Major neurocognitive disorder treatment
Major Neurocognitive Disorder: Signs and Symptoms
Major neurocognitive disorder — a new term for dementia — is an acquired deficit in your ability to think that’s severe enough to impact your daily functioning.
Neurocognitive disorders can lead to cognitive deficits in various domains involving attention, memory, language, or social skills, for instance.
Various medical conditions can lead to major neurocognitive disorder. Alzheimer’s disease is the most common type of major neurocognitive disorder.
While it’s not possible to “cure” the cognitive symptoms brought on by major neurocognitive disorder, various treatments — including medications, therapies such as skills training, and support options — can potentially slow down symptom progression.
What does neurocognitive mean?
“Neuro” is related to the nerves or nervous system, while “cognitive” relates to cognition.
In terms of major neurocognitive disorder, neurocognitive refers to an issue with how the brain functions. Neuro means that there’s a biological problem with the way the brain is functioning. Cognition is defined as thinking, or anything that the mind does to sense, organize, prepare, and perform tasks.
There are a variety of symptoms that may indicate major neurocognitive disorder. Some common symptoms, according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), include:
- a significant decline in one or more cognitive domains, compared to your previous abilities
- the cognitive change impairs your independence in daily life, such as paying bills, managing money, or taking medications
- the cognitive change does not exclusively occur as part of a delirium — a sudden state of confusion
- the cognitive decline cannot be better explained by another mental health condition
The cognitive symptoms that someone with a major neurocognitive disorder has may be reported by the person having them, someone close to them, or a medical professional.
Medical professionals can assess a person’s cognitive abilities using standardized neurological and psychological tests.
Mild neurocognitive disorder is a less severe form of major neurocognitive disorder. The difference in symptoms is that if you have a mild neurocognitive disorder, there’s only a modest cognitive decline from your previous level of performance.
If you have a mild neurocognitive disorder, you can still perform daily activities with independence. You can complete your usual complex activities, although they may require more effort than before.
The DSM-5 discusses groups of symptoms that individuals with major and mild neurocognitive disorders may have. Common symptoms among neurocognitive disorders include:
- anxiety
- depression
- elation
- agitation
- confusion
- insomnia (difficulty sleeping)
- hypersomnia (oversleeping)
- apathy
- wandering
- disinhibition
- hyperphagia (extreme hunger or eating)
- hoarding
- hallucinations
- delusions
Treatment for major neurocognitive disorder is primarily based on what symptoms you’re experiencing. For example, cognitive behavioral therapy (CBT) can help treat symptoms of anxiety and depression present with major neurocognitive disorder.
For example, cognitive behavioral therapy (CBT) can help treat symptoms of anxiety and depression present with major neurocognitive disorder.
Major neurocognitive disorder is a new name for dementia. The DSM-5 changed the name to major neurocognitive disorder in 2013.
The word ‘dementia’ comes from the Latin word for madness or ‘being out of one’s mind.’ The name change intends to remove stigma from the condition.
According to the DSM-5, major neurocognitive disorder occurs in around 1–2% of people at age 65, and 30% of people by age 85.
In comparison, mild neurocognitive disorder affects around 2–10% of people at age 65 and between 5–25% of people by age 85.
Around 6.2 million people in the United States are living with Alzheimer’s disease, the most common major neurocognitive disorder.
In the United States, Alzheimer’s disease is the sixth leading cause of death — and in people ages 65 and older, it’s the fifth leading cause of death.
The most significant predictor of developing major neurocognitive disorder is age.
Major neurocognitive disorder may be caused by a variety of factors noted in the DSM-5 as specifiers. These specifiers are:
- Alzheimer’s disease
- frontotemporal lobar degeneration
- Lewy body disease
- vascular disease
- traumatic brain injury
- substance or medication use
- HIV
- prion disease
- Parkinson’s disease
- Huntington’s disease
- another medical condition
- multiple etiologies
- unspecified
Females have a higher risk of developing a major neurocognitive disorder, especially Alzheimer’s disease. This may be because females live longer on average than males.
Major neurocognitive disorder is not currently curable. However, some treatments can alleviate symptoms or slow the progression of cognitive decline.
Treatment is mainly dependent on the specific cause.
In some cases, cognitive training may help improve cognition or slow down the progression of symptoms. This non-pharmacological treatment uses guided practices to improve memory, problem-solving, or attention. This type of skills training focuses on the improvement of specific cognitive functions.
This non-pharmacological treatment uses guided practices to improve memory, problem-solving, or attention. This type of skills training focuses on the improvement of specific cognitive functions.
One study in 2018 examined the pharmacological treatments of major neurocognitive disorders.
The researchers recommended that non-pharmacological treatments should be the first line of treatment for major neurocognitive disorders due to the risks and side effects linked with antipsychotics, such as mortality from stroke, myocardial infarction, or infection.
Doctors often prescribe antipsychotics as a treatment for major neurocognitive disorders.
Antipsychotics may be used to relieve mood instability, psychosis, agitation, and aggression in people with neurocognitive disorders. If used for these purposes, it’s important for the medical professional to work with the patient and their family to determine if this is the best course of action.
Standard antipsychotics that can be effective for symptoms include:
- risperidone (Risperdal)
- olanzapine (Zyprexa)
- quetiapine (Seroquel)
- aripiprazole (Abilify)
Not all patients respond to antipsychotics, but those that do respond generally find their symptoms improve in 1–4 weeks.
Consider talking with your doctor if you’re considering antipsychotic medications, as they can have significant side effects.
Other types of medications that have minor to moderate effects on treating symptoms of major neurocognitive disorder include:
- cholinesterase inhibitors
- memantine
- selective serotonin reuptake inhibitors (SSRIs)
Consider talking with a doctor to find the appropriate medications for you or your loved one.
If you or a loved one have been diagnosed with major neurocognitive disorder, support is available. You can reach the Alzheimer’s Association helpline 24/7 at 800-272-3900.
If you’re a caregiver for someone who has cognitive deficits, the Family Caregiver Alliance offers information on caring for adults with cognitive disorders and memory impairments.
While it’s not currently possible to reverse cognitive decline, treatments may slow down or help manage symptoms. Consider speaking with your doctor to assess treatment options that may be right for you.
Treatments for Neurocognitive Disorders | Abnormal Psychology
Learning Objectives
- Describe psychological perspectives and treatments for neurocognitive disorders
In this course, we have learned to assess and analyze disorders from multiple psychological perspectives, such as the psychodynamic, biological, humanistic, behavioral, and cognitive perspectives. Neurocognitive disorders, by definition, mostly relate to the cognitive perspective, as the cognitive perspective views psychological disorders as originating from an interruption, whether short or long, in our basic cognitive functions, i.e., memory processing, perception, problem-solving, and language. All the neurocognitive disorders have biological causes, as explained in the previous readings, because they are attributed to biological changes caused by Alzheimer’s, Parkinson’s, Huntington’s disease, HIV, traumatic brain injury (TBI), or other illnesses. The focus in understanding and treating neurocognitive disorders lies then in understanding the biological changes and what measures can be taken to slow cognitive decline.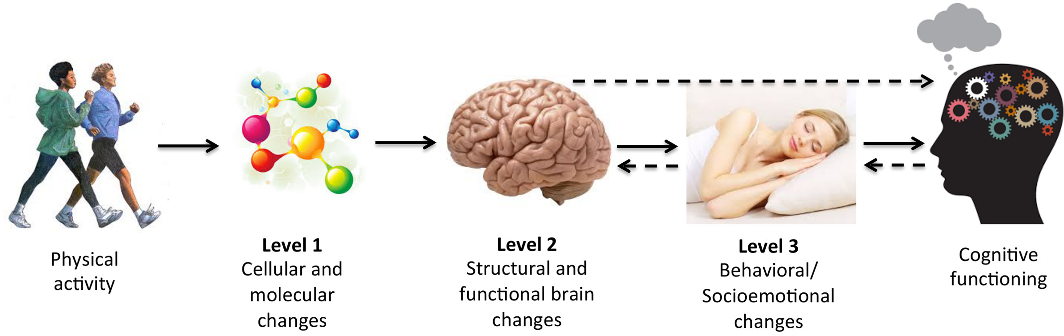 There is evidence that some measures are effective in preventing or slowing the progression of cognitive symptoms.
There is evidence that some measures are effective in preventing or slowing the progression of cognitive symptoms.
Recall that major neurocognitive disorder (MND) is a syndrome that progresses with significant deterioration of cognitive domains as compared to previous levels of cognitive performance in memory, speech, reasoning, intellectual function, and/or spatiotemporal perception, and may also be associated with changes in emotional behavior and difficulties at the functional level. The decline is initially noticed by the individual, the family, or the general practitioner (GP) who is usually responsible for the early diagnosis (American Psychiatric Association [APA], 2014).
MND may result from brain disorders, classified as primary (degenerative), or be a consequence of other conditions (secondary) (Emre, 2009). The most common types of MND are Alzheimer’s disease, vascular dementia (VaD), Lewy body dementia, and frontotemporal dementia (FD). In secondary MND (e.g., alcoholic dementia and infectious diseases), the symptoms may be treated and/or prevented. Therefore, a correct diagnosis is crucial for proper disease management and treatment. This is supported by a detailed collection of the person’s clinical history, neurological and neuropsychological examination, and the comprehensive use of laboratory and imaging tests. In primary MND, early diagnosis is equally crucial either to delay the progression of cognitive symptoms and to control/stabilize psychiatric manifestations (Ribeira et al., 2004).
Therefore, a correct diagnosis is crucial for proper disease management and treatment. This is supported by a detailed collection of the person’s clinical history, neurological and neuropsychological examination, and the comprehensive use of laboratory and imaging tests. In primary MND, early diagnosis is equally crucial either to delay the progression of cognitive symptoms and to control/stabilize psychiatric manifestations (Ribeira et al., 2004).
Predictive Factors of MND
MND is likely to develop in a continuous process (Brooks and Loewenstein, 2010). Individual factors affect the likelihood of developing MND and these factors predicting the development of the disease should be known. If known, preventive interventions may prevent or protect people at risk of MND.
Previous studies have identified predictive factors of MND, which can be grouped into the following:
- sociodemographic factors (e.g., sex, age, and years spent in education and social isolation)
- health factors (e.
 g., hearing loss, cardiovascular diseases, hypertension, diabetes, handgrip strength, and nutritional status)
g., hearing loss, cardiovascular diseases, hypertension, diabetes, handgrip strength, and nutritional status) - bio-behavioral factors (e.g., smoke, alcohol, and physical activity) (Helzner et al., 2009; Nagai et al., 2010; Polidori et al., 2012; Baumgart et al., 2015; Santana et al., 2015; Schwarzinger et al., 2018)
Given that most of these factors are all potentially modifiable (e.g., diabetes, cholesterol, depression, or malnutrition; Chen et al., 2017), the individual can play an active role in the prevention or management of MND, which creates the opportunity to allow for more efficient intervention. Primary prevention in the primary health care context is important for the course of MND and should focus on the identification of situations that increase the likelihood of occurrence or worsening of symptoms. However, few studies identify predictive factors associated with the severe stage of MND (Eshkoor et al., 2016).
A study by Sousa, Laetitia Teixeira, and Constança Paúl identified four predictors of MND including age, years spent in education, physical activity, and hand strength. Physical activity, hand strength, and education play a protective role (“the more the better”). On the other hand and as expected, while age increases, the risk of MND also increases.[1]
Physical activity, hand strength, and education play a protective role (“the more the better”). On the other hand and as expected, while age increases, the risk of MND also increases.[1]
There is extensive and persuasive evidence from mechanistic and well-designed prospective cohort studies that reducing the exposure to high blood pressure and hypertension in mid-life and to diabetes in mid-and late life, reducing tobacco use, and increasing the education level of populations can effectively reduce the dementia risk for populations (Prince et al, 2014).
Treating Neurocognitive Disorders
There is no cure for neurocognitive disorders or the diseases that cause them. Pharmacological approaches combined with behavioral and environmental interventions are most successful in treating neurocognitive disorders.
Figure 1. High blood pressure is a vascular risk factor for developing a neurocognitive disorder.
Antidepressants, antipsychotics, and other medications that treat memory loss and behavioral symptoms are also available and may help to treat the diseases.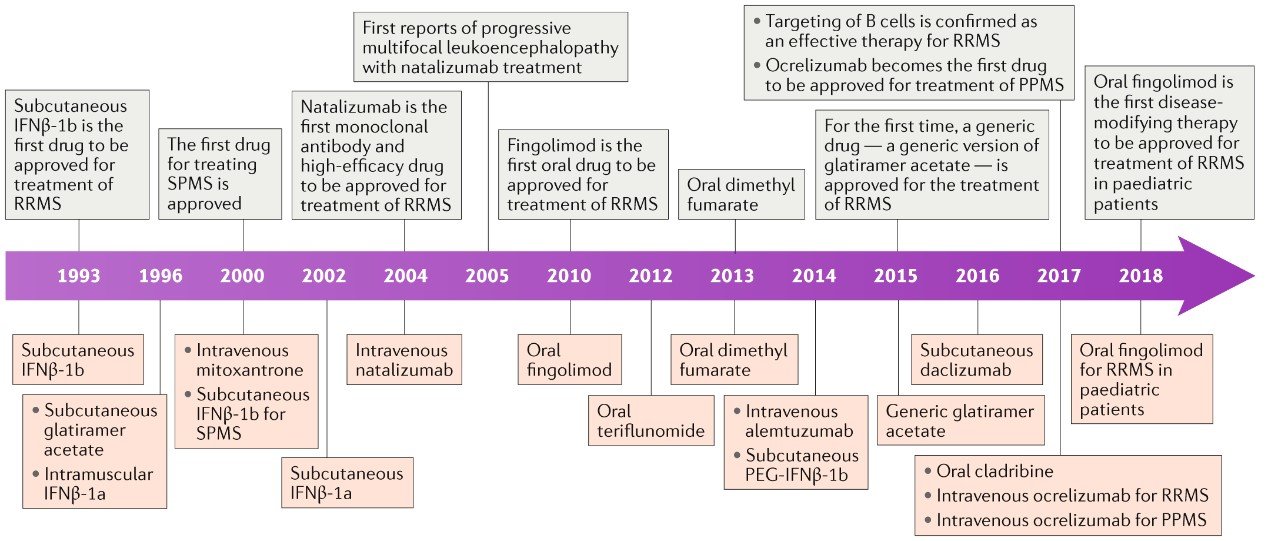 Ongoing psychotherapy and psychosocial support for patients and families are usually necessary for clear understanding and proper management of the disorder and to help maintain a better quality of life for caregivers and patients. In addition, speech therapy has been shown to help neurocognitive dementia patients with language impairment.
Ongoing psychotherapy and psychosocial support for patients and families are usually necessary for clear understanding and proper management of the disorder and to help maintain a better quality of life for caregivers and patients. In addition, speech therapy has been shown to help neurocognitive dementia patients with language impairment.
Studies suggest that diets with high Omega 3 content and low in saturated fats and sugars, along with regular exercise can increase the level of brain plasticity. Other studies have shown that mental exercise such as newly developed computerized brain training programs can also help build and maintain targeted specific areas of the brain. These studies have been very successful for those diagnosed with schizophrenia and can improve fluid intelligence and the ability to adapt and deal with new problems or challenges the first time encountered; in young people, it can still be effective in later life.
A person with amnesia may slowly be able to recall their memories or work with an occupational therapist to learn new information to replace what was lost or to use intact memories as a basis for taking in new information. If amnesia is caused by an underlying cause such as Alzheimer’s disease or infections, the cause may be treated but the amnesia may not be.
If amnesia is caused by an underlying cause such as Alzheimer’s disease or infections, the cause may be treated but the amnesia may not be.
Pharmacological Interventions
Targets for pharmacological treatment include
- cognitive impairment (e.g., memory loss, disorientation, and decrease in attention and problem-solving).
- behavioral symptoms (e.g., agitation and aggression).
- psychological symptoms (e.g., depression, anxiety, and psychosis).
There is a large body of evidence for the efficacy of cholinesterase inhibitors (ChEIs), such as donepezil, rivastigmine, and galantamine, in the treatment of mild to moderate Alzheimer’s disease (Institute for Quality and Efficiency in Healthcare, 2014). The use of each of these medications is associated with modest and short-term comparable improvements in cognitive function, global clinical state, and activities of daily living. However, the evidence base for cholinesterase inhibitors (ChEIs) in low- and middle-income countries is limited.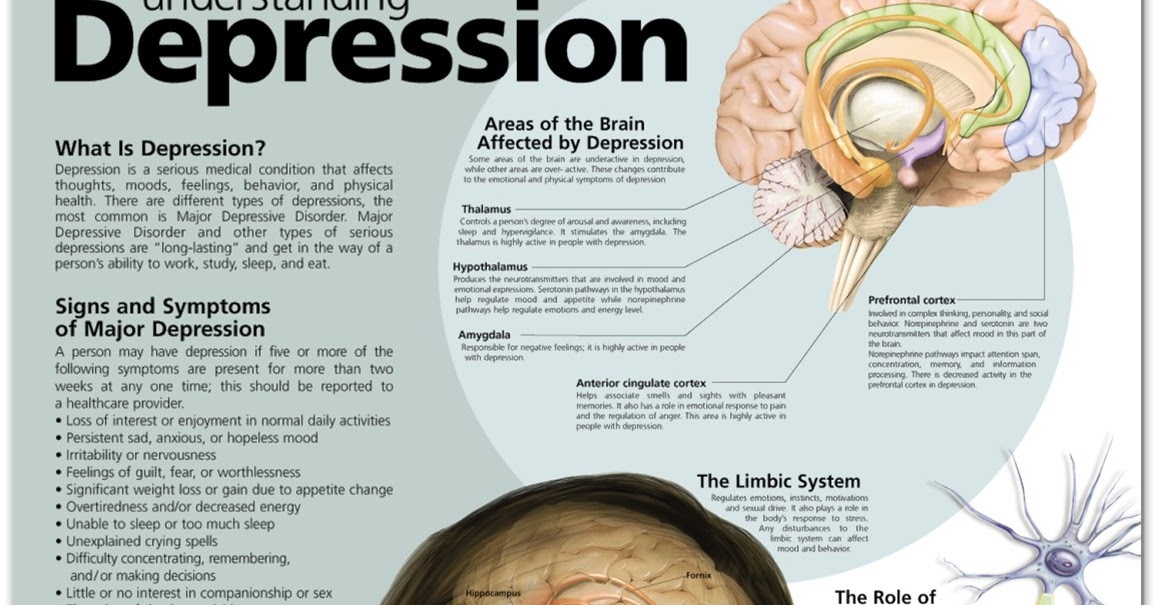 Moreover, the efficacy of this class of drugs in severe dementia is unclear, although behavioral symptom improvement was identified for galantamine (Institute for Quality and Efficiency in Healthcare, 2014). A fourth drug for the treatment of cognitive impairment, memantine, has a different mode of action and is well tolerated, but evidence for its efficacy is limited to people with moderate to severe dementia. Cholinesterase inhibitors (ChEIs) and memantine are less efficacious in vascular dementia than other forms. ChEIs and memantine’s efficacy in the treatment of behavioral disturbances is not established; manufacturer-sponsored licensing trials and post hoc analyses indicate small improvements.
Moreover, the efficacy of this class of drugs in severe dementia is unclear, although behavioral symptom improvement was identified for galantamine (Institute for Quality and Efficiency in Healthcare, 2014). A fourth drug for the treatment of cognitive impairment, memantine, has a different mode of action and is well tolerated, but evidence for its efficacy is limited to people with moderate to severe dementia. Cholinesterase inhibitors (ChEIs) and memantine are less efficacious in vascular dementia than other forms. ChEIs and memantine’s efficacy in the treatment of behavioral disturbances is not established; manufacturer-sponsored licensing trials and post hoc analyses indicate small improvements.
Figure 2. Haloperidol is an atypical antipsychotic medication that can be administered through injection.
Use of haloperidol and atypical antipsychotic medications for the treatment of agitation and behavioral and psychological symptoms of dementia (BPSD) indicate small treatment effects, most evident for aggression, although these must be weighed against the associated mortality risk (Kales et al, 2012).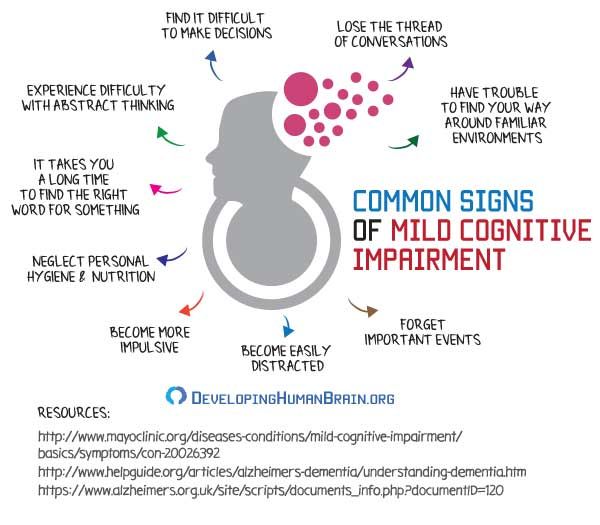 Atypical antipsychotic drugs have been widely prescribed for psychosis in dementia, but a meta-analysis of their efficacy indicated that only aripiprazole and risperidone had a statistically and clinically significant effect on psychiatric symptoms (Tan et al, 2015). An important caveat to the use of these medications in dementia is the associated increased risk of death and cerebrovascular adverse events. The literature on antipsychotic treatment in older people with dementia reveals that although improvement in the behavioral disturbance was minimal after six to 12 weeks, there was a significant increase in absolute mortality risk of approximately 1% (Banerjee et al, 2009). The literature suggests that prescribing antipsychotics in dementia beyond six to 12 weeks is likely to be substantially harmful in continued antipsychotic treatment in dementia. Therefore, many recommend nonpharmacological treatments, such as psychological and training interventions, to reduce BPSD rather than antipsychotic management (Duedon et al, 2009).
Atypical antipsychotic drugs have been widely prescribed for psychosis in dementia, but a meta-analysis of their efficacy indicated that only aripiprazole and risperidone had a statistically and clinically significant effect on psychiatric symptoms (Tan et al, 2015). An important caveat to the use of these medications in dementia is the associated increased risk of death and cerebrovascular adverse events. The literature on antipsychotic treatment in older people with dementia reveals that although improvement in the behavioral disturbance was minimal after six to 12 weeks, there was a significant increase in absolute mortality risk of approximately 1% (Banerjee et al, 2009). The literature suggests that prescribing antipsychotics in dementia beyond six to 12 weeks is likely to be substantially harmful in continued antipsychotic treatment in dementia. Therefore, many recommend nonpharmacological treatments, such as psychological and training interventions, to reduce BPSD rather than antipsychotic management (Duedon et al, 2009).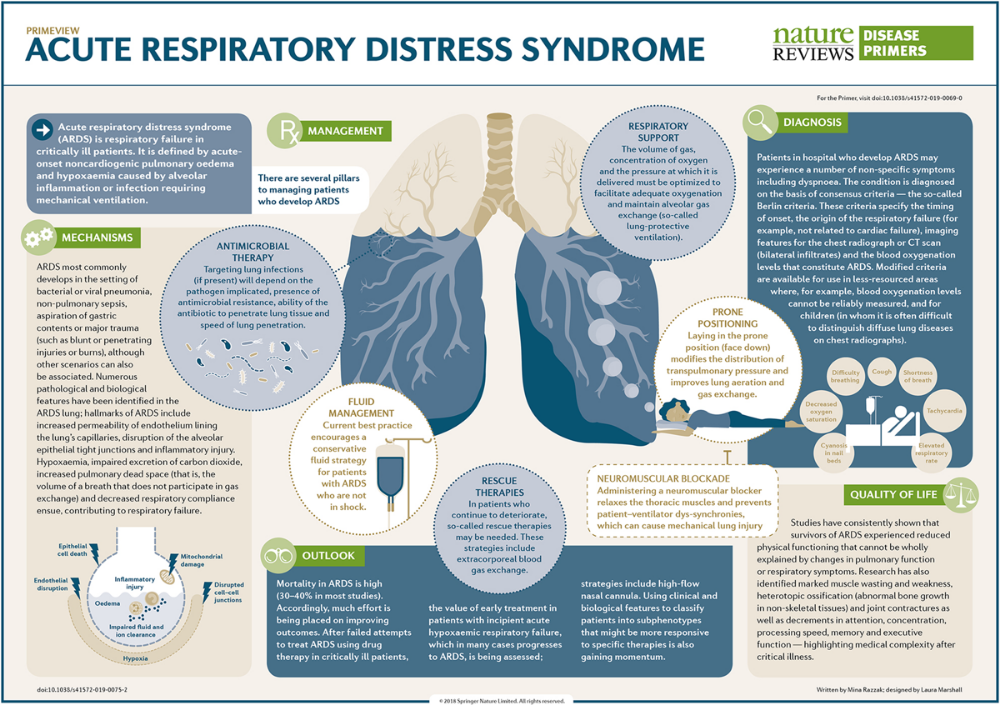 A meta-analysis of the efficacy of antidepressants in people with dementia was inconclusive (Leong, 2014). Antidepressants have been proposed for the treatment of BPSD with encouraging results (Henry et al, 2011).
A meta-analysis of the efficacy of antidepressants in people with dementia was inconclusive (Leong, 2014). Antidepressants have been proposed for the treatment of BPSD with encouraging results (Henry et al, 2011).
Nonpharmacological Interventions
A well-conducted randomized control trial of cognitive stimulation (reality orientation, games, and discussions based on information processing rather than knowledge) conducted in the United Kingdom as a group intervention, and a small pilot trial from Brazil, suggest that cognitive benefits from this intervention are similar to those for ChEIs (Aguirre et al, 2013). More specific cognitive training produced no benefits. Cognitive rehabilitation, an individualized therapy designed to enhance residual cognitive skills and the ability to cope with deficits, showed promise in uncontrolled case series in hospital incident command systems. A meta-analysis of four trials of reminiscence therapy (the discussion of past activities, events, and experiences) provides evidence for short-term improvement in cognition, mood, and caregiver strain, but the quality of these trials was poor (Bahar-Fuchs, 2013; Woods et al, 2005; Woods et al, 2012).
In terms of nonpharmacologic therapies, cognitive stimulation therapy has been shown to be cost-effective for people with mild-to-moderate dementia when delivered biweekly over seven weeks, though was found to have modest effects when continued for longer when added to administration of acetylcholinesterase inhibitors (D’Amico et al, 2015). An exercise intervention was found to have the potential to be cost effective when considering behavioral and psychological symptoms, but did not appear cost effective when considering quality-adjusted life year gains. The START (Strategies for Relatives) study, a randomized controlled trial to determine the clinical effectiveness and cost-effectiveness of a manual-based coping strategy program in promoting the mental health of carers of people with dementia, found the intervention to be cost effective with respect to the caregiver and patient outcomes, and National Institute for Health and Care Excellence (NICE) thresholds (Livingston et al, 2014). In a health economic analysis of resource costs and costs of formal care on a psychosocial intervention for family caregivers of persons with dementia, those in the intervention group reported a higher quality of life while their spouse was living at home (Dahlrup et al, 2014).
In a health economic analysis of resource costs and costs of formal care on a psychosocial intervention for family caregivers of persons with dementia, those in the intervention group reported a higher quality of life while their spouse was living at home (Dahlrup et al, 2014).
Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator (sometimes referred to as a brain pacemaker), which sends electrical impulses, through implanted electrodes, to specific targets in the brain (brain nuclei) for the treatment of movement and some neurocognitive disorders, including Parkinson’s disease and epilepsy. Deep brain stimulation (DBS) is used to manage some of the symptoms of Parkinson’s disease that cannot be adequately controlled with medications. Deep brain stimulation (DBS) is recommended for people who have Parkinson’s disease with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication, as long as they do not have severe neuropsychiatric problems. Four areas of the brain have been treated with neural stimulators in Parkinson’s disease. These are the globus pallidus internus, thalamus, subthalamic nucleus and the pedunculopontine nucleus. However, most DBS surgeries in routine practice target either the globus pallidus internus, or the subthalamic nucleus. Generally DBS is associated with 30–60% improvement in motor score evaluations.[2]
Four areas of the brain have been treated with neural stimulators in Parkinson’s disease. These are the globus pallidus internus, thalamus, subthalamic nucleus and the pedunculopontine nucleus. However, most DBS surgeries in routine practice target either the globus pallidus internus, or the subthalamic nucleus. Generally DBS is associated with 30–60% improvement in motor score evaluations.[2]
Try It
Glossary
deep brain stimulation: a neurosurgical procedure involving the placement of a medical device called a neurostimulator that sends electrical impulses, through implanted electrodes, to specific targets in the brain
- Sousa, Susana, Laetitia Teixeira, and Constança Paúl. “Assessment of Major Neurocognitive Disorders in Primary Health Care: Predictors of Individual Risk Factors.” Frontiers in Psychology 11 (2020): 1413. https://doi.org/10.3389/fpsyg.2020.01413. ↵
- Thakur KT, Albanese E, Giannakopoulos P, et al.
 Neurological Disorders. In: Patel V, Chisholm D, Dua T, et al., editors. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, Third Edition (Volume 4). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Mar 14. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK361950/ doi: 10.1596/978-1-4648-0426-7_ch5 ↵
Neurological Disorders. In: Patel V, Chisholm D, Dua T, et al., editors. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, Third Edition (Volume 4). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Mar 14. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK361950/ doi: 10.1596/978-1-4648-0426-7_ch5 ↵
Cognitive disorders without dementia: classification, underlying causes and treatment
About 90% of the area of the human cerebral cortex is involved in cognitive activity. Therefore, most neurological diseases with an interest in the brain are accompanied by certain cognitive disorders. Usually they are combined with changes in the emotional-behavioral sphere, being united by a common pathomorphological and pathophysiological substrate. A practicing neurologist needs to assess the presence and characteristics of cognitive and other neuropsychiatric disorders and take this information into account when diagnosing syndromic, topical and nosological diseases of the nervous system. Cognitive impairment is of equal importance to clinicians in other medical specialties. The target organ of many somatic diseases, in particular, diseases of the cardiovascular system that are widespread in the elderly, is the brain. In this case, the assessment of the state of the brain is extremely important for assessing the effectiveness of the control of the underlying disease and the choice of therapeutic tactics. nine0003
Cognitive impairment is of equal importance to clinicians in other medical specialties. The target organ of many somatic diseases, in particular, diseases of the cardiovascular system that are widespread in the elderly, is the brain. In this case, the assessment of the state of the brain is extremely important for assessing the effectiveness of the control of the underlying disease and the choice of therapeutic tactics. nine0003
The presence of cognitive impairments has an extremely negative impact on the quality of life of the patient and his immediate family, makes it difficult to treat concomitant diseases and carry out rehabilitation measures. Therefore, timely diagnosis and the earliest possible start of therapy for existing cognitive disorders are very important.
Definition and classification of cognitive impairments
According to the latest revision of the international recommendations for the diagnosis of mental disorders (Diagnostic and statistical manual of mental diseases - DSM-V), cognitive disorders include a decrease in comparison with the premorbid level of one or more higher brain functions that provide the processes of perception, storage, transformation and transmission of information (Table 1) [1]. nine0003
nine0003
It is important not only to establish cognitive decline and conduct its qualitative analysis, but also to quantify the severity of existing disorders. It is known that some drugs that are effective in severe cognitive impairment (dementia) have a much lesser effect on cognitive impairment that does not reach the degree of dementia. This is probably due to various neurochemical changes that are noted at the early and later stages of the pathological process [2–4]. nine0003
Dementia (or, according to DSM-V, severe neurocognitive impairment) is characterized by significant impairment of higher brain functions that interfere with the normal functioning of the patient. With dementia, due to severe cognitive impairment, the patient is at least partially deprived of independence and needs outside help in the most common life situations (for example, when orienting in the area, shopping in a store) (Table 2) [1].
In the treatment of patients with severe cognitive disorders, priority should be given to drugs with a symptomatic effect, which can reduce the severity of disorders and thereby improve the quality of life of patients and their relatives.
The diagnosis of non-dementia cognitive impairment is established in cases where, despite the existing intellectual defect, the patient retains independence in everyday life. At the same time, the patient may feel some difficulties in mental work, which is reflected in complaints. However, the patient overcomes these difficulties without resorting to outside help (Table 2) [1]. In the treatment of patients with non-dementia cognitive disorders, one should not only use symptomatic therapy, but also take measures to prevent dementia. nine0003
According to the classification of Academician N.N. Yakhno, non-dementia cognitive disorders are divided into mild and moderate (Table 3) [5]. At the same time, patients with moderate impairments may experience difficulties in the most complex and unusual activities for the patient. At the same time, patients with mild disorders are completely independent and independent in all types of activity, including the most difficult one.
In recent years, the attention of neurologists, psychiatrists and representatives of other neurosciences has increased to an even earlier stage of cognitive deficiency - the so-called subjective cognitive impairment. The wording "subjective cognitive impairment" (subjective memory impairment, cognitive complaints) is currently widely used both in the scientific literature and in everyday clinical practice as an independent diagnosis. This diagnosis is made if there are cognitive complaints, while the results of objective cognitive tests remain within the age norm. nine0003
The wording "subjective cognitive impairment" (subjective memory impairment, cognitive complaints) is currently widely used both in the scientific literature and in everyday clinical practice as an independent diagnosis. This diagnosis is made if there are cognitive complaints, while the results of objective cognitive tests remain within the age norm. nine0003
Patients may complain of increased forgetfulness, decreased concentration of attention, increased fatigue during mental work, and sometimes difficulty in finding the right word in a conversation. These complaints are a very urgent problem for the patient, which can serve as an independent or main reason for contacting a doctor. At the same time, the use of standard cognitive tests does not reveal any significant deviations from the accepted standards. Patients with subjective cognitive disorders fully maintain independence in everyday life. Cognitive difficulties are also invisible from the outside: relatives, colleagues and other persons always assess the patient's cognitive abilities as quite intact. nine0003
nine0003
Currently, the following international diagnostic criteria (2014) for the syndrome of subjective cognitive impairment are known [6]:
- the patient's complaints about a persistent deterioration in mental performance compared to the past, which arose for no apparent reason;
- the absence of any deviations from the age norm according to the data of cognitive tests used to diagnose Alzheimer's disease and other dementing diseases;
- cognitive complaints are not associated with any established diagnosis of a neurological, psychiatric disease or intoxication. nine0003
| Function | Definition |
|---|---|
| Perception (gnosis) | The ability to perceive and recognize information coming from the senses |
| Memory | The ability to capture, store and repeatedly reproduce the information received nine0044 |
| Psychomotor function (praxis) | The ability to create, save and execute motor programs |
| Speech | The ability to verbal communication, including understanding addressed speech, constructing one's own speech statement, reading and writing |
| Attention | The ability to respond in a timely manner to signals coming from the senses, to concentrate and maintain mental performance for the required time, to share information flows nine0044 |
| Control Functions | The ability to plan and control cognitive activity and behavior, including goal selection (goal setting), program design (programming), transition from one stage of the program to another (switchability, intellectual flexibility) and comparison of the result with the goal (control) |
| social intelligence | The ability to understand the emotions and logic of other people nine0044 |
The dissociation between patient complaints, test results, and patients' day-to-day functioning raises legitimate questions about the true nature of the complaints. These issues are still far from being resolved and are being actively studied. At the present stage of scientific knowledge, it seems that patients with subjective cognitive impairments represent a very heterogeneous group, which includes both patients with the earliest stages of the dementing process and patients with anxiety-depressive and hypochondria spectrum disorders. In some cases, the predominantly subjective nature of disorders is explained by methodological difficulties in objectifying cognitive status. Currently, there are no generally accepted recommendations on the use of specific methods for the diagnosis of dementia or non-dementia cognitive impairment. Therefore, in practice, tests of varying degrees of sensitivity, specificity and reproducibility are used. The use of tests with low sensitivity will lead to underdiagnosis of mild and moderate cognitive impairments and to overdiagnosis of so-called subjective impairments. nine0003
These issues are still far from being resolved and are being actively studied. At the present stage of scientific knowledge, it seems that patients with subjective cognitive impairments represent a very heterogeneous group, which includes both patients with the earliest stages of the dementing process and patients with anxiety-depressive and hypochondria spectrum disorders. In some cases, the predominantly subjective nature of disorders is explained by methodological difficulties in objectifying cognitive status. Currently, there are no generally accepted recommendations on the use of specific methods for the diagnosis of dementia or non-dementia cognitive impairment. Therefore, in practice, tests of varying degrees of sensitivity, specificity and reproducibility are used. The use of tests with low sensitivity will lead to underdiagnosis of mild and moderate cognitive impairments and to overdiagnosis of so-called subjective impairments. nine0003
The diagnosis of "subjective cognitive impairment" is often received by patients with a high premorbid intellectual level. The cognitive functions reduced as a result of a cerebral disease in comparison with the individual norm will formally be within the average statistical standard for a long time. Consequently, cognitive decline can remain formally unconfirmed for a long time, in other words, “subjective”.
The cognitive functions reduced as a result of a cerebral disease in comparison with the individual norm will formally be within the average statistical standard for a long time. Consequently, cognitive decline can remain formally unconfirmed for a long time, in other words, “subjective”.
Complaints of a cognitive nature may be due to anxiety-depressive disorders in the absence of an organic cerebral disease. Thus, patients with a high level of anxiety will be overly concerned about minor situational forgetfulness. In this case, the reason for going to the doctor is such widespread complaints, including among healthy people, such as “I don’t remember why I came to the room”, “I don’t remember what I put where”, “I didn’t recognize a familiar person or didn’t remember him surname", etc. nine0003
However, the greatest research interest in a heterogeneous group of patients with subjective cognitive impairments is caused by patients with reduced tolerance to mental stress, since this pathological phenomenon may indeed be the earliest clinical manifestation of the dementing process. As is known, at the very initial stages of a neurodegenerative or cerebrovascular disease, clinical symptoms may be absent, despite the presence of organic brain damage, sometimes significant. This is due to the so-called cerebral reserve, that is, the compensatory capabilities of the brain. The presence of such opportunities will lead to a false negative test result. At the same time, in everyday life, the patient may experience difficulties in special conditions when the cerebral reserve is depleted and cannot overcome the difficulties that arise, for example, in a state of fatigue or emotional stress. At present, the development of the "intellectual treadmill" methodology is being carried out very actively in the world. It will allow assessing the degree of tolerance to increased mental stress, which may decrease to the development of clinically defined cognitive disorders. nine0003
As is known, at the very initial stages of a neurodegenerative or cerebrovascular disease, clinical symptoms may be absent, despite the presence of organic brain damage, sometimes significant. This is due to the so-called cerebral reserve, that is, the compensatory capabilities of the brain. The presence of such opportunities will lead to a false negative test result. At the same time, in everyday life, the patient may experience difficulties in special conditions when the cerebral reserve is depleted and cannot overcome the difficulties that arise, for example, in a state of fatigue or emotional stress. At present, the development of the "intellectual treadmill" methodology is being carried out very actively in the world. It will allow assessing the degree of tolerance to increased mental stress, which may decrease to the development of clinically defined cognitive disorders. nine0003
| Severity of violations | Criterion |
|---|---|
| Mild Cognitive Impairment Syndrome (Mild Neurocognitive Impairment) | A slight decrease from the previous level of one or more cognitive functions (attention, executive functions, memory, speech, praxis, gnosis, social intelligence), which is confirmed by: - patient complaints, information from third parties, including the attending physician; - Neuropsychological tests or independent clinical assessment.  Cognitive impairment does not deprive the patient of independence in daily activities (including its complex types, for example, when performing financial transactions or taking medications). The patient remains independent, but daily activities may require more effort than before, or the use of special coping strategies. Cognitive impairments are present not only during delirium. Cognitive impairment is not associated with other psychiatric disorders such as depression or schizophrenia nine0044 |
| Severe neurocognitive impairment | Significant decrease from the previous level of one or more cognitive functions (attention, executive functions, memory, speech, praxis, gnosis, social intelligence), which is confirmed: - patient complaints, information from third parties, including the attending physician; - Neuropsychological tests or independent clinical assessment. Cognitive impairment deprives the patient of independence in everyday life (at least in its complex forms, for example, when performing financial transactions or taking medication). 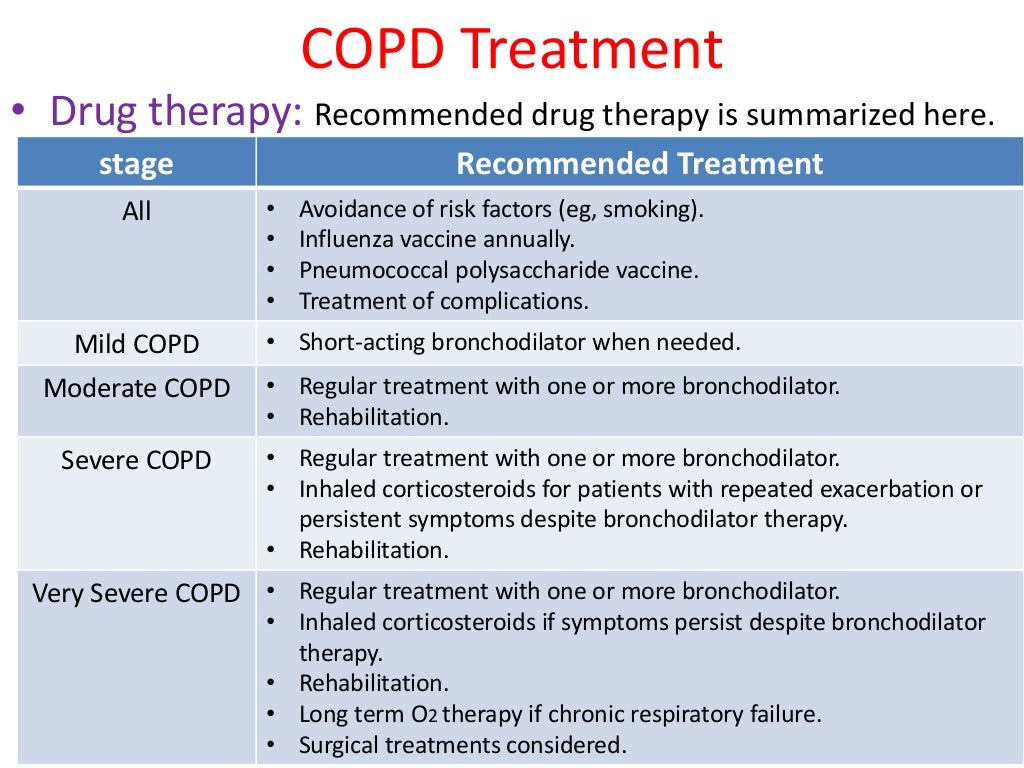 Cognitive impairments are present not only during delirium. Cognitive impairment is not associated with other psychiatric disorders such as depression or schizophrenia nine0044 Cognitive impairments are present not only during delirium. Cognitive impairment is not associated with other psychiatric disorders such as depression or schizophrenia nine0044 |
International studies show that the risk of developing dementing diseases among patients with subjective cognitive impairment is significantly higher than the average in the population [6]. Therefore, even isolated complaints that are not confirmed by cognitive tests should not be ignored by the attending physicians. They cannot serve as a basis for any specific clinical diagnosis, but their presence is an indication for active prevention, primarily non-pharmacological (mental and physical activity, optimization of nutrition and lifestyle). nine0003
Diagnosis of moderate cognitive impairment
As follows from the above criteria (Table 2), the diagnosis of the syndrome of moderate neurocognitive impairment is based, firstly, on complaints from patients and/or their relatives, and secondly, on objective test results. At the same time, it should be borne in mind that complaints of a cognitive nature are far from always straightforward. Usually, patients with the so-called amnestic type of the syndrome of moderate neurocognitive impairment complain of a decrease in memory or increased forgetfulness, in which progressive memory disorders predominate in their cognitive status. In such patients, Alzheimer's disease is most often established in the future. However, according to the analysis of specialized outpatient admission of patients with cognitive impairment, the most common cause of the syndrome of moderate cognitive impairment is cerebrovascular pathology. Thus, the experience of the first Russian clinic of memory disorders indicates that dyscirculatory encephalopathy or the consequences of acute cerebrovascular accidents cause 68% of moderate cognitive impairments [7]. nine0003
At the same time, it should be borne in mind that complaints of a cognitive nature are far from always straightforward. Usually, patients with the so-called amnestic type of the syndrome of moderate neurocognitive impairment complain of a decrease in memory or increased forgetfulness, in which progressive memory disorders predominate in their cognitive status. In such patients, Alzheimer's disease is most often established in the future. However, according to the analysis of specialized outpatient admission of patients with cognitive impairment, the most common cause of the syndrome of moderate cognitive impairment is cerebrovascular pathology. Thus, the experience of the first Russian clinic of memory disorders indicates that dyscirculatory encephalopathy or the consequences of acute cerebrovascular accidents cause 68% of moderate cognitive impairments [7]. nine0003
Vascular cognitive impairments in most cases are of the so-called subcortical-frontal type. At the same time, the memory for current events and life events practically does not suffer, and the cognitive status is dominated by a decrease in the concentration of attention and the pace of cognitive activity (bradyphrenia), a violation of frontal control functions (planning and control). A characteristic feature is also the frequent combination of cognitive and emotional-behavioral disorders: depression, apathy or affective lability. It should be emphasized that emotional-behavioral disorders in chronic cerebrovascular insufficiency are of an organic nature and are caused by the same brain damage (dysfunction of fronto-striatal connections) as cognitive impairment. The comorbidity of vascular depression and vascular cognitive impairment is at least 80% [8–11]. nine0003
A characteristic feature is also the frequent combination of cognitive and emotional-behavioral disorders: depression, apathy or affective lability. It should be emphasized that emotional-behavioral disorders in chronic cerebrovascular insufficiency are of an organic nature and are caused by the same brain damage (dysfunction of fronto-striatal connections) as cognitive impairment. The comorbidity of vascular depression and vascular cognitive impairment is at least 80% [8–11]. nine0003
| Violations | Description |
|---|---|
| Lungs | A decrease in cognitive abilities compared to a higher premorbid level of an individual, the decrease formally remains within the average statistical age norm or deviates slightly from it. It is usually reflected in the patient's complaints, but does not attract the attention of others. Does not cause difficulties in everyday life, even with its most complex forms nine0044 Does not cause difficulties in everyday life, even with its most complex forms nine0044 |
| Moderate | Decrease in cognitive abilities compared to both the individual and the average statistical age norm. It is reflected in the complaints of the individual and attracts the attention of others. Does not lead to significant difficulties in everyday life, although it may interfere with the most complex types of intellectual activity |
| heavy | Decrease in cognitive abilities, which leads to significant difficulties in everyday life: professional or social sphere, and in the most severe disorders - in self-care. Partial or complete loss of independence. Dependence on outside help nine0044 |
Patients with vascular cognitive disorders rarely complain of forgetfulness, since their memory is relatively intact. The structure of complaints is dominated by the so-called subjective neurological symptoms: headache, non-systemic dizziness, noise and heaviness in the head, increased fatigue, sleep disturbances.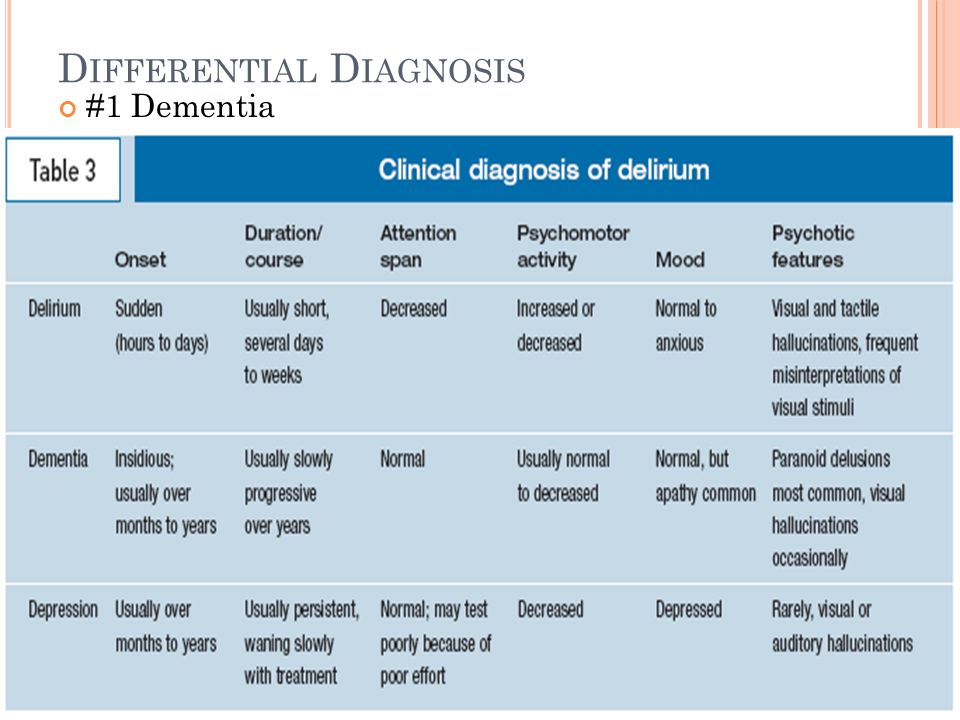 These symptoms are quite typical for the initial stages of dyscirculatory encephalopathy and in the recent past were considered as an important sign of chronic ischemic brain damage. It is now obvious that headache, dizziness and other unpleasant sensations in the head cannot be a direct result of cerebral ischemia. The pathogenesis of subjective neurological symptoms is more complex and is associated primarily with existing cognitive, emotional and motor disorders. Thus, headache most often has the character of a tension headache, which, as you know, is almost always caused by anxiety and / or depression. Sleep disturbances also have an emotional cause. Increased fatigue can either be a sign of depression (Table 4) or reflect a decrease in mental performance. In the latter case, this complaint is the subjective equivalent of cognitive disorders. Dizziness in chronic cerebrovascular insufficiency is usually non-systemic and is described as a feeling of unsteadiness when walking. Behind this feeling, as a rule, there are real imbalances due to damage to the fronto-striatal and fronto-cerebellar connections.
These symptoms are quite typical for the initial stages of dyscirculatory encephalopathy and in the recent past were considered as an important sign of chronic ischemic brain damage. It is now obvious that headache, dizziness and other unpleasant sensations in the head cannot be a direct result of cerebral ischemia. The pathogenesis of subjective neurological symptoms is more complex and is associated primarily with existing cognitive, emotional and motor disorders. Thus, headache most often has the character of a tension headache, which, as you know, is almost always caused by anxiety and / or depression. Sleep disturbances also have an emotional cause. Increased fatigue can either be a sign of depression (Table 4) or reflect a decrease in mental performance. In the latter case, this complaint is the subjective equivalent of cognitive disorders. Dizziness in chronic cerebrovascular insufficiency is usually non-systemic and is described as a feeling of unsteadiness when walking. Behind this feeling, as a rule, there are real imbalances due to damage to the fronto-striatal and fronto-cerebellar connections. nine0003
nine0003
Subjective neurological symptoms are almost always present in the initial stages of chronic cerebrovascular insufficiency. They cannot be the basis for a diagnosis, but should lead the doctor to suspect a chronic cerebrovascular disease. To confirm the diagnosis, a thorough assessment of the cognitive and emotional status using objective methods is required. At the stage of moderate (non-dementia) cognitive disorders, the most sensitive methods should be used, for example, the Montreal Cognitive Assessment Scale [12]. nine0003
Pathogenetic and symptomatic therapy of non-dementia cognitive impairments
To date, a unified generally accepted protocol for the management of patients with cognitive impairments that do not reach the severity of dementia has not been finally developed. Many international studies have failed to demonstrate that pharmacotherapy with drugs such as acetylcholinesterase inhibitors, piracetam, non-steroidal anti-inflammatory drugs prevents or reduces the risk of dementia [2–4].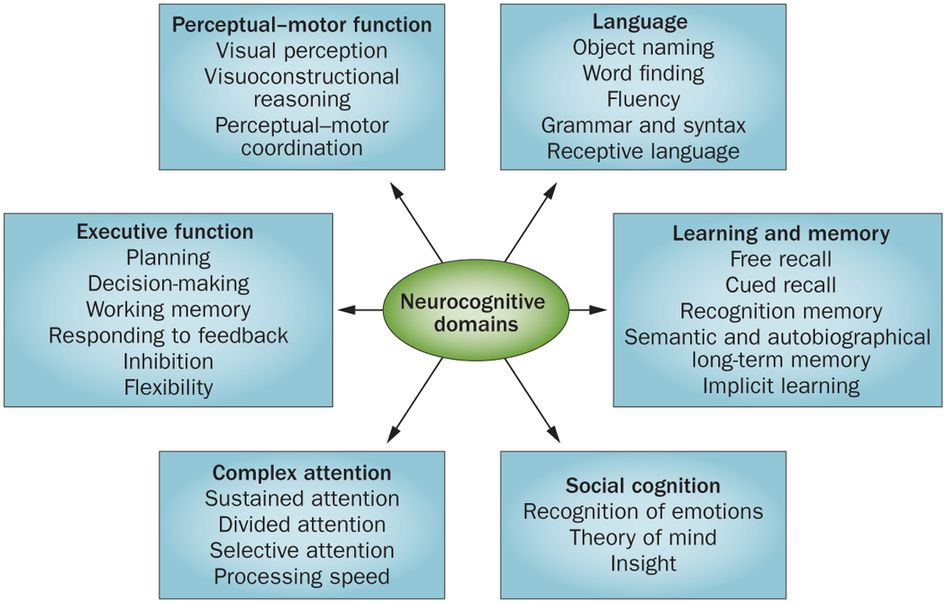 At the same time, the same studies showed the ability of some of the above drugs to reduce the severity of symptoms in patients with mild cognitive impairment syndrome. nine0003
At the same time, the same studies showed the ability of some of the above drugs to reduce the severity of symptoms in patients with mild cognitive impairment syndrome. nine0003
Currently, vasotropic and neurometabolic drugs, the dopaminergic and noradrenergic drug piribedil (Pronoran), and NMDA receptor blockers are widely used empirically in everyday clinical practice.
The results of a number of large studies and practical experience indicate the clinical effectiveness of the drug piribedil (Pronoran). Pronoran has a complex mechanism of action: it stimulates postsynaptic D2/D3 dopamine receptors and blocks presynaptic alpha-adrenergic receptors. At the same time, the blockade of presynaptic adrenergic receptors leads to an increase in cerebral noradrenergic activity. Thus, against the background of the use of this drug, the activity of two cerebral neurotransmitter systems increases: dopaminergic and noradrenergic. Both of these systems are directly involved in cognitive activity. At the same time, it is believed that dopaminergic stimulation of the prefrontal cortex, indirectly through the mesocortical dopaminergic pathway, plays an important role in attention processes and provides intellectual flexibility, that is, the ability to change the paradigm of behavior. Noradrenergic activation is important for the processes of memorization and reproduction of information, since it provides the optimal level of concentration and motivation for mnestic activity. With age, the synthesis and activity of both dopamine and norepinephrine decrease. Therefore, the correction of these neurotransmitter disorders against the background of the use of Pronoran helps to reduce the severity of age-associated disorders of attention and memory. In addition, due to the adrenoblocking and dopaminergic action, Pronoran also has a favorable vasotropic effect, which creates additional benefits in case of cognitive impairment of vascular etiology [13-16]. nine0003
At the same time, it is believed that dopaminergic stimulation of the prefrontal cortex, indirectly through the mesocortical dopaminergic pathway, plays an important role in attention processes and provides intellectual flexibility, that is, the ability to change the paradigm of behavior. Noradrenergic activation is important for the processes of memorization and reproduction of information, since it provides the optimal level of concentration and motivation for mnestic activity. With age, the synthesis and activity of both dopamine and norepinephrine decrease. Therefore, the correction of these neurotransmitter disorders against the background of the use of Pronoran helps to reduce the severity of age-associated disorders of attention and memory. In addition, due to the adrenoblocking and dopaminergic action, Pronoran also has a favorable vasotropic effect, which creates additional benefits in case of cognitive impairment of vascular etiology [13-16]. nine0003
| Criterion | Symptom |
|---|---|
| A. Main | Presence most of the time for at least the last two weeks of at least two of the following: - depressed mood or longing; - a decrease in interests or a loss of a sense of pleasure from those activities that previously gave positive emotions; nine0029 - decreased energy and increased fatigue |
| B. Additional | Presence of at least two of the following features: - decreased ability to concentrate; - low self-esteem and lack of self-confidence; - ideas of guilt and self-abasement; - a gloomy, pessimistic vision of the future; - suicidal thoughts or actions; nine0029 - sleep disorders; - appetite disorders |
In clinical practice, Pronoran is used to treat mild and moderate cognitive impairment that does not reach the severity of dementia in patients over 50 years of age. The drug can be prescribed both for vascular cognitive impairment and at the initial stages of the neurodegenerative process. A large number of clinical studies have been performed for this indication, including those using a double-blind method. So, in France at 1980s 14 clinical studies were conducted, in which more than 7 thousand patients with non-dementia cognitive impairments took part. It has been shown that Pronoran contributes to a significant improvement in memory, concentration and intellectual flexibility, that is, the ability to change the paradigm of behavior depending on external conditions [17, 18]. In 2001, the clinical efficacy of Pronoran was again demonstrated by D. Nagaradja and S. Jayashree. The authors used Pronoran in the syndrome of moderate cognitive impairment in accordance with modern diagnostic criteria. It was shown that against the background of the study drug, there was a more than two-fold increase in the frequency of cognitive improvement on the short mental status assessment scale compared with placebo, which was statistically and clinically significant [19].
The drug can be prescribed both for vascular cognitive impairment and at the initial stages of the neurodegenerative process. A large number of clinical studies have been performed for this indication, including those using a double-blind method. So, in France at 1980s 14 clinical studies were conducted, in which more than 7 thousand patients with non-dementia cognitive impairments took part. It has been shown that Pronoran contributes to a significant improvement in memory, concentration and intellectual flexibility, that is, the ability to change the paradigm of behavior depending on external conditions [17, 18]. In 2001, the clinical efficacy of Pronoran was again demonstrated by D. Nagaradja and S. Jayashree. The authors used Pronoran in the syndrome of moderate cognitive impairment in accordance with modern diagnostic criteria. It was shown that against the background of the study drug, there was a more than two-fold increase in the frequency of cognitive improvement on the short mental status assessment scale compared with placebo, which was statistically and clinically significant [19].
Currently, Russian specialists also have significant experience in using Pronoran in patients with cognitive impairments that do not reach the severity of dementia. Thus, as part of the PROMETHEUS study, 574 patients from 33 cities in 30 regions of Russia, including 336 women and 207 men, aged 60 to 89 years (mean age 69.5 ± 5.5 years) with mild or moderate cognitive impairments received Pronoran. . Patients with cognitive complaints who scored 25–27 points on the Mini Mental Status Scale or performed the clock drawing test with errors but did not meet the diagnostic criteria for dementia were selected for treatment. Against the background of therapy, a statistically significant improvement in cognitive functions was recorded, which was noted as early as the sixth week of treatment and further increased until the end of the 12-week observation. At the same time, one part of the patients received Pronoran monotherapy, and the other part received Pronoran in combination with vasotropic and / or neurometabolic drugs. There was no significant difference between these groups of patients, that is, the combination of Pronoran with vasotropic and neurometabolic therapy had no advantages over monotherapy with the study drug [20, 21]. nine0003
There was no significant difference between these groups of patients, that is, the combination of Pronoran with vasotropic and neurometabolic therapy had no advantages over monotherapy with the study drug [20, 21]. nine0003
In the largest Russian non-comparative study, more than 2,000 patients aged 50 to 94 years (mean age 64.9 ± 8.3 years) with a diagnosis of stage 1 or 2 dyscirculatory encephalopathy and with mild or moderate cognitive impairment received Pronoran therapy. violations. All patients took Pronoran for three months. According to the attending physicians, in 2/3 of cases there was a significant or moderate improvement in cognitive and other neurological functions [22]. According to some data, the magnitude of the therapeutic effect of dopamine and noradrenergic therapy in relation to non-dementia cognitive disorders may be greater than that of other vasotropic and neurometabolic drugs actively used in clinical practice. In the FUETE study, 189patients, including 139 women and 57 men, aged 42 to 82 years (mean age 63. 6 ± 8.5 years) with cognitive disorders that do not reach the severity of dementia, against the background of arterial hypertension and cerebral atherosclerosis. The patients were treated with various drugs, while the representatives of the therapeutic groups did not differ in age, level of education and clinical features of the underlying disease. Against the background of the therapy, there was a regression of both subjective and objective cognitive disorders in all compared therapeutic groups. At the same time, the severity of subjective improvement and objective dynamics of cognitive tests against the background of the use of Pronoran after two months of therapy were significantly greater compared with vasotropic and neurometabolic therapy (Fig. 1) [23]. nine0003
6 ± 8.5 years) with cognitive disorders that do not reach the severity of dementia, against the background of arterial hypertension and cerebral atherosclerosis. The patients were treated with various drugs, while the representatives of the therapeutic groups did not differ in age, level of education and clinical features of the underlying disease. Against the background of the therapy, there was a regression of both subjective and objective cognitive disorders in all compared therapeutic groups. At the same time, the severity of subjective improvement and objective dynamics of cognitive tests against the background of the use of Pronoran after two months of therapy were significantly greater compared with vasotropic and neurometabolic therapy (Fig. 1) [23]. nine0003
Regression of cognitive disorders, according to special tests, is the main criterion for the effectiveness of the therapy. However, as noted above, many patients with moderate cognitive impairment, primarily of a vascular nature, also complain of headache, non-systemic dizziness, noise, heaviness or other unpleasant sensations in the head, increased fatigue and sleep disturbances.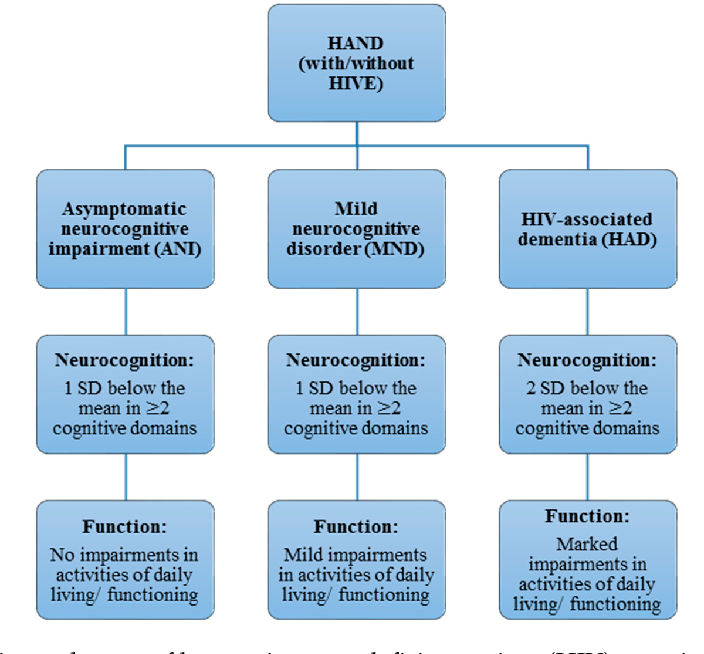 These complaints are of the same nature and are associated with both cognitive impairment and changes in the emotional status of patients at the initial stage of chronic cerebrovascular insufficiency. They significantly reduce the quality of life of patients and are often the main reason for visiting a neurologist. Therefore, the dynamics of subjective neurological symptoms in patients with a syndrome of moderate neurocognitive disorders of vascular etiology during therapy is extremely important for assessing the significance of the clinical effect and the degree of influence of therapy on the daily life of patients. Regression of subjective neurological symptoms contributes most to adherence to therapy. nine0003
These complaints are of the same nature and are associated with both cognitive impairment and changes in the emotional status of patients at the initial stage of chronic cerebrovascular insufficiency. They significantly reduce the quality of life of patients and are often the main reason for visiting a neurologist. Therefore, the dynamics of subjective neurological symptoms in patients with a syndrome of moderate neurocognitive disorders of vascular etiology during therapy is extremely important for assessing the significance of the clinical effect and the degree of influence of therapy on the daily life of patients. Regression of subjective neurological symptoms contributes most to adherence to therapy. nine0003
In the study by N.N. Yakhno et al. (2006) 29 patients with a diagnosis of "moderate cognitive impairment" against the background of discirculatory encephalopathy of the first or second stage received Pronoran for three months [24]. At the same time, no other vasotropic or neurometabolic drugs were used. During treatment with Pronoran, the frequency and severity of headache, dizziness, fatigue and subjective feeling of forgetfulness significantly decreased (Fig. 2). Other authors also reported on the weakening of subjective neurological symptoms during the use of Pronoran [17, 18]. Thus, dopamine and noradrenergic therapy contributes to a significant improvement in the well-being of patients, and, consequently, improves the quality of life and adherence to ongoing therapeutic measures. It can be summarized that to date, Pronoran has established itself as an effective drug that improves cognitive abilities and well-being in patients with the initial stages of organic cerebral diseases without dementia. In contrast to Parkinson's disease, in which significantly higher doses are used, for non-demented cognitive impairment, Pronoran is prescribed at a dose of 50 mg / day once a day. The recommended duration of therapy is at least three months. nine0003
During treatment with Pronoran, the frequency and severity of headache, dizziness, fatigue and subjective feeling of forgetfulness significantly decreased (Fig. 2). Other authors also reported on the weakening of subjective neurological symptoms during the use of Pronoran [17, 18]. Thus, dopamine and noradrenergic therapy contributes to a significant improvement in the well-being of patients, and, consequently, improves the quality of life and adherence to ongoing therapeutic measures. It can be summarized that to date, Pronoran has established itself as an effective drug that improves cognitive abilities and well-being in patients with the initial stages of organic cerebral diseases without dementia. In contrast to Parkinson's disease, in which significantly higher doses are used, for non-demented cognitive impairment, Pronoran is prescribed at a dose of 50 mg / day once a day. The recommended duration of therapy is at least three months. nine0003
Literature
Diagnostic and manual statistical of mental diseases. Ved. (DSM-V). London: American Psychiatric Association, 2013.
Ved. (DSM-V). London: American Psychiatric Association, 2013.
2. Jelic V., Kivipelto M., Winblad B. Clinical trials in mild cognitive impairment: lessons for the future // J. Neurol. neurosurgery. Psychiatry. 2006 Vol. 77. No. 4. P. 429-438.
3. Knopman D.S. Current treatment of mild cognitive impairment and Alzheimer's disease // Curr. Neurol. Neroci. Rep. 2006 Vol. 6. No. 5. P. 365–371. nine0029 4. Kurshner H.S. Mild cognitive impairment: to treat or not to treat? // Curr. Neurol. neurosci. Rep. 2005 Vol. 5. No. 6. P. 455–457.
5. Yakhno N.N. Cognitive disorders in a neurological clinic // Neurological journal. 2006. V. 11. Appendix No. 1. S. 4–12.
6. Jessen F., Amariglio R. E., van Boxtel M. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease // Alzheimer's Dement. 2014. Vol. 10. No. 6. P. 844–852. nine0029 7. Yakhno N.N., Preobrazhenskaya I.S., Zakharov V.V. Prevalence of cognitive impairment in neurological diseases (analysis of the work of a specialized outpatient clinic) // Neurology, neuropsychiatry, psychosomatics. 2012. No. 2. S. 30–34.
2012. No. 2. S. 30–34.
8. Yakhno N.N., Zakharov V.V., Lokshina A.B. Syndrome of moderate cognitive impairment in dyscirculatory encephalopathy // Journal of Neurology and Psychiatry. S.S. Korsakov. 2005. V. 105. No. 2. S. 13–17.
9. Yakhno N.N., Zakharov V.V., Lokshina A.B. Memory and attention disorders in the elderly // Journal of Neurology and Psychiatry. S.S. Korsakov. 2006. V. 106. No. 2. S. 58–62. nine0029 10. Yakhno N.N., Levin O.S., Damulin I.V. Comparison of clinical and MRI data in dyscirculatory encephalopathy. Message 2: cognitive impairment // Neurological journal. 2001. V. 6. No. 3. S. 10–19.
11. Voznesenskaya T.G. Non-cognitive neuropsychiatric disorders in cognitive impairment in the elderly // Neurological journal. 2010. V. 15. No. 2. S. 4–18.
12. www.mocatest.org.
13. DeKeyser J., Herregodts P., Ebinger G. The mesonocortical dopamine neuron system // Neurology. nineteen90 Vol. 40. No. 11. R. 1660-1662.
14. Aston-Jones G., Rajkowsky J. , Cohen J. Role of locus coeruleus in attention and behavioral flexibility // Biol. Psychiatry. 1999 Vol. 46. No. 9. P. 1309-1320.
, Cohen J. Role of locus coeruleus in attention and behavioral flexibility // Biol. Psychiatry. 1999 Vol. 46. No. 9. P. 1309-1320.
15. Backman L., Ginovart N., Dixon R. et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system // Am. J. Psychiatry. 2000 Vol. 157. No. 4. P. 635–637.
16. Volkow N., Wang G., Fowler J. et al. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging // Ann. Neurol. nineteen98 Vol. 44. No. 1. P. 143–147.
17. Bille J., Bukiwsky J.V., de Ferron A. et al. Decline cerebral et therapeutique: une etude clinique multicenrique de Trivastal 50 retard en Neuro-Geriatrie // Psych. Med. 1986 Vol. 18. P. 609–626.
18. Scholing W.E. A double-blind study using psychometric tests Trivastal versus a reference compound // Temp. Medical. 1982. P. 114b.
19. Nagaraia D., Jayashree S. Randomized study of the dopamine receptor agonist piribedil in the treatment of mild cognitive impairment // Am. J. Psychiatry. 2001 Vol. 158. No. 9. P. 1517-1519.
J. Psychiatry. 2001 Vol. 158. No. 9. P. 1517-1519.
20. Zakharov V.V. All-Russian Program for Research on the Epidemiology and Therapy of Cognitive Disorders in the Elderly (Prometheus) // Neurological Journal. 2006. V. 11. No. 2. S. 27–32.
21. Zakharov V.V. Prevalence and treatment of cognitive impairment in a neurological clinic (Results of the All-Russian study "PROMETHEUS") // Consilium Medicum. 2008. V. 10. No. 2. S. 114–117.
22. Zakharov V.V. Dopaminergic and noradrenergic therapy for cognitive impairment // Journal of Neurology and Psychiatry named after V.I. S.S. Korsakov. 2006. Vol. 106. No. 9. pp. 43–47.
23. Yakhno N.N., Zakharov V.V., Strachunskaya E.A. et al. Treatment of non-demented cognitive impairment in patients with arterial hypertension and cerebral atherosclerosis // Neurological journal. 2012. No. 4. S. 49–55.
24. Zakharov V.V., Lokshina A.B. The use of the drug "Pronoran" (piribedil) in mild cognitive disorders in elderly patients with dyscirculatory encephalopathy // Neurological journal. 2004. V. 9. No. 2. S. 30–35
2004. V. 9. No. 2. S. 30–35
Related articles
- Biofeedback method in the treatment of chronic headache and comorbid disorders
- Temporomandibular joint dysfunction in chronic migraine
- Central regulation of pain in patients with joint disease and approaches to therapy nine0257
Related videos
- Cognitive impairment
- Cognitive behavioral therapy for chronic pain. Part 1. Danilov A.B.
- School of Professor Zakharov V.V. "Cognitive impairments in clinical cases. Modern practical management algorithms" 02.11.21 nine0257
Neurocognitive aging and cognitive impairment
Advances in public health have led to an increase in life expectancy and a significant increase in the number of older people worldwide. This process is inevitably accompanied by an increase in the prevalence of chronic age-related diseases, primarily accompanied by the steady development of cognitive impairment. By 2050, the number of people living with dementia worldwide may triple (from 47 million to 132 million) [1]. A preventive approach to the problem of dementia involves an analysis of the causes and factors that predetermine the transformation of early pre-dementia cognitive impairment into dementia. This approach also requires studying the patterns of brain aging and identifying the determining processes of neurocognitive aging. nine0003
By 2050, the number of people living with dementia worldwide may triple (from 47 million to 132 million) [1]. A preventive approach to the problem of dementia involves an analysis of the causes and factors that predetermine the transformation of early pre-dementia cognitive impairment into dementia. This approach also requires studying the patterns of brain aging and identifying the determining processes of neurocognitive aging. nine0003
Patterns of neurocognitive aging
The cognitive changes that occur with aging are well understood. Some cognitive abilities, such as vocabulary, are resistant to brain aging and may even improve with age, but other functions, such as conceptualization, memory, and processing speed, gradually decline. There is considerable heterogeneity among older people in the rate of decline in some abilities, especially in perception scores and processing speed. nine0003
The concept of crystallized and fluid intelligence is often used to describe the nature of cognitive changes throughout life [1]. Crystallized intelligence refers to skills, abilities, and knowledge that are acquired and used frequently throughout life. Examples of this kind of ability are vocabulary and general awareness, which remain relatively stable or even improve in the sixth and seventh decades of life [2]. Because crystallized intelligence is driven by the accumulation of information based on life experiences, older people tend to be better at tasks that require this type of intellectual ability than younger people. In contrast, fluid intelligence refers to abilities that include problem solving abilities and reasoning about things that are less familiar and independent of the level of awareness. Rather, this type of intelligence involves the use of a person's innate ability to process and master new information, solve problems and manipulate facts. Executive functions, processing speed, memory, and psychomotor abilities are all forms of fluid intelligence. Many fluid cognitions, especially psychomotor functions, peak in the third decade of life and then decline in subsequent years.
Crystallized intelligence refers to skills, abilities, and knowledge that are acquired and used frequently throughout life. Examples of this kind of ability are vocabulary and general awareness, which remain relatively stable or even improve in the sixth and seventh decades of life [2]. Because crystallized intelligence is driven by the accumulation of information based on life experiences, older people tend to be better at tasks that require this type of intellectual ability than younger people. In contrast, fluid intelligence refers to abilities that include problem solving abilities and reasoning about things that are less familiar and independent of the level of awareness. Rather, this type of intelligence involves the use of a person's innate ability to process and master new information, solve problems and manipulate facts. Executive functions, processing speed, memory, and psychomotor abilities are all forms of fluid intelligence. Many fluid cognitions, especially psychomotor functions, peak in the third decade of life and then decline in subsequent years. At the same time, the rate of decline in individual cognitive functions (processing speed, attention, memory, speech, visual abilities and spatial orientation, executive functions, judgments) varies greatly in different individuals. nine0003
At the same time, the rate of decline in individual cognitive functions (processing speed, attention, memory, speech, visual abilities and spatial orientation, executive functions, judgments) varies greatly in different individuals. nine0003
The speed of information processing reflects the pace of cognitive actions, as well as motor reactions. These abilities begin to decline in the third decade of life, and this process is steadily progressing [2]. Many of the cognitive changes seen in the elderly are the result of a slowdown in the speed of information processing. This "slowdown" can adversely affect the performance of many neuropsychological tests designed to assess other cognitive functions (eg, fluency), i.e., a decrease in the speed of information processing may have consequences for the functioning of various cognitive domains. nine0003
Attention refers to the ability to focus on specific stimuli. Simple auditory attention span (also known as immediate memory), which is assessed by, for example, repeating a sequence of numbers, shows only a slight decline in later life. A more noticeable effect of age is observed when performing more complex tasks for attention, such as selective and divided attention [1]. Selective attention is the ability to focus on specific information in the environment while ignoring non-essential information. Selective attention is important for tasks such as talking in a noisy environment or driving a car. Divided attention is the ability to focus on multiple tasks at the same time, such as talking on the phone while cooking. Older people are also worse than younger people at working memory tasks, which means the ability to instantly hold information in memory while simultaneously manipulating it. nine0003
A more noticeable effect of age is observed when performing more complex tasks for attention, such as selective and divided attention [1]. Selective attention is the ability to focus on specific information in the environment while ignoring non-essential information. Selective attention is important for tasks such as talking in a noisy environment or driving a car. Divided attention is the ability to focus on multiple tasks at the same time, such as talking on the phone while cooking. Older people are also worse than younger people at working memory tasks, which means the ability to instantly hold information in memory while simultaneously manipulating it. nine0003
Memory is one of the most important functions, and complaints about its impairment are the most common among older people. Age-related changes in memory may be associated with a slower speed of information processing, a decrease in the ability to ignore irrelevant information, or use compensatory strategies to improve learning and recall [3, 4]. Moreover, the various components of the actual memory in the process of aging undergo various types of changes. There are two main types of memory - declarative and non-declarative. Declarative memory is the conscious recollection of facts and events. It includes semantic memory and episodic memory. Semantic memory reflects the amount of information, the use of language and practical knowledge, such as remembering the meaning of words. Episodic (also known as autobiographical) is the memory of personally experienced events in a specific place and time, which can be assessed based on the memorization of stories, lists of words or numbers. While the decline in semantic and episodic memory occurs consistently with normal aging, the rates of this decline differ. Episodic memory declines progrediently throughout life, while semantic memory shows a clear decline in later life [3]. Non-declarative (implicit) memory, which is associated with the memorization of motor and cognitive skills, remains largely unchanged throughout life.
Moreover, the various components of the actual memory in the process of aging undergo various types of changes. There are two main types of memory - declarative and non-declarative. Declarative memory is the conscious recollection of facts and events. It includes semantic memory and episodic memory. Semantic memory reflects the amount of information, the use of language and practical knowledge, such as remembering the meaning of words. Episodic (also known as autobiographical) is the memory of personally experienced events in a specific place and time, which can be assessed based on the memorization of stories, lists of words or numbers. While the decline in semantic and episodic memory occurs consistently with normal aging, the rates of this decline differ. Episodic memory declines progrediently throughout life, while semantic memory shows a clear decline in later life [3]. Non-declarative (implicit) memory, which is associated with the memorization of motor and cognitive skills, remains largely unchanged throughout life. These features of memory changes during aging are presented in the table. Effect of aging on different types of memory [1]
These features of memory changes during aging are presented in the table. Effect of aging on different types of memory [1]
In general, obvious changes in the ability to acquire new knowledge are observed throughout life, while successfully acquired information is retained for a long time in cognitively healthy older people. The ability to extract recently received information noticeably decreases with age [3].
Speech is one of the most complex cognitive functions. General speech abilities remain unchanged with aging. Vocabulary is stable and can even improve over time [4]. However, this does not apply to the ability to visually compare and name objects, this function remains stable until about 70 years old, and then decreases in subsequent years. The same applies to the possession of oral speech, i.e., the ability to search for words and generate them in a certain period of time, which deteriorates markedly with age [4]. nine0003
Vision and design involves the ability to perceive space in two and three dimensions. Visual design skills, which include the ability to connect individual parts into a whole, decline over time [5]. However, in general, visual-spatial abilities, i.e., perception and the ability to recognize familiar objects, to evaluate the physical location of objects both individually and in relation to each other, remain unchanged for a long time. nine0003
Visual design skills, which include the ability to connect individual parts into a whole, decline over time [5]. However, in general, visual-spatial abilities, i.e., perception and the ability to recognize familiar objects, to evaluate the physical location of objects both individually and in relation to each other, remain unchanged for a long time. nine0003
Executive functions that enable a person to carry out successful, independent, purposeful activities in an optimal way for him, include a wide range of cognitive functions, such as the ability of self-control, planning, organization of actions, reasoning, as well as mental flexibility and problem solving [1 ]. Research has shown that concept formation, abstraction, and mental flexibility decrease with age, especially after age 70 [2], as older people tend to think more concretely than younger people [6]. Aging also negatively affects reaction inhibition, that is, the ability to suppress an automatic response in favor of creating a new form of response. At the same time, abilities that require an accelerated motor component, reasoning using unfamiliar material, are especially susceptible to age-related influences [1]. Other types of executive functions, such as the ability to evaluate similarities, describe the meaning of proverbs, and reason about familiar facts, remain stable throughout life. nine0003
At the same time, abilities that require an accelerated motor component, reasoning using unfamiliar material, are especially susceptible to age-related influences [1]. Other types of executive functions, such as the ability to evaluate similarities, describe the meaning of proverbs, and reason about familiar facts, remain stable throughout life. nine0003
Neurobiological basis of cognitive impairment in old age
Studies of the neurobiology of aging in recent years have made it possible to identify some patterns of changes in neuroimaging characteristics that are associated with the process of physiological aging. Most of the described patterns of change have clear clinical correlates.
Decreased volume of gray matter is a well-studied phenomenon in the aging process. Significant changes are recorded after 20 years. The most pronounced atrophic changes are found in the prefrontal cortex. Age-dependent changes in the temporal lobes are less pronounced and are mainly due to a decrease in the volume of the hippocampus [7].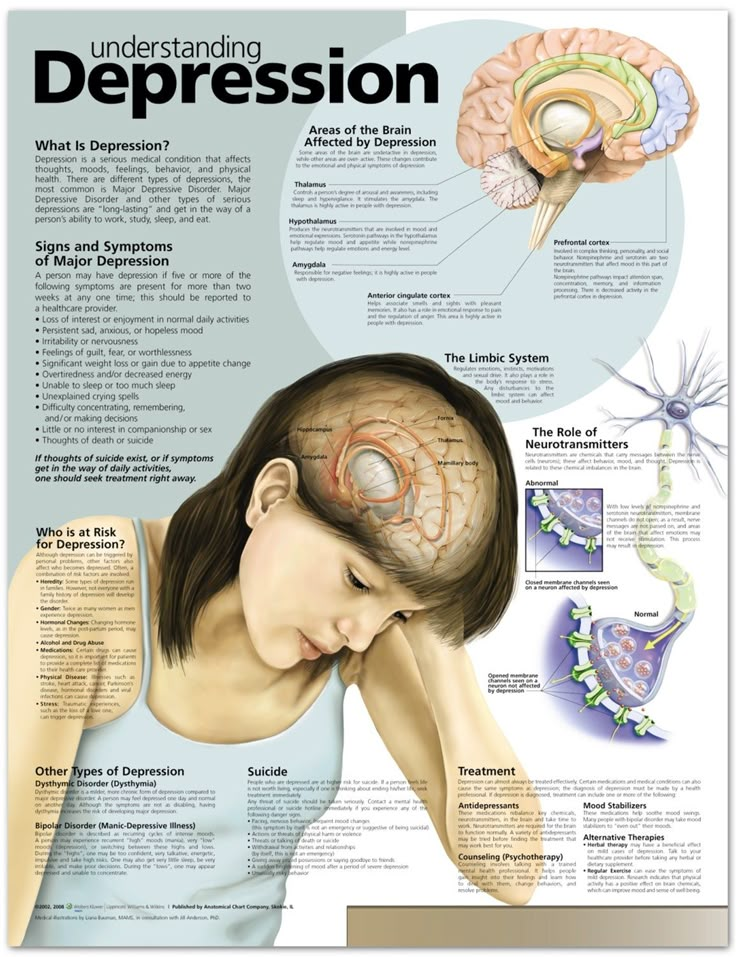 The entorhinal cortex, which serves as a relay center between the hippocampus and association areas, undergoes early changes more in Alzheimer's disease (AD) than in normal aging. nine0003
The entorhinal cortex, which serves as a relay center between the hippocampus and association areas, undergoes early changes more in Alzheimer's disease (AD) than in normal aging. nine0003
What are the possible causes of gray matter volume loss in normal aging? One of the possible explanations is the death of neurons proper, which is of fundamental importance, especially in the context of the vulnerability of the processes of their proliferation and differentiation and the likelihood of accumulation of mutations in old age [2]. Another proposed mechanism for gray matter volume loss is the accumulation of beta-amyloid protein in the brain. This mechanism causes neuronal death and correlates with cognitive decline in AD, but also determines the transition of mild cognitive impairment to dementia of the Alzheimer's type. Recent studies of functional neuroimaging using radionuclide identification of beta-amyloid plaques have made it possible to study this process in cognitively intact elderly people. Amyloid-beta is found in the cerebral cortex in approximately 20–30% of normal adults [8], which may be characteristic of those individuals at the highest risk of developing AD, given the correlations between high levels of beta-amyloid, a decrease in hippocampal volume and a decrease in the volume of episodic memory in cognitively intact individuals [9].
Amyloid-beta is found in the cerebral cortex in approximately 20–30% of normal adults [8], which may be characteristic of those individuals at the highest risk of developing AD, given the correlations between high levels of beta-amyloid, a decrease in hippocampal volume and a decrease in the volume of episodic memory in cognitively intact individuals [9].
Meanwhile, a decrease in the volume of gray matter in the elderly may be due not so much to the death of the neurons themselves, but to a decrease in their size and the number of connections between them [10]. The decrease in synaptic density observed during aging is detected in elderly people without cognitive decline and is accompanied by certain morphological changes in the cytoskeleton, including a decrease in the complexity of dendritic branching, a decrease in their length and density of the spiny apparatus, with a corresponding decrease in the efficiency of neurotransmission [11]. nine0003
Even more noticeable in the course of aging are the processes of a decrease in the volume of white matter [12]. According to autopsy studies of cognitively intact elderly subjects, in individuals over 70 years of age, the volume of white matter decreases by 16-20% compared with younger subjects. These processes mostly affect areas of the precentral gyrus, parahippocampal area, corpus callosum, i.e. those areas in which the degree of decrease in gray matter volume does not exceed 6% [13], which may explain the origin of age-dependent memory changes by a violation of the integrity of communications with structures hippocampus. Age-related disorders of white matter integrity are most pronounced in the anterior regions of the brain and correlate with deficits in executive functions [14]. nine0003
According to autopsy studies of cognitively intact elderly subjects, in individuals over 70 years of age, the volume of white matter decreases by 16-20% compared with younger subjects. These processes mostly affect areas of the precentral gyrus, parahippocampal area, corpus callosum, i.e. those areas in which the degree of decrease in gray matter volume does not exceed 6% [13], which may explain the origin of age-dependent memory changes by a violation of the integrity of communications with structures hippocampus. Age-related disorders of white matter integrity are most pronounced in the anterior regions of the brain and correlate with deficits in executive functions [14]. nine0003
In people over 65 years of age, AD is the most common etiology of moderate cognitive impairment (MCD) and mild dementia. Amnestic type of cognitive impairment is typical for AD at the stage of MCI or mild dementia. Another common variant of the progressive development of cognitive disorders is cerebrovascular disease (CVD). By the end of the 1990s, it became clear that various forms of CVD and traditional risk factors for stroke are associated with the development of dementia of late age. It has been found that up to 50% of AD cases may be associated with modifiable risk factors for stroke and cardiovascular disease [15]. Factors that may play a role in the pathogenesis of cognitive impairment and dementia of late age include arterial hypertension, diabetes mellitus, hyperglycemia, insulin resistance, hyperlipidemia, smoking, obesity, physical inactivity, dietary habits, coronary artery disease, atrial fibrillation, heart failure and some other factors. nine0003
By the end of the 1990s, it became clear that various forms of CVD and traditional risk factors for stroke are associated with the development of dementia of late age. It has been found that up to 50% of AD cases may be associated with modifiable risk factors for stroke and cardiovascular disease [15]. Factors that may play a role in the pathogenesis of cognitive impairment and dementia of late age include arterial hypertension, diabetes mellitus, hyperglycemia, insulin resistance, hyperlipidemia, smoking, obesity, physical inactivity, dietary habits, coronary artery disease, atrial fibrillation, heart failure and some other factors. nine0003
In recent years, more and more importance has been attached to the role of mixed (vascular-Alzheimer's) pathology in the origin of cognitive decline in old age [16]). Neuroimaging often detects silent infarctions or extensive white matter changes in older individuals with vascular risk factors, as was demonstrated in the large Rotterdam Study, in which the incidence of asymptomatic infarctions steadily increased with age [17].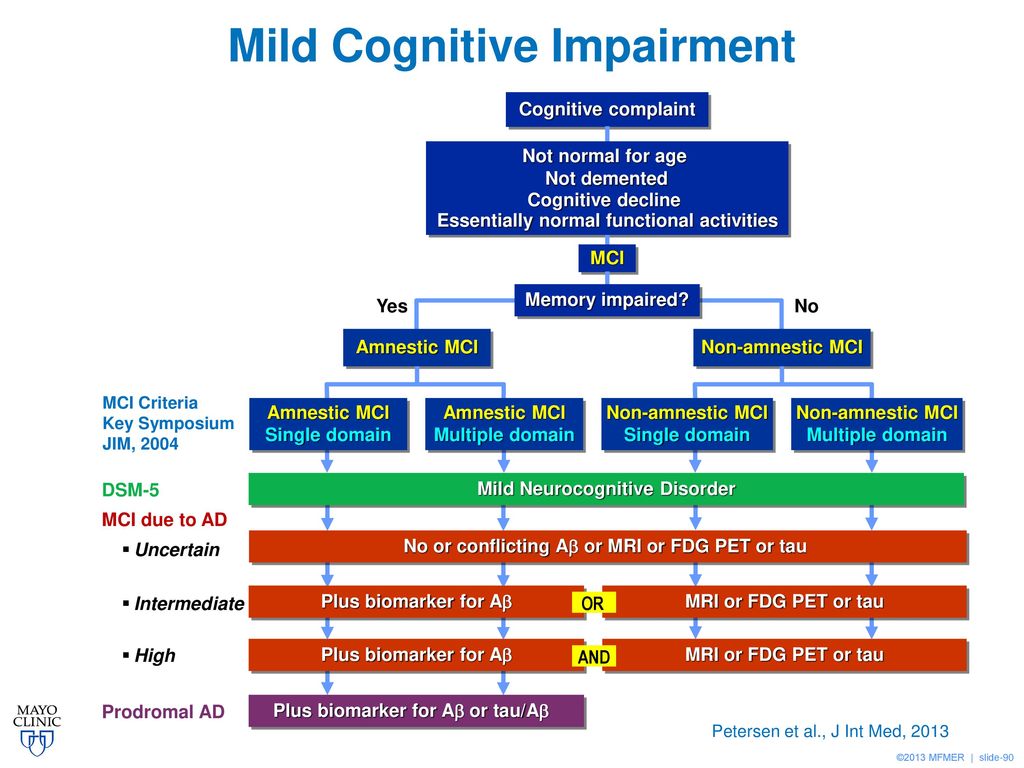 Although these patients did not have clinical signs of a previous stroke, they were more likely to have focal neurological symptoms. In general, asymptomatic heart attacks are about 5 times more common than symptomatic ones. Up to 64% of people aged 65 years and older with a history of stroke have some degree of cognitive impairment. A large systematic review of studies on the prevalence, incidence, and risk factors associated with pre-stroke and post-stroke dementia [18] found that 10% of patients had dementia before their first stroke, and 10% had dementia-level cognitive impairment for the first time shortly after their first stroke. , and more than 1/3 of patients developed dementia after a recurrent stroke. These data suggest the importance of both neurodegenerative and vascular factors in the development of cognitive impairment in the elderly. The role of vascular disorders in the development of neurodegenerative dementias is evidenced by the fact that the frequency of their detection in frontotemporal dementia is about 60%, and in AD it is more than 80% [19].
Although these patients did not have clinical signs of a previous stroke, they were more likely to have focal neurological symptoms. In general, asymptomatic heart attacks are about 5 times more common than symptomatic ones. Up to 64% of people aged 65 years and older with a history of stroke have some degree of cognitive impairment. A large systematic review of studies on the prevalence, incidence, and risk factors associated with pre-stroke and post-stroke dementia [18] found that 10% of patients had dementia before their first stroke, and 10% had dementia-level cognitive impairment for the first time shortly after their first stroke. , and more than 1/3 of patients developed dementia after a recurrent stroke. These data suggest the importance of both neurodegenerative and vascular factors in the development of cognitive impairment in the elderly. The role of vascular disorders in the development of neurodegenerative dementias is evidenced by the fact that the frequency of their detection in frontotemporal dementia is about 60%, and in AD it is more than 80% [19]. ]. The presence of a vascular component in these diseases doubles the chances of developing dementia, and the older the patients, the more often brain vascular pathology is detected [19].
]. The presence of a vascular component in these diseases doubles the chances of developing dementia, and the older the patients, the more often brain vascular pathology is detected [19].
Pre-dementia cognitive impairment and dementia risk
Alzheimer's-type pathology is the most common cause of cognitive decline in the elderly. Based on neuroimaging data, neuropathological and biochemical studies, it was found that the processes of neurodegeneration in AD begin years or even decades before the manifestation of cognitive impairment [20]. The detection of these early changes is critical for potential therapeutic interventions and the possible prevention of subsequent cognitive decline. nine0003
Attempts to identify patterns of cognitive decline in old age led to the formulation of the concept of RBM. The term MCI was first used to describe cognitive impairments that affect complex occupational and social functioning, but whose degree does not yet meet the criteria for dementia [21].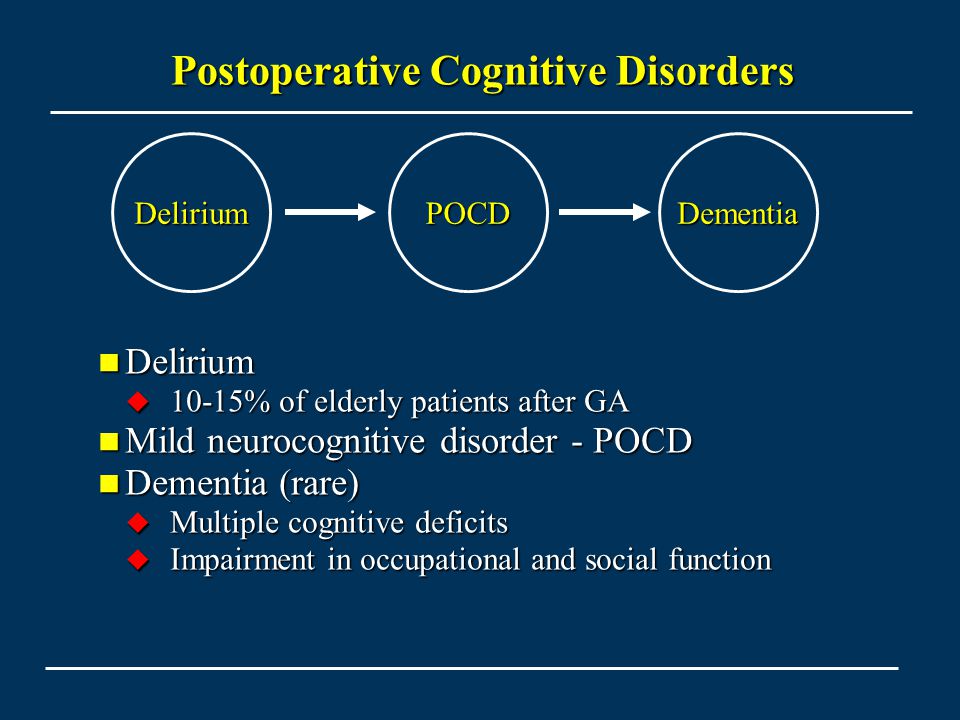 In 1999, R. Petersen et al. [21] defined MCI as “a syndrome of cognitive decline that goes beyond the expected norm for an individual for a given age and level of education, but which does not significantly affect daily activities.” Initially, the criteria for RBM included only memory functions, which are often the earliest symptom of AD. Subsequently, it became clear that decline in other cognitive domains can develop during or even before memory impairment, therefore, in order to emphasize the heterogeneity of the clinical picture and the multiplicity of etiological factors of MCI, the criteria were expanded into three subtypes: amnestic and non-amnestic, mono- and polysymptomatic [22]. According to one of the population studies [23], the amnestic type of MCI occurs in 5–10% of the elderly population. When using extended criteria, MCD prevalence rates are 8–25% among people over the age of 60 years [24]. nine0003
In 1999, R. Petersen et al. [21] defined MCI as “a syndrome of cognitive decline that goes beyond the expected norm for an individual for a given age and level of education, but which does not significantly affect daily activities.” Initially, the criteria for RBM included only memory functions, which are often the earliest symptom of AD. Subsequently, it became clear that decline in other cognitive domains can develop during or even before memory impairment, therefore, in order to emphasize the heterogeneity of the clinical picture and the multiplicity of etiological factors of MCI, the criteria were expanded into three subtypes: amnestic and non-amnestic, mono- and polysymptomatic [22]. According to one of the population studies [23], the amnestic type of MCI occurs in 5–10% of the elderly population. When using extended criteria, MCD prevalence rates are 8–25% among people over the age of 60 years [24]. nine0003
In a systematic review published in 2013 [25], the proportion of individuals who experience conversion of MCR to BA ranges from 7.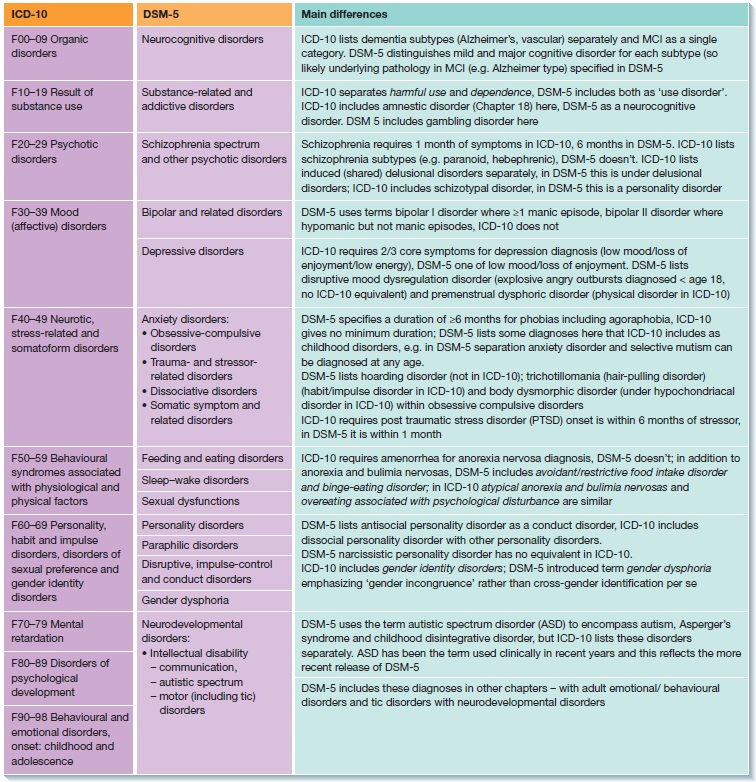 5 to 16.5% per year in clinical samples and from 5.4 to 11.5% - in the population. At the same time, the amnestic subtype of MCI is associated with a higher risk of asthma progression, which has been demonstrated in several longitudinal studies [20].
5 to 16.5% per year in clinical samples and from 5.4 to 11.5% - in the population. At the same time, the amnestic subtype of MCI is associated with a higher risk of asthma progression, which has been demonstrated in several longitudinal studies [20].
Long before the onset of apparent cognitive impairment, many patients experience subjective decline in memory and other cognitive functions. The presence of subjective complaints even at the stage of normal cognitive activity is associated with an increased risk of detecting positive biomarkers of AD and a more likely transformation into dementia [26]. Various terms have been used to describe these disorders. Since 2012, the term “subjective cognitive impairment” (SCI) has been more frequently used [27], which is used in cases where there are subjective complaints of a decrease in cognitive functions relative to the previously achieved level, which is normal for a given subject, while according to standard cognitive tests no deviations from the norm corresponding to a certain age, sex and level of education are detected [27]. SCI may represent a preclinical stage of AD. On the other hand, their appearance may be associated with a number of somatic and mental factors that may affect the cognitive functioning of older people. Thus, most often in clinical practice, a relationship is revealed between cognitive complaints, on the one hand, and depression, anxiety, personality traits, and sleep disorders, on the other [20]. Therefore, SCI is rather a non-specific syndrome that can have many causes. Meanwhile, on the basis of clinical and experimental studies, it is possible to distinguish the subtype of SCI plus, which is associated with a high risk of developing AD and includes a group of certain signs: subjective memory loss, the appearance of SCI within the last 5 years, the age of onset of SCI is 60 years, anxiety due to cognitive complaints, sensations of reduced performance relative to other individuals of the same age group, objective evidence of cognitive decline, the presence of other markers of AD (APOE ε4 genotype and neuroimaging data) [27].
SCI may represent a preclinical stage of AD. On the other hand, their appearance may be associated with a number of somatic and mental factors that may affect the cognitive functioning of older people. Thus, most often in clinical practice, a relationship is revealed between cognitive complaints, on the one hand, and depression, anxiety, personality traits, and sleep disorders, on the other [20]. Therefore, SCI is rather a non-specific syndrome that can have many causes. Meanwhile, on the basis of clinical and experimental studies, it is possible to distinguish the subtype of SCI plus, which is associated with a high risk of developing AD and includes a group of certain signs: subjective memory loss, the appearance of SCI within the last 5 years, the age of onset of SCI is 60 years, anxiety due to cognitive complaints, sensations of reduced performance relative to other individuals of the same age group, objective evidence of cognitive decline, the presence of other markers of AD (APOE ε4 genotype and neuroimaging data) [27].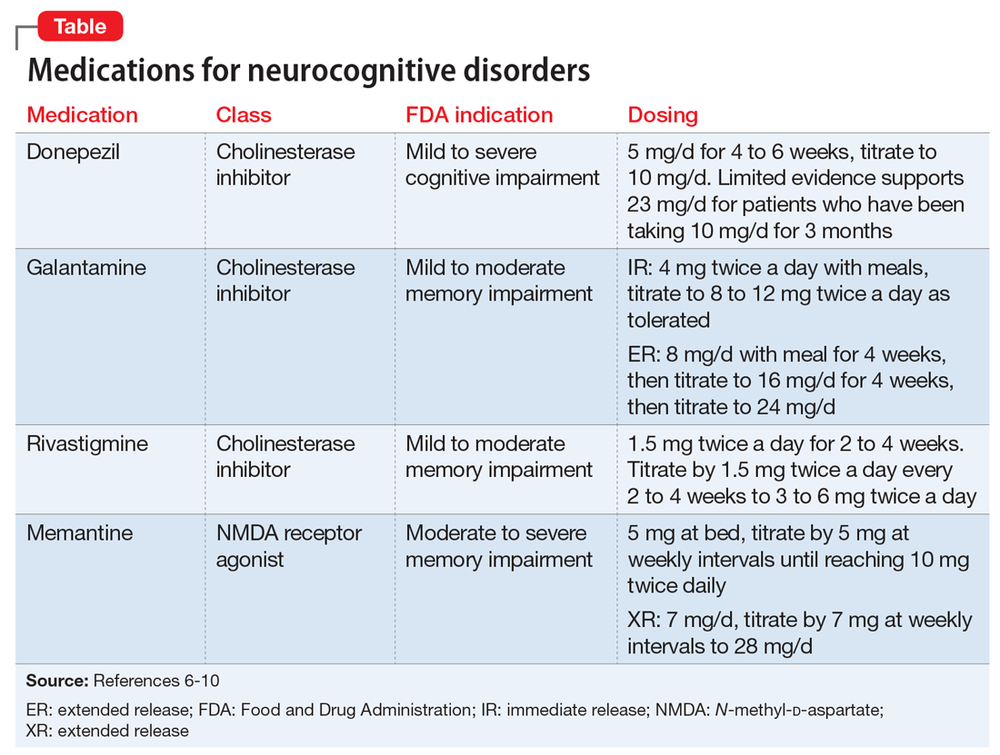 The presence of signs of SCI plus predicts a significantly higher risk of developing MCI over the next 13 months, which is 18.9% of cases compared with 5.6% of new cases of MCC, which were preceded only by individual symptoms of ICR [28]. In a study of individuals aged 75 years and older, F. Jessen et al. [26] showed a twofold increase in the risk of developing AD among patients with anxiety compared with patients without it. In addition, the presence of objective evidence of cognitive decline from people who know the subject closely predicts the likelihood of conversion to MCI and AD more effectively than the presence of only subjective complaints. The development of SCI is far ahead of the appearance of signs of SCI. As shown in one study, complaints of cognitive decline appear about 30 months earlier than these disorders are objectively confirmed by people who know these subjects closely [29].
The presence of signs of SCI plus predicts a significantly higher risk of developing MCI over the next 13 months, which is 18.9% of cases compared with 5.6% of new cases of MCC, which were preceded only by individual symptoms of ICR [28]. In a study of individuals aged 75 years and older, F. Jessen et al. [26] showed a twofold increase in the risk of developing AD among patients with anxiety compared with patients without it. In addition, the presence of objective evidence of cognitive decline from people who know the subject closely predicts the likelihood of conversion to MCI and AD more effectively than the presence of only subjective complaints. The development of SCI is far ahead of the appearance of signs of SCI. As shown in one study, complaints of cognitive decline appear about 30 months earlier than these disorders are objectively confirmed by people who know these subjects closely [29].
One of the most pressing issues in the problem of predicting the course of cognitive impairment in the elderly is the analysis of risk factors for the transformation of MCI into AD. Over the past 10 years, several biomarkers associated with the risk of developing dementia have been identified. These risk factors include specific beta-amyloid-associated biomarkers of neuronal injury and comorbid vascular disease. The carriage of the apolipoprotein E (APOE) ε4 allele, which is the most important genetic risk factor for developing AD, in individuals with MCI is associated with a faster transition to AD [30]. In individuals with verified MCI, a change in biomarkers in the cerebrospinal fluid — a decrease in the level of β-amyloid (Aβ42), an increase in the level of phosphorylated tau protein (P-tau) and the total content of tau protein (t-tau) — was associated with conversion to clinically significant B .AND. Specific neuroimaging methods also make it possible to predict the subsequent development of AD in patients with MCI [20]. Among the biomarkers of neuronal damage, the most significant are atrophic changes in the medial temporal structures and a decrease in brain metabolism in the temporo-parietal cortical regions [31].
Over the past 10 years, several biomarkers associated with the risk of developing dementia have been identified. These risk factors include specific beta-amyloid-associated biomarkers of neuronal injury and comorbid vascular disease. The carriage of the apolipoprotein E (APOE) ε4 allele, which is the most important genetic risk factor for developing AD, in individuals with MCI is associated with a faster transition to AD [30]. In individuals with verified MCI, a change in biomarkers in the cerebrospinal fluid — a decrease in the level of β-amyloid (Aβ42), an increase in the level of phosphorylated tau protein (P-tau) and the total content of tau protein (t-tau) — was associated with conversion to clinically significant B .AND. Specific neuroimaging methods also make it possible to predict the subsequent development of AD in patients with MCI [20]. Among the biomarkers of neuronal damage, the most significant are atrophic changes in the medial temporal structures and a decrease in brain metabolism in the temporo-parietal cortical regions [31]. nine0003
nine0003
In recent years, there has been a significant increase in interest in vascular risk factors and their role in the transformation of RBM. Separate studies have convincingly demonstrated a higher conversion of RBM to AD and other types of dementia in patients with diabetes mellitus and other traditional vascular risk factors. In addition, patients with good control of glucose levels, blood pressure, and dyslipidemia are significantly less likely to develop MCD into dementia compared to patients with poor control of these risk factors [32]. nine0003
Notably, traditional vascular risk factors may have different effects on cognitive decline at different ages. Thus, it has been shown that the presence of arterial hypertension in middle age increases the risk of developing cognitive impairment in later life, but the effect of hypertension in late age on the risk of cognitive decline was not so clear [33]. In addition, data from special clinical studies of the effect of antihypertensive therapy do not always show its positive effect on cognitive functions [33].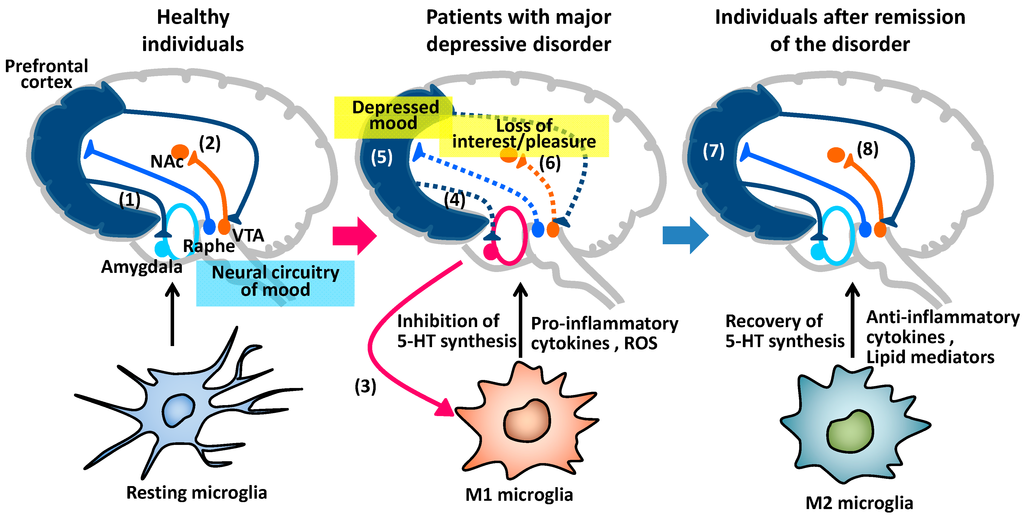 nine0003
nine0003
Therapeutic strategies for the correction of cognitive impairment in the elderly
The traditional approach to the management of patients with progressive cognitive impairment in the elderly involves the control and possible modification of the influence of risk factors for the development of dementia. The modern concept of “anti-aging strategy” for maintaining cognitive health [34] suggests the feasibility of using several key recommendations: 1) maintaining physical and social activity; 2) maintaining intellectual functioning; 3) treatment and prevention of cardiovascular and cerebrovascular diseases; 4) control of traditional risk factors; 5) compliance with sleep and wakefulness and correction of sleep disorders; 6) control of psychotic disorders associated with dementias. nine0003
Management of patients with MCI should primarily include advice on lifestyle and management of traditional risk factors for dementia. Despite the difficulties of verifying the positive impact of non-pharmacological strategies in this category of patients, their justification is beyond doubt. Thus, a study of individuals with TCS, which included mainly complaints of memory impairment, showed clear, albeit insignificant, benefits of physical exercise in slowing down the rate of cognitive decline [35]. Despite the paucity of large prospective studies on the effect of effective medical treatment on the risk of developing a cognitive defect, the validity of this approach is not objectionable. nine0003
Despite the difficulties of verifying the positive impact of non-pharmacological strategies in this category of patients, their justification is beyond doubt. Thus, a study of individuals with TCS, which included mainly complaints of memory impairment, showed clear, albeit insignificant, benefits of physical exercise in slowing down the rate of cognitive decline [35]. Despite the paucity of large prospective studies on the effect of effective medical treatment on the risk of developing a cognitive defect, the validity of this approach is not objectionable. nine0003
The rationale for the pharmacological treatment of MCI associated with AD is unfortunately not supported by data from randomized controlled trials. Several studies have been conducted with the use of cholinesterase inhibitors (AChE) in individuals with amnestic type of MCI, which, however, did not demonstrate their obvious effect [36]. Although one study showed a trend towards delayed progression of cognitive impairment with donepezil over 24 months, this effect did not persist at follow-up.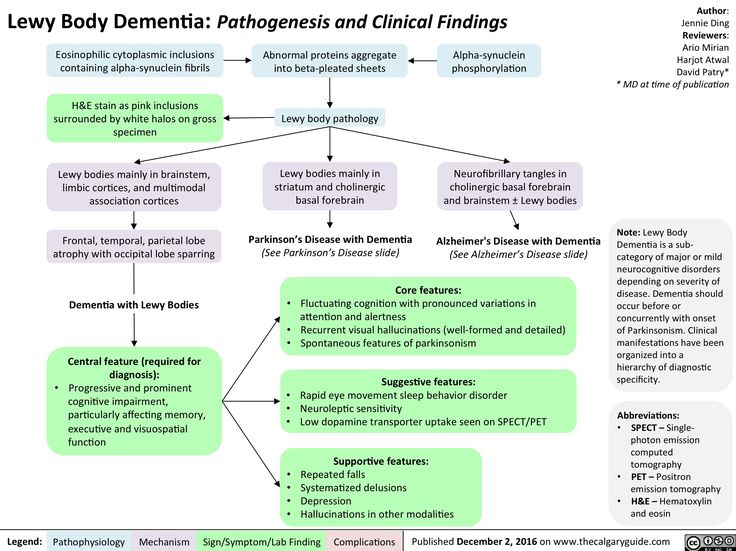 In general, to date, no drugs have been found in the treatment of patients with MCI that convincingly show the effect of slowing down the decline in cognitive functioning [37]. However, in the treatment of mild to moderate dementia, such therapy has tangible, albeit modest, advantages, so three representatives of ACEI — donepezil, rivastigmine, and galantamine — are approved for the treatment of mild dementia in AD [37]. Considering that cholinergic deficiency is a universal pathogenetic link in the development of both AD and vascular cognitive disorders (CCD), the clinical use of iAChE is rational. There is also some evidence that these drugs increase cerebral blood flow, so they are being considered as a potential treatment option for patients with IBS. However, in clinical trials, the effects of iAChE in SCR are controversial. Moreover, patients with vascular dementia do not show any benefit in terms of global functioning when treated with iAChE. Thus, no representative of iAChE has received FDA approval for the treatment of patients with vascular dementia [38].
In general, to date, no drugs have been found in the treatment of patients with MCI that convincingly show the effect of slowing down the decline in cognitive functioning [37]. However, in the treatment of mild to moderate dementia, such therapy has tangible, albeit modest, advantages, so three representatives of ACEI — donepezil, rivastigmine, and galantamine — are approved for the treatment of mild dementia in AD [37]. Considering that cholinergic deficiency is a universal pathogenetic link in the development of both AD and vascular cognitive disorders (CCD), the clinical use of iAChE is rational. There is also some evidence that these drugs increase cerebral blood flow, so they are being considered as a potential treatment option for patients with IBS. However, in clinical trials, the effects of iAChE in SCR are controversial. Moreover, patients with vascular dementia do not show any benefit in terms of global functioning when treated with iAChE. Thus, no representative of iAChE has received FDA approval for the treatment of patients with vascular dementia [38]. nine0003
nine0003
Another important direction in the pharmacotherapy of dementia in AD is the use of a drug that modulates glutamatergic neurotransmission. Glutamate is an excitatory neurotransmitter in the brain that is present in significant amounts in cortical and hippocampal neurons. The loss of cortical neurons in patients with dementia may be related to the toxic effects of glutamate. Glutamate activates N-methyl-D-aspartate (NMDA) receptors. In addition, excessive NMDA stimulation caused by ischemia leads to the activation of excitotoxicity processes. Memantine is a non-competitive NMDA receptor antagonist with moderate affinity that has been shown to prevent neurodegeneration and reduce learning disabilities in animal models of dementia [39].]. In addition, memantine reduces the degree of neuronal damage in models of global and focal cerebral ischemia. Clinical studies also demonstrate that memantine improves cognition and functionality in various stages of dementia [39].
J. Orgogozo et al. [40] conducted a phase III randomized, placebo-controlled trial to investigate the efficacy and tolerability of memantine in patients with mild to moderate manifestations of vascular dementia (VOD). This study was conducted in 50 centers in France, Belgium and Switzerland. After an initial 3-week titration period (5 mg/day at week 1, 10 mg/day at week 2, and 15 mg/day at week 3), patients received daily doses of memantine 20 mg/day or placebo at during the remaining 28 weeks of follow-up. Two main endpoints were used as performance parameters: ADAS-cog and CIBIC-plus. In total, 403 patients were included in the study. After 28 weeks, there was a significant improvement in the mean ADAS-cog scores in the memantine group compared to placebo (the mean score increase in the main group was 0.4, while in the placebo group the mean score decreased by 1.6, thus the difference in the groups was 2 ,0 points). The proportion of patients with improvement in CIBIC-plus scores was 60% in the memantine group compared to 52% in the placebo group.
Orgogozo et al. [40] conducted a phase III randomized, placebo-controlled trial to investigate the efficacy and tolerability of memantine in patients with mild to moderate manifestations of vascular dementia (VOD). This study was conducted in 50 centers in France, Belgium and Switzerland. After an initial 3-week titration period (5 mg/day at week 1, 10 mg/day at week 2, and 15 mg/day at week 3), patients received daily doses of memantine 20 mg/day or placebo at during the remaining 28 weeks of follow-up. Two main endpoints were used as performance parameters: ADAS-cog and CIBIC-plus. In total, 403 patients were included in the study. After 28 weeks, there was a significant improvement in the mean ADAS-cog scores in the memantine group compared to placebo (the mean score increase in the main group was 0.4, while in the placebo group the mean score decreased by 1.6, thus the difference in the groups was 2 ,0 points). The proportion of patients with improvement in CIBIC-plus scores was 60% in the memantine group compared to 52% in the placebo group. The results of this study showed that in patients with mild to moderate ODS, treatment with memantine 20 mg/day was characterized by a consistent improvement in cognitive function according to the results of the dynamics of various cognitive scales, although a significant improvement in global functioning was not observed. In this study, no significant side effects of memantine were reported and no differences were obtained in terms of tolerability from the placebo group [40]. Similar data were obtained by G. Wilcock et al. [41] in another 28-week, double-blind, randomized controlled trial of the efficacy and safety of memantine in mild to moderate ODS. This study was conducted at 54 centers in the UK and included 579patients who received memantine 20 mg or placebo for 6 months. The authors also demonstrated a significant improvement compared to placebo in the main group on the ADAS-cog scale, although there were no differences with placebo on the global clinical impression scale, as in the previous case [41].
The results of this study showed that in patients with mild to moderate ODS, treatment with memantine 20 mg/day was characterized by a consistent improvement in cognitive function according to the results of the dynamics of various cognitive scales, although a significant improvement in global functioning was not observed. In this study, no significant side effects of memantine were reported and no differences were obtained in terms of tolerability from the placebo group [40]. Similar data were obtained by G. Wilcock et al. [41] in another 28-week, double-blind, randomized controlled trial of the efficacy and safety of memantine in mild to moderate ODS. This study was conducted at 54 centers in the UK and included 579patients who received memantine 20 mg or placebo for 6 months. The authors also demonstrated a significant improvement compared to placebo in the main group on the ADAS-cog scale, although there were no differences with placebo on the global clinical impression scale, as in the previous case [41]. Thus, memantine generally demonstrates clinical efficacy in improving cognitive function in patients with mild to moderate ODS.
Thus, memantine generally demonstrates clinical efficacy in improving cognitive function in patients with mild to moderate ODS.
The study of the leading processes of neurocognitive aging is undoubtedly associated with the analysis of the role of neurotrophic factors, which are a key link in maintaining the functional activity of the brain. Natural neurotrophins are synthesized by neurons and glial cells and contribute to the proliferation, differentiation and maintenance of neuronal viability. Cortexin (GEROPHARM) has a similar effect of natural neurotrophins, which contains a balanced combination of polypeptides, amino- and ribonucleic acids (90% oligo- and short peptides and about 10% various amino acids) and trace elements [42].
The clinical efficacy of Cortexin was shown in a study involving 189 patients (mean age 64.3 ± 0.5 years) with chronic cerebral ischemia (CCI) stage I-II, who did not have clinically significant cognitive impairment (at least 30 points on a short mental status scale (MMSE) [43].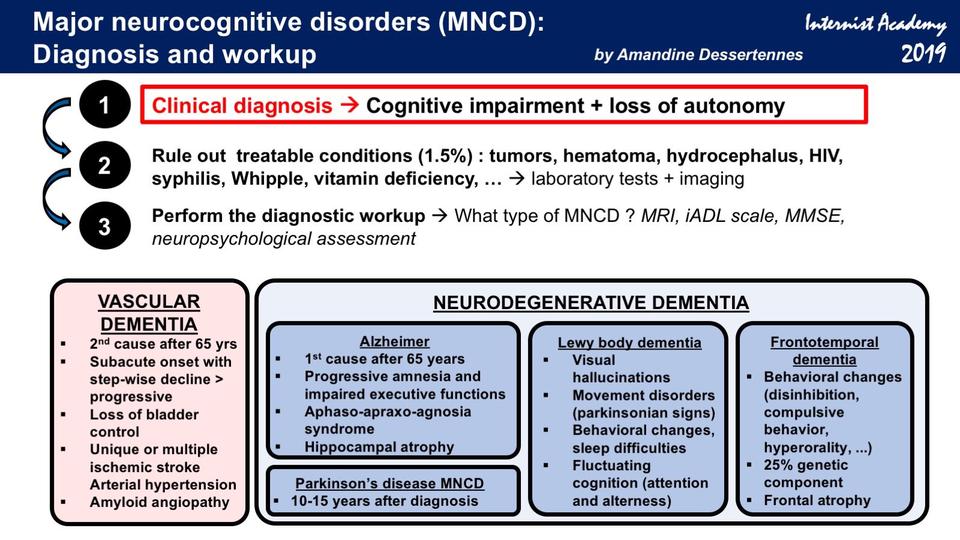 The patients were randomized into three groups: the 1st group included 69 patients who were prescribed intramuscular injection of 20 mg of Cortexin 1 time per day, the 2nd group consisted of 68 patients who received the drug at a dose of 10 mg 1 time per day, in Group 3 (comparisons) included 52 patients who received only basic therapy without the use of neuroprotective, antioxidant and cerebrovascular drugs. The therapy regimen included three courses lasting 10 days with an interval of 6 months. nine0003
The patients were randomized into three groups: the 1st group included 69 patients who were prescribed intramuscular injection of 20 mg of Cortexin 1 time per day, the 2nd group consisted of 68 patients who received the drug at a dose of 10 mg 1 time per day, in Group 3 (comparisons) included 52 patients who received only basic therapy without the use of neuroprotective, antioxidant and cerebrovascular drugs. The therapy regimen included three courses lasting 10 days with an interval of 6 months. nine0003
Analysis of therapeutic effects in the three analyzed groups showed significant differences between groups in a number of parameters. Thus, the average score of the “drawing clock” test in the 1st group increased by 40.4±12.5%, in the 2nd group by 27.1±5.5% ( p = 0.308), and in group 3 — by 11.5±2.4% ( p = 0.308, p = 0.031 and p = 0.292, respectively). Also more pronounced in the 1st group was the dynamics of the test "remembering 5 words": in the 1st group it was 67.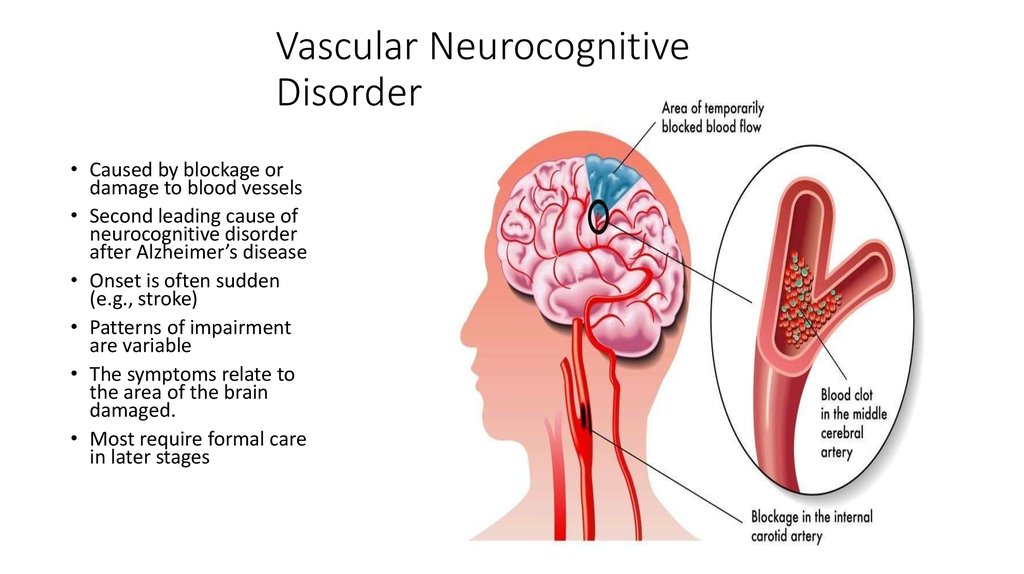 0±12.8%, in the 2nd group — 47.7±8.0%, in group 3 — 20.1±10.2% ( p = 0.371; p = 0.001; p = 0.001, respectively). Differences in efficacy were similar between groups and other analyzed parameters. Thus, the results of the study showed the presence of a dose-dependent effect of Cortexin in the course treatment of CCI with the use of 10 and 20 mg of the drug. It has been established that cortexin at a dosage of 20 mg has a more pronounced effect on neurological disorders in general, asthenic syndrome and dyssomnia. A pronounced effect of Cortexin 10 and 20 mg on cognitive disorders was noted, which did not have a dose-dependent effect. The antidepressant and anxiolytic effect of Cortexin is manifested only after the appointment of repeated courses of therapy. In addition, the antioxidant effect of the drug was confirmed. At the same time, the authors noted that the study drug was well tolerated: only 3 patients withdrew from the study during the 6-month treatment period [43].
0±12.8%, in the 2nd group — 47.7±8.0%, in group 3 — 20.1±10.2% ( p = 0.371; p = 0.001; p = 0.001, respectively). Differences in efficacy were similar between groups and other analyzed parameters. Thus, the results of the study showed the presence of a dose-dependent effect of Cortexin in the course treatment of CCI with the use of 10 and 20 mg of the drug. It has been established that cortexin at a dosage of 20 mg has a more pronounced effect on neurological disorders in general, asthenic syndrome and dyssomnia. A pronounced effect of Cortexin 10 and 20 mg on cognitive disorders was noted, which did not have a dose-dependent effect. The antidepressant and anxiolytic effect of Cortexin is manifested only after the appointment of repeated courses of therapy. In addition, the antioxidant effect of the drug was confirmed. At the same time, the authors noted that the study drug was well tolerated: only 3 patients withdrew from the study during the 6-month treatment period [43].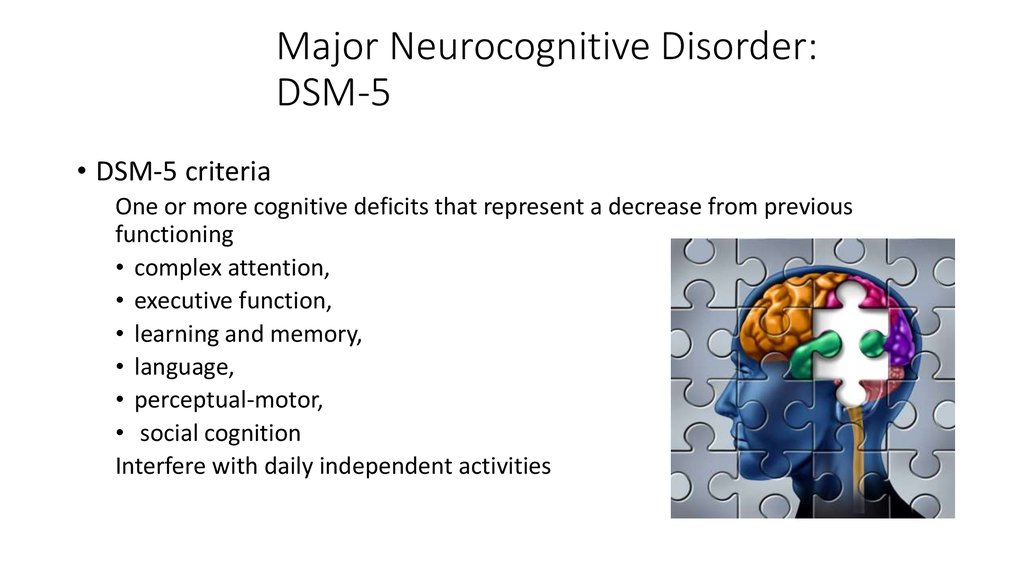 nine0003
nine0003
Based on the idea of the advantages of multimodal strategies in the correction of cognitive impairment, the experience of the combined use of cortexin and memantine (memantinol) in patients with CCI is of interest. A similar analysis was carried out as part of a multicentre observational program conducted in a routine outpatient setting [44]. The study included 495 patients with a verified diagnosis of CCI. The patients were randomized into two groups. Group 1 included 388 patients who received a 10-day course of treatment with Cortexin daily by intramuscular injection of 10 mg of the drug. Group 2 included 107 patients with more severe disorders who received combination therapy: a 10-day course of cortexin and additionally memantinol for 3 months. Memantinol prescription regimen was as follows: 1st week - 5 mg in the morning, 2nd week - 5 mg in the morning and afternoon, 3rd week - 5 mg in the morning and 10 mg in the afternoon, then - 10 mg in the morning and afternoon. Analysis of the dynamics of clinical parameters in the 1st group showed a significant ( p <0.05) increase in mean MMSE score from baseline 21.94±0.26 to 26.15±0.17 points on the 90th day of treatment. There was also a significant ( p <0.001) positive dynamics of the Mini-Kog test: by the 4th visit, this indicator increased by an average of 3 points compared to the 1st visit. The speech activity test indicators were also characterized by similar dynamics. In the 2nd group of patients, which included patients with more severe cognitive impairment, the dynamics of the indicators was more pronounced than in the 1st group (more than 2 times). Memory self-assessment scores showed positive dynamics, but no significant difference between the groups ( p >0.05). The same insignificant difference was also revealed when comparing the dynamics of the Mini-Kog test in the two groups ( p >0.05). The dynamics of the test for speech activity was more pronounced in patients of the 2nd group ( p <0.
Analysis of the dynamics of clinical parameters in the 1st group showed a significant ( p <0.05) increase in mean MMSE score from baseline 21.94±0.26 to 26.15±0.17 points on the 90th day of treatment. There was also a significant ( p <0.001) positive dynamics of the Mini-Kog test: by the 4th visit, this indicator increased by an average of 3 points compared to the 1st visit. The speech activity test indicators were also characterized by similar dynamics. In the 2nd group of patients, which included patients with more severe cognitive impairment, the dynamics of the indicators was more pronounced than in the 1st group (more than 2 times). Memory self-assessment scores showed positive dynamics, but no significant difference between the groups ( p >0.05). The same insignificant difference was also revealed when comparing the dynamics of the Mini-Kog test in the two groups ( p >0.05). The dynamics of the test for speech activity was more pronounced in patients of the 2nd group ( p <0.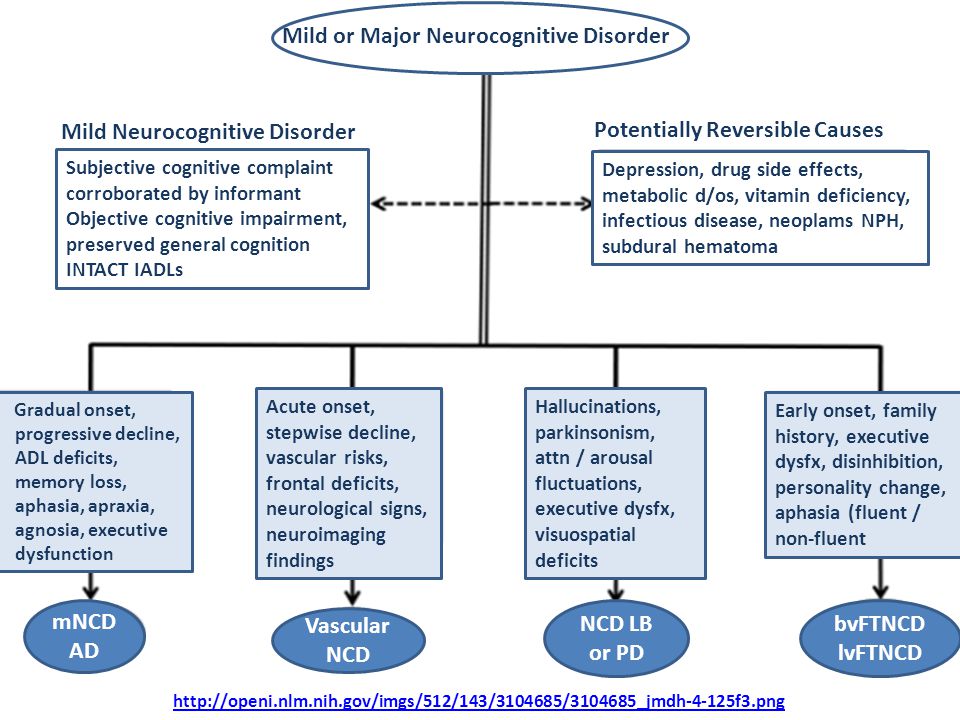 05). At the same time, the analysis of therapy tolerance in both groups did not reveal cases of withdrawal from the study due to side effects of the drugs taken [44].
05). At the same time, the analysis of therapy tolerance in both groups did not reveal cases of withdrawal from the study due to side effects of the drugs taken [44].
The data presented in this review show that one of the most important problems associated with population aging is cognitive impairment, which has the most pronounced impact on the daily functioning of older people. Dementia not only dramatically changes the lives of patients themselves, but also carries a heavy burden on families, friends, caregivers and the health care system as a whole. Cognitive impairment at various levels and dementia are multifactorial in nature. Their manifestation and rate of progression are determined by a variety of factors, many of which may be potentially modifiable. Given the multifactorial nature of the main types of cognitive impairment and dementia, the use of multiple strategies and multimodal pharmacological interventions may allow optimizing the correction of cognitive disorders and, possibly, changing the progression of the increase in cognitive impairment in the elderly.
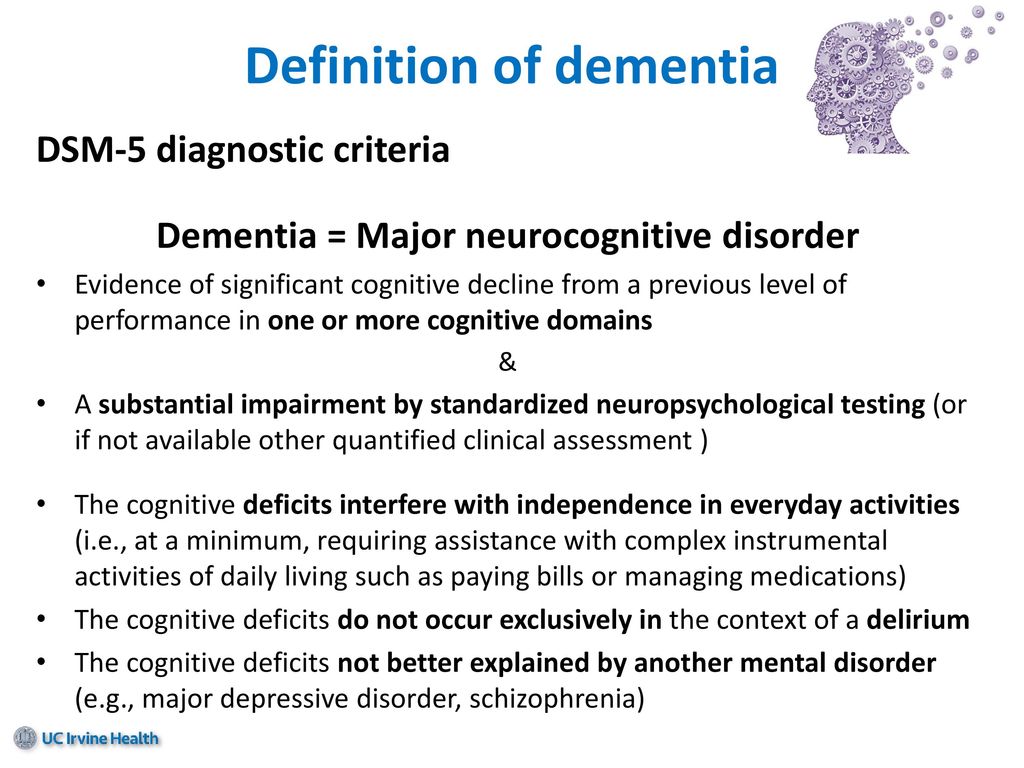 Diagnostic criteria for depression according to the International Classification of Diseases, 10th Revision
Diagnostic criteria for depression according to the International Classification of Diseases, 10th Revision