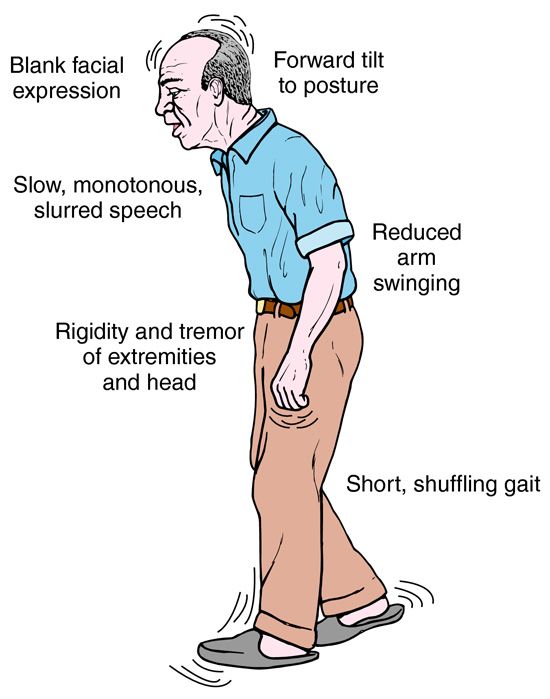
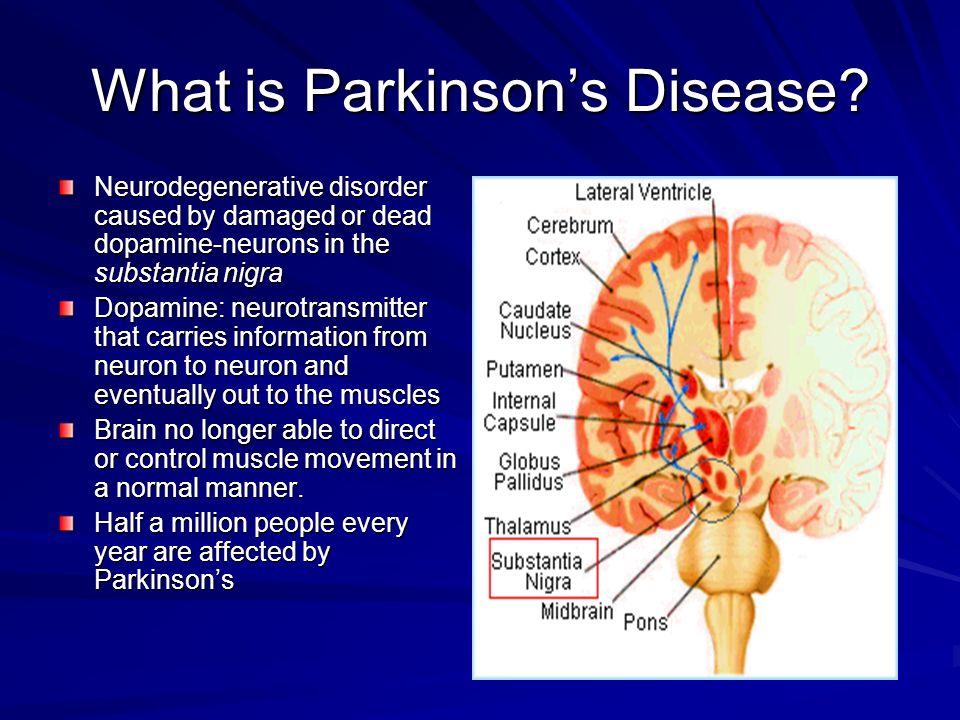
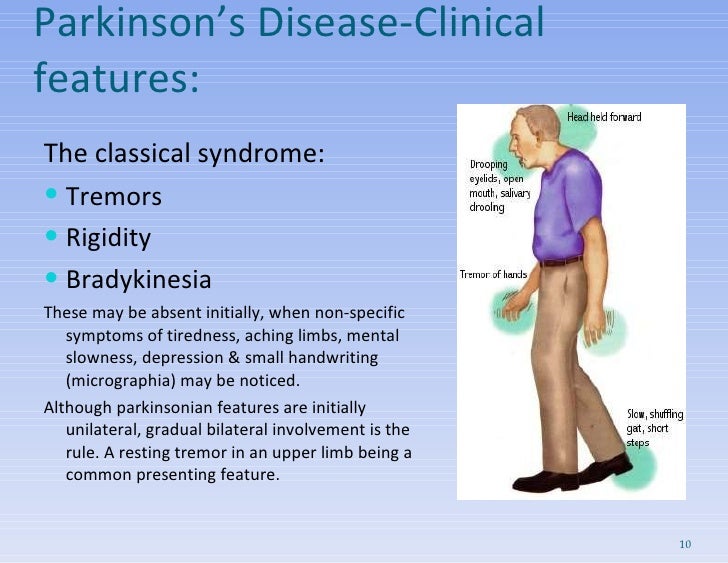
Parkinson disease surgery
Other Surgical Options | Parkinson's Foundation
In addition to deep brain stimulation (DBS) surgery and Duopa™ therapy, there are several less common surgical treatments that can help reduce some Parkinson’s disease (PD) symptoms. Like DBS and Duopa, these treatments are reserved for those who have exhausted medical treatment of Parkinson’s tremor or who live with extreme “on-off” times (motor fluctuations).
Focused Ultrasound
- Guided by magnetic resonance imaging (MRI), high-intensity, inaudible sound waves are emitted into the brain. Where these waves cross, they create high energy. This high energy creates heat, destroying a very specific area in the brain connected to tremor.
- Focused ultrasound uses computer software through an MRI. It is considered non-invasive because it does not involve incisions or holes in the skull.
- The Food and Drug Administration (FDA) has approved the procedure for those with Parkinson’s tremor refractory to medications.
-
- The person with Parkinson’s wears a special helmet called a transducer that allows focusing of ultrasound energy targeting certain areas in the brain.
- The care team uses an MRI to guide the waves and target areas, destroying the area in the brain responsible for tremor.
- Focusing the waves on a specific target and monitoring with MRI helps prevent damage in surrounding brain tissue.
-
- Many people are fully awake after surgery and can eat and drink right away.
- They can usually go home as soon as an hour later.
- The most common side effects include face numbness, arm numbness, weakness, poor balance and difficulty with speech and swallowing.
 Side effects are usually temporary but numbness (or tingling) may persist for some time after.
Side effects are usually temporary but numbness (or tingling) may persist for some time after.
-
- Focused ultrasound is not very common.
- This procedure is not recommended for those with very thick skulls or who cannot undergo an MRI.
- Additional research is needed to establish the long-term procedure benefits and risks and to determine the best candidates.
Thalamotomy
- A type of brain surgery in which the thalamus, a tiny area of the brain, is lesioned.
- Before surgery, detailed brain scans using a CT scan or MRI identify the precise location for treatment.
- Surgery on one side of the brain affects the opposite side of the body. If you have tremor in your right hand, for instance, the left side of your brain will be treated.
- The procedure can be repeated on the other side of the brain if needed, but it greatly increases the risk of speech and cognitive problems after surgery if both sides are done.
-
- During the surgery, the person with Parkinson's is awake, but the scalp area where instruments are inserted is numbed with a local anesthetic.
- The surgeon inserts a hollow probe through a small hole drilled in the skull to the target location.
- An extremely cold substance, liquid nitrogen, is circulated inside the probe. The cold probe lesions or destroys the targeted brain tissue.
- The probe is then removed and the wound is closed.
-
The surgery usually requires a two-day hospital stay.
 Most people recover completely within about six weeks. Because there are many risk factors, including underlying medical conditions, discuss the risks with your neurologist.
Most people recover completely within about six weeks. Because there are many risk factors, including underlying medical conditions, discuss the risks with your neurologist. -
- Thalamotomy is rarely done today.
- May be used to treat severe tremor on one side of the body (most often in an arm or leg) that does not respond to medications.
- Does not assist with slow movement, stiffness, speech problems or walking difficulties.
Pallidotomy
- In a pallidotomy, the surgeon lesions a tiny part of the globus pallidus by creating a scar.
- This reduces the brain activity in that area, which may help relieve movement symptoms such as tremor, stiffness and slowness.
- Before surgery, detailed brain scans using MRI identify the precise location for treatment.
- Most likely not a good option for treatment when a person has not responded to levodopa.
- Surgery on one side of the brain affects the opposite side of the body. If you have tremor in your right hand, for instance, the left side of your brain will be treated
The procedure can be repeated on the other side of the brain if needed. Pallidotomy may be considered when a person with advanced PD disease has:
- Severe motor fluctuations, such as dyskinesia and on-off responses, due to long-term levodopa treatment.
- Severe or disabling tremor, stiffness (rigidity) or slow movement (bradykinesia) medication can no longer control.

-
- The person is awake during the surgery, but the scalp area where instruments are inserted is numbed with a local anesthetic.
- The surgeon inserts a hollow probe through a small hole drilled in the skull to the target location.
- An extremely cold substance, liquid nitrogen, is circulated inside the probe.
- The cold probe lesions or destroys the targeted brain tissue. The probe is then removed, and the wound is closed.
-
The surgery usually requires a two-day hospital stay. Most people recover completely within about six weeks. Because there are many risk factors, including underlying medical conditions, discuss the risks with your neurologist.
-
- Doctors rarely perform pallidotomy today.
- Instead, doctors use DBS, a procedure that does not destroy brain tissue and has fewer risks than pallidotomy.
Subthalamotomy
- Subthalamotomy is a type of brain surgery in which the subthalamus, a tiny area of the brain, is destroyed.
- Before surgery, detailed brain scans using a CT scan or MRI identify the precise location for treatment.
- Surgery on one side of the brain affects the opposite side of the body. If you have tremor in your right hand, for instance, the left side of your brain will be treated.
- The procedure can be repeated on the other side of the brain if needed, but it greatly increases the risk of speech and cognitive problems after surgery.
-
- During the surgery, the person with Parkinson's is awake, but the scalp area where instruments are inserted is numbed with a local anesthetic.
- The surgeon inserts a hollow probe through a small hole drilled in the skull to the target location.
- An extremely cold substance, liquid nitrogen, is circulated inside the probe. The cold probe lesions or destroys the targeted brain tissue.
- The probe is then removed, and the wound is closed.
-
The surgery usually requires a two-day hospital stay. Most people recover completely within about six weeks. Because there are many risk factors, including underlying medical conditions, discuss the risks with your neurologist.

-
Subthalamotomy is rarely done today.
Page reviewed by Dr. Chauncey Spears, Clinical Assistant Professor at the University of Michigan.
Related Blog Posts
Advancing Research
Neuro Talk: Surgical Options
READ NOW
Advancing Research
What’s Hot in PD? Choosing Between Subcutaneous Apomorphine Infusions, Intestinal Pumps (Duopa) and Deep Brain Stimulation: Implications of the TOLEDO Trial
READ NOW
Science News
Possible New Key to Parkinson's Treatment
READ NOW
Deep Brain Stimulation (DBS) | Parkinson's Foundation
Deep brain stimulation (DBS) is a surgical therapy used to treat certain aspects of Parkinson’s disease (PD). This powerful therapy most addresses the movement symptoms of Parkinson’s and certain side effects caused by medications. DBS may also improve some non-motor symptoms, including sleep, pain, and urinary urgency. It is important to keep in mind that DBS can only help relieve symptoms, not cure or stop disease progression.
DBS may also improve some non-motor symptoms, including sleep, pain, and urinary urgency. It is important to keep in mind that DBS can only help relieve symptoms, not cure or stop disease progression.
The U.S. Food and Drug Administration (FDA) approved DBS surgery in:
- 1997 to treat Parkinson’s tremor
- 2002 to treat of advanced Parkinson's symptoms
- 2016 for the earlier stages of PD — for people who have had PD for at least four years and have motor symptoms not adequately controlled with medication.
DBS is the most important therapeutic advancement since the development of levodopa. It is most effective for people who experience disabling tremors, wearing-off spells, and medication-induced dyskinesias, with studies showing benefits lasting at least five years. It is not right for every person with Parkinson’s, as many issues, such as those with speech, swallow, thinking, or gait freezing, do not consistently respond to DBS therapy.
Like all brain surgeries, DBS does carry a small risk of infection, stroke, bleeding, or seizure. DBS surgery may be associated with reduced clarity of speech or even slight changes in finding the right word. A small number of people living with PD have experienced cognitive decline after DBS surgery, most so if there are concerns regarding cognition prior to surgery.
It is important that a person with Parkinson’s considering DBS surgery be informed about the procedure and be realistic in his or her expectations.
How does DBS work?
In DBS surgery, electrodes are inserted into a targeted area of the brain, using MRI (magnetic resonance imaging) and, at times, recordings of brain cell activity during the procedure. A second procedure is performed to implant an impulse generator battery (called an IPG), which is similar to a heart pacemaker and approximately the size of a stopwatch.
The IPG is placed under the collarbone or in the abdomen and delivers an electrical stimulation to targeted areas in the brain that control movement. Those who undergo DBS surgery are given a controller to turn the device on or off and review basic parameters such as battery life.
Those who undergo DBS surgery are given a controller to turn the device on or off and review basic parameters such as battery life.
DBS Facts
- DBS is a surgical procedure used to treat a variety of disabling neurological symptoms — most commonly the debilitating movement symptoms of Parkinson’s, such as tremor, stiffness, slowed movement, and slowed walking.
- DBS is not felt to damage healthy brain tissue or destroy nerve cells. Instead, the procedure is felt to interrupt problematic electrical signals from targeted areas in the brain.
- At present, the procedure is used only for people whose symptoms cannot be adequately controlled with medications.
The DBS System Consists of Three Components
-
Also called an electrode, the lead is a thin, insulated wire inserted through a small opening in the skull and implanted in a targeted area of the brain.
-
An insulated wire passed under the skin of the head, neck and shoulder, connecting the lead to the neurostimulator (IPG).
-
Also referred to as the battery pack or IPG, the neurostimulator is the third component and is usually implanted under the skin near the collarbone. In some cases, it may be implanted lower in the chest or under the skin over the abdomen.
Which brain targets should be used to implant the DBS lead?
- There are three brain targets that the FDA has approved for use in Parkinson’s: the subthalamic nucleus (STN) and the globus pallidus interna (GPi) are the most common.
- The target choice should be tailored to a person’s individual needs.
- There are many ongoing studies that will help refine target choice for individual people.
- Although the picture is not yet clear on the issue of target choice, the STN seems to provide more medication reduction, while GPi may be slightly safer for language and cognition.

FAQs
-
Although most people still need to take medication after undergoing DBS, many people experience considerable reduction of their PD symptoms and can greatly reduce their medications. The amount of reduction varies from person to person. The reduction in dose of medication can lead to decreased risk of side effects such as dyskinesia.
There is a 1 to 3% chance of infection, stroke, bleeding in the brain, or other complications associated with anesthesia. It is best to discuss associated risks with your neurologist and neurosurgeon, as diabetes and heart and lung conditions all may influence these risks and the decision to pursue surgery.
-
To determine if you are a good candidate, you:
- Have had PD symptoms for at least four years.
- Have “on/off” fluctuations despite consistent and regular medication dosing.

- Have bothersome dyskinesias. *Note: many with dyskinesias do not find these bothersome.
- Are unable to tolerate Parkinson’s medications due to side effects.
- Have tremor that is not adequately controlled with best medication trials.
- Continue to have a good response to medications, especially carbidopa-levodopa, although the duration of response may be insufficient.
- Have tried different combinations of medications under the supervision of a movement disorders neurologist.
- Have PD symptoms that interfere with daily activities.
Page reviewed by Dr. Chauncey Spears, Clinical Assistant Professor and Dr.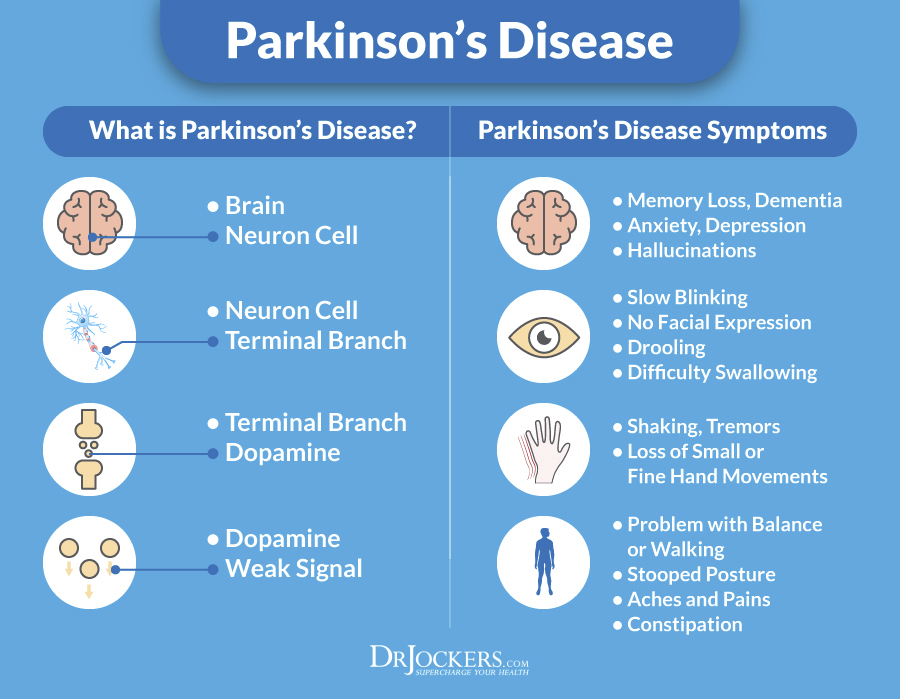 Amelia Heston, Movement Disorders Fellow at the University of Michigan.
Amelia Heston, Movement Disorders Fellow at the University of Michigan.
Related Blog Posts
Raise Awareness
8 Questions You’ve Always Wanted to Ask About Deep Brain Stimulation Surgery
READ NOW
Advancing Research
What’s Hot in PD? Choosing Between Subcutaneous Apomorphine Infusions, Intestinal Pumps (Duopa) and Deep Brain Stimulation: Implications of the TOLEDO Trial
READ NOW
Science News
Resting Tremor and DBS. Sooner Better Than Later?
READ NOW
Surgical treatment of Parkinson's disease in Israel
from March 1, 2022, Israel opened the borders for all foreign citizens, read more about the entry rules
Information for the new
Russian -speaking Israeli citizens
Employees of the International Department quickly:
- instruct how to collect documents and fill out the declarations necessary for the trip;
- organize face-to-face consultation with a doctor and all necessary procedures; nine0011
- help organize hospitalization to the clinic.

For all questions, please contact our single call center by phone 8(800)550-96-30.
Deep brain stimulation and stereotaxic surgery at the Hadassah Medical Center in Israel are surgically effective methods for the treatment of Parkinson's disease . They are resorted to in cases where drug therapy is unsuccessful and the symptoms of pathology lead to disability of a person. nine0002 There are three main types of surgery:
- stereotaxic surgery: destructive (destructive) operations - thalamotomy and pallidotomy;
- electrical stimulation of the deep parts of the brain;
- stem cell gene therapy (under development).
Most of the surgical interventions performed in the best clinics in Israel in the treatment of Parkinson's disease are neurostimulation . Less often, neurosurgeons have to resort to thalamotomy or pallidotomy, destructive operations during which certain brain cells are destroyed in various ways. nine0003
nine0003
Stereotactic surgery for Parkinson's disease
This type of surgical intervention in Israel is based on the destruction of certain areas of the central nervous system using radiofrequency electrosurgery (Gamma Knife, Cyber Knife, linear accelerator, proton accelerator).
Do you have any questions?
Contact us and get an answer
by phone: +972 2 560-97-99 (24/7) by email: [email protected] or by filling out the contact form nine0003
By clicking the "Send" button, I consent to the processing of personal data "More"
I agree to receive information materials by e-mail
For the most precise impact on the necessary areas of the brain and minimizing possible damage to surrounding tissues, all destructive manipulations are carried out in a stereotaxic way. Stereotaxis allows you to calculate and provide access to a specific point with an error of 1 mm.
nine0037 Deep brain stimulationDeep electrical stimulation of certain areas of the CNS is carried out as follows.
 Thin electrodes are applied to the subthalamic nuclei and the pale ball.
Thin electrodes are applied to the subthalamic nuclei and the pale ball. A low-frequency electric current is transmitted to the electrodes by a neurostimulator, a special device that is sewn under the skin of the chest. In most patients who underwent this manipulation, there is a significant decrease in tremor, motor and neurological disorders.
nine0002 The possibility of surgical treatment of Parkinson's disease in Israel and the choice of technique are determined purely individually for each.Readings:
- failure of drug therapy;
- rapid progression;
- under 75 years of age;
- no severe mental disorders;
- severe side effects when taking levodopa preparations;
- the duration of the disease with trembling paralysis is at least 5 years; nine0011
- absence of serious somatic changes and oncopathologies.
To increase the effectiveness of surgical treatment of Parkinson's disease, before deep brain stimulation in the Israeli clinic, the patient receives advice from the best specialists (parkinologist, neurosurgeon, neurologist) and undergoes prescribed examinations (MRI, CT) .
Deep stimulation at the Hadassah Medical Center in Israel includes two stages:
- Under local anesthesia using stereotaxic technique, 2 thin electrodes are inserted into the required areas. Their outer ends are sewn under the skin of the head. nine0011
- A week later, under general anesthesia, a pulse generator is placed under the skin of the chest and connected to the electrodes.
For more accurate information about the cost of treatment and special offers, click the button GET EXACT PRICES
Performing neurostimulation is not a reason to stop taking medications. The first switching on of the pulse generator occurs no earlier than 2-4 weeks after the intervention. It takes a few more weeks to select the optimal mode of operation of the device and the dosage of medicines. The battery for it lasts about 5 years and is then easily changed by the surgeon. nine0003
Treatment of Parkinson's disease in Israel: prognosis and organization of treatment
Surgical treatments for Parkinson's disease, carried out in Israeli medical clinics, help patients avoid severe disability, return them to their normal lives, significantly reduce the manifestations of the disease, as evidenced by the reviews left on our website.
 The emotional state improves and self-esteem increases.
The emotional state improves and self-esteem increases. To date, there is no method of radical cure for shaking paralysis, but the surgical treatment of Parkinson's disease, which patients receive in Israel at the Hadassah Medical Center, allows for many years to prolong life and avoid serious consequences, especially since our prices are much lower than in the leading centers of America and Europe .
Fill out an application or call us on the hotline, after which our consultants will contact you immediately. Employees of the coordinating service in Israel for work with foreign clients will answer all questions about the diagnosis, surgical treatment of Parkinson's disease, stay at the Hadassah Medical Center and individually determine the cost of the course .
Fill out the form and get expert advice!
Surgical treatments for Parkinson's disease | Tokarev
1. Shtok V.N., Levin O.S., Fedorova N. V. extrapyramidal disorders. M.: MIA, 2002:235.
V. extrapyramidal disorders. M.: MIA, 2002:235.
2. Bucy P.C. Surgical relief of tremor at rest. Ann. Surg. 1945;122:933–941.
3. Putnam T.J. Relief from unilateral paralysis agitans by section of the pyramidal tract. Arch. Neurol. Psychiatry. 1938;40:1049–1050.
4. Abel T.J., Walch T., Howard M.A.III. Russell Meyers (1905–1999): pioneer of functional and ultrasonic neurosurgery. J. Neurosurg. 2016;125:1589–1595. https://doi.org/10.3171/2015.9.JNS142811
5. Speelman J.D., Bosch D.A. Resurgence of functional neurosurgery for Parkinson's disease: A historical perspective. Movement Disorders. 1998;13(3):582–588. https://doi.org/10.1002/mds.870130336
6. Spiegel E.A., Wycis H.T. Pallidothalamotomy in chorea. Arch. Neurol. Psychiatry. 1950;64:295–296.
7. Spiegel E.A., Wycis H.T., Thur C. The stereoencephalotome (model III of our stereotaxic apparatus for operations on the human brain). J. Neurosurg. nineteen51;8:452–453.
8. Cooper I.S. Anterior choroidal artery ligation for involuntary movements. Science. 1953;118:193.
Anterior choroidal artery ligation for involuntary movements. Science. 1953;118:193.
9. Bravo G.J., Cooper I.S. A clinical and radiological correlation of the lesions produced by chemopallidectomy and thalamectomy. J. Neurol. neurosurgery. Psychiatry. 1959;22:1–10.
10. Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311:1670–1683. https://doi.org/10.1001/jama.2014.3654
11. Defer G.L., Widner H., Marié R.M., Rémy P., Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). mov. Discord. nineteen99;14:572–84. https://doi.org/10.1002/1531-8257(199907)14:43.0.co;2-c
12. Extrapyramidal disorders yesterday, today, tomorrow. [Sat. st]. Ed. prof. O.S. Levin. 2nd ed. M., 2015:408.
13. Akhmetzhanov V.K., Shashkin Ch.S., Jamantaeva B.D. Parkinson's disease. Pathophysiology of the extrapyramidal system. Modern ideas about the causes and pathogenesis of parkinsonism. Neurosurgery and neurology of Kazakhstan. 2016;2(43):44–51.
Neurosurgery and neurology of Kazakhstan. 2016;2(43):44–51.
14. Schuurman P.R., Bosch D.A., Bossuyt P.M.M., Bonsel G.J., Someren E.J.W., Rob Bie M.A., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N. Engl. J. Med. 2000;342:461–468. https://doi.org/10.1056/NEJM200002173420703
15. Bergman H., Wichmann T., DeLong M.R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438.
16. Aziz T.Z., Peggs D., Sambrook M.A., Crossman A.R. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. mov. Discord. 1991;6:288–292. https://doi.org/10.1159/000064599
17. Alvarez L., Macias R., Pavón N., López G., RodriguezOroz M.C., Rodriguez R. et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson's disease: results in 89patients followed up to 36 months. J. Neurol. neurosurgery. Psychiatry. 2009;80(9):979–85. https://dx.doi.org/10.1136/jnnp.2008.154948
neurosurgery. Psychiatry. 2009;80(9):979–85. https://dx.doi.org/10.1136/jnnp.2008.154948
18. Vitek J.L., Bakay R.A., Freeman A., Evatt M., Green J., McDonald W. et al. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann. Neurol. 2003;53:558–569. https://doi.org/10.1002/ana.10517
19. Eskandar E.N., Shinobu L.A., Penney J.B., Jr., Cosgrove G.R., Counihan T.J. Stereotactic pallidotomy performed without using microelectrode guidance in patients with Parkinson’s disease: surgical technique and 2-year results. J. Neurosurg. 2000;92:375–383. https://doi.org/10.1159/000068960
20. Shannon K.M., Penn R.D., Kroin J.S., Adler C.H., Janko K.A., York M. et al. Stereotactic pallidotomy for the treatment of Parkinson's disease. Efficacy and adverse effects at 6 months in 26 patients. Neurology. 1998;50:434–438. https://doi.org/10.1212/wnl.50.2.434
21. Rand R.W. Role of Cryosurgery and MRI for Parkinson's Disease. stereotact. Funct. neurosurgery. 1995;65(1–4):18–22. https://doi.org/10.1159/000098891
1995;65(1–4):18–22. https://doi.org/10.1159/000098891
22. Koller W.C., Pahwa R., Lyons K.E., Albanese A. Surgical treatment of Parkinson’s disease. J. Neurol. sci. nineteen99;167(1):1–10. https://doi.org/10.1016/S0022-510X(99)00139-2
23. Moosa S., Martínez-Fernández R., Elias W.J., Alamo M.D., Eisenberg H.M., Fishman P.S. The Role of High-Intensity Focused Ultrasound as a Symptomatic Treatment for Parkinson's Disease. mov. Discord. 2019;34(9):1243–1251. https://doi.org/10.1002/mds.27779. Epub 2019 Jul 10.
24. Ito H., Yamamoto K., Fukutake S., Odo T., Yamaguchi T., Taira T. Magnetic resonance imaging-guided focused ultrasound bilateral thalamotomy for essential tremor: A case report. Neurol. Clin. neurosci. 2020;8(6):1–3. https://doi.org/10.1111/ncn3.12438
25. Alvarez L., Macias R., Pavon N., López G., RodriguezOroz M.C., Rodriguez R. et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson's disease: results in 89 patients followed for up to 36 months. J. Neurol. neurosurgery. Psychiatry. 2009;80:979–985. https://doi.org/10.1136/jnnp.2008.154948
J. Neurol. neurosurgery. Psychiatry. 2009;80:979–985. https://doi.org/10.1136/jnnp.2008.154948
26. Krack P., Martinez-Fernandez R., Del Alamo M., Obeso J.A. Current applications and limitations of surgical treatments for movement disorders. mov. Discord. 2017;32:36–52. https://doi.org/10.1002/mds.26890
27. Niranjan A., Lunsford L.D., Kano H. Leksell Radiosurgery for Movement Disorders. Lexell Radiosurgery. Prog. Neurol. Surg. Basel, Karger, 2019;34:279–288. https://doi.org/10.1159/000493075
28. Duma C.M. Movement disorder radiosurgery - planning, physics and complication avoidance. Prog. Neurol. Surg. 2007;20:249–266. https://doi.org/10.1159/000100168
29. Young R.F., Li F., Vermeulen S., Clayton D.A., Hesselgesser R.D. Gamma Knife pallidotomy for treatment of Parkinson's disease: long term results, clinical study. Transl. Cancer Res. 2014;3:342–350. https://doi.org/10.3978/j.issn.2218-676X.2014.08.04
30. Cooper I.S. A Cryogenic Method for Physiologic Inhibition and Production of Lesions in the Brain. J. Neurosurg. 1962;19:853– 8. https://doi.org/10.3171/jns.1962.19.10.0853
J. Neurosurg. 1962;19:853– 8. https://doi.org/10.3171/jns.1962.19.10.0853
31. Kandel E.I. Functional and stereotaxic neurosurgery. M.: Medicine, 1981: 368 p.
32. Charles A., Fager M.D. Use of the Radio-Frequency Electrode in Stereotactic Surgery of Parkinson's Disease. Surgical Clinics of North America. 1965;45(3):705–713. https://doi.org/10.1016/s0039-6109(16)37593-4
33. Watkins E.S. Heat Gains in Brain During Electrocoagulative Lesions. J. Neurosurg. 1965;23(3):319–28. https://doi.org/10.3171/jns.1965.23.3.0319
34. Schreglmann S.R., Krauss J.K., Chang J.W., Bhatia K.P., Kägi G. Functional Lesional Neurosurgery for Tremor: A Systematic Review and Meta-Analysis. J. Neurol. neurosurgery. Psychiatry. 2018;89(7):717–726. https://doi.org/10.1136/jnnp-2017-316302. Epub 2018 Jan 11.
35. Ohye C., Higuchi Y., Shibazaki T., Hashimoto T., Koyama T., Hirai T. et al. Gamma Knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. neurosurgery. 2012;70:526–536. https://doi.org/10.1227/NEU.0b013e3182350893
neurosurgery. 2012;70:526–536. https://doi.org/10.1227/NEU.0b013e3182350893
36. Young R.F., Jacques S., Mark R., Kopyov O., Copcutt B., Posewitz A. et al. Gamma Knife thalamotomy for treatment of tremor: long-term results. J. Neurosurg. 2000;93(Suppl.3):128–135. https://doi.org/10.3171/jns.2000.93.supplement
37. Kooshkabadi A., Lunsford L.D., Tonetti D., Flickinger J.C., Kondziolka D. Gamma Knife thalamotomy for tremor in the magnetic resonance imaging era. J. Neurosurg .2013;118:713–718. https://doi.org/10.3171/2013.1.JNS121111
38. Niranjan A., Kondziolka D., Baser S., Heyman R., Lunsford L.D. Functional outcomes after Gamma Knife thalamotomy for essential tremor and MS-related tremor. Neurology. 2000;55:443–446. https://doi.org/10.1212/wnl.55.3.443
39. Campbell A.M., Glover J., Chiang V.L., Gerrard J., Yu J.B. Gamma Knife stereotactic radiosurgical thalamotomy for intractable tremor: a systematic review of the literature. radiother. oncol. 2015;114:296–301. https://doi.org/10.1016/j.radonc.2015.01.013
https://doi.org/10.1016/j.radonc.2015.01.013
40. Okun M.S., Stover N.P., Subramanian T., Gearing M., Wainer B.H., Holder C.A. et al. Complications of gamma knife surgery for Parkinson disease. Arch. Neurol. 2001;58:1995–2002. https://doi.org/10.1001/archneur.58.12.1995
41. Witjas T., Carron R., Krack P., Eusebio A., Vaugoyeau M., Hariz M. et al. A prospective single-blind study of Gamma Knife thalamotomy for tremor. Neurology. 2015;85:1562–1568. https://doi.org/10.1212/WNL.0000000000002087
42. Moser D. MR-guided focused ultrasound technique in functional neurosurgery: targeting accuracy. J. Ther. Ultrasound. 2013;1:3. https://doi.org/10.1186/2050-5736-1-3
43. Christian E., Yu C., Apuzzo M.L. Focused ultrasound: relevant history and prospects for the addition of mechanical energy to the neurosurgical armamentarium. World Neurosurgery. 2014;82:354–365. https://doi.org/10.1016/j.wneu.2014.06.021
44. Fry W.J., Mosberg Jr.W.H., Barnard J.W., Fry F. J. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 1954;11:471–478. https://doi.org/10.3171/jns.1954.11.5.0471
J. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 1954;11:471–478. https://doi.org/10.3171/jns.1954.11.5.0471
45. Jagannathan J., Sanghvi N.T., Crum L.A., Yen C.-P., Medel R., Dumont A.S. et al. High-intensity focused ultrasound surgery of the brain: part 1 — a historical perspective with modern applications. Neurosurgery 2009;64:201–210; discussion, 210–211. https://doi.org/10.1227/01.NEU.0000336766.18197.8E
46. Gallay M.N., Moser D., Jeanmonod D. Safety and accuracy of incisionless transcranial MR-guided focused ultrasound functional neurosurgery: single-center experience with 253 targets in 180 treatments. J. Neurosurg. 2018;May 1. https://doi.org/10.3171/2017.12.JNS172054
47. Bond A.E., Shah B.B., Huss D.S., Dallapiazza R.F., Warren A., Harrison M.B. et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremordominant Parkinson disease: a randomized clinical trial.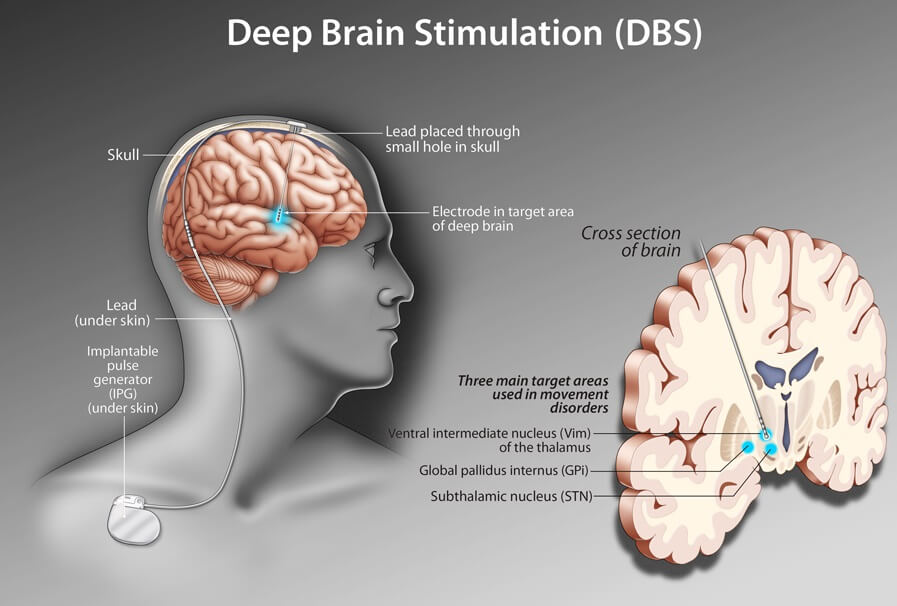 JAMA Neurol. 2017;74:1412–1418. https://doi.org/10.1001/jamaneurol.2017.3098
JAMA Neurol. 2017;74:1412–1418. https://doi.org/10.1001/jamaneurol.2017.3098
48. Jung N.Y., Rachmilevitch I., Sibiger O., Amar T., Zadicario E., Chang J.W. Factors Related to Successful Energy Transmission of Focused Ultrasound through a Skull: A Study in Human Cadavers and Its Comparison with Clinical Experiences. J. Korean Neurosurg. soc. 2019;62(6):712–722. https://doi.org/10.3340/jkns.2018.0226
49. Mazerolle E.L., Seasonsa G.M., Warwaruk-Rogers R., Romo P., Nordal R., Sevick R.J. et al. Focused ultrasound resolves persistent radiosurgery related change in a patient with tremor. Radiology Case Reports. 2019;14(10):1233–1236. https://doi.org/10.1016/j.radcr.2019.07.010
50. Zalyalova Z.A. Deep brain stimulation. How does it control movement in Parkinson's disease? Neurosurgery. 2019;21(3):93–99. https://doi.org/10.17650/1683-3295-2019-21-3-93-99
51. Benazzouz A., Hallett M. Mechanism of Action of Deep Brain Stimulation. Neurology. 2000;55(12Suppl.6):S13–6. PMID: 11188968.
PMID: 11188968.
52. Tomsky A.A., Bril E.V., Gamaleya A.A., Fedorova N.V., Levin O.S. Functional neurosurgery for Parkinson's disease in Russia. Annals of clinical and experimental neurology. 2019;13(4):10–15. https://doi.org/10.25692/ACEN.2019.4.2
53. Follett K.A., Weaver F.M., Stern M., Kwan Hur, Harris C.L., Luo P. et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J. Med. 2010;362:2077–2091. https://doi.org/10.1056/NEJMoa0907083
54. Voon V., Kubu C., Krack P., Houeto J.-L., Tröster A.I. Deep brain stimulation: Neuropsychological and neuropsychiatric issues. mov. Discord. 2006;21Suppl.14:S305-27. https://doi.org/10.1002/mds.20963
55. Vendette M., Gagnon J.-F., Decary A., Massicotte-Marquez J., Postuma R.B., Doyon J. et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson's disease without dementia. Neurology. 2007;69(19):1843–9. https://doi.org/10.1212/01.wnl.0000278114.14096.74
56.