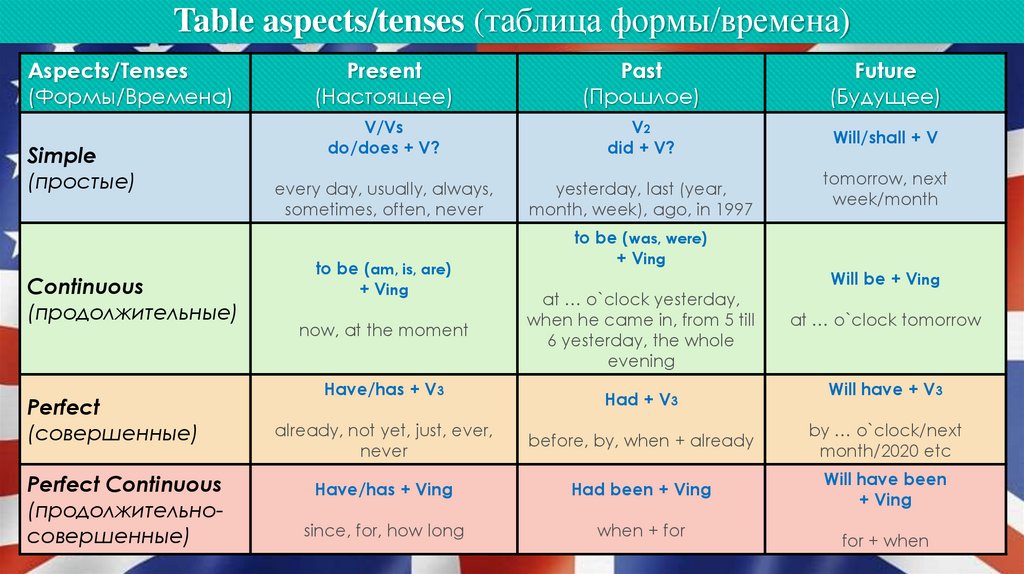
Nature vs. nurture schizophrenia
Nature and nurture interplay: schizophrenia
. 2004 Nov;31 Suppl 2:S189-93.
doi: 10.1055/s-2004-834565.
Peter McGuffin 1
Affiliations
Affiliation
- 1 Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry London, Great Britain.
- PMID: 15586309
- DOI: 10.1055/s-2004-834565
Peter McGuffin. Psychiatr Prax. 2004 Nov.
. 2004 Nov;31 Suppl 2:S189-93.
doi: 10.1055/s-2004-834565.
Author
Peter McGuffin 1
Affiliation
- 1 Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry London, Great Britain.
- PMID: 15586309
- DOI: 10.1055/s-2004-834565
Abstract
There is compelling evidence from family, twin and adoption studies of a substantial genetic contribution to schizophrenia. The mode of transmission is complicated and very rarely if ever involves a single gene. Rather schizophrenia results from multiple genes of small effect and their interplay with the environment. Perhaps because the overall size of the genetic effect is large, accounting for about 80 % of variance, definite environmental factors have been difficult to pin down. It has even been suggested that "the environment" consists entirely of epigenetic or stochastic phenomena that can never be detected by a standard epidemiological methods. Nevertheless, a variety of social stressors, including high expressed emotion in relatives and life events affect the course of illness and certain physical factors such as obstetric complications and cannabis smoking have been implicated in contributing to liability to the disorder. The recent discovery of several positional candidate genes that have been replicated as being associated with liability to schizophrenia holds considerable promise not just for a better understanding of the neurobiology but also for improved knowledge about risk prediction and gene-environment interplay.
Rather schizophrenia results from multiple genes of small effect and their interplay with the environment. Perhaps because the overall size of the genetic effect is large, accounting for about 80 % of variance, definite environmental factors have been difficult to pin down. It has even been suggested that "the environment" consists entirely of epigenetic or stochastic phenomena that can never be detected by a standard epidemiological methods. Nevertheless, a variety of social stressors, including high expressed emotion in relatives and life events affect the course of illness and certain physical factors such as obstetric complications and cannabis smoking have been implicated in contributing to liability to the disorder. The recent discovery of several positional candidate genes that have been replicated as being associated with liability to schizophrenia holds considerable promise not just for a better understanding of the neurobiology but also for improved knowledge about risk prediction and gene-environment interplay.
Similar articles
-
Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia.
Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Brans RG, et al. Arch Gen Psychiatry. 2008 Nov;65(11):1259-68. doi: 10.1001/archpsyc.65.11.1259. Arch Gen Psychiatry. 2008. PMID: 18981337
-
The environment and schizophrenia: the role of cannabis use.
Henquet C, Murray R, Linszen D, van Os J. Henquet C, et al. Schizophr Bull. 2005 Jul;31(3):608-12. doi: 10.1093/schbul/sbi027. Epub 2005 Jun 23. Schizophr Bull. 2005. PMID: 15976013
-
Mechanisms of gene-environment interactions in depression: evidence that genes potentiate multiple sources of adversity.
Wichers M, Schrijvers D, Geschwind N, Jacobs N, Myin-Germeys I, Thiery E, Derom C, Sabbe B, Peeters F, Delespaul P, van Os J. Wichers M, et al. Psychol Med. 2009 Jul;39(7):1077-86. doi: 10.1017/S0033291708004388. Epub 2008 Oct 6. Psychol Med. 2009. PMID: 18834553
-
Are there genetic influences on addiction: evidence from family, adoption and twin studies.
Agrawal A, Lynskey MT. Agrawal A, et al. Addiction. 2008 Jul;103(7):1069-81. doi: 10.1111/j.1360-0443.2008.02213.x. Epub 2008 May 20. Addiction. 2008. PMID: 18494843 Review.
-
[Risk factors of schizophrenia].
Suvisaari J. Suvisaari J. Duodecim. 2010;126(8):869-76. Duodecim. 2010. PMID: 20597333 Review. Finnish.

See all similar articles
Cited by
-
Early-Onset Schizophrenia With Predominantly Negative Symptoms: A Case Study of a Drug-Naive Female Patient Treated With Cariprazine.
Molnar MJ, Jimoh IJ, Zeke H, Palásti Á, Fedor M. Molnar MJ, et al. Front Pharmacol. 2020 Apr 23;11:477. doi: 10.3389/fphar.2020.00477. eCollection 2020. Front Pharmacol. 2020. PMID: 32390838 Free PMC article.
-
Genetic variations of PIP4K2A confer vulnerability to poor antipsychotic response in severely ill schizophrenia patients.
Kaur H, Jajodia A, Grover S, Baghel R, Gupta M, Jain S, Kukreti R. Kaur H, et al. PLoS One. 2014 Jul 15;9(7):e102556. doi: 10.1371/journal.pone.0102556. eCollection 2014.
 PLoS One. 2014. PMID: 25025909 Free PMC article.
PLoS One. 2014. PMID: 25025909 Free PMC article. -
Gene expression profiling in rodent models for schizophrenia.
Schijndel JE, Martens GJ. Schijndel JE, et al. Curr Neuropharmacol. 2010 Dec;8(4):382-93. doi: 10.2174/157015910793358132. Curr Neuropharmacol. 2010. PMID: 21629445 Free PMC article.
-
Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder.
Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Moreau MP, et al. Biol Psychiatry. 2011 Jan 15;69(2):188-93. doi: 10.1016/j.biopsych.2010.09.039. Biol Psychiatry. 2011. PMID: 21183010 Free PMC article.
-
New findings in the genetics of major psychoses.

Nöthen MM, Nieratschker V, Cichon S, Rietschel M. Nöthen MM, et al. Dialogues Clin Neurosci. 2010;12(1):85-93. doi: 10.31887/DCNS.2010.12.1/mnoethen. Dialogues Clin Neurosci. 2010. PMID: 20373670 Free PMC article. Review.
See all "Cited by" articles
MeSH terms
Schizophrenia: Nature vs Nurture - BC Schizophrenia Society
PERSON 1: When I was first diagnosed, I went and was trying to understand the contributing factors to having psychosis. And it listed genetics has been one of them, but maybe not one of the highest factors, but definitely one of them.
PERSON 2: My identical twin, who had passed away at the age of, uh, 26. So he has schizophrenia, but ironically, on the topic of genetics, he was able to take Ativan, and I was unable to take Ativan.
PERSON 3: I have mental illness in my family history. And sometimes it makes me nervous about having my own kids. I don’t know how I feel about passing on mental illness. But I also think, like who better of a parent to have than someone with mental illness, to help someone go through something like that.
And sometimes it makes me nervous about having my own kids. I don’t know how I feel about passing on mental illness. But I also think, like who better of a parent to have than someone with mental illness, to help someone go through something like that.
PERSON 4: I have an uncle who has schizophrenia. I remember, I did think he was crazy and I feel so awful that I thought that. Then I was diagnosed with schizophrenia. And it’s like, oh, that was a little bit of a reality check.
FAYDRA: My name is Faydra Aldridge. Welcome to Look Again: Mental Illness Re-Examined, a podcast about mental illness brought to you by the BC Schizophrenia Society and our BC partner organization. Researchers have estimated that about 80% of the risk of developing schizophrenia is hereditary. And yet, that doesn’t mean people with that genetic component will actually develop the disorder.
So today, we want to get into the why is that the case? We’re going to be looking at the role genetics play in the development and onset of schizophrenia. Is it all about your genes? Or are there other potential risks that can trigger this illness? So to help us understand some of these questions and many more, we’re going to be talking to two people.
Is it all about your genes? Or are there other potential risks that can trigger this illness? So to help us understand some of these questions and many more, we’re going to be talking to two people.
Dr. Robert Stowe is a behavioral neurologist in the University of British Columbia Neuropsychiatry Program. He’s also a member of an international genetic testing task force. He’s also a researcher involved in a clinical and genetic counseling research project, dealing with treatment resistant schizophrenia and schizoaffective disorder. It is called the MAGERS project at UBC in the Faculty of Medicine. We are going to be hearing more about that project in just a little while, but first let’s meet Courtney. Courtney Cook has her master’s in genetic counseling from UBC and works as a genetic counselor on the MAGERS project.
As part of her work with this project, she provides psychiatric genetic counseling, and does genome sequencing. Dr. Stowe and Courtney, welcome to the show.
- STOWE: Thank you very much Faydra, for that generous introduction.

FAYDRA: And we have a lot to unpack, so let’s get to it. Dr. Stowe, I really wanted to get into the work that you and Courtney have been doing around this MAGERS project. But first let’s back up and start talking about genetics and serious mental illness. Now we all know that psychiatric conditions are usually caused by a combination of environmental and biological factors. But how much of a role does genetics play when it comes to an illness like schizophrenia?
- STOWE: I think you’re quite correct in saying that on average, about 80% of the heritability of schizophrenia is attributable to genetic risk factors.
However, having said that, there is no known genetic condition that is a hundred percent predictive of the risk of schizophrenia. So there’s always some additional component.
FAYDRA: I find it fascinating Dr. Stowe. 80% is quite high. Why is it that some people get it, and some people don’t, with such a high percentage?
- STOWE: Heritability in some cases includes some environmental risk factors.
 So for example, if you look at identical twins, they will share the vast majority of their genome, over 99%. But if they’re exposed to a risk factor in utero, then that can account for some of the risks and it would be inherited by both of them. But it’s truly, the environmental piece is not then part of their genetics. There is a mechanism by which genes exert their influence in our bodies and our brains. And that’s called epigenetics. That is a type of genetic risk factor, but it’s not one that’s transmitted by our genes. It’s the way that our genes are expressed in the body, in the brain, that can be modified by environmental risks.
So for example, if you look at identical twins, they will share the vast majority of their genome, over 99%. But if they’re exposed to a risk factor in utero, then that can account for some of the risks and it would be inherited by both of them. But it’s truly, the environmental piece is not then part of their genetics. There is a mechanism by which genes exert their influence in our bodies and our brains. And that’s called epigenetics. That is a type of genetic risk factor, but it’s not one that’s transmitted by our genes. It’s the way that our genes are expressed in the body, in the brain, that can be modified by environmental risks.
FAYDRA: Courtney is going to fill us in. So Courtney let’s hear it. Let’s talk about the environmental factors that could potentially lead to an illness like schizophrenia.
COURTNEY: Absolutely. I think how I want to do that actually, is bringing it all in together with an analogy that we use with patients in the clinics.
It’s a way that psychiatric genetic counseling is really done. And it’s facilitated by the use of something called the mental illness jar analogy. So it’s really helpful for just conceptualizing all of this together. How do genes, environment, all of this fit into a picture that actually makes sense to the average person. So thinking of this analogy, you think of an empty glass jar.
And it’s facilitated by the use of something called the mental illness jar analogy. So it’s really helpful for just conceptualizing all of this together. How do genes, environment, all of this fit into a picture that actually makes sense to the average person. So thinking of this analogy, you think of an empty glass jar.
This is our mental illness jar. And everyone has a mental illness jar, which means that everyone has the possibility of developing a mental illness. So when it’s full all the way to the top, that’s when someone’s having an active episode of mental illness. And so thinking about the genetic factors first, or the role that genes play.
So thinking of that empty jar, you can have small balls in that jar. And each of those little balls is a genetic risk factor. Only having a small effect individually, fill up this charge with certain capacity. And each individual is going to have some of this genetic vulnerability to develop mental illness.
Some individuals are born with more balls in their jar. Other individuals are born with a lot less. The vast majority of the population is going to be somewhere in the middle. And that’s not the whole story. We also have this role of environment. You can think of the environmental, or the sort of life experience, as these triangles that get added into that jar.
Other individuals are born with a lot less. The vast majority of the population is going to be somewhere in the middle. And that’s not the whole story. We also have this role of environment. You can think of the environmental, or the sort of life experience, as these triangles that get added into that jar.
Like head trauma in childhood, the time of the year that someone’s born at, different stressful life experiences. So environmental sort of emotional stressors that someone might experience throughout their life. That those then get added into the jar. And some of those things where these protective factors. It’s the things that we know are good for us, but are always harder to do, like diet, exercise, good sleep, nutrition.
You can think of them as these rings that are getting stacked on to that jar. So making the jar larger, making it less likely to fill to the top. And these can be helpful for individuals with schizophrenia. But if they’re able to employ those protective factors that work for them, they may not be experiencing an active episode of mental illness.
So someone could be born with a high degree of genetic vulnerability, but if they don’t have those environmental factors throughout their life, they’re not going to go on to develop a mental illness. Conversely, someone could have a lot lower genetic vulnerability, but because they have all of these sort of known environmental risk factors, life stressors, throughout their life. That they might develop mental illness themselves.
- STOWE: So you can imagine that the environmental, the triangles, you can actually remove some of them. And so the triangles are mobile. They can go in or out of the jar. And temporarily, when you’re under a lot of stress, you’ve got more triangles in the jar and it goes over the top. And then with medications, with psychotherapy, and stress reduction, with various other approaches, you can take some of those triangles out of the jar.
FAYDRA: Courtney, can you back up and just tell us what it is and what exactly do you do?
COURTNEY: Genetic counseling. Helping individuals with genetic conditions adapt to the social, the familial implications of that condition. In the context of psychiatric, you know, counseling is a lot what we’re doing. Going through that jar model with people, helping them to understand the different factors that may have contributed to their illness, and really with the goal of helping individuals adapt to that.
Helping individuals with genetic conditions adapt to the social, the familial implications of that condition. In the context of psychiatric, you know, counseling is a lot what we’re doing. Going through that jar model with people, helping them to understand the different factors that may have contributed to their illness, and really with the goal of helping individuals adapt to that.
So helping with feelings of guilt, or shame, or blame that a lot of individuals with mental illness experience, but it wasn’t your fault that you developed a mental illness.
FAYDRA: You’re listening to Look Again: Mental Illness Re-Examined. A podcast brought to you by the BC Schizophrenia Society and BC partner organizations.
I’m your host Faydra Aldridge. This podcast would not be possible without the support of the entire community. From the bottom of our hearts, we want to thank you for caring about mental illness. Together we truly can make a difference.
And we’re back with Dr. Stowe and Courtney Cook. This episode, we’re getting into the genetics of schizophrenia. And there’s a lot to unpack when it comes to why someone develops a serious mental illness. There are so many factors to consider. Both environmental and biological. And it’s those biological factors, our DNA, which can affect brain function that Dr. Stowe and his team at UBC are looking at through the MAGERS project. I’d like to hear more about the genetic component of schizophrenia. Is there a single gene that causes this illness or are there a number of genes that can cause schizophrenia?
Stowe and Courtney Cook. This episode, we’re getting into the genetics of schizophrenia. And there’s a lot to unpack when it comes to why someone develops a serious mental illness. There are so many factors to consider. Both environmental and biological. And it’s those biological factors, our DNA, which can affect brain function that Dr. Stowe and his team at UBC are looking at through the MAGERS project. I’d like to hear more about the genetic component of schizophrenia. Is there a single gene that causes this illness or are there a number of genes that can cause schizophrenia?
- STOWE: So in the vast majority of cases, there doesn’t appear to be a single gene that contributes a major risk of illness.
We believe that individuals who have treatment resistant schizophrenia, and severe schizophrenia, are more likely to have rare mutations in a single gene. That contributes a very substantial part of their risk of schizophrenia. But for most individuals with schizophrenia, the genetic component is actually what we call polygenic risk. Which is basically the sum of many variations that are fairly common in the population. Each of which contributes a tiny amount of risk. But when you add them all up, they can put you over the threshold for susceptibility to schizophrenia.
Which is basically the sum of many variations that are fairly common in the population. Each of which contributes a tiny amount of risk. But when you add them all up, they can put you over the threshold for susceptibility to schizophrenia.
FAYDRA: That’s really fascinating. Here with our families and the people we work with, a lot of frustration around why have we not been able to come up with a treatment that can cure schizophrenia? But hearing you talk about the mutations within the gene makes sense to me, as to why it would be so difficult for people like yourselves to come up with basis and understanding which will hopefully lead to effective treatment.
- STOWE: We know that there are over a thousand genes, that actually where a common variation in these genes, actually contributes a little bit of risk to schizophrenia. And there are many different pathways that are targeted by these genes, or that the proteins that these genes encode function in. And so our project is basically what we call a precision medicine focused project.

It’s based on the assumption that everybody is different. We all are individuals. And individuals with schizophrenia have in many cases, different reasons why they ended up with their illness, both genetic and environmental. And so we think that actually studying individuals who have severe psychosis and are treatment resistant, will help us identify those rare and potent mutations in genes, that will help pinpoint the pathways that are operative to predispose them to illness.
And then, if we understand the pathway and we understand the mechanism, we may be able to come up with a treatment that’s individualized. Furthermore, identification of the pathway, even if it may not have benefit for an individual, will advance the field. Because then we can look for other genes that are involved in the same pathway, and see if they’re also targeted by some of these rare mutations or by common variation.
FAYDRA: Tell us, when did it start? Who’s involved? Let’s talk about the MAGERS project.
- STOWE: We recruited our first participant in 2016, and it’s really involved a very large group over 40 co-investigators. And they run the gamut from clinicians like myself and Courtney, to individuals at BC Genome Sciences Centre. There are individuals at the Royal Columbian Hospital Cytogenetics Lab. We have a group at BC Children’s Hospital. We have investigators at the Biomedical Research Centre at BC Children’s, and we’ve got a bioinformatics group at the Michael Smith Labs. So it’s really a very eclectic group.
FAYDRA: What are you hoping to achieve with this project? What would you like to see as the final outcome?
- STOWE: As I indicated earlier, individuals who harbor rare variants, can point to very important mechanisms, that may predispose to psychotic symptoms. We’re hoping to find some of those. And we have found some of those already. But we believe that individuals who are unfortunately afflicted with severe illness, or treatment resistant illness, are a very important population to focus in on.
 Because they’re more likely to harbor these rare variants, and the pharmaceutical industry has spent billions of dollars trying to mind the common variant, and they have largely been unsuccessful. So now I think it’s really important that we focus on the rare variants, and I think our patients, our participants, are very valuable in this, in this process. And they’re valued collaborators in the process. They’ve been very generous with their time and their support for the project.
Because they’re more likely to harbor these rare variants, and the pharmaceutical industry has spent billions of dollars trying to mind the common variant, and they have largely been unsuccessful. So now I think it’s really important that we focus on the rare variants, and I think our patients, our participants, are very valuable in this, in this process. And they’re valued collaborators in the process. They’ve been very generous with their time and their support for the project.
COURTNEY: Something that’s unique to the MAGERS project is this design, and the genetic counseling within the protocol of the study. So, well our participants at entry into the study received psychiatric genetic counseling, and that all the return of the different genetic testing results that we’ve done is under this sort of umbrella of genetic counseling.
Unfortunately, psychiatric genetic counseling isn’t widely available to individuals. There is a clinic in Vancouver here. But across Canada it’s not something widely available to the general population. And so they’re building in some of these outcome measures within this study. We want to be able to show that genetic counseling is effective and it’s helpful for this population.
And so they’re building in some of these outcome measures within this study. We want to be able to show that genetic counseling is effective and it’s helpful for this population.
- STOWE: And I just want to amplify a point that Courtney made about availability. And so just as psychiatric genetic counseling is not readily available, genome sequencing is not readily available either. It’s not covered by MSP under ordinary circumstances. And in fact, the agencies that do approve it under special circumstances, have been very resistant to ordering and to paying for it in individuals with psychiatric illness.
Even when we know that these individuals are likely to harbor these rare variants, and that it might have some impact on treatment. So another goal of the project is to really demonstrate that, at least in individuals with more severe illness, that we should be considering genome sequencing and making it available.
Which will not only, we think moves the field forward, but hopefully lead to some targeted treatments for some of the individuals. And I should point out the treatment doesn’t necessarily have to be a prescription medication. It could be a nutraceutical like a vitamin and mineral, depending on what the actual mechanism is. And we’re hoping to find treatments that are better tolerated and more effective, than some of the treatments we have.
And I should point out the treatment doesn’t necessarily have to be a prescription medication. It could be a nutraceutical like a vitamin and mineral, depending on what the actual mechanism is. And we’re hoping to find treatments that are better tolerated and more effective, than some of the treatments we have.
FAYDRA: Genetic testing is controversial. I’d like to get into that. Why is it so controversial in our country right now?
- STOWE: I think there are a number of possible reasons for that. The first is maybe a historical reason, and that there’s been a resistance to the idea that major psychiatric illnesses like schizophrenia are genetically driven.
And the concern is that we might blame everything on the genetics and forget about the importance of environment, and then downplay the importance of psychotherapy and mindfulness, then all these other strategies that can help promote wellness. Another is that it’s the wild west out there in terms of direct to consumer genetic testing.
So there are companies out there that will do your whole exome and sorting out the wheat from the chaff. Trying to figure out which ones are really important is technically difficult. And so there are risks of over interpretation. And one of the reasons we have genetic counselors like Courtney on the project, is because they’re very well-trained in trying to separate the wheat from the chaff. My colleagues on the International Society of Psychiatric Genetics taskforce on genetic testing, we don’t think that sequencing is advisable in most individuals with schizophrenia. Because the yield is going to be very low. But in individuals who have treatment resistant illness, or severe illness, that’s a different story. And there we think there is a role for this sort of testing. I think it is important to recognize that genes are not the whole story, and there’s a balance, but I think the controversy in the field will eventually resolve itself as we discover more of the genetic risk factor.
COURTNEY: Um, and just to really, I think really summarize what Bob was saying there, is that genetic testing isn’t necessarily going to be helpful, beneficial in every individual with schizophrenia. Um, that there’s really, there’s certain unique things that we’re looking for in the family history, in that medical history, that might raise our suspicion. That there might be one of these more significant genetic risk factors. One of these rare variants.
FAYDRA: What do you think is the connection between genetic testing and the stigma associated with schizophrenia?
COURTNEY: I think that’s not unique to psychiatric genetics either. There’s often this idea of if something’s inherited, or it’s genetic, or you’re born with it, that there’s more stigma. There’s more blame within a family.
I think one thing that we really try to emphasize with families, no one has control over their genetic information. That’s the way it is. You don’t control it. You pass on to your children, you don’t have control over what you’re born with. And yeah, like really enforcing that fact. Cause that’s often, it’s just it’s common people just have that.
And yeah, like really enforcing that fact. Cause that’s often, it’s just it’s common people just have that.
They internalize that at some point. And it’s really important for us to emphasize again, it’s nothing that we have control over.
- STOWE: I would just add to that. In our study, you know we’re doing pre-test genetic counseling as well. And that’s important, because it’s an opportunity to explore how an individual might react to a genetic finding.
And in some cases, we think in most cases, parents are relieved to hear that if a child has inherited a strong genetic risk factor, that it wasn’t bad parenting that contributed to the illness. On the other hand, there might be some parents who say “Oh, I had a brother with schizophrenia, or I had a grandfather, or an uncle with schizophrenia and I should have known better. I shouldn’t have had children. This is my fault.”
So it’s important I think, that individuals think about what the implications of genetic testing might mean for them, and whether it would be helpful or harmful. And we think in the vast majority of cases, there are ways to deliver that information that actually relieves guilt, and stigma, and blame and those sorts of things.
And we think in the vast majority of cases, there are ways to deliver that information that actually relieves guilt, and stigma, and blame and those sorts of things.
But everyone’s different. And we have to look at each individual differently from that perspective.
COURTNEY: Absolutely. No, that’s a huge part of genetic counseling is taking that individual’s values, their reactions, and applying it to the situation. Helping them to process those results, or how they would feel about this testing, before it’s done. So it is going to be beneficial for that person.
FAYDRA: So if there is a family member that has one uncle, or a brother, or sister that has schizophrenia, what would you say if they were thinking about getting genetic testing to see if they have the gene for schizophrenia?
- STOWE: First of all I would emphasize again, that there is no one gene from schizophrenia. And I think what I would advise, is psychiatric genetic counseling. And that’s available outside our project through the Adapt Clinic.
 And psychiatric genetic counseling can be helpful in family members understanding risk to children, for example, or if you have a sibling with schizophrenia, what’s your risk of developing the illness.
And psychiatric genetic counseling can be helpful in family members understanding risk to children, for example, or if you have a sibling with schizophrenia, what’s your risk of developing the illness.
And what they’ll do is actually look at the family history through three generations, and they can use that information to advise the individuals on what their risk might be in that situation. I don’t think anyone should go into genetic testing, or even whole genome sequencing, and assume that they’re going to get genetic, um, diagnosis.
Many individuals and families overestimate their genetic vulnerability. And so it’s often an individual may decide not to have children. Because they’re worried about they had an uncle, a grandfather, or somebody with schizophrenia and they’ve grossly overestimated the risk that their child would have schizophrenia.
So what would I advise in terms of genetic testing? If someone has treatment resistant illness, I think pharmacogenomic profiling can be helpful. And that is available on a sort of private pay basis. There are a variety of companies that do that even here in the Lower Mainland. And if the individual has had learning, or also has been diagnosed with as being on the autism spectrum, or if they have any unusual facial characteristics, or heart murmurs, or congenital heart disease. Or there are a variety of things that increase the risk that there is a single gene mutation or a chromosomal variant, then they could ask their psychiatrist or family physician to order a chromosomal microarray screening. Because that is actually available both at BC Children’s Hospital and for individuals who live in the Fraser Health region. And that’s covered, there’s no charge to patients for that testing.
And that is available on a sort of private pay basis. There are a variety of companies that do that even here in the Lower Mainland. And if the individual has had learning, or also has been diagnosed with as being on the autism spectrum, or if they have any unusual facial characteristics, or heart murmurs, or congenital heart disease. Or there are a variety of things that increase the risk that there is a single gene mutation or a chromosomal variant, then they could ask their psychiatrist or family physician to order a chromosomal microarray screening. Because that is actually available both at BC Children’s Hospital and for individuals who live in the Fraser Health region. And that’s covered, there’s no charge to patients for that testing.
That would be the first step. And then if that’s negative, but there are strong reasons to suspect that there’s a potent single gene mutation, that might in fact be transmitted through multiple effected individuals in the family, then the whole genome or whole exome sequencing might be worth looking at. But that would really require a consultation by a medical geneticist, typically.
But that would really require a consultation by a medical geneticist, typically.
Um, those are, unfortunately, they’ve got about a one-year waiting list at this point.
FAYDRA: Dr. Stowe, Courtney, it was such a pleasure speaking with both of you. Thank you so much for joining me today.
- STOWE: Thank you again for having us. It was a real pleasure and an honor.
COURTNEY: So appreciative of yourselves here at giving us this opportunity to talk about our project, and talk about the work we do.
FAYDRA: And a huge thank you to you, our audience for joining us for this episode.
Together, we can better understand and change the narrative around mental illnesses like schizophrenia. I hope you learned as much as I did today. And if you have any questions or any comments at all, tweet us at BC Schizophrenia. And to get our latest podcast episodes, be sure to hit follow on Apple Podcasts, Spotify, or anywhere where you listen to podcasts.
We hope you join us next episode. Talk to you soon.
Talk to you soon.
MALE VOICE: This podcast is brought to you by the BC Schizophrenia Society and the BC partners for mental health and substance use information. We’re a group of non-profit agencies providing good quality information to help individuals and families maintain or improve their mental wellbeing. The BC partners members are Anxiety Canada, BC Schizophrenia Society, Canadian Institute for Substance Use Research, Canadian Mental Health Association BC Division, Family Smart, Jesse’s Legacy and North Shore Family Services Program and Mood Disorders Association of BC. A branch of Lookout Housing and Health Society.
The BC partners are funded and stewarded by BC Mental Health and Substance use Services an agency of the Provincial Health Services Authority. For more information, visit heretohelp.bc.ca.
The largest study of schizophrenia has identified more than 100 genes that cause this disease
The largest study of schizophrenia has been carried out: Vera Golimbet, Doctor of Biology, told Gazeta.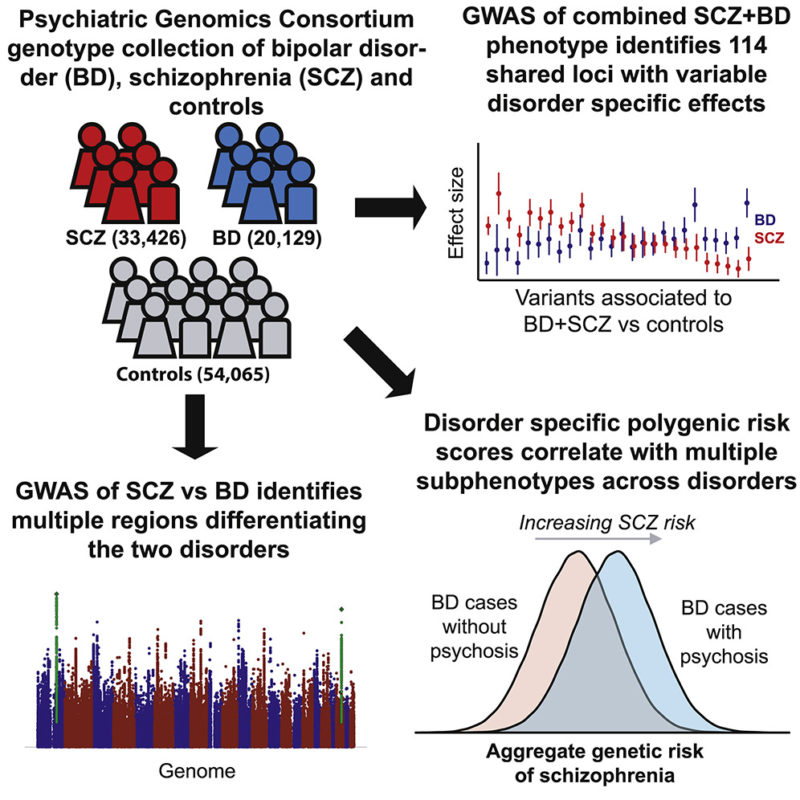 Ru about its results and the contribution of Russian geneticists to this work.
Ru about its results and the contribution of Russian geneticists to this work.
Schizophrenia is one of those mental illnesses that everyone has heard of. But why this disease happens and what links of brain mechanics break down in this case is still unclear even to doctors. The ambiguity of the biological nature entails the uncertainty of the diagnosis, therefore, anything can be squeezed into it, from dissent, which was used with might and main by the punitive Soviet psychiatry with a wonderful diagnosis " sluggish schizophrenia ”, to autism, which until recently, when a person reached the age of 18, suddenly turned into schizophrenia .
Schizophrenia is more complicated than it was said
Scientists managed to obtain molecular clues to the occurrence of schizophrenia by sequencing the genomes of patients ...
January 23 13:38
If the biological nature of the disease is unclear, then how to treat it is also unclear.
Therefore, until now, schizophrenia is treated by the same methods as 60 years ago, namely, antipsychotic drugs, or antipsychotics.(157).jpg)
They suppress, inhibit the processes of excitation in the central nervous system, extinguish psychoses, including hallucinations, paranoia. But this is only part of the symptoms of schizophrenia. At the same time, neuroleptics not only do not improve intellectual activity, social behavior, but, on the contrary, inhibit it. And as was recently shown, in high doses leads to a decrease in brain volume . No fundamentally new treatment has been invented during this time.
Schizophrenia affects on average every hundredth inhabitant of the planet. It often occurs in adolescence and young age, up to 20 years.
The economic burden of schizophrenia, for example, in the United States is $60 million, but for Russia it simply has not been calculated.
Since schizophrenia is largely dependent on heredity, its genetic nature, scientists have taken care not yesterday. So, in a study published in Nature six months ago, about which Gazeta.Ru wrote, methods of complete sequencing (reading DNA) of the genomes of patients and healthy volunteers managed to identify about 30 mutations associated with schizophrenia. Latest study, , the results of which are published in the latest issue of Nature , is much larger. Russian researchers from the Institute of Molecular Genetics of the Russian Academy of Sciences and the Scientific Center for Mental Health of the Russian Academy of Medical Sciences also took part in it.
Latest study, , the results of which are published in the latest issue of Nature , is much larger. Russian researchers from the Institute of Molecular Genetics of the Russian Academy of Sciences and the Scientific Center for Mental Health of the Russian Academy of Medical Sciences also took part in it.
Scientists analyzed nearly 37,000 DNA samples from schizophrenic patients compared to 113,000 DNA from healthy people, both European and Asian.
Antipsychotics dry out the brain
Taking antipsychotics, the main drug prescribed for patients with schizophrenia...
September 18 14:07
This is the result of several years of research by the Schizophrenia Working Group of the Psychiatric Genomics Consortium , an international, multidisciplinary collaboration founded in 2007. It includes about 500 researchers from more than 80 organizations in 25 countries. The work is coordinated by specialists from the Broad Institute of the Massachusetts General Hospital.
Scientists applied the so-called Genome-Wide Association Search (GWAS) . Two words about what it is. Throughout the genome (that is, DNA) there are regions in which one nucleotide can be replaced by another - these are the so-called polymorphic regions. They may differ for different people. This is single nucleotide polymorphism (SNP) - the same as point mutations. So, the variations of these parts of the genome are compared in a population of healthy people and in a population of patients with a disease. And they find which variations (SNPs) are associated with a given disease.
After reviewing nearly 10 million of these variations, geneticists have identified 108 of them, which are more common in the group of patients and, therefore, are associated with schizophrenia. Moreover, 83 of them have not yet been known.
Most of the genes in which these variations are found work in the brain, they are associated with the functions of neurons and synapses. In particular, these genes provide synaptic plasticity necessary for learning and memory, and are responsible for the functioning of calcium ion channels involved in the transmission of nerve signals. A mutation in any one gene causes a cascade of changes in the so-called signaling pathways, chains of biochemical reactions.
In particular, these genes provide synaptic plasticity necessary for learning and memory, and are responsible for the functioning of calcium ion channels involved in the transmission of nerve signals. A mutation in any one gene causes a cascade of changes in the so-called signaling pathways, chains of biochemical reactions.
“We are bad at treating”
Despite the difficult political situation, a major international conference on autism is taking place in Moscow. At...
June 03 14:08
Finding out these pathways will help find drug targets to correct these changes.
Among others, scientists have found a link between schizophrenia and the neurotransmitter 9 receptor gene0005 dopamine (DRD2), as well as genes involved in the metabolism of the neurotransmitter glutamate . Both substances are the most important transmitters of the nerve signal in synapses - contacts between neurons.
Thus, the biological picture of schizophrenia has become much more complete.
In particular, the connection of this disease with immune disorders was confirmed, because among the genes associated with schizophrenia there were many genes of the immune system.
Vera Golimbet, Doctor of Biology, Head of the Laboratory of Clinical Genetics of the Scientific Center for Mental Health of the Russian Academy of Medical Sciences, told Gazeta.Ru about the participation of Russian scientists in the work.
— What part of the work did you and your colleagues do?
- Our part was that we formed a sample of patients with schizophrenia and a sample of healthy people without mental illness, took DNA samples and prepared them for genotyping.
— How many Russian patients were there?
- About 500 people in each group - sick and healthy.
— What are the main features of this work?
- This is a meta-analysis of a large number of studies of patients from different ethnic groups.
Different psychos have common genes
For the first time in a genome-wide study on a large population, scientists have clarified the contribution of different gene variations. ..
August 12 17:30
The scientists conducting them have formed a large international consortium for schizophrenia research.
And managed to collect the largest amount of data, on the largest sample, than ever.
— What was the basis for genotyping?
— Genotyping, including our samples, was carried out on biochips; this method can immediately determine a large number of genetic polymorphisms. This was done by the Icelandic company deCode.
— Do you also conduct genotyping in the laboratory of clinical genetics?
Yes, but not at that level. Today, large international studies are a common practice because they are becoming more and more expensive, and this is the only way to study a disease with such a complex and unclear etiology as schizophrenia.
— Which of the results do you find most interesting?
- It was possible to prove that the genes of the dopamine system and the glutamate system are associated with schizophrenia. This is a good confirmation of other studies.
This is a good confirmation of other studies.
Many other genes associated with schizophrenia have also been found, among them genes for the immune system.
- Can new treatments be developed based on these findings?
- All research is done to find new molecules that are affected by the disease, and on the basis of this to develop new treatments. This is the main goal. But the period from the laboratory to the drug is quite long. This work is very important because it generalized and brought to a new level our knowledge about the genetic nature of schizophrenia.
background and current state of the problem
The problem of a possible connection between schizophrenia and epilepsy has been of interest to psychiatrists and neurologists for many years, almost since the isolation of dementia praecox as an independent nosological unit. The history of the isolation of epilepsy then totaled more than 20 centuries. The categorical thinking adopted at that time, with its characteristic dichotomous division in terms of the alleged etiology of mental disorders (endo- or exogenous, genetic or environmental, somato- or psychogenic, etc.), simplification and schematization of the disease process, divided researchers into two groups with answering the question - is it possible for the appearance of schizophrenia and epilepsy in one patient? Note that many of the participants in this discussion were specialists in both neurology and psychiatry.
The categorical thinking adopted at that time, with its characteristic dichotomous division in terms of the alleged etiology of mental disorders (endo- or exogenous, genetic or environmental, somato- or psychogenic, etc.), simplification and schematization of the disease process, divided researchers into two groups with answering the question - is it possible for the appearance of schizophrenia and epilepsy in one patient? Note that many of the participants in this discussion were specialists in both neurology and psychiatry.
In the problem under consideration, a large place is occupied by ideas about the biological antagonism of schizophrenia and epilepsy, based on a variety of observations. Suffice it to say that on the basis of a supposed antagonism, convulsive therapy was introduced into psychiatry. Research by I.P. Pavlova [1] showed that patients with epilepsy have a strong type of higher nervous activity, while patients with schizophrenia have a weak type. In these diseases, opposite changes in glia reactivity [2] and EEG [3] were found. There are also observations that if an epileptic paroxysm occurs after a stroke, then it causes some biochemical and humoral changes that counteract the onset of schizophrenia [4].
There are also observations that if an epileptic paroxysm occurs after a stroke, then it causes some biochemical and humoral changes that counteract the onset of schizophrenia [4].
The biological antagonism of these diseases to a certain extent is also evidenced by the fact that the diagnostic criteria for epilepsy, set out in the classification systems (ICD, DSM), are constantly expanding, while schizophrenia is narrowing. The point is that a number of symptoms that were previously considered characteristic only of schizophrenia are also found in other diseases, including epilepsy. As G. Huber wrote [5]: "There is not a single symptom or syndrome of schizophrenia that would not occur in patients with epilepsy, but this rule does not work in the opposite direction."
An important argument in favor of the existence of biological antagonism between schizophrenia and epilepsy was the inverse relationship between the appearance of psychotic symptoms and EEG features in patients with epilepsy, identified by H. Landolt in 1953 [6]. He called this phenomenon “forced normalization”, denoting an improvement in the EEG during the manifestation of psychotic symptoms in patients with epilepsy. A similar pattern on the background of drug therapy was observed by R. Restak [7], who noted that the normalization or decrease in convulsive activity on the EEG with the use of anticonvulsants leads to the precipitation of schizophrenia-like epileptic psychoses. Summarizing the studies conducted on this topic, D. Blumer et al. [8] suggested that “schizophrenia-like psychoses in epilepsy may be iatrogenic in nature and be caused by drugs with anticonvulsant properties.
Landolt in 1953 [6]. He called this phenomenon “forced normalization”, denoting an improvement in the EEG during the manifestation of psychotic symptoms in patients with epilepsy. A similar pattern on the background of drug therapy was observed by R. Restak [7], who noted that the normalization or decrease in convulsive activity on the EEG with the use of anticonvulsants leads to the precipitation of schizophrenia-like epileptic psychoses. Summarizing the studies conducted on this topic, D. Blumer et al. [8] suggested that “schizophrenia-like psychoses in epilepsy may be iatrogenic in nature and be caused by drugs with anticonvulsant properties.
The opposite point of view was expressed by the authors, who pointed out the possibility of schizophrenia and epilepsy in one patient. This kind of synergism between schizophrenia and epilepsy was expressed in terms of "epileptic schizophrenia", "psychotic epilepsy", "epileptic schizophrenic disorder". Both E. Kraepelin and E. Bleuler admitted that epileptiform convulsions can be observed in dementia praecox , and in this case the disease can take the form of epilepsy. They assumed that schizophrenia and epilepsy may have common links in pathogenesis and paid attention in this regard to the temporal region of the brain.
They assumed that schizophrenia and epilepsy may have common links in pathogenesis and paid attention in this regard to the temporal region of the brain.
The phenomenological similarity of chronic psychoses in epilepsy and schizophrenia was pointed out by many authors, including a number of well-known Russian psychiatrists [9-11]. V.N. Favorina [11] noted that in the late period of the course of epilepsy, its clinical manifestations are similar to severe end states in schizophrenia. These observations corresponded to the ideas of A.V. Snezhnevsky [12] about the equifinality of the development of mental illness - leveling the influence of the etiological factor at the final stage of the disease. The data of E.Ya. do not contradict this either. Shtenberg [13], who pointed out that the expansion of the cerebral ventricles and the development of cognitive disorders found in patients with schizophrenia are characteristic of organic brain damage that can manifest itself as epilepsy.
A compromise point of view on the possibility of the existence of schizophrenia and epilepsy in one patient was the idea of the existence of marginal, atypical or "hybrid" schizophrenia [14], which in the classification space can be between traditional forms of diseases, i.e. represent intermediate and transitional options . In this case, schizophrenia-like and epileptiform symptoms can be considered as the result of some kind of polysyndromic hereditary suffering.
Later, studies of epileptiform disorders in schizophrenia (schizoepilepsy) [15] and schizophrenia-like psychoses in epilepsy (epishizophrenia) [16] occupied a large place in psychiatry and neurology. Naturally, they reflected not only the peculiarities of approaches in these areas of medicine, but also the different ideas of individual authors about the influence of the sequence of appearance of these diseases on the features of their interaction and interaction.
By the beginning of the 20th century, some authors [17, 18] came to the conclusion that there were no sufficient grounds for isolating an independent disease, in which there is a combination of two endogenous diseases - schizophrenia and epilepsy. In their opinion, in such cases it is more about a random combination of different diseases, frequent in themselves, provoking and reinforcing each other, but this may also be a consequence of the imperfection of the classification of diseases, which admits that separate links can be distinguished from a continuous series of disorders. , dismembering the natural continuum into a set of discrete, unrelated units of pathology.
In their opinion, in such cases it is more about a random combination of different diseases, frequent in themselves, provoking and reinforcing each other, but this may also be a consequence of the imperfection of the classification of diseases, which admits that separate links can be distinguished from a continuous series of disorders. , dismembering the natural continuum into a set of discrete, unrelated units of pathology.
In the works relating to the last decades of the last century, three main directions can be distinguished in the analysis of the relationship between schizophrenia and epilepsy. The first of them is an assessment of the prevalence of schizophrenia-like psychoses in patients with epilepsy, highlighting clinical features. The second is the study of schizophrenia occurring in organically altered soil. The third is the identification of pathogenetic mechanisms that are universal for the occurrence of epilepsy and paroxysmal conditions in schizophrenia, based on the implementation of the concept of the paroxysmal brain or the model of amygdala kindling.
Within the framework of the first direction, a large number of studies have been conducted, the results of which indicate an increased incidence of psychosis in patients with epilepsy compared with the general population (1-2%). At the same time, a large scatter of the obtained data was noted, associated with differences in the criteria for selecting patients, especially when included in the group of schizophrenia-like psychoses with epilepsy.
Let us dwell on individual works concerning the frequency of development of psychoses, including schizophrenia-like ones, in epilepsy.
Frequency of "unclassifiable" psychoses? according to F. Gibbs and E. Gibbs [19] and M. Matsuura et al. [20], varies from 7 to 25% in patients with complex partial seizures and from 2.4 to 9.4% in patients with complex partial and generalized. J. Bruens [21] suggests that mixed convulsions weaken the strength of the considered association. N. Adachi et al. [22] noted a high correlation between temporal and frontal epilepsy, on the one hand, and schizophrenia-like psychoses, on the other.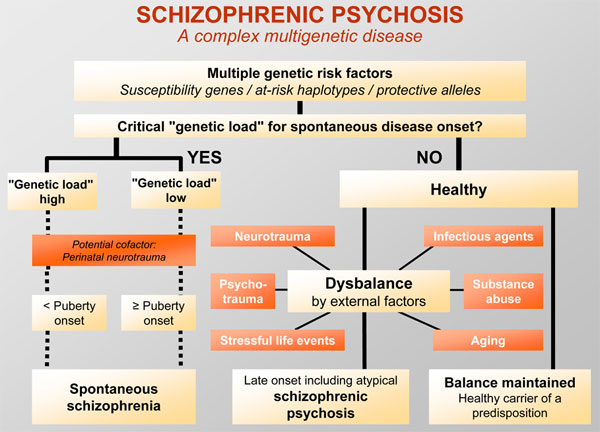 N. Kascella et al. [23] stated a high probability of developing chronic interictal psychoses in patients with recurrent postictal psychosis - "bimodal" psychosis.
N. Kascella et al. [23] stated a high probability of developing chronic interictal psychoses in patients with recurrent postictal psychosis - "bimodal" psychosis.
Depending on the assessment tools used, the prevalence of schizophrenia-like psychoses in complex partial and generalized seizures ranges from 0.8 to 70% using an unstructured clinical interview [24], 9.25% in cases of partial epilepsy using DSM-III criteria [25 ] and 50% in patients with partial and generalized epilepsy when applying the criteria of the Present State Examination scale [26]. Using the Operational Criteria Checklist for Psychotic Illness, the prevalence of schizophrenia-like psychoses in partial and generalized epilepsy ranged from 9up to 52% [27]. It has been noted that the prevalence of psychosis in patients with temporal lobe epilepsy is higher (19%) than in cases of primary generalized epilepsy (10%) [28]. At the same time, the incidence of schizophrenia-like psychoses correlates with the duration of epilepsy, as well as the age of patients by the time of its onset [29].
In one of the studies [30], higher rates of association of psychotic conditions with epilepsy were established compared with somatic diseases: the incidence of psychosis in men in the main group compared to the control group was 3 times higher (6.2 and 2.3% respectively). According to G. Gudmundsson [31], this kind of data not only indicates a close relationship between epilepsy and schizophrenia, but also possibly explains the victimization of patients with schizophrenia (a tendency to rash acts and provocative actions that lead to accidents, injuries).
Several studies of those years were devoted to answering the question of whether schizophrenia is a risk factor for the development of epilepsy. One such study [32] found that the prevalence of epilepsy and acute symptomatic seizures in patients diagnosed with schizophrenia and paranoid disorders according to DSM-III-R was not increased compared to the general population. However, in another study [33], a statistically significant relationship was found between schizophrenia and epileptic paroxysms. However, the methodological limitations of these studies until recent years left the issue under study open.
However, the methodological limitations of these studies until recent years left the issue under study open.
In the same years, many studies were conducted on the systematics and differential diagnosis of schizophrenia-like psychoses in epilepsy in comparison with schizophrenia. It was found that schizophrenia-like psychoses in epilepsy represent a heterogeneous group, with positive symptoms not differing from those observed in schizophrenia without epilepsy. According to some foreign researchers [34], it is difficult to clinically distinguish between psychotic states in epilepsy and schizophrenia, especially taking into account risk factors and personality changes observed in the long course of diseases. A different position in this respect is taken by domestic psychiatrists [35, 36], who, pointing to the endoform nature of mental disorders in epilepsy, emphasize that the psychopathological similarity of the external manifestations of the two diseases does not allow them to be identified, especially taking into account the fundamental differences in the nature of personality changes and the influence of therapy.
The second area of research - the study of schizophrenia (mainly in childhood and adolescence), occurring on organically altered soil, has acquired particular interest and practical importance due to increased convulsive readiness and a high frequency of epileptiform manifestations in children. Many authors [37-40] admit the influence of organically modified soil on the clinic and course of schizophrenia, especially when it comes to childhood and adolescence and early cerebral insufficiency. One of the manifestations of organically altered soil is known to be seizures and their equivalents. M.Sh. Vrono and A.L. Levin [41] made up a sample of 25 children in whom schizophrenic and schizophrenia-like symptoms were combined with various convulsive manifestations caused by residual effects of early organic brain damage. The epileptiform syndrome in these children was very persistent, and their relatives (mainly of the first degree of kinship) had personality traits of the epileptoid circle or minor epileptic signs (enuresis, stuttering, migraine).
In the foreign literature of the 60s of the last century, residual organic symptoms in children with schizophrenia were considered within the framework of the concept of "minimal brain dysfunction" or "minimal cerebral dysfunction". In contrast to the psychological model [42], in these works, organic brain damage was brought to the fore in the etiology of psychotic symptoms. At the same time, organic damage in a number of studies [43, 44] meant both asynchrony in the maturation of individual brain structures, and their actual organic damage.
Research in the second direction led to the search for exogenous organic factors in schizophrenia, which are reflected in such concepts as "pathologically altered soil", "organic microsymptoms", etc. [45]. In such cases, it was, according to G. Huber [46], symptomatic forms of schizophrenia. According to other researchers [47-49], such patients show spikes and slow-wave activity in the temporal lobes, paroxysmal dysrhythmia, functional disorders in the midbrain and brainstem, signs of disintegration of the cerebral cortex, the phenomenon of hypofrontality, discharges of hypersynchronous slow waves etc.
The third direction can be designated as "conceptual", since it was expressed in the formation of hypotheses that explain the biological essence of the connection between schizophrenia and epilepsy. It is a kind of theoretical foundation of the problem considered in this review.
The study of the neurophysiological mechanisms of paroxysmality as a universal phenomenon allowed A.M. Wayne [50] and V.A. Karlov [51] to reveal the general mechanisms of epileptic and non-epileptic seizures. The presence of a universal neurophysiological pattern was found, which is expressed by an increased total power of spontaneous EEG, an asymmetric predominance of the power of the θ-band in the right hemisphere, an increase in the amplitude of the general negative expectation wave, which in general indicates an imbalance of activation systems that contribute to the persistence of excitation of cortical neurons and the "readiness" of the brain to the development of paroxysmal conditions.
The term "paroxysm" appears in many descriptions of schizophrenia. In this regard, we can recall such clinical phenomena as “already seen”, “never seen”, “already experienced” - these are nothing more than short-term paroxysmal states; paroxysmal senestopathies have been described. A paroxysmal break in the train of thought is also known - sperrung; an acute paroxysm of sensual delirium has been described; acute paroxysm of hallucinatory-paranoid hypochondriacal syndrome, etc. G.O. Chikovani [52], studying the problem of paroxysmality in schizophrenia, singled out paroxysmal-like conditions, including those occurring according to the type of senostopathy and depersonalization disorders, ideational disorders, true hallucinations, the Kandinsky-Clerambault syndrome, the features of which were visual dysfunctions that give an exogenous color to psychosis (the predominance of true visual hallucinations in the evening and at night, the functional nature of verbal hallucinations), as well as the imagery of experiences, their visibility, brightness and expressiveness, a significant participation in their design of the element of imagination.
Another concept that considers the cerebral genesis of epileptic mechanisms in the development of mental disorders is the amygdala kindling model [53], the pathogenetic significance of which has been discussed in such conditions as schizophrenia-like psychoses, bipolar disorder, withdrawal syndrome, post-traumatic stress disorder and panic disorder.
The development of paroxysmal manifestations as a result of long-term periodic subthreshold nonspecific irritations of brain structures, which eventually lead to a decrease in the convulsive threshold and the launch of further self-developing paroxysmal activity, was associated with changes in the sensitivity of postsynaptic dopaminergic receptors and the turnover of GABA, aspartate and glutamate. In accordance with this hypothesis, there have been attempts [54, 55] to explain the positive correlation between the duration of epilepsy, the early onset of seizures, and the onset of psychosis. The occurrence of psychosis and electrical stimulation seizures was associated with an increase in dopaminergic neurotransmission.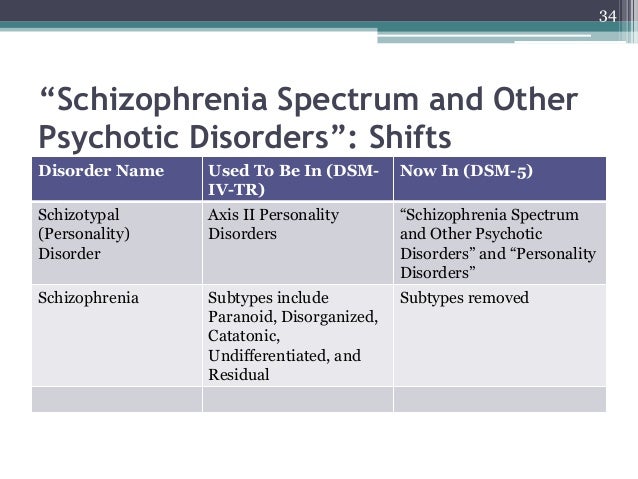
The problem of the relationship between schizophrenia and epilepsy has not become less relevant in recent years. In accordance with the data of epidemiological, neuropsychological, neuroanatomical, neurophysiological, genetic and molecular studies, the vector of scientific developments was again aimed at finding and establishing causal relationships between schizophrenia and epilepsy.
A group of scientists from the Taiwan Medical University [56] analyzed the database of the National Center for Health Insurance and Medical Statistics for 1998-2008 They selected 5195 patients with schizophrenia (versus 20,776 without schizophrenia) and 11,527 with epilepsy (versus 46,032 without epilepsy). The frequency of epileptic seizures in schizophrenia reached 7 people per 1000 (control - 1 person per 1000), and schizophrenia-like psychoses in epilepsy - 3.53 vs. higher, while men with epilepsy were more likely to develop schizophrenia later on. The results of the study are interpreted by the authors as a two-way relationship between these diseases, possibly arising from "general susceptibility". The presence of a two-way relationship between schizophrenia and epilepsy was shown on the basis of data from another epidemiological study [57], which included 6604 individuals with schizophrenia and 17,747 with epilepsy. Similar bilateral associations between epilepsy and schizophrenia were obtained as a result of a systematic review and meta-analysis of 215 articles, the data of which showed a high prevalence of psychoses comorbid with epilepsy, reaching 6%, and in temporal lobe epilepsy - 7% [58]. M. Matsuura et al. [59] believe that the differences in symptomatology between epileptic psychoses and schizophrenia are more quantitative than qualitative. Conflicting data relate to the association between the form of epilepsy and the incidence of psychosis. Some studies [60] have shown a higher incidence of psychosis in temporal lobe epilepsy, while others [61] have not.
The presence of a two-way relationship between schizophrenia and epilepsy was shown on the basis of data from another epidemiological study [57], which included 6604 individuals with schizophrenia and 17,747 with epilepsy. Similar bilateral associations between epilepsy and schizophrenia were obtained as a result of a systematic review and meta-analysis of 215 articles, the data of which showed a high prevalence of psychoses comorbid with epilepsy, reaching 6%, and in temporal lobe epilepsy - 7% [58]. M. Matsuura et al. [59] believe that the differences in symptomatology between epileptic psychoses and schizophrenia are more quantitative than qualitative. Conflicting data relate to the association between the form of epilepsy and the incidence of psychosis. Some studies [60] have shown a higher incidence of psychosis in temporal lobe epilepsy, while others [61] have not.
Some studies have focused on neuropsychological functioning in schizophrenia and epilepsy. So, D. Nathaniel-James et al. [62] examined 26 patients with schizophrenia and 12 with schizophrenia-like psychosis with epilepsy compared with 38 healthy and 12 patients with epilepsy without psychosis using tools such as the National adult reading test, Raven's progressive matrices, and California verbal learning. The results of the work confirmed the data existing in the literature that cognitive functioning in schizophrenia is characterized by a decrease in memory and executive functions. The profile of cognitive impairment in schizophrenia-like psychoses within epilepsy turned out to be similar to that observed in schizophrenia, but less pronounced, which, according to the authors, is in conflict with the concept of nosological independence of schizophrenia-like psychoses in epilepsy. Most likely, as stated in the article, this proves that epilepsy is a risk factor for the development of relatively benign forms of schizophrenia.
[62] examined 26 patients with schizophrenia and 12 with schizophrenia-like psychosis with epilepsy compared with 38 healthy and 12 patients with epilepsy without psychosis using tools such as the National adult reading test, Raven's progressive matrices, and California verbal learning. The results of the work confirmed the data existing in the literature that cognitive functioning in schizophrenia is characterized by a decrease in memory and executive functions. The profile of cognitive impairment in schizophrenia-like psychoses within epilepsy turned out to be similar to that observed in schizophrenia, but less pronounced, which, according to the authors, is in conflict with the concept of nosological independence of schizophrenia-like psychoses in epilepsy. Most likely, as stated in the article, this proves that epilepsy is a risk factor for the development of relatively benign forms of schizophrenia.
The high prevalence of schizophrenia-like psychoses in epilepsy suggests not only common mechanisms for the implementation of epilepsy and psychoses, but also common substrates for their implementation.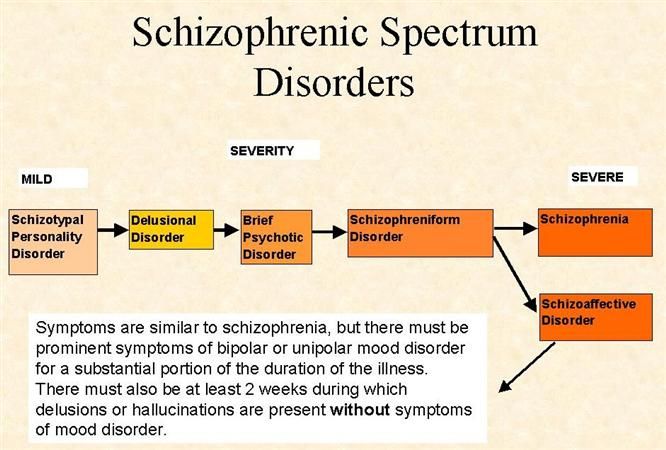 J. Stevens [63] in her neuromorphological work noted the prevalence of pathological changes in the hippocampus, hypothalamus, thalamus, pallidum and cerebellum in epilepsy and psychoses. In the view of many researchers [64, 65], the temporal lobe of the brain plays an important role in the connection between psychoses and epilepsy and schizophrenia-like symptoms.
J. Stevens [63] in her neuromorphological work noted the prevalence of pathological changes in the hippocampus, hypothalamus, thalamus, pallidum and cerebellum in epilepsy and psychoses. In the view of many researchers [64, 65], the temporal lobe of the brain plays an important role in the connection between psychoses and epilepsy and schizophrenia-like symptoms.
There are two hypotheses that there is a common biological substrate in schizophrenia and epilepsy. They suggest the presence of an anomaly in the development of the brain, clinically manifested by heterogeneous disorders, which, along with cognitive decline, developmental and communication disorders, autism spectrum disorders, attention deficit hyperactivity disorder, specific learning disorders and motor skills, include schizophrenia, bipolar disorder, cerebral palsy paralysis and epilepsy.
The first hypothesis suggests that a common factor in the development of schizophrenia and epilepsy is a violation of the development of the brain with the formation of cortical dysplasia.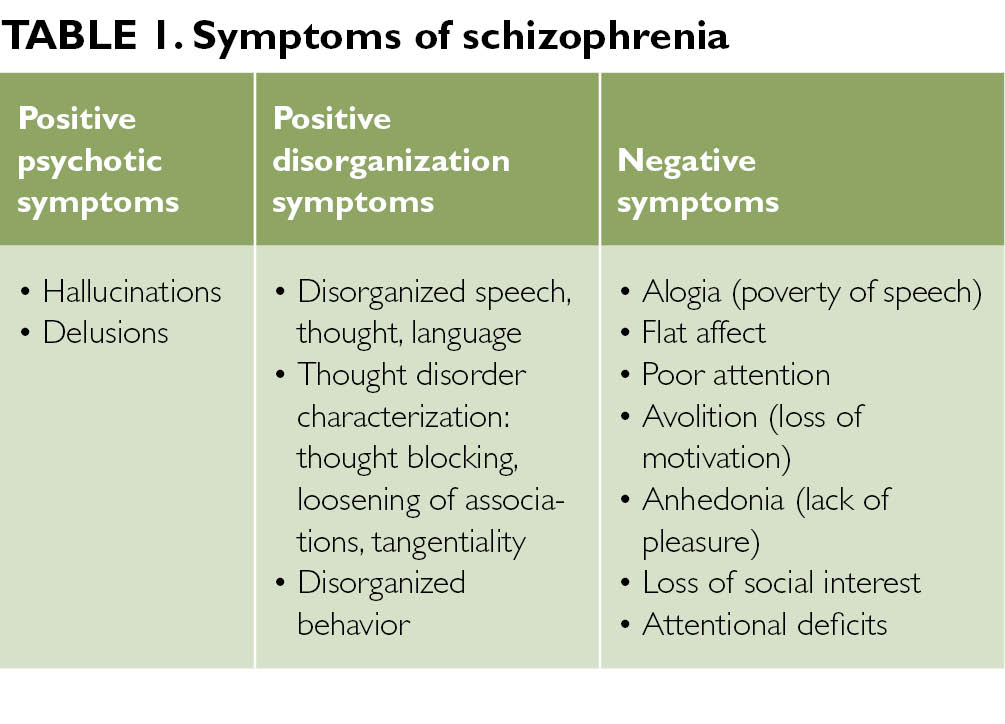 A neuromorphological study of temporal lobe epilepsy [66] revealed loss and signs of sclerosis of hippocampal cells in about 2/3 of patients and noted the frequency of concomitant tissue lesions (gliomas, hamartomas, and dystopias), which indicates defective neuroembryogenesis. Similar structural developmental anomalies are believed to underlie schizophrenia [67]. Attention is drawn to the disorganization of the layer of pyramidal cells, impaired neuronal migration in the hippocampus and synaptic reorganization [68], which are the result of either genetic “breakdowns” or pre-, perinatal or early development of a stroke. Evidence that schizophrenia-like psychosis develops more often in patients with epilepsy who have brain anomalies is consistent with the data presented. At the same time, epileptic activity can be aggravated by the described dysgenesis, “starting” the development of psychosis.
A neuromorphological study of temporal lobe epilepsy [66] revealed loss and signs of sclerosis of hippocampal cells in about 2/3 of patients and noted the frequency of concomitant tissue lesions (gliomas, hamartomas, and dystopias), which indicates defective neuroembryogenesis. Similar structural developmental anomalies are believed to underlie schizophrenia [67]. Attention is drawn to the disorganization of the layer of pyramidal cells, impaired neuronal migration in the hippocampus and synaptic reorganization [68], which are the result of either genetic “breakdowns” or pre-, perinatal or early development of a stroke. Evidence that schizophrenia-like psychosis develops more often in patients with epilepsy who have brain anomalies is consistent with the data presented. At the same time, epileptic activity can be aggravated by the described dysgenesis, “starting” the development of psychosis.
The second hypothesis suggests diffuse brain damage underlying both epilepsy and schizophrenia. In this regard, neuronal migration disorders are distinguished - congenital structural anomalies in the development of the brain, as well as signs of synaptic regeneration [69]. These data indicate that schizophrenia-like psychosis in epilepsy may be associated with both de- and regenerative changes in the brain, which may be the result of a CNS developmental disorder or a genetic defect [70].
In this regard, neuronal migration disorders are distinguished - congenital structural anomalies in the development of the brain, as well as signs of synaptic regeneration [69]. These data indicate that schizophrenia-like psychosis in epilepsy may be associated with both de- and regenerative changes in the brain, which may be the result of a CNS developmental disorder or a genetic defect [70].
One of the genealogical studies [71] analyzed the medical histories of parents and their children born in Helsinki between 1947 and 1990 to identify family “vulnerability” to schizophrenia and epilepsy. (the sample consisted of 9,653 families and 23,404 of their children). Individuals with epilepsy had a 5.5-fold increased risk of developing psychotic disorders, an almost 8.5-fold increased risk of developing schizophrenia, and a 6.3-fold increased risk of developing bipolar disorder. An association between epilepsy and psychosis in families has been revealed. Children whose parents suffered from epilepsy showed a 2-fold increase in the risk of developing psychosis compared with individuals who did not have such data. Children whose parents had psychosis found a 2.7-fold increased risk of developing generalized epilepsy compared to those whose parents did not suffer from psychosis. A retrospective analysis showed that these associations were not caused by the comorbidity of epilepsy and psychosis in parents, which confirms, according to the authors, the presence of an "overlap" of etiological, possibly genetic, factors between epilepsy and schizophrenia.
Children whose parents had psychosis found a 2.7-fold increased risk of developing generalized epilepsy compared to those whose parents did not suffer from psychosis. A retrospective analysis showed that these associations were not caused by the comorbidity of epilepsy and psychosis in parents, which confirms, according to the authors, the presence of an "overlap" of etiological, possibly genetic, factors between epilepsy and schizophrenia.
Specific variants of the genome structure are described as a predisposition to the development of schizophrenia, epilepsy and other neurodevelopmental disorders. Individuals with these genome variants may suffer from both schizophrenia and epilepsy, some have only schizophrenia or epilepsy, and still others do not have this pathology. This means that in this case we are not talking about a simple "toxic" effect of epileptic seizures on the central nervous system, but rather that one or more genes, the function of which is structurally impaired, play (play) a role in the pathogenesis of both epilepsy and psychosis . Such a pleomorphism of psychoneurological pathology, in which the state of individuals takes on a number of different forms during their own or descendants of the life cycle, is confirmed by the study of A. Jablensky et al. [72], regarding the observation of a family with schizophrenia from Western Australia, which showed that etiological homogeneity is compatible with phenotypic variability as a result of a change in the process lability threshold.
Such a pleomorphism of psychoneurological pathology, in which the state of individuals takes on a number of different forms during their own or descendants of the life cycle, is confirmed by the study of A. Jablensky et al. [72], regarding the observation of a family with schizophrenia from Western Australia, which showed that etiological homogeneity is compatible with phenotypic variability as a result of a change in the process lability threshold.
Most genetic research has focused on the genes that control brain development. Their results suggest that epilepsy and mental illness may represent distinct clinical variations in the overall etiological process. It is suggested that the general genetic constitution is responsible for the neurodevelopmental anomaly, while subsequent exposure to adverse modifiers (perhaps genetic, environmental or epigenetic) determines the specific phenotypic outcome. This hypothesis is supported by several lines of evidence. First, there is evidence that a microdeletion expressed during embryogenesis can lead to any form of epilepsy or schizophrenia [73]. Second, there is evidence that the accumulation of multiple genetic mutations leads to more severe or altered clinical phenotypes or a different phenotype [74]. Thirdly, there is evidence that one gene, affecting in different ways, can lead to various changes, in particular, that a genetic mutation can reflect not only the DNA mutation pattern, but also changes in the structure and function of proteins [75]. Fourthly, a deficit of neuronal migration and dysregulation of neurotransmitter systems in epilepsy and schizophrenia, especially dopamine and glutamate, have been found [76]. Fifth, there is evidence that older parental age at conception increases the risk of both schizophrenia and epilepsy in the offspring [77, 78]. Finally, the concordance rate in monozygotic twins in schizophrenia, bipolar disorder, and epilepsy is well below 100% [79, 80], which indicates the importance of non-genetic modifiers in disease manifestations.
Second, there is evidence that the accumulation of multiple genetic mutations leads to more severe or altered clinical phenotypes or a different phenotype [74]. Thirdly, there is evidence that one gene, affecting in different ways, can lead to various changes, in particular, that a genetic mutation can reflect not only the DNA mutation pattern, but also changes in the structure and function of proteins [75]. Fourthly, a deficit of neuronal migration and dysregulation of neurotransmitter systems in epilepsy and schizophrenia, especially dopamine and glutamate, have been found [76]. Fifth, there is evidence that older parental age at conception increases the risk of both schizophrenia and epilepsy in the offspring [77, 78]. Finally, the concordance rate in monozygotic twins in schizophrenia, bipolar disorder, and epilepsy is well below 100% [79, 80], which indicates the importance of non-genetic modifiers in disease manifestations.
Focused genetic studies have shown [81] that a heterozygous deletion of chromosome 16p13. 11 is associated with schizophrenia and epilepsy, as well as cognitive decline, autism, and attention deficit hyperactivity disorder. Certain genetic "breakdowns" that predispose to the development of schizophrenia also increase the risk of epilepsy. N. Cascella et al. [82] described the family in study LGI genes that control the migration of brain cells during fetal development and the regulation of the functions of the glutamatergic system, predisposing to the development of complex focal seizures with auditory hallucinations, which are observed in both epilepsy and schizophrenia. The authors conclude that the study of specific forms of genetically determined epilepsy can lead to the identification of features of molecular pathology in both epilepsy and schizophrenia.
11 is associated with schizophrenia and epilepsy, as well as cognitive decline, autism, and attention deficit hyperactivity disorder. Certain genetic "breakdowns" that predispose to the development of schizophrenia also increase the risk of epilepsy. N. Cascella et al. [82] described the family in study LGI genes that control the migration of brain cells during fetal development and the regulation of the functions of the glutamatergic system, predisposing to the development of complex focal seizures with auditory hallucinations, which are observed in both epilepsy and schizophrenia. The authors conclude that the study of specific forms of genetically determined epilepsy can lead to the identification of features of molecular pathology in both epilepsy and schizophrenia.
The studies cited in this review are not without methodological shortcomings. This concerns, first of all, the psychiatric standardization of clinical material, in which the terms "psychosis" and "schizophrenia" are often identified.