How to take xenical
Xenical Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Uses
This medication is used with a doctor-approved exercise, behavior change, and reduced-calorie diet program to help you lose weight. It is used by certain overweight people, such as those who are obese or have weight-related medical problems. Taking orlistat can also help keep you from gaining back weight you have lost. Losing weight and keeping it off can lessen the many health risks that come with obesity, including heart disease, diabetes, high blood pressure, and a shorter life.Dietary fats need to be broken down into smaller pieces before the body can absorb them. Orlistat works by blocking the enzyme that breaks down fats in your diet. This undigested fat then passes out of your body in your bowel movement. Orlistat does not block the absorption of calories from sugar and other non-fat foods, so you still need to restrict your total intake of calories.
How to use Xenical
If you are taking the over-the-counter product to self-treat, read all directions on the product package before taking this medication.
If your doctor has prescribed this medication, read the Patient Information Leaflet if available from your pharmacist before you start taking orlistat and each time you get a refill. Take this medication as directed by your doctor, by mouth with liquid sometime during each meal that contains fat or within 1 hour after the meal, usually 3 times daily. If you miss a meal or your meal contains no fat, skip that dose of the medication. To decrease the chance of unpleasant side effects, it is very important that no more than 30% of the calories in your diet come from fat. Your daily intake of fat, protein, and carbohydrates should be evenly spread over 3 main meals.
Do not increase your dose or use this drug more often or for longer than prescribed. Your condition will not improve any faster, and your risk of side effects will increase.
Because this drug can interfere with the absorption of certain vitamins (fat-soluble vitamins including A, D, E, K), a daily multivitamin supplement containing these nutrients is recommended. Take the multivitamin at least 2 hours before or 2 hours after taking orlistat (such as at bedtime).
Take the multivitamin at least 2 hours before or 2 hours after taking orlistat (such as at bedtime).
If you take cyclosporine, take it at least 3 hours before or after orlistat to make sure the full dose of cyclosporine is absorbed into your bloodstream. If you take levothyroxine, take it at least 4 hours before or after orlistat.
You should see some weight loss within 2 weeks after you start orlistat. Tell your doctor if your condition does not improve or if it worsens.
Side Effects
Changes in your bowel function often occur because of the unabsorbed fat. Fatty/oily stool, oily spotting, intestinal gas with discharge, a feeling of needing to have a bowel movement right away, increased number of bowel movements, or poor bowel control may occur. These side effects may get worse if you eat more fat than you should. If these effects last or get worse, notify your doctor promptly.
If your doctor has directed you to use this medication, remember that your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Many people using this medication do not have serious side effects.
Stop taking this medication and tell your doctor right away if any of these rare but serious side effects occur: symptoms of liver disease (such as nausea/vomiting that doesn't stop, severe stomach/abdominal pain, dark urine, yellowing eyes/skin), symptoms of kidney stones (such as back pain, pain when urinating, pink/bloody urine).
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects.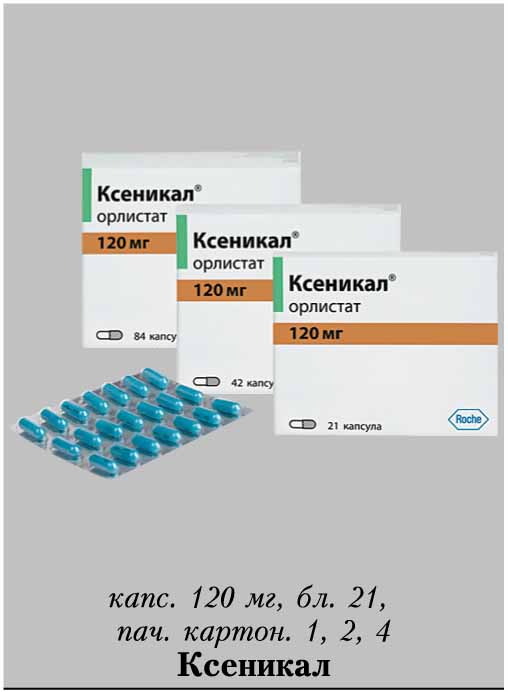 You may report side effects to Health Canada at 1-866-234-2345.
You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before taking orlistat, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: a certain digestive problem (chronic malabsorption syndrome), a certain gall bladder problem (cholestasis), underactive thyroid (hypothyroidism), kidney stones/problems (such as calcium oxalate kidney stones, hyperoxaluria), certain eating disorders (anorexia nervosa/bulimia), HIV infection, seizures.
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
If you are diabetic, weight loss may improve your blood sugar control.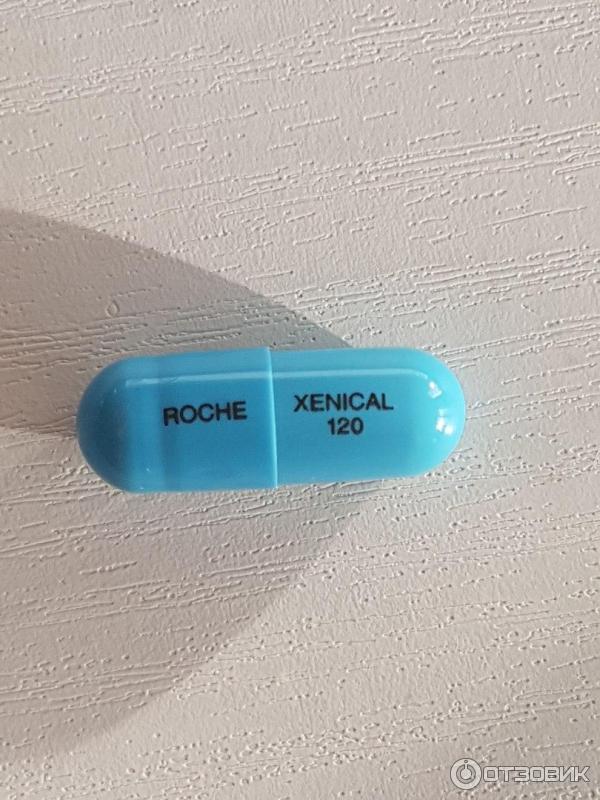 Be sure to check your blood sugar regularly and tell your doctor the results. Your doctor may need to adjust your diabetes medication, exercise program, or diet.
Be sure to check your blood sugar regularly and tell your doctor the results. Your doctor may need to adjust your diabetes medication, exercise program, or diet.
This medication must not be used during pregnancy. Weight loss offers no potential benefit to a pregnant woman and may harm an unborn baby. If you become pregnant or think you may be pregnant, tell your doctor right away.
It is unknown if this drug passes into breast milk. Consult your doctor before breast-feeding.
Interactions
See also How to Use section.
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: "blood thinners" (such as warfarin), HIV medications (such as atazanavir, efavirenz, emtricitabine, lopinavir, tenofovir), ritonavir.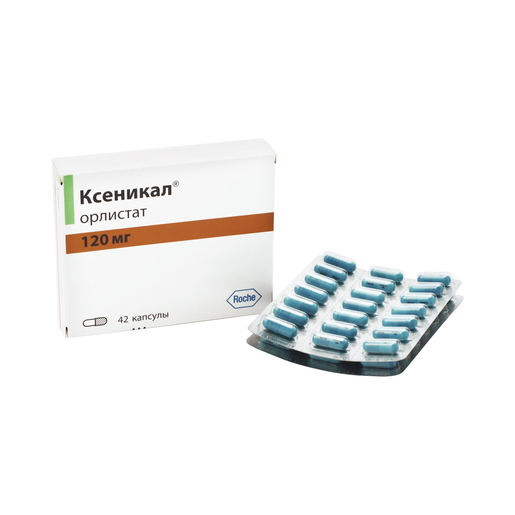
If you are taking medications to treat seizures, orlistat may cause these drugs to not work as well. Stop taking orlistat and tell your doctor right away if your seizures are happening more often or are getting worse.
Does Xenical interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
If this medication has been prescribed for you, do not share this medication with others.
For best results, this medication should be used along with a doctor-approved exercise program and diet plan. Consult your doctor or dietician for help designing an appropriate exercise and food plan for you.
If you miss a dose, take it as soon as you remember unless it has been more than 1-2 hours since your meal. In that case, skip the missed dose since most of the fat from your meal will already have been absorbed and the medication will not work. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company for more details.
Images
Next
Related Links
Drug Survey
Are you currently using Xenical?
This survey is being conducted by the WebMD marketing sciences department.
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
Xenical - Uses, Side Effects, Interactions
How does this medication work? What will it do for me?
Orlistat belongs to a class of medications known as anti-obesity agents, specifically gastrointestinal lipase inhibitors. When combined with a mildly reduced-calorie diet that contains no more than 30% of calories from fat, orlistat is used to help obese people lose weight and maintain weight loss.
Lipase is an enzyme that is needed to breakdown dietary fats into fatty acids, which is the form of fat that gets absorbed. Orlistat blocks the action of lipase and therefore prevents dietary fat from being absorbed. At the recommended dose, orlistat inhibits dietary fat absorption by approximately 30%.
This medication should only be used by people who have a body mass index (BMI) of 30 kg/m² or higher, or by people with a BMI of 27 kg/m² or higher with other health risk factors such as high blood pressure, type 2 diabetes, high cholesterol, or large waist measurement. BMI is not a direct measure of fat and these guidelines do not apply to athletes and pregnant women.
BMI is calculated by dividing your body weight in kilograms by the square of your height in metres. For example, if you weigh 150 lbs (68 kg) and are 5 ft 8 in (1.73 m) tall, divide 68 by (1.73×1.73), or 2.99. The result is a BMI of 22.7 kg/m².
Orlistat can also be used in combination with diabetes medications to improve blood glucose control in overweight or obese people with type 2 diabetes whose blood glucose levels are inadequately controlled with diet, exercise, and diabetes medications (e. g., glyburide, insulin, metformin).
g., glyburide, insulin, metformin).
This medication may be available under multiple brand names and/or in several different forms. Any specific brand name of this medication may not be available in all of the forms or approved for all of the conditions discussed here. As well, some forms of this medication may not be used for all of the conditions discussed here.
Your doctor may have suggested this medication for conditions other than those listed in these drug information articles. If you have not discussed this with your doctor or are not sure why you are taking this medication, speak to your doctor. Do not stop taking this medication without consulting your doctor.
Do not give this medication to anyone else, even if they have the same symptoms as you do. It can be harmful for people to take this medication if their doctor has not prescribed it.
What form(s) does this medication come in?
Each turquoise capsule, with "Xenical 120" printed in black ink, contains 120 mg of orlistat. Nonmedicinal ingredients: gelatin, indigo carmine, microcrystalline cellulose, povidone K30, sodium lauryl sulfate, sodium starch glycolate, talc, and titanium dioxide.
Nonmedicinal ingredients: gelatin, indigo carmine, microcrystalline cellulose, povidone K30, sodium lauryl sulfate, sodium starch glycolate, talc, and titanium dioxide.
How should I use this medication?
The recommended adult dose is 120 mg three times daily with each main meal (breakfast, lunch, and dinner) or up to 1 hour after the meal. If you occasionally miss a meal or the meal does not contain fat, do not take the dose of orlistat. Swallow the capsule whole with some water.
Orlistat should be taken with a mildly reduced-calorie diet that contains no more than 30% of calories from fat, as recommended by your doctor, dietitian, or other health care professional.
Many things can affect the dose of medication that a person needs, such as body weight, other medical conditions, and other medications. If your doctor has recommended a dose different from the ones listed here, do not change the way that you are taking the medication without consulting your doctor.
It is important to take this medication exactly as prescribed by your doctor. If you miss a dose, it can be taken up to 1 hour after a meal and still be effective. However, if it is greater than an hour when you remember, skip the missed dose and continue with your regular dosing schedule. Do not take a double dose to make up for a missed one. If you are not sure what to do after missing a dose, contact your doctor or pharmacist for advice.
Store this medication at room temperature in the original package, protect it from moisture, and keep it away from heat and out of the reach of children.
Do not dispose of medications in wastewater (e.g. down the sink or in the toilet) or in household garbage. Ask your pharmacist how to dispose of medications that are no longer needed or have expired.
Who should NOT take this medication?
Do not take this medication if you:
- are allergic to orlistat or any ingredients of the medication
- have cholestasis (a condition where bile excretion in the liver is stopped)
- have chronic malabsorption syndrome (a condition where you do not absorb nutrients from food properly)
What side effects are possible with this medication?
Many medications can cause side effects. A side effect is an unwanted response to a medication when it is taken in normal doses. Side effects can be mild or severe, temporary or permanent.
A side effect is an unwanted response to a medication when it is taken in normal doses. Side effects can be mild or severe, temporary or permanent.
The side effects listed below are not experienced by everyone who takes this medication. If you are concerned about side effects, discuss the risks and benefits of this medication with your doctor.
The following side effects have been reported by at least 1% of people taking this medication. Many of these side effects can be managed, and some may go away on their own over time.
Contact your doctor if you experience these side effects and they are severe or bothersome. Your pharmacist may be able to advise you on managing side effects.
- discoloured stools
- diarrhea
- gas with discharge
- inability to control bowel movements
- increases in bowel movements
- oily or fatty stools
- oily spotting of underclothes
- urgent need to have a bowel movement
Although most of the side effects listed below don't happen very often, they could lead to serious problems if you do not seek medical attention.
Check with your doctor as soon as possible if any of the following side effects occur:
- signs of kidney problems (e.g., increased urination at night, decreased urine production, blood in the urine, swelling of the hands or feet, painful urination)
- swelling of feet or ankles
- symptoms of gallstones (such as pain in the upper right part of the abdomen that may be accompanied by nausea and vomiting)
- symptoms of kidney stones (such as back pain and blood in the urine)
- symptoms of liver damage (e.g., abdominal pain, clay-coloured stools, dark urine, loss of appetite, yellow skin or eyes)
- symptoms of low blood sugar (e.g., cold sweat, cool pale skin, headache, fast heartbeat, weakness
Stop taking the medication and seek immediate medical attention if any of the following occur:
- bleeding from the rectum
- signs of an allergic reaction (e.g., hives, shortness of breath or difficulty breathing, swelling of the eyes, mouth, lips, or throat)
- signs of pancreatitis (e.
 g., abdominal pain on the upper left side, back pain, nausea, fever, chills, rapid heartbeat, swollen abdomen)
g., abdominal pain on the upper left side, back pain, nausea, fever, chills, rapid heartbeat, swollen abdomen)
Some people may experience side effects other than those listed. Check with your doctor if you notice any symptom that worries you while you are taking this medication.
Are there any other precautions or warnings for this medication?
Before you begin taking a medication, be sure to inform your doctor of any medical conditions or allergies you may have, any medications you are taking, whether you are pregnant or breast-feeding, and any other significant facts about your health. These factors may affect how you should use this medication.
Diabetes: If you have type 2 diabetes and are taking orlistat in combination with other diabetes medications, you may be more at risk of experiencing hypoglycemia (low blood sugar). If you experience hypoglycemia symptoms such as sweating, dizziness, shakiness, hunger, or confusion, contact your doctor. Your doctor may need to adjust the dose of your medication(s). You should continue to monitor your blood sugar levels regularly.
Your doctor may need to adjust the dose of your medication(s). You should continue to monitor your blood sugar levels regularly.
Fat intake: It is very important to follow recommended dietary guidelines while taking orlistat. The risk of side effects associated with the stomach (such as gas with discharge or oily spotting of underclothes) increases when orlistat is taken with a high-fat diet. Your daily intake of fat should be distributed over three main meals and should not be more than 30% of the total calories. Discuss with your doctor or dietitian if you have any concerns or questions regarding how to manage your diet.
Gastrointestinal problems: If you have bowel or rectal problems, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed. Bleeding from the rectum has been reported with the use of orlistat. If this occurs, contact your doctor as soon as possible.
If this occurs, contact your doctor as soon as possible.
Kidney stones: If you have a history of kidney stones, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Liver: There have been rare reports of severe liver injury in people taking orlistat. If you experience any symptoms such as loss of appetite, yellow skin or eyes, abdominal pain, dark urine, light-coloured stools, or pain in the upper right part of the abdomen, contact your doctor immediately.
Seizures: Orlistat may interact with seizure medications by decreasing the amount of seizure medication that your body absorbs. This can result in increased seizures. If you have a history of seizures, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Thyroid: Orlistat may affect how well your thyroid gland works, possibly by reducing the amount of iodine available from your diet for your body to produce thyroid hormone. If you have hypothyroidism (low thyroid hormone levels), your doctor should closely monitor your condition while you are taking orlistat, as it may affect thyroid levels.
Vitamins: Orlistat may reduce the absorption of beta-carotene and fat-soluble vitamins such as vitamin A, vitamin D, vitamin E, and vitamin K. Talk to your doctor or pharmacist about taking a multivitamin supplement. If you are already on a multivitamin, take it at least 2 hours before or after taking orlistat, or take it at bedtime.
Pregnancy: Orlistat has not been adequately studied in pregnant women. This medication should not be used during pregnancy unless the benefits outweigh the risks. If you become pregnant while taking this medication, contact your doctor immediately.
Breast-feeding: It is not known if orlistat passes into breast milk. If you are a breast-feeding mother and taking this medication, it may affect your baby. Talk to your doctor about whether you should continue breast-feeding.
If you are a breast-feeding mother and taking this medication, it may affect your baby. Talk to your doctor about whether you should continue breast-feeding.
Children: The safety and effectiveness of orlistat have not been established for use in children less than 12 years of age.
What other drugs could interact with this medication?
There may be an interaction between orlistat and any of the following:
- acetazolamide
- adefovir
- alfacalcidol
- amiodarone
- birth control pills
- calcitriol
- certain diabetes medications (e.g., glyburide, gliclazide, insulin, metformin)
- cobicistat
- cyclosporine
- fat-soluble vitamin supplements (e.g., vitamin A, vitamin D, vitamin E, and vitamin K)
- gabapentin
- HIV integrase strand inhibitors (e.g., bictegravir, dolutegravir, raltegravir)
- HIV non-nucleoside reverse transcriptase inhibitors (NNRTIs; e.
 g., efavirenz, etravirine, nevirapine)
g., efavirenz, etravirine, nevirapine) - HIV nucleoside reverse transcriptase inhibitors (NRTIs; e.g., abacavir, didanosine, lamivudine, tenofovir, zidovudine)
- HIV protease inhibitors (atazanavir, lopinavir, ritonavir)
- levothyroxine
- maraviroc
- propafenone
- seizure medications (e.g., carbamazepine, divalproex, lamotrigine, levetiracetam, phenytoin, phenobarbital, topiramate)
- stiripentol
- warfarin
If you are taking any of these medications, speak with your doctor or pharmacist. Depending on your specific circumstances, your doctor may want you to:
- stop taking one of the medications,
- change one of the medications to another,
- change how you are taking one or both of the medications, or
- leave everything as is.
An interaction between two medications does not always mean that you must stop taking one of them. Speak to your doctor about how any drug interactions are being managed or should be managed.
Medications other than those listed above may interact with this medication. Tell your doctor or prescriber about all prescription, over-the-counter (non-prescription) and herbal medications you are taking. Also tell them about any supplements you take. Since caffeine, alcohol, the nicotine from cigarettes, or street drugs can affect the action of many medications, you should let your prescriber know if you use them.
All material copyright MediResource Inc. 1996 – 2022. Terms and conditions of use. The contents herein are for informational purposes only. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Source: www.medbroadcast.com/drug/getdrug/Xenical
| 📜 Instructions for use Xenical ® 💊 Composition of Xenical ® ✅ Application of the drug Ksenical ® 📅 Storage conditions Ksenicig ® ⏳ The shelf life of Ksenicig 9000 ® Save Search for analogues Interaction Product description Xenical ® (Xenical ® ) Based on the official instructions for use of the drug and prepared for the electronic edition of the Vidal handbook 2011, update date: 2021. Marketing authorization holder:CHEPLAPHARM ARZNEIMITTEL GmbH (Germany)
Manufactured and packaged:DELPHARM MILANO, S.r.l. (Italy) or F.Hoffmann-La Roche Ltd (Switzerland)
Packaging and release quality control:DELPHARM MILANO, S.r.l. (Italy) or F.Hoffmann-La Roche Ltd (Switzerland) or RAINBOW PRODUCTION CJSC (Russia) ATX code: A08AB01 (Orlistat) Active substance: orlistat (orlistat) Rec. Dosage form
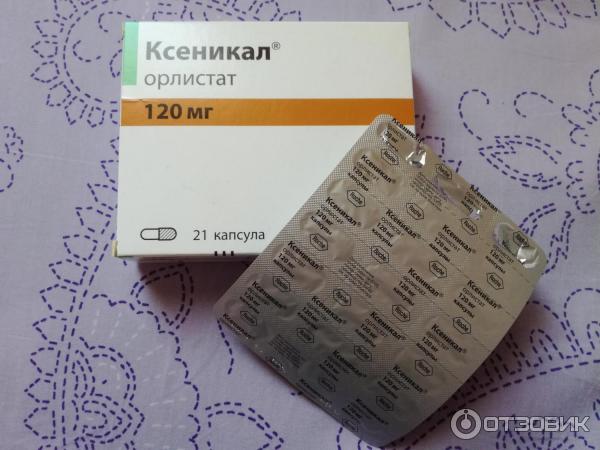
Release form, packaging and composition drug Xenical®Capsules hard gelatin, No. 1, opaque, turquoise; on the case the inscription "XENICAL 120" in black; the contents of the capsules are white or almost white pellets.
* composition Pellet: Orlistat - 120 mg, cellulose microcrystalline - 93.6 mg, carboxymethylcamal sodipid (lotion) - 7.2 mg, povididon, povididon. K-30 - 12 mg, sodium lauryl sulfate - 7. Excipients : talc - 0.24 mg. Composition of the capsule shell: gelatin, indigo carmine, titanium dioxide. 21 pcs. - blisters (1) - packs of cardboard. Clinical and pharmacological group: Anti-obesity drug - Gastrointestinal lipase inhibitor Pharmacotherapeutic group: Gastrointestinal lipase inhibitor Pharmacological action Xenical is a potent, specific and reversible long acting inhibitor of gastrointestinal lipases. Its therapeutic effect is carried out in the lumen of the stomach and small intestine and consists in the formation of a covalent bond with the active serine site of gastric and pancreatic lipases. The inactivated enzyme loses its ability to break down dietary fats in the form of triglycerides into absorbable free fatty acids and monoglycerides. Based on the results of fecal fat content, the effect of orlistat begins 24-48 hours after ingestion. After discontinuation of the drug, the fat content in the feces after 48-72 hours usually returns to the level that occurred before the start of therapy. Efficacy Obese patients In clinical studies, patients taking orlistat experienced greater weight loss compared to patients on diet therapy. Weight loss began already within the first 2 weeks after the start of treatment and lasted from 6 to 12 months, even in patients with a negative response to diet therapy. Over the course of 2 years, there was a statistically significant improvement in the profile of metabolic risk factors associated with obesity. In addition, compared with placebo, there was a significant reduction in body fat. Reduced risk of developing type 2 diabetes in obese patients In a 4-year clinical trial, orlistat was shown to significantly reduce the risk of developing type 2 diabetes (approximately 37% compared with placebo). Puberty obesity In a 1-year clinical study in obese adolescents, a decrease in body mass index was observed when taking orlistat compared with the placebo group, where there was even an increase in body mass index. In addition, patients in the orlistat group showed a decrease in fat mass, as well as waist and hip circumference, compared with the placebo group. Also, in patients treated with orlistat therapy, there was a significant decrease in diastolic blood pressure compared with the placebo group. Preclinical safety data Based on preclinical data, no additional risks to patients in terms of safety profile, toxicity, genotoxicity, carcinogenicity and reproductive toxicity have been identified. PharmacokineticsAbsorption In normal weight and obese volunteers, systemic exposure to the drug is minimal. After a single oral dose of the drug at a dose of 360 mg, unchanged orlistat in plasma could not be determined, which means that its concentrations are below the level of 5 ng / ml. In general, after administration of therapeutic doses, it was possible to detect unchanged orlistat in plasma only in rare cases, while its concentrations were extremely low (<10 ng / ml or 0.02 μmol). There were no signs of cumulation, which confirms that the absorption of the drug is minimal. Distribution V d cannot be determined because the drug is very poorly absorbed. In vitro, orlistat is more than 99% bound to plasma proteins (mainly lipoproteins and albumin). In minimal amounts, orlistat can penetrate red blood cells. Metabolism Animal data indicate that orlistat is metabolized primarily in the intestinal wall. In a study in obese individuals, it was found that approximately 42% of the minimum fraction of the drug that undergoes systemic absorption falls on the two main metabolites - M1 (four-membered hydrolyzed lactone ring) and M3 (M1 with a cleaved N-formilleucine residue). Molecules M1 and M3 have an open b-lactone ring and inhibit lipase extremely weakly (respectively, 1000 and 2500 times weaker than orlistat). Given this low inhibitory activity and low plasma concentrations (average 26 ng/mL and 108 ng/mL, respectively) following therapeutic doses, these metabolites are considered to be pharmacologically inactive. Excretion Studies in normal and overweight individuals have shown that the main route of elimination is excretion of unabsorbed drug with feces. About 97% of the accepted dose of the drug, with 83% in the form of unchanged orlistat. Cumulative renal excretion of all substances structurally related to orlistat is less than 2% of the administered dose. The time to complete elimination of the drug from the body (with feces and urine) is 3-5 days. The ratio of orlistat excretion pathways in volunteers with normal and overweight was the same. Both orlistat and the metabolites M1 and M3 may be excreted in the bile. Pharmacokinetics in special clinical groups Plasma concentrations of orlistat and its metabolites (M1 and M3) in children do not differ from those in adults when comparing the same doses of the drug. The daily excretion of fat in the feces was 27% of the intake with food with orlistat therapy and 7% with placebo. Indications of the drug Xenical®
Open list of ICD-10 codes
Dosing regimenLong-term therapy in obese or overweight patients, including those with obesity-associated risk factors, in combination with a moderately hypocaloric diet in adults and children over 12 years of age the recommended dose of orlistat is one 120 mg capsule with each main meal (during meals or no later than one hour after meals). In combination with hypoglycemic drugs (metformin, sulfonylurea derivatives and/or insulin) or a moderately hypocaloric diet in patients with type 2 diabetes mellitus who are overweight or obese : in adults mg with each main meal (during a meal or no later than one hour after a meal). If a meal is skipped or if the meal does not contain fat, Xenical can also be skipped. The drug should be taken in combination with a balanced, moderately hypocaloric diet containing no more than 30% of calories in the form of fat. An increase in the dose of orlistat above the recommended one (120 mg 3 times / day) does not lead to an increase in its therapeutic effect. The efficacy and safety of Xenical in patients with impaired liver and / or kidney function , as well as in elderly and pediatric patients (under 12 years of age) have not been studied. Side effectsClinical data The following categories are used to describe the frequency of adverse reactions: very often (≥1/10), often (≥1/100, <1/10), infrequently (≥1/1000 , <1/100), rarely (≥1/10000, <1/1000) and very rarely (<1/10000), including isolated cases. Adverse reactions to orlistat occurred mainly from the gastrointestinal tract and were due to the pharmacological action of the drug, which prevents the absorption of dietary fats. Very often there were such phenomena as oily discharge from the rectum, gas with some discharge, imperative urge to defecate, steatorrhea, increased defecation, loose stools, flatulence, pain or discomfort in the abdomen. Their frequency increases with increasing fat content in the diet. Patients should be informed about the possibility of adverse reactions from the gastrointestinal tract and taught how to eliminate them by better dietary compliance, especially in relation to the amount of fat contained in it. The use of a low-fat diet reduces the likelihood of gastrointestinal side effects and thus helps patients control and regulate their fat intake. As a rule, these adverse reactions are mild and transient. They occurred in the early stages of treatment (in the first 3 months), and most patients had no more than one episode of such reactions. Gastrointestinal side effects that are common during treatment with Xenical are soft stools, rectal pain or discomfort, fecal incontinence, bloating, tooth damage, and gum disease. Also very common: headaches, upper respiratory infections, influenza; often: lower respiratory tract infections, urinary tract infections, dysmenorrhea, anxiety, weakness. In patients with type 2 diabetes, the nature and frequency of adverse events were comparable to those in patients without diabetes with overweight and obesity. The only new side effects in patients with type 2 diabetes were hypoglycemic conditions, which occurred with a frequency of >2% and an incidence of ≥1% compared with placebo (which could result from improved carbohydrate metabolism compensation), and often - bloating. In the 4-year clinical study, the overall safety profile did not differ from that obtained in the 1- and 2-year studies. At the same time, the overall incidence of adverse events from the gastrointestinal tract decreased annually over a 4-year period of taking the drug. Post-marketing surveillance Rare cases of allergic reactions have been described, the main clinical symptoms of which were pruritus, rash, urticaria, angioedema, bronchospasm and anaphylaxis. Very rare cases of bullous rash, increased activity of transaminases and alkaline phosphatase, as well as isolated, possibly serious, cases of hepatitis have been described (a causal relationship with taking the drug Xenical ® or pathophysiological mechanisms of development have not been established). With the simultaneous administration of Xenical and anticoagulants, there have been cases of a decrease in prothrombin, an increase in the values of the international normalized ratio (INR) and unbalanced anticoagulant therapy, which led to a change in hemostatic parameters. Cases of rectal bleeding, diverticulitis, pancreatitis, cholelithiasis and oxalate nephropathy have been reported (frequency unknown). While taking orlistat and antiepileptic drugs, there have been cases of seizures (see section "Interaction with other drugs"). Contraindications for use
Use in pregnancy and lactation Category B product. In the absence of a teratogenic effect in animals, a similar effect in humans should not be expected. However due to lack of clinical data Xenical should not be given to pregnant women. Excretion of orlistat in breast milk has not been studied, so it should not be taken during breastfeeding. Special instructionsXenical is effective in terms of long-term weight control (weight loss and maintenance at a new level, prevention of re-gain of body weight). Treatment with Xenical leads to an improvement in the profile of risk factors and diseases associated with obesity, including hypercholesterolemia, type 2 diabetes mellitus, impaired glucose tolerance, hyperinsulinemia, arterial hypertension, and to a decrease in visceral fat. When used in combination with hypoglycemic drugs such as metformin, sulfonylurea derivatives and/or insulin in patients with type 2 diabetes mellitus who are overweight (body mass index (BMI) ≥28 kg/m 2 ) or obese (BMI ≥30 kg / m 2 ), Xenical in combination with a moderately hypocaloric diet provides an additional improvement in the compensation of carbohydrate metabolism. In clinical studies, the concentrations of vitamins A, D, E, K and betacarotene remained within the normal range in most patients during four years of orlistat therapy. The patient should receive a balanced, moderately hypocaloric diet containing no more than 30% of calories in the form of fat. A diet rich in fruits and vegetables is recommended. The daily intake of fats, carbohydrates and proteins must be divided into three main meals. The likelihood of adverse reactions from the gastrointestinal tract may increase if Xenical is taken on the background of a diet rich in fats (for example, 2000 kcal / day, of which more than 30% are in the form of fat, which equals approximately 67 g of fat). Daily fat intake should be divided into three main meals. If Xenical is taken with a meal that is very rich in fat, the likelihood of gastrointestinal reactions is increased. In patients with type 2 diabetes mellitus, weight loss during treatment with Xenical is accompanied by improved carbohydrate metabolism compensation, which may allow or require a reduction in the dose of hypoglycemic drugs (eg, sulfonylurea derivatives). OverdoseIn clinical studies in subjects with normal body weight and obese patients, single doses of 800 mg or multiple doses of 400 mg 3 times / day for 15 days were not accompanied by the appearance of significant adverse events. In addition, obese patients have experience with the use of orlistat 240 mg 3 times / day for 6 months, which was not accompanied by a significant increase in the frequency of adverse events. In cases of overdose of the drug Xenical, either no adverse events were reported, or adverse events did not differ from those observed when taking the drug at therapeutic doses. In the event of a severe overdose of Xenical, it is recommended to observe the patient for 24 hours. Based on human and animal studies, any systemic effects that could be associated with the lipase-inhibiting properties of orlistat should be rapidly reversible. Drug interactions No interactions with amitriptyline, atorvastatin, biguanides, digoxin, fibrates, fluoxetine, losartan, phenytoin, oral contraceptives, phentermine, pravastatin, warfarin, nifedipine sibutramine or alcohol (based on drug interaction studies). When taken simultaneously with Xenical, there was a decrease in the absorption of vitamins D, E and betacarotene. If multivitamins are recommended, they should be taken at least 2 hours after taking Xenical or at bedtime. With the simultaneous administration of Xenical and cyclosporine, a decrease in plasma concentrations of cyclosporine was noted, therefore, more frequent determination of plasma cyclosporine concentrations while taking cyclosporine and Xenical is recommended. With oral administration of amiodarone during therapy with Xenical, a decrease in the systemic exposure of amiodarone and deethylamiodarone (by 25-30%) was noted, however, due to the complex pharmacokinetics of amiodarone, the clinical significance of this phenomenon is not clear. The addition of Xenical to long-term amiodarone therapy may reduce the therapeutic effect of amiodarone (no studies have been conducted). Co-administration of Xenical and acarbose should be avoided due to lack of pharmacokinetic data. Concurrent use of orlistat and antiepileptic drugs has been associated with seizures. A causal relationship between the development of seizures and orlistat therapy has not been established. However, patients should be monitored for possible changes in the frequency and/or severity of seizures. Storage conditions of the drug Xenical®The drug should be stored at a temperature not exceeding 25 ° C in a place protected from moisture, out of the reach of children. Shelf life of Xenical®Shelf life - 3 years. Do not use after the expiry date stated on the packaging. Terms of saleThe drug is dispensed by prescription. Keep | ||||||||||||||
How to lose weight? With XENICAL it's easy
In August, APTEKA Weekly told its readers about XENICAL (orlistat), which is designed to reduce excess body weight (No. 31 (252), No. 33 (254). This drug has a fundamentally new mechanism of action.
31 (252), No. 33 (254). This drug has a fundamentally new mechanism of action.
Many have already used the information line "TIME TO LOSE WEIGHT" (tel.: 8-800-504-45-50; calls within Ukraine are free) and consulted with a specialist on the problem of reducing excess body weight. The representative office of Hoffmann-La Roche continues to receive phone calls. Today we bring to your attention the answers to the most frequently asked questions that arise when using XENICAL.
|
WHY DOES PEOPLE GET BETTER?
The main reason for this is that more calories enter the body with food than are consumed, that is, a person eats more than he needs. Fats are an important source of calories in food, since burning 1 g of fat releases 2 times more energy than burning 1 g of proteins or carbohydrates.
Fats contained in food are absorbed by the body only after they are broken down in the intestines by a special pancreatic enzyme - lipase. Lipase enters the intestines with the start of a meal and acts so effectively that all fats that come with food are usually absorbed by 100%. Therefore, as a solution to the problem of reducing excess body weight, scientists from the F. Hoffmann-La Roche chose lipase neutralization.
Lipase enters the intestines with the start of a meal and acts so effectively that all fats that come with food are usually absorbed by 100%. Therefore, as a solution to the problem of reducing excess body weight, scientists from the F. Hoffmann-La Roche chose lipase neutralization.
HOW IS XENICAL DIFFERENT FROM FOOD SUPPLEMENTS?
The fundamental difference between this drug and food supplements is that XENICAL is not a mixture of various substances, the mechanism of action of which is unknown, but a MEDICINE containing one known active ingredient orlistat with a fundamentally new, well-studied mechanism of action.
Most nutritional supplements have only a temporary effect of reducing body weight, either by acting on the hunger center in the brain, or by increasing heat transfer, or by acting as a diuretic or laxative. Therefore, after stopping the intake of nutritional supplements, body weight increases rapidly, reaching the same levels.
WHAT “DANGERS” ARE IN TOUCH FOR A PERSON WHO DECIDED TO LOSE WEIGHT AND STARTED TO TAKE XENICAL?
1. After starting XENICAL, some patients consume more carbohydrates and proteins, as a result of which the calorie content of food increases significantly. At the same time, they believe that "with XENICAL you can eat everything and in unlimited quantities." This is a delusion that can nullify all the efforts of the patient. XENICAL inhibits the absorption of fats, but does not affect the metabolism of proteins and carbohydrates. Unfortunately, the “ideal drug” for weight loss has not yet been created, taking which the patient could not limit himself to food.
2. Experience has shown that some patients who started taking XENICAL stop taking the drug after 2-3 weeks. In most cases, this is due to the fact that people expect to get results quickly. Unfortunately, rapid weight loss without harm to health is simply impossible - what has accumulated over the years cannot disappear in a week. The decrease in body weight when taking XENICAL occurs gradually (on average by 1.5–4 kg per month).
The decrease in body weight when taking XENICAL occurs gradually (on average by 1.5–4 kg per month).
3. When taking XENICAL, unsplit fat molecules remain in the lumen of the small intestine and mix with feces. An increase in the content of fats in the feces may be accompanied by such phenomena as the passage of gases, an increase in defecation, the stool becomes "fatty". These reactions are not caused by XENICAL itself, but by fats that are not absorbed in the intestines. These phenomena disappear when the amount of fat in the diet is limited.
WHAT HOSPITALS CAN HELP PATIENTS WITH OVERWEIGHT?
Patients who are determined to lose weight need advice and ongoing support from an experienced specialist. The doctor will draw up an individual plan for reducing body weight, give advice on how to properly keep a "food diary", which is necessary for the patient for self-control.
In the Kyiv City Clinical Endocrinological Hospital, patients with overweight and metabolic disorders are treated (Kyiv, Pushkinskaya St.
 10.29
10.29  INN WHO registered
INN WHO registered 
 2 mg.
2 mg. 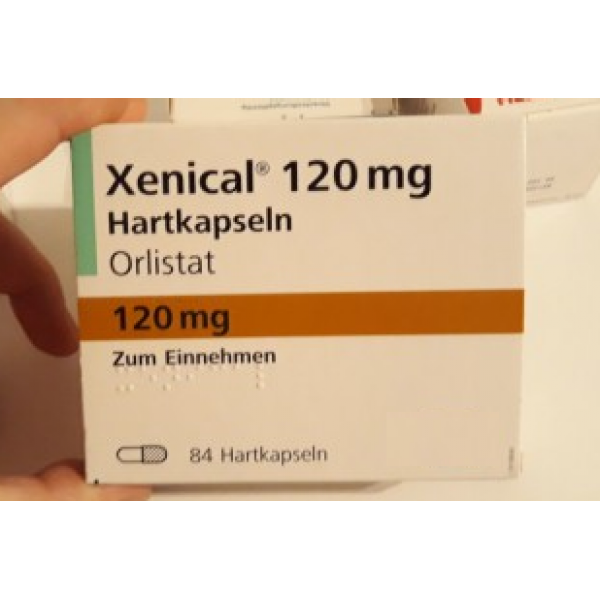 Because undigested triglycerides are not absorbed, the resulting decrease in calorie intake leads to weight loss. Thus, the therapeutic effect of the drug is carried out without absorption into the systemic circulation.
Because undigested triglycerides are not absorbed, the resulting decrease in calorie intake leads to weight loss. Thus, the therapeutic effect of the drug is carried out without absorption into the systemic circulation.  Orlistat is effective in preventing re-gain of body weight. Re-gaining body weight, no more than 25% of the lost, was observed in about half of the patients, and in half of these patients, re-gaining body weight was not observed or even a further decrease was noted. 9orlistat treated with diet therapy alone. Weight loss occurred mainly due to a decrease in the amount of fat in the body. It should be noted that before the start of the study, despite the use of hypoglycemic agents, patients often had insufficient glycemic control. However, with orlistat therapy, a statistically and clinically significant improvement in glycemic control was observed. In addition, against the background of orlistat therapy, a decrease in doses of hypoglycemic agents, insulin concentrations, and a decrease in insulin resistance were observed.
Orlistat is effective in preventing re-gain of body weight. Re-gaining body weight, no more than 25% of the lost, was observed in about half of the patients, and in half of these patients, re-gaining body weight was not observed or even a further decrease was noted. 9orlistat treated with diet therapy alone. Weight loss occurred mainly due to a decrease in the amount of fat in the body. It should be noted that before the start of the study, despite the use of hypoglycemic agents, patients often had insufficient glycemic control. However, with orlistat therapy, a statistically and clinically significant improvement in glycemic control was observed. In addition, against the background of orlistat therapy, a decrease in doses of hypoglycemic agents, insulin concentrations, and a decrease in insulin resistance were observed.  The rate of risk reduction was even greater in patients with baseline glucose intolerance (approximately 45%). The orlistat group experienced more significant weight loss compared to the placebo group. Maintenance of body weight at a new level was observed throughout the entire study period. Moreover, compared with placebo, patients treated with orlistat showed a significant improvement in their metabolic risk factor profile.
The rate of risk reduction was even greater in patients with baseline glucose intolerance (approximately 45%). The orlistat group experienced more significant weight loss compared to the placebo group. Maintenance of body weight at a new level was observed throughout the entire study period. Moreover, compared with placebo, patients treated with orlistat showed a significant improvement in their metabolic risk factor profile.  Animal studies have also shown no teratogenic effect. Due to the absence of a teratogenic effect in animals, it is unlikely to be detected in humans.
Animal studies have also shown no teratogenic effect. Due to the absence of a teratogenic effect in animals, it is unlikely to be detected in humans. 


 The daily intake of fats, carbohydrates and proteins must be divided into three main meals.
The daily intake of fats, carbohydrates and proteins must be divided into three main meals. 

 A multivitamin may be prescribed to ensure adequate intake of all nutrients.
A multivitamin may be prescribed to ensure adequate intake of all nutrients. 
 However, it is necessary to monitor the INR indicators during concomitant therapy with warfarin or other oral anticoagulants.
However, it is necessary to monitor the INR indicators during concomitant therapy with warfarin or other oral anticoagulants.