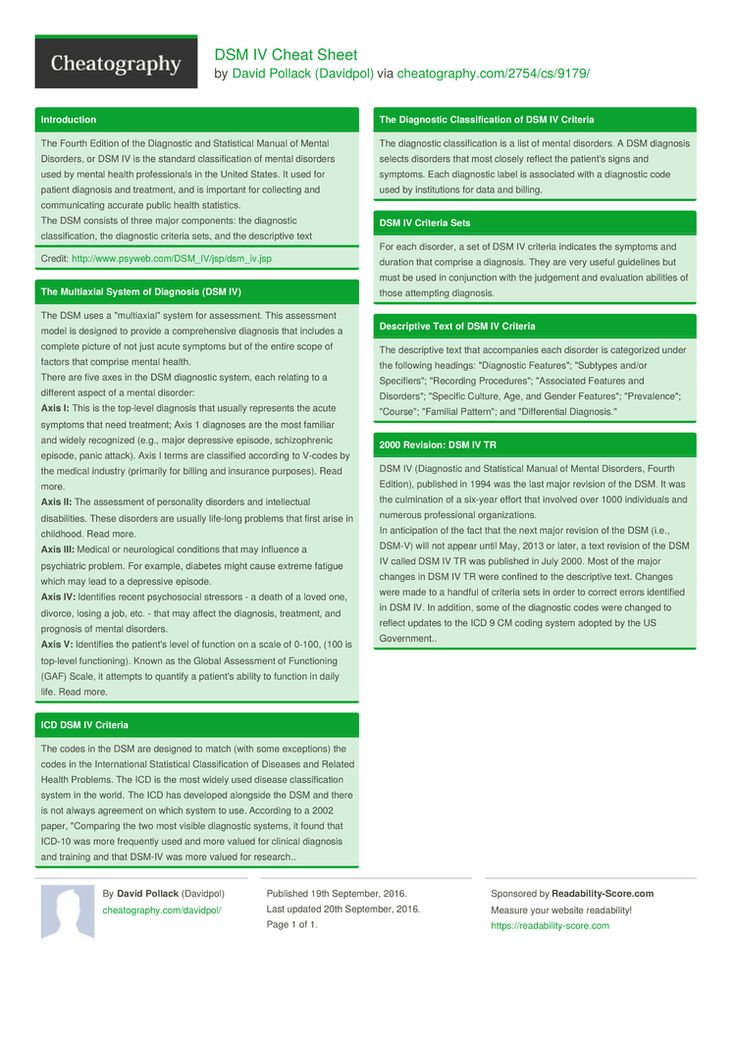
Strattera in pregnancy
What You Need to Know
Is Atomoxetine Safe to Take While Pregnant?
Atomoxetine is a prescription medication classified as a norepinephrine reuptake inhibitor. It’s sold under the trade name Strattera and is also available as a generic medication. Atomoxetine is prescribed to treat symptoms of attention deficit hyperactivity disorder (ADHD). It’s prescribed to children, teens, and adults, but not usually children under the age of six. Atomoxetine has some benefits compared to other treatments for ADHD, such as a lower abuse potential. It’s also possible to stop using atomoxetine without experiencing withdrawal symptoms. Common side effects of atomoxetine include nausea, dry mouth, loss of appetite, insomnia, fatigue, and headache. These are fairly common side effects with most ADHD medications. There is a black box warning about the risk of suicidal thoughts and behaviors with the use of atomoxetine as well. As with most ADHD medicines, atomoxetine should be used in conjunction with a full treatment plan that includes social and psychological treatments.
Is atomoxetine safe to take while pregnant? Most ADHD medicines, including atomoxetine, are classified as category C drugs by the FDA. This classification means that there is not be definitive research that either links atomoxetine to harm during pregnancy or rules it out. A doctor will usually weigh the benefits and the potential risks of the medication and then make a decision as to whether the medicine should be discontinued or prescribed during pregnancy. Ideally, this conversation happens before a woman becomes pregnant; however, that doesn’t always happen and may also occur during her pregnancy.
In some animal studies, ADHD medicines have resulted in an increased risk of spina bifida and skeletal abnormalities. In some cases, the use of ADHD drugs during pregnancy has been linked to birth defects and even death of the fetus.
When a doctor is trying to determine whether or not a pregnant patient should continue taking an ADHD medication, some factors should be part of that decision –the specifics of her ADHD diagnosis, her level of functionality without medicine, how long she’s been taking medicine, and what the demands are in her work life that could be affected by not taking ADHD medication. Some doctors may recommend not using medication during the first trimester but then using it in the second and third trimester. This is possible because most of the fetal development happens during the first trimester.
Some doctors may recommend not using medication during the first trimester but then using it in the second and third trimester. This is possible because most of the fetal development happens during the first trimester.
If a pregnant woman decides she absolutely doesn’t want to take atomoxetine during pregnancy, she and her healthcare provider may explore different options. However, it’s important to never stop taking a prescription medication without speaking with a doctor first. If a doctor does say that it’s okay to stop using atomoxetine, natural alternatives may be suggested. For example, certain vitamins, such as the use of a B-complex, may be helpful. Many adults with ADHD find talk therapy like cognitive-behavioral therapy to be helpful. Relaxation techniques, exercise, and meditation may all provide some benefits. Mindfulness psychotherapy, neurofeedback, and acupuncture are all also alternative ADHD treatments that some people recommend. All of these options should be openly and honestly discussed with your healthcare provider, who will likely have suggestions as well.
To learn more about addiction treatment and recovery, including during pregnancy, contact our caring, compassionate professionals at The Recovery Village. We believe in the power of recovery for everyone.
Read Previous
How Long Is Strattera In Your System
Read Next
Mixing Strattera & Alcohol
Medical Disclaimer
The Recovery Village aims to improve the quality of life for people struggling with substance use or mental health disorder with fact-based content about the nature of behavioral health conditions, treatment options and their related outcomes. We publish material that is researched, cited, edited and reviewed by licensed medical professionals. The information we provide is not intended to be a substitute for professional medical advice, diagnosis or treatment. It should not be used in place of the advice of your physician or other qualified healthcare providers.
Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy
- Journal List
- Clin Epidemiol
- v.
 7; 2015
7; 2015 - PMC4317061
Clin Epidemiol. 2015; 7: 139–147.
Published online 2015 Jan 29. doi: 10.2147/CLEP.S72906
,1,2,2,3,4,4,4,5,6,7 and 1
Author information Copyright and License information Disclaimer
- Supplementary Materials
Objective
To determine if prenatal exposure to methylphenidate (MPH) or atomoxetine (ATX) increases the risk of adverse pregnancy outcomes in women with attention deficit/hyperactivity disorder (ADHD).
Materials and methods
This was a population-based cohort study of all pregnancies in Denmark from 1997 to 2008. Information on use of ADHD medication, ADHD diagnosis, and pregnancy outcomes was obtained from nationwide registers.
Results
We identified 989,932 pregnancies, in which 186 (0.02%) women used MPH/ATX and 275 (0.03%) women had been diagnosed with ADHD but who did not take MPH/ATX. Our reference pregnancies had no exposure to MPH/ATX and no ADHD diagnosis. Exposure to MPH/ATX was associated with an increased risk of spontaneous abortion (SA; ie, death of an embryo or fetus in the first 22 weeks of gestation) (adjusted relative risk [aRR] 1.55, 95% confidence interval [CI] 1.03–2.36). The risk of SA was also increased in pregnancies where the mother had ADHD but did not use MPH/ATX (aRR 1.56, 95% CI 1.11–2.20). The aRR of Apgar scores <10 was increased among exposed women (aRR 2.06, 95% CI 1.11–3.82) but not among unexposed women with ADHD (aRR 0.99, 95% CI 0.48–2.05).
Conclusion
MPH/ATX was associated with a higher risk of SA, but our study indicated that it may at least partly be explained by confounding by indication. Treatment with MPH/ATX was however associated with low Apgar scores <10, an association not found among women with ADHD who did not use MPH/ATX.
Keywords: attention deficit/hyperactivity disorder, ADHD, methylphenidate, atomoxetine, pregnancy outcomes
The central stimulants methylphenidate (MPH) and atomoxetine (ATX) are the most commonly used drugs in the treatment of attention deficit/hyperactivity disorder (ADHD) in Western countries.1,2 The number of children diagnosed with ADHD has risen dramatically over the past two decades, and some remain on treatment into adulthood.3–7 The use of ADHD medication among women of fertile age has increased almost 100-fold during the past 12 years in Denmark, but little is known about the safety of these drugs during pregnancy.2,8,9
Animal studies have shown that high doses of MPH given during pregnancy are associated with an increased risk of malformations in the offspring. Decreased food intake was found among exposed animals, which is consistent with the anorexigenic effect of this medication. 10–12 Human studies and case reports of the effects of MPH on pregnancy outcomes have found no major adverse outcomes, but most of these studies are insufficiently powered to identify even strong fetotoxic effects.13–16 A recent meta-analysis of four cohort studies revealed a total of four malformations among 180 first-trimester-exposed children, which did not exceed the expected number.17 A Danish study of 222 pregnancies exposed to MPH during the first trimester showed no increased risk of major malformations.18 A recent Danish study found a two-fold-increased risk of spontaneous abortion (SA) among women who used ADHD medication (MPH, modafinil, or ATX) during pregnancy compared with unexposed pregnancies.9 Therefore, these studies suggest that ADHD medication may be associated with adverse birth outcomes, and warrant further studies of other pregnancy outcomes, such as birth weight, gestational age (GA), and Apgar score.
10–12 Human studies and case reports of the effects of MPH on pregnancy outcomes have found no major adverse outcomes, but most of these studies are insufficiently powered to identify even strong fetotoxic effects.13–16 A recent meta-analysis of four cohort studies revealed a total of four malformations among 180 first-trimester-exposed children, which did not exceed the expected number.17 A Danish study of 222 pregnancies exposed to MPH during the first trimester showed no increased risk of major malformations.18 A recent Danish study found a two-fold-increased risk of spontaneous abortion (SA) among women who used ADHD medication (MPH, modafinil, or ATX) during pregnancy compared with unexposed pregnancies.9 Therefore, these studies suggest that ADHD medication may be associated with adverse birth outcomes, and warrant further studies of other pregnancy outcomes, such as birth weight, gestational age (GA), and Apgar score.
In pharmacoepidemiological studies, it is always a challenge to disentangle the effects of the medication from the effects of the underlying disease (confounding by indication). We aimed to examine the effects of the underlying disease (ADHD) and its treatment (MPH and ATX) on pregnancy outcomes in a nationwide cohort study from Denmark.
Study population
The cohort comprised all clinically recognized pregnancies in Denmark with estimated time of conception and an observed pregnancy outcome in the period from February 1, 1997 to December 31, 2008. Information was obtained from Danish administrative health registries, including the Danish Medical Birth Registry (MBR) and the Danish National Hospital Discharge Register (NHDR), and data were linked through the personal identification number unique to each person with a permanent address in Denmark. When investigating birth outcomes, we included only singleton births.
Central stimulant exposure
In Denmark, ADHD medication can only be purchased in authorized pharmacies with a prescription from a physician, usually a psychiatrist. We obtained information on all redeemed prescriptions in Denmark from the Registry of Medicinal Product Statistics, and included information on MPH/ATX on all redeemed prescriptions from January 1, 1997 to December 31, 2008. The pregnancy period was defined from the estimated date of conception based on GA to the date of the outcome. For SA, the exposure window spanned from 30 days before the estimated day of conception until the day prior to abortion or gestational age 152 days (22 completed weeks). For live births and stillbirths, the exposure window spanned from 30 days before the estimated day of conception until the day prior to birth. MPH was defined according to the Anatomical Therapeutic Chemical (ATC) code N06BA04 and ATX with ATC code N06BA09.
We obtained information on all redeemed prescriptions in Denmark from the Registry of Medicinal Product Statistics, and included information on MPH/ATX on all redeemed prescriptions from January 1, 1997 to December 31, 2008. The pregnancy period was defined from the estimated date of conception based on GA to the date of the outcome. For SA, the exposure window spanned from 30 days before the estimated day of conception until the day prior to abortion or gestational age 152 days (22 completed weeks). For live births and stillbirths, the exposure window spanned from 30 days before the estimated day of conception until the day prior to birth. MPH was defined according to the Anatomical Therapeutic Chemical (ATC) code N06BA04 and ATX with ATC code N06BA09.
Maternal ADHD diagnosis
We received information on hospital contact for psychiatric illnesses from the Danish Psychiatric Central Registry (DPCR). Women were coded as having ADHD if they were recorded with an International Classification of Diseases (ICD)-10 code F90 in the DPCR from the date of conception until the end of the index pregnancy.
Women who had redeemed a prescription for MPH/ATX within the exposure window were defined as “the exposed ADHD cohort”. Women with an ADHD diagnosis who had not redeemed a prescription for MPH/ATX within the exposure window were defined as “the unexposed ADHD cohort”.
Pregnancy outcomes
The following codes were used: abortions (ICD-10 O02.0–O06.9) were coded as spontaneous (ICD-10 O02.0–O03.9), induced (ICD-10 O04.0–O05.2 and O05.5–O06.9), or induced due to fetal disease (ICD-10 O05.3 and O05.4). Molar or ectopic pregnancies (ICD-10 O00.0–O01.9) were excluded from the main analyses. Furthermore, failed induced abortions (ICD-10 O07) were disregarded, as we assumed that a failed induced abortion would be followed by successful induced abortion, a stillbirth, or a live birth.
Live births and stillbirths were identified in the MBR. Stillbirth was defined as an intrauterine death occurring from 22 completed weeks of gestations and onwards. The Danish lower boundary of stillbirth shifted from 28 weeks to 22 weeks in 2004. We therefore recoded all SAs as stillbirths if they occurred from gestational week 22 to gestational week 28, regardless of calendar year. The MBR provided information on Apgar score at 5 minutes, birth weight, gestational age, and neonatal death (defined as death within the first 28 days after birth). Low birth weight was defined as a weight below 2,500 g. Preterm birth was defined as a birth that took place after less than 37 weeks of pregnancy.
We therefore recoded all SAs as stillbirths if they occurred from gestational week 22 to gestational week 28, regardless of calendar year. The MBR provided information on Apgar score at 5 minutes, birth weight, gestational age, and neonatal death (defined as death within the first 28 days after birth). Low birth weight was defined as a weight below 2,500 g. Preterm birth was defined as a birth that took place after less than 37 weeks of pregnancy.
We defined those small for GA (SGA) as children with a weight below the 10th percentile for the gestational birth week, and we used the estimated mean birth weight per GA week using the population of children with nonimputed GA. Missing information on GA for abortions, stillbirths, and live births was replaced by the median over the nonmissing values from each category respectively.
We obtained information on major congenital malformations (ICD-10 Q0–Q99, D215, D821, D1810, P350, P351, and P371, except Q25.0 for GA <37 weeks) through the NHDR. For abortions, the NHDR included ICD-10 codes with information on GA. In cases of abortion before 12 weeks, GA is normally based on the last menstrual period, and for late gestations ultrasound scans are used to determine GA. For stillbirths or live births, the MBR contained information on GA at birth, normally based on ultrasound estimates combined with the last menstrual period.
For abortions, the NHDR included ICD-10 codes with information on GA. In cases of abortion before 12 weeks, GA is normally based on the last menstrual period, and for late gestations ultrasound scans are used to determine GA. For stillbirths or live births, the MBR contained information on GA at birth, normally based on ultrasound estimates combined with the last menstrual period.
We coded pregnancies hierarchically to avoid misclassification due to repeated contacts. Any stillbirth or live birth resulted in recoding of any other end point in the index pregnancy period. Any coded SA occurring after the diagnosis of an induced abortion was coded as an SA, because the diagnosis of an induced abortion may be coded on outpatient visits days or weeks prior to the surgical procedure.
Covariates
Information on the following variables was obtained from Statistics Denmark and subsequently coded as shown in parentheses: maternal age at conception (continuous), cohabitation at the time of conception (yes/no), income at the time of conception (< median, ≥ median), and education at the time of conception (<10, 10–12, >12 years). The woman’s parity was categorized as either nulliparous or multiparous. From the Registry of Medicinal Product Statistics, we obtained information on the use of other drugs redeemed during the pregnancy period: antipsychotics (yes/no) (ATC code N05A), antiepileptic drugs (yes/no) (ATC code N03A) and antidepressants (yes/no) (ATC code N06A). These drugs were combined into a composite variable for any antipsychotic, antiepileptic, or antidepressant drug use during pregnancy (yes/no). From the DPCR, we obtained information on history of psychiatric comorbidity, defined as having the following diagnosis ever or ongoing: severe mental disorder (yes/no) (ICD-8 296.1–296.8, 298.1, and 295; ICD-10 F20, F30, and F31), depression (yes/no) (ICD-8 296.0, 298.0, or 300.4; ICD-10 F32 and F33), misuse (yes/no) (ICD-10 F10–F19), and epilepsy (yes/no) (ICD-8 345; ICD-10 G40 and G41). These diagnoses were combined into a composite variable for comorbidity (yes/no). Information on maternal smoking during pregnancy (yes/no) was only available if the index pregnancy resulted in a live or stillborn child.
The woman’s parity was categorized as either nulliparous or multiparous. From the Registry of Medicinal Product Statistics, we obtained information on the use of other drugs redeemed during the pregnancy period: antipsychotics (yes/no) (ATC code N05A), antiepileptic drugs (yes/no) (ATC code N03A) and antidepressants (yes/no) (ATC code N06A). These drugs were combined into a composite variable for any antipsychotic, antiepileptic, or antidepressant drug use during pregnancy (yes/no). From the DPCR, we obtained information on history of psychiatric comorbidity, defined as having the following diagnosis ever or ongoing: severe mental disorder (yes/no) (ICD-8 296.1–296.8, 298.1, and 295; ICD-10 F20, F30, and F31), depression (yes/no) (ICD-8 296.0, 298.0, or 300.4; ICD-10 F32 and F33), misuse (yes/no) (ICD-10 F10–F19), and epilepsy (yes/no) (ICD-8 345; ICD-10 G40 and G41). These diagnoses were combined into a composite variable for comorbidity (yes/no). Information on maternal smoking during pregnancy (yes/no) was only available if the index pregnancy resulted in a live or stillborn child.
Statistics
Relative risks (RRs) for SA, preterm birth, SGA, low birth weight, and Apgar score <10 were estimated by using binominal regression with robust variance estimation to allow for correlations of pregnancy outcomes in each woman. RRs for SA were adjusted for maternal age, education, cohabitation, comorbidity, and comedication; according to a propensity score-adjustment simulation, this seemed reasonable to do, even though the rule of thumb of a minimum of ten events per parameter was not satisfied (see Supplementary materials). We did not perform RR analysis when fewer than five exposed events were observed; this is indicated with “NA” (not applicable) in . We used linear regression to study the associations between exposure to ADHD medication and birth weight and GA at birth, respectively. Robust variance estimation was used in the case of birth weight, while confidence intervals (CIs) for GA were bootstrapped based on 1,000 replications, due to departures from the normality assumption. 19 Mean differences for birth weight and GA were adjusted for maternal age, smoking, and parity. Analyses were performed using Stata 12 (StataCorp, USA).
19 Mean differences for birth weight and GA were adjusted for maternal age, smoking, and parity. Analyses were performed using Stata 12 (StataCorp, USA).
Table 3
Risk estimates for pregnancy and birth outcomes according to ADHD medication and ADHD diagnosis during pregnancy compared with pregnant women with no ADHD medication use and no ADHD diagnosis
| Outcome | Women who used ADHD medication during pregnancy Association (95% CI) | Women who did not use ADHD medication during pregnancy but had an ADHD diagnosis Association (95% CI) |
|---|---|---|
| Spontaneous abortion | ||
| Crude relative risk | 1. 87 (1.26–2.78) 87 (1.26–2.78) | 1.42 (1.02–1.97) |
| Adjusted relative riska | 1.55 (1.03–2.36) | 1.56 (1.11–2.20) |
| Birth weight | ||
| Crude mean difference | −0.13 (−145 to 144) | −235 (−365 to −105) |
| Adjusted mean differenceb | 76 (−69 to 221) | −91 (−226 to 45) |
| GA | ||
| Crude mean difference | −0. 64 (−4.02 to 2.74)c 64 (−4.02 to 2.74)c | −2.77 (−6.03 to 0.49)b |
| Adjusted mean differencea | −0.32 (−3.86 to 3.21)b | −2.67 (−6.04 to 0.71)b |
| Preterm birth | ||
| Crude relative risk | NAd | 1.95 (1.07–3.54) |
| Adjusted relative riska | NAc | 1.82 (1.01–3.29) |
| SGA | ||
| Crude relative risk | NAc | 1. 82 (1.11–2.99) 82 (1.11–2.99) |
| Adjusted relative riska | NAc | 1.19 (0.73–1.96) |
| Low birth weighte | ||
| Crude relative risk | NAc | 2.24 (1.17–4.28) |
| Adjusted relative riska | NAc | 1.79 (0.94–3.38) |
| Apgar score <10 | ||
| Crude relative risk | 2. 04 (1.10–3.77) 04 (1.10–3.77) | 1.06 (0.54–2.08) |
| Adjusted relative riska | 2.06 (1.11–3.82) | 0.99 (0.48–2.05) |
Open in a separate window
Notes:
aAdjusted for maternal age, education, cohabitation, comorbidity (history of severe mental illness, depression, misuse, or epilepsy), and comedication (reimbursed prescription for antidepressants, antipsychotics, or antiepileptics during pregnancy). According to a propensity score-adjustment simulation, this seemed reasonable, even though the rule of thumb of a minimum of ten events per parameter was not satisfied
badjusted for maternal age, smoking, and parity
cbootstrap CI based on 1,000 simulations
dNA indicates that relative risk analysis was not performed, due to fewer than five events
edefined as <2,500 g compared to ≥2,500 g.
Abbreviations: ADHD, attention deficit/hyperactivity disorder; CI, confidence interval; SGA, small for gestational age; NA, not applicable.
The study population consisted of 989,932 pregnant women, of whom 186 (0.02%) used MPH and/or ATX (the exposed ADHD cohort) and 275 (0.03%) who had a history of an ADHD diagnosis but received no treatment with MPH/ATX (the unexposed ADHD cohort) during pregnancy. The reference group consisted of women without MPH/ATX use and no ADHD diagnosis. In the exposed ADHD cohort, 67 (35%) had a hospital diagnosis of ADHD, 166 (89%) used MPH, 18 (9.7%) used ATX, and two (1.1%) used both ATX and MPH.
Compared with the reference group, women in the exposed ADHD cohort and women in the unexposed ADHD cohort were more likely to be younger, single, have less education, low income, take comedication, have comorbidity, smoke, and be nulliparous ().
Table 1
Characteristics of the 989,932 pregnant women in the cohort according to ADHD-medication use and ADHD hospital contact
| Variable | Women who used ADHD medication during pregnancy (n=186) | Women who did not use ADHD medication during pregnancy, but had an ADHD diagnosis (n=275) | Women who did not use ADHD medication during pregnancy and did not have an ADHD diagnosis (n=989,471) |
|---|---|---|---|
| Maternal age, mean (SD) | 26. 5 (7.2) 5 (7.2) | 21.3 (4.4) | 30.2 (5.5) |
| Missing, n (%) | 0 | 0 | 47 (<0.1) |
| Cohabitation, n (%) | |||
| Yes | 76 (40.9) | 122 (44.4) | 755,701 (76.4) |
| No | 110 (59.1) | 153 (55. 6) 6) | 218,635 (22.1) |
| Missing | 0 | 0 | 15,135 (1.5) |
| Income, n (%) | |||
| Low, <50% | 130 (69.9) | 248 (90.2) | 493,063 (49.8) |
| High, ≥50% | 56 (30.1) | 27 (9.8) | 490,169 (49.5) |
| Missing | 0 | 0 | 6,239 (0. 6) 6) |
| Education, n (%) | |||
| <10 years | 57 (30.6) | 146 (53.1) | 108,639 (11.0) |
| 10–12 years | 72 (38.7) | 95 (34.5) | 305,868 (30.9) |
| >12 years | 44 (23.7) | 10 (3.6) | 539,746 (54.5) |
| Missing | 13 (7. 0) 0) | 24 (8.7) | 35,218 (3.6) |
| Comedication, n (%) | |||
| Antipsychotics | 17 (9.1) | 25 (9.1) | 3,096 (0.3) |
| Antidepressants | 57 (30.6) | 35 (12.7) | 21,700 (2.2) |
| Antiepileptics | 7 (3.8) | 10 (3. 6) 6) | 4,637 (0.5) |
| History of comorbid conditions, n (%) | |||
| Severe mental illness | 3 (1.6) | 17 (6.2) | 2,696 (0.3) |
| Depression | 27 (14.5) | 48 (17.5) | 13,382 (1.4) |
| Misuse | 18 (9.7) | 46 (16.7) | 5,498 (0. 6) 6) |
| Epilepsy | 6 (3.3) | 17 (6.2) | 11,552 (1.2) |
| Parity, n (%)a | |||
| 0 | 26 (49.1) | 71 (69.6) | 298,089 (43.0) |
| ≥1 | 27 (50.9) | 31 (30.4) | 395,030 (56.9) |
| Missing | 0 | 0 | 554 (0. 1) 1) |
| Maternal smoking, n (%)a | |||
| Yes | 22 (41.5) | 64 (62.7) | 127,523 (18.4) |
| No | 30 (56.6) | 30 (29.4) | 533,314 (76.9) |
| Missing | 1 (1.9) | 8 (7.8) | 32,836 (4.7) |
Open in a separate window
Note:
aOnly information on pregnancies resulting in live births – 53, 102, and 693,673, respectively.
Abbreviations: ADHD, attention deficit/hyperactivity disorder; SD, standard deviation.
Congenital abnormalities occurred in two children (3.8%) from the exposed ADHD cohort, in seven children (6.9%) in the unexposed ADHD cohort, and in 39,557 children (5.7%) in the reference group ().
Table 2
Pregnancy and birth outcomes according to ADHD medication use and ADHD hospital contact
| Outcome | Women who used ADHD medication during pregnancy (n=186) | Women who did not use ADHD medication during pregnancy, but had an ADHD diagnosis (n=275) | Women who did not use ADHD medication during pregnancy and did not have an ADHD diagnosis (n=989,471) |
|---|---|---|---|
| Live birth, n (%) | 53 (28. 5) 5) | 102 (37.1) | 693,673 (70.1) |
| Stillbirth | 0 | 1 (0.4) | 3,517 (0.4) |
| Abortion | 133 (71.5) | 172 (62.5) | 292,296 (29.5) |
| Spontaneous abortion | 18 (9.7) | 26 (9.5) | 114,672 (11.6) |
| Induced abortion | 115 (61. 8) 8) | 146 (53.1) | 177,624 (18.0) |
| Birth weight, g, mean (SD)a | 3,535.4 (525.9) | 3,300.2 (615.8) | 3,535.5 (569.7) |
| Birth weight, n (%)a | |||
| <2,500 g | 1 (1.9) | 8 (7.8) | 23,691 (3.4) |
| 2,500–4,500 g | 48 (96. 2) 2) | 93 (91.2) | 629,644 (90.8) |
| >4,500 g | 1 (1.9) | 1 (1.0) | 23,259 (3.4) |
| Missing | 0 | 0 | 17,079 (2.5) |
| GA, mean (SD)a | 277.5 (12.3) | 275.4 (15.7) | 278.1 (13.0) |
| Missing (imputed) | 0 | 0 | 14,076 (2. 0) 0) |
| Preterm birth, n (%)a | 3 (5.7) | 10 (9.8) | 34,211 (4.9) |
| SGA, n (%)a | 4 (7.5) | 18 (17.6) | 65,499 (9.4) |
| Apgar score, n (%)a | |||
| <10 | 8 (15.1) | 8 (7.8) | 50,184 (7.2) |
| 10 | 45 (84. 9) 9) | 94 (92.2) | 626,672 (90.3) |
| Missing | 0 | 0 | 16,817 (2.4) |
| Congenital abnormalities, n (%)a | 2 (3.8) | 7 (6.9) | 39,557 (5.7) |
| Neonatal death, n (%)a | 0 | 0 | 1,829 (0. 3) 3) |
Open in a separate window
Note:
aOnly information on pregnancies resulting in live births – 53, 102, and 693,673, respectively.
Abbreviations: ADHD, attention deficit/hyperactivity disorder; SD, standard deviation; SGA, small for gestational age.
Compared to women in the reference cohort, the risk of SA was 55% higher for women in the exposed ADHD cohort ((adjusted relative risk [aRR] 1.55, 95% CI 1.03–2.36) and 56% higher for women in the unexposed ADHD cohort (aRR 1.56, 95% CI 1.11–2.20) after adjustment for maternal age, education, cohabitation, comorbidity, and comedication ().
Children of women in the unexposed ADHD cohort tended to have a lower mean birth weight than both the reference group of children born to women without an ADHD diagnosis who did not use MPH/ATX and the children born to women who used MPH/ATX, but the difference decreased and was not statistically significant after adjusting for maternal age, smoking, and parity (). The children of women in the exposed and unexposed ADHD cohorts also tended to have a shorter GA than the reference group, albeit the difference did not reach statistical significance. We found three preterm births among children in the exposed ADHD cohort and ten preterm births among children in the unexposed ADHD cohort. This number was too small to allow calculation of crude RR (cRR) for the exposed ADHD cohort or adjustment for confounding in the unexposed ADHD cohort. However, the unexposed ADHD cohort showed a significantly increased cRR of preterm birth, SGA, and low birth weight (<2,500 g) compared with the reference pregnancies, but after adjustment for smoking, age, and parity, only the aRR for preterm birth was statistically significant. The aRR of Apgar score <10 at 5 minutes was significantly increased in the exposed ADHD cohort, but not in the unexposed ADHD cohort ().
The children of women in the exposed and unexposed ADHD cohorts also tended to have a shorter GA than the reference group, albeit the difference did not reach statistical significance. We found three preterm births among children in the exposed ADHD cohort and ten preterm births among children in the unexposed ADHD cohort. This number was too small to allow calculation of crude RR (cRR) for the exposed ADHD cohort or adjustment for confounding in the unexposed ADHD cohort. However, the unexposed ADHD cohort showed a significantly increased cRR of preterm birth, SGA, and low birth weight (<2,500 g) compared with the reference pregnancies, but after adjustment for smoking, age, and parity, only the aRR for preterm birth was statistically significant. The aRR of Apgar score <10 at 5 minutes was significantly increased in the exposed ADHD cohort, but not in the unexposed ADHD cohort ().
In this nationwide cohort study, we found that women in the exposed ADHD cohort as well as women in the unexposed ADHD cohort had an increased risk of SA. Children born of women using MPH/ATX had an increased risk of being born with a low Apgar score (<10). Children born of women in the unexposed ADHD cohort had a higher crude risk of being born preterm, with a low birth weight or being SGA compared with the reference group of women without an ADHD diagnosis who did not use MPH/ATX. This indicates that the underlying disease or lifestyle factors related to the disease may play an important role for these associations. In the exposed ADHD cohort, we did not have enough events to calculate the cRRs for being born preterm, SGA, or with a low birth weight. A recent Danish study also used nationwide registers to study the risk of SA following exposure to ADHD-medication use during pregnancy.9 The study had more exposed cases than our study, because the study period was longer and because they included modafinil as ADHD medicine, although this medication is only approved in Denmark for the treatment of narcolepsy.20,21 The authors found a two-fold-higher risk of SA among exposed women compared to unexposed women.
Children born of women using MPH/ATX had an increased risk of being born with a low Apgar score (<10). Children born of women in the unexposed ADHD cohort had a higher crude risk of being born preterm, with a low birth weight or being SGA compared with the reference group of women without an ADHD diagnosis who did not use MPH/ATX. This indicates that the underlying disease or lifestyle factors related to the disease may play an important role for these associations. In the exposed ADHD cohort, we did not have enough events to calculate the cRRs for being born preterm, SGA, or with a low birth weight. A recent Danish study also used nationwide registers to study the risk of SA following exposure to ADHD-medication use during pregnancy.9 The study had more exposed cases than our study, because the study period was longer and because they included modafinil as ADHD medicine, although this medication is only approved in Denmark for the treatment of narcolepsy.20,21 The authors found a two-fold-higher risk of SA among exposed women compared to unexposed women. Case-crossover analyses revealed that SA was not more likely to occur in pregnancies exposed to ADHD medication than in unexposed pregnancies in the same women. The authors concluded that the association may be attributable to factors related to the underlying disorder (ADHD), rather than exposure to the medication itself.9 A recent review, which included four cohort studies and 180 exposed cases, found malformations in four of the children exposed to MPH in fetal life, which yielded an RR of 0.6 (95% CI 0.2–1.6).17 Furthermore, studies on Danish data found no increased risk of malformation, albeit they were both limited by few exposed cases.9,18 We found very few cases of congenital abnormalities, and did not have enough cases to calculate RRs.
Case-crossover analyses revealed that SA was not more likely to occur in pregnancies exposed to ADHD medication than in unexposed pregnancies in the same women. The authors concluded that the association may be attributable to factors related to the underlying disorder (ADHD), rather than exposure to the medication itself.9 A recent review, which included four cohort studies and 180 exposed cases, found malformations in four of the children exposed to MPH in fetal life, which yielded an RR of 0.6 (95% CI 0.2–1.6).17 Furthermore, studies on Danish data found no increased risk of malformation, albeit they were both limited by few exposed cases.9,18 We found very few cases of congenital abnormalities, and did not have enough cases to calculate RRs.
The risk of being born with an Apgar score <10 was increased two-fold in the offspring of women in the exposed ADHD cohort; a similar effect was not found in the unexposed ADHD cohort, which suggests that the ADHD medication may play a causal role for the associated risk of low Apgar score. The Apgar score is a measure of heart rate, respiratory efforts, muscle tone, reflex irritability, and color measured 1 and 5 minutes after birth, with a maximum of 2 points for each parameter and a total of 10 points possible. A low Apgar score is associated with increased mortality and adverse health, especially the score at 5 minutes.22,23 Children in the unexposed ADHD group had a higher risk of being born with a low birth weight (<2,500 g), but after adjustment for relevant confounders, this effect was attenuated along with the difference in mean birth weight and no longer significant. Exposure to MPH/ATX during pregnancy or having an ADHD diagnosis during pregnancy was not associated with a lower GA at birth.
The Apgar score is a measure of heart rate, respiratory efforts, muscle tone, reflex irritability, and color measured 1 and 5 minutes after birth, with a maximum of 2 points for each parameter and a total of 10 points possible. A low Apgar score is associated with increased mortality and adverse health, especially the score at 5 minutes.22,23 Children in the unexposed ADHD group had a higher risk of being born with a low birth weight (<2,500 g), but after adjustment for relevant confounders, this effect was attenuated along with the difference in mean birth weight and no longer significant. Exposure to MPH/ATX during pregnancy or having an ADHD diagnosis during pregnancy was not associated with a lower GA at birth.
We included all pregnancies in Denmark during the study period, and followed all pregnancies without any loss to follow-up. Selection bias is therefore an unlikely explanation for the associations observed. Information on MPH and ATX did not rely on the memory of the women, but was collected automatically from compulsory reporting of medications redeemed at all Danish pharmacies. Therefore, the information about the redeemed drugs was registered accurately. However, we lacked information on the actual usage of the prescribed medication, which could have masked a harmful effect of the medication, although compliance with nervous system drugs appears to be high.24
Therefore, the information about the redeemed drugs was registered accurately. However, we lacked information on the actual usage of the prescribed medication, which could have masked a harmful effect of the medication, although compliance with nervous system drugs appears to be high.24
The information on GA at birth and birth weight in Danish registers is known to have high validity, but the registration of early SA is incomplete.25 The results may be biased if the registration or reporting of early SAs were different among exposed and unexposed women. Underreporting of SA among exposed women would for example lead to an underestimation of risk of SA associated with ADHD treatment.
In spite of being one of the largest epidemiological studies on the adverse effects of MHP and ATX in pregnancy, our study was limited by a small sample size and therefore low statistical precision. We consequently had to keep our statistical models simple, which makes residual confounding an important issue. Due to the small sample size, we had few cases with low birth weight, preterm birth, and Apgar score <10, and we were not able to fully adjust for confounders. However, we found that when adjusting for maternal age, parity, and smoking in the analysis of birth weight and GA at birth, the differences were attenuated compared with the reference group, which indicates that these variables were important confounders for both birth weight and GA at birth. After adjustment, we found that the aRRs for SA decreased in the exposed ADHD cohort, but increased in the unexposed ADHD cohort, although both remained above a 50% increased risk. Furthermore, we had only limited information on potential confounders like lifestyle factors, diet, and somatic and psychiatric comorbidity, which may also influence risks associated with medicine exposure in pregnancy. We had no information on the occurrence or severity of symptoms in our exposed and unexposed ADHD cohort or on the effect of MPH/ATX on these symptoms.
Due to the small sample size, we had few cases with low birth weight, preterm birth, and Apgar score <10, and we were not able to fully adjust for confounders. However, we found that when adjusting for maternal age, parity, and smoking in the analysis of birth weight and GA at birth, the differences were attenuated compared with the reference group, which indicates that these variables were important confounders for both birth weight and GA at birth. After adjustment, we found that the aRRs for SA decreased in the exposed ADHD cohort, but increased in the unexposed ADHD cohort, although both remained above a 50% increased risk. Furthermore, we had only limited information on potential confounders like lifestyle factors, diet, and somatic and psychiatric comorbidity, which may also influence risks associated with medicine exposure in pregnancy. We had no information on the occurrence or severity of symptoms in our exposed and unexposed ADHD cohort or on the effect of MPH/ATX on these symptoms. If there was a discrepancy of the symptoms between the two cohorts, it could have affected the pregnancy outcomes.
If there was a discrepancy of the symptoms between the two cohorts, it could have affected the pregnancy outcomes.
Confounding by indication is a major challenge in pharmacoepidemiological studies, because it is difficult to disentangle the indication for the treatment from the underlying disease. Women in the unexposed ADHD cohort had at least as high a risk of SA as those in the exposed ADHD cohort after adjustment for confounders, which indicates that factors related to the underlying disease may play a causal role in the risk of adverse birth outcome, although confounding from other factors cannot be ruled out.
We found an increased risk of SA among the women in the exposed ADHD cohort as well as among the unexposed ADHD cohort, which suggests that the underlying ADHD disorder may explain at least a part of the association between MPH/ATX and risk of SA. Treatment with MPH/ATX was associated with low Apgar score, an association not found among the unexposed women with ADHD, suggesting a direct effect of MPH/ATX in pregnancy. However, the sample size was small, and larger studies with more clinical information are needed to corroborate these findings.
However, the sample size was small, and larger studies with more clinical information are needed to corroborate these findings.
Simulation
The simulation outlined here investigates whether the rule of thumb of a minimum of ten events per predictor variable in a binary regression analysis can be relaxed in the particular covariate pattern seen when studying the effect of attention deficit/hyperactivity disorder (ADHD) medication in pregnancy on spontaneous abortion.1 A study by Vittinghoff and McCulloch suggested that this rule may in some instances be relaxed, although the situations considered in Vittinghoff and McCulloch do not cover the regression models considered in the present study.2 This simulation was done with the dual purpose of assessing whether 1) propensity-score adjustment for a number of covariates is appropriate, and/or whether 2) adjustment for a number of covariates in a binary regression is appropriate when studying the effect of ADHD medication in pregnancy on spontaneous abortion. The question that seeks answering is how many covariates to include in the analysis while ensuring that the procedure for constructing the confidence interval achieves a 95% level of coverage.
The question that seeks answering is how many covariates to include in the analysis while ensuring that the procedure for constructing the confidence interval achieves a 95% level of coverage.
A prioritized list of potential confounders to be added one at a time in the two situations outlined is made and simulated as follows: 1) mothers’ age as normally distributed, with a mean of 30 and a standard deviation of 5.5; 2) education as multinomially distributed, with probabilities 0.11, 0.32, and 0.57 corresponding to low, medium, and high education, respectively, and afterward coded as two binary variables for medium versus low and high versus low education; 3) cohabitation as a binary variable with a probability of 0.2; 4) comorbidity as a binary variable with a probability of 0.05; and 5) comedication as a binary variable with a probability of 0.05. All simulated potential confounders are thereafter standardized to have a mean of 0 and a standard deviation of 1.
A logistic regression of ADHD medication on spontaneous abortion is fitted on the real data set, which yields a coefficient value of 0. 65. Next, logistic regressions of mothers’ age on ADHD medication and on spontaneous abortion are both fitted on the real data set, which yields coefficient values of −0.20 and 0.24, respectively. Similarly, logistic regressions for the other potential confounders on both ADHD medication and spontaneous abortion are fitted, which yield coefficient values of 0.10 and −0.06 for medium versus low education, −0.38 and −0.08 for high versus low education, 0.54 and 0.12 for cohabitation, 0.33 and 0.05 for comorbidity, and 0.47 and 0.05 for comedication. Baseline coefficients are chosen as −10 and −1.8, respectively.
65. Next, logistic regressions of mothers’ age on ADHD medication and on spontaneous abortion are both fitted on the real data set, which yields coefficient values of −0.20 and 0.24, respectively. Similarly, logistic regressions for the other potential confounders on both ADHD medication and spontaneous abortion are fitted, which yield coefficient values of 0.10 and −0.06 for medium versus low education, −0.38 and −0.08 for high versus low education, 0.54 and 0.12 for cohabitation, 0.33 and 0.05 for comorbidity, and 0.47 and 0.05 for comedication. Baseline coefficients are chosen as −10 and −1.8, respectively.
As one wishes to report risk ratios for spontaneous abortion using binomial regression with log link, data on binary ADHD-medication exposure and binary spontaneous abortion outcome are simulated under this model, while adding covariates successively using the coefficient values reported, ie, the log-probability model for spontaneous abortion firstly consists of only baseline and ADHD medication. Secondly, the model consists of both baseline and ADHD medication and standardized mothers’ age, and so forth, until finally the full model consists of baseline, ADHD medication, standardized mothers’ age, standardized education, standardized cohabitation, standardized comorbidity, and standardized comedication.
Secondly, the model consists of both baseline and ADHD medication and standardized mothers’ age, and so forth, until finally the full model consists of baseline, ADHD medication, standardized mothers’ age, standardized education, standardized cohabitation, standardized comorbidity, and standardized comedication.
Propensity scores were calculated in two ways: 1) as the predicted values from a multiple logistic regression of exposure on standardized covariates, and 2) as the predicted values from a multiple linear regression of exposure on standardized covariates. The propensity-score adjustment was done as a binomial regression, with propensity score adjusted linearly. Coverage probability is calculated based on 1,000 replications.
As seen from Table S1, appropriate coverage probabilities are achieved even when all the covariates are included. Propensity scores can thus be calculated using all covariates considered, and can subsequently be used in a propensity score-adjustment analysis of the effect of ADHD medication on spontaneous abortion. Here, both logistic and linear regression can be used to calculate propensity scores; convergence is more often achieved when using linear regression.
Here, both logistic and linear regression can be used to calculate propensity scores; convergence is more often achieved when using linear regression.
However, when simulating data and using the individual covariates instead of combining these into a propensity score, similar appropriate coverage probabilities, as for the propensity score-adjustment simulation. were achieved (Table S1). Therefore, in the setup outlined, relevant for evaluating the effect of ADHD medication in pregnancy on spontaneous abortion, the rule of thumb of a minimum of ten events per predictor variable may be relaxed.
Table S1
Coverage probabilities in two propensity adjustments and a binomial regression adjustment based on 1,000 replications. The number of times of convergence was not achieved, ie, where the model could not be fitted to data at the first 100 iterations, is shown in parentheses
| Adjustment | Propensity adjustment with scores calculated using logistic regression | Propensity adjustment with scores calculated using linear regression | Binomial regression adjustment |
|---|---|---|---|
| None | 94. 9% (0) 9% (0) | 94.9% (0) | 94.9% (0) |
| Mothers’ age | 95.5% (7) | 95.6% (2) | 95.6% (2) |
| Mothers’ age and education | 96.1% (5) | 96.2% (0) | 95.9% (4) |
| Mothers age, education, and cohabitation | 93.5% (1) | 93.6% (0) | 93. 7% (22) 7% (22) |
| Mothers age, education, cohabitation, and comorbidity | 95.1% (54) | 95.1% (0) | 95.5% (40) |
| Mothers age, education, cohabitation, comorbidity, and comedication | 86.5% (829) | 95.5% (0) | 95.5% (128) |
Open in a separate window
References
1. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. [PubMed] [Google Scholar]
2. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. [PubMed] [Google Scholar]
Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. [PubMed] [Google Scholar]
We thank Morten Pilegaard for help with preparation of the manuscript. The study was supported by the Danish Epilepsy Association. Thank you to the Fonden til Styrkelse af Psykiatrisk Forskning ved Børne – Og Ungdomspsykiatrisk Center for financial support.
Disclosure
The authors report no conflicts of interest in this work.
1. Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31. [PubMed] [Google Scholar]
2. Pottegård A, Bjerregaard BK, Glintborg D, Kortegaard LS, Hallas J, Moreno SI. The use of medication against attention deficit hyperactivity disorder in Denmark: a drug use study from a patient perspective. Eur J Clin Pharmacol. 2013;69(3):589–598. [PubMed] [Google Scholar]
3. Sclar DA, Robinson LM, Bowen KA, Schmidt JM, Castillo LV, Organov AM. Attention-deficit/hyperactivity disorder among children and adolescents in the United States: trend in diagnosis and use of pharmacotherapy by gender. Clin Pediatr (Phila) 2012;51(6):584–589. [PubMed] [Google Scholar]
Sclar DA, Robinson LM, Bowen KA, Schmidt JM, Castillo LV, Organov AM. Attention-deficit/hyperactivity disorder among children and adolescents in the United States: trend in diagnosis and use of pharmacotherapy by gender. Clin Pediatr (Phila) 2012;51(6):584–589. [PubMed] [Google Scholar]
4. Robison LM, Skaer TL, Sclar DA, Gailin RS. Is attention deficit hyperactivity disorder increasing among girls in the US? Trends in diagnosis and the prescribing of stimulants. CNS Drugs. 2002;16(2):129–137. [PubMed] [Google Scholar]
5. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24–31. [PubMed] [Google Scholar]
6. Hechtman L. Predictors of long-term outcome in children with attention-deficit hyperactivity disorder. Pediatr Clin North Am. 1999;46(5):1039–1052. [PubMed] [Google Scholar]
7. Hinshaw SP, Owens EB, Zalecki C, et al. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into early adulthood: continuing impairment includes elevated risk for suicide attempts and self-injury. J Consult Clin Psychol. 2012;80(6):1041–1051. [PMC free article] [PubMed] [Google Scholar]
J Consult Clin Psychol. 2012;80(6):1041–1051. [PMC free article] [PubMed] [Google Scholar]
8. McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC Pediatr. 2012;12:78. [PMC free article] [PubMed] [Google Scholar]
9. Haervig K, Mortensen L, Hansen A, Strandberg-Larsen K. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemoiol Drug Saf. 2014;23(5):526–533. [PubMed] [Google Scholar]
10. Teo SK, Stirling DI, Thomas SD, Hoberman AM, Christian MS, Khetani VD. The perinatal and postnatal toxicity of D-methylphenidate and D,L-methylphenidate in rats. Reprod Toxicol. 2002;16(4):353–366. [PubMed] [Google Scholar]
11. Teo SK, Stirling DI, Thomas SD, Hoberman AM, Christian MS, Khetani VD. D-methylphenidate and D,L-methylphenidate are not developmental toxicants in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol. 2003;68(2):162–171. [PubMed] [Google Scholar]
Birth Defects Res B Dev Reprod Toxicol. 2003;68(2):162–171. [PubMed] [Google Scholar]
12. Beckman DA, Schneider M, Youreneff M, Tse FLS. Developmental toxicity assessment of d,l-methylphenidate and d-methylphenidate in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol. 2008;83(5):489–501. [PubMed] [Google Scholar]
13. Smithells RW. Thalidomide and malformations in Liverpool. Lancet. 1962;1(7242):1270–1273. [PubMed] [Google Scholar]
14. Kopelman AE, McCullar FW, Heggeness L. Limb malformations following maternal use of haloperidol. JAMA. 1975;231(1):62–64. [PubMed] [Google Scholar]
15. Heinonen OP, Slone D, Shapiro S. Birth Defects and Drugs in Pregnancy: Maternal Drug Exposure and Congenital Malformations. Littleton (MA): Publishing Sciences Group; 1977. [Google Scholar]
16. Debooy VD, Seisha MM, Tenenbein M, Casiro OG. Intravenous pentazocine and methylphenidate abuse during pregnancy. Am J Dis Child. 1993;147(10):1062–1065. [PubMed] [Google Scholar]
17. Dideriksen D, Pottegård A, Hallas J, Aagaard L, Damkier P. First trimester in utero exposure to methylphenidate. Basic Clin Pharmacol Toxicol. 2013;112(2):73–76. [PubMed] [Google Scholar]
Dideriksen D, Pottegård A, Hallas J, Aagaard L, Damkier P. First trimester in utero exposure to methylphenidate. Basic Clin Pharmacol Toxicol. 2013;112(2):73–76. [PubMed] [Google Scholar]
18. Pottegård A, Hallas J, Andersen JT, et al. First-trimester exposure to methylphenidate: a population-based cohort study. J Clin Psychiatry. 2014;75(1):e88–e93. [PubMed] [Google Scholar]
19. Efron B, Tibshirani RJ. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75. [Google Scholar]
20. pro.medicin.dk [homepage on the internet] Modafinil “Mylan”. Copenhagen: Dansk Lægemiddel Information; 2014. [Accessed October 7, 2014]. [updated January 16, 2014]. Available from: http://pro.medicin.dk/Medicin/Praeparater/6716. [Google Scholar]
21. pro.medicin.dk [homepage on the internet] Modiodal: modafinil. Copenhagen: Dansk Lægemiddel Information; 2014. [Accessed October 7, 2014]. [updated May 21, 2014]. Available from: http://pro.medicin.dk/Medicin/Praeparater/2520. [Google Scholar]
Available from: http://pro.medicin.dk/Medicin/Praeparater/2520. [Google Scholar]
22. Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. New Engl J Med. 2001;344(7):467–471. [PubMed] [Google Scholar]
23. Sun Y, Vestergaard M, Pedersen C, Christensen J, Olsen J. Apgar scores and long-term risk of epilepsy. Epidemiology. 2006;17(3):296–301. [PubMed] [Google Scholar]
24. Olesen C, Søndergaard C, Thrane N, Nilensen GL, Jong-van den Berg L, Olsen J. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12(5):497–501. [PubMed] [Google Scholar]
25. Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49(8):893–897. [PubMed] [Google Scholar]
Articles from Clinical Epidemiology are provided here courtesy of Dove Press
instruction, composition, use in pregnancy
Home : Good to know : Drugs and medicines in pharmacies
Manual
Brand name: Strattera ATC code: N06BA09
Show more details
- N : Drugs affecting the nervous system
- N06 : Psychoanaleptics
- N06B : Psychostimulants and nootropics
- N06BA : Sympathomimetics0012
- N06BA09 : Atomoxetine
Anatomical Therapeutic Chemical Classification (ATC) from Lat. Anatomical Therapeutic Chemical (ATC) is an internationally accepted drug classification system developed under the auspices of WHO. Each drug will be assigned a code of 5 levels: action on the anatomical organ (level 1), main therapeutic and pharmacological action (levels 2-4) and chemical structure (level 5)
Anatomical Therapeutic Chemical (ATC) is an internationally accepted drug classification system developed under the auspices of WHO. Each drug will be assigned a code of 5 levels: action on the anatomical organ (level 1), main therapeutic and pharmacological action (levels 2-4) and chemical structure (level 5)
Pharmacotherapeutic group: Psychomotor stimulants
Depending on the field of medical application, pharmacological action, as well as on the therapeutic effect, drugs are assigned a pharmacotherapeutic group.
International non-proprietary name: Atomoxetine
The International Nonproprietary Name (INN) is the name of the drug recommended by the World Health Organization, or, in its absence, the common common name. It must not be used as a trade name for a medicinal product.
Composition of the medicinal product
Dosage form and packaging:
Capsules of 25 mg No. 7 (7x1) in blisters in a box
7 (7x1) in blisters in a box
Composition:
1 capsule contains: atomoxetine hydrochloride equivalent to 25 mg atomoxetine
Shelf life and storage conditions :
Keep out of the reach of children at temperatures between 15° and 25°C. Shelf life - 3 years.
Dispensing category: prescription
Producer: Lilly S.A. (packer)/Lilli del Caribe Inc. (Spain/Puerto Rico (USA)) / Lilly S.A./Lilly del Caribe Inc.
Registration: UA/9415/01/03
Registration period: from 2009-02-25 to 2014-02-25
Applicant: Lilly S.A. (Spain) / Lilly S.A.
Where to buy - price in pharmacies in Kyiv
See prices in pharmacies in Kyiv
Dosage and administration
Indications:
Strattera is a centrally acting sympathomimetic drug that improves brain metabolism. Strattera inhibits the activity of norepinephrine transporters. The active substance of the drug is atomoxetine, which is not a psychostimulant and amphetamine derivative. Atomoxetine has minimal affinity for other noradrenergic receptors or for other transporters or neurotransmitter receptors.
The active substance of the drug is atomoxetine, which is not a psychostimulant and amphetamine derivative. Atomoxetine has minimal affinity for other noradrenergic receptors or for other transporters or neurotransmitter receptors.
Indication: Attention Deficit Hyperactivity Disorder (ADHD) in children 6 years of age and older, adolescents and adults.
Dosage and administration:
The drug is taken orally, regardless of food intake or during meals, 1 time per day, in the morning. In the event of adverse events when taking the drug once a day, it can be recommended to take it twice a day, dividing the dose into morning and evening. For children and adolescents weighing over 70 kg and adults, the recommended maintenance dose is 80 mg. The recommended maximum daily dose is 120 mg.
Overdose:
Overdose may cause drowsiness, agitation, hyperactivity, behavioral disturbances and gastrointestinal symptoms; mydriasis, tachycardia, dry mouth, convulsions. Symptomatic and supportive treatment is recommended.
Symptomatic and supportive treatment is recommended.
Use in pregnancy and lactation
Strattera should only be used during pregnancy if the expected benefit to the mother greatly outweighs the potential risk to the fetus. If necessary, the appointment of the drug during lactation requires caution.
Side effects and contraindications
Side effects:
When using Strattera in children and adolescents during treatment, abdominal pain, weight loss, loss of appetite, nausea, vomiting, orthostatic hypotension, influenza, irritability, frequent mood changes, dizziness, mydriasis, palpitations, dermatitis, rash, itching on the skin, fatigue. In adult patients, Strattera may cause decreased appetite, insomnia, tachycardia, sleep disturbance, dizziness, hot flashes, cold hands and feet, constipation, flatulence, abdominal pain, difficulty urinating, weakness, chills, drowsiness, ejaculatory disturbance, or its absence, prostatitis, erectile dysfunction and menstrual cycle.
Contraindications:
Strattera is contraindicated in angle-closure glaucoma; severe damage to the heart; simultaneous use with MAO inhibitors; hypersensitivity to the drug.
The drug should be used with caution in patients with arterial hypertension, tachycardia, cardiovascular diseases, severe physical overload, concomitant use of psychostimulants, sudden cardiac death in a family history, cerebrovascular accident, convulsive seizures in anamnesis, as well as in conditions that may lead to arterial hypotension.
Special warnings:
Caution is required when using Strattera with drugs that lower the seizure threshold (antidepressants, antipsychotics, mefloquine, tramadol).
The combination of the drug with MAO inhibitors is contraindicated.
When taking the drug, care should be taken when driving dangerous mechanical means, incl. by car.
Similar medicines
Important!
Listed below are drugs with the same level 3 or 4 ATC code, or the same pharmacotherapeutic group.
Before replacing the drug with an analogue, be sure to consult your doctor!
- N : Nervous system drugs
- N06 : Psychoanaleptics
- N06B : Psychostimulants and nootropics
- N06BA : Centrally acting sympathomimetics
Sympathomimetics of central action
- Concerta: price, instructions
Instructions for use, composition, side effects and other detailed information on this page for ease of perception is given in a free translation of the manufacturer's official instructions. This material is for informational purposes only. We do not manufacture or sell drugs. Remember: the need to use the drug, methods and doses are determined only by your doctor. Self-medication can be dangerous for your health!
Instructions for use, Classification, Articles »Handbook of LS
Each capsule contains:
for a dosage of 10 mg:
Capsule contents:
Active substance - ATOMOCOTINTINTINA hydrochloride, equivalent .
Excipients - dimethicone 1.15 mg/capsule, pregelatinized starch 217.42 mg/capsule.
Capsule shell - titanium dioxide 1.554 mg/capsule, sodium lauryl sulfate 0.069 mg/capsule, gelatin qs 50 mg/capsule.
For a dosage of 18 mg:
Capsule content:
Active substance - Atomoxetine hydrochloride equivalent to atomoxetine base, 18 mg/capsule.
Excipients - dimethicone 1.15 mg/capsule, pregelatinized starch 208.28 mg/capsule.
Capsule shell - titanium dioxide 0.932 mg/capsule, sodium lauryl sulfate 0.070 mg/capsule, gelatin qs 50 mg/capsule, iron oxide yellow 0.173 mg/capsule.
For a dosage of 25 mg:
Capsule content:
Active substance - Atomoxetine hydrochloride, equivalent to atomoxetine base, 25 mg/capsule.
Excipients - dimethicone 1.15 mg/capsule, pregelatinized starch 200.28 mg/capsule.
Capsule shell - titanium dioxide 1. 230 mg/capsule, sodium lauryl sulfate 0.070 mg/capsule, gelatin qs 50 mg/capsule, indigo carmine dye 0.029 mg/capsule.
230 mg/capsule, sodium lauryl sulfate 0.070 mg/capsule, gelatin qs 50 mg/capsule, indigo carmine dye 0.029 mg/capsule.
For a dosage of 40 mg:
Capsule contents:
Active substance - Atomoxetine hydrochloride, equivalent to atomoxetine base. 40 mg/capsule.
Excipients - dimethicone 1.15 mg/capsule, pregelatinized starch 183.14 mg/capsule.
Capsule shell - titanium dioxide 0.745 mg/capsule, sodium lauryl sulfate 0.071 mg/capsule, gelatin qs 50 mg/capsule, indigo carmine dye 0.072 mg/capsule.
For a dosage of 60 mg:
Capsule content:
Active substance - Atomoxetine hydrochloride, equivalent to atomoxetine base, 60 mg/capsule.
Excipients - dimethicone 1.55 mg/capsule, pregelatinized starch 239.89 mg/capsule.
Capsule shell - titanium dioxide 0.376 mg/capsule, sodium lauryl sulfate 0.089 mg/capsule, gelatin qs 63 mg/capsule, iron oxide yellow 0. 326 mg/capsule, indigo carmine dye 0.036 mg/capsule.
326 mg/capsule, indigo carmine dye 0.036 mg/capsule.
Due to insufficient experience with the use of atomoxetine during pregnancy, the drug should be prescribed during pregnancy only if the potential benefit to the patient significantly outweighs the potential risk to the fetus.
It is not known if atomoxetine is excreted in breast milk. Care must be taken when prescribing the drug to a nursing woman.
Special instructions: Symptoms of ADHD in the form of impaired attention and hyperactivity (identified in more than one social environment, for example, both at home and at school) can manifest as lack of concentration, distractibility, excessive impatience, impulsivity, disorganization, restlessness and other similar behavioral disorders. The diagnosis of ADHD must meet accepted diagnostic criteria.
The diagnosis of ADHD must meet accepted diagnostic criteria.
Suicidal thoughts and behavior. While taking the drug in clinical studies in children and adolescents, the likelihood of developing suicidal thoughts increased. In 12 clinical trials in 2200 patients (including 1357 patients treated with atomoxetine and 851 patients treated with placebo), of which 0.37% of patients in the atomoxetine group developed suicidal thoughts (5 of 1357 patients), in the placebo group suicidal thoughts were not identified. In these clinical studies, one suicide attempt was reported, but there were no completed suicides. All reactions were observed in children aged 12 years during the first month from the start of treatment. It is not known whether the risk of developing suicidal thoughts persists over a longer period of treatment. In similar studies in adult patients, an increase in the risk of developing suicidal thoughts was not observed.
Parents and caregivers of children and adolescents treated with Strattera® should be closely monitored for unusual behavioral changes, worsening of symptoms, and suicidal ideation, especially during the first months of treatment and during changes (both increases and decreases ) dose of the drug.
The following symptoms have been reported with Strattera®: anxiety, agitation, panic attacks, insomnia, irritability, aggressiveness, impulsivity, akathisia, hypomania, mania. Despite the fact that the relationship between the occurrence of these symptoms and the appearance of suicidal phenomena has not been identified, there is an assumption that such symptoms may be harbingers of the development of suicidal thoughts. In the event of the appearance of these symptoms, it may be necessary to change the dosing regimen, including the possible withdrawal of the drug, especially if they occur suddenly and are severe.
Parents and caregivers should be warned to monitor patients for agitation, irritability, unusual behavioral changes, and other symptoms described above, as well as suicidal thoughts, on a daily basis and to report such symptoms to the attending physician immediately.
Allergic reactions. In rare cases, patients taking atomoxetine experienced allergic reactions in the form of rash, angioedema, urticaria.
Monoamine oxidase inhibitors (MAOIs). Atomoxetine should not be used for at least 2 weeks after MAOIs have been discontinued. Treatment with MAOIs should not begin within 2 weeks after the abolition of atomoxetine.
Influence on the cardiovascular system. Atomoxetine may affect heart rate (HR) and blood pressure (BP). It is recommended to measure heart rate and blood pressure before starting treatment and then periodically repeat measurements during treatment in order to identify a clinically significant increase in these indicators. Most patients receiving atomoxetine experienced a slight increase in heart rate (less than 10 bpm on average) and/or an increase in blood pressure (less than 5 mmHg on average), which were not clinically significant. However, data from clinical studies of ADHD indicate that some patients (about 5-10% of children and adults) experience clinically significant changes in heart rate (by 20 bpm or more) and blood pressure (by 15-20 mmHg). Art and more). Atomoxetine should be used with caution in patients with comorbidities such as arterial hypertension, tachycardia, other cardiovascular and cerebrovascular disorders, in which the patient's condition may worsen in the event of an increase in blood pressure or heart rate. This drug should not be used in patients with severe cardiovascular disease.
Art and more). Atomoxetine should be used with caution in patients with comorbidities such as arterial hypertension, tachycardia, other cardiovascular and cerebrovascular disorders, in which the patient's condition may worsen in the event of an increase in blood pressure or heart rate. This drug should not be used in patients with severe cardiovascular disease.
In addition, atomoxetine should be used with caution in patients with congenital or acquired prolongation of the QT interval (including due to the use of a concomitant drug), or a hereditary history of prolongation of the QT interval. The development of orthostatic hypotension has also been reported, so atomoxetine should be used with caution in patients with those diseases in which arterial hypotension or sudden changes in blood pressure or heart rate can be observed.
Impaired liver or kidney function. Very rarely, spontaneous observations of liver damage have been reported, which manifested as an increase in the activity of liver enzymes and an increase in the concentration of bilirubin in the blood with the development of jaundice. Also, in very rare cases, severe liver damage up to the development of acute liver failure has been reported. Atomoxetine should be discontinued and not restarted in patients with jaundice or laboratory evidence of liver damage.
Also, in very rare cases, severe liver damage up to the development of acute liver failure has been reported. Atomoxetine should be discontinued and not restarted in patients with jaundice or laboratory evidence of liver damage.
In clinical studies, adult patients with ADHD treated with atomoxetine had higher rates of urinary retention compared to placebo. Complaints of urinary retention can potentially be regarded as a result of the use of atomoxetine.
Seizures. Use with caution in patients with a history of seizures. It is necessary to stop taking atomoxetine in the event of the development of seizures that cannot be explained by reasons other than the use of atomoxetine.
Use in children. There is insufficient data on the safety and efficacy of atomoxetine in children under 6 years of age. The effectiveness of treatment with atomoxetine for more than 18 months and the safety of treatment with it for more than 2 years have not been systematically evaluated. Use in the elderly. The safety and efficacy of atomoxetine in elderly patients have not been studied.
Use in the elderly. The safety and efficacy of atomoxetine in elderly patients have not been studied.
Aggressive behavior or hostility. Aggressive behavior or hostility is often observed in patients with ADHD. There is no unequivocal evidence that atomoxetine can cause aggressive behavior or hostility. However, in clinical studies, aggressive behavior or hostility was observed more often in children, adolescents and adults taking atomoxetine (no statistically significant difference compared with the placebo group). Patients receiving treatment for ADHD should be monitored for aggressive behavior or hostility.
Priapism. Rare cases of priapism (persistent painful or painless erection lasting more than 4 hours) have been reported in adults and children during post-marketing surveillance. In cases of suspected priapism, medical attention should be provided immediately.
Effect on height and body weight. In general, growth and corresponding weight gain in children treated with atomoxetine were slower than normal during the first 9-12 months from the start of treatment. Anthropometric parameters (body weight and height) after two years of treatment were close to normal. It is necessary to monitor growth rates in patients taking atomoxetine.
Anthropometric parameters (body weight and height) after two years of treatment were close to normal. It is necessary to monitor growth rates in patients taking atomoxetine.
Psychotic or manic episodes. In children and adolescents without a history of psychotic or manic episodes, atomoxetine, when taken at normal doses, may cause the development of symptoms of these conditions, including hallucinations, delusions, and manic mood disorder. In the event of the occurrence of the described symptoms, the possible role of taking atomoxetine in their development should be assessed and the issue of canceling therapy should be decided. Similar symptoms were observed in approximately 0.2% of cases (in 4 patients out of 1939 treated with atomoxetine for several weeks at usual doses) of atomoxetine therapy compared with no such cases among 1056 patients treated with placebo, according to the results of a pooled analysis of multiple short-term placebo-controlled studies.
Bipolar disorder. In general, in patients treated for ADHD with concomitant bipolar disorder, special attention should be paid to the possibility of inducing mixed/manic episodes under the influence of this therapy. It cannot be definitely stated whether the symptoms described above can be due to such an induction. In this regard, patients with existing symptoms of depression should be carefully examined to determine the presence or absence of the risk of developing bipolar disorder prior to the appointment of atomoxetine therapy. Such examination should include a detailed medical history, including a family history of suicide, bipolar disorder, and depression.
In general, in patients treated for ADHD with concomitant bipolar disorder, special attention should be paid to the possibility of inducing mixed/manic episodes under the influence of this therapy. It cannot be definitely stated whether the symptoms described above can be due to such an induction. In this regard, patients with existing symptoms of depression should be carefully examined to determine the presence or absence of the risk of developing bipolar disorder prior to the appointment of atomoxetine therapy. Such examination should include a detailed medical history, including a family history of suicide, bipolar disorder, and depression.
Urinary retention. In adult patients with ADHD who participated in controlled studies, the frequency of urinary retention (1.7%, 9/540) and difficulty urinating (5.6%, 30/540) was higher in patients taking atomoxetine, according to compared with patients receiving placebo (0%, 0/402; 0.5%, 2/402, respectively). Two adult patients taking atomoxetine withdrew prematurely from the study due to urinary retention, while no such cases were noted among those receiving placebo. Complaints of urinary retention or difficulty urinating should be considered as potentially related to atomoxetine.
Two adult patients taking atomoxetine withdrew prematurely from the study due to urinary retention, while no such cases were noted among those receiving placebo. Complaints of urinary retention or difficulty urinating should be considered as potentially related to atomoxetine.
Special Populations - In controlled studies in children with ADHD and comorbid chronic motor tics or Tourette's syndrome, patients treated with atomoxetine did not experience worsening of tics compared with patients treated with placebo. In controlled studies of adolescents with ADHD and comorbid major depressive disorder, patients treated with atomoxetine showed no worsening of depressive symptoms compared to patients treated with placebo. In two controlled studies (one in children and one in the adult population) of patients with ADHD and comorbid anxiety disorders, patients treated with atomoxetine did not experience an increase in anxiety compared with patients taking placebo. During post-marketing use, there have been rare reports of anxiety and depression or depressed mood, as well as very rare reports of tics. The following symptoms have been noted while taking atomoxetine: anxiety, agitation, panic attacks, insomnia, irritability, impulsivity, akathisia, hypomania and mania.
The following symptoms have been noted while taking atomoxetine: anxiety, agitation, panic attacks, insomnia, irritability, impulsivity, akathisia, hypomania and mania.
Parents and loved ones should carefully monitor the occurrence of all of the above symptoms and suicidal thoughts in children and adolescents taking atomoxetine, and immediately report this to the attending physician.
Influence on the ability to drive transport. cf. and fur.:Taking the drug may be accompanied by drowsiness. In this regard, patients taking atomoxetine should be careful when driving dangerous mechanical means, including a car, until they are sure that atomoxetine does not cause any violations.
Children and adolescents
Headache, abdominal pain 6 and decreased appetite are the most commonly reported adverse events with atomoxetine (19%, 18% and 16%, respectively) in placebo-controlled studies in children, but they generally do not require discontinuation of the drug (discontinuation rates for the following indications are 0. 1% for headache, 0.2% for abdominal pain, and 0.0% for decreased appetite). Abdominal pain and decreased appetite are usually temporary.
1% for headache, 0.2% for abdominal pain, and 0.0% for decreased appetite). Abdominal pain and decreased appetite are usually temporary.
In clinical studies of ADHD in children and adolescents at the beginning of therapy, there was a lag in the average rate of weight gain and growth from the age norm among those treated with atomoxetine due to decreased appetite. Subsequently, after a long period of time, body weight and height in these patients returned to the average age norm, determined taking into account anthropometric data before the start of treatment with atomoxetine.
Nausea, vomiting and drowsiness 1 may occur in approximately 10% and 11% of patients, respectively, especially during the first month of treatment. However, these episodes were usually mild to moderate, transient, and did not lead to treatment discontinuation in a significant number of cases (discontinuation rates <0.5%).
In placebo-controlled studies in children and adults treated with atomoxetine, there was an increase in heart rate, an increase in systolic and diastolic pressure compared with placebo.
Patients treated with atomoxetine experienced orthostatic hypotension (0.2%) and syncope (0.8%) due to its effect on sympathetic tone. Atomoxetine should be used with caution in any condition that may lead to a decrease in blood pressure.
The following are side effects and laboratory abnormalities reported in clinical studies in children and adolescents.
Metabolic and nutritional disorders
Very common (> 10%): decreased appetite
Common (1-10%): anorexia (loss of appetite)
Nervous system disorders
Very common (> 10%): drowsiness 1 , headache
Common (1-10%): dizziness
Uncommon (0.1-1%): syncope 2 , tremor
mood, insomnia 3 , depression 4
Uncommon (0.1-1%): suicidal thoughts and behavior, aggressive behavior, hostility, emotional instability
Visual disturbances
(0.1-1%): conjunctivitis
Cardiac and vascular disorders
Very common (> 10%): increased blood pressure 5 , increased heart rate 5
Uncommon (0. 1-1%): palpitations, sinus tachycardia
1-1%): palpitations, sinus tachycardia
Gastrointestinal disorders
Very common (> 10%): abdominal pain 6 , nausea, vomiting
Common (1-10%): constipation, dyspepsia
Skin and subcutaneous tissue disorders
Common (1-10%): rash, itching
General disorders
Common (1-10%): fatigue, weight loss, irritability
Uncommon (0.1-1%): asthenia
1 Includes sedation.
2 Includes vasovagal syncope
3 Includes difficulty falling asleep, restless sleep and early awakening.
4 Includes major depressive disorder, depressive symptoms, depressive mood, and dysphoria.
5 Based on heart rate and blood pressure measurements.
6 Includes abdominal discomfort, epigastric pain and discomfort, stomach discomfort.
The following side effects were observed in at least 2% of children and adolescents with low activity of the CYP2D6 isoenzyme, statistically significantly more often than in patients with high activity of the CYP2D6 isoenzyme: weight loss (7. 3% and 4.4%, respectively) , constipation (6.8% and 4.3%, respectively), insomnia (11% and 6.1%, respectively), depression (6.5% and 4.1%, respectively), tremor (4.5% and 0, 9%, respectively), restless sleep (2.8% and 1.3%, respectively), fainting (2.5% and 0.7%, respectively), conjunctivitis (2.5% and 1.2%, respectively), early morning awakening (2 .3% and 0.8%, respectively), mydriasis (2.0% and 0.6%, respectively), sedation (3.9% and 2.1%, respectively).
3% and 4.4%, respectively) , constipation (6.8% and 4.3%, respectively), insomnia (11% and 6.1%, respectively), depression (6.5% and 4.1%, respectively), tremor (4.5% and 0, 9%, respectively), restless sleep (2.8% and 1.3%, respectively), fainting (2.5% and 0.7%, respectively), conjunctivitis (2.5% and 1.2%, respectively), early morning awakening (2 .3% and 0.8%, respectively), mydriasis (2.0% and 0.6%, respectively), sedation (3.9% and 2.1%, respectively).
Adults
In clinical studies in adults, the most common side effects during the administration of atomoxetine were from the gastrointestinal tract, the nervous system and the psyche. The most common side effects (>5%): loss of appetite (14.9%), insomnia (11.3%), headache (16.3%), dry mouth (18.4%) and nausea (26.7%). In most cases, all of the listed side effects were mild or moderate. The most commonly reported severe side effects were nausea, insomnia, fatigue, and headache.
Complaints of urinary retention and difficulty starting urination could potentially be associated with atomoxetine.
The following are side effects and laboratory abnormalities reported in adult clinical studies.
Metabolic and nutritional disorders
Very common (> 10%>): loss of appetite
Nervous system disorders
Very common (> 10%): headache %): dizziness, change in taste, paresthesia, drowsiness 4 , tremor
Mental disorders
Very often (> 10%>): insomnia 1
%): agitation 1-1. libido, sleep disturbance 1
Uncommon (0.1-1.0%): orgasm disturbance, anxiety, suicidal thoughts and behavior, aggressive behavior, hostility, emotional instability -1.0%): blurred vision
Cardiac and vascular disorders
Very common (>10%): increased blood pressure 2 , increased heart rate 2
Common (1-10%): palpitations, tachycardia, flushing (of blood), redness of the skin
Very common (> 10%): dry mouth, nausea
Common (1-10%): abdominal pain 3 , constipation, dyspepsia, flatulence, vomiting
Skin and subcutaneous tissues
Common (1-10%): rash, hyperhidrosis
Uncommon (0. 1-1.0%): pruritus, urticaria
1-1.0%): pruritus, urticaria
Musculoskeletal disorders
Uncommon (0.1-1.0%): muscle spasms
Renal disorders and urinary tract
Common (1-10%): dysuria, urinary retention, difficulty initiating urination 5 , frequent urination
Uncommon (0.1-1.0%): painful urge to urinate
Genital and breast disorders
often (1-10 %): dysmenorrhea 6 , ejaculation disruption 7 , erectile dysfunction 7 , prostatitis 7 , testicles in the testes 7
is incredible (0.1-1 %) : absence of ejaculation, menstrual disorders 6
General disorders
Common (1-10%): fatigue, chills, weight loss, asthenia, anxiety, irritability, thirst
Uncommon (0.1-1 %): feeling cold
1 Includes difficulty falling asleep, restless sleep and early awakening.
2 Data based on heart rate and blood pressure measurements.
3 Includes abdominal discomfort, epigastric pain and discomfort, stomach discomfort.
4 Includes sedation
5 Includes jet reduction
6 Frequency based on female patients
7 Frequency based on data from male patients
The following side effects were observed in at least 2% of adult patients with low CYP2D6 isoenzyme activity, statistically significantly more often than in patients with high CYP2D6 isoenzyme activity: blurred vision (3 .9% and 1.3%, respectively), dry mouth (34.5% and 17.4%, respectively), constipation (11.3% and 6.7%, respectively), anxiety (4.9% and 1 .9% respectively), loss of appetite (23.2% and 14.7% respectively), tremor (5.4% and 1.2% respectively), insomnia (19.2% and 11.3%, respectively), sleep disturbances (6.9% and 3.4%, respectively), restless sleep (5.4% and 2.7%, respectively), early awakening (3.0% and 0 .9%, respectively), urinary retention (5. 9% and 1.2%, respectively), erectile dysfunction (20.9% and 8.9%, respectively), ejaculation disorder (6.1% and 2.2%, respectively) , increased sweating (14.8% and 6.8%, respectively), cold in the extremities (3.0% and 0.5%, respectively).
9% and 1.2%, respectively), erectile dysfunction (20.9% and 8.9%, respectively), ejaculation disorder (6.1% and 2.2%, respectively) , increased sweating (14.8% and 6.8%, respectively), cold in the extremities (3.0% and 0.5%, respectively).
Spontaneous (post-marketing) messages
Cardiac and vascular disorders
Uncommon (0.1-1%): prolongation of the QT interval
Rare (>0.01% and <0.1%): increased blood pressure
Very rare (< 0.01%): peripheral vascular reactions and/or Raynaud's syndrome and risk of exacerbation of Raynaud's syndrome
Liver and biliary disorders
Uncommon (0.1-1%): increased bilirubin concentration in children and adolescents
Rarely (>0.01% and <0.1%): abnormal liver function tests, jaundice, hepatitis, liver damage, acute liver failure; an increase in the concentration of bilirubin in adults.
Renal and urinary tract disorders
Very rare (<0.01%>): painful or prolonged erection, pain in the vulva in men, difficulty urinating in children and adolescents, urinary retention in children and adolescents
Nervous system disorders
Uncommon (0.