How long does pristiq take to work
Desvenlafaxine (Pristiq) | NAMI: National Alliance on Mental Illness
Brand names:
- Pristiq®
- Tablets (extended release): 25 mg, 50 mg, 100 mg
- Khedezla®
- Tablets (extended release): 50 mg, 100 mg
- Desvenlafaxine
- Tablets (extended release): 25 mg, 50 mg, 100 mg
Generic name: Desvenlafaxine (des ven la FAX een)
All FDA black box warnings are at the end of this fact sheet. Please review before taking this medication.
What Is Desvenlafaxine And What Does It Treat?
Desvenlafaxine is an antidepressant medication that works in the brain. It is approved for the treatment of major depressive disorder (MDD).
Symptoms of depression include:
- Depressed mood - feeling sad, empty, or tearful
- Feeling worthless, guilty, hopeless, and helpless
- Loss of interest or pleasure in your usual activities
- Sleep and eat more or less than usual (for most people it is less)
- Low energy, trouble concentrating, or thoughts of death (suicidal thinking)
- Psychomotor agitation (‘nervous energy’)
- Psychomotor retardation (feeling like you are moving and thinking in slow motion)
- Suicidal thoughts or behaviors
What Is The Most Important Information I Should Know About Desvenlafaxine?
Do not stop taking desvenlafaxine, even when you feel better. With input from you, your health care provider will assess how long you will need to take the medicine.
Missing doses of desvenlafaxine may increase your risk for relapse in your symptoms.
Stopping desvenlafaxine abruptly may result in one or more of the following withdrawal symptoms: irritability, nausea, feeling dizzy, vomiting, nightmares, headache, and/or paresthesias (prickling, tingling sensation on the skin).
Depression is also a part of bipolar illness. People with bipolar disorder who take antidepressants may be at risk for "switching" from depression into mania. Symptoms of mania include "high" or irritable mood, very high self-esteem, decreased need for sleep, pressure to keep talking, racing thoughts, being easily distracted, frequently involved in activities with a large risk for bad consequences (for example, excessive buying sprees).
Medical attention should be sought if serotonin syndrome is suspected. Please refer to serious side effects for signs/symptoms.
Are There Specific Concerns About Desvenlafaxine And Pregnancy?
If you are planning on becoming pregnant, notify your health care provider to best manage your medications. People living with MDD who wish to become pregnant face important decisions. Untreated MDD has risks to the fetus, as well as the mother. It is important to discuss the risks and benefits of treatment with your doctor and caregivers. For women who take antidepressant medications during weeks 13 through the end of their pregnancy (second and third trimesters), there is a risk that the baby can be born before it is fully developed (before 37 weeks).
Caution is advised with breastfeeding since desvenlafaxine does pass into breast milk.
What Should I Discuss With My Health Care Provider Before Taking Desvenlafaxine?
- Symptoms of your condition that bother you the most
- If you have thoughts of suicide or harming yourself
- Medications you have taken in the past for your condition, whether they were effective or caused any adverse effects
- If you experience side effects from your medications, discuss them with your provider.
 Some side effects may pass with time, but others may require changes in the medication.
Some side effects may pass with time, but others may require changes in the medication. - Any other psychiatric or medical problems you have, including a history of bipolar disorder
- All other medications you are currently taking (including over the counter products, herbal and nutritional supplements) and any medication allergies you have
- Other non-medication treatment you are receiving, such as talk therapy or substance abuse treatment. Your provider can explain how these different treatments work with the medication.
- If you are pregnant, plan to become pregnant, or are breastfeeding
- If you drink alcohol or use drugs
How Should I Take Desvenlafaxine?
Desvenlafaxine is usually taken one time per day with or without food.
Typically patients begin at a low dose of medicine and the dose is increased slowly over several weeks.
The dose usually ranges from 50 mg to 400 mg. Only your healthcare provider can determine the correct dose for you.
The tablets should be swallowed whole. They should not be chewed, crushed, or broken.
If you are taking desvenlafaxine, you should not take other medications that include venlafaxine (Effexor®).
Consider using a calendar, pillbox, alarm clock, or cell phone alert to help you remember to take your medication. You may also ask a family member or a friend to remind you or check in with you to be sure you are taking your medication.
What Happens If I Miss A Dose of Desvenlafaxine?
If you miss a dose of desvenlafaxine take it as soon as you remember, unless it is closer to the time of your next dose. Discuss this with your health care provider. Do not double your next dose or take more than what is prescribed.
What Should I Avoid While Taking Desvenlafaxine?
Avoid drinking alcohol or using illegal drugs while you are taking antidepressant medications. They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g. , sedation) of the medication.
, sedation) of the medication.
What Happens If I Overdose With Desvenlafaxine?
If an overdose occurs, call your doctor or 911. You may need urgent medical care. You may also contact the poison control center at 1-800-222-1222.
A specific treatment to reverse the effects of desvenlafaxine does not exist.
What Are The Possible Side Effects of Desvenlafaxine?
Common side effects
Headache, nausea, vomiting, diarrhea, constipation, dry mouth, increased sweating, decreased appetite, tremor, feeling nervous, restless, fatigue, or having trouble sleeping (insomnia). These will often improve over the first week or two as you continue to take the medication.
Sexual side effects, such as problems with orgasm or ejaculatory delay, and blood pressure increases often do not improve over time.
Rare/serious side effects
Increased heart rate, low blood pressure, increased salivation, irregular menstrual cycle, increased frequency of urination, changes in taste, increased liver enzymes, low sodium (symptoms of low sodium levels may include headache, weakness, difficulty concentrating and remembering), teeth grinding, difficulty urinating, angle closure glaucoma (symptoms of angle closure glaucoma may include eye pain, changes in vision, swelling or redness in or around eye), serotonin syndrome (symptoms may include shivering, diarrhea, confusion, severe muscle tightness, fever, seizures, and death), hypertensive crisis (severely elevated blood pressure), myocardial infarction (heart attack), Stevens-Johnson syndrome (rash)
Are There Any Risks For Taking Desvenlafaxine For Long Periods Of Time?
To date, there are no known problems associated with long term use of desvenlafaxine. It is a safe and effective medication when used as directed.
It is a safe and effective medication when used as directed.
What Other Medications May Interact With Desvenlafaxine?
Desvenlafaxine should not be taken with or within 2 weeks of taking monoamine oxidase inhibitors (MAOIs). These include phenelzine (Nardil®), tranylcypromine (Parnate®), isocarboxazid (Marplan®), rasagiline (Azilect®), and selegiline (Emsam®).
Although rare, there is an increased risk of serotonin syndrome when desvenlafaxine is used with other medications that increase serotonin, such as other antidepressants, migraine medications called “triptans” (e.g., Imitrex®), some pain medications (e.g., tramadol (Ultram®), the antibiotic linezolid (Zyvox®), and amphetamines.
The following medication may increase the levels and effects of desvenlafaxine: ketoconazole (Nizoral®).
Desvenlafaxine may increase the effects of other medications that can cause bleeding (e.g., ibuprofen (Advil®, Motrin®), warfarin (Coumadin®) and aspirin).
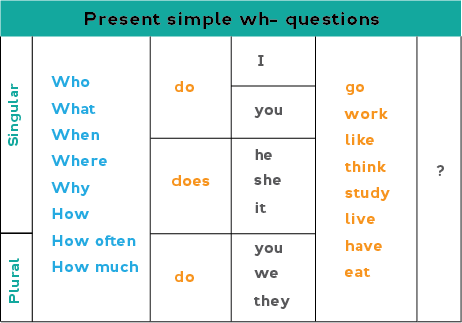
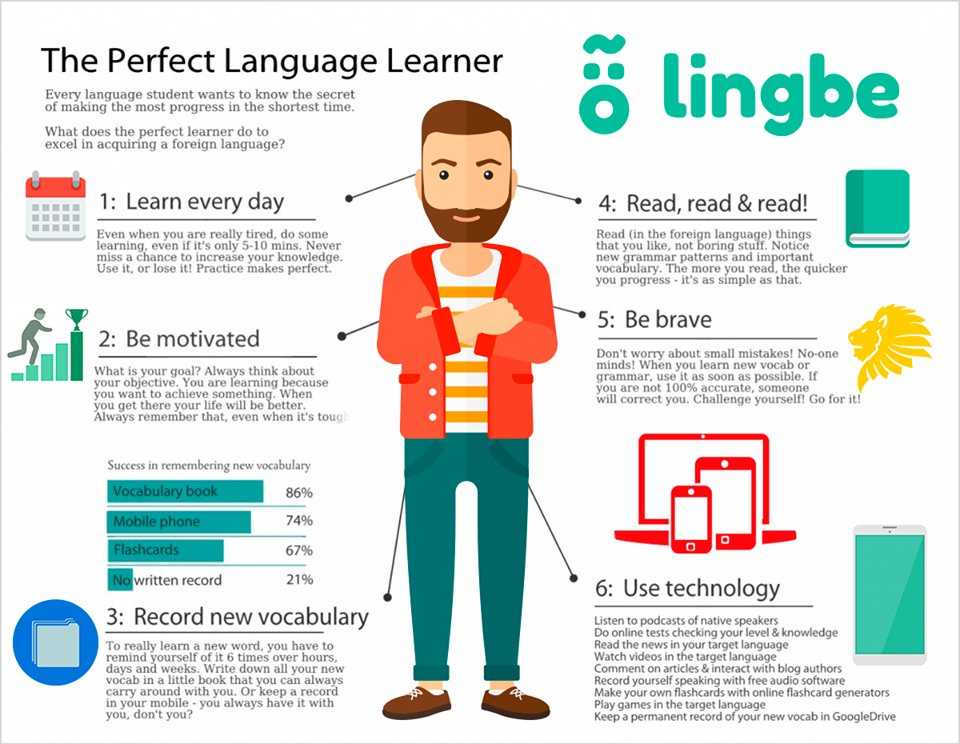
How Long Does It Take For Desvenlafaxine To Work?
Sleep, energy, or appetite may show some improvement within the first 1-2 weeks. Improvement in these physical symptoms can be an important early signal that the medication is working. Depressed mood and lack of interest in activities may need up to 6-8 weeks to fully improve.
Summary of FDA Black Box Warnings
Suicidal thoughts or actions in children and adults
Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications. This risk may persist until significant remission occurs.
In short-term studies, antidepressants increased the risk of suicidality in children, adolescents, and young adults when compared to placebo. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24. Adults age 65 and older taking antidepressants have a decreased risk of suicidality. Patients, their families, and caregivers should be alert to the emergence of anxiety, restlessness, irritability, aggressiveness and insomnia. If these symptoms emerge, they should be reported to the patient’s prescriber or health care professional. All patients being treated with antidepressants for any indication should watch for and notify their health care provider for worsening symptoms, suicidality and unusual changes in behavior, especially during the first few months of treatment.
Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24. Adults age 65 and older taking antidepressants have a decreased risk of suicidality. Patients, their families, and caregivers should be alert to the emergence of anxiety, restlessness, irritability, aggressiveness and insomnia. If these symptoms emerge, they should be reported to the patient’s prescriber or health care professional. All patients being treated with antidepressants for any indication should watch for and notify their health care provider for worsening symptoms, suicidality and unusual changes in behavior, especially during the first few months of treatment.
Provided by
(December 2020)
©2020 The College of Psychiatric and Neurologic Pharmacists (CPNP) and the National Alliance on Mental Illness (NAMI). CPNP and NAMI make this document available under the Creative Commons Attribution-No Derivatives 4. 0 International License. Last Updated: January 2016.
0 International License. Last Updated: January 2016.
This information is being provided as a community outreach effort of the College of Psychiatric and Neurologic Pharmacists. This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
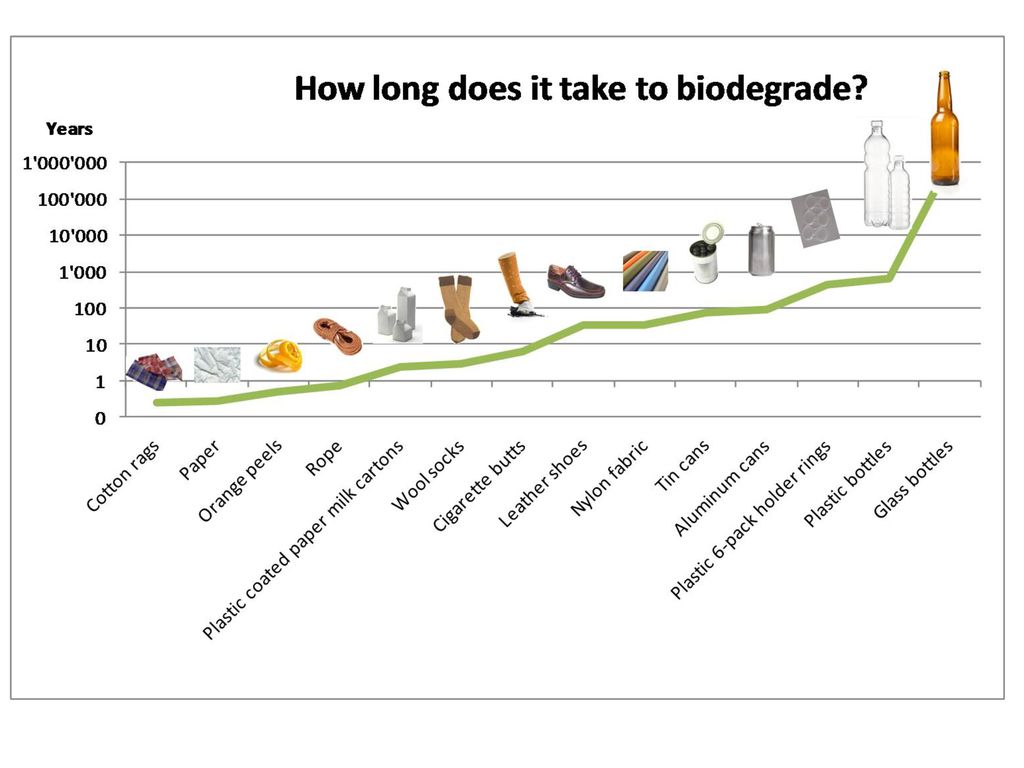
How Long Does Pristiq Stay in Your System?
Pristiq (Desvenlafaxine) is a selective serotonin reuptake inhibitor that was approved in 2008 by the FDA and is primarily prescribed to treat depression. While many people have found this medication to be effective, there are those who claim the the drug has caused a variety of side effects including dizziness, fatigue, headaches, insomnia, nausea, and other medical conditions. As a result, some people decide to discontinue taking Pristiq (Desvenlafaxine) after it is prescribed. One of the most common questions asked by people who take the medication is how long the treatment lasts. The answer depends on a variety of factors that vary from person to person. For an individual who takes 10 mg of Pristiq (Desvenlafaxine), however, the drug will remain in their system for roughly a week.
While many people have found this medication to be effective, there are those who claim the the drug has caused a variety of side effects including dizziness, fatigue, headaches, insomnia, nausea, and other medical conditions. As a result, some people decide to discontinue taking Pristiq (Desvenlafaxine) after it is prescribed. One of the most common questions asked by people who take the medication is how long the treatment lasts. The answer depends on a variety of factors that vary from person to person. For an individual who takes 10 mg of Pristiq (Desvenlafaxine), however, the drug will remain in their system for roughly a week.
There are important pieces of information to know about Pristiq (Desvenlafaxine), including the following prescription facts:
- Pristiq (Desvenlafaxine) is often used for depression and mood disorders, but is also prescribed for anxiety disorders, such as attention deficit hyperactivity disorder, chronic neuropathic pain, and obsessive-compulsive disorder.
- If you notice improvements in your condition, you should not stop taking the medication before speaking with a physician.
- The normal dose of Pristiq (Desvenlafaxine) is 50 mg daily. Higher doses including amounts of up to 400 mg a day have been prescribed but are not common.
- If you miss a dose of Pristiq (Desvenlafaxine), do not double doses because this can have very serious adverse consequences.
There are few regulations that apply to Pristiq (Desvenlafaxine). The drug is not listed on the DEA’s controlled substance list. Many people have argued, however, that this lack of regulation is responsible for some people misusing Pristiq (Desvenlafaxine). It is important to understand, however, that the medication can show up as false positives during substance use tests.
Desvenlafaxine is a synthetic SSRI containing venlafaxine. While venlafaxine is used in popular products like Effexor, desvenlafaxine is most commonly used in Pristiq.
Like other SSRIs, medical researchers are still trying to determine exactly how Pristiq (Desvenlafaxine) works. Most physicians, however, believe that medication works by blocking serotonin from being reabsorbed into a person’s brain. Preventing reabsorption in this manner is important because after the process occurs, serotonin stops being helpful at helping to control a person’s mood. It is important to note that it is yet to be definitively determined that this is how SSRIs work.
Most physicians, however, believe that medication works by blocking serotonin from being reabsorbed into a person’s brain. Preventing reabsorption in this manner is important because after the process occurs, serotonin stops being helpful at helping to control a person’s mood. It is important to note that it is yet to be definitively determined that this is how SSRIs work.
The approximate half-life of Pristiq (Desvenlafaxine) is 11 hours. This means that a person who takes 50 mg of the drug is expected to have 50% or 25 mg 11 hours after ingesting 50 mg. 11 hours after the drug was reduced to 50%, the remains of Pristiq (Desvenlafaxine) would be reduced to 12.5 mg. This division continues until traces of the drug in a person’s system are not noticeable. A drug’s half-life is slightly extended if a person is a long-term user of Pristiq (Desvenlafaxine), but after a week has elapsed the drug should become unrecognizable in a person’s system.
There are a variety of factors that determine how a person is affected by withdrawal from Pristiq (Desvenlafaxine) which include the following:
- Age.
 The length of time that it takes Pristiq (Desvenlafaxine) to be cleansed from a person’s system tends to increase among older individuals. Younger people are able to eliminate traces of the medication in their system much faster.
The length of time that it takes Pristiq (Desvenlafaxine) to be cleansed from a person’s system tends to increase among older individuals. Younger people are able to eliminate traces of the medication in their system much faster. - Body Mass. The greater a person’s body mass, the quicker that they can excrete Pristiq (Desvenlafaxine) through their system.
- Dosage. People who take larger doses of Pristiq (Desvenlafaxine) can, sometimes, take longer to eliminate traces of the drug in their system versus people who take smaller doses.
It should be noted that there are many other physiology factors that influence the exact speed and manner in which a person’s body is cleansed from the medication.
Drug testing has become popular among companies in a variety of different industries. As a result, workers are often curious about how Pristiq (Desvenlafaxine) will remain in their system and how the drug might impact a drug test. Unfortunately, there are no exact answers to how long Pristiq (Desvenlafaxine) will remain in a person’s system. The only thing of which is sure is, if a person takes 10 mg of the medication, traces of the drug will not be in their system after a week.
Unfortunately, there are no exact answers to how long Pristiq (Desvenlafaxine) will remain in a person’s system. The only thing of which is sure is, if a person takes 10 mg of the medication, traces of the drug will not be in their system after a week.
If you are trying to learn more about Pristiq (Desvenlafaxine), the Recovery Village has highly trained staff waiting on standby to answer your questions.
Read Previous
Mixing Pristiq and Alcohol
Medical Disclaimer
The Recovery Village aims to improve the quality of life for people struggling with substance use or mental health disorder with fact-based content about the nature of behavioral health conditions, treatment options and their related outcomes. We publish material that is researched, cited, edited and reviewed by licensed medical professionals. The information we provide is not intended to be a substitute for professional medical advice, diagnosis or treatment. It should not be used in place of the advice of your physician or other qualified healthcare providers.
Pristiq vs Effexor: Differences, Similarities & What's Best for You - Drug Vs. Friend
Home >> Drug Vs. Friend >> Pristiq vs Effexor: Differences, Similarities & Which is Best for You
Drug Vs. Friend
Drug Overview and Key Differences | Conditions of treatment | Efficiency | Insurance coverage and cost comparison | Side effects | Drug Interactions | Warnings | FAQ
More than 16 million American adults have depression (major depressive disorder). Pristiq and Effexor are two popular medications designed to treat depression. state of mental health. Effexor XR (Extended Edition) also treats Generalized Anxiety Disorder, Panic Disorder and Social Anxiety. Both drugs are approved by the US Food and Drug Administration (FDA). Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer, makes both drugs in proprietary forms. nine0005
Pristiq and Effexor belong to a group of drugs called SNRIs (serotonin and norepinephrine reuptake inhibitors). They work by regulating the reuptake of the neurotransmitters serotonin and norepinephrine in the CNS (central nervous system), thereby improving symptoms of depression.
They work by regulating the reuptake of the neurotransmitters serotonin and norepinephrine in the CNS (central nervous system), thereby improving symptoms of depression.
Note that the generic name for Pristika is desvenlafaxine and the generic name for Effexor is venlafaxine. These drugs are very similar. When the effexor (venlafaxine) is metabolized, it is converted to the active metabolite, desvenlafaxine. nine0005
Although Pristiq and Effexor are SNRIs, they have some differences which we will describe below.
What are the main differences between Pristiq and Effexor?
Pristiq and Effexor are SNRI antidepressants available in branded and generic forms. Both drugs are approved for use in adults.
Pristiq is available as an extended edition tablet. Dosage may vary, but the standard dose is 50 mg per day.
Dosage may vary, but the standard dose is 50 mg per day.
Effexor is available in tablet form, as well as extended release capsules and extended release tablets. A typical dose is 75 or 150 mg per day (XR formulation). nine0042 Desvenlafaxine succinate (desvenlafaxine)
Want the best price on Pristiq?
Sign up for Pristiq Price Alerts and be notified when the price changes!
Receive price alerts
Conditions treated by Pristiq and Effexor
Pristiq (what is Pristiq?) Indicated for the treatment of major depressive disorder (MDD) in adults. Sometimes Pristiq is prescribed. not intended for other purposes.
Sometimes Pristiq is prescribed. not intended for other purposes.
Effexor (immediate release) is indicated for the treatment of major depressive disorder. Effexor XR (what is Effexor?) is indicated for major depressive disorder, generalized anxiety disorder, social anxiety disorder and panic disorder.
| Panic disorder | Off label | Yes (XR form only) |
Is Pristiq or Effexor more effective?
A meta-analysis examined the safety and efficacy of Pristiq and Effexor. The researchers concluded that both drugs were similar in terms of effectiveness in treating depression as well as side effects. However, patients taking Pristiq experienced less nausea than patients taking Effexor. nine0005
Consult your physician for medical advice. Only your healthcare provider can determine which medicine is best for you based on your medical condition and medical history, as well as any medicines you are taking that may interact with Pristiq or Effexor.
Get a pharmacy discount card
Pristiq and Effexor coverage and cost comparison
Insurance plans and Medicare Part D usually cover Pristiq. The regular price for a typical prescription for generic 30.50 mg tablets is around $380. A free SingleCare card can bring the price down to under $60. nine0012 Plans and Medicare Part D usually cover Effexor XR (the most commonly prescribed type of Effexor). The regular price for a typical prescription for 30 150mg capsules is about $140. You can use the free SingleCare card to bring the price down to about $15.
Because insurance plans vary and are subject to change, contact your health plan for up-to-date coverage information.
| Pristiq | Effexor | |||
| Usually covered by insurance? | yes | yes | ||
| Usually covered by Medicare Part D? | yes | yes | ||
| Standard dosage | Extended release tablets 30, 50 mg0005 The most common side effects of Effexor XR are nausea, drowsiness, dry mouth, sweating, sexual problems, decreased appetite and constipation. Other side effects may occur. Check with your healthcare professional for a complete list of side effects. Side effects0042 Yes | 10% | Yes | Eleven% |
| Libido decrease | yes | yes | 5% | |
| Problems with ejaculation with ejaculation | Yes, | Yes, | Yes, 9004 | 10% |
| Impotence / Erectile dysfunction | Yes | 3% | Yes |
* COMMUNITIONS FOR EFFEXOR XR, the most often prescribed drug EFFEXOR. nine0012 Source: DailyMed (Pristiq), DailyMed (Effexor XR)
Drug Interactions Pristiq vs Effexor
The use of SNRI antidepressants with MAO inhibitors may increase the risk of serotonin syndrome, which can be life-threatening. Pristiq or Effexor must be separated from the MAOI for seven to 14 days, depending on which drug was discontinued first. Pristiq or Effexor should not be taken with other serotonin-raising medications such as other SNRI or SSRI antidepressants, migraine triptans, and opioids for the same reason. In addition, the dextromethorphan cough found in Robitussin-DM as well as many other cough and cold products should be avoided as it can also cause serotonin syndrome when combined with Pristiq or Effexor. nine0005
In addition, the dextromethorphan cough found in Robitussin-DM as well as many other cough and cold products should be avoided as it can also cause serotonin syndrome when combined with Pristiq or Effexor. nine0005
Other drugs that may interact with Pristiq or Effexor include NSAIDs (non-steroidal anti-inflammatory drugs) such as aspirin or ibuprofen and anticoagulants (blood thinners) such as warfarin. Avoid drinking alcohol while taking Pristiq or Effexor.
If Pristiq is taken with a drug that is metabolized by the cytochrome P 2D6 enzyme, the level of the other drug may reach too high and become toxic. Dosage adjustment may be required. The effexor does not have this interaction. nine0005
Other warnings include:
- Pristiq and Effexor are not approved for pediatric patients.
- Serotonin syndrome is a serious, life-threatening emergency caused by the accumulation of too much serotonin. Patients taking Pristiq or Effexor should be closely monitored for signs and symptoms of serotonin syndrome such as hallucinations, seizures, changes in blood pressure and agitation.
 Patients should seek emergency medical attention if any of these symptoms occur. Patients taking other drugs that increase serotonin levels (triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, dextromethorphan, amphetamines, St. John's wort and MAOIs) have a higher risk of developing serotonin syndrome. nine0646
Patients should seek emergency medical attention if any of these symptoms occur. Patients taking other drugs that increase serotonin levels (triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, dextromethorphan, amphetamines, St. John's wort and MAOIs) have a higher risk of developing serotonin syndrome. nine0646 - Pristiq or Effexor may increase blood pressure. Check your blood pressure regularly. If you have high blood pressure or heart problems, check with your doctor before taking Pristiq or Effexor.
- SNRIs may increase the risk of bleeding. The risk is increased when taking aspirin, NSAIDs or warfarin at the same time.
- Activation of mania or hypomania may occur. In patients with bipolar disorder, antidepressants may induce a mixed/manic episode. nine0646
- Avoid or use SNRIs with caution in patients with untreated anatomically narrow angles (angle-closure glaucoma). Ask your doctor if you are at risk.
- When you stop taking Pristiq or Effexor, ask your doctor to schedule a gradual dose reduction.
 Abrupt discontinuation may cause withdrawal symptoms such as nausea, tremors, confusion, and convulsions. Gradually reducing the dose may help avoid these symptoms. nine0646
Abrupt discontinuation may cause withdrawal symptoms such as nausea, tremors, confusion, and convulsions. Gradually reducing the dose may help avoid these symptoms. nine0646 - Hyponatremia (low sodium levels) may occur due to syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Patients may experience headaches, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to a fall. More serious cases may occur. Patients should seek emergency care if symptoms develop and stop taking SNRIs.
- Do not drive or operate machinery until you know how Pristiq or Effexor affects you. nine0646
- Talk to your doctor about using Pristiq or Effexor if you have a history of seizures.
- Rarely, rash and allergic/systemic anaphylaxis reactions or angioedema have been reported. If you develop a rash or allergic symptoms, stop taking Pristiq or Effexor and seek medical attention immediately. Do not take Pristiq or Effexor if you are allergic to any of the ingredients.
- These drugs have been associated in rare cases of interstitial lung disease and eosinophilic pneumonia. If you are taking Pristiq or Effexor and you experience shortness of breath, cough, or chest discomfort, seek immediate medical attention. nine0646
- Pristiq or Effexor should be used during pregnancy only if the benefit to the mother outweighs the risk to the baby. Stopping the medication may cause a relapse of depression or anxiety. Therefore, patients should be assessed on an individual basis. Your healthcare provider may weigh the risks and benefits of using an SNRI during pregnancy. Newborns exposed to SNRIs in the third trimester developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. If you are already taking Pristiq or Effexor and find out you are pregnant, contact your doctor immediately. nine0646
- Pristiq: Swallow the tablet whole with water. Do not chew, crush, dissolve, or split tablets.
- Effexor XR: Swallow the capsule whole with water.
 Do not split, crush, chew, or place the capsule in water. Alternatively, you can open the capsule, sprinkle it on a spoonful of applesauce and swallow the mixture immediately, followed by a glass of water.
Do not split, crush, chew, or place the capsule in water. Alternatively, you can open the capsule, sprinkle it on a spoonful of applesauce and swallow the mixture immediately, followed by a glass of water.
Frequently asked questions about Pristiq and Effexor
What is Pristiq?
Pristiq is an SNRI antidepressant. Pristiq treats depression in adults. The generic name is desvenlafaxine.
What is Effexor?
Effexor is also an SNRI antidepressant. Effexor treats depression in adults. Effexor XR (extended release) treats depression, social anxiety disorder, panic disorder, and generalized anxiety disorder. Effexor's generic name is venlafaxine.
Are Pristiq and Effexor the same thing? nine0124
Medicines are very similar. When Effexor is metabolized in the body, it is converted to desvenlafaxine, the active ingredient in Pristiq. The two drugs are similar but have some differences, such as dose, price, side effects, and drug interactions.
Is Pristiq or Effexor better?
Both drugs are similar in efficacy. Your healthcare provider can advise you on which drug is right for you.
Can I use Pristiq or Effexor while pregnant? nine0124
Consult your doctor. He or she will weigh the benefits of taking an antidepressant against the risk to the child. Newborns treated with certain antidepressants, including SNRIs or SSRIs (selective serotonin reuptake inhibitors such as Prozac), developed serious complications during the third trimester of pregnancy.
If you are already taking Pristiq or Effexor and find out you are pregnant, contact your OB/GYN right away. If you are breastfeeding, please also consult your OB/GYN. nine0005
Can I use Pristiq or Effexor with alcohol?
No. Pristiq or Effexor should not be taken with alcohol because the combination may increase the risk of respiratory depression (slowed breathing, lack of oxygen) and increase sedation and drowsiness, as well as impair alertness. The combination can also exacerbate anxiety and depression.
The combination can also exacerbate anxiety and depression.
Does Pristiq help with anxiety?
Although Pristiq is only indicated for the treatment of depression, some clinicians prescribe it off-label for anxiety. However, in clinical trials, 3% of patients who took Pristiq 50 mg (recommended dose) experienced anxiety as a side effect. Some need to try different medications to see what works best. Consult with your healthcare provider for more information. nine0005
Pristiq Mood Stabilizer?
Medicines classified as mood stabilizers are commonly used to treat bipolar disorder. Pristiq is indicated for the treatment of depression. Taking Pristiq may improve your mood, but it is not classified as a mood stabilizer. Pristiq is an SNRI antidepressant.
Venlafaxine is an SNRI?
Yes. Effexor (venlafaxine) is an SNRI. Other SNRIs for depression include Pristiq (desvenlafaxine), Fetzima (levomilnacipran), and Cymbalta (duloxetine). nine0005
| Depression Ambulance | [May. 14th, 2012| 06:11pm ] 14th, 2012| 06:11pm ] Neurosciences |
| All existing antidepressants leave much to be desired. Their effect is felt only a few days after the start of the reception, and for many they do not help at all. Pharmacologists are actively looking for more effective drugs against depression. By the time a young woman named blueberry octopus spoke on the Experience Project website about her struggles with anxiety and anxiety, she had been taking antidepressants for three years. At first she was on Paxil, one of the selective serotonin reuptake inhibitors (SSRIs), but decided to stop because it suppressed sexual desire. Then I switched to the anti-anxiety drug Xanax. The sexual disturbances disappeared, but at the price of a return of mental symptoms. Went back to paxil, then switched to leksapro (another SSRI) and then to pristiq, a serotonin-norepinephrine reuptake inhibitor (SNRI). She is now taking another SSRI drug, Zoloft, plus Wellbutrin (SSRI's cousin, which affects dopamine and norepinephrine activity), which counteracts the sexual side effects of Zoloft. “I didn’t notice a noticeable effect from taking wellbutrin, but perhaps because my dose is too low,” the patient writes. I'm going to see a psychiatrist next week. Who knows, maybe they can help me." We are dealing with a typical case of selection of an antidepressant for the treatment of not only depression as such, but also similar disorders by trial and error. Andrew Solomon, author of The Noonday Demon, a popular book on depression, called the tactic "playing darts." nine0005 SNRIs, which have dominated the antidepressant market since their introduction in the 1980s and 1990s, do not help everyone: about a third of patients do not benefit from them. In addition, while doing a great job today, these drugs sometimes suddenly refuse to work tomorrow. The urgent need for effective "immediate-acting" antidepressants is clear, and yet there is no light at the end of the tunnel. Pharmaceutical giant GlaxoSmithKline has announced its intention to shut down all psychiatric research because development is too expensive, laborious and time consuming. Individual university laboratories and small pharmaceutical companies do not give up. Whether their efforts will be crowned with success, no one knows. It is only clear that there is no need to wait for an ambulance for approximately 15 million people suffering from depression. In pursuit of speed In search of fast-acting antidepressants, researchers have turned to substances that instantly elevate mood. They intend to find out the mechanism of their action on the metabolism of serotonin, one of the brain's key signaling molecules. One such substance is ketamine. Ketamine is an anesthetic, analgesic and relaxant at the same time. Among drug users, it is known as Special K. Among other things, ketamine has an effect on the mind and causes hallucinations, and experiments on rodents have shown that it is toxic to nerve cells. Clearly, ketamine itself is by no means a suitable antidepressant. But the best “hint” in search of an answer to the question of how to speed up the action of antidepressants cannot be found. According to Ronald Duman and George Aghajanian of Yale University, as early as two hours after the administration of ketamine, laboratory rats increase the production of proteins necessary for the formation of new synapses - structures through which signals are transmitted from one nerve cell to the other in the prefrontal cortex. "Research over the past decade has shown that depression is accompanied by atrophy of the prefrontal region of the brain and the hippocampus," says Duman, director of the Yale University Laboratory of Molecular Psychiatry. "Ketamine quickly eliminates this anomaly." Duman and colleagues also examined the brains of rats just a few hours after the ketamine injection to see if synaptic spines appeared earlier than 24 hours after the injection. Duman found that ketamine activates the enzyme mTOR by injecting animals with a substance that suppresses its activity. Since ketamine is not suitable for therapeutic use, the search began for other mTOR activators . Ketamine was known to work by preventing glutamate (a key excitatory neurotransmitter in the brain) from binding to the so-called NMDA receptor (N-methyl-D-aspartate receptor) on the neuron's surface. Therefore, Duman and colleagues tested the effectiveness of another NMDA inhibitor and found that it also affects the activity of mTOR rapidly induces dendritic spine formation and relieves depression in mice. Now the scientist is testing other substances for the ability to block NMDA receptors, hoping that among them there will be safe, fast-acting antidepressants. Another quick mood booster, scopolamine, is already widely used, but for a different purpose: it is part of a plaster to prevent motion sickness. Back in the 1970s. it was found that an increase in the level of acetylcholine can cause depression. When patients with bipolar disorders (alternating bouts of mania and depression), while in the manic phase, started taking some drug that enhances the signaling activity of acetylcholine, they developed symptoms of depression within an hour - lowered mood, lethargy, etc. And when such patients were given a drug that increases the level of acetylcholine in the brain, depression intensified. nine0005 Given these facts, it would be worthwhile to study the pathways of acetylcholine inactivation. Unfortunately, interest in this area disappeared with the advent of the era of serotonin reuptake inhibitors. Many have decided that SSRIs are good because they do not affect the neural networks of the brain, which include acetylcholine, while the "old" antidepressants give a lot of side effects, since they affect the choliergic system, in particular, muscarinic receptors containing a whole network of acetylcholine receptors scattered throughout the brain. So, we return to the need to find a substance that specifically affects muscarinic receptors, which would cause mild side effects and quickly exert an antidepressant effect. This is exactly what scopolamine does. A clinical trial in 22 depressed patients by Maura Furey of the Department of Experimental Therapy and Pathophysiology of the National Institute of Mental Health found that intravenous administration of scopolamine relieved patients for three days and made them feel better for the next few days. everything is better. Within a month, every two out of three patients had symptoms almost disappeared, and a third went into remission. This state of affairs persisted for two weeks after discontinuation of the drug. nine0005 The Institute is now looking for a pharmaceutical company to conduct full trials to be able to market a new fast-acting antidepressant. Fury is extremely concerned that so far there have been no applicants. Fury is now looking for the best way to administer scopolamine. Intravenous injections in this case are inappropriate. When using skin patches, the desired concentration of the drug in the blood is not achieved. Oral administration is not suitable, since most of the drug is excreted through the gastrointestinal tract. There is a lot of work to be done. nine0005 Help everyone! Another disadvantage of the antidepressants used today is that they do not work for everyone. To solve the problem, the researchers focused on new mechanisms of action. So, some of them explore the second type of acetylcholine receptors - nicotinic ones. In particular, the small pharmaceutical firm Targacept of Winston-Salem, North Carolina, has focused on studying the behavior of drug TC-5214 that blocks one of this type of receptor. It can be used in addition to another antidepressant to enhance the effect. In one trial involving 265 volunteers who did not respond to citalopram (celexa), patients were given in addition TC-5214 or a placebo. Those who received placebo improved their standard depression score (Hamilton scale) by 7.75 points, while those who received TC-5214 improved by 13.75. AstraZeneca then approached Targacept with a proposal to conduct a deeper trial (Phase III). The first two attempts involving 614 patients failed; no improvement after eight months of use TS-5214 was not observed compared with placebo. But firms are going to repeat the tests with the involvement of 1.3 thousand volunteers living in different parts of the world, and hope for success. Another way to overcome resistance to all known antidepressants is more radical. It consists in acting on a completely different biological process, and not on those associated with any receptors. This refers to neurogenesis (the formation of new neurons), specifically in the hippocampus, a small structure at the base of the brain where adult neurogenesis occurs. It has long been known that the hippocampus is involved in the development of depression. Post-mortem examination of the brain of patients with clinical depression often revealed atrophy of this area and a significant decrease in its volume. SSRI and SNRI not only prevent serotonin reuptake, but also contribute to the replenishment of the hippocampal cell pool. The process is very slow, which is partly why the action of drugs in this class is delayed so much. Researchers at Neuralstern Pharmaceutical Company in Rockville, Md., say they have found another way to stimulate neurogenesis and keep it going after therapy is stopped. nine0005 Pharmacologists came to him while conducting experiments on a culture of neural stem cells of the hippocampus - according to them, the only culture of its kind in the world. They tested more than 10,000 substances for their ability to affect cell culture in order to find those that increase the rate of proliferation of target cells. Neuralstern is currently in Phase 1 clinical trials (safety testing) of a new drug - NS1-189 - in tablet form. If everything goes according to plan, then efficiency tests will begin at the end of the year. At the same time, it is planned to use magnetic resonance methods to visualize neurogenesis. But even if the hopes placed on NS1-189 are justified, we should not expect a quick practical result. “It is unlikely that a person with epilepsy will immediately stop the seizures,” says Karl Johe (Karl Johe), an employee of the firm Neuralstern. - It is necessary that changes in cells occur at the genetic level. Deep Dive Chronic inflammation, a condition associated with diseases as diverse as cancer, atherosclerosis, and diabetes, has recently been found to be related to depression, and this immediately opened up new possibilities in its treatment. The relationship between depression and inflammation, usually occurring in response to entry into the body of an infectious agent, has been demonstrated in various ways. Thus, it has been shown that the blood of depressed patients contains many cytokines, small protein molecules that regulate the inflammatory process. In addition, about a decade ago, it was discovered that patients with skin cancer who received cytokines developed depression. "I was once counseling one such patient," says Andrew Miller, director of psychiatric oncology at the Emory University Cancer Institute, "and I was struck by the similarity of his symptoms with my clinically depressed patients." A particularly nasty property of cytokines is their ability to suppress SSRI- and SNRL-stimulated neurogenesis "By blocking neurogenesis, you're pulling the rug out from under the feet of antidepressants," says Miller. This explains why depressed patients with a pronounced inflammatory response are less amenable to antidepressant treatment than others. In 2006, an article appeared in the Lancet announcing that the experimental psoriasis drug etanercept often alleviates symptoms of depression even when the underlying disease does not show any improvement. Apparently, this is due to the neutralization of the cytokine TNF-alpha. nine0005 Miller was intrigued by these amazing results and decided - after detailed discussion with colleagues - to conduct clinical trials of one of the cytokine antagonists, remicade, an anti-inflammatory drug already used in the clinic to treat patients suffering from rheumatoid arthritis and other autoimmune diseases. Renewed attempts to influence depression through serotonin receptors, but on the other hand - by increasing their number. A completely radical approach, the use of gene therapy, is also not ruled out. Recently, preliminary data have appeared on its successful use in the treatment of patients with parkinsonism. The p11 gene was chosen as the target gene: it encodes a protein involved in the transport of serotonin receptors from the cytoplasm to the cell surface. In the absence of this protein, the receptor remains inside the cell and does not respond to serotonin. In 2006, Paul Greengard and colleagues at Rockefeller University showed that rodents with depressive symptoms had lower levels of protein p11 . Mice with the non-functional p11 gene obtained from Greengard's laboratory showed clear signs of depression. It was necessary to show that the symptoms are at least alleviated if a full-fledged p11 gene is inserted into the animal genome. Such an experiment was carried out by Michael Kaplitt, head of the Laboratory of Molecular Neurosurgery at Weill-Cornell Medical College, who has already done the same with Parkinson's patients. An inactivated adeno-associated virus was used as a vector; gene p11 was injected directly into the nucleus of p11 deficient mice, and their depressive symptoms soon disappeared. Each neurophysiologist has his own "favorite" part of the brain. For Kaplitt, this is the nucleus accumbens. “I prefer to work on the surrendered area because it contains reward and pleasure centers that are affected by dopamine,” he explains. Finally, the third reason is that the nucleus accumbens has already been used as a target for the treatment of depression by deep-brain stimulation ( DBS , deep-brain stimulation). According to Kaplitt, in situ gene therapy is easier than the DBS method, because "instead of electrodes, you put a small catheter into the brain - and no iron!" (With deep stimulation, in addition to electrodes, you also need an electrical stimulator implanted in the region of the collarbone and generating electrical impulses.) Caplitt and colleagues made sure that the viral vector is safe and works p11 -gene can be delivered through it to the proper place. | |
 nine0005
nine0005  It happens that the effect has to wait for weeks, as a result, the patient does not receive support in the most difficult period for him. According to a report published in 2006 in the American Journal of Psychiatry, among depressed people over 66 years of age, the frequency of suicide attempts is five times higher in the first five months of taking SSRI than in the subsequent period. nine0005
It happens that the effect has to wait for weeks, as a result, the patient does not receive support in the most difficult period for him. According to a report published in 2006 in the American Journal of Psychiatry, among depressed people over 66 years of age, the frequency of suicide attempts is five times higher in the first five months of taking SSRI than in the subsequent period. nine0005  Many, in a desperate attempt to get rid of mental anguish, decide on such risky experiments as electrical stimulation using electrodes inserted into the brain. nine0005
Many, in a desperate attempt to get rid of mental anguish, decide on such risky experiments as electrical stimulation using electrodes inserted into the brain. nine0005  It is in this area, located just above eye level, that disorders are observed in depression. 24 hours after the injection of ketamine, new synaptic spines began to appear along the dendrites - processes of nerve cells that receive signals from other neurons. The more they became, the faster the nerve impulses were transmitted and the less pronounced were the symptoms of depression in rats. nine0005
It is in this area, located just above eye level, that disorders are observed in depression. 24 hours after the injection of ketamine, new synaptic spines began to appear along the dendrites - processes of nerve cells that receive signals from other neurons. The more they became, the faster the nerve impulses were transmitted and the less pronounced were the symptoms of depression in rats. nine0005  When the rats were then given ketamine, the symptoms of depression did not disappear. Thus, in order for ketamine to perform its function, active mTOR is needed.
When the rats were then given ketamine, the symptoms of depression did not disappear. Thus, in order for ketamine to perform its function, active mTOR is needed.  Scopolamine acts on a different neural circuit in the brain than ketamine: it prevents the binding of acetylcholine, a neurotransmitter that mediates memory processes, to the so-called muscarinic receptors. nine0005
Scopolamine acts on a different neural circuit in the brain than ketamine: it prevents the binding of acetylcholine, a neurotransmitter that mediates memory processes, to the so-called muscarinic receptors. nine0005  nine0005
nine0005 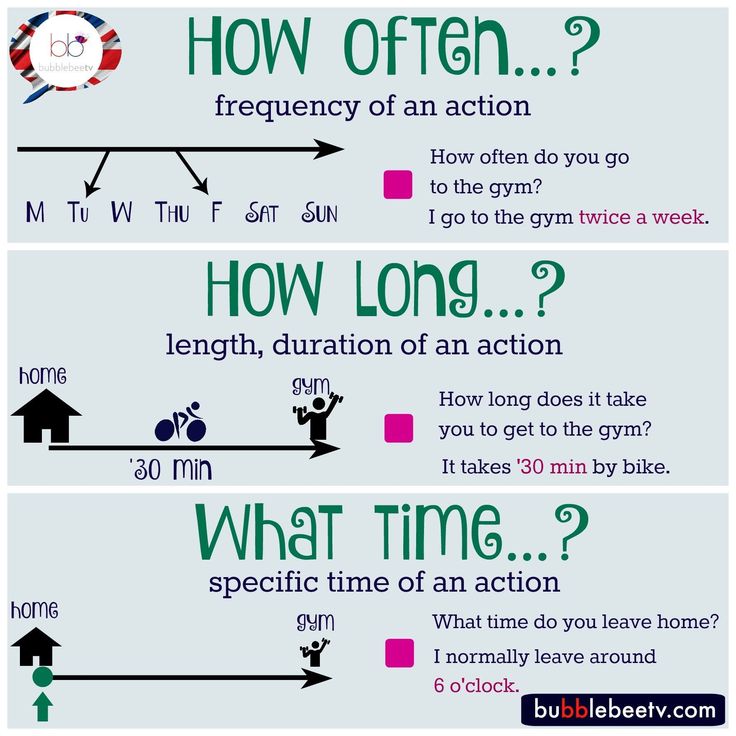 “After all, I saw with my own eyes how good the new drug is!” she exclaims.
“After all, I saw with my own eyes how good the new drug is!” she exclaims.  nine0005
nine0005  nine0005
nine0005 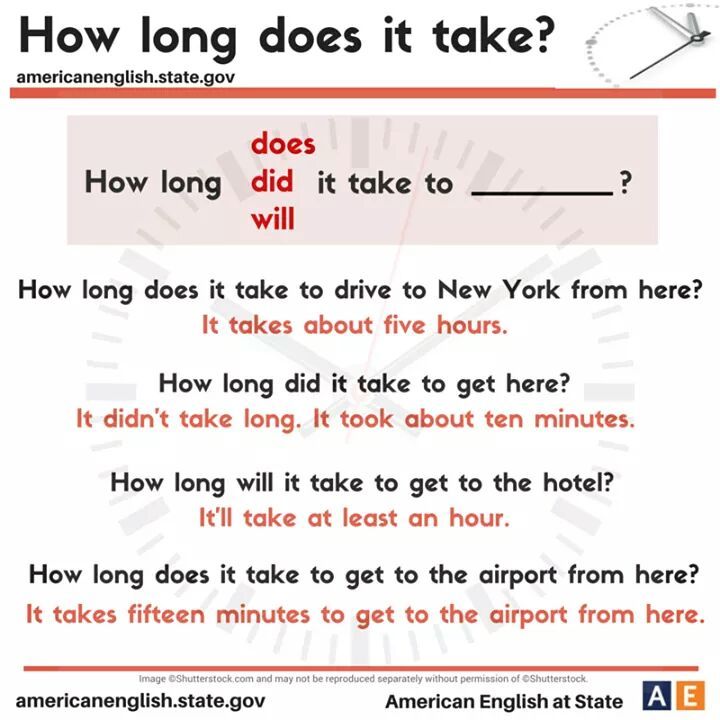 Of the 200 substances selected, about a dozen could stimulate neurogenesis. In 2004, animal experiments began; drugs were injected into healthy mice. Those that stimulated neurogenesis better than others were administered to rodents with symptoms of depression, and based on the results of the experiments, the most effective drug was selected. nine0005
Of the 200 substances selected, about a dozen could stimulate neurogenesis. In 2004, animal experiments began; drugs were injected into healthy mice. Those that stimulated neurogenesis better than others were administered to rodents with symptoms of depression, and based on the results of the experiments, the most effective drug was selected. nine0005  Hippocampal atrophy lasts for years, and it takes a long time to reverse the process.” However, Joe hopes that the effect of the treatment will be long-term, so take NS1-189 will not have to constantly, but with long breaks.
Hippocampal atrophy lasts for years, and it takes a long time to reverse the process.” However, Joe hopes that the effect of the treatment will be long-term, so take NS1-189 will not have to constantly, but with long breaks.  nine0005
nine0005  Unfortunately, Miller did not receive the money for the trials from the National Institutes of Health until five years later. The results of the studies, which inspire optimism, are promised to be reported in the near future. nine0005
Unfortunately, Miller did not receive the money for the trials from the National Institutes of Health until five years later. The results of the studies, which inspire optimism, are promised to be reported in the near future. nine0005  A similar picture is observed in people suffering from clinical depression.
A similar picture is observed in people suffering from clinical depression.  One of the most common symptoms of depression, anhedonia (the inability to enjoy life), is particularly distressing and may be related to the dopamine signaling system. Another reason why Caplitt is particularly interested in studying the nucleus accumbens is that, according to magnetic resonance studies, this area is associated with many other areas of the brain associated with depression. nine0005
One of the most common symptoms of depression, anhedonia (the inability to enjoy life), is particularly distressing and may be related to the dopamine signaling system. Another reason why Caplitt is particularly interested in studying the nucleus accumbens is that, according to magnetic resonance studies, this area is associated with many other areas of the brain associated with depression. nine0005