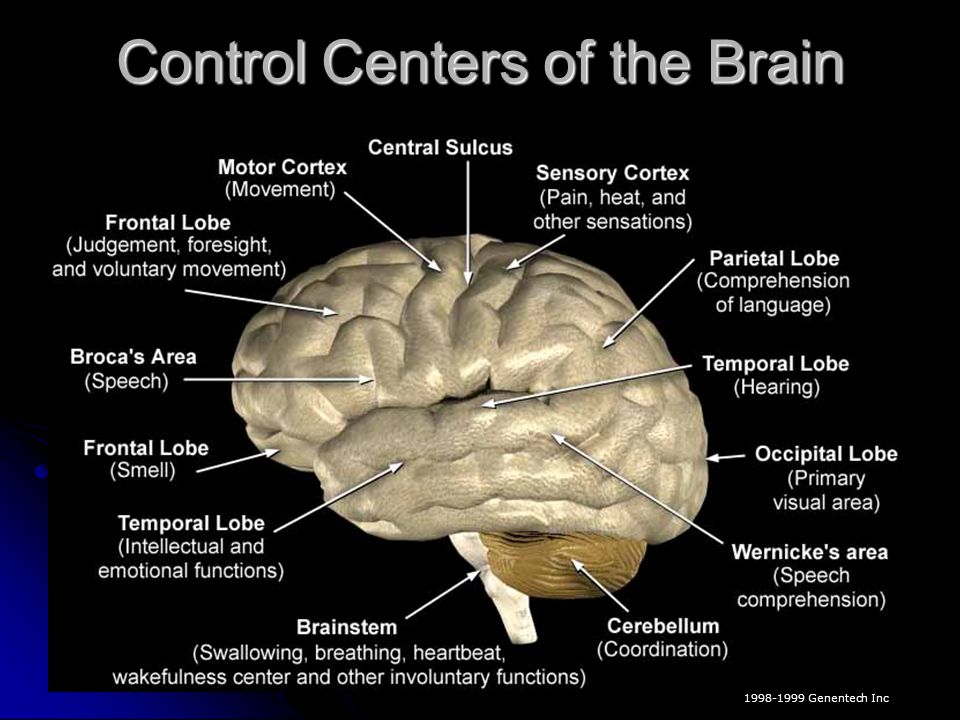
What does buspirone do to the brain
Buspirone: review of its pharmacology and current perspectives on its mechanism of action
Review
. 1986 Mar 31;80(3B):1-9.
doi: 10.1016/0002-9343(86)90325-6.
A S Eison, D L Temple Jr
- PMID: 2870639
- DOI: 10.1016/0002-9343(86)90325-6
Review
A S Eison et al. Am J Med. .
. 1986 Mar 31;80(3B):1-9.
doi: 10.1016/0002-9343(86)90325-6.
Authors
A S Eison, D L Temple Jr
- PMID: 2870639
- DOI: 10.
1016/0002-9343(86)90325-6
Abstract
Buspirone is a novel anxiolytic agent unrelated to the benzodiazepines in structure or pharmacologic properties. Extensive clinical studies have shown buspirone to be effective in the treatment of anxiety, with efficacy comparable to diazepam or clorazepate. Buspirone exhibits a unique pharmacologic profile in that it alleviates anxiety without causing sedation or functional impairment and does not promote abuse or physical dependence. Furthermore, preclinical studies have shown that buspirone does not possess anticonvulsant or muscle relaxant properties and does not interact significantly with central nervous system depressants. Biochemical and electrophysiologic studies indicate that buspirone alters monoaminergic and GABAergic systems in a manner different from that of the benzodiazepines. The uniform depressant action of the benzodiazepines upon serotonergic, noradrenergic, and dopaminergic cell firing may result from their facilitatory effect on gamma-aminobutyric acid and its known inhibitory influence in these monoaminergic areas. Unlike the benzodiazepines, buspirone exerts a differential influence upon monoaminergic neuronal activity, suppressing serotonergic activity while enhancing dopaminergic and noradrenergic cell firing. The mechanism of action of buspirone challenges the notion that only one neurotransmitter mediates anxiety. The interaction with multiple neurotransmitters at multiple brain sites suggests that buspirone may alter diverse activities within a "neural matrix of anxiety." In contrast to the benzodiazepines, buspirone orchestrates activity within this neural matrix to achieve effective treatment of anxiety while preserving arousal and attentional processes.
Unlike the benzodiazepines, buspirone exerts a differential influence upon monoaminergic neuronal activity, suppressing serotonergic activity while enhancing dopaminergic and noradrenergic cell firing. The mechanism of action of buspirone challenges the notion that only one neurotransmitter mediates anxiety. The interaction with multiple neurotransmitters at multiple brain sites suggests that buspirone may alter diverse activities within a "neural matrix of anxiety." In contrast to the benzodiazepines, buspirone orchestrates activity within this neural matrix to achieve effective treatment of anxiety while preserving arousal and attentional processes.
Similar articles
-
Pharmacological and clinical effects of buspirone.
Taylor DP, Eison MS, Riblet LA, Vandermaelen CP. Taylor DP, et al. Pharmacol Biochem Behav. 1985 Oct;23(4):687-94. doi: 10.
 1016/0091-3057(85)90438-1. Pharmacol Biochem Behav. 1985. PMID: 2866549 Review.
1016/0091-3057(85)90438-1. Pharmacol Biochem Behav. 1985. PMID: 2866549 Review. -
Buspirone hydrochloride: a unique new anxiolytic agent. Pharmacokinetics, clinical pharmacology, abuse potential and clinical efficacy.
Goldberg HL. Goldberg HL. Pharmacotherapy. 1984 Nov-Dec;4(6):315-24. doi: 10.1002/j.1875-9114.1984.tb03385.x. Pharmacotherapy. 1984. PMID: 6151170 Review.
-
Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic.
Goa KL, Ward A. Goa KL, et al. Drugs. 1986 Aug;32(2):114-29. doi: 10.2165/00003495-198632020-00002. Drugs. 1986. PMID: 2874976 Review.
-
Preclinical pharmacology of buspirone hydrochloride.
Skolnick P, Paul SM, Weissman BA. Skolnick P, et al. Pharmacotherapy. 1984 Nov-Dec;4(6):308-14. doi: 10.1002/j.1875-9114.1984.tb03384.x. Pharmacotherapy. 1984. PMID: 6151169 Review.
-
Neuropharmacology of buspirone.
Riblet LA, Eison AS, Eison MS, Taylor DP, Temple DL, VanderMaelen CP. Riblet LA, et al. Psychopathology. 1984;17 Suppl 3:69-78. doi: 10.1159/000284133. Psychopathology. 1984. PMID: 6150510
See all similar articles
Cited by
-
6-Hydroxydopamine lesion and levodopa treatment modify the effect of buspirone in the substantia nigra pars reticulata.
Vegas-Suárez S, Pisanò CA, Requejo C, Bengoetxea H, Lafuente JV, Morari M, Miguelez C, Ugedo L.
 Vegas-Suárez S, et al. Br J Pharmacol. 2020 Sep;177(17):3957-3974. doi: 10.1111/bph.15145. Epub 2020 Jul 6. Br J Pharmacol. 2020. PMID: 32464686 Free PMC article.
Vegas-Suárez S, et al. Br J Pharmacol. 2020 Sep;177(17):3957-3974. doi: 10.1111/bph.15145. Epub 2020 Jul 6. Br J Pharmacol. 2020. PMID: 32464686 Free PMC article. -
Psychopharmacology: From serendipitous discoveries to rationale design, but what next?
Robinson E. Robinson E. Brain Neurosci Adv. 2018 Nov 22;2:2398212818812629. doi: 10.1177/2398212818812629. eCollection 2018 Jan-Dec. Brain Neurosci Adv. 2018. PMID: 32166162 Free PMC article. Review.
-
5-HT1A Receptor Function Makes Wound Healing a Happier Process.
Sadiq A, Menchetti I, Shah A, Jeschke MG, Belo C, Carlos-Alcalde W, Hayat MQ, Amini-Nik S. Sadiq A, et al. Front Pharmacol. 2018 Dec 11;9:1406. doi: 10.3389/fphar.2018.01406. eCollection 2018.
 Front Pharmacol. 2018. PMID: 30618734 Free PMC article.
Front Pharmacol. 2018. PMID: 30618734 Free PMC article. -
Behavioral and neurobiological mechanisms of punishment: implications for psychiatric disorders.
Jean-Richard-Dit-Bressel P, Killcross S, McNally GP. Jean-Richard-Dit-Bressel P, et al. Neuropsychopharmacology. 2018 Jul;43(8):1639-1650. doi: 10.1038/s41386-018-0047-3. Epub 2018 Mar 27. Neuropsychopharmacology. 2018. PMID: 29703994 Free PMC article. Review.
-
Human motoneurone excitability is depressed by activation of serotonin 1A receptors with buspirone.
D'Amico JM, Butler AA, Héroux ME, Cotel F, Perrier JM, Butler JE, Gandevia SC, Taylor JL. D'Amico JM, et al. J Physiol. 2017 Mar 1;595(5):1763-1773. doi: 10.1113/JP273200. Epub 2016 Dec 17.
 J Physiol. 2017. PMID: 27859267 Free PMC article. Clinical Trial.
J Physiol. 2017. PMID: 27859267 Free PMC article. Clinical Trial.
See all "Cited by" articles
Publication types
MeSH terms
Substances
Effects of buspirone on plasma neurotransmitters in healthy subjects
. 1998;105(6-7):561-73.
doi: 10.1007/s007020050079.
F Lechin 1 , B van der Dijs, H Jara, B Orozco, S Baez, M Benaim, M Lechin, A Lechin
Affiliations
Affiliation
- 1 Section of Psychopharmacology, Instituto de Medicina Experimental, Universidad Central de Venezuela.
- PMID: 9826102
- DOI: 10.1007/s007020050079
F Lechin et al. J Neural Transm (Vienna). 1998.
. 1998;105(6-7):561-73.
doi: 10.1007/s007020050079.
Authors
F Lechin 1 , B van der Dijs, H Jara, B Orozco, S Baez, M Benaim, M Lechin, A Lechin
Affiliation
- 1 Section of Psychopharmacology, Instituto de Medicina Experimental, Universidad Central de Venezuela.
- PMID: 9826102
- DOI: 10.
 1007/s007020050079
1007/s007020050079
Abstract
Buspirone is an anxiolytic drug which exerts several central effects. It antagonizes presynaptic inhibitory DA2 autoreceptors at dopaminergic neurons and acts as an agonist for 5-HT1A inhibitor autoreceptors at serotonergic cells. Thus, buspirone respectively enhances and depresses the firing rates of both type of neurons. At doses which correlate with dopaminergic stimulation, but not 5-HT inhibition, buspirone also increases the firing rates of the central noradrenergic cells. We measured levels of circulating neurotransmitters before and up to 240 minutes after the oral administration of 20 mg of buspirone in 32 healthy volunteers. Buspirone significantly increased levels of noradrenaline, dopamine, and free serotonin but did not affect levels of adrenaline, tryptophane, or platelet serotonin. Small but significant drops in systolic blood pressure and heart rate were observed after buspirone ingestion. Atropine administration before buspirone ingestion annulled the free serotonin increase as well as systolic blood pressure-heart rate decrease. We found significant positive correlations between noradrenaline and dopamine levels. The strength and significance of these correlations were increased by using the noradrenaline/adrenaline ratio instead of noradrenaline absolute values. This finding indicates that increases in both noradrenaline and dopamine arise from sympathetic nerves rather than the adrenal glands. We also found significant negative correlations between free serotonin increases and systolic blood pressure-heart rate decreases. Our results indicate that buspirone stimulates central sympathetic activity. These acute effects of buspirone are reflected in an increased peripheral neural sympathetic activity, but not adrenal sympathetic activity in healthy individuals. In addition, buspirone increases free serotonin plasma concentrations and decreases systolic blood pressure plus heart rate levels through mechanisms associated with parasympathetic activation.
Atropine administration before buspirone ingestion annulled the free serotonin increase as well as systolic blood pressure-heart rate decrease. We found significant positive correlations between noradrenaline and dopamine levels. The strength and significance of these correlations were increased by using the noradrenaline/adrenaline ratio instead of noradrenaline absolute values. This finding indicates that increases in both noradrenaline and dopamine arise from sympathetic nerves rather than the adrenal glands. We also found significant negative correlations between free serotonin increases and systolic blood pressure-heart rate decreases. Our results indicate that buspirone stimulates central sympathetic activity. These acute effects of buspirone are reflected in an increased peripheral neural sympathetic activity, but not adrenal sympathetic activity in healthy individuals. In addition, buspirone increases free serotonin plasma concentrations and decreases systolic blood pressure plus heart rate levels through mechanisms associated with parasympathetic activation.
Similar articles
-
Effects of amantadine on circulating neurotransmitters in healthy subjects.
Lechin F, van der Dijs B, Pardey-Maldonado B, Rivera JE, Baez S, Lechin ME. Lechin F, et al. J Neural Transm (Vienna). 2010 Mar;117(3):293-9. doi: 10.1007/s00702-010-0371-1. Epub 2010 Feb 4. J Neural Transm (Vienna). 2010. PMID: 20131070 Free PMC article.
-
Acute effects of tianeptine on circulating neurotransmitters and cardiovascular parameters.
Lechin F, van der Dijs B, Hernández G, Orozco B, Rodríguez S, Baez S. Lechin F, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2006 Mar;30(2):214-22. doi: 10.1016/j.pnpbp.2005.10.013. Epub 2005 Nov 21. Prog Neuropsychopharmacol Biol Psychiatry. 2006. PMID: 16303223 Clinical Trial.
-
Dose related effects of buspirone on serum electrolyte balance, plasma osmolality and systolic blood pressure (SBP) in rats.
Farooq R, Haleem DJ, Haleem MA. Farooq R, et al. Pak J Pharm Sci. 2007 Oct;20(4):295-9. Pak J Pharm Sci. 2007. PMID: 17604252
-
Buspirone: review of its pharmacology and current perspectives on its mechanism of action.
Eison AS, Temple DL Jr. Eison AS, et al. Am J Med. 1986 Mar 31;80(3B):1-9. doi: 10.1016/0002-9343(86)90325-6. Am J Med. 1986. PMID: 2870639 Review.
-
Buspirone: an update on a unique anxiolytic agent.
Jann MW. Jann MW. Pharmacotherapy.
 1988;8(2):100-16. doi: 10.1002/j.1875-9114.1988.tb03543.x. Pharmacotherapy. 1988. PMID: 3041384 Review.
1988;8(2):100-16. doi: 10.1002/j.1875-9114.1988.tb03543.x. Pharmacotherapy. 1988. PMID: 3041384 Review.
See all similar articles
Cited by
-
Efficacy of Buspirone Augmentation of Escitalopram in Patients with Major Depressive Disorder with and without Atypical Features: A Randomized, 8 Week, Multicenter, Open-Label Clinical Trial.
Shin C, Ko YH, Shim SH, Kim JS, Na KS, Hahn SW, Lee SH. Shin C, et al. Psychiatry Investig. 2020 Aug;17(8):796-803. doi: 10.30773/pi.2020.0017. Epub 2020 Aug 6. Psychiatry Investig. 2020. PMID: 32750760 Free PMC article.
-
Conserved Serotonergic Background of Experience-Dependent Behavioral Responsiveness in Zebrafish (Danio rerio).
Varga ZK, Pejtsik D, Biró L, Zsigmond Á, Varga M, Tóth B, Salamon V, Annus T, Mikics É, Aliczki M.
 Varga ZK, et al. J Neurosci. 2020 Jun 3;40(23):4551-4564. doi: 10.1523/JNEUROSCI.2178-19.2020. Epub 2020 Apr 29. J Neurosci. 2020. PMID: 32350040 Free PMC article.
Varga ZK, et al. J Neurosci. 2020 Jun 3;40(23):4551-4564. doi: 10.1523/JNEUROSCI.2178-19.2020. Epub 2020 Apr 29. J Neurosci. 2020. PMID: 32350040 Free PMC article. -
Anorexia nervosa depends on adrenal sympathetic hyperactivity: opposite neuroautonomic profile of hyperinsulinism syndrome.
Lechin F, van der Dijs B, Pardey-Maldonado B, Rivera JE, Baez S, Lechin ME. Lechin F, et al. Diabetes Metab Syndr Obes. 2010 Sep 9;3:311-7. doi: 10.2147/DMSOTT.S10744. Diabetes Metab Syndr Obes. 2010. PMID: 21437100 Free PMC article.
-
Effects of amantadine on circulating neurotransmitters in healthy subjects.
Lechin F, van der Dijs B, Pardey-Maldonado B, Rivera JE, Baez S, Lechin ME. Lechin F, et al. J Neural Transm (Vienna).
 2010 Mar;117(3):293-9. doi: 10.1007/s00702-010-0371-1. Epub 2010 Feb 4. J Neural Transm (Vienna). 2010. PMID: 20131070 Free PMC article.
2010 Mar;117(3):293-9. doi: 10.1007/s00702-010-0371-1. Epub 2010 Feb 4. J Neural Transm (Vienna). 2010. PMID: 20131070 Free PMC article. -
Role of the serotonergic system in reduced pulmonary function after exposure to methamphetamine.
Wells SM, Buford MC, Porter VM, Brunell HL, Bunderson-Schelvan M, Nevin AB, Cardozo-Pelaez F, Holian A. Wells SM, et al. Am J Respir Cell Mol Biol. 2010 May;42(5):537-44. doi: 10.1165/rcmb.2009-0121OC. Epub 2009 Jun 18. Am J Respir Cell Mol Biol. 2010. PMID: 19541843 Free PMC article.
See all "Cited by" articles
Publication types
MeSH terms
Substances
Use of buspirone in clinical practice
Buspirone (spitomin) is a non-benzodiazepine anxiolytic, which is a derivative of azapirone. Azapirones are a class of drugs with high affinity for serotonin 5-HT1A receptors located on the body and in the endings of serotonergic neurons, as well as in the dendrites of postsynaptic neurons with which serotonergic endings contact. The anti-anxiety effect of buspirone is not associated with an effect on GABA-benzodiazepine receptors. However, in terms of anxiolytic activity, buspirone is comparable to such benzodiazepines as diazepam, lorazepam or alprazolam, but unlike them, it does not cause drug dependence, cognitive and psychomotor impairment, pronounced sedative and muscle relaxant effects [1].
Azapirones are a class of drugs with high affinity for serotonin 5-HT1A receptors located on the body and in the endings of serotonergic neurons, as well as in the dendrites of postsynaptic neurons with which serotonergic endings contact. The anti-anxiety effect of buspirone is not associated with an effect on GABA-benzodiazepine receptors. However, in terms of anxiolytic activity, buspirone is comparable to such benzodiazepines as diazepam, lorazepam or alprazolam, but unlike them, it does not cause drug dependence, cognitive and psychomotor impairment, pronounced sedative and muscle relaxant effects [1].
Mechanism of action of buspirone
The mechanism of the anxilytic action of buspirone, which predetermines its features, is mainly due to the effect on serotonin receptors. Buspirone has a high affinity for presynaptic serotonin (5-HT1A) receptors, of which it is an agonist, as well as for postsynaptic serotonin receptors, for which it can be considered as a partial (partial) agonist [1].
Partial agonists are ligands that bind to receptors but are only able to cause their partial activation. If a partial agonist interacts with free receptors, it causes incomplete (50%) cell activation. If a partial agonist acts on a tissue whose receptors are already activated by a full agonist, then due to the displacement of the latter from their connection with the receptor, the cell response decreases and, therefore, it has a blocking effect.
Thus, due to its receptor action, buspirone reduces the synthesis and release of serotonin, reduces the activity of serotonergic neurons in the raphe nuclei ("serotonin stabilizer"). Buspirone is also able to interact with postsynaptic 5-HT2 receptors, but has a low affinity for them. Being a partial agonist, due to its postsynaptic action, it can theoretically reduce serotonergic activity and cause an anxiolytic effect, and due to its action on somatodendritic serotonin presynaptic autoreceptors, it can theoretically increase serotonergic activity and cause an antidepressant effect.
To a lesser extent, the drug selectively blocks pre- and postsynaptic D2-dopamine receptors. Moreover, it was originally developed as an antipsychotic, but later it turned out that it has a low affinity for dopamine receptors, and its presynaptic action prevails over the postsynaptic one, which prevents the neuroleptic effect.
Experimental studies have indicated a decrease in serotonin turnover with the introduction of low doses of buspirone, which reflects the stimulation of presynaptic autoreceptors. However, repeated administration of buspirone reduces the response of somatodendritic 5-HT1A receptors, since the decrease in 5-HT metabolism was less pronounced.
Buspirone can act as a partial agonist at postsynaptic serotonin 5-HT1A receptors in the hippocampus and prefrontal cortex, as well as presynaptic autoreceptors on the bodies of serotonergic neurons. Since the effect of azapirones develops over several days, it does not appear to be related to their direct action on receptors. Animal studies suggest that the anxiolytic effect of these drugs is due to their action on presynaptic receptors, and the antidepressant effect is due to the action on postsynaptic receptors.
Animal studies suggest that the anxiolytic effect of these drugs is due to their action on presynaptic receptors, and the antidepressant effect is due to the action on postsynaptic receptors.
Decreased serotonin circulation also occurs in the striatum, which may be accompanied by an inhibitory effect on dopaminergic transmission. This effect is manifested in the fact that the co-administration of buspirone can attenuate apomorphine sensitization. Thus, buspirone at a relatively low dose can counteract the side effect of apomorphine. However, the introduction of buspirone at a higher dose caused sensitization of motor behavior [2].
This effect may be due to blockade of presynaptic dopamine autoreceptors. The introduction of a relatively high dose of buspirone in the experiment caused a sharp increase in the synthesis and metabolism of dopamine in the striatum.
The experiment has shown that the administration of buspirone in the brain dialysate reduces the level of serotonin by 50%, but increases the level of dopamine by 100% and norepinephrine by 140%, which indicates a complex effect of buspirone on monoaminergic neurotransmission [3].
However, in addition to acting on autoreceptors, buspirone also has another point of application, since the administration of haloperidol, which blocks D1- and D2-postsynaptic mesolimbic and striatal receptors, did not block the dopaminomimetic effect of buspirone. It has been shown that the antagonistic effect of the drug on D2 receptors is 15 times weaker than the effect on 5-HT1A receptors. Buspirone is a relatively weak blocker of postsynaptic dopamine receptors, even at high doses. It binds with higher affinity to D3 and D4 receptors than to D2 receptors. The action on presynaptic dopamine receptors is stronger than the blockade of postsynaptic receptors. Only in super-high doses, postsynaptic striatal D1- and D2-receptors are blocked quite actively. However, weak postsynaptic dopaminergic antagonism weakens the presynaptic dopaminomimetic effect of the drug, avoiding stereotypy [2, 3].
Use of buspirone in generalized anxiety and other affective disorders
The greatest experience with the use of buspirone has been accumulated in generalized anxiety disorder (GAD).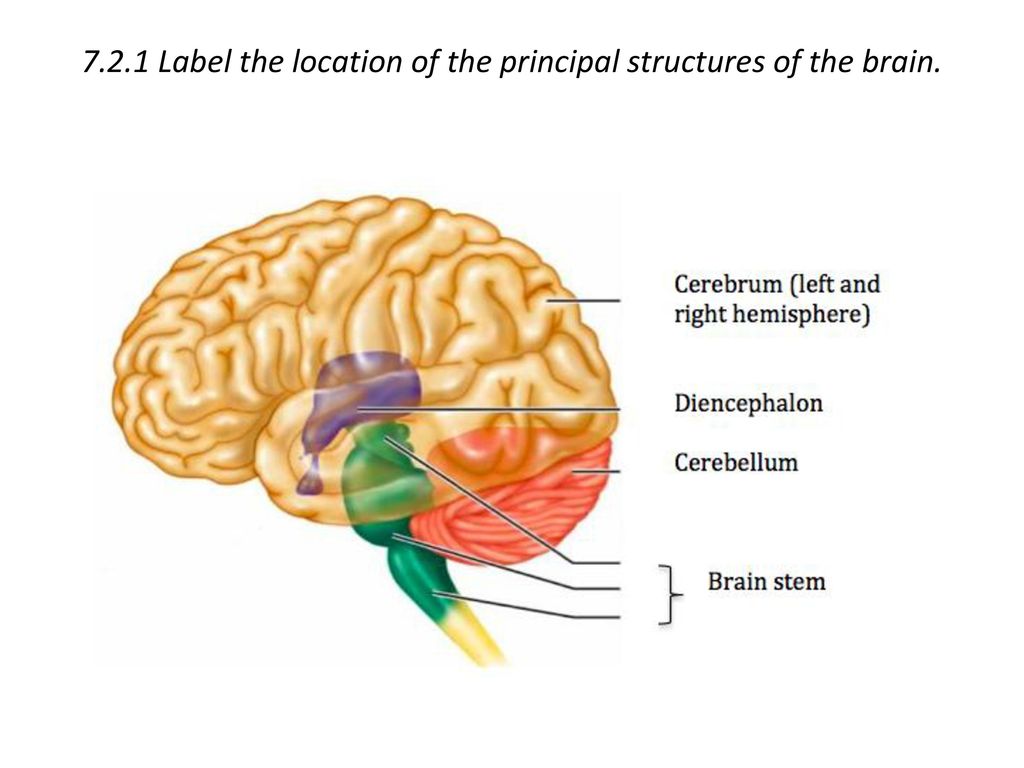 Clinical studies of the last 30 years have been accompanied by constant improvement in the nosological structure of anxiety disorders. At the beginning of the 20th century, the understanding of anxiety disorders was rather vague, but over time their place among other mental disorders has been more clearly defined, partly under the influence of pharmacological research.
Clinical studies of the last 30 years have been accompanied by constant improvement in the nosological structure of anxiety disorders. At the beginning of the 20th century, the understanding of anxiety disorders was rather vague, but over time their place among other mental disorders has been more clearly defined, partly under the influence of pharmacological research.
Symptoms of GAD are chronic and often observed in patients who visit general practitioners. Typically, such patients present vague somatic complaints of fatigue, muscle pain or tension, and sleep disturbances.
Buspirone is the first FDA-approved non-benzodiazepine anxiolytic for the treatment of GAD, based on studies showing that it is comparable in efficacy to benzodiazepines.
In accordance with DSM-V [4] criteria, GAD is characterized by:
A. Excessive anxiety and worry (anxious expectations) that occurs most of the days for at least 6 months and manifests itself in a variety of events or activities (for example, in the performance of professional duties or while studying at school).
B. Difficulty in controlling anxiety.
C. Presence most of the days for 6 months at least 3 of the following symptoms: restlessness or restlessness; fast fatiguability; difficulty concentrating and being distracted; irritability; muscle tension; sleep disturbance.
D. Clinically significant distress or impairment of social, occupational, or other activities caused by anxiety, restlessness, or physical (psychomotor) symptoms.
E. These disorders that cannot be explained by a physiological effect of a psychoactive substance or medical illness.
E. These disorders that cannot be explained by another mental illness.
Buspirone has also been successfully used for the prophylactic treatment of migraine and alcoholism in individuals with comorbid anxiety disorder, as well as for the treatment of anxious depression, anxiety and agitation in patients with traumatic brain injury [5, 6].
The direct antidepressant effect of buspirone, independent of its anxiolytic action, has also been demonstrated.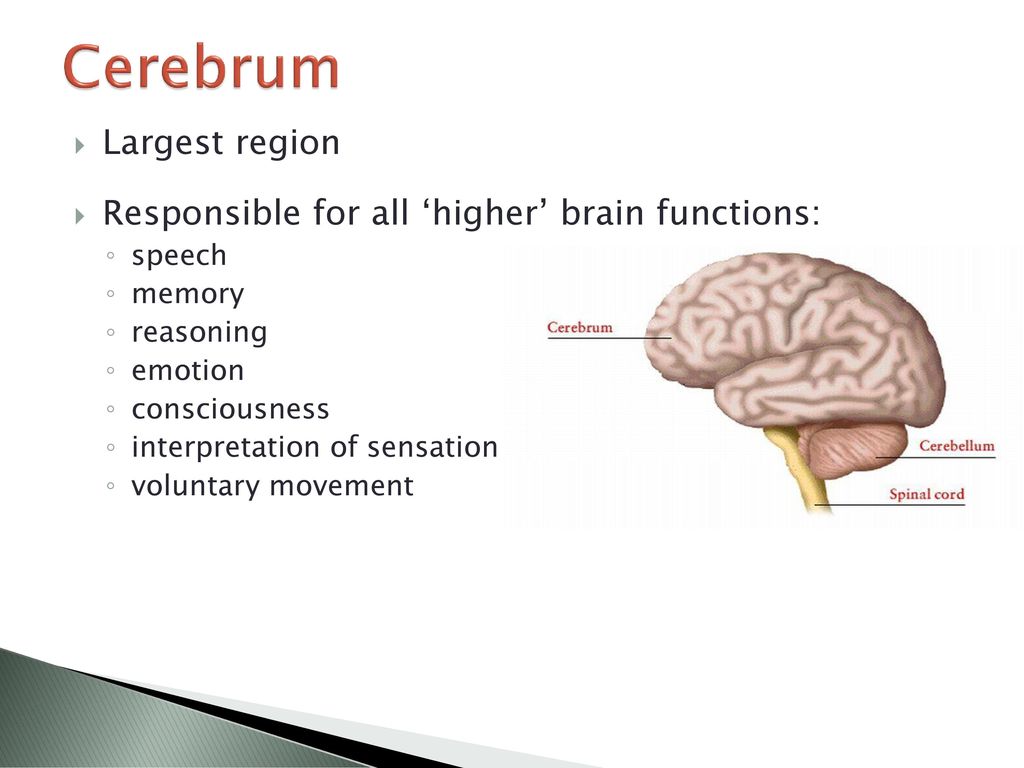 It may be due to the fact that the sensitivity of 5-HT1A autoreceptors in depression is increased, while the properties of postsynaptic 5-HT1A and 5-HT2A receptors do not change in untreated patients with depression.
It may be due to the fact that the sensitivity of 5-HT1A autoreceptors in depression is increased, while the properties of postsynaptic 5-HT1A and 5-HT2A receptors do not change in untreated patients with depression.
Data on the effectiveness of buspirone in obsessive-compulsive disorder are contradictory, but some studies show a positive effect of the drug, especially when combined with other psychopharmacological drugs, primarily antidepressants. An open study showed that the addition of buspirone (at a dose of 30-90 mg/day) to selective serotonin reuptake inhibitors (SSRIs) increases the effectiveness of the treatment of body dysmorphic disorder in almost half of patients, mainly in those who had a partial effect on serotonergic antidepressants [7, 8].
Buspirone can be considered as an alternative to antidepressants in the treatment of social phobia. The effect of buspirone on social phobia has been shown in a placebo-controlled study. When added to SSRIs, buspirone may correct some of their side effects.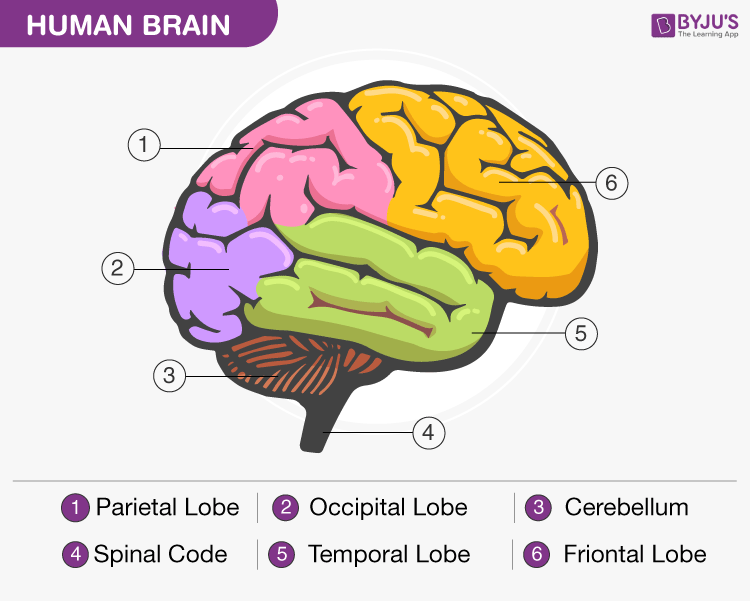 For example, it has been shown that at a dose of 15-60 mg/day it can reduce sexual dysfunction and bruxism [8].
For example, it has been shown that at a dose of 15-60 mg/day it can reduce sexual dysfunction and bruxism [8].
Antiaggressive effect of buspirone
Separate mention should be made of the anti-aggressive effect of buspirone in agitation and aggressive actions in patients with dementia, attention deficit hyperactivity disorder, and traumatic brain injury [8]. At the same time, the effect of buspirone at a dose of 15–60 mg/day was noted both in relation to verbal (60%) and physical (90%) aggression [9–12].
Effect of buspirone on cognitive functions
Data on the effect of buspirone on cognitive functions are contradictory, some studies show a moderate positive effect on regulatory functions, while others show no clear effect [13]. In any case, unlike benzodiazepines and sedatives, buspirone does not have a negative effect on cognitive functions, which distinguishes it from other drugs used to correct anxiety disorders [14].
Use of buspirone for the treatment of dependence syndrome
Due to its dopaminergic action, buspirone can be used to treat dependence syndrome. Initially, this quality was found in relation to addiction to cocaine. It has been experimentally shown that intramuscular administration of buspirone blocks D2 and D3 receptors, while oral administration of buspirone blocks D3 receptors more selectively, occupying more than 80% of the receptors. Thus, for the treatment of addiction, higher doses may be required - 2-3 times higher than for the treatment of anxiety (up to a maximum of 60 mg). As shown by previous studies, these doses are safe and well tolerated [15]. Correction of withdrawal anxiety under the influence of buspirone may also contribute to the treatment of alcohol and tobacco dependence [16-18].
Use of buspirone in extrapyramidal disorders
Experimental and clinical studies indicate that buspirone may be effective in the treatment of tardive dyskinesia.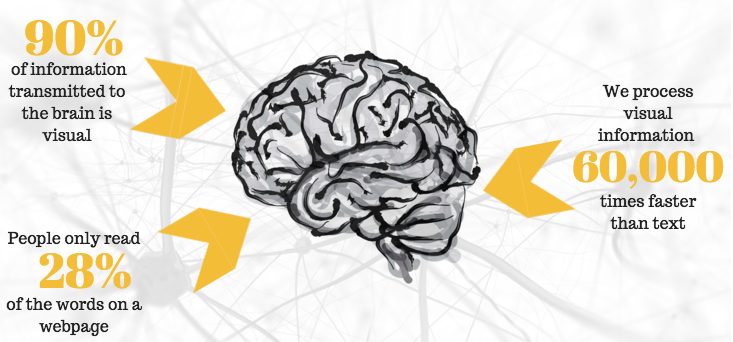 Tardive dyskinesia is characterized by involuntary movements involving the muscles of the head, limbs, or trunk. It usually occurs against the background of long-term therapy with antipsychotics, often manifesting itself or intensifying after their cancellation. Tardive dyskinesia is usually resistant to various treatments. Although the evidence base for buspirone in tardive dyskinesia is low, it may be an alternative to others (particularly dopaminergic drugs) with dangerous side effects. The mechanism of action of buspirone in tardive dyskinesia is unclear [19, 20].
Tardive dyskinesia is characterized by involuntary movements involving the muscles of the head, limbs, or trunk. It usually occurs against the background of long-term therapy with antipsychotics, often manifesting itself or intensifying after their cancellation. Tardive dyskinesia is usually resistant to various treatments. Although the evidence base for buspirone in tardive dyskinesia is low, it may be an alternative to others (particularly dopaminergic drugs) with dangerous side effects. The mechanism of action of buspirone in tardive dyskinesia is unclear [19, 20].
Buspirone may also be effective in patients with Parkinson's disease suffering from dyskinesias on the background of long-term levodopa therapy. The mechanism of action of the drug is not clear enough in this case either, but the therapeutic effect depends on the dose. In recent years, using pathomorphological and imaging methods, it has been shown that the serotonergic system is involved in the pathological process in Parkinson's disease, in particular, in the pathogenesis of a number of non-motor symptoms, as well as medicinal dyskinesias. Because of this, drugs that act on the serotonergic system can be effective against dyskinesias and non-motor symptoms such as depression, chronic fatigue, and psychotic disorders [21].
Because of this, drugs that act on the serotonergic system can be effective against dyskinesias and non-motor symptoms such as depression, chronic fatigue, and psychotic disorders [21].
It has already been mentioned that 5-HT1A receptor agonists regulate striatal concentrations of dopamine formed from exogenous levodopa, whereby buspirone can reduce drug-induced dyskinesias ("peak dose") in Parkinson's disease.
In a clinical study [22], it was shown that consecutive administration of the drug for 3 days (at a dose of 15-30 mg / day), but not a single dose of the drug at a dose of 10 and 20 mg, led to a 20% reduction in dyskinesias (without an increase in symptoms parkinsonism). In another study, a 3-week dose of buspirone at a dose of 20 mg/day reduced the severity of drug-induced dyskinesias by 71% without any negative effect on the symptoms of parkinsonism. Thus, buspirone can be considered as a potentially effective strategy in the correction of drug-induced dyskinesias.
These clinical data are experimentally confirmed - at a dose of 2 mg / kg, buspirone attenuated violent movements by 45% in mice with an experimental model of dopaminergic dyskinesias. Administration of buspirone to rats at a dose of 0.25-2.5 mg/kg 30 min before levodopa reduced dyskinesias and improved locomotor activity. In the experiment, buspirone reduced catalepsy in rats induced by 6-OHDA and haloperidol.
The effectiveness of buspirone was also shown in dyskinesias that occurred after transplantation of fetal midbrain tissue. It is believed that transplantation dyskinesias are associated with serotonergic neurotransmission [23]. Correction of dyskinesias is more effective with a high dose of 5-HT1A agonist, which more completely blocks mediator release. Dopaminergic reinnervation after transplantation causes persistent dyskinesias due to the uncontrolled release of dopamine induced by serotonin. The release of dopamine occurs in this case with a lower intensity than with "peak dose" dyskinesia. Accordingly, the effect in this situation is more pronounced with systemic administration of lower doses of 5-HT1A agonists [22, 23].
Accordingly, the effect in this situation is more pronounced with systemic administration of lower doses of 5-HT1A agonists [22, 23].
Buspirone can be effective in hereditary spinocerebellar ataxia, which is explained by the pronounced serotonergic innervation of the cerebellum [24]. It is suggested that this effect may also be associated with an effect on the noradrenergic pathways. This assumption has been confirmed in several studies showing that buspirone reduces mild to moderate cerebellar ataxia by 37%. The positive effect of buspirone on the severity of ataxia was confirmed in a small placebo-controlled study [25, 26].
Practical aspects of the use of buspirone (Spitomin)
The main indications for the use of buspirone (spitomin) registered in the Russian Federation are generalized anxiety disorder (GAD), panic disorder, autonomic dysfunction syndrome, alcohol withdrawal syndrome (as adjuvant therapy), depressive disorders (as adjuvant therapy, but not prescribed for monotherapy depression). The literature describes the use of buspirone for various anxiety conditions, tobacco withdrawal, depression, as well as autism, obsessive-compulsive and premenstrual syndromes (in which the drug acts as an adjuvant). The mechanism of action makes it possible to use it for the treatment of resistant depression (in combination with an antidepressant) [27].
The literature describes the use of buspirone for various anxiety conditions, tobacco withdrawal, depression, as well as autism, obsessive-compulsive and premenstrual syndromes (in which the drug acts as an adjuvant). The mechanism of action makes it possible to use it for the treatment of resistant depression (in combination with an antidepressant) [27].
The therapeutic (anxiolytic) effect develops gradually, manifests itself after 7-14 days and reaches a maximum after 4 weeks.
The goal of treatment for mood disorders may be to achieve complete remission or at least symptomatic relief, and to prevent possible relapses if possible. In chronic anxiety disorders, long-term maintenance therapy with buspirone may be required for long-term symptom control.
An important advantage of buspirone is that, unlike classical anxiolytics such as benzodiazepines, it does not adversely affect psychomotor functions, does not cause tolerance, drug dependence and withdrawal symptoms, and does not potentiate the effect of alcohol. However, during the period of treatment with buspirone, alcohol should be excluded [28].
However, during the period of treatment with buspirone, alcohol should be excluded [28].
The absence of a therapeutic effect within 6-8 weeks requires an increase in the dose, but in some cases indicates resistance to the drug. In the absence of an effect, the drug is usually replaced with another agent (benzodiazepine or antidepressant). With a partial effect, an antidepressant, sedative or hypnotic (with insomnia) can be added to enhance its action. Especially often, buspirone is used in combination with an SSRI or a selective serotonin and norepinephrine reuptake inhibitor (SNRI) [29].
Use in persons under 18 years of age is contraindicated, according to the current instructions in the Russian Federation for buspirone. In children and adolescents from 6 to 18 years of age, no efficacy studies have been conducted in relation to anxiety disorder. Pregnancy and breastfeeding are also contraindications, although there is no direct evidence of danger.
Buspirone is generally well tolerated. Side effects, if they are observed, usually occur at the beginning of the course of treatment and then disappear, despite the continuation of the drug. In some cases, a dose reduction is necessary. As a side effect, the drug can cause dizziness, headache, weakness, sleep disturbance, decreased attention, extrapyramidal disorders (very rarely), confusion, nausea, dry mouth, diarrhea, vomiting, constipation, weight loss, weight gain, myalgia, muscle spasms, rash, sweating, paresthesia. In case of an overdose, vomiting, dizziness, miosis, depression of consciousness are possible. The drug is carefully combined with antipsychotics, cardiac glycosides, antihypertensive and antidiabetic agents, oral contraceptives. Interaction with MAO inhibitors can lead to serotonin syndrome or hypertensive crisis with more limited symptoms, therefore, the simultaneous use of buspirone and MAO inhibitors is contraindicated in the instructions for medical use.
Side effects, if they are observed, usually occur at the beginning of the course of treatment and then disappear, despite the continuation of the drug. In some cases, a dose reduction is necessary. As a side effect, the drug can cause dizziness, headache, weakness, sleep disturbance, decreased attention, extrapyramidal disorders (very rarely), confusion, nausea, dry mouth, diarrhea, vomiting, constipation, weight loss, weight gain, myalgia, muscle spasms, rash, sweating, paresthesia. In case of an overdose, vomiting, dizziness, miosis, depression of consciousness are possible. The drug is carefully combined with antipsychotics, cardiac glycosides, antihypertensive and antidiabetic agents, oral contraceptives. Interaction with MAO inhibitors can lead to serotonin syndrome or hypertensive crisis with more limited symptoms, therefore, the simultaneous use of buspirone and MAO inhibitors is contraindicated in the instructions for medical use.
In the Russian Federation, the drug is presented in the form of 10 mg divided tablets. The initial dose is 5 mg / day, if necessary, it is increased by 5 mg every 5 days. The average daily dose is 15 mg. The maximum single dose is 30 mg, the maximum daily dose is 60 mg. The daily dose is usually divided into 2-3 doses. The advantages of the drug include safety, lack of dependence and withdrawal syndrome, sexual dysfunction and weight gain. Moreover, buspirone is able to reduce sexual dysfunction caused by generalized anxiety and serotonergic antidepressants [28, 30].
The initial dose is 5 mg / day, if necessary, it is increased by 5 mg every 5 days. The average daily dose is 15 mg. The maximum single dose is 30 mg, the maximum daily dose is 60 mg. The daily dose is usually divided into 2-3 doses. The advantages of the drug include safety, lack of dependence and withdrawal syndrome, sexual dysfunction and weight gain. Moreover, buspirone is able to reduce sexual dysfunction caused by generalized anxiety and serotonergic antidepressants [28, 30].
The peculiarities of the drug include the gradual onset of the full clinical effect (within 4 weeks), while benzodiazepines act more quickly.
Buspirone is metabolized primarily by the microsomal enzyme CYP450 3A4. T½ is approximately 2-3 hours. Particular care should be taken when combined with CYP450 3A4 inhibitors (for example, diltiazem, verapamil, itraconazole, erythromycin), which can reduce the clearance of buspirone and increase its level in the blood. You should also avoid drinking large amounts of grapefruit and grapefruit juice at the same time. The dose of buspirone should also be reduced when using HIV protease inhibitors or ketoconazole. Conversely, CYP450 3A4 inducers (eg carbamazepine and rifampicin) may increase the clearance of buspirone. Buspirone increases blood levels of nordiazepam, the active metabolite of diazepam, which can cause dizziness, headache, and nausea.
The dose of buspirone should also be reduced when using HIV protease inhibitors or ketoconazole. Conversely, CYP450 3A4 inducers (eg carbamazepine and rifampicin) may increase the clearance of buspirone. Buspirone increases blood levels of nordiazepam, the active metabolite of diazepam, which can cause dizziness, headache, and nausea.
In mild to moderate renal and hepatic insufficiency, liver cirrhosis, heart failure, in the elderly, the drug is prescribed in smaller doses. When discontinuing the drug, a gradual dose reduction is usually not necessary. In severe renal, hepatic insufficiency, uncontrolled epilepsy, glaucoma, myasthenia gravis, the drug is contraindicated.
Thus, buspirone, with its unique neurotransmitter effects, significantly expands the possibilities of treatment for a wide range of mental and neurological disorders - from anxiety disorders to dyskinesia in Parkinson's disease and ataxia. The relative safety of the drug makes its use in clinical practice very promising.
Spitomin (buspirone). A new "classic" in the treatment of anxiety disorders
partners Keywords / keywords: Spitomin, Neuropsychiatry, Anxiolytics, Psychiatry, Anxiety disorders
Introduction to the problem
According to large-scale epidemiological studies conducted in the United States and Europe in 2005 and 2010, anxiety disorders of varying severity are observed in 21-30% of the population 1 . According to the WHO, the problem is in the top 10 most significant health problems 2 .
It has been proven that anxiety disorders are characterized by a chronic course 3 and a high degree of comorbidity with other mental and behavioral disorders. First of all, this concerns depression, which in more than 50% of cases 4 accompanies panic disorder or generalized anxiety disorder.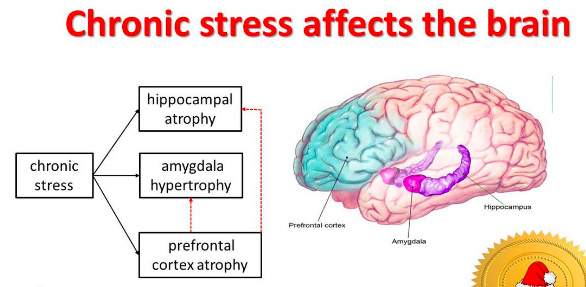 Often, the combination of anxiety and depressive disorders leads to a more severe course of the disease and resistance to standard treatment.
Often, the combination of anxiety and depressive disorders leads to a more severe course of the disease and resistance to standard treatment.
Therapy of anxiety-depressive disorders, which include generalized anxiety disorder (GAD), panic disorder with agoraphobia, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), social phobias, as a rule, long-term and combined, requires careful selection of doses , their episodic correction and monitoring of side effects.
Complex therapy should be directed to:
- elimination of mental and somatic symptoms (anxiety, restlessness, muscle tension, sleep disturbances, pain syndrome, blood pressure fluctuations, arrhythmias, etc.),
- treatment of comorbid conditions,
- improving the quality of life,
- achieving complete remission (relapse prevention).
Among the means of pharmacotherapy today, combinations of tricyclic antidepressants, antidepressants of the SSRI and SNRI groups, benzodiazepines are most often used.
Existing problems in the classical treatment of anxiety disorders
Antidepressants
The therapeutic effect of SSRIs, like other antidepressants, is realized mainly from the 2nd–3rd week of therapy, and the 1st week may be marked by an increase in anxiety, which is subjectively difficult to tolerate by patients 5 . In addition, there is the possibility of developing, despite a generally favorable tolerability profile, potentially dangerous side effects (serotonin syndrome), as well as a withdrawal syndrome 6 .
Benzodiazepines
It is generally recognized that tranquilizers of this group have pronounced undesirable effects: muscle relaxant, sedative, excessive hypnotic, amnestic, negative effects on psychomotor functions, a high risk of developing addiction and withdrawal syndrome 7 .
Pregabalin
Recently, the psychotropic activity of the drug pregabalin, a structural analogue of GABA, has been discussed in the literature. It reduces the stimulation of postsynaptic neurons, causing anxiolytic, anticonvulsant and analgesic effects. However, when using the drug for 8 weeks, only 52% of patients had a 50% reduction in symptoms according to the Hamilton anxiety scale (HAM-A) 8 .
It reduces the stimulation of postsynaptic neurons, causing anxiolytic, anticonvulsant and analgesic effects. However, when using the drug for 8 weeks, only 52% of patients had a 50% reduction in symptoms according to the Hamilton anxiety scale (HAM-A) 8 .
Taking into account the goals of therapy and existing problems, a list of requirements was determined that the main clinical and pharmacological characteristics of anxiolytics must meet:
- a distinct anxiolytic effect, both in relation to the mental and somatic components of anxiety;
- lack of sedative, muscle relaxant and additive properties;
- the action of the drug should be aimed not only at eliminating the mental symptoms of the disorder, but also at treating comorbid conditions;
- decrease in maladaptation;
- improving the quality of life.
Spitomin (buspirone), the only representative of the class of serotonergic anxiolytics, azapirones, is perhaps most consistent with these criteria.
Spitomin (buspirone): mechanism of action
Buspirone differs in its chemical structure from all other previously known anxiolytics and belongs to the azaspirone class.
Its anxiolytic action is associated with stimulation of presynaptic serotonin autoreceptors, and, unlike SSRIs, does not depend on the nature of the basal release of serotonin 9 . Buspirone has proven to be an effective pharmacotherapy for the most difficult treatable forms of anxiety disorders and was the first nonbenzodiazepine anxiolytic to be approved by the FDA in 1986, for the treatment of patients with GAD 10 .
Spitomin (buspirone) occupies a special place among all neuropharmacological agents and can be considered as a drug that combines the properties of an anxiolytic and antidepressant, which is of great value in the treatment of mixed anxiety-depressive disorders.
Spitomin (buspirone) affects:
- Serotonin neurotransmission system.
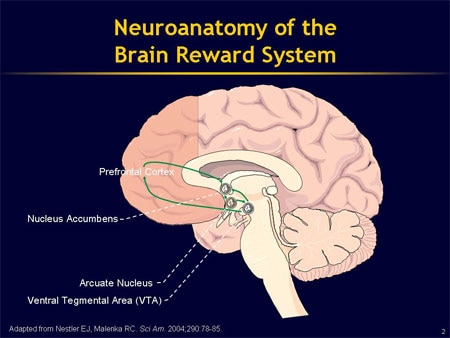 When it is hyperactive, it stimulates presynaptic 5-HT1A receptors that block the release of serotonin into the synapse (antiserotonergic effect). When it is weakened, it stimulates postsynaptic 5-HT1A receptors (activates serotonergic processes) 11 .
When it is hyperactive, it stimulates presynaptic 5-HT1A receptors that block the release of serotonin into the synapse (antiserotonergic effect). When it is weakened, it stimulates postsynaptic 5-HT1A receptors (activates serotonergic processes) 11 . - Dopamine neurotransmission system. By selectively blocking pre and postsynaptic D2 receptors, it increases the rate of excitation of dopamine neurons in the midbrain 12 and enhances its independent antidepressant effect.
- Norepinephrine system of neurotransmission. The active metabolites of buspirone have an adrenergic effect 13 . The associated general mood elevation explains the antidepressant effect in SSRI-resistant patients.
Spitomin (buspirone) does not affect either GABA or benzodiazepine receptors, and therefore does not have the side effects of such tranquilizers. Nevertheless, in terms of its effectiveness, by the 7-14th day of therapy, the drug is comparable to typical benzodiazepines.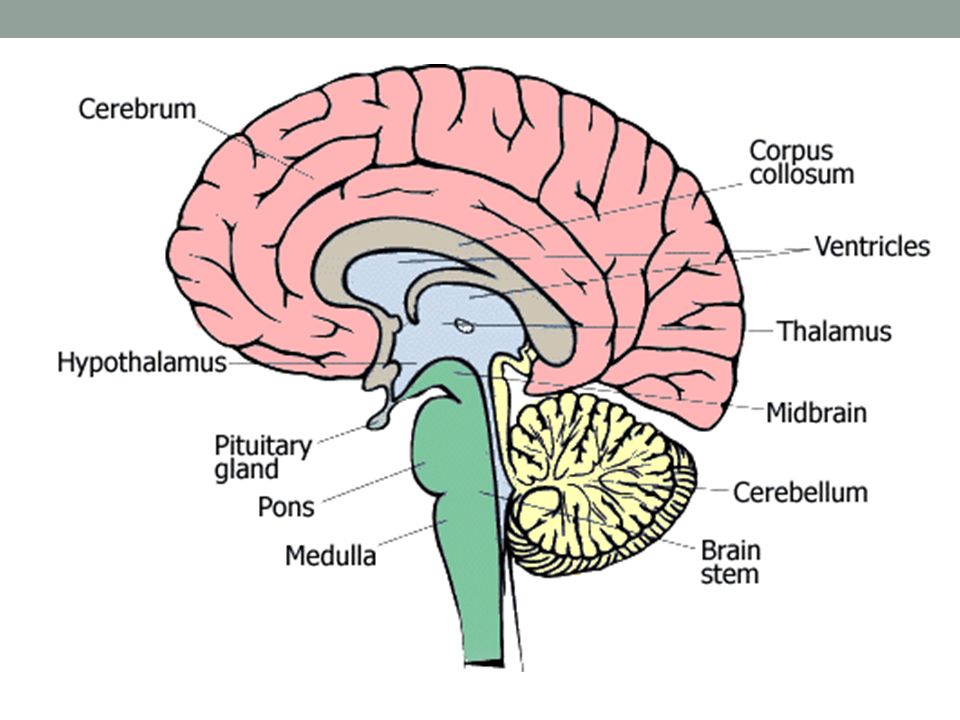
Indications for use
Spitomin (buspirone) has shown its clinical efficacy in the treatment of the following diseases:
- Generalized Anxiety Disorder (GAD).
- panic disorder.
- Syndrome of autonomic dysfunction.
- Alcohol withdrawal syndrome (as adjuvant therapy).
- Depressive disorders (as adjuvant therapy).
Clinical efficacy
Based on numerous clinical studies (Murphy, Owen and Tyrer; Schramm et al., Pato et al., Goodman et al., Jenike et al., Robinson et al., etc.), buspirone has been proven to be a very promising and safe agent for long-term mono- or combination therapy.
For generalized anxiety disorder
The anxiolytic effect of buspirone does not differ from that of diazepam (after use for 3 or more weeks) 14 . Buspirone is much more effective than benzodiazepines in reducing depressive symptoms associated with anxiety disorder.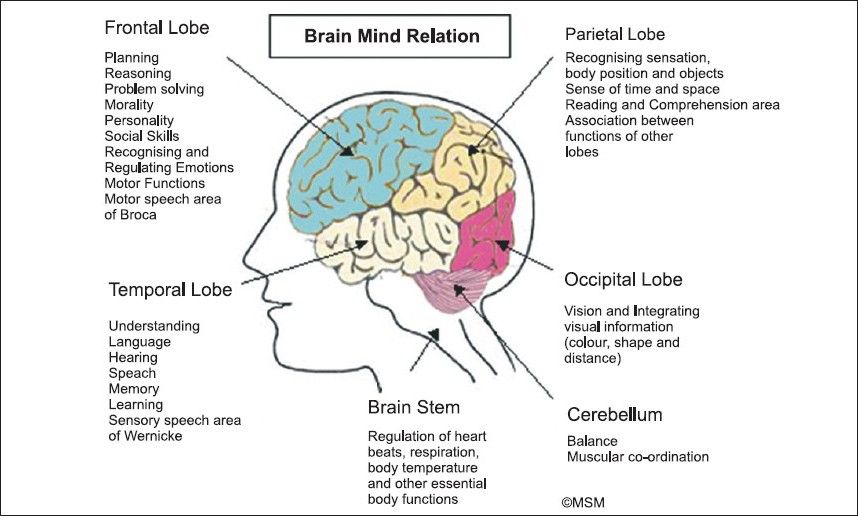 Adverse events of a sedative nature when taking buspirone occur much less frequently than with benzodiazepines 15 .
Adverse events of a sedative nature when taking buspirone occur much less frequently than with benzodiazepines 15 .
For anxiety and depression
Buspirone was 1.3 times more effective than placebo in reducing anxiety and 1.6 times better than placebo in reducing depressive symptoms in the treatment of GAD with depressive symptoms 16 . The tolerability of buspirone is comparable to placebo.
For obsessive-compulsive disorder (OCD)
- Clomipramine and buspirone show the same result on the OCD scale and the depression scale.
- Buspirone added to OCD therapy with fluoxetine improves the effectiveness of treatment.
- Simultaneous administration of buspirone and antipsychotics is well suited for patients who are not amenable to therapy with selective serotonin reuptake inhibitors.
For the treatment of depression
Numerous open studies have unambiguously proven the positive effect of buspirone on depressive symptoms, including resistant depression. When buspirone was added to antidepressant therapy, there was a significant improvement in the condition, compared with the placebo group. Some symptoms such as depressed mood, unwillingness to work, lethargy improved after the first week of taking the drug.
When buspirone was added to antidepressant therapy, there was a significant improvement in the condition, compared with the placebo group. Some symptoms such as depressed mood, unwillingness to work, lethargy improved after the first week of taking the drug.
Buspirone, already an effective antidepressant in its own right, when added to enhance classical antidepressant therapy, presumably via the serotonergic and noradrenergic systems, may improve treatment outcomes 17 . Buspirone is an effective and safe agent for improving the results of antidepressant therapy.
In the treatment of panic disorder
Buspirone significantly reduces the severity of generalized anxiety, agoraphobia, panic attacks and depression in a short time 18 . The positive effect of buspirone has been demonstrated in relation to both cognitive and somatic components of anxiety.
For anxiety disorder in the elderly
The use of buspirone by elderly people suffering from anxiety disorder is more favorable than benzodiazepine treatment. Often, in the treatment of such patients, a prolonged anxiolytic effect is achieved while maintaining muscle tone, normal breathing, and the speed of cognitive and psychomotor reactions. In addition, it is important that buspirone improves mood.
Often, in the treatment of such patients, a prolonged anxiolytic effect is achieved while maintaining muscle tone, normal breathing, and the speed of cognitive and psychomotor reactions. In addition, it is important that buspirone improves mood.
For alcohol dependence
In the buspirone group, compared with placebo, there was a significant improvement in the following psychopathological parameters: anxiety disorder, depression, irritability, interpersonal relationships, and global psychopathological score. A meta-analysis has shown that buspirone improves the treatment of alcohol dependence and anxiety disorders. The researchers concluded that the main effect of buspirone on patients with alcohol dependence is not in reducing alcohol consumption, but in a positive effect on psychopathological symptoms. It improves mood, which is very important in the treatment of anxiety disorders, does not cause addiction, and does not adversely affect the psychomotor reaction.
Output
Thus, Spitomin (buspirone) can successfully replace classic drugs that cause intolerance or side effects, or supplement therapy to achieve a more complete and faster effect.
Buspirone is a powerful selective serotonergic anxiolytic, which, due to its pharmacodynamics, has a pronounced anxiolytic effect without disturbing cognitive functions and psychomotor reaction speed, which is perfect for both the elderly and active business people.
1 Kessler RC, Chiu WT, Demler O et al. Prevalence, severity and comorbidity of 12-month DSM-IV disorders in NCSR. Arch Gen Psychiatr 2005; 62:617–27; Terrie E, HonaLee H, Avshalom C et al. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a Birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry 2007;
2 Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. NY: Guilford Press 2002; European Status Report on Alcohol and Health 2010. WHO Euro, Copenhagen;
WHO Euro, Copenhagen;
3 Hirschfeld RM. Placebo response in the treatment of panic disorder. Bull Menning Clin 1996; 60(2): 76–86;. Keller MB. The clinical course of panic disorder and depression. J Clin Psychiat 1992; 53 (Suppl.): 5–8;
4 Kalinin V.V. / Anxiety disorders - Rijeka: In Tech, 2011 - 323p;
5 Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr 2005; 10(1): 49–56;
6 Schatzberg AF, Haddad P, Kaplan EM et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiat 1997; 58 (Suppl. 7): 5–10;
7 Avedisova A.S., Yastrebov D.V., Kostacheva E.A. Pharmacoepidemiological analysis of outpatient prescription of benzodiazepine tranquilizers in psychiatric institutions. Ros. Psychiatrist. magazine 2005; 4:10–2;
8 Instructions for use for pregabalin (Lyrica).
9 Baldwin D, I. Anderson, D. Nutt et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2005; 19: 567–96
10 Loane C, Politis M. Buspirone: what is it all about? Brain Res. 2012 Jun 21; 1461: 111-8
11 Anon A. Buspiron bei Angst und Depression // Pharm Ztg. - 1996. - Vol. 34. – P. 43.
12 Instructions for medical use.
13 S.G. Burchinsky, Buspiron: new possibilities for the treatment of anxiety and depressive disorders in neurological practice. Institute of Gerontology, AMS of Ukraine, Kyiv
14 RickelsK., Wiseman K., Norstad N., et al. Buspirone and diazepam in anxiety: a controlled Study// J. Clin. Psychiatry, 1982, 43(12), 81-86;
15 BY HAROLD L. GOLDBERG, M.D., AND RICHARD J. FINNERTY, Ph.D. he Comparative Efficacy of Buspirone and Diazepam in the Treatment of Anxiety.