Allergic reaction to effexor
Side effects of venlafaxine - NHS
Like all medicines, venlafaxine can cause side effects in some people, but many people have no side effects, or only minor ones.
Some of the common side effects of venlafaxine will gradually improve as your body gets used to it.
Common side effects
These common side effects of venlafaxine happen in more than 1 in 100 people. There are things you can do to help cope with them:
Feeling sick (nausea)Try taking venlafaxine with or after food. It may also help if you avoid rich or spicy food. If it carries on, tell your doctor.
Sweating and hot flushesTry wearing loose clothing and using or a fan, where possible. If there is no improvement after a week, speak to your doctor.
Make sure you rest, and drink plenty of fluids. It’s also best to avoid drinking too much alcohol. Ask your pharmacist to recommend a painkiller.
Headaches usually go away after the first week of taking venlafaxine. Talk to your doctor if they last longer than a week or are severe.
A dry mouthChew sugar-free gum or sugar-free sweets.
Feeling dizzyIf venlafaxine makes you feel dizzy when you stand up, try getting up very slowly or stay sitting down until you feel better. If you begin to feel dizzy, lie down so that you do not faint, then sit until you feel better.
Do not drive, ride a bike or use tools or machinery if you feel dizzy.
Feeling sleepyDo not drive, ride a bike or use tools or machinery if you're feeling sleepy. Cut down the amount of alcohol you drink as this will make you feel more tired.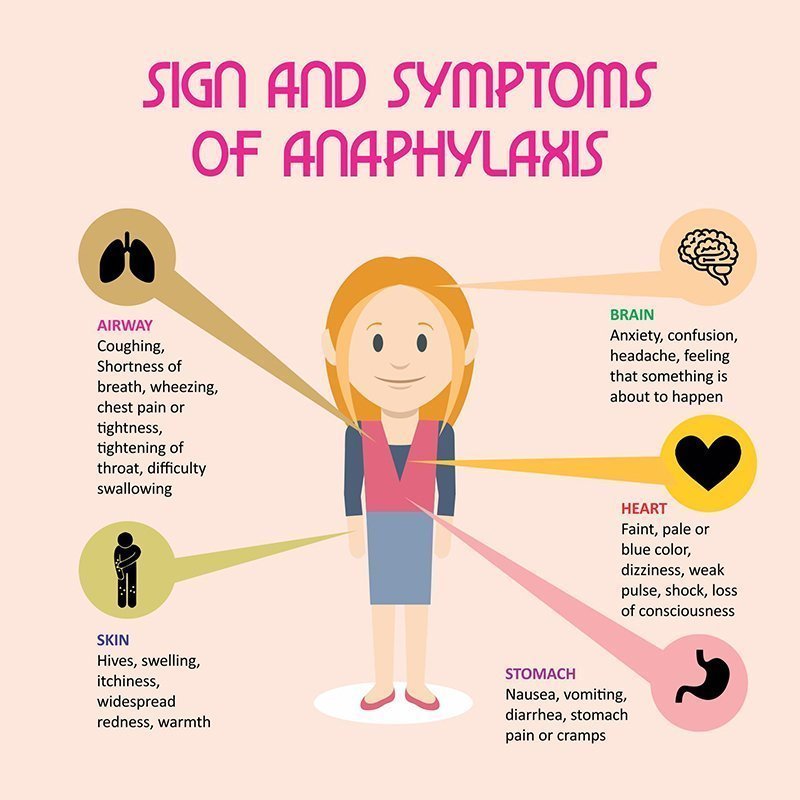 If this symptom does not go away after a week or two, ask your doctor for advice.
If this symptom does not go away after a week or two, ask your doctor for advice.
Take venlafaxine first thing in the morning.
ConstipationGet more fibre into your diet such as fresh fruit and vegetables and cereals, and drink plenty of water. Try to exercise, for example, by going for a daily walk or run. If this does not help, talk to your pharmacist or doctor.
If this advice does not help and any of these side effects bother you, talk to your doctor or pharmacist.
Serious side effects
It's not common, but some people (less than 1 in 100) may have serious side effects when taking venlafaxine.
Book an appointment with your doctor if:
- you gain weight or lose weight without trying
- you have changes in your periods such as heavy bleeding, spotting, or bleeding between periods
Call your doctor or contact 111 now if:
- you get constant headaches, long-lasting confusion, weakness, or frequent muscle cramps – these can all be signs of low sodium levels in your blood you have feelings of overwhelming happiness (euphoria), excessive enthusiasm or excitement, or a feeling of restlessness that means you cannot sit or stand still
- you have unexplained muscle pain or weakness
- the whites of your eyes or skin turn yellow, although this may be less obvious on black or brown skin – this can be a sign of liver problems
- you have any changes in your eyesight, like blurred vision or dilated pupils
- you cough up blood or have blood in your pee
- you have black or red poo, or blood in your vomit – these can be signs of bleeding in the stomach
- you are bleeding from the gums, or have bruises that appear without a reason or that get bigger
- you get shortness of breath, or a fast or irregular heart beat
- you have thoughts about harming yourself or ending your life
Go to 111. nhs.uk or call 111.
nhs.uk or call 111.
Immediate action required: Call 999 now if:
- you get chest pain or pressure in your chest, shortness of breath, or a fast or irregular heart beat
Serious allergic reaction
In rare cases, it's possible to have a serious allergic reaction (anaphylaxis) to venlafaxine.
Immediate action required: Call 999 or go to A&E now if:
- you get a skin rash that may include itchy, red, swollen, blistered or peeling skin
- you're wheezing
- you get tightness in the chest or throat
- you have trouble breathing or talking
- your mouth, face, lips, tongue or throat start swelling
You could be having a serious allergic reaction and may need immediate treatment in hospital.
Long-term side effects
For most people, venlafaxine is safe to take for a long time and there are no lasting effects.
A few people may get sexual side effects, such as problems getting an erection or a lower sex drive. In some cases these can continue even after stopping the medicine. Speak to your doctor if you are worried.
Other side effects
These are not all the side effects of venlafaxine. For a full list, see the leaflet inside your medicine packet.
Information:
You can report any suspected side effect using the Yellow Card safety scheme.
Visit Yellow Card for further information.
Page last reviewed: 10 February 2022
Next review due: 10 February 2025
Side Effects and What to Do about Them
If you have certain mental health conditions, your doctor might suggest Effexor XR (venlafaxine) as a treatment option for you.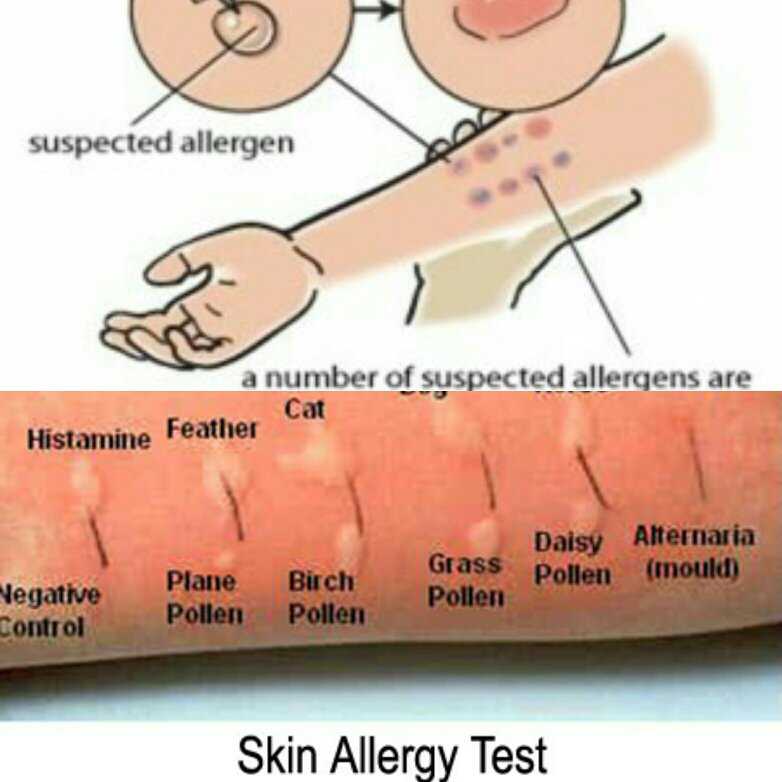
Effexor XR is a prescription medication that’s used in adults to treat:
- major depressive disorder
- generalized anxiety disorder
- social anxiety disorder
- panic disorder
Effexor XR helps relieve the symptoms of these conditions. The drug comes as a capsule that you take by mouth once daily. If Effexor XR works for you, your doctor will likely recommend that you take it long term.
Effexor XR is an extended-release (XR) drug, which means it slowly releases the active ingredient over a prolonged period of time.
For more information about Effexor XR, including details about its uses, see this in-depth article on the drug.
Like other drugs, Effexor XR can cause mild or serious side effects. Keep reading to learn more.
Some people may experience mild or serious side effects during their Effexor XR treatment. Examples of Effexor XR’s commonly reported side effects include:
- nausea
- feeling tired
- sweating*
- constipation
- sexual side effects
* To learn more about this side effect, see “Side effects explained” below.
Effexor XR may cause mild side effects in some people. Examples of mild side effects that have been reported with Effexor XR include:
- nausea
- feeling tired
- sweating*
- constipation
- dry mouth
- unusual dreams
- loss of appetite
- headache
- weight gain or weight loss*
* To learn more about this side effect, see “Side effects explained” below.
In most cases, these side effects should be temporary. And some may be easily managed, too. But if you have any symptoms that are ongoing or that bother you, talk with your doctor or pharmacist. And don’t stop using Effexor XR unless your doctor recommends it.
Effexor XR may cause mild side effects other than the ones listed above. See the Effexor XR medication guide for details.
Note: After the Food and Drug Administration (FDA) approves a drug, it tracks side effects of the medication. If you’d like to notify the FDA about a side effect you’ve had with Effexor XR, visit MedWatch.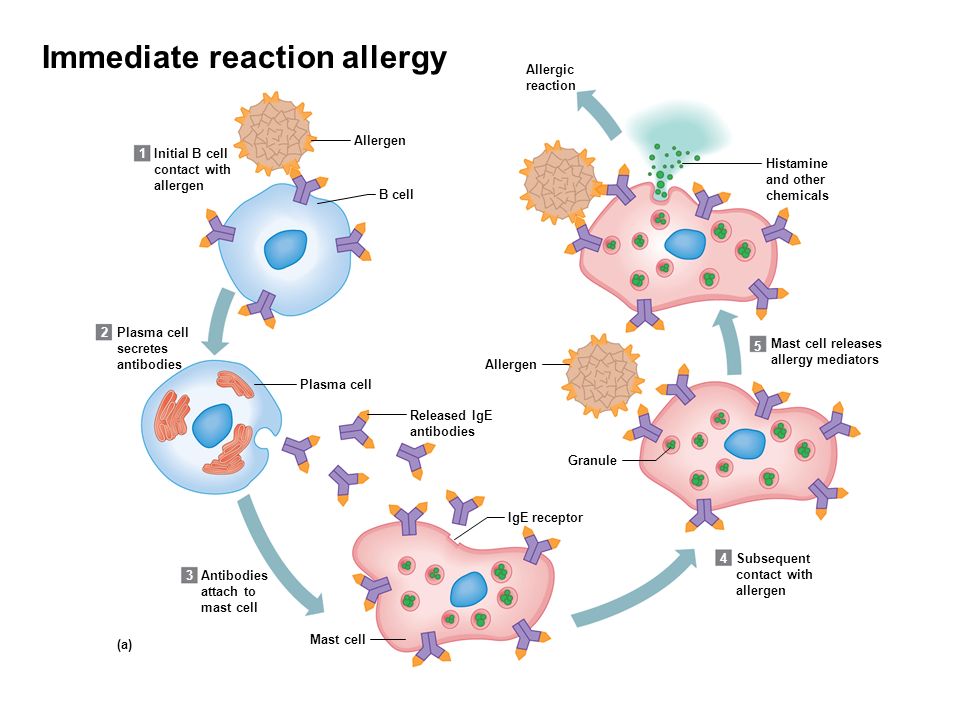
In rare cases, some people may develop serious side effects from taking Effexor XR. Serious side effects that have been reported with Effexor XR include:
- suicidal thoughts or behaviors*
- serotonin syndrome
- sexual side effects, such as erectile dysfunction and abnormal ejaculation
- high blood pressure†
- unusual bleeding
- eye problems, such as closed-angle glaucoma
- mania or hypomania
- allergic reaction†‡
- low sodium in your blood
- seizures
- lung problems, such as pneumonia
- high cholesterol
If you develop serious side effects while taking Effexor XR, call your doctor right away. If the side effects seem life threatening or if you think you’re having a medical emergency, immediately call 911 or your local emergency number.
* Effexor XR has a boxed warning for this side effect. This is the most serious warning from the Food and Drug Administration (FDA). To learn more, see the “Side effects explained” section below.
† To learn more about this side effect, see “Side effects explained” below.
‡ An allergic reaction is possible after using Effexor XR. But it’s not clear whether this side effect occurred in studies.
You may experience more side effects in your first week of treatment with Effexor XR. But not everyone will experience side effects, and side effects can be different for each person.
You may be more likely to experience suicidal thoughts and behaviors* during the first week of treatment. If you notice symptoms, be sure to talk with your doctor.
When you first start taking a new medication, your body has to get used to it. So in the first week, you may experience more side effects. It takes about 3 days for Effexor XR to reach a consistent level in your blood. During the time that your body is adjusting, you may have a higher risk for side effects.
Side effects that you experience may also depend on other medical conditions you have or other medications you take. If you have questions about what to expect when you first start taking Effexor XR, talk with your doctor or pharmacist.
If you have questions about what to expect when you first start taking Effexor XR, talk with your doctor or pharmacist.
* Effexor XR has a boxed warning for this side effect. This is the most serious warning from the Food and Drug Administration (FDA). To learn more, see the “Side effects explained” section below.
Get answers to some frequently asked questions about Effexor XR’s side effects.
Is my risk for side effects higher the first week I’m taking Effexor XR?
It’s possible. You may experience more side effects in your first week of treatment with Effexor XR. But not everyone will experience side effects, and side effects can be different for each person.
When you first start taking a new medication, your body has to get used to it. So in the first week, you may experience more side effects. It takes about 3 days for Effexor XR to reach a consistent level in your blood. During this time that your body is adjusting, you may have a higher risk for side effects.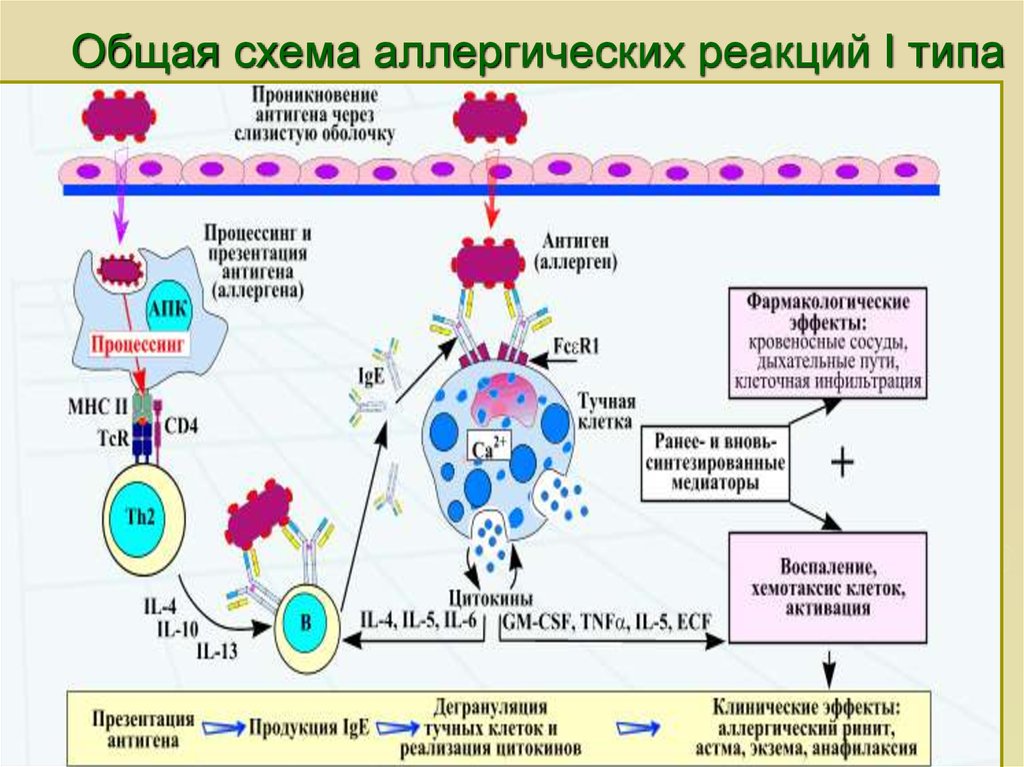
Side effects that you experience may also depend on other medical conditions you have or other medications you take. If you have questions about what to expect when you first start taking Effexor XR, talk with your doctor or pharmacist.
Are there any long-term side effects of Effexor XR?
Yes, long-term side effects of Effexor XR are possible. Examples include weight gain, weight loss, and eye problems such as closed-angle glaucoma.
It’s possible that taking Effexor XR for a longer period of time may raise your risk for long-term side effects. But this isn’t the case for everyone, as side effects of Effexor XR can vary from person to person.
If you’re concerned about long-term side effects of Effexor XR, talk with your doctor or pharmacist.
Do side effects from Effexor XR vary depending on the strength I take (37.5 mg, 75 mg, or 150 mg)?
It’s possible. You may have an increased risk for side effects if you’re taking a higher dose of Effexor XR. This is because there’s more medication in your body, and it may have a greater effect on you.
This is because there’s more medication in your body, and it may have a greater effect on you.
Effexor XR is available in strengths of 37.5 milligrams (mg), 75 mg, and 150 mg. In most cases, the maximum recommended dose of Effexor XR is 225 mg per day. In some cases, your doctor may prescribe a dose as high as 300 mg per day. But this is not an FDA-approved dose.
You should always take the dosage your doctor prescribes for you. They’ll determine the best dosage to fit your needs.
If you’re experiencing side effects from Effexor XR, talk with your doctor. They may want to lower your dose to see if this helps reduce your side effects.
Will Effexor XR cause withdrawal symptoms if I stop taking it?
Effexor XR can cause withdrawal symptoms if treatment stops suddenly.
Examples of withdrawal symptoms that may occur if you stop taking Effexor XR include:
- agitation or irritability
- confusion
- dizziness
- anxiety
- headache
- insomnia (trouble sleeping)
- seizures
If you’re interested in stopping your Effexor XR treatment, talk with your doctor first. They’ll likely want to decrease your dose slowly to help reduce the risk of you experiencing withdrawal symptoms.
They’ll likely want to decrease your dose slowly to help reduce the risk of you experiencing withdrawal symptoms.
In most cases, your doctor will decrease your dose by 75 milligrams per week until you’re not taking the drug anymore. But be sure to follow your doctor’s recommendation for decreasing your dose and stopping treatment.
Because some withdrawal symptoms may be serious, you should not stop taking Effexor XR without first talking with your doctor. They can help you safely stop your treatment.
Can side effects occur if I miss a dose of Effexor XR?
Yes, it’s possible that missing a dose of Effexor XR may cause certain side effects, including withdrawal symptoms.
If you miss a dose of Effexor XR, take it as soon as you remember. But if it’s almost time for your next dose, skip your missed dose and take your next dose on your regular schedule. You shouldn’t take two doses of Effexor XR to make up for a missed dose. Doing so could raise your risk for side effects.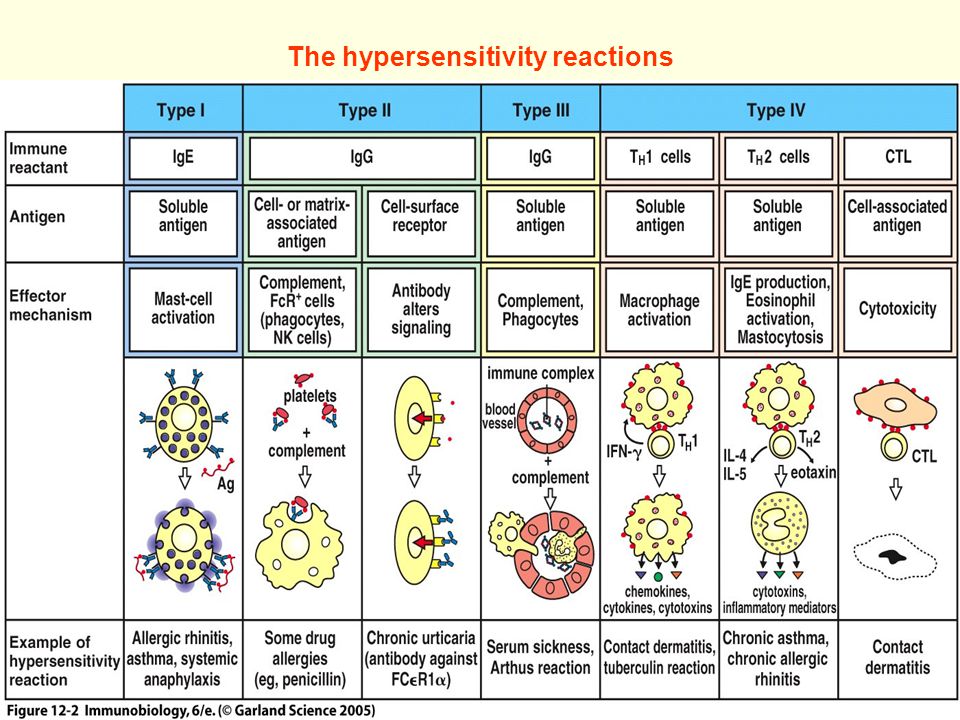
How long do side effects from Effexor XR last?
It depends. Some side effects, such as nausea, may occur when you first start taking Effexor XR but may go away after you take it for a while. Other side effects, including decreased appetite and changes in weight, may last throughout your treatment.
If you’re concerned about certain side effects of Effexor XR, talk with your doctor. They can discuss your risk for these side effects and how long they may last if you do experience them. Your doctor may also be able to treat your side effects so they don’t last as long.
Learn more about some of the side effects Effexor XR may cause.
Weight gain or weight loss
Weight gain or weight loss can occur in people taking Effexor XR. But these were not common side effects reported in studies of Effexor XR.
What might help
If you’re concerned about any unexpected weight gain or weight loss you experience while taking Effexor XR, talk with your doctor.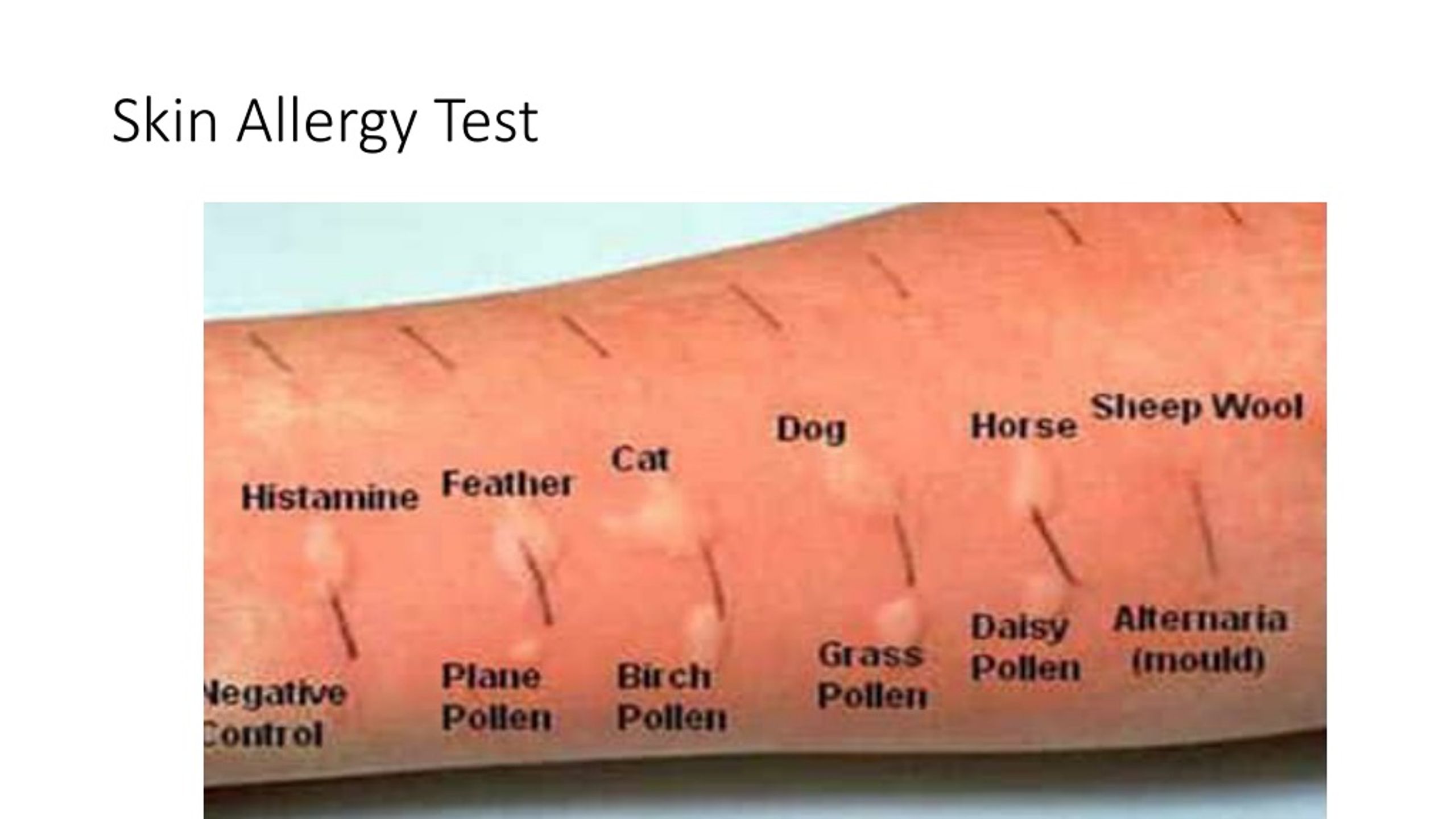 They can help determine what your next steps should be.
They can help determine what your next steps should be.
Sweating
You may experience sweating during your Effexor XR treatment. Sweating was one of the most common side effects that people taking Effexor XR reported in studies.
What might help
If you’re sweating more than usual while taking Effexor XR and it’s bothersome to you, talk with your doctor. They may be able to recommend ways to decrease this side effect. In some cases, they may recommend a different medication to treat your mental health condition.
Suicidal thoughts and behaviors in children and young adults
Effexor XR has a boxed warning for the risk of suicidal thoughts and behaviors in children and in young adults (ages 18 to 24 years). A boxed warning is the most serious warning from the Food and Drug Administration (FDA). It alerts doctors and patients about drug effects that may be dangerous.
All antidepressant drugs carry this boxed warning about suicidal thoughts and behaviors. These side effects can occur within the first few months of starting treatment or whenever the dose is increased or decreased.
These side effects can occur within the first few months of starting treatment or whenever the dose is increased or decreased.
It’s important to note that Effexor XR is not approved for anyone younger than 18 years old.
What might help
It’s important that you tell your doctor if you notice any new or worsening symptoms of depression or suicidal thoughts or behavior. These may include:
- suicidal thoughts or suicide attempts
- violence or aggression
- anxiety or panic attacks
- feeling restless or irritable
- insomnia (trouble sleeping)
- changes in behavior or mood
If you notice any of these symptoms, talk with your doctor. If the side effects seem life threatening or if you think you’re having a medical emergency, immediately call 911 or your local emergency number.
If you’re a young adult taking Effexor XR, your doctor will likely monitor you more closely during treatment for any symptoms of depression and suicidal thoughts or behaviors.
SUICIDE PREVENTIONIf you think someone is at immediate risk of self-harm or hurting another person:
- Call 911 or your local emergency number.
- Stay with the person until help arrives.
- Remove any guns, knives, medications, or other things that may cause harm.
- Listen, but don’t judge, argue, threaten, or yell.
If you or someone you know is considering suicide, get help from a crisis or suicide prevention hotline. Try the National Suicide Prevention Lifeline at 800-273-8255.
High blood pressure
Effexor may increase your blood pressure. In clinical studies, some people who didn’t already have high blood pressure developed the condition after starting treatment with Effexor XR.
If you already have high blood pressure, Effexor XR may make it worse.
What might help
Before you start taking Effexor XR, tell your doctor about any blood pressure concerns you have or if you’re taking medication to lower your blood pressure.
If you have untreated high blood pressure, your doctor will likely want to treat it before starting your Effexor XR treatment. This is because the drug can also increase your blood pressure, which may be unsafe if your blood pressure is already high.
Your doctor will also check and monitor your blood pressure throughout your treatment.
Allergic reaction
Like most drugs, Effexor XR can cause an allergic reaction in some people. But it’s not clear whether this side effect occurred in studies.
Symptoms can be mild or serious and can include:
- skin rash
- itchiness
- flushing (temporary warmth, redness, or deepening of skin color)
- swelling under your skin, typically in your eyelids, lips, hands, or feet
- swelling of your mouth, tongue, or throat, which can make it hard to breathe
Allergic reactions can also affect the eyes. It’s important that you tell your doctor if you notice any problems with your eyes. Symptoms may include:
- changes in your vision
- eye pain
- redness or swelling in or around your eye
What might help
If you have mild symptoms of an allergic reaction, such as a mild rash, call your doctor right away.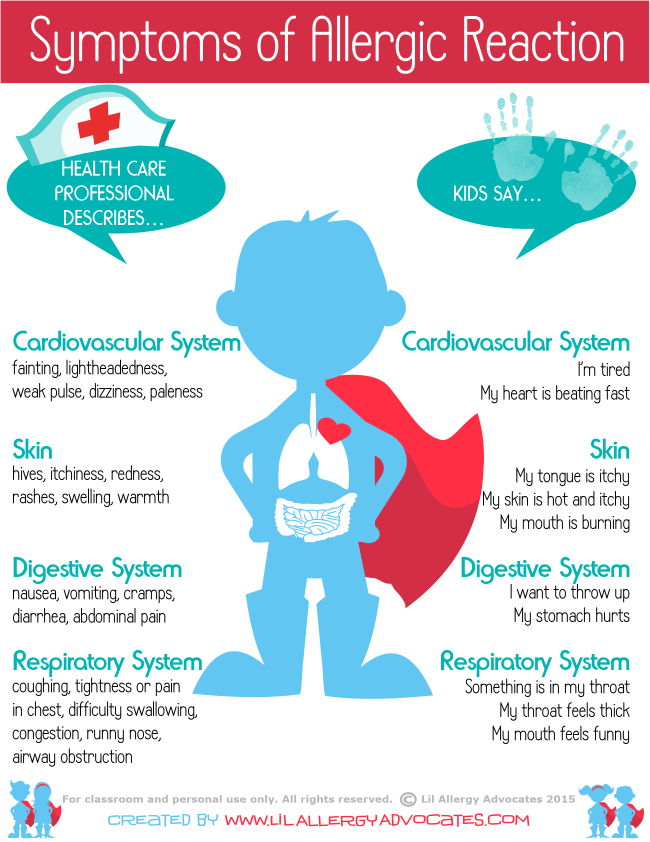 They may suggest an over-the-counter oral antihistamine, such as Benadryl (diphenhydramine), or a topical product, such as hydrocortisone cream, to manage your symptoms.
They may suggest an over-the-counter oral antihistamine, such as Benadryl (diphenhydramine), or a topical product, such as hydrocortisone cream, to manage your symptoms.
If your doctor confirms you had a mild allergic reaction to Effexor XR, they’ll decide if you should continue using it.
If you have symptoms of a severe allergic reaction, such as swelling or trouble breathing, call 911 or your local emergency number right away. These symptoms could be life threatening and require immediate medical care.
If your doctor confirms you had a serious allergic reaction to Effexor XR, they may have you switch to a different treatment.
Keeping track of side effectsDuring your Effexor XR treatment, consider keeping notes on any side effects you’re having. Then, you can share this information with your doctor. This is especially helpful to do when you first start taking new drugs or using a combination of treatments.
Your side effect notes can include things such as:
- what dose of drug you were taking when you had the side effect
- how soon after starting that dose you had the side effect
- what your symptoms were from the side effect
- how it affected your daily activities
- what other medications you were also taking
- any other information you feel is important
Keeping notes and sharing them with your doctor will help your doctor learn more about how the drug affects you.
Your doctor can use this information to adjust your treatment plan if needed.
Before you start taking Effexor XR, talk with your doctor about any medical conditions you have or any medications you take. They can determine if this medication may be a safe treatment option for you.
Details about this drug’s warnings are below.
Boxed warning: Suicidal thoughts and behaviors in children and young adults
Effexor XR has a boxed warning for suicidal thoughts and behaviors in children and in young adults (ages 18 to 24 years). A boxed warning is the most serious warning from the Food and Drug Administration (FDA). It alerts doctors and patients about drug effects that may be dangerous.
If you’re a young adult taking Effexor XR, your doctor will likely monitor you more closely during treatment for any symptoms of depression and suicidal thoughts or behaviors.
It’s important to note that Effexor XR is not approved for anyone younger than 18 years old.
To learn more, see the “Side effects explained” section above.
Other warnings
Effexor XR may not be right for you if you have certain medical conditions or other factors that affect your health. Talk with your doctor about your health history before you take Effexor XR. The list below includes factors to consider.
High blood pressure or other heart problems. Before taking Effexor XR, tell your doctor if you have high blood pressure. This drug may cause your blood pressure to increase even more. Your doctor will check your blood pressure before you start treatment. If it’s not well managed, your doctor will likely recommend treating your high blood pressure before you start taking Effexor XR. Even if your blood pressure is well managed, your doctor may want to monitor you more closely during your Effexor XR treatment. This is to make sure your blood pressure doesn’t get too high.
Allergic reaction. If you’ve had an allergic reaction to Effexor XR or any of its ingredients, you shouldn’t take Effexor XR.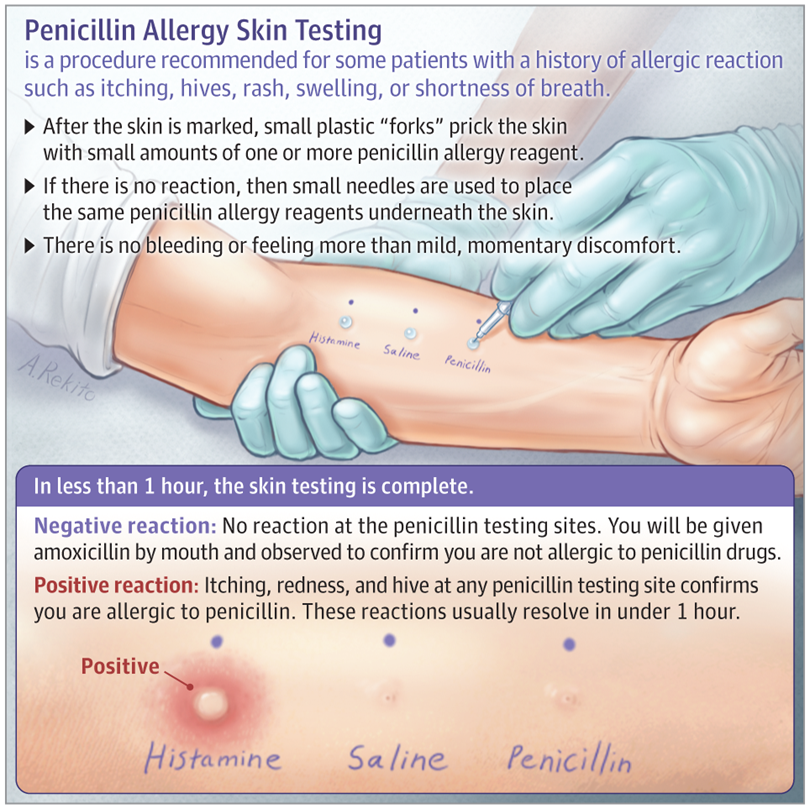 Ask your doctor what other medications are better options for you.
Ask your doctor what other medications are better options for you.
Bipolar disorder or mania. Effexor XR may make your symptoms of bipolar disorder or mania worse. If you have one of these conditions, your doctor may recommend a different medication to treat your condition, or they may monitor you more closely during your treatment.
Glaucoma. Effexor XR may cause an eye condition called closed-angle glaucoma. If you already have closed-angle glaucoma, Effexor XR may make your condition worse. Before taking Effexor XR, talk with your doctor about any eye conditions you have.
Liver problems. Effexor XR may cause elevated liver enzymes, which may be a sign of a liver condition. If you already have a liver problem, Effexor XR may make your condition worse. Before you start treatment with Effexor XR, tell your doctor about any liver conditions you have.
Kidney problems. Effexor XR is removed from your body through your kidneys. If your kidneys aren’t working properly, your body may not be able to get rid of the drug as quickly as it should. This can cause levels of the drug to build up in your body, which can raise your risk for side effects. If you have any kidney problems, talk with your doctor before taking Effexor XR. They can determine if this drug is safe for you to take.
If your kidneys aren’t working properly, your body may not be able to get rid of the drug as quickly as it should. This can cause levels of the drug to build up in your body, which can raise your risk for side effects. If you have any kidney problems, talk with your doctor before taking Effexor XR. They can determine if this drug is safe for you to take.
Seizures or convulsions. Effexor XR may raise your risk for seizures. If you have a seizure condition or have had seizures or convulsions before, Effexor XR may raise your risk for seizures even more. Talk with your doctor about whether Effexor XR is safe for you to take.
Low sodium in your blood. Effexor XR can decrease the amount of sodium in your blood. If you already have low sodium levels, this can be dangerous. Low sodium levels can cause confusion, fatigue, seizures, and even coma. If you have low sodium levels in your blood or have had this condition before, tell your doctor before taking Effexor XR.
High cholesterol. Effexor XR may cause your cholesterol levels to increase. If you already have high cholesterol, this drug can make it worse. Talk with your doctor to see if this drug is right for you.
Bleeding problems. Effexor XR may raise your risk for bleeding. If you have any conditions that could also cause bleeding problems, or if you’re taking medications that may affect your blood, tell your doctor before taking Effexor XR. They may recommend a different medication for you, or they may monitor you more closely throughout your treatment.
Alcohol use and Effexor XR
You should not drink alcohol while taking Effexor XR. Alcohol may raise your risk for certain side effects from Effexor XR.
If you have concerns about avoiding alcohol during your Effexor XR treatment, talk with your doctor.
Pregnancy and breastfeeding while taking Effexor XR
Below are details about using Effexor XR during pregnancy or while breastfeeding.
Pregnancy
It’s not known if Effexor XR is safe to take during pregnancy. If you’re pregnant or planning to become pregnant, talk with your doctor before taking Effexor XR.
Breastfeeding
Taking Effexor XR while breastfeeding is not recommended. Effexor XR passes into breast milk, and it may affect a child who is breastfed. If you’re currently breastfeeding or planning to breastfeed, talk with your doctor about your options.
Effexor XR may be an effective treatment for certain mental health conditions. But some people may experience side effects with this drug. Most side effects are mild, but some may be serious.
It’s important to discuss potential side effects with your doctor before taking Effexor XR so you know what to watch for. Here are some questions you may want to ask your doctor:
- How should I treat any side effects that I experience?
- Do I have a higher risk for side effects based on my other medical conditions?
- What should I do if I become pregnant while taking Effexor XR?
To get information on different conditions and tips for improving your health, subscribe to any of Healthline’s newsletters. You may also want to check out the online communities at Bezzy. It’s a place where people with certain conditions can find support and connect with others.
You may also want to check out the online communities at Bezzy. It’s a place where people with certain conditions can find support and connect with others.
Q:
If I’m taking a monoamine oxidase inhibitor (MAOI), how long should I wait between stopping the MAOI and starting treatment with Effexor XR so that I don’t experience side effects?
Anonymous
A:
A: If you’re taking Effexor XR with an MAOI such as the antidepressant Nardil (phenelzine) or the antibiotic Zyvox (linezolid), you should not take these medications within 7 days of stopping Effexor XR unless your doctor recommends it. It’s also important that you stop taking an MAOI at least 2 weeks before starting your Effexor XR treatment.
Before you stop taking any medications, be sure to check with your doctor first.
The Healthline Pharmacist TeamAnswers represent the opinions of our medical experts.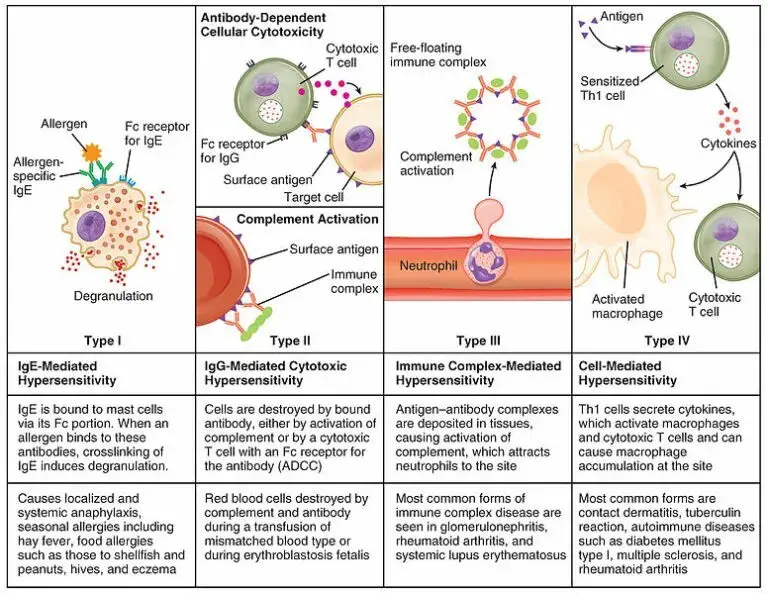 All content is strictly informational and should not be considered medical advice.
All content is strictly informational and should not be considered medical advice.
Disclaimer: Healthline has made every effort to make certain that all information is factually correct, comprehensive, and up to date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or another healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
Allergy to vaccines | Remedium.ru
Contents
- Vaccine allergies
- Clinical manifestations of allergy to vaccines
- Allergy to vaccine components
- Can a person with allergies be given the vaccine?
- How do you diagnose a vaccine allergy?
- Procedure for vaccination of patients with vaccine allergy
- References
Vaccines play a huge role in preventing a range of bacterial and viral diseases, including COVID-19, a new viral infection caused by SARS-CoV-2, which has caused a pandemic in the world.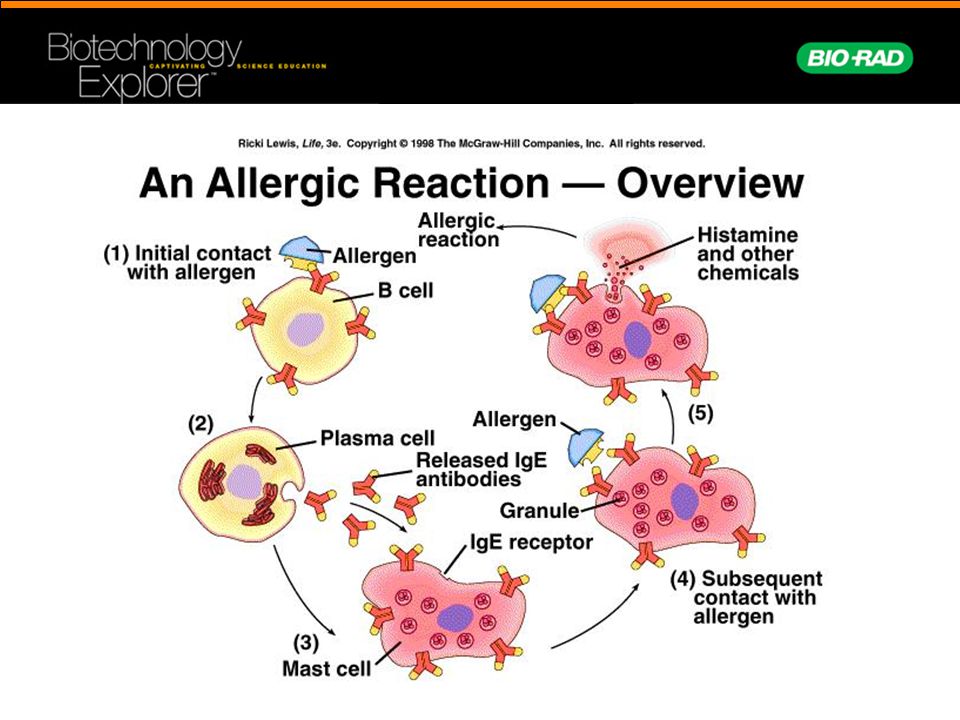 A number of vaccines have been created against COVID-19 in Russia - Sputnik V, Sputnik Light, Kovivak, Epivaccorona, but people are often afraid of severe side effects, including an allergy to vaccines. Severe allergic reactions to vaccines are rare but difficult to predict, and their existence significantly hinders the advancement of the idea of vaccination.
A number of vaccines have been created against COVID-19 in Russia - Sputnik V, Sputnik Light, Kovivak, Epivaccorona, but people are often afraid of severe side effects, including an allergy to vaccines. Severe allergic reactions to vaccines are rare but difficult to predict, and their existence significantly hinders the advancement of the idea of vaccination.
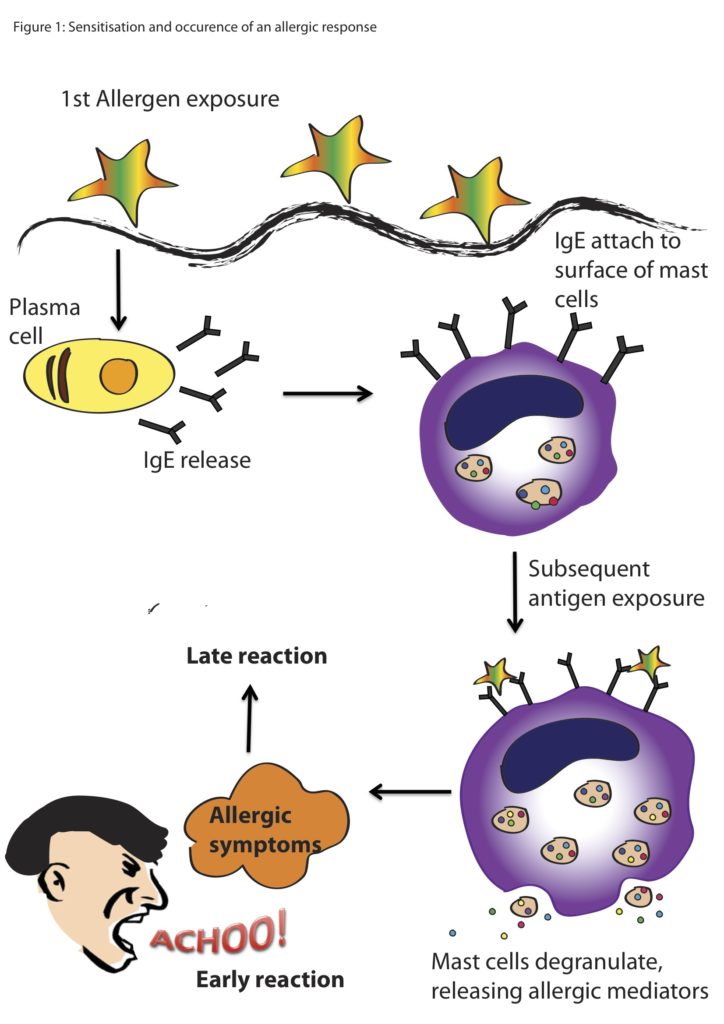
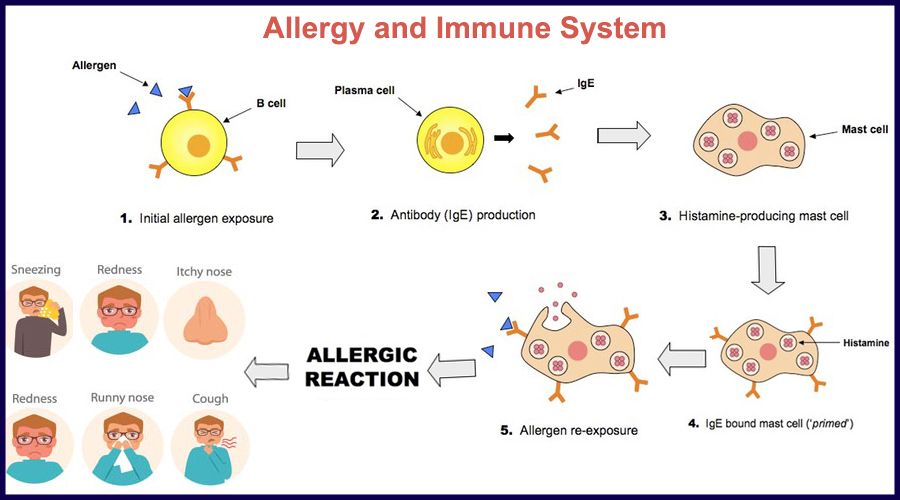
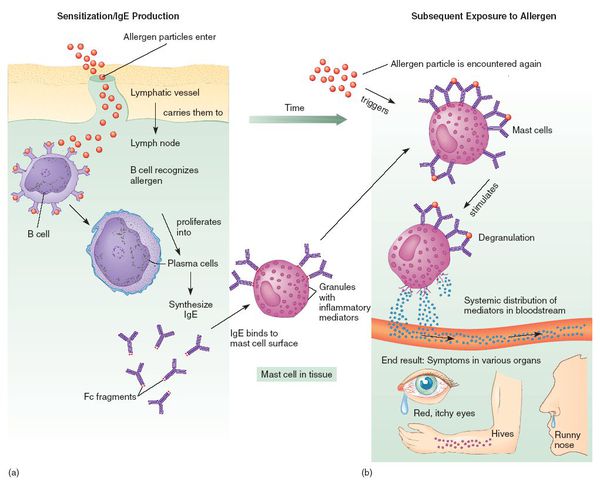
The World Allergy Organization has recommended that immunological reactions to drugs (including vaccines) be classified according to the time of onset of symptoms]. This system defines two main types of reactions: immediate and delayed. This approach is intended to distinguish immunoglobulin E (IgE) mediated (type I immunological reactions), which cause many immediate reactions, from other types, as these reactions carry the risk of life-threatening anaphylaxis if the patient is re-exposed. nine0022
- Immediate reactions begin within the first hour after administration and may manifest within the first few minutes.
 IgE-mediated reactions are most likely during this time period.
IgE-mediated reactions are most likely during this time period. - Delayed reactions appear hours or days after ingestion. These reactions can be caused by several different mechanisms, but they are rarely associated with IgE.
An immediate, IgE-mediated allergic reaction can include various combinations of 40 potential symptoms and signs. nine0022
The most common symptoms and signs:
- Skin symptoms including redness, itching, urticaria and angioedema.
- Respiratory symptoms including nasal discharge, nasal congestion, change in quality of voice, feeling of closing or choking, stridor, cough, wheezing and shortness of breath.
- Cardiovascular symptoms including syncope, syncope, altered mental status, palpitations and hypotension. Anaphylaxis (anaphylactic shock after vaccination) is the most severe form of an IgE-mediated allergic reaction. nine0006
Anaphylaxis is defined as a systemic allergic reaction that develops rapidly and can lead to death.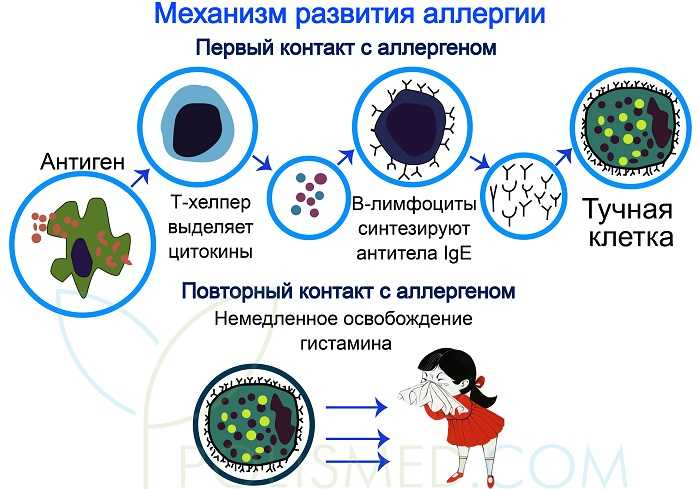 Anaphylactic reactions to vaccines are rare, with rates ranging from 0.65 to 1.31 per million vaccine doses in active surveillance studies.
Anaphylactic reactions to vaccines are rare, with rates ranging from 0.65 to 1.31 per million vaccine doses in active surveillance studies.
Anaphylactic shock occurs most often within the first 30 minutes after vaccine administration, although onset can sometimes be delayed up to several hours. Late onset reactions are usually less severe. Reactions that occur hours or days after vaccine administration may be associated with delayed absorption of the allergenic component. Some of these late reactions may not be causally related to the vaccination, but to another allergen that the person was exposed to after the vaccine was given. nine0022
Vaccine reactions . Not to be confused with complications after the introduction of vaccines (post-vaccination complications, PVO). They can be both immunological and non-immunological in nature.
- Fever, fever is common after the introduction of the vaccine and does not prevent the introduction of subsequent doses of the same drug (revaccination, booster).

- Local reactions to vaccination, such as swelling and redness at the injection site, are common and resolve on their own. This should not be considered as a reason not to administer further doses of the vaccine. nine0006 Local reactions can be treated with cool compresses within the first hours of symptom onset, paracetamol, or non-steroidal anti-inflammatory drugs (NSAIDs) if pain or swelling is bothersome. However, antipyretics should not be administered prophylactically, "just in case", as several studies have shown that these drugs can reduce the immune response to vaccination.
- Serum sickness and reactions , similar to serum sickness , includes rash, fever, malaise, joint tenderness or polyarthritis occurring one to two weeks after vaccination, or sooner if the patient has received the vaccine more than once.
- Other rare reactions - delayed immunologic reactions include rare cases of persistent pruritic nodules at the injection site of aluminum-containing vaccines, which may be associated with delayed-type hypersensitivity to aluminum.
 Another rare reaction is encephalopathy. Some of these more serious reactions may be contraindications to further doses of specific vaccines. nine0006
Another rare reaction is encephalopathy. Some of these more serious reactions may be contraindications to further doses of specific vaccines. nine0006
The introduction of the vaccine may cause vasovagal reaction (loss of consciousness), especially in patients prone to this reaction. Vasovagal reactions are characterized by hypotension, pallor, sweating, weakness, nausea, vomiting, bradycardia, and, in severe cases, loss of consciousness.
Vasovagal reactions may mimic anaphylaxis since both conditions may include hypotension and collapse. However, skin signs and symptoms are completely different. Syncope is usually preceded by pallor, while anaphylaxis often begins with flushing and may also include pruritus, urticaria, and angioedema. In anaphylaxis, tachycardia is more common than bradycardia. For patients who report syncope in the past in response to vaccination, it is reasonable to administer vaccines in the future in the supine position. nine0022
This phrase is listed as a contraindication in the prescribing information for many vaccines.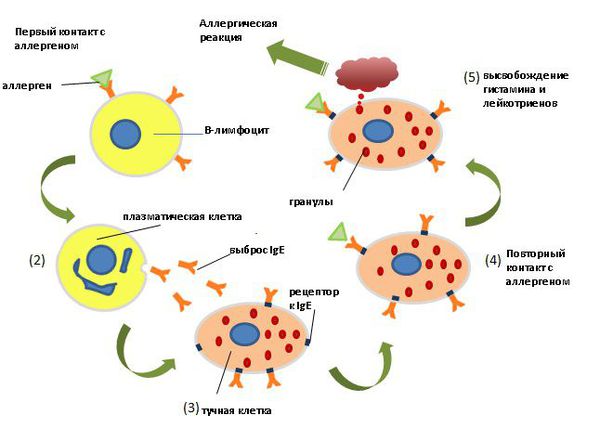 But what exactly does it mean, what substances can we talk about? Important: the listed list is general for the vaccine industry, this does not mean that all the substances and compounds listed are absolutely in all vaccines, a specific list of excipients in each vaccine is reflected in its instructions for medical use.
But what exactly does it mean, what substances can we talk about? Important: the listed list is general for the vaccine industry, this does not mean that all the substances and compounds listed are absolutely in all vaccines, a specific list of excipients in each vaccine is reflected in its instructions for medical use.
nine0046 Gelatine . Added to many vaccines as a stabilizer, may cause anaphylactic reactions to vaccines containing it. Often these people are sensitized to alpha-gal (galactose-alpha-1,3-galactose), a carbohydrate allergen that causes an allergy to mammalian meat. Some patients had a clinically significant allergy to beef and pork.
Before administering any gelatin-containing vaccine, a history of allergy to gelatin and products containing gelatin (for example, marshmallows and marmalade sweets) should be ascertained. However, a negative allergy history does not rule out the possibility of anaphylaxis, so gelatin allergy testing may be required. nine0022
nine0022
Individuals who react to gelatin by mouth should be evaluated by an allergist prior to administering the vaccine. If there is a history of an immediate allergic reaction to gelatin and this is confirmed by skin tests or serum IgE tests to gelatin, it is advisable to perform a skin test in such patients with gelatin-containing vaccines before their administration. If the skin test for the vaccine is negative, it can be administered in the usual way, but after that the patient must remain under observation for at least 30 minutes. If vaccine skin tests are positive, the vaccine can be administered in graduated doses. The use of gelatin-free vaccines, if available, is recommended as an alternative. nine0022
The frequency of allergic reactions to gelatin in vaccines was particularly high in Japan, a phenomenon subsequently attributed in part to the genetic characteristics of the population. Japanese manufacturers have removed gelatin from some vaccines and switched to more thoroughly hydrolyzed gelatin in others, which has drastically reduced the frequency of reactions.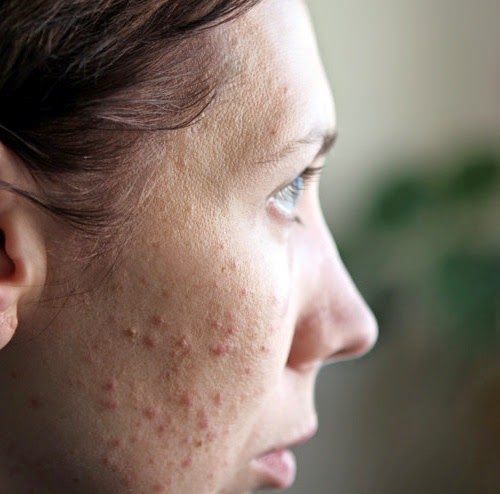 These strategies have been applied in different ways in other countries, so reactions to gelatin in vaccines still occur.
These strategies have been applied in different ways in other countries, so reactions to gelatin in vaccines still occur.
Egg white is present in yellow fever vaccines, MMR, and some influenza and rabies vaccines. However, this amount is only potentially of clinical significance in yellow fever vaccine. MMR and rabies vaccines can be safely administered in the usual way to patients with egg allergy.
Before administering the yellow fever vaccine, a history of allergy after ingestion of eggs, raw or cooked, should be ascertained, and those with a positive history should be examined by an allergist. Such patients should undergo a skin test with yellow fever vaccine prior to administration. If the skin test for the vaccine is negative, the vaccine can be administered in the usual way, but after that the patient must remain under observation for at least 30 minutes. If vaccine skin tests are positive, the vaccine can be administered in graduated doses. nine0022
Cow's milk .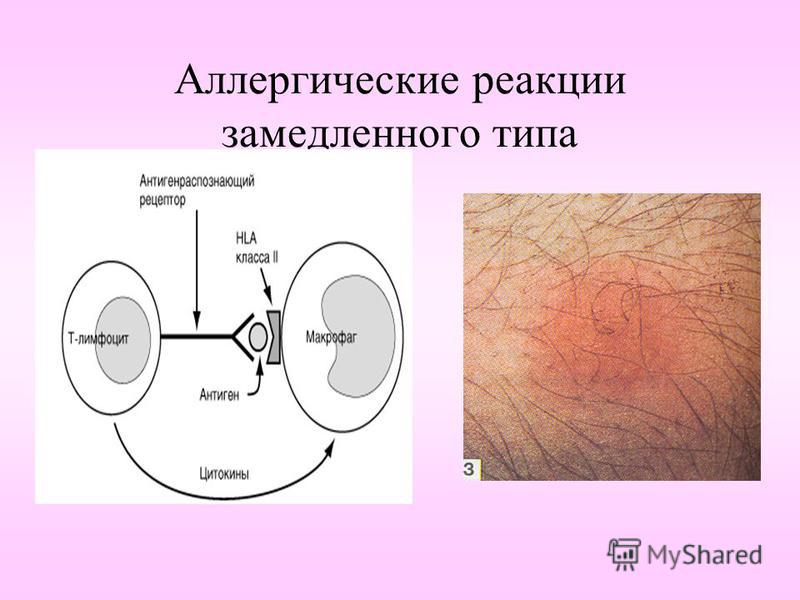 Casein, an allergenic protein found in cow's milk, has been previously implicated in diphtheria, tetanus, and pertussis vaccine (DTaP or Tdap) anaphylaxis in a small number of children with severe milk allergy. Such vaccines are prepared in a medium derived from cow's milk protein, and nanogram amounts of residual casein have been demonstrated in these formulations. However, the vast majority of patients with severe milk allergy do not have allergic reactions to such vaccines. nine0022
Casein, an allergenic protein found in cow's milk, has been previously implicated in diphtheria, tetanus, and pertussis vaccine (DTaP or Tdap) anaphylaxis in a small number of children with severe milk allergy. Such vaccines are prepared in a medium derived from cow's milk protein, and nanogram amounts of residual casein have been demonstrated in these formulations. However, the vast majority of patients with severe milk allergy do not have allergic reactions to such vaccines. nine0022
Thimerosal, aluminum, and phenoxyethanol are added to some vaccines as preservatives, although the use of thimerosal (which contains mercury) in vaccines has declined sharply due to theoretical concerns about cumulative mercury exposure in children. These preservatives have not been documented to cause immediate allergic reactions to vaccines, and immediate skin testing is not usually indicated.
Some antimicrobials can be added in trace amounts to vaccines, most commonly neomycin, polymyxin B, and streptomycin. Although there are no specific reports of vaccine-induced anaphylaxis caused by these drugs, rare patients who have confirmed anaphylactic reactions caused by these antibiotics should not receive vaccines containing them without prior evaluation by an allergist.
Although there are no specific reports of vaccine-induced anaphylaxis caused by these drugs, rare patients who have confirmed anaphylactic reactions caused by these antibiotics should not receive vaccines containing them without prior evaluation by an allergist.
Latex . The "rubber" in vaccine vial stoppers or syringe plungers can be either dry natural rubber (DNR) latex or synthetic rubber. Those made from latex carry a theoretical risk to latex allergic patients, either as a result of the liquid vaccine solution extracting latex allergens from the cork on physical contact, or by passing the needle through the cork and retaining the latex allergen in or on the needle. One report of an anaphylactic reaction after administering hepatitis B vaccine to a latex allergic patient was associated with rubber in cork. A review of > 160,000 reports from the US Vaccine Adverse Event Reporting System (VAERS) found only 28 cases of possible immediate allergic reactions after receiving a DNR-containing vaccine that could be caused by other ingredients. The content of latex in the packaging of the vaccine can be found in the instructions for medical use. nine0022
The content of latex in the packaging of the vaccine can be found in the instructions for medical use. nine0022
Patients with latex anaphylaxis can safely receive vaccines from vials with DNR-free stoppers. If the only product available has a latex stopper, the stopper should be removed and the vaccine drawn directly from the vial without passing the needle through the stopper. If the only available vaccine contains packaged latex, which cannot be avoided, such as in a pre-filled syringe, the vaccine can still be administered, but the patient should be observed for at least 30 minutes thereafter. nine0022
Unlike patients with latex-induced anaphylaxis, patients with latex contact allergy, such as hand dermatitis, can safely receive vaccines from vials with latex stoppers.
Yeast . Some vaccines contain yeast protein, including hepatitis B vaccines (up to 25 mg per dose) and 4- and 9-valent human papillomavirus vaccines (<7 mcg per dose), but adverse reactions to these, if any, are rarely fixed.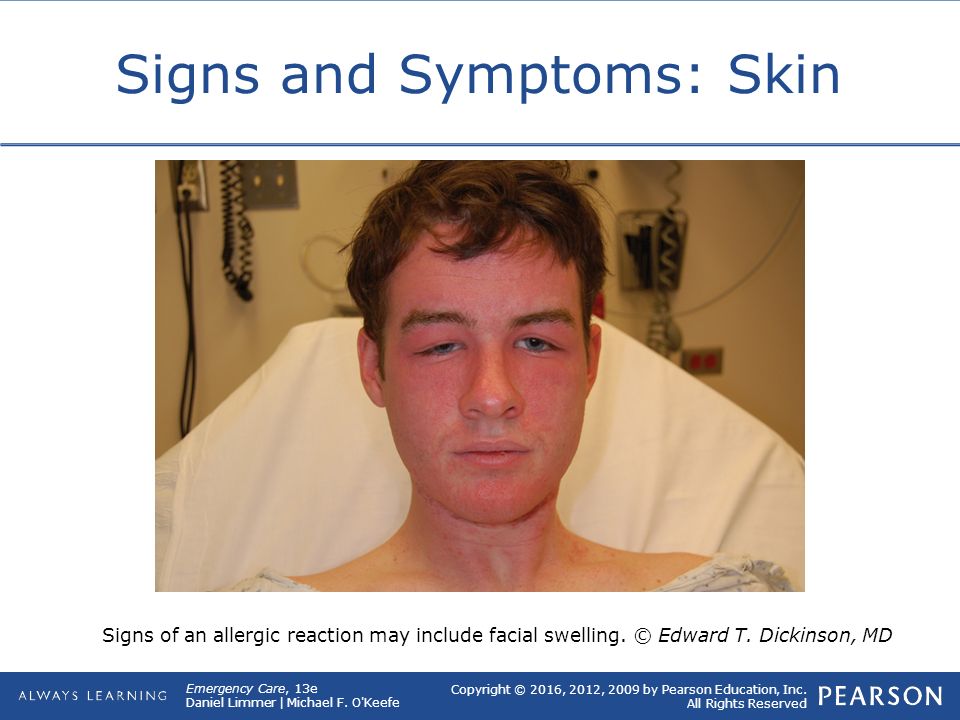 Yeast allergy itself is very rare, but if a patient has a history of clinical reactivity to baker's or brewer's yeast and a positive skin test for Saccharomyces cerevisiae, a skin test would be appropriate before vaccine administration. If skin tests are negative, the vaccine can be administered in the usual way, but after that the patient must be observed for at least 30 minutes. If skin tests are positive, the vaccine can be administered in graduated doses. nine0022
Yeast allergy itself is very rare, but if a patient has a history of clinical reactivity to baker's or brewer's yeast and a positive skin test for Saccharomyces cerevisiae, a skin test would be appropriate before vaccine administration. If skin tests are negative, the vaccine can be administered in the usual way, but after that the patient must be observed for at least 30 minutes. If skin tests are positive, the vaccine can be administered in graduated doses. nine0022
Dextran causes allergic reactions to a specific brand of MMR vaccine previously used in Italy and Brazil. The reactions were associated with the presence of IgG to dextran, and it was assumed that the mechanism is the activation of the complement system and the release of anaphylatoxin. This brand of vaccine has been withdrawn from the market, although dextran is found sporadically in other vaccines (such as some rotavirus vaccines).
The diagnostic approach is different for a patient who has reacted to a vaccine component such as gelatin, eggs, latex, or yeast, but not to the active substance itself. A common situation is the flu vaccination of a patient with an egg allergy, which is successful and without complications. nine0022
A common situation is the flu vaccination of a patient with an egg allergy, which is successful and without complications. nine0022
What clinical questions should be answered before a patient with allergies is vaccinated?
- Do the nature of manifestations and time of onset of anaphylactic anaphylaxis correspond? Does this look like an IgE mediated clinical picture?
- Is there a history of such a reaction? It is important to obtain this information and understand whether it was caused by the antigenic material of the vaccine or by the excipients. The various components of the vaccine are clearly listed in the instructions for medical use supplied by the manufacturers. If the reaction occurs after the first dose of the vaccine, the likelihood that the immunizing agent itself is the allergen is greatly reduced. In this clinical situation, healthcare professionals should also be aware of allergic reactions to food, especially to gelatin. nine0006
- Is there a need for future doses of this vaccine or other vaccines with common components? Following a history of a vaccine reaction shortly after administration that is consistent with an IgE-mediated reaction, the clinician should determine whether future doses of the suspected vaccine or other vaccines with common components are required.
 Given the potential for cross-reaction with common components in other vaccines and with food, careful evaluation is warranted even if additional doses of the potentially allergenic vaccine are not required. nine0006
Given the potential for cross-reaction with common components in other vaccines and with food, careful evaluation is warranted even if additional doses of the potentially allergenic vaccine are not required. nine0006
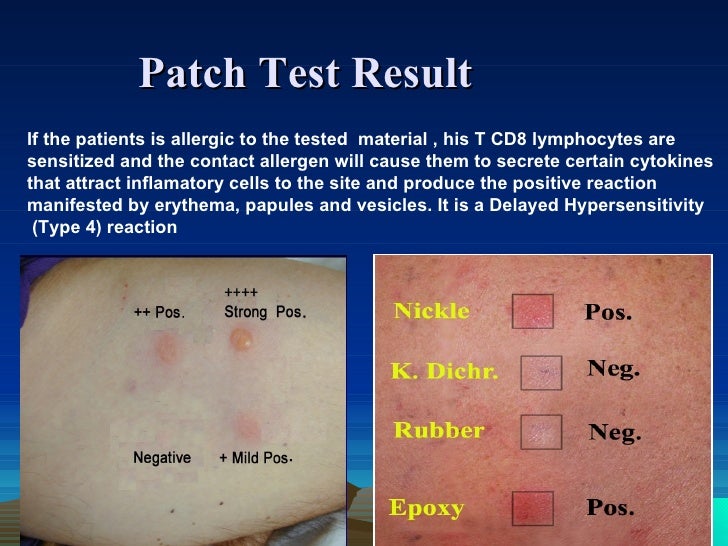
If the patient is to receive additional doses of the vaccine, skin testing should be done using the same vaccine. Proper performance and interpretation of skin tests requires expertise in this procedure, including the use of appropriate positive and negative controls. In addition, skin tests themselves can rarely cause anaphylactic reactions in people with severe allergies. Thus, skin testing should only be performed by individuals, such as allergists, who are trained in the interpretation and treatment of possible test reactions, and only in settings where anaphylactic reactions can be recognized and treated quickly. nine0022
The vaccine must first be tested by skin prick method. A full vaccine may be used if the history of the reaction has not been truly life-threatening; in this case, it is advisable to dilute the vaccine before skin injection.
If the full shot is negative, an intradermal test should be performed with the vaccine diluted 1:100 in 0.9% (isotonic) saline, again with appropriate positive and negative controls. nine0022
Interpretation of skin tests for vaccines. Allergic history is critical to the interpretation of skin tests for vaccines. Clinically irrelevant positive skin tests are possible, as with skin tests for any allergen. The presence of a positive skin test for a vaccine does not necessarily indicate a subsequent reaction, although it should be considered a significant risk factor.
Vaccines can induce IgE production in the first days or weeks after vaccination, although the clinical significance of this is unclear. Specific IgE to diphtheria (DT) and tetanus toxoids was induced by vaccination and was found to persist for at least two years after immunization. This specific IgE was not associated with allergic reactions following vaccination. nine0022
Irritant (false positive) reactions to skin tests are also possible, especially with intradermal tests at concentrations of 1:10 or undiluted. One study in 20 adult volunteers with no history of vaccine reactions evaluated skin prick response and intradermal testing with 10 common vaccines. Irritant reactions were not observed in skin prick testing and were uncommon after intradermal testing at a concentration of 1:100. However, they were common at higher intradermal concentrations (1:10 or undiluted). Thus, intradermal testing of vaccines should only be done at a concentration of 1:100. nine0022
One study in 20 adult volunteers with no history of vaccine reactions evaluated skin prick response and intradermal testing with 10 common vaccines. Irritant reactions were not observed in skin prick testing and were uncommon after intradermal testing at a concentration of 1:100. However, they were common at higher intradermal concentrations (1:10 or undiluted). Thus, intradermal testing of vaccines should only be done at a concentration of 1:100. nine0022
delayed reactions. Delayed-type hypersensitivity reactions have been observed at the site of a skin test (especially intradermal) with the vaccine. These reactions are not relevant for the diagnosis of IgE-mediated vaccine allergy.
Testing of vaccine components. If the suspected vaccine contains eggs, gelatin, latex, or Saccharomyces cerevisiae yeast, the patient should also undergo skin testing for the following:
Skin injections: nine0022
- Saccharomyces cerevisiae egg and yeast extracts for skin testing.
- Gelatin extract can be prepared by dissolving 1 teaspoon of candied gelatin powder of any color or flavor in 5 ml of saline to create a skin test solution.
- Standardized extracts of natural rubber latex (NRL).
Measurement of allergen-specific IgE in serum:
- Specific IgE to eggs, gelatin, latex and yeast can be measured in serum using immunoassays. The sensitivity of the ImmunoCAP and Immulite latex tests is approximately 80%. nine0006
Most patients with previous reactions to vaccines can be safely vaccinated in the future with appropriate precautions.
General strategy: if the intradermal test with a vaccine is negative, then the likelihood that the patient has IgE to any vaccine is negligible, and the vaccine can be administered in the usual way. However, it is prudent to observe such patients for at least 30 minutes and to have an anaphylactic shock bed in close proximity. nine0022
If skin tests of a vaccine or vaccine component are positive, and if the vaccine is deemed necessary, it can still be administered using a graduated dose protocol (split administration using the Bezredko method).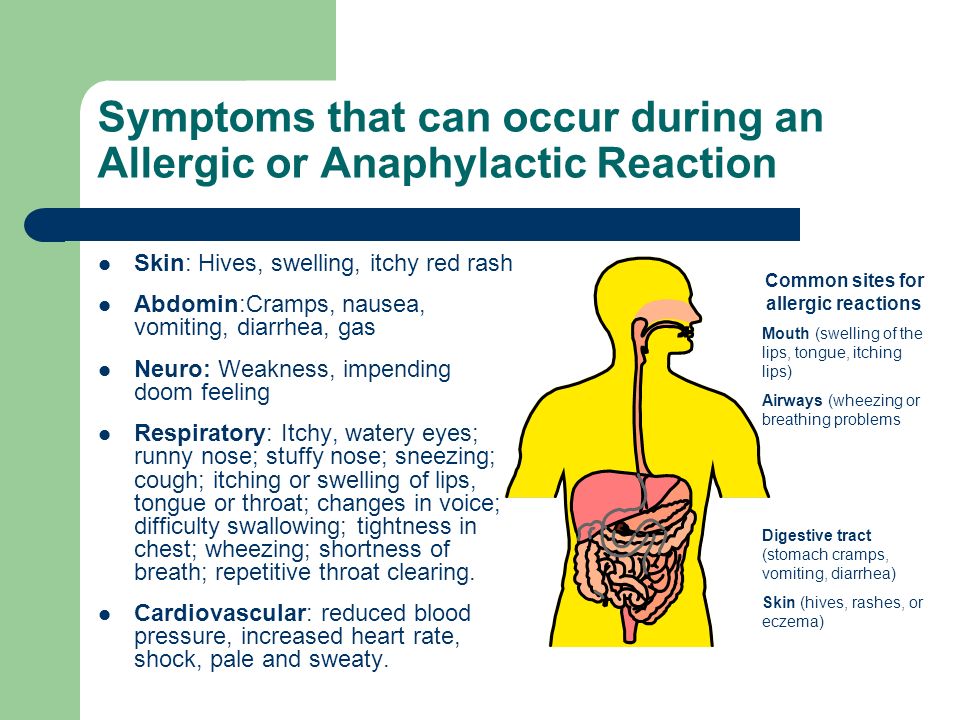 Successful administration of vaccines to patients with positive skin tests using differentiated protocols has been described for several different vaccines.
Successful administration of vaccines to patients with positive skin tests using differentiated protocols has been described for several different vaccines.
Administering a vaccine to an allergic person, even with such a graduated dose protocol, still carries the risk of an anaphylactic reaction and should only be given after written informed consent has been obtained. In addition, the introduction should be carried out in the presence of personnel and equipment for the recognition and treatment of anaphylaxis. nine0022
References
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003 // J Allergy Clin Immunol 2004; 113:832.
- Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium // J Allergy Clin Immunol 2006; 117:391.
- Bohlke K, Davis RL, Marcy SM, et al. Risk of anaphylaxis after vaccination of children and adolescents // Pediatrics 2003; 112:815.
- McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults // J Allergy Clin Immunol 2016; 137:868.
- Patja A, Mäkinen-Kiljunen S, Davidkin I, et al. Allergic reactions to measles-mumps-rubella vaccination // Pediatrics 2001; 107:E27. nine0005 Cheng DR, Perrett KP, Choo S, et al. Pediatric anaphylactic adverse events following immunization in Victoria, Australia from 2007 to 2013 // Vaccine 2015; 33:1602.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP) // MMWR Recomm Rep 2011; 60:1.
- Broder KR, Cortese MM, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) // MMWR Recomm Rep 2006; 55:1.
 nine0006
nine0006 - Kretsinger K, Broder KR, Cortese MM, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC) , for use of Tdap among health-care personnel // MMWR Recomm Rep 2006; 55:1.
- Talbot EA, Brown KH, Kirkland KB, et al. The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: Experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak // Vaccine 2010; 28:8001. nine0006
- Beytout J, Launay O, Guiso N, et al. Safety of Tdap-IPV given one month after Td-IPV booster in healthy young adults: a placebo-controlled trial // Hum Vaccin 2009; 5:315.
- Centers for Disease Control and Prevention (CDC).
 Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010 // MMWR Morb Mortal Wkly Rep 2011; 60:13. nine0005 Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminum after vaccination with aluminum-adsorbed vaccines-prognosis and outcome after booster vaccination // Eur J Pediatr 2013; 172:171.
Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010 // MMWR Morb Mortal Wkly Rep 2011; 60:13. nine0005 Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminum after vaccination with aluminum-adsorbed vaccines-prognosis and outcome after booster vaccination // Eur J Pediatr 2013; 172:171. - Kang LW, Crawford N, Tang ML, et al. Hypersensitivity reactions to human papillomavirus vaccine in Australian schoolgirls: retrospective cohort study // BMJ 2008; 337:a2642.
- Kelso JM, Greenhawt MJ. Adverse reactions to vaccines in infectious diseases. In: Middleton's allergy: Principles and practice, 8th ed, Adkinson NF, Bochner BS, Burks AW, et al (Eds), Elsevier, Philadelphia 2014.
- Centers for Disease Control and Prevention (CDC). Syncope after vaccination--United States, January 2005-July 2007 // MMWR Morb Mortal Wkly Rep 2008; 57:457.
- Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin // J Allergy Clin Immunol 1993; 91:867.
- Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines // J Allergy Clin Immunol 1996; 98:1058.
- Sakaguchi M, Yamanaka T, Ikeda K, et al. IgE-mediated systemic reactions to gelatin included in the varicella vaccine // J Allergy Clin Immunol 1997; 99:263.
- Sakaguchi M, Miyazawa H, Inouye S. Specific IgE and IgG to gelatin in children with systemic cutaneous reactions to Japanese encephalitis vaccines // Allergy 2001; 56:536.
- Lasley M.V. Anaphylaxis after booster influenza vaccine due to gelatin allergy // Pediatr Asthma Allergy Immunol 2007; 20:201. nine0006
- Stone CA Jr, Hemler JA, Commins SP, et al. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism // J Allergy Clin Immunol 2017; 139:1710.
- Kumagai T, Yamanaka T, Wataya Y, et al. A strong association between HLA-DR9 and gelatin allergy in the Japanese population // Vaccine 2001; 19:3273.
- Sakaguchi M, Nakayama T, Kaku H, et al. Analysis of HLA in children with gelatin allergy // Tissue Antigens 2002; 59:412.
- Kuno-Sakai H, Kimura M. Removal of gelatin from live vaccines and DTaP-an ultimate solution for vaccine-related gelatin allergy // Biologicals 2003; 31:245.
- O'Brien TC, Maloney CJ, Tauraso NM. Quantitation of residual host protein in chicken embryo-derived vaccines by radial immunodiffusion // Appl Microbiol 1971; 21:780.
- Kelso JM. Raw egg allergy-a potential issue in vaccine allergy // J Allergy Clin Immunol 2000; 106:990.
- Kattan JD, Konstantinou GN, Cox AL, et al. Anaphylaxis to diphtheria, tetanus, and pertussis vaccines among children with cow's milk allergy // J Allergy Clin Immunol 2011; 128:215.
- Grabenstein JD. Clinical management of hypersensitivities to vaccine components // Hosp Pharm 1997; 32:77.

- Heidary N, Cohen D.E. Hypersensitivity reactions to vaccine components // Dermatitis 2005; 16:115.
- Patrizi A, Rizzoli L, Vincenzi C, et al. Sensitization to thimerosal in atopic children // Contact Dermatitis 1999; 40:94.
- Lee-Wong M, Resnick D, Chong K. A generalized reaction to thimerosal from an influenza vaccine // Ann Allergy Asthma Immunol 2005; 94:90.
- Audicana MT, Muñoz D, del Pozo MD, et al. Allergic contact dermatitis from mercury antiseptics and derivatives: study protocol of tolerance to intramuscular injections of thimerosal // Am J Contact Dermat 2002; 13:3.
- Lear JT, English JS. Anaphylaxis after hepatitis B vaccination // Lancet 1995; 345:1249.
- Russell M, Pool V, Kelso JM, Tomazic-Jezic VJ. Vaccination of persons allergic to latex: a review of safety data in the Vaccine Adverse Event Reporting System (VAERS) // Vaccine 2004; 23:664.
- Hamilton RG, Brown RH, Veltri MA, et al. Administering pharmaceuticals to latex-allergic patients from vials containing natural rubber latex closures // Am J Health Syst Pharm 2005; 62:1822.
- DiMiceli L, Pool V, Kelso JM, et al. Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (VAERS) // Vaccine 2006; 24:703. nine0006
- Zanoni G, Puccetti A, Dolcino M, et al. Dextran-specific IgG response in hypersensitivity reactions to measles-mumps-rubella vaccine // J Allergy Clin Immunol 2008; 122:1233.
- Martín-Muñoz MF, Pereira MJ, Posadas S, et al. Anaphylactic reaction to diphtheria-tetanus vaccine in a child: specific IgE/IgG determinations and cross-reactivity studies // Vaccine 2002; 20:3409.
- Skov PS, Pelck I, Ebbesen F, Poulsen LK. Hypersensitivity to the diphtheria component in the Di-Te-Pol vaccine. A type I allergic reaction demonstrated by basophil histamine release // Pediatr Allergy Immunol 1997; 8:156.
- Begin P, Vu MQ, Picard M, et al. Spontaneous resolution of diphtheria-tetanus vaccine hypersensitivity in a pediatric population // Allergy 2011; 66:1508.
- Systemic adverse effects of hepatitis B vaccines are rare // Prescrire Int 2004; 13:218.
- Nelson MR, Oaks H, Smith LJ, et al. Anaphylaxis complicating routine childhood immunization: Hemophilus Influenza b conjugated vaccine // Pediatr Asthma Allergy Immunol 2000; 14:315. nine0006
- Brotherton JM, Gold MS, Kemp AS, et al. Anaphylaxis following quadrivalent human papillomavirus vaccination // CMAJ 2008; 179:525.
- Nagao M, Fujisawa T, Ihara T, Kino Y. Highly increased levels of IgE antibodies to vaccine components in children with influenza vaccine-associated anaphylaxis // J Allergy Clin Immunol 2016; 137:861.
- Takahashi H, Pool V, Tsai TF, Chen RT. Adverse events after Japanese encephalitis vaccination: review of post-marketing surveillance data from Japan and the United States. The VAERS Working Group // Vaccine 2000; 18:2963.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs // N Engl J Med 1995; 332:1262.
- Ball R, Braun MM, Mootrey GT, Vaccine Adverse Event Reporting System Working Group. Safety data on meningococcal polysaccharide vaccine from the Vaccine Adverse Event Reporting System // Clin Infect Dis 2001; 32:1273.
- Ponvert C, Ardelean-Jaby D, Colin-Gorski AM, et al. Anaphylaxis to the 23-valent pneumococcal vaccine in child: a case-control study based on immediate responses in skin tests and specific IgE determination // Vaccine 2001; nineteen:4588.
- Ponvert C, Scheinmann P, de Blic J. Anaphylaxis to the 23-valent pneumococcal vaccine: a second explored case by means of immediate-reading skin tests with pneumococcal vaccines // Vaccine 2010; 28:8256.
- Centers for Disease Control (CDC). Systemic allergic reactions following immunization with human diploid cell rabies vaccine // MMWR Morb Mortal Wkly Rep 1984; 33:185.
- Swanson MC, Rosanoff E, Gurwith M, et al. IgE and IgG antibodies to beta-propiolactone and human serum albumin associated with urticarial reactions to rabies vaccine // J Infect Dis 1987; 155:909.
- Carey AB, Meltzer EO. Diagnosis and "desensitization" in tetanus vaccine hypersensitivity // Ann Allergy 1992; 69:336.
- Mayorga C, Torres MJ, Corzo JL, et al. Immediate allergy to tetanus toxoid vaccine: determination of immunoglobulin E and immunoglobulin G antibodies to allergenic proteins // Ann Allergy Asthma Immunol 2003; 90:238.
- Slater JE, Rabin RL, Martin D. Comments on cow's milk allergy and diphtheria, tetanus, and pertussis vaccines // J Allergy Clin Immunol 2011; 128:434; author reply 435.
- Begier EM, Burwen DR, Haber P, et al. Postmarketing safety surveillance for typhoid fever vaccines from the Vaccine Adverse Event Reporting System, July 1990 through June 2002 // Clin Infect Dis 2004; 38:771.
- Hoyt RE, Herip DS. Severe systemic reactions attributed to the acetone-inactivated parenteral typhoid vaccine // Mil Med 1996; 161:339.
- Wise RP, Salive ME, Braun MM, et al. Postlicensure safety surveillance for varicella vaccine // JAMA 2000; 284:1271.
 nine0006
nine0006 - Kelso JM, Mootrey GT, Tsai TF. Anaphylaxis from yellow vaccine fever // J Allergy Clin Immunol 1999; 103:698.
- Tseng HF, Liu A, Sy L, et al. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study // J Intern Med 2012; 271:510.
- Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update // J Allergy Clin Immunol 2012; 130:25. nine0005 Mark A, Björkstén B, Granström M. Immunoglobulin E responses to diphtheria and tetanus toxoids after booster with aluminum-adsorbed and fluid DT-vaccines // Vaccine 1995; 13:669.
- Aalberse RC, van Ree R, Danneman A, Wahn U. IgE antibodies to tetanus toxoid in relation to atopy // Int Arch Allergy Immunol 1995; 107:169.
- Wood RA, Setse R, Halsey N, Clinical Immunization Safety Assessment (CISA) Network Hypersensitivity Working Group. Irritant skin test reactions to common vaccines // J Allergy Clin Immunol 2007; 120:478.
 nine0006
nine0006 - Bierman CW, Shapiro GG, Pierson WE, et al. Safety of influenza vaccination in allergic children // J Infect Dis 1977; 136 Suppl:S652.
- Miller JR, Orgel HA, Meltzer EO. The safety of egg-containing vaccines for egg-allergic patients // J Allergy Clin Immunol 1983; 71:568.
- Kelso JM, Cockrell GE, Helm RM, Burks AW. Common allergens in avian meats // J Allergy Clin Immunol 1999; 104:202.
Keywords: vaccine allergy vaccine allergy to vaccine allergy to coronavirus vaccine allergy to vaccine satellite analysis for vaccine components symptoms of allergy to vaccine
Latest Articles
29.12.2022
You can't wait to operate. Rules for surgical punctuation
28.12.2022
Chronic and acute periodontitis: types, treatment nine0330
27. 12.2022
12.2022
Osteoarthritis of the joints of the hands. Review of clinical guidelines
12/26/2022
Teeth whitening
23.12.2022 nine0022
Risks of developing sarcopenia after bariatric surgery in patients with type 2 diabetes mellitus
12/21/2022
Postcovid syndrome: focus on neuropsychiatric disorders
Allergen c88 - mepivacaine/polocaine, IgE
Quantitative determination in the blood of specific class E immunoglobulins to the local anesthetic mepivacaine/polocaine, which are markers in the event of an allergic reaction to it. Allergy to this drug is rare and can manifest as skin rash, itching around the injection site, rhinitis, and in severe cases as Quincke's edema and anaphylactic shock. The determination of specific immunoglobulin E to this allergen in an increased amount indicates the presence of sensitization of the body to it. nine0022
The determination of specific immunoglobulin E to this allergen in an increased amount indicates the presence of sensitization of the body to it. nine0022
The study is carried out only on the 1st type of allergic reactions. A negative test result cannot guarantee the absence of another type of allergic reaction.
Synonyms Russian
Class E specific immunoglobulins to mepivacaine/polocaine.
Synonyms English
Allergen c88 - Mepivacaine/Polocaine; IgE.
Research method
ELISA. nine0022
Units
IU/ml (international unit per milliliter).
Which biomaterial can be used for research?
Venous blood.
How to properly prepare for the examination?
- Do not smoke for 30 minutes before the test.
General information about the study
Mepivacaine/polocaine is an amide-type local anesthetic with a fairly strong and rapid action. It is used to create terminal, infiltration, conduction anesthesia. When the drug enters the cells of the nerve fiber, it reversibly blocks the entry of sodium into them, which leads to the cessation of the propagation of the nerve impulse and the absence of pain sensitivity. Mepivacaine is used in dentistry, for tracheal intubation, endoscopic research methods (bronchoscopy, FGDS). The drug is also known under trade names - Mepivastezin, Mepidont, Isocaine, Scandonest, etc.
It is used to create terminal, infiltration, conduction anesthesia. When the drug enters the cells of the nerve fiber, it reversibly blocks the entry of sodium into them, which leads to the cessation of the propagation of the nerve impulse and the absence of pain sensitivity. Mepivacaine is used in dentistry, for tracheal intubation, endoscopic research methods (bronchoscopy, FGDS). The drug is also known under trade names - Mepivastezin, Mepidont, Isocaine, Scandonest, etc.
One of the possible side effects of the anesthetic is the development of an allergic reaction in susceptible individuals. Allergy develops according to the type of immediate hypersensitivity (in a few minutes or hours) and can be manifested by local symptoms: redness, itching, burning at the injection site. Possible skin rashes in the form of urticaria or allergic dermatitis. With more severe development, signs of allergic rhinitis, rhinoconjunctivitis, and bronchial asthma appear. The most severe manifestations of an allergic reaction: angioedema (angioedema) and anaphylactic shock. nine0022
nine0022
It should be noted that an allergic reaction to mepivacaine/polocaine can be combined with an allergy to other drugs from the group of local anesthetics.
What is research used for?
- Diagnosis of immediate allergic reactions to mepivacine/polocaine;
- differential diagnosis of the causes of allergic reactions in children and adults against the background of the use of local anesthetics.
When is the test scheduled? nine0360
- When planning local anesthesia using mepivacaine/polocaine in patients with suspected allergy to local anesthetics;
- when examining children and adults with urticaria, pruritus, allergic rhinitis, conjunctivitis, bronchial asthma, angioedema, anaphylactic shock after the use of local anesthetics.
What do the results mean?
Reference values: 0.00 - 0.35 IU/ml.
Reasons for positive result:
- immediate hypersensitivity to mepivacaine/polocaine.
Reasons for the negative result:
- no IgE sensitization to this allergen;
- long-term restriction or exclusion of contact with the allergen.
Important Notes
- Lack of sensitization to an allergen does not rule out allergy to other allergen sources. In some cases, further allergy testing may be required to clarify sensitization to other possible allergens. nine0006
- Performing this test is safe for the patient compared to skin tests (in vivo), as it eliminates the patient's exposure to the allergen. Taking antihistamines and age characteristics do not affect the quality and accuracy of the study.
Also recommended
- Serum total immunoglobulins E (IgE)
- Allergen c308 - cefuroxime, IgE, ELISA
- Allergen c122 - nystatin, IgE, ELISA
- Allergochip ImmunoCAP ISAC (112 allergenic components)
Who orders the examination?
Allergist-immunologist, pediatrician, internist, surgeon, dentist, general practitioner.