How stress affects the brain
Neurobiological and Systemic Effects of Chronic Stress
1. Lazarus RS, Folkman S. Stress, appraisal and coping, New York: Springer Verlag, 1984. [Google Scholar]
2. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010; 1186: 125–145. [PubMed] [Google Scholar]
3. Theall KP, Brett ZH, Shirtcliff EA, et al. Neighborhood disorder and telomeres: connecting children's exposure to community level stress and cellular response. Soc Sci Med 2013; 85: 50–58. [PMC free article] [PubMed] [Google Scholar]
4. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009; 16: 300–317. [PMC free article] [PubMed] [Google Scholar]
5. Dhabhar FS, Malarkey WB, Neri E, et al. Stress-induced redistribution of immune cells – from barracks to boulevards to battlefields: a tale of three hormones – Curt Richter Award winner. Psychoneuroendocrinology 2012; 37: 1345–1368.
[PMC free article] [PubMed] [Google Scholar]
6. Dhabhar FS, Saul AN, Daugherty C, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun 2010; 24: 127–137. [PMC free article] [PubMed] [Google Scholar]
7. Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 2005; 97: 1760–1767. [PMC free article] [PubMed] [Google Scholar]
8. Crum AJ, Salovey P, Achor S. Rethinking stress: the role of mindsets in determining the stress response. J Pers Soc Psychol 2013; 104: 716–733. [PubMed] [Google Scholar]
9. McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013; 79: 16–29. [PMC free article] [PubMed] [Google Scholar]
10. Gray JD, Rubin TG, Hunter RG, et al. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 2014; 19: 1171–1178. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
11. De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement 2014; 10(1 Suppl): S26–S32. [PubMed] [Google Scholar]
12. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022. [PMC free article] [PubMed] [Google Scholar]
13. McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature 1968; 220: 911–912. [PubMed] [Google Scholar]
14. Reul JM, DeKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985; 117: 2505–2511. [PubMed] [Google Scholar]
15. McEwen BS. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res 2016; 1645: 50–54. [PubMed] [Google Scholar]
16. Cameron HA, Gould E. The control of neuronal birth and survival. In: Shaw CA. (ed). Receptor dynamics in neural development. Pharmacology and toxicology: basic and clinical aspects, 1st New York: CRC Press, 1996, pp. 141–157. [Google Scholar]
In: Shaw CA. (ed). Receptor dynamics in neural development. Pharmacology and toxicology: basic and clinical aspects, 1st New York: CRC Press, 1996, pp. 141–157. [Google Scholar]
17. McEwen BS. Stress and hippocampal plasticity. Ann Rev Neurosci 1999; 22: 105–122. [PubMed] [Google Scholar]
18. Vyas A, Mitra R, Rao BSS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [PMC free article] [PubMed] [Google Scholar]
19. Bennur S, Shankaranarayana Rao BS, Pawlak R, et al. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 2007; 144: 8–16. [PubMed] [Google Scholar]
20. Lau T, Bigio B, Zelli D, et al. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Mol Psychiatry 2017; 22: 227–234. [PMC free article] [PubMed] [Google Scholar]
21. Rao RP, Anilkumar S, McEwen BS, et al. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 2012; 72: 466–475. [PMC free article] [PubMed] [Google Scholar]
Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 2012; 72: 466–475. [PMC free article] [PubMed] [Google Scholar]
22. Krishnan V, Han M-H, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404. [PubMed] [Google Scholar]
23. Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2004; 125: 1–6. [PubMed] [Google Scholar]
24. Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874. [PMC free article] [PubMed] [Google Scholar]
25. Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 2006; 27: 244–250. [PubMed] [Google Scholar]
26. Popoli M, Yan Z, McEwen BS, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012; 13: 22–37. [PMC free article] [PubMed] [Google Scholar]
Popoli M, Yan Z, McEwen BS, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012; 13: 22–37. [PMC free article] [PubMed] [Google Scholar]
27. Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci 2009; 1179: 167–178. [PubMed] [Google Scholar]
28. Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-Psychopharm Biol Psychiat 2010; 34: 791–797. [PMC free article] [PubMed] [Google Scholar]
29. Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 2009; 106: 3543–3548. [PMC free article] [PubMed] [Google Scholar]
30. Wei Q, Lu X-Y, Liu L, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856. [PMC free article] [PubMed] [Google Scholar]
31. Jacobson L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res 2014; 1583: 109–121. [PMC free article] [PubMed] [Google Scholar]
Jacobson L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res 2014; 1583: 109–121. [PMC free article] [PubMed] [Google Scholar]
32. Szyf M, Weaver ICG, Champagne FA, et al. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrin 2005; 26: 139–162. [PubMed] [Google Scholar]
33. McGowan PO, Sasaki A, D'Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neurosci 2009; 12: 241–243. [PMC free article] [PubMed] [Google Scholar]
34. Zohar J, Yahalom H, Kozlovsky N, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol 2011; 21: 796–809. [PubMed] [Google Scholar]
35. Schelling G, Roozendaal B, De Quervain DJ-F. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann NY Acad Sci 2004; 1032: 158–166. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
36. Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 2008; 105: 5573–5578. [PMC free article] [PubMed] [Google Scholar]
37. Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA 2011; 108: 16074–16079. [PMC free article] [PubMed] [Google Scholar]
38. Liston C, Cichon JM, Jeanneteau F, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 2013; 16: 698–705. [PMC free article] [PubMed] [Google Scholar]
39. Lamont EW, Robinson B, Stewart J, et al. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 2005; 102: 4180–4184. [PMC free article] [PubMed] [Google Scholar]
40. Reddy AB, Maywood ES, Karp NA, et al.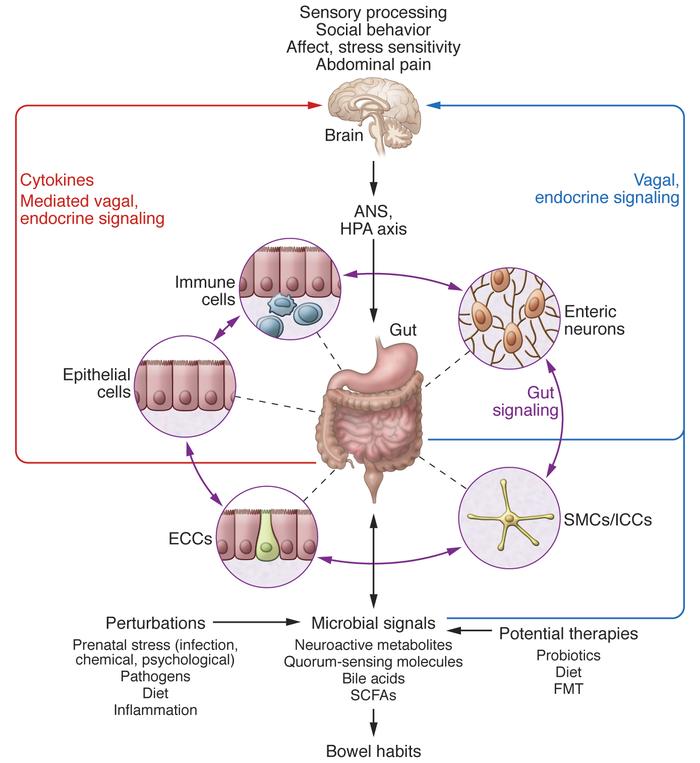 Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 2007; 45: 1478–1488. [PubMed] [Google Scholar]
Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 2007; 45: 1478–1488. [PubMed] [Google Scholar]
41. Karatsoreos IN, Bhagat SM, Bowles NP, et al. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 2010; 151: 2117–2127. [PMC free article] [PubMed] [Google Scholar]
42. Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem 1993; 61: 1957–1960. [PubMed] [Google Scholar]
43. Karst H, Berger S, Turiault M, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 2005; 102: 19204–19207. [PMC free article] [PubMed] [Google Scholar]
44. Treccani G, Musazzi L, Perego C, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry 2014; 19: 433–443. [PubMed] [Google Scholar]
Mol Psychiatry 2014; 19: 433–443. [PubMed] [Google Scholar]
45. Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98. [PubMed] [Google Scholar]
46. Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampus CA3 pyramidal neurons. Brain Res 1992; 588: 341–344. [PubMed] [Google Scholar]
47. Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex 2011; 21: 2366–2373. [PMC free article] [PubMed] [Google Scholar]
48. Arendt T, Stieler J, Strijkstra AM, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 2003; 23: 6972–6981. [PMC free article] [PubMed] [Google Scholar]
49. Kinoshita Y, Hunter RG, Gray JD, et al. Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc Natl Acad Sci USA 2014; 111: 16130–16135. [PMC free article] [PubMed] [Google Scholar]
Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc Natl Acad Sci USA 2014; 111: 16130–16135. [PMC free article] [PubMed] [Google Scholar]
50. Sapolsky RM, Krey LC, McEwen BS. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. EndocrRev 1986; 7: 284–301. [PubMed] [Google Scholar]
51. Nasca C, Zelli D, Bigio B, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA 2015; 112: 14960–14965. [PMC free article] [PubMed] [Google Scholar]
52. Nasca C, Xenos D, Barone Y, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 2013; 110: 4804–4809. [PMC free article] [PubMed] [Google Scholar]
53. Chattarji S, Tomar A, Suvrathan A, et al. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 2015; 18: 1364–1375. [PubMed] [Google Scholar]
Nat Neurosci 2015; 18: 1364–1375. [PubMed] [Google Scholar]
54. Pereira AC, Lambert HK, Grossman YS, et al. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci USA 2014; 111: 18733–18738. [PMC free article] [PubMed] [Google Scholar]
55. Pereira AC, Gray JD, Kogan JF, et al. Age and Alzheimer's disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry 2017; 22: 296–305. [PMC free article] [PubMed] [Google Scholar]
56. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904. [PubMed] [Google Scholar]
57. Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging 2011; 32: 1942–1948. [PMC free article] [PubMed] [Google Scholar]
58. Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and medial prefrontal gyrus metabolism in women receiving hormone therapy. Psychiatry Res 2014; 223: 28–36. [PubMed] [Google Scholar]
Psychiatry Res 2014; 223: 28–36. [PubMed] [Google Scholar]
59. Kenna H, Hoeft F, Kelley R, et al. Fasting plasma insulin and the default mode network in women at risk for Alzheimer's disease. Neurobiol Aging 2013; 34: 641–649. [PMC free article] [PubMed] [Google Scholar]
60. Grillo CA, Piroli GG, Lawrence RC, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 2015; 64: 3927–3936. [PMC free article] [PubMed] [Google Scholar]
61. Grillo CA, Piroli GG, Kaigler KF, et al. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res 2011; 222: 230–235. [PMC free article] [PubMed] [Google Scholar]
62. Grillo CA, Piroli GG, Evans AN, et al. Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: effects of dietary restriction. Physiol Behav 2011; 104: 235–241. [PMC free article] [PubMed] [Google Scholar]
63. Grillo CA, Mulder P, Macht VA, et al.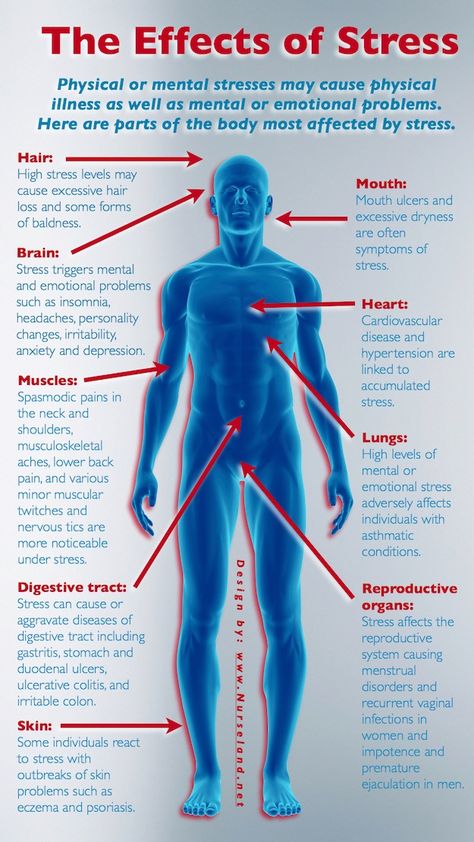 Dietary restriction reverses obesity-induced anhedonia. Physiol Behav 2014; 128: 126–132. [PMC free article] [PubMed] [Google Scholar]
Dietary restriction reverses obesity-induced anhedonia. Physiol Behav 2014; 128: 126–132. [PMC free article] [PubMed] [Google Scholar]
64. Yao J, Irwin RW, Zhao L, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 14670–14675. [PMC free article] [PubMed] [Google Scholar]
65. D'Alessio D. Is GLP-1 a hormone: whether and when?. J Diabetes Investig 2016; 7(Suppl 1): 50–55. [PMC free article] [PubMed] [Google Scholar]
66. McIntyre RS, Powell AM, Kaidanovich-Beilin O, et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res 2013; 237: 164–171. [PubMed] [Google Scholar]
67. Bigio B, Mathe AA, Sousa VC, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: implications for treatment resistance. Proc Natl Acad Sci USA 2016; 113: 7906–7911. [PMC free article] [PubMed] [Google Scholar]
68. Halfon N, Larson K, Lu M, et al. Lifecourse health development: past, present and future. Matern Child Health J 2014; 18: 344–365. [PMC free article] [PubMed] [Google Scholar]
Halfon N, Larson K, Lu M, et al. Lifecourse health development: past, present and future. Matern Child Health J 2014; 18: 344–365. [PMC free article] [PubMed] [Google Scholar]
69. Allfrey VG. Changes in chromosomal proteins at times of gene activation. Fed Proc 1970; 29: 1447–1460. [PubMed] [Google Scholar]
70. Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol 2008; 86: 305–341. [PMC free article] [PubMed] [Google Scholar]
71. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience 2014; 275: 420–435. [PubMed] [Google Scholar]
72. Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev 2007; 87: 799–823. [PubMed] [Google Scholar]
73. Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats.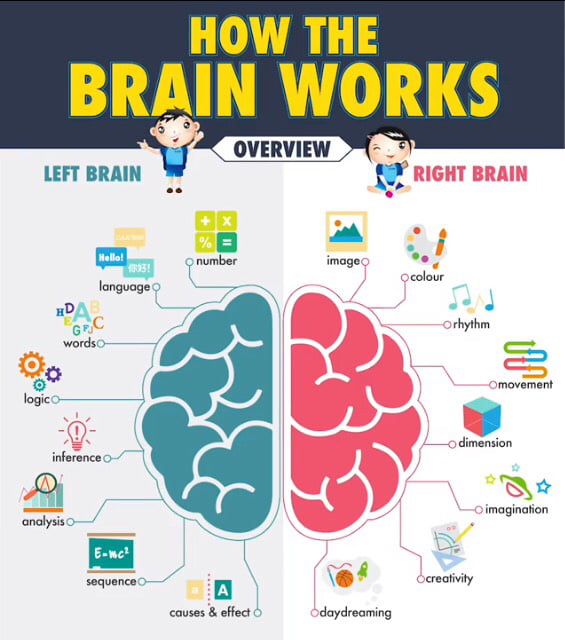 Behav Brain Res 2012; 229: 280–288. [PubMed] [Google Scholar]
Behav Brain Res 2012; 229: 280–288. [PubMed] [Google Scholar]
74. Nasca C, Bigio B, Zelli D, et al. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 2015; 20: 755–763. [PMC free article] [PubMed] [Google Scholar]
75. Freund J, Brandmaier AM, Lewejohann L, et al. Emergence of individuality in genetically identical mice. Science 2013; 340: 756–759. [PubMed] [Google Scholar]
76. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005; 102: 10604–10609. [PMC free article] [PubMed] [Google Scholar]
77. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998; 14: 245–258. [PubMed] [Google Scholar]
78. Levine S, Haltmeyer G, Kara G, et al.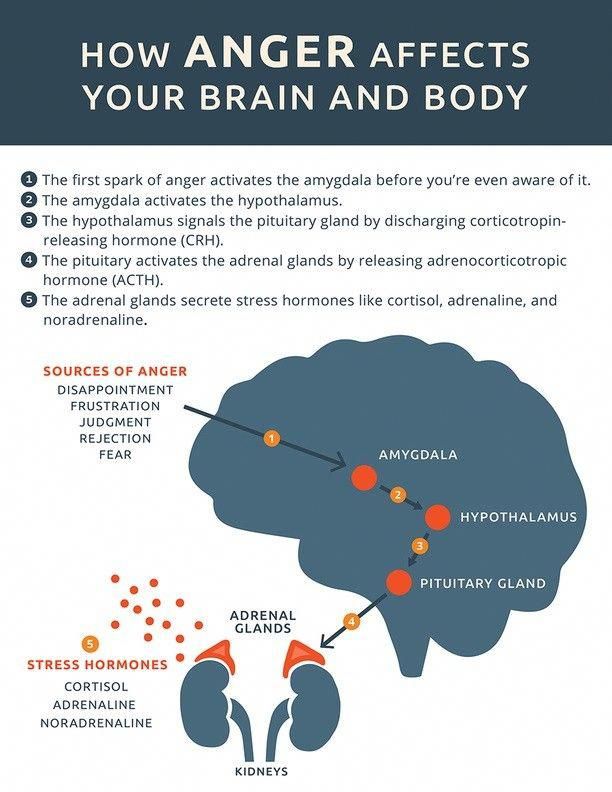 Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2: 55–59. [Google Scholar]
Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2: 55–59. [Google Scholar]
79. Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 2005; 7: 103–123. [PMC free article] [PubMed] [Google Scholar]
80. Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA 2003; 100: 16131–16136. [PMC free article] [PubMed] [Google Scholar]
81. Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav 2006; 50: 454–462. [PubMed] [Google Scholar]
82. Akers KG, Yang Z, DelVecchio DP, et al. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS One 2008; 3: e2840. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
83. Tang AC, Akers KG, Reeb BC, et al. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA 2006; 103: 15716–15721. [PMC free article] [PubMed] [Google Scholar]
84. Parker KJ, Buckmaster CL, Sundlass K, et al. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA 2006; 103: 3000–3005. [PMC free article] [PubMed] [Google Scholar]
85. Isgor C, Kabbaj M, Akil H, et al. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 2004; 14: 636–648. [PubMed] [Google Scholar]
86. Rice CJ, Sandman CA, Lenjavi MR, et al. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008; 149: 4892–4900. [PMC free article] [PubMed] [Google Scholar]
87. Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci 2006; 8: 1004–1006. [PMC free article] [PubMed] [Google Scholar]
Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci 2006; 8: 1004–1006. [PMC free article] [PubMed] [Google Scholar]
88. Kaufman D, Smith ELP, Gohil BC, et al. Early appearance of the metabolic syndrome in socially reared bonnet macaques. J Clin Endocrin & Metab 2005; 90: 404–408. [PubMed] [Google Scholar]
89. Coplan JD, Smith ELP, Altemus M, et al. Variable foraging demand rearing: Sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiat 2001; 50: 200–204. [PubMed] [Google Scholar]
90. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301: 386–389. [PubMed] [Google Scholar]
91. Spinelli S, Schwandt ML, Lindell SG, et al. The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Dev Psychopathol 2012; 24: 157–165. [PMC free article] [PubMed] [Google Scholar]
Dev Psychopathol 2012; 24: 157–165. [PMC free article] [PubMed] [Google Scholar]
92. Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science 2002; 297: 851–854. [PubMed] [Google Scholar]
93. Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann NY Acad Sci 2006; 1094: 52–62. [PubMed] [Google Scholar]
94. Obradovic J, Bush NR, Stamperdahl J, et al. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev 2010; 81: 270–289. [PMC free article] [PubMed] [Google Scholar]
95. Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 2005; 17: 271–301. [PubMed] [Google Scholar]
96. Galea LAM, McEwen BS, Tanapat P, et al. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 1997; 81: 689–697. [PubMed] [Google Scholar]
Neuroscience 1997; 81: 689–697. [PubMed] [Google Scholar]
97. Luine V, Villegas M, Martinez C, et al. Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639: 167–170. [PubMed] [Google Scholar]
98. Luine VN, Beck KD, Bowman RE, et al. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinology 2007; 19: 743–751. [PubMed] [Google Scholar]
99. Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res 2001; 904: 279–289. [PubMed] [Google Scholar]
100. Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 1998; 95: 4066–4071. [PMC free article] [PubMed] [Google Scholar]
101. Wood GE, Shors TJ, Beylin AV. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci 2001; 115: 175–187. [PubMed] [Google Scholar]
Behav Neurosci 2001; 115: 175–187. [PubMed] [Google Scholar]
102. Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA 2002; 99: 13955–13960. [PMC free article] [PubMed] [Google Scholar]
103. Leuner B, Mendolia-loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiat 2004; 56: 964–970. [PMC free article] [PubMed] [Google Scholar]
104. Shansky RM, Hamo C, Hof PR, et al. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 2010; 20: 2560–2567. [PMC free article] [PubMed] [Google Scholar]
105. Bangasser DA, Curtis A, Reyes BA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 2010; 15: 877, 896–904. [PMC free article] [PubMed] [Google Scholar]
106. Bangasser DA, Zhang X, Garachh V, et al. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 2011; 103: 342–351. [PMC free article] [PubMed] [Google Scholar]
Bangasser DA, Zhang X, Garachh V, et al. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 2011; 103: 342–351. [PMC free article] [PubMed] [Google Scholar]
107. Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7: 477–484. [PubMed] [Google Scholar]
108. McEwen BS, Lasley EN. The end of sex as we know it. Cerebrum The Dana Forum on Brain Science 2005; Vol. 7, New York, NY: Dana Press. [Google Scholar]
109. McEwen BS. Introduction: the end of sex as we once knew it. Physiol Behav 2009; 97: 143–145. [PMC free article] [PubMed] [Google Scholar]
110. Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry 2007; 164: 1530–1538. [PubMed] [Google Scholar]
111. Meites J. Short history of neuroendocrinology and the international society of neuroendocrinology. Neuroendocrinology 1992; 56: 1–10. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
112. Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nature Neurosci 2002; 5: 933–934. [PubMed] [Google Scholar]
113. Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology 2010; 35: 67–82. [PubMed] [Google Scholar]
114. McEwen BS, McEwen CA. Social, psychological, and physiological reactions to stress, New York: John Wiley & Sons, Inc, 2015. [Google Scholar]
115. Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 2009; 163: 1135–1143. [PMC free article] [PubMed] [Google Scholar]
116. Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci 2010; 21: 848–856. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
117. McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med 1998; 338: 171–179. [PubMed] [Google Scholar]
118. Anda RF, Butchart A, Felitti VJ, et al. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med 2010; 39: 93–98. [PubMed] [Google Scholar]
119. McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health 2011; 101(Suppl 1): S131–S139. [PMC free article] [PubMed] [Google Scholar]
120. Evans GW, Gonnella C, Marcynyszyn LA, et al. The role of chaos in poverty and children's socioemotional adjustment. Psychol Sci 2005; 16: 560–565. [PubMed] [Google Scholar]
121. Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res 2006; 1110: 166–174. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
122. Hart B, Risley TR. Meaningful differences in the everyday experience of young American Children, Baltimore, MD: Brookes Publishing Company, 1995, pp. 304. [Google Scholar]
123. Hanson JL, Chandra A, Wolfe BL, et al. Association between income and the hippocampus. PLoS One 2011; 6: e18712. [PMC free article] [PubMed] [Google Scholar]
124. Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci 2007; 2: 161–173. [PMC free article] [PubMed] [Google Scholar]
125. Gianaros PJ, Horenstein JA, Hariri AR, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci 2008; 3: 91–96. [PMC free article] [PubMed] [Google Scholar]
126. Adler NE, Boyce TW, Chesney MA, et al. Socioeconomic Inequalities in Health. JAMA 1993; 269: 3140–3145. [PubMed] [Google Scholar]
127. Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108: 14324–14329. [PMC free article] [PubMed] [Google Scholar]
Proc Natl Acad Sci USA 2011; 108: 14324–14329. [PMC free article] [PubMed] [Google Scholar]
128. Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev 2011; 35: 1562–1592. [PMC free article] [PubMed] [Google Scholar]
129. Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health 2010; 100: 933–939. [PMC free article] [PubMed] [Google Scholar]
130. Tomasdottir MO, Sigurdsson JA, Petursson H, et al. Self reported childhood difficulties, adult multimorbidity and allostatic load. A cross-sectional analysis of the Norwegian HUNT study. PLoS One 2015; 10: e0130591. [PMC free article] [PubMed] [Google Scholar]
131. Rasgon NL, McEwen BS. Insulin resistance-a missing link no more. Mol Psychiatry 2016; 21: 1648–1652. [PubMed] [Google Scholar]
132. Waddington CH. The epigenotype. Endeavoour 1942; 1: 18–20. [Google Scholar]
[Google Scholar]
133. Acheson SD. Independent inquiry into inequalities in health report, London: The Stationary Office, 1998. [Google Scholar]
134. McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA 2012; 109(Suppl 2): 17180–17185. [PMC free article] [PubMed] [Google Scholar]
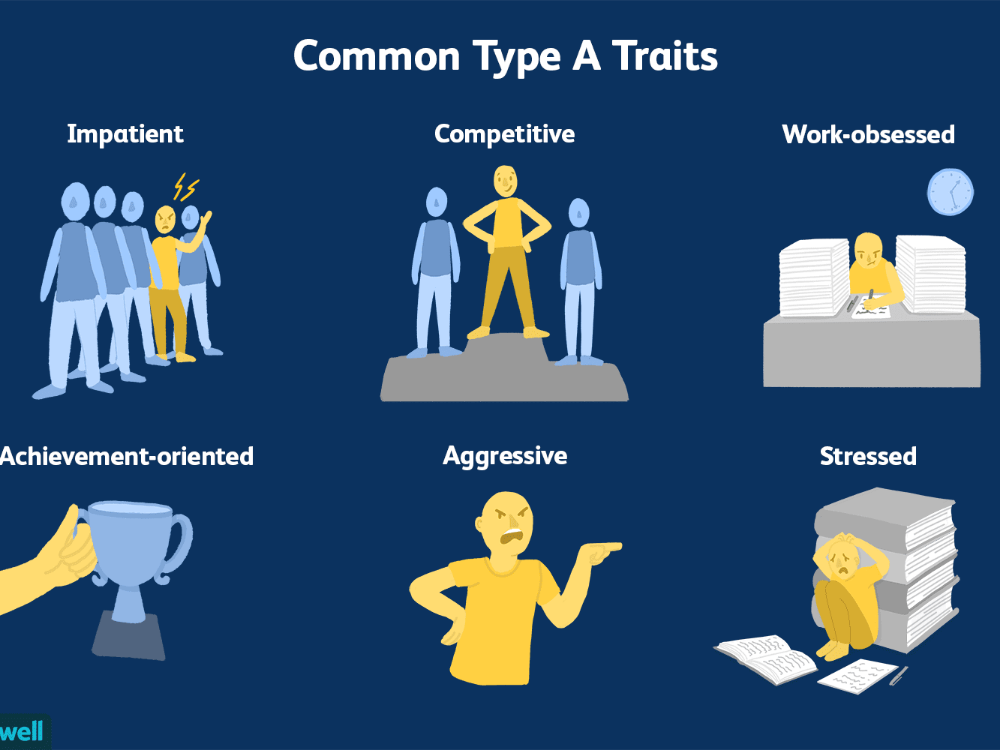
6 Ways Stress Affects Your Brain
The brain is a fascinating and complex organ. It’s the primary control center for our whole body, and it can be affected by stress in many different ways. Stress itself is an important part of life – it helps us prepare for danger or respond to emergencies. But when we’re constantly stressed out, that’s when our brain starts to pay the price. This blog post will explore how stress affects your brain, both positively and negatively, so you can develop strategies to reduce your brain’s vulnerability to its harmful effects.
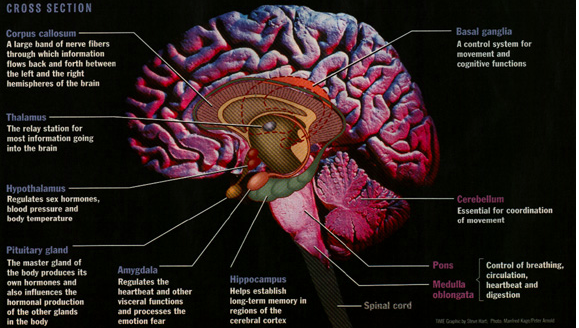
For starters, it is important to understand how our body processes stress. In the simplest terms, stress is basically the “fight or flight” response to a perceived threat. This activates the amygdala, or “fear center” of the brain, and causes a cascade of events. These include the production of the stress hormone cortisol, an increase in glucose levels, increased heart rate, and an increase in blood flow to the muscles in the arms and legs. After the threat has passed, then the body will eventually return to normal.
This activates the amygdala, or “fear center” of the brain, and causes a cascade of events. These include the production of the stress hormone cortisol, an increase in glucose levels, increased heart rate, and an increase in blood flow to the muscles in the arms and legs. After the threat has passed, then the body will eventually return to normal.
In the case of chronic stress, however, the fear center of the brain is constantly activated, meaning that the body is in a constant state of stress. Cortisol levels are also constantly elevated, which can eventually start to cause problems with digestion, sleeping, and the immune system. Not only that, but when one part of the brain is constantly engaged, it is postulated that the other parts of the brain may not have enough energy to carry out their own functions properly. As a result, here are six ways that stress can affect the brain:
Impairs MemoryOne effect of chronic stress that researchers have observed is memory impairment.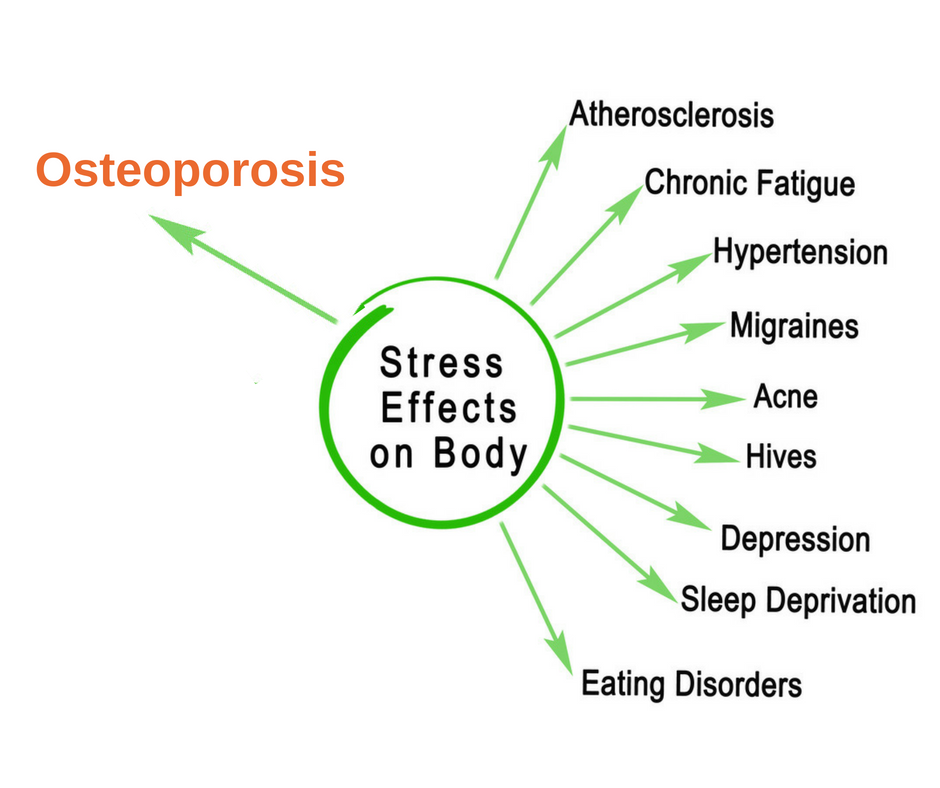 Specifically, it has been noted that people who are stressed tend to be more forgetful and less likely to remember specific information. Researchers believe that even minor stress, such as being late to work, can cause you to forget simple things like where your keys are. One study performed on older rats even noted that high levels of cortisol caused short-term memory declines. According to Dr. Kerry Ressler, chief scientific officer at McLean Hospital and professor of psychiatry at Harvard Medical School, “The basic idea is that the brain is shunting its resources because it’s in survival mode, not memory mode”.
Specifically, it has been noted that people who are stressed tend to be more forgetful and less likely to remember specific information. Researchers believe that even minor stress, such as being late to work, can cause you to forget simple things like where your keys are. One study performed on older rats even noted that high levels of cortisol caused short-term memory declines. According to Dr. Kerry Ressler, chief scientific officer at McLean Hospital and professor of psychiatry at Harvard Medical School, “The basic idea is that the brain is shunting its resources because it’s in survival mode, not memory mode”.
Your brain is composed of both gray matter and white matter. Gray matter is used for decision-making and problem-solving, while white matter is used to connect regions of the brain and communicate information. It has been noted that during times of chronic stress, the myelin sheaths that make up white matter become overproduced, while less gray matter is produced. When this happens, there can be an imbalance in gray and white matter. In some cases, this results in permanent changes to the brain’s structure.
When this happens, there can be an imbalance in gray and white matter. In some cases, this results in permanent changes to the brain’s structure.
An imbalance between white and gray matter can also play a role in the development of mental illness. The theory is that having excess myelin in certain areas of the brain interferes with the timing and balance of communication. It was also noted that chronic stress can negatively alter hippocampal function. The hippocampus is involved in memory, specifically spatial memory, memory consolidation, and memory transfer.
Stress Kills Brain CellsIt has been suggested by researchers that chronic stress can even kill new neurons in the brain’s hippocampus. The hippocampus is one of only two locations where neurons are produced. Despite the fact that the formation of new neurons does not seem to be affected, research shows that new neurons produced during periods of stress are more likely to die within a week.
While the overall volume of the brain tends to remain about the same, it has been found that chronic stress in otherwise healthy individuals can cause areas of the brain associated with emotions, metabolism, and memory to shrink. Chronic stress also made people more likely to experience brain shrinkage when exposed to intense stressors. This means that people under constant stress may find it harder to deal with future stress.
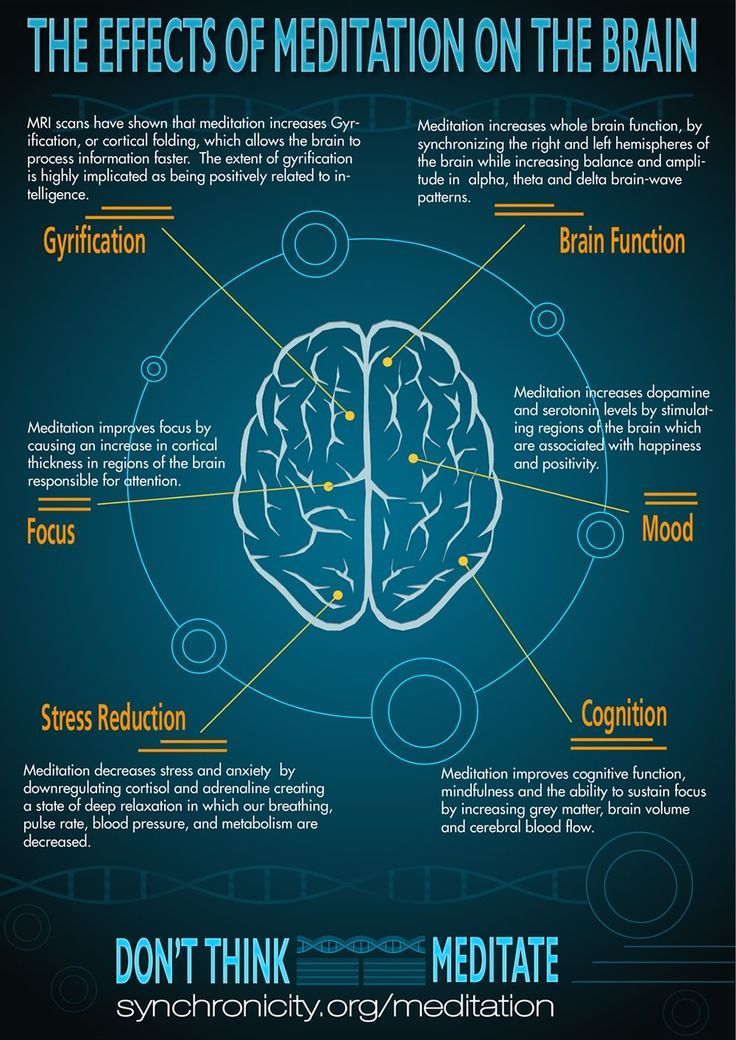
Improves Cognitive FunctionStress is not all bad for your brain. In fact, moderate stress can actually improve brain performance by strengthening the connection between neurons in the brain. This helps to improve memory and attention span in order to make you more productive overall. This is why some people tend to perform “better under pressure”.
Dr. Kashouty, a diplomate of the American Board of Psychiatry and Neurology (ABPN), practices general neurology with fellowship trained specialization in clinical neurophysiology. Dr. Kashouty finds the form and function of the nerves and muscles the most interesting part of neurology, which is what led him to specialize in neurophysiology with more emphasis on neuromuscular conditions. He treats all neurological diseases, but his main focus is to treat and manage headaches, movement disorders and neuromuscular diseases.
Dr. Kashouty finds the form and function of the nerves and muscles the most interesting part of neurology, which is what led him to specialize in neurophysiology with more emphasis on neuromuscular conditions. He treats all neurological diseases, but his main focus is to treat and manage headaches, movement disorders and neuromuscular diseases.
How stress affects our brain, or why it is bad to be nervous for a long time
We face stress every day due to deadlines at work, family problems, information noise and other factors. But we are far from always aware of how dangerous it is to health. Stress increases the risk of developing serious diseases and harms all body systems. The brain and cognitive abilities suffer the most: memory, attention, decision-making and planning, logical thinking. Together with Adrian Landry, Director of Educational Content at the Novakid online English school, we are looking into how stress affects cognitive functions and what can help you cope with it.
 nine0003
nine0003
Adrian Landry
Director of Educational Content, Novakid Online School of English
Short-term stress
Short-term stress triggers the fight-or-flight response in the body. Its mechanism was formed a very long time ago - back in those prehistoric times, when a person had to literally escape from predators. Today, the cause of stress is usually not saber-toothed tigers, but traffic jams or conflicts with others. But the reaction works automatically. nine0009
The fight-or-flight mechanism in the brain triggers the amygdala, or amygdala, a region of the nervous tissue responsible for detecting threats and feeling anger and fear. Under stress, the amygdala enlarges and commands the body to adapt to the encounter with danger. A person's heart rate increases, digestive processes slow down, the skin turns pale - blood leaves the skin so that in the event of a battle there is no massive blood loss. In addition, visual acuity and hearing are reduced, and attention becomes tunnel-focused exclusively on the source of stress. nine0009
nine0009
It is commonly believed that short-term stress increases our cognitive abilities. Once in a stressful situation, a person makes every effort to get out of it and, as a result, becomes more collected and concentrated. This is true, but only if the stress is directly related to a specific problem.
Let's say you have to pass a driver's license test. If you're only worried about your test results in the run-up to the tests, but otherwise you're doing well in life, short-term stress really activates your memory and concentration. However, if it’s not the exam itself that makes you nervous, but, say, the prospect of layoffs at work, it will be extremely difficult for you to focus on driving. nine0009
Scientist Dan Ariely in his book Positive Irrationality. How to capitalize on your illogical actions” describes an experiment his team conducted in India. A group of subjects were asked to play a few simple children's puzzle games. Certain bonuses were offered for the victory. The amount of remuneration ranged from modest amounts to very solid ones - equal to the participant's salary for several months. It turned out that the higher the amounts were at stake, the worse the subjects coped with the tasks. The prospect of a small win did not make people nervous or affect their level of concentration. But the chance to hit the big jackpot brought the body into a stressful state and worsened cognitive abilities. nine0009
The amount of remuneration ranged from modest amounts to very solid ones - equal to the participant's salary for several months. It turned out that the higher the amounts were at stake, the worse the subjects coped with the tasks. The prospect of a small win did not make people nervous or affect their level of concentration. But the chance to hit the big jackpot brought the body into a stressful state and worsened cognitive abilities. nine0009
The fact is that short-term stress turns off the prefrontal cortex of the brain, which is responsible for cognitive abilities. Namely, for memory, planning, logical thinking, the ability to find solutions and set goals. In ordinary life, the prefrontal cortex allows us to function normally, work and learn. But with the “fight or flight” reaction, the need for these abilities in the body simply disappears - work with a specific threat comes to the fore. At this moment, we need cognitive functions in a minimal amount: only those that will help to cope with the source of stress. nine0009
nine0009
Chronic stress
If the brain can get at least some benefit from a short-term stressful situation, then long-term stress literally destroys it.
Stress increases the production of cortisol. Long-term excess of the concentration of this hormone negatively affects the hippocampus. This part of the brain is responsible for the perception of information, memory and neurogenesis - the formation of new neurons. The hippocampus plays the role of a data processing center: it distributes information between short-term and long-term memory, makes decisions about what knowledge to keep and what not. Under the influence of cortisol, the hippocampus is gradually destroyed. As a result, a person's memory worsens: at first it becomes more difficult to memorize new things, and then to remember already known ones. A decrease in the number of neurons and a slowdown in neurogenesis reduce the ability to learn and master new skills. nine0009
Chronic stress and increased production of cortisol also destroy dendritic junctions, through which information is transmitted between neurons.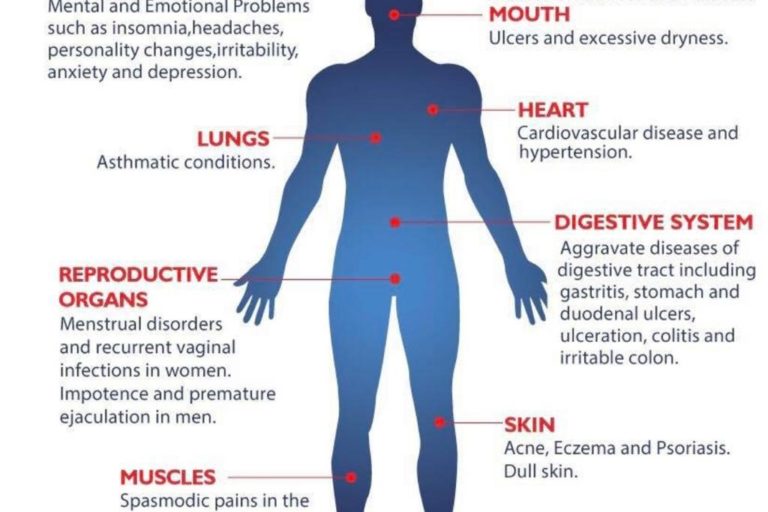 The number and quality of dendrites also affect our memory capacity and processing speed. The fewer of them, the worse and slower we begin to think. Even simple decisions are difficult for a person: what to wear, what dish to cook for dinner, and so on.
The number and quality of dendrites also affect our memory capacity and processing speed. The fewer of them, the worse and slower we begin to think. Even simple decisions are difficult for a person: what to wear, what dish to cook for dinner, and so on.
Blame it on the amygdala we talked about earlier. Under chronic stress, it is always in an excited state and sees danger or threat everywhere - even if there are no objective reasons for this. But even worse, the amygdala tries its best to "pull the blanket" over itself and makes us constantly afraid and think about the source of stress. As a result, it becomes more and more difficult to switch to everyday activities. nine0009
Finally, prolonged stress destroys the prefrontal cortex, which is directly responsible for cognitive abilities. In a normal state, its nerve connections are reliably protected by the myelin sheath. It not only protects, but also participates in a chain reaction between neurons at the moments when we think or make decisions. Cortisol thins the myelin sheath and deprives neurons of protection. It becomes more difficult for the brain to get to the necessary information and formulate a command for this or that action. nine0009
Cortisol thins the myelin sheath and deprives neurons of protection. It becomes more difficult for the brain to get to the necessary information and formulate a command for this or that action. nine0009
Can stress be beneficial?
It is believed that life without stress does not exist, and this is true. Normally, during the day, 80% of the time a person should be in a state of calm, 20% should feel a sense of joy and happiness, another 20% should feel stress. The study of scientists from the University of Georgia, conducted as part of the Human Connectome Project, is devoted to the positive effect of moderate stress on the brain.
1200 young people took part in the experiment. They filled out a questionnaire in which they assessed their level of stress and tension. After that, they were offered to take tests to assess cognitive abilities. It turned out that those participants who periodically experienced mild stress in everyday life coped best with the tasks. Those who almost never faced stressful situations had worse results.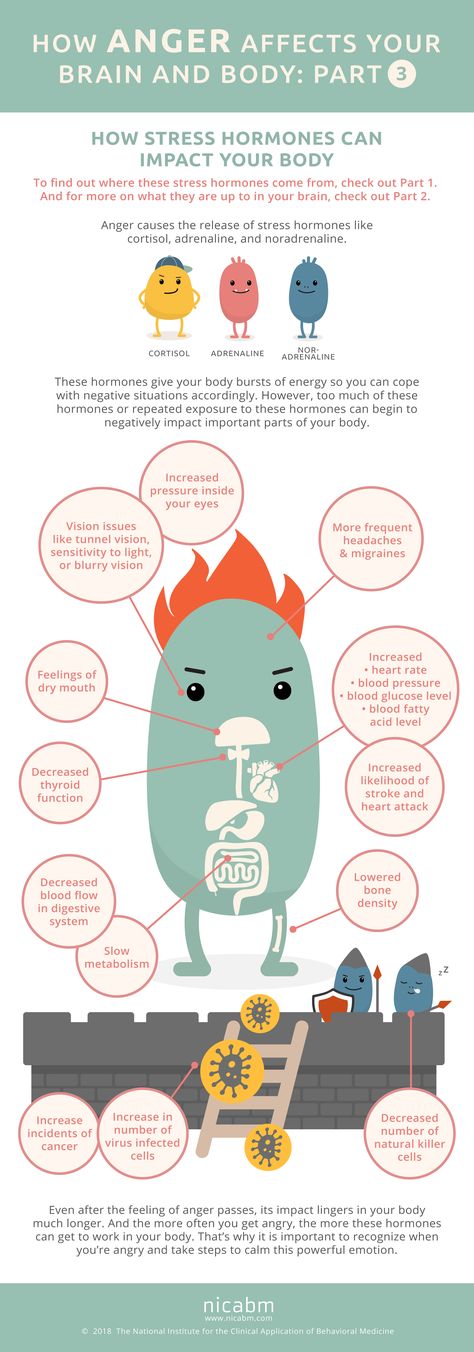 nine0009
nine0009
Thus, it can be assumed that a moderate level of stress in everyday life makes us more resistant to severe stressful situations and experiences. However, it is important to understand that we are talking only about mild short-term stress, which is not capable of seriously harming the brain.
How to reduce stress?
-
Moderate physical activity. Sweating in the gym for several hours a day is not at all necessary. An excellent alternative would be swimming, yoga and even ordinary walks. nine0009
-
Nutrition. Avoiding fast carbohydrates and foods with a high glycemic index will help reduce stress levels. This includes sweets, pastries, soda, and favorite fast food. Such products provoke jumps in blood sugar, which additionally stimulate the brain and bring it into a state of overexcitation.
-
Learning new things. Foreign languages are great here. Although studying under stress seems like something difficult and not very productive, it is a great opportunity to switch the brain.
 Regularity in classes can be one of the steps to establishing a routine and daily routine. Finally, learning foreign languages stimulates neurogenesis, which means that it can at least partially compensate the brain for neurons lost due to chronic stress. nine0009
Regularity in classes can be one of the steps to establishing a routine and daily routine. Finally, learning foreign languages stimulates neurogenesis, which means that it can at least partially compensate the brain for neurons lost due to chronic stress. nine0009 -
Self-monitoring. Stress factors, such as problems at work or information noise, will not disappear on their own. However, we can change our attitude towards them, learn to negotiate with ourselves and turn off the involuntary inclusion of the “fight or flight” reaction. This can be achieved with the help of breathing practices, physical activity, meditation, normalization of the daily routine and diet.
Alexandra Smarakova
Tags
#well-being
#erudition
How does stress affect the brain?
Back in 2020, alarming publications began to appear about the worldwide epidemic of stress. At that time we thought that we were going through a period of high turbulence, but we knew that sooner or later it would pass and the life of most of us would return to its former or almost former course.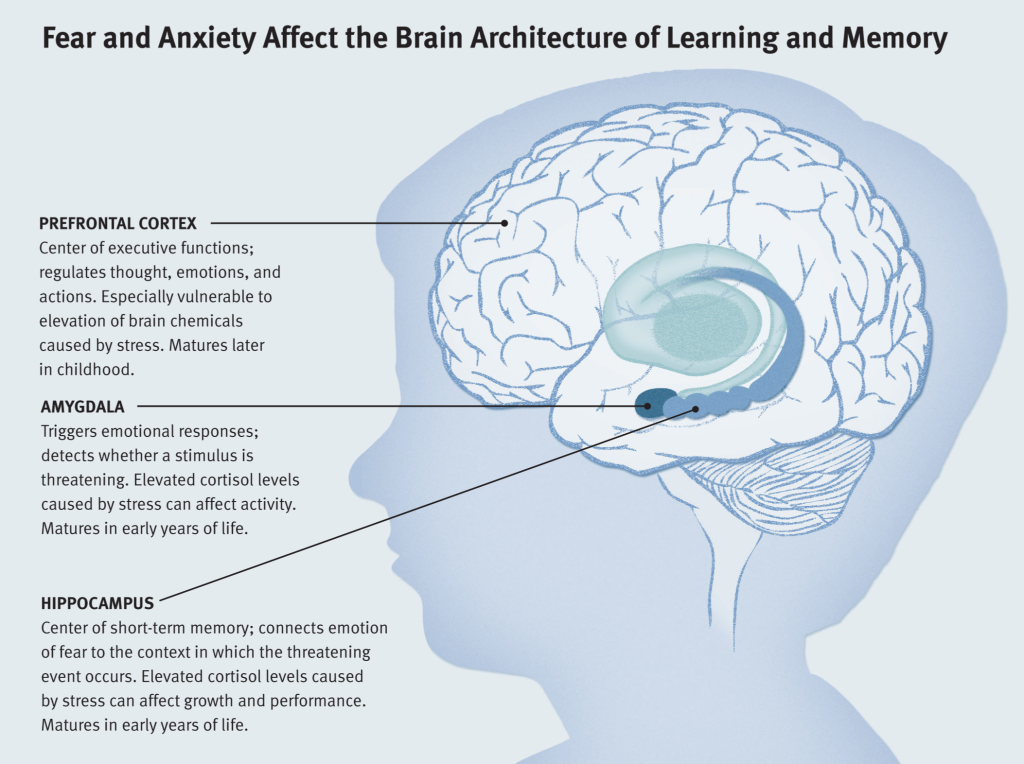 Two years later, we can say that the pandemic has been replaced by a period of global change, and the level of stress has only increased. And in this situation, you need to adapt, be effective at work and at school, take care of loved ones, take care of children, devote time to your health, appearance, home and hobbies. All of these tasks require intellectual effort in one way or another in a situation where the effect of stress on the brain is significant. nine0077
Two years later, we can say that the pandemic has been replaced by a period of global change, and the level of stress has only increased. And in this situation, you need to adapt, be effective at work and at school, take care of loved ones, take care of children, devote time to your health, appearance, home and hobbies. All of these tasks require intellectual effort in one way or another in a situation where the effect of stress on the brain is significant. nine0077
In this article, we will look at the key aspects of the effect of stress on the brain, as well as give recommendations on what to do in this situation and how to support the nervous system and brain under the circumstances.
How does stress affect the brain?
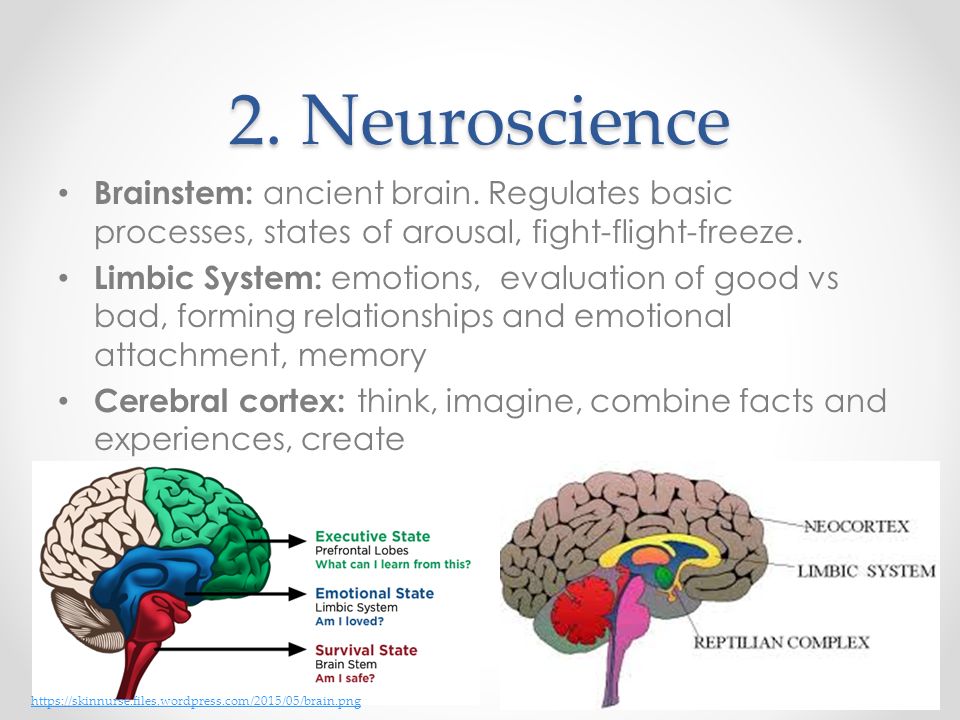
The influence of stress and stress hormones on the brain occurs throughout life, and we are forced to look for ways to adapt to stressful loads of any level, but our body is far from always able to go through this adaptation painlessly. Studies have shown 1 , the brain is a key organ of the stress response because it determines what is threatening and therefore potentially stressful, as well as physiological and behavioral responses, which can be either adaptive or destructive. Stress includes a two-way communication between the brain and the cardiovascular, immune and other systems through nervous and endocrine mechanisms 2 . In addition to the fight-or-flight response to acute stress, there are events in everyday life that cause a kind of chronic stress and, over time, lead to wear and tear on the body (allostatic load 3 ). However, stress-related hormones protect the body in the short term and promote adaptation (allostasis). The brain is a target of stress, and the hippocampus was the first area of the brain other than the hypothalamus to be recognized as a target for glucocorticoids.
Studies have shown 1 , the brain is a key organ of the stress response because it determines what is threatening and therefore potentially stressful, as well as physiological and behavioral responses, which can be either adaptive or destructive. Stress includes a two-way communication between the brain and the cardiovascular, immune and other systems through nervous and endocrine mechanisms 2 . In addition to the fight-or-flight response to acute stress, there are events in everyday life that cause a kind of chronic stress and, over time, lead to wear and tear on the body (allostatic load 3 ). However, stress-related hormones protect the body in the short term and promote adaptation (allostasis). The brain is a target of stress, and the hippocampus was the first area of the brain other than the hypothalamus to be recognized as a target for glucocorticoids.
In order to reduce the negative impact of stress on the brain, compensate for its consequences, and also support the nervous system, in addition to the obvious concern for one’s own health and informational hygiene, it is necessary to provide the brain with a sufficient amount of nutrients, micro- and macronutrients, which are consumed faster in a situation of severe or ongoing stress.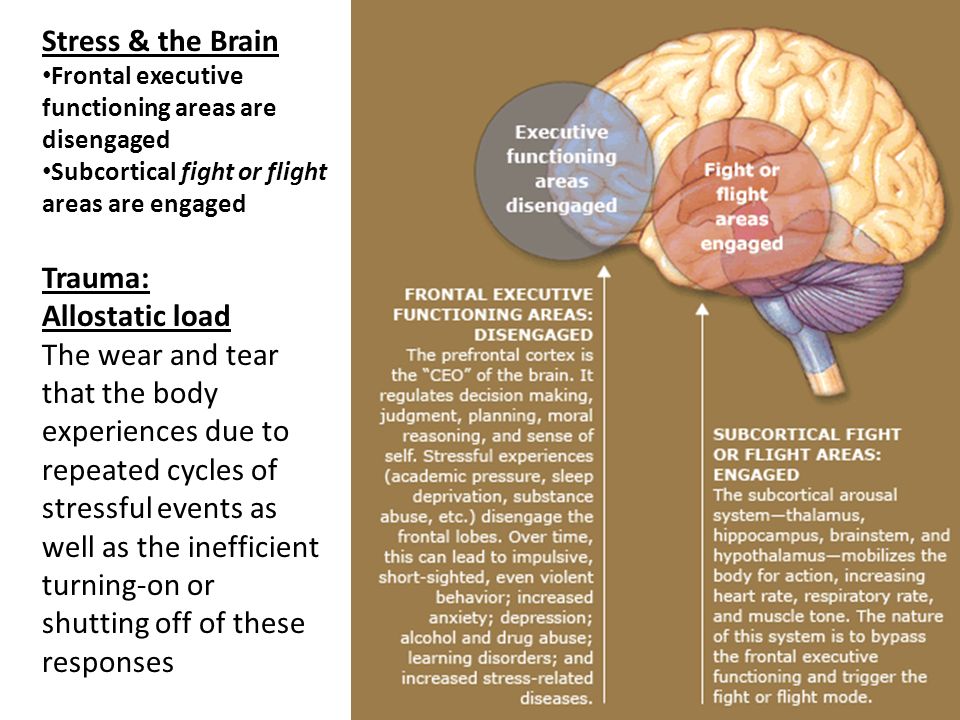 and in large quantities. nine0009
and in large quantities. nine0009
What vitamins are needed for the brain?
To compensate for the effect of stress on the brain, it is necessary to take basic trace elements, these are:
- B vitamins,
- magnesium,
- amino acid glycine.
This simple set, where all dietary supplements are combined with each other, is quite enough to close the basic deficits in a stressful situation and maintain cognitive functions in a normal state. nine0009
We talked about this combination in more detail in the article “How to stay in a resource in a stressful situation?”.
In this article, we will focus on innovative solutions from Evalar: the combination of Huperzine A and Phosphatidylserine. Both substances are presented in dietary supplements from the Cognivia series, which have recently appeared in the company's assortment.
Both drugs are complex solutions that can not only improve cognitive function, but also reduce the impact of stress on the brain.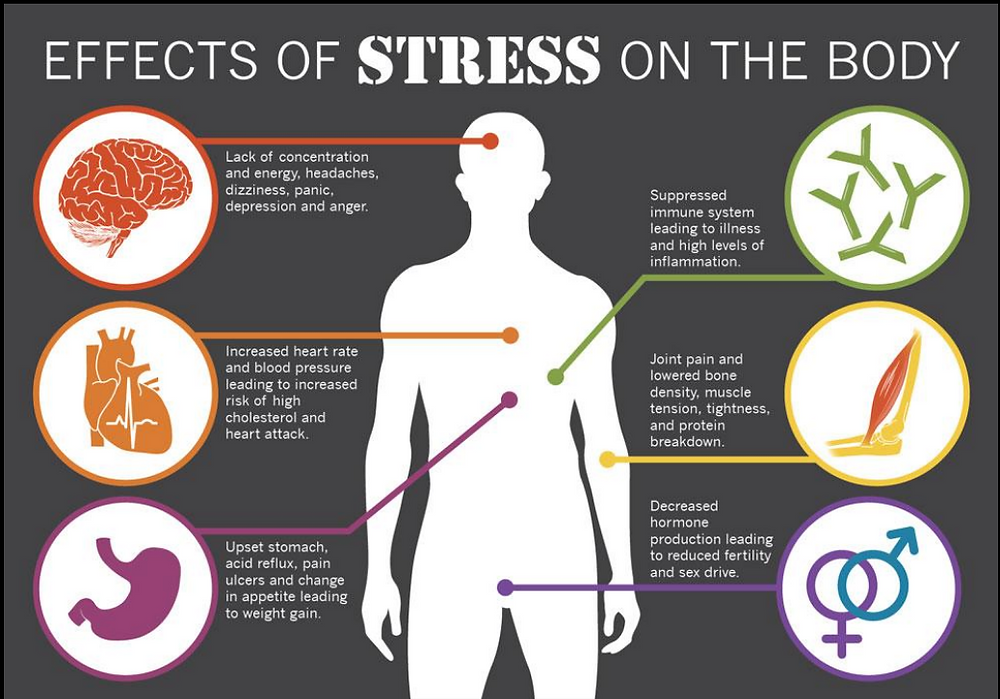 nine0009
nine0009
Hot drink "Cognivia" contains a key active ingredient - Huperzine A. Magnesium, B vitamins, theanine and other trace elements enhance its action. As part of "Kogniviya phosphatidylserine" , which is available in capsules, in addition to phosphatidylserine, also contains coenzyme Q10 and phosphatidylcholine. Thus, the drugs perfectly complement each other and are comfortable when taken together. Just two packs, and in your diet - more than 10 nutrients necessary for the nervous system. nine0009
Huperzine A, Phosphatidylserine and Phosphatidylcholine - Common and Differences
How do Huperzine A, Phosphatidylserium and Phosphatidylcholine work? All three micronutrients target the synthesis and maintenance of adequate levels of acetylcholine, an important neurotransmitter in the central nervous system that affects alertness, memory, and learning 4 .
In addition to this general function, there are some differences.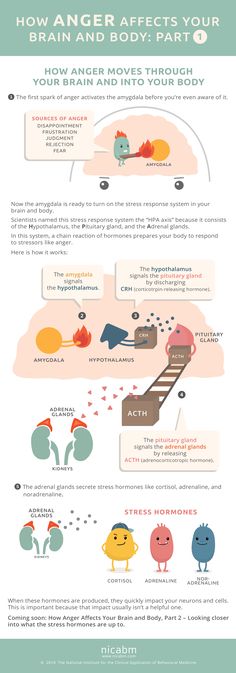 So, for example, Huperzine A has neuroprotective properties. It promotes the growth of neurons by stimulating the production of a protein that maintains their vitality. Prevents or reduces cell damage due to lack of oxygen. Protects against seizures.
So, for example, Huperzine A has neuroprotective properties. It promotes the growth of neurons by stimulating the production of a protein that maintains their vitality. Prevents or reduces cell damage due to lack of oxygen. Protects against seizures.
Due to its mechanism of action, Huperzine A supports memory and erudition. Used for both short-term brain stimulation (for example, when taking exams) and for longer-term tasks (for memory impairment associated with natural aging) 5 .
Phosphatidylserine is the main phospholipid in the brain. Forms the inner layer of cell membranes, ensures the normal functioning of the brain and nervous system. Carries out the transport of nutrients into the cell, stimulates metabolic processes, promotes the activation of glucose metabolism in the brain and increases the resistance of nerve cells to ischemic damage 6 .
Phosphatidylcholine is a phospholipid that is involved in memory, metabolism, slows down the aging process of the cell.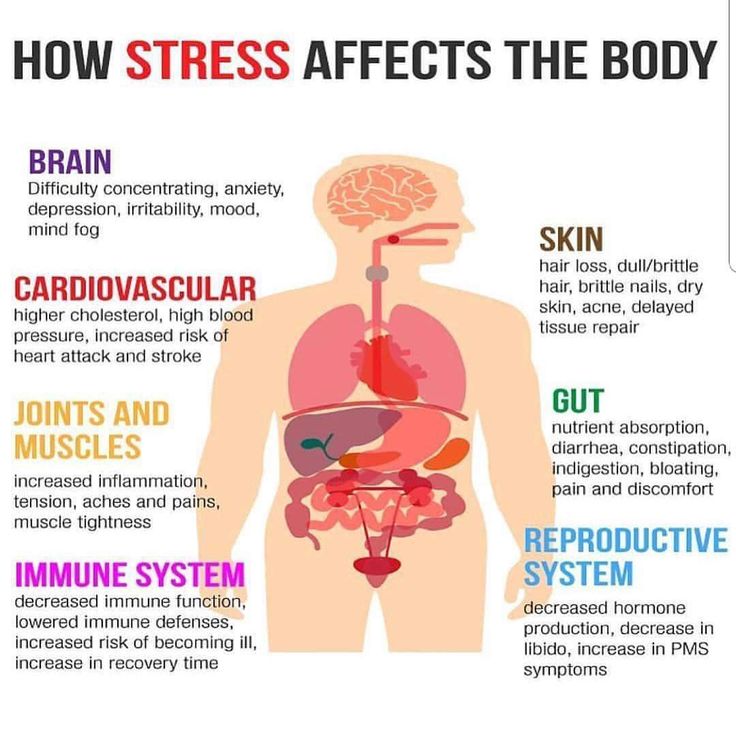 nine0009
nine0009
How to take vitamins for the brain during stress?
Above was a detailed description of how stress affects the brain. If we summarize and simplify all this information, then the effect of severe stress on the brain can be described, in fact, in one phrase: cognitive decline. Someone becomes inattentive and distracted, someone suffers from memory (both short-term and long-term), someone slows down the speed of information processing - there are many options. nine0009
If you notice such manifestations in yourself, then a course intake of essential trace elements can help offset the negative effects of stress on the brain. It is enough to remember that all the drugs recommended in this article should be drunk in a course of at least a month, then you can feel a sufficient effect. However, some users reported that glycine and magnesium act on them quite quickly: from 1-4 applications. Therefore, if necessary, you can make their reception situational. B vitamins are best taken in the morning, and glycine and magnesium in the evening, shortly before bedtime. nine0009
B vitamins are best taken in the morning, and glycine and magnesium in the evening, shortly before bedtime. nine0009
As for the complex of two dietary supplements "Cognivia", here you should not count on such a quick effect as with magnesium or glycine. For a monthly course, you will need three packs of dietary supplement "Kogniviya" (in a sachet) and one pack of "Kogniviya phosphatidylserine" . To achieve a more pronounced effect, a course of at least three months is required.
But whatever you choose, please remember that you can take dietary supplements and drugs together only with the permission of a doctor. nine0009
1. Physiology and neurobiology of stress and adaptation: central role of the brain, Bruce S McEwen
2. Protective and damaging effects of stress mediators: central role of the brain, Bruce S McEwen
3. Stress- and allostasis-induced brain plasticity, Bruce S McEwen, Peter J Gianaros
4.