Saphris dosage for sleep
Effectiveness, Ease of Use, and Satisfaction
Show ratings & reviews for
3.6 Overall Rating
Share Your Experience
Effectiveness
Tooltip iconSee more
Ease of Use
Tooltip iconSee more
Satisfaction
Tooltip iconSee more
Most voted positive review
16 People found this comment helpful
First med, I tried that makes me feel normal w/o side affects! I been suffering with bipolar for years and would recommend this med; to anyone with this condition.
Shared reviews and ratings
SORT BYCondition: Mania associated with Bipolar Disorder
Overall rating 4.3
EffectivenessEase of UseSatisfactioni've been taking Saphris for 9y now. I take 10mg. I experimented with taking 5mg but that was not effective. During 2 episodes over the 9y i went up to 20mg. The meds take a long time - 3 weeks - to take into effect. My main learning from this med is that it lets me get a full 8 hours of sleep. If i forget to take Saphris at night, i notice i get 3 hrs of sleep. If I take Saphris in the midlle of thenight then I get back to sleep pretty fast. I have not noticed any mood benefits from this meds. I think the sound sleep I get lelts me have a less anxious day and a good day next day. Read More Read Less
Report this post
Fill 3Created with Sketch.Condition: Other
Overall rating 3.7
EffectivenessEase of UseSatisfactionI read all the reviews but didn't see anyone experience depersonalization which I have. My partner says living with me is like living with a ghost. ..I don't talk or interact anymore. It does help me sleep but at what cost. I have weight gain, muscle problems, no motivation. Now I understand it may be very hard to phase off taking this drug. Not sure my doctor was aware of that when he prescribed it for me.
..I don't talk or interact anymore. It does help me sleep but at what cost. I have weight gain, muscle problems, no motivation. Now I understand it may be very hard to phase off taking this drug. Not sure my doctor was aware of that when he prescribed it for me.
Report this post
Fill 3Created with Sketch.Condition: Additional Medications to Treat Depression
Overall rating 4.7
EffectivenessEase of UseSatisfactionThis is a total gamechanger for me MDD. Within 3 days I felt as close to normal as I have ever been. Yes, the taste is a little off but I don't think it is that bad.
1
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.
Condition: Other
Overall rating 3.3
EffectivenessEase of UseSatisfactionI take saphris for severe insomnia. The pill works most nights in allowing me to fall asleep. I often will wake up after sleeping two to three hours but usually can fall back asleep with little difficulty. The medication is sublingual and dissolves quickly. I've gained 15 pounds since I've been on the medication.
ShapeCreated with Sketch.1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Mania associated with Bipolar Disorder
Overall rating 2.7
EffectivenessEase of UseSatisfactionMy doctor gave me this medication on yesterday. I took the medication and in less than 15mins I was very dizzy and couldnt control my body functions. I could barely move my eyes. It put me to sleep for 12hours however my body couldnt move.Someone else can have this medication but it wont be me.
I took the medication and in less than 15mins I was very dizzy and couldnt control my body functions. I could barely move my eyes. It put me to sleep for 12hours however my body couldnt move.Someone else can have this medication but it wont be me.
1
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 3.7
EffectivenessEase of UseSatisfactionThis medication has been a Godsend, except it is the most difficult medicine to administer I've ever had. You cannot eat ten minutes after administration (and ten minutes before according to my doctor). It's supposed to sit in your mouth for three minutes, but last night I sneezed after ingesting so who knows if I got the full dosage or not. The tin foil packaging can pop the pill out on to the floor, and at $6 a pill (depending on insurance coverage), you can't waste any. Ask your doctor about timing dosages with intimacy. Still, other medications are awful, they deaden compared to the effects of this, and I have been taking these medications since the age of 19.Read More Read Less
The tin foil packaging can pop the pill out on to the floor, and at $6 a pill (depending on insurance coverage), you can't waste any. Ask your doctor about timing dosages with intimacy. Still, other medications are awful, they deaden compared to the effects of this, and I have been taking these medications since the age of 19.Read More Read Less
2
ShapeCreated with Sketch.1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 5.0
EffectivenessEase of UseSatisfactionEven Though I Have Only Been On This Medication For A Short Time I Am Ecstatic With The Results! I Have Been Over Medicated For Years For Bi Polar Disorder Wit Several Hospitalizations Resulting From Psychotic Episodes As One Of The Side Effects. I Have Never Had Regular Sleep Patterns, Nor I Been Able To Stay Asleep For More Than A Couple Hours. This Medication Lets Me Get Uninterrupted Sleep For 6-8 Hours EVERY Night! It Has Also Replaced 6 Other Medications I Had Been Taking! I Hope These GREAT Results Continues! Read More Read Less
I Have Never Had Regular Sleep Patterns, Nor I Been Able To Stay Asleep For More Than A Couple Hours. This Medication Lets Me Get Uninterrupted Sleep For 6-8 Hours EVERY Night! It Has Also Replaced 6 Other Medications I Had Been Taking! I Hope These GREAT Results Continues! Read More Read Less
2
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 2.7
EffectivenessEase of UseSatisfactionI've only been on Saphris a few days and first time I took it I totally crashed out sleeping... Was so confused the next morning that I literally felt like I lost a day's time. Adding to consistently sore muscles? uhhh. .. thanks, but no thanks!
.. thanks, but no thanks!
3
ShapeCreated with Sketch.1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 4.3
EffectivenessEase of UseSatisfactionThe medication is very helpful. But it tastes TERRIBLE and my doctor says that almost every patient that he has on it complains about the taste. He also said that him and several of his patients including me have even called the manufacturer about the taste. I wish there was either an additional form that wasn't sublingual or if they could have a verity of flavor options. Especially since you are not supposed to eat or drink (even water) for ten minutes after taking it. To be honest so I don't have to deal with the taste as much I will just swallow it or put it in a small class of carbonated-flavored water, soda, or juice. Other than the taste the medication is really helpful. Read More Read Less
To be honest so I don't have to deal with the taste as much I will just swallow it or put it in a small class of carbonated-flavored water, soda, or juice. Other than the taste the medication is really helpful. Read More Read Less
4
ShapeCreated with Sketch.1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 5.0
EffectivenessEase of UseSatisfaction1
ShapeCreated with Sketch.3
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Mania associated with Bipolar Disorder
Overall rating 1. 0
0
This medication ruined my life. I completely lost my appetite, couldn't hardly walk, was too weak to do anything and it cost me my career. I started doing better immediately after I stopped taking it after I complained to my doctor several times. I do not recommend this medication under any condition. It is horrid.
ShapeCreated with Sketch.1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Additional Medications to Treat Depression
Overall rating 3.7
EffectivenessEase of UseSatisfactionWhen I first started Saphris, I thought it was a miracle drug. I was prescribed Saphris 2.5mg to boost the effects of my Lexapro 30mg. Within a day of taking my first dose of Saphris, my depression lifted completely and I felt better than I had in years. Unfortunately I developed a Type 1 allergic reaction with wheezing, puffy face and hands, dry skin and mouth, incredible thirst and itching and had to stop Saphris after just a few days. Coming off of Saphris is no picnic. DO NOT drink alcohol while discontining Saphris - I had two glasses of wine one night and was so suicidal I almost drove to the hospital. Two days later, I am having vivid nightmares and "exploding head" sensations and I still feel fragile and confused. I'm taking Claritin D and Pepcid AC to help with the puffiness and itchiness and I am still not comfortable. Can't wait to get this med out of my system.Read More Read Less
Within a day of taking my first dose of Saphris, my depression lifted completely and I felt better than I had in years. Unfortunately I developed a Type 1 allergic reaction with wheezing, puffy face and hands, dry skin and mouth, incredible thirst and itching and had to stop Saphris after just a few days. Coming off of Saphris is no picnic. DO NOT drink alcohol while discontining Saphris - I had two glasses of wine one night and was so suicidal I almost drove to the hospital. Two days later, I am having vivid nightmares and "exploding head" sensations and I still feel fragile and confused. I'm taking Claritin D and Pepcid AC to help with the puffiness and itchiness and I am still not comfortable. Can't wait to get this med out of my system.Read More Read Less
1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Schizophrenia
Overall rating 4. 0
0
The first month gave me some problems with side effects. After that, no problems that I can remember. I take it at bedtime along with another AP for sleep. It does a good job of putting me to sleep almost immediately. Apparently, it continues its work during the following day.
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Additional Medications to Treat Depression
Overall rating 1.3
EffectivenessEase of UseSatisfactionOMG. This was among the most terrifying experiences of my life. My doctor gave me sample to try to handle general depressing. The side effects took 3 to 4 months to kick in and no benefit meanwhile. The side effects were that my vision was so blurry that when I went to an optomitrist to she couldn't believe how fast my vision was detriorating as by the time the glasses were ready, they had to be redone. She asked if I was on any new medications and this was when I finally figured it out. It left me with permanent eye sight damage months later! To compound, it has the effect of your handwriting getting smaller and smaller until you can't write! Watch out for this stuff as if you're depressed, it could cause you to totally lose your mind when you can't see or write to compound your situation!Read More Read Less
The side effects were that my vision was so blurry that when I went to an optomitrist to she couldn't believe how fast my vision was detriorating as by the time the glasses were ready, they had to be redone. She asked if I was on any new medications and this was when I finally figured it out. It left me with permanent eye sight damage months later! To compound, it has the effect of your handwriting getting smaller and smaller until you can't write! Watch out for this stuff as if you're depressed, it could cause you to totally lose your mind when you can't see or write to compound your situation!Read More Read Less
Report this post
Fill 3Created with Sketch.Condition: Other
Overall rating 4.0
EffectivenessEase of UseSatisfactionI have a difficult time getting to sleep and I wake several times through the night. This medication has helped my condition. I used to always feel tired even if I'd been in bed for 12hrs. Now I can wake up and feel rested after about 8hrs. This medication is strong and I take half the dose prescribed otherwise I get the shakes and feel itchy all over.
This medication has helped my condition. I used to always feel tired even if I'd been in bed for 12hrs. Now I can wake up and feel rested after about 8hrs. This medication is strong and I take half the dose prescribed otherwise I get the shakes and feel itchy all over.
Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 4.0
EffectivenessEase of UseSatisfactionAfter many years of trial and error taking every new drug and having been hospitalized many times because of the side effects. Ive been using sasphris for about 4 years. If i miss adose psychotic mania looms ahead. I do get some very bothersome side effects. Facils ticks,grinding my teeth. Pacing pacing this one bothers everyone a great deal.i take klonipin to help the akathesia but if i take enough "Kpin" to even me out it causes such bad amnesia. But still a great drug for me
Pacing pacing this one bothers everyone a great deal.i take klonipin to help the akathesia but if i take enough "Kpin" to even me out it causes such bad amnesia. But still a great drug for me
3
ShapeCreated with Sketch.1
thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Schizophrenia
Overall rating 5.0
EffectivenessEase of UseSatisfactionI herd voice and seen things scene I was a kid and now with this I have a new live. I have put on weight but this seems to be the only thing that has taken the voice and seeing things away. I take the black cherry in the morning and then at dinner. I'm able to do more then I ever have and my friends notice a difference in me and it feels so good. I'm even able to listen to music again and TV or movies. So the weight gain something I'm learning to deal with and eat better meals. I can sleep also which is a big change. Now I'm getting about 7 to 8 hours of sleep and waking up rested. So I feel this med is will though the try!!!!!!!!! Cj Read More Read Less
I'm even able to listen to music again and TV or movies. So the weight gain something I'm learning to deal with and eat better meals. I can sleep also which is a big change. Now I'm getting about 7 to 8 hours of sleep and waking up rested. So I feel this med is will though the try!!!!!!!!! Cj Read More Read Less
1
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Mania associated with Bipolar Disorder
Overall rating 3.3
EffectivenessEase of UseSatisfactionweight gain, feeling like I am dragging around a 1000 lb weight. No motivation. Worked great the first week, then began to feel as if I was in a dream. Like i wasn't really here. I swear I began to have hallucinations.
Report this post
Fill 3Created with Sketch.Condition: Other
Overall rating 1.7
EffectivenessEase of UseSatisfactionI had a terrible reaction to this drug almost immediately. I couldn't stand up without passing out within 2-3 minutes, consistently. I remember thinking to myself "if this is what everyday felt like, I'd rather be dead." I had to go to the ER, as it dumped my blood pressure way below my normal, which is already low.
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.Condition: Bipolar I Disorder with Most Recent Episode Mixed
Overall rating 1. 7
7
I was on this medicine for 1 week and ended up very ill. I had diarrhea and vomiting so bad that I lost 10 pounds in three days. I would not recommend this medicine at all. My tongue was numb for about 20 minutes and had a stomach ache for the first three days. I ended up in the hospital and missed 2 days of work!
4
ShapeCreated with Sketch.thumb_up copy 5Created with Sketch.Report this post
Fill 3Created with Sketch.IMPORTANT INFORMATION ABOUT USER-GENERATED CONTENT ON WEBMD
The opinions expressed in WebMD User-generated content areas like communities, reviews, ratings, or blogs are solely those of the User, who may or may not have medical or scientific training. These opinions do not represent the opinions of WebMD. User-generated content areas are not reviewed by a WebMD physician or any member of the WebMD editorial staff for accuracy, balance, objectivity, or any other reason except for compliance with our Terms and Conditions.
Read More
SAPHRIS® (asenapine) Dosing & Administration for Adult Bipolar Mania
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SAPHRIS is not approved for treatment of patients with dementia-related psychosis.
Contraindications: SAPHRIS is contraindicated in patients with severe hepatic impairment (Child-Pugh C) or known hypersensitivity to SAPHRIS or its formulation components. Reactions have included anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash.
Cerebrovascular Adverse Events, Including Stroke: In clinical trials with antipsychotic drugs, elderly subjects with dementia
had a higher incidence of stroke (including fatal stroke) and transient ischemic attack vs placebo. SAPHRIS is not approved for the treatment
of patients with dementia-related psychosis.
SAPHRIS is not approved for the treatment
of patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome (NMS): NMS, a potentially fatal symptom complex, has been reported with antipsychotics. NMS may cause hyperpyrexia, muscle rigidity, delirium, and autonomic instability. If NMS is suspected, immediately discontinue SAPHRIS and provide intensive symptomatic treatment and monitoring.
Tardive Dyskinesia (TD): Risk of developing TD (a syndrome of potentially irreversible, involuntary dyskinetic movements) and the
likelihood it will become irreversible increase as the duration of treatment and total cumulative dose of antipsychotic drugs, including SAPHRIS,
given to the patient increase. The syndrome can develop, after relatively brief treatment periods, even at low doses, and may also occur after
discontinuation of treatment. Prescribe SAPHRIS in a manner most likely to minimize TD. If signs and symptoms of TD appear, drug discontinuation
should be considered.
If signs and symptoms of TD appear, drug discontinuation
should be considered.
Metabolic Changes: Atypical antipsychotics, including SAHPRIS, have caused metabolic changes, including the following:
- Hyperglycemia and Diabetes Mellitus: Hyperglycemia, in some cases associated with ketoacidosis, hyperosmolar coma, or death, has been reported in patients treated with atypical antipsychotics. Hyperglycemia has been reported with SAPHRIS. Assess fasting plasma glucose before or soon after initiation of treatment, and monitor periodically during long-term treatment.
-
Dyslipidemia: Atypical antipsychotics cause adverse alterations in lipids have been observed in patients treated
with atypical antipsychotics. Before or soon after initiation of antipsychotics, obtain a baseline fasting lipid profile and monitor
periodically during treatment.

- Weight Gain: Weight gain has been observed with atypical antipsychotics, including SAPHRIS. Monitor weight at baseline and frequently thereafter.
Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing, and rash, have been observed in patients treated with SAPHRIS. In several cases, these reactions occurred after the first dose.
Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects: SAPHRIS has caused orthostatic hypotension and syncope. Generally
the risk is greatest during initial titration and when increasing the dose. Monitor orthostatic vital signs and monitor patients who are vulnerable to
hypotension (elderly patients, patients with dehydration, hypovolemia, concomitant treatment with antihypertensive medications, patients with
cardiovascular and/or cerebrovascular disease). Use SAPHRIS cautiously with other drugs that can cause hypotension, bradycardia, or respiratory or
central nervous system depression. Monitor orthostatic vital signs, and consider a dose reduction if hypotension occurs.
Use SAPHRIS cautiously with other drugs that can cause hypotension, bradycardia, or respiratory or
central nervous system depression. Monitor orthostatic vital signs, and consider a dose reduction if hypotension occurs.
Falls: SAPHRIS may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures, or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotics and recurrently for patients on long-term therapy.
Leukopenia, Neutropenia, and Agranulocytosis: : Leukopenia/neutropenia have been reported with antipsychotics, including SAPHRIS.
Agranulocytosis (including fatal cases) has been reported with other antipsychotics. Monitor complete blood count in patients with pre-existing low
white blood cell count (WBC) or absolute neutrophil count or history of drug-induced leukopenia or neutropenia. Discontinue SAPHRIS at the first sign
of a clinically significant decline in WBC and in severely neutropenic patients.
Discontinue SAPHRIS at the first sign
of a clinically significant decline in WBC and in severely neutropenic patients.
QT Prolongation: In an adult QT study, SAPHRIS was associated with increases in the QTc interval from 2 to 5 msec vs placebo. No SAPHRIS patients had QTc increases of ≥60 msec or a QTc of ≥500 msec. Avoid SAPHRIS in combination with other drugs known to prolong QTc interval, in patients with congenital prolongation of QT interval or a history of cardiac arrhythmias, and in circumstances that may increase occurrence of torsades de pointes and/or sudden death in association with the use of drugs that prolong QTc interval.
Hyperprolactinemia: Like other drugs that antagonize dopamine D2 receptors, SAPHRIS can elevate prolactin levels and the elevation can
persist during chronic administration. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating
compounds.
Seizures: Use SAPHRIS with caution in patients with history of seizures or with conditions that lower the seizure threshold.
Potential for Cognitive and Motor Impairment: Somnolence was reported with SAPHRIS. Caution patients about performing activities requiring mental alertness (eg, operating hazardous machinery or a motor vehicle).
Body Temperature Regulation: Appropriate care is advised when using SAPHRIS in patients who will experience conditions that increase body temperature, eg, exercising strenuously, extreme heat, concomitant anticholinergics, or dehydration.
Dysphagia: Esophageal dysmotility and aspiration have been associated with antipsychotics. Dysphagia has been reported with SAPHRIS. Use SAPHRIS cautiously in patients at risk for dysphagia.
Drug Interactions: Monitor blood pressure and adjust antihypertensive drugs when taken with SAPHRIS. Based on clinical response,
SAPHRIS dose reduction may be necessary when used with strong CYP1A2 inhibitors (fluvoxamine). Reduce paroxetine (CYP2D6 substrate and inhibitor)
dose by half when taken with SAPHRIS.
Based on clinical response,
SAPHRIS dose reduction may be necessary when used with strong CYP1A2 inhibitors (fluvoxamine). Reduce paroxetine (CYP2D6 substrate and inhibitor)
dose by half when taken with SAPHRIS.
Pregnancy: Advise patients to notify their healthcare provider of a known or suspected pregnancy. SAPHRIS may cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. Based on animal data, SAPHRIS may cause fetal harm. The National Pregnancy Registry for Atypical Antipsychotics monitors pregnancy outcomes in women exposed to antipsychotics, including SAPHRIS, during pregnancy. For information, contact 1-866-961-2388 or http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Adverse Reactions: In clinical trials, the most commonly observed adverse reactions in adults
treated with SAPHRIS (5 and 10 mg BID) or pediatric patients (ages 10-17) treated with SAPHRIS (2. 5, 5, and 10 mg BID) vs placebo were:
5, 5, and 10 mg BID) vs placebo were:
- Adult schizophrenia: somnolence (13% vs 7%), akathisia (6% vs 3%), and oral hypoesthesia (5% vs 1%)
- Adult bipolar I (monotherapy): somnolence (23% vs 5%), oral hypoesthesia (10% vs 1%), dizziness (8% vs 4%), extrapyramidal symptoms excluding akathisia (8% vs 4%), and akathisia (6% vs 2%)
- Adult bipolar I (adjunctive): somnolence (22% vs 10%) and oral hypoesthesia (5% vs 0%)
- Pediatric bipolar I (monotherapy): somnolence (49% vs 12%), oral hypoesthesia (27% vs 4%), fatigue (9% vs 5%), increased appetite (8% vs 2%), dizziness (7% vs 3%), dysgeusia (6% vs 2%), nausea (6% vs 3%), and increased weight (3% vs 0%)
Postmarketing Experience: Application site reactions, primarily sublingual, have been reported (eg, oral ulcers, blisters,
peeling/sloughing, and inflammation). Choking has been reported, sometimes associated with oropharyngeal muscular dysfunction or hypoesthesia.
Choking has been reported, sometimes associated with oropharyngeal muscular dysfunction or hypoesthesia.
Indications and Usage
In bipolar I disorder: SAPHRIS (asenapine) is indicated for the acute treatment of manic or mixed episodes of bipolar I disorder as monotherapy in adults or pediatric patients (ages 10-17) or as adjunctive therapy with either lithium or valproate in adults.
In schizophrenia: SAPHRIS is indicated for the acute and maintenance treatment of schizophrenia in adults.
Please also see the full Prescribing Information, including Boxed Warning.
1. SAPHRIS (asenapine) Prescribing Information. Irvine, CA: Allergan USA, Inc.
2. Findling RL, Landbloom RL, Szegedi A, Koppenhaver J, Braat S, Zhu Q, et al. J Am Acad Child
Adolesc Psychiatry.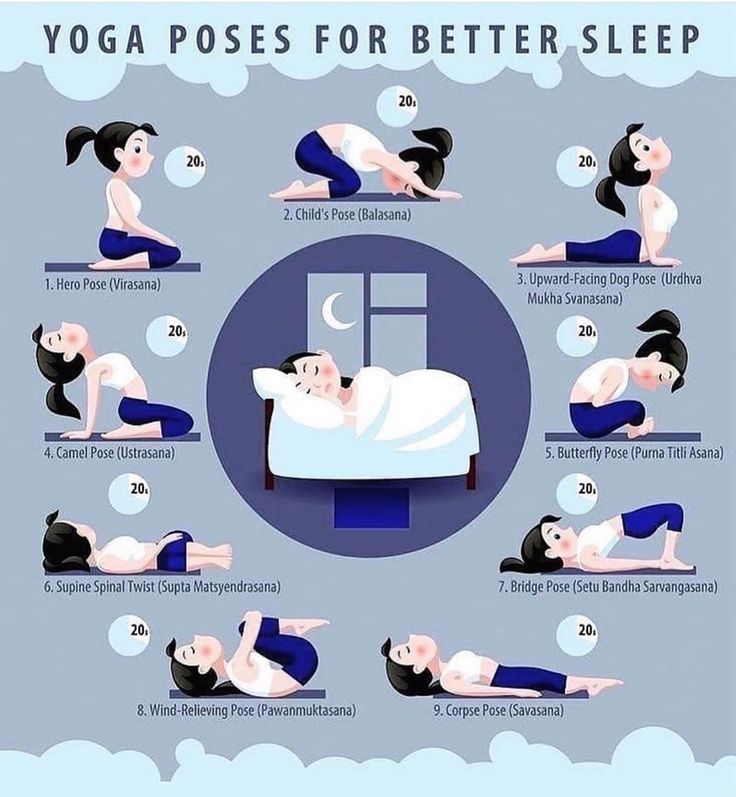 2015;54:1032-1041 [reprint]. 3.
Data on file. Allergan. 4. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale
for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. 5. Spearing
MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) scale for use
in bipolar illness (BP): the CGI-BP. Psych Res. 1997;73:159-171. 6. American Psychiatric
Association. Practice Guideline for the Treatment of Patients with Bipolar Disorder. Second Edition. Washington,
DC: American Psychiatric Association; 2002.
2015;54:1032-1041 [reprint]. 3.
Data on file. Allergan. 4. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale
for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. 5. Spearing
MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) scale for use
in bipolar illness (BP): the CGI-BP. Psych Res. 1997;73:159-171. 6. American Psychiatric
Association. Practice Guideline for the Treatment of Patients with Bipolar Disorder. Second Edition. Washington,
DC: American Psychiatric Association; 2002.
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an
increased risk of death. SAPHRIS is not approved for treatment of patients with dementia-related
psychosis.
SAPHRIS is not approved for treatment of patients with dementia-related
psychosis.
Contraindications: SAPHRIS is contraindicated in patients with severe hepatic impairment (Child-Pugh C) or known hypersensitivity to SAPHRIS or its formulation components. Reactions have included anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash.
Cerebrovascular Adverse Events, Including Stroke: In clinical trials with antipsychotic drugs, elderly subjects with dementia had a higher incidence of stroke (including fatal stroke) and transient ischemic attack vs placebo. SAPHRIS is not approved for the treatment of patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome (NMS): NMS, a potentially fatal symptom complex, has been reported with antipsychotics. NMS may
cause hyperpyrexia, muscle rigidity, delirium, and autonomic instability. If NMS is suspected, immediately discontinue SAPHRIS and provide
intensive symptomatic treatment and monitoring.
If NMS is suspected, immediately discontinue SAPHRIS and provide
intensive symptomatic treatment and monitoring.
Tardive Dyskinesia (TD): Risk of developing TD (a syndrome of potentially irreversible, involuntary dyskinetic movements) and the likelihood it will become irreversible increase as the duration of treatment and total cumulative dose of antipsychotic drugs, including SAPHRIS, given to the patient increase. The syndrome can develop, after relatively brief treatment periods, even at low doses, and may also occur after discontinuation of treatment. Prescribe SAPHRIS in a manner most likely to minimize TD. If signs and symptoms of TD appear, drug discontinuation should be considered.
Metabolic Changes: Atypical antipsychotics, including SAHPRIS, have caused metabolic changes, including the following:
-
Hyperglycemia and Diabetes Mellitus: Hyperglycemia, in some cases associated with ketoacidosis, hyperosmolar coma,
or death, has been reported in patients treated with atypical antipsychotics.
 Hyperglycemia has been reported with SAPHRIS. Assess
fasting plasma glucose before or soon after initiation of treatment, and monitor periodically during long-term treatment.
Hyperglycemia has been reported with SAPHRIS. Assess
fasting plasma glucose before or soon after initiation of treatment, and monitor periodically during long-term treatment.
- Dyslipidemia: Atypical antipsychotics cause adverse alterations in lipids have been observed in patients treated with atypical antipsychotics. Before or soon after initiation of antipsychotics, obtain a baseline fasting lipid profile and monitor periodically during treatment.
- Weight Gain: Weight gain has been observed with atypical antipsychotics, including SAPHRIS. Monitor weight at baseline and frequently thereafter.
Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue,
dyspnea, wheezing, and rash, have been observed in patients treated with SAPHRIS. In several cases, these reactions occurred after the first dose.
In several cases, these reactions occurred after the first dose.
Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects: SAPHRIS has caused orthostatic hypotension and syncope. Generally the risk is greatest during initial titration and when increasing the dose. Monitor orthostatic vital signs and monitor patients who are vulnerable to hypotension (elderly patients, patients with dehydration, hypovolemia, concomitant treatment with antihypertensive medications, patients with cardiovascular and/or cerebrovascular disease). Use SAPHRIS cautiously with other drugs that can cause hypotension, bradycardia, or respiratory or central nervous system depression. Monitor orthostatic vital signs, and consider a dose reduction if hypotension occurs.
Falls: SAPHRIS may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently,
fractures, or other injuries.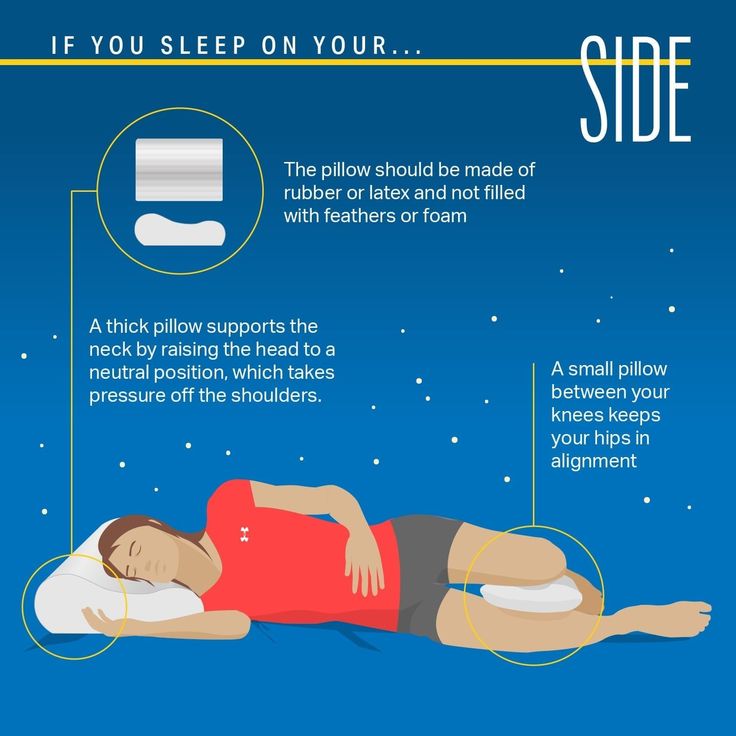 For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk
assessments when initiating antipsychotics and recurrently for patients on long-term therapy.
For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk
assessments when initiating antipsychotics and recurrently for patients on long-term therapy.
Leukopenia, Neutropenia, and Agranulocytosis: : Leukopenia/neutropenia have been reported with antipsychotics, including SAPHRIS. Agranulocytosis (including fatal cases) has been reported with other antipsychotics. Monitor complete blood count in patients with pre-existing low white blood cell count (WBC) or absolute neutrophil count or history of drug-induced leukopenia or neutropenia. Discontinue SAPHRIS at the first sign of a clinically significant decline in WBC and in severely neutropenic patients.
QT Prolongation: In an adult QT study, SAPHRIS was associated with increases in the QTc interval from 2 to 5 msec vs placebo. No SAPHRIS
patients had QTc increases of ≥60 msec or a QTc of ≥500 msec. Avoid SAPHRIS in combination with other drugs known to prolong QTc interval, in patients with
congenital prolongation of QT interval or a history of cardiac arrhythmias, and in circumstances that may increase occurrence of torsades de pointes and/or
sudden death in association with the use of drugs that prolong QTc interval.
Avoid SAPHRIS in combination with other drugs known to prolong QTc interval, in patients with
congenital prolongation of QT interval or a history of cardiac arrhythmias, and in circumstances that may increase occurrence of torsades de pointes and/or
sudden death in association with the use of drugs that prolong QTc interval.
Hyperprolactinemia: Like other drugs that antagonize dopamine D2 receptors, SAPHRIS can elevate prolactin levels and the elevation can persist during chronic administration. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds.
Seizures: Use SAPHRIS with caution in patients with history of seizures or with conditions that lower the seizure threshold.
Potential for Cognitive and Motor Impairment: Somnolence was reported with SAPHRIS. Caution patients about performing activities requiring
mental alertness (eg, operating hazardous machinery or a motor vehicle).
Caution patients about performing activities requiring
mental alertness (eg, operating hazardous machinery or a motor vehicle).
Body Temperature Regulation: Appropriate care is advised when using SAPHRIS in patients who will experience conditions that increase body temperature, eg, exercising strenuously, extreme heat, concomitant anticholinergics, or dehydration.
Dysphagia: Esophageal dysmotility and aspiration have been associated with antipsychotics. Dysphagia has been reported with SAPHRIS. Use SAPHRIS cautiously in patients at risk for dysphagia.
Drug Interactions: Monitor blood pressure and adjust antihypertensive drugs when taken with SAPHRIS. Based on clinical response,
SAPHRIS dose reduction may be necessary when used with strong CYP1A2 inhibitors (fluvoxamine). Reduce paroxetine (CYP2D6 substrate and inhibitor)
dose by half when taken with SAPHRIS.
Pregnancy: Advise patients to notify their healthcare provider of a known or suspected pregnancy. SAPHRIS may cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. Based on animal data, SAPHRIS may cause fetal harm. The National Pregnancy Registry for Atypical Antipsychotics monitors pregnancy outcomes in women exposed to antipsychotics, including SAPHRIS, during pregnancy. For information, contact 1-866-961-2388 or http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Adverse Reactions: In clinical trials, the most commonly observed adverse reactions in adults treated with SAPHRIS (5 and 10 mg BID) or pediatric patients (ages 10-17) treated with SAPHRIS (2.5, 5, and 10 mg BID) vs placebo were:
- Adult schizophrenia: somnolence (13% vs 7%), akathisia (6% vs 3%), and oral hypoesthesia (5% vs 1%)
- Adult bipolar I (monotherapy): somnolence (23% vs 5%), oral hypoesthesia (10% vs 1%), dizziness (8% vs 4%), extrapyramidal symptoms excluding akathisia (8% vs 4%), and akathisia (6% vs 2%)
- Adult bipolar I (adjunctive): somnolence (22% vs 10%) and oral hypoesthesia (5% vs 0%)
- Pediatric bipolar I (monotherapy): somnolence (49% vs 12%), oral hypoesthesia (27% vs 4%), fatigue (9% vs 5%), increased appetite (8% vs 2%), dizziness (7% vs 3%), dysgeusia (6% vs 2%), nausea (6% vs 3%), and increased weight (3% vs 0%)
Postmarketing Experience: Application site reactions, primarily sublingual, have been reported (eg, oral ulcers, blisters,
peeling/sloughing, and inflammation). Choking has been reported, sometimes associated with oropharyngeal muscular dysfunction or hypoesthesia.
Choking has been reported, sometimes associated with oropharyngeal muscular dysfunction or hypoesthesia.
Indications and Usage
In bipolar I disorder: SAPHRIS (asenapine) is indicated for the acute treatment of manic or mixed episodes of bipolar I disorder as monotherapy in adults or pediatric patients (ages 10-17) or as adjunctive therapy with either lithium or valproate in adults.
In schizophrenia: SAPHRIS is indicated for the acute and maintenance treatment of schizophrenia in adults.
Please also see the full Prescribing Information, including Boxed Warning.
For healthcare professionals licensed in Vermont or licensed healthcare
professionals who regularly practice in Vermont: Allergan corporate policy prohibits
the downloading, printing and/or acceptance of copay cards by healthcare professionals licensed
in Vermont, or licensed healthcare professionals who regularly practice in Vermont.
By clicking "Confirm" below you confirm that you are neither a healthcare professional licensed in Vermont, nor a licensed healthcare professional who regularly practices in Vermont.
Scale ranges from 0 (no manic symptoms) to 60 (maximum score) and includes the following symptoms:
- Elevated mood
- Increased motor activity-energy
- Sexual interest
- Sleep
- Irritability
- Speech
- Language-thought disorder
- Content
- Disruptive-aggressive behavior
- Appearance
- Insight
The efficacy of SAPHRIS was based on the YMRS total score, not on any individual component of the scale.
Total PANSS scores can range from 30 to 210.
7 positive symptoms
- delusions
- conceptual disorganization
- hallucinatory behavior
- excitement
- grandiosity
- suspiciousness
- hostility
7 negative symptoms
- blunted affect
- emotional withdrawal
- poor rapport
- passive-apathetic social withdrawal
- difficulty in abstract thinking
- lack of spontaneity & flow of conversation
- stereotyped thinking
16 cognitive or general psychopathology symptoms
- somatic concern
- anxiety
- guilt feelings
- tension
- mannerisms & posturing
- depression
- motor retardation
- uncooperativeness
- unusual thought content
- disorientation
- poor attention
- lack of judgment & insight
- disturbance of volition
- poor impulse control
- preoccupation
- active social avoidance
The efficacy of SAPHRIS was based on the PANSS total score,
not on any individual component of the scale.
| 7 positive symptoms | 7 negative symptoms | 16 cognitive or general psychopathology symptoms |
|---|---|---|
|
|
|
|
The efficacy of SAPHRIS was based on the PANSS total score, not on any individual component of the scale. |
Melipramine | Dictionary of a psychiatrist-narcologist│Medicines
The drug is known as an antidepressant, which is widely used in psychiatry and psychotherapy to correct the emotional state. The effect of the drug does not appear immediately, but as a result of long-term use for a period of 2 to 8 weeks. Due to this mild effect, the drug is used in the treatment of mental disorders in children.
Indications for the use of melipramine
Antidepressants should only be taken on prescription. With some diagnoses, during the treatment course with melipramine, the patient must be under round-the-clock supervision of medical personnel. Most often, the drug is prescribed for mental disorders of the psyche, such as:
- all types of depression
- panic disorder
- Sleep incontinence in children (over 6 years)
The dosage is determined strictly individually. The age of the patient must be taken into account. Doctors are especially careful in calculating the daily dose in relation to patients of senile and minor age. On average, the initial dose is 25-75 g per day, subsequently it should not exceed 300 mg per day. The drug is recommended to be taken once after meals and before going to bed. Cancel the drug gradually. The duration of the course should not exceed 3 months.
The age of the patient must be taken into account. Doctors are especially careful in calculating the daily dose in relation to patients of senile and minor age. On average, the initial dose is 25-75 g per day, subsequently it should not exceed 300 mg per day. The drug is recommended to be taken once after meals and before going to bed. Cancel the drug gradually. The duration of the course should not exceed 3 months.
Contraindications to the use of melipramine
During pregnancy and lactation, treatment with melipramine is prohibited, since there is a possibility of effects on the fetus. The use of the drug should be prescribed exclusively according to the available indications. The drug should not be used in the presence of the following contraindications:
- hypersensitivity to the components of melipramine
- use of MAO inhibitors
- cardiac disorders
- heart attack
- diseases of the kidneys and liver
- urinary retention
- angle-closure glaucoma
- lactose intolerance
- under 6 years old
Before prescribing melipramine, the doctor carefully reviews the patient's medical history. In the first weeks of treatment with antidepressants, an increase in suicidal tendencies was noted, which are eliminated when remission is achieved. Maintenance therapy with a drug should not exceed six months.
In the first weeks of treatment with antidepressants, an increase in suicidal tendencies was noted, which are eliminated when remission is achieved. Maintenance therapy with a drug should not exceed six months.
Side effects
Not all patients have side effects. Such processes can be affected by the prescribed dosage. In this case, the doctor either cancels the remedy or reduces the dose. If the patient, as a result of taking melipramine, has complex mental and neurological reactions, treatment should be temporarily suspended. Among the most common side effects, physicians distinguish:
- tachycardia
- ECG changes
- arrhythmia
- hot flashes
- paresthesia
- tremor
- confusion
- dizziness
- headache
- disorientation
- hallucinations
- anxiety
- weakness
- sleep disorders
- libido disorders
- fatigue
- agitation
- anxiety
- alarm
- vomiting
- dry mouth
- constipation
- increased sweating
- weight gain
- anorexia
- skin rash
There are cases of overdose, which is expressed by an increase in side effects.