcohol or drugs in order to get enough sleep. Estimates vary, but it’s believed as many as 38% of shift workers have this disorder. It is equally prevalent among the sexes and different racial groups.
Jet Lag
Most people experience jet lag after flights that pass over multiple time zones. The condition, marked by temporary sleep disturbances and daytime fatigue, represents a transition period during which a person’s internal clock needs to synchronize with local time. Jet lag symptoms typically begin one to two days after the flight and can persist for up to a week or two.
The condition, marked by temporary sleep disturbances and daytime fatigue, represents a transition period during which a person’s internal clock needs to synchronize with local time. Jet lag symptoms typically begin one to two days after the flight and can persist for up to a week or two.
Eastbound travel tends to produce more severe jet lag than westbound travel; northbound and southbound travel usually results in little to no jet lag unless the plane crosses two or more time zones.
Additionally, the severity of symptoms often correlates to the number of time zones crossed; for many people, the body will require one day of adjustment for each time zone.
Jet lag is usually not a serious condition, but it can propel people into a downward spiral if they do not practice healthy sleep hygiene during this post-flight period. Persistent symptoms can lead to insomnia and other more serious sleep disorders.
Other Circadian Rhythm Sleep Disorders
Disorders in this category are usually tied to underlying health conditions.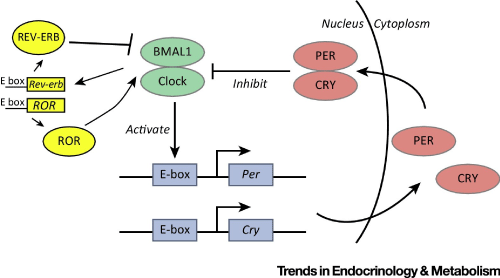
They resemble the other circadian rhythm sleep disorders listed above in terms of general symptoms, including insomnia and excessive daytime sleepiness, but patients do not meet the diagnostic criteria.
These are rare cases that typically require tailored care from a doctor or sleep specialist.
Circadian Rhythm Sleep Disorder Treatment
Treatment of circadian rhythm sleep disorders depends on the patient’s specific diagnosis. Most treatments emphasize the importance of good sleep hygiene, a healthy sleep environment, and a consistent sleep-wake schedule. These factors can improve entrainment and reduce sleep deprivation for people with these disorders.
Circadian rhythm sleep disorder treatment may include melatonin supplements. These supplements should be prescribed by a doctor and administered at specific times to induce feelings of sleepiness. Properly timed melatonin doses can effectively reorient your circadian rhythm and entrainment schedule. Always consult with a doctor before taking melatonin to ensure you are healthy enough to do so.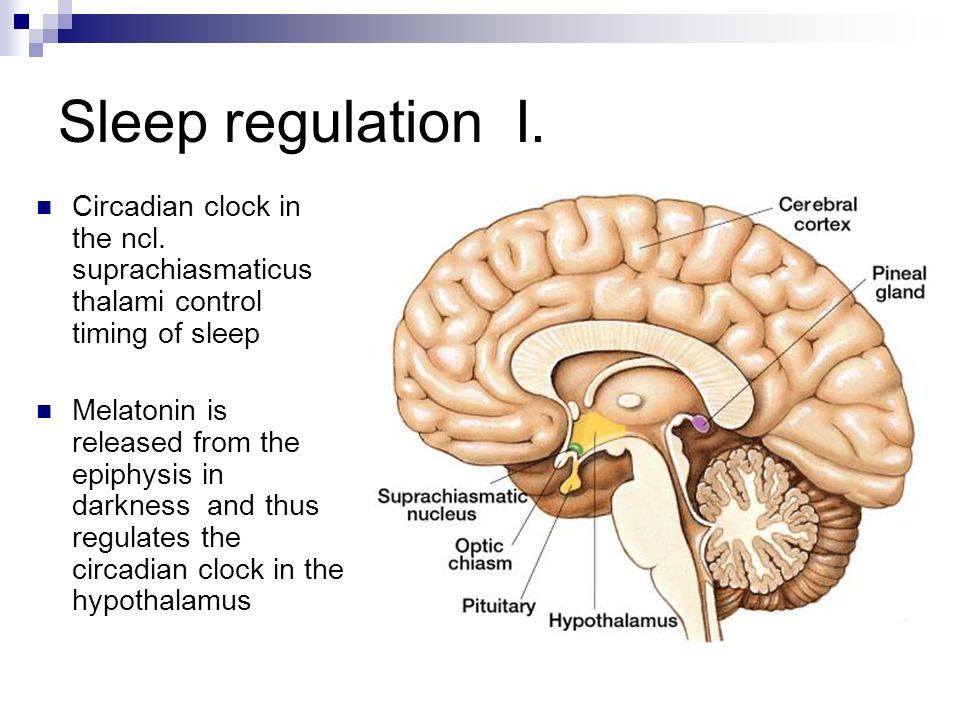
Timed bright light exposure in the morning can aid people with delayed sleep-wake phase disorder, whereas the same exposure in the evening can be used to treat those with advanced sleep-wake phase disorder. This type of light therapy can encourage a healthy shift in circadian rhythm.
For people with shift work disorder, timed light exposure during their shift can be helpful. These patients can also benefit from a regimen of napping before work and moderate caffeine intake during their shift. Coping strategies for staying awake during their shift and sleeping during the day can also be effective. These strategies include avoiding bright light during the day, bright light exposure at work, and maintaining an optimal sleep environment. Melatonin supplements or hypnotics can serve as sleep aids during the day, but these are a temporary fix and will not correct circadian misalignment.
- Was this article helpful?
- YesNo
About Our Editorial Team
Danielle Pacheco
Staff Writer
Danielle writes in-depth articles about sleep solutions and holds a psychology degree from the University of British Columbia.
Dr. Anis Rehman
Endocrinologist
MD
Dr. Rehman, M.D., is a board-certified physician in Internal Medicine as well as Endocrinology, Diabetes, and Metabolism.
References
+5 Sources
-
1.
National Heart, Blood, and Lung Institute. (n.d.). Sleep Deprivation and Deficiency. Retrieved September 3, 2020. https://www.nhlbi.nih.gov/health-topics/sleep-deprivation-and-deficiency
-
2.
The National Institute for Occupational Safety and Health (NIOSH). (2020, April 1). Interim NIOSH Training for Emergency Responders: Reducing Risks Associated with Long Work Hours. Centers for Disease Control. Retrieved September 3, 2020. https://www.cdc.gov/niosh/emres/longhourstraining/clock.html
-
3.
National Institutes of Health. (2020, July 13). Circadian Rhythms. Retrieved September 3, 2020. https://www.nigms.nih.gov/education/fact-sheets/Pages/circadian-rhythms.
 aspx
aspx -
4.
National Institute on Aging (NIA). (2017, May 17). Tips for Coping with Sundowning. Retrieved September 3, 2020. https://www.nia.nih.gov/health/tips-coping-sundowning
-
5.
Dodson, E., & Zee, P. (2010). Therapeutics for Circadian Rhythm Sleep Disorders. Sleep Medicine Clinics, 5(4), 701–715. Retrieved September 3, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3020104/
See More
Non-24-Hour Sleep-Wake Disorder - NORD (National Organization for Rare Disorders)
Non-24-Hour Sleep-Wake Disorder
NORD gratefully acknowledges James S.P. Fadden, MA, Vice-President, Circadian Sleep Disorders Network, and Katherine Sharkey, MD, PhD, Associate Professor of Medicine, Rhode Island Hospital/Alpert Medical School of Brown University, Departments of Medicine and Psychiatry and Human Behavior, for assistance in the preparation of this report.
Synonyms of Non-24-Hour Sleep-Wake Disorder
- circadian rhythm sleep disorder, free-running type
- free-running disorder
- hypernychthemeral syndrome
- N24
- non-24
- non-24-hour disorder
- non-24-hour sleep-wake cycle disorder
- non-24-hour sleep-wake syndrome
Signs & Symptoms
Causes
All life on earth has evolved in conditions of a 24-hour day-night (light-dark) cycle. Organisms have evolved mechanisms to time their cellular and metabolic processes to anticipate this daily rhythm. As a result, within nearly all cells of the human body there is a biological clock based on a cycle of DNA and protein synthesis. Clock gene activity has been found within white blood cells and cells of the heart, brain, liver and many other tissues.
Organisms have evolved mechanisms to time their cellular and metabolic processes to anticipate this daily rhythm. As a result, within nearly all cells of the human body there is a biological clock based on a cycle of DNA and protein synthesis. Clock gene activity has been found within white blood cells and cells of the heart, brain, liver and many other tissues.
The individual cellular clocks run on a cycle that is close to 24 hours. This is known as a circadian rhythm (“circa-” = about and “dian” = pertaining to a day). But because the clocks are not exact, the clocks of individual cells can drift apart from each other or from the earth’s day-night cycle. To keep these clocks in time there is a master clock located in the brain. In the same way that the conductor of an orchestra keeps the musicians playing in time with each other, this master clock keeps the body’s cellular clocks to the same time cycle.
The master clock is located in what is called the suprachiasmatic nucleus (SCN), located in a part of the brain called the hypothalamus which regulates many basic body functions.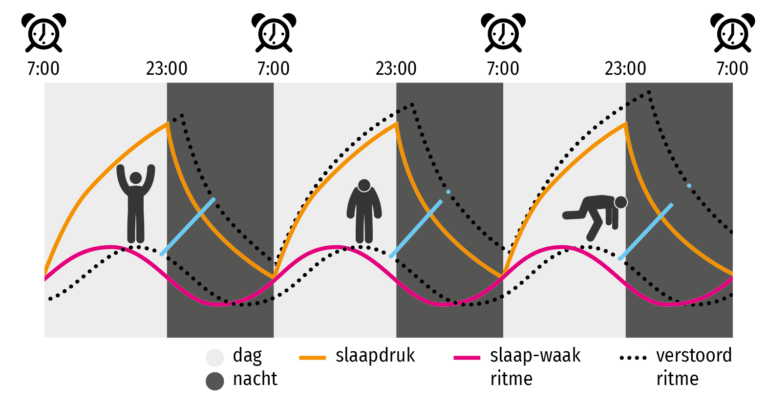 The SCN is composed of about 20,000 closely networked cells whose rhythms are coordinated so that the firing rate of the cells varies together in a near-24-hour rhythm. The firing of SCN cells is then transmitted directly and indirectly to many other regions of the brain which then pass on this clock signal to the rest of the body by neurochemical and hormonal means.
The SCN is composed of about 20,000 closely networked cells whose rhythms are coordinated so that the firing rate of the cells varies together in a near-24-hour rhythm. The firing of SCN cells is then transmitted directly and indirectly to many other regions of the brain which then pass on this clock signal to the rest of the body by neurochemical and hormonal means.
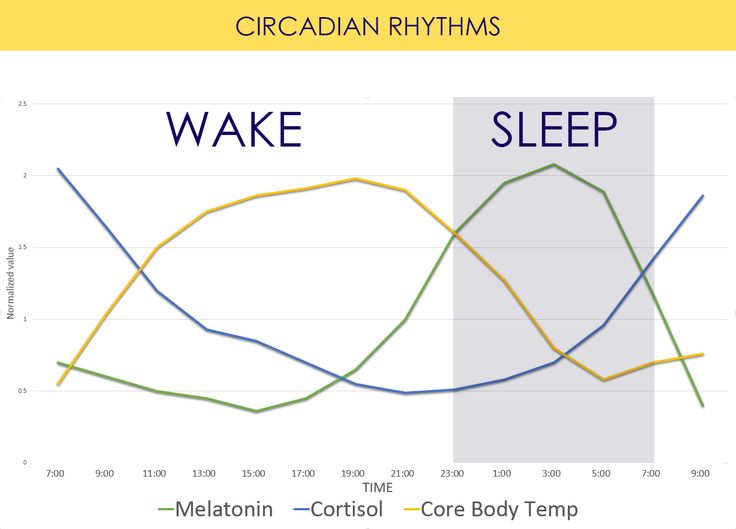
Two of the best characterized rhythms driven by the clock signal are the body temperature cycle and the production of the hormone melatonin. The SCN regulates body temperature via connections to other areas of the hypothalamus. Body temperature varies in a wave-like pattern, which reaches a maximum during the day and a minimum (or nadir) during the night.
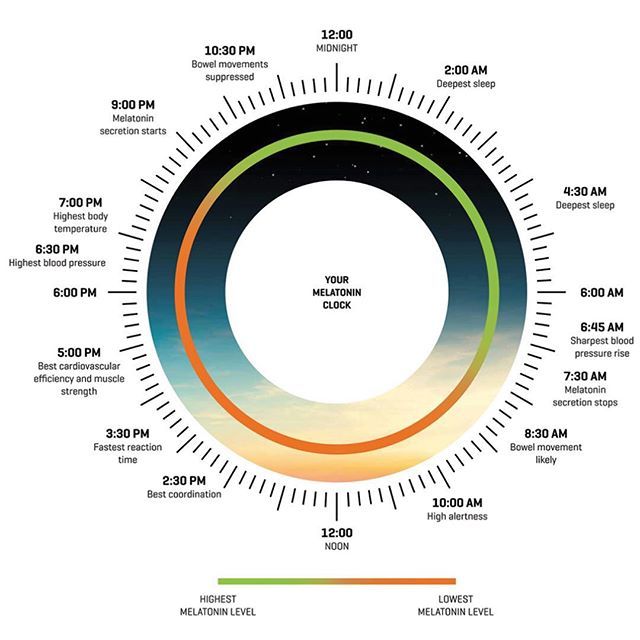
The SCN also sends a nerve signal that follows a complex poly-synaptic pathway via the cervical spinal ganglia to regulate the activity of the pineal gland, which is responsible for the production of melatonin. Melatonin, sometimes called “the hormone of darkness,” is produced during the dark at night. It is secreted by the pineal into the cerebrospinal fluid and then travels in the bloodstream to reach the cells of the body. It acts upon specific melatonin receptors to directly regulate cell functions. It also reinforces the temperature cycle by facilitating the nocturnal drop in body temperature. Among other effects, this drop in body temperature helps ready the brain and body for sleep.
It is secreted by the pineal into the cerebrospinal fluid and then travels in the bloodstream to reach the cells of the body. It acts upon specific melatonin receptors to directly regulate cell functions. It also reinforces the temperature cycle by facilitating the nocturnal drop in body temperature. Among other effects, this drop in body temperature helps ready the brain and body for sleep.
While the SCN serves to coordinate the cell clocks throughout the body, there is still a need to coordinate the SCN clock to the earth’s 24-hour period. If left to itself, the SCN keeps a rhythm that is close to but not exactly 24 hours. In healthy humans the intrinsic period of the SCN clock averages about 24.2 hours. If there were no way to correct this cycle to equal 24 hours the clock in the SCN would drift by several minutes each day until it no longer kept correct time or stayed “entrained.”
The primary means to keep the SCN clock set properly is via light-dark exposure. Specialized cells in the retina of the eye, which are different from the cells used for vision, register the exposure to light and dark and transmit this signal by a nerve path known as the retinohypothalamic tract to the SCN. When the eyes are exposed to light in the early morning hours this sends a signal that advances the clock in the SCN to an earlier time, thereby providing the necessary daily entrainment. When light falls on the eyes late at night, a delay signal is sent to the SCN. A graph of the effect of light at different times of day and night is known as a phase-response curve and can be used to predict the effects of light on the biological clock. If the SCN clock runs longer than 24 hours, it tends to become delayed relative to the day-night cycle, but morning light exposure will reset it. If the SCN clock runs shorter than 24 hours, late night light exposure will delay it a bit. By this means, the SCN clock is kept in time with the light and dark cycle of day and night. In healthy individuals routine exposure to morning light works to keep circadian rhythms entrained.
When the eyes are exposed to light in the early morning hours this sends a signal that advances the clock in the SCN to an earlier time, thereby providing the necessary daily entrainment. When light falls on the eyes late at night, a delay signal is sent to the SCN. A graph of the effect of light at different times of day and night is known as a phase-response curve and can be used to predict the effects of light on the biological clock. If the SCN clock runs longer than 24 hours, it tends to become delayed relative to the day-night cycle, but morning light exposure will reset it. If the SCN clock runs shorter than 24 hours, late night light exposure will delay it a bit. By this means, the SCN clock is kept in time with the light and dark cycle of day and night. In healthy individuals routine exposure to morning light works to keep circadian rhythms entrained.
The retinal cells that register light for circadian functions use a pigment known as melanopsin as a light sensor. Because melanopsin is particularly sensitive to blue light, light of that color has a greater effect in circadian rhythms. Red, orange and yellow light have much less effect. Green light can also affect rhythms under certain circumstances.
Red, orange and yellow light have much less effect. Green light can also affect rhythms under certain circumstances.
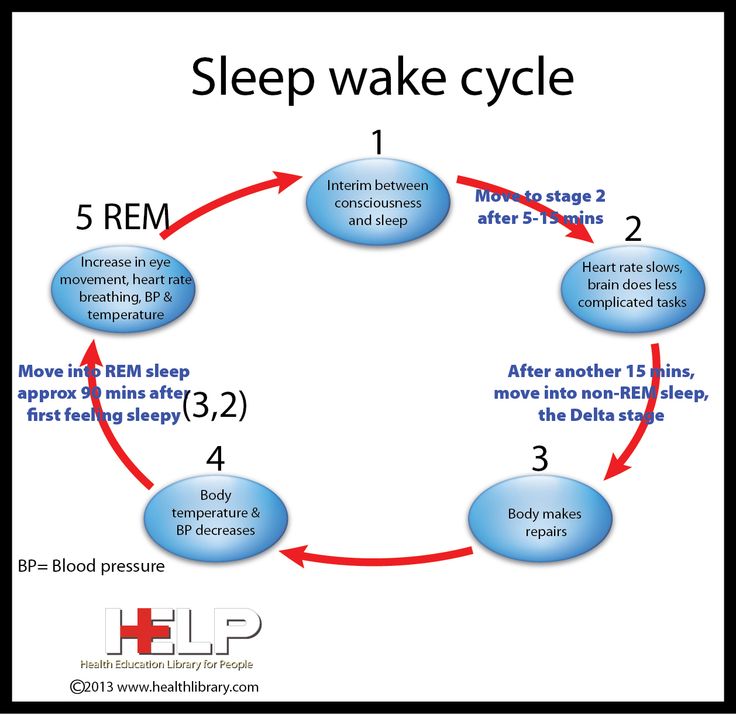
Among the most important of the body rhythms controlled by the SCN is that of the sleep-wake cycle. This cycle is controlled by two processes known as the homeostatic process and the circadian process. During sleep the brain and body repair themselves and accumulate energy and metabolic resources for the activities of the day. During the day, while the person is awake, these resources are gradually consumed. The gradual loss of energy during the day produces a drive to sleep in order to restore that energy. This is known as the homeostatic sleep drive. If the homeostatic process were the only one involved, a person would wake up fully energized and then gradually wind down over the course of the day, like a battery losing power. This would mean an uneven level of alertness during the day, with dangerously low alertness in the afternoon and evening. To counterbalance this, the SCN also regulates alertness by what is known as the circadian process. As the day goes on, and energy winds down, the SCN compensates for this by sending a stronger alertness signal to the brain and body. This alertness signal reaches a peak in the two hours just before bedtime. This zone of maximum alertness is known as the “forbidden zone for sleep” since the alertness signal makes sleep nearly impossible during that zone. When the usual bedtime is reached, the SCN begins to turn down its alertness signal to allow the body to sleep. In order to prevent early awakening, before the night’s sleep is done, the circadian alertness signal declines further across the night.
As the day goes on, and energy winds down, the SCN compensates for this by sending a stronger alertness signal to the brain and body. This alertness signal reaches a peak in the two hours just before bedtime. This zone of maximum alertness is known as the “forbidden zone for sleep” since the alertness signal makes sleep nearly impossible during that zone. When the usual bedtime is reached, the SCN begins to turn down its alertness signal to allow the body to sleep. In order to prevent early awakening, before the night’s sleep is done, the circadian alertness signal declines further across the night.
This complex interplay between the circadian process and the homeostatic process allows the human organism to have a relatively level state of alertness during the day (with the occasional exception of a mid-afternoon nap period) and allows a 7-9 hour period of consolidated sleep at night.
When all is working well, light signals registered in the eyes keep the SCN on track with the 24-hour day-night cycle and the SCN in turn coordinates the clocks in the pineal gland and in cells throughout the body. All the clocks keep a 24-hour cycle in sync with each other like the members of a well-conducted orchestra. The circadian alertness signal then combines with the homeostatic process resulting in an individual who can sleep through the night and maintain alertness during the day.
All the clocks keep a 24-hour cycle in sync with each other like the members of a well-conducted orchestra. The circadian alertness signal then combines with the homeostatic process resulting in an individual who can sleep through the night and maintain alertness during the day.
But there are a number of things that can go wrong with this system and result in a circadian disorder such as N24.
1. Blindness. The most well-understood cause of N24 is what occurs in blind individuals. Persons who are completely blind (no perception of light) will not register the light signals which are needed to fine-tune the body clock to a 24-hour day. If the SCN clock starts to drift away from 24 hours, a blind person has no intrinsic way to bring it back in sync without medical treatment. Since the inherent rhythm of the SCN is not always precisely 24 hours, a blind person’s circadian timing system will slowly drift over time. They will cycle over time between periods of nighttime sleep and periods of daytime sleep. In the vast majority of cases the sleep rhythm gradually delays so the period is over 24 hours, but there are a few cases of gradual advances and a less-than-24-hour period. The length of the circadian period in blind persons with N24 is typically in the range of 23.8 to 25 hours.
In the vast majority of cases the sleep rhythm gradually delays so the period is over 24 hours, but there are a few cases of gradual advances and a less-than-24-hour period. The length of the circadian period in blind persons with N24 is typically in the range of 23.8 to 25 hours.
2. Alterations in Light Sensitivity. In some sighted individuals there may be a subsensitivity or insensitivity to the effects of light on the circadian system. The vision-producing areas of the eye and brain may function well, but the separate cell pathway that transmits the circadian light signal may not. If they are totally insensitive to the circadian effects of light, their condition, from a circadian point of view, is not different from that of a blind person. If they are subsensitive to light, light may produce some effect on their rhythms but it may not be strong enough to correct circadian drift in their particular lighting environment.
Conversely, some patients with delayed sleep phase disorder, a condition related to N24, have been shown to be supersensitive to the effects of light. If they are exposed to normal room light in the evening it may produce an exaggerated delay in their circadian rhythms. If this delay becomes cumulative, the result is N24.
If they are exposed to normal room light in the evening it may produce an exaggerated delay in their circadian rhythms. If this delay becomes cumulative, the result is N24.
3. Environment. Environmental exposure to light may also play a role. Healthy individuals, when kept in isolation without time cues and allowed to turn their lights on and off when they choose, will often fall into a non-24-hour rhythm. The length of the rhythm is not only longer than the intrinsic 24.2-hour cycle of the SCN, but may be up to 25 hours or more in length. This is because self-selected light exposure late in the day has a delaying effect. However, this cannot be the sole cause of N24 since light does not lead to N24 in all persons in a non-isolated environment. In contrast, persons with N24 cannot maintain a 24-hour schedule even in a non-isolated environment with normal time cues.
4. Hormonal Factors. In some cases the hormone melatonin may be involved in the development or perpetuation of N24. Some patients with N24 produce less melatonin than normal, which can be problematic since melatonin helps link sleep to the day-night cycle. On the other hand, too much melatonin may also cause problems. The antidepressant fluvoxamine, which greatly increases melatonin levels by inhibiting its metabolism, has been reported to cause DSPD, which is closely related to N24. Some individuals have an abnormality in their ability to metabolize melatonin, which can lead to higher-than-normal daytime levels that may result in circadian clock dysfunction.
Some patients with N24 produce less melatonin than normal, which can be problematic since melatonin helps link sleep to the day-night cycle. On the other hand, too much melatonin may also cause problems. The antidepressant fluvoxamine, which greatly increases melatonin levels by inhibiting its metabolism, has been reported to cause DSPD, which is closely related to N24. Some individuals have an abnormality in their ability to metabolize melatonin, which can lead to higher-than-normal daytime levels that may result in circadian clock dysfunction.
5. Differences in Cellular Clock Function. Other studies of the causes of circadian rhythm disorders have focused on the cellular clock itself. Studies in healthy subjects show a correlation between the period of the cellular clock and the phase of entrainment. Morning persons have a shorter clock period than evening persons. N24 may be an extension of extreme “eveningness” in which the cellular rhythm may be too far from 24 hours for normal light exposure to correct it, a situation known as being “outside the range of entrainment”.
The period of the human biological clock can be measured in two ways. First one may examine the period under the usual living conditions of the subject. Under those conditions the period of a normal person is 24 hours. The timing of their sleep wake cycle does not change over time. A person with N24 by definition will have a period that is longer than 24 hours, sometimes as long as 25-26 hours.
Under normal circumstances the circadian clock is affected by outside factors, especially light. But under special experimental conditions (constant routines and forced desynchrony) scientists can cancel out these outside effects and find what is called the intrinsic period of the clock. This is the time the clock would keep if it were isolated from outside influences. For normal subjects the intrinsic period of the clock is around 24.2 hours. Daily exposure to normal light compensates for the 0.2 difference and allows normal subjects to stay on a 24-hour day.
Three small studies have looked at the intrinsic period of N24 patients. One study of 6 patients found a 24.5 hour period, a study of 4 patients reported 24.9 hours, and a case report of a single patient also found a 24.5 hour period. Thus these N24 patients require an adjustment of at least 0.5 to 0.9 hours per day to remain in a 24-hour cycle. Normal light exposure may not be enough to make this adjustment. When combined with other factors that push the clock later this may make entrainment to a 24-hour day impossible.
One study of 6 patients found a 24.5 hour period, a study of 4 patients reported 24.9 hours, and a case report of a single patient also found a 24.5 hour period. Thus these N24 patients require an adjustment of at least 0.5 to 0.9 hours per day to remain in a 24-hour cycle. Normal light exposure may not be enough to make this adjustment. When combined with other factors that push the clock later this may make entrainment to a 24-hour day impossible.
Other studies have also looked at the clock within muscle cells (fibroblasts) extracted and grown in culture. The period of cells in culture is correlated with the intrinsic period of the person from whom the cells were sampled. This shows that the clock period is determined on a cellular level. For N24 patients this suggests that at least some may be manifesting a fundamental malfunction of the biochemical basis of the circadian clock, which results in a longer intrinsic period.
While the intrinsic period of N24 patients is longer than average, it overlaps with the period found in a few extreme evening type subjects who do not have clinical N24. Thus, while the long intrinsic period is clearly a major contributing factor to the development of N24, there may also be additional factors involved, which make the difference between an extreme evening chronotype and free-running N24.
Thus, while the long intrinsic period is clearly a major contributing factor to the development of N24, there may also be additional factors involved, which make the difference between an extreme evening chronotype and free-running N24.
6. Differences in the Regulation of Sleep. Another possible set of causes of N24 is related to the homeostatic and circadian regulation of sleep. On average patients with N24 have a slightly greater sleep requirement than normal. In some cases this can be extreme. While a healthy person may sleep 8 hours and be awake for 16 hours, if someone needs 12 hours of sleep and then is awake for a normal 16 hours, their day will last 28 hours total. The change in the sleep cycle will in turn change the timing of light exposure, perpetuating an N24 cycle. Similarly, if someone is deficient in the homeostatic drive for sleep they may sleep a normal 8 hours but require 20 hours of awake time before sufficient homeostatic pressure accumulates to permit sleep, again resulting in a 28 hour day.
The timing of sleep in relation to internal circadian rhythms, also known as the phase angle between sleep and circadian rhythms, is abnormal in many cases of N24. Here phase angle is described in terms of the relationship between sleep timing and the circadian rhythm of body temperature. In healthy individuals the body temperature starts to drop shortly before sleep onset and reaches a minimum late in the sleep period — usually about 2 hours before waking. Persons with N24 tend to fall asleep very late relative to their temperature cycle and so the time between the temperature minimum and time of waking (sleep offset) may be 4 hours or more, even up to 8 hours in extreme cases. Since the body’s response to light-dark exposure is synched with the internal rhythms (such as core temperature) rather than the sleep-cycle per se, N24s with an abnormal relationship between sleep and circadian rhythms will sleep through the phase advance portion of their clock and not get the light they need on a daily basis to reset their clock. At the same time since they are awake late relative to their temperature cycle, they are exposed to light during the phase delay portion of the phase response curve. This tends to push their circadian rhythm in the direction of a much longer than normal day. This amplifies the effect of the already prolonged intrinsic period of N24 patients.
At the same time since they are awake late relative to their temperature cycle, they are exposed to light during the phase delay portion of the phase response curve. This tends to push their circadian rhythm in the direction of a much longer than normal day. This amplifies the effect of the already prolonged intrinsic period of N24 patients.
The circadian regulation of sleepiness is also important. Even healthy individuals have a “forbidden zone for sleep” that occurs an hour or two before normal bedtime and is associated with the maximum circadian alertness signal. In persons with N24 this forbidden zone occurs too late in the day and is too strong to permit sleep on a 24-hour cycle.
This pattern may be reinforced by certain effects of sleep and wake on alertness. When individuals wake after a prolonged period of sleep, they are often in a state of reduced alertness known as sleep inertia. In people with N24 this state of sluggishness and grogginess may be very powerful and persist for many hours. The longer they are awake the more alert they become. (This may be explained by an observation that brain cell circuits become more excitable with longer time awake.) When it comes time for them to sleep (if they are trying to stay on a 24-hour cycle) their alertness will have reached a high point and their heightened state of energy, even if brief, will not permit them to fall asleep at a normal time. In addition, patients with N24 may not want to try to fall asleep at this time because they finally feel awake, alert and productive.
The longer they are awake the more alert they become. (This may be explained by an observation that brain cell circuits become more excitable with longer time awake.) When it comes time for them to sleep (if they are trying to stay on a 24-hour cycle) their alertness will have reached a high point and their heightened state of energy, even if brief, will not permit them to fall asleep at a normal time. In addition, patients with N24 may not want to try to fall asleep at this time because they finally feel awake, alert and productive.
7. Development. Development of the brain, and in particular the circadian and sleep centers, is another factor. In pervasive developmental disorders such as autism a relatively high frequency of occurrence of N24 and other circadian rhythm and sleep disorders has been noted. It is assumed that the circadian and sleep centers of the brain did not properly develop or are affected by other neurochemical or anatomical deficits. It may be that other N24s who do not have pervasive developmental disorders may have impaired development limited to the sleep and circadian brain centers.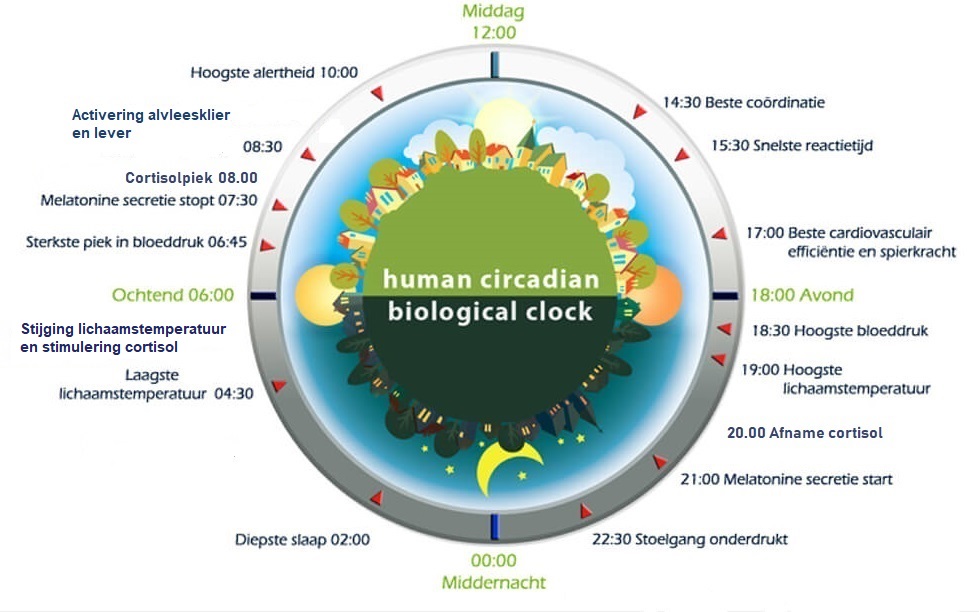
8. Trauma. Physical damage to the brain, such as occurs from head injury has been noted to lead to N24 in previously healthy individuals. It is assumed that the head injury damages the sleep and circadian centers of the brain such as the hypothalamus or pineal gland. Similarly, brain tumors have been noted to lead to the development of N24. Circadian sleep disorders have been noted in survivors of tumors affecting the pons and the hypothalamus. Craniopharyngiomas are particularly likely to lead to sleep disorders. In some cases the damage is due to the tumor itself and in other cases to the effects of radiation treatment to the head. In one case an aneurysm near the SCN resulted in transient N24. There has also been a report of N24 following chemotherapy for Hodgkin’s lymphoma.
Under the heading of physical abnormalities, any factor that leads to total blindness, whether via genes, disease or injury, can lead to secondary N24.
9. Iatrogenic. N24 can also arise from attempts at treatment of the more common disorder, delayed sleep phase disorder (DSPD). One of the widely used treatments for DSPD is chronotherapy, in which the patient is instructed to gradually delay their bedtime and wake time up to three hours a day until they go around the clock to a more socially acceptable sleep-wake schedule. In essence this means temporarily adopting an N24 schedule. Unfortunately, in some patients, once an N24 schedule has been established it becomes nearly impossible to break. They have exchanged one circadian rhythm disorder, DSPD, for an even more disabling one, N24. There are several reasons why the N24 pattern is hard to break out of once established. One involves the timing of sleep relative to the temperature rhythm mentioned above. The other involves what is called the plasticity of the circadian system. That means that once an organism has been placed on a particular cycle, including a non-24-hour cycle, the circadian clock remembers that cycle and tries to continue it. The risk of N24 after chronotherapy has been known since the 1990s but many doctors continue to be unaware of the risk when recommending chronotherapy.
One of the widely used treatments for DSPD is chronotherapy, in which the patient is instructed to gradually delay their bedtime and wake time up to three hours a day until they go around the clock to a more socially acceptable sleep-wake schedule. In essence this means temporarily adopting an N24 schedule. Unfortunately, in some patients, once an N24 schedule has been established it becomes nearly impossible to break. They have exchanged one circadian rhythm disorder, DSPD, for an even more disabling one, N24. There are several reasons why the N24 pattern is hard to break out of once established. One involves the timing of sleep relative to the temperature rhythm mentioned above. The other involves what is called the plasticity of the circadian system. That means that once an organism has been placed on a particular cycle, including a non-24-hour cycle, the circadian clock remembers that cycle and tries to continue it. The risk of N24 after chronotherapy has been known since the 1990s but many doctors continue to be unaware of the risk when recommending chronotherapy.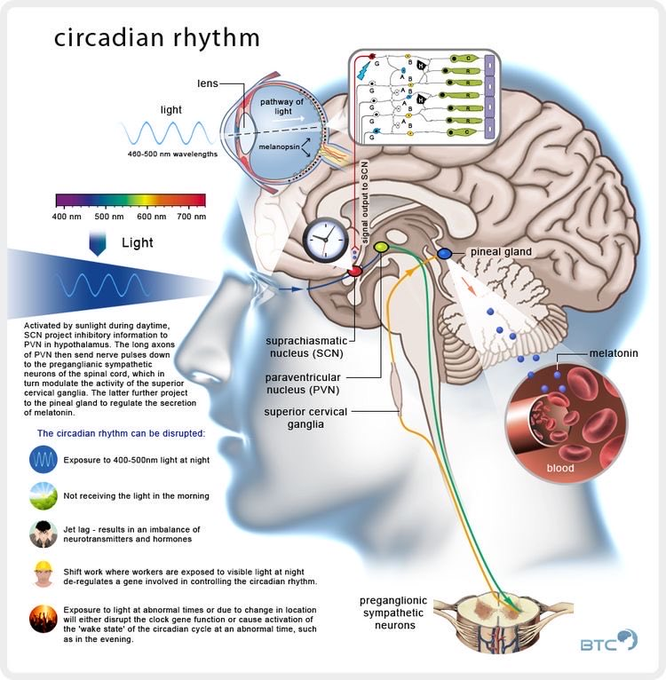
10. Genetics. There is increasing evidence of a genetic component to N24. In most cases it is not a simple inherited genetic condition (Medelian inheritance). Most patients with N24 do not have parents or close relatives with the condition. However, there do seem to be several genetic factors which can predispose someone to the development of N24.
One study found specific genetic changes (single nucleotide polymorphisms, SNPs) in the gene BHLHE40 in 4 patients with N24. As this gene encodes components of the cellular clock, such mutations may affect clock function leading to the abnormalities noted in N24.
A separate study of 67 N24 patients found an association with polymorphisms in the PER3 gene. PER3 also encodes a crucial component of the circadian clock. The same polymorphisms were associated with extreme evening chronotype – a genetic predisposition to functioning better late in the day, a tendency which is also noted in Non-24s. Variations in the PER3 gene (both SNPs and repeat numbers) are believed to affect free-running period (in animals), the homeostatic drive for sleep (in humans) and the response to light (in humans). All of these factors have been hypothesized, with some evidence, to be abnormal in N24.
All of these factors have been hypothesized, with some evidence, to be abnormal in N24.
DSPD, a condition related to N24, has been linked to the presence of a mutation in the CRY1 gene, which plays a role in the circadian clock, in a study of one family.
Several genome-wide association studies – genetic screenings of over 100,000 persons — have shown genetic associations with human chronotypes. While these studies did not involve N24 patients specifically, N24 is closely related to extreme evening chronotype, suggesting some of the same genetic factors may be relevant.
Taken together, both the specific studies of genes in Non-24 and the more general genetic studies of circadian rhythms strongly suggest that some individuals may have a genetic predisposition to the development of N24.
Affected Populations
While the total number of people living with N24 is unknown, researchers assume that more blind people are affected than sighted people. It is estimated that 55-70% of all people who are totally blind have N24. People who lack any light perception (for example those whose eyes are enucleated) are more likely to be affected than those with some retinal function. The frequency of N24 among the sighted is unknown but the world-wide medical literature provides case studies of roughly 100 sighted individuals with N24. Fifty-seven of these cases appear in a single Japanese study. The Circadian Sleep Disorders Network (see under “organizations”) has 98 members who have indicated they or a family member have N24. The Facebook N24 group has over 500 members but it is not known how many are actual patients. As the condition is not widely known, there may be a significant number of undiagnosed cases.
People who lack any light perception (for example those whose eyes are enucleated) are more likely to be affected than those with some retinal function. The frequency of N24 among the sighted is unknown but the world-wide medical literature provides case studies of roughly 100 sighted individuals with N24. Fifty-seven of these cases appear in a single Japanese study. The Circadian Sleep Disorders Network (see under “organizations”) has 98 members who have indicated they or a family member have N24. The Facebook N24 group has over 500 members but it is not known how many are actual patients. As the condition is not widely known, there may be a significant number of undiagnosed cases.
In published cases of sighted patients, around 75% are male, although it is not known if this is representative of the ratio in the overall patient population. Studies in healthy adults show that on average men have longer circadian periods than women. Among support groups the numbers of male and female patients are roughly equal. The most frequent age of onset is late teens or early twenties, although N24 can manifest at a much younger or older age. The disorder appears to be life-long. Insufficient data exists to determine whether N24 is progressive. Anecdotal evidence offered by long-term sufferers indicates a worsening of symptoms with age, along with an increase in the day length, however this may be due to the interaction between N24 and age-induced sleep disruptions. Clinical research on changes in the manifestation of N24 throughout the life cycle is absent at present.
The most frequent age of onset is late teens or early twenties, although N24 can manifest at a much younger or older age. The disorder appears to be life-long. Insufficient data exists to determine whether N24 is progressive. Anecdotal evidence offered by long-term sufferers indicates a worsening of symptoms with age, along with an increase in the day length, however this may be due to the interaction between N24 and age-induced sleep disruptions. Clinical research on changes in the manifestation of N24 throughout the life cycle is absent at present.
N24 was first described in the medical literature by Eliott, Mills, and Waterhouse in 1970.
Diagnosis
Initial diagnosis is based on home sleep logs kept by the patient that show a non-24-hour sleep pattern. This is usually more easily distinguished if the patient’s sleep times are not constrained by social or occupational obligations.
Confirmation of diagnosis may be obtained by the use of an actigraph, a device worn on the wrist that registers movement which is used to track the timing of sleep. The actigraph should be worn for sufficient time for the sleep cycle to complete at least one pass around the clock, typically several weeks.
The actigraph should be worn for sufficient time for the sleep cycle to complete at least one pass around the clock, typically several weeks.
Documenting a non-24-hour pattern of melatonin secretion may be a useful confirmation of the diagnosis, though this procedure is currently more commonly used for research purposes.
Clinical Testing and Work-Up
Sleep logs and actigraphy are the main means for initial work up and follow up. Polysomnography (an overnight sleep study) is not necessary for diagnosis of N24 but may be used to rule out related disorders. For polysomnography to be useful, it must be done at a time when the patient’s cycle allows him or her to sleep.
Standard Therapies
Treatment
In 2014, The U.S. Food and Drug Administration (FDA) approved Hetlioz (tasimelteon), a melatonin receptor agonist, to treat N24. Hetlioz, manufactured by Vanda Pharmaceuticals, Inc., is the first FDA approved treatment for the disorder. The effectiveness of Hetlioz was evaluated in two clinical trials of totally blind individuals with N24.
The effectiveness of Hetlioz was evaluated in two clinical trials of totally blind individuals with N24.
The most widely recommended treatments for sighted patients involve exposure to specific regimens of light (phototherapy) and dark (scototherapy).
Phototherapy usually involves the use of a lightbox. The lightbox is used in the early morning, typically for a duration of 2 hours, in order to stabilize the sleep cycle. Light treatment is best started when the patient’s cycle already has them arising at the desired wake time. Light is registered by special cells in the retina of the eye which send a signal to the brain via the retinohypothalamic tract. This signal suppresses the output of melatonin and shifts the timing of sleep. A phase-response curve determines the best time for light exposure.
Dark therapy (scototherapy) is accomplished by avoiding light exposure late in the day. Even ordinary room light may have phase-delaying effect so patients should remain in dim light or use special dark goggles that reduce light exposure during the evening and night.
A combination of light and dark therapy is believed to be more effective than either alone. If entrainment to a 24-hour cycle is achieved with light and dark therapy, the patient must maintain the treatment regimen or entrainment will be lost.
The hormone melatonin may be used to stabilize the sleep-wake cycle. Melatonin is usually taken about 4-6 hours before the desired sleep time. While melatonin is often effective in blind patients with N24, it is rarely successful as the sole treatment in sighted patients.
Investigational Therapies
Early case reports suggested that vitamin B12 could successfully treat some cases of N24; however, a double-blind placebo-controlled trial found it was not significantly better than placebo for treatment of N24 or DSPD.
Blue light plays a particular role in affecting circadian rhythms. Blue-enriched light has been used in treatment of the related condition, DSPD, and may be useful for N24, although there are no published cases or trials.
Conversely, avoidance of blue light using goggles which block out all blue (and sometimes green) light has become a widely used treatment among patients with N24 with anecdotal success, but as of yet there are no published studies of this approach. In addition to, or in place of goggles, patients may use special red or amber lights (which do not put out blue or green light) in the evening for illumination. They do not use standard room light and avoid sunlight by using shades or shutters in the evening.
There is considerable ongoing research on the basic biology and molecular genetics of circadian rhythms. Drugs which alter the timing of the biological clock are a promising avenue for future study but as of yet none are near being ready for clinical use. Research on the circadian and homeostatic control of sleep timing in healthy subjects and patients with N24 and related disorders may also offer clues to future treatments.
Information on current clinical trials is posted on the Internet at www. clinicaltrials.gov. All studies receiving U.S. Government funding, and some supported by private industry, are posted on this government web site.
clinicaltrials.gov. All studies receiving U.S. Government funding, and some supported by private industry, are posted on this government web site.
For information about clinical trials being conducted at the NIH Clinical Center in Bethesda, MD, contact the NIH Patient Recruitment Office:
Toll-free: (800) 411-1222
TTY: (866) 411-1010
Email: [email protected]
Some current clinical trials also are posted on the following page on the NORD website:
https://rarediseases.org/for-patients-and-families/information-resources/news-patient-recruitment/
For information about clinical trials sponsored by private sources, contact:
www.centerwatch.com
For information about clinical trials conducted in Europe, contact:
https://www.clinicaltrialsregister.eu/
References
TEXTBOOKS
Kryger MH, Roth T, and Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017: 420-421.
American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2005:126-136.
International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2005:126-136.
Meier-Ewert K, Okawa M. Sleep-Wake Disorders. New York, NY: Plenum Press; 1997:53-66.
JOURNAL ARTICLES
Patke A, Murphy P, Onur E, et al. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017 Apr 6, Volume 169 , Issue 2 , 203 – 215.e13.
Micic G, Lovato N, Gradisar M, et al. Circadian Melatonin and Temperature Taus in Delayed Sleep-wake Phase Disorder and Non-24-hour Sleep-wake Rhythm Disorder Patients: An Ultradian Constant Routine Study. J Biol Rhythms. 2016 Aug;31(4):387-405.
Jones SE, Tyrrell J, Wood AR, et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS Genet. 2016 Aug 5;12(8).
Lane JM, Vlasac I, Anderson SG, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun. 2016 Mar 9;7:10889.
Nat Commun. 2016 Mar 9;7:10889.
Garbazza C, Bromundt V, Eckert A, et al. Non-24-Hour Sleep-Wake Disorder Revisited – A Case Study. Front Neurol. 2016 Feb 29;7:17.
Uchiyama M, Lockley SW. Non-24-Hour Sleep-Wake Rhythm Disorder in Sighted and Blind Patients. Sleep Med Clin. 2015 Dec;10(4):495-516.
Lockley SW, Dressman MA, Licamele L, et al. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. 2015 Oct 31;386(10005):1754-64.
Auger RR, Burgess HJ, Emens JS, et al. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med.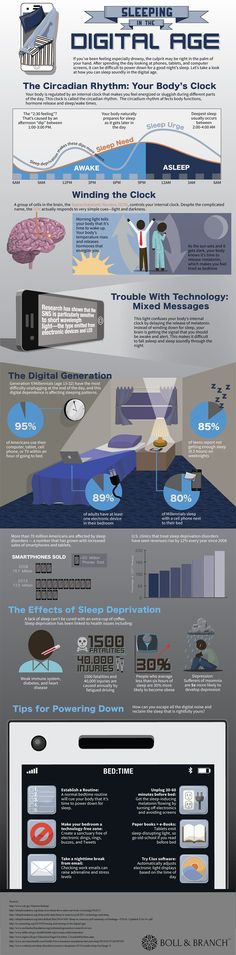 2015 Oct 15;11(10):1199-236.
2015 Oct 15;11(10):1199-236.
Fadden, J. What You Need to Know About Non-24. Sleep Review 2015 Aug;16(7):22-25. Online version: http://www.sleepreviewmag.com/2015/05/need-know-non-24/
Lee D, Shin WC. Forced entrainment by using light therapy, modafinil and melatonin in a sighted patient with non-24-hour sleep-wake disorder. Sleep Med. 2015 Feb;16(2):305-7.
Kripke DF, Klimecki WT, Nievergelt CM, et al. Circadian polymorphisms in night owls, in bipolars, and in non-24-hour sleep cycles. Psychiatry Investig. 2014 Oct;11(4):345-62.
Hida A, Kitamura S, Katayose Y, et al. Screening of clock gene polymorphisms demonstrates association of a PER3 polymorphism with morningness-eveningness preference and circadian rhythm sleep disorder. Sci Rep. 2014 Sep 9;4:6309.
O’Neill B, Gardani M, Findlay G, et al. Challenging behaviour and sleep cycle disorder following brain injury: a preliminary response to agomelatine treatment. Brain Inj. 2014;28(3):378-81.
Huber R, Mäki H, Rosanova M, et al.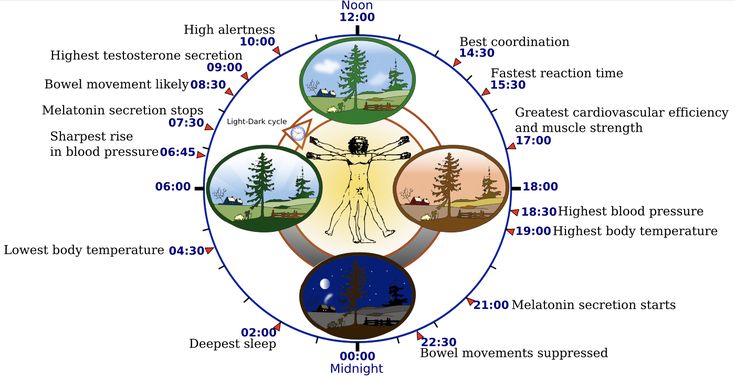 Human cortical excitability increases with time awake. Cereb Cortex. 2013 Feb;23(2):332-8.
Human cortical excitability increases with time awake. Cereb Cortex. 2013 Feb;23(2):332-8.
Kitamura S, Hida A, Enomoto M, et al. Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type. Biol Psychiatry. 2013 Jan 1;73(1):63-9.
Zhu L, Zee PC. Circadian rhythm sleep disorders. Neurol Clin. 2012 Nov;30(4):1167-91.
Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;13;108 Suppl 3:15602-8.
Uchimaya M, Lockley SW. Non-24-hour sleep-wake syndrome in sighted and blind patients. Sleep Med Clin 2009;4:195-211.
Pagani L, Semenova EA, Moriggi E, et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS One 2010;5(10):e13376.
Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Standards of Practice Committee of the American Academy of Sleep Medicine.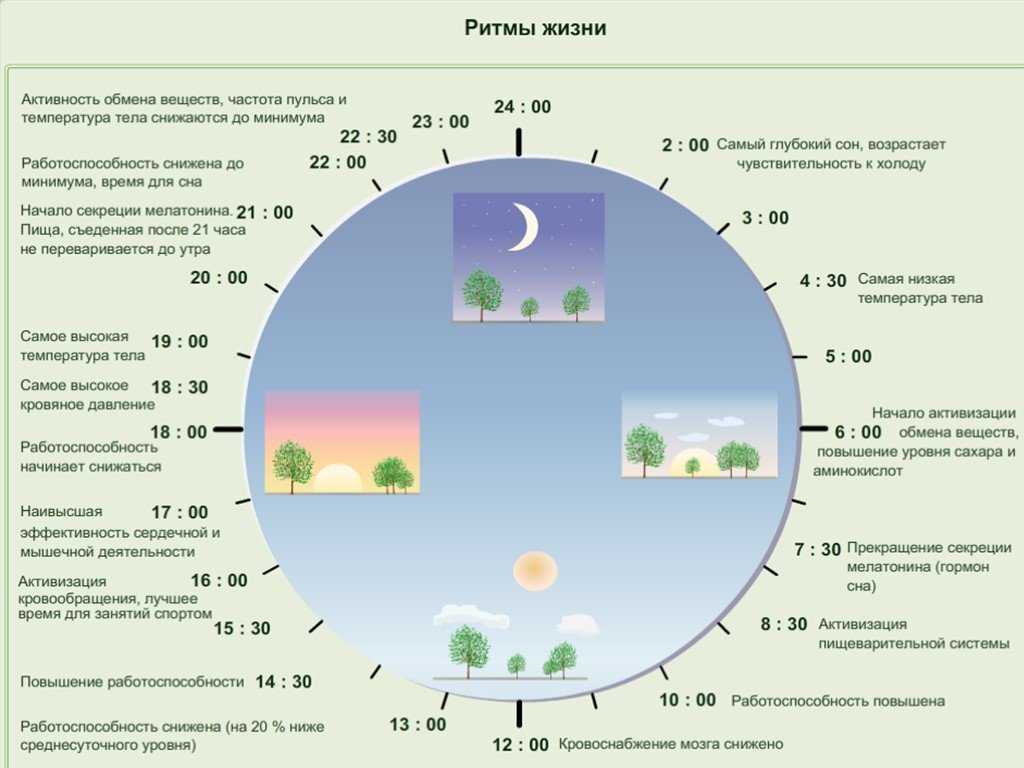 Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007;30(11):1445-59.
Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007;30(11):1445-59.
Okawa M, Uchiyama M. Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24 sleep-wake syndrome. Sleep Medicine Reviews 2007;11:485-496.
Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 2007;30(11):1484-501.
Dagan Y, Ayalon L. Case study: psychiatric misdiagnosis of non-24-hours sleep-wake schedule disorder resolved by melatonin. J Am Acad Child Adolesc Psychiatry 2005;44(12):1271-5.
Hayakawa T, Uchiyama M, Kamei Y, et al. Clinical analyses of sighted patients with non-24-h sleep wake syndrome: a study of 57 consecutively diagnosed cases. Sleep 2005;28(8):949.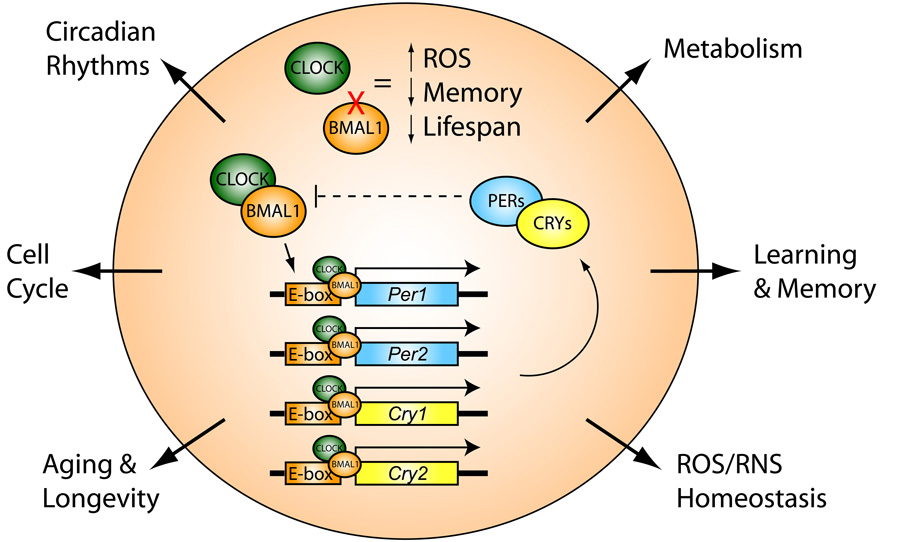
Boivin DB, Caliyurt O, James FO, et al. Association between delayed sleep phase and hypernyctohemeral syndromes: a case study. Sleep 2004;27(3):417-21.
Boivin DB, James FO, Santo JB, et al. Non-24-hour sleep-wake syndrome following a car accident. Neurology 2003;60:1841-3.
Dagan Y. Circadian Rhythm Sleep Disorders (CRSD) in psychiatry–a review. Isr J Psychiatry Relat Sci 2002;39(1):19-27.
Uchiyama M, Shibui K, Hayakawa T, et al. Larger phase angle between sleep propensity and melatonin rhythms in sighted humans with non-24-hour sleep-wake syndrome. Sleep 2002;25:83-8.
Dagan Y, Abadi J. Sleep-wake schedule disorder disability: a lifelong untreatable pathology of the circadian time structure. Chronobiol Int 2001 Nov;18(6):1019-27.
Hermish H, Lemberg H, Abadi J, et al. Circadian rhythm sleep disorders as a possible side effect of fluvoxamine. CNS Spectrum 2001;6:511–513.
Shibui K, Uchiyama M, Iwama H, et al. Periodic fatigue symptoms due to desynchronization in a patient with non-24-h sleep-wake syndrome.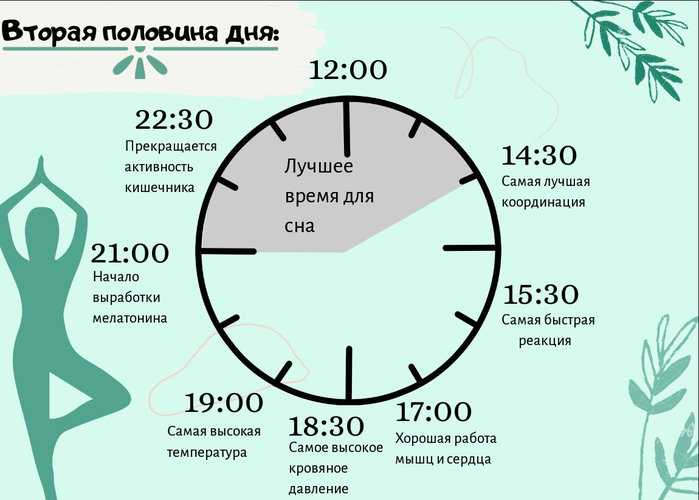 Psychiatry Clin Neurosci 1998;52:477-81.
Psychiatry Clin Neurosci 1998;52:477-81.
Oren DA, Giesen HA, Wehr TA. Restoration of detectable melatonin after entrainment to a 24-hour schedule in a ‘free-running’ man. Psychoneuroendocrinology 1997;22(1):39-52.
McArthur AJ, Lewy AJ, Sack RL. Non-24-hour sleep-wake syndrome in a sighted man: circadian rhythm studies and efficacy of melatonin treatment. Sleep 1996;19:544-53.
Uchiyama M, Okawa M, Ozaki S, et al. Delayed phase jumps of sleep onset in a patient with non-24-hour sleep-wake syndrome. Sleep 1996;19:637-40.
Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: clinical features of sleep-wake rhythm disorders. Psychiatry Clin Neurosci 1996;50:195-201.
Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: therapeutic effects of vitamin B12, bright light therapy, chronotherapy and hypnotics. Psychiatry Clin Neurosci. 1996;50(4):203-9.
Emens J, Brotman D, Czeisler C, Evaluation of the Intrinsic Period of the Circadian Pacemaker in a Patient with a Non-24-Hour Sleep-Wake Schedule Disorder.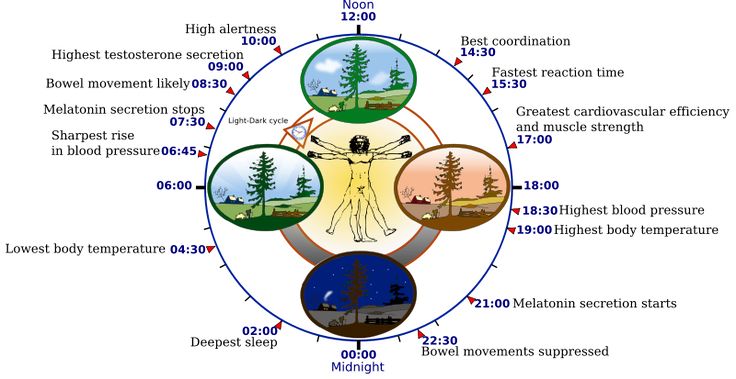 Sleep Research 1993 23:256.
Sleep Research 1993 23:256.
Oren AD, Wehr TA. Hypernyctohemeral syndrome after chronotherapy for delayed sleep phase syndrome. N Engl J Med. 1992;327(24):1762.
Hoban TM, Sack RL, Lewy AJ, et al. Entrainment of a free-running human with bright light? Chronobiol Int 1989;6:347-53.
Eastman CI, Anagnopoulos CA, Cartwright RD. Can bright light entrain a free-runner? Sleep Res 1988;17:372.
Sugita Y, Ishikawa H, Mikami A, et al. Successful treatment for a patient with hypernychthemeral syndrome. Sleep Res 1987;16:642.
Wollman M, Lavie P. Hypernychthemeral sleep-wake cycle: some hidden regularities. Sleep 1986;9: 324-34.
Kamgar-Parsi B, Wehr TA, Gillin JC. Successful treatment of human non-24-hour sleep-wake syndrome. Sleep 1983;6:257-64.
Weber AL, Cary MS, Connor N, et al. Human non-24-hour sleep-wake cycles in an everyday environment. Sleep 1980;2:347-54.
Kokkoris CP, Weitzman ED, Pollak CP, et al. Longterm ambulatory temperature monitoring in a subject with a hypernychthemeral sleep-wake cycle disturbance. Sleep 1978;1:177-90.
Sleep 1978;1:177-90.
Eliott AL, Mills JN, Waterhouse JM. A man with too long a day. J Physiol 1970;212:30-1.
INTERNET
Fadden, J. Can Chronotherapy Cause Non-24 (Q&A). Circadian Sleep Disorders Network. Last modified Aug 21, 2016. http://www.circadiansleepdisorders.org/info/N24chrono.php. Accessed July 31, 2016.
DSPS – A Sleep Disorder. Charting the Course of N24. Updated October 27, 2010. http://delayed2sleep.wordpress.com/2010/10/27/60-charting-the-course-of-n24/. Accessed July 31, 2017.
Years Published
2013, 2017
The information in NORD’s Rare Disease Database is for educational purposes only and is not intended to replace the advice of a physician or other qualified medical professional.
The content of the website and databases of the National Organization for Rare Disorders (NORD) is copyrighted and may not be reproduced, copied, downloaded or disseminated, in any way, for any commercial or public purpose, without prior written authorization and approval from NORD. Individuals may print one hard copy of an individual disease for personal use, provided that content is unmodified and includes NORD’s copyright.
Individuals may print one hard copy of an individual disease for personal use, provided that content is unmodified and includes NORD’s copyright.
National Organization for Rare Disorders (NORD)
55 Kenosia Ave., Danbury CT 06810 • (203)744-0100
Circadian rhythm disorder is a risk factor for obesity and chronic anovulation in women of reproductive age
Circadian rhythm disorder is a risk factor for obesity and chronic anovulation in women of reproductive ageAndreeva E.N.
National Medical Research Center of Endocrinology of the Ministry of Health of Russia;
Moscow State University of Medicine and Dentistry named after A.I. A.I. Evdokimov” of the Ministry of Health of Russia
Grigoryan O.R.
National Medical Research Center of Endocrinology of the Ministry of Health of Russia
Sheremetyeva E.V.
National Medical Research Center of Endocrinology of the Ministry of Health of Russia
Absatarova Yu.S.
National Medical Research Center of Endocrinology of the Ministry of Health of Russia
Fursenko V.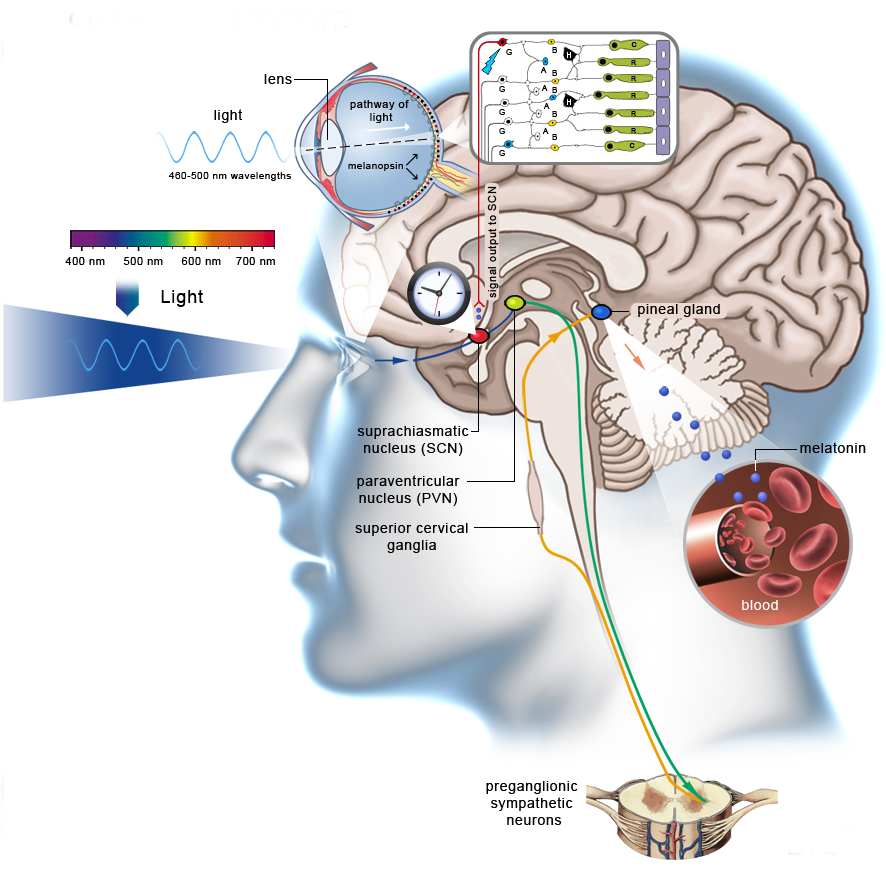 A.
A.
National Medical Research Center for Endocrinology of the Russian Ministry of Health
Circadian rhythm disorder is a risk factor for obesity and chronic anovulation in women of reproductive age
Journal: reproduction problems. 2020;26(5): 36‑42
DOI 10.17116/repro20202605136
How to quote
Andreeva E.N., Grigoryan O.R., Sheremetyeva E.V., Absatarova Yu.S., Fursenko V.A. Disruption of circadian rhythms is a risk factor for the development of obesity and chronic anovulation in women of reproductive age. Problems of reproduction. 2020;26(5):36‑42.
Andreeva EN, Grigoryan OR, Sheremetyeva EV, Absatarova YuS, Fursenko VA. Circadian rhythm disturbance is a risk factor for obesity and chronic anovulation in women of reproductive age. Russian Journal of Human Reproduction. 2020;26(5):36‑42. (In Russ.).
https://doi.org/10.17116/repro20202605136
Authors:
Andreeva E.N.
Federal State Budgetary Institution "National Medical Research Center for Endocrinology" of the Ministry of Health of Russia;
Moscow State University of Medicine and Dentistry named after A. I. A.I. Evdokimov" of the Ministry of Health of Russia
I. A.I. Evdokimov" of the Ministry of Health of Russia
All authors (5)
Read metadata
In the last decade, interest has been growing in the physiological effects of the hormonal regulation of the sleep-wake system, in particular, in the pineal (pineal gland) hormone melatonin. Melatonin is one of the key mediators of the influence of the pineal gland on the endocrine, immune and other systems of the body, including the regulation of the sleep-wake system. Sleep disorders are closely related to circadian rhythm disorders - jet lag, shift work disorder, weekend insomnia and others. Sleep deprivation along with the presence of a TV in the sleeping room (bedroom) and watching TV before bed are considered as possible causes of obesity. Obesity is a key link in the metabolic syndrome. By 2025, about 50% of women on our planet will be obese. Identification of early markers and risk factors for the development of metabolic syndrome in women of reproductive age is one of the priority areas of endocrine gynecology. Obesity and disruption of circadian rhythms, both individually and in combination, are aggravating factors for chronic anovulation syndrome, which, in turn, leads to infertility, the risk of pathological pregnancy and a threat to the health of the unborn child, as well as the risk of developing cancer in children. women of reproductive age.
Obesity and disruption of circadian rhythms, both individually and in combination, are aggravating factors for chronic anovulation syndrome, which, in turn, leads to infertility, the risk of pathological pregnancy and a threat to the health of the unborn child, as well as the risk of developing cancer in children. women of reproductive age.
Keywords:
obesity
sleep
reproduction
ovulation
circadian rhythms
metabolic syndrome
9Eeva.N. National Medical Research Center of Endocrinology of the Ministry of Health of Russia;
Moscow State University of Medicine and Dentistry named after A.I. A.I. Evdokimov” of the Ministry of Health of Russia
- SPIN RSCI: 1239-2937
- Scopus AuthorID: 7102485410
- ResearcherID: B-5889-2017
- ORCID: 0000-0001-8425-0020
Grigoryan O.R.
National Medical Research Center of Endocrinology of the Ministry of Health of Russia
Sheremetyeva E.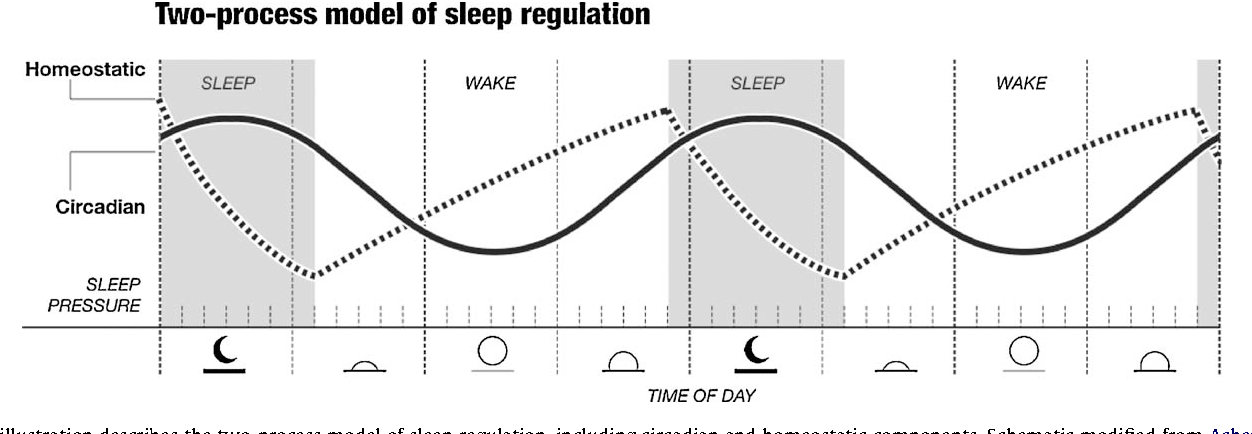 V.
V.
National Medical Research Center for Endocrinology of the Russian Ministry of Health
- SPIN RSCI: 9413-5136
- Scopus AuthorID: 57193433618
- ORCID: 0000-0001-7177-0254
Absatarova Yu.S.
National Medical Research Center of Endocrinology of the Ministry of Health of Russia
Fursenko V.A.
National Medical Research Center for Endocrinology of the Russian Ministry of Health
References:
- Vitaterna M, King D, Chang A, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719-725. https://doi.org/10.1126/science.8171325
- Cagnacci A. Melatonin in relation to physiology in adult humans. Journal of Pineal Research. 1996;21(4):200-213. https://doi.org/10.1111/j.1600-079x.1996.tb00287.x
- Melatonin: prospects for clinical use. Ed. Rapoport S.I.
 Moscow: IMA-PRESS; 2012.
Moscow: IMA-PRESS; 2012. - Smythe G, Lazarus L. Growth Hormone Regulation by Melatonin and Serotonin. Nature. 1973;244(5413):230-231. https://doi.org/10.1038/244230a0
- Andreeva E.N., Sheremetyeva E.V., Fursenko V.A. Obesity is a threat to Russia's reproductive potential. Obesity and metabolism. 2019;16(3):20-28. https://doi.org/10.14341/omet10340
- Volel B.A., Ragimova A.A., Burchakov D.I., Burchakova M.N., Kuznetsova I.V. Stress related menstrual disorders. Consilium Medicum. 2016;18(6):8-13.
- Kamdar B, Tergas A, Mateen F, Bhayani N, Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Research and Treatment. 2013;138(1):291-301. https://doi.org/10.1007/s10549-013-2433-1
- Yuan X, Zhu C, Wang M, Mo F, Du W, Ma X. Night Shift Work Increases the Risks of Multiple Primary Cancers in Women: A Systematic Review and Meta-analysis of 61 Articles. Cancer Epidemiology Biomarkers and Prevention. 2018;27(1):25-40.
 https://doi.org/10.1158/1055-9965.epi-17-0221
https://doi.org/10.1158/1055-9965.epi-17-0221 - The putative 82 causes of obesity. The downey obesity report. Accessed April 7, 2020. https://www.downeyobesityreport.com/2013/02/the-putative-82-causes-of-obesity
- Smirnova V.O. Possibilities of correcting the components of the metabolic syndrome with melatonin: diss. ... cand. honey. Sciences. Volgograd; 2018.
- Cappuccio F, Taggart F, Kandala N, Currie A, Peile E, Stranges S, Miller MA. Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults. Sleep. 2008;31(5):619-626. https://doi.org/10.1093/sleep/31.5.619
- De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. International Journal of Epidemiology. 2009; 38(3):848-854. https://doi.org/10.1093/ije/dyn360
- Jetten AM. Retinoid-Related Orphan Receptors (RORs): Critical Roles in Development, Immunity, Circadian Rhythm, and Cellular Metabolism.
 Nuclear Receptor Signaling. 2009;7(1):e003. https://doi.org/10.1621/nrs.07003
Nuclear Receptor Signaling. 2009;7(1):e003. https://doi.org/10.1621/nrs.07003 - Maemura K, Takeda N, Nagai R. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Role of the Biological Clock in Cardiovascular Diseases. Journal of Pharmacological Sciences. 2007; 103(2):134-138. https://doi.org/10.1254/jphs.fmj06003x2
- Sisson S, Camhi S, Church T, Martin CK, Tudor-Locke C, Bouchard C, Earnest CP, Smith SR, Newton RL Jr, Rankinen T, Katzmarzyk PT . Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metabolic Syndrome and Related Disorders. 2009;7(6):529-536. https://doi.org/10.1089/met.2009.0023
- Tsfasman A.Z., Gorokhov V.D., Alpaev D.V. The circadian rhythm of melatonin during night sleep deprivation. Problems of endocrinology. 2013;59(2):40-44. https://doi.org/10.14341/probl201359240-44
- Cipolla-Neto J, Amaral F, Afeche S, Tan D, Reiter R. Melatonin, energy metabolism, and obesity: a review.
 Journal of Pineal Research. 2014;56(4):371-381. https://doi.org/10.1111/jpi.12137
Journal of Pineal Research. 2014;56(4):371-381. https://doi.org/10.1111/jpi.12137 - Reiter R, Tan D, Korkmaz A, Ma S. Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Annals of Medicine. 2012;44(6):564-577. https://doi.org/10.3109/07853890.2011.586365
- Waldhauser F, Frisch H, Waldhauser M, Weiszenbacher G, Zeitlhuber U, Wurtman R. Fall in nocturnal serum melatonin during prepuberty and pubescence. The Lancet. 1984;323(8373):362-365. https://doi.org/10.1016/s0140-6736(84)
- -4
- Díaz López B, Díaz Rodríguez E, Urquijo C, Fernández Alvarez C. Melatonin Influences on the Neuroendocrine-Reproductive Axis. Annals of the New York Academy of Sciences. 2005;1057(1):337-364. https://doi.org/10.1196/annals.1356.026
- Roy D, Belsham D. Melatonin Receptor Activation Regulates GnRH Gene Expression and Secretion in GT1-7 GnRH Neurons. Journal of Biological Chemistry. 2001;277(1):251-258. https://doi.
 org/10.1074/jbc.m1088
org/10.1074/jbc.m1088 - Baker F, Driver H. Circadian rhythms, sleep, and the menstrual cycle. Sleep medicine. 2007;8(6):613-622. https://doi.org/10.1016/j.sleep.2006.09.011
- Lim A, Huang Z, Chua S, Kramer M, Yong E. Sleep Duration, Exercise, Shift Work and Polycystic Ovarian Syndrome-Related Outcomes in a Healthy Population: A Cross-Sectional Study. PLOS One. 2016;11(11):e0167048. https://doi.org/10.1371/journal.pone.0167048
- Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care for Women International. 2002;23(6-7):703-714. https://doi.org/10.1080/073993302449
- Lee K. Self-Reported Sleep Disturbances in Employed Women. Sleep. 1992;15(6):493-498. https://doi.org/10.1093/sleep/15.6.493
- Preston FS, Bateman SC, Short RV, Wilkinson RT. Effects of flying and of time changes on menstrual cycle length and on performance in airline stewardesses. Aerospace Medicine. 1973;44(4):438-443.
- Messing K, Saurel-Cubizolles M, Bourgine M, Kaminski M. Menstrual-cycle characteristics and work conditions of workers in poultry slaughterhouses and canneries. Scandinavian Journal of Work, Environment and Health. 1992;18(5):302-309. https://doi.org/10.5271/sjweh.1572
- Uehata T, Sasakawa N. The fatigue and maternity disturbances of night workwomen. Journal of Human Ergology. 1982;11(Suppl):465-474.
- Baumgartner A, Dietzel M, Saletu B, Wolf R, Campos-Barros A, Gräf KJ, Kürten I, Mannsmann U. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Research. 1993;48(2):153-178. https://doi.org/10.1016/0165-1781(93)
-j
- Axelsson G, Rylander R, Molin I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. British Journal of Industrial Medicine. 1989;46(6):393-398.
 https://doi.org/10.1136/oem.46.6.393
https://doi.org/10.1136/oem.46.6.393 - Michels K, Mendola P, Schliep K, Yeung EH, Ye A, Dunietz GL, Wactawski-Wende J, Kim K, Freeman JR, Schisterman EF, Mumford SL. The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiology International. 2019;37(2): 260-271. https://doi.org/10.1080/07420528.2019.1694938
- Andreeva E.N., Grigoryan O.R., Sheremetyeva E.V., Absatarova Yu.S. Comorbidity: Polycystic ovary syndrome and obesity. Tutorial. Moscow: AMP Group; 2020.
- Spinedi E, Cardinali D. The Polycystic Ovary Syndrome and the Metabolic Syndrome: A Possible Chronobiotic-Cytoprotective Adjuvant Therapy. International Journal of Endocrinology. 2018;1349688. https://doi.org/10.1155/2018/1349868
- Andreeva E.N., Absatarova Yu.S., Sheremetyeva E.V., Derkach D.A., Ponomareva T.A., Tyulpakov A.N. , Ioutsi V.A., Ilyin A.V., Murvatov K.D. Analysis of the informativeness of the determination of melatonin in polycystic ovary syndrome.
 Obesity and metabolism. 2016;13(4):15-20. https://doi.org/10.14341/omet2016415-20
Obesity and metabolism. 2016;13(4):15-20. https://doi.org/10.14341/omet2016415-20 - Lowes D, Webster N, Murphy M, Galley H. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. British Journal of Anesthesia. 2013;110(3):472-480. https://doi.org/10.1093/bja/aes577
- Soares J, Masana M, Ershahin Ç, Dubocovich M. Functional Melatonin Receptors in Rat Ovaries at Variable Stages of the Estrous Cycle. Journal of Pharmacology and Experimental Therapeutics. 2003; 306(2):694-702. https://doi.org/10.1124/jpet.103.049916
- Reiter R, Tamura H, Tan D, Xu X. Melatonin and the circadian system: contributions to successful female reproduction. Fertility and Sterility. 2014;102(2):321-328. https://doi.org/10.1016/j.fertnstert.2014.06.014
- Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N.
 Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. Journal of Pineal Research. 2008; 44(3):280-287. https://doi.org/10.1111/j.1600-079x.2007.00524.x
Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. Journal of Pineal Research. 2008; 44(3):280-287. https://doi.org/10.1111/j.1600-079x.2007.00524.x - Cappuccio F, D’Elia L, Strazzullo P, Miller M. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care. 2009;33(2):414-420. https://doi.org/10.2337/dc09-1124
- Van Cauter E. Sleep disturbances and insulin resistance. Diabetic Medicine. 2011;28(12):1455-1462. https://doi.org/10.1111/j.1464-5491.2011.03459.x
- Shreeve N, Cagampang F, Sadek K, Tolhurst M, Houldey A, Hill CM, Brook N, Macklon N, Cheong Y. Poor sleep in PCOS; is melatonin the culprit? human reproduction. 2013;28(5):1348-1353. https://doi.org/10.1093/humrep/det013
- Shabani A, Foroozanfard F, Kavossian E, Aghadavod E, Ostadmohammadi V, Reiter RJ, Eftekhar T, Asemi Z. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome : A randomized, double-blind, placebo-controlled trial.
 Journal of Affective Disorders. 2019;250:51-56. https://doi.org/10.1016/j.jad.2019.02.066
Journal of Affective Disorders. 2019;250:51-56. https://doi.org/10.1016/j.jad.2019.02.066 - Cardinali DP, Cano P, Jiménez-Ortega V, Esquifino AI. Melatonin and the Metabolic Syndrome: Physiopathologic and Therapeutical Implications. neuroendocrinology. 2011;93(3):133-142. https://doi.org/10.1159/000324699
- Cardinali DP, Hardeland R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. neuroendocrinology. 2017;104(4):382-397. https://doi.org/10.1159/000446543
- Rapoport S.I., Molchanov A.Yu., Golichenkov V.A., Burlakova O.V., Suprunenko E.A., Savchenko E.S. Metabolic syndrome and melatonin. Clinical medicine. 2013;91(11):8-14.
Close metadata
Circadian rhythms are fluctuations in human biological rhythms that occur with different intensity and cyclicality and are determined by the regime of day and night, i.e. This is the biological clock of every person. Circadian rhythms are the most important mechanism in the regulation of the whole organism; they provide homeostasis, dynamic balance and the process of adaptation in biological systems.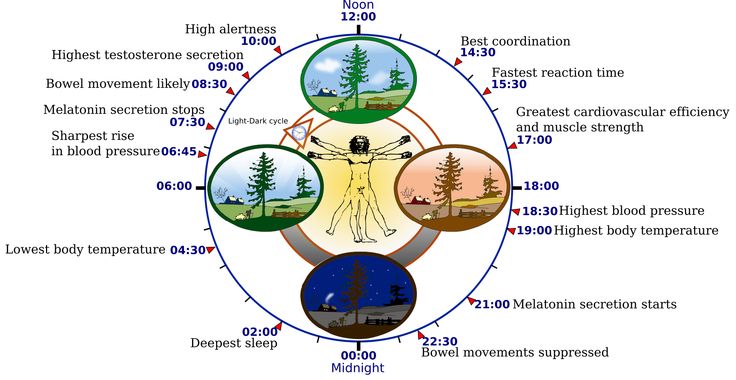 These rhythms have an endogenous genetic nature and are closely related to external environmental factors [1].
These rhythms have an endogenous genetic nature and are closely related to external environmental factors [1].
Each person experiences peaks and falls in circadian rhythms during the day, due to life-support processes in the body, for example, changes in body temperature, changes in pulse rate, respiration, etc. [2]. An important function of circadian rhythms is the identification of day and night patterns. Violation of circadian rhythms occurs due to desynchronization of endogenous (early or late falling asleep) and exogenous (change of time zone, change in daily work schedule) factors of biological rhythms. Circadian rhythm disturbances are associated with such sleep disorders as delayed sleep phase syndrome, shift work sleep disorders, jet lag (including social) [2]. In turn, the risk of developing diseases such as type 2 diabetes mellitus (DM), obesity, cardiovascular diseases, menstrual disorders, infertility, oncological diseases, etc. is now actively associated with circadian rhythm disorders and sleep disorders.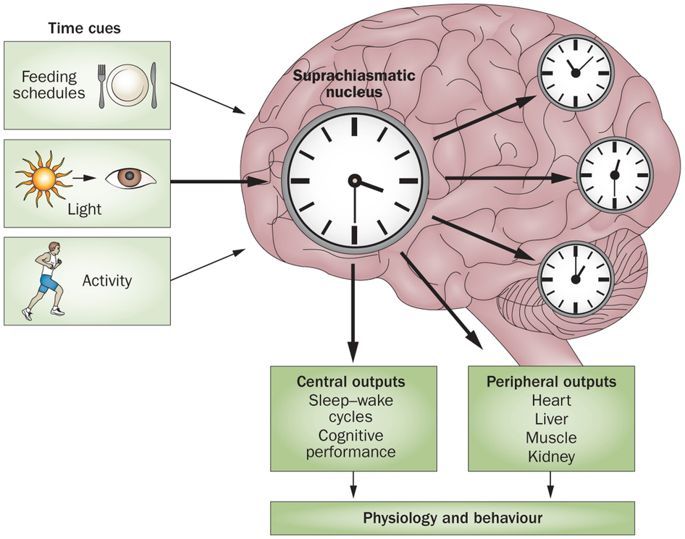 [ 3].
[ 3].
Circadian rhythm disorders and metabolic syndrome
Currently, one of the causes of metabolic syndrome (MS) is called desynchronosis, which is formed as a result of disturbances in the sleep-wake cycle and a decrease in the synthesis of the natural biosynchronizer melatonin at night. Melatonin is not only a natural regulator of the normal circadian rhythm, but also a moderator of the synthesis of growth hormone, which is a metabolic activator in humans, which accelerates catabolic processes [4].
Obesity is a key link in MS. The frequency of obesity in women of reproductive age is 25-27%. By 2025, about 50% of women on our planet will be obese. An increase in body weight in a woman not only leads to a decrease in fertility, but also negatively affects the course of pregnancy, increasing the risk of developing gestational diabetes, eclampsia, pathology of labor, and is also a risk factor for the development of diseases in the newborn [5].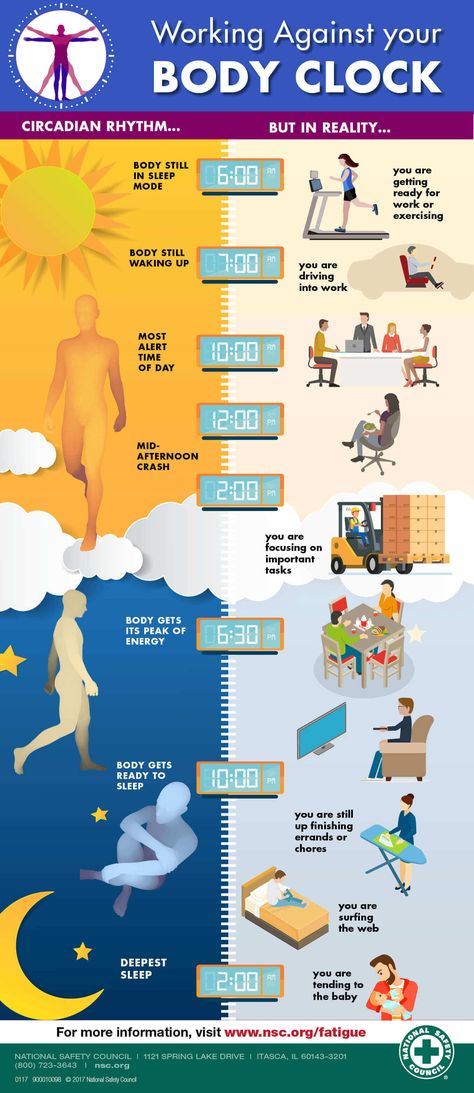 Violation of circadian rhythms in women of reproductive age first contributes to the disruption of the regularity of the menstrual cycle, and then leads to chronic anovulation syndrome [6], which, in turn, increases the risk of developing such a socially significant disease as breast cancer [7, 8].
Violation of circadian rhythms in women of reproductive age first contributes to the disruption of the regularity of the menstrual cycle, and then leads to chronic anovulation syndrome [6], which, in turn, increases the risk of developing such a socially significant disease as breast cancer [7, 8].
The head of the American Obesity Association M. Downey in 2013 named sleep deprivation along with the presence of a TV in the sleeping room (bedroom) and watching TV before bed as one of the possible causes of obesity [9]. Recent studies have shown that disruption of the rhythm "sleep-wakefulness" affects the metabolic health of a person, including the control of body weight [10].
A meta-analysis (2008) found an association between short sleep duration in patients and obesity, confirming the association of habitually short sleep duration with an increased risk of developing obesity (1.55 times). The same researchers concluded that an increase in sleep duration per hour was accompanied by a decrease in body mass index by 0.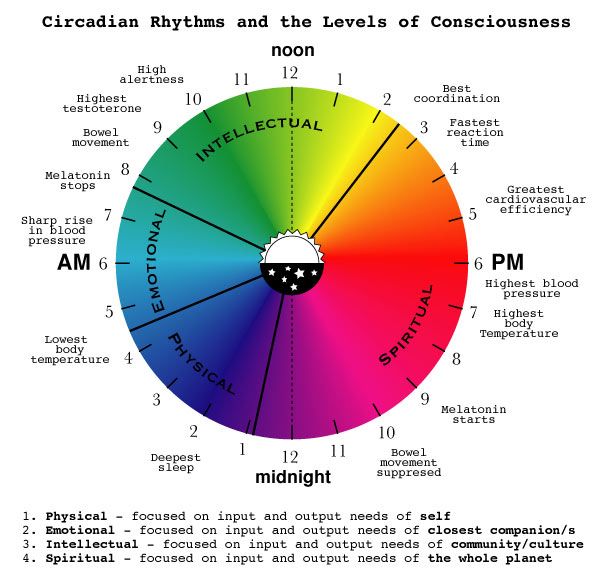 35 kg/m 2 [11]. Recent studies show that people with chronically short sleep duration have a higher risk of developing obesity compared to people who sleep 7–8 hours a day [10].
35 kg/m 2 [11]. Recent studies show that people with chronically short sleep duration have a higher risk of developing obesity compared to people who sleep 7–8 hours a day [10].
The alternation of the circadian cycle of day and night is an important regulator of various physiological rhythms in both animals and humans [10]. Currently, much attention is paid to the study of the activity of melatonin, one of the leading markers of the circadian rhythm and the regulator of the most important metabolic processes in humans.
Works of the last decade show that a decrease in nocturnal secretion or a violation of the pulse pattern of epiphyseal melatonin production can affect the increase in the prevalence of MS, type 2 diabetes, and oncological diseases [12–15]. The hormone melatonin was discovered in 1958 by A.B. Lerner. Changes in melatonin concentration have a noticeable diurnal rhythm: typically high levels of the hormone during the night and low levels during the day.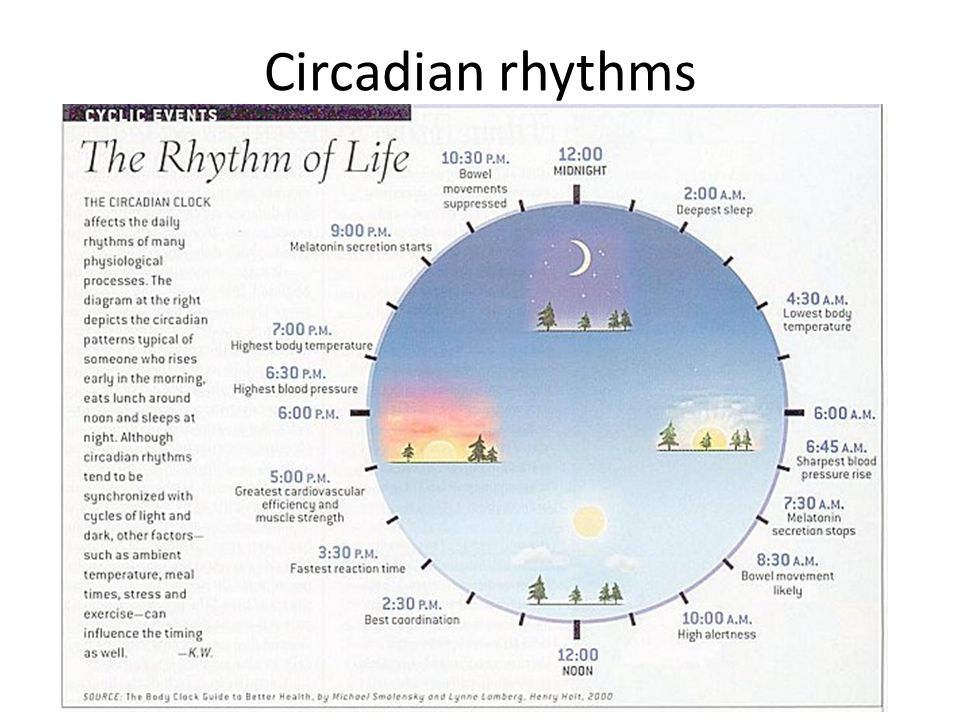 The maximum values of melatonin concentration in human blood are observed between midnight and 5 o'clock in the morning local solar time. This hormone is produced by the main secretory cells of the pineal gland, pinealocytes, and ensures the daily and seasonal rhythm of the physiological activity of organs and tissues, adapting their work to changing environmental conditions [3]. Changing the lifestyle of women in modern big cities (increasing the duration of waking hours during the daytime, and sometimes at night), artificial lighting, the constant presence of light from TV screens, computer monitors, smartphones, phones disrupt the cyclical circadian rhythms and cause the development of desynchronosis, which , in turn, leads to a change in the daily pattern of melatonin production. In the work of A.Z. Tsfasman et al. it is shown that deprivation 1 nocturnal sleep led to a disruption in the circadian rhythm of melatonin production: synthesis at night sharply decreased and reached the daytime level, whereas in a normal lifestyle with sleep at night, its nocturnal synthesis was 63–75% of the daily production [16].
The maximum values of melatonin concentration in human blood are observed between midnight and 5 o'clock in the morning local solar time. This hormone is produced by the main secretory cells of the pineal gland, pinealocytes, and ensures the daily and seasonal rhythm of the physiological activity of organs and tissues, adapting their work to changing environmental conditions [3]. Changing the lifestyle of women in modern big cities (increasing the duration of waking hours during the daytime, and sometimes at night), artificial lighting, the constant presence of light from TV screens, computer monitors, smartphones, phones disrupt the cyclical circadian rhythms and cause the development of desynchronosis, which , in turn, leads to a change in the daily pattern of melatonin production. In the work of A.Z. Tsfasman et al. it is shown that deprivation 1 nocturnal sleep led to a disruption in the circadian rhythm of melatonin production: synthesis at night sharply decreased and reached the daytime level, whereas in a normal lifestyle with sleep at night, its nocturnal synthesis was 63–75% of the daily production [16]. In recent years, possible associations between melatonin deficiency and obesity have been shown [17, 18].
In recent years, possible associations between melatonin deficiency and obesity have been shown [17, 18].
Circadian rhythms, melatonin, women's reproductive health
The very fact that melatonin synthesis rhythm disturbance can be the cause of various diseases, including obesity, as shown in the work of J. Cipolla-Neto et al. The authors drew attention to the fact that the violation of biorhythms in the human body, including the metabolic balance, can lead to disadaptation in conditions of circadian rhythm disorders [17].
Research by A. Brzezinski et al. showed that melatonin can change the pulsatile secretion of luteinizing hormone (LH) and regulate folliculogenesis [3]. A high nocturnal concentration of melatonin significantly reduces the concentration of LH in blood plasma due to the prevention of its secretion [19]. It has been repeatedly shown in recent studies that this pineal gland hormone is involved in maintaining the normal maturation of follicles due to an increased concentration (by 3 times) in granulosa cells and follicular fluid (compared to blood levels) [3].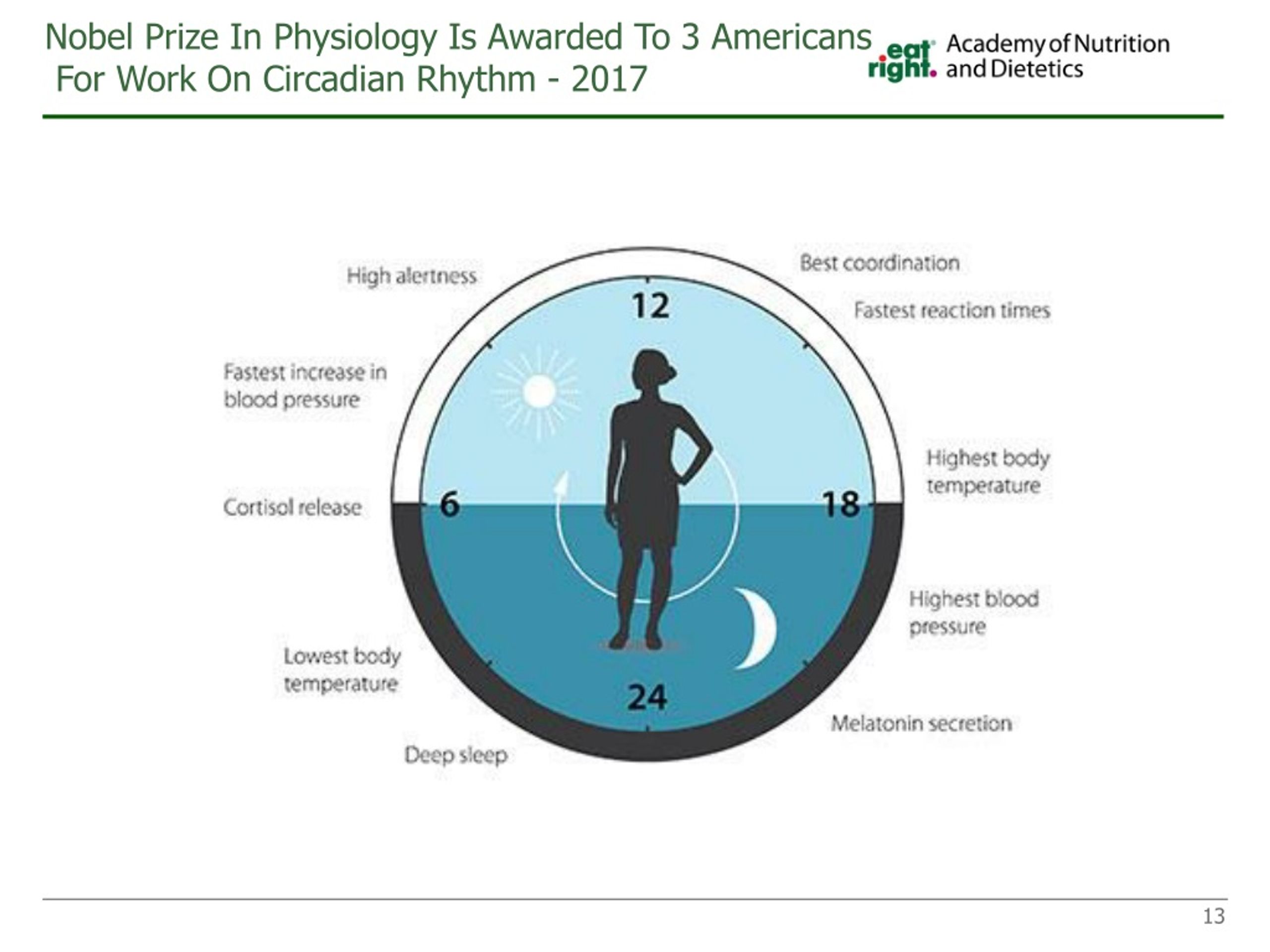 Melatonin regulates the secretion of gonadotropic hormones (LH, follicle-stimulating hormone, FSH), as well as prolactin in accordance with the daily photoperiod 2 [20]. According to D. Roy and D. Belsham, gene expression and pulsatile secretion of gonadotropin-releasing hormone (GnRH), which controls the secretion of LH and FSH, is melatonin-dependent and has a 24-hour cycle [21]. In women of reproductive age with a high concentration of melatonin in the blood serum, suppression of the function of the hypothalamus (as part of the development of hypothalamic functional amenorrhea) was noted with a reduced level of pulsatile secretion of GnRH and LH, which affects the entire process of folliculogenesis. In blind women, melatonin levels during the day are higher than in sighted women, and basal and peak levels of LH and FSH are significantly lower compared to sighted women [3].
Melatonin regulates the secretion of gonadotropic hormones (LH, follicle-stimulating hormone, FSH), as well as prolactin in accordance with the daily photoperiod 2 [20]. According to D. Roy and D. Belsham, gene expression and pulsatile secretion of gonadotropin-releasing hormone (GnRH), which controls the secretion of LH and FSH, is melatonin-dependent and has a 24-hour cycle [21]. In women of reproductive age with a high concentration of melatonin in the blood serum, suppression of the function of the hypothalamus (as part of the development of hypothalamic functional amenorrhea) was noted with a reduced level of pulsatile secretion of GnRH and LH, which affects the entire process of folliculogenesis. In blind women, melatonin levels during the day are higher than in sighted women, and basal and peak levels of LH and FSH are significantly lower compared to sighted women [3].
Since the early 2000s, there has been a lot of discussion about the impact of sleep and sleep disturbances on ovulatory function.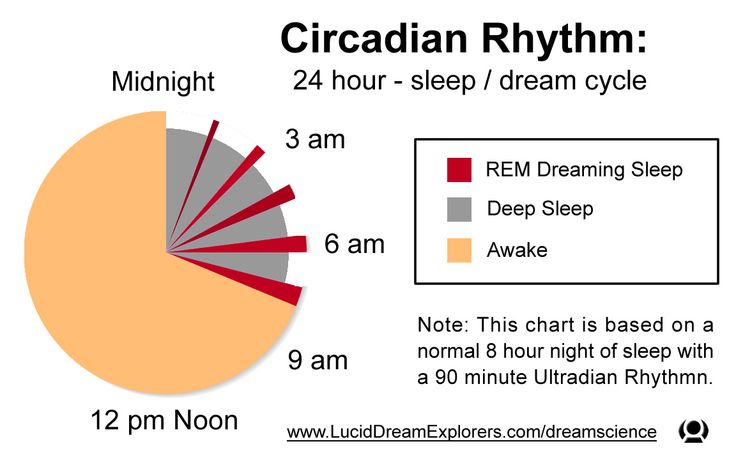
Ovulation is also part of a woman's endogenous circadian rhythm; chronic anovulation affects the woman's menstrual cycle [22]. A. Lim et al. showed that if in women of reproductive age sleep lasts less than 6 hours, then the menstrual cycle will be disrupted by the type of "delay" or abnormal uterine bleeding [23]. Nurses, flight attendants, and women with shift work have more frequent cases of menstrual irregularities and non-pregnancy associated with chronic anovulation syndrome [24–28]. Menstrual irregularities in these women may be associated with changes in LH pulsation and amplitude as a result of circadian rhythm and sleep disturbances [29], as well as with the "stress" of the hypothalamic-pituitary-ovarian axis. Sleep has a direct suppressive effect on LH secretion, contributing to a decrease in the level of this gonadotropic hormone at night in a woman of reproductive age in the early follicular phase of the menstrual cycle [22]. Since women who work at night or with sleep disturbances have shorter and more superficial, interrupted sleep, ovarian dysfunction may be associated with impaired production and amplitude of gonadotropic hormones through an effect on GnRH [22].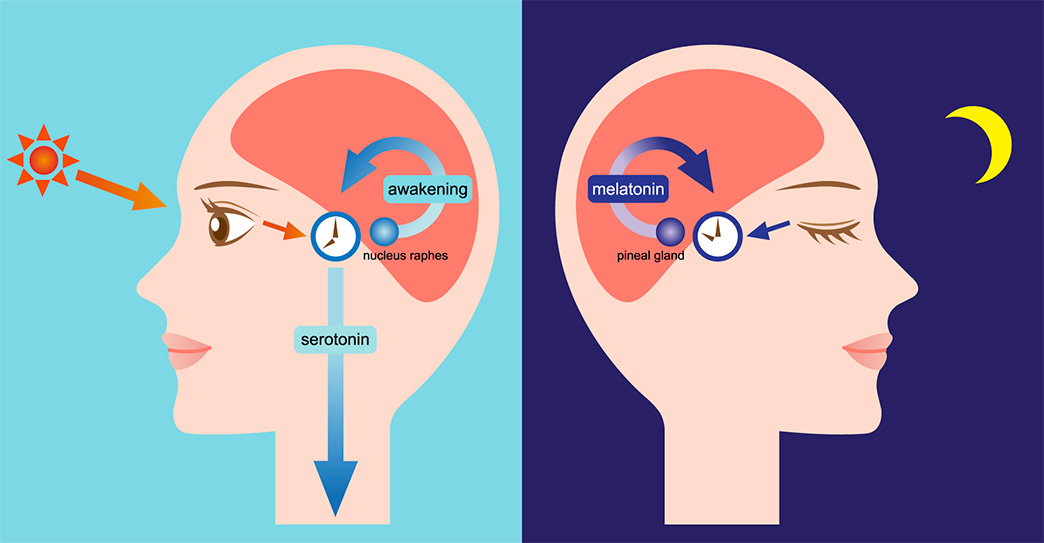 In turn, such menstrual irregularities may be a risk factor for infertility, small fetal size for gestational age, and preterm birth [30].
In turn, such menstrual irregularities may be a risk factor for infertility, small fetal size for gestational age, and preterm birth [30].
K. Michels (2019) analyzed in detail the differences in the average concentrations of gonadotropins, the magnitude of the shift in time and the amplitude of the peaks of gonadotropins, as well as the risk of developing sporadic anovulation associated with the nature of sleep and work at night. With an increase in the duration of daily sleep for every hour, the average concentrations of estradiol increased by 3.9%, progesterone in the luteal phase - by 9.4%. Sleeping less than 7 hours per day is associated with an earlier rise in FSH and LH peak levels. Women who worked nights or shifts had lower testosterone levels compared to women who worked during the day. Women with morning chronotype 3 had an earlier increase in estradiol levels during the menstrual cycle and possibly an earlier increase in LH levels. Women with the evening chronotype had a later PH peak.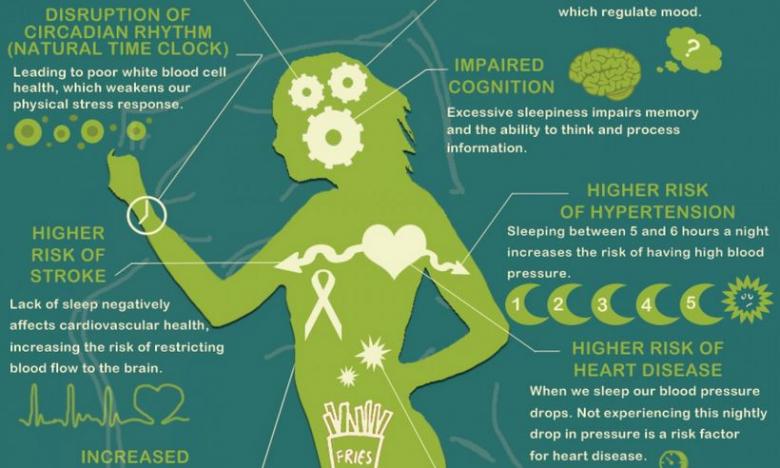 An increase in the risk of developing sporadic anovulation has been shown with a decrease in the amount of sleep, especially for the group with the morning chronotype [31].
An increase in the risk of developing sporadic anovulation has been shown with a decrease in the amount of sleep, especially for the group with the morning chronotype [31].
An illustrative model showing the relationship between circadian rhythm disorders, melatonin and ovulatory function in a woman is a patient with polycystic ovary syndrome (PCOS) and MS. More than half of women with PCOS are overweight or obese and insulin resistant (IR) [32]. Dysfunction of white adipose tissue has been identified as the main factor contributing to the development of IR in PCOS [33]. The etiology and pathogenesis of the syndrome are still actively discussed. In recent years, the authors of studies have analyzed the role of melatonin in the pathogenesis of PCOS, which, along with its effect on cycle « sleep-wake » has additional functions, incl. immunostimulatory effect [34]. It is known that melatonin is synthesized in the ovaries, endometrium, and placenta, while its production does not depend on the circadian rhythm [35].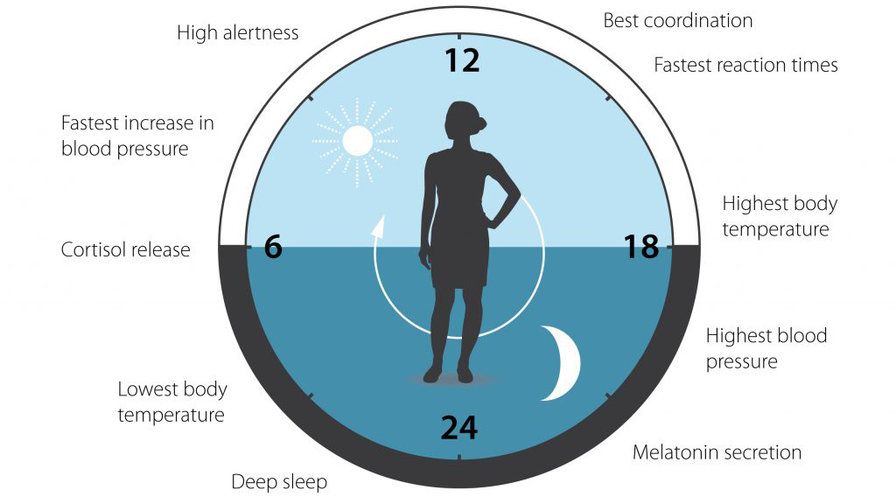 The level of melatonin in the follicular fluid is three times higher than in a blood sample [34]. The closer to ovulation, the higher the level of follicular melatonin, and it does not enter the general circulation [34]. In the work of J. Soares et al. it has been shown that the level of melatonin is higher in large follicles than in small ones; this allowed the authors to suggest that its increase in preovulatory follicles may stimulate ovulation. Together with FSH, melatonin plays an important role in folliculogenesis, providing follicle growth, which is confirmed by an increase in the number of atretic follicles in the ovaries after removal of the epiphysis in mice [36]. In the experiment of R. Reiter et al. a direct effect of melatonin on the synthesis of follicular steroids has been shown, however, this depends on the duration of treatment, the experimental model (cell or follicular culture) [37]. Therefore, in relation to folliculogenesis, melatonin is a “protector” of follicles from oxidative stress, has a protective effect against atresia, promoting ovulation [33].
The level of melatonin in the follicular fluid is three times higher than in a blood sample [34]. The closer to ovulation, the higher the level of follicular melatonin, and it does not enter the general circulation [34]. In the work of J. Soares et al. it has been shown that the level of melatonin is higher in large follicles than in small ones; this allowed the authors to suggest that its increase in preovulatory follicles may stimulate ovulation. Together with FSH, melatonin plays an important role in folliculogenesis, providing follicle growth, which is confirmed by an increase in the number of atretic follicles in the ovaries after removal of the epiphysis in mice [36]. In the experiment of R. Reiter et al. a direct effect of melatonin on the synthesis of follicular steroids has been shown, however, this depends on the duration of treatment, the experimental model (cell or follicular culture) [37]. Therefore, in relation to folliculogenesis, melatonin is a “protector” of follicles from oxidative stress, has a protective effect against atresia, promoting ovulation [33].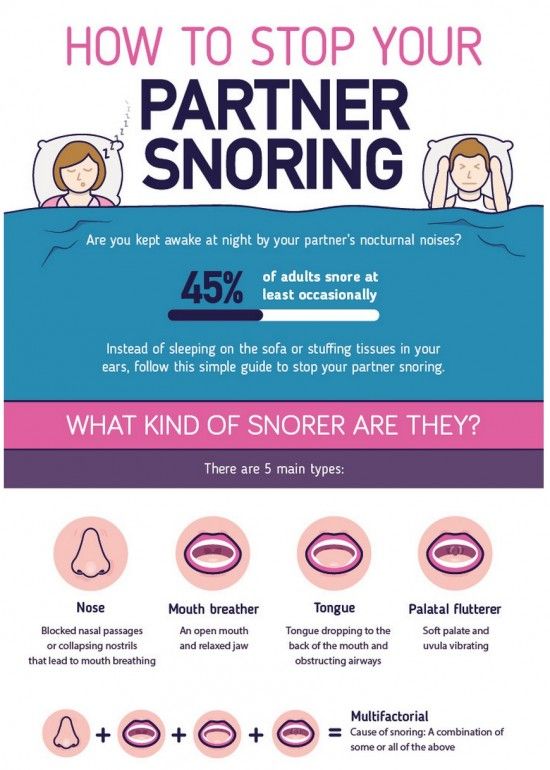
One of the socially significant possible outcomes of PCOS is infertility due to chronic anovulation. When using melatonin in protocols of assisted reproductive technologies, Japanese authors showed that it can improve the quality of human oocytes and embryos [38].
Despite high blood levels (due to negative feedback) in women with PCOS, ovarian melatonin levels may be reduced compared to healthy control women. Decreased levels of melatonin in the follicular fluid impair folliculogenesis, which can lead to anovulation in women [34].
Meta-analysis by F. Cappuccio et al. [39], and an experimental study by E. Van Cauter [40], demonstrate that sleep disturbances and chronic sleep deprivation at night can be risk factors for the development of MS and IR in healthy adults. Mutations in the melatonin receptor gene are also associated not only with an increased risk of developing PCOS itself, but also with impaired insulin secretion and increased fasting glucose levels [33].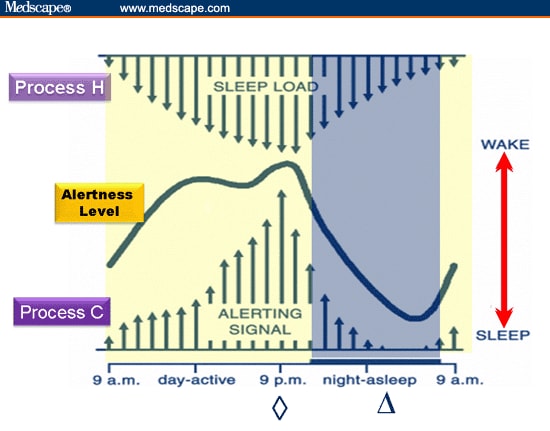 Genomic association studies have shown that polymorphisms in the genes encoding human melatonin receptors (MTNR1A and MTNR1B) are involved in the pathogenesis of type 2 DM [33]. Metabolic disorders that are characteristic of women with PCOS in reproductive age are also found in some physiological or pathophysiological conditions associated with a decrease in the level of melatonin in the blood: aging, shift work, high ambient light levels at night [34]. Somnological disorders are a fairly common pathology in PCOS [33, 34]. One of the epigenetic factors affecting the quality of sleep may be changes and fluctuations in melatonin levels. In the work of N. Shreeve et al. showed that in the group of women with PCOS there was a higher proportion of women with "bad sleep" (according to self-reports) compared with the control group. Women with PCOS felt more lethargic during the day due to disturbed sleep compared to women in the control group [41]. At night, the level of 6-sulfatoxymelatonin (a metabolite of melatonin in the urine) was 1.
Genomic association studies have shown that polymorphisms in the genes encoding human melatonin receptors (MTNR1A and MTNR1B) are involved in the pathogenesis of type 2 DM [33]. Metabolic disorders that are characteristic of women with PCOS in reproductive age are also found in some physiological or pathophysiological conditions associated with a decrease in the level of melatonin in the blood: aging, shift work, high ambient light levels at night [34]. Somnological disorders are a fairly common pathology in PCOS [33, 34]. One of the epigenetic factors affecting the quality of sleep may be changes and fluctuations in melatonin levels. In the work of N. Shreeve et al. showed that in the group of women with PCOS there was a higher proportion of women with "bad sleep" (according to self-reports) compared with the control group. Women with PCOS felt more lethargic during the day due to disturbed sleep compared to women in the control group [41]. At night, the level of 6-sulfatoxymelatonin (a metabolite of melatonin in the urine) was 1.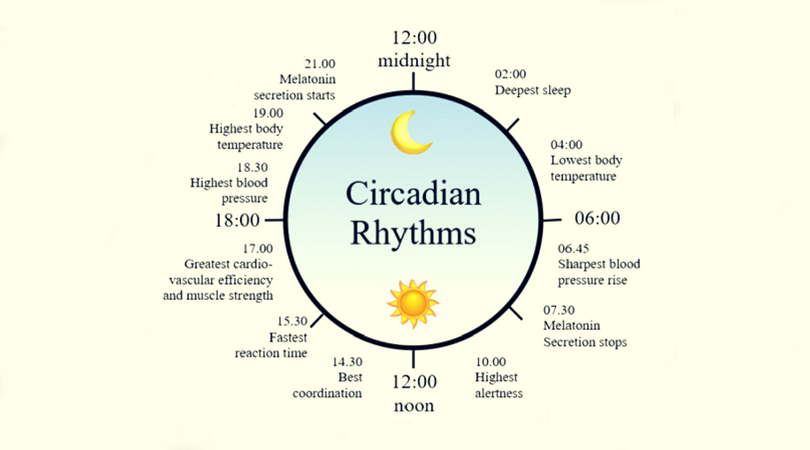 6 times higher in women in the PCOS group than in women in the control group. Elevated nocturnal melatonin levels in PCOS women could potentially act as a free radical scavenger given the high levels of oxidative stress common in women with PCOS [23, 34]. In many studies, low levels of melatonin as part of a sleep disorder have been identified as a risk factor for the development of MS in women with PCOS [23, 33].
6 times higher in women in the PCOS group than in women in the control group. Elevated nocturnal melatonin levels in PCOS women could potentially act as a free radical scavenger given the high levels of oxidative stress common in women with PCOS [23, 34]. In many studies, low levels of melatonin as part of a sleep disorder have been identified as a risk factor for the development of MS in women with PCOS [23, 33].
A. Shabani et al. showed that taking melatonin for 12 weeks in women with PCOS had a positive effect on mental health parameters (decrease in anxiety intensity, improved sleep), lowered insulin levels, HOMA-IR levels, total cholesterol and low-density lipoprotein [42 ]. Similar results were demonstrated by E. Spinedi et al.: melatonin therapy can have a protective effect on the development of MS in PCOS and be used in combination with the main pathogenetic therapy of this disease [33]. Therefore, in the literature of recent years, melatonin, as the main chronobiotic, occupies a special place in the prevention and treatment of MS [33, 43, 44]. A circadian rhythm of fluctuations in the concentration of insulin in the blood with a maximum in the daytime has been described [45].
A circadian rhythm of fluctuations in the concentration of insulin in the blood with a maximum in the daytime has been described [45].
Biological rhythms are a universal and necessary tool for adapting an organism to the environment and cover all manifestations of life from the functions of subcellular structures, cells, tissues, organs to complex behavioral reactions of an organism, populations, ecological systems. In recent years, more and more attention has been drawn to the data on the important regulatory role of the pineal gland and its main hormone melatonin in the physiology of the woman's body, including its effect on reproductive health (menstrual and ovulatory functions, the onset and course of pregnancy). However, melatonin is produced not only in the pineal gland, its synthesis is found in almost all organs, including the gonads. The action of extrapineal melatonin is usually auto- and/or paracrine. Melatonin regulates the secretion of gonadotropic hormones and prolactin in accordance with circadian rhythms. The amplitude of secretion of gonadotropin-releasing hormone, which controls the production of luteinizing and follicle-stimulating hormones, and gene expression are regulated by melatonin and are cyclical with a period of 24 hours. Living in megacities negatively affects the quantity and quality of night sleep. City dwellers are more likely to suffer from insomnia than rural dwellers. This is due to the disorganization of the circadian daily rhythms of sleep and wakefulness. Violation of the photoperiod with an increase in the light gap, light exposure (TV, smartphones, phones, laptops/computers) at night can lead to menstrual disorders, chronic anovulation syndrome, infertility, pathological pregnancy.
The amplitude of secretion of gonadotropin-releasing hormone, which controls the production of luteinizing and follicle-stimulating hormones, and gene expression are regulated by melatonin and are cyclical with a period of 24 hours. Living in megacities negatively affects the quantity and quality of night sleep. City dwellers are more likely to suffer from insomnia than rural dwellers. This is due to the disorganization of the circadian daily rhythms of sleep and wakefulness. Violation of the photoperiod with an increase in the light gap, light exposure (TV, smartphones, phones, laptops/computers) at night can lead to menstrual disorders, chronic anovulation syndrome, infertility, pathological pregnancy.
In recent years, circadian rhythm disorders have been actively discussed as one of the possible causes of the development of the metabolic syndrome, especially in combination with physical inactivity. Melatonin carries out a change in physiological processes in the body and controls the recovery processes in organs and tissues. Pineal hormone improves sensitivity to inulin, reducing its level to basal, and does not allow compensatory hyperinsulinemia with an increase in glucose levels. The circadian rhythm of fluctuations in the concentration of insulin in the blood with a maximum in the daytime is described. The formation and progression of the metabolic syndrome may be due to desynchronosis. Recently, there has been an increase in the number of works that describe the positive effect of the use of melatonin in combination therapy for various pathological conditions. Based on all of the above data, it can be concluded that melatonin may be an important component in the treatment of such a complex condition as metabolic syndrome in women of reproductive age.
Pineal hormone improves sensitivity to inulin, reducing its level to basal, and does not allow compensatory hyperinsulinemia with an increase in glucose levels. The circadian rhythm of fluctuations in the concentration of insulin in the blood with a maximum in the daytime is described. The formation and progression of the metabolic syndrome may be due to desynchronosis. Recently, there has been an increase in the number of works that describe the positive effect of the use of melatonin in combination therapy for various pathological conditions. Based on all of the above data, it can be concluded that melatonin may be an important component in the treatment of such a complex condition as metabolic syndrome in women of reproductive age.
Participation of authors:
Research concept and design — Andreeva E.N., Sheremetyeva E.V.
Collection and processing of material — Grigoryan O.R., Sheremetyeva E.V., Absatarova Yu.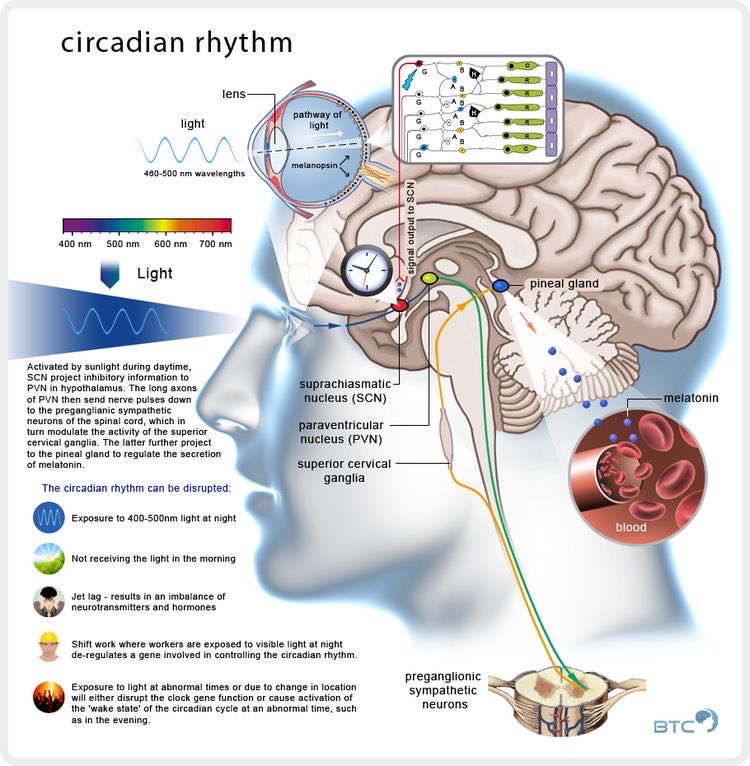 S., Fursenko V.A.
S., Fursenko V.A.
Text writing — Sheremetyeva E.V., Grigoryan O.R., Absatarova Yu.S.
Editing — Andreeva E.N., Grigoryan O.R.
The work was carried out within the framework of the State task "Central and peripheral pathophysiological mechanisms of the development of adipose tissue diseases, taking into account clinical and hormonal characteristics" 2020-2022.
The authors declare no conflicts of interest.
1 Deprivation is a negative mental state caused by the deprivation of the opportunity to satisfy the most essential necessities of life (such as sleep, food, housing, sex, communication between the child and mother or father, etc.) or the deprivation of such benefits as which a person has long been accustomed to.
2 Photoperiod - the ratio between the dark and light hours of the day.
3 Chronotype (from the Greek χρόνος - time) - the individual characteristics of the daily rhythms of the human body. The human chronotype determines the organization of the physiological functions of the body and its ability to adapt, and can be used as a universal criterion for the general functional state of the body. Usually, three main human chronotypes are distinguished: early (morning, "larks"), intermediate (normal, "pigeons") and late (evening, "owls").
The human chronotype determines the organization of the physiological functions of the body and its ability to adapt, and can be used as a universal criterion for the general functional state of the body. Usually, three main human chronotypes are distinguished: early (morning, "larks"), intermediate (normal, "pigeons") and late (evening, "owls").
Diagnosis and treatment of sleep disorders
For many centuries, sleep has been viewed as a state of rest and reduced reactivity to external stimuli. But recently an increasing number of facts have been accumulating that do not fit into the usual ideas about sleep and make this definition incorrect. What sign of sleep can be considered its necessary condition? The main sign of sleep in the first place is its rhythm. The rhythmic alternation of sleep phases makes it possible to distinguish normal sleep from monotonous "dream-like states". As part of "normal sleep" should alternate 1-2-3-4 stages of slow sleep, and at the end - REM (paradoxical) sleep. Such a radical change in ideas about the nature of sleep was the result of the emergence in the second half of the 20th century of the science of sleep - somnology. Currently, somnology is one of the fastest growing branches of the science of the nervous system.
Such a radical change in ideas about the nature of sleep was the result of the emergence in the second half of the 20th century of the science of sleep - somnology. Currently, somnology is one of the fastest growing branches of the science of the nervous system.
Currently, doctors and researchers have proven that even small chronic disturbances in sleep and wakefulness are very dangerous for health and are fraught with serious consequences. They can be one of the most important causes of industrial injuries, incidents and disasters, provoking the so-called "human factor".
At the same time, the lifestyle of a modern person, especially in large cities, is poorly consistent with the requirements of "sleep hygiene". The abundance of bright electric light, increased noise levels, working with a computer, watching television programs at night - all this negatively affects sleep.
The situation continues to worsen, in connection with which in many countries centers for the correction of sleep disorders, institutes for the study of sleep are being created, drug-free methods of treatment are being developed. One of the most important areas in "sleep medicine" is the creation of effective and harmless drugs of a new generation.
One of the most important areas in "sleep medicine" is the creation of effective and harmless drugs of a new generation.
What threatens sleep disturbance?
- Chronic lack of sleep reduces long-term concentration and memory.
- Lack of sleep affects carbohydrate metabolism and the hormonal system. Currently, many studies confirm that sleep deprivation and metabolic disorders are linked. Obesity, diabetes, and cardiovascular disease are on the rise, in part because we are sleeping less and more erratically. In recent years, several experiments have shown that people who sleep very little or poorly are more likely to be obese than others. Hormones that regulate appetite play a decisive role in this. Those who sleep too little have increased levels of the hunger hormone ghrelin in the blood, and the amount of leptin, which curbs appetite, is lowered.
- Lack of sleep weakens the body's immune system. With reduced sleep, the human body produces significantly less growth hormone.
 This means that lack of sleep reduces the possibility of regeneration for the system of internal organs. If organs do not have the time and material to replace old or diseased cells with new ones, they will perform less well and their resistance to disease will decrease.
This means that lack of sleep reduces the possibility of regeneration for the system of internal organs. If organs do not have the time and material to replace old or diseased cells with new ones, they will perform less well and their resistance to disease will decrease.
How much sleep does a person need?
As a result of a study conducted at the American Institute of Health, it is shown that on average a person needs 8 hours and 15 minutes of sleep.
In summer, when daylight hours are longer, we need slightly less sleep than in winter. But each person has a different need for sleep. It is genetically determined and depends on many factors. Any number between 5 and 10 hours is considered normal.
What to do if sleep is disturbed?
According to the general opinion of experts, sleep hygiene is very important. These are specific and easily enforceable rules that lead to physical and mental readiness for normal sleep:
- Limit your time in bed.
 Go to bed when you really want to sleep, and get up in the morning as soon as you feel you've had enough sleep.
Go to bed when you really want to sleep, and get up in the morning as soon as you feel you've had enough sleep. - Use the bed only for sleeping. Remove the TV from the bedroom, do not eat in bed. If you wake up in the middle of the night and cannot fall asleep, get up at the latest in half an hour and find some quiet activity - reading or doing housework. Don't go back to bed until you feel really sleepy.
- Maintain consistent sleep hours. Get up and go to bed at the same time, including on weekends. Compliance with the diet and sports at the same hours also contribute to the synchronization of internal rhythms, their stabilization and strengthening, which has a good effect on the quality of sleep.
- Remove the clock from the bedroom. This will help to avoid looking at the clock at night and the feeling of rushing time that it causes.
- Sleep mostly at night. Avoid daytime sleep, and if this is difficult for you, limit its duration to 1 hour. After 15.
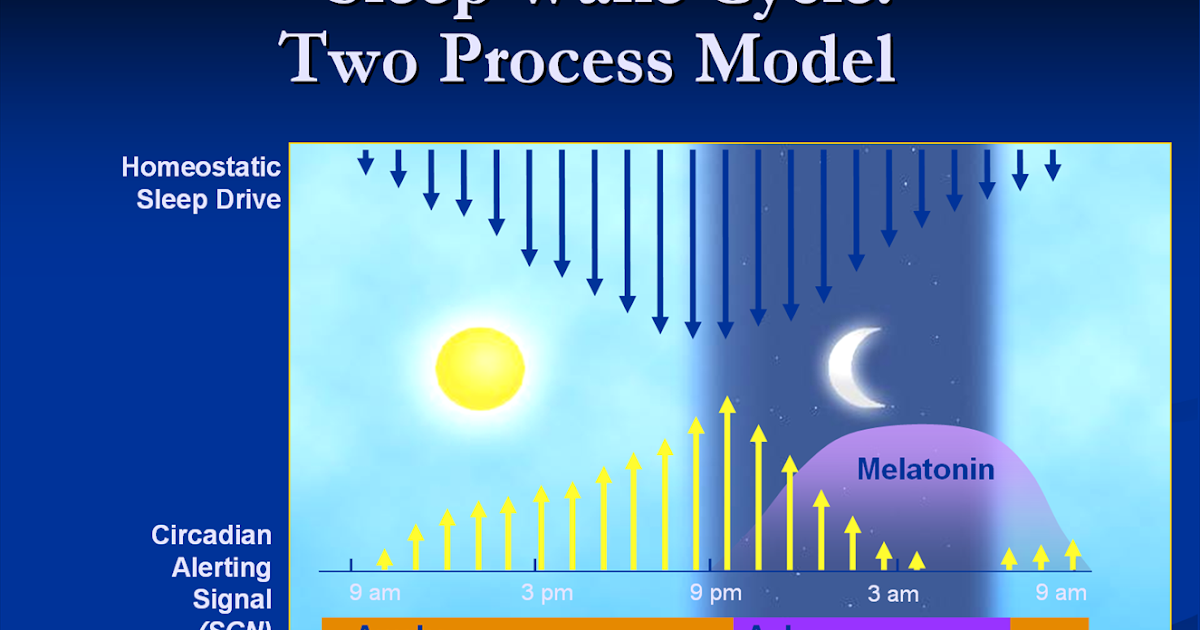 00 you should not sleep.
00 you should not sleep. - Make your day more active. Try regularly, preferably daily, to play sports and generally move more - if possible in the fresh air. It is important not to overstrain in the last hours before bedtime. In the evenings, especially just before bedtime, any strenuous activity that activates blood circulation and requires special concentration is prohibited, including hot and very cold baths, mopping, sports and computer work.
- At least 3-4 hours before bedtime, do not drink coffee, tea, Coca-Cola and do not take caffeinated and other stimulant medications. During the day, caffeine should also be consumed in moderation, otherwise the withdrawal syndrome begins at night. Cigarettes have the same effect.
- Do not drink alcohol 4-6 hours before bedtime. Alcohol interferes with long sleep.
- Do not overeat at night. A heavy dinner can deprive you of sleep for a long time.
- The bedroom should be comfortable, the temperature is about 18 C, ventilate the room well and cover the windows tightly from the light.
- If all these measures do not help, do not be afraid to ask the advice of a doctor - a specialist in sleep disorders - a somnologist.
Sleeping pills
In no case do not take any sleeping pills and sedatives, even harmless and sold without a prescription, without a doctor's prescription. The main disadvantage of potent sedatives is that the brain gets used to them sooner or later. When you try to refuse them, a withdrawal syndrome occurs. Patients suffer from increased anxiety and very poor sleep. Another thing is if the therapy was prescribed by a somnologist. The doctor knows exactly in what dosage one or another sleeping pill or sedative can be taken. Along with pills for chronic insomnia, behavioral therapy is always used: relaxation, sleep hygiene and adherence to the regimen.
The International Classification of Sleep Disorders (ICSD) is the most widely used classification system for sleep disorders. The third version of ICSD (ICSD-3) includes seven major sleep disorders:
1.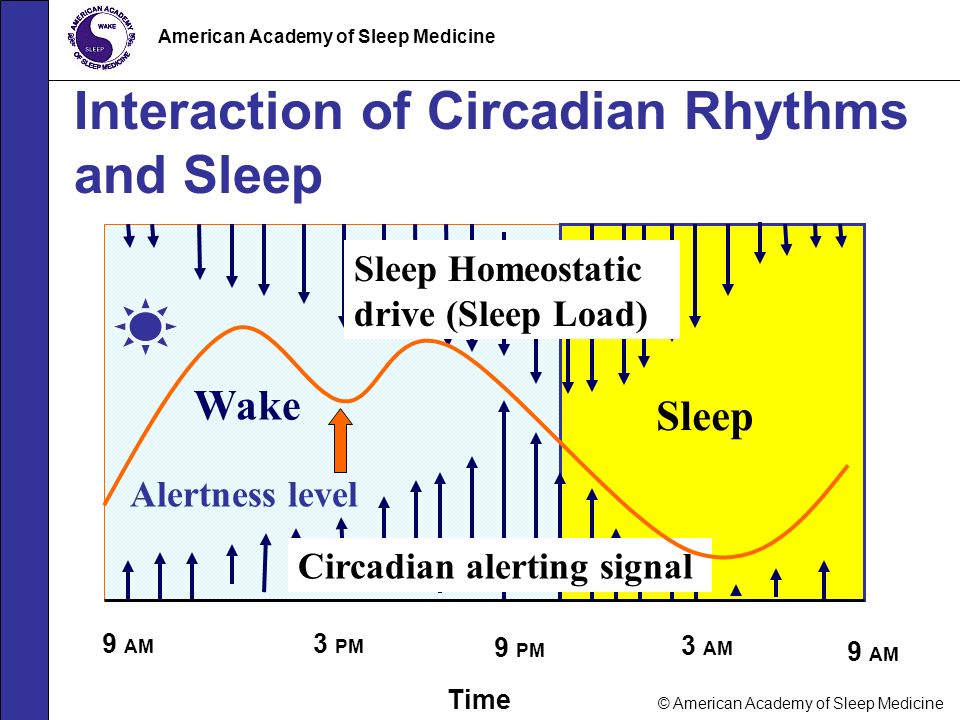 Insomnia (insomnia). Includes conditions characterized by problems falling asleep, maintaining sleep, or poor sleep quality.
Insomnia (insomnia). Includes conditions characterized by problems falling asleep, maintaining sleep, or poor sleep quality.
2. Breathing disorders associated with sleep.
Central sleep apnea syndrome.
- Central sleep apnea with Chain-Stokes respiration
- Central sleep apnea due to somatic disease without respiration Chain-Stokes
- Central apnea due to high-amplitude periodic breathing,
- Central sleep apnea due to drugs or substances,
- Primary central sleep apnea,
- Primary neonatal apnea,
- Primary sleep apnea of premature babies,
- Central apnea resulting from treatment.
Obstructive sleep apnea syndrome.
- Adult obstructive sleep apnea,
- Obstructive sleep apnea in children.
Hypoventilation disorders associated with sleep.
- Obesity-hypoventilation syndrome,
- Syndrome of congenital central alveolar hypoventilation in children,
- Late onset central hypoventilation syndrome,
- Idiopathic central hypoventilation,
- Sleep-related hypoventilation due to use of drugs or substances,
- Sleep-related hypoventilation due to medical conditions.

Sleep-related hypoxemia.
3. Hypersomnia of central origin.
- Narcolepsy (type 1 and type 2).
- Idiopathic hypersomnia.
- Klein-Levin syndrome.
- Sleep deprivation syndrome (including voluntary sleep deprivation).
- Hypersomnia associated with the use of drugs, somatic and mental illness.
4. Disorders of the circadian rhythm of sleep and wakefulness (chronic or ongoing sleep disturbance due to a discrepancy between the environment and the individual sleep-wake cycle in a person).
- Sleep disorders associated with shift work;
- Jet lag syndrome.
- A sleep-wake disorder characterized by earlier falling asleep and earlier waking up (more common in the elderly).
- Irregular sleep-wake rhythm, characterized by the absence of a definite circadian rhythm. This disorder is commonly associated with childhood disruption and neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and Huntington's disease.
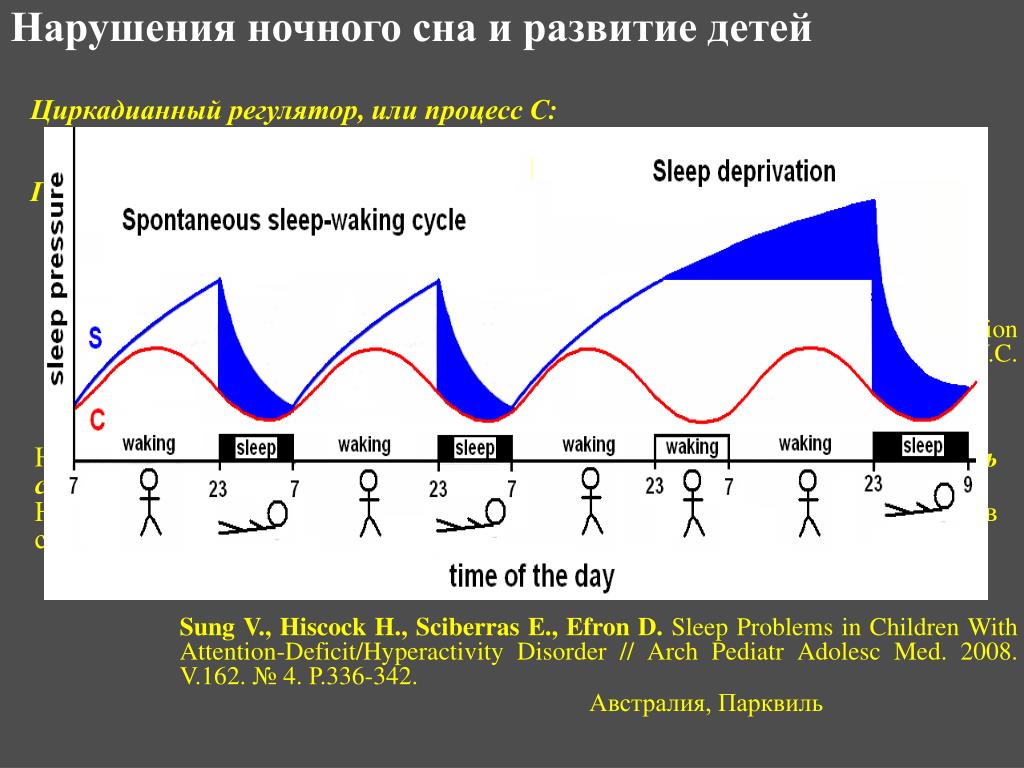
- Non-24-hour sleep-wake rhythm disorder, characterized by excessive sleepiness or insomnia, which occurs because the internal circadian rhythm is not adapted to the 24-hour daily cycle. In most cases, this disorder affects blind people who are not able to determine circadian rhythms.
- Circadian arrhythmias can also occur due to somatic, psychiatric, or neurological disorders.
5. Parasomnias (abnormal movements, sleep behavior, nightmares).
Non-rapid eye movement (NREM) parasomnias of awakening disorders:
- confused awakenings,
- sleepwalking,
- nightmares in sleep,
- eating disorders associated with sleep.
Rapid eye movement (REM) associated parasomnias:
- sleep paralysis,
- nightmares.
Non-sleep stage related parasomnias:
- dream hallucinations
- nocturnal enuresis
- parasomnias associated with somatic diseases and medications.
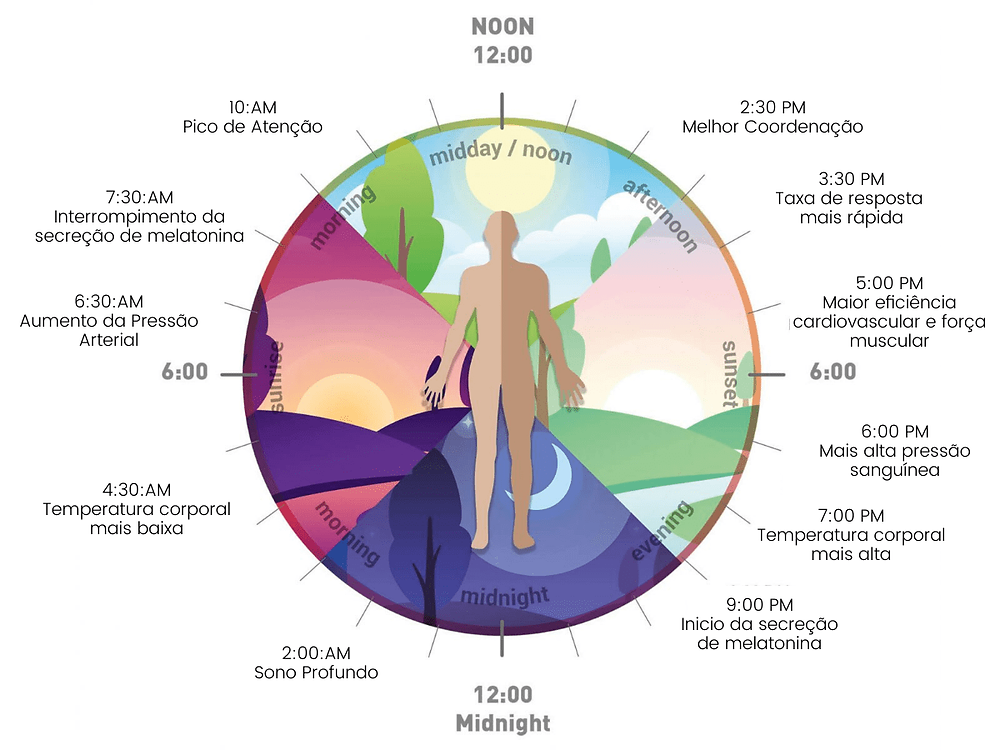
6. Sleep-related movement disorders (characterized by simple, stereotypical movements that interfere with sleep)
- Restless legs syndrome,
- Periodic limb movements,
- Sleep related crumpy syndrome,
- Sleep-related bruxism (teeth grinding),
- Sleep related rhythmic movement disorders
7. Other sleep disorders
This category includes sleep disorders that do not meet the classification, as well as cases where there is more than one sleep disorder, or cases where there is insufficient evidence to support a diagnosis.
To provide professional medical care to patients with sleep disorders, it is necessary to conduct a comprehensive examination by a specialist. The Clinic of Neurology and Neurosurgery of the European Medical Center offers patients the diagnosis of sleep disorders according to the most modern protocols using the polysomnography method, the gold standard in somnology.