Is divalproex the same as depakote
FDA criteria for generics – Depakote® (divalproex sodium)
- FOR U.S. HEALTHCARE PROFESSIONALS
- Important Safety Information
- Medication Guide
- Prescribing Information
- Patient Site
- Important Safety Information
- Prescribing Information
FOR U. S. HEALTHCARE
PROFESSIONALS
Each Rx refill is a possibility for pharmacy to switch from brand to generic formulation
When patients are switched to a generic substitute, they may not get the same generic manufacturer with each refill if there are multiple generic manufacturers.
According to a 2017 analysis of over 80,000 valproate patients,1*
more than 2/3 of new generic valproate patients received valproate from more than two different manufacturers in a 12-month span1
*Only patients who were on therapy for 4 quarters were considered in this analysis. n=80,459.
When can a generic be considered therapeutically equivalent?
Depakote is a reference listed drug
- The brand name is considered the innovator drug
- It’ s the standard to which all its generic versions must be shown to be bioequivalent
-
The generic drug is a pharmaceutical equivalent of the reference drug.
 They have the same:2
They have the same:2- Active ingredients
- Dosage form
- Route of administration
- Strength
- Generic drugs presenting with a known actual or potential bioequivalence problem can be considered therapeutically equivalent if the problem is resolved by adequate in vivo and/or in vitro evidence supporting bioequivalence.2
- Per the FDA, a generic drug that is classified as therapeutically equivalent can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the reference product.3
WRITE DAW†
Ensure patients receive the branded Depakote you prescribe.
HELP YOUR PATIENTS SAVE
Eligible patients could pay as little as $5/month.‡
LEARN MOREHELP PATIENTS SAVE TRIPS
Converting to a 90-day Rx may save patients trips to the pharmacy.
†Or your state’s legal language. ‡Up to $100/month savings for eligible patients.
ELIGIBILITY
Available to patients with commercial prescription insurance coverage for Depakote who meet eligibility criteria. Copay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law or by the patient’s health insurance provider. If at any time a patient begins receiving prescription drug coverage under any such federal, state, or government-funded healthcare program, patient will no longer be able to use the Depakote Savings Card and patient must call OPUS Health at 800. 364.4767 to stop participation. Patients residing in or receiving treatment in certain states may not be eligible. Patients may not seek reimbursement for value received from the Depakote Savings Card from any third-party payers. Offer subject to change or discontinuance without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. Please see full Terms and Conditions.
364.4767 to stop participation. Patients residing in or receiving treatment in certain states may not be eligible. Patients may not seek reimbursement for value received from the Depakote Savings Card from any third-party payers. Offer subject to change or discontinuance without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. Please see full Terms and Conditions.
TERMS AND CONDITIONS
Pharmacist Instructions
- Submit the copay card authorized for all commercially insured patients by the patient’s primary insurance as a secondary transaction to OPUS Health.
- When you use this card, you are confirming that you have not submitted and will not submit a claim for this prescription for reimbursement under any federal, state, or government-funded healthcare program, such as Medicare (including Part D), Medicare Advantage, Medicaid, Medigap, Veterans Affairs, the Department of Defense, or TRICARE.

- Pharmacists with questions please call OPUS Health at 800.364.4767.
INDICATIONS
4-6Mania
DEPAKOTE® ER (divalproex sodium) extended-release tablets, for oral use, is a valproate and is indicated for the treatment of acute manic or mixed episodes associated with bipolar disorder, with or without psychotic features.
DEPAKOTE® (divalproex sodium) delayed-release tablets, for oral use, is a valproate and is indicated for the treatment of the manic episodes associated with bipolar disorder.
A manic episode is a distinct period of abnormally and persistently elevated, expansive, or irritable mood. Typical symptoms of mania include pressure of speech, motor hyperactivity, reduced need for sleep, flight of ideas, grandiosity, poor judgment, aggressiveness, and possible hostility. A mixed episode is characterized by the criteria for a manic episode in conjunction with those for a major depressive episode (depressed mood, loss of interest or pleasure in nearly all activities).
The efficacy of DEPAKOTE ER is based in part on studies of DEPAKOTE in this indication, and was confirmed in a 3-week trial with patients meeting DSM-IV-TR criteria for bipolar I disorder, manic or mixed type, who were hospitalized for acute mania.
The efficacy of DEPAKOTE was established in 3-week trials with patients meeting DSM-III-R criteria for bipolar disorder who were hospitalized for acute mania.
The effectiveness of valproate for long-term use in mania, i.e., more than 3 weeks, has not been demonstrated in controlled clinical trials. Therefore, healthcare providers who elect to use DEPAKOTE or DEPAKOTE ER for extended periods should continually reevaluate the long-term risk-benefits of the drug for the individual patient.
Epilepsy
DEPAKOTE ER is indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures. DEPAKOTE ER is also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures in adults and children 10 years of age or older, and adjunctively in adults and children 10 years of age or older with multiple seizure types that include absence seizures.
DEPAKOTE ER is also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures in adults and children 10 years of age or older, and adjunctively in adults and children 10 years of age or older with multiple seizure types that include absence seizures.
DEPAKOTE is indicated as monotherapy and adjunctive therapy in the treatment of patients with complex partial seizures that occur either in isolation or in association with other types of seizures. DEPAKOTE is also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures.
DEPAKOTE® Sprinkle Capsules (divalproex sodium delayed release capsules), for oral use, are indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures. DEPAKOTE Sprinkle Capsules are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures.
DEPAKOTE Sprinkle Capsules are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures.
Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present.
Migraine
DEPAKOTE and DEPAKOTE ER are indicated for prophylaxis of migraine headaches. There is no evidence that DEPAKOTE ER or DEPAKOTE is useful in the acute treatment of migraine headaches.
Important Limitations
Because of the risk to the fetus of decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations, which may occur very early in pregnancy, valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable.
Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable.
For prophylaxis of migraine headaches, valproate is contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception.
IMPORTANT SAFETY INFORMATION
4-6Warning: Life-Threatening Adverse Reactions
Hepatotoxicity
General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate and its derivatives. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When DEPAKOTE (divalproex sodium) delayed-release tablets, for oral use, DEPAKOTE ER (divalproex sodium) extended-release tablets, for oral use, or DEPAKOTE Sprinkle Capsules (divalproex sodium delayed release capsules), for oral use, is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
Patients With Mitochondrial Disease: There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e. g., Alpers Huttenlocher Syndrome). DEPAKOTE, DEPAKOTE ER, and DEPAKOTE Sprinkle Capsules are contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and in children under two years of age who are clinically suspected of having a mitochondrial disorder. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, DEPAKOTE, DEPAKOTE ER, or DEPAKOTE Sprinkle Capsules should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with DEPAKOTE, DEPAKOTE ER, or DEPAKOTE Sprinkle Capsules for the development of acute liver injury, with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice.
g., Alpers Huttenlocher Syndrome). DEPAKOTE, DEPAKOTE ER, and DEPAKOTE Sprinkle Capsules are contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and in children under two years of age who are clinically suspected of having a mitochondrial disorder. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, DEPAKOTE, DEPAKOTE ER, or DEPAKOTE Sprinkle Capsules should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with DEPAKOTE, DEPAKOTE ER, or DEPAKOTE Sprinkle Capsules for the development of acute liver injury, with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice.
Fetal Risk
Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores and neurodevelopmental disorders following in utero exposure.
Valproate is therefore contraindicated for prophylaxis of migraine headaches in pregnant women and in women of childbearing potential who are not using effective contraception [see Contraindications (4)]. Valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable.
Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable. In such situations, effective contraception should be used [see Warnings and Precautions (5.2, 5.3, 5.4)].
A Medication Guide describing the risks of valproate is available for patients [see Patient Counseling Information (17)].
Pancreatitis
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated.
Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated.
- Valproate should not be administered to patients with hepatic disease or dysfunction. Immediately discontinue drug if significant hepatic dysfunction is suspected or apparent. Progression of dysfunction has occurred in spite of discontinuation of valproate.
- Valproate is contraindicated in patients known to have mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG; e.g., Alpers Huttenlocher Syndrome) and in children under two years of age who are suspected of having a POLG-related disorder.
 POLG-related disorder symptoms may include unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus at presentation, developmental delays, psychomotor regression, axonal sensorimotor neuropathy, myopathy cerebellar ataxia, ophthalmoplegia, or complicated migraine with occipital aura.
POLG-related disorder symptoms may include unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus at presentation, developmental delays, psychomotor regression, axonal sensorimotor neuropathy, myopathy cerebellar ataxia, ophthalmoplegia, or complicated migraine with occipital aura. - Valproate is contraindicated for use in prophylaxis of migraine headaches in women who are pregnant and in women of childbearing potential who are not using effective contraception.
- Valproate is contraindicated in patients with known hypersensitivity to the drug.
- Valproate is contraindicated in patients with known urea cycle disorders (UCDs). Hyperammonemic encephalopathy, sometimes fatal, has been reported following initiation of valproate. Symptoms of unexplained hyperammonemic encephalopathy during valproate therapy require prompt treatment (including discontinuation of valproate).
- Valproate can cause decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations (e.
 g., craniofacial defects, hypospadias, cardiovascular and limb malformations). In epileptic mothers, the rate of congenital malformations with in utero exposure is about four times higher compared to the rate with in utero exposure to other anti-seizure monotherapies. Lower cognitive test scores, including decreased IQ scores, were associated with in utero valproate exposure compared with in utero exposure to either another or no antiepileptic drug. Unless other medications have failed to provide adequate symptom control or are otherwise unacceptable, women of childbearing potential should not receive valproate.
g., craniofacial defects, hypospadias, cardiovascular and limb malformations). In epileptic mothers, the rate of congenital malformations with in utero exposure is about four times higher compared to the rate with in utero exposure to other anti-seizure monotherapies. Lower cognitive test scores, including decreased IQ scores, were associated with in utero valproate exposure compared with in utero exposure to either another or no antiepileptic drug. Unless other medications have failed to provide adequate symptom control or are otherwise unacceptable, women of childbearing potential should not receive valproate. - Valproate can increase the risk of suicidal thoughts or behavior. Patients treated with any AED should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or unusual changes in mood or behavior. Counsel patients and families to be alert for and to immediately report depression, any unusual changes in mood or behavior, or suicidal thoughts, behavior, or acts of self-harm.

- Thrombocytopenia is dose-related. Decreases in other cell lines and myelodysplasia have also been associated with valproate. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of ≥110 ug/mL in females and ≥135 ug/mL in males or at 50 mg/kg/d. Check complete blood counts and coagulation times before starting therapy or surgery, at periodic intervals, and during pregnancy. Reduce dose or discontinue if hemorrhage, bruising, or hemostasis/coagulation disorder occur.
- Hyperammonemia has been associated with valproate; it may be present despite normal liver function tests and should be considered if hypothermia occurs. Asymptomatic elevations of ammonia are more common and require close monitoring. Discontinue valproate if ammonia increases.
- Concomitant administration of topiramate and valproate has been associated with hyperammonemia (with or without encephalopathy) in patients who have tolerated either drug alone.

- Hypothermia has been associated with valproate therapy both in conjunction with, and in the absence of, hyperammonemia. It can occur after starting topiramate treatment or after increasing the daily dose of topiramate. Consider stopping valproate if hypothermia develops.
- Drug Reaction with Eosinophilia and Systemic Syndrome (DRESS) / multi-organ hypersensitivity reactions have been reported and may be fatal or life-threatening. Patients typically present with fever, rash, and lymphadenopathy associated with other organ system involvement, e.g., hematologic abnormalities. Early symptoms may not include rash. Regardless, immediately evaluate and discontinue therapy for any signs of hypersensitivity.
- Carbapenem antibiotics (such as ertapenem, imipenem, meropenem) may reduce serum valproate concentrations to subtherapeutic levels, resulting in loss of seizure control.
- In a clinical trial, somnolence was associated with valproate in some elderly dementia patients along with reduced nutritional intake; weight loss; and a trend to have a lower baseline albumin concentration, higher BUN, and lower valproate clearance.
 Discontinuation occurred in some patients.
Discontinuation occurred in some patients. - Valproate may interact with drugs capable of enzyme induction; check valproate and concomitant drug levels periodically in the early course of therapy.
- Rare reports of medication residue in the stool have occurred, some in patients with anatomic (ileostomy or colostomy) or functional gastrointestinal disorders with shortened GI transit times or in the context of diarrhea. If medication residue occurs, monitor plasma valproate levels and patient’s clinical condition; alternative treatment may be considered.
ADVERSE EVENTS
4-6- Most common adverse reactions (reported >5%) are abdominal pain, abnormal thinking, alopecia, amblyopia/blurred vision, amnesia, anorexia, asthenia, ataxia, back pain, bronchitis, constipation, depression, diarrhea, diplopia, dizziness, dyspepsia, dyspnea, ecchymosis, emotional lability, fever, flu syndrome, headache, increased appetite, infection, insomnia, nausea, nervousness, nystagmus, peripheral edema, pharyngitis, rash, rhinitis, somnolence, thrombocytopenia, tinnitus, tremor, vomiting, weight gain, and weight loss.
Keep DEPAKOTE and all other medications where children cannot reach them.
REFERENCES
- IQVIA LAAD Rx Dataset, January 2017 – September 2019; IQVIA Analysis, Dec 2019.
- US Food and Drug Administration, Orange Book Preface. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079068.htm#TEC. Accessed July 1, 2020.
- US Food and Drug Administration, Drug Approvals and Databases, Drugs@FDA Glossary of Terms. http://www.fda.gov/drugs/informationondrugs/ucm079436.htm#TE. Accessed July 1, 2020.
- Depakote Delayed-Release Tablets [package insert]. North Chicago, IL: AbbVie Inc.
- Depakote ER [package insert]. North Chicago, IL: AbbVie Inc.
- Depakote Sprinkle Capsules [package insert]. North Chicago, IL: AbbVie Inc.
If you have any questions about AbbVie's Depakote.com website that have not been answered, click here. This website and the information contained herein is intended for use by US residents only, is provided for informational purposes only, and is not intended to replace a discussion with a healthcare provider. All decisions regarding patient care must be made with a healthcare provider and consider the unique characteristics of each patient.
This website and the information contained herein is intended for use by US residents only, is provided for informational purposes only, and is not intended to replace a discussion with a healthcare provider. All decisions regarding patient care must be made with a healthcare provider and consider the unique characteristics of each patient.
- Contact us
- Important Safety Information
- Medication Guide
- Prescribing Information
- Site Map
- Privacy Policy
- Terms of Use
- Cookies Settings
©2020 AbbVie Inc.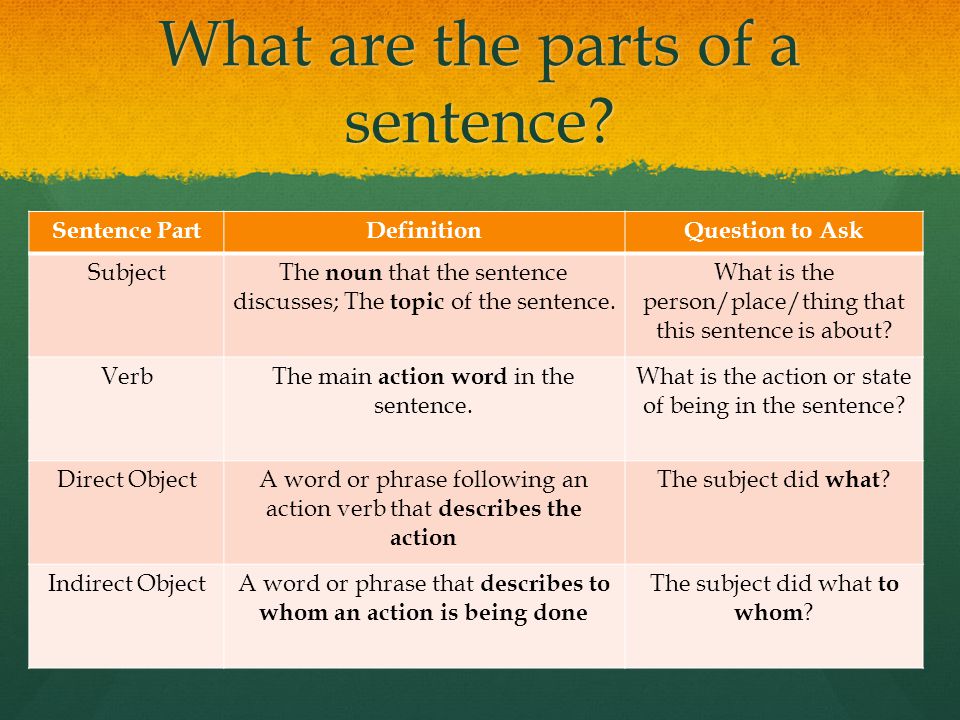 North Chicago, IL 60064 US-DPKT-200006 July 2020
North Chicago, IL 60064 US-DPKT-200006 July 2020
INDICATIONS
4-6Mania
DEPAKOTE® ER (divalproex sodium) extended-release tablets, for oral use, is a valproate and is indicated for the treatment of acute manic or mixed episodes associated with bipolar disorder, with or without psychotic features.
DEPAKOTE® (divalproex sodium) delayed-release tablets, for oral use, is a valproate and is indicated for the treatment of the manic episodes associated with bipolar disorder.
IMPORTANT SAFETY INFORMATION
4-6Warning: Life-Threatening Adverse Reactions
Hepatotoxicity
General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate and its derivatives. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months.
What is Depakote & What is It Used For?
The medication Depakote (divalproex sodium) is an anticonvulsant. Doctors prescribe it to treat seizure disorders and to prevent migraine headaches. People also use it to manage the manic phase of bipolar disorder. Depakote side effects can be serious and sometimes fatal. These include birth defects in children whose mothers take the medications while pregnant.
Depakote is an anticonvulsant. Doctors prescribe it to treat conditions that affect the brain.
Depakote contains the active ingredient divalproex sodium. This is a combination of sodium valproate and valproic acid.
The medication comes in a white powder form. Drugmaker AbbVie Inc. makes it into a tablet.
Patients take Depakote by mouth. The drug’s maker tells patients to swallow Depakote whole, not to crush or chew it.
Valproate in Depakote is effective in managing and stabilizing moods. But the chemical substance can cause serious side effects.
Depakote lawsuits blame the drug for causing birth defects in children whose mothers took Depakote during pregnancy.
Depakote (Divalproex Sodium) Uses
The U.S. Food and Drug Administration approved Depakote in 1983 to treat epilepsy. Doctors may sometimes prescribe divalproex sodium with other medications to treat types of seizures.
The FDA expanded Depakote uses to include the treatment of manic episodes associated with bipolar disorder. Divalproex sodium also helps prevent migraines headaches.
What Depakote Does for the Brain
Divalproex sodium works by increasing the amount of gamma-aminobutyric acid (GABA) in the brain. The body produces GABA naturally. It is a chemical neurotransmitter — or brain messenger.
Faster than normal electrical impulses in the brain can cause erratic impulses. These impulses overstimulate the nerves. GABA helps to calm and relax nerves. Relaxing nerves can stabilize moods.
Increasing GABA can also prevent abnormal brain signals that lead to seizures.
One 2022 clinical research trial reported on the effects of Depakote on posttraumatic irritability after traumatic brain injury. Results showed a significant and sustained reduction of posttraumatic irritability. However, the study authors noted that treatment efficacy was not related to measures of anxiety, posttraumatic stress disorder, sedation, or veteran versus nonveteran status. Alcohol use did not change as a result of treatment.
Types of Depakote: Depakote DR vs Depakote ER vs Depakote Sprinkle Capsules
Divalproex sodium comes in Depakote tablets and Depakote ER (extended-release) tablets. It’s also available in Depakote Sprinkle Capsules (delayed-release capsules).
Depakote ER and the delayed-release formulation of Depakote are not interchangeable.
Depakote tablets are delayed release. They have a coating to keep the medication from dissolving too soon after it’s swallowed. These tablets come in three sizes: 125 mg, 250 mg and 500 mg. They are prescribed to be taken twice a day.
Depakote delayed release 250mg
Depakote delayed release 500mg
Depakote ER tablets are made to slowly deliver the medication over 24 hours. They are taken just once a day. They come in 250 mg and 500 mg. Depakote ER does not have a coating.
Depakote extended release 250mg
Depakote extended release 500mg
Depakote Sprinkle Capsules are approved only for the treatment of epilepsy. They are available in 125 mg. They work similar to Depakote delayed release, except that they are in capsules rather than tablets. Patients who have trouble swallowing may open the capsules and sprinkle the medication on soft food.
A closely related medication, Depakene (valproic acid), comes in capsule and liquid form. Another variety of valproic acid, Stavzor, is no longer manufactured.
In 2008, the FDA approved the first generic version of Depakote. Collectively, these medications are known as valproate products.
Depakote Dosages
Depakote dosage can vary. It depends on the patient and the condition for which a doctor prescribes it.
For example, when taken to control mania, the recommended initial dose is 750 mg a day taken in divided doses for Depakote. For Depakote ER, the recommended initial dose is 25 mg once a day, up to a maximum of 60 mg a day.
The initial Depakote therapy is 10 mg to 15 mg for treating epilepsy in patients age 10 and older. Doctors can then increase the dosage by 5 mg to 10 mg each week. This continues until the doctor determines that the medication is doing what it’s supposed to.
With Depakote ER, patients ages 10 and older should start the same with a maximum dose of 60 mg a day.
If a patient forgets to take Depakote at the usual time, he or she can take it upon remembering. But if it’s close to the next dose, patients can skip the forgotten dose and resume the normal schedule. Do not double up.
Depakote Interactions
Valporic acid in Depakote may interact with a number of different medications. Depakote interactions may increase or decrease the effects of the medications. Some can be fatal.
Valproic acid can increase the serum concentrations of diazepam in patients taking that medication. Antacids containing magnesium and aluminum hydroxide may increase valproic acid an average of 12 percent.
Patients with urea cycle disorders who are being treated with sodium phenylbutyrate should not receive valproic acid. This interaction can be fatal.
Supplements and food can also interact with Depakote. For example, high doses of folate can decrease the effectiveness of Depakote.
High doses of the herb Ginkgo biloba also could decrease the effectiveness of Depakote.
Drugs that can interact with Depakote include:
- Alprazolam
- Amphetamine
- Benzphetamine
- Bupropion
- Butabarbital
- Carbamazepin
- Cholestyramine
- Darunavir
- Doripenem
- Ertapenem
- Fosamprenavir
- Haloperidol
- Lamotrigine
- Loxapin
- Meropenem
- Pemoline
- Propofol
- Ritonavir
- Sodium Phenylbutyrate
- Thiothixene
- Vorinostat
- Warfarin
Common Side Effects of Depakote
A common side effect of Depakote and Depakene is nausea, especially during the first month of treatment. This can be overcome by starting on a low dosage and slowly increasing the amount. Taking the medication on a full stomach can also help.
This can be overcome by starting on a low dosage and slowly increasing the amount. Taking the medication on a full stomach can also help.
Common side effects associated with Depakote include:
- Nausea
- Drowsiness
- Abdominal pain
- Diarrhea
- Vomiting
- Low platelet count
- Tremors
- Hair loss
These side effects may be mild or moderate. You should talk to your doctor about any side effects because they could be signs of something serious.
Depakote Serious Side Effects
Taking Depakote has been linked to some serious and even deadly side effects.
Liver damage is one of the side effects that may be fatal. This is especially true in children under 2. This risk is highest in the first months of taking the medication. The risk may still be present even after the Depakote medication is stopped.
Another potentially fatal side effect is inflammation of the pancreas, or pancreatitis. This is when digestive enzymes start digesting the pancreas itself.
When pregnant women take Depakote, their unborn babies are at risk of developing birth defects. These defects can affect the brain, spinal cord, heart, arms, legs and penis. These babies also risk developing lower intelligence. Women who are or may become pregnant should not take Depakote.
Depakote Black Box Warnings
The FDA requires that Depakote’s label have black box warnings. A black box warning is the agency’s most serious type of warning. It calls attention to serious or life-threatening risks.
Depakote’s boxed warnings are for hepatotoxicity, fetal risk and pancreatitis.
Depakote Precautions
Depakote manufacturer AbbVie warns that certain people should not take Depakote or Depakene. This includes people with liver problems, and women who are or may become pregnant.
Talk to your doctor before taking Depakote or Depakene if you:
- Have liver problems
- Have or think you have a genetic liver problem caused by a mitochondrial disorder (e.g. Alpers-Huttenlocher syndrome)
- Are allergic to divalproex sodium, valproic acid, sodium valproate, or any of the ingredients in Depakote or Depakene
- Have a genetic problem called urea cycle disorder
- Are or may become pregnant
Depakote Facts
Please seek the advice of a medical professional before making health care decisions.
TELL US WHAT YOU THINK
Did You Find Drugwatch Helpful?
Yes No
Thank you for your feedback. Do you have any thoughts you'd like to share about Drugwatch.
 com?
com?This article changed my life!
This article was informative
I have a question
How can we improve this page?
This article contains incorrect information
This article doesn't have the information I'm looking for
I have a question
How can we improve this page?
Thank You for Your Feedback
We appreciate your feedback. One of our content team members will be in touch with you soon.
We appreciate your feedback. One of our content team members will be in touch with you soon.
Valproic Acid and Derivatives: Pediatric Medication
Pediatric Medicine
ShareProvided by Lexicomp ® , this document contains all the information you need to know about this medicine, including indications, directions for use, side effects, and when your healthcare provider should be contacted.
Trade names: USA
Depacon [DSC]; Depakene [DSC]; Depakote; Depakote ER; Depakote Sprinkles
Trade names: Canada
APO-Divalproex; APO-Valproic Acid; Depakene; epival; MYLAN-Divalproex; PMS-Valproic; PMS-Valproic Acid; SANDOZ Valproic [DSC]; TEVA-Divalproex [DSC]
Warning
All Forms:
- The use of this drug was accompanied by violations of the liver.
 In some cases, these violations have led to death. In most cases, liver problems occurred within the first 6 months after starting this drug. Call your child's doctor right away if your child shows signs of liver problems such as dark urine, fatigue, lack of appetite, nausea or abdominal pain, light-colored stools, vomiting, yellowing of the skin or eyes. In patients suffering from seizures, there may be a loss of control over seizures. Do your child's blood test as directed by their doctor. nine0006
In some cases, these violations have led to death. In most cases, liver problems occurred within the first 6 months after starting this drug. Call your child's doctor right away if your child shows signs of liver problems such as dark urine, fatigue, lack of appetite, nausea or abdominal pain, light-colored stools, vomiting, yellowing of the skin or eyes. In patients suffering from seizures, there may be a loss of control over seizures. Do your child's blood test as directed by their doctor. nine0006 - The risk of life-threatening liver problems is increased in children under 2 years of age. The risk is highest in patients taking more than 1 anti-seizure drug or who have a metabolic disorder, severe epilepsy with mental retardation, or congenital brain disease. Consult your doctor.
- Patients with a genetic liver disorder are at an increased risk of developing liver failure due to a mitochondrial disorder such as Alpers-Huttenlocher syndrome. Your child may need genetic testing to detect this disease.
 If a child has or may have a mitochondrial disorder, do not give this drug without consulting your doctor. nine0006
If a child has or may have a mitochondrial disorder, do not give this drug without consulting your doctor. nine0006 - This drug can cause serious and sometimes deadly problems with the pancreas (pancreatitis). Such a violation can occur in children at any time during treatment with this drug. Symptoms of pancreatitis include abdominal pain, nausea, vomiting, or decreased appetite. If your child develops any of these symptoms, contact their doctor immediately.
If your child is or may be sexually active:
- If your daughter is pregnant or may become pregnant, check with her doctor to make sure this drug is right for her. She must use birth control while taking this drug to prevent pregnancy.
If your daughter is pregnant or likely to be pregnant:
- This drug can cause serious birth defects if taken during pregnancy. It can also lower a child's IQ and may increase the risk of autism or ADHD. If your daughter becomes pregnant while taking this drug, call her doctor right away.
 nine0006
nine0006 - Do not give this migraine prevention drug to your daughter if she is pregnant or not using birth control to prevent pregnancy.
All oral preparations:
- This drug comes with a separate patient information leaflet called Patient Drug Information. Read it carefully each time you take this drug. If you have any questions about this drug, ask your doctor, pharmacist, or other health care professional. nine0006
What is this drug used for?
- Used to treat seizures.
- It is used to prevent migraine.
- This medicine is used to treat bipolar disorder.
- This drug can be given to children for other indications. Consult your doctor.
What do I need to tell the doctor BEFORE my child takes this drug?
- If your child has an allergy to this drug, any of its ingredients, other drugs, foods, or substances. Tell the doctor about the allergy and how it manifested itself in the child.
 nine0006
nine0006 - If your child suffers from any of the following: liver disease or a urea cycle disorder.
This list of drugs and conditions that may interact with this drug is not exhaustive.
Talk to your doctor or pharmacist about all medicines your child is taking (prescription and over-the-counter, natural, and vitamins) and any health problems. You need to make sure that this drug is safe to use for your child's illnesses and in combination with other drugs he or she is already taking. Do not start, stop taking, or change the dosage of any drug your child is taking without the doctor's approval. nine0003
What do I need to know or do while my child is taking this drug?
For all uses of this drug:
- Tell all health care providers who care for your child that your child is taking this drug. These are your child's doctors, nurses, pharmacists and dentists.
- Have your child avoid tasks or activities that require attention until you see how this drug works for your child.
 This includes cycling, playing sports, or using items such as scissors, lawn mowers, electric scooters, toy cars, or motorized vehicles. nine0006
This includes cycling, playing sports, or using items such as scissors, lawn mowers, electric scooters, toy cars, or motorized vehicles. nine0006 - Perform blood tests as directed by your doctor. Consult with your doctor.
- Alcohol can interact with this drug. Make sure your child does not drink alcohol.
- Check with your child's doctor before using marijuana, other forms of cannabis, or prescription or over-the-counter drugs that may slow your child's progress.
- This drug may affect the results of some lab tests. Tell all health care workers and laboratory workers who provide your child's health care that your child is taking this drug. nine0006
- If your child is unable to eat or drink as usual, check with their healthcare provider. These situations include illness, fasting, certain procedures or surgery.
- Some brands of this drug contain peanut butter. If your child is allergic to peanuts, ask your pharmacist to check the peanut butter content of your child's brand of peanut butter.
 nine0006
nine0006 - Your child may become more prone to bleeding. Make sure your child is careful to avoid injury. Make sure your child uses a soft toothbrush.
- This drug has been associated with increased levels of ammonia in the blood. This can lead to certain brain disorders. Some fatalities. Consult your doctor.
- In some individuals, certain brain disorders were not accompanied by an increase in the level of ammonia in the blood. In some cases, these brain disorders resolved after discontinuation of treatment with this drug. However, in some cases they did not go completely. Consult your doctor. nine0006
- There was a serious reaction that could be deadly. In most cases, this reaction was accompanied by symptoms such as fever, rash, inflammation of the lymph nodes, and dysfunction of various organs such as the liver, kidneys, blood, heart, muscles, joints and lungs. If you have any questions, please consult your doctor.
- This drug may affect a man's ability to have a child.
 Consult your doctor. nine0006
Consult your doctor. nine0006
If your daughter is breastfeeding:
- Tell your doctor if your daughter is breastfeeding. You will need to be counseled about the possible risks to the child.
Anti-seizure:
- Talk to your doctor if your seizures change or get worse after you start taking this drug.
What side effects should I report to my child's doctor right away? nine0017
WARNING/CAUTION: Although rare, some people may have very serious and sometimes deadly side effects of this drug. Call your child's doctor right away or get medical help if your child has any of the following signs or symptoms that could be associated with a very bad side effect:
- Signs of an allergic reaction, such as rash, hives, itching, red and swollen skin with blisters or peeling, possibly accompanied by fever, wheezing or wheezing, tightness in the chest or throat, difficulty breathing, swallowing or speaking, unusual hoarseness, swelling in the mouth, face, lips, tongue or throat.
 nine0006
nine0006 - Signs of infection, such as fever, chills, very bad pain in the throat, ear, or sinuses, cough, more sputum or change in color, pain when urinating, mouth sores, or a sore that doesn't heal.
- Signs of high ammonia levels such as irregular heartbeat, trouble breathing, confusion, pale skin, bradycardia, seizures, sweating, vomiting, or muscle twitches.
- Chest pain. nine0006
- Swelling of the hands or feet.
- Vision change.
- Impairment or loss of memory.
- Balance change.
- Difficulties with walking.
- Any unexplained bruising or bleeding.
- The appearance of purple spots on the skin or redness of the skin.
- Difficulty urinating or a change in the amount of urine produced.
- Swelling of the gland.
- Muscle pain or weakness. nine0006
- Pain or inflammation in the joints.
- Shiver.
- Loss of control over eye movements.
- Tinnitus.
- Chills.
- Like other seizure medicines, this medicine can rarely increase the risk of suicidal ideation or behavior. This risk may be higher in people who have tried or had suicidal thoughts in the past. Call your doctor right away if you develop or worsen symptoms such as depression, nervousness, anxiety, irritability, panic attacks, or other mood or behavioral disturbances. If you have suicidal thoughts or attempted suicide, contact your doctor immediately. nine0006
What are some other side effects of this drug?
Any drug can cause side effects. However, for many people, side effects are either minor or non-existent. Contact your child's doctor or seek medical attention if any of these or other side effects bother your child or if they persist:
- Headache.
- Constipation, diarrhea, abdominal pain, nausea, vomiting, or decreased appetite. nine0006
- Increased appetite.
- Feeling dizzy, tired or weak.

- Sleep disorders.
- Weight gain or loss.
- Hair loss.
- Nervous tension and agitation.
- Flu-like symptoms.
This list of possible side effects is not exhaustive. If you have any questions about side effects, ask your child's doctor. Talk to your child's doctor about side effects. nine0003
You can report side effects to the National Health Board.
What is the best way to give this drug?
Give this drug to your child as directed by your doctor. Read all the information provided to you. Strictly follow all instructions.
All oral preparations:
- Give this drug with or without food. If the drug causes stomach upset, give it with food. nine0006
- Keep giving this drug as directed by your child's doctor or other health care provider, even if your child is feeling well.
- Do not stop giving this drug to your child abruptly without talking to the doctor.
 This can increase the risk of seizures. If needed, this drug should be stopped gradually for your child as directed by the doctor. nine0005 If your child is taking cholestyramine, you may need to give it at a different time than this drug. Consult with a pharmacist.
This can increase the risk of seizures. If needed, this drug should be stopped gradually for your child as directed by the doctor. nine0005 If your child is taking cholestyramine, you may need to give it at a different time than this drug. Consult with a pharmacist.
Tablets and capsules:
- Ask your child to swallow whole. Ask your child not to chew, break, or crush the tablet.
- Give this drug with a full glass of water.
- If your child has difficulty swallowing, check with your doctor. nine0006
Long acting tablets:
- If you or your child see particles of this drug in your child's stool, contact your doctor.
Split capsule:
- The child may swallow the capsule whole or mix the contents of the capsule with certain foods such as applesauce. Make sure the child takes the mixture immediately. Do not store for future use.
- If you or your child see particles of this drug in your child's stool, contact your doctor.
 nine0006
nine0006
Fluid:
- Liquid doses should be measured with caution. Use the dispenser that comes with the medication. If the dispenser is not provided in the package, ask the pharmacist for a dosing agent for this drug.
Injection:
- This drug is administered by infusion intravenously continuously for a certain time.
What if my child misses a dose of medication? nine0017
All oral preparations:
- Give the missed dose as soon as possible.
- If it is time for your child to take the next dose, do not take the missed dose and then go back to your child's normal schedule.
- Do not give a double dose at the same time or additional doses.
Injection:
- Contact your child's doctor to find out the next steps. nine0006
How do I store and/or discard this drug?
All oral preparations:
- Store at room temperature in a dry place.
 Do not store in the bathroom.
Do not store in the bathroom.
Injection:
- If you need to store this drug at home, check with your child's doctor, nurse, or pharmacist about how to store it.
All editions:
- Keep all medicines in a safe place. Keep all medicines out of the reach of children and pets.
- Dispose of unused or expired drugs. Do not empty into a toilet or sewer unless instructed to do so. If you have any questions about disposing of medicines, ask your pharmacist. Drug disposal programs may be in place in your area. nine0006
General information about medicines
- If your child's symptoms or health problems do not improve, or worsen, contact your child's doctor.
- Do not share your child's medicine with others and do not give anyone else's medicine to your child.
- Some medicines may come with other patient information leaflets. If you have questions about this drug, talk with your child's doctor, nurse, pharmacist, or other health care professional.
 nine0006
nine0006 - If you think you have overdosed, call a poison control center or get medical help right away. Be prepared to tell or show what drug you took, how much, and when it happened.
Consumer Use of Information and Limitation of Liability
This summary information includes a summary of the diagnosis, treatment, and/or drug product. It is not intended to be a comprehensive source of data and should be used as a tool to help the user understand and/or evaluate potential diagnostic and treatment options. It does NOT include all information about conditions, treatments, medications, side effects, or risks that may apply to a particular patient. It should not be considered medical advice or a substitute for medical advice, diagnosis or treatment provided by a physician based on a medical examination and assessment of the patient's specific and unique circumstances. Patients should consult with their physician for full information about their health, medical issues, and treatment options, including any risks or benefits regarding the use of medications. This information is not a guarantee that a treatment or drug is safe, effective, or approved for a particular patient. UpToDate, Inc. and its subsidiaries disclaim any warranties or liabilities related to this information or its use. The use of this information is subject to the Terms of Use found at https://www.wolterskluwer.com/en/know/clinical-effectiveness-terms. nine0003
This information is not a guarantee that a treatment or drug is safe, effective, or approved for a particular patient. UpToDate, Inc. and its subsidiaries disclaim any warranties or liabilities related to this information or its use. The use of this information is subject to the Terms of Use found at https://www.wolterskluwer.com/en/know/clinical-effectiveness-terms. nine0003
Copyright
© UpToDate, Inc. and its affiliates and/or licensors, 2022. All rights reserved.
Depakote instructions, description and reviews
Pronunciation
Generic name: divalproex sodium (val PRO ex dye)
Trade name: Depakote, Depakote ER, Depakote Sprinkles
Depakote (divalproex sodium in the body) affects chemicals, which may be involved in prolapsed seizures.
Depakote is used to treat various types of seizures. It is sometimes used in conjunction with other seizures.
Depakote is also used to treat manic episodes associated with bipolar disorder (manic depression) and to prevent migraine headaches.
Depakote may also be used for purposes not listed in this medication guide.
Do not use Depakote to prevent migraine headaches if you are pregnant. nine0003
If you are taking Depakote for seizures or manic episodes: Do not start or stop taking the medicine during pregnancy without your doctor's advice. Divalproex sodium may harm the unborn baby, but seizures during pregnancy can harm both mother and baby.
Slideshow Epilepsy in adults: a guide for healthcare professionals
You should not use Depakote if you have liver disease, a urea cycle disorder, or a genetic disorder such as Alpers disease or Alper-Hattenlocher syndrome (especially in a child younger than 2 years old). nine0003
Depakote can cause liver failure, which can be fatal, especially in children under 2 years of age and in people with liver problems caused by a genetic mitochondrial (MYE-toe-KON-dree-al) disorder.
Call your doctor right away if a person taking this medicine has early signs of liver or pancreas problems such as: loss of appetite, stomach pain (may spread to the back), ongoing nausea or vomiting, dark urine, swelling a Face or jaundice (yellowing of the skin or eyes). nine0003
nine0003
You should not use Depakote if you are allergic to divalproex sodium, or if you have:
-
liver disease;
-
Urea cycle disorder; or
-
Genetic mitochondrial (MYE-toe-KON-dree-al) disorder such as Alpers disease or Alper-Huttenlocher syndrome, especially in a child younger than 2 years of age.
Depakote can cause liver failure, which can be fatal, especially in children under 2 years of age and in people with liver problems caused by a genetic mitochondrial disorder. nine0003
To make sure this medicine is safe for you, tell your doctor if you have:
-
Liver problems caused by a genetic mitochondrial disorder;
-
History of depression, mental illness, or suicidal thoughts or actions;
-
Family history of urinary tract disorder or infant death of unknown cause; or
-
HIV or CMV (cytomegalovirus).
Some young people have suicidal thoughts when they first take Depakote. Your doctor will need to check your progress at regular visits while you are using this medicine. Your family or other caregivers should also be aware of changes in your mood or symptoms.
Your doctor will need to check your progress at regular visits while you are using this medicine. Your family or other caregivers should also be aware of changes in your mood or symptoms.
Do not use Depakote to prevent migraine headaches if you are pregnant.
If you are taking Depakote for seizures or manic episodes: This medicine may harm an unborn baby or cause birth defects and may affect cognition (reasoning, intelligence, problem solving) later in a child's life. However, having seizures during pregnancy can harm both mother and baby. Do not start or take medicine during pregnancy without your doctor's advice. nine0003
Use effective birth control while using Depakote, and tell your doctor right away if you become pregnant.
Tell your doctor if you start or stop using estrogen-containing hormonal contraception (birth control pills, injections, implants, skin patches, and vaginal rings). Estrogen may interact with divalproex sodium and make it less effective in preventing seizures.
Seizure control is very important during pregnancy. The benefit of preventing seizures may outweigh any risks associated with Depakote. There may be other seizures that are safer to use during pregnancy. Follow your doctor's instructions about taking this medication while you are pregnant. nine0003
Divalproex sodium may pass into breast milk and may harm a nursing baby. Tell your doctor if you are breastfeeding a baby.
Take Depakote exactly as prescribed by your doctor. Follow all directions on the prescription label. Your doctor may occasionally change your dose to make sure you get the best results. Do not take this medicine in larger or smaller amounts, or for longer than recommended.
Drink plenty of water while you are taking this drug. Your dose may need to be changed if you are not getting enough fluids every day. nine0003
You can open a Deprakote capsule and sprinkle it on a spoonful of pudding or applesauce to make it easier to swallow. Swallow this mixture right away.
Do not crush, chew, break or open a sustained release or extended release tablet or capsule. Swallow it all.
You may need frequent blood tests while using Depakote.
Wear a medical alert tag or carry identification that says you are taking Depakote. Any doctor, dentist, or emergency care provider who treats you should know that you are taking seizures. nine0003
If you need surgery, tell the surgeon that you are using Depakote.
Do not stop using Depakote suddenly, even if you feel well. Stopping suddenly can lead to a serious, life-threatening type of seizure. Follow your doctor's instructions for dose reduction.
Store at room temperature away from moisture and heat.
See also: Dosing Information (more details)
Take the missed dose as soon as you remember. Skip the missed dose if it is almost time for your next scheduled dose. Do not take extra medicine to make up for a missed dose. nine0003
Read also about the drug Differ in.
Seek emergency medical attention or call the Poison Help Line at 1-800-222-1222.
Drinking alcohol may cause certain side effects of Depakote.
Depakote may interfere with your thinking or reactions. Be careful if you drive or do anything that requires your attention.
Avoid exposure to sunlight or tanning beds. Divalproex sodium may make it easier for you to tan. Wear protective clothing and use sunscreen (SPF 30 or higher) when you are outside. nine0003
Read also about Norvir.
Get emergency medical help if you have any of these signs of an allergic reaction: to Depakote hives; fever, swollen glands, sores in the neck, difficulty breathing; Swelling of the face, lips, tongue, or throat.
Call your doctor right away if a person taking this medicine has early signs of liver or pancreas problems such as: loss of appetite, stomach pain (may spread to the back), ongoing nausea or vomiting, dark urine, swelling a Face or jaundice (yellowing of the skin or eyes). nine0003
nine0003
Report any new or worsening symptoms to your doctor, such as: mood or behavior changes, depression, anxiety, panic attacks, trouble sleeping, or if you feel impulsive, irritable, agitated, hostile, aggressive, restless, hyperactive (mentally or physically) Or thinking about suicide or hurting yourself.
If you have any of these side effects, call your doctor right away:
-
Confusion, tiredness, feeling cold, vomiting, change in your mental state;
-
easy bruising, unusual bleeding (nose, mouth, or gums), purple or red spots under your skin;
-
Severe drowsiness;
-
Worse convulsions;
-
signs of inflammation in your body - swollen glands, flu symptoms, severe tingling or numbness, muscle weakness, chest pain, new or worsening cough with fever, difficulty breathing; or
-
Severe skin reaction - fever, sore throat, swelling in your face or tongue, burning in your eyes, skin pain, and then a red or purple skin rash that spreads (especially to the face or upper body ) and causes swelling and peeling.

Common Depakote side effects may include:
-
Moderate nausea or vomiting, mild abdominal pain, diarrhea;
-
Headache, slight dizziness, weakness, tremor; nine0003
-
Problems with balance or walking;
-
Blurred vision, double vision; or
-
Appetite changes, weight gain.
This is not a complete list of side effects and others may occur. Ask your doctor about side effects. You can report side effects to the FDA at 1-800-FDA-1088.
See also: Side effects (more details)
It is sometimes not safe to use certain medicines at the same time. Some drugs can raise or lower your blood levels of divalproex, which may cause side effects or make Depakote less effective. Divalproex sodium can also affect the levels of some other medicines, making them less effective or causing more side effects. nine0003
Many drugs can interact with divalpro sodium. This includes prescription and over-the-counter medicines, vitamins, and herbal products.