Trileptal high feeling
Mental Health Medications | NAMI: National Alliance on Mental Illness
Download PDF
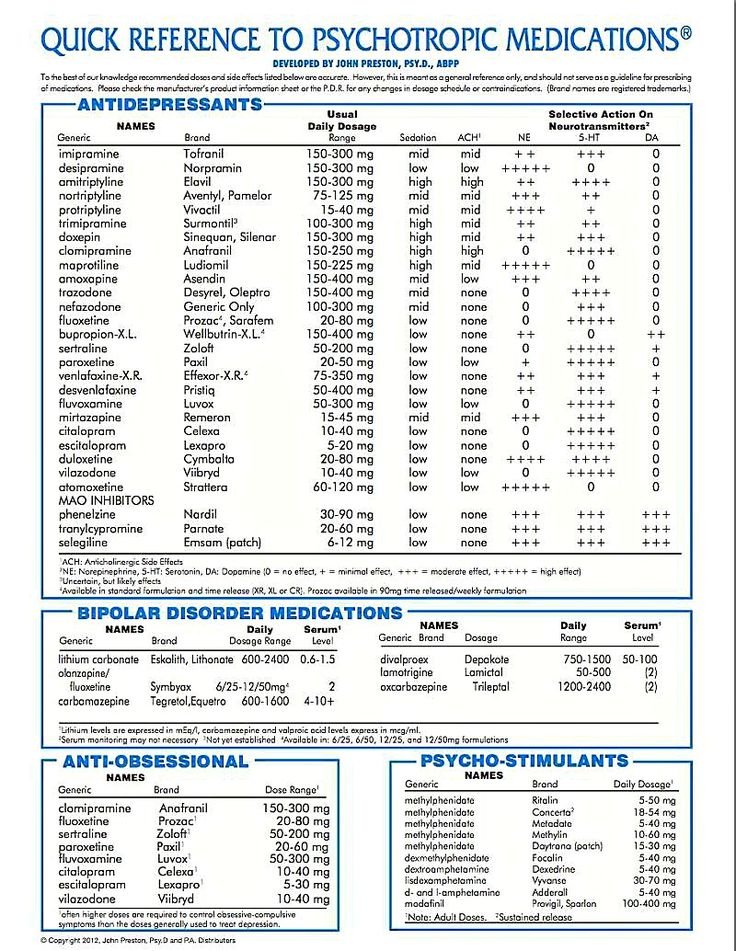
Generic name: oxcarbazepine (ox car BAZ e peen)
- Tablets: 150 mg, 300 mg, 600 mg
- Oral suspension: 300 mg/5 mL
Brand names:
- Trileptal®,
- Tablets: 150 mg, 300 mg, 600 mg
- Oral susension: 300 mg/5 mL
- Oxtellar XR®
- Extended-release tablets: 150 mg, 300 mg, 600 mg
All FDA black box warnings are at the end of this fact sheet. Please review before taking this medication.
What Is Oxcarbazepine And What Does It Treat?
Oxcarbazepine is an antiepileptic medication that works in the brain to prevent and control seizures. It is approved for the treatment of partial seizures.
Oxcarbazepine may also be helpful when prescribed “off-label” for nerve pain or as a mood stabilizer for bipolar disorder. “Off-label” means that it hasn’t been approved by the Food and Drug Administration for this condition. Your mental health provider should justify his or her thinking in recommending an “off-label” treatment. They should be clear about the limits of the research around that medication and if there are any other options.
Bipolar disorder involves episodes of depression and/or mania.
Symptoms of depression include:
- Depressed mood — feeling sad, empty, or tearful
- Feeling worthless, guilty, hopeless, or helpless
- Loss of interest or pleasure in normal activities
- Sleep and eat more or less than usual (for most people it is less)
- Low energy, trouble concentrating, or thoughts of death (suicidal thinking)
- Psychomotor agitation (‘nervous energy’)
- Psychomotor retardation (feeling like you are moving in slow motion)
Symptoms of mania include:
- Feeling irritable or “high”
- Having increased self esteem
- Feeling like you don’t need to sleep
- Feeling the need to continue to talk
- Feeling like your thoughts are too quick (racing thoughts)
- Feeling distracted
- Getting involved in activities that are risky or could have bad consequences (e.
 g., excessive spending)
g., excessive spending)
What Is The Most Important Information I Should Know About Oxcarbazepine?
Bipolar disorder requires long-term treatment. Do not stop taking oxcarbazepine, even when you feel better. With input from you, your health care provider will assess how long you will need to take the medicine. Missing doses of oxcarbazepine may increase your risk for a relapse in your mood symptoms.
Do not stop taking oxcarbazepine or change your dose without talking to with your healthcare provider first.
In order for oxcarbazepine to work properly, it should be taken every day as ordered by your healthcare provider.
Periodically, your healthcare provider may ask you to provide a blood sample to make sure the appropriate level of medication is in your body and to assess for side effects, such as changes in blood cell counts and sodium levels.
Are There Specific Concerns About Oxcarbazepine And Pregnancy?
If you are planning on becoming pregnant, notify your healthcare provider so that he/she can best manage your medications. People living with bipolar disorder who wish to become pregnant face important decisions. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
People living with bipolar disorder who wish to become pregnant face important decisions. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
Oxcarbazepine has been associated with an increased risk of craniofacial defects and heart malformations. There may be precautions to decrease the risk of this effect. Do not stop taking oxcarbazepine without first speaking to your healthcare provider. Discontinuing mood stabilizer medications during pregnancy has been associated with a significant increase in symptom relapse.
Regarding breast-feeding, caution is advised since oxcarbazepine does pass into breast milk.
What Should I Discuss With My Health Care Provider Before Taking Oxcarbazepine?
- Symptoms of your condition that bother you the most
- If you have thoughts of suicide or harming yourself
- Medications you have taken in the past for your condition, whether they were effective or caused any adverse effects
- If you experience side effects from your medications, discuss them with your provider.
 Some side effects may pass with time, but others may require changes in the medication.
Some side effects may pass with time, but others may require changes in the medication. - Any other psychiatric or medical problems you have
- All other medications you are currently taking (including over the counter products, herbal and nutritional supplements) and any medication allergies you have
- Other non-medication treatment you are receiving, such as talk therapy or substance abuse treatment. Your provider can explain how these different treatments work with the medication.
- If you are pregnant, plan to become pregnant, or are breast-feeding
- If you drink alcohol or use illegal drugs
How Should I Take Oxcarbazepine?
Oxcarbazepine is usually taken once or twice a day with or without food.
Typically patients begin at a low dose of medicine and the dose is increased slowly over several weeks.
The dose usually ranges from 900-1200 mg per day. People taking this medication for seizure control may take up to 2400 mg daily. Only your healthcare provider can determine the correct dose for you.
Only your healthcare provider can determine the correct dose for you.
Oxcarbazepine suspension: Measure your dose with a dosing spoon or oral syringe, which you can get from your pharmacy. Before using, shake the bottle well to ensure the medicine is mixed thoroughly. Your dose can be mixed in a small glass of water just prior to taking or may be swallowed directly from the dosing spoon.
Use a calendar, pillbox, alarm clock, or cell phone alert to help you remember to take your medication. You may also ask a family member a friend to remind you or check in with you to be sure you are taking your medication.
What Happens If I Miss A Dose Of Oxcarbazepine?
If you miss a dose of oxcarbazepine, take it as soon as you remember, unless it is closer to the time of your next dose. Discuss this with your healthcare provider. Do not double your dose or take more than what is prescribed.
What Should I Avoid While Taking Oxcarbazepine?
Avoid drinking alcohol or using illegal drugs while you are taking oxcarbazepine. They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g., sedation, dizziness) of the medication.
They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g., sedation, dizziness) of the medication.
What Happens If I Overdose With Oxcarbazepine?
If an overdose occurs call your doctor or 911. You may need urgent medical care. You may also contact the poison control center at 1-800-222-1222.
A specific treatment to reverse the effects of oxcarbazepine does not exist.
What Are The Possible Side Effects Of Oxcarbazepine?
Common side effects
- Dizziness
- Drowsiness
- Vision changes
- Nausea/vomiting
- Headache
- Fatigue
- Uncoordinated movements
- Tremor
Rare/serious side effects
Oxcarbazepine can cause a decrease in the body’s sodium level. Some signs of low sodium include nausea, tiredness, lack of energy, headache, confusion, or more frequent or more severe seizures.
Oxcarbazepine may also cause allergic reactions or serious problems which may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
You may or may not have a rash with these types of reactions.
In rare cases (<1%) a severe, spreading rash with blistering of the skin in patches over the entire body along with fever, headache and cough can occur (Stevens-Johnson syndrome). Although this is rare with oxcarbazepine, discontinuation of this medication is necessary. Rare cases of severe allergic reactions have been reported. Symptoms include swelling of the face, eyes, lips, or tongue, difficulty swallowing or breathing. If you experience any of these side effects, it is important to seek medical care immediately.
Studies have found that individuals who take antiepileptic medications including oxcarbazepine may be twice as likely to have suicidal thoughts or behaviors as individuals who take placebo (inactive medication). These thoughts or behaviors are rare and occurred in approximately 1 in 500 patients taking the antiepileptic class of medications. If you experience any thoughts or impulses to hurt yourself, you should contact your doctor immediately.
Are There Any Risks For Taking Oxcarbazepine For Long Periods Of Time?
To date, there are no known problems associated with long term use of oxcarbazepine. It is a safe and effective medication when used as directed.
It is important to note that some of the side effects listed above (particularly changes in blood sodium, rash, and suicidal thoughts) may continue to occur or worsen if you continue taking the medication. It is important to follow up with your doctor for blood work and to contact your doctor immediately if you notice any skin rash or changes in mood or behavior.
What Other Medications May Interact With Oxcarbazepine?
The seizure medications phenobarbital and phenytoin may decrease the effects of oxcarbazepine.
Oxcarbazepine may increase the effects of phenobarbital and phenytoin.
Oxcarbazepine may decrease the level and effects of:
- Oral contraceptives
- Anti-rejection medications used in organ transplants, like tacrolimus (Prograf®) and cyclosporine (Neoral®, Sandimmune®)
- Certain blood pressure medications, such as amlodipine (Norvasc®) or felodipine (Plendil®)
How Long Does It Take For Oxcarbazepine To Work?
It is very important to tell your doctor how you feel things are going during the first few weeks after you start taking oxcarbazepine and whether or not you are experiencing any side effects from the medication. It will probably take several weeks to see big enough changes in your symptoms to decide if oxcarbazepine is the right medication for you.
It will probably take several weeks to see big enough changes in your symptoms to decide if oxcarbazepine is the right medication for you.
Mood stabilizer treatment is generally needed lifelong for persons with bipolar disorder. Your doctor can best discuss the duration of treatment you need based on your symptoms and illness.
Summary of FDA Black Box Warnings
Currently there are no FDA black box warnings for oxcarbazepine.
Provided by
(January 2023)
©2022 The American Association of Psychiatric Pharmacists (AAPP) and the National Alliance on Mental Illness (NAMI). AAPP and NAMI make this document available under the Creative Commons Attribution-No Derivatives 4.0 International License. Last Updated: January 2016.
This information is being provided as a community outreach effort of the American Association of Psychiatric Pharmacists. This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The American Association of Psychiatric Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The American Association of Psychiatric Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
Oxcarbazepine: 7 things you should know
Print SaveMedically reviewed by Carmen Fookes, BPharm. Last updated on Dec 30, 2022.
1. How it works
- Oxcarbazepine is an anticonvulsant that reduces the frequency of certain types of seizures.
- Once in the body, it is metabolized in the liver to another compound, called MHD, which also helps to reduce seizure frequency. The exact way oxcarbazepine and its active metabolite exert their antiseizure activity is not known; however, it is thought to be by stabilizing nerve membranes, which reduces repetitive firing.
- Oxcarbazepine belongs to the class of medicines known as dibenzazepine carboxamides.
2. Upsides
- May be used for the treatment and prevention of partial seizures in adults, either alone or in addition to other anti-seizure medicines.
- May be used as the sole therapy for the treatment and prevention of partial seizures in children over the age of four, or in addition to other medications for epilepsy in children over the age of two.
- It may be used off-label (not an FDA-approved use) to treat other conditions such as bipolar disorder or migraine.
- Oxcarbazepine does not appear to cause dependence or tolerance, although a slow withdrawal is recommended to avoid precipitating seizures. The abuse potential of oxcarbazepine is unknown.
- Oxcarbazepine is available in tablet form or as a suspension.
- Brand names of oxcarbazepine include Trileptal and Oxtellar.
- Cost-saving, generic oxcarbazepine is available.
3.
 Downsides
DownsidesIf you are between the ages of 18 and 60, take no other medication or have no other medical conditions, side effects you are more likely to experience include:
- Central nervous system (CNS) side effects, including a slowing in the speed of information processing, difficulty with concentration, dizziness, speech and language problems, sleepiness and fatigue, and coordination difficulties including muscle movement disorders and gait disturbances.
- Nausea, vomiting, double vision, headache, and nystagmus (rapid side-to-side eye movements) are also common. Anaphylaxis (serious allergic reactions) is rare.
- Oxcarbazepine can inhibit some liver enzymes (notably CYP2C19 and CYP3A4/5), which may affect plasma levels of some other medications, including oral contraceptives and felodipine.
- Although dosages greater than 1200 mg/day in adults have shown greater efficacy, most people cannot tolerate the CNS side effects that occur with these dosages.

- The dosage of oxcarbazepine needs reducing in people with kidney disease.
- Oxcarbazepine may cause low sodium levels (hyponatremia), usually during the first three months of therapy, although hyponatremia may occur at any time. May be life-threatening. Hyponatremia generally resolves once oxcarbazepine has been discontinued.
- The risk of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (both serious dermatological reactions) may be greater in people carrying the HLA-B 1502 allele (predominantly found in people from China, the Philippines, Malaysia, Thailand, India, and Korea).
- As with other drugs used to treat epilepsy or depression, oxcarbazepine has been associated with a higher risk of suicidal thoughts and behaviors. Monitor for the emergence of worsening mood or unusual behaviors.
- Oxcarbazepine is best withdrawn slowly on discontinuation unless it is an emergency. Sudden discontinuation has been associated with an increase in seizure frequency and status epilepticus.

- May not be suitable for everybody including those with liver or kidney disease, previous mental health issues, or planning to become pregnant.
- There are no controlled data in human pregnancy. Animal studies have reported higher rates of structural abnormalities, spontaneous abortions, growth retardation, fetal malformations, decreased fetal body weight, and maternal toxicity in fetuses of animals exposed to oxcarbazepine. Oxcarbazepine and its active metabolite, MHD, cross the human placenta and oxcarbazepine is structurally related to carbamazepine, which is a known human teratogen. Do not use during pregnancy unless the benefits outweigh the risks. If a woman inadvertently becomes pregnant while taking oxcarbazepine, encourage her to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling the toll-free number 1-888-233-2334 or online at http://www.aedpregnancyregistry.org/. Oxcarbazepine and its active metabolite (MHD) are found in breastmilk, but the effects on a breastfed infant are unknown.

Note: In general, seniors or children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures) or people who take other medications are more at risk of developing a wider range of side effects. View complete list of side effects
Oxcarbazepine is an antiseizure medication that is used either alone or in combination with other antiseizure medications to reduce seizure frequency in people with partial seizures and sometimes other seizure types. CNS side effects (including concentration difficulties, sleepiness, and confusion) are the main side effects associated with its use.
5. Tips
- May be taken with or without food.
- Oxcarbazepine suspension may be substituted for oxcarbazepine tablets in people or children who have difficulty swallowing (the dosages are equivalent).
- Oxcarbazepine may make you sleepy or impair your reaction times and affect your ability to drive or operate machinery.
 Do not drive or operate machinery if oxcarbazepine affects you in this way. Alcohol may make these effects worse.
Do not drive or operate machinery if oxcarbazepine affects you in this way. Alcohol may make these effects worse. - Talk to your doctor if you experience any signs of hyponatremia (low blood sodium levels) such as confusion, headache, nausea, tiredness, or an increase in seizure frequency.
- Seek urgent medical advice if you develop any allergic-type reactions including facial swelling or a rash. 25-30% of people who are allergic to carbamazepine will also be allergic to oxcarbazepine. Also seek urgent advice if you develop any unusual bruising or bleeding, swollen lymph nodes, abdominal pain, painful mouth sores, yellowing of the skin, frequent or persistent infections, or different or more frequent seizures.
- If you are taking hormonal contraceptives, talk with your doctor. Your dosage may need to be increased or an alternative method of contraception may be needed.
- Do not stop oxcarbazepine suddenly, unless your doctor has told you to do so. It is best to discontinue oxcarbazepine slowly.

- Tell your doctor if you are pregnant, intending to become pregnant, or breastfeeding because oxcarbazepine may not be suitable for you.
6. Response and effectiveness
- Oxcarbazepine is completely absorbed after oral administration with over 70% being converted to its active metabolite, MHD. MHD is responsible for more antiseizure effects than oxcarbazepine. Peak concentrations of MHD are reached within 3 to 13 hours (average time of 4.5 hours).
- Food does not affect the absorption of oxcarbazepine. Reductions in seizure frequency have been noted within ten days of regular administration of oxcarbazepine.
7. Interactions
Medicines that interact with oxcarbazepine may either decrease its effect, affect how long it works for, increase side effects, or have less of an effect when taken with oxcarbazepine. An interaction between two medications does not always mean that you must stop taking one of the medications; however, sometimes it does. Speak to your doctor about how drug interactions should be managed.
Speak to your doctor about how drug interactions should be managed.
Common medications that may interact with oxcarbazepine include:
- antidepressants, such as citalopram, sertraline, St John’s Wort, and monoamine oxidase inhibitors
- cyclosporine
- HIV combination drugs such as elvitegravir/cobicistat
- other medications for seizure control such as phenytoin or phenobarbital
- other medications that cause drowsiness, such as anti-anxiety medications, muscle relaxants, narcotics, psychiatric medications, sedating antihistamines, or sleeping pills
- oral contraceptives (oxcarbazepine may make them less effective)
- proton pump inhibitors such as lansoprazole, omeprazole, or pantoprazole
- some heart medications, such as amiodarone, calcium channel blockers (eg, amlodipine, felodipine, nifedipine).
Alcohol may also increase drowsiness associated with oxcarbazepine, so is best avoided.
Note that this list is not all-inclusive and includes only common medications that may interact with oxcarbazepine. You should refer to the prescribing information for oxcarbazepine for a complete list of interactions.
You should refer to the prescribing information for oxcarbazepine for a complete list of interactions.
More about oxcarbazepine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (346)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Support group
- Drug class: dibenzazepine anticonvulsants
- Breastfeeding
- En español
Patient resources
- Drug Information
- Oxcarbazepine Extended-Release Tablets
- Oxcarbazepine Tablets
- Oxcarbazepine Oral Suspension
Other brands
Trileptal, Oxtellar XR
Professional resources
- Prescribing Information
Related treatment guides
- Bipolar Disorder
- Anxiety
- Borderline Personality Disorder
- Cyclothymic Disorder
References
- Oxcarbazepine. Revised 11/2022. Major Pharmaceuticals.
 https://www.drugs.com/pro/oxcarbazepine.html
https://www.drugs.com/pro/oxcarbazepine.html
Further information
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use oxcarbazepine only for the indication prescribed.
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Copyright 1996-2023 Drugs.com. Revision date: December 30, 2022.
Medical Disclaimer
Keep for yourself Search for analogues Interaction Description of the active ingredients of the preparation Trileptal ® (Trileptal ® ) The scientific information provided is general and cannot be used to make decisions. Update date: 2020.05.07 Marketing authorization holder:NOVARTIS PHARMA, AG (Switzerland)
Manufactured:NOVARTIS PHARMA STEIN, AG (Switzerland) ATX code: N03AF02 (Oxcarbazepine) Active substance: oxcarbazepine (oxcarbazepine) Rec.INN registered by WHO Dosage form
Release form, packaging and composition Trileptal®Film-coated tablets yellow, oval, slightly biconvex, scored on both sides, marked "TE/TE" on one side and "CG/CG" on the other. Excipients : colloidal silicon dioxide, microcrystalline cellulose, hypromellose, crospovidone, magnesium stearate, macrogol 8000, talc, titanium dioxide (E171), purified water, iron oxide yellow (E172). 10 pcs. - blisters (1) - packs of cardboard. Clinical and pharmacological group: Anticonvulsant drug Pharmacotherapeutic group: Anticonvulsant Pharmacological action Antiepileptic. The implementation of the anticonvulsant action is facilitated by an increase in the conductivity of potassium ions and modulation of calcium channels activated by a high membrane potential. There was no significant interaction with brain neurotransmitters or receptor binding. Experimental studies have shown that oxcarbazepine and its metabolite have a pronounced anticonvulsant effect. The efficacy of oxcarbazepine in epileptic seizures has been demonstrated both in monotherapy and in the use of oxcarbazepine as part of combination therapy in children and adults. PharmacokineticsAfter oral administration, oxcarbazepine is completely absorbed and largely metabolized to form the pharmacologically active metabolite, the 10-monohydroxy derivative. After a single dose of oxcarbazepine, depending on the dosage form used, C max metabolite in plasma is 24.9-34 µmol / l, the average time to reach it is about 4.5-6 hours. Pharmacokinetic studies have shown that 2 % oxcarbazepine and 70% MGP; the rest is accounted for by secondary metabolites, rapidly excreted from the blood plasma. Binding of the metabolite to plasma proteins, mainly albumin, is about 40%. In the therapeutic range, the degree of binding does not depend on the concentration of the drug in the blood serum. Oxcarbazepine and MGP do not bind to α 1 -acid glycoprotein. Apparent V d - 49 l. From ss MHD in blood plasma is achieved on days 2-3 when taking oxcarbazepine 2 times / day. In the equilibrium state, the pharmacokinetic parameters of MGP are linear and dose-dependent in the range of daily doses of 300 mg - 2400 mg. Oxcarbazepine is rapidly metabolized by hepatic cytosolic enzymes to the pharmacologically active MHD metabolite, which undergoes further glucuronidation. Minor amounts of MHP (about 4% of the dose) are oxidized to form an inactive metabolite - 10, 11-dihydroxy derivative (DHP). Oxcarbazepine is excreted as metabolites mainly by the kidneys (95%), less than 1% is excreted unchanged. Approximately 80% of excreted metabolites are MHD, of which 49% are glucuronides and 27% are unchanged MGP. DGP is excreted unchanged (about 3%), oxcarbazepine conjugates account for 13%. About 4% of the dose is excreted in the feces. Oxcarbazepine is rapidly excreted from the blood plasma, the apparent T 1/2 is 1.3 - 2.3 hours. The apparent T 1/2 MGP averages 9.3 ± 1.8 hours. With CC less than 30 ml / min after a single dose of 300 mg of oxcarbazepine T 1/2 IHL increases to 19 hours, and AUC doubles. Weight-adjusted clearance of MHD in children decreases with age and body weight, approaching that of adults. After taking oxcarbazepine in a single dose of 300 mg or multiple doses of 600 mg / day in healthy volunteers aged 60-82 years, plasma C max and AUC values for MHD were 30-60% higher compared with the same indicators in young volunteers (18-32 years old), which is associated with an age-related decrease in CC. Pregnant women experience a number of physiological changes that can lead to a gradual decrease in plasma levels of MHD during pregnancy. Indications of the active substances of the drug Trileptal®Simple and complex partial epileptic seizures with or without secondary generalization in adults and children aged 1 month and older. Generalized tonic-clonic epileptic seizures in adults and children aged 2 years and older. Open list of ICD-10 codes
Dosage regimen The method of administration and dosing regimen of a particular drug depends on its form of release and other factors. The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed. Taken orally. Initial dose - 8-10 mg / kg body weight / day. Further, the dose is adjusted depending on the treatment regimen, the age of the patient, the effectiveness of treatment, and kidney function. For patients with impaired renal function (CC less than 30 ml / min), adjustment of the initial dose and dosing regimen is required. Side effectsMost common (≥ 10%) : drowsiness, headache, dizziness, diplopia, nausea, vomiting, fatigue. From the side of the hematopoietic system: sometimes - leukopenia; very rarely - suppression of bone marrow hematopoiesis, agranulocytosis, aplastic anemia, neutropenia, pancytopenia, thrombocytopenia. From the immune system: very rarely - hypersensitivity reactions accompanied by fever and rash (including multiple organ disorders). With the development of hypersensitivity reactions, damage to the circulatory and lymphatic systems (eosinophilia, thrombocytopenia, lymphadenopathy, splenomegaly), muscles and joints (myalgia, swelling in the joints, arthralgia), nervous system (encephalopathy), kidneys (proteinuria, interstitial nephritis, renal failure) is possible. From the side of metabolism: often - hyponatremia; very rarely - clinically significant hyponatremia (sodium concentration <125 mmol / l - usually during the first 3 months of drug therapy; in some patients - more than 1 g after the start of treatment), leading to the development of such manifestations and symptoms as seizures, confusion, decreased level of consciousness, encephalopathy, visual disturbances (including blurred vision), nausea, vomiting, folic acid deficiency; very rarely - hypothyroidism. From the side of the central nervous system: very often - drowsiness, headache, dizziness; often - ataxia, tremor, nystagmus, impaired attention, amnesia; confusion, depression, apathy, agitation, emotional lability. From the senses: very often - diplopia; often - visual impairment, blurred vision, vertigo. From the side of the cardiovascular system: very rarely - arrhythmias, AV blockade, arterial hypertension. From the digestive system: very often - nausea, vomiting; often - diarrhea, constipation, abdominal pain; sometimes - an increase in the activity of liver enzymes, an increase in the concentration of alkaline phosphatase in the blood; very rarely - pancreatitis and / or increased levels of lipase and / or amylase, hepatitis. Dermatological reactions: often - rash, alopecia, acne. Allergic reactions: sometimes - urticaria; very rarely - angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell's syndrome), erythema multiforme. Other: very common - feeling tired; often - asthenia; very rarely - systemic lupus erythematosus. In children under 4 years of age: very often (11%) - drowsiness; often (≥1%–<10%) - ataxia, irritability, vomiting, lethargy, fatigue, nystagmus, tremor, loss of appetite, increased concentration of uric acid in the blood. Contraindications for useChildren under 3 years of age; hypersensitivity to oxcarbazepine. Pregnancy and lactationLimited experience with pregnancy. Reports suggest that oxcarbazepine use during pregnancy may be associated with the development of birth defects (eg, cleft palate). In experimental studies with the use of oxcarbazepine in toxic doses, an increase in embryonic mortality, slowing down and impaired development and growth of the fetus was noted. If the patient plans to become pregnant or becomes pregnant while using oxcarbazepine, and if there is a question about the use of oxcarbazepine during pregnancy, the expected benefits of therapy and the possible risk to the fetus, especially in the first trimester of pregnancy, should be carefully weighed. Use the lowest effective dose of oxcarbazepine during pregnancy. When clinically effective in women of childbearing age, oxcarbazepine should be used as monotherapy. Effective antiepileptic treatment should not be interrupted during pregnancy, as disease progression may adversely affect the mother and fetus. Folic acid deficiency is known to develop during pregnancy. Antiepileptic drugs can exacerbate this deficiency, which is one of the possible causes of fetal developmental disorders, so additional folic acid supplementation is recommended. When used during pregnancy, it must be taken into account that physiological changes occurring in the body of a pregnant woman can lead to a gradual decrease in the concentration of the active metabolite in the blood plasma. To achieve maximum control of the symptoms of the disease, it is necessary to regularly evaluate the clinical effect of oxcarbazepine and determine the concentration of the metabolite in the blood plasma. Determination of the concentration of MHD in blood plasma is also recommended in the postpartum period, especially if the dose of oxcarbazepine was increased during pregnancy. There are reports that the use of antiepileptic drugs during pregnancy can lead to increased bleeding in newborns. As a precautionary measure, vitamin K 1 is recommended during the last few weeks of pregnancy and in newborns whose mothers received oxcarbazepine. Oxcarbazepine and MHD cross the placental barrier and are excreted in breast milk. The concentration ratio in milk and plasma was 0.5 for both substances. Since the effect on newborns of oxcarbazepine and MHD, received with mother's milk, is not known, oxcarbazepine should not be used during breastfeeding. Special instructions Use with caution in patients with known hypersensitivity to carbamazepine, as in this group of patients, approximately 25-30% of cases may develop hypersensitivity reactions to oxcarbazepine. In patients with no history of hypersensitivity to carbamazepine, it is also possible to develop hypersensitivity reactions to oxcarbazepine, including multiple organ disorders. Use with caution in patients with severe hepatic impairment. In patients with impaired renal function and low serum sodium levels, or in patients receiving concomitant treatment with drugs that promote sodium excretion from the body (diuretics, drugs that affect the secretion of ADH), the concentration should be determined before starting therapy with oxcarbazepine sodium in blood serum. In the future, the concentration of sodium in the blood serum should be monitored 2 weeks after the start of therapy and then monthly for 3 months or as needed. With particular attention to these risk factors should be treated in elderly patients. If it is necessary to prescribe diuretics and other drugs that reduce the concentration of sodium in the blood serum, patients receiving oxcarbazepine should follow the same recommendations. If clinical symptoms appear that suggest hyponatremia, serum sodium concentration should be measured. If symptoms of severe bone marrow suppression develop, consideration should be given to discontinuing oxcarbazepine. Patients receiving anticonvulsants rarely experienced episodes of suicidal behavior and thoughts. The mechanism of increased risk of suicide in this category of patients has not been established. Therefore, at all stages of treatment, careful monitoring of patients receiving oxcarbazepine is necessary. Weight should be monitored in all patients with heart failure to detect fluid retention in a timely manner. With fluid retention or with the progression of symptoms of heart failure, the concentration of sodium in the blood serum should be determined. If hyponatremia occurs, fluid intake should be limited. When using oxcarbazepine, in very rare cases, a violation of cardiac conduction is possible, therefore, during the period of treatment, careful monitoring of patients with previous conduction disorders (AV blockade, arrhythmia) is necessary. Dermatological reactions have been observed in both children and adults with oxcarbazepine and developed on average 19 days after the start of treatment. There are isolated reports of recurrence of skin reactions when oxcarbazepine is restarted. With the development of skin reactions against the background of the use of oxcarbazepine, consideration should be given to its cancellation and the appointment of another antiepileptic agent. If hepatitis is suspected, consider discontinuing oxcarbazepine. As with all antiepileptic drugs, oxcarbazepine should be discontinued gradually due to the risk of increased seizure frequency. Influence on the ability to drive vehicles and mechanisms Patients who experience dizziness, drowsiness or other disorders of the central nervous system during the use of oxcarbazepine should not drive vehicles or work with mechanisms during the period of treatment. Drug interactions Oxcarbazepine and its pharmacologically active metabolite MHD are inhibitors of cytochrome CYP2C19. As inducers of CYP3A4 and CYP3A5, oxcarbazepine and MHD reduce plasma concentrations of drugs metabolized by these isoenzymes: dihydropyridine calcium antagonists, oral contraceptives, and antiepileptic drugs (eg, carbamazepine). With simultaneous use with oxcarbazepine, a decrease in plasma concentrations of other drugs that are substrates of CYP3A4 and CYP3A5 enzymes (for example, drugs of the immunosuppressant group - cyclosporine) is also possible. Because MHD is a weak inducer of UDP-glucuronyl transferase in vitro, it is therefore unlikely that it would interfere in vivo with the metabolism of drugs excreted as glucuronic acid conjugates (eg, valproic acid and lamotrigine). In vitro studies have confirmed the weak inducing ability of oxcarbazepine and MGP in relation to the isoenzymes of the CYP2B and CYP3A4 enzyme subsystems. The inducing effect of oxcarbazepine and MHD on other CYP isoenzymes is unknown. Plasma concentration of phenytoin increases by up to 40% with simultaneous use of oxcarbazepine at a dose of 1200 mg / day and above. Therefore, when using oxcarbazepine at such doses, a reduction in the dose of phenytoin may be required. The increase in serum concentrations of phenobarbital with simultaneous use with oxcarbazepine is insignificant (15%). Simultaneous administration of strong inducers of cytochrome P450 isoenzymes (i. Oxcarbazepine has been shown to interact with ethinyl estradiol and levonorgestrel. The average AUC values for them decreased by 48-52% and 35-52%, respectively. Interaction studies of oxcarbazepine with other oral or implantable contraceptives have not been conducted. Thus, the simultaneous use of oxcarbazepine and hormonal contraceptives may lead to a decrease in the effectiveness of the latter. Co-administration of oxcarbazepine and felodipine may result in a 28% decrease in felodipine AUC, although plasma concentrations remain within the therapeutic range. On the other hand, with simultaneous use with verapamil, a decrease in the concentration of MHD in the blood serum by 20% is possible. This reduction has no clinical significance. Cimetidine, erythromycin, dextropropoxyphene do not affect the pharmacokinetic parameters of MHD; viloxazine slightly affects the concentration of MHD in plasma (the concentration of MHD increases by 10% after repeated co-administration). Oxcarbazepine may increase the sedative effect of ethanol. Keep If you want to place a link to the description of this drug - use this code Trileptal ® . Description of the drug in the reference book Vidal. |
Pharmacy on Lenina
Trade name: Trileptal (Trileptal)
International name: Oxcarbazepine (Oxcarbazepine)
Pharmacological group: antiepileptic
ATC pharmacological group: N03AF02. Oxcarbazepine
Pharmacological action: analgesic, antiepileptic, tranquilizing
Pharmacodynamics: Antiepileptic drug, the exact mechanism of action and its active metabolite 10-monohydroxy derivative (MHP) is unknown. Oxcarbazepine and MGP block voltage-dependent Na+ channels, which leads to stabilization of the membranes of overexcited neurons, inhibition of the occurrence of serial discharges of neurons, and a decrease in synaptic conduction of impulses. Increased K+ conductivity and modulation of Ca2+ channels activated by high membrane potential are also involved in the antiepileptic effect of the drug.
Pharmacokinetics: Completely absorbed after oral administration Rapidly metabolized to form a pharmacologically active metabolite - MGP. Eating does not affect the speed and completeness of absorption. Communication with MGP proteins - 40% (mainly with albumin). In the therapeutic dose range, the degree of binding does not depend on the concentration of the drug in the blood serum. Oxcarbazepine and MGP do not bind to alpha1-acid glycoprotein. The volume of distribution of MHD is 49 liters.
Cmax of the active metabolite - 34 µmol / l, TCmax - 4.5 hours (3-13 hours) after a single dose of 600 mg. In blood plasma, only 2% of oxcarbazepine, 70% of MHD is determined, and the rest are secondary metabolites that are rapidly excreted from blood plasma.
Css MHD in blood plasma is achieved in 2-3 days when taking oxcarbazepine 2 times a day.
Steady-state pharmacokinetic parameters are linear and dose-dependent in the range of daily doses of 300-2400 mg
Oxcarbazepine is rapidly metabolized by liver cytosolic enzymes to the pharmacologically active metabolite MHD, which undergoes further glucuronidation. About 4% of the dose is hydroxylated to form an inactive metabolite - 10,11-dihydroxy derivative (DHP). T1 / 2 - 1.3-2.3 hours T1 / 2 IHL - 9.3 ± 1.8 hours. Excreted mainly by the kidneys (95%) as metabolites, 1% is excreted unchanged. About 80% of the excreted metabolites are MHD, of which 49% are glucuronides and 27% are unchanged MHD. DGP is excreted unchanged (about 3%), oxcarbazepine conjugates account for 13%. About 4% of the dose is excreted through the intestines.
Mild to moderate hepatic insufficiency does not affect the pharmacokinetic parameters of oxcarbazepine and MHD. Pharmacokinetics in severe hepatic impairment has not been studied.
There is a linear relationship between renal clearance of MHD and CK. With CC less than 30 ml / min after a single dose of 300 mg of oxcarbazepine, T1 / 2 MHD increases to 19 hours, AUC - 2 times.
Following a single dose of 5-15 mg/kg dose-adjusted body weight, the AUC for MHD is 30% lower in children 2 to 5 years of age compared to children 6 to 12 years of age. In children with normal renal function, the renal clearance of MHD is higher than in adults, and T1 / 2 is reduced by 10-50% (up to 5-9 hours) compared with adults.
After taking a single (300 mg) or multiple (600 mg / day) dose in patients aged 60-82 years, Cmax and AUC for MHD are 30-60% higher, which is associated with an age-related decrease in CC.
There were no differences in pharmacokinetic parameters depending on sex in childhood, adulthood or old age.
Indications for use: Monotherapy (as a drug of first choice), as well as in combination therapy:
simple and complex partial epileptic seizures (with or without loss of consciousness), incl. with secondary generalization,
generalized tonic-clonic epileptic seizures.
Contraindications: Hypersensitivity, lactation.
Dosage regimen: Inside, regardless of food intake, with a small amount of liquid. The suspension can be taken undiluted directly from the syringe or diluted with a small amount of water.
In the case of monotherapy or in combination with other antiepileptic drugs, the course of treatment begins with a clinically effective dose divided into 2 doses. The dose may be increased depending on the response to therapy. When replacing other antiepileptic drugs with oxcarbazepine at the beginning of taking oxcarbazepine, the dose of the drug being replaced should be gradually reduced. When oxcarbazepine is used as add-on therapy in combination therapy, dose reduction of concomitant antiepileptic drugs and/or slower dose escalation of oxcarbazepine may be required.
Monotherapy: The initial dose for adults is 600 mg/day (8-10 mg/kg/day) divided into 2 doses. An adequate therapeutic response is observed in the dose range of 600-2400 mg per day. The dose may be gradually increased at weekly intervals by no more than 600 mg/day until the desired therapeutic response is achieved. In stationary conditions, a rapid increase in the dose to 2400 mg per day within 48 hours is possible. . An adequate therapeutic response is observed in the dose range of 600-2400 mg per day. In the presence of clinical indications, the dose may be gradually increased by no more than 600 mg / day at weekly intervals until the desired therapeutic response is achieved. There is limited experience with daily doses up to 4200 mg.
The recommended starting dose for children over 2 years of age is 8-10 mg/kg of body weight per day, divided into 2 doses, both in monotherapy and as part of combination therapy. In combination with other antiepileptic drugs, an adequate therapeutic effect is observed at a maintenance dose of 30 mg/kg of body weight per day. To achieve the desired therapeutic response, a gradual increase in dose by 10 mg / kg / day per week is possible up to a maximum daily dose of 46 mg / kg of body weight.
Dosing adjustment is not required in patients with mild to moderate hepatic impairment.
In renal failure (CC less than 30 ml / min), the recommended initial dose is 300 mg / day, which is slowly increased until the desired therapeutic response is achieved.
Side effects: Very often - more than 10%, often - 1-10%, sometimes - 0.1-1%, rarely - 0.01-0.1%, very rarely - less than 0.01%.
Most common: in adults - drowsiness, headache, dizziness, diplopia, nausea, vomiting, fatigue (more than 10%), in children (from 1 month to 4 years) - drowsiness (11%), often - ataxia , irritability, vomiting, lethargy, fatigue, nystagmus, tremor, loss of appetite, hyperuricemia.
From the nervous system: very often - dizziness, headache, drowsiness, often - asthenia, fatigue, agitation, amnesia, agitation, apathy, ataxia, impaired concentration, disorientation, depression, emotional lability, nystagmus, tremor.
From the CCC: very rarely - arrhythmia, AV blockade.
From the digestive system: very often - nausea, vomiting, often - constipation, abdominal pain, diarrhea, sometimes - increased activity of hepatic transaminases and / or alkaline phosphatase, very rarely - pancreatitis and / or increased activity of lipase and / or amylase.
On the part of the hematopoietic organs: sometimes - leukopenia, very rarely - thrombocytopenia, inhibition of bone marrow hematopoiesis, agranulocytosis, aplastic anemia, neutropenia, pancytopenia.
From the side of water and electrolyte metabolism: often - asymptomatic hyponatremia, very rarely - clinically manifested hyponatremia (convulsions, confusion, encephalopathy, blurred vision, nausea, vomiting).
From the side of the skin: often - acne, alopecia, rash, sometimes - urticaria, very rarely - erythema multiforme (including Stevens-Johnson syndrome), Lyell's syndrome, SLE.
From the senses: very often - diplopia, often - blurred vision, dizziness.
Allergic reactions: very rarely - multiple organ disorders, incl. with fever and rash: damage to the circulatory and lymphatic systems (eosinophilia, thrombocytopenia, lymphadenopathy, splenomegaly), muscles and joints (myalgia, swelling in the joints, arthralgia), nervous system (encephalopathy), kidneys (proteinuria, interstitial nephritis, renal failure) , lungs (shortness of breath, pulmonary edema, bronchospasm, interstitial inflammation), changes in the activity of "liver" enzymes, angioedema.
Other: very rarely - clinically significant hyponatremia (Na + - more than 125 mmol / l), as a rule, during the first 3 months of therapy (in some patients - after more than 1 year).
Overdose
Symptoms: drowsiness, dizziness, nausea, vomiting, hypokinesia, hyponatremia, ataxia, nystagmus.
Treatment: symptomatic and supportive. Gastric lavage and activated charcoal to reduce absorption (in case of recent drug intake). There is no specific antidote.
Interaction: Oxcarbazepine and MGP are inhibitors of cytochrome CYP2C19. Co-administration of high doses of oxcarbazepine and drugs metabolized by CYP2C19 (phenobarbital, phenytoin) may lead to an interaction. For some patients, it may be necessary to reduce the dose of drugs - substrates of CYP2C19. Oxcarbazepine and MHD interact weakly or not at all with CYP1A2, CYP2A6, CYP2C9, CYP2D9, CYP2E1, CYP4A4 and CYP4C11.
Oxcarbazepine and MGP are inducers of cytochromes CYP3A4 and CYP3A5 involved in the metabolism of dihydropyridine derivatives of BMCC, oral contraceptives and antiepileptic drugs (including carbamazepine). Co-administration of oxcarbazepine and CYP3A4 and CYP3A5 substrates may reduce plasma concentrations of the latter.
In vitro, MHD is a weak inducer of UDP-glucuronyltransferase and, therefore, it is unlikely that in vivo it is able to influence the metabolism of drugs excreted as conjugates (including valproic acid and lamotrigine), however, it may be necessary to increase the doses of drugs used simultaneously metabolized by the CYP3A4 system or UDP-glucouronyltransferase. If it is necessary to cancel oxcarbazepine, the doses of these drugs should be adequately reduced.
In vivo plasma concentrations of phenytoin increase by up to 40% when oxcarbazepine doses of 1200 mg/day and above are given concomitantly, so a dose reduction of phenytoin may be required. At the same time, phenytoin can reduce the concentration of oxcarbazepine by 29-35%.
Oxcarbazepine increases the concentration of phenobarbital by 15% when administered simultaneously. In turn, phenobarbital can reduce the concentration of oxcarbazepine by 30-31%.
Oxcarbazepine can reduce the concentration of carbamazepine by 22%, while carbamazepine can reduce the concentration of oxcarbazepine by 40%.
Clobazam and felbamate do not affect the concentration of oxcarbazepine. The effect of oxcarbazepine on the concentration of these drugs has not been studied.
Valproic acid may reduce the concentration of oxcarbazepine by 18%.
Simultaneous administration of powerful P450 inducers (including carbamazepine, phenytoin and phenobarbital) reduces plasma concentrations of MHD (by 29-40%). No phenomena of autoinduction were revealed.
Reduces the AUC of ethinyl estradiol and levonorgestrel by 48-52 and 35-52%, respectively. There are no data for other oral or implantable contraceptives. However, the simultaneous administration of oxcarbazepine and hormonal contraceptives may lead to a decrease in the effectiveness of the latter.
Reduces the AUC of felodipine by 28%, however, the plasma concentration remains within the therapeutic range.
Enhances the sedative effect of ethanol.
Verapamil may reduce MHD levels by 20% (not clinically significant).
Cimetidine, erythromycin, dextropropoxyphene do not affect the pharmacokinetic parameters of MHD. There were no interactions with warfarin with single or course administration of oxcarbazepine.
Special instructions: Allergic reactions may develop in patients without a history of hypersensitivity to carbamazepine. In the event of an allergic reaction, the drug should be discontinued immediately.
Hyponatremia (serum Na + concentration below 125 mmol / l) is usually not clinically manifested and does not require correction of the dosing regimen, observed in 2.7% of patients. Na+ levels return to normal when oxcarbazepine is discontinued (dose reduced) or fluid intake is restricted. In patients with impaired renal function associated with hyponatremia, or during simultaneous therapy with diuretics, drugs that affect the secretion of ADH, the concentration of Na + in the blood plasma should be determined before starting therapy with oxcarbazepine, and 2 weeks after the start of therapy, and then monthly or as needed. If it is necessary to simultaneously prescribe saluretics and other drugs that cause hyponatremia, the same recommendations should be followed. When clinical symptoms of hyponatremia appear, the serum Na+ concentration should be determined.
During the period of treatment, it is necessary to monitor body weight in patients with CHF for the timely detection of fluid retention. In case of fluid retention or progression of symptoms of CHF, serum Na + concentration should be determined. In the case of hyponatremia, the amount of fluid consumed should be limited.
During the period of treatment, careful monitoring of patients with a history of rhythm and conduction disturbances is necessary.
If hepatitis is suspected during treatment, the drug should be discontinued.
Women of childbearing age taking oral contraceptives should be warned that the simultaneous administration of the drug may reduce their effectiveness, therefore, the additional use of non-hormonal methods of contraception is recommended.
Experience with the drug during pregnancy is limited. There are separate reports of a possible relationship between taking the drug and congenital deformities (including cleft palate). It is necessary to carefully compare the expected benefits of therapy and its possible complications, especially in the first pregnancy, if necessary, the appointment during pregnancy. The minimum effective dose should be used. In women of childbearing age, the drug is recommended to be used as monotherapy. The patient should be warned about possible fetal developmental disorders and the need for antenatal diagnosis.
Antiepileptic drugs can increase folic acid deficiency during pregnancy (one of the possible causes of fetal developmental disorders), therefore, additional folic acid intake is recommended during the treatment period.
The use of antiepileptic drugs during pregnancy can lead to increased bleeding in newborns, so vitamin K is recommended in the last weeks of pregnancy and the newborn.
Oxcarbazepine and MHD cross the placental barrier and are excreted in breast milk. During the period of treatment, it is necessary to stop breastfeeding.
The drug should be discontinued gradually (risk of increased frequency of epileptic seizures).
During the period of treatment, it is necessary to refrain from engaging in potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions.
Precautions
History of hypersensitivity to carbamazepine (25-30% may develop cross-allergic reactions to oxcarbazepine),
pregnancy.
The use of the drug in children under 2 years of age has not been studied.
Renewal date 06/24/2010
Producer: Novartis Farma S.p.A., Italy
Marketing authorization holder: Novartis Pharma AG, Switzerland
Release form: film-coated tablets 150, 300, 600 mg (blisters)
Composition: oxcarbazepine 1003/15 /600 mg
Belongs to Vital and Essential Drugs
Shelf life: 3 years
Registration data: P No.
 decisions about the use of a particular drug.
decisions about the use of a particular drug.  , coated film coated, 300 mg: 10, 20, 30 or 50 pcs.
, coated film coated, 300 mg: 10, 20, 30 or 50 pcs.  Pharmacological activity is primarily due to the action of the metabolite - monohydroxy derivative (MHP) oxcarbazepine. The mechanism of action of oxcarbazepine and its metabolite is mainly associated with the blockade of voltage-dependent sodium channels, which leads to stabilization of overexcited neuronal membranes, inhibition of the occurrence of serial neuronal discharges and a decrease in synaptic conduction of impulses.
Pharmacological activity is primarily due to the action of the metabolite - monohydroxy derivative (MHP) oxcarbazepine. The mechanism of action of oxcarbazepine and its metabolite is mainly associated with the blockade of voltage-dependent sodium channels, which leads to stabilization of overexcited neuronal membranes, inhibition of the occurrence of serial neuronal discharges and a decrease in synaptic conduction of impulses. 
 Weight-adjusted clearance in children aged 1 month to 4 years is 93% higher than in adults. As a result, it is expected that the AUC of MHD in children of this age group will be 2 times less than that in adults when using the same doses (when adjusted for body weight). Weight-adjusted clearance in children aged 4 to 12 years is 43% higher than in adults. The estimated AUC of MHD in children of this age group is 2/3 of that in adults when using the same doses (when adjusted for body weight). It is assumed that in children aged 13 years and older, due to the increase in body weight, the clearance of MHD, adjusted for body weight, corresponds to the clearance of MHD in adults.
Weight-adjusted clearance in children aged 1 month to 4 years is 93% higher than in adults. As a result, it is expected that the AUC of MHD in children of this age group will be 2 times less than that in adults when using the same doses (when adjusted for body weight). Weight-adjusted clearance in children aged 4 to 12 years is 43% higher than in adults. The estimated AUC of MHD in children of this age group is 2/3 of that in adults when using the same doses (when adjusted for body weight). It is assumed that in children aged 13 years and older, due to the increase in body weight, the clearance of MHD, adjusted for body weight, corresponds to the clearance of MHD in adults. 

 ), lungs (shortness of breath, pulmonary edema, bronchospasm, interstitial inflammation), abnormal liver function tests, angioedema, anaphylactic reactions.
), lungs (shortness of breath, pulmonary edema, bronchospasm, interstitial inflammation), abnormal liver function tests, angioedema, anaphylactic reactions. 


 In the event of an immediate hypersensitivity reaction, oxcarbazepine should be discontinued immediately and alternative therapy instituted.
In the event of an immediate hypersensitivity reaction, oxcarbazepine should be discontinued immediately and alternative therapy instituted.  For other patients, measurement of serum sodium concentration can be performed during routine blood tests.
For other patients, measurement of serum sodium concentration can be performed during routine blood tests.