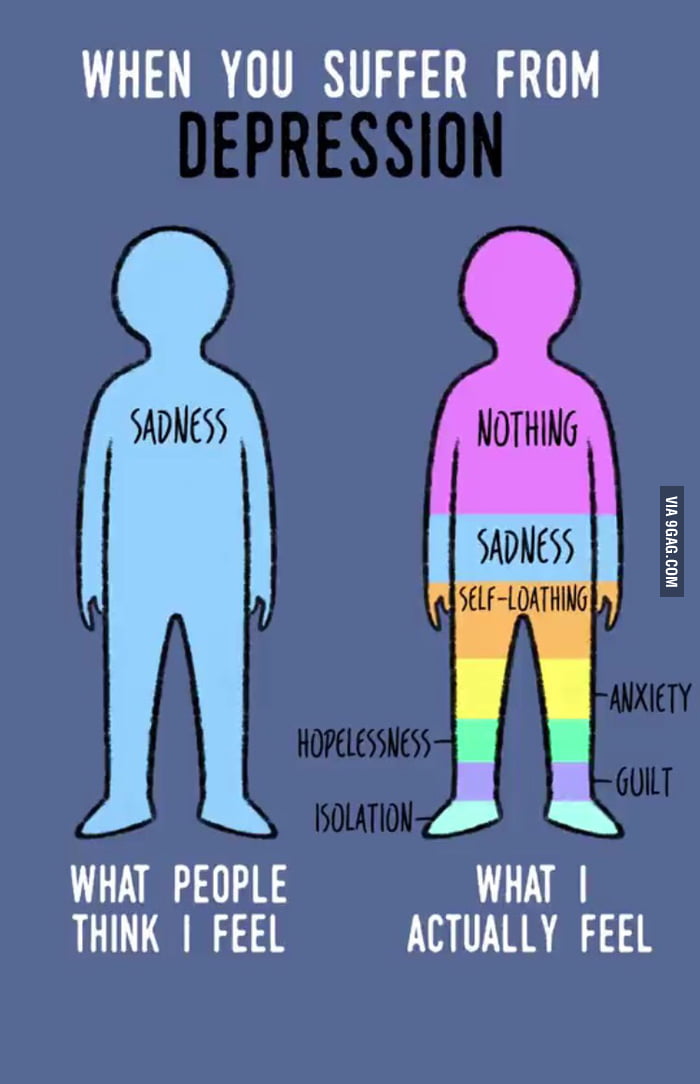
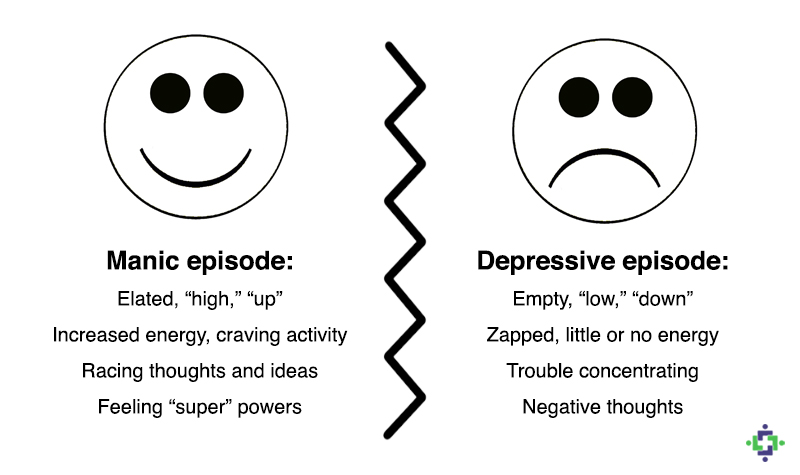
Venlafaxine dosage 300 mg
Side Effects, Dosage, Uses, and More
Highlights for venlafaxine
- Venlafaxine oral tablet is only available as a generic drug. It comes in both an immediate-release and an extended-release form.
- Venlafaxine also comes as an extended-release oral capsule.
- Venlafaxine oral tablet is used to treat depression (immediate-release tablet and extended-release tablet). It’s also used to treat social anxiety disorder (extended-release tablet only).
FDA warning: Suicidal behavior warning
Other warnings
- Serotonin syndrome warning: This drug may cause a possibly life threatening condition called serotonin syndrome. Symptoms of serotonin syndrome include:
- hallucinations and delusions
- agitation
- coma
- fast heart rate
- changes in blood pressure
- dizziness
- loss of consciousness
- seizures
- shakiness
- muscle tremor or stiff muscles
- sweating
- nausea
- vomiting
- High blood pressure warning: Venlafaxine may increase your blood pressure.
Your doctor will likely make sure your blood pressure is normal before you start taking venlafaxine. They will check your blood pressure regularly during your treatment.
- Increased bleeding warning: This drug may increase your risk for bleeding or bruising if used with aspirin, nonsteroidal anti-inflammatory drugs such as ibuprofen or naproxen, or the blood thinner warfarin. Talk with your doctor if you’re taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding.
Venlafaxine is a prescription drug. It comes as an oral tablet and an oral capsule.
Venlafaxine oral tablet comes in immediate-release and extended-release forms. Both forms are only available as generic drugs. Generic drugs usually cost less than brand-name drugs.
Why it’s used
Venlafaxine oral tablet is used to treat depression (immediate-release tablet and extended-release tablet). It’s also used to treat social anxiety disorder (extended-release tablet only).
Venlafaxine may be used as part of a combination therapy. This means you may need to take it with other medications to treat your condition.
How it works
Venlafaxine belongs to a class of antidepressant drugs called serotonin norepinephrine reuptake inhibitors (SNRIs). A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions.
SNRIs work by increasing the levels of substances called serotonin and norepinephrine in your brain. Having more serotonin and norepinephrine in your brain can improve your symptoms of depression and anxiety.
Venlafaxine oral tablet may cause drowsiness. It may also affect your ability to make decisions, think clearly, or react quickly. You should not drive, use heavy machinery, or do things that require you to be alert until you know you can function normally. Venlafaxine may also cause other side effects.
More common side effects
The more common side effects of venlafaxine can include:
- unusual dreams
- sexual problems, such as:
- decreased interest in sex
- impotence (not being able to get or keep an erection)
- trouble having an orgasm
- loss of appetite
- constipation
- nausea or vomiting
- dry mouth
- tiredness
- trouble sleeping or change in sleep habits
- yawning
- tremor or shaking
- dizziness
- blurry vision
- sweating
- feeling anxious, nervous, or jittery
- headache
- increased heart rate
If these effects are mild, they may go away within a few days or a couple of weeks.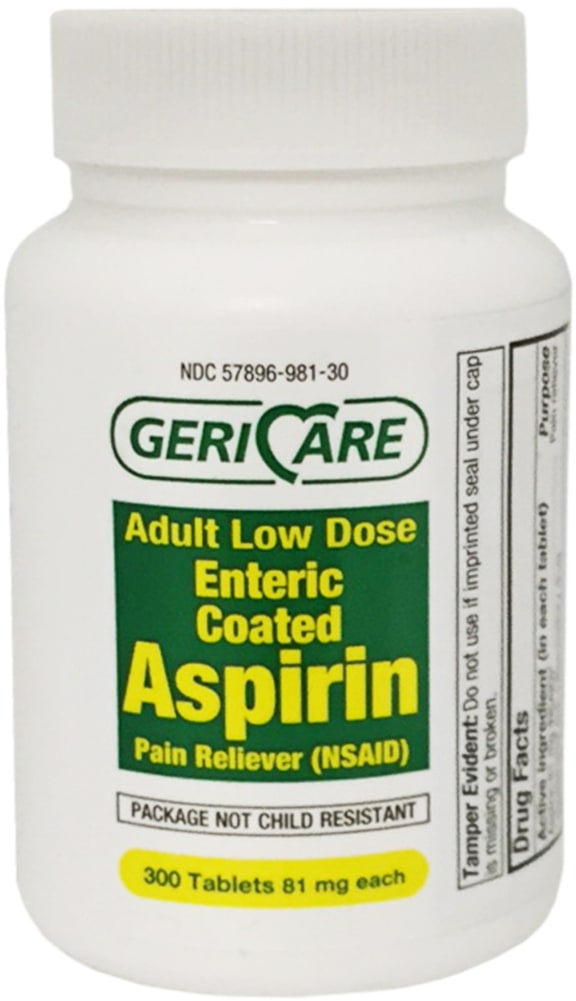 If they’re more severe or don’t go away, talk to your doctor or pharmacist.
If they’re more severe or don’t go away, talk to your doctor or pharmacist.
Serious side effects
Call your doctor right away if you have serious side effects. Call 911 if your symptoms feel life threatening or if you think you’re having a medical emergency. Serious side effects and their symptoms can include the following:
- Attempting suicide
- Acting on dangerous impulses
- Aggressive or violent behavior
- Thoughts about suicide or dying
- New or worsened depression
- New or worsened anxiety or panic attacks
- Agitation, restlessness, anger, or irritability
- Trouble sleeping
- Serotonin syndrome. Symptoms can include:
- agitation
- hallucinations (seeing or hearing something that isn’t there)
- coma
- changes in your mental status
- coordination problems
- muscle twitching or overactive reflexes
- fast heart rate
- high or low blood pressure
- sweating
- fever
- nausea
- vomiting
- diarrhea
- muscle stiffness
- High blood pressure.
 Symptoms can include:
Symptoms can include: - headache
- chest pain
- Mania. Symptoms can include:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
- Seizures
- Eye problems. Symptoms can include:
- eye pain
- vision changes
- enlarged pupils
- swelling or redness in or around your eyes
- Low sodium levels. Symptoms can include:
- headache
- weakness
- feeling unsteady
- confusion
- problems concentrating
- thinking or memory problems
- Bruising easily
- Frequent nosebleeds
- Frequent bleeding from your gums while brushing your teeth or flossing
- Dark, tar-like stool
- Bleeding from wounds that’s hard to stop
- Lung disease or pneumonia. Symptoms can include:
- shortness of breath that gets worse
- cough
- chest discomfort
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.
However, because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.
Venlafaxine oral tablet can interact with other medications, vitamins, or herbs you may be taking. An interaction is when a substance changes the way a drug works. This can be harmful or prevent the drug from working well.
To help avoid interactions, your doctor should manage all of your medications carefully. Be sure to tell your doctor about all medications, vitamins, or herbs you’re taking. To find out how this drug might interact with something else you’re taking, talk to your doctor or pharmacist.
Examples of drugs that can cause interactions with venlafaxine are listed below.
Drugs you should not use with venlafaxine
Do not take these drugs with venlafaxine. When used with venlafaxine, these drugs can cause dangerous effects in your body. Examples of these drugs include:
When used with venlafaxine, these drugs can cause dangerous effects in your body. Examples of these drugs include:
- Monoamine oxidase inhibitors (MAOIs), including linezolid and methylene blue. Unless directed by your doctor, do not start venlafaxine within 2 weeks of stopping an MAOI and do not take an MAOI within 7 days of stopping venlafaxine. Taking venlafaxine and an MAOI too close together in time may cause serious or life threatening side effects. These side effects can include high fever, uncontrolled muscle spasms, and stiff muscles. Other side effects can include sudden changes in your heart rate or blood pressure, confusion, and passing out.
- Drugs for weight loss, such as phentermine. Using venlafaxine with drugs such as phentermine may lead to excessive weight loss, serotonin syndrome, and heart problems such as rapid heart rate and high blood pressure.
Interactions that increase your risk of side effects
Taking venlafaxine with certain medications raises your risk of side effects. Examples of these drugs include:
Examples of these drugs include:
- Cimetidine. Taking this drug with venlafaxine raises your risk of high blood pressure or liver disease. These risks are greater if you are a senior.
- Haloperidol. Taking this drug with venlafaxine raises your risk of QT prolongation. This is a heart condition with symptoms such as dizziness and an irregular heart rhythm.
- Warfarin. Taking this drug with venlafaxine raises your risk of bleeding. Your doctor will monitor you closely, especially when starting or stopping your venlafaxine therapy. Tell your doctor right away if you notice any abnormal bleeding or bruising.
- Anti-inflammatory drugs such as aspirin, ibuprofen, naproxen, and ketoprofen. Taking any of these drugs with venlafaxine raises your risk of bleeding. Your doctor will monitor you closely, especially when starting or stopping your venlafaxine therapy. Tell your doctor right away if you notice any abnormal bleeding or bruising.
- Drugs such as ritonavir, clarithromycin, or ketoconazole. Drugs such as ritonavir, clarithromycin, or ketoconazole can slow the breakdown of drugs in your body. If you take any of these drugs with venlafaxine, the amount of venlafaxine may build up in your body. This would increase your risk of side effects.
- Drugs that cause drowsiness, such as zolpidem, lorazepam, and diphenhydramine. Taking any of these drugs with venlafaxine may make the sleepiness from venlafaxine even worse.
- Other drugs that can increase serotonin levels, such as fluoxetine, paroxetine, citalopram, duloxetine, lithium, and tramadol. Venlafaxine increases your levels of serotonin. Taking it with any of these drugs may increase your serotonin levels even more. If your serotonin levels are too high, a life threatening condition called serotonin syndrome can occur. Your doctor will monitor you closely when starting or increasing your dosage of either drug.
- Certain drugs for migraine, called triptans, such as sumatriptan, rizatriptan, and zolmitriptan. Venlafaxine increases your levels of serotonin. Taking it with any of these drugs may increase your serotonin levels even more. If your serotonin levels are too high, a life threatening condition called serotonin syndrome can occur. Your doctor will monitor you closely when starting or increasing your dosage of either drug.
Interactions that can make your drugs less effective
When certain drugs are used with venlafaxine, they may not work as well. This is because the amount of these drugs in your body may be decreased. Examples of these drugs include:
- Metoprolol. Metoprolol may be less effective when you take it with venlafaxine. This may cause your blood pressure to rise. Talk to your doctor before taking these drugs together.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you are taking.
However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you are taking.
Venlafaxine oral tablet comes with several warnings.
Allergy warning
Venlafaxine can cause a severe allergic reaction. Symptoms can include:
- trouble breathing
- swelling of your face, tongue, eyes, or mouth
- rash, hives, or blisters, alone or with joint paint or fever
If you have an allergic reaction, call your doctor or local poison control center right away. If your symptoms are severe, call 911 or go to the nearest emergency room.
Don’t take this drug again if you’ve ever had an allergic reaction to it. Taking it again could be fatal (cause death).
Alcohol interaction warning
Do not drink alcohol with venlafaxine. Drinking alcohol raises your risk of sleepiness from venlafaxine. This may affect your ability to make decisions, think clearly, and react quickly. If you drink alcohol, talk to your doctor.
Warnings for people with certain health conditions
For people with liver disease: If you have a history of liver disease, your liver may not process this drug as quickly as it should. This could lead to a buildup of this drug in your body. Your doctor may start you on a reduced dosage. If they increase your dosage later, they will monitor you closely.
For people with kidney disease: If you have kidney disease or a history of kidney disease, you may not be able to clear this drug from your body well. This may increase the levels of venlafaxine in your body. This can cause more side effects. Your doctor may start you on a low dosage and monitor you closely if they increase your dosage.
For people with heart problems: Venlafaxine can increase your heart rate, especially if you’re taking doses greater than 200 mg per day. If you have heart failure or if you’ve recently had a heart attack, your heart may not be able to tolerate this side effect.
For people with hyperthyroidism: Hyperthyroidism can increase your heart rate. Venlafaxine can also increase your heart rate. If you have hyperthyroidism and take venlafaxine, your heart rate may increase to a dangerous level. You are especially at risk if you take venlafaxine doses greater than 200 mg per day.
For people with a history of seizures: Venlafaxine raises your risk of seizures. If you have a seizure, stop taking venlafaxine and call your doctor right away.
For people with increased eye pressure (glaucoma): Venlafaxine can widen your pupils and block the flow of fluid in your eye. These effects can increase the pressure in your eyes. People with a history of increased eye pressure or glaucoma should have their eye pressure checked regularly while taking venlafaxine. Do not take venlafaxine if you have uncontrolled angle-closure glaucoma.
People with a history of increased eye pressure or glaucoma should have their eye pressure checked regularly while taking venlafaxine. Do not take venlafaxine if you have uncontrolled angle-closure glaucoma.
Warnings for other groups
For pregnant women: Venlafaxine is a category C pregnancy drug. That means two things:
- Research in animals has shown adverse effects to the fetus when the mother takes the drug.
- There haven’t been enough studies done in humans to be certain how the drug might affect the fetus.
Talk to your doctor if you’re pregnant or plan to become pregnant. This drug should only be used if the potential benefit justifies the potential risk to the fetus. Call your doctor right away if you become pregnant while taking this drug.
For women who are breastfeeding: Venlafaxine may pass into breast milk and cause side effects in a child who is breastfed. Talk to your doctor about breastfeeding your child. You may need to decide whether to stop breastfeeding or stop taking this medication.
You may need to decide whether to stop breastfeeding or stop taking this medication.
For seniors: The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, more of a drug stays in your body for a longer time. This raises your risk of side effects. Older adults may be at higher risk than younger people for low sodium levels in their blood when taking venlafaxine.
For children: This drug should not be used in people younger than 18 years of age.
All possible dosages and drug forms may not be included here. Your dosage, drug form, and how often you take the drug will depend on:
- your age
- the condition being treated
- how severe your condition is
- other medical conditions you have
- how you react to the first dose
Forms and strengths
Generic: Venlafaxine
- Form: immediate-release oral tablet
- Strengths: 25 mg, 37.
 5 mg, 50 mg, 75 mg, 100 mg
5 mg, 50 mg, 75 mg, 100 mg - Form: extended-release oral tablet
- Strengths: 37.5 mg, 75 mg, 150 mg, 225 mg
Dosage for depression
Adult dosage (ages 18 years and older)
- Immediate-release oral tablets:
- Typical starting dosage: 75 mg total per day, taken in two or three divided doses
- Dosage increases: If needed, your doctor may increase your dosage to 150 mg per day.
- Typical maximum dosage: 225 mg per day. If you have more severe depression, your doctor may prescribe a dosage as high as 375 mg per day, taken in three divided doses.
- Extended-release oral tablets:
- Typical starting dosage: 75 mg per day, taken in a single dose in the morning or evening. Some patients should start at a lower dosage of 37.5 mg per day for 4–7 days.
- Dosage increases: If needed, your doctor may increase your dosage.
 They may increase it every 4 days by 75 mg until you reach 225 mg per day.
They may increase it every 4 days by 75 mg until you reach 225 mg per day. - Typical maximum dosage: 225 mg per day.
Child dosage (ages 0–17 years)
This medication should not be used in people younger than 18 years of age.
Dosage for social anxiety disorder
Adult dosage (ages 18 years and older)
- Extended-release oral tablets:
- Typical dosage: 75 mg per day, given in a single dose in the morning or evening.
- Maximum dosage: 75 mg per day.
Child dosage (ages 0–17 years)
This medication should not be used in people younger than 18 years of age.
Special dosage considerations
People with liver problems: People with mild to moderate liver problems should take about half of the typical dose. People with severe liver disease or cirrhosis may need an even lower dosage. Your doctor can tell you more.
Your doctor can tell you more.
People with kidney problems: People with mild to moderate kidney problems should take 75% of the typical dosage. People who are on dialysis should take half of the typical dosage. Your doctor can tell you more.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this list includes all possible dosages. This information is not a substitute for medical advice. Always to speak with your doctor or pharmacist about dosages that are right for you.
Venlafaxine oral tablet is used for long-term treatment. It comes with serious risks if you don’t take it as prescribed.
If you stop taking the drug suddenly or don’t take it at all: Your depression or anxiety may not get better and may get worse. Do not stop venlafaxine without talking to your doctor. Stopping venlafaxine too quickly can cause serious symptoms such as:
- anxiety
- irritability
- tiredness
- restlessness
- trouble sleeping
- headache
- sweating
- dizziness
- tingling or “pins and needles” feeling
- shaking
- confusion
- nightmares
- nausea
- vomiting
- diarrhea
If this happens, your doctor may have you start taking venlafaxine again and decrease your dosage slowly.
If you miss doses or don’t take the drug on schedule: Your medication may not work as well or may stop working completely. For this drug to work well, a certain amount needs to be in your body at all times.
If you take too much: You could have dangerous levels of the drug in your body. This can lead to death. Symptoms of an overdose of this drug can include:
- fast heart rate
- unusual sleepiness
- enlarged pupils
- seizure
- vomiting
- heart rhythm changes
- low blood pressure
- muscle aches or pains
- dizziness
If you think you’ve taken too much of this drug, call your doctor or seek guidance from the American Association of Poison Control Centers at 800-222-1222 or through their online tool. But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
What to do if you miss a dose: Take your dose as soon as you remember. If you remember just a few hours before your next scheduled dose, take only one dose. Never try to catch up by taking two doses at once. This could result in dangerous side effects.
If you remember just a few hours before your next scheduled dose, take only one dose. Never try to catch up by taking two doses at once. This could result in dangerous side effects.
How to tell if the drug is working: The symptoms of your depression or anxiety should be less severe or happen less often.
Keep these considerations in mind if your doctor prescribes venlafaxine oral tablet for you.
General
- Take venlafaxine with food.
- You can cut or crush the immediate-release tablet, but do not cut or crush the extended-release tablet.
Storage
- Store the immediate-release oral tablet at room temperature between 68°F and 77°F (20°C and 25°C).
- Store the extended-release oral tablet at temperatures between 59°F and 86°F (15°C and 30°C).
- Keep this drug away from light.
- Don’t store this medication in moist or damp areas, such as bathrooms.
Refills
A prescription for this medication is refillable. You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
Travel
When traveling with your medication:
- Always carry your medication with you. When flying, never put it into a checked bag. Keep it in your carry-on bag.
- Don’t worry about airport X-ray machines. They can’t hurt your medication.
- You may need to show airport staff the pharmacy label for your medication. Always carry the original prescription-labeled container with you.
- Don’t put this medication in your car’s glove compartment or leave it in the car. Be sure to avoid doing this when the weather is very hot or very cold.
Availability
Not every pharmacy stocks this drug. When filling your prescription, be sure to call ahead to make sure your pharmacy carries it.
Insurance
Many insurance companies require a prior authorization for this drug. This means your doctor may need to get approval from your insurance company before your insurance company will pay for the prescription.
There are other drugs available to treat your condition. Some may be better suited for you than others. Talk to your doctor about other drug options that may work for you.
Disclaimer: Healthline has made every effort to make certain that all information is factually correct, comprehensive, and up-to-date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
Tolerability of high-dose venlafaxine in depressed patients
Comparative Study
. 2004 Jun;18(2):200-4.
2004 Jun;18(2):200-4.
doi: 10.1177/0269881104042621.
C Louise Harrison 1 , Nicol Ferrier, Allan H Young
Affiliations
Affiliation
- 1 School of Neurology, Neurobiology and Psychiatry, Psychiatry, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
- PMID: 15260908
- DOI: 10.1177/0269881104042621
Comparative Study
C Louise Harrison et al. J Psychopharmacol. 2004 Jun.
. 2004 Jun;18(2):200-4.
doi: 10.1177/0269881104042621.
Authors
C Louise Harrison 1 , Nicol Ferrier, Allan H Young
Affiliation
- 1 School of Neurology, Neurobiology and Psychiatry, Psychiatry, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
- PMID: 15260908
- DOI: 10.1177/0269881104042621
Abstract
High doses of antidepressants are often used for treatment-resistant depression. Venlafaxine, a dual serotonin and noradrenaline reuptake inhibitor, has been shown to have a tolerable side-effect profile in previous studies using doses of up to 375 mg/day. We investigated the tolerability of higher than currently recommended doses of venlafaxine using the UKU side-effect rating scale. Seventy outpatients fulfilling DSM-IV criteria for major depressive disorder were recruited into two demographically matched groups according to their daily dosage of venlafaxine: high dose n = 35 (> or = 375 mg/day, range 375-600 mg, average 437 mg/day) or standard dose n = 35 (< 375 mg/day, range 75-300 mg, average 195 mg/day. Clinical characteristics were noted and the UKU side-effect rating scale was administered to a subsample of patients. The most frequently reported complaints in both groups were increased fatigue (48%), concentration difficulties (48%), sleepiness/sedation (37%), failing memory (44.4%) and weight gain (29.6%). Apart from weight gain, the complaints were found to be experienced significantly more severely by the high-dose group. Six patients discontinued venlafaxine due to intolerable side-effects but only two of these patients were on a high dose.
We investigated the tolerability of higher than currently recommended doses of venlafaxine using the UKU side-effect rating scale. Seventy outpatients fulfilling DSM-IV criteria for major depressive disorder were recruited into two demographically matched groups according to their daily dosage of venlafaxine: high dose n = 35 (> or = 375 mg/day, range 375-600 mg, average 437 mg/day) or standard dose n = 35 (< 375 mg/day, range 75-300 mg, average 195 mg/day. Clinical characteristics were noted and the UKU side-effect rating scale was administered to a subsample of patients. The most frequently reported complaints in both groups were increased fatigue (48%), concentration difficulties (48%), sleepiness/sedation (37%), failing memory (44.4%) and weight gain (29.6%). Apart from weight gain, the complaints were found to be experienced significantly more severely by the high-dose group. Six patients discontinued venlafaxine due to intolerable side-effects but only two of these patients were on a high dose. There was a tendency for mildly raised blood pressure in 10% of patients on an average dose of 342 mg/day. However, no difference between the two groups was found. This preliminary open study demonstrates that venlafaxine is tolerated at higher than British National Formulary recommended doses (i.e. up to 600 mg daily). However, increased frequency and severity of reported side-effects in the high-dose group are not associated with increased rates of discontinuation.
There was a tendency for mildly raised blood pressure in 10% of patients on an average dose of 342 mg/day. However, no difference between the two groups was found. This preliminary open study demonstrates that venlafaxine is tolerated at higher than British National Formulary recommended doses (i.e. up to 600 mg daily). However, increased frequency and severity of reported side-effects in the high-dose group are not associated with increased rates of discontinuation.
Similar articles
-
Venlafaxine extended release versus citalopram in patients with depression unresponsive to a selective serotonin reuptake inhibitor.
Lenox-Smith AJ, Jiang Q. Lenox-Smith AJ, et al. Int Clin Psychopharmacol. 2008 May;23(3):113-9. doi: 10.1097/YIC.0b013e3282f424c2. Int Clin Psychopharmacol. 2008. PMID: 18408525 Clinical Trial.
-
Combined treatment with venlafaxine and tricyclic antidepressants in depressed patients who had partial response to clomipramine or imipramine: initial findings.

Gómez Gómez JM, Teixidó Perramón C. Gómez Gómez JM, et al. J Clin Psychiatry. 2000 Apr;61(4):285-9. J Clin Psychiatry. 2000. PMID: 10830150 Clinical Trial.
-
Adequacy of venlafaxine dose prescribing in major depression and hospital resources implications.
Vanoli A, Lane C, Harrison C, Steen N, Young A. Vanoli A, et al. J Psychopharmacol. 2008 Jun;22(4):434-40. doi: 10.1177/0269881107086178. J Psychopharmacol. 2008. PMID: 18635723
-
Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study.
Alevizos B, Alevizos E, Leonardou A, Zervas I. Alevizos B, et al. Psychiatriki. 2012 Apr-Jun;23(2):143-8. Psychiatriki. 2012. PMID: 22796912 Clinical Trial.

-
Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients.
Thase ME. Thase ME. J Clin Psychiatry. 1998 Oct;59(10):502-8. doi: 10.4088/jcp.v59n1002. J Clin Psychiatry. 1998. PMID: 9818630
See all similar articles
Cited by
-
Factors related to the improvement in quality of life for depressed inpatients treated with fluoxetine.
Yang WC, Lin CH, Wang FC, Lu MJ. Yang WC, et al. BMC Psychiatry. 2017 Aug 25;17(1):309. doi: 10.1186/s12888-017-1471-3. BMC Psychiatry. 2017. PMID: 28841824 Free PMC article. Clinical Trial.
-
Serotonergic modulation of hippocampal theta activity in relation to hippocampal information processing.
Olvera-Cortés ME, Gutiérrez-Guzmán BE, López-Loeza E, Hernández-Pérez JJ, López-Vázquez MA. Olvera-Cortés ME, et al. Exp Brain Res. 2013 Oct;230(4):407-26. doi: 10.1007/s00221-013-3679-x. Epub 2013 Aug 30. Exp Brain Res. 2013. PMID: 23990128 Review.
-
Metabolic syndrome and migraine.
Sachdev A, Marmura MJ. Sachdev A, et al. Front Neurol. 2012 Nov 19;3:161. doi: 10.3389/fneur.2012.00161. eCollection 2012. Front Neurol. 2012. PMID: 23181051 Free PMC article.
-
[Psychotropic drugs and diabetes].
Ress C, Tschoner A, Kaser S, Ebenbichler CF. Ress C, et al. Wien Med Wochenschr. 2011 Nov;161(21-22):531-42. doi: 10.1007/s10354-011-0004-9. Epub 2011 Jul 29. Wien Med Wochenschr.
 2011. PMID: 21792529 Review. German.
2011. PMID: 21792529 Review. German. -
Tolerability and safety of fluvoxamine and other antidepressants.
Westenberg HG, Sandner C. Westenberg HG, et al. Int J Clin Pract. 2006 Apr;60(4):482-91. doi: 10.1111/j.1368-5031.2006.00865.x. Int J Clin Pract. 2006. PMID: 16620364 Free PMC article. Review.
Publication types
MeSH terms
Substances
clinical effect, tolerability and personalized indications for prescription
At the present stage of development and improvement of methods for the treatment of endogenous depression, more and more attention is paid to the participation of monoamine neurotransmitters in the pathogenesis of the disease and the peculiarities of the impact on these links of newly created psychotropic antidepressant drugs [1, 2]. The pharmacological action of different classes of antidepressants introduced into psychiatric practice is due to different mechanisms of their interaction with certain brain receptors and participation in various metabolic processes. The search for thymoanaleptics that interact with both serotonergic and noradrenergic receptors, i.e. with a dual mechanism of action, led to the creation of a new class of antidepressants - selective serotonin and norepinephrine reuptake inhibitors (SNRIs). The appearance of antidepressants with a dual mechanism of action was accompanied by an increase in the effectiveness of the treatment of depressive conditions and made it possible to achieve remission in 45% of patients [2, 3]. nine0003
The pharmacological action of different classes of antidepressants introduced into psychiatric practice is due to different mechanisms of their interaction with certain brain receptors and participation in various metabolic processes. The search for thymoanaleptics that interact with both serotonergic and noradrenergic receptors, i.e. with a dual mechanism of action, led to the creation of a new class of antidepressants - selective serotonin and norepinephrine reuptake inhibitors (SNRIs). The appearance of antidepressants with a dual mechanism of action was accompanied by an increase in the effectiveness of the treatment of depressive conditions and made it possible to achieve remission in 45% of patients [2, 3]. nine0003
One of the SNRIs is venlafaxine, the first thymoanaleptic of the third generation. In many studies [4-6], it is evaluated as a "reference" in the group of these compounds.
Venlafaxine and its main metabolite, O-desmethylvenlafaxine, are potent inhibitors of serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake. Both the main drug and its specified metabolite reduce β-adrenergic reactivity. Venlafaxine has no affinity for muscarinic, cholinergic, histamine, and α1-adrenergic, opioid, benzodiazepine, orencyclidine and N-methyl-α-aspartate receptors in the brain; while the ratio of monoamine transfer (serotonin/norepinephrine) with venlafaxine is 30 (compared to 9,4 for another SNRI, duloxetine). This indicates a significant predominance of the serotonergic effect in the action of venlafaxine, which is similar in strength to selective serotonin reuptake inhibitors (SSRIs). A group of authoritative experts in the field of psychopharmacotherapy showed [7] that venlafaxine, along with escitalopram and clomipramine, can be considered as one of the most effective antidepressants.
Both the main drug and its specified metabolite reduce β-adrenergic reactivity. Venlafaxine has no affinity for muscarinic, cholinergic, histamine, and α1-adrenergic, opioid, benzodiazepine, orencyclidine and N-methyl-α-aspartate receptors in the brain; while the ratio of monoamine transfer (serotonin/norepinephrine) with venlafaxine is 30 (compared to 9,4 for another SNRI, duloxetine). This indicates a significant predominance of the serotonergic effect in the action of venlafaxine, which is similar in strength to selective serotonin reuptake inhibitors (SSRIs). A group of authoritative experts in the field of psychopharmacotherapy showed [7] that venlafaxine, along with escitalopram and clomipramine, can be considered as one of the most effective antidepressants.
Venlafaxine is a racemic mixture of two enantiomers: the S-enantiomer, which is a more potent inhibitor of serotonin reuptake, and the R-enantiomer, a potent norepinephrine reuptake inhibitor. Due to this structure, the drug in neuronal synapses eliminates the deficiency of both serotonin and norepinephrine, which provides a more balanced effect on neurotransmission and synergism of psychopharmacological effects. The features of venlafaxine should also include the high selectivity of its pharmacological action, which leads to a favorable profile of its tolerability and safety [8-10]. nine0003
The features of venlafaxine should also include the high selectivity of its pharmacological action, which leads to a favorable profile of its tolerability and safety [8-10]. nine0003
To date, data have been accumulated on the dose-dependence of the therapeutic effect of venlafaxine. Possessing a wide range of therapeutic action, venlafaxine consistently includes serotonergic, noradrenergic and dopaminergic receptors in the spectrum of its neurochemical activity [11]. When comparing the effectiveness of venlafaxine with SSRI antidepressants in a meta-analysis of 34 randomized double-blind trials [12], which included 8000 patients with depressive disorders of varying severity, statistically significant advantages of venlafaxine as a means to achieve remission were established. nine0003
The relatively short half-life necessitates strict adherence to the daily regimen of the drug [13, 14]. Many years of successful world experience in the use of venlafaxine indicates a dose-dependent mechanism of its action, which makes it possible to use the drug as an alternative to SSRIs and tricyclic antidepressants in the treatment of both therapeutically resistant depression and acute primary depression, both in inpatient and outpatient practice [7, 14— nineteen]. A favorable tolerability profile and a low incidence of side effects during venlafaxine therapy are emphasized [14].
A favorable tolerability profile and a low incidence of side effects during venlafaxine therapy are emphasized [14].
Venlafaxine has been shown to be highly effective in a wide range of depressive conditions within recurrent and bipolar affective disorders [14], as well as in generalized anxiety, somatogenic and post-stroke depression [4, 20].
A number of authors [5, 21, 22] note the early onset of the antidepressant action of venlafaxine and its positive effect on the manifestations of the thymic component of depression, anxiety, agitation, motor retardation, and cognitive functions. However, the issues of the effect of venlafaxine on depressive states of various structures remain insufficiently developed [23]. nine0017
The aim of the study was to evaluate the clinical effect of venlafaxine during course treatment of patients with various depressive states of endogenous nature.
Material and methods
We examined 32 patients, 15 women and 17 men, aged 18 to 57 years (mean age 31. 75 years) with endogenous depression, who underwent inpatient treatment in the clinical departments of the Department for the Study of Endogenous Mental Disorders and Affective States of the Federal State Budgetary Institution Scientific Center for Mental health" RAS. nine0003
75 years) with endogenous depression, who underwent inpatient treatment in the clinical departments of the Department for the Study of Endogenous Mental Disorders and Affective States of the Federal State Budgetary Institution Scientific Center for Mental health" RAS. nine0003
The study was open naturalistic in design. The average duration of the disease from its first manifestation was 7.9 years, the average number of depressive episodes transferred before inclusion in the study was 3.3. The duration of the current depressive episode prior to Velaxin was on average 4.5 months.
According to the nosological affiliation, the patients were distributed as follows: 22 (68.6%) patients were diagnosed with endogenous affective diseases - manic-depressive psychosis (MDP), cyclothymia; in 5 (15.6%) depression developed in the dynamics of low-progressive schizophrenia, and in 5 (15.6%) there was post-psychotic depression in the framework of paroxysmal schizophrenia. According to ICD-10, the depressive state of patients was diagnosed according to the following headings: F31. 3—F31.4; F32.0-F32.2; F33.0-F33.2; F21; F20.4. Typologically, depressive states were defined as melancholy (3 patients, 9.4%), anxious (11 cases; 34.4%) and apatho-adynamic (18 patients; 56.2%). Thus, in accordance with the two-level psychopathological model of depression [24], its first 2 types were defined as typical, representing positive affectivity (dreary and anxious depressions), apatho-adynamic depressions as atypical and related to negative affectivity.
3—F31.4; F32.0-F32.2; F33.0-F33.2; F21; F20.4. Typologically, depressive states were defined as melancholy (3 patients, 9.4%), anxious (11 cases; 34.4%) and apatho-adynamic (18 patients; 56.2%). Thus, in accordance with the two-level psychopathological model of depression [24], its first 2 types were defined as typical, representing positive affectivity (dreary and anxious depressions), apatho-adynamic depressions as atypical and related to negative affectivity.
According to the sum of scores on the Hamilton Depression Scale (HAM-D) before treatment: mild depressive disorders were registered only in 2 (6.25%) people, moderate - in 13 (40.6%), severe depression was in 17 (53.15%), i.e., the vast majority of patients had moderate to severe depression. nine0003
The study used venlafaxine, sold under the name Velaxin in 75 mg tablets by Egis (Hungary). The drug was administered orally, starting with 37.5-75 mg per day. In the future, based on the condition of the patients, over the next days the daily dose was increased, bringing it to the maximum for this patient, but not more than 300 mg. On average, the highest mean dose of Velaxin used was 182.1-198.2 mg per day. The drug was taken twice, in the morning and in the evening. According to the study protocol, Velaxin therapy was 56 days (8 weeks). nine0003
On average, the highest mean dose of Velaxin used was 182.1-198.2 mg per day. The drug was taken twice, in the morning and in the evening. According to the study protocol, Velaxin therapy was 56 days (8 weeks). nine0003
To assess the severity of depressive symptoms and determine the therapeutic effect, in addition to the method of clinical observation, the HAM-D-24 scale containing 24 signs was used. The severity of depressive symptoms was assessed before the start of the course of treatment (day 0), then on days 1, 3, 5 of therapy, and then at the end of each of the 8 weeks of course treatment. The effectiveness of the antidepressant action of Velaxin was assessed by the degree of reduction of HAM-D scores in % in relation to the assessment before treatment in the following gradations: reduction of scores to 20% - as "insignificant", by 21-50% - as "moderate", by 51- 80% - as "good" and 81-100% - as a "significant" effect (including "practical recovery"). A decrease in the total score for HAM-D to 6 or less meant a complete "exit" to remission. Maintaining the HAM-D depression severity score at the same level or increasing the total score was regarded as no effect or “worsening” of the condition. To assess the spectrum of the antidepressant action of Velaxin, the degree of reduction in the average total score of individual signs identified in the HAM-D, conditionally characterizing melancholic or melancholy (points 1-3, 22-24), apatho-adynamic (points 7 and 8) and anxious (points nineand 10) manifestations in the structure of a depressive state.
Maintaining the HAM-D depression severity score at the same level or increasing the total score was regarded as no effect or “worsening” of the condition. To assess the spectrum of the antidepressant action of Velaxin, the degree of reduction in the average total score of individual signs identified in the HAM-D, conditionally characterizing melancholic or melancholy (points 1-3, 22-24), apatho-adynamic (points 7 and 8) and anxious (points nineand 10) manifestations in the structure of a depressive state.
In addition, the severity of the depressive state and the degree of its improvement over time were assessed within the above timeframes using the CGI clinical impression scale and its CGI-S and CGI-I subscales. To register side effects, the UKU interview scale was used, which consists of 4 subscales that make it possible to distinguish mental, neurological, vegetative and so-called other side effects.
Results
During the prescribed 56-day course of treatment with Velaxin, 31 (96. 9%) people were recognized as responders, of which "insignificant" effect from the treatment was observed in 1 (3.1%) patients, "moderate" - in 3 (9, 4%), "good" - in 2 (6.25%), and 25 (78.1%) patients showed a "significant" therapeutic effect. Thus, a decrease in the intensity of depressive disorders by 50% or more during treatment with Velaxin was found in the vast majority of cases - in 27 (84.4%) patients, which is shown in Fig. nineteen0003
9%) people were recognized as responders, of which "insignificant" effect from the treatment was observed in 1 (3.1%) patients, "moderate" - in 3 (9, 4%), "good" - in 2 (6.25%), and 25 (78.1%) patients showed a "significant" therapeutic effect. Thus, a decrease in the intensity of depressive disorders by 50% or more during treatment with Velaxin was found in the vast majority of cases - in 27 (84.4%) patients, which is shown in Fig. nineteen0003
Rice. 1. The severity of the therapeutic effect of Velaksin in endogenous depression. On the abscissa - the degree of reduction according to HAM-D,%; along the y-axis - % of patients.
In general, the effectiveness of Velaxin, assessed on the 56th day of therapy, was quite high: the average total depression score according to HAM-D was reduced by 85.9%. Already by the end of the 1st week of therapy with Velaxin, a positive therapeutic response to the drug was observed: the reduction in depression was at the level of a “slight” effect, but approached a 20% level of reduction in disorders bordering on a “moderate” improvement. A distinct "moderate" improvement (reduction of the HAM-D score by 33.2%) was observed by the 14th day of treatment; between the 21st and 28th days of treatment, the therapeutic effect reached the “good” range with a decrease in HAM-D scores by 50% or more, and by the 6th week, the effect of Velaxin treatment was already approaching the “significant” border (up to 76.7% reduction in the severity of symptoms of depression). In the next 7-8 weeks of therapy, an indisputable "significant" improvement in the condition of patients was found (reduction of disorders by more than 80%), up to "recovery" (Fig. 2). In 20 out of 31 patients (in 64.5% of cases) who completed the course of treatment with Velaxin, depression was completely reduced and there was an "exit" to remission (their total HAM-D score became 6 or lower). nine0003
A distinct "moderate" improvement (reduction of the HAM-D score by 33.2%) was observed by the 14th day of treatment; between the 21st and 28th days of treatment, the therapeutic effect reached the “good” range with a decrease in HAM-D scores by 50% or more, and by the 6th week, the effect of Velaxin treatment was already approaching the “significant” border (up to 76.7% reduction in the severity of symptoms of depression). In the next 7-8 weeks of therapy, an indisputable "significant" improvement in the condition of patients was found (reduction of disorders by more than 80%), up to "recovery" (Fig. 2). In 20 out of 31 patients (in 64.5% of cases) who completed the course of treatment with Velaxin, depression was completely reduced and there was an "exit" to remission (their total HAM-D score became 6 or lower). nine0003
Rice. 2. Spectrum of therapeutic action of Velaxin in endogenous depression (HAM-D score). Here and in Figures 3, 4 and 5 (the last one shows the right axis): along the y-axis - the average score in % of the score before the start of therapy, along the abscissa - the day of therapy.
The severity of the condition, assessed by the CGI-S subscale, decreased on average during treatment with Velaxin from 4.7 to 1.6 points by the end of the study (from “significantly pronounced” to less than “mildly expressed”, i.e. to almost no disorders). Moreover, a noticeable decrease in the severity of symptoms was noted between the 7th and 21st days of treatment, when the intensity of depression manifestations consistently decreased on average to "moderately" and "weakly" pronounced. From the 5th week of therapy, the severity of depressive disorders became "very weak", and on the 7th-8th week of the course therapy there was a practical "exit" from depression with the "absence" of depressive symptoms (the average level of their severity was 1.6 points). During the 56-day course of therapy, an assessment of the degree of improvement in the depressive state on the CGI-I subscale showed a “deterioration” in the mental state (5th level of assessment) only in 1 (3.1%) person, remained “unchanged” (4th level). assessment level) also in 1 (3.1%) patient, "slight improvement" (3rd level) - also in 1 (3.1%), "pronounced" improvement (2nd level) was observed in 4 (12 .6%), the remaining 25 (78.1%) patients showed "significant" improvement (grade 1). nine0003
assessment level) also in 1 (3.1%) patient, "slight improvement" (3rd level) - also in 1 (3.1%), "pronounced" improvement (2nd level) was observed in 4 (12 .6%), the remaining 25 (78.1%) patients showed "significant" improvement (grade 1). nine0003
It was found that in the spectrum of the antidepressant action of Velaxin, all 3 components of its psychotropic activity, in accordance with the final results of treatment, are represented almost equally with a slight advantage in terms of antidepressant action (see Fig. 2). Thus, the degree of reduction in the average total score of disorders on points 1-3 and 22-24 of HAM-D (reflecting the actual thymoleptic effect of the drug) was 88.2%, on points 7 and 8 (stimulating effect) - 83.7%, and on paragraphs 9and 10 (anti-anxiety action) - 85.1%. However, the implementation of all 3 components of the action of Velaxin was different in time and had its own characteristics. Thus, thymoleptic and especially anxiolytic effects were detected most quickly during treatment. They developed almost in parallel, reaching the level of reduction in the severity of disorders according to HAM-D by 32.8 and 37.1%, respectively, already by the 14th day of therapy (“moderate” effect). At the same time, the stimulating effect of Velaxin was somewhat "late" in comparison with the anti-anxiety and anti-sadness effects, reaching similar values ("moderate" effect - reduction of disorders by 36.9%) only by the 21st day of the study.
They developed almost in parallel, reaching the level of reduction in the severity of disorders according to HAM-D by 32.8 and 37.1%, respectively, already by the 14th day of therapy (“moderate” effect). At the same time, the stimulating effect of Velaxin was somewhat "late" in comparison with the anti-anxiety and anti-sadness effects, reaching similar values ("moderate" effect - reduction of disorders by 36.9%) only by the 21st day of the study.
Later, in the period from the 21st to the 28th days of treatment, the thymoleptic and anti-anxiety components of action in the spectrum of antidepressant activity of Velaxin leveled off in terms of the rate of development and increased to the range of a distinct "good" effect (reduction of the score by 60.2 and 59 .2%). But already from the 5th week of treatment, the thymoleptic effect proper began to surpass the anxiolytic effect in terms of the depth of reduction of disorders according to the corresponding HAM-D items: the thymoleptic effect was manifested at the “significant” level already at the 6th week, and the anti-anxiety effect only after the 7th week course therapy.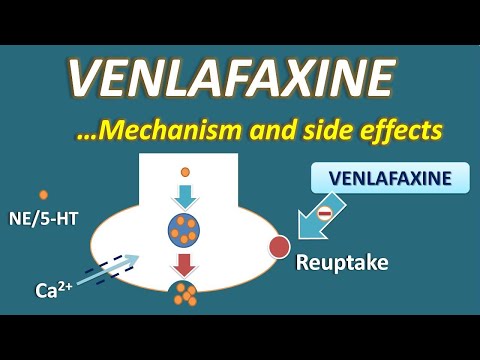 The formation of the stimulating effect was “delayed” in terms of the degree of manifestations compared with the other two components of the action of Velaxin at week 1, and its “significant” severity was determined only at the 8th week of treatment. And only at this stage of treatment, as already mentioned, the depth of the stimulating effect of Velaxin approximately coincided with the severity of the thymoleptic and anxiolytic effects. nine0003
The formation of the stimulating effect was “delayed” in terms of the degree of manifestations compared with the other two components of the action of Velaxin at week 1, and its “significant” severity was determined only at the 8th week of treatment. And only at this stage of treatment, as already mentioned, the depth of the stimulating effect of Velaxin approximately coincided with the severity of the thymoleptic and anxiolytic effects. nine0003
In accordance with the established data on the features of the implementation of individual components of the antidepressant action in the spectrum of psychotropic activity of Velaxin, its therapeutic efficacy was analyzed depending on the syndromic type of depression, i.e. on the dominant affect that determines the picture of the depressive state (Fig. 3). It was found that with the leading anxiety affect, the reduction in the average total score for HAM-D was 85.5% by the 56th day of treatment; with the predominance of apato-adynamic affect, the effectiveness of Velaxin was similar - the average total score for HAM-D decreased by 85. 9%. The highest efficacy of Velaxin was observed in the treatment of patients with the leading melancholy affect in the picture of depression - the reduction in the average total score according to HAM-D was 91.1%. But with a relatively similar degree of reduction in depressive symptoms, differences were also noted in the dynamics of the implementation of the antidepressant effect of the drug. So, with leading anxious and melancholy affects, i.e. in patients with positive affectivity and the most typical manifestations of depression, the reduction of depressive symptoms proceeded at an equal rate, reaching 21.1% (with anxiety) and 22.5% (with melancholy depression) already by the 7th day of therapy. Subsequently, the decrease in the average total score for HAM-D was more intense and reached more than 50% reduction in symptoms of depression by the 28th day of treatment. Treatment with Velaxin in patients with negative affectivity (atypical apatho-adynamic depression) improved more slowly.
9%. The highest efficacy of Velaxin was observed in the treatment of patients with the leading melancholy affect in the picture of depression - the reduction in the average total score according to HAM-D was 91.1%. But with a relatively similar degree of reduction in depressive symptoms, differences were also noted in the dynamics of the implementation of the antidepressant effect of the drug. So, with leading anxious and melancholy affects, i.e. in patients with positive affectivity and the most typical manifestations of depression, the reduction of depressive symptoms proceeded at an equal rate, reaching 21.1% (with anxiety) and 22.5% (with melancholy depression) already by the 7th day of therapy. Subsequently, the decrease in the average total score for HAM-D was more intense and reached more than 50% reduction in symptoms of depression by the 28th day of treatment. Treatment with Velaxin in patients with negative affectivity (atypical apatho-adynamic depression) improved more slowly. The reduction of the average total score on HAM-D by 20% or more ("moderate" effect) occurred in them only at the 2nd-3rd week of therapy; and only by the 4th week a “good” effect of Velaxin was recorded (reduction of disorders by 61.3%). By the 7th-8th week of treatment, the improvement in the condition was already “significant” (more than 80% reduction in disorders), it coincided in severity with the therapeutic effect of Velaxin in anxious depressions and somewhat “lagged behind” the effect in melancholy depression. nine0003
The reduction of the average total score on HAM-D by 20% or more ("moderate" effect) occurred in them only at the 2nd-3rd week of therapy; and only by the 4th week a “good” effect of Velaxin was recorded (reduction of disorders by 61.3%). By the 7th-8th week of treatment, the improvement in the condition was already “significant” (more than 80% reduction in disorders), it coincided in severity with the therapeutic effect of Velaxin in anxious depressions and somewhat “lagged behind” the effect in melancholy depression. nine0003
Rice. Fig. 3. Dynamics of Velaxin efficacy in different types of endogenous depression (HAM-D score).
An analysis of the effectiveness of Velaxin depending on the nosological affiliation of depression also revealed some features (Fig. 4): the smallest decrease in the average total score for HAM-D by the end of the course of treatment was in patients with low-progressive schizophrenia, it was equal to 82.3%. In these same patients, the slowest rate of reduction of depressive disorders was also noted - by the 7th day it was only 15. 2%, and a reduction of 50% or more (the border of the "good" effect) was achieved only by the 28th day; A "significant" effect was found in patients with low-progressive schizophrenia only after the 7th week of Velaxin treatment. In patients with an affective disease (MDP, cyclothymia), the reduction in the mean total HAM-D score at the end of course therapy was close in value (86.6%), and the rate of decrease in depressive symptoms was similar: by the 7th day, this indicator reached 18 .2%, and by the 4th week of therapy it was already 59.4% ("good" effect). The best result of therapy was achieved in patients with postpsychotic depression with paroxysmal schizophrenia - 90.4% reduction of disorders on the 56th day of treatment. Despite the fact that by the end of the 1st week of the study, the rate of reduction of depressive manifestations reached gradations of "insignificant" improvement (19.2%), in the future, the decrease in symptoms of depression was more intense than with MDP, demonstrating a "good" effect already by 14 Day 1 of treatment (symptom reduction score -53.
2%, and a reduction of 50% or more (the border of the "good" effect) was achieved only by the 28th day; A "significant" effect was found in patients with low-progressive schizophrenia only after the 7th week of Velaxin treatment. In patients with an affective disease (MDP, cyclothymia), the reduction in the mean total HAM-D score at the end of course therapy was close in value (86.6%), and the rate of decrease in depressive symptoms was similar: by the 7th day, this indicator reached 18 .2%, and by the 4th week of therapy it was already 59.4% ("good" effect). The best result of therapy was achieved in patients with postpsychotic depression with paroxysmal schizophrenia - 90.4% reduction of disorders on the 56th day of treatment. Despite the fact that by the end of the 1st week of the study, the rate of reduction of depressive manifestations reached gradations of "insignificant" improvement (19.2%), in the future, the decrease in symptoms of depression was more intense than with MDP, demonstrating a "good" effect already by 14 Day 1 of treatment (symptom reduction score -53. 9%), and by the 28th day it was already 76.5%, that is, it was almost on the border of "significant". However, it should be noted that in these patients, the severity of depressive disorders was initially significantly lower, at the level of "moderate" severity, than in other, more "severe" depressions (23 points according to HAM-D versus 31.3 points for affective illness and 31.6 in low-progressive schizophrenia).
9%), and by the 28th day it was already 76.5%, that is, it was almost on the border of "significant". However, it should be noted that in these patients, the severity of depressive disorders was initially significantly lower, at the level of "moderate" severity, than in other, more "severe" depressions (23 points according to HAM-D versus 31.3 points for affective illness and 31.6 in low-progressive schizophrenia).
Rice. 4. Antidepressant effect of Velaxin in different nosological groups of patients (HAM-D score). nine0003
All the indicated patterns of development of the therapeutic effect of Velaxin indicate the absence of a direct dependence on the value of its daily dose. As can be seen from Fig. 5, the therapeutic effect began to appear and increase from the 3rd–4th week of treatment (reduction of depression symptoms by 50% or more according to HAM-D), with an increase in the average daily dose to the maximum (182.1–198.2 mg per day) . But in the future, despite the stabilization and even a slight decrease in the achieved maximum average daily dose of Velaxin (up to 190. 3 mg per day), response to therapy continued to increase at a faster rate, to a level of "significant" improvement,
3 mg per day), response to therapy continued to increase at a faster rate, to a level of "significant" improvement,
Rice. 5. The ratio of the therapeutic effect (HAM-D score) and the daily dose of Velaxin. On the left y-axis - the average daily dose of the drug (mg).
During course therapy with Velaxin, 45 adverse events of various types were recorded in 21 (65.6%) patients; in 5 they were single, and in 16 they were combined. The most frequent were mental and vegetative adverse events noted on the UKU scale, they occurred in 40% of patients. 1 patient dropped out of the study ahead of schedule (after 2 weeks of treatment) due to the development of acute affective-delusional psychosis, requiring a change in therapy. Neurological and other side effects occurred in only 8.9and 11.1% of patients. The most common among the individual side effects were such as "agitation/anxiety" (in 15.5% of patients), decreased sleep duration and nausea (in 11.1%), tachycardia and hyperhidrosis (in 6. 6%), sedation and constipation (in 4.4%). All other side effects occurred in isolated cases. The frequency of side effects during course treatment with Velaxin was highest on the 2nd week of treatment, when the average daily dose of the drug was insignificant (157.7 mg per day). The most frequent during this period were mental and autonomous adverse events. But in the future, despite the increase in the average daily dose, the incidence of side effects gradually decreased. The exception was "other" side effects, the frequency of which practically remained at the same level until the end of treatment. These were mainly violations of sexual functions, which developed in accordance with an increase in the daily dose of Velaxin. nine0003
6%), sedation and constipation (in 4.4%). All other side effects occurred in isolated cases. The frequency of side effects during course treatment with Velaxin was highest on the 2nd week of treatment, when the average daily dose of the drug was insignificant (157.7 mg per day). The most frequent during this period were mental and autonomous adverse events. But in the future, despite the increase in the average daily dose, the incidence of side effects gradually decreased. The exception was "other" side effects, the frequency of which practically remained at the same level until the end of treatment. These were mainly violations of sexual functions, which developed in accordance with an increase in the daily dose of Velaxin. nine0003
It should be noted that, in general, all adverse events were mild or approached moderate in the first 2 weeks of treatment, and then the average degree of their severity, as assessed by UKU, decreased to a “mild” level, despite a gradual increase in the average daily doses of Velaxin. At the same time, mental side effects were initially the most severe: the average UKU score for their severity at the 1st–2nd week of treatment was 1.9–2.0. However, in the course of further course treatment, the severity of adverse mental symptoms decreased from the 3rd week. The severity of some other adverse events slightly increased compared to the initial one: if on the 2nd week of treatment their severity corresponded on average to the UKU of a “mild” degree (1 point), then in the future they reached 1.5 points, and at the same time their degree severity increased correspondingly with an increase in the mean dose of Velaxin. nine0003
At the same time, mental side effects were initially the most severe: the average UKU score for their severity at the 1st–2nd week of treatment was 1.9–2.0. However, in the course of further course treatment, the severity of adverse mental symptoms decreased from the 3rd week. The severity of some other adverse events slightly increased compared to the initial one: if on the 2nd week of treatment their severity corresponded on average to the UKU of a “mild” degree (1 point), then in the future they reached 1.5 points, and at the same time their degree severity increased correspondingly with an increase in the mean dose of Velaxin. nine0003
Discussion
The results of the clinical use of Velaxin in endogenous depression showed its high antidepressant activity. In 84.4% of patients, there was a clear positive therapeutic effect with a decrease in the severity of disorders (according to HAM-D) by 50% or more. Of these, the majority (78.1%) achieved a "significant" therapeutic effect with a reduction in the HAM-D score by more than 80%, and in 62. 5% of patients, at the end of the 8-week course treatment with Velaxin, a good quality remission occurred with complete "out" of depression. The distinct antidepressant properties of Velaxin are also evidenced by the inversion of depressive affect into hypomanic affect in 2 patients by the end of the course of treatment. It is very important that the therapeutic effect of Velaxin manifests itself early, already at the 2nd week of treatment it is determined at the level of "moderate" (up to 50% reduction in the severity of symptoms according to HAM-D), and by the 3rd-4th week of therapy, the reduction of the HAM score -D reached 50% or more ("good" effect). nine0003
5% of patients, at the end of the 8-week course treatment with Velaxin, a good quality remission occurred with complete "out" of depression. The distinct antidepressant properties of Velaxin are also evidenced by the inversion of depressive affect into hypomanic affect in 2 patients by the end of the course of treatment. It is very important that the therapeutic effect of Velaxin manifests itself early, already at the 2nd week of treatment it is determined at the level of "moderate" (up to 50% reduction in the severity of symptoms according to HAM-D), and by the 3rd-4th week of therapy, the reduction of the HAM score -D reached 50% or more ("good" effect). nine0003
According to the clinical characteristics, Velaxin can be legitimately classified as a balanced antidepressant. In the spectrum of its antidepressant activity, all 3 components of the antidepressant effect are approximately equally represented with a certain predominance (in terms of depth of effect) of the thymoleptic effect, an earlier anxiolytic effect and a “lag” in the timing of the manifestation of the stimulating effect. These properties of the antidepressant activity of Velaxin ensured its high therapeutic efficacy in the treatment of syndromic different types of depressive states - anxiety, apatho-adynamic and, primarily, melancholic. By the end of the course of treatment, the effectiveness of Velaxin in anxiety and apatho-adynamic depression "stopped" at the level of 85.5 and 85.9, respectively.% reduction of disorders according to HAM-D, and with melancholy depressions was deeper at this stage of treatment and reached 91.1% reduction of the corresponding symptoms.
These properties of the antidepressant activity of Velaxin ensured its high therapeutic efficacy in the treatment of syndromic different types of depressive states - anxiety, apatho-adynamic and, primarily, melancholic. By the end of the course of treatment, the effectiveness of Velaxin in anxiety and apatho-adynamic depression "stopped" at the level of 85.5 and 85.9, respectively.% reduction of disorders according to HAM-D, and with melancholy depressions was deeper at this stage of treatment and reached 91.1% reduction of the corresponding symptoms.
Features of the spectrum of therapeutic action of Velaxin depend on the nosological affiliation of the disease and the form of its course. In cases where the basis of the clinical essence of an endogenous disease was a distinct property of phase- or paroxysmal formation (MDP, cyclothymia, paroxysmal schizophrenia), the effectiveness of Velaxin in the treatment of depression was higher than in cases of diseases in which depression developed as part of exacerbation against the background of continuous (sluggish) ) course (low-progressive schizophrenia). But in general, the depth of therapeutic improvement in all these diseases was quite high, respectively, at the level of 86.6; nine0.4% and 82.3% reduction in HAM-D depressive symptom severity score.
But in general, the depth of therapeutic improvement in all these diseases was quite high, respectively, at the level of 86.6; nine0.4% and 82.3% reduction in HAM-D depressive symptom severity score.
The results of the study substantiate the expediency of prescribing long-term courses of treatment with Velaxin, since the formation of its therapeutic effect at the level of "good" begins only from the 3rd-4th week of the course of therapy, and the "significant" effect is achieved only at the 2nd month of treatment. The data obtained allow us to recommend moderate daily doses of the drug to achieve a "significant" therapeutic effect and "exit" into remission: doses of 180-19 can be considered optimal.8 mg, i.e. 2-3 tablets per day (75 mg each), since a further increase in the daily dose does not lead to a clear increase in the clinical effect.
In general, Velaxin is well tolerated, rare side effects associated with its administration are mild or moderate and develop mainly in the first 2 weeks of treatment; they do not require discontinuation of the drug and do not affect the regimen of therapy. Despite ongoing treatment and an increase in daily doses of Velaxin, the severity and frequency of adverse events are decreasing, which may justify their dose-independence. It is important to note that such severe side effects as urinary retention, lowering blood pressure, dry mouth, vomiting, convulsions, orthostatism, which are usually characteristic of tricyclic antidepressants, did not occur during treatment with Velaxin. nine0003
Despite ongoing treatment and an increase in daily doses of Velaxin, the severity and frequency of adverse events are decreasing, which may justify their dose-independence. It is important to note that such severe side effects as urinary retention, lowering blood pressure, dry mouth, vomiting, convulsions, orthostatism, which are usually characteristic of tricyclic antidepressants, did not occur during treatment with Velaxin. nine0003
Possessing a wide range and depth of antidepressant action, as well as good tolerance, Velaxin can be recommended as a drug of choice in the treatment of nosologically various endogenous diseases that occur with a picture of depression, both moderate and severe. Velaxin, a drug with a distinct antidepressant activity of balanced action, at the present stage of the development of psychopharmacotherapy can rightly be classified as an active antidepressant as a highly effective representative of the third generation of this class of psychotropic compounds. nine0003
nine0003
The use of Venlafaxine in modern psychiatric practice: advantages and prospects
partners Keywords / keywords: Venlafaxine, Depression, Dose-dependent effect, Prolonged-action capsules, Compliance, Anxiety, Velaxin
The use of Venlafaxine in modern psychiatric practice: advantages and prospects
The features of Venlafaxine use in outpatient psychiatric practice were studied. It was found that the drug is currently used in a wide range of mental disorders with anxiety and depression symptoms (organic, "endogenous", neurotic, etc.). At the same time, its dose-dependent effect allows you to select the dosage that best suits the clinical picture of a particular patient. It is shown that the use of ancient-action capsules Venlafaxine promotes greater patient adherence to prescribed treatment and improve its effectiveness.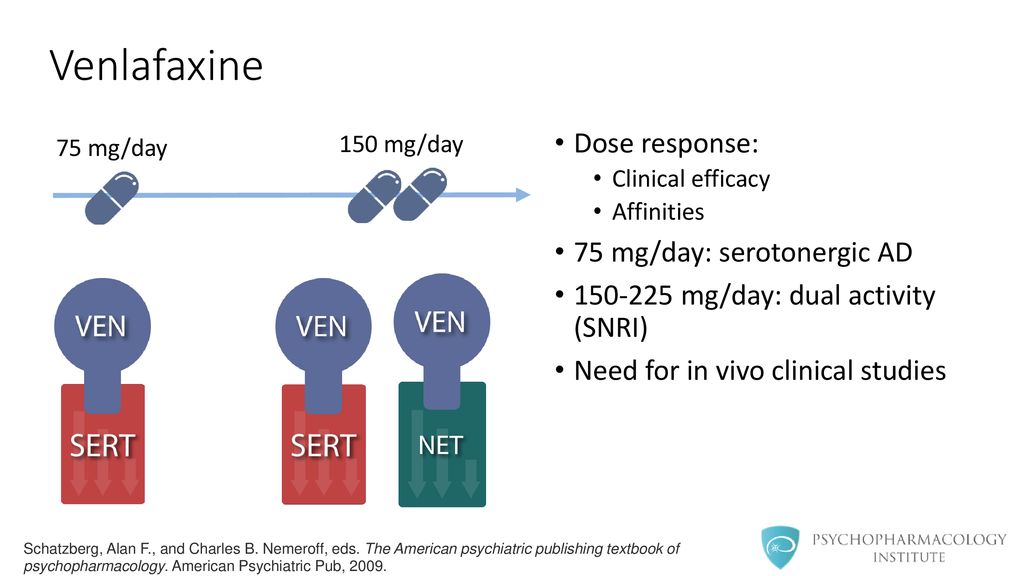 nine0003
nine0003
Introduction . At the present stage of development of psychiatry, the problem of the pathology of the affective sphere, especially depressive and anxiety disorders, is of particular relevance [2,3]. Currently, depression is one of the leading causes of a decrease in the quality of life [5]. Thus, according to the World Health Organization (2006), by 2020 they will come out on top in the world in terms of prevalence, overtaking cardiovascular diseases [3,11].
In most developed countries over the past forty years, there has been a steady increase in depressive spectrum disorders (RDS) [5]. The proportion of depressive and anxiety disorders is up to 40% of the volume of registered mental pathology in the world and up to 20% in European countries [1,10]. At the same time, their growth occurs mainly due to non-psychotic forms. About 60% of patients in the polyclinic network have RDS of varying severity [6]. Depression is observed not only in affective, but also in a number of other mental disorders: reactions to severe stress and adaptation disorders, disorders caused by degenerative-dystrophic and atrophic processes of the brain, addictive pathology [8]. nine0003
nine0003
Major depression produces maximum disability rates of up to 10%, which is more than 2 times higher than such common causes of disability as iron deficiency anemia, chronic obstructive pulmonary disease and osteoarthritis [4, 10]. This fact is largely associated with two obstacles to obtaining effective care: late diagnosis of depression and the appointment of inadequate therapy [3, 4].
Currently, a large number of antidepressant drugs are used in psychiatric practice, differing both in their chemical structure and in the mechanism of action [4,10]. Among them, a special place is occupied by the selective serotonin and norepinephrine reuptake inhibitor (SNRI) Venlafaxine, with the use of which new possibilities for the treatment of anxiety and depressive disorders have appeared in practicing psychiatrists. nine0003
Study aims to investigate the use of venlafaxine in modern outpatient psychiatric practice and evaluate its efficacy in the treatment of anxiety and depressive disorders.
Material and research methods . The material was the results of a multicenter observational study of the use of Venlafaxine in outpatient psychiatric practice, conducted in eight regions of Russia (Moscow, Northwestern, Central, Siberian, Urals, Volga, Southern, Far Eastern). The study included 2730 patients with the following diagnoses: obsessive-compulsive disorder, generalized anxiety disorder, sociophobic disorder, affective disorders in schizophrenia, organic mental disorders (anxiety, depressive), mixed anxiety and depressive disorder, recurrent depressive disorder, neurotic (panic disorder, agoraphobia, a withdrawal state caused by the use of psychoactive substances. The mean age of the patients was 42.8 ± 21.6 years. The assessment of the mental state of patients was carried out for 8 weeks of therapy. nine0003
The study of adherence to treatment of patients was assessed using the Morisky-Green compliance scale (Morisky D.E., Green L.W., Levine D. M., 1985), where the highest adherence to recommendations was rated at 1 point, and the lowest - at 4 points. Statistical processing of the results of the study was carried out using the Statistika 10.0 for Windows application package. Group mean values, dispersion of research results, standard deviation, minimum and maximum values of indicators were calculated by the Descriptive Statistics module. The critical significance level for testing hypotheses was less than 0.05 (p<0.05). nine0003
M., 1985), where the highest adherence to recommendations was rated at 1 point, and the lowest - at 4 points. Statistical processing of the results of the study was carried out using the Statistika 10.0 for Windows application package. Group mean values, dispersion of research results, standard deviation, minimum and maximum values of indicators were calculated by the Descriptive Statistics module. The critical significance level for testing hypotheses was less than 0.05 (p<0.05). nine0003
Results and discussion . Currently, there are a large number of hypotheses of depressive disorders (genetic, chronobiological, GABAergic, thyroid, immune, etc.). Modern molecular genetic research is developing in the direction of the so-called genetic mapping, i.e. search for certain regions of chromosomes that may be associated with the hereditary transmission of the disease or its distant signs [9,11]. Studies of disorders of various aspects of metabolism (lipid, carbohydrate, mineral) indicate that the source of affective disorders is a violation of the activity of the hypothalamus in regulating metabolism and the endocrine balance of the body [2,8]. nine0003
nine0003
The hypothesis of the neurophysiological foundations of affective disorders is based on the fact that the anatomical substrate of emotions is the brain structures that are part of the so-called limbic system (the hippocampus with pathways, the transparent septum, the nuclei of the amygdala complex, the hypothalamus, as well as a number of nuclei that lie in the reticular formation of the trunk of the bridge and midbrain) [4,10]. The function of these structures is largely provided by the corresponding neurotransmission mediators, in particular, by such neurotransmitters as serotonin and norepinephrine [11]. nine0003
Biochemical hypotheses of affective disorders are grouped around disorders of water and electrolyte metabolism, monoamine metabolism and hormonal disorders. Of great interest is the melatonin theory of depression. Its founder is A. Lewi (1980), who proved that during seasonal affective disorders, the secretion of the pineal gland hormone, melatonin, changes [6].
The main hypothesis of the pathogenesis of affective psychoses, in particular endogenous depressions, is currently the monoamine (serotonin) hypothesis. According to this hypothesis, the disease is based on dysfunction of the central serotonergic neurotransmission [7,12]. Indirectly, the hypothesis of monoamine deficiency in RDS is supported by the rapid effect of psychostimulants. Thus, amphetamine and cocaine are powerful activators of the release of monoamine neurotransmitters into the synaptic cleft. These substances have an immediate euphoric and psychostimulant effect, but in severe depression often there is not an alleviation of depressive symptoms, but the development of arousal. Some studies indicate that the immediate clinical response to a single injection of amphetamine correlates with the course effectiveness of SSRIs [9,eleven].
In general, the monoamine hypothesis of depression has now formed the basis of a clinical and pathological model [4], according to which the development of mild (undifferentiated) depressions is associated with a deficiency in synaptic endings of serotonin, moderate (melancholic) - serotonin and norepinephrine, and the most severe (psychotic) ) - serotonin, norepinephrine and dopamine (Fig.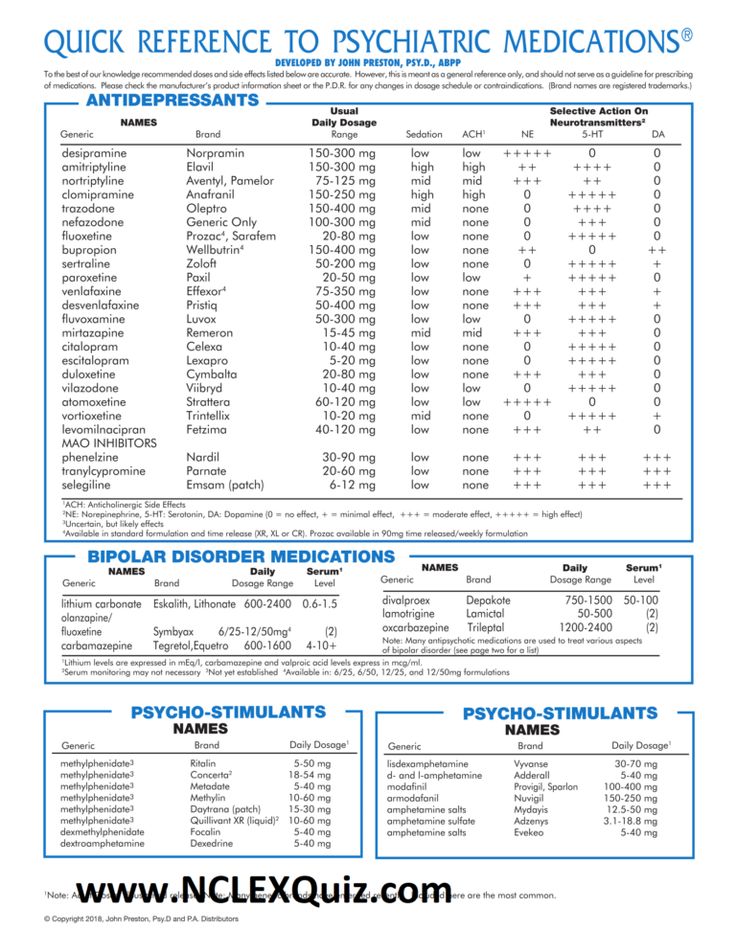 1).
1).
In this regard, the problem of choosing an antidepressant drug that would not only have high therapeutic efficacy against depressive symptoms of various etiologies, but also be as convenient as possible for the patient and contribute to higher compliance with maintenance therapy in outpatient practice is currently highly relevant. nine0003
Tricyclic antidepressants (TCAs) and new generation drugs (SSRIs, SNRIs) have a different range of side effects, which can be a decisive factor when choosing a drug in individual clinical cases. So, for TCAs, anticholinergic (dry mouth, constipation, blurred vision, urinary retention, tachycardia), cardiovascular (α-adrenergic blockade, orthostatic hypotension, bradyarrhythmia, tachycardia), antihistamine (sedation) side effects, weight gain and neurological symptoms (mild myoclonus, convulsive states in overdose, delirium in elderly patients). nine0003
Fig. 1. Clinical and pathological model of depression (Mosolov S. N., 2012)
N., 2012)
The above clinical and pathological model of depression largely corresponds to the mechanism of the neurotransmitter effect of the selective serotonin and norepinephrine reuptake inhibitor venlafaxine, which has a dose-dependent effect. At the same time, at a dosage of up to 150 mg / day, a predominantly serotonergic effect is realized, in the range of 150-225 mg / day - serotonergic and noradrenalergic, and at higher dosages, an increase in dopaminergic transmission occurs. The unique mechanism of action of Venlafaxine allows its use in a fairly wide range of mental disorders, in the phenomenological structure of which depressive symptoms are revealed ("endogenous", neurotic, organic). nine0003
An observational study of the prescription of Venlafaxine by psychiatrists in outpatient psychiatric practice confirmed its wide therapeutic range (Fig. 2).
Fig. 2. The frequency of Venlafaxine use in outpatient psychiatric practice for various mental disorders (%).
Thus, venlafaxine was most often used for mixed anxiety and depressive disorder (23.7%), generalized anxiety disorder (18.3%), panic disorder (14.1%), recurrent depression (12.5%) and organic mental disorders. (11.7%). Less commonly, the drug was prescribed for obsessive-compulsive disorder (8.7%), social phobia (5.9%) and schizophrenia spectrum disorders (5.1%).
At the same time, the dosages of the drug depended on the specific nosological form of the mental disorder, and the highest doses were used for recurrent depressive disorders (Fig. 3). Thus, more than half of the patients took Venlafaxine at a dosage of 150 mg/day and higher, which was due to the severity of depressive disorders, and the use of the drug at such dosages contributed to the enhancement of both serotonergic and norepinephrine transmission, which is a necessary intrasynaptic mechanism in the treatment of these conditions. nine0003
Fig. 3. Frequency of use of the main dosages of Venlafaxine in recurrent depressive disorder (%).
In turn, with organic mental disorders (depressive, anxiety), high dosages were also used. In 6.0% of cases they amounted to 300 mg/day, in 72.0% - 150 mg/day (Fig. 4). The use of lower dosages was noted only in 22.0% of patients.
Fig. 4. The frequency of use of the main dosages of Venlafaxine in organic mental disorders (%). nine0141
In turn, in patients with neurotic disorders (panic, agoraphobic), other ratios were noted (Fig. 5). High doses of venlafaxine (300 mg/day) were prescribed in 5.0% of patients, and 150 mg/day in 44.0%. The majority of patients in this group took the lowest dosage of the drug - 75 mg / day.
Fig. 5. Frequency of application of the main dosages of Venlafaxine in neurotic disorders (panic, agoraphobic) (%). nine0141
Thus, the dose-dependent effect used allowed practitioners to use Venlafaxine in outpatient practice for a fairly wide range of mental disorders with anxiety and depressive symptoms.
At the next stage, using the Morisky-Green compliance scale (Morisky D.E., Green L.W., Levine D.M., 1985), the compliance of the studied patients with the prescribed treatment was studied depending on the form of the drug release. At the same time, tableting form of Venlafaxine (37.5 mg and 75 mg) for a double dose and prolonged capsules of Velaxin (75 mg and 150 mg) for a single dose were evaluated (Fig. 6). nine0003
Fig. 6. Assessment of patient compliance, Morisky-Green scale, points (1 - high, 4 - low)
The study showed that the average score on this scale in the group of patients who took the tablet form of Venlafaxine for a double dose was (3.2 ± 1.1 points), and in the group of patients who were prescribed Venlafaxine (Velaxin) of prolonged action with a single dose (1. 6 ± 0.9 points). This indicated a greater compliance of the patients of the second group and their greater adherence to the prescribed treatment. nine0003
It should be noted that the modern possibilities of medical science do not allow to identify individual biological markers of the effectiveness of antidepressant therapy. However, the creation of multivariate statistical models of depressive disorders based on the assessment of a number of parameters (psychometric, neurohumoral, psychophysiological) can contribute to a more accurate assessment of the severity of psychopathological disorders and the appointment of more differentiated treatment using drugs with a dose-dependent effect. nine0003
However, the creation of multivariate statistical models of depressive disorders based on the assessment of a number of parameters (psychometric, neurohumoral, psychophysiological) can contribute to a more accurate assessment of the severity of psychopathological disorders and the appointment of more differentiated treatment using drugs with a dose-dependent effect. nine0003
Conclusion. The study showed that at present Venlafaxine is an effective drug in outpatient psychiatric practice and is used by practitioners for a wide range of mental disorders with anxiety and depressive symptoms (organic mental, "endogenous", neurotic). At the same time, the dose-dependent effect of the drug makes it possible to personalize the prescribed therapy and select the necessary dosage corresponding to the mental state of the patient. Along with this, the use of Venlafaxine (Velaxin) prolonged capsules contributes to greater adherence of patients to the prescribed treatment and, as a result, to an increase in its effectiveness. nine0003
nine0003
Literature
- Alexandrovsky Yu.A. Borderline mental disorders / Yu.A. Alexandrovsky. – M.: GEOTAR-Media, 2007. – 720 p.
- Andrukh P.G. Features of modern forms of anxiety and depressive disorders / Andrukh P.G. // Archive of psychiatry. - 2004. - S. 202-206.
- Antropov Yu.A. Fundamentals of the diagnosis of mental disorders / Yu.A. Antropov, A.Yu. Antropov, N.G. Neznanov. - M.: GEOTAR-Media, 2010. - 384 p. nine0203
- Biological methods of therapy of mental disorders (evidence-based medicine - clinical practice) / Pod. ed. Professor S.N. Mosolov. - M .: Publishing house "Social and political thought", 2012. - 1080 p.
- Krasnov V.N. Affective Spectrum Disorders / V.N. Krasnov. - M.: Practical medicine, 2011. - 432 p.
- Krasnov V.N. Improving the methods of early diagnosis of mental disorders (based on interaction with primary health care specialists) / V.
 N. Krasnov, T.V. Dovzhenko, A.E. Bobrov. - M.: Medpraktika, 2008. - 136 p. nine0203
N. Krasnov, T.V. Dovzhenko, A.E. Bobrov. - M.: Medpraktika, 2008. - 136 p. nine0203 - Polyvyanaya M. Yu. Evaluation of the quality of life of the mentally ill / M. Yu. Polyvyanaya // Archives of Psychiatry. - 2004. -T. 10, no. 29. - P. 5-9.
- Smulevich A.B. Depression in general medicine / A. B. Smulevich - M .: Medical Information Agency, 2001. - 256 p.
- Tiganov A.S. New in the study of the pathogenesis and therapy of endogenous depression / A.S. Tiganov and [others] // Journal of Neurology and Psychiatry. S.S. Korsakov. - 2012. - T. 112, No. 11. - S. 65-72. nine0203
- Chaban O.S. Neurotic and endogenous depressions / O.S. Chaban, S.G. Polshkova // NeuroNews. - 2008. - No. 3. - P. 10-15.
- Möller H.-J. Antidepressants: Controversies about their efficacy in depression, their effect on suicidality and their place in a complex psychiatric treatment approach // The World Journal of Biological Psychiatry.