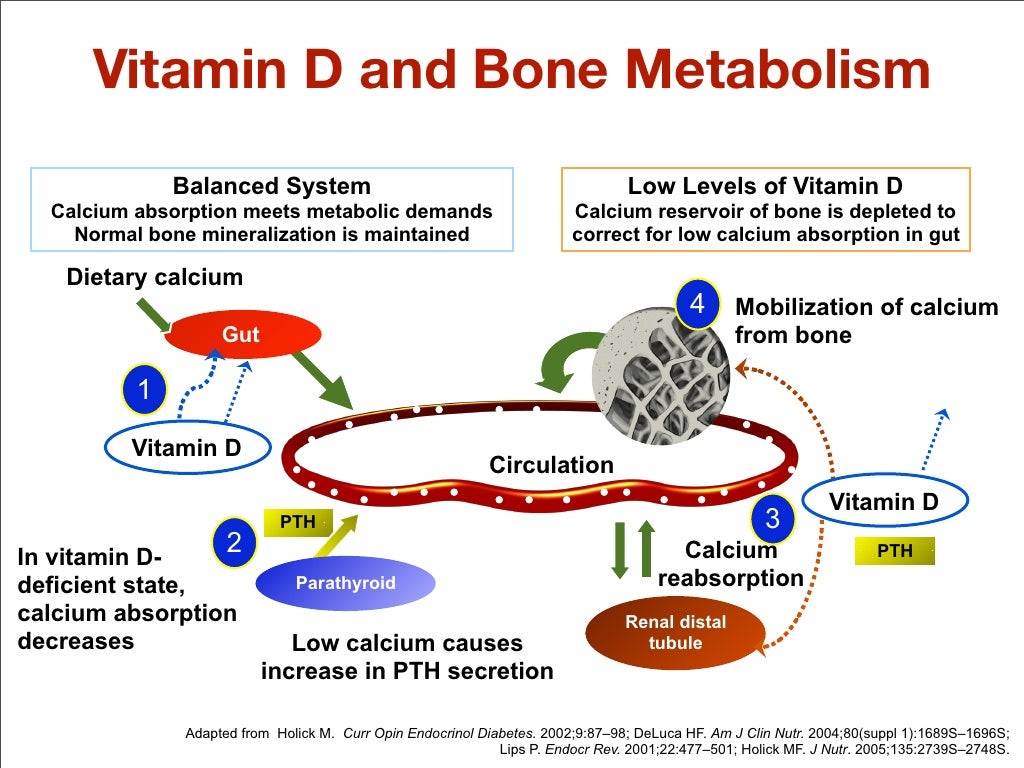
Can low ferritin cause anxiety
Association of ferritin levels with depression, anxiety, sleep quality, and physical functioning in patients with fibromyalgia syndrome: a cross-sectional study
Croat Med J. 2019 Dec; 60(6): 515–520.
doi: 10.3325/cmj.2019.60.515
,1,2,3,4 and 5
Author information Article notes Copyright and License information Disclaimer
Aim
To determine the frequency of ferritin deficiency in individuals with fibromyalgia syndrome (FMS) and to evaluate the association of ferritin level with depression, anxiety, sleep quality, and physical functioning.
Methods
This cross-sectional study, conducted from 2016 to 2017, compared the frequency of ferritin deficiency between 100 non-anemic fibromyalgia patients and 100 non-anemic individuals without FMS. Serum ferritin level of <30 ng/mL indicated iron deficiency. FMS patients filled out demographic questionnaire, Fibromyalgia Impact Questionnaire, Beck Anxiety Inventory, Beck Depression Inventory, and Pittsburg Sleep Quality Index.
Results
Median serum ferritin level was 20.95 ng/mL. A total of 64% of patients and 42% of controls had iron deficiency. Beck Anxiety Inventory, Beck Depression Inventory, and Pittsburgh Sleep Quality Index scores were not associated with ferritin levels. FMS patients with poor sleep quality had significantly higher Beck Depression Inventory, Beck Anxiety Inventory, and Fibromyalgia Impact Questionnaire scores (P < 0.05). In individuals with poor sleep quality, lower ferritin levels also correlated with higher Beck Depression Inventory scores (r = -0.277, P < 0.05). Sleep quality was not significantly associated with age, body mass index, duration of diagnosis, and serum ferritin levels.
Conclusions
Patients with fibromyalgia syndrome have a rather high prevalence of non-anemic iron deficiency. No associations were found between serum ferritin level and anxiety, depression, sleep quality, and physical functioning.
ClinicalTrials. gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03825393","term_id":"NCT03825393"}}NCT03825393.
gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03825393","term_id":"NCT03825393"}}NCT03825393.
Fibromyalgia syndrome (FMS) is a musculoskeletal disease characterized by chronic pain, fatigue, anxiety, memory loss, and morning stiffness (1). FMS prevalence in the general population is 2.7% and is three times greater in women (2). While the risk factors for the disease include vitamin and mineral deficiencies, its exact etiology and pathogenesis are unclear. The onset and prognosis of FMS are determined by interactions between neuroendocrine, immunological, and metabolic factors, and the diagnosis is usually established in middle-aged patients (2,3).
Serum ferritin concentration (cut-off <30 ng/mL) is the most sensitive and specific test for iron deficiency (4-6). The symptoms specific to a decreased activity of iron-containing enzymes are weakness, fatigue, lack of concentration, lower work performance, and impaired oxygen transport to body tissues. However, it is not known to what extent these non-hematological effects of iron deficiency emerge before anemia occurs (7). Iron deficiency in FMS causes chronic fatigue, myalgia, decreased endurance, and sleep disorders (8,9). It lowers the pain threshold, making increased pain sensitivity a potential key factor in the pathophysiology of fibromyalgia (10). A case-control study by Ortancil et al (11) showed FMS patients had lower serum ferritin levels than healthy control groups. To the best of our knowledge, no study has so far evaluated the association between ferritin levels and sleep in FMS patients. Therefore, the aim of the present study was to determine the frequency of non-anemic ferritin deficiency in individuals with FMS and to evaluate the association of ferritin levels with anxiety, depression, sleep quality, and physical functioning, as well as the interactions between clinical parameters in FMS patients.
Iron deficiency in FMS causes chronic fatigue, myalgia, decreased endurance, and sleep disorders (8,9). It lowers the pain threshold, making increased pain sensitivity a potential key factor in the pathophysiology of fibromyalgia (10). A case-control study by Ortancil et al (11) showed FMS patients had lower serum ferritin levels than healthy control groups. To the best of our knowledge, no study has so far evaluated the association between ferritin levels and sleep in FMS patients. Therefore, the aim of the present study was to determine the frequency of non-anemic ferritin deficiency in individuals with FMS and to evaluate the association of ferritin levels with anxiety, depression, sleep quality, and physical functioning, as well as the interactions between clinical parameters in FMS patients.
This cross-sectional study included women older than 18 who presented to Physical Medicine and Rehabilitation polyclinic and whose ferritin levels had been measured in the previous four weeks at the Tokat State Hospital in the 2016-2017 period.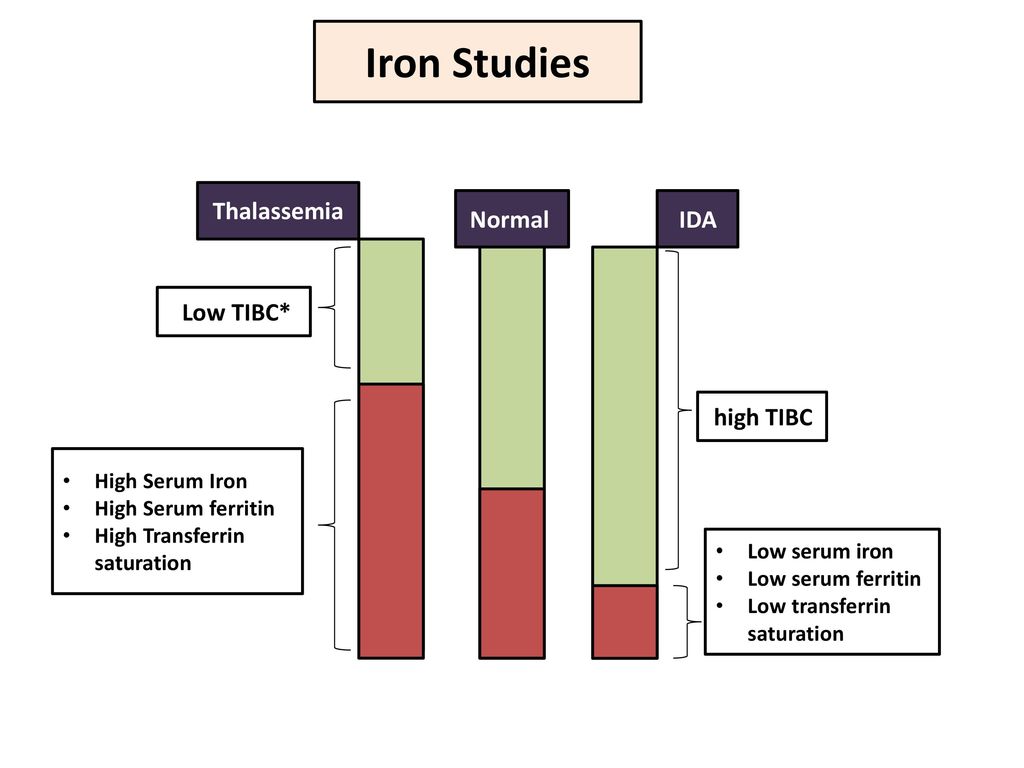 Exclusion criteria were parenteral or enteral iron use in the last four weeks, hemoglobin level less than 12 g/dL, infection, malignity, active inflammatory arthritis, serious skin variations, serious peripheral vascular disease, iron storing disorder, and pregnancy or lactation. After the exclusion criteria were applied, 100 participants in the patient group were selected among 137 non-anemic FMS patients who were diagnosed based on 2011 FMS diagnostic criteria of the American Rheumatology College (12). The selection was performed by simple random sampling with random number generator. The control group included 100 women selected by a simple random sampling among 153 non-anemic women without FMS. The study was approved by the Ethics Committee for Clinical Research of Gaziosmanpasa University (17-KAEK-093), and all participants gave informed consent. Serum ferritin levels were obtained from hospital information system records. Only FMS patients filled out a demographic questionnaire, Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI), Fibromyalgia Impact Questionnaire (FIQ), and Pittsburgh Sleep Quality Index (PSQI).
Exclusion criteria were parenteral or enteral iron use in the last four weeks, hemoglobin level less than 12 g/dL, infection, malignity, active inflammatory arthritis, serious skin variations, serious peripheral vascular disease, iron storing disorder, and pregnancy or lactation. After the exclusion criteria were applied, 100 participants in the patient group were selected among 137 non-anemic FMS patients who were diagnosed based on 2011 FMS diagnostic criteria of the American Rheumatology College (12). The selection was performed by simple random sampling with random number generator. The control group included 100 women selected by a simple random sampling among 153 non-anemic women without FMS. The study was approved by the Ethics Committee for Clinical Research of Gaziosmanpasa University (17-KAEK-093), and all participants gave informed consent. Serum ferritin levels were obtained from hospital information system records. Only FMS patients filled out a demographic questionnaire, Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI), Fibromyalgia Impact Questionnaire (FIQ), and Pittsburgh Sleep Quality Index (PSQI).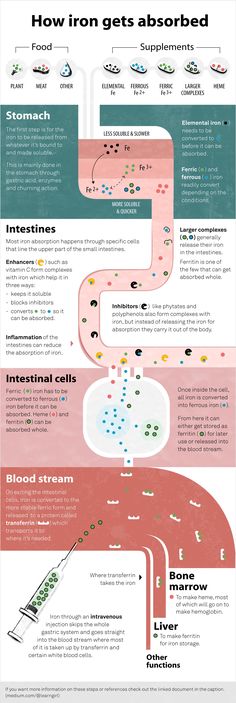
FIQ is a 10-item evaluation tool measuring the status, prognosis, and outcomes of FMS patients. The total score ranges from 0 to 100, with a higher score indicating a greater effect of FMS on functionality. The validity and reliability of FIQ in Turkish population were assessed by Sarmer et al (13).
BDI is a 21-item questionnaire evaluating the presence and severity of depression. A higher score indicates more severe depression (0-9 points: minimum depression; 10-18 points: slight depression; 19-29 points: moderate depression; 30-63 points: severe depression). The validity and reliability of BDI in Turkish population were assessed by Hisli et al (14).
BAI is a short, 21-item questionnaire evaluating the severity of anxiety. The total score ranges from 0 to 63 (0-9 points: normal; 10-18 points: slight to moderate anxiety; 19-29 points: moderate to severe anxiety; and 30-63 points: very severe anxiety). The validity and reliability of BAI in Turkish population were assessed by Ulusoy et al (15).
PSQI is an 18-item questionnaire measuring sleep quality and disorders in a period of one month. PSQI measures subjective sleep quality, duration and latency of sleep, sleep disorders, habitual sleep efficiency, daytime dysfunction, and sleep medications use. A score of 5 or higher indicates poor sleep, while a score lower than 5 indicates good sleep quality. The validity and reliability of PSQI in Turkish population was assessed by Ağargün et al (16).
Serum ferritin level was measured with Roche Cobas e801 autoanalyzer (Roche Diagnostics, Mannheim, Germany) in our hospital laboratory with use of electrochemiluminescence immunoassay method. Serum ferritin level lower than 30 ng/mL indicated iron deficiency.
Statistical analysis
General characteristics of study groups were summarized by use of descriptive statistics. Normality of distribution was assessed with the Shapiro-Wilk’s test. Continuous variables are expressed as mean ± standard deviation for normally distributed variables and median and range for non-normally distibuted variables, while categorical variables are expressed as counts and percentages. Group means were compared with use of the independent-samples t test or Mann-Whitney U test, where appropriate. Correlations between quantitative variables were evaluated with Pearson correlation coefficient. The level of statistical significance was set at P < 0.05. Statistical analyses were carried out using SPSS software, version 19.0. (IBM Corp, Armonk, NY, USA).
Group means were compared with use of the independent-samples t test or Mann-Whitney U test, where appropriate. Correlations between quantitative variables were evaluated with Pearson correlation coefficient. The level of statistical significance was set at P < 0.05. Statistical analyses were carried out using SPSS software, version 19.0. (IBM Corp, Armonk, NY, USA).
Control and patient groups did not significantly differ in mean age (42.24 ± 8.03 and 40.44 ± 8.47 years, respectively). Average body mass index (BMI) in the patient group was 29.56 ± 5.68 kg/m2. Average duration of diagnosis was 21.5 months in 73% of patients, while 27% of patients were diagnosed during their visit to our outpatient clinic (). Significantly more patients than controls had serum ferritin level lower than 30 ng/mL (64 vs 42, P = 0.002). Control group had a significantly higher ferritin level (median 34.91, IQR 17.87-55.12 ng/mL] than FMS patients (median 20.95, IQR 11.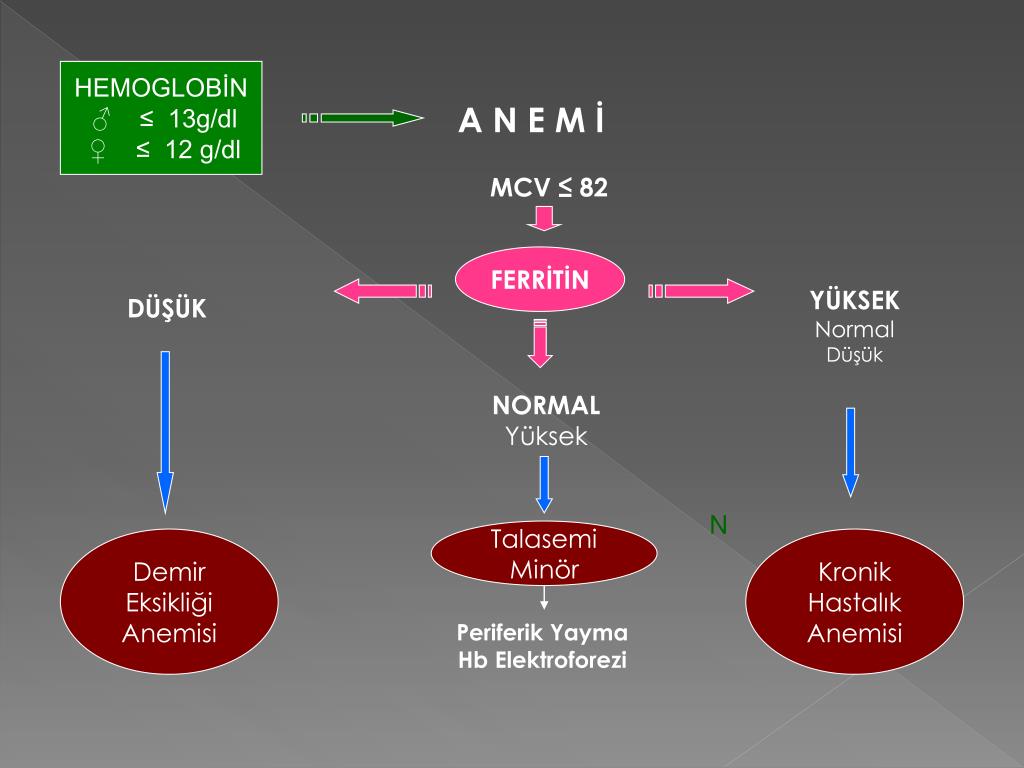 75-39.55 ng/mL, P < 0.001).
75-39.55 ng/mL, P < 0.001).
Table 1
Quantitative variables in individuals with fibromyalgia syndrome
| Mean ± standard deviation | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 42.24 ± 8.03 | 21.00 | 59.00 |
| Height (cm) | 1.61 ± 0.08 | 1.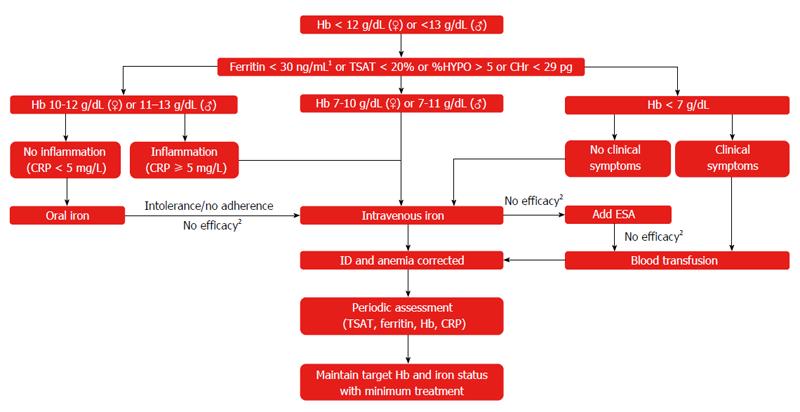 15 15 | 1.82 |
| Weight (kg) | 75.7 ± 12.2 | 40.00 | 117.00 |
| Body mass index (kg/m2) | 29.56 ± 5.68 | 15.62 | 52.93 |
| Duration of diagnosis in months (median and IQR*) | 0 (0-1.25) | 0.00 | 120. 00 00 |
| Ferritin (ng/mL, median and IQR) | 20.95 (11.75-39.55) | 2.75 | 93.00 |
| Fibromyalgia Impact Questionnaire | 61.69 ± 17.31 | 14.63 | 95.63 |
| Beck Depression Inventory | 15.73 ± 9.76 | 0.00 | 45.00 |
| Beck Anxiety Inventory | 22. 58 ± 11.14 58 ± 11.14 | 0.00 | 54.00 |
| Pittsburgh Sleep Quality Index | 8.3 ± 3.77 | 1.00 | 18.00 |
Open in a separate window
*IQR – interquartile range.
FMS patients were further divided into iron-deficiency and non-deficiency group. The groups did not differ in BMI, duration of diagnosis, FIQ, BDI, BAI, and PSQI scores, although iron deficiency group had significantly lower average age (P = 0.047). It also had relatively higher BDI, BAI, and PSQI scores, but the differences were not significant ().
Table 2
Distribution of quantitative variables according to iron deficiency (<30 ng/mL) in patients with fibromyalgia syndrome
| Variables* | Patients with ferritin level | P† | |
|---|---|---|---|
| <30 ng/mL (n = 64) | ≥30 ng/mL (n = 36) | ||
| Age (years) | 41. 05 ± 7.69 05 ± 7.69 | 44.36 ± 8.28 | 0.047 |
| Height (cm) | 1.61 ± 0.09 | 1.60 ± 0.07 | 0.709 |
| Weight (kg) | 75.08 ± 12.03 | 76.81 ± 12.59 | 0.500 |
| Body mass index (kg/m2) | 29.31 ± 6.00 | 30. 01 ± 5.11 01 ± 5.11 | 0.560 |
| Duration of diagnosis (months) | 0 (0-1.75) | 0 (0-1.25) | 0.818‡ |
| Fibromyalgia Impact Questionnaire | 61.52 ± 17.4 | 62.00 ± 17.39 | 0.895 |
| Beck Depression Inventory | 17.03 ± 10.66 | 13. 42 ± 7.51 42 ± 7.51 | 0.075 |
| Beck Anxiety Inventory | 23.34 ± 11.55 | 21.22 ± 10.40 | 0.363 |
| Pittsburgh Sleep Quality Index | 8.36 ± 3.81 | 8.19 ± 3.75 | 0.835 |
Open in a separate window
*Data are presented as mean ± standard deviation or median and interquartile range.
†Independent samples t test.
‡Mann-Whitney U test.
According to the PSQI score, FMS patients were divided into poor sleep and good sleep quality group. Poor sleep quality group had significantly higher BDI, BAI, and FIQ scores (P < 0.05) (). Sleep quality was poor in 76.57% (n = 49) of individuals with iron deficiency and in 77.7% (n = 28) of individuals with no deficiency (P = 0.999).
Poor sleep quality group had significantly higher BDI, BAI, and FIQ scores (P < 0.05) (). Sleep quality was poor in 76.57% (n = 49) of individuals with iron deficiency and in 77.7% (n = 28) of individuals with no deficiency (P = 0.999).
Table 3
Distribution of quantitative variables based on sleep quality in patients with fibromyalgia syndrome
| Variables* | Patients with sleep quality | P† | |
|---|---|---|---|
| poor (n = 77) | good (n = 23) | ||
| Age (years) | 43. 00 ± 7.91 00 ± 7.91 | 39.70 ± 8.06 | 0.083 |
| Height (cm) | 1.61 ± 0.08 | 1.59 ± 0.08 | 0.408 |
| Weight (kg) | 76.99 ± 11.96 | 71.39 ± 12.27 | 0.053 |
| Body mass index (kg/m2) | 29.96 ± 5.80 | 28.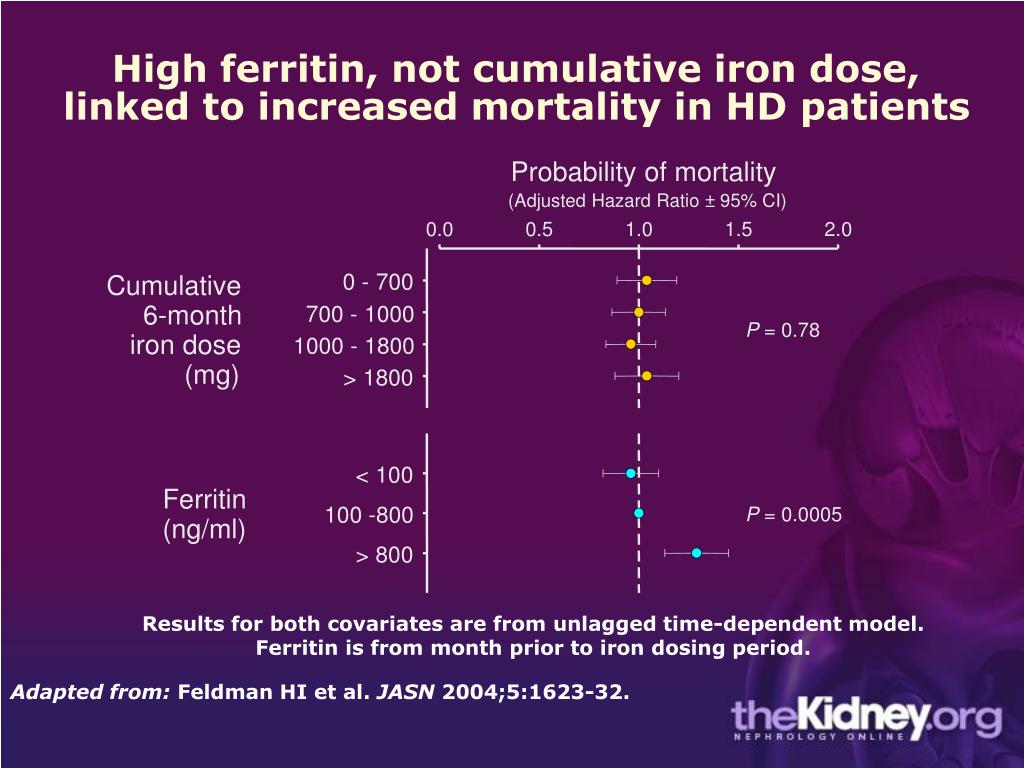 23 ± 5.16 23 ± 5.16 | 0.203 |
| Duration of diagnosis (months) | 0 (0-1) | 0 (0-2.5) | 0.912‡ |
| Fibromyalgia Impact Questionnaire | 28.32 ± 21.14 | 25.52 ± 21.72 | 0.580 |
| Beck Depression Inventory | 65.99 ± 15.36 | 47.31 ± 15.90 | <0. 001 001 |
| Beck Anxiety Inventory | 16.78 ± 9.87 | 12.22 ± 8.71 | 0.049 |
| Pittsburgh Sleep Quality Index | 24.78 ± 10.83 | 15.22 ± 8.94 | <0.001 |
Open in a separate window
*Data are presented as mean ± standard deviation or median and interquartile range.
†Independent samples t test.
‡Mann-Whitney U test.
A significant correlation between BDI and PSQI (r = 0.368, P < 0.05) was observed in participants with iron deficiency but not in those with no deficiency. In poor sleep quality group, BDI scores increased significantly with decreasing ferritin levels (r = -0.277, P < 0.05).
In poor sleep quality group, BDI scores increased significantly with decreasing ferritin levels (r = -0.277, P < 0.05).
The present study showed that iron deficiency was significantly more frequent in FMS patients than in controls. Similarly, Ortancil et al (11) found the FMS incidence risk to be 5.9 times higher in patients with ferritin levels lower than 50 ng/mL, suggesting an association of a relative decrease in iron reserve with FMS (11). Another study found a high FMS prevalence in patients with iron deficiency anemia (17). In addition, FMS patients in our study had lower median ferritin level than the control group. Similarly, another study found that women with FMS compared with controls also had lower calcium, magnesium, iron, and manganese hair concentrations (18). Contrary to these findings, in a study by Mader et al (19) FMS patients did not have lower serum iron, and low ferritin levels were not associated with FMS.
Mice fed on iron-deficient diet (10) had a decreased pain threshold, which was accompanied by elevated c-Fos expression in immunoreactive cells in the ipsilateral dorsal horn, indicating that iron deficiency indirectly increases cell activity at the spinal cord level (10). In FMS, which is characterized by chronic widespread pain, IV iron supplement considerably improved pain and fatigue index (20). In this disease, iron deficiency-related changes could also be observed in response to pain at the central nervous system level.
In FMS, which is characterized by chronic widespread pain, IV iron supplement considerably improved pain and fatigue index (20). In this disease, iron deficiency-related changes could also be observed in response to pain at the central nervous system level.
FMS patients with iron deficiency in the present study had higher depression and anxiety levels and poor sleep quality, although the differences were not significant. FMS patients are known to suffer from symptoms that considerably affect their life quality, such as fatigue, stiffness, susceptibility to cold, cognitive disorder, sensitivity to outside factors, sleep disorders, anxiety, and depression (21). This might be explained by the fact that these patients have lower biogenic amine metabolites, such as dopamine, norepinephrine, and serotonin, in the cerebrospinal fluid (22,23). Iron is necessary for neurotransmitter synthesis, and iron stores deficiencies decrease biogenic amine production (8,11). Impairments in serotonergic, GABAergic, dopaminergic, and other neurotransmitter and neuropeptide systems in the cerebrospinal fluid have also been shown in depression (24). Shukla et al (25) found low levels of serotonin and its metabolite, 5-hydroxyindoleacetic acid, in the brains of rats suffering from iron deficiency. Besides, increased activity and stereotypic behaviors, attributed to impairments in dopaminergic neurons were observed in rats with non-anemic iron deficiency (26). Although depression and anxiety levels in the present study were higher in individuals with iron deficiency, no association was found between iron deficiency and FIQ scores. In contrast, another study found an association between low ferritin scores and low total FIQ scores (19).
Shukla et al (25) found low levels of serotonin and its metabolite, 5-hydroxyindoleacetic acid, in the brains of rats suffering from iron deficiency. Besides, increased activity and stereotypic behaviors, attributed to impairments in dopaminergic neurons were observed in rats with non-anemic iron deficiency (26). Although depression and anxiety levels in the present study were higher in individuals with iron deficiency, no association was found between iron deficiency and FIQ scores. In contrast, another study found an association between low ferritin scores and low total FIQ scores (19).
We did not observe any effect of age, BMI, duration of diagnosis, or serum ferritin level on sleep quality. While in patients with chronic obstructive lung disease sleep quality deteriorated with lower ferritin levels (27), in our patients, it deteriorated with the increase in depression. Sleeping problems may exacerbate FMS symptoms, thus increasing the risk of both depression and impaired physical and social functioning (28). In a study by Miró et al, 99% of FMS individuals had poor sleep quality, which was a significant predictor of pain, fatigue, and maladaptive social functioning (29). Sleep and pain in FMS have a two-way relationship, which could interact with depressive symptoms. Sleep disorder, difficulty falling asleep, and deterioration in sleep quality could increase pain in FMS patients (30). Sleep disorders were also associated with low ferritin levels in children with attention deficit hyperactivity disorder (31).
In a study by Miró et al, 99% of FMS individuals had poor sleep quality, which was a significant predictor of pain, fatigue, and maladaptive social functioning (29). Sleep and pain in FMS have a two-way relationship, which could interact with depressive symptoms. Sleep disorder, difficulty falling asleep, and deterioration in sleep quality could increase pain in FMS patients (30). Sleep disorders were also associated with low ferritin levels in children with attention deficit hyperactivity disorder (31).
In the present study, individuals with poor sleep quality had higher depression when they had low ferritin levels. Another study found iron deficiency in 29.5% of individuals with depression, which was by 15% higher compared with healthy individuals (32). This finding could be explained by the fact that iron is needed for the synthesis of neurotransmitters involved in depression pathogenesis. On the other hand, Hunt et al (33) found no association between depression and ferritin levels.
Individuals with poor sleep quality in our study had poor physical functioning and higher anxiety and depression levels. In another study, sleep problems in FMS were reported to affect pain, depression, and anxiety (34). Sleep quality considerably affects health-related life quality in FMS, which is why it was proposed that sleep quality should be improved to increase the health-related life quality in these patients (35).
Despite the low number of patients in certain subgroups (BAI), this study contributed to better understanding of iron deficiency in FMS. Iron deficiency was quite common in individuals with FMS diagnosis. Also, it was reported to lower pain threshold and increase pain perception in FMS. Although no association was observed between iron deficiency and sleep quality, depression, anxiety, and physical functioning in individuals with iron deficiency, impaired sleep quality was associated with depression. In conclusion, sleep disorder, a frequently observed symptom in FMS, decreases the quality of life and increases depression and anxiety levels. Additionally, the results of our study point to the fact that FMS patients should have their ferritin levels evaluated, since iron deficiency treatment could prevent the deterioration of their clinical condition.
Additionally, the results of our study point to the fact that FMS patients should have their ferritin levels evaluated, since iron deficiency treatment could prevent the deterioration of their clinical condition.
Funding None.
Ethical approval given by the Ethics Committee for Clinical Research of Gaziosmanpasa University (17-KAEK-093).
Declaration of authorship SO, HS, and FO conceived and designed the study; SO and AÇT acquired the data; SO, AÇT, and FÖ analyzed and interpreted the data; SO, HS, and FO drafted the manuscript; SO, AÇT, and FO critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
1. Oza MJ, Garud MS, Gaikwad AB, Kulkarni YA. Fibromyalgia syndrome: role of obesity and nutrients. Nutrition and Functional Foods for Healthy Aging. 2017;53-63. [Google Scholar]
2. Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356. doi: 10.1007/s11916-013-0356-5. [PubMed] [CrossRef] [Google Scholar]
3. Makrani AH, Afshari M, Ghajar M, Forooghi Z, Moosazadeh M. Vitamin D and fibromyalgia: a meta-analysis. Korean J Pain. 2017;30:250–7. doi: 10.3344/kjp.2017.30.4.250. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
4. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–43. doi: 10.1056/NEJMra1401038. [PubMed] [CrossRef] [Google Scholar]
5. Lopez A, Cacoub P, Macdougall IC, Peyrin Biroulet L. Iron deficiency anemia. Lancet. 2016;387:907–16. doi: 10.1016/S0140-6736(15)60865-0. [PubMed] [CrossRef] [Google Scholar]
6. Steinbicker AU, Muckenthaler MU. Out of balance—systemic iron homeostasis in iron-related disorders. Nutrients. 2013;5:3034–61. doi: 10.3390/nu5083034. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Nutrients. 2013;5:3034–61. doi: 10.3390/nu5083034. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. Soppi ET. Iron deficiency without anemia–a clinical challenge. Clin Case Rep. 2018;6:1082–6. doi: 10.1002/ccr3.1529. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51:157–70. doi: 10.1111/j.1753-4887.1993.tb03096.x. [PubMed] [CrossRef] [Google Scholar]
9. Gerwin RD. A review of myofascial pain and fibromyalgia – factors that promote their persistence. Acupunct Med. 2005;23:121–34. doi: 10.1136/aim.23.3.121. [PubMed] [CrossRef] [Google Scholar]
10. Dowling P, Klinker F, Amaya F, Paulus W, Liebetanz D. Iron-deficiency sensitizes mice to acute pain stimuli and formalin-induced nociception. J Nutr. 2009;139:2087–92. doi: 10.3945/jn.109.112557. [PubMed] [CrossRef] [Google Scholar]
11. Ortancil O, Sanli A, Eryuksel R, Basaran A, Ankarali H. Association between serum ferritin level and fibromyalgia syndrome.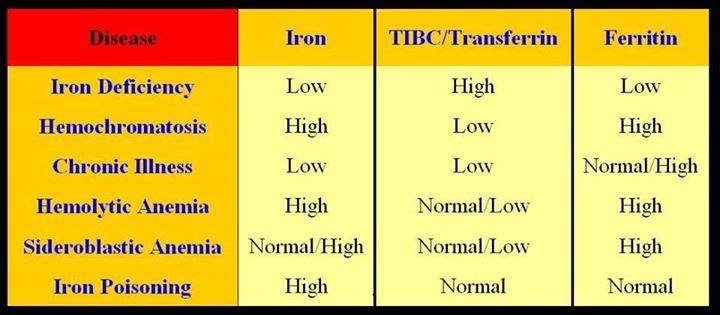 Eur J Clin Nutr. 2010;64:308–12. doi: 10.1038/ejcn.2009.149. [PubMed] [CrossRef] [Google Scholar]
Eur J Clin Nutr. 2010;64:308–12. doi: 10.1038/ejcn.2009.149. [PubMed] [CrossRef] [Google Scholar]
12. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–22. doi: 10.3899/jrheum.100594. [PubMed] [CrossRef] [Google Scholar]
13. Sarmer S, Ergin S, Yavuzer G. The validity and reliability of the Turkish version of the Fibromyalgia Impact Questionnaire. Rheumatol Int. 2000;20:9–12. doi: 10.1007/s002960000077. [PubMed] [CrossRef] [Google Scholar]
14. Hisli N. A study on validity and reliability test of the Beck Depression Scale. J Psychol. 1988;6:118–22. [Google Scholar]
15. Ulusoy M, Sahin NH, Erkmen H. Turkish version of the Beck Anxiety Inventory: psychometric properties. J Cogn Psychother. 1988;12:163–72. [Google Scholar]
16. Ağargün MY, Kara H, Anlar O.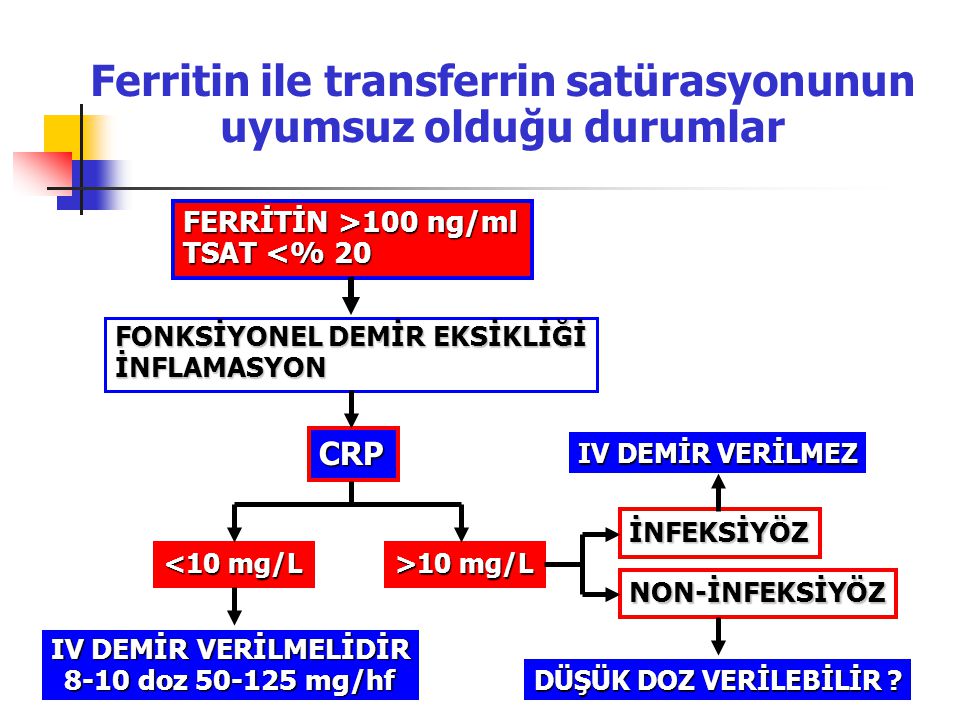 The validity and reliability of the Pittsburgh Sleep Quality Index. Turk Psikiyatr Derg. 1996;7:107–11. [Google Scholar]
The validity and reliability of the Pittsburgh Sleep Quality Index. Turk Psikiyatr Derg. 1996;7:107–11. [Google Scholar]
17. Pamuk GE, On P, Set T, Harmandar O, Yesil N. An increased prevalence of fibromyalgia in iron deficiency and thalassemia minor and associated factors. Clin Rheumatol. 2008;27:1103–8. doi: 10.1007/s10067-008-0871-7. [PubMed] [CrossRef] [Google Scholar]
18. Kim YS, Kim KM, Lee DJ, Kim BT, Park SB, Cho DY. Women with fibromyalgia have lower levels of calcium, magnesium, iron and manganese in hair mineral analysis. J Korean Med Sci. 2011;26:1253–7. doi: 10.3346/jkms.2011.26.10.1253. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Mader R, Koton Y, Buskila D, Herer P, Elias M. Serum iron and iron stores in non-anemic patients with fibromyalgia. Clin Rheumatol. 2012;31:595–9. doi: 10.1007/s10067-011-1888-x. [PubMed] [CrossRef] [Google Scholar]
20. Boomershine CS, Koch TA, Morris D. A blinded, randomized, placebo-controlled study to investigate the efficacy and safety of ferric carboxymaltose in iron-deficient patients with fibromyalgia. Rheumatol Ther. 2018;5:271–81. doi: 10.1007/s40744-017-0088-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Rheumatol Ther. 2018;5:271–81. doi: 10.1007/s40744-017-0088-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
21. Mease PJ, Arnold LM, Crofford LJ, Williams DA, Russel IJ, Humphrey L. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum. 2008;59:952–60. doi: 10.1002/art.23826. [PubMed] [CrossRef] [Google Scholar]
22. Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–6. doi: 10.1002/art.1780350509. [PubMed] [CrossRef] [Google Scholar]
23. Legangneux E, Mora JJ, Spreux-Varoquaux O, Thorin I, Herrou M, Alvado G, et al. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and (3H) imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology (Oxford) 2001;40:290–6. doi: 10.1093/rheumatology/40.3.290. [PubMed] [CrossRef] [Google Scholar]
24. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–7. doi: 10.1038/87865. [PubMed] [CrossRef] [Google Scholar]
Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–7. doi: 10.1038/87865. [PubMed] [CrossRef] [Google Scholar]
25. Shukla A, Agarwal KN, Chansuria JP, Taneja V. Effect of latent iron deficiency on 5-hydroxytryptamine metabolism in rat brain. J Neurochem. 1989;52:730–5. doi: 10.1111/j.1471-4159.1989.tb02515.x. [PubMed] [CrossRef] [Google Scholar]
26. Hunt JR, Zito CA, Erjavec J, Johnson LK. Severe or marginal iron deficiency affects spontaneous physical activity in rats. Am J Clin Nutr. 1994;59:413–8. doi: 10.1093/ajcn/59.2.413. [PubMed] [CrossRef] [Google Scholar]
27. Cavalcante AG, de Bruin PF, de Bruin VM, Pereira ED, Cavalcante MM, Nunes DM, et al. Restless legs syndrome, sleep impairment, and fatigue in chronic obstructive pulmonary disease. Sleep Med. 2012;13:842–7. doi: 10.1016/j.sleep.2012.03.017. [PubMed] [CrossRef] [Google Scholar]
28. Amutio A, Franco C, Sánchez-Sánchez LC, Pérez-Fuentes MDC, Gázquez-Linares JJ, Van Gordon W, et al.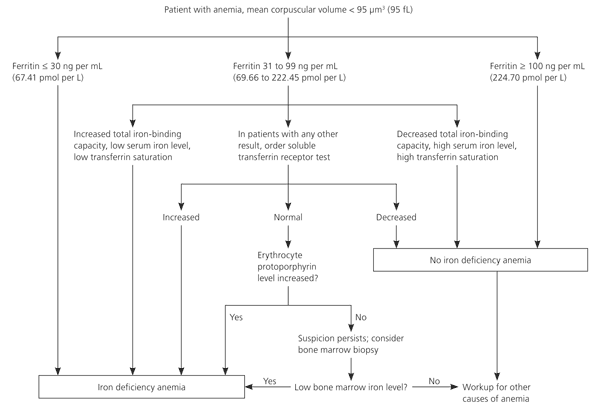 Effects of mindfulness training on sleep problems in patients with fibromyalgia. Front Psychol. 2018;9:1365. doi: 10.3389/fpsyg.2018.01365. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Effects of mindfulness training on sleep problems in patients with fibromyalgia. Front Psychol. 2018;9:1365. doi: 10.3389/fpsyg.2018.01365. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
29. Miró E, Martínez MP, Sánchez AI, Prados G, Medina A. When is pain related to emotional distress and daily functioning in fibromyalgia syndrome? The mediating roles of self-efficacy and sleep quality. Br J Health Psychol. 2011;16:799–814. doi: 10.1111/j.2044-8287.2011.02016.x. [PubMed] [CrossRef] [Google Scholar]
30. Keskindag B, Karaaziz M. The association between pain and sleep in fibromyalgia. Saudi Med J. 2017;38:465. doi: 10.15537/smj.2017.5.17864. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
31. Abou-Khadra MK, Amin OR, Shaker OG, Rabah TM. Parent-reported sleep problems, symptom ratings, and serum ferritin levels in children with attention-deficit/hyperactivity disorder: a case control study. BMC Pediatr. 2013;13:217. doi: 10.1186/1471-2431-13-217. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
32. Shariatpanaahi MV, Shariatpanaahi ZV, Moshtaaghi M, Shahbaazi SH, Abadi A. The relationship between depression and serum ferritin level. Eur J Clin Nutr. 2007;61:532. doi: 10.1038/sj.ejcn.1602542. [PubMed] [CrossRef] [Google Scholar]
Shariatpanaahi MV, Shariatpanaahi ZV, Moshtaaghi M, Shahbaazi SH, Abadi A. The relationship between depression and serum ferritin level. Eur J Clin Nutr. 2007;61:532. doi: 10.1038/sj.ejcn.1602542. [PubMed] [CrossRef] [Google Scholar]
33. Hunt JR, Penland JG. Iron and depression in premenopausal women. An MMPI study. Behav Med. 1999;25:62–8. doi: 10.1080/08964289909595738. [PubMed] [CrossRef] [Google Scholar]
34. Diaz-Piedra C, Catena A, Miro E, Martinez MP, Sanchez AI, Buela-Casal G. The impact of pain on anxiety and depression is mediated by objective and subjective sleep characteristics in fibromyalgia patients. Clin J Pain. 2014;30:852–9. doi: 10.1097/AJP.0000000000000040. [PubMed] [CrossRef] [Google Scholar]
35. Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62:145–51. doi: 10.1016/j.jpsychores.2006.09.013. [PubMed] [CrossRef] [Google Scholar]
8 Causes Behind Your Fatigue, Anxiety, and Depression
If you suffer from fatigue, anxiety, depression, or a combination of the three, you’ve probably been told by your doctor that it’s due to a chemical imbalance in your brain.
Many doctors jump to prescribe antidepressants, with 13 percent of Americans over the age of 12 reporting they took an antidepressant in the last month, according to the latest report from the National Center for Health Statistics. In some cases, antidepressants might be beneficial , but in other cases they may be masking the underlying cause of your symptoms.
Unfortunately, most doctors don’t dig deep enough to find out what may be the real cause. At Parsley Health , we use advanced testing to look at several variables that have been associated with anxiety, depression, and fatigue. Once we determine the root cause and develop a personalized treatment plan, we’re often able to reduce or eliminate the need for medications that treat these conditions.
8 overlooked causes behind fatigue, anxiety, and depression.Ready to understand what’s causing your symptoms and start feeling your best? Ask your doctor about getting tested for certain nutritional deficiencies and imbalances. Here’s what could be at the root.
Here’s what could be at the root.
Vitamin B12 is found in animal products, so vegans and vegetarians are most at risk for being deficient, as well as people with gastrointestinal issues, who may have difficulty absorbing the vitamin, and people on heartburn and reflux medications. The important vitamin is needed in order to make red blood cells and DNA and plays a major role in nerve function and mood. B12 helps to maintain the myelin sheath surrounding nerve cells, which make it possible for cells to communicate.
But when levels are low, this communication becomes compromised, which can lead to neurological changes. B12 is also involved in the production of serotonin and other neurotransmitters that regulate mood, so low levels could cause changes in the nervous system.
One study in the journal BMC Psychiatry followed 115 people with depression for six months and found that those with higher levels of B12 had a greater chance of recovery from depression, leading scientists to believe there is an association between B12 and mental health. Low levels of B12 have even been linked to decreased brain volume and cognition .
Low levels of B12 have even been linked to decreased brain volume and cognition .
Your thyroid gland produces hormones involved in metabolism and growth, releasing the hormones only when needed. But when your thyroid gets out of whack (from things like stress, diet, and environmental factors), your thyroid can overproduce or underproduce these hormones, leading to a range of physical and mental symptoms.
In a large 2015 study of people diagnosed with thyroid conditions, researchers linked an overactive thyroid (hyperthyroidism) to anxiety, while an underactive thyroid (hypothyroidism) was associated with depression. Another study found that 60 percent of patients with hypothyroidism reported symptoms of depression, while 63 percent reported symptoms of anxiety.
Researchers still aren’t exactly sure what underlying mechanism links thyroid disorders with depression and anxiety—there may be several —but the relationship between thyroid hormones and mood regulation is likely stronger than previously thought.
The connection between your brain and gut isn’t just a one-way street (think: butterflies in your stomach when you feel anxious). A growing body of research is pointing to the bidirectional superhighway between your mind and stomach and its implication on mental health.
In one recent animal study , researchers discovered that levels of miRNA (molecules in the brain) were altered in mice raised without gut bacteria, as compared to normal mice. Prior research has suggested a link between anxiety-like behaviors and a change in miRNA, underscoring the importance of a healthy microbiome.
Another study in Psychosomatic Medicine of healthy women examined their gut bacteria composition and found that those with more of a certain bacteria group displayed higher levels of anxiety, stress, and irritability when they were shown certain negative images.
The research is still just scratching the surface, but one strong theory proposed by a scientist at Brown suggests that when the gut microbiota is unbalanced, the intestinal wall becomes permeable. This allows bacteria to pass into the bloodstream, increasing the risk for psychiatric disorders through several pathways. Cleaning up your diet and including probiotic-rich foods are key factors in good gut health.
4. You’re iron deficient.Iron is essential in the production of hemoglobin, a protein that allows red blood cells to carry oxygen to your tissues and muscles. So when you have low levels of iron, less oxygen gets to your cells, keeping them from functioning properly and often leading to fatigue, weakness, and even anxiety and depression. Eventually, the lack of oxygen in your cells caused by this failure to produce enough hemoglobin can lead to anemia, a condition that can cause excessive fatigue, shortness of breath, dizziness, headaches, and more. But beyond physical symptoms, there may also be an association between low iron and anxiety, depression, or other mental health issues. A large 2020 study in BMC Psychiatry found that people with iron deficiency anemia had a significantly higher incidence and risk of anxiety disorders, depression, sleep disorder, and psychotic disorders.
But beyond physical symptoms, there may also be an association between low iron and anxiety, depression, or other mental health issues. A large 2020 study in BMC Psychiatry found that people with iron deficiency anemia had a significantly higher incidence and risk of anxiety disorders, depression, sleep disorder, and psychotic disorders.
More research is needed to understand the exact mechanisms behind this association, scientists point out, but we do have some clues. Iron deficiency has been found to affect neurological functioning and development, largely due to its role in many of the brain’s processes regulating mood, emotions, and psychological behaviors. The amount of iron in your brain is controlled by the blood-brain barrier, a collection of blood vessels that regulate the movement of ions, molecules, and cells between the blood and the brain in order to maintain homeostasis. This barrier tightly controls the amount of iron that’s able to pass through and enter the brain based on how much of the mineral is present in your body—so if your body is deficient in iron, the less iron that’s able to enter the brain. And since iron is an essential part of the systems and circuits in the brain that can dictate psychological behaviors, low iron levels in the body may make you more at risk for anxiety and depression. Research in the European Journal of Clinical Nutrition has shown that average ferritin level (a marker of stored iron) was significantly lower in depressed people.
And since iron is an essential part of the systems and circuits in the brain that can dictate psychological behaviors, low iron levels in the body may make you more at risk for anxiety and depression. Research in the European Journal of Clinical Nutrition has shown that average ferritin level (a marker of stored iron) was significantly lower in depressed people.
Even though iron naturally occurs in the environment, iron deficiency is one of the most common nutrient deficiencies, affecting an estimated 25 percent of people worldwide . Women require more than double the amount of iron than men (and more during pregnancy), yet one out of every five women of childbearing age has iron-deficiency anemia, according to the National Heart, Lung, and Blood Institute . If you’re looking to increase your iron intake through your diet, iron-rich foods include grass-fed beef, chicken, and oysters. Since iron is most bioavailable in meat sources, if you eat plant-based it’s best to add some source of vitamin C (like lemon juice) to your plant source of iron (like spinach) to help with absorption.
At Parsley Health, testing for iron is part of the routine lab work recommended for every member. This provides the data needed to understand if there is a connection between your low iron and anxiety or depression. If you do have low iron levels, our doctors and health coaches will work with you to optimize your iron intake through your diet, helping you reach ferritin levels between 30-200ng/mL. If you’re not able to reach optimal levels through diet alone, your doctor may prescribe an iron supplement and will work with you to resolve any underlying causes that could lead to iron deficiency, like a hormonal imbalance or a gut issue.
5. You’re lacking in Vitamin D.If you’ve got a job that keeps you indoors most of the day, chances are you may be part of the 42 percent of Americans with a vitamin D deficiency. Vitamin D is created by your body when sunlight hits the skin and is found in fatty fishes like salmon and tuna, eggs, and mushrooms.
Most people associate low vitamin D with wintertime and seasonal depression, but it can hit at any time of year. A thorough review of studies analyzing depression and vitamin D concluded that lower vitamin D levels were found in people with depression compared to controls.
Scientists also found that when depressed individuals received vitamin D supplementation for one year, they had significant improvements in symptoms of depression compared with depressed people taking a placebo. The area of the brain associated with depression is also a site of vitamin D receptors, posing one possible explanation for the link between the two.
6. You have unstable blood sugar.You don’t have to be diabetic to have a blood sugar problem. We help many of our patients here at Parsley Health pull the brakes on the rollercoaster of symptoms they’ve been experiencing from spikes and dips in blood sugar.
Take a typical on-the-go breakfast for many people: a pastry and sugary coffee drink. That wave of sugar creates a rapid spike in your blood sugar and causes your pancreas to release insulin to stabilize it. As your blood sugar returns to normal, you crash, feeling tired. You’ll also feel hungry again pretty quickly, leading you to reach for carbs and perpetuating the cycle.
All that sugar adds up. When people consumed 40g of added sugar a day in the form of a can of soda for three weeks, they showed at least a 60 percent increase in high-sensitivity C-reactive protein , a marker for inflammation . Inflammation has been associated with many chronic diseases, including depression. Learning to eat a combination of fiber, protein, and healthy fat at each meal and never skipping a meal can help you maintain steady blood sugar throughout the day and tame inflammation.
7. You drink alcohol in excess.
You might think happy hour is the ultimate way to unwind from your day, but the temporary effects of alcohol are just that—temporary. When you drink, alcohol increases dopamine, a feel-good chemical, and binds to and alters the neurotransmitter receptor GABA, which increases the effect of GABA and can have a calming effect in the moment. But it also interrupts other mood-regulating neurotransmitters, like serotonin. When these alcohol-induced effects wear off, you may experience a range of anxiety symptoms.
In the long term, excessive drinking makes neurons less excitable and permanently changes the mRNA and protein levels in GABA receptors. This makes the receptors less sensitive, so the brain needs more GABA. Without it, symptoms of anxiety can result. This doesn’t necessarily mean you have to completely remove alcohol from your life, but take at least three nights off per week from drinking and always drink in moderation.
Everything else aside, you could be the picture-perfect vision of health but still struggle with depression. A study of 460,000 people in Nature Genetics used data from 23andMe to discover 15 regions on the human genome associated with risk of major depression. These areas represent irregularities that people with reported depression had when compared to people who did not report depression.
One such gene mutation that’s been widely studied is methylenetetrahydrofolate reductase (MTHFR). MTHFR produces an essential enzyme that converts folate into an accessible form which plays a role in mood-regulating neurotransmitter production. Research has found an association between a MTHFR mutation and depression and other mental health disorders.
In some cases, scientists believe it may be possible to use folate as a treatment option for MTHFR-associated depression and anxiety. Another gene, NKPD1, was also recently linked to depressive symptoms . Researchers think it may account for up to four percent of the heritable risk for depression.
Another gene, NKPD1, was also recently linked to depressive symptoms . Researchers think it may account for up to four percent of the heritable risk for depression.
Iron deficiency anemia - what will a blood test for iron show? part 1
One test is not enough to diagnose iron deficiency anemia. It is necessary to evaluate a set of indicators: ferritin, transferrin, serum iron, hemoglobin, total iron-binding capacity. Iron deficiency can be latent, but the level of hemoglobin in the general blood test (CBC) does not cause concern. However, iron stores in the body are often depleted by that time (read more about ferritin in the article “Ferritin analysis: why is it needed?”). What does a serum iron test show?
What is iron?
The serum iron test is often referred to as the iron test and is the same test. However, it should not be confused with ferritin or hemoglobin. Iron is a trace element that is part of hemoglobin and other respiratory pigments that ensure the transport and delivery of oxygen to tissues. Iron is responsible for hematopoiesis and redox reactions of the body, is involved in the synthesis of collagen, the functioning of the immune system, and the metabolism of porphyrin. Fe deficiency leads to impaired hemoglobin synthesis and oxygen transport in the body.
Iron is responsible for hematopoiesis and redox reactions of the body, is involved in the synthesis of collagen, the functioning of the immune system, and the metabolism of porphyrin. Fe deficiency leads to impaired hemoglobin synthesis and oxygen transport in the body.
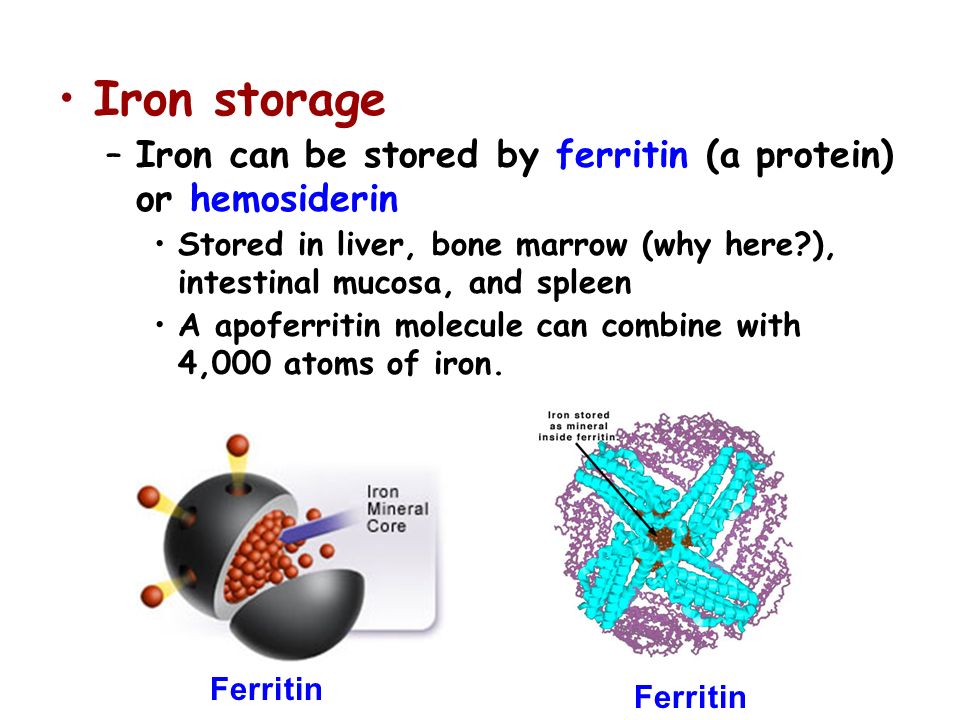
Man receives iron from food and is absorbed in the intestines. And here the transport protein ferritin, which carries and stores iron, is important.
Ferritin and serum iron are different tests,” explains Lyubava Kazakova, general practitioner at ELISA ICC. - The indicator of iron in the body can vary depending on the diet, and ferritin shows the supply of this substance. For example, serum iron may be one today and another tomorrow. It changes after eating or iron supplements. Ferretin does not change so dramatically. Also, ferritin can increase during inflammatory processes in the body. Thus, sometimes there is a false impression that the reserves of the substance are normal, but in fact this is a mistake.
Therefore, when diagnosing iron deficiency anemia, a complex of analyzes is needed.
A serum iron test is required to assess the amount of iron in the body at the time of the test. As we have already said, the main source of trace elements is food. Heme iron - meat and fish, non-heme - vegetables and fruits. It is important to understand that only 1-5% of iron is absorbed by the body from plant foods. From products of animal origin, this figure reaches 35%. The process of absorption of the substance is "controlled" by the intestine. Excess microelement is deposited in the body in reserve. But the level of ferrum in serum varies throughout the day, and also depends on age and gender.
Norm of iron content in the blood
The level of Fe in the blood of men and women is different. For physiological reasons, in women the indicator will be lower (menstrual cycle and pregnancy), in men it will be higher. The average for men is 14.3–25.1 µmol/l, for women it is 10. 7–21.5 µmol/l.
7–21.5 µmol/l.
Regardless of gender, the older a person is, the lower his level of iron in the body.
What reduces the level of ferrum in the blood?
- Age. The older the person, the lower the serum iron concentration.
- Menstruation and pregnancy.
- Chronic fatigue and stress.
- Sleep deficiency.
- Physical overvoltage.
Anemia. How to increase the level of iron in the blood?
Impaired intake, absorption or loss of iron can lead to iron deficiency anemia. Anemia is not an independent diagnosis, more often it is a consequence of chronic diseases: tumor-like formations, polyps, intestinal diverticulosis, uterine fibroids, endometriosis, gastric ulcer ... Before treatment, it is necessary to find out the cause of iron deficiency.
Causes of iron deficiency anemia:
- Nutritional deficiencies (older people are more likely to suffer, in whose diet there are more products of dairy and vegetable origin than animal).
- Diseases (gastric ulcer, duodenal ulcer, various polyps, tumors. In oncology, hemoglobin decreases, which is associated with increased destruction of iron in the body).
- Menorrhagia in women (heavy menstruation).
Symptoms:
- General weakness.
- Increased fatigue.
- Hair loss and brittle nails.
- Rapid heartbeat.
- Increased irritability, anxiety.
- Light sleep.
- Problems with memory and concentration.
- Dysphagia (impaired swallowing of dry food).
Therapy should be an integrated approach: dietary changes plus the use of synthetic forms of iron. In some diseases, for example, resection of the small intestine, the problem of iron deficiency cannot be solved by changing the diet. Parenteral administration of drugs is required.
What increases the level of iron in the blood?
- Products containing iron.
- Taking iron supplements.
There is a category of people who do not consume products of animal origin and are sure that hemoglobin can be raised by plant products with a high iron content, says Lyubava Kazakova. - Yes, there is iron in buckwheat, green vegetables, pomegranates. But from plant foods, an average of 1-5% of iron is absorbed. This is due to the fact that these products contain iron in a trivalent form, and when it enters the body, it must be converted to a divalent form.
There is a lot of controversy about pomegranate juice, which increases hemoglobin. But if you drink 3 glasses of juice a day, then the harm from this will be more than good. Pomegranate juice is highly acidic, which can exacerbate chronic gastritis and other gastrointestinal problems. However, the pomegranate contains a large amount of vitamin C and B, which improve the absorption of the trace element. That is, if you have animal products (meat and offal), green vegetables and pomegranates in your diet, this will improve the absorption of iron.
As for meat, you don't have to eat it every day! It will be enough 1-2 times a week, and on other days replace it with fish and eggs.
Is it possible to increase the level of a microelement in the blood with a hematogen? Hematogen is a food supplement, a bar, which includes black food albumin (powder from the blood of cattle). It should be understood that this is a supplement, not a medicine. It is impossible to cure anemia and increase the level of iron in the blood with hematogen alone. The bar can be considered as a source of useful trace elements and vitamins, but not as an alternative to iron-containing preparations.
Why doesn't my Fe level rise if you take iron supplements or eat iron-rich foods? This may be due to the fact that substances that interfere with the absorption of iron enter the body: tea, coffee, calcium preparations.
Pregnancy anemia, tests to detect anemia, high iron levels - continue article
More about anemia on our website:
Sequelae of anemia if left untreated, video
Symptoms of anemia in children
ANEMIA and PREGNANCY, OUTPATIENT PRACTICE
Anemia of pregnancy is a series of anemic conditions that occur during pregnancy, complicate its course and usually disappear soon after childbirth or after its termination. Since the prevalence of anemia in pregnant women is much higher than in non-pregnant women, it is logical to assume that most of these anemias are related to the pregnancy itself. Isolation in the ICD-10 of anemia in pregnant women (this is code O 99.0) in a separate section emphasizes the peculiarity of this group of anemias, which consists in the existence of physiological and pathophysiological changes characteristic of pregnancy that contribute to the development of anemia.
Since the prevalence of anemia in pregnant women is much higher than in non-pregnant women, it is logical to assume that most of these anemias are related to the pregnancy itself. Isolation in the ICD-10 of anemia in pregnant women (this is code O 99.0) in a separate section emphasizes the peculiarity of this group of anemias, which consists in the existence of physiological and pathophysiological changes characteristic of pregnancy that contribute to the development of anemia.
The most common consequences of anemia in pregnancy are miscarriage, premature birth, intrauterine growth retardation and an increased risk of low birth weight newborns. The development of anemia in the 1st and 2nd trimesters of pregnancy is associated with a two-fold increase in the risk of preterm birth.
Most researchers believe that iron stores in the fetus do not depend on the iron content in the mother's body. The transfer of iron from the mother through the placenta is regulated by the needs of the fetus, even against the concentration gradient, and mainly occurs in the 3rd trimester of pregnancy. That is why the development of iron deficiency is possible only in premature babies.
That is why the development of iron deficiency is possible only in premature babies.
These adverse effects of anemia in pregnancy are generally associated with hemoglobin levels < 90g/l. With a hemoglobin level of 90-110 g / l in the second half of pregnancy, the prognosis for a woman and a child is favorable. At the same time, an increase in the concentration of Hb above 120 g / l in this period of gestation is fraught with a high risk of complications (in particular, preeclampsia).
Any pregnancy results in an increase in plasma volume, which averages 1250 ml. This is approximately 1.5 times the plasma volume in non-pregnant women. This condition is one of the main reasons for the relative decrease in Hb levels in pregnant women.
Today, the lower limit of the normal concentration of Hb in pregnant women is 110 g / l, Hb from 90 to 110 g / l is anemia of 1 degree, from 70 to 90 g / l - anemia of 2 degrees, < 70 g / l - anemia 3 tbsp.
According to WHO, 35-75% of pregnant women in the world are anaemic every year. In domestic obstetric practice, the prevalence of iron deficiency in pregnant women is considered to be high.
In domestic obstetric practice, the prevalence of iron deficiency in pregnant women is considered to be high.
Pregnancy anemia is multifactorial, and iron deficiency is an important, but by no means the only, cause of anemia during pregnancy.
4. Hemolytic anemias outside of hemoglobinopathies
The most common types of anemia in pregnancy include iron deficiency anemia (IDA) and folic deficiency anemia, and less frequent are aplastic, megaloblastic, hemolytic anemias.
Factors that predispose to the development of IDA in pregnant women include frequent bleeding with placenta previa; anemia that existed in the patient's mother during pregnancy and prematurity of the patient, as well as seasonality and related changes in the composition of food (vitamin deficiency in the winter-spring period).
Anemia of inflammation - in recent years, there has been an increase in the number of women with urogenital infections (colpitis, cervicitis, bacterial vaginosis, pyelonephritis, etc. ), which often occur latently. At the same time, about 30% of pregnant women with urogenital infections approach childbirth in a state of anemia, despite the repeated correction with iron preparations. This anemia is defined as "hypochromic anemia without iron deficiency" with normal or elevated iron stores in the body.
), which often occur latently. At the same time, about 30% of pregnant women with urogenital infections approach childbirth in a state of anemia, despite the repeated correction with iron preparations. This anemia is defined as "hypochromic anemia without iron deficiency" with normal or elevated iron stores in the body.
Complaints with anemia during pregnancy are usually rare, usually in the presence of concomitant pathology. The most characteristic are complaints of weakness, dizziness, fatigue, in more severe cases, shortness of breath, anxiety and impaired consciousness.
Examination and treatment at the outpatient stage of patients with anemia in pregnancy is carried out in accordance with the Order of the Ministry of Health of the Russian Federation dated 01.11.2012. No. 572n.
Examination at the outpatient stage:
1) Completed general clinical blood test once a month with leukocyte count, count of reticulocytes and platelets.
2) ECG in each trimester.
3) Biochemical blood test (total protein, serum iron, ferritin, transferrin, total and direct bilirubin).
4) Consultation with a general practitioner (hematologist) and further follow-up (1-2 times a month).
5) Clarification of the diagnosis and resolution of the issue of the possibility of continuing the pregnancy up to 10 weeks.
6) Bone marrow puncture (as prescribed by a hematologist).
7) CTG and dopplerometry in dynamics.
Outpatient treatment:
1) Diet rich in proteins, iron, vitamins and folates.
2) Preparations containing iron (in accordance with the Decree of the Government of the Russian Federation of December 26, 2015 N 2724-r, which contains a list of vital and essential drugs for medical use for 2016) - these are oral preparations of 3-valent iron - iron 3 hydroxide polymaltose (maltofer, fenyuls, ferrum lek). It is not recommended to stop taking iron supplements after normalization of hemoglobin levels.
3) Treatment of underlying and concomitant diseases.
Indications for hospitalization:
1) Deterioration of the pregnant woman's condition, lack of effect from outpatient treatment of pregnancy complications.
2) Planned hospitalization for delivery at 38-39 weeks.
Prevention of anemia in pregnancy.
To maintain a normal iron balance during pregnancy, it is necessary that:
1. iron stores in the woman's body by the beginning of pregnancy are adequate;
2. The pregnant woman's diet contained sufficient bioavailable iron to ensure high levels of intestinal absorption in the 2nd half of pregnancy.
The daily iron requirement of a pregnant woman is estimated at 27 mg. Lower intake in women with inadequate iron stores may lead to anemia. Iron absorption is greatly increased in the presence of ascorbic acid. Therefore, it is most beneficial to consume foods containing iron in combination with foods fortified with vitamin C.
WHO recommends 60 mg iron per day for all pregnant women in areas where the prevalence of IDA is less than 20% and 120 mg where it is above this value.
Prevention of iron deficiency during pregnancy planning is an ideal form of prevention of IDA in pregnant women.
Primary prevention of IDA in pregnant women aims to reduce the prevalence of iron deficiency during pregnancy and prevent the adverse effects it can cause on the woman and fetus.
2014
1803
382
21,2
2015
1782
462
26
Statistical data on the incidence of anemia for 6 months. 2015 and 6 months 2016
| Finished pregnancy | Anemia of pregnancy | % | |
| 6 months 2015 | 845 | 155 | 18% |
| 6 months. 2016 | 1118 | 250 | 22. |
If analyzed the above incidence of anemia in pregnant women over the past 3 years, then the following could be said, in 2013. and in 2015 % of this pathology among pregnant women remained approximately at the same level, in 2014 there was a slight decrease in this indicator to 21.2%.
Management of patients with anemia of pregnancy in the w/c No. 2.
No. 572n.
2. When the diagnosis of anemia of pregnancy is established, the patient is referred for a consultation with a general practitioner f/c.
3. With anemia 2-3 tbsp. and not amenable to correction of anemia 1 tbsp. the woman is sent for a consultation with a hematologist.
4. Within the framework of the Birth certificate program, we prescribe free oral iron preparations to patients with anemia of pregnancy.
5. In accordance with the order of the Ministry of Health of the Republic of Kazakhstan of 21.01. No. 54 “On providing adequate nutrition for pregnant women, nursing mothers, as well as children under the age of 3 years in the Ryazan region”, we issue certificates for receiving monthly monetary compensation before childbirth to pregnant women diagnosed with anemia of 2-3 tbsp.
 3%
3%