Allergic reaction to fluoxetine
Side effects of fluoxetine - NHS
Like all medicines, fluoxetine can cause side effects in some people, but many people have no side effects or only minor ones.
Some of the common side effects will gradually improve as your body gets used to it.
Common side effects
These common side effects of fluoxetine happen in more than 1 in 100 people. There are things you can do to help cope with them:
Feeling sick (nausea)Try taking fluoxetine with or after food. It may also help to stick to simple meals and avoid rich or spicy food.
HeadachesMake sure you rest and drink plenty of fluids. Try not to drink too much alcohol. Ask your pharmacist to recommend a painkiller.
Headaches usually go away after the first week of taking fluoxetine. Talk to your doctor if they last longer than a week or are severe.
Take fluoxetine first thing in the morning.
DiarrhoeaDrink plenty of water or other fluids to avoid dehydration. Signs of dehydration include peeing less than usual or having dark, strong-smelling pee.
Do not take any other medicines to treat diarrhoea without speaking to a pharmacist or doctor.
If you take contraceptive pills and you have severe diarrhoea for more than 24 hours, your contraceptive pills may not protect you from pregnancy. Check the pill packet to find out what to do.
Feeling tired or weakIf fluoxetine makes you feel tired or weak, stop what you’re doing and sit or lie down until you feel better.
Do not drive, ride a bike or use tools or machinery if you’re feeling tired.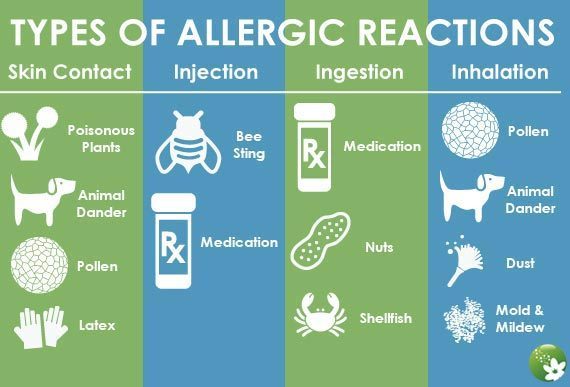 It’s best not to drink alcohol as it will make you feel worse.
It’s best not to drink alcohol as it will make you feel worse.
If these symptoms do not go away after a week or two, ask your doctor for advice.
Speak to a doctor or pharmacist if the advice on how to cope does not help and a side effect is still bothering you or does not go away.
Serious side effects
It happens rarely (in less than 1 in 100 people), but some people may have serious side effects when taking fluoxetine.
Book an appointment with your doctor if you:
- gain weight gain or lose weight without trying
- get changes in your periods such as heavy bleeding, spotting, or bleeding between periods
Call a doctor or contact 111 straight away if you:
- get feelings of overwhelming happiness (euphoria), excessive enthusiasm or excitement, or a feeling of restlessness that means you cannot sit or stand still
- are bleeding from the gums or get bruises that appear without a reason or that get bigger
- are coughing up blood, or have blood in your pee
- have black or red poo, or blood in your vomit – these can be signs of bleeding in your stomach
Go to 111.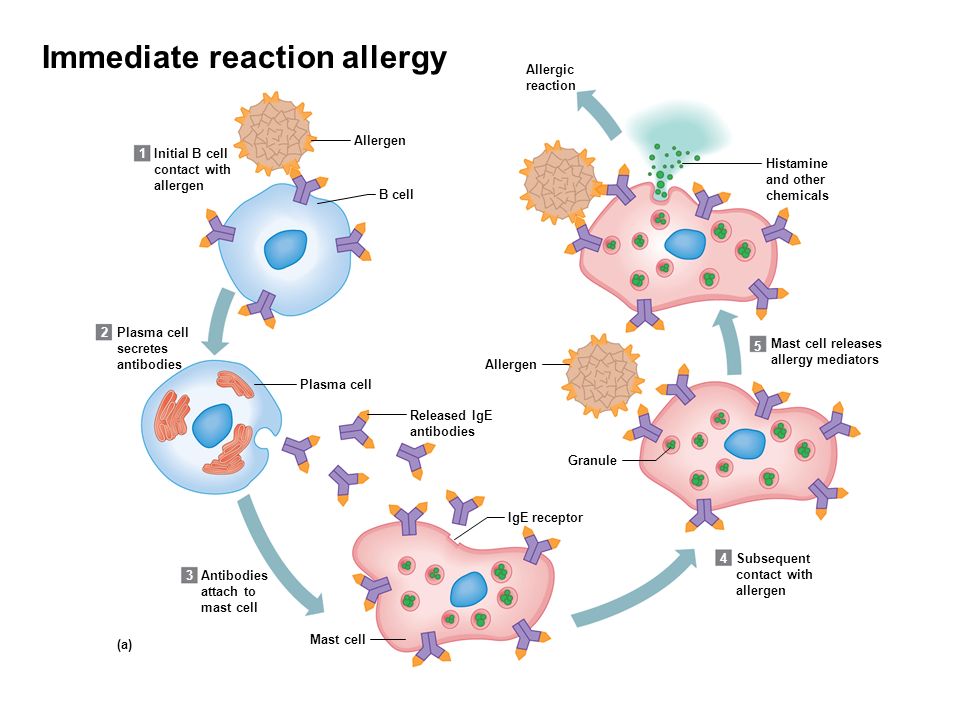 nhs.uk or call 111.
nhs.uk or call 111.
Immediate action required: Call 999 or go to A&E now if:
- you get chest pain or pressure, or shortness of breath
- you get severe dizziness or pass out
- you get painful erections that last longer than 2 hours – this may happen even when you are not having sex
- you have a fit or seizure
- you get headaches, have trouble focusing, have memory problems, cannot think clearly, have a seizure or fit, or lose your balance – these can be signs of low sodium levels
- you have thoughts about harming yourself or ending your life
- you get any severe bleeding, or bleeding that you cannot stop, such as cuts or nosebleeds that do not stop within 10 minutes
Serious allergic reaction
In rare cases, it's possible to have a serious allergic reaction (anaphylaxis) to fluoxetine.
Immediate action required: Call 999 or go to A&E now if:
- you get a skin rash that may include itchy, red, swollen, blistered or peeling skin
- you're wheezing
- you get tightness in the chest or throat
- you have trouble breathing or talking
- your mouth, face, lips, tongue or throat start swelling
You could be having a serious allergic reaction and may need immediate treatment in hospital.
Long-term side effects
Sexual side effects, such as problems getting an erection or a lower sex drive, have been reported after taking fluoxetine for a long time. In some cases, these can continue even after stopping the medicine.
There do not seem to be any lasting harmful effects from taking fluoxetine for many months and years but if you are worried, speak to your doctor.
Other side effects
These are not all the side effects of fluoxetine. For a full list, see the leaflet inside your medicine packet.
Information:
You can report any suspected side effect using the Yellow Card safety scheme.
Visit Yellow Card for further information.
Page last reviewed: 10 February 2022
Next review due: 10 February 2025
Fluoxetine-Induced Hypersensitivity | JAMA Dermatology
Fluoxetine-Induced Hypersensitivity | JAMA Dermatology | JAMA Network [Skip to Navigation]This Issue
- Download PDF
- Full Text
-
Share
Twitter Facebook Email LinkedIn
- Cite This
- Permissions
Article
June 1994
Kenneth Beer, MD; John Albertini, MD; Maria Medenica, MD; et al Shail Busbey, MD
Author Affiliations
Section of Dermatology Department of Medicine The University of Chicago 5841 S Maryland Ave, MC 5067 Chicago, IL 60637
Arch Dermatol.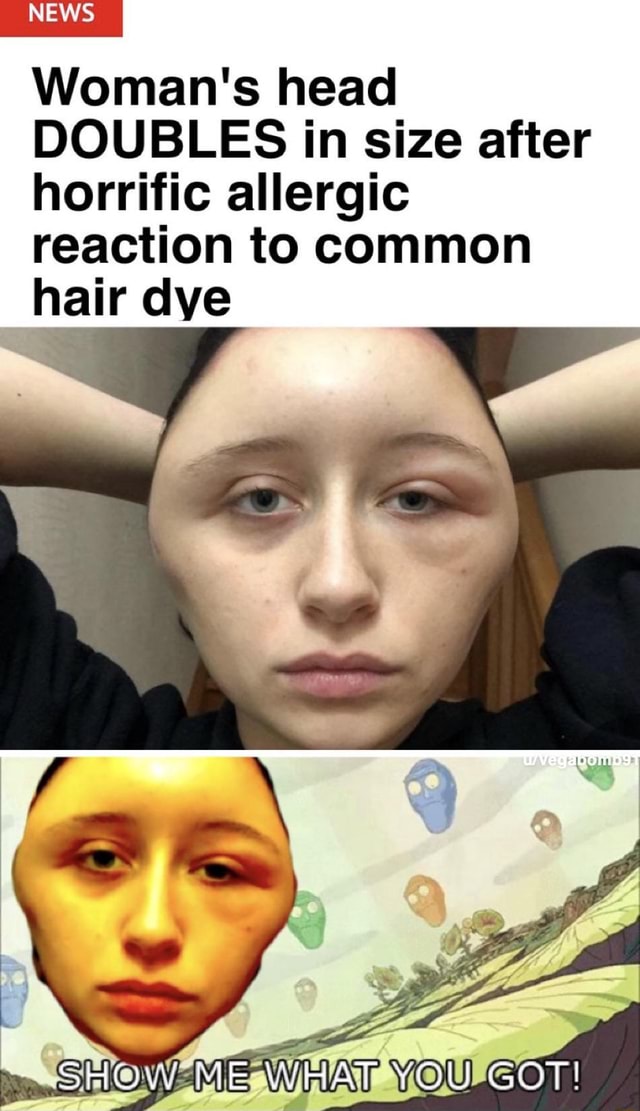 1994;130(6):803-804. doi:10.1001/archderm.1994.01690060141027
1994;130(6):803-804. doi:10.1001/archderm.1994.01690060141027
Full Text
Abstract
We report what we believe to be the first case of hypersensitivity to fluoxetine (Prozac) associated with a rash, eosinophilia, and arthralgias. Our patient responded to cessation of the medication and administration of steroids. On rechallenge, she developed dermatitis but not eosinophilia. The mechanism of the hypersensitivity is unknown.
Fluoxetine is a commonly prescribed antidepressant. According to the manufacturer, approximately 4% of patients taking the medication will develop a rash (personal communication, Eli Lilly Inc, Indianapolis, Ind). To our knowledge, this case represents the first reported instance of a fluoxetine-induced dermatitis associated with eosinophilia and arthralgias.
Report of a Case.
A 44-year-old white woman with an 8-week history of a pruritic eruption presented to the dermatology clinic.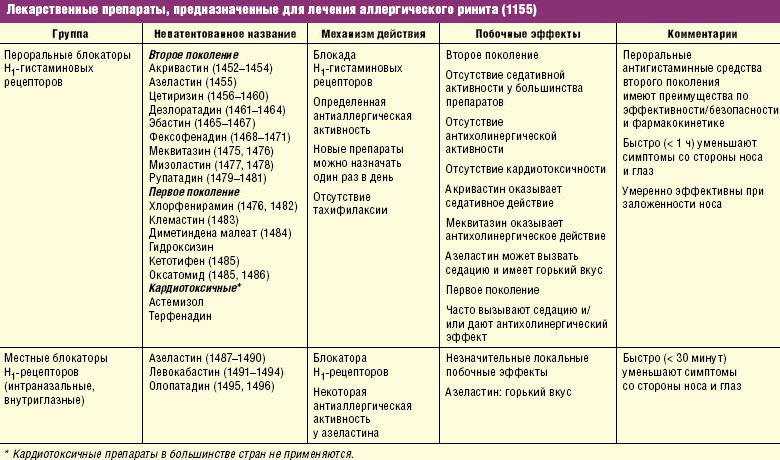 She denied arthralgias, photosensitivity, diarrhea, headaches, oral ulcers, or Raynaud's symptoms. She had cats in her home. She denied eating uncooked fish, undercooked meats, or foreign travel.Her medications included imipramine, which she
She denied arthralgias, photosensitivity, diarrhea, headaches, oral ulcers, or Raynaud's symptoms. She had cats in her home. She denied eating uncooked fish, undercooked meats, or foreign travel.Her medications included imipramine, which she
Full Text
Add or change institution
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Bleeding and Transfusion
- Cardiology
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life
- Environmental Health
- Equity, Diversity, and Inclusion
- Ethics
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Geriatrics
- Global Health
- Guide to Statistics and Methods
- Guidelines
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Informatics
- Health Policy
- Hematology
- History of Medicine
- Humanities
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Melanoma
- Mobile Health and Telemedicine
- Narrative Medicine
- Nephrology
- Neurology
- Neuroscience and Psychiatry
- Notable Notes
- Nursing
- Nutrition
- Nutrition, Obesity, Exercise
- Obesity
- Obstetrics and Gynecology
- Occupational Health
- Oncology
- Ophthalmic Images
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Pediatrics
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Poetry
- Population Health
- Preventive Medicine
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Radiology
- Regulatory Agencies
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgery
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Toxicology
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Urology
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Violence
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Save Preferences
Privacy Policy | Terms of Use
| 💊 The composition of the drug Fluoxetine ✅ Use of Fluoxetine Save Search for analogues Interaction Description of the active ingredients of the preparation fluoxetine (Fluoxetine) The scientific information provided is general and cannot be used to make decisions. decisions about the use of a particular drug. nine0006 Update date: 2020. Marketing authorization holder:OZON, OOO (Russia) ATX code: N06AB03 (Fluoxetine) Active substance: fluoxetine (fluoxetine) Rec.INN WHO registered Dosage form
Release form, packaging and composition Fluoxetine
7 pcs. - blisters (1) - packs of cardboard. Clinical and pharmacological group: Antidepressant Pharmacotherapeutic group: Antidepressant Pharmacological action Antidepressant, propylamine derivative. Pharmacokinetics Absorbed from the gastrointestinal tract. Weakly metabolized during the "first pass" through the liver. Eating does not affect the degree of absorption, although it may slow down its rate. C max in plasma is achieved after 6-8 hours. C ss in plasma is achieved only after continuous administration for several weeks. Protein binding 94.5%. Easily penetrates through the BBB. T 1/2 fluoxetine is 2-3 days, norfluoxetine is 7-9 days. Excreted by the kidneys 80% and through the intestines - about 15%. Indications of the active substances of the drug fluoxetineDepression of various origins, obsessive-compulsive disorders, bulimic neurosis. Open list of ICD-10 codes
Dosage regimenThe route of administration and dosing regimen of a particular drug depends on its form of release and other factors. The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed. nine0043 Initial dose - 20 mg 1 time / day in the morning; if necessary, the dose can be increased after 3-4 weeks. The frequency of admission is 2-3 times / day. The maximum daily oral dose of for adults is 80 mg. Side effectsFrom the side of the central nervous system: possible anxiety, tremor, nervousness, drowsiness, headache, sleep disturbances. From the digestive system: possible diarrhea, nausea. nine0006 From the side of metabolism: increased sweating, hypoglycemia, hyponatremia (especially in elderly patients and with hypovolemia) are possible. From the reproductive system: decreased libido. Allergic reactions: possible skin rash, itching. Other: pain in the joints and muscles, shortness of breath, fever. Contraindications for useGlaucoma, bladder atony, severe renal dysfunction, benign prostatic hyperplasia, concomitant administration of MAO inhibitors, convulsive syndrome of various origins, epilepsy, pregnancy, lactation, hypersensitivity to fluoxetine. nine0006 Use in pregnancy and lactationContraindicated in pregnancy and lactation. Use in hepatic impairmentUse with extreme caution in patients with hepatic impairment. Use in impaired renal functionContraindicated in severe renal impairment. Use with extreme caution in patients with moderate and mild renal impairment. Use in childrenThe safety of fluoxetine in children has not been established. Use in elderly patients Elderly patients require dosage adjustment. Special instructionsUse with extreme caution in patients with impaired liver and kidney function, with a history of epileptic seizures, cardiovascular diseases. In patients with diabetes mellitus, changes in blood glucose levels are possible, which requires correction of the dosing regimen of hypoglycemic drugs. When used in debilitated patients while taking fluoxetine, the likelihood of developing epileptic seizures increases. nine0006 With the simultaneous use of fluoxetine and electroconvulsive therapy, prolonged epileptic seizures may develop. Fluoxetine can be used no earlier than 14 days after discontinuation of MAO inhibitors. The period after the abolition of fluoxetine before the start of therapy with MAO inhibitors should be at least 5 weeks. Elderly patients require dosage adjustment. The safety of fluoxetine in children has not been established. Do not drink alcohol during treatment. Influence on the ability to drive vehicles and mechanisms During the period of treatment, one should refrain from potentially hazardous activities that require increased attention and rapid psychomotor reactions. Drug interactionsWhen used simultaneously with drugs that have a depressant effect on the central nervous system, with ethanol, a significant increase in the inhibitory effect on the central nervous system, as well as an increase in the likelihood of convulsions, is possible. nine0006 With simultaneous use with MAO inhibitors, furazolidone, procarbazine, tryptophan, serotonin syndrome may develop (confusion, hypomania, restlessness, agitation, convulsions, dysarthria, hypertensive crisis, chills, tremor, nausea, vomiting, diarrhea). With simultaneous use, fluoxetine inhibits the metabolism of tricyclic and tetracyclic antidepressants, trazodone, carbamazepine, diazepam, metoprolol, terfenadine, phenytoin, which leads to an increase in their concentration in blood serum, an increase in their therapeutic and side effects. nine0006 With simultaneous use, inhibition of the biotransformation of drugs metabolized with the participation of the CYP2D6 isoenzyme is possible. When used simultaneously with hypoglycemic agents, their action may be enhanced. There have been reports of increased effects of warfarin when co-administered with fluoxetine. When used simultaneously with haloperidol, fluphenazine, maprotiline, metoclopramide, perphenazine, periciazine, pimozide, risperidone, sulpiride, trifluoperazine, cases of extrapyramidal symptoms and dystonia have been described; with dextromethorphan - a case of the development of hallucinations is described; with digoxin - a case of increasing the concentration of digoxin in the blood plasma. nine0006 When used simultaneously with lithium salts, an increase or decrease in the concentration of lithium in the blood plasma is possible. With simultaneous use, an increase in the concentration of imipramine or desipramine in the blood plasma by 2-10 times is possible (may persist for 3 weeks after fluoxetine is discontinued). When used simultaneously with propofol, a case has been described in which spontaneous movements were observed; with phenylpropanolamine - a case is described in which dizziness, weight loss, hyperactivity were observed. Co-administration may enhance the effects of flecainide, mexiletine, propafenone, thioridazine, zuclopenthixol. Keep |
FGBNU NTSPZ. ‹‹Depression in General Medicine: A Guide for Physicians››
One of the main arguments in favor of the use of first-line antidepressants in the treatment of affective disorders in general medical practice is the minimal (compared to second-line drugs) severity of side effects. nine0006
Differences in the complications of thymoanaleptic therapy are clearly seen when comparing the main manifestations of the undesirable effect of representatives of first-line antidepressants (SSRIs, SSOZS) and TCAs, which is clearly demonstrated by the data given in Table. fourteen.
Nevertheless, it is impossible to completely exclude complications even when using sparing psychotropic drugs. The greatest likelihood of developing side effects of psychopharmacotherapy occurs in patients with somatic diseases, as well as in elderly people who show hypersensitivity to psychotropic drugs; in these contingents, even with careful dose titration, in addition to the main thymoanaleptic effect, side effects may also occur.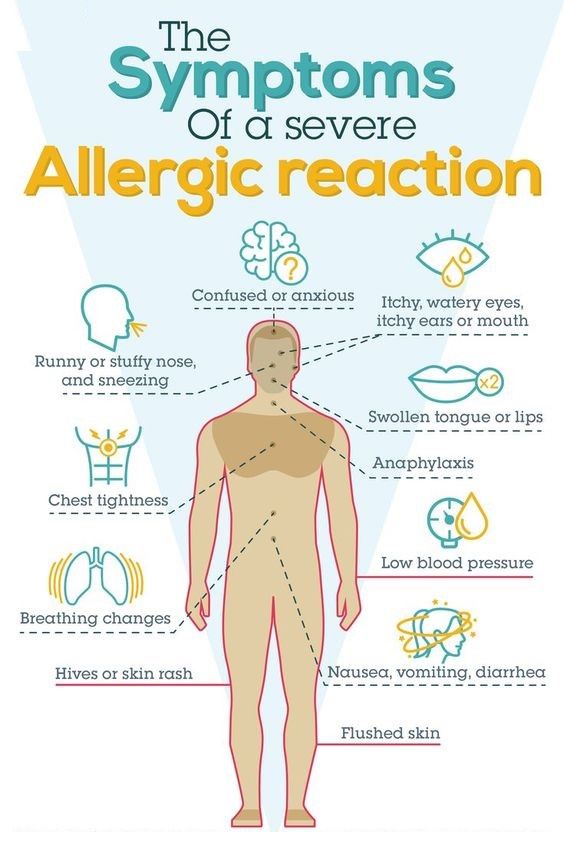 nine0006
nine0006
The main side effects of antidepressants include anticholinergic disorders of the central and autonomic nervous system, cardiovascular system, complications from the hematopoietic organs, metabolic and endocrine disorders (weight changes, sexual dysfunction, allergic reactions).
Side effects often appear in the initial stages of treatment (in the first 2 weeks) and sometimes persist for 3-4 weeks of therapy, and then reverse development. For more persistent and at the same time severe disorders, dose reduction is indicated, and, if necessary, therapy is discontinued. nine0006
Treatment with tianeptine (SSOZS) is accompanied by a minimum of undesirable effects. Side effects of the drug are most often limited to complaints of dry mouth, nausea, and drowsiness during the day. Only in some cases, the phenomena of transient orthostatic hypotension, dizziness, headaches, skin allergic reactions are also observed. The most common side effects of SSRIs are:
- nausea,
- dry mouth
- loss of appetite, nine0006
- vomit,
- diarrhea,
- constipation.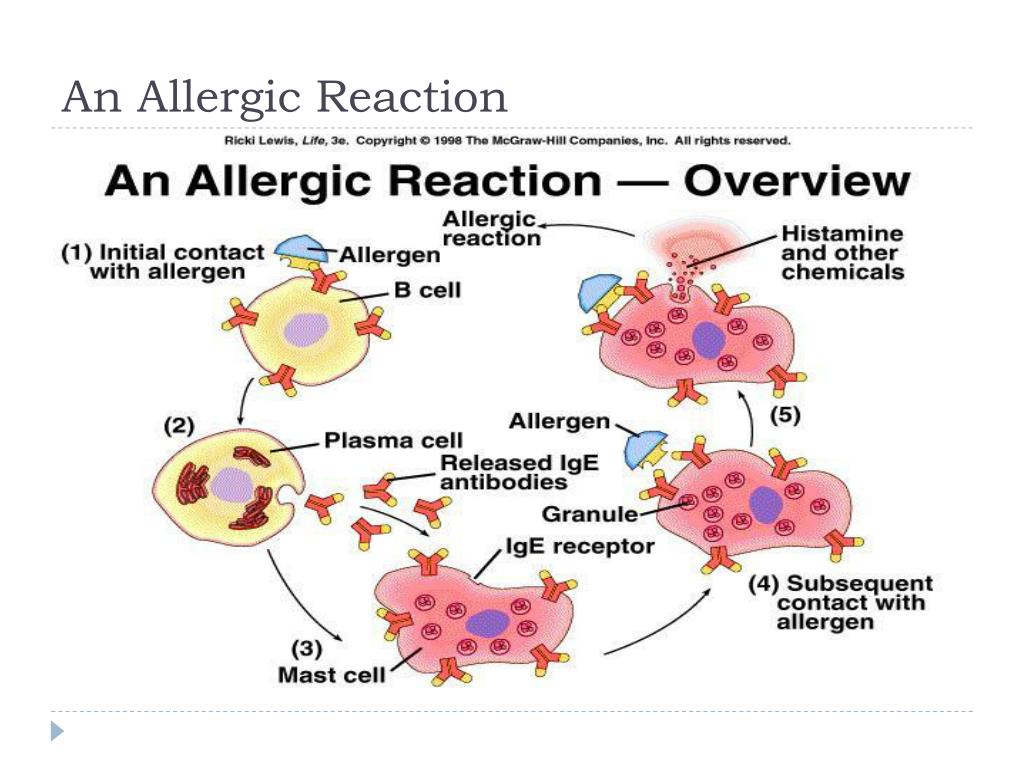
Along with this, undesirable effects from the autonomic and central nervous system are possible:
- dizziness,
headaches (citalopram)
- insomnia,
- increase (or appearance) of anxiety,
- nervousness
- a sense of inner tension.
The latter appear in the first weeks of treatment or with increasing doses. nine0006
There are transient extrapyramidal disorders in the form of tremor. As for other disorders (parkinsonism, akathisia, dyskinesias), judging by the data of a number of publications, they are recorded only in individual cases. The use of fluoxetine and paroxetine may be accompanied by increased bleeding and even bleeding.
During SSRI therapy, neurotoxic reactions (serotonin syndrome) affecting the gastrointestinal tract and nervous system are possible (colic in the abdomen , flatulence, loose stools, nausea, vomiting; tremor, dysarthria, muscle hypertonicity, hyperreflexia, myoclonic twitching, ataxia).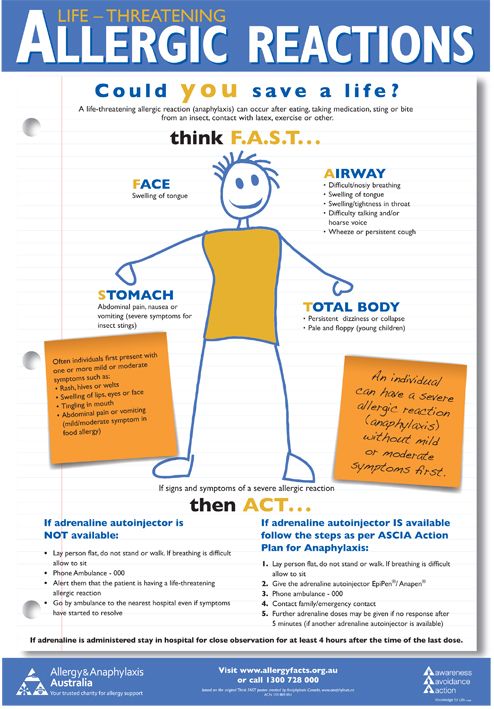 In more severe cases, hyperthermia, confusion, symptoms of disorientation join [Malin D.I., 2000]. nine0006
In more severe cases, hyperthermia, confusion, symptoms of disorientation join [Malin D.I., 2000]. nine0006
Severe complications often occur in the process of drug interaction with combined use:
- SSRIs and MAOIs,
- SSRIs and OIMAO-A (moclobemide),
- TCA (anafranil) and OIMAO-A.
Along with side effects, the effects of antidepressants associated with overdose are of great importance (especially in the context of a general medical network). The risk of deliberately taking large amounts of drugs for suicidal purposes poses a safety advantage to first-line antidepressants. This is evidenced by comparative data on the safety of some first-line and second-line antidepressants, presented in Table. fifteen. nine0006
The safety of first-line drugs is indicated by the data of C. Las-mier et al. (1991) in relation to tianeptine. Taking large doses of this drug (from 12 to 60 tablets) (12 cases of suicidal use of tianeptine are summarized) does not lead to death and is not accompanied by significant (compared with the norm) deviations of the clinical and electrocardiological parameters of the cardiovascular system.
Table 15. Safety of antidepressants [based on R. Priest, D. Baldwin, 1994]
| Preparations | Hazard measure (number of deaths from overdose per 1 million prescriptions) | Degree of danger |
| First line antidepressants | ||
| Fluoxetine (Prozac) Fluvoxamine (Fevarin) Mianserin (Lerivon) | Less than 10 | nine0414 |
| Second line antidepressants | ||
| Clomipramine (Anafranil) Maprotiline (Ludiomil) Trazadone (Tritgiko) | More than 10 | Potentially dangerous |
| Imipramine (Melipramine) Phenelzine (Nardil) | Over 20 | Dangerous |
| Amitriptyline Trianylcypramine (transamine) | Over 40 | Very dangerous |
With an overdose of milnacipran (ixel), vomiting, hyperventilation, and tachycardia are observed.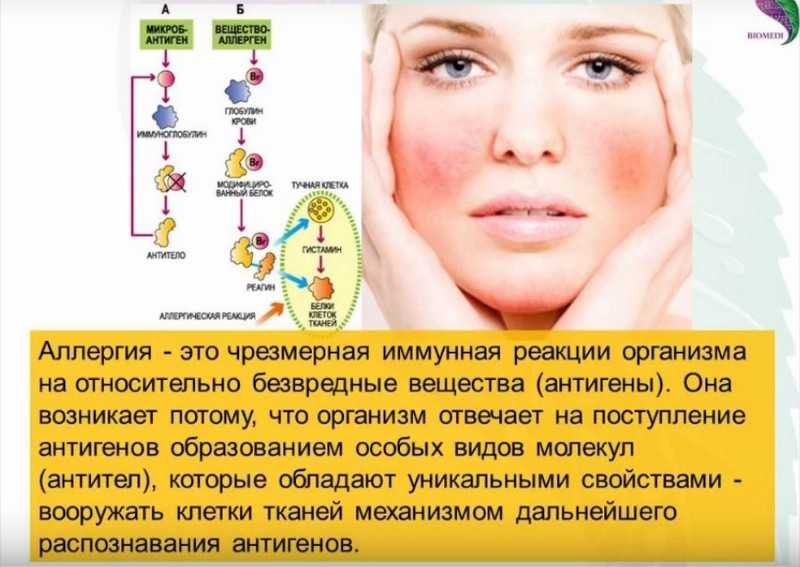
 10 mg: blisters of 7, 10, 14 or 20 pcs, containers of 7, 10, 14, 20, 28, 30, 40, 50 or 100 pcs. (21151)
10 mg: blisters of 7, 10, 14 or 20 pcs, containers of 7, 10, 14, 20, 28, 30, 40, 50 or 100 pcs. (21151)  06.09
06.09 


 - polymer containers (20) - packs of cardboard.
- polymer containers (20) - packs of cardboard.  The mechanism of action is associated with selective blockade of neuronal reuptake of serotonin in the CNS. Fluoxetine is a weak antagonist of cholino-, adreno- and histamine receptors. Unlike most antidepressants, fluoxetine does not appear to cause a decrease in the functional activity of postsynaptic β-adrenergic receptors. Helps improve mood, reduces feelings of fear and tension, eliminates dysphoria. Does not cause sedation. When taken in average therapeutic doses, it practically does not affect the functions of the cardiovascular and other systems. nine0006
The mechanism of action is associated with selective blockade of neuronal reuptake of serotonin in the CNS. Fluoxetine is a weak antagonist of cholino-, adreno- and histamine receptors. Unlike most antidepressants, fluoxetine does not appear to cause a decrease in the functional activity of postsynaptic β-adrenergic receptors. Helps improve mood, reduces feelings of fear and tension, eliminates dysphoria. Does not cause sedation. When taken in average therapeutic doses, it practically does not affect the functions of the cardiovascular and other systems. nine0006  It is metabolized in the liver by demethylation to form the main active metabolite of norfluoxetine. nine0006
It is metabolized in the liver by demethylation to form the main active metabolite of norfluoxetine. nine0006  2
2 


 nine0006
nine0006