Sertraline lowest dosage
How and when to take sertraline
Dosage and strength
Sertraline is available as 25mg, 50mg or 100mg tablets.
The usual dose of sertraline is 50mg a day in adults. But your doctor may start you on a lower dose, then increase it gradually to a maximum dose of 200mg a day.
If you have liver problems, your doctor might give you a lower dose.
The usual dose of sertraline for children aged 6 to 12 is 25mg a day, but this may be increased to 50mg a day after a week.
The usual dose of sertraline for children aged 13 to 17 is 50mg a day.
Children aged 6 to 17 might have their dose increased up to 200mg a day, if needed.
How to take itTake sertraline once a day. You can take it with or without food.
You can choose to take sertraline at any time, as long as you stick to the same time every day.
If you have trouble sleeping, it's best to take it in the morning.
How long to take it forOnce you're feeling better it's likely that you'll continue to take sertraline for several more months. Stopping before that time can make depression come back.
Most doctors recommend that you take antidepressants for 6 months to a year after you no longer feel depressed.
If you forget to take itIf you occasionally forget to take a dose, skip the missed dose and take your next dose the next day at the usual time.
Never take 2 doses at the same time to make up for a forgotten one.
If you forget doses often, it may help to set an alarm to remind you. You could also ask your pharmacist for advice on other ways to help you remember to take your medicine.
The amount of sertraline that can lead to an overdose varies from person to person.
Taking too much can cause symptoms such as:
- being sick (vomiting)
- shaking
- feeling sleepy
- feeling dizzy
- fast heart rate
- fits or seizures
Urgent advice: Contact 111 for advice now if:
- you take more than your prescribed dose of sertraline
Go to 111.nhs.uk or call 111
If you need to go to A&E, do not drive yourself. Get someone else to drive you or call for an ambulance.
Take the sertraline packet, or the leaflet inside it, plus any remaining medicine with you.
If you have been feeling better for 6 months or more, your doctor may suggest coming off sertraline.
Your doctor will probably recommend reducing your dose gradually over several weeks, or longer if you have been taking sertraline for a long time.
This is to help prevent any withdrawal symptoms you might get as a reaction to coming off the medicine.
These can include:
- feeling dizzy
- feeling sick
- numbness or tingling in the hands or feet
- trouble sleeping
- feeling agitated or anxious
- headaches
- shaking
Page last reviewed: 2 February 2022
Next review due: 2 February 2025
When Should You Increase Your Zoloft Dosage To Treat Anxiety?
Content
- Overview
- What is Zoloft?
- What is the proper Zoloft dosage for anxiety?
- When to increase your Zoloft dose for anxiety?
- Talk to your doctor first
- Factors that could impact whether you need a higher dose
- Zoloft’s effects on anxiety
- The lowdown
If you’ve been prescribed Zoloft to treat your anxiety, but it’s not quite doing the job, you might find it tempting to increase your dosage.
However, Zoloft is a psychiatric medicine, so it’s crucial you don’t take more or less than the dosage prescribed. However, your doctor may be able to prescribe a stronger dose if needed, if they determine that this is the right course of action.
Have you considered clinical trials for Anxiety?
We make it easy for you to participate in a clinical trial for Anxiety, and get access to the latest treatments not yet widely available - and be a part of finding a cure.
Check your eligibility
Zoloft, or sertraline as it is known under its generic name, is a psychiatric medicine used largely in the treatment of depression and anxiety.
Taken orally, Zoloft belongs to a group of antidepressants called selective serotonin reuptake inhibitors, or SSRIs. SSRIs are a commonly prescribed antidepressant that improves mood by blocking the mechanisms that absorb serotonin, allowing it to be available to the brain for longer.
This is important because serotonin is known for its ability to induce feelings of happiness. In fact, serotonin is often referred to as the “happy hormone.”
In fact, serotonin is often referred to as the “happy hormone.”
Starting Zoloft
The appropriate dosage of Zoloft will be determined by a doctor and is likely to differ depending on the patient and their individual needs.
Typically, 50 milligrams once a day is a standard starting dosage for adults with depression, obsessive-compulsive disorder, and premenstrual dysphoric disorder. Some conditions tend to start with a lower dose of 25 milligrams once a day, like panic disorder.
Maximum dose
Your Zoloft dose must stay within a safe range, so you’re unlikely to be prescribed anything higher than 150-200 milligrams per day. In the end, your dosage will depend on the condition being treated and your individual needs.
Children
Children can also benefit from Zoloft but will follow a different dosage regimen. This dose will be at the discretion of your doctor and is based on your child’s age, size, and therapeutic need. For instance, for children aged 6 to 12 years of age with obsessive-compulsive disorder, a standard starting dose is 25 milligrams once a day.
If you have concerns or questions about your dosing, make sure to discuss it with your doctor before making any changes to your medication regimen.
If you’re taking Zoloft, but aren’t feeling any improvement in your anxiety levels, it can be tempting to simply up your dose – but it’s important not to.
Zoloft is not an instant relief medication. SSRIs do not exert instant effects like, pain relief does. Zoloft (and other SSRIs) is a little more complicated and it takes longer for its full effects to be felt.
These medications take time for their effectiveness to peak. Zoloft works by blocking the reuptake of serotonin and it takes repetitive dosing and time for the medication to be able to exert its effects on your system. It can take around two to six weeks before you start to experience a reduction in your anxiety symptoms.
If after this period you still haven’t seen any benefits then you may want to talk to your doctor about altering your Zoloft dose.
While Zoloft can have a positive impact on anxiety, it can also come with some minor side effects. Zoloft can also increase your risk of serious side effects if you have an existing medical condition or are taking other medication.
Therefore, increasing your Zoloft dose without approval from your doctor should not be done under any circumstances. This can have serious detrimental effects on your health and wellbeing and can result in coma and seizures.
Zoloft side effects and drug interactions
The following side effects¹ and potential drug interactions² highlight why it’s important to follow your Zoloft prescription and avoid increasing your dosage without your doctor's approval.
Potential side effects of Zoloft include:
Nausea
Loss of appetite or indigestion
Diarrhea
Excessive sweating
Tiredness or fatigue
Sexual performance issues, e.g. reduced sex drive, failure to ejaculate
Sleep disturbances, e.
 g. sleepiness or insomnia
g. sleepiness or insomnia Shaking hands
Increased agitation and irritability
Suicidal ideations
Impulsive and dangerous behavior
Worsening of depression and/or anxiety symptoms
Violent behavior
Seizures
Manic behavior, e.g. fast-paced and racing thoughts, extreme high and low emotions, excessive energy, and talking grandiose thinking.
An allergic reaction (typical symptoms include trouble breathing, a rash or hives, and swelling).
Eye problems, e.g. blurred vision, red eyes, pain in the eyes.
Serotonin syndrome includes symptoms such as hallucinations, agitated behavior, loss of consciousness/coma, increased heart rate, vomiting, and rigid muscles
There are also specific side effects that can affect children taking Zoloft:
Nose bleeds
Urine problems, e.g. frequently needing to urinate or leaking urine
Changes to menstruation, specifically heavier periods
Agitation or fidgeting
Aggressive or irritable behavior that is out of the norm
Changes to physical development.
 You may see your child’s growth rate start to slow down and/or weight gain.
You may see your child’s growth rate start to slow down and/or weight gain.
Conditions that can increase your risk of Zoloft side effects:
Glaucoma: People with glaucoma can experience an increase in glaucoma attacks if they take Zoloft and should therefore talk to their doctor to determine if the SSRI is appropriate.
Seizure conditions: Since seizures are already a potential side effect of Zoloft, the medication should be considered carefully in people with a seizure condition lest it increases their frequency or severity³.
Liver issues: Patients with liver problems should take care when starting Zoloft as they can experience stronger effects than normal, due to their liver’s inability to break down the medication.
Bipolar disorder: Bipolar disorder is a possible contraindication for Zoloft as you may experience a higher risk of manic symptoms.
Kidney issues: Much like the liver, if your kidneys aren’t functioning well then you may not be able to filter medications properly, resulting in higher levels of Zoloft in your system.
Side effects that can occur when Zoloft is taken alongside certain medications:
Increased risk of serotonin syndrome can occur when Zoloft is taken with monoamine oxidase inhibitors, linezolid, intravenous methylene blue, triptans, lithium, fentanyl, tramadol, and St John’s wort.
Heart problems can occur when Zoloft is taken with pimozide.
Bruising and bleeding can occur when taken with nonsteroidal anti-inflammatory medications, e.g. aspirin.
A build-up of medications (including Zoloft) can occur in your system when taken with cimetidine, tricyclic antidepressants.
While it’s important to stick to the dose recommended by your doctor, there are legitimate and safe reasons why your doctor may increase your Zoloft dose.
For instance, your doctor may increase your Zoloft dose if you aren’t seeing a significant reduction in your anxiety symptoms, so long as it is safe.
The risks of altering your Zoloft dosage without medical approval are substantial. However, with a prescribed dosage and professional advice, Zoloft can have a positive impact on your anxiety and improve your quality of life.
Research tells us that Zoloft can effectively reduce anxiety scores. One study⁴ found that Zoloft managed to significantly improve anxiety, worry, and depressive symptoms in patients (60 years and older), with an anxiety diagnosis (generalized anxiety disorder, panic disorder, agoraphobia, or social phobia).
Zoloft is also an effective treatment option for children suffering from anxiety. A 2018 study found that SSRIs significantly reduced anxiety symptoms⁵ in children. The study also found that a combination of Zoloft and cognitive behavioral therapy was a particularly effective treatment option.
What To Expect When You Take Anxiety Medication
Zoloft is an effective treatment for managing anxiety. Your doctor will prescribe Zoloft if they believe it is a good fit, and may gradually increase your dose until you start to see results or reach a maximum dose.
However, since Zoloft can have some serious side effects and can interact negatively with other drugs, do not increase your dosage until you have consulted a medical professional.
Active substance SERTRALINUM | Compendium - drug reference book
- Pharmacological properties
- Indications SERTRALINE
- Application of SERTRALINE
- Contraindications
- Side effects
- Special instructions
- Interactions
- Overdose
- Diagnosis
- Recommended alternatives
- Trade names
Medicinal preparations containing the active substance SERTRALINE
Prices in pharmacies
Serlift
tablets covered with a shell 100 mg blister, No. 28
SUN
Prices in pharmacies
CELLOFT 25
tablets 25 mg blister, No. 30
Health
.
Sertraloft 50
film-coated tablets 50 mg blister, № 30
Health
Pharmacy prices
Sertraloft 100
film-coated tablets, № 100 mg0029
Health
Prices in pharmacies
Stimalon ®
tablets covered with a shell 50 mg blister, No. 30
EGIS
Price in pharmacies
stimulota ® 10029 tablets, covered scrapp No. 28 EGIS Prices in pharmacies Emoto
Ersel Pharma
Price in pharmacies
Emoton
Tablets covered with film shell 50 mg Blista, No. 30
Ersel Pharma
Pharmacy prices
specific serotonin (5-HT) reuptake inhibitor in neurons in vitro . It has little effect on the reuptake of norepinephrine and dopamine. At clinical doses, sertraline blocks the uptake of serotonin in human platelets. It does not have a stimulating, sedative or anticholinergic effect and does not have cardiotoxicity. Due to selective inhibition of 5-HT uptake, sertraline does not enhance catecholaminergic activity. Sertraline has no affinity for muscarinic (cholinergic), serotonin, dopamine, adrenergic, histamine, GABA or benzodiazepine receptors. Long-term use of sertraline in animals led to a decrease in the activity of norepinephrine receptors in the brain; other clinically effective antidepressant and anti-obsessional drugs have a similar effect. nine0029
Due to selective inhibition of 5-HT uptake, sertraline does not enhance catecholaminergic activity. Sertraline has no affinity for muscarinic (cholinergic), serotonin, dopamine, adrenergic, histamine, GABA or benzodiazepine receptors. Long-term use of sertraline in animals led to a decrease in the activity of norepinephrine receptors in the brain; other clinically effective antidepressant and anti-obsessional drugs have a similar effect. nine0029
Unlike tricyclic antidepressants, sertraline does not cause weight gain in the treatment of depression or obsessive-compulsive disorder (OCD); in some patients, it even decreases. The development of drug dependence has not been established.
Sertraline is actively biotransformed during the first pass through the liver. The main metabolite, N-desmethylsertraline, is almost 20 times less active than sertraline in vitro and is actually inactive in models of depression in vivo . T ½ N-desmethylsertraline varies within 62–104 hours. Sertraline and N-desmethylsertraline are actively biotransformed in the body; the resulting metabolites are excreted in the feces and urine in equal amounts. Unchanged sertraline is excreted in the urine in small amounts (<0.2%). Eating does not significantly affect the bioavailability of sertraline.
Sertraline and N-desmethylsertraline are actively biotransformed in the body; the resulting metabolites are excreted in the feces and urine in equal amounts. Unchanged sertraline is excreted in the urine in small amounts (<0.2%). Eating does not significantly affect the bioavailability of sertraline.
depression (symptomatic treatment), including accompanied by anxiety, with or without a history of mania. After achieving a clinical effect, maintenance therapy with sertraline can prevent relapses of depression. nine0029
Sertraline is also indicated for the treatment of OCD, including in children. Long-term (up to 2 years) treatment of OCD with sertraline is quite effective, safe and well tolerated after the initial effect is achieved.
Sertraline is also used to treat panic disorders with or without agoraphobia.
sertraline is prescribed once a day in the morning or evening, regardless of the meal. The usual therapeutic dose is 50 mg/day.
Treatment of panic disorders should be started at 25 mg/day, after 1 week of use the dose of sertraline is increased to 50 mg/day. Such a scheme helps to reduce the frequency of occurrence of negative reactions characteristic of panic disorder at an early stage of treatment. nine0029
Such a scheme helps to reduce the frequency of occurrence of negative reactions characteristic of panic disorder at an early stage of treatment. nine0029
The daily dose of the drug for all indications can be increased from 50 mg/day (with insufficient therapeutic effect) to 200 mg/day over several weeks.
Initial therapeutic effect may develop within 7 days; however, it usually takes 2-4 weeks or more to achieve the full effect (in the treatment of OCD). Maintenance dose for long-term treatment should be minimally effective; in the future, it is corrected depending on the therapeutic effect. Like many drugs, sertraline should be used with caution in patients with renal or hepatic impairment. nine0029
The safety and efficacy of sertraline in children aged 6–17 years has been established. The initial dose for the treatment of OCD in children aged 13-17 years is 50 mg / day, for children aged 6-12 years, a dose of 25 mg / day is prescribed initially, after 1 week it is increased to 50 mg / day. If necessary, in the future, the dose can be increased from 50 mg / day (with insufficient therapeutic efficacy) to 200 mg / day. When increasing the dose to prevent overdose, the lower body weight in children compared with adults should be taken into account. Considering 24 hour T ½ sertraline, the dose should be changed no more than once a week.
If necessary, in the future, the dose can be increased from 50 mg / day (with insufficient therapeutic efficacy) to 200 mg / day. When increasing the dose to prevent overdose, the lower body weight in children compared with adults should be taken into account. Considering 24 hour T ½ sertraline, the dose should be changed no more than once a week.
simultaneous treatment with MAO inhibitors, hypersensitivity to sertraline.
possible nausea and other dyspeptic symptoms, unstable stool or diarrhea, tremor, dizziness, insomnia, drowsiness, increased sweating, dry mouth and sexual dysfunction in men (mainly delayed ejaculation), withdrawal syndrome (rare cases), asymptomatic increased activity of ALT and AST (usually during the first 9weeks of treatment and quickly disappear after the abolition of sertraline), hyponatremia, platelet dysfunction, sometimes reactions that cannot be distinguished from the manifestations of the course of the underlying disease: paresthesia, hypoesthesia, symptoms of depression, hallucinations, aggressive behavior, agitation, anxiety and psychosis.
during treatment with antidepressants or anti-obsessional agents, there may be cases of exacerbation of mania or hypomania, convulsions. If seizures occur, in all cases, sertraline should be discontinued. nine0029
Patients with depression are prone to suicidal attempts, therefore, at the beginning of treatment, such patients should be under strict medical supervision.
The safety and efficacy of sertraline in children under 6 years of age have not been established. Sertraline should only be used during pregnancy if the expected benefit to the mother outweighs the potential risk to the fetus. Women of reproductive age during treatment with sertraline should use adequate methods of contraception. There is no information on the penetration of sertraline into breast milk, so it is not recommended to prescribe it during breastfeeding. nine0029
severe reactions (symptoms resembling serotonin syndrome) are possible in patients receiving sertraline in combination with MAO inhibitors. Symptoms of the interaction of selective serotonin reuptake inhibitors and MAO inhibitors include: hyperthermia, rigidity, muscle cramps, autonomic instability, changes in mental state (confusion, irritability and excessive arousal with the development of delirium and coma). In this regard, sertraline should not be used in combination with MAO inhibitors and should not be administered within 14 days after stopping treatment with them. You can prescribe an MAO inhibitor no earlier than 14 days after the abolition of sertraline. nine0029
Symptoms of the interaction of selective serotonin reuptake inhibitors and MAO inhibitors include: hyperthermia, rigidity, muscle cramps, autonomic instability, changes in mental state (confusion, irritability and excessive arousal with the development of delirium and coma). In this regard, sertraline should not be used in combination with MAO inhibitors and should not be administered within 14 days after stopping treatment with them. You can prescribe an MAO inhibitor no earlier than 14 days after the abolition of sertraline. nine0029
When prescribing sertraline with other serotonergic drugs (tryptophan, fenfluramine), care must be taken and, if possible, such a combination should be avoided due to the existing possibility of pharmacodynamic interaction.
Substitution of other antidepressants or anti-obsessional drugs with sertraline should be done with extreme caution, especially when replacing long-acting drugs (eg fluoxetine).
Co-administration of sertraline 200 mg/day does not potentiate the effects of alcohol, carbamazepine, haloperidol, or phenytoin on cognitive and psychomotor function in healthy individuals; however, concomitant use of sertraline and alcohol should be avoided.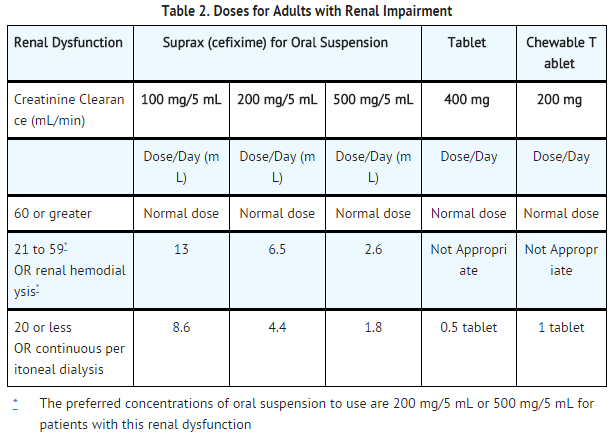 nine0029
nine0029
Co-administration of sertraline with diazepam or tolbutamide results in a small but statistically significant change in some pharmacokinetic parameters. Cimetidine causes a significant decrease in the clearance of sertraline in their combined use. The clinical significance of this effect is unknown. Sertraline had no effect on the β-adrenergic blocking activity of atenolol. Signs of interaction of sertraline at a dose of 200 mg/day with glibenclamide and digoxin were not identified.
Co-administration of sertraline 200 mg/day with warfarin resulted in a slight but statistically significant increase in prothrombin time; the clinical significance of this effect is unknown. In this regard, the prothrombin time should be carefully monitored at the beginning of sertraline therapy and after its withdrawal. nine0029
Caution should be exercised when sertraline is co-administered with drugs such as lithium, the effect of which may be mediated by serotonergic mechanisms.
possible death if the dose of sertraline is exceeded in combination with other drugs and / or alcohol. There is no specific antidote for sertraline. It is advisable to wash the stomach, prescribe enterosorbents, control vital functions, ensure airway patency and adequate ventilation of the lungs, and carry out symptomatic and supportive therapy. Given the large volume of distribution of sertraline, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are ineffective. nine0029
404 Not Found
Effective Date: November 12, 2015
These Online Terms of Use govern your access to websites controlled by Veropharm, including its subsidiaries and affiliates (collectively, "Veropharm ”) that link to these Online Terms of Use (collectively, the “Veropharm Websites”). These Online Terms of Use do not apply to Veropharm's websites that do not link to these Online Terms of Use and third party websites to which Veropharm's websites may be linked. Your use of the Veropharm websites is subject to these Online Terms of Use and the Veropharm Websites Privacy Policy. nine0029
nine0029
To the extent permitted by applicable law, Veropharm reserves the right to amend these Online Terms of Use to reflect technological advances, legal and regulatory changes, and good business practices. If Veropharm makes changes to these Online Terms of Use, the updated version of the Online Terms of Use will reflect the changes and we will notify you by updating the effective date of the Online Terms of Use above. nine0029
By accessing and using the Veropharm websites, you agree that you have read, understood and agreed to be bound by these Online Terms of Use in their current version, which you had the opportunity to review when accessing the company's websites " Veropharm. If you do not agree to these Online Terms of Use or are dissatisfied with the operation of the Veropharm websites, your sole and exclusive remedy, to the extent permitted by applicable law, is to stop using this Veropharm website. nine0029
Disclaimer
You acknowledge and agree that:
a. While we always strive to present the latest developments related to our products and services and other information about Veropharm on Veropharm's websites, the information is provided "AS IS" and may contain technical inaccuracies, typographical errors or be out of date. Veropharm reserves the right to add, remove or change information contained on Veropharm's websites at any time without prior notice. nine0029
While we always strive to present the latest developments related to our products and services and other information about Veropharm on Veropharm's websites, the information is provided "AS IS" and may contain technical inaccuracies, typographical errors or be out of date. Veropharm reserves the right to add, remove or change information contained on Veropharm's websites at any time without prior notice. nine0029
b. Veropharm makes no representations or warranties of any kind or nature regarding the information or data posted on Veropharm's websites.
To the extent permitted by applicable law, Veropharm hereby disclaims any and all representations or warranties, expressed or implied, statutory, contractual or otherwise, and in no event shall Veropharm be liable for any damages of any kind or nature, including, without limitation, direct, indirect, special (including loss of profits), consequential or incidental, damages arising out of or in connection with the existence or use of the Veropharm websites, and/or information or information posted on the websites of Veropharm, regardless of whether Veropharm anticipated the possibility of such damage. nine0029
nine0029
in. Veropharm is not responsible for and makes no warranties regarding the accuracy, effectiveness, timeliness or acceptability of any information or information obtained from third parties, including hyperlinks to or from third party websites. Except as expressly provided on Veropharm's websites, Veropharm does not edit, review, or otherwise control content submitted by third parties on bulletin boards, chat rooms, or other similar forums posted on the company's websites. Veropharm. In this regard, such information should be considered suspicious and is not confirmed by Veropharm. nine0029
Veropharm's websites may contain forward-looking statements that reflect Veropharm's expectations of future events and business developments. Forward-looking statements involve risks and uncertainties. Actual developments or results may differ materially from those anticipated and depend on a variety of factors, including (but not limited to) the successful completion of ongoing development programs, the results of current or future clinical studies, ongoing commercial product introduction, regulatory approval of pharmaceuticals, credibility and compliance. the validity of patents, the stability of commercial relationships and general economic conditions. Veropharm intends to update its websites regularly, but does not assume any obligation to update any content on the websites. nine0029
the validity of patents, the stability of commercial relationships and general economic conditions. Veropharm intends to update its websites regularly, but does not assume any obligation to update any content on the websites. nine0029
Your use of
You understand, acknowledge and agree that:
a. By using the Veropharm websites, you agree not to modify or destroy our electronic information posted on the Veropharm websites or on any of our servers. In addition, you also agree not to attempt to circumvent the security measures of Veropharm's websites and to comply with all applicable local, state, federal and international laws, rules and regulations. nine0029
b. You grant Veropharm the right to use any materials that you upload or otherwise transmit to Veropharm websites in accordance with these Online Terms of Use and the Veropharm Websites Privacy Policy , by any means that Veropharm deems preferable, including, but not limited to, copying, displaying, reproducing or publishing in any format, modifying materials, incorporating into other materials or making works based on these materials. nine0029
nine0029
in. Except as specifically provided and agreed in advance by Veropharm, a confidential relationship between Veropharm and a user of the Veropharm websites will not arise if the user of the Veropharm websites sends any or verbal, written or electronic communication from Veropharm (feedback, questions, comments, suggestions, ideas, etc.).
If any Veropharm website requires or asks for such information, and this information contains personally identifiable information (for example, name, address, telephone number, email address), Veropharm intends to obtain, use and store this information with the consent of the respective user in accordance with the provisions specified in the Policy regarding the processing of personal data on the websites of Veropharm. nine0029
Otherwise, such communications and any information provided in their context will be treated as non-confidential and Veropharm shall have the right to reproduce, publish or otherwise use this information for any purpose whatsoever, including, without restrictions, research, development, production, use or sale of products involving the implementation of this information. The person who sent any information to Veropharm is fully responsible for its content, including its reliability, accuracy and the fact that it does not violate anyone's rights, including property rights. nine0029
The person who sent any information to Veropharm is fully responsible for its content, including its reliability, accuracy and the fact that it does not violate anyone's rights, including property rights. nine0029
Product labeling
Veropharm's local websites contain general information about Veropharm and its products that are officially registered in the respective territory and are in free circulation, promotional materials that strictly comply with local legislation, as well as other scientific information that we consider useful for posting on the relevant website for your personal non-commercial purposes or to enhance your professional level if you are a medical, pharmaceutical or other healthcare professional. nine0029
However, please note that the local websites of the respective country may contain links to foreign websites of Veropharm. In such a case, product names, descriptions and labels may be more closely related or created under the laws of a country other than your country of permanent residence. Some products may not be available in all countries or may be available under different brand names, strengths, or indications. Many of the products listed can only be dispensed on the prescription of a local healthcare professional. nine0029
Some products may not be available in all countries or may be available under different brand names, strengths, or indications. Many of the products listed can only be dispensed on the prescription of a local healthcare professional. nine0029
Except as otherwise agreed in advance by Veropharm, directors, employees, agents or representatives of Veropharm, its subsidiaries and affiliates shall not participate in medical consultations, diagnosis, treatment or other medical services that could to create any kind of relationship, such as "doctor-patient", through the websites of Veropharm. In any case, no information about our products posted on our websites should be considered and understood as direct advice from a specialist or a substitute for such advice from an appropriate specialist (physician). nine0029
Please note that Veropharm products have contraindications for use, so before using them, you should carefully read the instructions for their use and seek medical advice.
Under no circumstances should the information posted on the websites of Veropharm be used for self-diagnosis of your health and possible diseases.
In accordance with the requirements of the legislation of the Russian Federation, the information located in some sections of the Veropharm websites may be intended exclusively for medical and pharmaceutical workers, as well as other workers in the healthcare system. In this case, access to such sections may be restricted in accordance with the rules specified in the Terms of Use section on prescription drugs and medical devices, the use of which requires special training. nine0029
To enter such sections, Veropharm reserves the right to ask you to answer some questions related to medicine or pharmaceuticals and / or provide information by performing additional registration on websites for the purpose of confirming the actual status of a medical, pharmaceutical worker or other healthcare professional at the time of visiting the relevant website.
Intellectual property
The information, documents and related graphics published on the Veropharm websites (hereinafter referred to as the “Information”) are the exclusive property of Veropharm, except for information provided by a third party associated with Veropharm » contractual relationship. Permission to use the Information is granted on the condition that (1) all copies cite the original source and the above copyright notice; (2) Information will be used for informational non-commercial purposes and only for personal use; (3) The information will not be changed in any way; (4) the graphics displayed on this Veropharm website will not be used in isolation from the accompanying text. nine0029
Permission to use the Information is granted on the condition that (1) all copies cite the original source and the above copyright notice; (2) Information will be used for informational non-commercial purposes and only for personal use; (3) The information will not be changed in any way; (4) the graphics displayed on this Veropharm website will not be used in isolation from the accompanying text. nine0029
Veropharm is not responsible for content provided by a third party, and you may not use or distribute such materials without the permission of their copyright holders. Except as permitted above, no license or right, express or implied, is granted to anyone under any of Veropharm's patents, trademarks or other proprietary rights.
Veropharm's trademarks, trade names, trade dress or products may not be used on VEROPHARM's websites without the prior written permission of VEROPHARM. nine0029
PRIVACY AND SECURITY
Veropharm is committed to maintaining the confidentiality of your information transmitted through this website.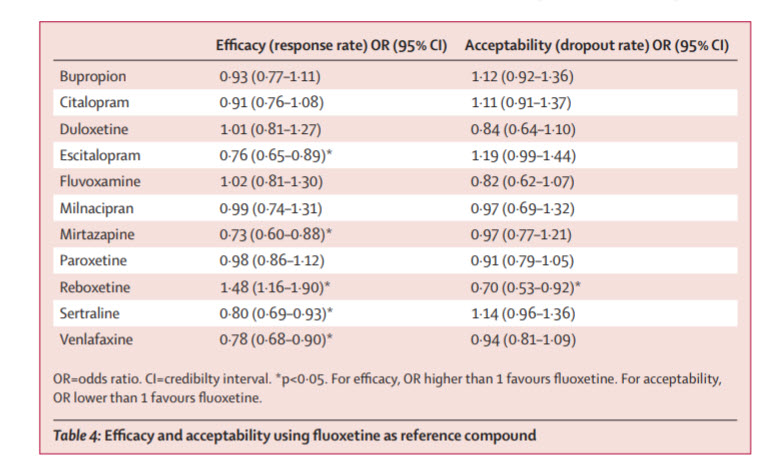 We recognize the importance of privacy for our consumers and visitors to Veropharm's websites. Our use of personal data is governed by our Policy regarding the processing of personal data on Veropharm's websites, to which you have confirmed your consent by starting to use Veropharm's websites. nine0029
We recognize the importance of privacy for our consumers and visitors to Veropharm's websites. Our use of personal data is governed by our Policy regarding the processing of personal data on Veropharm's websites, to which you have confirmed your consent by starting to use Veropharm's websites. nine0029
You hereby acknowledge and agree that when submitting your personal data to Veropharm's websites, despite the fact that Veropharm has effective security measures in place to prevent unauthorized access or interference, the absolute confidentiality of your personal data provided on the websites of Veropharm cannot be entirely dependent on the measures taken by Veropharm.
In the unlikely event that, despite our efforts, tampering or unauthorized access occurs, Veropharm will not be liable for such tampering or unauthorized access, to the extent permitted by applicable law, or for any direct, indirect, special , incidental or consequential damages (also lost profits) from which the consumer or user will suffer, even if Veropharm was previously warned of the possibility of such damage, Veropharm does not guarantee, expressly or impliedly, that the information, provided by the user is not subject to tampering or unauthorized access, and provides no implied warranties as to merchantability or fitness for a particular purpose. nine0029
nine0029
Each user is solely responsible for maintaining the confidentiality of their password.
Limitations of Liability
Veropharm does not assume any liability with respect to materials, information or opinions presented, sent or otherwise found on Veropharm's websites. You may rely on the accuracy of these materials, information and opinions solely at your own risk. Veropharm shall not be liable for harm and/or damage resulting from the use of Veropharm's websites or materials presented on them. nine0029
Veropharm's websites, site content, products and services provided on or through Veropharm's websites are provided on an "as is" and "as available" basis, with all its consequences. In no event shall Veropharm or its suppliers or, as appropriate, their directors, employees or agents (hereinafter referred to as "persons associated with Veropharm" be liable for any damages of any kind arising out of or in connection with your use of, or inability to use, the Veropharm websites. 0029
0029
As well as the materials of the Sites, the services provided on or through the Sites, or on any linked Sites, including any special, indirect, punitive, incidental, punitive or consequential damages, including (but (but not limited to) harm, loss of profit or damage arising from delay, suspension of services, viruses, deletion of files or electronic messages, errors, omissions or other inaccuracies on the Veropharm websites or in the materials of the sites, regardless of whether whether this is due to any omissions on the part of Veropharm and whether Veropharm was warned of the possibility of such damage. nine0029
Please note that additional legal notices, disclaimers and other terms and conditions may apply to Veropharm websites.
General Provisions
You hereby agree that these Online Terms of Use and the Privacy Policy on Veropharm's websites constitute a single, indivisible agreement. You hereby agree that by reviewing these Online Terms of Use and the Policy regarding the processing of personal data on the Veropharm websites. You agree to be bound by them, and you acknowledge that these Terms of Online Use, as well as other terms of operation of the Veropharm Websites, are governed by, among other things, the laws of the State of Illinois and other federal laws of the United States. nine0029
You agree to be bound by them, and you acknowledge that these Terms of Online Use, as well as other terms of operation of the Veropharm Websites, are governed by, among other things, the laws of the State of Illinois and other federal laws of the United States. nine0029
The laws of the State of Illinois will govern these Online Terms of Use to the extent that the laws of the State of Illinois do not conflict with the mandatory laws of the Russian Federation, in particular consumer protection laws. In the event that the competent judicial authorities determine that any provision of these Online Terms of Use is invalid or unenforceable, you agree that the remaining provisions of these Online Terms of Use will remain in full force and effect. nine0029
In connection with the foregoing, any of your actions aimed at your use of the Veropharm websites through your access or other use of the websites and the information contained therein, you acknowledge that you have read these Terms of Online Use and fully agree with such Online Terms of Use.