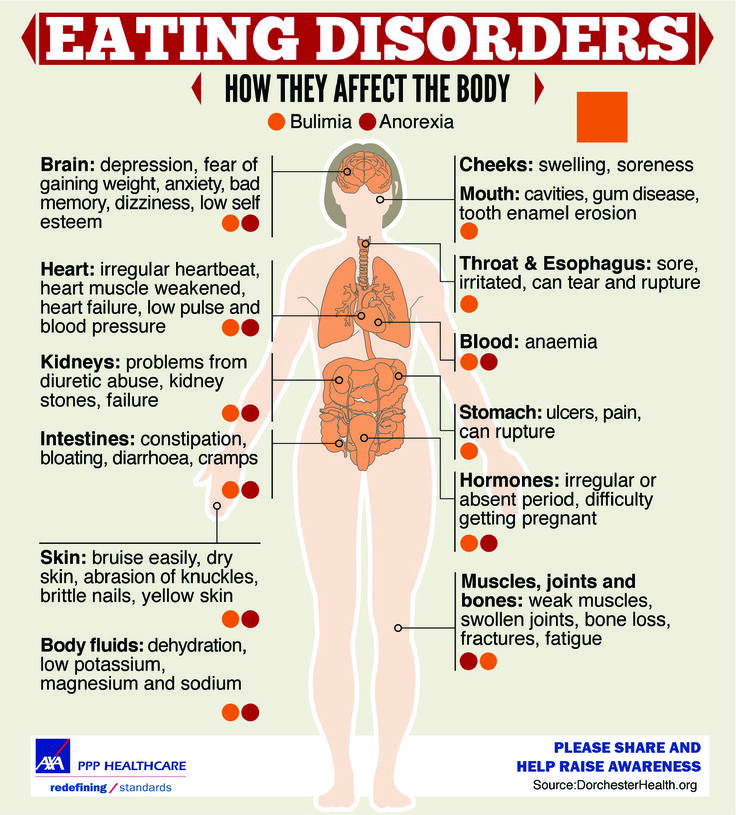
Can guaifenesin cause heart palpitations
Side Effects, Dosage, Precautions, Uses
1. Is Guaifenesin safe?
Yes, Guaifenesin is generally a safe oral expectorant. It is considered the first line of treatment for cough, as the side effects are very rare. No severe allergic reaction has been seen with the use of Guaifenesin if taken under the recommended dosage.
2. Is Guaifenesin safe for heart patients?
Guaifenesin is generally safe for heart patients. This drug is not known to elevate blood pressure. If an overdose of Guaifenesin is taken, it may cause severe effects on the heart and increase heart rate. For heart patients or any previous history of cardiac illness, take the drug under medical supervision.
3. Does Guaifenesin make you cough more?
Guaifenesin will not cause severe and frequent coughing but can cause your cough to be productive (cough with phlegm or mucus). The drug helps to thin the secretion from your lungs and loosen the mucus. This effect can result in a productive cough that aims to clear excessive secretions from the airways.
4. Does Guaifenesin keep you awake?
No, Guaifenesin usually does not keep you awake. But in some individuals, it is reported that it is harder for them to sleep after intake of Guaifenesin, which is not a common side effect of this drug. If you experience this, you are advised to take the recommended dose in the morning and early afternoon. Never take the dose too close to bedtime.
5. Is Guaifenesin safe for pregnancy?
Guaifenesin can be used during pregnancy if your doctor recommends it. But it should be avoided during the first trimester. Experts highly recommend that Guaifenesin formulation be alcohol-free and not be used too frequently by pregnant women.
6. When to take Guaifenesin for fertility?
The scientific study on Guaifenesin for fertility needs more validation. It is not clear that thinning of cervical mucus alone can make you fertile. So based on the theory, if you want to try Guaifenesin for fertility, you may start taking it just before you are due to ovulate. Get in touch with our medical experts at Yashoda Hospitals to learn more about Guaifenesin and fertility.
Get in touch with our medical experts at Yashoda Hospitals to learn more about Guaifenesin and fertility.
7. Who should not take Guaifenesin?
- Children under the age of 4
- People with persistent chronic cough due to tobacco smoking, asthma or emphysema
- People with chronic bronchitis or significant respiratory depression
- Guaifenesin can alter the urine laboratory test; discontinue its intake at least 48 hours before the collection of urine
- If you are pregnant or breastfeeding, you should talk to your doctor about whether or not to take Guaifenesin
8. Is it okay to take Guaifenesin every day?
Cough medicines are usually taken for a short time until your symptoms clear up. In patients with chronic respiratory symptoms or chronic bronchitis, Guaifenesin is advisable for long term use in a recommended dosage. This medicine has contributed to a rapid improvement in the symptoms. However, always seek advice from a physician for long-term use. Regular visits to your doctor are necessary to screen for side effects.
Regular visits to your doctor are necessary to screen for side effects.
9. Does Guaifenesin dry up mucus?
No, Guaifenesin does not dry up mucus; it only loosens or thins the mucus already present in the respiratory tract. It increases the volume of bronchial secretions and decreases the mucus viscosity. So this makes it easier to expel mucus or phlegm by coughing.
10. Is Guaifenesin bad for kidneys?
Guaifenesin is generally a safe cough medication to take in patients with kidney disease. However, it is essential to remember that you must take only the recommended dosage per day. If you are suffering from existing renal impairments, it is always advisable to seek a doctor's suggestion.
Common and Rare Side Effects for Guaifenesin DAC
COMMON side effects
If experienced, these tend to have a Severe expression i
Sorry, we have no data available. Please contact your doctor or pharmacist.
If experienced, these tend to have a Less Severe expression i
INFREQUENT side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
RARE side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
Full Drug Information
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Related Links
Drug Survey
Yes, In the past 3 months
Yes, In the past 6 months
Yes, In the past year
Haven't purchased but considering
Don't plan to purchase
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
Today on WebMD
instructions for use, home delivery
Description
Round, flat-cylindrical tablets, white or almost white, with a bevel and one-sided notch.
Active substances
Bromhexine + Gweifenzin + Salbutamol
Form of release
tablets
BROMGEKB drug contains:
Acting substances are Bromhekenzin, Habeaphenin and Salbutamol. nine0005
Each tablet contains 8 mg bromhexine (as hydrochloride), 100 mg guaifenesin, 2.41 mg salbutamol (as sulfate), corresponding to 2 mg salbutamol.
The other ingredients (auxiliaries) are calcium hydrogen phosphate, corn starch, colloidal silicon dioxide, magnesium stearate, talc.
Pharmacological effect
Combined expectorant
BromgeComb is a combined preparation containing fixed doses of bromhexine, guaifenesin and salbutamol. nine0005
nine0005
The action of the drug BromgeComb is due to the effects of its components: salbutamol expands the bronchi and facilitates breathing, bromhexine and guaifenesin contribute to liquefaction of sputum and facilitate its expectoration.
Indications
BromgeComb is used in adults and children over 6 years of age for the symptomatic treatment of productive (wet) cough associated with various diseases of the respiratory system, including:
If there is no improvement or if you feel worse after 4-5 days, you should consult a doctor.
Contraindications
Do not take BromgeComb:
Children and adolescents
BromgeComb is not intended for use in children under 6 years of age.
Precautions
Talk to your doctor or pharmacist before taking BromgeComb, especially if you have: . You need to tell your doctor if you develop chest pain or shortness of breath while taking BromgeComb, as these may be signs of myocardial ischemia (insufficient supply of oxygen to the heart muscle) associated with salbutamol,
Use in pregnancy and lactation
If you are pregnant or breastfeeding, think you may become pregnant or plan to become pregnant, consult your doctor before using this medicine.
BromgeComb is contraindicated during pregnancy and during breastfeeding. nine0005
Dosage and Administration
Always take your medicine exactly as directed in this package leaflet or as advised by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist for advice.
If in doubt, ask your doctor or pharmacist for advice.
Recommended adult dose
1 tablet 3 times a day.
Recommended doses for children and adolescents
Children over 12 years old - 1 tablet 3 times a day.
Children aged 6 to 12 years - 1/2 or 1 tablet 3 times a day. nine0005
Route and/or route of administration
For oral administration.
Duration of therapy
Do not take the drug for more than 4-5 days.
If you forget to take a tablet of BromgeComb
If you forget to take a dose of the drug, take it as soon as you remember. However, if it is almost time for the next dose, skip the forgotten dose. Take your next dose at the usual time. Do not take a double dose to make up for a missed dose. nine0005
If you have any questions about the use of this medicine, ask your doctor or pharmacist.
Side effects
Like all medicines, BromgeComb can cause side effects, although not everyone gets them.
Serious adverse reactions
Stop taking the drug immediately and contact your doctor if you notice any of the following serious adverse reactions:
Very rare - may affect up to 1 in 10,000 people:
Unknown (frequency of occurrence cannot be estimated from the available data):
Tell your doctor if you notice any of the following adverse reactions:
Very common - may affect more than 1 in 10 people:

Common - may affect up to 1 in 10 people:
Rare - may affect up to 1 in 1,000 people:
Very rare - may affect up to 1 in 10,000 people:
Call your doctor right away if you have any side effects not listed in this package insert.
Reporting adverse reactions
If you experience any adverse reactions, talk to your doctor or pharmacist. This recommendation applies to any possible adverse reactions, including those not listed in this package insert. You can also report adverse reactions directly (see below). By reporting adverse reactions, you help to get more information about the safety of the drug.
Overdose
If you took more BromgeComb than you should have
If you (or your child) accidentally take more BromgeComb tablets than you should, contact your doctor immediately.
Overdose symptoms include:
Interactions with other drugs
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. nine0005
In particular:
diuretics, guanethidine, reserpine or methyldopa (to treat high blood pressure),
tricyclic antidepressants or monoamine oxidase inhibitors (to treat depression),
glucocorticosteroids,
if you require dental or surgical intervention),
drugs for the treatment of diabetes,
digoxin (for the treatment of heart disease),
beta-blockers such as propranolol,
methylxanthines such as theophylline (for the treatment of diseases of the respiratory system).
When taken simultaneously, Bromhexine, which is part of the drug BromgeComb, promotes the penetration of antibiotics (erythromycin, cephalexin, oxytetracycline) into the lung tissue.
BromgeComb should not be taken concomitantly with drugs that suppress coughs, as this makes it difficult to expectorate thin sputum, as well as with combination cold medicines. nine0005
nine0005
Some drugs (diuretics, digoxin, methylxanthines, glucocorticosteroids) may increase the risk of hypokalemia, a condition in which there is a low level of potassium in the blood (see sections 3 and 4). This condition can lead to cardiac arrhythmias. If you are taking any of these medicines, please consult your doctor before taking BromgeComb.
Special instructions
Very rare cases of severe skin reactions associated with the use of bromhexine have been reported. If you develop a skin rash (including sores on mucous membranes such as the mouth, throat, nose, eyes, or genital area), stop taking BromgeComb and contact your doctor immediately. nine0005
Guaifenesin can color urine pink and also distort laboratory tests (5-hydroxyindoleacetic and vanillylmandelic acids can be detected in urine).
Long-term use of high doses of BromgeComb may cause the formation of kidney stones.
Read this leaflet completely before taking this medicine because it contains important information for you.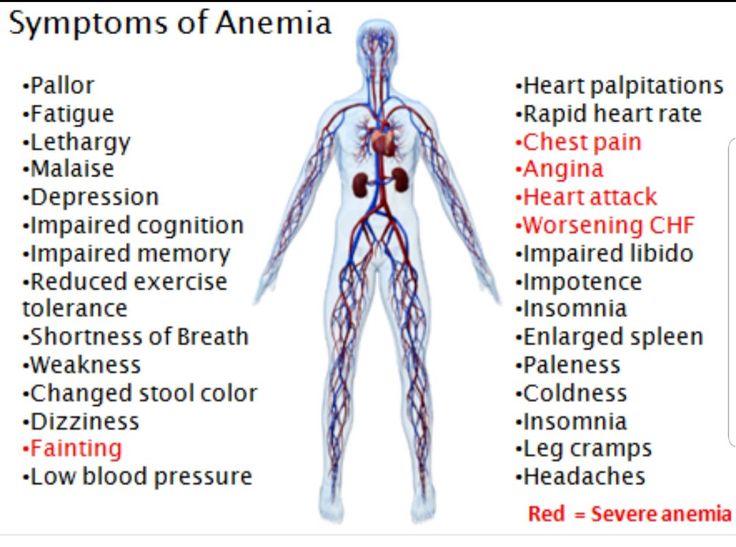
Keep the leaflet. You may need to read it again. nine0005
If you have any further questions, ask your doctor or pharmacist.
The drug has been prescribed specifically for you. Do not pass it on to other people. It can harm them even if their symptoms are the same as yours. If you experience any adverse reactions, contact your doctor or pharmacist. This recommendation applies to any possible adverse reactions, including those not listed in section 4 of the package insert. nine0005
Influence on the ability to drive vehicles. cf. and fur.:
Considering the possibility of developing adverse reactions during treatment with the drug (for example, dizziness, drowsiness and others), it is recommended to refrain from activities that require increased concentration of attention and speed of reactions, including driving vehicles and working with mechanisms.
Storage conditions
Keep the drug out of the reach of the child so that the child could not see it.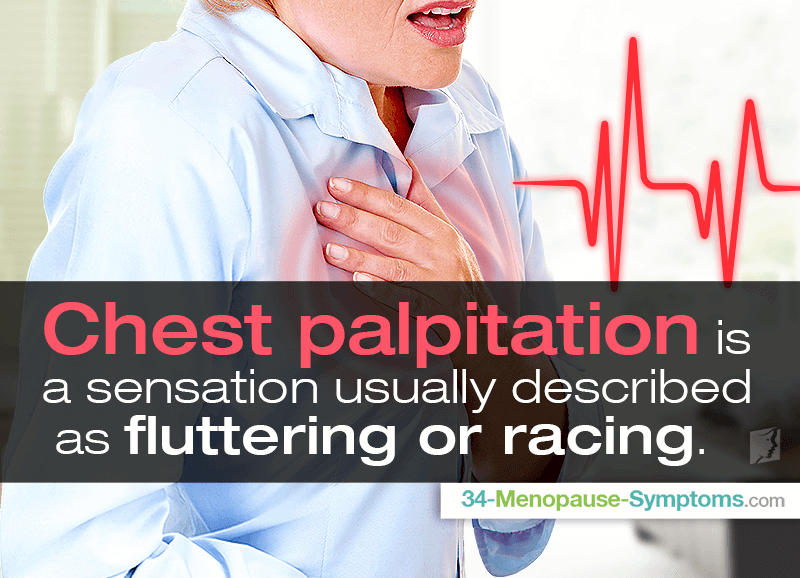 nine0005
nine0005
Store at temperatures below 25 °C in the original packaging (blister pack in a pack).
Prescription
Yes
Bromhexine + Guaifenesin + Salbutamol
Dosage form
Syrup
Composition per 1 ml:
Active ingredients:
- Salbutamol sulfate - 0.24 mg nine0049
- corresponds to salbutamol - 0.2 mg
- Bromhexine hydrochloride - 0.4 mg
- Guaifenesin - 10 mg
Excipients:
- Sucrose (sugar) - 500 mg
- Sorbitol (70% solution) - 263 mg
- Glycerol (glycerin) - 125 mg nine0049
- Propylene glycol - 62 mg
- Sodium benzoate - 2 mg
- Citric acid monohydrate - 2.
 4 mg
4 mg - Sorbic acid - 1 mg
- Dye sunset yellow (FCF) - 0.028 mg
- Levomenthol (menthol) - 0.1 mg
- Blackcurrant flavor - 3 mg nine0049
- Flavoring pineapple - 1 mg
- Purified water - up to 1 ml
Description
Transparent viscous orange liquid with a characteristic odor. Slight opalescence is allowed.
Pharmacotherapeutic group: combined expectorant.
ATX code nine0372 : R05C
Pharmacological properties
The combined drug has a bronchodilator, expectorant and mucolytic effect. Salbutamol is a bronchodilator that stimulates beta 2 - adrenoreceptors of the bronchi, blood vessels and myometrium. Prevents or eliminates bronchospasm, reduces resistance in the airways, increases the vital capacity of the lungs. Causes expansion of the coronary arteries, does not lower blood pressure. Bromhexine is a mucolytic agent. It has an expectorant effect, improves sputum discharge. Guaifenesin is a mucolytic agent that reduces the surface tension of the structures of the bronchopulmonary apparatus; stimulates the secretory cells of the bronchial mucosa that produce neutral polysaccharides, depolymerizes acid mucopolysaccharides, reduces the viscosity of sputum, facilitates the removal of sputum and promotes the transition of an unproductive cough into a productive one. nine0005
Prevents or eliminates bronchospasm, reduces resistance in the airways, increases the vital capacity of the lungs. Causes expansion of the coronary arteries, does not lower blood pressure. Bromhexine is a mucolytic agent. It has an expectorant effect, improves sputum discharge. Guaifenesin is a mucolytic agent that reduces the surface tension of the structures of the bronchopulmonary apparatus; stimulates the secretory cells of the bronchial mucosa that produce neutral polysaccharides, depolymerizes acid mucopolysaccharides, reduces the viscosity of sputum, facilitates the removal of sputum and promotes the transition of an unproductive cough into a productive one. nine0005
Pharmacokinetics
Salbugamol: when taken orally, absorption is high. Food intake reduces the rate of absorption, but does not affect bioavailability. Communication with plasma proteins - 10%. Penetrates through the placenta.
It undergoes first pass metabolism in the liver and in the intestinal wall, by means of phenol sulfotransferase it is inactivated to 4-o-sulfate ester.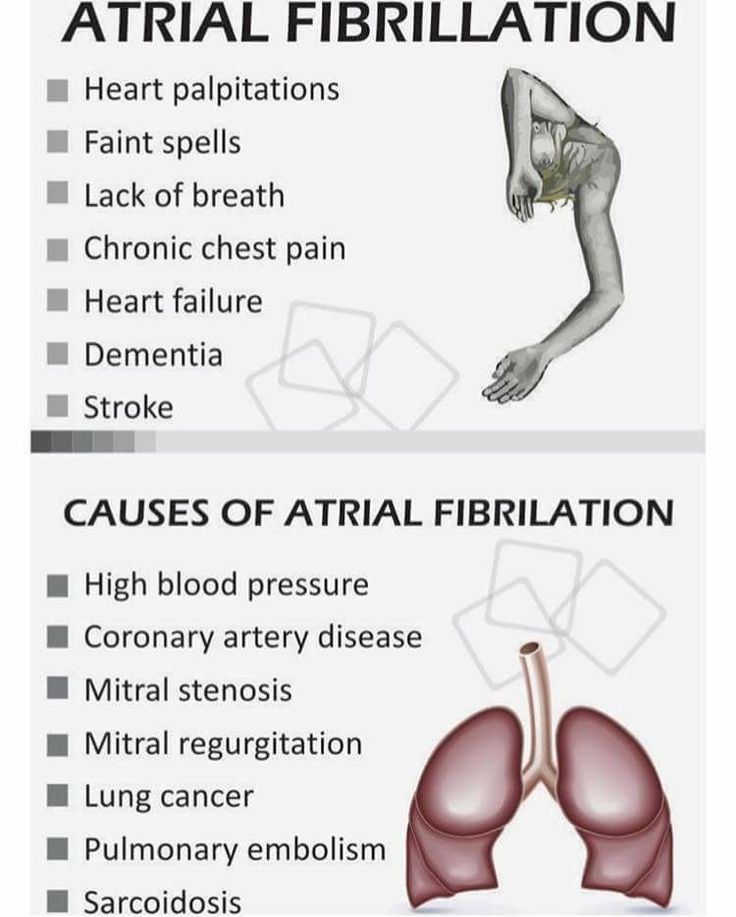 The half-life (T1 / 2) is 3.8-6 hours. It is excreted by the kidneys (69-90%), mainly as an inactive phenol sulfate metabolite (60%) for 72 hours and with bile (4%). The bioavailability of orally administered salbutamol is about 50%. nine0005
The half-life (T1 / 2) is 3.8-6 hours. It is excreted by the kidneys (69-90%), mainly as an inactive phenol sulfate metabolite (60%) for 72 hours and with bile (4%). The bioavailability of orally administered salbutamol is about 50%. nine0005
Bromhexine: when taken orally, it is almost completely (99%) absorbed in the gastrointestinal tract (GIT) within 30 minutes. Bioavailability is low (the effect of the primary "passage" through the liver). Penetrates through the placental and blood-brain barriers. In the liver, it undergoes demethylation and oxidation, and is metabolized to the pharmacologically active ambroxol. T1 / 2 - 15 hours (due to slow reverse diffusion from tissues). Excreted by the kidneys. In chronic renal failure, excretion of metabolites is impaired. With repeated use, it can accumulate. nine0005
Guaifenesin: absorption from the gastrointestinal tract is fast (25-30 minutes after ingestion). T1 / 2 - 1 hour Penetrates into tissues containing acid mucopolysaccharides.
Approximately 60% of the administered drug is metabolized in the liver. It is excreted by the lungs (with sputum) and by the kidneys both unchanged and in the form of inactive metabolites.
Indications for use
As part of the combined therapy of acute and chronic bronchopulmonary diseases, accompanied by the formation of a difficult-to-separate viscous secretion: nine0005
- bronchial asthma;
- tracheobronchitis;
- obstructive bronchitis;
- pneumonia;
- emphysema;
- whooping cough;
- pneumoconiosis;
- pulmonary tuberculosis, etc.
Contraindications nine0004
- hypersensitivity to the components of the drug;
- pregnancy, breastfeeding period;
- tachyarrhythmia, myocarditis;
- heart defects;
- decompensated diabetes mellitus;
- thyrotoxicosis;
- glaucoma;
- liver or kidney failure; nine0049
- peptic ulcer of the stomach and duodenum in the acute stage;
- children's age up to 2 years;
- sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption.
Carefully prescribed to patients with diabetes mellitus, arterial hypertension, peptic ulcer of the stomach and duodenum in remission, hyperthyroidism, severe diseases of the cardiovascular system; with diseases of the bronchi, accompanied by excessive accumulation of secretions; children's age from 2 to 6 years; should not be used in combination with beta-blockers. nine0005
Use during pregnancy and during breastfeeding
The drug is contraindicated during pregnancy and during breastfeeding.
Dosage and administration
inside. Adults and children over 12 years of age are prescribed 10 ml (2 teaspoons) 3 times / day. Children aged 2 to 6 years - 5 ml (1 teaspoon) 3 times / day, from 6 to 12 years - 5-10 ml (1-2 teaspoons) 3 times / day. Shake before use. nine0005
Side effect
The assessment of the frequency of occurrence of adverse reactions was made on the basis of the following criteria: very frequent ( > 1/10), frequent (from > 1/100 to < 1/10), infrequent (from > 1/1000 to < 1/100), rare (from > 1/10,000 to < 1/1,000), very rare (< 1/10,000), frequency unknown (no frequency estimate available). nine0005
nine0005
Immune system disorders.
Rarely: allergic reactions (rash, urticaria), bronchospasm (as a sign of a hypersensitivity reaction).
Frequency unknown: anaphylactic reactions, including anaphylactic shock, angioedema, pruritus, severe skin reactions, including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis and acute generalized exanthematous pustulosis. nine0005
Nervous System Disorders
Rarely: when used in high doses, headache, dizziness, increased nervous excitability, sleep disturbance, drowsiness, tremor, convulsions are sometimes observed.
Cardiovascular disorders
Rare: palpitations, low blood pressure, collapse. nine0005
Frequency unknown: myocardial ischemia.
Gastrointestinal disorders
Rare: nausea, vomiting, diarrhea, exacerbation of peptic ulcer of the stomach and duodenum.
Renal and urinary tract disorders
Rare: Pink coloration of urine is possible.
Overdose
It is possible to increase the manifestations of the described side effects. Treatment is symptomatic.
Interaction with other drugs
Other beta-2-agonists and theophylline increase the effect of salbutamol and increase the likelihood of side effects.
The drug is not prescribed simultaneously with drugs containing codeine, and other antitussives, as this makes it difficult to expel liquefied sputum. nine0005
Bromhexine, which is part of the drug, promotes the penetration of antibiotics (erythromycin, cephalexin, oxytetracycline) into the lung tissue.
It is not recommended to use the drug simultaneously with non-selective beta-adrenergic blockers, such as propranolol.
Salbutamol, which is part of the drug, is not recommended for patients who receive monoamine oxidase inhibitors (MAOIs) and / or tricyclic antidepressants. nine0005
Diuretics and glucocorticosteroid preparations enhance the hypokalemic effect of salbutamol.
It is not recommended to take an alkaline drink simultaneously with the drug.
special instructions
With post-registration use of salbutamol, there have been a small number of reports of rare cases of myocardial ischemia associated with the use of this drug. There have been reports of severe skin reactions such as erythema multiforme, Stevens-Johnson syndrome (SSD), toxic epidermal necrolysis (TEN) and acute generalized exanthematous pustulosis (AGEP) associated with the use of ambroxol (the active metabolite of bromhexine). If symptoms of a progressive skin reaction occur (sometimes associated with lesions of the mucous membranes of the mouth, throat, nose, eyes, genitals), stop using the drug immediately and consult a doctor. It is not recommended to take alkaline solutions simultaneously with the drug. Guaifenesin turns urine pink. nine0005
It is not recommended to take alkaline solutions simultaneously with the drug. Guaifenesin turns urine pink. nine0005
Influence on the ability to drive vehicles, mechanisms
Not studied. Given the profile of side effects (dizziness, drowsiness, and others), it is recommended during treatment to refrain from driving vehicles and mechanisms, from engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Release form nine0004
Syrup.
50 ml, 60 ml, 70 ml, 80 ml, 90 ml, 100 ml, 120 ml, 150 ml or 200 ml of the drug in dark glass vials sealed with polyethylene stoppers and screw-on plastic caps or sealed with polyethylene or polymeric polyethylene closures, or corked with aluminum caps.
50 ml, 60 ml, 70 ml, 80 ml, 90 ml, 100 ml, 120 ml, 150 ml or 200 ml of the drug in polyethylene polymer vials, sealed with polyethylene stoppers and screw-on plastic caps or sealed with polyethylene or polymeric closures.