Trileptal dosage for bipolar
Research, Dosage, and Side Effects
Trileptal may be an effective treatment for bipolar disorder, but more research is needed to support its benefits.
Trileptal (brand name of the generic drug oxcarbazepine) is an antiseizure drug that doctors routinely prescribe as a second-line option to control seizures in adults and children.
Some research suggests Tripeltal could also help treat symptoms of bipolar disorder, and it’s sometimes used off-label for this purpose. However, more studies are needed before experts know its true benefits and risks when used in this way.
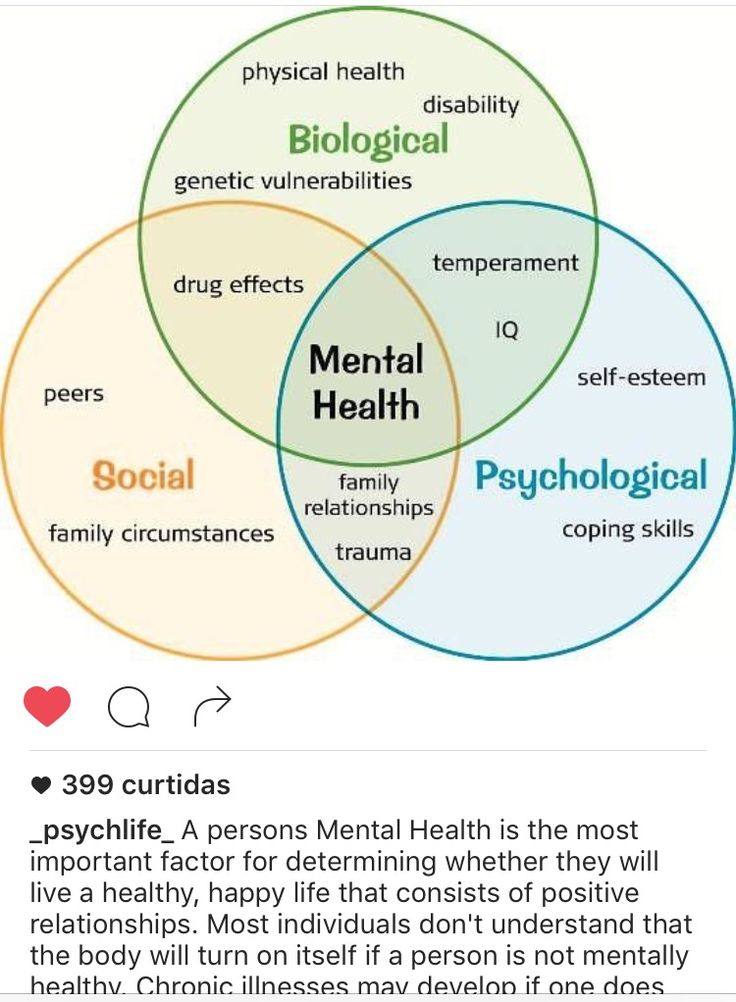
Bipolar disorder involves episodes of mania, depression, or both. Treatment plans often include talk therapy along with medications, which might include a mood stabilizer, an antiseizure medication, or an antipsychotic.
Medical professionals may prescribe Trileptal for the following conditions:
- seizures in adults and children
- trigeminal neuralgia (severe facial pain) in multiple sclerosis
- maintenance treatment of bipolar disorder
However, the Food and Drug Administration (FDA) has not approved Trileptal for bipolar disorder or trigeminal neuralgia, as research is generally lacking for both conditions. It’s only been FDA-approved for use in adult and childhood seizure disorders.
Trileptal has a similar action as carbamazepine, which is the drug it’s derived from. Carbamazepine has been studied more than Trileptal as a potential treatment for bipolar disorder. As a result, carbamazepine was previously approved by the FDA for hypomania, mild-to-moderate mania, and bipolar disorder with mixed features.
Recent research on Trileptal’s effectiveness for bipolar disorder is very limited. Most of the research for both Trileptal and carbamazepine on bipolar disorder was performed in the 1980s and 1990s.
However, a 2022 study looked at the use of oxcarbazepine in people with bipolar disorder who were hospitalized during episodes of mania. They were given between 900 mg and 2400 mg of oxcarbazepine per day, or they were given sodium valproate. The researchers reported similar improvements in mania symptoms over a 6-week period for both drugs.
Additionally, findings from a 2021 literature review indicated the following potential benefits of oxcarbazepine for bipolar disorder:
- may be effective for mild-to-moderate mania
- may have mild-to-moderate effectiveness for depressive episodes
- may be useful as an add-on therapy
- may be particularly helpful for hostile behavior
Researchers noted that in addition to more studies, better quality studies are needed to confirm these potential benefits.![]()
First-line treatments for bipolar disorder commonly include antipsychotics or mood stabilizers, which alter certain mood-related brain chemicals like dopamine. Antiseizure drugs like Trileptal are used less frequently, but can also be considered.
Valproate is a well-known antiseizure drug that doctors more commonly give people for mania in bipolar disorder.
Similar to Valproate, Trileptal works by lowering the amount of sodium in your brain. Sodium plays a critical role in the nervous system, helping nerve impulses carry throughout the body.
By altering sodium levels, Trileptal is thought to slow down the rate of nerve impulses between the neurons in the brain. This helps explain its effectiveness for seizures, where nerve impulses are overactive.
But like Valproate, researchers aren’t exactly sure why Trileptal is potentially effective for bipolar disorder.
Since Trileptal is not FDA-approved for use in bipolar disorder, there are no standard dosage recommendations.
According to the National Alliance on Mental Illness, the typical dose range for oxcarbazepine is 900–1200 mg. People will usually begin at a low dose, then increase the dose slowly over a few weeks. If you take this medication for seizures, you may take up to 2400 mg per day.
The most common side effects of Trileptal or oxcarbazepine are:
- dizziness
- confusion
- fatigue
- rash
- visual changes
- nausea or vomiting
- headache
- uncoordinated body movements
More serious side effects can also include:
- low sodium levels (hyponatremia)
- skin reactions
- suicidal thoughts or actions
- issues with blood cells
- worsened seizures
Low sodium is a particularly concerning symptom. It can cause serious complications such as seizures. If you’re interested in trying Trileptal, your doctor will check your sodium levels routinely, especially during the first 3 months of use.
Additionally, if you want to stop using Trileptal, it’s important to talk with your provider first. It is unsafe to abruptly discontinue this medication.
It is unsafe to abruptly discontinue this medication.
Although it’s sometimes used off-label for bipolar disorder, it seems that Trileptal has a long way to go before it gains FDA approval.
If you’re living with bipolar disorder and are searching for treatment options, you can talk with a healthcare professional about all of your choices.
To learn more about bipolar disorder and for additional help, you can:
- visit the National Alliance on Mental Illness (NAMI) for videos, podcasts, and information
- use the American Psychology Association’s website to find a psychiatrist
- read about natural medicines that can help manage the symptoms of bipolar disorder
- visit Psych Central’s bipolar disorder resource hub
- check out Psych Central’s How to Find Mental Health Support resource
Research, Dosage, and Side Effects
Trileptal may be an effective treatment for bipolar disorder, but more research is needed to support its benefits.
Trileptal (brand name of the generic drug oxcarbazepine) is an antiseizure drug that doctors routinely prescribe as a second-line option to control seizures in adults and children.
Some research suggests Tripeltal could also help treat symptoms of bipolar disorder, and it’s sometimes used off-label for this purpose. However, more studies are needed before experts know its true benefits and risks when used in this way.
Bipolar disorder involves episodes of mania, depression, or both. Treatment plans often include talk therapy along with medications, which might include a mood stabilizer, an antiseizure medication, or an antipsychotic.
Medical professionals may prescribe Trileptal for the following conditions:
- seizures in adults and children
- trigeminal neuralgia (severe facial pain) in multiple sclerosis
- maintenance treatment of bipolar disorder
However, the Food and Drug Administration (FDA) has not approved Trileptal for bipolar disorder or trigeminal neuralgia, as research is generally lacking for both conditions. It’s only been FDA-approved for use in adult and childhood seizure disorders.
It’s only been FDA-approved for use in adult and childhood seizure disorders.
Trileptal has a similar action as carbamazepine, which is the drug it’s derived from. Carbamazepine has been studied more than Trileptal as a potential treatment for bipolar disorder. As a result, carbamazepine was previously approved by the FDA for hypomania, mild-to-moderate mania, and bipolar disorder with mixed features.
Recent research on Trileptal’s effectiveness for bipolar disorder is very limited. Most of the research for both Trileptal and carbamazepine on bipolar disorder was performed in the 1980s and 1990s.
However, a 2022 study looked at the use of oxcarbazepine in people with bipolar disorder who were hospitalized during episodes of mania. They were given between 900 mg and 2400 mg of oxcarbazepine per day, or they were given sodium valproate. The researchers reported similar improvements in mania symptoms over a 6-week period for both drugs.
Additionally, findings from a 2021 literature review indicated the following potential benefits of oxcarbazepine for bipolar disorder:
- may be effective for mild-to-moderate mania
- may have mild-to-moderate effectiveness for depressive episodes
- may be useful as an add-on therapy
- may be particularly helpful for hostile behavior
Researchers noted that in addition to more studies, better quality studies are needed to confirm these potential benefits.
First-line treatments for bipolar disorder commonly include antipsychotics or mood stabilizers, which alter certain mood-related brain chemicals like dopamine. Antiseizure drugs like Trileptal are used less frequently, but can also be considered.
Valproate is a well-known antiseizure drug that doctors more commonly give people for mania in bipolar disorder.
Similar to Valproate, Trileptal works by lowering the amount of sodium in your brain. Sodium plays a critical role in the nervous system, helping nerve impulses carry throughout the body.
By altering sodium levels, Trileptal is thought to slow down the rate of nerve impulses between the neurons in the brain. This helps explain its effectiveness for seizures, where nerve impulses are overactive.
But like Valproate, researchers aren’t exactly sure why Trileptal is potentially effective for bipolar disorder.
Since Trileptal is not FDA-approved for use in bipolar disorder, there are no standard dosage recommendations.
According to the National Alliance on Mental Illness, the typical dose range for oxcarbazepine is 900–1200 mg. People will usually begin at a low dose, then increase the dose slowly over a few weeks. If you take this medication for seizures, you may take up to 2400 mg per day.
The most common side effects of Trileptal or oxcarbazepine are:
- dizziness
- confusion
- fatigue
- rash
- visual changes
- nausea or vomiting
- headache
- uncoordinated body movements
More serious side effects can also include:
- low sodium levels (hyponatremia)
- skin reactions
- suicidal thoughts or actions
- issues with blood cells
- worsened seizures
Low sodium is a particularly concerning symptom. It can cause serious complications such as seizures. If you’re interested in trying Trileptal, your doctor will check your sodium levels routinely, especially during the first 3 months of use.
Additionally, if you want to stop using Trileptal, it’s important to talk with your provider first. It is unsafe to abruptly discontinue this medication.
It is unsafe to abruptly discontinue this medication.
Although it’s sometimes used off-label for bipolar disorder, it seems that Trileptal has a long way to go before it gains FDA approval.
If you’re living with bipolar disorder and are searching for treatment options, you can talk with a healthcare professional about all of your choices.
To learn more about bipolar disorder and for additional help, you can:
- visit the National Alliance on Mental Illness (NAMI) for videos, podcasts, and information
- use the American Psychology Association’s website to find a psychiatrist
- read about natural medicines that can help manage the symptoms of bipolar disorder
- visit Psych Central’s bipolar disorder resource hub
- check out Psych Central’s How to Find Mental Health Support resource
New gold standard | www.mgzt.ru
Within the framework of the VI All-Russian Congress of Neurologists held in Yaroslavl, a symposium "New possibilities for the use of antiepileptic drugs in neurology" was held, which was organized by Novartis Pharma Services Inc. The presidium of the scientific meeting included Academician of the Russian Academy of Medical Sciences Nikolai Yakhno, Chairman of the Russian Anti-Epileptic League Professor Gagik Avakyan, Secretary of the Russian Anti-Epileptic League Professor Alla Gekht, Chief Expert Pediatric Neurologist of the Ministry of Health and Social Development of Russia Professor Larisa Kalinina. nine0004
The presidium of the scientific meeting included Academician of the Russian Academy of Medical Sciences Nikolai Yakhno, Chairman of the Russian Anti-Epileptic League Professor Gagik Avakyan, Secretary of the Russian Anti-Epileptic League Professor Alla Gekht, Chief Expert Pediatric Neurologist of the Ministry of Health and Social Development of Russia Professor Larisa Kalinina. nine0004
Differences and advantages
Professor Konstantin Mukhin (Russian State Medical University) opened the symposium with the report “Clinical experience with Trileptal (oxcarbazepine) in symptomatic focal epilepsy”. He spoke in detail about the history of creation and requirements for antiepileptic drugs, which include high efficiency in the treatment of epilepsy, a wide range of effects on various seizures, the absence of seizure aggravation, and good tolerance. nine0005 The speaker dwelled on the general principles of epilepsy treatment - the effectiveness of monotherapy, prescribing drugs depending on the form of the disease, the nature of the attacks and the age of the patients. It was emphasized that if a drug was not effective enough, it should be gradually withdrawn and replaced by another.
It was emphasized that if a drug was not effective enough, it should be gradually withdrawn and replaced by another.
Pharmacoeconomic data were presented, according to which, per inpatient, only antiepileptic drugs in Germany were spent per month in 1986 - 28 euros, in 1992 - 33 euros, in 2001 - 70 euros (when new generation drugs entered clinical practice), in 2002 - 92 euros. The author stressed that this figure will grow due to the cost of modern drugs for the treatment of epilepsy. However, the costs to society associated with the disability of patients with epilepsy are much greater, which is why the availability of new effective drugs is important.
Trileptal (oxcarbazepine) is a drug that has become firmly established in clinical practice and has been used in some countries for 10 years. It preferentially blocks voltage-gated sodium and calcium channels, peaking at 4.5 hours after oral administration of 600 mg. Trileptal has a linear pharmacokinetics, unlike, for example, otdifenin and phenytoin, where, due to non-linear pharmacokinetics, even with a small increase in dose, it is possible to quickly reach a peak concentration and get a toxic effect. nine0005 The binding of Trileptal to plasma proteins is low - 40%. This is especially important in pediatric practice, where drugs with high plasma protein binding must be used with caution.
nine0005 The binding of Trileptal to plasma proteins is low - 40%. This is especially important in pediatric practice, where drugs with high plasma protein binding must be used with caution.
The fundamental difference between Trileptal and drugs of the carbamazepine group is a different metabolic pathway with the formation of a completely different metabolite - monohydroxy derivative. Thus, during the transformation of Trileptal, the body does not form 10,11 epoxide, the active metabolite of carbamazepine, which is traditionally associated with its main side effects. A side effect that is possible when taking both carbamazepine and Trileptal is hyponatremia, that is, a decrease in the level of sodium in the blood plasma of less than 120 mmol / l. Hyponatremia occurs more often in adults, especially in the elderly; in pediatric practice, hyponatremia is rare. nine0005 The half-life is on average 2.5 hours for Trileptal itself (oxcarbazepine) and 9.5 hours for its metabolite, monohydroxy derivative, which allows you to take the drug 2 times a day. 95% of the drug is metabolized in the kidneys.
95% of the drug is metabolized in the kidneys.
An important advantage of oxcarbazepine is that, unlike the drugs of the carbamazepine group, it is a weak inducer of microsomal liver enzymes and does not affect the metabolism of other drugs. Thus, it is convenient to combine oxcarbazepine with other antiepileptic drugs, knowing that their concentration will not change significantly. However, caution should be exercised when using oxcarbazepine while taking oral contraceptives. nine0005
Trileptal is currently positioned as a starting monotherapy for symptomatic focal forms of epilepsy in adults and children over 2 years of age. In the near future, Trileptal suspension for very young children will be approved for use.
The second indication is the use as adjunctive therapy for symptomatic forms of focal epilepsy. Trileptal is contraindicated in patients with typical absence seizures or myoclonus within idiopathic generalized forms of epilepsy. nine0005 Dosages for children - from 8 to 46 mg / kg of body weight per day, the average dose is 20-30 mg / kg of body weight per day in two divided doses. For adults - 10-45 mg per kg of body weight per day, an average of 1000 mg / day. The starting dose ranges from 150 mg/day in young children to 300 mg/day in older children, followed by a weekly increase in dose from 150 to 200 mg. For adults, it is also possible to titrate the dose from 150 mg with a further weekly increase in the dose by 600 mg.
For adults - 10-45 mg per kg of body weight per day, an average of 1000 mg / day. The starting dose ranges from 150 mg/day in young children to 300 mg/day in older children, followed by a weekly increase in dose from 150 to 200 mg. For adults, it is also possible to titrate the dose from 150 mg with a further weekly increase in the dose by 600 mg.
According to clinical studies, in 90% of patients with partial epilepsy, a decrease in the frequency of seizures by more than 50% was observed, and in 60% of patients with newly diagnosed epilepsy, complete relief of seizures was observed with Trileptal monotherapy. nine0005 The drug demonstrates the highest percentage of retention on therapy - up to 91% of patients continue to take Trileptal during the year. Interruption of therapy due to side effects - only 3%. An important point - if necessary, it is possible to replace other antiepileptic drugs with Trileptal "overnight", that is, switching drugs can be one-step.
Therapy of neuropathic pain
Professor Valery Alekseev (Moscow Medical Academy named after I. M. Sechenov) in the report "Anticonvulsants in the treatment of neuropathic pain" spoke in detail about the functioning of the nociceptive and antinociceptive systems, the mechanisms of pain formation, and gave characteristics to various types of pain. nine0005 The therapy strategy for neuropathic pain is based on a complex effect both on the peripheral components of the system that conduct pain, and on the regulation of behavior and the emotional sphere. All anticonvulsants used to treat neuropathic pain are conditionally divided into three groups: blockers of voltage-gated sodium channels; drugs that enhance GABA-ergic activity; drugs that block the release of activating amino acids (glutamate and aspartate). Trileptal blocks voltage-dependent sodium and calcium channels, opens potassium channels, that is, creates the effect of membrane hyperpolarization, as a result, the peripheral nociceptive stimulus is not perceived, according to the “busy here” principle, and finally blocks the release of activating amino acids, which complements the overall effect against the formation of neuropathic pain .
M. Sechenov) in the report "Anticonvulsants in the treatment of neuropathic pain" spoke in detail about the functioning of the nociceptive and antinociceptive systems, the mechanisms of pain formation, and gave characteristics to various types of pain. nine0005 The therapy strategy for neuropathic pain is based on a complex effect both on the peripheral components of the system that conduct pain, and on the regulation of behavior and the emotional sphere. All anticonvulsants used to treat neuropathic pain are conditionally divided into three groups: blockers of voltage-gated sodium channels; drugs that enhance GABA-ergic activity; drugs that block the release of activating amino acids (glutamate and aspartate). Trileptal blocks voltage-dependent sodium and calcium channels, opens potassium channels, that is, creates the effect of membrane hyperpolarization, as a result, the peripheral nociceptive stimulus is not perceived, according to the “busy here” principle, and finally blocks the release of activating amino acids, which complements the overall effect against the formation of neuropathic pain . It is important that with the combined use of a wide range of drugs with an effect on peripheral receptor apparatus, Trileptal has practically no interaction with other drugs, that is, it can be actively used in complex treatment. nine0005 There are data on the use of Trileptal in traumatic lesions of the spinal cord, complex regional pain syndrome type 1, postherpetic neuralgia, trigeminal neuralgia, diabetic neuropathy with pain syndrome.
It is important that with the combined use of a wide range of drugs with an effect on peripheral receptor apparatus, Trileptal has practically no interaction with other drugs, that is, it can be actively used in complex treatment. nine0005 There are data on the use of Trileptal in traumatic lesions of the spinal cord, complex regional pain syndrome type 1, postherpetic neuralgia, trigeminal neuralgia, diabetic neuropathy with pain syndrome.
Mood corrector
Prof. Olga Vorobieva (MMA named after I.M. Sechenov) made a presentation on “Normothymic action of antiepileptic drugs”.
After the introduction of the first anticonvulsants to the market, it became clear that in addition to the effect on epileptic seizures, they affect psychopathological syndromes. nine0005 Primarily, anticonvulsants can be used for mood disorders.
Mood disorders are divided into bipolar and depressive, these disorders are extremely numerous. And if neurologists do not encounter bipolar disorders of the 1st type, when manias are replaced by depression, in their practice, they constantly encounter bipolar disorder of the 2nd type, in which mania does not reach the degree of manic psychosis. These are shallow, mild depressions, mood disorders induced by drugs used in neurology or caused by somatic suffering, including neurological. nine0005 Anticonvulsants stabilize mood and are commonly referred to as mood correctors. Bipolar disorder is characterized by alternating manic and depressive episodes, with each successive episode requiring a lesser trigger. The gaps between the phases shorten, similar to the epilepsy model, where each seizure paves the way for the next.
These are shallow, mild depressions, mood disorders induced by drugs used in neurology or caused by somatic suffering, including neurological. nine0005 Anticonvulsants stabilize mood and are commonly referred to as mood correctors. Bipolar disorder is characterized by alternating manic and depressive episodes, with each successive episode requiring a lesser trigger. The gaps between the phases shorten, similar to the epilepsy model, where each seizure paves the way for the next.
First-generation anticonvulsants have proven to be truly mood stabilizing, highly effective for acute mania and long-term treatment of bipolar disorder. Modern anticonvulsants entering the market do not have such a stabilizing effect. Some of them are effective against PTSD, post-stress anxiety, some are effective against depression, but not enough to be called mood correctors. New generation drugs have a narrower, selective psychotropic spectrum. nine0005 Looking at the level of sales of anticonvulsants, it is clear that first-generation drugs remain very attractive. Therefore, now some new drugs are being developed on the basis of molecules of the first generation of anticonvulsants. Trileptal is very close in action to carbamazepine and retains its effects with a much more favorable tolerability spectrum. Trileptal gives fewer allergic reactions compared to carbamazepine and, which is very important for the treatment of psychopathological syndromes, interacts significantly less with psychotropic drugs, it has better performance in combined treatment, no matter what it is prescribed with - anticonvulsants or psychotropic drugs. nine0005 Trileptal (oxcarbazepine) is similar in its mechanisms of action and clinical effects to carbamazepine and can be used as a mood corrector, for the treatment of manic-depressive psychosis, while mainly for the treatment of mania (for the correction of depression in doctors, there is already a fairly large arsenal of drugs) . As a mood corrector, the drug has been investigated in four large controlled trials, where dosages from 900 to 2300 mg per day were used.
Therefore, now some new drugs are being developed on the basis of molecules of the first generation of anticonvulsants. Trileptal is very close in action to carbamazepine and retains its effects with a much more favorable tolerability spectrum. Trileptal gives fewer allergic reactions compared to carbamazepine and, which is very important for the treatment of psychopathological syndromes, interacts significantly less with psychotropic drugs, it has better performance in combined treatment, no matter what it is prescribed with - anticonvulsants or psychotropic drugs. nine0005 Trileptal (oxcarbazepine) is similar in its mechanisms of action and clinical effects to carbamazepine and can be used as a mood corrector, for the treatment of manic-depressive psychosis, while mainly for the treatment of mania (for the correction of depression in doctors, there is already a fairly large arsenal of drugs) . As a mood corrector, the drug has been investigated in four large controlled trials, where dosages from 900 to 2300 mg per day were used. Its action was compared with antipsychotics (haloperidol), lithium and placebo. With regard to haloperidol, oxcarbazepine has a similar efficacy, and in some aspects looks more attractive, the same with lithium. nine0005 Trileptal (oxcarbazepine) is used as a mood corrector at dosages of 900-1200 mg per day. Neurologists deal with incomplete syndromes and use mainly the dosage of 900 mg per day. Psychiatrists use dosages sometimes significantly higher than 1200 mg per day.
Its action was compared with antipsychotics (haloperidol), lithium and placebo. With regard to haloperidol, oxcarbazepine has a similar efficacy, and in some aspects looks more attractive, the same with lithium. nine0005 Trileptal (oxcarbazepine) is used as a mood corrector at dosages of 900-1200 mg per day. Neurologists deal with incomplete syndromes and use mainly the dosage of 900 mg per day. Psychiatrists use dosages sometimes significantly higher than 1200 mg per day.
Albert KHISAMOV , corr. "MG".
‹ Where are Piglet and I going? Up I forgot what candles are lit on New Year's Eve... ›
Antiepileptic drugs outside of epilepsy (use of anticonvulsants in the treatment of pain syndromes)
Anticonvulsants, or anticonvulsants, according to one of the definitions - a group of drugs of different chemical structure that have the ability to prevent or relieve convulsions. Anticonvulsants include a number of substances with hypnotic and sedative effects (for example, bromides, chloral hydrate, phenobarbital), as well as substances that have a selective anticonvulsant effect [3]. The first description of the use of potassium bromide as an anticonvulsant dates back to 1857, and synthetic selective antiepileptic therapy dates back to 1912, when the antiepileptic properties of phenobarbital were discovered, discovered when prescribing the drug as a hypnotic. The next important step in the development of the pharmacology of anticonvulsants was the discovery of phenytoin, which was synthesized in 1908, and studied and described as an antiepileptic agent without a hypnotic effect in 1938 by Merrit and Putnam. Further attempts to find a less toxic drug for the relief of convulsive syndromes led to the discovery of ethosuximide in 1958 g. In the 1960s, carbamazepine, valproic acid, and benzodiazepines entered the practice of treating epilepsy. A new era in the development of antiepileptic drugs (AEDs) begins in 1975, when a program was established in the United States to study the latest anticonvulsant molecules. Since 1990, 16 new AEDs have been registered in Europe and the United States, constituting the current generation of anticonvulsants, such as: tiagabine, vigabatrin, gabapentin, topiramate, oxcarbazepine, felbamate, zonisamide, levetiracetam, pregabalin, lacosamide, rufinamide, and others.
The first description of the use of potassium bromide as an anticonvulsant dates back to 1857, and synthetic selective antiepileptic therapy dates back to 1912, when the antiepileptic properties of phenobarbital were discovered, discovered when prescribing the drug as a hypnotic. The next important step in the development of the pharmacology of anticonvulsants was the discovery of phenytoin, which was synthesized in 1908, and studied and described as an antiepileptic agent without a hypnotic effect in 1938 by Merrit and Putnam. Further attempts to find a less toxic drug for the relief of convulsive syndromes led to the discovery of ethosuximide in 1958 g. In the 1960s, carbamazepine, valproic acid, and benzodiazepines entered the practice of treating epilepsy. A new era in the development of antiepileptic drugs (AEDs) begins in 1975, when a program was established in the United States to study the latest anticonvulsant molecules. Since 1990, 16 new AEDs have been registered in Europe and the United States, constituting the current generation of anticonvulsants, such as: tiagabine, vigabatrin, gabapentin, topiramate, oxcarbazepine, felbamate, zonisamide, levetiracetam, pregabalin, lacosamide, rufinamide, and others. molecules are under development [11]. nine0045
molecules are under development [11]. nine0045
In the years since the introduction of the first anticonvulsants, an extensive evidence base and experience in the use of these drugs in the treatment of various forms of epilepsy has emerged, while at the same time a number of points of application for these drugs in the treatment of other diseases have emerged. The literature contains the results of a study of the effectiveness of AEDs in the following diseases and syndromes: neuropathic pain and fibromyalgia, migraine, anxiety and bipolar disorders, schizophrenia, essential tremor [21]. nine0045
Prerequisites for the use of anticonvulsants in neuropathic pain
Neuropathic pain, according to the current definition, is a pain syndrome caused by damage to the somatosensory nervous system due to a variety of reasons [33]. The pathophysiological basis for the occurrence of neuropathic pain syndrome is disturbances in the mechanisms of generation and conduction of a nociceptive signal in nerve fibers, as well as the processes of controlling the excitability of nociceptive neurons in the structures of the brain and spinal cord. Due to nerve damage, the number of sodium and calcium channels on the nerve fiber membrane increases, and zones of generation of ectopic impulses appear. The increased impulsation from the periphery also disorganizes the work of the central structures, resulting in the so-called “central sensitization” [1, 2]. nine0045
Due to nerve damage, the number of sodium and calcium channels on the nerve fiber membrane increases, and zones of generation of ectopic impulses appear. The increased impulsation from the periphery also disorganizes the work of the central structures, resulting in the so-called “central sensitization” [1, 2]. nine0045
A number of pathophysiological mechanisms characteristic of neuropathic pain have analogues in epilepsy. For example, similarities have been found between the phenomenon of “winding up” that occurs in the posterior horns of the spinal cord in neuropathic pain, central sensitization, and the phenomenon of “kindling” or the formation of “pathological excitation generators” in hippocampal neurons in epilepsy. It is believed that both phenomena, along with other mechanisms, arise due to the activation of NMDA receptors. Not surprisingly, anticonvulsants show their effectiveness both in terms of reducing the number of epileptic seizures and in terms of suppressing the intensity of the pain syndrome [13, 17, 37]. nine0045
nine0045
Mechanisms of action of anticonvulsants in pain syndromes
The mechanisms of action of anticonvulsants in neuropathic pain are different. The anti-pain effect of drugs such as carbamazepine, phenytoin, oxcarbazepine, lamotrigine, valproate, topiramate is usually explained by a decrease in high-frequency repetitive neuron firing by blocking voltage-dependent sodium and calcium channels in peripheral nerves. A number of drugs (eg, phenobarbital, tiagabine, topiramate, vigabatrin, and valproate) increase nerve transmission of pain-suppressing impulses or directly block nerve transmission of excitatory impulses. Pregabalin and gabapentin have a fundamentally different mechanism of action associated with the effect on the alpha-2-delta subunit of voltage-gated calcium channels and the suppression of central sensitization processes in the dorsal horn of the spinal cord and in the brain. Summarized data on the mechanisms of action of anticonvulsants are presented in tab. 1.
1.
In addition to those listed in Table. 1 proposed mechanisms of action of anticonvulsants, their possible indirect effects on various neurotransmitter systems and effects on intracellular processes are also considered. Today, the question is being discussed whether the mechanism of realization of the therapeutic effect of the same anticonvulsant in different conditions: epilepsy, pain syndromes, anxiety, etc. is the same through similar mechanisms or these mechanisms differ [21]. nine0045
The first publication on the use of anticonvulsants for the treatment of neuropathic pain appeared in 1942, when M. Bergouignan showed the effectiveness of phenytoin in the treatment of neuropathic pain in trigeminal neuralgia [8]. 20 years later, since the early 1960s, carbamazepine was successfully introduced into the practice of treating the same trigeminal neuralgia, which became one of the first drugs officially registered for the treatment of this condition [9]. The beginning of the 1990s was characterized by a kind of boom in the study of antidepressants, which led to the emergence of new effective and safer drugs. A large number of evidence-based randomized controlled trials have been published, allowing further meta-analysis of the data obtained. Summarized results of systematic reviews and meta-analyses are presented in tab. 2 [17]. Attention is drawn to a large number of studies on the effectiveness of the latest generation of anticonvulsants - pregabalin and gabapentin.
The beginning of the 1990s was characterized by a kind of boom in the study of antidepressants, which led to the emergence of new effective and safer drugs. A large number of evidence-based randomized controlled trials have been published, allowing further meta-analysis of the data obtained. Summarized results of systematic reviews and meta-analyses are presented in tab. 2 [17]. Attention is drawn to a large number of studies on the effectiveness of the latest generation of anticonvulsants - pregabalin and gabapentin.
Based on the meta-analysis and systematic reviews, such authoritative international organizations as the European Federation of Neurological Societies, the Group for the Study of Neuropathic Pain of the International Association for the Study of Pain, etc., published recommendations for the treatment of neuropathic pain and identified several directions (lines) of therapy for this condition . In most documents, first-line drugs are distinguished, i.e. those drugs with which to start treatment, and if ineffective or intolerable, change to another drug of the same line, as well as second and third line drugs, and some recommendations even suggest the allocation of fourth-line drugs. The authors of the recommendations agree on one thing: to date, among anticonvulsants, only pregabalin and gabapentin are first-line drugs in the treatment of neuropathic pain. Evidence for the effectiveness of carbamazepine is only available for trigeminal neuralgia [32] (Table 3) .
those drugs with which to start treatment, and if ineffective or intolerable, change to another drug of the same line, as well as second and third line drugs, and some recommendations even suggest the allocation of fourth-line drugs. The authors of the recommendations agree on one thing: to date, among anticonvulsants, only pregabalin and gabapentin are first-line drugs in the treatment of neuropathic pain. Evidence for the effectiveness of carbamazepine is only available for trigeminal neuralgia [32] (Table 3) .
It is important to emphasize that the considered approach to the treatment of neuropathic pain is based on evidence obtained in studies that took into account not only the experience of each specialist, but also summarized the relevant world data [32]. The same positions are reflected in the recommendations of a group of Russian experts [4].
In Russia today a huge number of anticonvulsants are registered - more than 200 trade names of various forms of release as of 03/01/13.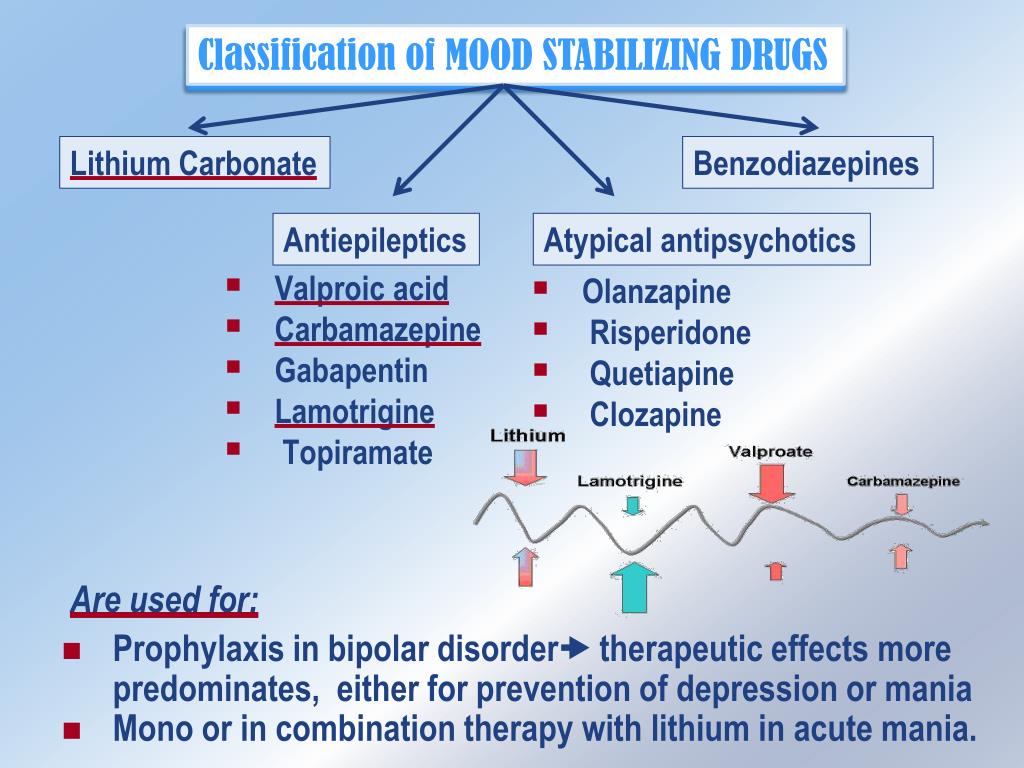 Most of these drugs are indicated for the treatment of various forms of epilepsy as monotherapy or in combination with other drugs. Neuropathic pain is among the indications for use only in a very limited number of drugs in this group. These include: phenytoin, which is registered for the treatment of trigeminal neuralgia, and carbamazepine, indicated for the treatment of trigeminal neuralgia, glossopharyngeal neuralgia, and painful diabetic polyneuropathy. Pregabalin (lyrica) and its predecessor gabapentin are the only drugs registered for the treatment of all types of neuropathic pain in adults [1] . Let's consider each of these drugs in more detail.
Most of these drugs are indicated for the treatment of various forms of epilepsy as monotherapy or in combination with other drugs. Neuropathic pain is among the indications for use only in a very limited number of drugs in this group. These include: phenytoin, which is registered for the treatment of trigeminal neuralgia, and carbamazepine, indicated for the treatment of trigeminal neuralgia, glossopharyngeal neuralgia, and painful diabetic polyneuropathy. Pregabalin (lyrica) and its predecessor gabapentin are the only drugs registered for the treatment of all types of neuropathic pain in adults [1] . Let's consider each of these drugs in more detail.
Phenytoin (Difenin) is one of the very first AEDs. In addition to anticonvulsant and analgesic, it has a membrane-stabilizing, antiarrhythmic and hypotensive effect. Its bioavailability is low and is 20-50%. It is metabolized in the liver and is an inducer of the isoenzymes CYP3A4, CYP3A5. As an anesthetic, the drug is indicated for the treatment of only trigeminal neuralgia, in which it is prescribed 100-300 mg 1-3 times a day. Of the side effects, ataxia, nystagmus, dizziness, tremor, muscle weakness, nausea, vomiting, toxic hepatitis and others are most characteristic. Very rarely, the development of Stevens-Johnson syndrome and acute epidermal necrolysis is possible. The drug should be prescribed with caution in patients with algoholism, diabetes mellitus, chronic heart and liver failure. In addition, the drug is characterized by a high risk of adverse events due to drug interactions [1]. nine0045
Of the side effects, ataxia, nystagmus, dizziness, tremor, muscle weakness, nausea, vomiting, toxic hepatitis and others are most characteristic. Very rarely, the development of Stevens-Johnson syndrome and acute epidermal necrolysis is possible. The drug should be prescribed with caution in patients with algoholism, diabetes mellitus, chronic heart and liver failure. In addition, the drug is characterized by a high risk of adverse events due to drug interactions [1]. nine0045
Carbamazepine (tegretol, finlepsin, actinerval, zeptol, etc.) belongs to the first generation of AEDs and is similar in chemical structure to tricyclic antidepressants. Carbamazepine has a wide range of indications, including conditions accompanied by neuropathic pain, trigeminal neuralgia, glossopharyngeal neuralgia, and painful diabetic polyneuropathy, among other diseases. Most well-designed studies have been conducted in patients with trigeminal neuralgia and facial pain, where the drug has proven effective. Data on its effectiveness in other types of neuropathic pain are limited (few trials conducted and patients included). In this regard, in a number of recommendations for the treatment of neuropathic pain, carbamazepine is recommended as a first-line drug only for trigeminal neuralgia [17]. Carbamazepine is usually taken with or without food, with a small amount of liquid. In adults, treatment is recommended to start with a dose of 100-200 mg 1 or 2 times a day, gradually increasing it by no more than 200 mg per day, until pain relief (on average, 800-1200 mg per day), and then reduce it to minimum effective dose. In some cases, a dose of 1600 mg per day may be required, which requires careful titration and monitoring of the patient. Carbamazepine is contraindicated in atrioventricular blockade, hematopoietic disorders, acute intermittent porphyria, including history, as well as simultaneously with monoamine oxidase inhibitors. nine0045
Data on its effectiveness in other types of neuropathic pain are limited (few trials conducted and patients included). In this regard, in a number of recommendations for the treatment of neuropathic pain, carbamazepine is recommended as a first-line drug only for trigeminal neuralgia [17]. Carbamazepine is usually taken with or without food, with a small amount of liquid. In adults, treatment is recommended to start with a dose of 100-200 mg 1 or 2 times a day, gradually increasing it by no more than 200 mg per day, until pain relief (on average, 800-1200 mg per day), and then reduce it to minimum effective dose. In some cases, a dose of 1600 mg per day may be required, which requires careful titration and monitoring of the patient. Carbamazepine is contraindicated in atrioventricular blockade, hematopoietic disorders, acute intermittent porphyria, including history, as well as simultaneously with monoamine oxidase inhibitors. nine0045
When using carbamazepine, a number of serious side effects have been registered, such as Stevens-Johnson syndrome (544 cases were registered in the world in 1999), acute epidermal necrolysis, agranulocytosis, aplastic anemia, hepatitis, impaired renal function, endocrine disorders. The most common side effects (10% or more) of carbamazepine include: dizziness, ataxia, drowsiness, urticaria, multi-organ hypersensitivity reactions, vasculitis, leukopenia, nausea, vomiting, edema, weight gain. The risk of developing these phenomena is increased when taking into account adverse drug interactions. With caution, carbamazepine should be prescribed in elderly patients [5, 6]. nine0045
The most common side effects (10% or more) of carbamazepine include: dizziness, ataxia, drowsiness, urticaria, multi-organ hypersensitivity reactions, vasculitis, leukopenia, nausea, vomiting, edema, weight gain. The risk of developing these phenomena is increased when taking into account adverse drug interactions. With caution, carbamazepine should be prescribed in elderly patients [5, 6]. nine0045
Therefore, carbamazepine should only be used under regular medical supervision. Before starting treatment, it is recommended to do a urinalysis, a detailed biochemical blood test, including the level of urea. These indicators should be monitored first weekly and then monthly. During treatment, it is also necessary to regularly monitor liver function, blood status, including the concentration of electrolytes in the serum [14].
Oxcarbazepine (trileptal) is structurally similar to carbamazepine. It is believed that the effectiveness of these drugs in epilepsy is quite comparable, but oxcarbazepine is better tolerated. For example, when using the drug, not a single case of Stevens-Johnson syndrome has been registered. Several clinical studies have been conducted with oxcarbazepine in trigeminal neuralgia with positive results. Recommended dosing regimen 900-1800 mg per day. A number of recommendations for the treatment of neuropathic pain indicate that oxcarbazepine is the first-line drug for trigeminal neuralgia. Oxcarbazepine is not registered in Russia for this indication [17].
For example, when using the drug, not a single case of Stevens-Johnson syndrome has been registered. Several clinical studies have been conducted with oxcarbazepine in trigeminal neuralgia with positive results. Recommended dosing regimen 900-1800 mg per day. A number of recommendations for the treatment of neuropathic pain indicate that oxcarbazepine is the first-line drug for trigeminal neuralgia. Oxcarbazepine is not registered in Russia for this indication [17].
Gabapentin is recognized in a number of international recommendations as a first-line drug for the treatment of neuropathic pain [32]. In addition to the original drug Neurontin, a number of generics have been registered in Russia: gapentek, tebantin, gabagamma, convalis, etc. In 9 large randomized placebo-controlled trials, the effectiveness of gabapentin in postherpetic neuralgia and diabetic polyneuropathy was shown. One placebo-controlled study was conducted for phantom pain, Guillain-Barré syndrome, neuropathic pain of various etiologies, neuropathic pain caused by cancer, and spinal cord injury. According to the results of clinical trials, the drug showed its superiority over placebo in pain relief and its good tolerability by patients [26]. nine0045
According to the results of clinical trials, the drug showed its superiority over placebo in pain relief and its good tolerability by patients [26]. nine0045
The mechanism of action of gabapentin is based on the ability to bind to the additional subunit of the alpha-2-delta voltage-dependent Ca2 + channels, which is located on the extracellular side of the channel. Strong binding at this site reduces calcium influx to nerve endings, leading to inhibition of the release of a number of neurotransmitters, including glutamate and substance P.
In the absence of neurotransmitters in the synaptic cleft, the impulse propagation to the next neuron is blocked [13, 23]. nine0045
Gabapentin can be taken with or without food. There is no need to measure serum concentrations to optimize treatment. The drug should be titrated starting at 300 mg per day and then increasing the dose by 300 mg per day to a target daily dose of 1800-3600 mg (the titration period to the maximum dose usually takes 12 days). The time between successive doses of the drug should not exceed 8 hours. Elderly patients may require dose adjustment, as they often have impaired renal function. The most common side effects include dizziness, drowsiness and ataxia. To avoid falls, the doctor needs to warn patients, especially the elderly, about the possibility of these side effects. Other characteristic side effects are nystagmus, diarrhea, headache, nausea, peripheral edema and asthenia are less common. nine0045
The time between successive doses of the drug should not exceed 8 hours. Elderly patients may require dose adjustment, as they often have impaired renal function. The most common side effects include dizziness, drowsiness and ataxia. To avoid falls, the doctor needs to warn patients, especially the elderly, about the possibility of these side effects. Other characteristic side effects are nystagmus, diarrhea, headache, nausea, peripheral edema and asthenia are less common. nine0045
Pregabalin (lyric) is currently positioned in all international and Russian guidelines for the treatment of neuropathic pain and painful diabetic polyneuropathy as a first-line drug [4, 32]. Pregabalin, like gabapentin, belongs to a class of drugs that have a high affinity for alpha-2-delta protein in the central nervous system. Pregabalin is a derivative of GABA and is essentially its analogue. In studies on the background of taking the drug, a decrease in the release of a number of neurotransmitters (including glutamate, norepinephrine and substance P) in hyperexcited neurons was demonstrated. This is believed to be caused by modulation of the function of the alpha-2-delta subunit of voltage-gated calcium channels. Pregabalin, by reducing the release of neurotransmitters, thus slows down the transmission of a nerve impulse to the next neuron, which results in a reduction in pain. It is important to note that pregabalin has an effect only under conditions of hyperexcitation of neurons in pathological conditions; its modulating action leads to the transition of neurons to the normal state [13, 18, 23]. nine0045
This is believed to be caused by modulation of the function of the alpha-2-delta subunit of voltage-gated calcium channels. Pregabalin, by reducing the release of neurotransmitters, thus slows down the transmission of a nerve impulse to the next neuron, which results in a reduction in pain. It is important to note that pregabalin has an effect only under conditions of hyperexcitation of neurons in pathological conditions; its modulating action leads to the transition of neurons to the normal state [13, 18, 23]. nine0045
Despite the similarities between pregabalin and gabapentin, the pharmacokinetic profile of pregabalin has some differences compared to the profile of gabapentin. Unlike gabapentin, pregabalin has a linear pharmacokinetics, which ensures the predictability of changes in plasma concentrations of the drug with increasing or decreasing doses. Pregabalin is rapidly absorbed into the blood and has a higher bioavailability (90%) compared to gabapentin (33-66%). As a result, the drug is effective in smaller doses, and the likelihood of side effects is much less. A meta-analysis of conducted randomized clinical trials of both drugs showed that the effectiveness of pregabalin is similar to that of gabapentin in reducing pain intensity, but at significantly lower dosages. It has been demonstrated that the maximum dose of gabapentin (3600 mg) is as effective as the average therapeutic dose of pregabalin - 450 mg (for pregabalin the maximum dose is 600 mg) [36]. In addition, pregabalin is characterized by a shorter time to reach maximum concentration with a long period of absorption. This circumstance allows the use of a two-time dose of the drug, in contrast to gabapentin, which is required to be taken 3 times a day. The time to reach a stable concentration of pregabalin is shorter, therefore, a shorter titration period is required, and the therapeutic concentration and, accordingly, the clinical effect are reached faster [1]. nine0045
A meta-analysis of conducted randomized clinical trials of both drugs showed that the effectiveness of pregabalin is similar to that of gabapentin in reducing pain intensity, but at significantly lower dosages. It has been demonstrated that the maximum dose of gabapentin (3600 mg) is as effective as the average therapeutic dose of pregabalin - 450 mg (for pregabalin the maximum dose is 600 mg) [36]. In addition, pregabalin is characterized by a shorter time to reach maximum concentration with a long period of absorption. This circumstance allows the use of a two-time dose of the drug, in contrast to gabapentin, which is required to be taken 3 times a day. The time to reach a stable concentration of pregabalin is shorter, therefore, a shorter titration period is required, and the therapeutic concentration and, accordingly, the clinical effect are reached faster [1]. nine0045
Pregabalin has been shown to be highly effective in neuropathic pain in clinical trials. In total, more than 10,000 patients were treated in them. The main studies on the effectiveness of pregabalin have been conducted in postherpetic neuralgia and painful diabetic neuropathy, since these diseases have been accepted by the US Food and Drug Administration - FDA - as standard models of neuropathic pain. All studies, 4 in postherpetic neuralgia, 5 in diabetic neuropathy, and 1 in these conditions, were randomized, double-blind, placebo-controlled, up to 13 weeks in duration. The dosage of pregabalin was flexible or fixed at 75 to 600 mg per day. In all studies without exception, at all doses, pregabalin showed a significantly higher efficacy compared to placebo, up to 60% or more; it not only reduced the intensity of pain, but also normalized sleep and improved the quality of life of patients. According to the results of a somewhat later meta-analysis, the effect of the drug was observed already by the end of the 2nd or the beginning of the 3rd day of therapy, continuing until the end of the study, and the number of responders with a 50% or more reduction in pain intensity was about 65%.
The main studies on the effectiveness of pregabalin have been conducted in postherpetic neuralgia and painful diabetic neuropathy, since these diseases have been accepted by the US Food and Drug Administration - FDA - as standard models of neuropathic pain. All studies, 4 in postherpetic neuralgia, 5 in diabetic neuropathy, and 1 in these conditions, were randomized, double-blind, placebo-controlled, up to 13 weeks in duration. The dosage of pregabalin was flexible or fixed at 75 to 600 mg per day. In all studies without exception, at all doses, pregabalin showed a significantly higher efficacy compared to placebo, up to 60% or more; it not only reduced the intensity of pain, but also normalized sleep and improved the quality of life of patients. According to the results of a somewhat later meta-analysis, the effect of the drug was observed already by the end of the 2nd or the beginning of the 3rd day of therapy, continuing until the end of the study, and the number of responders with a 50% or more reduction in pain intensity was about 65%.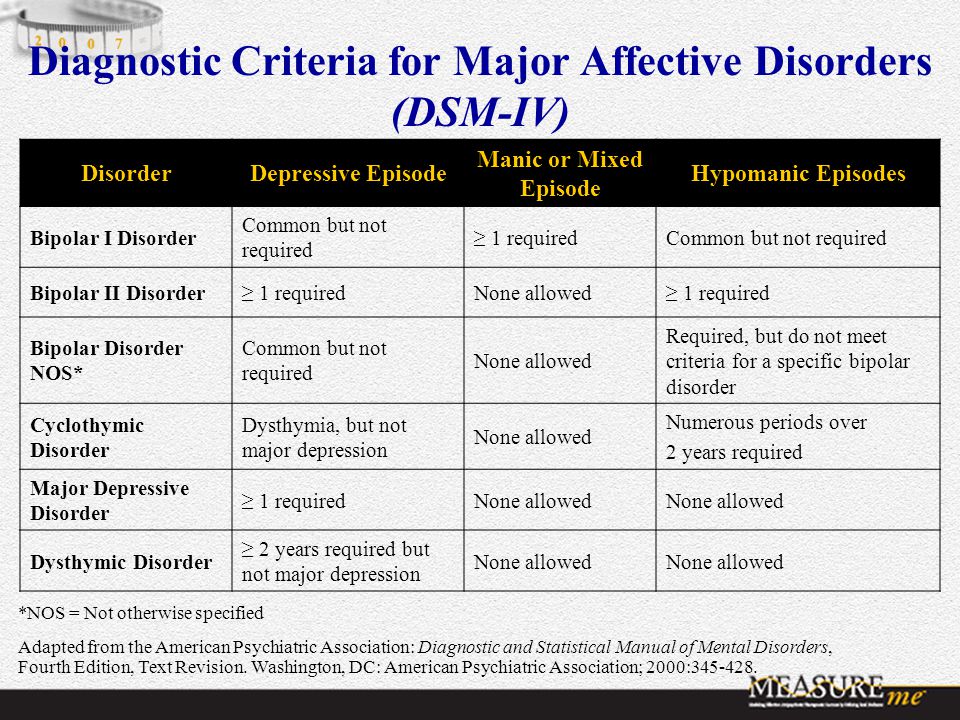 Some of the studies described above were continued in the open phase, the average duration of pregabalin use was 15 months, and the maximum duration was 3.5 years. These data allow us to speak about pregabalin as an effective and safe agent for long-term treatment of neuropathic pain [22, 25, 28, 29].
Some of the studies described above were continued in the open phase, the average duration of pregabalin use was 15 months, and the maximum duration was 3.5 years. These data allow us to speak about pregabalin as an effective and safe agent for long-term treatment of neuropathic pain [22, 25, 28, 29].
There is extensive post-marketing experience with pregabalin in the literature. Thus, in Germany, it was studied in the treatment of 10,300 patients with such forms of pathology as diabetic polyneuropathy, back pain with a neuropathic component, postherpetic and trigeminal neuralgia, alcoholic polyneuropathy, various other polyneuropathies and neuropathic pain due to a tumor. Significant reduction in pain intensity was noted as early as the 1st week of therapy. By the 6th week of treatment with pregabalin, pain intensity decreased by an average of 62%, while patients improved their sleep and mood [24]. nine0045
There is experience with the use of the drug in patients with severe, not relieved by other means, pain.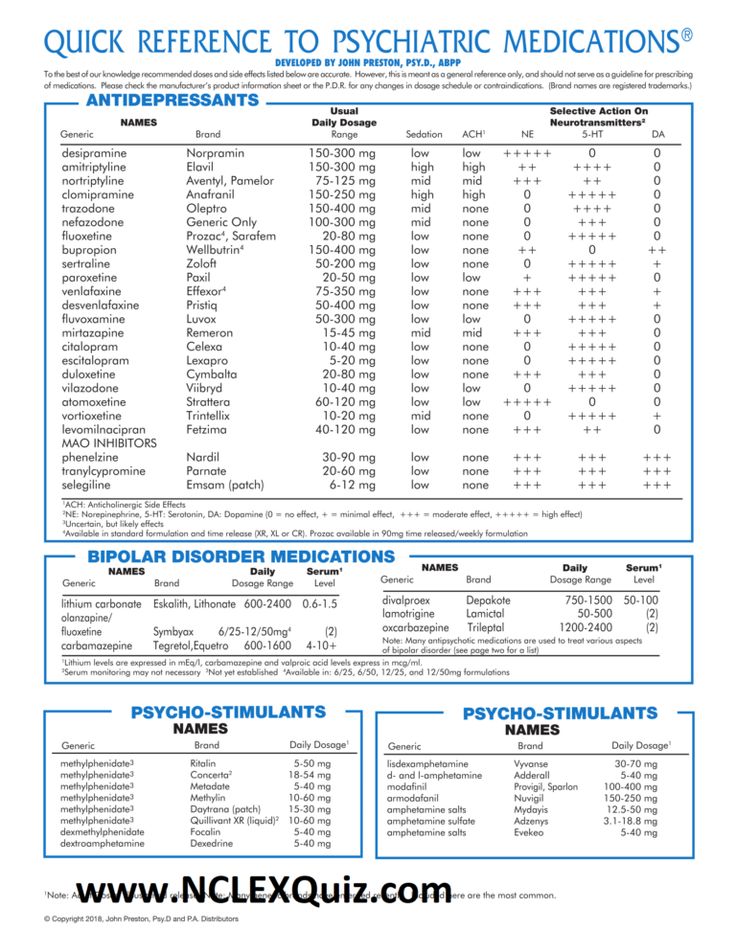 In one open-label study [24], in patients with pain refractory to gabapentin, tricyclic antidepressants, or other drugs, pregabalin significantly reduced visual analog scale pain and anxiety and sleep disturbances [25].
In one open-label study [24], in patients with pain refractory to gabapentin, tricyclic antidepressants, or other drugs, pregabalin significantly reduced visual analog scale pain and anxiety and sleep disturbances [25].
The drug was also studied in central neuropathic pain in patients with spinal cord injury in a 12-week randomized placebo-controlled trial. Against the background of pregabalin therapy, pain intensity in this group of patients decreased starting from the 1st week of therapy, and the effect persisted until the end of the course of treatment. In parallel with anesthesia, the use of the drug caused a persistent improvement in sleep. In another study, pregabalin was more effective than placebo in patients with central post-stroke pain [30, 34]. nine0045
As already mentioned, pregabalin is well tolerated. The most common side effects are dizziness and drowsiness. According to data from clinical studies, dizziness and drowsiness while taking pregabalin are transient in nature, occurring after 1–2 days of administration and stopping at 2–4 weeks of therapy [25].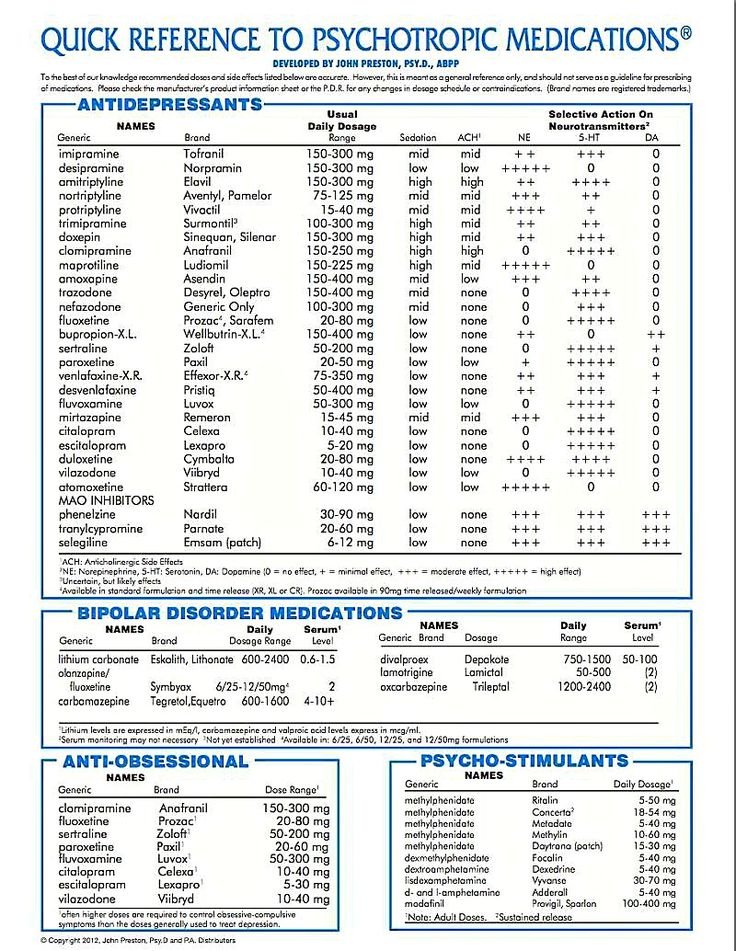 Other side effects include: ataxia, dysarthria, impaired attention, euphoria, irritability, diplopia, dry mouth, fatigue, edema, transient weight gain. Pregabalin is not metabolized in the liver and does not bind to plasma proteins, so the risk of interaction with other drugs is minimal. nine0045
Other side effects include: ataxia, dysarthria, impaired attention, euphoria, irritability, diplopia, dry mouth, fatigue, edema, transient weight gain. Pregabalin is not metabolized in the liver and does not bind to plasma proteins, so the risk of interaction with other drugs is minimal. nine0045
The range of daily therapeutic doses of pregabalin is 300-600 mg divided into 2 doses. The drug can be taken regardless of food intake. In Russia, the drug is sold in capsules of 25, 75, 150 and 300 mg. In the treatment of neuropathic pain, the recommended starting dose is 150 mg per day. Depending on the effect and tolerability, the dose can be increased to 300 mg per day after 3-7 days. If necessary, you can increase the dose to a maximum (600 mg per day) after another 7 days. In patients with impaired renal function, treatment is recommended to be started and carried out at lower doses. According to the experience gained in clinical studies, in case of discontinuation of the drug, it is recommended to reduce the dose gradually over a week.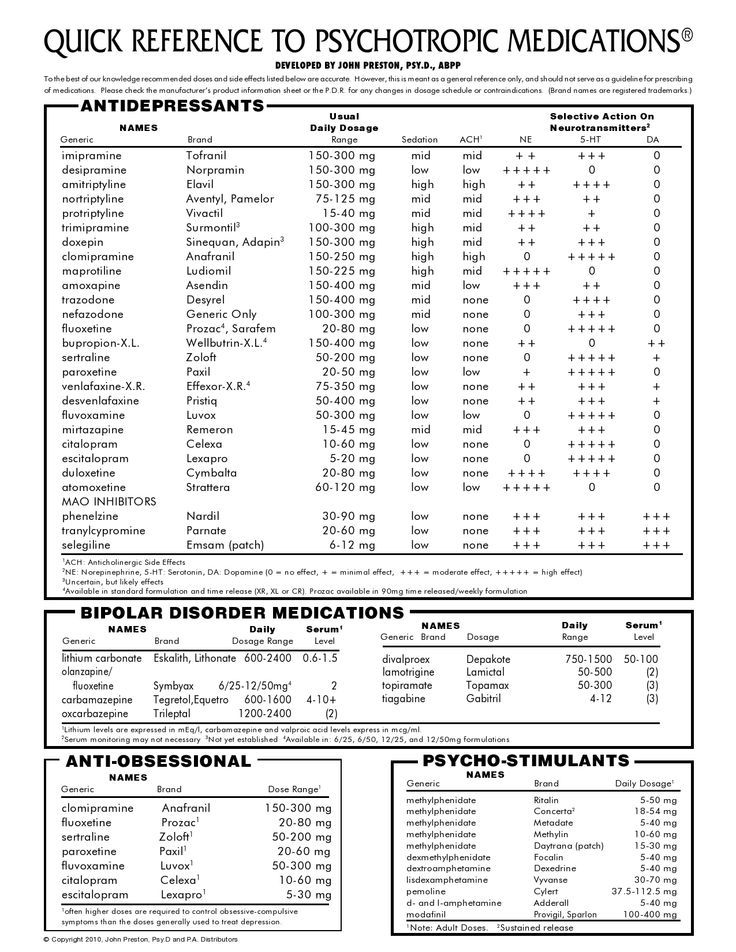 nine0045
nine0045
Other anticonvulsants for the treatment of neuropathic pain . Lamotrigine (lamiktal, convulsan, lamolep, lamictor) is a second-generation anticonvulsant and is registered for the treatment of epilepsy and bipolar disorder. In neuropathic pain, the effectiveness of the drug has been shown in the painful form of diabetic neuropathy, spinal injury, trigeminal neuralgia, central post-stroke pain, however, in some other studies, conflicting results have been obtained. Topiramate (Topamax, Topiromax, Maxitopyr, etc.) has been shown to be effective in reducing pain in diabetic neuropathy and spinal injury, but in other studies the effect was insufficient. Lacosamide (Vimpat), which at the stage of clinical trials instilled certain hopes for effectiveness, did not live up to expectations in relation to the treatment of pain syndromes. The absence of neuropathic pain in the official list of indications for all three drugs should also be taken into account [18, 20, 38, 39].
Anticonvulsant use for migraine, anxiety disorders and fibromyalgia
The use of anticonvulsants for migraine is only considered in the context of prophylactic treatment of attacks. According to the recommendations of the American Academy of Neurology, in order to start preventive therapy, there should be at least 2 such attacks per month, or the attacks should be severe, refractory to treatment and leading to maladjustment of the patient [31]. Among the possible mechanisms of the preventive action of anticonvulsants, their influence on the GABA-glutamate system or on intracellular mediators (ATP, c-AMP, protein kinase C, etc.) by affecting the calcium or sodium channels of the synapse is considered. In the United States, topiramate and valproate are registered by the FDA for the prevention of migraine, but in Russia these indications do not appear [31, 37]. nine0045
The hypothetical possibility of using most anticonvulsants in anxiety disorders is associated with an effect on GABA transmission. The most proven anti-anxiety effects are tiagabine, gabapentin, and pregabalin, with the latter officially registered in both the US and Russia for the treatment of generalized anxiety disorder. The mechanism of the anxiolytic action of pregabalin is associated with presynaptic inhibition of the release of excitatory neurotransmitters, and more than 7 randomized placebo-controlled trials have been published as evidence of its effectiveness [35]. nine0045
The most proven anti-anxiety effects are tiagabine, gabapentin, and pregabalin, with the latter officially registered in both the US and Russia for the treatment of generalized anxiety disorder. The mechanism of the anxiolytic action of pregabalin is associated with presynaptic inhibition of the release of excitatory neurotransmitters, and more than 7 randomized placebo-controlled trials have been published as evidence of its effectiveness [35]. nine0045
One disease that is also to some extent treatable with anticonvulsants is fibromyalgia, or as it is also called, the syndrome of individual hypersensitivity to pain, a disease that is most difficult to treat. Of the currently known anticonvulsants in his treatment, only pregabalin has proven its effectiveness, studied both in short, 8-week studies and in long-term studies lasting more than a year. The presence of sufficient evidence allowed the European League against Rheumatism to recommend the inclusion of this drug in the complex therapy of fibromyalgia.![]() The mechanism of action of pregabalin in fibromyalgia is associated with the effect on the processes of central sensitization [12, 19].
The mechanism of action of pregabalin in fibromyalgia is associated with the effect on the processes of central sensitization [12, 19].
Thus, the use of anticonvulsants today is possible not only for epilepsy, but also for many other diseases and syndromes. Among these forms of clinical pathology, neuropathic pain stands out, in which anticonvulsants are especially well studied. The first-line drugs for such pain are pregabalin and gabapentin; with trigeminal neuralgia - carbamazepine. Topiramate and valproate are registered in the United States for the prevention of migraine attacks, and pregabalin is increasingly being used to treat generalized anxiety disorder. Pregabalin is also the first and only anticonvulsant in the United States and Russia to date registered for the treatment of such a difficult condition as fibromyalgia. Conducted in recent years in the world of research on the latest anticonvulsants allow us to hope for further expansion of the scope of these drugs in a variety of pain syndromes.