Gene testing for mental illness
What Can They Tell Me About My Mental Health?
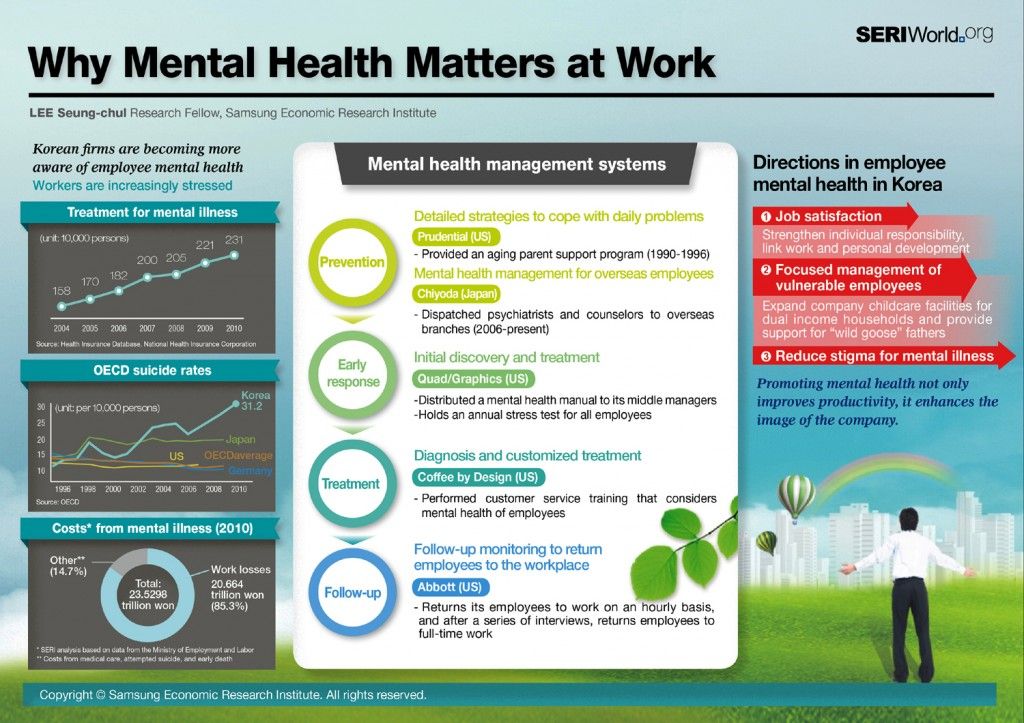
Overview
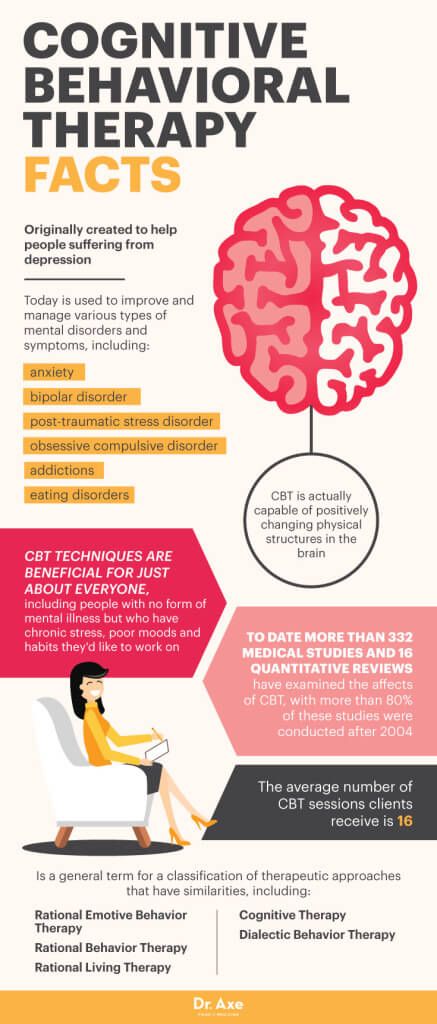
Mental disorders are health conditions that affect how a person thinks, feels, and acts. These disorders can impact a person’s life in significant ways, including how they cope with life events, earn a living, and relate to others.
“Why did this happen?” That is a common question that patients and their families have following a psychotic episode, a suicide attempt, or the diagnosis of a mental disorder.
Research conducted and funded by the National Institute of Mental Health (NIMH) has found that many mental disorders are caused by a combination of biological, environmental, psychological, and genetic factors. In fact, a growing body of research has found that certain genes and gene variations are associated with mental disorders. So, what is the best way to “look at your genes” and determine your personal risk?
Your Family Health History
Your family health history may be one of your best clues for determining your risk of developing a mental disorder and many other common illnesses. Certain mental disorders tend to run in families, and having a close relative with a mental disorder could mean you are at a higher risk.
If a family member has a mental disorder, it does not necessarily mean you will develop one. Many other factors also play a role. But knowing your family’s mental health history can help you determine whether you are at a higher risk for certain disorders, help your doctor to recommend actions for reducing your risk, and enable both you and your doctor to look for early warning signs.
To gain a better understanding of your family health history, it may help to talk to your blood relatives, keep a record of your family history, talk with a mental health professional, or visit a genetic counselor.
Talk to Your Blood Relatives
The first step in creating a family health history is to talk to your blood relatives. The most helpful information comes from “first-degree” relatives—parents, brothers, sisters, and children. Information from “second-degree” relatives—such as nieces, nephews, half-brothers, half-sisters, grandparents, aunts, and uncles—also can be helpful.
Don't worry if you cannot get complete information on every relative. Some people may not want to talk. Others may be unable to remember information accurately. That’s okay. Whatever information you can collect will be helpful.
Keep a Record of Your Family History
Free print and online tools can help you create a family health history. One tool, created by the U.S. Surgeon General, is "My Family Health Portrait". It helps organize the information in your family health history. You can download and print “My Family Health Portrait” and use it to record information about your family’s health. Once you fill in the information, you can keep it for your records, share the completed form with your doctor or other health care provider, or share it with family members.
As a family grows or family members are diagnosed with health conditions, new or updated information can be added. It may take a little time and effort, but this legacy can improve the health of your family for generations to come.
Talk With a Mental Health Professional
If you have mental illness in your family, you may want to consult with a mental health professional who can help you understand risk factors and preventive factors. Asking questions and providing information to your health care provider can improve your care. Talking with your doctor can build trust and may lead to better results, safety, and satisfaction. For tips and information about speaking with your doctor, visit the NIMH Taking Control of Your Mental Health webpage and the Agency for Healthcare Research and Quality webpage for patients and consumers.
Visit a Genetic Counselor
Genetic counseling can give you information about how genetic conditions might affect you or your family. The genetic counselor or other health care professional will collect your personal and family health history. They can use this information to determine how likely it is that you or a family member has a genetic condition. Based on this information, the genetic counselor can help you decide whether a genetic test might be right for you or your relative. Genetic testing often is done before or during pregnancy and soon after the birth of children, or if your doctor suspects you may have a rare disease for which specific genes are known to be the cause.
Genetic testing often is done before or during pregnancy and soon after the birth of children, or if your doctor suspects you may have a rare disease for which specific genes are known to be the cause.
To learn more about genetic counseling, visit the Genetic Counseling FAQ page of the National Human Genome Research Institute website and the Centers for Disease Control and Prevention’s Genetic Counseling webpage.
Your Genes
Genes are segments of DNA found in almost every cell and are passed down from parents to children. Some diseases are caused by genetic mutation(s) or by a permanent change in one or more specific genes.
In other diseases, including many mental disorders, gene variants play a role in increasing or decreasing a person’s risk of developing a disease or condition. Research is advancing our understanding of the role of genetics in mental health. Although there are common genetic variants associated with rare disorders, no gene variant can predict with certainty that a person will develop a mental disorder. In many cases, even the most well-researched genetic variant may contribute to a person’s risk only by very small amounts. Knowing that you have one of these gene variants won’t tell you nearly as much about your risk as your family history can. For more information, visit the website of the National Human Genome Research Institute.
In many cases, even the most well-researched genetic variant may contribute to a person’s risk only by very small amounts. Knowing that you have one of these gene variants won’t tell you nearly as much about your risk as your family history can. For more information, visit the website of the National Human Genome Research Institute.
Genetic Testing Versus Genome Scans
Clinical or Diagnostic Genetic Testing
Doctors order clinical or diagnostic genetic testing for people they think are at high risk of one of the rare diseases for which specific genes are known to be the cause. In clinical or diagnostic testing, doctors search for a single gene or a few genes that research has strongly associated with a specific disease. The results enable patients and their doctors to make informed health care decisions together. There are many different types of genetic tests. Genetic tests may help to:
- Identify gene changes that may increase the risk of developing a disease.
- Diagnose disease.
- Identify gene changes that are implicated in an already diagnosed disease.
- Determine the severity of a disease.
- Guide doctors in deciding on the best medicine or treatment to use for certain individuals, such as during cancer treatment.
If a disease runs in your family, your health care professional can tell you if it’s the kind of illness that can be detected through genetic testing. Your health care professional can help you make decisions about whether to be tested and can help you understand test results and their implications.
Direct-to-Consumer Genome Scans
Direct-to-consumer genome scans are different from clinical or diagnostic genetic testing. For a fee, anyone can mail a saliva sample to companies that sell the scan—without a prescription or a health care provider’s advice. Advertisements say that the company then can provide information, based on gene variations, about a person’s risks of developing specific diseases.
You can learn about the various types of genetic tests and genetic counseling by visiting the National Human Genome Research Institute website.
Can Genetic Testing Help Predict My Risk of Developing a Mental Disorder?
The short answer to this question is no. Currently, genetic tests cannot accurately predict your risk of developing a mental disorder. Although research is underway, scientists don’t yet know all the gene variations that contribute to mental disorders, and those that are known, so far, raise the risk by very small amounts.
One day, genetic research may make it possible to provide a more complete picture of a person’s risk of getting a particular mental disorder or to diagnose it, based on his or her genes. Although recent studies have begun to identify the genetic markers associated with certain mental disorders and eventually may lead to better screening and more personalized treatment, it is still too early to use genetic tests or genome scans to diagnose or treat mental disorders accurately.
NIMH Research on Genetics
NIMH, a part of the National Institutes of Health (NIH), funds and conducts research to help answer important scientific questions about mental illnesses. Through research, NIMH works to determine what is promising, what helps and why, what doesn’t work, and what is safe.
For example, the Genomics Research Branch in the NIMH Division of Neuroscience and Basic Behavioral Science and the Human Genetics Branch in the NIMH Intramural Research Program are currently studying and supporting research on the human genetic variations that contribute to the risk for mood and anxiety disorders, such as bipolar disorder and panic disorder, so that better ways to diagnose and treat these disorders can be developed.
Research investigating these topics will help the field take steps toward better screening and personalized treatment. You can learn more about ongoing research efforts by visiting the NIMH website (search term: Genetics).
Federal Resources
For information about how genes affect your risk for developing a disease or disorder, visit:
Centers for Disease Control and Prevention: Family Health History
National Human Genome Research Institute
MedlinePlus: Genetic Disorders
Participating in Clinical Research
Clinical trials are research studies that look at new ways to prevent, detect, or treat diseases and conditions. Although individuals may benefit from being part of a clinical trial, participants should be aware that the primary purpose of a clinical trial is to gain new scientific knowledge so that others may be better helped in the future.
Researchers at NIMH and around the country conduct clinical trials with patients and healthy volunteers. Talk to your health care provider about clinical trials, their benefits and risks, and whether one is right for you. For more information, visit NIMH’s Clinical Trials webpage.
For more information, visit NIMH’s Clinical Trials webpage.
Finding Help
Behavioral Health Treatment Services Locator
The Substance Abuse and Mental Health Services Administration (SAMHSA) provides this online resource for locating mental health treatment facilities and programs in your state. For additional resources, visit NIMH's Help for Mental Illnesses webpage.
Talking to Your Health Care Provider About Your Mental Health
Communicating well with your doctor or health care provider can improve your care and help you both make good choices about your health. Read our Tips for Talking With Your Health Care Provider to help prepare for and get the most out of your visit. For additional resources, including questions to ask your doctor, visit the Agency for Healthcare Research and Quality website.
Reprints
This publication is in the public domain and may be reproduced or copied without permission from NIMH. Citation of NIMH as a source is appreciated. To learn more about using NIMH publications, please contact the NIMH Information Resource Center at 1-866‑615‑6464, email [email protected], or refer to our reprint guidelines.
Citation of NIMH as a source is appreciated. To learn more about using NIMH publications, please contact the NIMH Information Resource Center at 1-866‑615‑6464, email [email protected], or refer to our reprint guidelines.
For More Information
MedlinePlus (National Library of Medicine) (En español)
ClinicalTrials.gov (En español)
U.S. Department of Health and Human Services
National Institutes of Health
NIH Publication No. 20-MH-4298
Revised 2020
GeneSight DNA Test for Psychiatric & Depression Medication
GeneSight® Tests: Psychotropic and MTHFR
The GeneSight Psychotropic Test
The GeneSight Psychotropic test analyzes how your genes may affect your outcomes with medications commonly prescribed to treat depression, anxiety, ADHD, and other mental health conditions. The GeneSight Psychotropic test provides your clinician with information about which medications may require dose adjustments, may be less likely to work for you or may have an increased risk of side effects based on your genetic makeup.
The GeneSight Psychotropic Test
The GeneSight Psychotropic test analyzes how your genes may affect your outcomes with medications commonly prescribed to treat depression, anxiety, ADHD, and other mental health conditions. The GeneSight Psychotropic test provides your clinician with information about which medications may require dose adjustments, may be less likely to work for you or may have an increased risk of side effects based on your genetic makeup.
Medications on the GeneSight Psychotropic Report
Genes on the GeneSight Psychotropic Report
PK
Pharmacokinetic Genes
Pharmacokinetic genes tell us what the body does to the medication. These genes provide information on how a patient may break down certain medications.
PD
Pharmacodynamic Genes
Pharmacodynamic genes tell us what the medication does to the body. These genes provide information on likelihood of response and/or risk of side effects for certain medications.
+
Additional Genotypes
These genotypes are included on the report for informational purposes only.
The GeneSight® MTHFR Test
MTHFR is an enzyme required to convert folic acid and dietary folate into its active form, which is called l-methylfolate. L-methylfolate plays an important role in making neurotransmitters, such as serotonin, dopamine, and norepinephrine, which help regulate mood. The GeneSight MTHFR test shows whether a person has variation in MTHFR, which would limit their ability to create l-methylfolate.
- GeneSight Psychotropic
-
The GeneSight Psychotropic Test
The GeneSight Psychotropic test analyzes how your genes may affect your outcomes with medications commonly prescribed to treat depression, anxiety, ADHD, and other mental health conditions. The GeneSight Psychotropic test provides your clinician with information about which medications may require dose adjustments, may be less likely to work for you or may have an increased risk of side effects based on your genetic makeup.
The GeneSight Psychotropic Test
The GeneSight Psychotropic test analyzes how your genes may affect your outcomes with medications commonly prescribed to treat depression, anxiety, ADHD, and other mental health conditions. The GeneSight Psychotropic test provides your clinician with information about which medications may require dose adjustments, may be less likely to work for you or may have an increased risk of side effects based on your genetic makeup.
Medications on the GeneSight Psychotropic Report
Genes on the GeneSight Psychotropic Report
PK
Pharmacokinetic Genes
Pharmacokinetic genes tell us what the body does to the medication. These genes provide information on how a patient may break down certain medications.
PD
Pharmacodynamic Genes
Pharmacodynamic genes tell us what the medication does to the body. These genes provide information on likelihood of response and/or risk of side effects for certain medications.
+
Additional Genotypes
These genotypes are included on the report for informational purposes only.
- GeneSight MTHFR
-
The GeneSight® MTHFR Test
MTHFR is an enzyme required to convert folic acid and dietary folate into its active form, which is called l-methylfolate. L-methylfolate plays an important role in making neurotransmitters, such as serotonin, dopamine, and norepinephrine, which help regulate mood. The GeneSight MTHFR test shows whether a person has variation in MTHFR, which would limit their ability to create l-methylfolate.
How does it work?
The GeneSight test must be ordered by your doctor or nurse practitioner. The test is a simple cheek swab taken in your healthcare provider’s office or can be sent by your doctor to be taken in the convenience of your home.
Step 1
Your doctor collects a DNA sample by painlessly swabbing the inside of your cheek OR you can collect the sample at home using our patient collection kit.
Step 2
The sample is sent to our lab for analysis.
Step 3
After we receive your sample, your doctor will typically get test results in about 2 days.
Step 4
Your doctor can contact our Medical Affairs team for a consultation. Your doctor can then review the results with you on your next visit.
How does it work?
The GeneSight test must be ordered by your doctor or nurse practitioner. The test is a simple cheek swab taken in your healthcare provider’s office or can be sent by your doctor to be taken in the convenience of your home.
Step 1
Your doctor collects a DNA sample by painlessly swabbing the inside of your cheek OR you can collect the sample at home using our patient collection kit.
Step 2
The sample is sent to our lab for analysis.
Step 3
After we receive your sample, your doctor will typically get test results about 2 days.
Step 4
Your doctor can contact our Medical Affairs team for a consultation. Your doctor can then review the results with you on your next visit.
Educational Resources
16
Understanding the GeneSight test algorithm The GeneSight test uses a unique combinatorial algorithm, which evaluates how variations in multiple genes may influence an individual’s outcomes with certain medications. The algorithm…
Read More
It is more common than you might think for women to suffer from major depressive disorder. However, depression can and should be treated. Please view this infographic to learn more…
Read More
By identifying the presence of the gene variations in HLA-B*1502 and HLA-A*3101, the GeneSight® Psychotropic pharmacogenomic test can predict if a patient may be at an increased risk of severe…
Read More
Genes and madness: genetic testing in psychiatry
Genes and madness: genetic testing in psychiatryContents
Contents
- Origin of abnormality
- Compatibility check
- Many knowledge - many sorrows? nine0008
August 25, 2017
Popular science competition "Bio/mol/text" -2017
Overview
Genes are the foundation on which our entire organism is built. Changes in the foundation, its defects can lead to serious consequences, for example, to mental disorders.
Changes in the foundation, its defects can lead to serious consequences, for example, to mental disorders.
The genetics of consciousness
-
Author
- Viktor Lebedev
-
Editors
- Vera Bashmakova
- Andrey Panov
Topics
- "Bio/mol/text"-2017
- Genetics nine0009
- Medicine
- Psychogenetics
Bio/mol/text contest entry: Psychiatry has been aloof from all major branches of medicine for many years. While traumatologists took x-rays and therapists studied blood tests, psychiatrists simply talked to their patients and watched them. Now genetics with its modern technologies comes to the aid of psychiatrists. nine0003
While traumatologists took x-rays and therapists studied blood tests, psychiatrists simply talked to their patients and watched them. Now genetics with its modern technologies comes to the aid of psychiatrists. nine0003
This work was published in the nomination "Biomedicine today and tomorrow" of the competition "bio/mol/text"-2017.
The general sponsor of the competition is the Diaem company: the largest supplier of equipment, reagents and consumables for biological research and production.
The sponsor of the audience award and the partner of the nomination "Biomedicine Today and Tomorrow" was the company "Invitro".
"Book" sponsor of the competition - "Alpina non-fiction"
Psychiatry has always lacked objectivity. A few decades ago, a patient might have had an electroencephalogram or pneumoventriculography to rule out a neurological disorder. And if it was confirmed, then the patient passed into the hands of neurosurgeons or neurologists. Psychiatrists continued to work with those patients in whom nothing significant could be found.
Psychiatrists continued to work with those patients in whom nothing significant could be found.
With the increase and complexity of the arsenal of medical research, psychiatry seems to have begun to claim its use on a regular basis, but in reality this is not so. For example, we know that the progression of Alzheimer's disease [1] is accompanied by a decrease in the volume of the hippocampus, the brain regions associated with memory [2]. It is also known that treatment with anti-dementia drugs can slow down the loss of hippocampal substance [3]. The question arises: how often does a patient with dementia undergo an MRI of the brain with the calculation of the volume of the hippocampus? The answer lies on the surface: almost never. nine0003
Mental illness is not taken from scratch. It is often possible to identify a provoking social factor in the development of a disorder, but one should not forget about biological factors. Each of us has genes on the basis of which the complex life of our cells, including neurons, is built. In order to understand how applicable to reality genetic testing in psychiatry can be, it is necessary to evaluate its effectiveness and usefulness before its introduction into widespread practice. We need answers to a few questions. Their approximate list might look like this:
In order to understand how applicable to reality genetic testing in psychiatry can be, it is necessary to evaluate its effectiveness and usefulness before its introduction into widespread practice. We need answers to a few questions. Their approximate list might look like this:
- Do genetic factors influence the development of mental disorders? Psychiatric disorders is a large group of diseases that includes affective pathologies (depression, bipolar affective disorder [BAD], anxiety disorders), diseases with psychotic symptoms (schizophrenia, confusional states) and cognitive impairment (mental retardation, dementia). Obviously, for each disease, the genetic contribution will be different. For this reason, it is necessary to understand under what pathologies it will be maximum. nine0009
- Can these factors be inherited, that is, pass from generation to generation? Through genetic research, we can understand the origin of genetic disorders in mental disorders.
 Were they passed on from parents, grandparents? Or did the observed changes occur in the patient himself (mutations de novo )? Only extensive fundamental research will help us find answers to these questions.
Were they passed on from parents, grandparents? Or did the observed changes occur in the patient himself (mutations de novo )? Only extensive fundamental research will help us find answers to these questions. - Is it possible to single out a specific gene or group of genes that have a noticeable effect on the development of pathology? nine0025 The search for scientists leads to different results. They can find a specific gene responsible for the development of a disease, or they can find several genes that affect the disease itself or individual symptoms.
- Can we find some genetic factors that determine the effect of antipsychotics, antidepressants and other drugs in mental disorders? Pharmacogenetic testing is the determination of genetic factors associated with the characteristics of drug metabolism, the development of side effects when taking it. Pharmacogenetic testing can be useful in predicting side effects and patient response to a drug.
 nine0009
nine0009 - Is it ethically reasonable to conduct genetic testing for mental disorders? The data obtained by scientists can be interesting, and the way they are obtained is extremely exciting, but it is important for us to evaluate the usefulness and applicability of this information. We cannot do research just for the sake of knowledge; it is important for us that they are cost-effective and do not harm the patient and his family.
The list can be supplemented depending on the circumstances, but the general vector of reflection is clear. The proposed study must be informative, and its application must be economically justified and morally acceptable. If we can combine these factors together, then genetic testing for psychiatric disorders will make sense. nine0003
The origin of the abnormality
Basic genetic research in mental disorders can be of considerable benefit. Molecular methods will help in the classification of mental disorders, in clarifying their relationships, just as they have been useful in determining and clarifying the degree of relatedness in plants, animals and microorganisms. The cost of genetic research is gradually decreasing [4], and their availability for the average user and the healthcare system as a whole is growing (Fig. 1). This means that extensive genetic research will become more and more part of the daily practice of researchers and physicians. nine0003
The cost of genetic research is gradually decreasing [4], and their availability for the average user and the healthcare system as a whole is growing (Fig. 1). This means that extensive genetic research will become more and more part of the daily practice of researchers and physicians. nine0003
Figure 1. The cost of genome research has dropped significantly over the past 15 years, outstripping even the pace of Moore's Law.
Diagnosis of mental disorders is based on the patient's complaints and the results of the examination, and not on the data of instrumental studies. The RDoC project ( Research Domain Criteria ) is currently being implemented in the United States, which aims to identify links between specific genetic variants and features of the functioning of a normal brain and a brain affected by a mental disorder [5]. The accumulation of data on this project may lead to a change in the classification of mental disorders, approaches to their diagnosis and treatment. nine0003
nine0003
Currently, genetic testing methods for psychiatric disorders are mainly limited to looking for chromosomal abnormalities (such as Down's syndrome) or identifying monogenic diseases (such as gangliosidosis) (Fig. 2).
Figure 2. Karyotype of a patient with Down syndrome. Circled in red is an additional 21st chromosome (trisomy on the 21st chromosome).
site pinterest.com
These disorders in the DNA structure have been well known for decades, and their detection has become part of the usual medical practice. The problem with mental disorders is that for the vast majority of them, it is impossible to find a specific gene responsible for the development of the disease. Mental disorders are polygenic diseases, the development of which is associated with a violation of the function of several genes at once, as well as changes in the network of their interaction. In addition, a significant part of the cases of the disease, for example, in schizophrenia, is associated with the occurrence of mutations de novo , which are not so easy to determine [6].
This leads to the fact that in the field of genetic research, new methods appear that allow a fresh look at this component of pathogenesis [7]:
- part of DNA that codes for proteins. Since only 1% of the entire nuclear DNA sequence is used to encode proteins, this approach is faster and cheaper than whole genome sequencing. nine0009
- Whole genome sequencing ( whole genome sequencing ) studies not only the coding sequence of nuclear DNA, but also promoter regions, enhancers, and mitochondrial DNA. This method provides a huge amount of information, but its usefulness in each case is evaluated differently.
- RNA sequencing ( RNA-seq ) evaluates the structure of messenger RNA, which is not a direct copy of the coding DNA. This is the advantage of the method: it is able to evaluate not the genetic sequence itself, but how it is embodied in the course of the cell. nine0009
In addition to these methods, it is possible to study proteins that function inside nerve cells and their interactions. Transcriptome analysis is promising for studying the genetics of mental disorders. A transcriptome is the totality of all RNA that is produced in a cell. Thanks to their study, we will find out which proteins, in what variants and in what quantity are produced by the cell. Alternative splicing occurs in the brain more often than in other organs [8], so the DNA sequence itself is not able to give us enough information about which proteins are synthesized based on it. nine0003
Transcriptome analysis is promising for studying the genetics of mental disorders. A transcriptome is the totality of all RNA that is produced in a cell. Thanks to their study, we will find out which proteins, in what variants and in what quantity are produced by the cell. Alternative splicing occurs in the brain more often than in other organs [8], so the DNA sequence itself is not able to give us enough information about which proteins are synthesized based on it. nine0003
In turn, the sum of genetic disorders can give more than each of them separately. Such network interactions are already known in schizophrenia [9] and late-onset Alzheimer's disease [10]. An extensive study in 2014 found about 4,000 genes associated with the occurrence of autism spectrum disorders, attention deficit hyperactivity disorder (ADHD), schizophrenia, and X-linked intellectual development disorders [11]. From a number of these genes, 435 of the most significant were identified, which contained a total of 789nonsynonymous single nucleotide substitutions (SNPs). As further analysis showed, the detected genetic variants could affect a number of important processes inside the nerve cell (Fig. 3). Similar "overlaps" have been found in other studies. For example, a number of identical genes are involved in the development of borderline personality disorder and bipolar affective disorder, for example, DPYD and PKP4 [12]. In addition, when calculating the polygenic risk of developing some mental disorders, their genetic proximity was found (Fig. 4). nine0003
As further analysis showed, the detected genetic variants could affect a number of important processes inside the nerve cell (Fig. 3). Similar "overlaps" have been found in other studies. For example, a number of identical genes are involved in the development of borderline personality disorder and bipolar affective disorder, for example, DPYD and PKP4 [12]. In addition, when calculating the polygenic risk of developing some mental disorders, their genetic proximity was found (Fig. 4). nine0003
Figure 3. A variety of mental disorders have common molecular bases at the protein level. Presents 13 protein modules involved in key processes within neurons. Modules with significant differences in the frequency of primary candidate genes are indicated with an asterisk. Lines with numbers represent protein interactions between modules. Legend: ASD - Autism Spectrum Disorder, ADHD - Attention Deficit Hyperactivity Disorder, SZ - schizophrenia, XLID - X-linked disorders of intellectual development.
To see the picture in full size, click on it.
[11], drawing adapted
Figure 4. When calculating the polygenic risk of bipolar affective disorder, schizophrenia and depression, it was found that some of the genes responsible for the development of one disease are involved in the pathogenesis of other mental disorders. Legend: Mean standardized PRS - average standardized polygenic risk score, BOR - borderline personality disorder, BIP - bipolar affective disorder, SCZ - schizophrenia, MDD - depression.
Departure from the search for specific genes leads to the search for links between endophenotypes in schizophrenia and changes in large areas of the genome [13]. An endophenotype is a set of behavioral or physiological traits that is stable and stably associated with specific changes in genetic data. At the same time, the signs themselves do not reach the level of a symptom. Most likely, the term "feature" fits them. To endophenotypes, for example, scientists include impaired recognition of emotions and problems with how the patient controls his movements. Problems with recognizing emotions turned out to be associated with changes in the region of chromosome 1p36, where the gene encoding the serotonin type 6 receptor is located. This receptor is a target for typical and atypical antipsychotics, drugs that show high efficacy in the treatment of schizophrenia. nine0003
Most likely, the term "feature" fits them. To endophenotypes, for example, scientists include impaired recognition of emotions and problems with how the patient controls his movements. Problems with recognizing emotions turned out to be associated with changes in the region of chromosome 1p36, where the gene encoding the serotonin type 6 receptor is located. This receptor is a target for typical and atypical antipsychotics, drugs that show high efficacy in the treatment of schizophrenia. nine0003
In general, the situation with genetic research in the origin of psychiatric pathology is disappointing. Too many genes influence the development of mental disorders. At the same time, they have too weak effects that require new mathematical methods for their analysis. Too complex studies are required to determine these weak links: they are not yet widely available in wide practice.
Compatibility testing
Genetic research in mental disorders is not limited to finding the causes of the disease and their differences. Now pharmacogenetic testing is gaining more and more popularity - the detection of features of enzymes that are involved in the metabolism of drugs. There are amazing workers in our body - a family of enzymes called "cytochrome p450". This family includes more than 50 enzymes, 6 of which are involved in the metabolism of about 90% of all medicines [14]. Here are the strikers of the metabolic front: CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A5. CYP2C19 and CYP2D6 are of great importance for the metabolism of psychotropic drugs.
Now pharmacogenetic testing is gaining more and more popularity - the detection of features of enzymes that are involved in the metabolism of drugs. There are amazing workers in our body - a family of enzymes called "cytochrome p450". This family includes more than 50 enzymes, 6 of which are involved in the metabolism of about 90% of all medicines [14]. Here are the strikers of the metabolic front: CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A5. CYP2C19 and CYP2D6 are of great importance for the metabolism of psychotropic drugs.
For example, 85% of antidepressants and 40% of antipsychotics are metabolized by the CYP2D6 enzyme [15]. This is reflected in the frequency of development of specific side effects when using psychotropic drugs. Patients with high CYP2D6 activity receiving antipsychotic therapy are more prone to the development of tardive (tardive) dyskinesia than those in whom this enzyme is less active [16]. Tardive dyskinesia is a specific syndrome caused by long-term use of neuroleptics and persisting after their withdrawal. With its development, the patient develops violent, repetitive movements of the tongue and lips. In severe forms, other muscle groups are involved: the patient experiences violent movements of the torso and limbs. These problems can be combined with restlessness, tremors, and drug-induced parkinsonism. Correcting tardive dyskinesia with medication is a difficult task. For this reason, efforts are usually made to prevent it. There is evidence that indicates that insufficient activity of CYP2D6 can lead to neuroleptic malignant syndrome [17]. After taking antipsychotics, the patient begins to complain about a rise in temperature. He has a pronounced increase in muscle tone, pronounced changes in pulse rate and blood pressure, as well as impaired consciousness of varying degrees. This is one of those rare conditions in psychiatry that by itself can lead to the death of the patient. nine0003
With its development, the patient develops violent, repetitive movements of the tongue and lips. In severe forms, other muscle groups are involved: the patient experiences violent movements of the torso and limbs. These problems can be combined with restlessness, tremors, and drug-induced parkinsonism. Correcting tardive dyskinesia with medication is a difficult task. For this reason, efforts are usually made to prevent it. There is evidence that indicates that insufficient activity of CYP2D6 can lead to neuroleptic malignant syndrome [17]. After taking antipsychotics, the patient begins to complain about a rise in temperature. He has a pronounced increase in muscle tone, pronounced changes in pulse rate and blood pressure, as well as impaired consciousness of varying degrees. This is one of those rare conditions in psychiatry that by itself can lead to the death of the patient. nine0003
Existing pharmacogenetic data in psychiatry are associated with the work of CYP2C19 and CYP2D6 enzymes [18]. Genetic testing can determine the rate at which enzymes work, depending on which allele of a gene is present in a person and in how many copies. Functional variants of CYP2D6 include variants of CYP2D6*1, CYP2D6*2 in any combination with other alleles. If this gene has additional copies, then the enzyme works too actively, which puts its owner into the group of "ultrafast" metabolizers. "Intermediate" and "slow" CYP2D6 metabolizers may have an increased risk of side effects, and therefore require lower doses of drugs in the treatment of mental disorders. By CYP2C19 enzyme activityhumans can also be classified into "ultra-fast", "extensive", "intermediate", and "slow" metabolisers. The prevalence of different alleles varies depending on the race to which the patient belongs.
Genetic testing can determine the rate at which enzymes work, depending on which allele of a gene is present in a person and in how many copies. Functional variants of CYP2D6 include variants of CYP2D6*1, CYP2D6*2 in any combination with other alleles. If this gene has additional copies, then the enzyme works too actively, which puts its owner into the group of "ultrafast" metabolizers. "Intermediate" and "slow" CYP2D6 metabolizers may have an increased risk of side effects, and therefore require lower doses of drugs in the treatment of mental disorders. By CYP2C19 enzyme activityhumans can also be classified into "ultra-fast", "extensive", "intermediate", and "slow" metabolisers. The prevalence of different alleles varies depending on the race to which the patient belongs.
The enzymes CYP2C19 and CYP2D6 play a key role in the metabolism of tricyclic antidepressants, among which amitriptyline must be mentioned. It is widely used by psychiatrists, neurologists and therapists for a wide variety of diseases. In the course of metabolism, amitriptyline undergoes transformations leading to a change in its effect on the mental state (Fig. 5) [18]. Amitriptyline as a tertiary amine has a pronounced effect on the serotonin system. Through the action of CYP2C19, it turns into nortriptyline, which actively interferes with the work of norepinephrine transmission. The more active the enzyme works, the weaker the “serotonin” component of the effect of amitriptyline and the stronger the norepinephrine one. Since CYP2D6 is involved in the metabolism of tricyclic antidepressants, its activity affects the ability of drugs to affect the patient's condition. In "ultra-rapid" CYP2D6 metabolizers, it is recommended to start therapy not with this class of drugs. If a patient with such metabolic features receives tricyclic therapy, then it is necessary to regularly evaluate the concentration of the drug in plasma. For "slow" metabolizers, the recommendations are the same. If they have to take tricyclic antidepressants, then the initial dose should be 50% of the recommended starting dose.
In the course of metabolism, amitriptyline undergoes transformations leading to a change in its effect on the mental state (Fig. 5) [18]. Amitriptyline as a tertiary amine has a pronounced effect on the serotonin system. Through the action of CYP2C19, it turns into nortriptyline, which actively interferes with the work of norepinephrine transmission. The more active the enzyme works, the weaker the “serotonin” component of the effect of amitriptyline and the stronger the norepinephrine one. Since CYP2D6 is involved in the metabolism of tricyclic antidepressants, its activity affects the ability of drugs to affect the patient's condition. In "ultra-rapid" CYP2D6 metabolizers, it is recommended to start therapy not with this class of drugs. If a patient with such metabolic features receives tricyclic therapy, then it is necessary to regularly evaluate the concentration of the drug in plasma. For "slow" metabolizers, the recommendations are the same. If they have to take tricyclic antidepressants, then the initial dose should be 50% of the recommended starting dose. Similar recommendations exist for CYP2C19[eighteen]. At the same time, one should not think that genetic testing of the enzymes under discussion is some kind of exotic. In 2005, the FDA approved the AmpliChip CYP450 system, which provides data on the genetics of these enzymes. Separate studies of the CYP2C19 and CYP2D6 genes are also available in our country.
Similar recommendations exist for CYP2C19[eighteen]. At the same time, one should not think that genetic testing of the enzymes under discussion is some kind of exotic. In 2005, the FDA approved the AmpliChip CYP450 system, which provides data on the genetics of these enzymes. Separate studies of the CYP2C19 and CYP2D6 genes are also available in our country.
Figure 5. In humans, amitriptyline is converted by CYP2C19 to nortriptyline, the active metabolite of amitriptyline. CYP2D6 is involved in the conversion of both molecules to an inactive form. nine0003
Another example of already existing pharmacogenetic testing aimed at preventing rare but dangerous side effects is screening for the HLA-B*1502 marker in people of Asian descent. Carbamazepine therapy in patients with this gene increases the risk of developing Stevens-Johnson syndrome, a potentially fatal skin lesion in which epidermal cells separate from the dermis [19]. The FDA recommends testing the HLA-B*1502 marker before initiating carbamazepine therapy. nine0003
nine0003
Many knowledge - many sorrows?
Whenever it comes to the introduction and use of a diagnostic method in medical practice, it is necessary to evaluate its usefulness. If we start doing genetic tests on patients with mental disorders, will it be useful for them? Will we get any meaningful information for the diagnosis and treatment of this group of diseases?
At the current stage of development of the genetics of mental disorders, their usefulness is doubtful. There are too many uncertain and poorly applicable results in studies even with a large sample. The main reason for this is the fundamental difference between mental disorders and monogenic diseases. In the classic case of a monogenic disease (for example, Huntington's disease), it is enough to analyze the structure of one gene to understand whether the patient has this disorder or not. With the same certainty, it will work to predict the development of Huntington's disease in a potential carrier of the gene. One can recall the Thirteenth from the TV series House M.D., which found out by one analysis whether she would develop Huntington's disease or not [20]. nine0003
One can recall the Thirteenth from the TV series House M.D., which found out by one analysis whether she would develop Huntington's disease or not [20]. nine0003
Mental disorders have a different genetic basis. These are polygenic diseases, the development of which is associated with a change in the work of many genes at the same time. The identification of each of these genes will not bring important diagnostic information for the doctor, at least at the current stage of development of this field of knowledge. There is also little point now in predicting the risk of developing a mental disorder based on genetic studies in each person and relatives of already known patients. In the development of mental disorders, environmental factors play an important role - socio-economic status, serious upheavals in life, place of residence of a person. The vague information that a person who does not suffer from a mental disorder will receive will lead to an increase in his anxiety about his health, and this excessive anxiety can provoke the onset of his illness ("I was worried that I would go crazy, and in the end I went crazy") . The other side of identifying such genetic risks in a person is a negative attitude towards him from others (“He has the genes for schizophrenia, which means that he can become a schizophrenic at any moment”). This will significantly complicate the life of a person. Although the risk itself may be insignificant, all the words and decisions of a person can be perceived through the prism of his potential insanity. A person with a calculated risk of a mental disorder can be recognized as sick without the disease itself. The usefulness here, of course, is doubtful. nine0003
The other side of identifying such genetic risks in a person is a negative attitude towards him from others (“He has the genes for schizophrenia, which means that he can become a schizophrenic at any moment”). This will significantly complicate the life of a person. Although the risk itself may be insignificant, all the words and decisions of a person can be perceived through the prism of his potential insanity. A person with a calculated risk of a mental disorder can be recognized as sick without the disease itself. The usefulness here, of course, is doubtful. nine0003
This problem is well illustrated by studies of the 5-HTTLPR (serotonin transporter) gene, which is often associated with the risk of antisocial behavior (aggression, crimes against property). A short version of the gene with a small number of repeats in its structure makes a person vulnerable to external manifestations of threatening situations and provokes increased activity of the hypothalamic-pituitary-adrenal system [21]. As a result, a teenager with a short version of the 5-HTTLPR gene will be more likely to exhibit antisocial behavior than someone with a long version of this gene. So far, everything is clear: let's take all teenagers and test them for this gene. Then we will form a risk group and keep a close watch on it in order to prevent crimes and these young people from going to prison. As they say, it was smooth on paper, but they forgot about the ravines. The "ravine" in this case is the environmental factors, in particular, the socio-economic conditions in which the teenager grows up. If we are talking about disadvantaged areas, then in them economic and social factors (unemployment, level of education) have a predominant influence on antisocial behavior [22]. This means that in order to prevent crime, interventions are needed at the level of the entire locality, measures that improve the social climate as a whole. The influence of 5-HTTLPR gene variants becomes noticeable under favorable socioeconomic conditions.
As a result, a teenager with a short version of the 5-HTTLPR gene will be more likely to exhibit antisocial behavior than someone with a long version of this gene. So far, everything is clear: let's take all teenagers and test them for this gene. Then we will form a risk group and keep a close watch on it in order to prevent crimes and these young people from going to prison. As they say, it was smooth on paper, but they forgot about the ravines. The "ravine" in this case is the environmental factors, in particular, the socio-economic conditions in which the teenager grows up. If we are talking about disadvantaged areas, then in them economic and social factors (unemployment, level of education) have a predominant influence on antisocial behavior [22]. This means that in order to prevent crime, interventions are needed at the level of the entire locality, measures that improve the social climate as a whole. The influence of 5-HTTLPR gene variants becomes noticeable under favorable socioeconomic conditions. It turns out that "genetic" hooligans should be looked for not in slums and ghettos, but among the representatives of the middle class and wealthy citizens, which is somewhat discouraging. nine0003
It turns out that "genetic" hooligans should be looked for not in slums and ghettos, but among the representatives of the middle class and wealthy citizens, which is somewhat discouraging. nine0003
Development of genetic research in the field of mental disorders will continue. Thanks to the research, we have data on pharmacogenetics that allow us to choose the treatment wisely in order to avoid side effects and achieve a good result of therapy. The calculation of the risks of developing the disease and the diagnosis of mental disorders still remain a difficult and unattainable task in practice. Let's hope this changes over time.
- In the ruins of memory: the present and future of Alzheimer's disease; nine0009
- Jack C.R. Jr., Petersen R.C., Xu Y., O'Brien P.C., Smith G.E., Ivnik R.J. et al. (2000). Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology . 55 , 484–89;
- Mamoru Hashimoto, Hiroaki Kazui, Keiji Matsumoto, Yoko Nakano, Minoru Yasuda, Etsuro Mori.
 (2005). Does Donepezil Treatment Slow the Progression of Hippocampal Atrophy in Patients With Alzheimer's Disease?. AJP . 162 , 676-682;
(2005). Does Donepezil Treatment Slow the Progression of Hippocampal Atrophy in Patients With Alzheimer's Disease?. AJP . 162 , 676-682; - DNA sequencing costs: data. (2016). National Human Genome Research Institute ;
- Research Domain Criteria (RDoC). National Institute of Mental Health ;
- Hui Liu, Simon C. Heath, Christina Sobin, J. Louw Roos, Brandi L. Galke, et. al. (2002). Genetic variation at the 22q11PRODh3/DGCR6locus presents an unusual pattern and increases susceptibility to schizophrenia. PNAS . 99 , 3717-3722;
- U Demkow, T Wolańczyk. (2017). Genetic tests in major psychiatric disorders—integrating molecular medicine with clinical psychiatry—why is it so difficult?. Transl Psychiatry . 7 , e1151;
- Qun Pan, Ofer Shai, Leo J Lee, Brendan J Frey, Benjamin J Blencowe.
 (2008). Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet . 40 , 1413-1415;
(2008). Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet . 40 , 1413-1415; - C Chen, L Cheng, K Grennan, F Pibiri, C Zhang, et. al. (2013). Two gene co-expression modules differentiate psychotics and controls. Mol Psychiatry . 18 , 1308-1314;
- Bin Zhang, Chris Gaiteri, Liviu-Gabriel Bodea, Zhi Wang, Joshua McElwee, et. al. (2013). Integrated Systems Approach Identifies Genetic Nodes and Networks in Late-Onset Alzheimer's Disease. Cell . nine0024 153 , 707-720;
- A S Cristino, S M Williams, Z Hawi, J-Y An, M A Bellgrove, et. al. (2014). Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Mol Psychiatry . 19 , 294-301;
- S H Witt, F Streit, M Jungkunz, J Frank, S Awasthi, et.
 al. (2017). Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. nine0241 Transl Psychiatry . 7 , e1155;
al. (2017). Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. nine0241 Transl Psychiatry . 7 , e1155; - Tiffany A. Greenwood, Neal R. Swerdlow, Raquel E. Gur, Kristin S. Cadenhead, Monica E. Calkins, et. al. (2013). Genome-Wide Linkage Analyzes of 12 Endophenotypes for Schizophrenia From the Consortium on the Genetics of Schizophrenia. AJP . 170 , 521-532;
- Lynch T. and Price A. (2007). The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. nine0064 Am. fam. Physician . 76 , 391–396;
- Needham D., Teed N., Pippins J. (2011). CYP2D6 and CYP2C19 genetic testing for psychiatric drug response. Personalized Medicine Portal ;
- Maju M Koola, Evangelia M Tsapakis, Padraig Wright, Shubulade Smith, Robert W Kerwin (RIP), et.
 al. (2014). Association of tardive dyskinesia with variation in CYP2D6: Is there a role for active metabolites?. J Psychopharmacol . 28 , 665-670;
al. (2014). Association of tardive dyskinesia with variation in CYP2D6: Is there a role for active metabolites?. J Psychopharmacol . 28 , 665-670; - Agnieszka Butwicka, Szymańska Krystyna, Włodzimierz Retka, Tomasz Wolańczyk. (2014). Neuroleptic malignant syndrome in an adolescent with CYP2D6 deficiency. Eur J Pediatr . 173 , 1639-1642;
- J K Hicks, J J Swen, C F Thorn, K Sangkuhl, E D Kharasch, et. al. (2013). Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants. nine0241 Clin Pharmacol Ther . 93 , 402-408;
- P Brent Ferrell, Howard L McLeod. (2008). Carbamazepine,HLA-B*1502and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics . 9 , 1543-1546;
- How to save the Thirteenth? (Prospects for the treatment of Huntington's disease);
- Jorim J.
 Tielbeek, Richard Karlsson Linnér, Koko Beers, Danielle Posthuma, Arne Popma, Tinca J. C. Polderman. (2016). Meta-analysis of the serotonin transporter promoter variant (5-HTTLPR) in relation to adverse environment and antisocial behavior. nine0241 Am. J. Med. Genet. . 171 , 748-760;
Tielbeek, Richard Karlsson Linnér, Koko Beers, Danielle Posthuma, Arne Popma, Tinca J. C. Polderman. (2016). Meta-analysis of the serotonin transporter promoter variant (5-HTTLPR) in relation to adverse environment and antisocial behavior. nine0241 Am. J. Med. Genet. . 171 , 748-760; - Catherine Tuvblad, Martin Grann, Paul Lichtenstein. (2006). Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. J Child Psychol & Psychiat . 47 , 734-743.
Comments
How does genetics affect the risk of getting a mental disorder?
According to recent research, 10.7% of people worldwide suffer from mental illness. The leading position is occupied by anxiety disorder (3.8%), depression (3.4%), disorders caused by the use of alcohol (1.4%) and drugs (0.9%). However, since the World Health Organization (WHO) announced the spread of coronavirus infection, the prevalence of mental illness has increased and varies in different countries.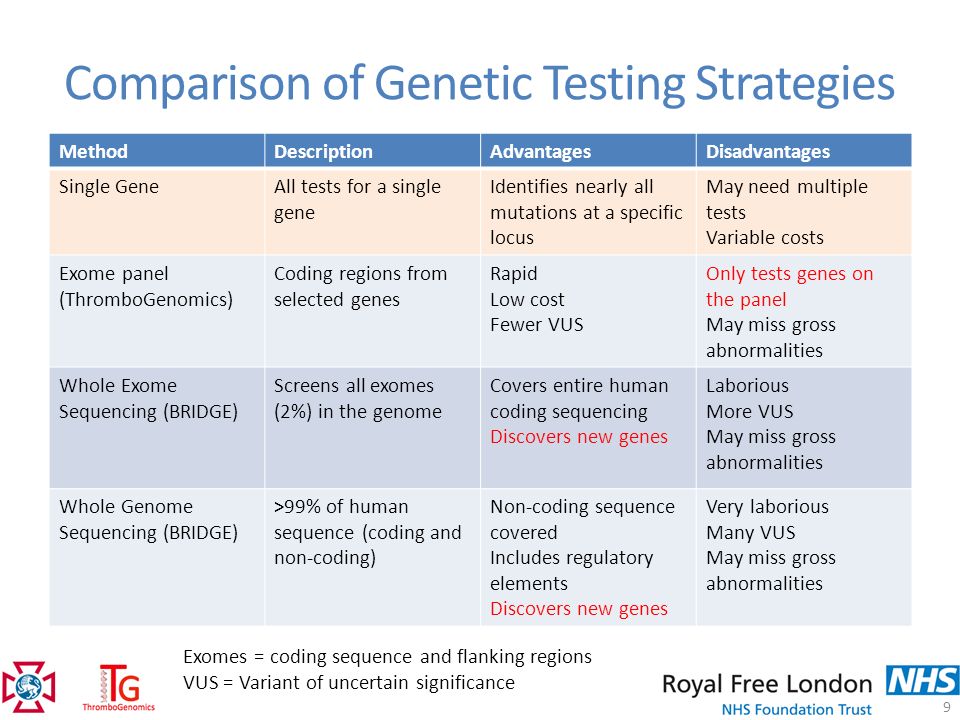 In this material, we understand what factors lead to the onset of mental disorders and how to reduce the risks.
In this material, we understand what factors lead to the onset of mental disorders and how to reduce the risks.
Contents
- What are mental disorders
- Brain, genes and environment: how is it all connected?
- Genes nine0007 Brain
- Environment
What are mental disorders?
Psychiatric disorders are a broad spectrum of conditions that include cognitive, emotional and behavioral changes that interfere with a person's normal functioning. The diagnosis of a mental disorder is established only by a psychiatrist based on the international classification of diseases (ICD-10).
Mental disorders include a wide range of diseases: affective disorders (depression, bipolar disorder), personality disorders, psychogenic diseases (neurosis), schizophrenia, and so on. Each of them corresponds to specific symptoms that are observed for a long time. So, for example, paranoid schizophrenia is characterized by delusions, often accompanied by auditory hallucinations, and depression is determined by low mood, a decrease in activity and energy, and changes in appetite. nine0003
nine0003
Bad mood and unwillingness to do something is often found in everyday life, but it is far from always worth raising the alarm. These manifestations are considered symptoms of mental disorders only if there is a persistent, long-term nature and a negative impact on the professional or social life of a person.
That is, a bad mood can become a symptom if it persists and interferes with daily and professional functioning.
Brain, genes and environment: how is it all connected?
Genes
To assess the role of heritability in the occurrence of mental disorders, studies were carried out on relatives of patients with various pathologies. The results show that parents, brothers and sisters of patients with schizophrenia can inherit the disease with a probability of 6% - 12%, depression 11% - 19%. Studies of monozygotic twins have shown an even greater percentage of inheritance of schizophrenia (44%), depression (from 40% to 50% according to various studies), anxiety disorder (30 - 50%). All these results clearly demonstrate the influence of the genetic factor in the occurrence of mental disorders. nine0003
All these results clearly demonstrate the influence of the genetic factor in the occurrence of mental disorders. nine0003
If you look deeper into the issue, it turns out that the genetic risk of mental illness is due to the influence of several genes and their variations, and not the breakdown of any one gene. For example, according to the results of one study with a sample of 80,000 people, it was found that there are about 100 genes that affect the risk of developing schizophrenia. A similar picture can be seen for other psychopathologies.
Brain
The identified candidate genes that potentially affect the risk of various mental disorders are mostly not specific to any particular disease. In most cases, these are genes associated with the functioning of brain cells and mediators, that is, chemicals that carry signals between cells. Thus, variations in candidate genes lead to disturbances in the functioning of nerve cells, mediators, and neural networks in general. Such a chain of changes underlies the symptoms of mental illness, which manifest themselves in the emotional, cognitive and behavioral spheres. nine0003
Such a chain of changes underlies the symptoms of mental illness, which manifest themselves in the emotional, cognitive and behavioral spheres. nine0003
For example, a modification in the candidate gene SLC6A4 associated with the work of serotonin, one of the mediators of the nervous system, leads to impaired recognition of emotions, which is noted in schizophrenia.
Environment
Let's go back to the twin studies. As we saw earlier, genetic studies do not explain all cases of mental disorders. However, the combination of genetic and environmental factors most fully reveals the nature of psychopathology. A wide group of factors that increase the risk of developing mental illness include various adverse effects on a person coming from outside: stress, episodes of physical or sexual violence, traumatic brain injury, low socioeconomic status, and so on. nine0003
Thus, the nature of the occurrence of mental disorders is complex and does not fit into the framework of the laws of inheritance described by scientists.
It is much more likely that variations in candidate genes create biological vulnerabilities that, together with adverse environmental influences, lead to mental disorders.
How to reduce the risk of mental illness?
There is no sure way to prevent mental illness, but you can control some environmental factors and how you react to them. So, among the main protective factors are:
Possess skills to overcome difficulties and problems;
Emotional self-regulation skills;
Good relationships with others, in the family, at work;
Financial stability;
Sports.
Learn more about your genetics, unique traits and risks with the Atlas Genetic Test.
American Mental Wellness Association
Efimova et.al., The effect of the serotonin transporter 5-HTTLPR polymorphism on the recognition of facial emotions in schizophrenia, 2014
Lohoff FW. Overview of the genetics of major depressive disorder, 2010
McGue et.