Highest dosage of ritalin
Ritalin, Concerta (methylphenidate) dosing, indications, interactions, adverse effects, and more
albuterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
aluminum hydroxide decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
amitriptyline, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate will decrease the level or effect of amlodipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
amoxapine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
apomorphine, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
arformoterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
aripiprazole increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
armodafinil increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
asenapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
aspirin/citric acid/sodium bicarbonate decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will increase the level or effect of atomoxetine by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of azilsartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of benazepril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
benzhydrocodone/acetaminophen, methylphenidate. Either increases effects of the other by serotonin levels. Use Caution/Monitor. Coadministration of drugs that affect the serotonergic neurotransmitter system may result in serotonin syndrome. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment.
bromocriptine, methylphenidate.
Either increases effects of the other by pharmacodynamic synergism.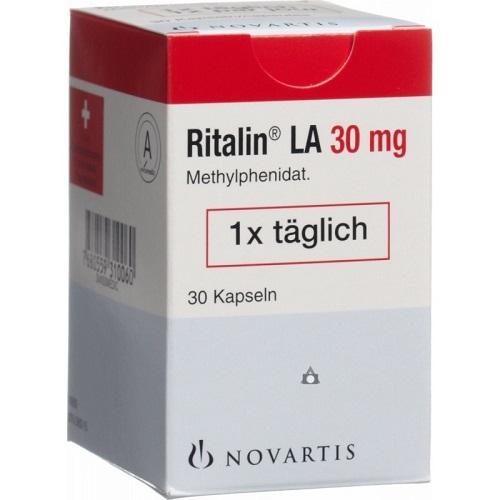 Use Caution/Monitor. Potential for additive CNS stimulation.
Use Caution/Monitor. Potential for additive CNS stimulation.
caffeine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
calcium carbonate decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of candesartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of captopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
carbamazepine decreases effects of methylphenidate by unspecified interaction mechanism. Use Caution/Monitor. Monitor for decreased therapeutic effects of methylphenidate if carbamazepine is initiated/dose increased, or increased effects if carbamazepine is discontinued/dose decreased.
cariprazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
chlorpromazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
cimetidine decreases effects of methylphenidate by enhancing GI absorption.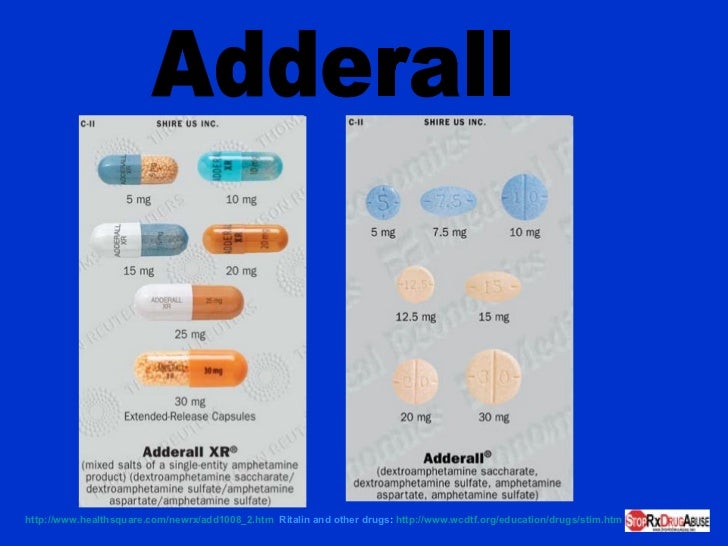 Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of clevidipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
clomipramine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
clozapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
cocaine topical increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
desipramine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
dexfenfluramine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
dexlansoprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
dexmethylphenidate increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
dextroamphetamine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
didanosine will decrease the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Use Caution/Monitor. Interaction specifically associated with Ritalin LA.
methylphenidate will decrease the level or effect of diltiazem by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
dobutamine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
Use Caution/Monitor.
dopamine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
dopexamine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
doxepin, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate will increase the level or effect of dronabinol by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of enalapril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects.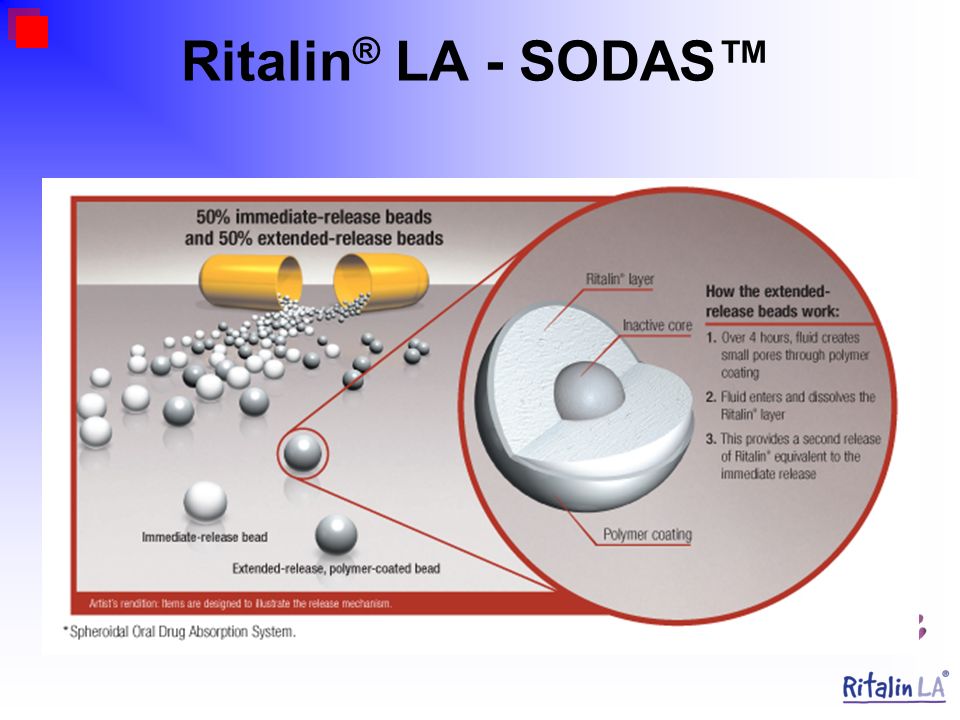 Monitor BP.
Monitor BP.
ephedrine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
epinephrine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate, epinephrine inhaled. Either increases effects of the other by sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
epinephrine racemic and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of eprosartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
esketamine intranasal, methylphenidate.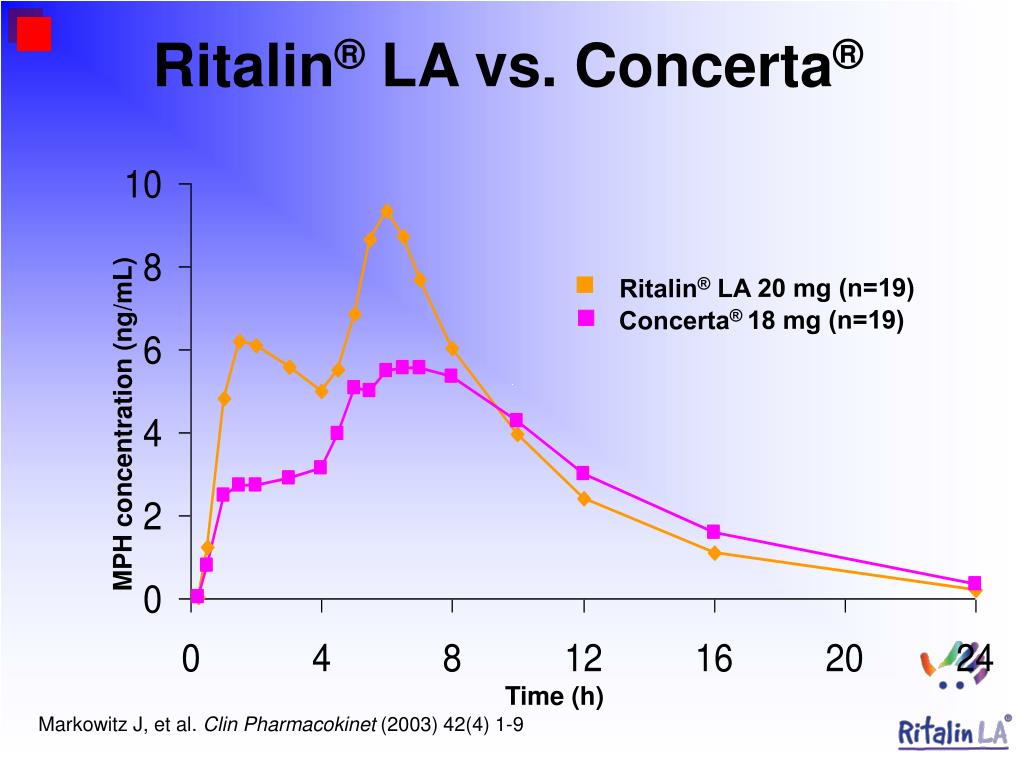 Either increases toxicity of the other by sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor. Closely monitor blood pressure with concomitant use of esketamine nasal with stimulants. .
Either increases toxicity of the other by sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor. Closely monitor blood pressure with concomitant use of esketamine nasal with stimulants. .
esomeprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
famotidine will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Applies only to extended release formulation
famotidine decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of felodipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
fenfluramine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
fluphenazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
only.
fluphenazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
formoterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of fosinopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will increase the level or effect of fosphenytoin by unknown mechanism. Use Caution/Monitor. Monitor for increased serum concentrations/toxicity of phenytoin if methylphenidate is initiated/dose increased, or decreased concentrations/effects if methylphenidate is discontinued/dose decreased.
green tea, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Green tea may include caffeine. Caffeine is a CNS-stimulant and additive effects may be seen when coadministered with other CNS stimulants. Caffeine should be avoided or used cautiously.
Other (see comment). Use Caution/Monitor.
Comment: Green tea may include caffeine. Caffeine is a CNS-stimulant and additive effects may be seen when coadministered with other CNS stimulants. Caffeine should be avoided or used cautiously.
haloperidol increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
hydralazine, methylphenidate. Mechanism: pharmacodynamic antagonism. Use Caution/Monitor. Sympathomimetics can antagonize the activity of some antihypertensive agents.
hydrocodone, methylphenidate.
Either increases effects of the other by serotonin levels. Use Caution/Monitor. Coadministration of drugs that affect the serotonergic neurotransmitter system may result in serotonin syndrome. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment.
ibuprofen/famotidine will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Applies only to extended release formulation
iloperidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
imipramine, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate decreases effects of iohexol by unspecified interaction mechanism. Modify Therapy/Monitor Closely. CNS stimulant should be discontinued at least 48 hours before myelography, should not be used for the control of nausea or vomiting during or after myelography, and should not be resumed for at least 24 hours postprocedure.
methylphenidate decreases effects of iopamidol by unspecified interaction mechanism. Modify Therapy/Monitor Closely. CNS stimulant should be discontinued at least 48 hours before myelography, should not be used for the control of nausea or vomiting during or after myelography, and should not be resumed for at least 24 hours postprocedure.
methylphenidate will decrease the level or effect of irbesartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
isoproterenol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of isradipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
lansoprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
levalbuterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
levodopa, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
lisdexamfetamine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of lisinopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of losartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
loxapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
loxapine inhaled increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
lurasidone, methylphenidate.
Either increases toxicity of the other by Other (see comment). Use Caution/Monitor.
Comment: Potential for additive CNS effects.
lurasidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
magnesium oxide decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
metaproterenol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methamphetamine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
Use Caution/Monitor. Risk of acute hypertensive episode.
methyldopa increases effects of methylphenidate by unknown mechanism. Use Caution/Monitor.
modafinil increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
methylphenidate will decrease the level or effect of moexipril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
molindone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of nadolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nicardipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nifedipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nimodipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of nisoldipine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
nizatidine will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Use Caution/Monitor.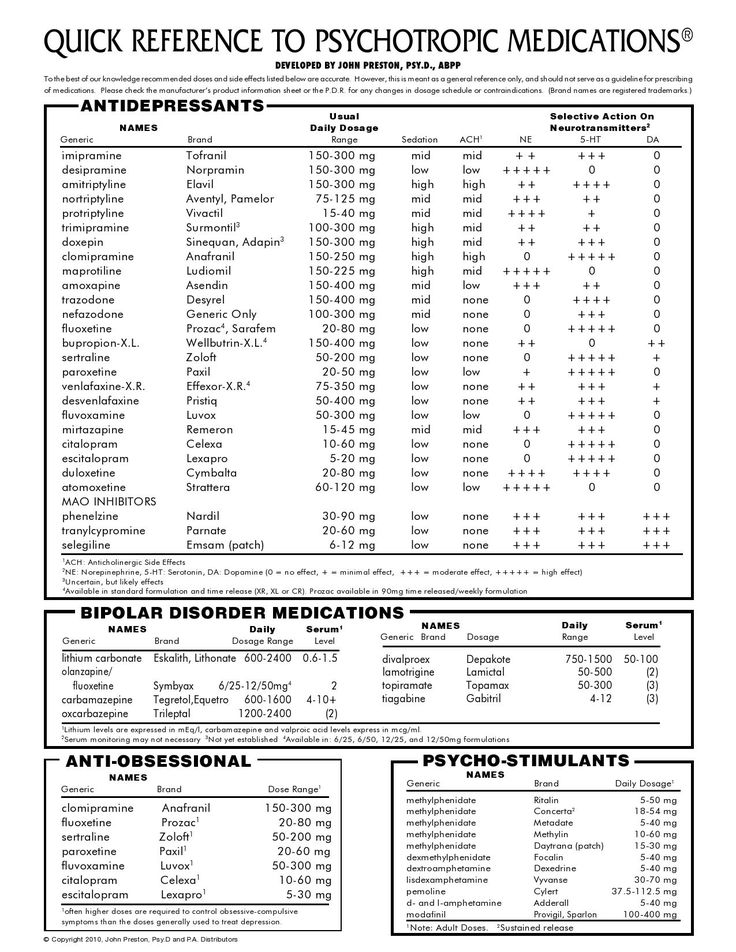 Applies only to extended release formulation
Applies only to extended release formulation
nizatidine decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
norepinephrine and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
nortriptyline, methylphenidate. Other (see comment). Use Caution/Monitor. Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
olanzapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of olmesartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
omeprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
oxytocin increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor.
paliperidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
pantoprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of penbutolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of perindopril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
perphenazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
perphenazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will increase the level or effect of phenobarbital by unknown mechanism. Use Caution/Monitor. Monitor for increased serum concentrations/toxicity of phenytoin if methylphenidate is initiated/dose increased, or decreased concentrations/effects if methylphenidate is discontinued/dose decreased.
methylphenidate will decrease the level or effect of phenoxybenzamine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of phentolamine by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will increase the level or effect of phenytoin by unknown mechanism. Use Caution/Monitor. Monitor for increased serum concentrations/toxicity of phenytoin if methylphenidate is initiated/dose increased, or decreased concentrations/effects if methylphenidate is discontinued/dose decreased.
pimavanserin increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
pimozide increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
pirbuterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
pramipexole, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
methylphenidate will decrease the level or effect of prazosin by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
procarbazine increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
prochlorperazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
promazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
promethazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
methylphenidate will decrease the level or effect of propranolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
protriptyline, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
quetiapine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of quinapril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
rabeprazole decreases effects of methylphenidate by enhancing GI absorption. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Since the characteristics of methylphenidate extended release capsules (Ritalin LA) are pH dependent, coadministration of antacids or acid suppressants could alter the release of methylphenidate. Consider separating the administration of the antacid and the methylphenidate extended-release capsules may be avoided.
methylphenidate will decrease the level or effect of ramipril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
risperidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
ropinirole, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
rotigotine, methylphenidate. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Potential for additive CNS stimulation.
methylphenidate will decrease the level or effect of sacubitril/valsartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
salmeterol and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
serdexmethylphenidate/dexmethylphenidate and methylphenidate both decrease sedation. Use Caution/Monitor.
serdexmethylphenidate/dexmethylphenidate and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
serdexmethylphenidate/dexmethylphenidate increases effects of methylphenidate by pharmacodynamic synergism. Use Caution/Monitor. Risk of acute hypertensive episode.
sodium zirconium cyclosilicate will increase the level or effect of methylphenidate by increasing gastric pH. Applies only to oral form of both agents. Modify Therapy/Monitor Closely. Check specific recommendations for drugs that exhibit pH-dependent solubility that may affect their systemic exposure and efficacy. In general, administer drugs at least 2 hr before or after sodium zirconium cyclosilicate. Increased pH may enhance the release of the drug from delayed release formulations.
methylphenidate and solriamfetol both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
methylphenidate will decrease the level or effect of sotalol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
sufentanil SL, methylphenidate. Either increases effects of the other by serotonin levels. Use Caution/Monitor. Coadministration of drugs that affect the serotonergic neurotransmitter system may result in serotonin syndrome. If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment.
methylphenidate will decrease the level or effect of telmisartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of terazosin by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
terbutaline and methylphenidate both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Use Caution/Monitor.
thioridazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
thiothixene increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
methylphenidate will decrease the level or effect of timolol by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of trandolapril by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate increases toxicity of trazodone by Other (see comment). Modify Therapy/Monitor Closely. Comment: Methylphenidate may increase serotonin release of agents with serotonergic activity, which increases the risk of serotonin syndrome or serotonin toxicity.
trifluoperazine, methylphenidate. Mechanism: unknown. Use Caution/Monitor. Risk of cardiac arrhythmia or sudden death, more likely w/thioridazine than other phenothiazines. Interaction more likely in certain predisposed pts. only.
trifluoperazine increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
trimipramine, methylphenidate. Other (see comment). Use Caution/Monitor.
Comment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.
methylphenidate will decrease the level or effect of valsartan by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate will decrease the level or effect of verapamil by pharmacodynamic antagonism. Use Caution/Monitor. Methylphenidate may diminish antihypertensive effects. Monitor BP.
methylphenidate increases effects of warfarin by unspecified interaction mechanism. Use Caution/Monitor.
ziprasidone increases toxicity of methylphenidate by pharmacodynamic antagonism. Use Caution/Monitor. Closely monitor for signs of altered clinical response to either methylphenidate or an antipsychotic when using these drugs in combination.
Evaluation of Methylphenidate Safety and Maximum-Dose Titration Rationale in Attention-Deficit/Hyperactivity Disorder
1. de Zwaan M, Gruss B, Müller A, et al.. The estimated prevalence and correlates of adult ADHD in a German community sample. Eur Arch Psychiatry Clin Neurosci. 2012;262(1):79-86. doi: 10.1007/s00406-011-0211-9 [PubMed] [CrossRef] [Google Scholar]
2. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490-499. doi: 10.1007/s13311-012-0135-8 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Sawyer MG, Reece CE, Sawyer ACP, Johnson SE, Lawrence D. Has the prevalence of child and adolescent mental disorders in Australia changed between 1998 and 2013 to 2014? J Am Acad Child Adolesc Psychiatry. 2018;57(5):343-350.e5. doi: 10.1016/j.jaac.2018.02.012 [PubMed] [CrossRef] [Google Scholar]
4. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
5. Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73(7):941-950. doi: 10.4088/JCP.11m07529 [PubMed] [CrossRef] [Google Scholar]
6. Biederman J, Quinn D, Weiss M, et al.. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs. 2003;5(12):833-841. doi: 10.2165/00148581-200305120-00006 [PubMed] [CrossRef] [Google Scholar]
7. Arnold LE, Hodgkins P, Caci H, Kahle J, Young S. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS One. 2015;10(2):e0116407. doi: 10.1371/journal.pone.0116407 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Subcommittee on Attention-Deficit/Hyperactivity Disorder Steering Committee on Quality Improvement and Management ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1023-1029. doi: 10.1542/peds.2011-2654 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. J Am Acad Child Adolesc Psychiatry. 1997;36(6):754-763. doi: 10.1097/00004583-199706000-00011 [PubMed] [CrossRef] [Google Scholar]
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666. doi: 10.1542/peds.100.4.662 [PubMed] [CrossRef] [Google Scholar]
11. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237. doi: 10.2165/00023210-200822030-00003 [PubMed] [CrossRef] [Google Scholar]
12. Taylor E, Döpfner M, Sergeant J, et al.. European Clinical Guidelines for Hyperkinetic Disorder–first upgrade. Eur Child Adolesc Psychiatry. 2004;13(suppl 1):I7-I30. doi: 10.1007/s00787-004-1002-x [PubMed] [CrossRef] [Google Scholar]
13. CONCERTA (methylphenidate HCl) extended-release tablets CII. Food and Drug Administration. 2007. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021121s014lbl.pdf. Accessed November 8, 2016.
14. Ritalin hydrochloride methylphenidate hydrochloride tablets USP. Food and Drug Administration. 2007. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/010187s069,018029s040,021284s011lbl.pdf. Accessed November 14, 2016.
15. eTG Complete. Attention deficit hyperactivity disorder. Therapeutic Guidelines Limited. http://online.tg.org.au. Accessed November 14, 2016.
16. The Royal Australian College of General Practitioners; The Pharmaceutical Society of Australia . Australasian Society for Clinical and Experimental Pharmacologists and Toxicologists. Methylphenidate. Australian Medicines Handbook. Rundle Mall, SA: Australian Medicines Handbook Pty Ltd; 2016. [Google Scholar]
17. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [PubMed] [CrossRef] [Google Scholar]
18. Storebø OJ, Ramstad E, Krogh HB, et al.. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev. 2015;(11):CD009885. doi: 10.1002/14651858.CD009885.pub2 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
20. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
21. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
22. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
23. World Health Organization International Classification of Diseases, Ninth Revision (ICD-9). Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
24. World Health Organization International Statistical Classification of Diseases, Tenth Revision (ICD-10). Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [PubMed] [CrossRef] [Google Scholar]
26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8(2):157-159. doi: 10.2307/1164923 [CrossRef] [Google Scholar]
29. Arnold LE, Lindsay RL, Conners CK, et al.. A double-blind, placebo-controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2004;14(4):542-554. doi: 10.1089/cap.2004.14.542 [PubMed] [CrossRef] [Google Scholar]
30. Findling RL, Bukstein OG, Melmed RD, et al.. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(1):149-159. doi: 10.4088/JCP.v69n0120 [PubMed] [CrossRef] [Google Scholar]
31. Findling RL, Quinn D, Hatch SJ, Cameron SJ, DeCory HH, McDowell M. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2006;15(8):450-459. doi: 10.1007/s00787-006-0565-0 [PubMed] [CrossRef] [Google Scholar]
32. Greenhill LL, Findling RL, Swanson JM; ADHD Study Group . A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002;109(3):E39. doi: 10.1542/peds.109.3.e39 [PubMed] [CrossRef] [Google Scholar]
33. Law SF, Schachar RJ. Do typical clinical doses of methylphenidate cause tics in children treated for attention-deficit hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 1999;38(8):944-951. doi: 10.1097/00004583-199908000-00009 [PubMed] [CrossRef] [Google Scholar]
34. Riggs PD, Winhusen T, Davies RD, et al.. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry. 2011;50(9):903-914. doi: 10.1016/j.jaac.2011.06.010 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Simonoff E, Taylor E, Baird G, et al.. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry. 2013;54(5):527-535. doi: 10.1111/j.1469-7610.2012.02569.x [PubMed] [CrossRef] [Google Scholar]
36. Wilens TE, McBurnett K, Bukstein O, et al.. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2006;160(1):82-90. doi: 10.1001/archpedi.160.1.82 [PubMed] [CrossRef] [Google Scholar]
37. Wolraich ML, Greenhill LL, Pelham W, et al.. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):883-892. doi: 10.1542/peds.108.4.883 [PubMed] [CrossRef] [Google Scholar]
38. Alsen S, Resmi H, Ozek H, Tufan AE, Bulbul M, Pekcanlar AA. Plasma norepinephrine and dopamine levels in prepubertal male children with attention-deficit hyperactivity disorder do not change with 8 weeks of methylphenidate treatment. Klinik Psikofarmakol Bülteni. 2015;25(3):259-266. doi: 10.5455/bcp.20150802050349 [CrossRef] [Google Scholar]
39. Arnold LE, Bozzolo DR, Hodgkins P, et al.. Switching from oral extended-release methylphenidate to the methylphenidate transdermal system: continued attention-deficit/hyperactivity disorder symptom control and tolerability after abrupt conversion. Curr Med Res Opin. 2010;26(1):129-137. doi: 10.1185/03007990903437412 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
40. Blader JC, Pliszka SR, Jensen PS, Schooler NR, Kafantaris V. Stimulant-responsive and stimulant-refractory aggressive behavior among children with ADHD. Pediatrics. 2010;126(4):e796-e806. doi: 10.1542/peds.2010-0086 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
41. Charles L, Schain R, Zelniker T. Optimal dosages of methylphenidate for improving the learning and behavior of hyperactive children. J Dev Behav Pediatr. 1981;2(3):78-81. doi: 10.1097/00004703-198109000-00003 [PubMed] [CrossRef] [Google Scholar]
42. Childress AC, Kollins SH, Cutler AJ, Marraffino A, Sikes CR. Efficacy, safety, and tolerability of an extended-release orally disintegrating methylphenidate tablet in children 6-12 years of age with attention-deficit/hyperactivity disorder in the laboratory classroom setting. J Child Adolesc Psychopharmacol. 2017;27(1):66-74. doi: 10.1089/cap.2016.0002 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
43. Cho SC, Kim BN, Cummins TD, Kim JW, Bellgrove MA. Norepinephrine transporter -3081(A/T) and alpha-2A-adrenergic receptor MspI polymorphisms are associated with cardiovascular side effects of OROS-methylphenidate treatment. J Psychopharmacol. 2012;26(3):380-389. doi: 10.1177/0269881111405356 [PubMed] [CrossRef] [Google Scholar]
44. Chou WJ, Chen SJ, Chen YS, et al.. Remission in children and adolescents diagnosed with attention-deficit/hyperactivity disorder via an effective and tolerable titration scheme for osmotic release oral system methylphenidate. J Child Adolesc Psychopharmacol. 2012;22(3):215-225. doi: 10.1089/cap.2011.0006 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Connor DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr (Phila). 2000;39(1):15-25. doi: 10.1177/000992280003900102 [PubMed] [CrossRef] [Google Scholar]
46. Correia Filho AG, Bodanese R, Silva TL, Alvares JP, Aman M, Rohde LA. Comparison of risperidone and methylphenidate for reducing ADHD symptoms in children and adolescents with moderate mental retardation. J Am Acad Child Adolesc Psychiatry. 2005;44(8):748-755. doi: 10.1097/01.chi.0000166986.30592.67 [PubMed] [CrossRef] [Google Scholar]
47. Findling RL, Katic A, Rubin R, Moon E, Civil R, Li Y. A 6-month, open-label, extension study of the tolerability and effectiveness of the methylphenidate transdermal system in adolescents diagnosed with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010;20(5):365-375. doi: 10.1089/cap.2009.0122 [PubMed] [CrossRef] [Google Scholar]
48. Findling RL, Wigal SB, Bukstein OG, et al.. Long-term tolerability of the methylphenidate transdermal system in pediatric attention-deficit/hyperactivity disorder: a multicenter, prospective, 12-month, open-label, uncontrolled, phase III extension of four clinical trials. Clin Ther. 2009;31(8):1844-1855. doi: 10.1016/j.clinthera.2009.08.002 [PubMed] [CrossRef] [Google Scholar]
49. Firestone P, Kelly MJ, Goodman JT, Davey J. Differential effects of parent training and stimulant medication with hyperactives: a progress report. J Am Acad Child Psychiatry. 1981;20(1):135-147. doi: 10.1016/S0002-7138(09)60723-8 [PubMed] [CrossRef] [Google Scholar]
50. Hoare P, Remschmidt H, Medori R, et al.. 12-Month efficacy and safety of OROS MPH in children and adolescents with attention-deficit/hyperactivity disorder switched from MPH. Eur Child Adolesc Psychiatry. 2005;14(6):305-309. doi: 10.1007/s00787-005-0486-3 [PubMed] [CrossRef] [Google Scholar]
51. Kemner JE, Starr HL, Ciccone PE, Hooper-Wood CG, Crockett RS. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther. 2005;22(5):498-512. doi: 10.1007/BF02849870 [PubMed] [CrossRef] [Google Scholar]
52. Kim SW, Lee JH, Lee SH, Hong HJ, Lee MG, Yook K-H. ABCB1 c.2677G>T variation is associated with adverse reactions of OROS-methylphenidate in children and adolescents with ADHD. J Clin Psychopharmacol. 2013;33(4):491-498. doi: 10.1097/JCP.0b013e3182905a8d [PubMed] [CrossRef] [Google Scholar]
53. Kral MC, Lally MD, Boan AD. Effectiveness and side effect profile of stimulant medication for the treatment of attention-deficit/hyperactivity disorder in youth with epilepsy. J Child Adolesc Psychopharmacol. 2017;27(8):735-740. doi: 10.1089/cap.2016.0186 [PubMed] [CrossRef] [Google Scholar]
54. Kronenberger WG, Giauque AL, Lafata DE, Bohnstedt BN, Maxey LE, Dunn DW. Quetiapine addition in methylphenidate treatment-resistant adolescents with comorbid ADHD, conduct/oppositional-defiant disorder, and aggression: a prospective, open-label study. J Child Adolesc Psychopharmacol. 2007;17(3):334-347. doi: 10.1089/cap.2006.0012 [PubMed] [CrossRef] [Google Scholar]
55. Kurlan R, Goetz CG, McDermott MP, et al.; Tourette’s Syndrome Study Group . Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527-536. doi: 10.1212/WNL.58.4.527 [PubMed] [CrossRef] [Google Scholar]
56. Loo SK, Bilder RM, Cho AL, et al.. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1. doi: 10.1016/j.jaac.2016.04.020 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
57. Maayan L, Paykina N, Fried J, Strauss T, Gugga SS, Greenhill L. The open-label treatment of attention-deficit/hyperactivity disorder in 4- and 5-year-old children with beaded methylphenidate. J Child Adolesc Psychopharmacol. 2009;19(2):147-153. doi: 10.1089/cap.2008.053 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
58. McGough JJ, McBurnett K, Bukstein O, et al.. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16(3):351-356. doi: 10.1089/cap.2006.16.351 [PubMed] [CrossRef] [Google Scholar]
59. Na K-S, Lee SI, Hong SD, et al.. Effect of osmotic-release oral system methylphenidate on learning skills in adolescents with attention-deficit/hyperactivity disorder: an open-label study. Int Clin Psychopharmacol. 2013;28(4):184-192. doi: 10.1097/YIC.0b013e3283612509 [PubMed] [CrossRef] [Google Scholar]
60. Newcorn JH, Kratochvil CJ, Allen AJ, et al.; Atomoxetine/Methylphenidate Comparative Study Group . Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721-730. doi: 10.1176/appi.ajp.2007.05091676 [PubMed] [CrossRef] [Google Scholar]
61. Newcorn JH, Stein MA, Cooper KM. Dose-response characteristics in adolescents with attention-deficit/hyperactivity disorder treated with OROS methylphenidate in a 4-week, open-label, dose-titration study. J Child Adolesc Psychopharmacol. 2010;20(3):187-196. doi: 10.1089/cap.2009.0102 [PubMed] [CrossRef] [Google Scholar]
62. Palumbo DR, Sallee FR, Pelham WE Jr, Bukstein OG, Daviss WB, McDermott MP. Clonidine for attention-deficit/hyperactivity disorder: I. efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(2):180-188. doi: 10.1097/chi.0b013e31815d9af7 [PubMed] [CrossRef] [Google Scholar]
63. Purper-Ouakil D, Cortese S, Wohl M, et al.. Temperament and character dimensions associated with clinical characteristics and treatment outcome in attention-deficit/hyperactivity disorder boys. Compr Psychiatry. 2010;51(3):286-292. doi: 10.1016/j.comppsych.2009.08.004 [PubMed] [CrossRef] [Google Scholar]
64. Radziuk AL, Kieling RR, Santos K, Rotert R, Bastos F, Palmini AL. Methylphenidate improves the quality of life of children and adolescents with ADHD and difficult-to-treat epilepsies. Epilepsy Behav. 2015;46:215-220. doi: 10.1016/j.yebeh.2015.02.019 [PubMed] [CrossRef] [Google Scholar]
65. Sayer GR, McGough JJ, Levitt J, et al.. Acute and long-term cardiovascular effects of stimulant, guanfacine, and combination therapy for attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(10):882-888. doi: 10.1089/cap.2015.0264 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
66. Song DH, Choi S, Joung YS, et al.. Titrating optimal dose of osmotic-controlled release oral delivery (OROS)-methylphenidate and its efficacy and safety in Korean children with ADHD: a multisite open labeled study. Psychiatry Investig. 2012;9(3):257-262. doi: 10.4306/pi.2012.9.3.257 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
67. Soutullo C, Banaschewski T, Lecendreux M, et al.. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743-751. doi: 10.1007/s40263-013-0086-6 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
68. Su Y, Li H, Chen Y, et al.. Remission rate and functional outcomes during a 6-month treatment with osmotic-release oral-system methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2015;35(5):525-534. doi: 10.1097/JCP.0000000000000389 [PubMed] [CrossRef] [Google Scholar]
69. Su Y, Yang L, Stein MA, Cao Q, Wang Y. Osmotic release oral system methylphenidate versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in Chinese youth: 8-week comparative efficacy and 1-year follow-up. J Child Adolesc Psychopharmacol. 2016;26(4):362-371. doi: 10.1089/cap.2015.0031 [PubMed] [CrossRef] [Google Scholar]
70. Tucker JD, Suter W, Petibone DM, et al.. Cytogenetic assessment of methylphenidate treatment in pediatric patients treated for attention deficit hyperactivity disorder. Mutat Res. 2009;677(1-2):53-58. doi: 10.1016/j.mrgentox.2009.05.005 [PubMed] [CrossRef] [Google Scholar]
71. Wang Y, Zheng Y, Du Y, et al.. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry. 2007;41(3):222-230. doi: 10.1080/00048670601057767 [PubMed] [CrossRef] [Google Scholar]
72. Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L. Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR™) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized, double-blind study. CNS Drugs. 2015;29(4):331-340. doi: 10.1007/s40263-015-0241-3 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
73. Wilens T, Pelham W, Stein M, et al.. ADHD treatment with once-daily OROS methylphenidate: interim 12-month results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry. 2003;42(4):424-433. doi: 10.1097/01.CHI.0000046814.95464.7D [PubMed] [CrossRef] [Google Scholar]
74. Wilens TE, Boellner SW, López FA, et al.. Varying the wear time of the methylphenidate transdermal system in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(6):700-708. doi: 10.1097/CHI.0b013e31816bffdf [PubMed] [CrossRef] [Google Scholar]
75. Geladé K, Bink M, Janssen TW, van Mourik R, Maras A, Oosterlaan J. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur Child Adolesc Psychiatry. 2017;26(4):457-468. doi: 10.1007/s00787-016-0902-x [PMC free article] [PubMed] [CrossRef] [Google Scholar]
76. Tannock R, Schachar RJ, Carr RP, Logan GD. Dose-response effects of methylphenidate on academic performance and overt behavior in hyperactive children. Pediatrics. 1989;84(4):648-657. [PubMed] [Google Scholar]
77. Horn WF, Ialongo NS, Pascoe JM, et al.. Additive effects of psychostimulants, parent training, and self-control therapy with ADHD children. J Am Acad Child Adolesc Psychiatry. 1991;30(2):233-240. doi: 10.1097/00004583-199103000-00011 [PubMed] [CrossRef] [Google Scholar]
78. Schachar R, Tannock R. Childhood hyperactivity and psychostimulants: a review of extended treatment studies. J Child Adolesc Psychopharmacol. 1993;3(2):81-97. doi: 10.1089/cap.1993.3.81 [PubMed] [CrossRef] [Google Scholar]
79. Stein MA, Sarampote CS, Waldman ID, et al.. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003;112(5):e404. doi: 10.1542/peds.112.5.e404 [PubMed] [CrossRef] [Google Scholar]
Ritalin 10 mg tablets; Virgo: 10 mg; package: ;
lv|en|RU
With this diagnostic code, the condition compensates % of the price
- General information
- Pharmacist
Instructions for use
Before starting treatment, your doctor will perform various tests at each dose change and every six months thereafter, or at each visit, to make sure methylphenidate is still reasonably safe and helpful. These checks will include:
- measure blood pressure and heart rate and record the results in a table each time the dose is changed, and then every six months or at each visit;
- Determining height, weight and appetite and tabulating the results each time the dose is changed and then every six months or at each visit.
- assessment of mental symptoms each time the dose is changed and every six months thereafter or at each visit.
Dose titration
Careful dose titration is required at the start of methylphenidate therapy. Dose titration should begin with the lowest possible dose.
Always take this medicine exactly as your doctor has told you. If you are not sure, talk to your doctor or pharmacist. The usual dose is 20 to 30 mg in 2 to 3 divided doses, but some patients may require a higher or lower dose. The highest recommended daily dose is 60 mg.
If you or your child do not feel better after taking this medicine, your doctor may decide that other treatment is needed. Tell your doctor if your child's condition does not improve after one month of treatment. nine0021
Ritalin 10 mg tablets
Ritalin is used to treat Attention Deficit Hyperactivity Disorder (ADHD) in adolescents and children 6 years of age and older who have not responded well to other non-pharmaceutical drugs.
Ritalin should be used in combination with other treatments as part of an extensive treatment program. An extensive treatment program usually includes psychological, educational and social measures, as well as medication, and aims to stabilize children with ADHD with symptoms that may include chronically short attention span, difficulty concentrating, emotional lability, impulsivity, moderate to severe hyperactivity, medical history. minor neurological signs and abnormal electroencephalography (EEG). Learning may or may not be disrupted. The diagnosis cannot be made on the basis of one or more symptoms. To make an adequate diagnosis, it is necessary to use medical and specialized psychology, education and social resources. nine0003
Ritalin should only be started and used under the supervision of a specialist in behavioral disorders in children and/or adolescents.
Ritalin therapy is not intended for all children with ADHD and the decision to use this medication should be based on a careful assessment of the severity and chronicity of the child's symptoms according to age. Use of Ritalin should always be done as directed, according to licensed indications, and in accordance with prescribing/diagnosis instructions. nine0003
Additional text
Ritalin with food
Taking Ritalin with food can help relieve stomach pain, nausea, or vomiting.
Ritalin with alcohol
You or your child should not drink alcohol while taking this medicine because alcohol can make the side effects of this medicine worse. Please note that some foods and medicines also contain alcohol.
Pregnancy and lactation
Talk to your doctor or pharmacist before taking Ritalin if you or your child has:
- sexually active. Your doctor will discuss contraception with you;
- pregnant or think you are pregnant. Your doctor will decide if you or your daughter should take methylphenidate;
- breastfeeding or planning to breastfeed. You or your daughter should not breastfeed during treatment with Ritalin. The active ingredient in Ritalin can be excreted in breast milk.
Driving and using machines
Dizziness, drowsiness and visual disturbances may occur when taking methylphenidate. If these side effects occur, it may be dangerous to do any dangerous activity, such as driving a car, operating machinery, cycling, or climbing trees, until you are sure that it will not affect you or your child.
Ritalin contains lactose and wheat starch.
Ritalin contains lactose - each tablet contains 40 mg lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine. nine0015 Ritalin also contains wheat starch - each tablet contains 48 mg of wheat starch. This medicine is suitable for people with celiac disease. Should not be used in patients with wheat allergy (other than celiac disease).
- Reimbursable medicines
| Drugs | packaging | Price | There is a surcharge |
The price may vary depending on the prescription and the diagnosis code.
Price may be higher than max. pharmacy price, non-residents or ind. orders.
If the state reimburses 100%, the pharmacy must pay 0.71 euros per prescription.
- Other products
| Drugs | packaging | nine0086 PriceThere is a surcharge |
- Similar drugs
| 222 Drugs | packaging | Price | Compensation |
- Other products nine0011
- Pharmacological action
- Pharmacokinetics
- Readings
- Contraindications
- Pregnancy and breastfeeding
- Dosage and Administration
- Side effects
- Overdose
- Interaction
- Sports medicine
- Classification
Methylphenidate - instructions for use
MethylphenidateInstruction:
Methylphenidate (Ritalin) is a non-amphetamine psychostimulant, norepinephrine-dopamine reuptake inhibitor.
Pharmacokinetics
Half-life (T ½ ) - 2-3 hours.
Indications
Attention deficit hyperactivity disorder, narcolepsy, depression.
Contraindications
Due to the stimulating effect, it is contraindicated in psychosis, severe anxiety, mental stress, agitation. In addition, it is relatively contraindicated in patients with glaucoma, tics, as well as persons with indications of Tourette's syndrome in a family history, there are also contraindications for people suffering from hypertension, vascular diseases, heart disease. Pregnancy, lactation. nine0003
Pregnancy and lactation
FDA fetal category C.
Contraindicated.
Dosage and Administration
Orally (by mouth).
Children over 6 years of age, attention deficit hyperactivity disorder: Initial dose 300 mcg/kg or 2. 5–5 mg twice daily (before breakfast and lunch), increased by 100 mcg/kg/day as needed or 5-10 mg / day with an interval of 1 week; maintenance dose 0.5-1 mg/kg/day; maximum dose 2 mg/kg/day or 60 mg/day. nine0003
Adults, narcolepsy: 10 mg 2-3 times a day, maximum dose 60 mg/day.
Side effects
The most common side effects are anxiety and insomnia; both are dose dependent. Other side effects include allergic reactions, anorexia, nausea, dizziness, headache, depression, dyskinesia, tachycardia, angina, heart rhythm disturbances, abdominal pain, visual disturbances, nervousness, drowsiness. Taking a high dose of methylphenidate can lead to the development of psychosis. With prolonged use of the drug, weight loss is possible. Long-term use in high doses sometimes leads to a delay in the growth of the child. Methylphenidate may be habit-forming. nine0003
Overdose
In case of overdose of the drug, convulsions, hyperthermia, tachycardia, loss of appetite, the occurrence of hallucinations, hyperexcitability, emotional imbalance, mydriasis (severe dilation of the pupils), epileptic seizures and headaches are possible. With an overdose of the drug several times - intense hallucinations comparable to hallucinations from cocaine, arterial hypertension, intracranial hemorrhages, destruction of blood vessels and brain damage. With chronic abuse, methylphenidate can lead to psychosis. nine0003
Interactions
Methylphenidate may slow down the metabolism of coumarin anticoagulants, anticonvulsants (such as phenobarbital, phenytoin or primidone), as well as phenylbutazone and tricyclic antidepressants. Therefore, the doses of these drugs, if they are prescribed together with methylphenidate, should be reduced.
Methylphenidate may reduce the antihypertensive effect of antihypertensive agents.
Sports medicine
Athletes should be made aware that methylphenidate may cause anti-doping rule violations and positive results in doping controls.
Methylphenidate is classified as S6 Stimulants on the WADA Prohibited List.