Newest antidepressants on the market
Newest Antidepressants: Brexanolone, Esketamine, Agomelatine
Antidepressants are medications that can help relieve depression symptoms, like fatigue and emotional numbness.
Several different kinds of antidepressants exist, but the most commonly prescribed are selective serotonin reuptake inhibitors (SSRIs).
Fluoxetine (Prozac) entered the market in 1988 as the first SSRI, and for the next 30 years, many experts considered SSRIs the “modern” antidepressant.
In 2019, the Food and Drug Administration (FDA) approved two new antidepressants, brexanolone and esketamine. There’s also been renewed interest in agomelatine, an antidepressant not currently available in the United States.
Read on to learn more about these new antidepressants, including how they compare to SSRIs, their side effects, and how to try them.
In 2019, the FDA approved brexanolone (Zulresso) as the first drug specifically designed to treat moderate to severe postpartum depression (PPD).
Experts consider some SSRIs safe to take while pregnant or nursing, but these medications may not lead to much improvement for several weeks. When you have PPD, symptoms don’t just affect your own well-being — they can also have long-term effects on your bond with your baby.
Brexanolone, however, begins to take effect immediately. According to two randomized clinical trials published in 2018, this medication can significantly reduce PPD symptoms — benefits that held when researchers followed up with participants 30 days after treatment.
Learn more about treatment for postpartum depression.
How it works
Brexanolone raises brain levels of the neurotransmitter gamma aminobutyric acid (GABA).
In a nutshell, GABA dampens the chemical activity in your neurons, almost like a dimmer switch for certain cells.
Scientists aren’t sure exactly how brexanolone treats PPD symptoms, but one theory suggests that with PPD, your GABA levels don’t recover quickly enough from pregnancy to manage your stress. Essentially, cortisol hormones rise unchecked, contributing to symptoms of depression. Brexanolone, then, may offer a “reset” by restoring your GABA levels.
Essentially, cortisol hormones rise unchecked, contributing to symptoms of depression. Brexanolone, then, may offer a “reset” by restoring your GABA levels.
You receive this medication as a one-time IV treatment over the course of 2 and a half days. You’ll remain in your healthcare center for the entire 60-hour treatment for monitoring.
Safety and side effects
Like other drugs that affect GABA levels, brexanolone can cause sedation. Roughly 1 in 4 people experience sedation-related side effects in the first 24 hours of treatment.
You may feel:
- extremely sleepy, even during the day
- unfocused and distractible
- dizzy or faint
Your care team will check on you every 2 hours for extreme symptoms like fainting. If you experience serious side effects, they’ll stop the infusion. Sedation-related symptoms should stop within 15 minutes after the IV infusion stops.
How to get a prescription
You can only receive this treatment from approved healthcare centers, and you’ll need a doctor’s referral to join the treatment program.
The treatment can cost up to $34,000, though health insurance can help offset some of the cost. Companies like Aetna and Cigna require pre-authorization, so you’ll want to make sure your insurance provider covers the treatment before you check in to your clinic.
Keep in mind, too, that most insurance companies only cover one round of treatment, as research has yet to explore the potential benefits of additional rounds of treatment.
The company that makes brexanolone also offers several financial assistance programs, which could be worth considering if the price tag puts it out of reach.
Esketamine (Spravato) is a chemical cousin of the anesthetic ketamine. The FDA approved esketamine in 2019 to treat treatment-resistant depression, or depression that persists after you try at least two different antidepressant treatments.
During clinical trials, doctors gave participants a nasal esketamine spray or a placebo spray. All participants also took an oral antidepressant they hadn’t tried before. Compared to people who took an oral antidepressant and used a placebo spray, those who used the esketamine spray reported greater symptom relief and longer symptom-free periods.
Compared to people who took an oral antidepressant and used a placebo spray, those who used the esketamine spray reported greater symptom relief and longer symptom-free periods.
How it works
Esketamine sets off a chain reaction of chemicals that ultimately raises your levels of brain-derived neurotrophic factor (BDNF). BDNF helps your neurons make new connections, which in turn enables you to form memories, learn new information, and develop different habits.
Depression typically involves low BDNF levels, and your brain may have difficulty adapting to changes. Esketamine helps restore BDNF levels, along with overall brain plasticity.
As with brexanolone, you have to take esketamine in the presence of a healthcare professional. Your doctor or clinician will give you a dose between 56–84 milligrams (mg), which you spray into your nostrils. You then relax in a chair for 2 hours. Your care team will monitor your blood pressure and heart rate during this time.
This medication works quickly, with many people noticing relief right away. Treatment requires multiple sessions, typically twice a week for the first 28 days and then spaced out over time. The effects usually last until your next dose.
Treatment requires multiple sessions, typically twice a week for the first 28 days and then spaced out over time. The effects usually last until your next dose.
Safety and side effects
In clinical trials, participants tended to report mild to moderate side effects. You may feel sleepy, dizzy, or a bit “out of it” during your treatment session. These side effects often go away within 90 minutes after you take your dose.
On rare occasions, people have reported more severe side effects like:
- vomiting
- anxiety and confusion
- worsening depression or suicidal thoughts
Esketamine can also lead to significant increases in blood pressure during the treatment session, which is why you’ll need monitoring for 2 hours. If you have hypertension or another vascular condition, make sure to tell your doctor before receiving treatment.
How to get a prescription
You can only receive this treatment at an approved healthcare center, so you’ll need to ask your doctor or psychiatrist if you’d like to try esketamine.
The course of treatment instead depends on the severity of your symptoms — and how much you’re able to pay. As of 2021, a standard 56-mg dose of esketamine costs $590, and a large dose of 84 mg costs $885.
The initial month of therapy is often the most expensive since treatment guidelines recommend twice-weekly treatment for the first month. This first month can cost anywhere from $4,800 and $6,800 dollars.
To date, no official guidelines set an ideal length of treatment.
According to the company that produces Spravato, some insurance programs cover most of the cost of esketamine. You’ll only need to pay a $10 copay per session until you hit the benefits cap of $7,150 per year.
Agomelatine (Valdoxan), an oral antidepressant, has been available in some other countries since 2009, though you can’t get this medication in the United States.
You take agomelatine as a 25-mg pill once a day at bedtime. If your depression symptoms don’t respond, a doctor may increase the dose to 50 mg per day.
Agomelatine may have particular benefit for depression that:
- involves disruptions in your circadian rhythm (sleep-wake cycle)
- involves anhedonia, or difficulty feeling pleasure
- happens with a health condition, such as Parkinson’s disease or type II diabetes
- happens with seasonal changes
- occurs as part of bipolar disorder
How it works
Agomelatine has two main effects on your brain. It increases activity at melatonin nerve receptors, which helps you sleep. It also decreases activity at specific serotonin receptors and helps increase dopamine and norepinephrine in the frontal cortex.
Increasing melatonin levels can improve sleep-related issues. In a small 2018 study that included 24 young adults, researchers found that the more agomelatine shifted the participants’ circadian rhythms, the more their depression symptoms improved.
Experts don’t yet know exactly how decreasing serotonin fits into the picture.
That said, older animal research from 2014 suggests boosting melatonin and decreasing serotonin binding to receptors simultaneously may help protect newly created neurons by shielding them from the damage caused by chronic stress.
Safety and side effects
Agomelatine may cause:
- nausea and vomiting
- constipation, stomach pain, and diarrhea
- drowsiness or difficulty sleeping
- headaches
But many people only experience mild side effects while taking agomelatine, which may partially explain the renewed interest in this antidepressant.
After all, if you don’t experience severe side effects, you’ll probably feel more inclined to continue taking the medication.
How agomelatine comparesA 2018 review including 522 trials with a total of 116,477 participants compared 21 antidepressants.
When the review authors considered dropout rates specifically due to adverse reactions, they discovered all included antidepressants had a higher dropout rate than the placebo — except agomelatine.
Incidentally, researchers also listed agomelatine as one of seven most effective antidepressants among those studied.
With many antidepressants, you may experience flu-like symptoms if you suddenly stop taking the medication. This phenomenon, called discontinuation syndrome, doesn’t occur with agomelatine. You can easily stop even long-term agomelatine use.
This phenomenon, called discontinuation syndrome, doesn’t occur with agomelatine. You can easily stop even long-term agomelatine use.
So, you might wonder: If this medication works so well, what’s holding up approval in the United States?
Agomelatine does have the potential to cause one very severe side effect: liver toxicity. The medication raises the levels of liver amino acids called transaminases, causing liver damage for up to 4.6% of people who take it. People taking this medication need to have their liver function tested at weeks 3, 6, 12, and 24 of treatment.
Other antidepressants can have a similar impact on your liver, but at much lower rates:
- placebo: 2.1%
- escitalopram (Lexapro): 1.4%
- paroxetine (Paxil): 0.6%
- fluoxetine (Prozac): 0.4%
How to get a prescription
Currently, you can’t get a prescription for agomelatine in the United States or Canada, as regulatory agencies have deemed the risk of liver injury too high to allow the drug on the market.
It’s possible that agomelatine could become more widely available in the future if researchers identify a way to minimize the risk of liver toxicity.
Doctors in Europe and Australia may prescribe this medication.
Brexanolone and esketamine appear to have the most benefit for postpartum depression and treatment-resistant depression, respectively. These medications also come with a high price tag that can make them harder to access.
Agomelatine can effectively treat a wider range of depression subtypes. But it also carries a risk of liver toxicity, and it hasn’t been approved for use in the United States.
To sum up, these medications likely won’t replace SSRIs as the first line of treatment for other types of depression anytime soon. Still, their existence opens up possibilities for future advances in depression treatment.
Emily Swaim is a freelance health writer and editor who specializes in psychology. She has a BA in English from Kenyon College and an MFA in writing from California College of the Arts. In 2021, she received her Board of Editors in Life Sciences (BELS) certification. You can find more of her work on GoodTherapy, Verywell, Investopedia, Vox, and Insider. Find her on Twitter and LinkedIn.
In 2021, she received her Board of Editors in Life Sciences (BELS) certification. You can find more of her work on GoodTherapy, Verywell, Investopedia, Vox, and Insider. Find her on Twitter and LinkedIn.
Newest Antidepressants: Brexanolone, Esketamine, Agomelatine
Antidepressants are medications that can help relieve depression symptoms, like fatigue and emotional numbness.
Several different kinds of antidepressants exist, but the most commonly prescribed are selective serotonin reuptake inhibitors (SSRIs).
Fluoxetine (Prozac) entered the market in 1988 as the first SSRI, and for the next 30 years, many experts considered SSRIs the “modern” antidepressant.
In 2019, the Food and Drug Administration (FDA) approved two new antidepressants, brexanolone and esketamine. There’s also been renewed interest in agomelatine, an antidepressant not currently available in the United States.
Read on to learn more about these new antidepressants, including how they compare to SSRIs, their side effects, and how to try them.
In 2019, the FDA approved brexanolone (Zulresso) as the first drug specifically designed to treat moderate to severe postpartum depression (PPD).
Experts consider some SSRIs safe to take while pregnant or nursing, but these medications may not lead to much improvement for several weeks. When you have PPD, symptoms don’t just affect your own well-being — they can also have long-term effects on your bond with your baby.
Brexanolone, however, begins to take effect immediately. According to two randomized clinical trials published in 2018, this medication can significantly reduce PPD symptoms — benefits that held when researchers followed up with participants 30 days after treatment.
Learn more about treatment for postpartum depression.
How it works
Brexanolone raises brain levels of the neurotransmitter gamma aminobutyric acid (GABA).
In a nutshell, GABA dampens the chemical activity in your neurons, almost like a dimmer switch for certain cells.
Scientists aren’t sure exactly how brexanolone treats PPD symptoms, but one theory suggests that with PPD, your GABA levels don’t recover quickly enough from pregnancy to manage your stress. Essentially, cortisol hormones rise unchecked, contributing to symptoms of depression. Brexanolone, then, may offer a “reset” by restoring your GABA levels.
You receive this medication as a one-time IV treatment over the course of 2 and a half days. You’ll remain in your healthcare center for the entire 60-hour treatment for monitoring.
Safety and side effects
Like other drugs that affect GABA levels, brexanolone can cause sedation. Roughly 1 in 4 people experience sedation-related side effects in the first 24 hours of treatment.
You may feel:
- extremely sleepy, even during the day
- unfocused and distractible
- dizzy or faint
Your care team will check on you every 2 hours for extreme symptoms like fainting. If you experience serious side effects, they’ll stop the infusion. Sedation-related symptoms should stop within 15 minutes after the IV infusion stops.
Sedation-related symptoms should stop within 15 minutes after the IV infusion stops.
How to get a prescription
You can only receive this treatment from approved healthcare centers, and you’ll need a doctor’s referral to join the treatment program.
The treatment can cost up to $34,000, though health insurance can help offset some of the cost. Companies like Aetna and Cigna require pre-authorization, so you’ll want to make sure your insurance provider covers the treatment before you check in to your clinic.
Keep in mind, too, that most insurance companies only cover one round of treatment, as research has yet to explore the potential benefits of additional rounds of treatment.
The company that makes brexanolone also offers several financial assistance programs, which could be worth considering if the price tag puts it out of reach.
Esketamine (Spravato) is a chemical cousin of the anesthetic ketamine. The FDA approved esketamine in 2019 to treat treatment-resistant depression, or depression that persists after you try at least two different antidepressant treatments.
During clinical trials, doctors gave participants a nasal esketamine spray or a placebo spray. All participants also took an oral antidepressant they hadn’t tried before. Compared to people who took an oral antidepressant and used a placebo spray, those who used the esketamine spray reported greater symptom relief and longer symptom-free periods.
How it works
Esketamine sets off a chain reaction of chemicals that ultimately raises your levels of brain-derived neurotrophic factor (BDNF). BDNF helps your neurons make new connections, which in turn enables you to form memories, learn new information, and develop different habits.
Depression typically involves low BDNF levels, and your brain may have difficulty adapting to changes. Esketamine helps restore BDNF levels, along with overall brain plasticity.
As with brexanolone, you have to take esketamine in the presence of a healthcare professional. Your doctor or clinician will give you a dose between 56–84 milligrams (mg), which you spray into your nostrils. You then relax in a chair for 2 hours. Your care team will monitor your blood pressure and heart rate during this time.
You then relax in a chair for 2 hours. Your care team will monitor your blood pressure and heart rate during this time.
This medication works quickly, with many people noticing relief right away. Treatment requires multiple sessions, typically twice a week for the first 28 days and then spaced out over time. The effects usually last until your next dose.
Safety and side effects
In clinical trials, participants tended to report mild to moderate side effects. You may feel sleepy, dizzy, or a bit “out of it” during your treatment session. These side effects often go away within 90 minutes after you take your dose.
On rare occasions, people have reported more severe side effects like:
- vomiting
- anxiety and confusion
- worsening depression or suicidal thoughts
Esketamine can also lead to significant increases in blood pressure during the treatment session, which is why you’ll need monitoring for 2 hours. If you have hypertension or another vascular condition, make sure to tell your doctor before receiving treatment.
How to get a prescription
You can only receive this treatment at an approved healthcare center, so you’ll need to ask your doctor or psychiatrist if you’d like to try esketamine.
The course of treatment instead depends on the severity of your symptoms — and how much you’re able to pay. As of 2021, a standard 56-mg dose of esketamine costs $590, and a large dose of 84 mg costs $885.
The initial month of therapy is often the most expensive since treatment guidelines recommend twice-weekly treatment for the first month. This first month can cost anywhere from $4,800 and $6,800 dollars.
To date, no official guidelines set an ideal length of treatment.
According to the company that produces Spravato, some insurance programs cover most of the cost of esketamine. You’ll only need to pay a $10 copay per session until you hit the benefits cap of $7,150 per year.
Agomelatine (Valdoxan), an oral antidepressant, has been available in some other countries since 2009, though you can’t get this medication in the United States.
You take agomelatine as a 25-mg pill once a day at bedtime. If your depression symptoms don’t respond, a doctor may increase the dose to 50 mg per day.
Agomelatine may have particular benefit for depression that:
- involves disruptions in your circadian rhythm (sleep-wake cycle)
- involves anhedonia, or difficulty feeling pleasure
- happens with a health condition, such as Parkinson’s disease or type II diabetes
- happens with seasonal changes
- occurs as part of bipolar disorder
How it works
Agomelatine has two main effects on your brain. It increases activity at melatonin nerve receptors, which helps you sleep. It also decreases activity at specific serotonin receptors and helps increase dopamine and norepinephrine in the frontal cortex.
Increasing melatonin levels can improve sleep-related issues. In a small 2018 study that included 24 young adults, researchers found that the more agomelatine shifted the participants’ circadian rhythms, the more their depression symptoms improved.
Experts don’t yet know exactly how decreasing serotonin fits into the picture.
That said, older animal research from 2014 suggests boosting melatonin and decreasing serotonin binding to receptors simultaneously may help protect newly created neurons by shielding them from the damage caused by chronic stress.
Safety and side effects
Agomelatine may cause:
- nausea and vomiting
- constipation, stomach pain, and diarrhea
- drowsiness or difficulty sleeping
- headaches
But many people only experience mild side effects while taking agomelatine, which may partially explain the renewed interest in this antidepressant.
After all, if you don’t experience severe side effects, you’ll probably feel more inclined to continue taking the medication.
How agomelatine comparesA 2018 review including 522 trials with a total of 116,477 participants compared 21 antidepressants.
When the review authors considered dropout rates specifically due to adverse reactions, they discovered all included antidepressants had a higher dropout rate than the placebo — except agomelatine.

Incidentally, researchers also listed agomelatine as one of seven most effective antidepressants among those studied.
With many antidepressants, you may experience flu-like symptoms if you suddenly stop taking the medication. This phenomenon, called discontinuation syndrome, doesn’t occur with agomelatine. You can easily stop even long-term agomelatine use.
So, you might wonder: If this medication works so well, what’s holding up approval in the United States?
Agomelatine does have the potential to cause one very severe side effect: liver toxicity. The medication raises the levels of liver amino acids called transaminases, causing liver damage for up to 4.6% of people who take it. People taking this medication need to have their liver function tested at weeks 3, 6, 12, and 24 of treatment.
Other antidepressants can have a similar impact on your liver, but at much lower rates:
- placebo: 2.1%
- escitalopram (Lexapro): 1.
 4%
4% - paroxetine (Paxil): 0.6%
- fluoxetine (Prozac): 0.4%
How to get a prescription
Currently, you can’t get a prescription for agomelatine in the United States or Canada, as regulatory agencies have deemed the risk of liver injury too high to allow the drug on the market.
It’s possible that agomelatine could become more widely available in the future if researchers identify a way to minimize the risk of liver toxicity.
Doctors in Europe and Australia may prescribe this medication.
Brexanolone and esketamine appear to have the most benefit for postpartum depression and treatment-resistant depression, respectively. These medications also come with a high price tag that can make them harder to access.
Agomelatine can effectively treat a wider range of depression subtypes. But it also carries a risk of liver toxicity, and it hasn’t been approved for use in the United States.
To sum up, these medications likely won’t replace SSRIs as the first line of treatment for other types of depression anytime soon. Still, their existence opens up possibilities for future advances in depression treatment.
Still, their existence opens up possibilities for future advances in depression treatment.
Emily Swaim is a freelance health writer and editor who specializes in psychology. She has a BA in English from Kenyon College and an MFA in writing from California College of the Arts. In 2021, she received her Board of Editors in Life Sciences (BELS) certification. You can find more of her work on GoodTherapy, Verywell, Investopedia, Vox, and Insider. Find her on Twitter and LinkedIn.
Avelity: A New Antidepressant for the Most Severe Depressions
Main
Avelity (Auvelity, dextromethorphan + bupropion, 45mg/105mg) is a new oral drug for the treatment of major depressive disorder in adults.
Unlike all existing oral antidepressants, Aveliti, also known under the code name AXS-05, is characterized by a rapid and long-lasting therapeutic effect. Improvement of depressive symptoms is noted literally in a week, remission occurs in two. "Avelity" is able to successfully cope with the symptoms of even a severe form of depression in most patients.
The medical need for new antidepressants that are effective and safe is extremely high due to the continuing increase in the prevalence of depression worldwide.
Thus, in the US alone in 2020, there were 21 million adults who had experienced at least one episode of major depressive disorder in the previous year. Between March and April 2021, an estimated 85 million American adults are experiencing major depressive symptoms, driven in large part by the ongoing COVID-19 pandemic..
Avility was developed by Axsome Therapeutics.
The U.S. Food and Drug Administration (FDA) approved Avility in mid-August 2022.
"Aveliti" will go on sale at the beginning of the fourth quarter of 2022.
The price of "Aveliti" will be determined in the coming weeks.
Aksam is looking for a commercial partner to bring the drug to the market outside the US.
The American regulator had to decide on the approval of "Aveliti" at the end of August 2021. However, the FDA requested additional data from Aksam to resolve the incompleteness of the registration dossier related to analytical methods in the section "Chemical properties, production process and quality control". At that time, the stock quotes of the company lost almost 40%, later winning back the fall, although not in full.
However, the FDA requested additional data from Aksam to resolve the incompleteness of the registration dossier related to analytical methods in the section "Chemical properties, production process and quality control". At that time, the stock quotes of the company lost almost 40%, later winning back the fall, although not in full.
Axam intends to expand the range of indications for Avality by proving its applicability in the treatment of agitation in Alzheimer's disease and as an adjunct in the treatment of nicotine dependence for those who want to quit smoking.
What is Major Depressive Disorder? symptoms. In severe cases, the patient may resort to suicide. [12]
Depression is a worldwide disease and one of the leading causes of disability. Depression affects approximately 3.8% of the world's population, and about 280 million people suffer from it. In low- and middle-income countries, the problem of depression is particularly acute: factors such as lack of resources, lack of trained health workers, and social stigma associated with mental disorders pose obstacles to the effective treatment of depression. It is not uncommon for people with depression to be misdiagnosed in countries of all income levels. [3]
It is not uncommon for people with depression to be misdiagnosed in countries of all income levels. [3]
Major depressive disorder is difficult to treat: nearly two-thirds of patients do not adequately respond to existing first-line oral antidepressants. Most initial treatment failures also end in the failure of all subsequent lines of therapy. [four]
Patients with clinical depression are diagnosed with treatment-resistant depression if they do not respond to two or more lines of antidepressant therapy of different pharmacological groups.
Currently approved oral antidepressants act predominantly through monoaminergic mechanisms. Since achieving a clinically significant response requires a long time of their continuous use (up to 6-8 weeks), and the therapy itself is accompanied by adverse events, this seriously affects the adherence of patients to treatment: they often stop therapy prematurely. [4] [5] [6]
That is why there is a high unmet medical need for fundamentally new pharmacotherapeutic options for the treatment of clinical depression: such drugs must be well tolerated, act quickly, and provide a stable clinical effect. [7]
[7]
Avelity: the combined mechanism of action of dextromethorphan with bupropion
The oral combination drug Avelity (Auvelity, dextromethorphan + bupropion, 45 mg/105 mg), codenamed AXS-05, is an N-receptor antagonist methyl-D-aspartate (NMDA) with multimodal activity.
Dextromethorphan, being a noncompetitive NMDA receptor antagonist, also works as a sigma-1 receptor agonist and an inhibitor of serotonin and norepinephrine transport proteins.
Antagonism of the NMDA receptor, which is an ionotropic glutamate receptor, causes an antidepressant effect realized by modulation of glutamate neurotransmission due to changes in the inhibitory tone of interneurons and/or a direct effect on the postsynaptic NMDA receptor. Sigma-1 receptor agonism modulates glutamate and monoamine signaling. [one]
NMDA receptor antagonism with ketamine is well known to produce a rapid and significant antidepressant effect. [2] However, the use of ketamine is accompanied by a number of difficulties, including parenteral administration, potential for abuse, psychotomimetic side effects, and a narrow therapeutic window. However, March 2019Janssen, part of Johnson & Johnson, succeeded in bringing Spravato (esketamine) nasal spray to the market for treatment-resistant depression and then adding a treatment in August 2020 major depressive disorder with acute suicidal ideation and behavior. Esketamine is the S(+) enantiomer of ketamine.
However, March 2019Janssen, part of Johnson & Johnson, succeeded in bringing Spravato (esketamine) nasal spray to the market for treatment-resistant depression and then adding a treatment in August 2020 major depressive disorder with acute suicidal ideation and behavior. Esketamine is the S(+) enantiomer of ketamine.
The similarity between dextromethorphan and ketamine in terms of receptor pharmacology and pharmacodynamic effects suggests that dextromethorphan has antidepressant efficacy without the limitations of ketamine. However, whether the antidepressant effect is due to NMDA receptor antagonism is still unclear. [3] [4]
Bupropion serves as a bioavailability booster for dextromethorphan, which is metabolized too rapidly in the human body to achieve plasma concentrations necessary to achieve desired psychotherapeutic effects.
Since bupropion inhibits cytochrome P450 2D6 (CYP2D6) and dextromethorphan is a substrate for this enzyme, the CYP2D6-mediated metabolism of dextromethorphan is suppressed, which is reflected by an increase in its plasma level and an extension of the half-life. Thus, the antidepressant effect is endowed with a stable character. [5]
Thus, the antidepressant effect is endowed with a stable character. [5]
Bupropion is a dual norepinephrine and dopamine reuptake inhibitor. [6]
In addition, dextromethorphan and bupropion are nicotinic acetylcholine receptor antagonists.
The complex antidepressant mechanism of action of "Aveliti" is tied to a number of signaling cascades related to synaptic plasticity, and is provided by strengthening synaptic connections and improving communication interactions between brain cells. The drug increases brain levels of serotonin, norepinephrine and dopamine, which are key neurotransmitters involved in mood regulation.
"Aveliti" also has anti-inflammatory properties. [7] [8] [9] [10] [11] There is an association between depression and inflammation: patients with depression have elevated serum levels of interleukin 6 (IL-6), interleukin 1 (IL-1), C-reactive protein (CRP), tumor necrosis factor (TNF) and other inflammatory biomarkers. [12] [13] [14] [15] [16]
Avality: efficacy and safety in the treatment of major depressive disorder
Main study
The GEMINI (NCT04019704) phase III clinical trial (randomized, double-blind, placebo-controlled, multicentre) enrolled adult patients (n=327) with moderate-severe (total MADRS score ≥ 25) major depressive disorder.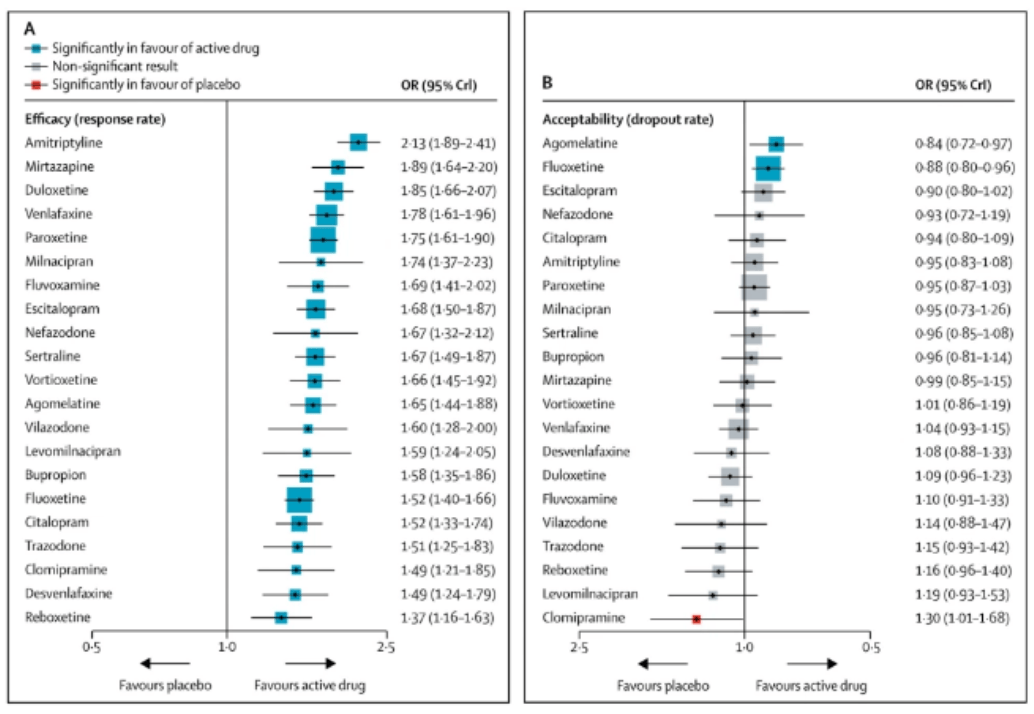
Baseline characteristics of the subjects: average age 42 years, women 66%, average total MADRS score is 33.
Mosmedpreparaty
Patients were given placebo or dextromethorphan plus buprion twice daily for 6 weeks; participants received only one dose for the first three days.
The primary endpoint of treatment efficacy was stated as change in total score on the Montgomery-Osberg Depression Rating Scale (MADRS) [higher score = more severely depressed] after 6 weeks.
The Avelity group showed a decrease in the total MADRS score by 16.6 points on average versus 11.9 points in the placebo group (p=0.002).
Those who received dextromethorphan plus buprion showed a statistically significant (p=0.007) difference with the control group already at the 1st week of treatment: the total MADRS score decreased by an average of 7.3 points - against 4.9item.
The use of "Aveliti" provided access to clinical remission (no clinically significant symptoms of depression and an overall MADRS score ≤ 10) after 2, 3, 4 and 6 weeks of therapy in 17%, 24%, 32% and 40% of patients versus 8%, 11%, 12% and 17% in the placebo group (p=0. 013, p=0.002, p<0.001, p<0.001).
013, p=0.002, p<0.001, p<0.001).
Avality resulted in an early and sustained clinical response (≥ 50% reduction in total MADRS score) after 1, 2, 3, 4 and 6 weeks of treatment in 15%, 28%, 42%, 49% and 54% of patients versus 7%, 17%, 25%, 27% and 34% in the placebo group (p=0.035, p=0.020, p=0.001, p<0.001, p<0.001).
The combination of dextromethorphan and buprion had a statistically significant effect on improvement scores on the Global Clinical Impression of Improvement (CGI-I), Global Clinical Impression of Disease Severity (CGI-S), Patient Global Impression of Improvement (PGI) -I), on the Short Questionnaire of Satisfaction with Quality of Life (Q-LES-Q-SF), on the Rapid Self-Questionnaire for Depressive Symptoms (QIDS-SR-16).
The safety profile of "Avelity" was characterized by acceptable tolerability. Among the most common adverse events that occurred during treatment: dizziness (in 16% of patients in the Avility group vs. 6% in the placebo group), nausea (13% vs. 9%), headache (8% vs. 4%), diarrhea (7% vs 3%), drowsiness (7% vs 3%), dry mouth (6% vs 2%). All adverse reactions were mild to moderate in severity.
6% in the placebo group), nausea (13% vs. 9%), headache (8% vs. 4%), diarrhea (7% vs 3%), drowsiness (7% vs 3%), dry mouth (6% vs 2%). All adverse reactions were mild to moderate in severity.
The Avelity Prescribing Information contains a black box warning about an increased risk of suicidal thoughts and behavior in children, adolescents and young adults (18–24 years) taking antidepressants. This patient population should be carefully monitored for clinical deterioration and suicidal tendencies. Avality is not approved for use in pediatric patients.
Longitudinal Study #1
The COMET (NCT04039022) phase III (non-randomized, open label, multicenter) clinical trial enrolled adult patients (n=611) with moderate to severe (MADRS overall score ≥ 25) major depressive disorder.
Baseline characteristics of subjects: mean age 42, female 62%, mean total MADRS score of 33.
For 12 months, patients were administered twice daily dextromethorphan with buprion.
Long-term treatment of major depressive disorder with the drug "Aveliti" made it possible to achieve the following indicators of the effectiveness of therapy.
Mean reductions in total MADRS score were 9.1, 14.0, and 21.1 points at 1, 2, and 6 weeks. After 6 and 12 months this score decreased by 23.9 points and 23.0 points .
19%, 40%, and 73% of patients were clinically responding to treatment for depression after 1, 2, and 6 weeks of therapy. Clinical response was fair for 85% and 83% of subjects after 6 and 12 months of treatment.
Clinical remission of depression was recorded for 8%, 22% and 53% of patients after 1, 2 and 6 weeks of treatment. This status was reached by 69% and 69% of participants after 6 and 12 months of therapy.
Clear improvements in depressive symptoms, as assessed by the CGI-I scale, were noted in 27%, 50% and 83% of patients after 1, 2 and 6 weeks of treatment. The same is registered for 87% and 93% of patients after 6 and 12 months of therapy.
The same is registered for 87% and 93% of patients after 6 and 12 months of therapy.
Clinical response to treatment for depression on the Sheehan Disability Scale (SDS; total score ≤ 12) assessing functionality at work/school, social life, family life/home responsibilities, documented for 42.9%, 55.1% and 70.7% of patients after 1, 2 and 6 weeks of treatment. This was true for 81% and 76% of subjects after 6 and 12 months of therapy.
The safety profile of "Avelity" was characterized by acceptable tolerability. Among the most common adverse events that occurred during treatment were dizziness (in 13% of patients), nausea (12%), headache (9%), dry mouth (7%), loss of appetite (6%). All adverse reactions were mild to moderate in severity.
Longitudinal study #2
The EVOLVE (non-randomised, open-label, multicentre) clinical trial enrolled adult patients (n=186) with moderate to severe (MADRS ≥ 25 total score) major depressive disorder who were previously treated with at least one antidepressant during the current episode of depression and whose treatment has not justified itself.
Baseline characteristics of the subjects: the average total MADRS score was 32.
Patients were administered twice daily dextromethorphan with buprion for up to 15 months.
Appointment of "Aveliti" led to a rapid improvement in depressive symptoms. So, the average decrease in the total MADRS score was 9.1±7.6 (p<0.001), 13.3±8.6 (p<0.001) and 20.4±7.8 (p<0.001) after 1, 2 and 6 weeks respectively. At the same time, a clinical response was recorded for 17.7%, 39.0% and 74.2% of patients. 9 went into remission of major depressive disorder0085 5.7%, 16.2% and 46.0% of subjects .
The antidepressant effect of "Aveliti" was characterized by long-term and stability throughout the 12 months of therapy. Thus, the remission status was fair for 65% and 68% of patients after 6 and 12 months of treatment.
Functional remission of major depressive disorder, according to the SDS scale (total score ≤ 6), was achieved in 18%, 31% and 40% of patients after 1, 2 and 6 weeks of treatment.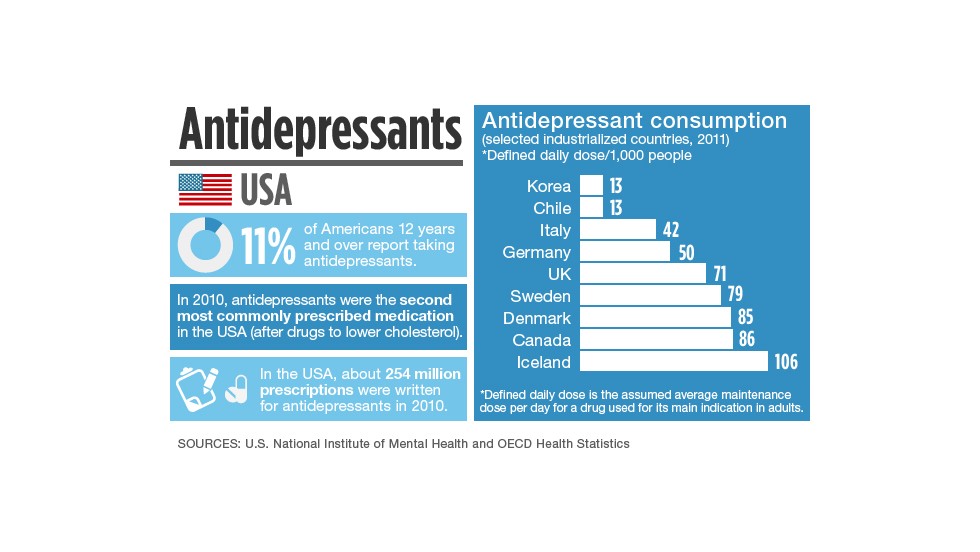 After 6 and 12 months of therapy, 54% and 59% of subjects .
After 6 and 12 months of therapy, 54% and 59% of subjects .
Avality provided relief from anxiety, as measured by the Hamilton Anxiety Scale (HAM-A), in 36%, 51%, and 58% of patients who achieved remission for this clinical indicator (total score ≤ 7) after 1 , 2 and 6 weeks of treatment. Remission after 6 and 12 months of therapy was recorded for 75% and 78% of subjects .
Long-term administration of "Aveliti" was characterized by acceptable tolerability. Among the most common adverse events were: nausea (in 8.9% of patients), headache (7.5%), dry mouth (6.2%), insomnia (5.5%), dizziness (5.5%).
Complementary study
The ASCEND (NCT03595579) phase II clinical trial (randomized, double-blind, active control, multicenter) enrolled adult patients (n=80) with moderate-severe (total MADRS score ≥ 25) major depressive disorder. disorder.
Baseline characteristics of subjects: mean age 37. 5 years, women 64%, mean total MADRS score is 32.
5 years, women 64%, mean total MADRS score is 32.
Mosmedpreparaty
Participants were given either dextromethorphan with bupropion or bupropion alone, twice daily for 6 weeks.
The use of "Aveliti" provided a statistically significant decrease in the total MADRS score: for the entire course of treatment, its decrease was an average of 13.7 points - against 8.8 points in the bupropion group (p<0.001).
At weeks 1, 2, and 6, patients treated with dextromethorphan with bupropion showed a reduction in total MADRS score of 7.4, 12.5, and 17.3 points vs. 4.5, 7.8 and 12.1 points in the control group (p=0.169, p=0.024, p=0.013).
Clinical remission was recorded at week 6 in 47% of subjects in the dextromethorphan plus bupropion group versus 16% in the control group (p=0.004).
Appointment "Aveliti" led to an improvement in other indicators of the effectiveness of the treatment of depression, including scores on the MADRS-6 subscale (major symptoms of depression), on the CGI-I scale, on the CGI-S scale.
Avility's safety profile was acceptable. There were no side effects associated with psychotomimetic disorders, weight gain, increased sexual dysfunction.
Aveliti: Market Prospects
Major depressive disorder killed many experimental drugs. This disease is very difficult to treat, and therefore it is surprising how dextrometrophan with bupropion managed to easily pass three clinical trials important for regulatory approval. Moreover, the therapeutic efficacy of "Aveliti" was consistent in all three studies, which is almost impossible to achieve in the case of almost any mental disorder. The well-known problem of drug testing in neuropsychiatric disorders, when in one clinical trial an experimental drug works with due efficiency, while in another, even if completely similar in design, it fails.
Thus, Avelity, validated in the phase III pivotal GEMINI (NCT04019704), phase III long-term COMET (NCT04039022) and phase II supplementary ASCEND (NCT03595579) clinical trial, demonstrated a reduction in total MADRS score of 16. 6, 21.1 and 17.3 points after 6 weeks of treatment, or, as a percentage of baseline, by 50%, 64%, 54%. 40%, 53% and 47% of patients went into clinical remission of depression, respectively. And all this without any negative effects.
6, 21.1 and 17.3 points after 6 weeks of treatment, or, as a percentage of baseline, by 50%, 64%, 54%. 40%, 53% and 47% of patients went into clinical remission of depression, respectively. And all this without any negative effects.
Long-term use of dextrometrofan with bupropion improved the proportion of patients reaching clinical remission status to 69% for six months of treatment. And again, no negative side effects.
Aksam would like to reinforce confidence in the therapeutic viability of Avelity by proving that it also successfully copes with therapeutically resistant depression (when two or more lines of therapy with different standard antidepressants do not help), but it did not turn out very smoothly. The Phase III STRIDE-1 (NCT02741791) clinical trial comparing the drug to bupropion failed to demonstrate a statistically significant overweight of the experimental drug. The problem was not with Aveliti itself, but with study design flaws that led to insufficient statistical power. Subsequent MERIT (NCT04608396) phase II confirmed that "Avelity" is capable of delaying the onset and preventing the recurrence of depressive symptoms. The COMET-TRD cohort in COMET (NCT04039022) also showed adequate efficacy. However, Axam is not currently considering dextrometrofan with bupropion as a drug for treatment-resistant depression.
Subsequent MERIT (NCT04608396) phase II confirmed that "Avelity" is capable of delaying the onset and preventing the recurrence of depressive symptoms. The COMET-TRD cohort in COMET (NCT04039022) also showed adequate efficacy. However, Axam is not currently considering dextrometrofan with bupropion as a drug for treatment-resistant depression.
Axam is convinced that Avility is suitable for the treatment of other clinically relevant patient groups: major depressive disorder not responding to one line of standard antidepressant therapy and major depressive disorder with suicidal tendencies. Corresponding COMET-AU and COMET-SI cohorts in COMET (NCT04039022) this was confirmed: after 6 weeks of treatment, remission was registered in 40% and 50% of patients; suicidal tendencies disappeared in 60% of patients already in the first week of therapy.
At the same time, Axam continues to test Aveliti in the treatment of agitation in Alzheimer's disease and the treatment of nicotine addiction. For example, in the phase II/III ADVANCE (NCT03226522) clinical trial, baseline total scores on the Cohen-Mansfield Excitability Assessment Inventory (CMAI) decreased by almost half, and in the phase II clinical trial NCT03471767, dextromethorphan with bupropion provided a greater reduction in the number of cigarettes smoked and a greater reduction smoking intensity versus bupropion alone, an approved drug for quit smokers.
For example, in the phase II/III ADVANCE (NCT03226522) clinical trial, baseline total scores on the Cohen-Mansfield Excitability Assessment Inventory (CMAI) decreased by almost half, and in the phase II clinical trial NCT03471767, dextromethorphan with bupropion provided a greater reduction in the number of cigarettes smoked and a greater reduction smoking intensity versus bupropion alone, an approved drug for quit smokers.
Industry observers estimate that Aveliti will cross the threshold of half a billion dollars in annual sales only by 2026. Such restrained forecasts are obviously due to delays in the regulatory verdict. Obviously, once the drug is approved, financial expectations will improve dramatically. What can I say if, before the regulatory problems, the sales forecast for Avility was extremely optimistic: in 2026, the drug sales should have reached the level of 1.3 billion dollars a year.
In terms of intellectual property protection, Aveliti is doing well: the proprietary combination of dextromethorphan with buprion is securely patented until 2037–2040.
Important information
Mosmedpreparaty.ru is a specialized research and reference analytical service of the Mosmedpreparaty group of companies, targeted at key events in the global pharmaceutical, biotechnology, medicine and healthcare industries.
- Nothing on Mosmedpreparaty.ru is not an advertisement or promotion of medicines, methods of treatment, medical services.
- Information and publications Mosmedpreparaty.ru are for educational and educational purposes only.
- Medical information broadcast by Mosmedpreparaty.ru is intended only for specialists in the field of healthcare and the field of drug circulation.
- Medical information contained in Mosmedpreparaty.ru is not intended to be used as a substitute for consultation with a healthcare professional.
- Nothing on Mosmedpreparaty.ru should be construed as providing medical advice or recommendations and should not form the basis for making any decision or taking any action without the participation of a healthcare professional.

Presence on the web resource Mosmedpreparaty.ru and familiarization with its contents means that you have read the "User Agreement" and accepted its terms.
Fighting depression: 10 modern drugs
{{if type === 'partner-stocks'}}
{{/if}}
{{/if}} {{each list}}${this} {{if isGorzdrav}}
Delete
{{/if}}
{{/each}} {{/if}} Search by drug, disease, substance: DERMAKOSMETIKA, SOLGAR, NaturAge, Voltaren, KagocelHome
Articles
Fighting depression: 10 modern drugs
Depression is an urgent problem, the number of visits to doctors is growing every year. It can be solved by contacting a psychotherapist and taking antidepressants . These are drugs that regulate the production of hormones and biochemical processes in the body. It is strictly forbidden to prescribe them to yourself, as these are complex drugs with certain restrictions, side effects . The doctor must authorize their appointment and control the intake. We will tell you which of them are the most effective and common in medicine, how many they have pluses and minuses.
It can be solved by contacting a psychotherapist and taking antidepressants . These are drugs that regulate the production of hormones and biochemical processes in the body. It is strictly forbidden to prescribe them to yourself, as these are complex drugs with certain restrictions, side effects . The doctor must authorize their appointment and control the intake. We will tell you which of them are the most effective and common in medicine, how many they have pluses and minuses.
What is meant by
depression Doctors have known it since ancient Greece and Egypt. Hippocrates described it as melancholy - a condition that is accompanied by anxiety, despondency, insomnia, refusal of food, irritability. Most often, the cause is childhood trauma or severe, frequent stress in adulthood. There are many provoking factors: the death of a loved one, deterioration of living conditions, alcoholism, brain diseases. Such cases are referred to as psychogenic depression.
Such cases are referred to as psychogenic depression.
The second type is endogenous. The problem appears not from large external shocks, but because of internal causes. A person is constantly dissatisfied with himself, subjecting himself to criticism. Many patients have panic attacks, haunted by a feeling of fear, anxiety.
How long the period of depression lasts
Many people mistake ordinary periods of low mood for depression. If they do not last long and are quickly replaced by periods of recovery, then we are not talking about a depressive state. The problem is obvious when the symptoms persist for months and dramatically change a person's life. Then you need to see a doctor.
What happens to the body
The most common theory is that there is a malfunction of neurotransmitters located in the brain. These substances transmit signals from neuron to neuron and are responsible for a person's mood. Dysfunction leads to a slowdown in the rate of this transmission and a decrease in the number of neurotransmitters themselves. Serotonin, which is called the "hormone of happiness", suffers the most. For clarity, this biochemical process can be compared, for example, with a drop in blood sugar levels in diabetes mellitus.
Dysfunction leads to a slowdown in the rate of this transmission and a decrease in the number of neurotransmitters themselves. Serotonin, which is called the "hormone of happiness", suffers the most. For clarity, this biochemical process can be compared, for example, with a drop in blood sugar levels in diabetes mellitus.
How is
treated depressionDepression has been treated in different ways. In the ancient world - emetics and laxatives. In the Renaissance - wine and sunbathing. In the Age of Enlightenment - external stimuli, for example, insects. The 19th century brought new recipes - in particular, a solution of camphor in tartaric acid. The treatment also included the use of drugs, which are now no longer allowed for sale, and some are recognized as narcotic.
Obviously, all these drugs had no effect on increasing the amount of serotonin. And the treatment is precisely to normalize its production. This was done after creating modern antidepressants , which have a minimum of side effects, are safe for the body and are not addictive. These are drugs, the action of which is aimed specifically at balancing the disturbed balance of neurotransmitters: serotonin, norepinephrine, dopamine.
These are drugs, the action of which is aimed specifically at balancing the disturbed balance of neurotransmitters: serotonin, norepinephrine, dopamine.
Prescription
If a healthy person takes antidepressants , there will be no effect . For a depressed patient, taking them will help:
- improve psychological state;
- get rid of irritability;
- panic fear;
- increase mental and physical activity;
- overcome a dreary mood.
Psychiatrists prescribe antidepressants for chronic back pain, headaches. And also with irritable bowel syndrome, incontinence and other cases when the body stops producing its own painkillers. Medication helps restore pain suppression mechanisms.
You can only take these drugs with a doctor's prescription, as many of them are strong stimulants. Self-prescribing may cost dearly - the condition may worsen. Only a doctor will correctly calculate how many medicines to take per day. In parallel with the treatment by a psychiatrist, a neurologist, a consultation of a psychotherapist is required.
Self-prescribing may cost dearly - the condition may worsen. Only a doctor will correctly calculate how many medicines to take per day. In parallel with the treatment by a psychiatrist, a neurologist, a consultation of a psychotherapist is required.
Precautions
- Prescribed drug start drinking with a small dose - the first couple of days they take a quarter of a tablet. Gradually increase the dose to normal. So the body adapts better. Finish the course by reducing the dose.
- The first effect of appears only 2 weeks after the start of administration. Steady action - after six months. All this time, should be taken without any gaps or breaks.
-
Products are not combined with melatonin, St. John's wort, products and dietary supplements based on sibutramine, 5-HTP. Their combination can raise serotonin to dangerous levels.
 Also, you can not combine them with monoamine oxidase inhibitors, for example, Cipralex. When writing a prescription, the doctor takes these points into account.
Also, you can not combine them with monoamine oxidase inhibitors, for example, Cipralex. When writing a prescription, the doctor takes these points into account. - Drinking antidepressants is better in parallel with visits to a psychotherapist. If the drugs normalize the biochemical processes in the body, then this doctor will help normalize the psychological state after depression.
The best antidepressants
In medicine, they have long argued that some drugs give only a placebo effect. The purpose of the study was to find out which of them are the most effective and valid . The project involved 116 thousand patients, and its results were published by the authoritative edition of the Lancet. We offer a list of the best.
1. Agomelatine
New generation drug. Agomelatine is used for severe depressive disorders, high levels of anxiety. Enhances the release of dopamine and norepinephrine, stimulates melatonin receptors. The standard therapeutic dose is 25-50 mg 1 time / day. Helps to restore the normal structure of sleep, get rid of anxiety and panic attacks attacks
The standard therapeutic dose is 25-50 mg 1 time / day. Helps to restore the normal structure of sleep, get rid of anxiety and panic attacks attacks
Pros
+ Does not adversely affect attention and memory.
+ No lethargy during the day.
+ No sexual deviations.
+ No relation to blood pressure.
+ Do not reduce dosage upon discontinuation.
Cons
— In 1-10% of cases, increased sweating, diarrhea, constipation.
- Possible increased fatigue, drowsiness.
- There are no evidence-based safety studies in people with renal or hepatic insufficiency, therefore, such patients are advised to refrain from taking drugs with active ingredient agomelatine.
2. Amitriptyline
Tricyclic antidepressant. Moreover, the World Health Organization considers Amitriptyline the most reliable in this group. The standard dose is 200-250 mg / day. The action is to block the reuptake of neurotransmitters. A good remedy for moderate to severe disorders of the endogenous type. Additionally, it has a sedative and hypnotic effect. Effective in the treatment of neuropathic pain, for the prevention of migraine.
A good remedy for moderate to severe disorders of the endogenous type. Additionally, it has a sedative and hypnotic effect. Effective in the treatment of neuropathic pain, for the prevention of migraine.
Pluses
+ Drugs with active substance amitriptyline are inexpensive.
+ High reliability, minimum side effects.
+ Relatively safe during breastfeeding.
Cons
- Possible side effect in the form of blurred vision, dry mouth.
- Lowering blood pressure.
- Some patients experience constipation.
- Drowsiness.
3. Escitalopram
Belongs to a group of modern serotonin reuptake inhibitors (SSRIs). Most often, it is recommended to take for anxiety, panic attacks. It is taken once, the standard dose is 10 mg per day. Escitalopram has a milder effect and is prescribed to patients for whom tricyclic drugs are contraindicated.
Pluses
+ A lasting effect occurs after 3 months.
+ Indicated for patients with disorders of the cardiovascular system.
+ Soft action.
Cons
- In some patients, the functions of the gastrointestinal tract are disturbed, which is most often expressed in diarrhea.
- Anxiety may increase during the first 2 weeks, therefore it is recommended to start treatment with low doses and gradually increase them.
- Contraindicated in pregnancy and lactation.
4. Mirtazapine
A drug of the tetracyclic group. Mirtazapine - good stimulant for anxious depressions, has a moderate sedative effect. The average amount is 30 mg / day, it must be consumed once. Usually it is prescribed to patients who lose interest in life, cease to experience joy, pleasure. Effective in the correction of sleep, in particular, early awakenings.
Pros
+ Earlier onset of action than SSRIs (1 week).
+ Works well with most generic drugs.
+ Full effect in 4 weeks.
+ Does not affect sexual function.
Cons
- The active substance mirtazapine is contraindicated in diabetes mellitus, arterial hypotension, increased intraocular pressure.
— During the admission period, you must drive carefully and engage in potentially dangerous types of work.
- 18% of patients experience drowsiness, 15% dry mouth, 5% weight loss. Other side effects occur in 1-3% of cases.
5. Paroxetine
Belongs to the SSRI group, is used most often for severe anxiety, panic, social phobia, nightmares, stress after trauma. Paroxetine can resolve the problems of anxious depression, anxiety-phobic disorders. Take once a day at a dose of 20 mg.
Pros
+ The most powerful stimulant among SSRIs.
+ Anxiety and insomnia pass quickly.
+ Minimal side effects in the form of vomiting, diarrhea.
+ Suitable for patients with cardiovascular problems.
Cons
— Not suitable for patients with severe motor, mental inhibition.
- Reduces libido.
- Harmful to the fetus when taken during pregnancy.
6. Fluoxetine
One of the most commonly used antidepressants in the SSRI group. Known as Prozac. Fluoxetine is also known as a good mood stimulant. Patients have a feeling of fear, tension, anxiety, gloomy dislike for others. Depending on the indications, the average daily dose is 20-60 mg.
Pluses
+ There is practically no effect on the work of the heart.
+ Does not cause sedation.
+ Effective for patients with motor retardation and excessive daytime sleepiness.
Cons
- May cause weight loss.
— Hypoglycemia is possible in diabetes mellitus.
- Contraindicated in severe renal impairment.
7. Fluvoxamine
Another SSRI drug. Fluvoxamine is similar to Prozac Fluoxetine but is fast acting and may cost cheaper. The effect is in a more active slowing down of the reuptake of serotonin by neurons. It is indicated for depression of various origins, as well as obsessive-compulsive disorders. The average daily dose is 100 mg.
Fluvoxamine is similar to Prozac Fluoxetine but is fast acting and may cost cheaper. The effect is in a more active slowing down of the reuptake of serotonin by neurons. It is indicated for depression of various origins, as well as obsessive-compulsive disorders. The average daily dose is 100 mg.
Pros
+ Lower price than traditional Prozac.
+ Faster action than him.
+ Relatively minor side effects (diarrhea, dry mouth, drowsiness).
Cons
- Contraindicated in diabetes.
- Pregnant women - with caution, lactation - prohibited.
- Causes nausea in some patients.
8. Sertraline
One of the widely used and universal drugs of the SSRI group. They treat almost any depressive condition, panic disorder, social phobia. However, in severe clinical cases, sertraline may not be effective enough. The standard dose is 50 mg/day.
Pros
+ No cardiotoxicity.
+ The patient's psychomotor activity does not change.
+ Does not increase body weight.
+ Combines well with other groups of antidepressants.
Cons
— In the first 2 weeks there may be problems with sleep, diarrhea.
- Side effects of a sexual nature.
- Contraindicated in pregnant women.
9. Escitalopram
The drug is classified as an SSRI. Its difference is in its effectiveness in depression, which is accompanied by involuntary movements (tic, tremor, chewing, smacking). Escitalopram is prescribed to patients with panic, anxiety, phobias, obsessive thoughts or actions. The daily dose is 20 mg.
Pros
+ Effective in tardive dyskinesia.
+ One of the most powerful SSRIs.
+ More pronounced thymoleptic effect (improvement of mood) compared to many antidepressants of the same group.
Cons
- In some patients, anxiety increases within 2 weeks after starting treatment.
- Possible gastrointestinal disorders, insomnia, agitation.