How does tricyclic antidepressants work
Tricyclic Antidepressants - StatPearls - NCBI Bookshelf
Continuing Education Activity
Tricyclic antidepressants are a class of medications used in the management and treatment of major depressive disorder. This activity reviews the indications, actions, and contraindications for tricyclic antidepressants as a valuable agent in treating major depressive disorder. This activity will highlight the mechanism of action, adverse event profile, monitoring, relevant interactions, off-label uses, and other key elements of tricyclic antidepressant therapy pertinent for interprofessional team members in the management of patients with major depressive disorder and related conditions.
Objectives:
Identify the most common adverse effects associated with tricyclic antidepressant therapy.
Outline the indications of tricyclic antidepressant therapy.
Review the mechanism of action of tricyclic antidepressant therapy.
Summarize the importance of collaboration and communication amongst the interprofessional team to enhance the delivery of care for patients receiving tricyclic antidepressant therapy.
Access free multiple choice questions on this topic.
Indications
Tricyclic antidepressants (TCAs) are a drug class that was first released to the market in 1959 as a pharmacotherapy for major depressive disorder (MDD).[1] Today, TCAs are Food and Drug Administration (FDA) approved to treat various illnesses, depending on the formulation.[2] TCAs that have FDA approval to treat MDD include amitriptyline, amoxapine, doxepin, desipramine, nortriptyline, protriptyline, imipramine, and trimipramine.[3][4] Clomipramine is FDA approved for obsessive-compulsive disorder (OCD) in ages ten and older.[5]
In treating major depressive disorder, TCAs display equivocal efficacy with selective serotonin reuptake inhibitors (SSRIs), but TCAs cause more significant adverse effects due to their anticholinergic activity and a lower threshold for overdose.[6][7] For these reasons, TCAs are typically not used as first-line treatment of MDD.[8] The off-label (non-FDA) uses of TCAs include migraine prophylaxis, OCD, insomnia, anxiety, and chronic pain, especially neuropathic pain conditions such as myofascial pain, diabetic neuropathy, and postherpetic neuralgia.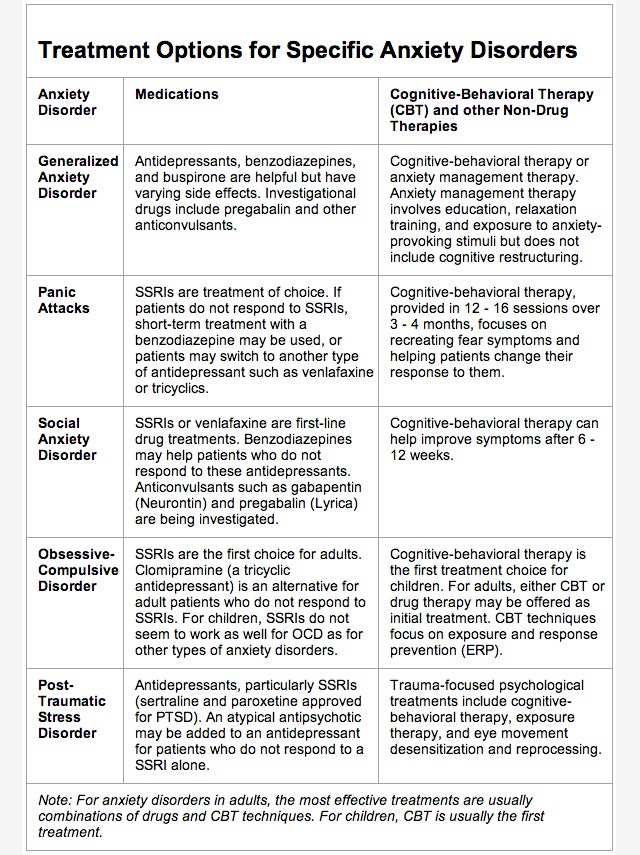 [9][10][11][12][13][14][15] Specifically, for migraine prophylaxis, doxepin and amitriptyline are most commonly used.[16][17] TCAs are also the second-line treatment for fibromyalgia after the failure of preferred treatments, such as pregabalin, duloxetine, and milnacipran.[18][19] Nocturnal enuresis of children is also treatable with TCAs after the failure of first-line therapy desmopressin.[20]
[9][10][11][12][13][14][15] Specifically, for migraine prophylaxis, doxepin and amitriptyline are most commonly used.[16][17] TCAs are also the second-line treatment for fibromyalgia after the failure of preferred treatments, such as pregabalin, duloxetine, and milnacipran.[18][19] Nocturnal enuresis of children is also treatable with TCAs after the failure of first-line therapy desmopressin.[20]
Mechanism of Action
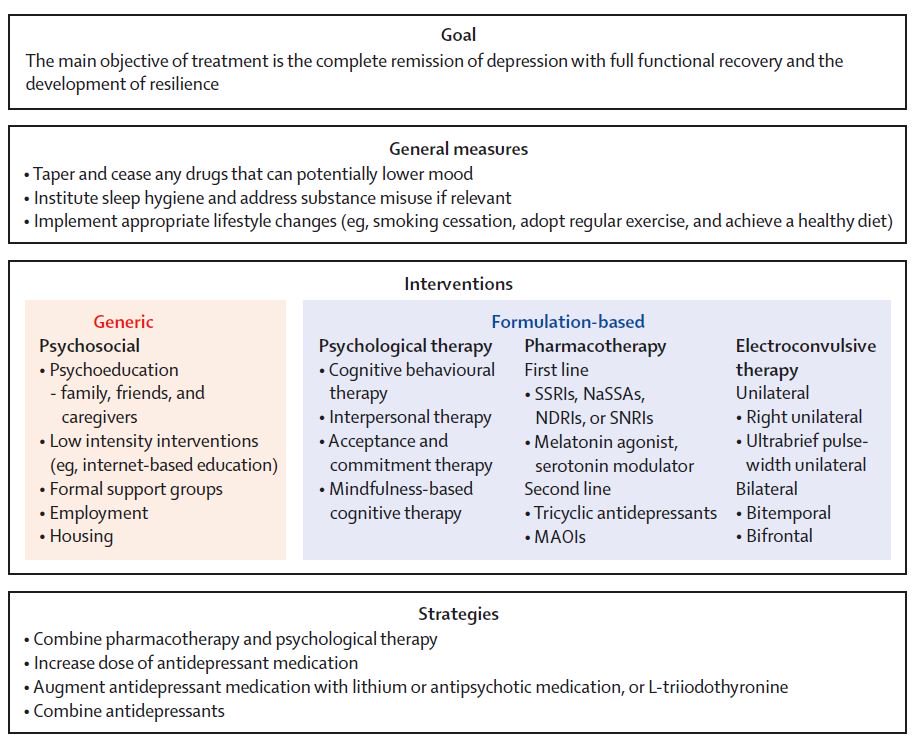
Tricyclic antidepressants act on approximately five different neurotransmitter pathways to achieve their effects. They block the reuptake of serotonin and norepinephrine in presynaptic terminals, which leads to increased concentration of these neurotransmitters in the synaptic cleft. The increased concentrations of norepinephrine and serotonin in the synapse likely contribute to its anti-depressive effect. Additionally, they act as competitive antagonists on post-synaptic alpha cholinergic (alpha1 and alpha2), muscarinic, and histaminergic receptors (h2).[21][22] Its structure greatly influences the affinity of the TCA for each of these receptors.
The chemical structure of a TCA consists of a three-ringed structure with an attached secondary or tertiary amine. Secondary amines include desipramine, nortriptyline, and protriptyline, while tertiary amines consist of amitryptiline, clomipramine, doxepin, imipramine, and trimipramine. Tertiary amines tend to have greater blockage of serotonin reuptake, while secondary amines have greater blockage of norepinephrine uptake.[23] The combination of different amine structures and variations in chemical composition contribute to the multitude of adverse effects seen with TCA usage as these factors affect TCA-receptor affinity and binding.[24]
Administration
TCAs are available in the form of oral tablets, capsules, and solutions. Although intravenous (IV) use of some TCAs, such as clomipramine, has taken place in clinical trials, IV is not a routine route of administration for the administration of TCAs.[25] Topical creams and transdermal patches have been studied for the use of some TCAs, like imipramine and doxepin, as well.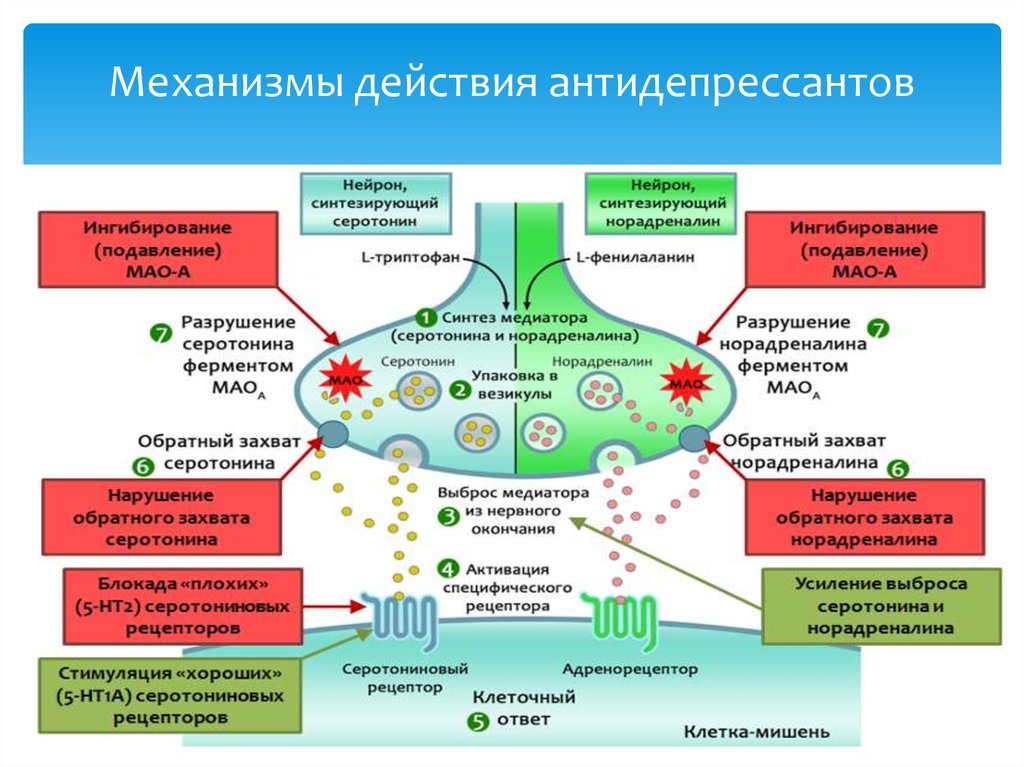 However, oral administration (PO) remains the usual route of administration for TCAs.[26] The exact dosages for each of the different TCA tablets vary; however because TCAs have a high risk of adverse effects, initial dosing is low and is gradually increased based on the level of response.[27] If patients are unresponsive at a low dose, they may respond at higher doses, especially since TCAs have shown increased efficacy at higher dosages compared with high-dose SSRIs.[27] Blood monitoring of TCA concentrations is available; however, there is mixed evidence on its effect on treatment outcomes.[7] In general, patients are given an oral dose of a TCA once a day due to the long half-life and sedative effects of the drug class.[24]
However, oral administration (PO) remains the usual route of administration for TCAs.[26] The exact dosages for each of the different TCA tablets vary; however because TCAs have a high risk of adverse effects, initial dosing is low and is gradually increased based on the level of response.[27] If patients are unresponsive at a low dose, they may respond at higher doses, especially since TCAs have shown increased efficacy at higher dosages compared with high-dose SSRIs.[27] Blood monitoring of TCA concentrations is available; however, there is mixed evidence on its effect on treatment outcomes.[7] In general, patients are given an oral dose of a TCA once a day due to the long half-life and sedative effects of the drug class.[24]
Adverse Effects
TCAs have varying degrees of receptor affinities, leading to several adverse effects. The most common adverse effects include constipation, dizziness, and xerostomia.[28] Due to its blockade of cholinergic receptors, these drugs can lead to blurred vision, constipation, xerostomia, confusion, urinary retention, and tachycardia. [29] Due to the blockade of alpha-1 adrenergic receptors, it can cause orthostatic hypotension and dizziness.[28][29] TCA-induced histamine blockade (h2) may lead to sedation, increased appetite, weight gain, and confusion.[30][28]
[29] Due to the blockade of alpha-1 adrenergic receptors, it can cause orthostatic hypotension and dizziness.[28][29] TCA-induced histamine blockade (h2) may lead to sedation, increased appetite, weight gain, and confusion.[30][28]
TCAs may also cause cardiovascular complications, including arrhythmias, such as QTc prolongation, ventricular fibrillation, and sudden cardiac death in patients with preexisting ischemic heart disease.[31] Therefore, the examination of a patient's cardiac health is important before TCA prescription. there is evidence of TCAs increasing the risk of seizures in those with epilepsy, and use requires caution in this population.[32] TCAs may cause mild liver enzyme elevation; however, acute hepatitis rarely has an association with the use of tricyclic antidepressants.[30][33]
TCA use has been shown to lead to an increased risk of suicidal ideation and behavior in individuals age 24 or less. Therefore, individuals started on TCAs of age 24 or less should be followed closely to assess for thoughts and behaviors related to suicide. [33]
[33]
The use of TCAs during pregnancy has correlated with congenital eye, ear, face, and neck defects.[34] Specifically, clomipramine has associations with more severe neonatal symptoms such as cardiac defects.[35] Therefore, TCAs are not generally considered safe to use during pregnancy. Regarding breastfeeding, all TCAs, except for doxepin, have not been associated with adverse effects from breastfeeding and therefore are regarded as safe.[36] Nortriptyline appears to be the safest for use during breastfeeding due to its non-sedating profile.[35]
Contraindications
Tricyclic antidepressants are contraindicated in several populations and with the concomitant use of various medications. TCAs should not be prescribed if there is a family history of QTc interval prolongation or sudden cardiac death. Hypersensitivity reactions to a TCA drug are considered an absolute contraindication. However, patients with a hypersensitivity reaction to a particular TCA drug may be prescribed a different member of the class with caution. TCAs should not be used concomitantly with monoamine oxidase inhibitors (MAOI), e.g., phenelzine, due to the risk of developing serotonin syndrome.[37] Also, patients must have discontinued MAOI for at least 14 days before starting a TCA medication. Combining TCAs with SSRIs is not advised as a combination of these agents has been shown to increase plasma concentrations of TCAs and the risk of serotonin syndrome.[38][39]
TCAs should not be used concomitantly with monoamine oxidase inhibitors (MAOI), e.g., phenelzine, due to the risk of developing serotonin syndrome.[37] Also, patients must have discontinued MAOI for at least 14 days before starting a TCA medication. Combining TCAs with SSRIs is not advised as a combination of these agents has been shown to increase plasma concentrations of TCAs and the risk of serotonin syndrome.[38][39]
TCA use requires caution in individuals with angle-closure glaucoma as its anticholinergic effects may increase the risk of an acute ocular crisis.[40] TCAs should be used with caution in patients with a history of seizures, as TCAs may lower the seizure threshold, and in patients with urinary retention, as its anticholinergic properties may worsen this symptom.[29][32] Clinicians should generally avoid TCAs in patients with coronary artery disease (CAD). However, CAD is not an absolute contraindication.[41] Because cytochrome P450 enzymes metabolize TCAs in the liver, caution is necessary when prescribing TCAs to patients with hepatic injury.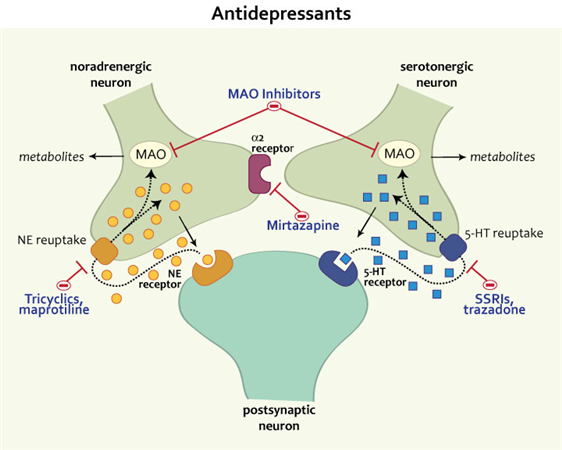 Specifically, among the TCAs, clomipramine has been shown to have the highest rate of drug-induced liver injury, so clomipramine is not preferred in patients with non-optimal liver function.[42]
Specifically, among the TCAs, clomipramine has been shown to have the highest rate of drug-induced liver injury, so clomipramine is not preferred in patients with non-optimal liver function.[42]
Monitoring
Overall, the therapeutic index of TCAs is narrow, and the therapeutic range for each specific TCA is dependent on the drug prescribed. Because of the narrow therapeutic index of TCAs, patients should be monitored closely for symptoms of toxicity, i.e., QRS-widening on electrocardiogram (ECG), tremors, confusion, muscle rigidity, and coma.[23]
All patients starting a TCA need screening for pre-existing cardiac conditions, including prolonged QTc intervals, heart disease, and a family history of arrhythmias. Patients who test positive for pre-existing heart conditions may need additional evaluation by a cardiologist before initiating treatment. Additionally, these patients require regular monitoring for the presence of new cardiac symptoms. Patients with low potassium blood concentrations should have periodical monitoring to reduce the risk of arrhythmias. [31] In patients over the age of 50, obtaining an ECG is recommended.
[31] In patients over the age of 50, obtaining an ECG is recommended.
All patients starting a TCA or presently taking a TCA should be monitored for worsening depressive symptoms or new-onset suicidal thoughts or behaviors. It may be helpful to monitor the blood concentrations of TCAs in non-adherent patients, have decreased tolerability or little response to the drug. However, there is mixed evidence on the effectiveness of blood concentration monitoring on clinical outcomes.[7]
Toxicity
Tricyclic antidepressants (TCAs) generally have a narrow therapeutic index and, therefore, are prone to induce toxicity if intentional overdose occurs. They have demonstrated higher rates of death per one million prescriptions when compared to other antidepressants due to higher rates of suicide by deliberate overdose.[43] Older TCAs, such as desipramine, nortriptyline, and trimipramine, have been shown to induce toxicity at lower doses than newer TCAs, such as amitriptyline.
Overdose symptoms include ECG abnormalities such as QTc prolongation and widened QRS complex, hypotension, seizures, tremors, coma, xerostomia, urinary retention, and respiratory depression. [44] Death from TCAs most commonly occurs due to hypotension or arrhythmias.[45] Treatment of TCA overdose requires the stabilization of the patient and efficiently addressing the acute complications. Activated charcoal may be given for TCA overdose to prevent the absorption of the drug, but activated charcoal administration must be within 2 hours of TCA ingestion.[46] Patients with a prolonged QRS interval should receive sodium bicarbonate to increase the pH of the serum and reduce the concentration of active free TCA medication.[47] In cases of TCA-induced cardiotoxicity that is non-responsive to sodium bicarbonate, lidocaine may be an option as a reversal agent.[45] Patients with TCA-induced seizures should receive benzodiazepines for treatment.[48] Patients that develop hypotension need IV crystalloids, and patients with signs of respiratory compromise should receive respiratory support. Patients with hypotension that is non-responsive to crystalloids should have a trial of norepinephrine.
[44] Death from TCAs most commonly occurs due to hypotension or arrhythmias.[45] Treatment of TCA overdose requires the stabilization of the patient and efficiently addressing the acute complications. Activated charcoal may be given for TCA overdose to prevent the absorption of the drug, but activated charcoal administration must be within 2 hours of TCA ingestion.[46] Patients with a prolonged QRS interval should receive sodium bicarbonate to increase the pH of the serum and reduce the concentration of active free TCA medication.[47] In cases of TCA-induced cardiotoxicity that is non-responsive to sodium bicarbonate, lidocaine may be an option as a reversal agent.[45] Patients with TCA-induced seizures should receive benzodiazepines for treatment.[48] Patients that develop hypotension need IV crystalloids, and patients with signs of respiratory compromise should receive respiratory support. Patients with hypotension that is non-responsive to crystalloids should have a trial of norepinephrine. [49]
[49]
TCAs should not be used concomitantly with monoamine oxidase inhibitors (MAOI) due to the risk of developing serotonin syndrome.[37] Patients with serotonin syndrome present with dilated pupils, hyperreflexia, myoclonus, diarrhea, tremors, and confusion. treatment for serotonin syndrome includes cooling, discontinuation of serotonergic drugs, and cyproheptadine.
Enhancing Healthcare Team Outcomes
Tricyclic antidepressants (TCAs) are a class of antidepressants that are commonly prescribed for off-label use today. Although TCAs may be prescribed for MDD, they are seldom prescribed as a first-line treatment due to their unfavorable adverse effect profile, i.e., anticholinergic, antihistamine, and antiadrenergic effects, and because there are many safer alternatives available, such as SSRIs. However, TCAs may be prescribed for MDD if more conservative antidepressant pharmacotherapy has failed. Regardless of the indications for TCAs, patients who require treatment with TCAs need the involvement of an interprofessional team to help maintain patient safety.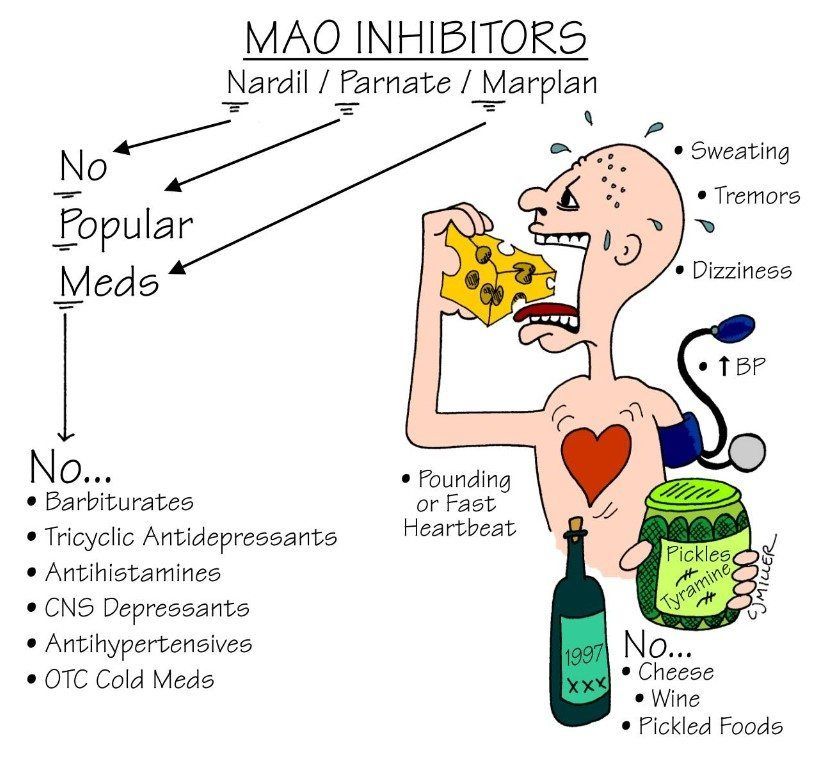
Coordination between a patient's primary care physician, psychiatrist, and possibly cardiologist if cardiac function abnormalities are present is essential for maintaining patient stability and preventing adverse outcomes. Patient monitoring for suicidal intent is necessary to avoid toxicity and overdose.[50] Communication between a cardiologist and a prescribing physician is essential to prevent cardiotoxic outcomes in patients with predisposing risk factors for cardiac dysfunction.[31]
Pharmacists should have involvement in the care of patients prescribed TCAs. Pharmacists can confirm the appropriate dosage and can recognize critical drug-drug interactions. Also, TCAs primarily undergo metabolism in the liver, so pharmacists can reduce the risk of interaction with other drugs by identifying the concomitant drugs that influence hepatic metabolism.[51] Nurses can assist in assessing patient adherence and monitoring patients for adverse effects. Open and frequent communication between all interprofessional healthcare team members should occur to ensure the best possible patient care outcomes. [Level 5]
[Level 5]
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Dopheide JA. Recognizing and treating depression in children and adolescents. Am J Health Syst Pharm. 2006 Feb 01;63(3):233-43. [PubMed: 16434782]
- 2.
Stokes PRA, Jokinen T, Amawi S, Qureshi M, Husain MI, Yatham LN, Strang J, Young AH. Pharmacological Treatment of Mood Disorders and Comorbid Addictions: A Systematic Review and Meta-Analysis: Traitement Pharmacologique des Troubles de L'humeur et des Dépendances Comorbides: Une Revue Systématique et une Méta-Analyse. Can J Psychiatry. 2020 Nov;65(11):749-769. [PMC free article: PMC7564307] [PubMed: 32302221]
- 3.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): Apr 27, 2018. Antidepressant Agents.
 [PubMed: 31643899]
[PubMed: 31643899]- 4.
Boafo A, Greenham S, Sullivan M, Bazaid K, Suntharalingam S, Silbernagel L, Magner K, Robillard R. Medications for sleep disturbance in children and adolescents with depression: a survey of Canadian child and adolescent psychiatrists. Child Adolesc Psychiatry Ment Health. 2020;14:10. [PMC free article: PMC7063733] [PubMed: 32175006]
- 5.
Del Casale A, Sorice S, Padovano A, Simmaco M, Ferracuti S, Lamis DA, Rapinesi C, Sani G, Girardi P, Kotzalidis GD, Pompili M. Psychopharmacological Treatment of Obsessive-Compulsive Disorder (OCD). Curr Neuropharmacol. 2019;17(8):710-736. [PMC free article: PMC7059159] [PubMed: 30101713]
- 6.
Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000 Apr;58(1):19-36. [PubMed: 10760555]
- 7.
Thorstrand C. Clinical features in poisonings by tricyclic antidepressants with special reference to the ECG.
 Acta Med Scand. 1976;199(5):337-44. [PubMed: 5853]
Acta Med Scand. 1976;199(5):337-44. [PubMed: 5853]- 8.
von Wolff A, Hölzel LP, Westphal A, Härter M, Kriston L. Selective serotonin reuptake inhibitors and tricyclic antidepressants in the acute treatment of chronic depression and dysthymia: a systematic review and meta-analysis. J Affect Disord. 2013 Jan 10;144(1-2):7-15. [PubMed: 22963896]
- 9.
Xu XM, Liu Y, Dong MX, Zou DZ, Wei YD. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017 Jun;96(22):e6989. [PMC free article: PMC5459715] [PubMed: 28562550]
- 10.
Clomipramine in the treatment of patients with obsessive-compulsive disorder. The Clomipramine Collaborative Study Group. Arch Gen Psychiatry. 1991 Aug;48(8):730-8. [PubMed: 1883256]
- 11.
Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992 May 07;326(19):1250-6.
 [PubMed: 1560801]
[PubMed: 1560801]- 12.
Hayashida KI, Obata H. Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System. Int J Mol Sci. 2019 Feb 14;20(4) [PMC free article: PMC6412536] [PubMed: 30769838]
- 13.
Pilowsky I, Hallett EC, Bassett DL, Thomas PG, Penhall RK. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982 Oct;14(2):169-179. [PubMed: 6757842]
- 14.
Pal US, Kumar L, Mehta G, Singh N, Singh G, Singh M, Yadav HK. Trends in management of myofacial pain. Natl J Maxillofac Surg. 2014 Jul-Dec;5(2):109-16. [PMC free article: PMC4405950] [PubMed: 25937719]
- 15.
Berger A, Dukes E, Edelsberg J, Stacey B, Oster G. Use of tricyclic antidepressants in older patients with diabetic peripheral neuropathy. Clin J Pain. 2007 Mar-Apr;23(3):251-8. [PubMed: 17314585]
- 16.
Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians.
 Arch Intern Med. 2006 May 08;166(9):1021-6. [PubMed: 16682577]
Arch Intern Med. 2006 May 08;166(9):1021-6. [PubMed: 16682577]- 17.
Punay NC, Couch JR. Antidepressants in the treatment of migraine headache. Curr Pain Headache Rep. 2003 Feb;7(1):51-4. [PubMed: 12525271]
- 18.
Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother. 2015 Jun;16(9):1347-68. [PubMed: 26001183]
- 19.
Häuser W, Bernardy K, Uçeyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA. 2009 Jan 14;301(2):198-209. [PubMed: 19141768]
- 20.
Gepertz S, Nevéus T. Imipramine for therapy resistant enuresis: a retrospective evaluation. J Urol. 2004 Jun;171(6 Pt 2):2607-10; discussion 2609-10. [PubMed: 15118430]
- 21.
Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015 Feb;23(1):1-21.
 [PMC free article: PMC4428540] [PubMed: 25643025]
[PMC free article: PMC4428540] [PubMed: 25643025]- 22.
Richelson E. Antimuscarinic and other receptor-blocking properties of antidepressants. Mayo Clin Proc. 1983 Jan;58(1):40-6. [PubMed: 6130192]
- 23.
Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007 Jul;151(6):737-48. [PMC free article: PMC2014120] [PubMed: 17471183]
- 24.
Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Gründer G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Müller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry. 2011 Sep;44(6):195-235. [PubMed: 21969060]
- 25.
Pallanti S, Hollander E, Bienstock C, Koran L, Leckman J, Marazziti D, Pato M, Stein D, Zohar J.
 , International Treatment Refractory OCD Consortium. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002 Jun;5(2):181-91. [PubMed: 12135542]
, International Treatment Refractory OCD Consortium. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002 Jun;5(2):181-91. [PubMed: 12135542]- 26.
Sandig AG, Campmany AC, Campos FF, Villena MJ, Naveros BC. Transdermal delivery of imipramine and doxepin from newly oil-in-water nanoemulsions for an analgesic and anti-allodynic activity: development, characterization and in vivo evaluation. Colloids Surf B Biointerfaces. 2013 Mar 01;103:558-65. [PubMed: 23261580]
- 27.
Adli M, Baethge C, Heinz A, Langlitz N, Bauer M. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005 Dec;255(6):387-400. [PubMed: 15868067]
- 28.
Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis.
 CMAJ. 1998 Nov 17;159(10):1245-52. [PMC free article: PMC1229819] [PubMed: 9861221]
CMAJ. 1998 Nov 17;159(10):1245-52. [PMC free article: PMC1229819] [PubMed: 9861221]- 29.
Güloglu C, Orak M, Ustündag M, Altunci YA. Analysis of amitriptyline overdose in emergency medicine. Emerg Med J. 2011 Apr;28(4):296-9. [PubMed: 20923818]
- 30.
David DJ, Gourion D. [Antidepressant and tolerance: Determinants and management of major side effects]. Encephale. 2016 Dec;42(6):553-561. [PubMed: 27423475]
- 31.
Fanoe S, Kristensen D, Fink-Jensen A, Jensen HK, Toft E, Nielsen J, Videbech P, Pehrson S, Bundgaard H. Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J. 2014 May 21;35(20):1306-15. [PubMed: 24644307]
- 32.
Johannessen Landmark C, Henning O, Johannessen SI. Proconvulsant effects of antidepressants - What is the current evidence? Epilepsy Behav. 2016 Aug;61:287-291. [PubMed: 26926001]
- 33.
Drug interactions with selective serotonin reuptake inhibitors, especially with other psychotropics.
 Prescrire Int. 2001 Feb;10(51):25-31. [PubMed: 11503857]
Prescrire Int. 2001 Feb;10(51):25-31. [PubMed: 11503857]- 34.
Bérard A, Zhao JP, Sheehy O. Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: an updated analysis of the Quebec Pregnancy Cohort. BMJ Open. 2017 Jan 12;7(1):e013372. [PMC free article: PMC5278249] [PubMed: 28082367]
- 35.
Gentile S. Tricyclic antidepressants in pregnancy and puerperium. Expert Opin Drug Saf. 2014 Feb;13(2):207-25. [PubMed: 24383525]
- 36.
Lanza di Scalea T, Wisner KL. Antidepressant medication use during breastfeeding. Clin Obstet Gynecol. 2009 Sep;52(3):483-97. [PMC free article: PMC2902256] [PubMed: 19661763]
- 37.
Schipper P, Vanmolkot L, Peeters FP. [Combining a classic monoamine oxidase inhibitor with a tricyclic antidepressant in therapy-resistant depression: a case report and literature review]. Tijdschr Psychiatr. 2016;58(12):886-890. [PubMed: 27976787]
- 38.
Dunner DL. Combining antidepressants. Shanghai Arch Psychiatry. 2014 Dec;26(6):363-4. [PMC free article: PMC4311111] [PubMed: 25642112]
- 39.
Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995 Nov;167(5):575-80. [PubMed: 8564311]
- 40.
Lieberman E, Stoudemire A. Use of tricyclic antidepressants in patients with glaucoma. Assessment and appropriate precautions. Psychosomatics. 1987 Mar;28(3):145-8. [PubMed: 3432532]
- 41.
Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000 Jan;108(1):2-8. [PubMed: 11059434]
- 42.
Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, Greil W, Grohmann R, Kasper S. Drug-Induced Liver Injury during Antidepressant Treatment: Results of AMSP, a Drug Surveillance Program.
 Int J Neuropsychopharmacol. 2016 Apr;19(4) [PMC free article: PMC4851269] [PubMed: 26721950]
Int J Neuropsychopharmacol. 2016 Apr;19(4) [PMC free article: PMC4851269] [PubMed: 26721950]- 43.
Henry JA. A fatal toxicity index for antidepressant poisoning. Acta Psychiatr Scand Suppl. 1989;354:37-45. [PubMed: 2589102]
- 44.
Starkey IR, Lawson AA. Poisoning with tricyclic and related antidepressants--a ten-year review. Q J Med. 1980 Winter;49(193):33-49. [PubMed: 6776584]
- 45.
Pentel PR, Benowitz NL. Tricyclic antidepressant poisoning. Management of arrhythmias. Med Toxicol. 1986 Mar-Apr;1(2):101-21. [PubMed: 3784839]
- 46.
Hultén BA, Adams R, Askenasi R, Dallos V, Dawling S, Heath A, Volans G. Activated charcoal in tricyclic antidepressant poisoning. Hum Toxicol. 1988 Jul;7(4):307-10. [PubMed: 3410479]
- 47.
Ramasubbu B, James D, Scurr A, Sandilands EA. Serum alkalinisation is the cornerstone of treatment for amitriptyline poisoning. BMJ Case Rep. 2016 Apr 11;2016:10.1136/bcr-2016-214685.
 [PMC free article: PMC4840635] [PubMed: 27068728]
[PMC free article: PMC4840635] [PubMed: 27068728]- 48.
Woolf AD, Erdman AR, Nelson LS, Caravati EM, Cobaugh DJ, Booze LL, Wax PM, Manoguerra AS, Scharman EJ, Olson KR, Chyka PA, Christianson G, Troutman WG., American Association of Poison Control Centers. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-33. [PubMed: 17453872]
- 49.
Tran TP, Panacek EA, Rhee KJ, Foulke GE. Response to dopamine vs norepinephrine in tricyclic antidepressant-induced hypotension. Acad Emerg Med. 1997 Sep;4(9):864-8. [PubMed: 9305427]
- 50.
Kerr GW, McGuffie AC, Wilkie S. Tricyclic antidepressant overdose: a review. Emerg Med J. 2001 Jul;18(4):236-41. [PMC free article: PMC1725608] [PubMed: 11435353]
- 51.
Rudorfer MV, Potter WZ. Metabolism of tricyclic antidepressants. Cell Mol Neurobiol. 1999 Jun;19(3):373-409. [PubMed: 10319193]
SSRIs, MAOIs, Tricyclics, and More
Written by R. Morgan Griffin
Morgan Griffin
In this Article
- Understanding Antidepressants: Basic Brain Chemistry
- Reuptake Inhibitors: SSRIs, SNRIs, and NDRIs
- Other Antidepressants: Tetracyclics and SARIs
- Older Antidepressants: Tricyclics and MAOIs
- Treatment-Resistant Depression: Getting the Most from Your Antidepressants
If you have treatment-resistant depression, you might have already picked up some of the antidepressant drug lingo -- you know your SSRIs, your SNRIs and your MAOIs. But do you really know how these drugs help?
If you don't, you're not alone. The truth is that even experts aren't completely sure how antidepressants work. There's just a lot we don't know about how the brain functions.
The most important thing you need to know when you’re living with treatment-resistant depression is that antidepressants often can help. To help you understand all your medication options, here are the facts about different types of antidepressants -- along with some tips on how to use them most effectively.
Understanding Antidepressants: Basic Brain Chemistry
If you've read up on antidepressants -- in newspapers and magazines, or on the Web -- you might see depression explained simply as a "chemical imbalance" or a "serotonin deficiency." Unfortunately, it's not that simple. We really don't know what causes depression or how it affects the brain. We don't exactly know how antidepressants improve the symptoms.
That said, many researchers believe that the benefits of antidepressants stem from how they affect certain brain circuits and the chemicals (called neurotransmitters) that pass along signals from one nerve cell to another in the brain. These chemicals include serotonin, dopamine, and norepinephrine. In various ways, different antidepressants seem to affect how these neurotransmitters behave. Here's a rundown of the main types of antidepressants.
Reuptake Inhibitors: SSRIs, SNRIs, and NDRIs
Some of the most commonly prescribed antidepressants are called reuptake inhibitors.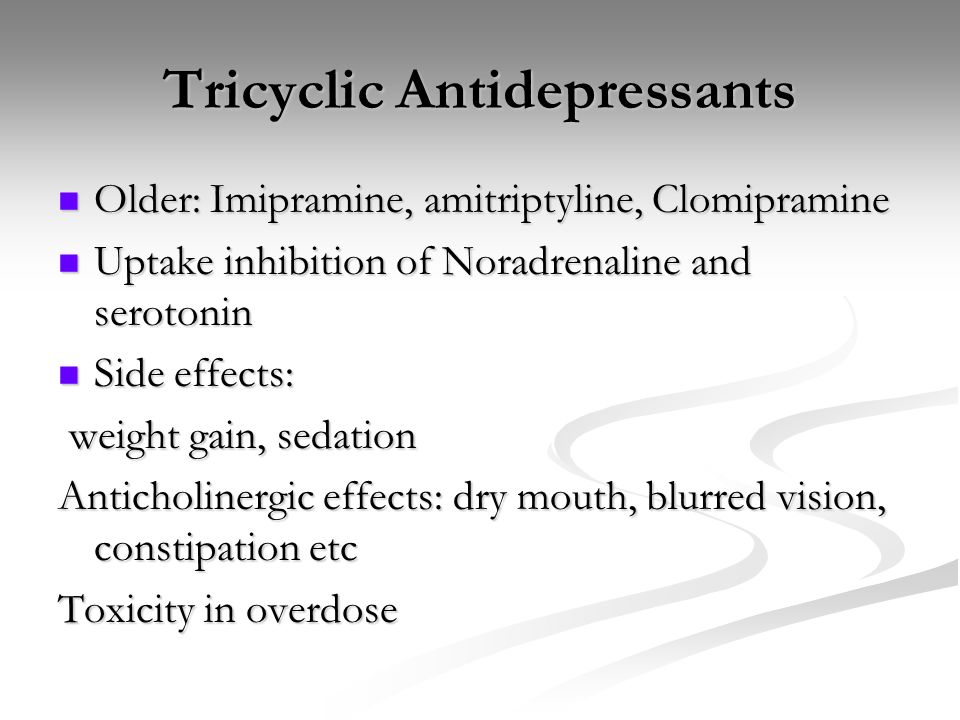 What's reuptake? It's the process in which neurotransmitters are naturally reabsorbed back into nerve cells in the brain after they are released to send messages between nerve cells. A reuptake inhibitor prevents this from happening. Instead of getting reabsorbed, the neurotransmitter stays -- at least temporarily -- in the gap between the nerves, called the synapse.
What's reuptake? It's the process in which neurotransmitters are naturally reabsorbed back into nerve cells in the brain after they are released to send messages between nerve cells. A reuptake inhibitor prevents this from happening. Instead of getting reabsorbed, the neurotransmitter stays -- at least temporarily -- in the gap between the nerves, called the synapse.
What's the benefit? The basic theory goes like this: keeping levels of the neurotransmitters higher could improve communication between the nerve cells -- and that can strengthen circuits in the brain which regulate mood.
Different kinds of reuptake inhibitors target different neurotransmitters. There are three types:
- Selective serotonin reuptake inhibitors (SSRIs) are some of the most commonly prescribed antidepressants available. They include citalopram (Celexa), escitalopram (Lexapro), fluvoxamine (Luvox), paroxetine (Paxil), Prozac, and sertraline (Zoloft). Another drug, olanzapine (Symbyax), is approved by the FDA specifically for treatment-resistant depression.
 It’s a combination of the SSRI antidepressant fluoxetine (Prozac) and another drug approved for bipolar disorder and schizophrenia called olanzapine (Zyprexa). Aripiprazole (Abilify), quetiapine (Seroquel), and brexpiprazole (Rexulti) have been FDA approved as add-on therapy to antidepressants for depression. Plus, doctors often use other drugs in combination for treatment-resistant depression. Also, the drugs vilazodone (Viibryd) and vortioxetine (Trintellix - formelrly called Brintellix) are among the newest antidepressants that affect serotonin. Both drugs affect the serotonin transporter (like an SSRI) but also affect other serotonin receptors to relieve major depression.
It’s a combination of the SSRI antidepressant fluoxetine (Prozac) and another drug approved for bipolar disorder and schizophrenia called olanzapine (Zyprexa). Aripiprazole (Abilify), quetiapine (Seroquel), and brexpiprazole (Rexulti) have been FDA approved as add-on therapy to antidepressants for depression. Plus, doctors often use other drugs in combination for treatment-resistant depression. Also, the drugs vilazodone (Viibryd) and vortioxetine (Trintellix - formelrly called Brintellix) are among the newest antidepressants that affect serotonin. Both drugs affect the serotonin transporter (like an SSRI) but also affect other serotonin receptors to relieve major depression. Serotonin and norepinephrine reuptake inhibitors (SNRIs) are among the newer types of antidepressant. As the name implies, they block the reuptake of both serotonin and norepinephrine. They include duloxetine (Cymbalta), venlafaxine (Effexor), desvenlafaxine ER (Khedezla), levomilnacipran (Fetzima), and desvenlafaxine (Pristiq).
Norepinephrine and dopamine reuptake inhibitors (NDRIs) are another class of reuptake inhibitors, but they're represented by only one drug: bupropion (Wellbutrin). It affects the reuptake of norepinephrine and dopamine.
Other Antidepressants: Tetracyclics and SARIs
- Tetracyclics are another class of antidepressant with drugs such as amoxapine ( Asendin), maprotiline (Ludiomil), and mirtazapine (Remeron). Although it affects neurotransmitters, Remeron doesn't prevent reuptake in the same way. Instead, it seems to stop neurotransmitters from binding with specific receptors on the nerves. Because the norepinephrine and serotonin don't bind to the receptors, they seem to build up in the areas between the nerve cells. As a result, the neurotransmitter levels rise.
- Serotonin antagonist and reuptake inhibitor (SARIs) appear to act in two ways. They prevent the reuptake of serotonin. But they also prevent serotonin particles that are released in a synapse from binding at certain undesired receptors and redirect them instead to other receptors that can help nerve cells within mood circuits function better.
 Examples include nefazodone (Serzone) and trazodone.
Examples include nefazodone (Serzone) and trazodone.
Older Antidepressants: Tricyclics and MAOIs
These drugs were among the first to be used for depression. Although they're effective, they can have serious side effects and can be especially dangerous in overdose. Nowadays, many doctors only turn to these drugs when newer -- and better tolerated -- medicines haven't helped. Tricyclics and MAOIs might not be the best approach for someone who was just diagnosed. But they can sometimes be very helpful for people with treatment-resistant depression, or certain forms of depression (such as depression with anxiety).
- Tricyclic antidepressants (TCAs) include amitriptyline (Elavil), desipramine (Norpramin), imipramine (Tofranil), and nortriptyline (Pamelor). Like reuptake inhibitors, tricyclics seem to block the reabsorption of serotonin and epinephrine back into nerve cells after these chemicals are released into a synapse. Because of the potential side effects, your doctor might periodically check your blood pressure, request an EKG, or recommend occasional blood tests to monitor the level of tricyclics in your system.
 These medicines might not be safe for people with certain heart rhythm problems.
These medicines might not be safe for people with certain heart rhythm problems. - Monoamine oxidase inhibitors (MAOIs) include selegiline (Emsam), isocarboxazid (Marplan), phenelzine (Nardil), and tranylcypromine (Parnate). These drugs seem to work a little differently. Monoamine oxidase is a natural enzyme that breaks down serotonin, epinephrine, and dopamine. MAOIs block the effects of this enzyme. As a result, the levels of those neurotransmitters might get a boost.
The downside is that MAOIs also prevent the body's ability to break down other medicines metabolized by this enzyme (such as Sudafed, or stimulants) -- raising the risk for high blood pressure -- as well as an amino acid called tyrosine, which is found in certain foods like aged meats and cheeses. MAOIs also shouldn't be combined with other medicines that can raise serotonin (such as certain migraine medicines, or other antidepressants), because that can cause a buildup of excessive serotonin (called "serotonin syndrome"), which could be life threatening.
- Nutraceuticals or “medical food” which includes l-methylfolate (Deplin). This is a prescription strength form of folate, also known as one of the essential B vitamins, B9. Depression is often related to low levels of folate which affect the neurotransmitters that control moods and l-methylfolate has proven to be effective in stimulating the production of neurotransmitters.
Treatment-Resistant Depression: Getting the Most from Your Antidepressants
It's worth remembering that a lot of what we think about antidepressants is still speculative. We don't really know if low levels of serotonin or other neurotransmitters "cause" depression, or if raising those levels will resolve it. We don't know enough about brain chemistry to say what's "balanced" or "unbalanced." It's possible that antidepressants have other unknown effects, and that their benefits don't have as much to do with neurotransmitter levels as they might with other effects, such as regulating genes that control nerve cell growth and function.
This might not sound very reassuring, especially if you're relying on antidepressants to help you feel better. But remember: even though experts don't have all the answers about how they work, we do know that they can work. Studies have established that antidepressants can help many people feel better, and that's what's really important.
We also have a lot of research into how people with depression -- including treatment-resistant depression -- can get the most of their medicine.
When taking an antidepressant for treatment-resistant depression, you have to be patient. Some people start an antidepressant and expect that it will work right away. After all, when you drink a few cups of coffee, or a few glasses of wine, you feel it pretty fast. People naturally expect the same kind of instant results with antidepressants.
But that's just not how antidepressants work. No one knows exactly why, but they can take weeks or months before they gain their full effect. When you're taking an antidepressant, it's important to adjust your expectations and to try to be patient.
When you're taking an antidepressant, it's important to adjust your expectations and to try to be patient.
Guide to antidepressants: how do they work?
EN
18 +
Help Found
Treatment
Depression of the Health Guardian Guest
Davydovspid.p.Centr
13 July 2022
20000
Speksenter
Speaddo -sprifs. Unfortunately, for many of our readers, this is a pressing issue. This time, with the help of Maria Danina, Candidate of Psychological Sciences, our authors have compiled a short guide to antidepressants. Their types, principles of operation and device. All basic concepts in one text.
Depression is quite common: according to WHO, more than 300 million people of all ages and genders suffer from it worldwide.
As defined by the American Psychiatric Association, this serious illness can take many forms, vary in severity from person to person, and coexist with other conditions that cause similar symptoms, such as anxiety disorders.
Since the causes of depression are related to disorders of the brain, it is useless to call on patients with depression to "pull themselves together" - its symptoms cannot be overcome by willpower, just as it is impossible to influence the brain by willpower.
“Mood is our subjective experience, that is, what we can become aware of and what we can give an account of. At the heart of any psychological phenomenon are complex and multi-level processes that take place in our nervous system. In particular, neurochemical ones,” explains Maria Danina, senior researcher at the Laboratory of the Scientific Foundations of Psychotherapy, Candidate of Psychological Sciences.
According to her, according to the monoamine theory, three different monoamine neurotransmitters contribute to depressive symptoms. Moreover, neurotransmitters do not "trigger" the disease by themselves, but affect certain processes, which, in fact, lead to depression.
First, it is dopamine, which is involved in the motivation system. A lack of dopamine can lead to anhedonia—that is, the inability to experience pleasure.
A lack of dopamine can lead to anhedonia—that is, the inability to experience pleasure.
Secondly, it is norepinephrine. It is involved in the regulation of our daily activities. Its deficiency is associated with psychomotor retardation (when a person begins to move and speak more slowly than usual).
And thirdly, serotonin. Which is involved in the control of the sensitivity of the pain system, suppresses pain signals and negative emotions.
Lack of serotonin leads to increased pain, negative emotions and anxiety. In addition, due to a lack of serotonin, obsessive states develop, associated with constantly recurring thoughts about the past and the causes of one's current state, which are difficult to get rid of - psychiatrists call this rumination.
“In addition to lowering the level of neurotransmitters, other groups of biologically active substances, such as glucocorticoids, can contribute to depression,” says Maria Danina. - Let's say that the level of the famous "stress hormone" cortisol also increases with depression - and this in turn leads to a lack of dopamine. Monoamine levels can be affected by thyroid hormones and the sex hormones estrogen and progesterone.”
Monoamine levels can be affected by thyroid hormones and the sex hormones estrogen and progesterone.”
However, many factors can trigger clinical depression, such as hereditary predisposition, low self-esteem, difficult life circumstances, severe stress, and chronic diseases. And therefore, we cannot “shift all the blame” only on violations in the brain, emphasizes Maria Danina.
Neurotransmitters and their effects depending on deficiency or excessLife in the "serotonin pit"
An alternative to the monoamine theory, which from the point of view of many scientists can no longer be considered the main one, is the biopsychosocial model of depression. In accordance with it, the development of the disease is influenced not only by biological, but also by psychological factors.
“A person may experience loss (grief) or severe frustration of their needs (stress). As a result, his neurochemistry also changes,” explains Danina. “But this is the so-called normal “mourning process. ” Doctors talk about reactive depression only if, even after months, a person continues to experience all the same unpleasant sensations, and over time, his condition only worsens.
” Doctors talk about reactive depression only if, even after months, a person continues to experience all the same unpleasant sensations, and over time, his condition only worsens.
In some people, however, the functional characteristics of their neurons make them more prone to depression. Such patients "start" the process does not require external circumstances. And their depression has a non-reactive "endogenous" character.
In both cases, in addition to psychotherapy, doctors prescribe antidepressants, that is, drugs that can affect neurotransmitters in the brain, as medical help.
There are five classes in total. Each of which affects the brain in its own way.
1. Selective serotonin reuptake inhibitors (SSRIs).
SSRI drugs increase serotonin levels. This helps to more accurately regulate mood and gradually overcome moderate to severe depression. These drugs do their job by "forbidding" neurons to absorb serotonin from the synaptic gap between neurons. As a result, neurons are able to communicate better.
As a result, neurons are able to communicate better.
SSRIs have fewer side effects than other classes of antidepressants. For example, in case of an overdose, they do not disrupt cardiac conduction and do not lead to convulsions.
However, the side effects are still there and quite serious - from increased suicidal thoughts to sexual dysfunction, drowsiness, dry mouth, dizziness and headache.
In addition, all drugs that increase the level of serotonin in the brain can cause serotonin syndrome - a situation where the neurotransmitter becomes too much.
Serotonin and signal transmission through nerve cells.In serotonin syndrome, a person becomes overexcited, restless, suffers from sweating and increased heart rate. That is why being treated simultaneously with two antidepressants that increase serotonin levels is dangerous to life and health.
2. Selective serotonin and norepinephrine reuptake inhibitors (SNRIs).
SNRIs work in much the same way as SSRIs, with one difference.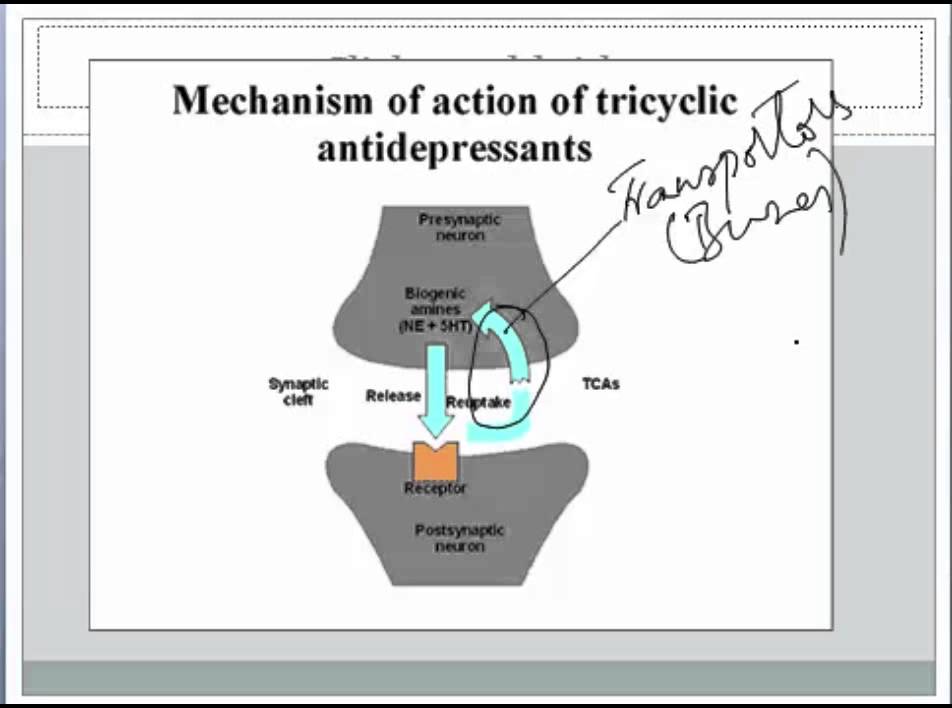 Drugs “forbid” neurons to absorb not only serotonin, but also norepinephrine, a neurotransmitter that is involved in the regulation of emotions and thought processes.
Drugs “forbid” neurons to absorb not only serotonin, but also norepinephrine, a neurotransmitter that is involved in the regulation of emotions and thought processes.
And since norepinephrine works in close conjunction with serotonin and dopamine, SNRIs are thought to increase attention, focus, and improve memory.
The indications and side effects of SNRIs are very similar to those of SSRIs. There are few advantages of this class of drugs over SSRIs, but for some patients they are better suited.
3. Atypical antidepressants.
This class includes drugs with different modes of action that have only one thing in common - these drugs are not like SSRIs and SNRIs.
Although some drugs in this class also "prohibit" neurons from taking up serotonin, like reuptake inhibitors, some of them can act on receptors for other neurotransmitters. This allows you to fine-tune the sensitivity of neurons to one or another substance.
It is worth noting that the components of some atypical antidepressants - say, bupropion - are listed in the note to the list of psychotropic substances subject to control in the Russian Federation. And although this tool is approved by reputable international medical organizations - for example, the US Food and Drug Administration (FDA), it cannot be legally purchased in Russia.
And although this tool is approved by reputable international medical organizations - for example, the US Food and Drug Administration (FDA), it cannot be legally purchased in Russia.
The side effects of this class of drugs depend on the specific drug. For example, some atypical antidepressants make you sleepy, while others, on the contrary, invigorate. To choose the right medicine, the doctor must take into account the peculiarities of the legislation, individual symptoms and the patient's health status.
4. Tricyclic antidepressants (TCAs).
All drugs in this class are thought to increase the levels of norepinephrine and serotonin in the brain, much like SNRIs do.
But TCAs have a very important difference - they are able to act on brain receptors that bind to other substances. In overdose, this leads to severe side effects, such as seizures and complete heart block. And although TCAs are quite effective, drugs from this group today are trying to use as little as possible.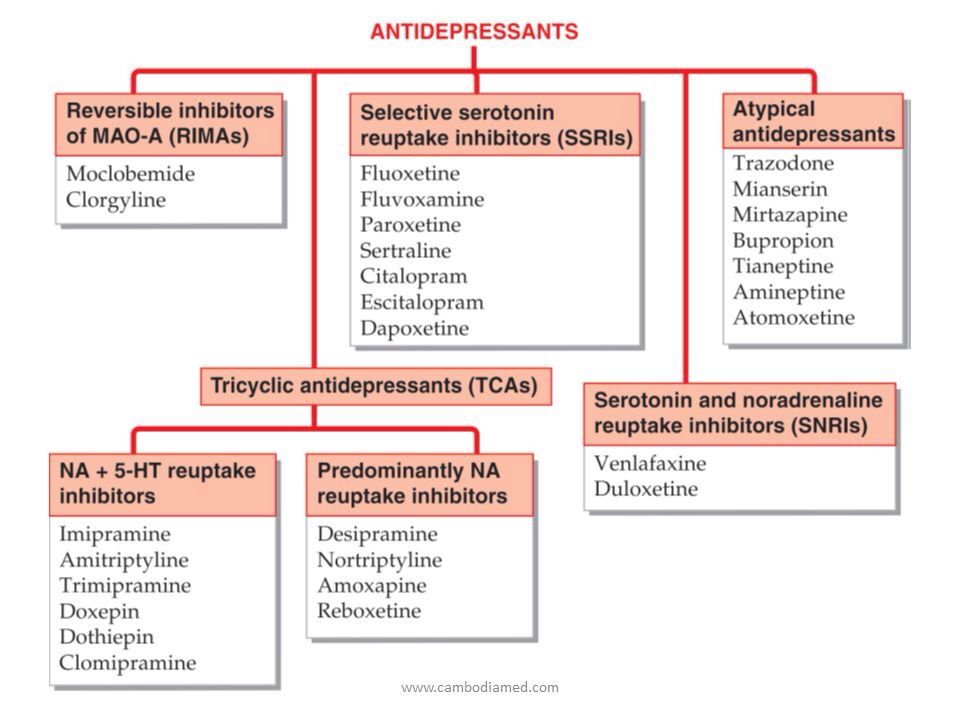
5. Monoamine oxidase inhibitors (MAOIs).
This class of drugs "turns off" monoamine oxidase, an enzyme that breaks down norepinephrine, serotonin, and dopamine in the brain. As a result, neurotransmitters are not destroyed and remain in the synaptic cleft for a long time.
Unfortunately, MAOIs have many side effects, including high blood pressure, weight gain, swelling, sexual dysfunction, and suicidal thoughts. So today, drugs of this class are used only under strict medical supervision and as a last resort - if other antidepressants have not helped.
Is it possible to do without antidepressants?
In medicine, there is the concept of a balance of benefits and harms. This is exactly what the old medical saying about medicines says, which "cure one thing - cripple another." When deciding to prescribe an antidepressant (or any other powerful drug), the doctor always thinks about whether it will benefit a particular patient.
related
Society
"You will burn in paradise!" Is depression really permanent?
- DepressionMedicineRussia
Of course, antidepressants are drugs with serious side effects, the abuse of which will not lead to anything good. However, this does not mean that the life of a person with depression without antidepressants will be any better.
What happens if you stop taking the antidepressants prescribed by your doctor? The biggest risk is the return of depression. Moreover, 80% of patients who abruptly stop taking antidepressants develop a withdrawal syndrome. This condition appears within a few days after discontinuation and can last up to two weeks.
From a medical point of view, it is not too dangerous, but the sensations can be quite unpleasant.
Withdrawal symptoms can be divided into four groups:
- gastrointestinal: nausea, vomiting, diarrhea and abdominal cramps;
- somatic: headaches, lethargy and sweating;
- sleep related: insomnia or excessive sleepiness, nightmares;
- affective: restlessness, agitation and bad mood.

To avoid withdrawal syndrome, the doctor should gradually reduce the dose of antidepressants. This may take several weeks or even several months.
Moreover, not only giving up antidepressants on your own, but also “prescribing” them to yourself without consulting a specialist is harmful.
“If you have symptoms of depression that do not go away for more than two weeks, this is a reason to consult a psychiatrist. The sooner the better, because early treatment gives a better prognosis,” Maria Danina sums up.
Sometimes doctors have to try several different antidepressants to find the right one for you. But it will not be possible to do without a specialist: only during a personal examination, the doctor can confirm the diagnosis and prescribe treatment, she emphasizes.
Where to go for depression?
There are several exits:
- neuropsychiatric dispensary at the place of residence (free of charge),
- psychiatric hospital (free of charge),
- private clinic (extra charge),
- private doctor (for a fee).
Of all the options described for the treatment of depression, the last three options are preferred.
Also, whether you have a confirmed diagnosis of depression, are on medication, or only suspect you have an illness, you may need support - informational or emotional.
At the time of publication of the article for people who suspect they have depression, there are:
- Twenty-four-hour hotline of the Moscow Service for Psychological Assistance to the Population: from a landline for Moscow residents - 051, free of charge; from a mobile phone - 8-495-051 *, only the services of a telecom operator are paid according to the tariff plan.
- Emergency medical and psychological assistance in crisis situations - (495) 205-05-50.
- Emergency psychological help - (495) 575-87-70.
- Hotline of the Psychological Assistance Ministry of Emergencies of Russia - (499) 216-50-50.
Subscribe to the AIDS.Center channel in Yandex. Zen
Zen
let's be friends?
- read
- read
- enter
- read
read also
Society
Anton Kostin "We must act - in spite of anxiety." Interview with the famous St. Petersburg psychiatrist Anton Kostin
- DepressionChildrenMedicinePsychiatryPsychology
Society "Granny of great sex": how a German female pilot created the world's first sex empire
- PoliticsSex
EpidemicAnother year of fighting HIV: AIDS.CENTER summed up
- HIVAIDS2022HIV Trends 2022CharityLife with HIVHealthcareRussiaHarm ReductionEpidemicEpidemiological situation
How do antidepressants work? | Healthy life | Health
Anna Shatokhina
Estimated reading time: 4 minutes
4190
Category: Diseases and treatment
There are several types of depressive states. The most common option is a reactive depressive state as a reaction of the psyche to any negative impact. People self-diagnose this disorder in themselves, calling it "depression", writing off the autumn blues, problems at work and stress, as well as chronic fatigue. In this case, psychotherapy is most effective. Another option is endogenous depression (endogenous - arising from the inside), which is a serious disease and occurs as if "by itself", which is treated under the supervision of doctors and with the appointment of special drugs, since its development is associated with an imbalance of neurotransmitters.
The most common option is a reactive depressive state as a reaction of the psyche to any negative impact. People self-diagnose this disorder in themselves, calling it "depression", writing off the autumn blues, problems at work and stress, as well as chronic fatigue. In this case, psychotherapy is most effective. Another option is endogenous depression (endogenous - arising from the inside), which is a serious disease and occurs as if "by itself", which is treated under the supervision of doctors and with the appointment of special drugs, since its development is associated with an imbalance of neurotransmitters.
Therapy in this case is usually offered, including with the help of antidepressants. But not everyone understands exactly how these drugs work.
“It is believed that the limbic system of the brain is responsible for our emotional background, and the main neurotransmitter that is necessary for its normal functioning is serotonin. Many are familiar with the statement that serotonin is the “hormone of happiness.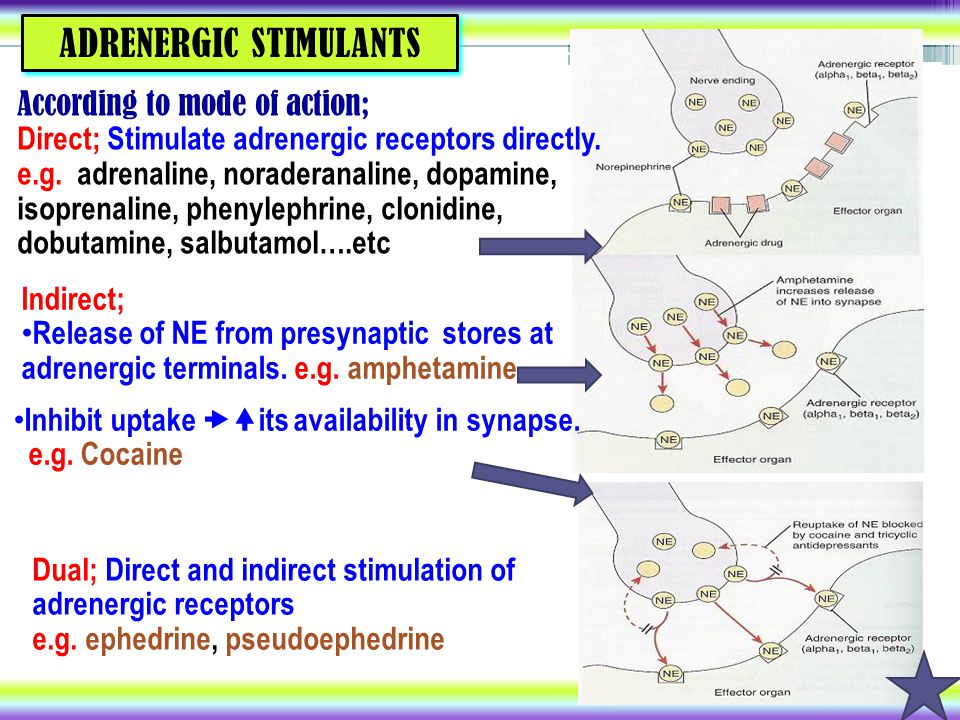 ” It is believed that one of the mechanisms for the development of endogenous depression, which occurs on its own and covers a person, is just a deficiency in the production of serotonin, ”says neurologist, chief freelance neurologist of the Ministry of Health of the Moscow Region Rinat Bogdanov .
” It is believed that one of the mechanisms for the development of endogenous depression, which occurs on its own and covers a person, is just a deficiency in the production of serotonin, ”says neurologist, chief freelance neurologist of the Ministry of Health of the Moscow Region Rinat Bogdanov .
Accordingly, it is necessary to work with this problem. And antidepressants are aimed at eliminating it. As Rinat Bogdanov notes, the mechanism of action of most antidepressants is to increase, through various actions, primarily the amount of serotonin in the synaptic system. Here it is worth understanding in more detail how signals are transmitted from neuron to neuron. “Signal transmission occurs through special contacts called synapses (from Greek this word is translated as “contact”, “connection”, “connection”). The end of one neuron releases a neurotransmitter (“transmitter” in Latin means “intermediary”), for example, the same serotonin, and this neurotransmitter acts on the receptors (translated from Latin “receiving”) of another neuron.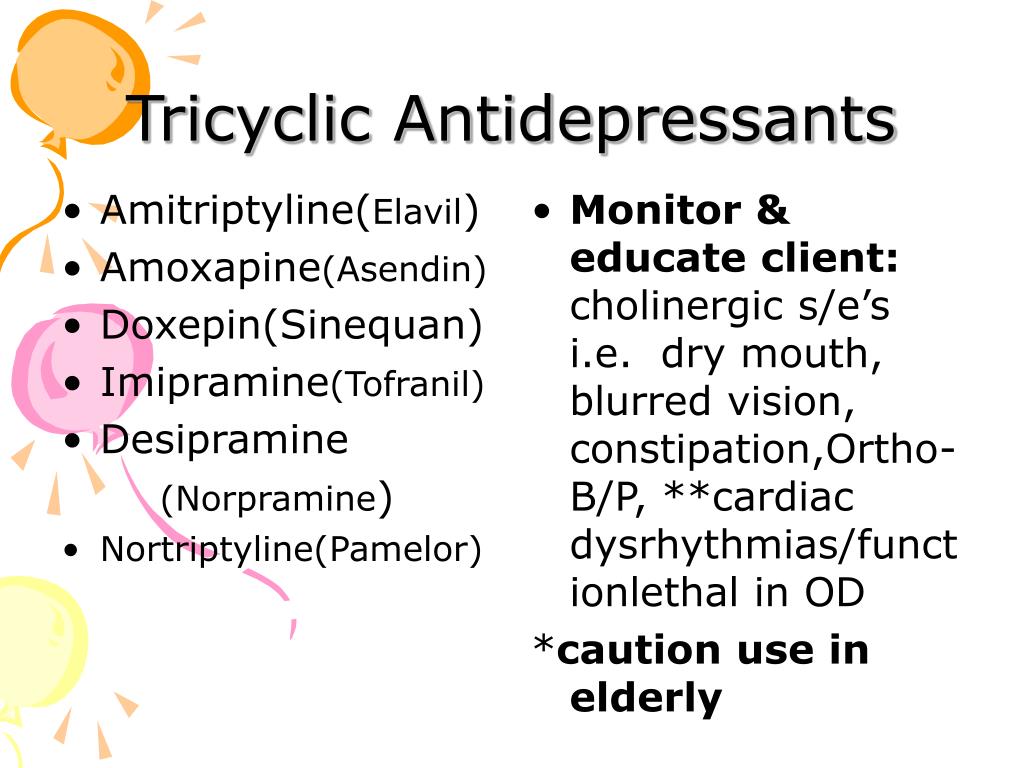 And this gap into which it is secreted is called a synapse, ”the neurologist notes.
And this gap into which it is secreted is called a synapse, ”the neurologist notes.
It is worth noting here, says the doctor, that other neurotransmitters also take part in the work of the emotional sphere: dopamine and norepinephrine. All three neurotransmitters are called monoamines because of their similar chemical composition and can be converted into each other under the influence of different enzymes. And this is where one of the principles of action of antidepressants is hidden.
“Antidepressants increase the amount of these neurotransmitters at the junctions between nerve cells. Normally, under the influence of an electrical signal, the neurotransmitter is released into the synaptic cleft, affects the ending of another neuron, and the excess is absorbed by the enzyme pump back into the ending of the first neuron, thereby preserving the neurotransmitter, and the previous neuron does not need to synthesize it again. Therefore, the first mechanism of action of antidepressants is the blockade of monoamine reuptake, ”says the neurologist.
This is exactly how tricyclic antidepressants work, which are “the most powerful”, as they block the reuptake of all three monoamines (serotonin, noradrenaline and dopamine), but at the same time, as expected, they also have the maximum range of side effects. In this regard, these drugs are used necessarily under the supervision of a physician, usually a psychiatrist, often in stationary conditions, where patients with the most severe depression are admitted.
To reduce side effects, antidepressants have been developed that selectively block the reuptake of one or two neurotransmitters.
“These are selective reuptake inhibitors. According to the mechanism of action, they can be “weaker” than the first group, but their plus is in fewer side effects. This is the most common group of antidepressants. They can block only the reuptake of serotonin (as they are called selective serotonin reuptake inhibitors - SSRIs) or only norepinephrine and serotonin (selective serotonin and norepinephrine reuptake inhibitors - SSRIs).