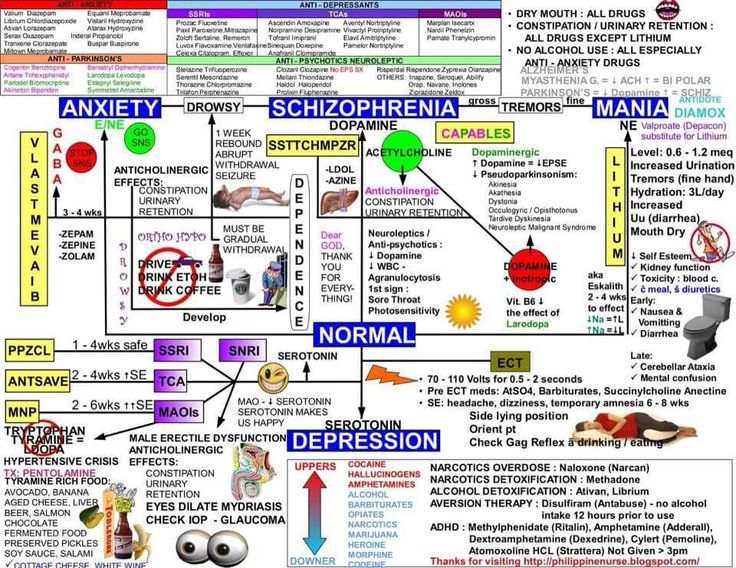
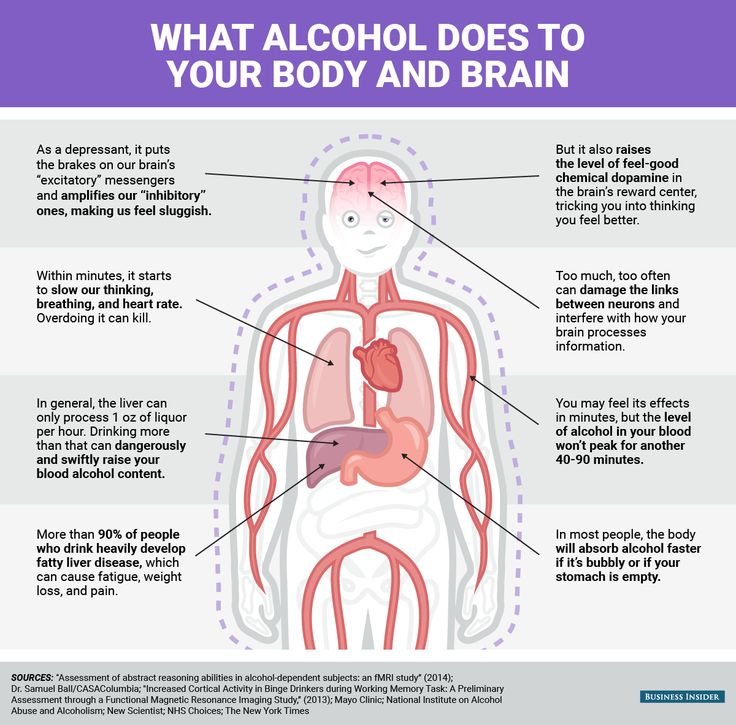
Is effexor an antipsychotic
Facts, Side Effects, Cost, Dosing
What is Effexor?
Effexor is an antidepressant medication known as a serotonin-norepinephrine reuptake inhibitor. Effexor is used to treat depression, and the extended-release version of the medication is used to treat generalized anxiety disorder, social anxiety disorder, and panic disorder.
When did the U.S. Food and Drug Administration (FDA) approve the medication?
Effexor was first approved by the FDA in 1993.
Is there a generic version of Effexor?
Yes, the generic version of Effexor is known as venlafaxine and is sold in the U.S.
Are there any major differences between Effexor and other mental health medications?
Effexor is a serotonin-norepinephrine reuptake inhibitor (SNRI). SNRIs work by increasing levels of serotonin and norepinephrine in the brain. The extended-release version of the medication is also prescribed to treat anxiety disorders. If you have bipolar disorder and take an SNRI, you may be at risk for triggering a manic episode if you are not also taking a mood stabilizer.
Talk to your doctor about your specific symptoms, other health concerns, and other medications you take so they can make the best recommendation for your condition and symptoms.
Can children take Effexor?
The safety and efficacy of Effexor has not been established for children.
Are there potential interaction issues for people taking Effexor and any other drugs?
Talk to your doctor if you take MAO inhibitors. There are hundreds of drugs which are known to interact with Effexor in major, moderate, or mild ways, so let your doctor know what other medications you are taking before you begin taking the medication. Some of these include anticoagulants, other antidepressants, anxiety medications, weight loss medications, pain medications, seizure medications, migraine medications, cimetidine, clozapine, diuretics, duloxetine, haloperidol, imipramine, indinavir, ketoconazole, linezolid, lithium, methadone, methylene blue, phentermine, ritonavir, sedatives, sibutramine, sleeping medications, tramadol, and tranquilizers.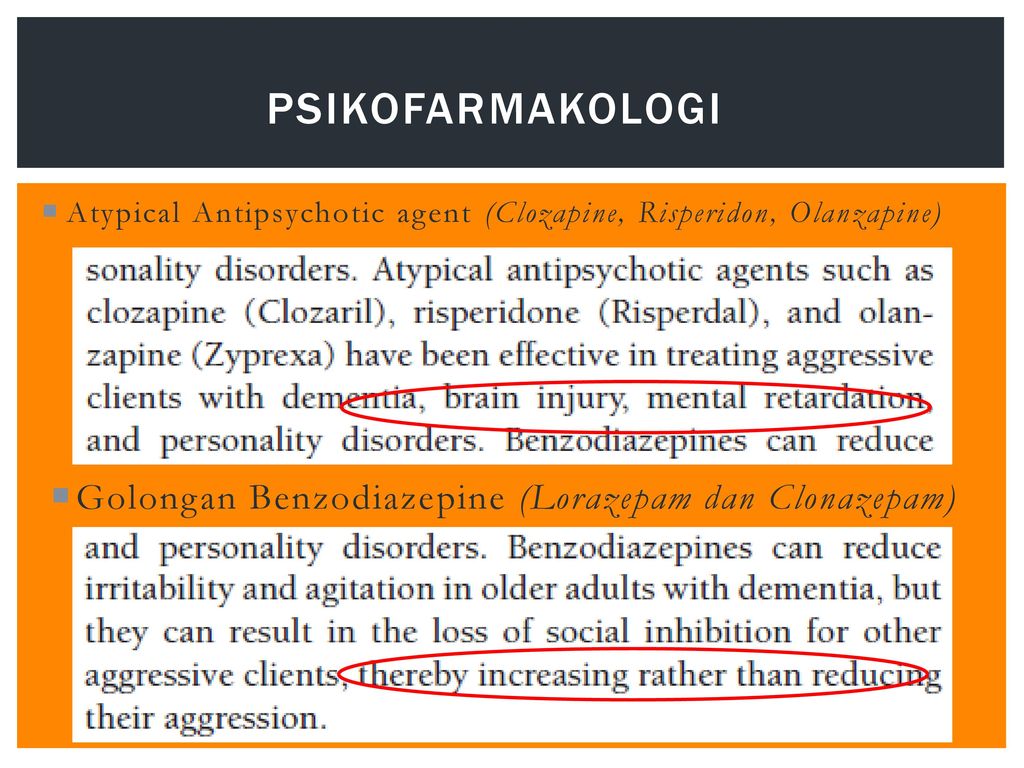
Are there any other medical conditions that would make someone ineligible for Effexor therapy?
Talk to your doctor about other medical conditions before you take Effexor, such as heart attack, heart disease, high blood pressure, high cholesterol, seizures, liver disease, heart disease, kidney disease, or thyroid disease. Also tell your doctor if you are allergic to any medications, have a history of abusing medication, or a history of suicidal thoughts or behavior.
What is the typical dose that would be prescribed to someone taking Effexor?
Dosage will vary depending on the condition being treated and whether you are taking Effexor tablets or extended-release capsules.
What do I do if I miss a dose?
Take the dose of Effexor when you remember, but skip the missed dose if it is almost time for your next dose. You should never take extra doses of the medication to make up for missed doses, and you should not take more than one dose per day if you take extended-release capsules.
How long does it take for Effexor to reach full efficacy?
It may take several weeks or longer for the medication to be fully effective and for initial side effects to decrease. If patients are going to respond, they generally notice some improvement in 1-2 weeks, but it can take up to 8 weeks to experience benefit. If someone has not experienced benefit by week 8, they will likely not experience any benefit. Also, response may be gradual, with improvement continuing over several months. Response rates go up with antidepressant lifestyle changes such as incorporating a healthy diet, regular routines, mindfulness and exercise.
What side effects can Effexor cause?
Common side effects can include:
It also is recommended that you wait to drive or operate machinery until you know how the medication affects you. It is also recommended that people avoid alcohol and illegal drugs while on the medication, as they can worsen adverse effects. Report side effects to your doctor immediately. Serious side effects can include rash, hives, seizures, chest pain, difficulty breathing or swallowing, irregular heartbeat, eye pain, fever, confusion, bleeding or bruising, coordination problems, muscle stiffness, hallucinations, and coma. You can also report side effects to the FDA at 1-800-FDA-1088 or online.
Serious side effects can include rash, hives, seizures, chest pain, difficulty breathing or swallowing, irregular heartbeat, eye pain, fever, confusion, bleeding or bruising, coordination problems, muscle stiffness, hallucinations, and coma. You can also report side effects to the FDA at 1-800-FDA-1088 or online.
What are the potential psychological side effects of taking Effexor?
A small percentage of teens and young adults who take antidepressants experience an increase in suicidal thoughts and behavior. Seek medical help if you experience these thoughts or other changes in behavior or mood.
What are the potential long-term effects of taking Effexor?
Effexor may cause angle-closure glaucoma, so talk to your doctor about the risks and about setting up an eye examination.
Is it safe for a woman who is pregnant, about to become pregnant, or nursing to take Effexor?
Birth defects and fetal harm are possible when Effexor is taken during pregnancy, but the risk is very low. The drug may be transferred via breast milk. Therefore, talk to your doctor about the risks and benefits if you are pregnant, planning to become pregnant, or are nursing before you take Effexor. You and your health care provider will want to weigh the risks of stopping an antidepressant regimen versus continuing to take medication, or tapering off just before birth.
The drug may be transferred via breast milk. Therefore, talk to your doctor about the risks and benefits if you are pregnant, planning to become pregnant, or are nursing before you take Effexor. You and your health care provider will want to weigh the risks of stopping an antidepressant regimen versus continuing to take medication, or tapering off just before birth.
Can symptoms occur if Effexor is discontinued?
It’s important not to discontinue use of the drug before talking with your doctor. Withdrawal symptoms of Effexor can include anxiety, agitation, vertigo, nausea, diarrhea, vomiting, sleep problems, nightmares, headache, fatigue, and dry mouth. If the drug needs to be discontinued, it should be done very slowly over months if at all possible.
What should I do if I overdose on Effexor?
An overdose of Effexor could be fatal, so seek immediate help or call the Poison Help Line at 1-800-222-1222 if you overdose. Overdose symptoms can include dizziness, vomiting, nausea, muscle pain, sleepiness, hot and cold spells, tingling or numbness in hands and feet, increased pupil size, seizures, changes in heartbeat, and coma.
Is Effexor habit-forming?
Effexor is not habit-forming, but it is not recommended that you discontinue use of the drug before talking with your doctor, as withdrawal symptoms can occur.
How much does Effexor cost?
According to goodrx.com, 30 capsules of 75 mg generic venlafaxine ER cost approximately $130. 30 capsules of 75 mg Effexor XR cost approximately $400. However, goodrx and some other websites may offer coupons for Effexor or its generic, dramatically lowering the cost.
Are there any disadvantages to Effexor?
The biggest disadvantages of Effexor are the potential side effects. Talk to your doctor about what medication is best for you.
DISCLAIMER: The information contained herein should NOT be used as a substitute for the advice of an appropriately qualified and licensed physician or other healthcare provider. This article mentions drugs that were FDA-approved and available at the time of publication and may not include all possible drug interactions or all FDA warnings or alerts. The author of this page explicitly does not endorse this drug or any specific treatment method. If you have health questions or concerns about interactions, please check with your physician or go to the FDA site for a comprehensive list of warnings.
The author of this page explicitly does not endorse this drug or any specific treatment method. If you have health questions or concerns about interactions, please check with your physician or go to the FDA site for a comprehensive list of warnings.
- FDA – Effexor
- NIH - Venlafaxine
Notes: This article was originally published October 21, 2021 and most recently updated October 22, 2021.
Approved and Off-Label Uses, Dosages and Warnings
Effexor (venlafaxine) was the first antidepressant in the class of drugs known as serotonin-norepinephrine reuptake inhibitors (SNRIs). It is available in an extended-release formula to treat depression, and anxiety and panic disorders. It works by regulating levels of serotonin and norepinephrine in the brain.
The U.S. Food and Drug Administration approved Effexor in 1993 to treat major depressive disorder in adults. An extended-release version called Effexor XR won FDA approval in 1997. Both formulas contain the same active ingredient, venlafaxine.
Wyeth Pharmaceuticals Inc. manufactured both the original and extended-release versions until Pfizer acquired the company in 2009. Today, Pfizer only markets the extended-release version, which is also approved to treat generalized anxiety disorder, social anxiety disorder and panic disorder.
The FDA approved the first generic version in 2010. Generic versions of the original formula are still available.
Venlafaxine is a serotonin-norepinephrine reuptake inhibitor, or SNRI. It helps block the reabsorption of both serotonin and norepinephrine, two chemicals in the brain that transmit nerve signals.
Effexor blocks the reabsorption of serotonin and norepinephrine.
Scientists believe maintaining proper levels of serotonin in the brain prevents depression and anxiety, and they also think boosting norepinephrine reduces neuropathic pain.
Findings from one 2022 clinical research trial noted that high doses of venlafaxine are associated with changes in different forms of plasticity in discrete brain areas. In particular, the hippocampus plays a crucial role in venlafaxine-mediated antidepressant effects by promoting new nerve development or regulating the excitatory/inhibitory balance.
In particular, the hippocampus plays a crucial role in venlafaxine-mediated antidepressant effects by promoting new nerve development or regulating the excitatory/inhibitory balance.
Research suggests venlafaxine may be more effective and cause fewer side effects than selective serotonin reuptake inhibitors, a slightly older class of antidepressants that focuses solely on serotonin and includes Prozac, Paxil and Zoloft.
Taking Effexor Safely
Pfizer discontinued the original version of Effexor in part because it required two or three doses per day while the extended-release version allows for a single daily dose for most patients.
Suggested dosages for venlafaxine are significantly higher compared to many SSRI antidepressants. The capsules come in 37.5 mg, 75 mg and 150 mg strengths.
Effexor XR 37.5mg
Effexor XR 75mg
Effexor XR 150mg
The medication guide instructs patients to take the drug with food at the same time each day. The medicine can make you sleepy, or make it harder for you to think clearly or react quickly. So, until you know how you will respond to the medication, it’s important to avoid driving, operating heavy machinery or doing other dangerous activities.
The medicine can make you sleepy, or make it harder for you to think clearly or react quickly. So, until you know how you will respond to the medication, it’s important to avoid driving, operating heavy machinery or doing other dangerous activities.
Interactions
Mixing venlafaxine with therapies that also affect serotonin levels, such as other antidepressants, linezolid, lithium, tramadol and St. John’s wort, can cause a condition called serotonin syndrome that raises blood pressure, heart rate and body temperature.
Let your doctor know of all prescription and over-the-counter medications and supplements you have taken or plan to take, including aspirin, migraine medications, amphetamines, antipsychotics and blood thinners.
Don’t start using Effexor XR within two weeks of taking a medication known as an MAOI, and don’t take an MAOI within a week of stopping venlafaxine.
Taking the medication with blood thinners or nonsteroidal anti-inflammatory drugs like Advil and Motrin may increase the risk of bleeding side effects. The medication guide also advises against drinking alcohol while taking the drug.
The medication guide also advises against drinking alcohol while taking the drug.
Recommended Dosages
Dosage and administration can be complicated and is detailed in a chart on the package insert. The recommended starting dose for major depressive disorder, generalized anxiety disorder and social phobia is 75 mg per day, but doctors may instruct some patients to start with 37.5 mg to allow their body to adjust to the medication. The recommended starting dose for patients with panic disorder is 35 mg per day.
Depending on need and how a patient tolerates venlafaxine, a doctor may increase the dose to 150 mg per day and then up to 225 mg per day. This is accomplished using 75 mg increments at intervals of no less than four days.
The maximum recommended dose for moderately depressed patients is 225 mg per day, but patients with severe depression may respond better to higher doses.
One study referenced in the drug’s label found severely depressed patients responded to an average dose of 350 mg per day.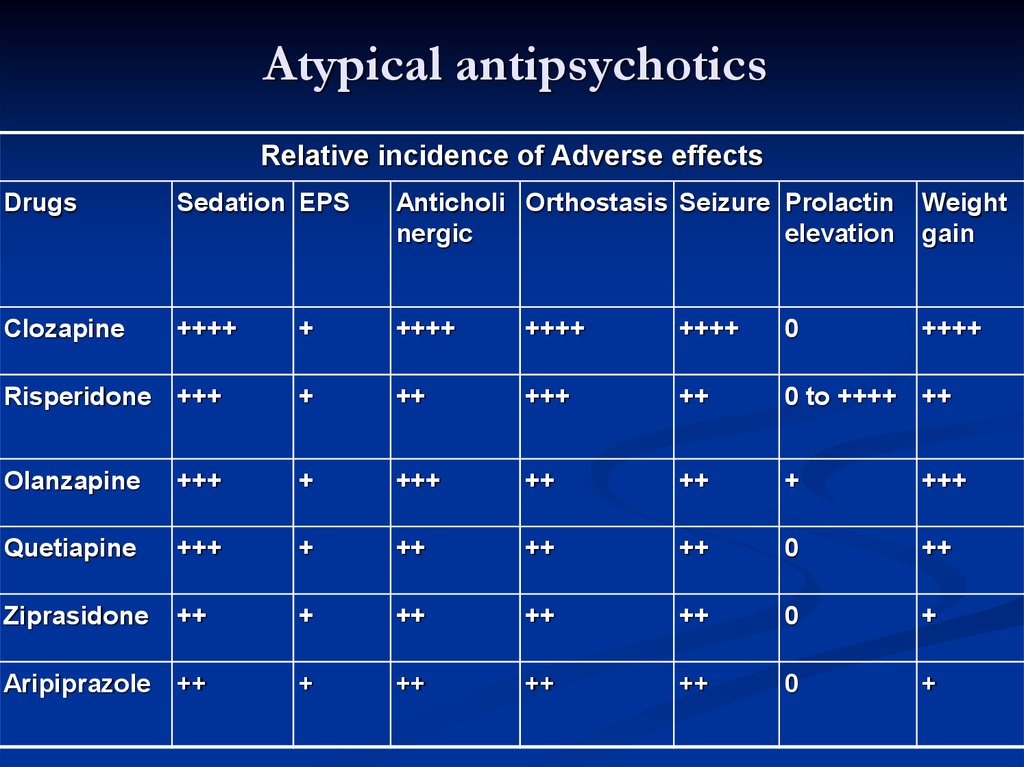 But it’s unknown whether such patients need that high of a dose, and the label notes experience with venlafaxine doses higher than 225 mg per day is “very limited.”
But it’s unknown whether such patients need that high of a dose, and the label notes experience with venlafaxine doses higher than 225 mg per day is “very limited.”
Adjusting the Dose for Kidney or Liver Problems
The dose a doctor prescribes is unique to each patient. Do not change your dosage without consulting your doctor or pharmacist.
The medication guide recommends doctors reduce the total daily dose by 50 percent in patients with mild to moderate liver failure, also known as hepatic impairment. It may be necessary to reduce the dose even more than 50 percent in some patients.
A reduced dose is also recommended for patients with kidney problems. The medication guide says doctors should reduce the dose by 25 percent for patients with a history of kidney failure and by 50 percent for patients undergoing hemodialysis.
Stopping Treatment
It’s unclear how long patients with depression, generalized anxiety disorder, social phobia or panic disorders should continue to take Effexor. During clinical studies for depression, the drug was effective up to 52 weeks without having to adjust the dose. The drug’s label advises doctors to periodically reassess the need for the medication.
During clinical studies for depression, the drug was effective up to 52 weeks without having to adjust the dose. The drug’s label advises doctors to periodically reassess the need for the medication.
Never stop taking venlafaxine without a doctor’s direction. Abruptly stopping the drug or decreasing the dose too quickly can cause serious withdrawal symptoms, including extreme fatigue, confusion, dizziness, headaches and shock-like electrical sensations.
Side Effects and Warnings
Among the most unwanted effects of venlafaxine are those related to sexual dysfunction such as lack of interest in sex, difficulty becoming aroused and the inability to achieve orgasm. Other reactions commonly reported in clinical trials include nausea, dry mouth, constipation and sweating.
The more serious Effexor side effects, however, involve pregnant or nursing women, children, teens and young adults. Venlafaxine is not approved for use in pediatric patients, and the medication guide warns pregnant or nursing women not to take the drug without discussing the risks with their doctor.
Newborns whose mothers took the drug in the third trimester of pregnancy may experience serious problems right after delivery, including seizures and problems feeding and breathing. And the drug may pass through breast milk and harm a nursing baby.
Still, the drug’s strongest warning is a black box warning for suicidal thoughts and behaviors in patients under the age of 25.
Off-Label Uses
Although the FDA approved Effexor XR to treat four illnesses, doctors may prescribe the drug for other conditions. These unapproved treatments are called off-label uses.
A 2017 Canadian study published in The British Medical Journal looked at adults who visited certain physicians between January 2003 and September 2015 and were prescribed an antidepressant through an electronic prescribing system.
Researchers found nearly a third of the 106,850 antidepressant prescriptions written for 20,920 patients were for off-label uses. And researchers determined only about 16 percent of off-label uses were backed up by medical research.
The researchers found SNRIs such as venlafaxine and SSRIs were the less likely to be prescribed for off-label uses. But Effexor has been used to treat conditions for which it has never been approved.
Diabetic Neuropathy
A 2010 review published by American Family Physician called SNRI antidepressants like venlafaxine “a promising category of antidepressants for treatment of diabetic peripheral neuropathic pain.” It cited a 2004 study in the journal Pain that found high doses of venlafaxine significantly reduced pain in diabetic neuropathy patients.
It also pointed to a 2007 Cochrane review that looked at three small studies on treating diabetic neuropathy with the drug. It found venlafaxine’s effectiveness at reducing pain was similar to that of tricyclic antidepressants such as amoxapine or desipramine.
“[SNRIs] are a promising category of antidepressants for treatment of diabetic peripheral neuropathic pain.”
However, a 2017 Cochrane review analyzed six trials involving neuropathic pain in general. It questioned the limits of the studies and cited considerable risk of bias in them.
It questioned the limits of the studies and cited considerable risk of bias in them.
“We found little compelling evidence to support the use of venlafaxine in neuropathic pain,” the researchers wrote in their conclusion.
Migraines
Effexor is also prescribed off-label to treat debilitating migraines. In April 2012, a neurologist from the Jefferson Headache Center at Thomas Jefferson University in Philadelphia presented new migraine treatment guidelines at the annual meeting of the American Academy of Neurology that formalized the drug’s role. The guidelines were published in the journal Neurology.
Hot Flashes
Venlafaxine has also been used for the off-label treatment of hot flashes associated with menopause. A study published in the Journal of the American Medical Association in 2006 found women who took the drug reported 60 percent fewer hot flashes. The study found the drug worked best at high doses, which meant an increased risk of side effects. Women should carefully weigh those risks against the benefits.
Effexor XR Facts
Please seek the advice of a medical professional before making health care decisions.
TELL US WHAT YOU THINK
Did You Find Drugwatch Helpful?
Yes No
Thank you for your feedback. Do you have any thoughts you'd like to share about Drugwatch.com?
This article changed my life!
This article was informative
I have a question
How can we improve this page?
This article contains incorrect information
This article doesn't have the information I'm looking for
I have a question
How can we improve this page?
Thank You for Your Feedback
We appreciate your feedback. One of our content team members will be in touch with you soon.
We appreciate your feedback. One of our content team members will be in touch with you soon.
Antipsychotics: pharmacological group
Description
Antipsychotics include drugs intended for the treatment of psychosis and other severe mental disorders. The group of antipsychotic drugs includes a number of phenothiazine derivatives (chlorpromazine, etc.), butyrophenones (haloperidol, droperidol, etc.), diphenylbutylpiperidine derivatives (fluspirilene, etc.), etc.
Neuroleptics have a multifaceted effect on the body. Their main pharmacological features include a kind of calming effect, accompanied by a decrease in reactions to external stimuli, a weakening of psychomotor arousal and affective tension, suppression of fear, and a decrease in aggressiveness. They are able to suppress delusions, hallucinations, automatism and other psychopathological syndromes and have a therapeutic effect in patients with schizophrenia and other mental illnesses.
Antipsychotics in normal doses do not have a pronounced hypnotic effect, but they can cause a drowsy state, promote the onset of sleep and increase the effect of hypnotics and other sedatives (sedatives). They potentiate the action of drugs, analgesics, local anesthetics and weaken the effects of psychostimulant drugs.
In some antipsychotics, the antipsychotic effect is accompanied by a sedative effect (aliphatic derivatives of phenothiazine: chlorpromazine, promazine, levomepromazine, etc.), while in others (piperazine derivatives of phenothiazine: prochlorperazine, trifluoperazine, etc.; some butyrophenones) - activating (energizing). Some neuroleptics relieve depression.
In the physiological mechanisms of the central action of neuroleptics, the inhibition of the reticular formation of the brain and the weakening of its activating effect on the cerebral cortex are essential. A variety of effects of neuroleptics are also associated with the impact on the occurrence and conduction of excitation in different parts of the central and peripheral nervous system.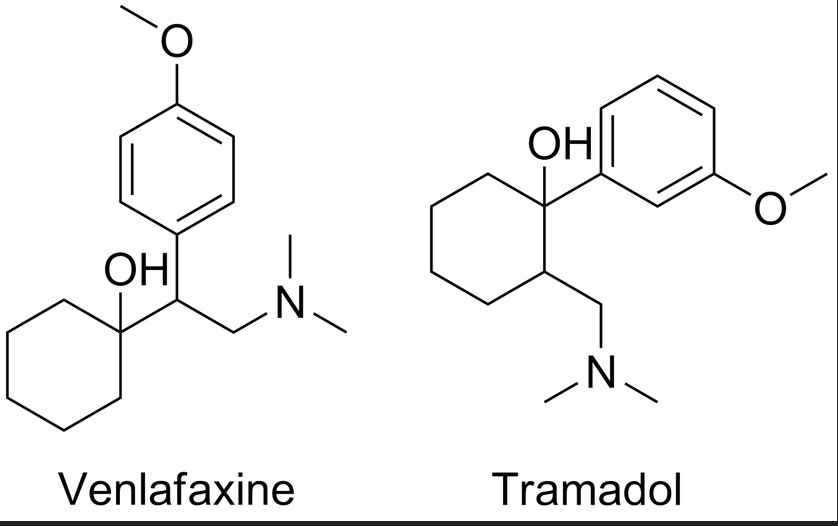
Antipsychotics change neurochemical (mediator) processes in the brain: dopaminergic, adrenergic, serotonergic, GABAergic, cholinergic, neuropeptide and others. Different groups of antipsychotics and individual drugs differ in their effect on the formation, accumulation, release and metabolism of neurotransmitters and their interaction with receptors in different brain structures, which significantly affects their therapeutic and pharmacological properties.
Antipsychotics of different groups (phenothiazines, butyrophenones, etc.) block dopamine (D 2 ) receptors of different brain structures. It is believed that this causes mainly antipsychotic activity, while the inhibition of central noradrenergic receptors (in particular, in the reticular formation) is only sedative. Not only the antipsychotic effect of neuroleptics, but also the neuroleptic syndrome caused by them (extrapyramidal disorders), explained by the blockade of dopaminergic structures of the subcortical formations of the brain (substance nigra and striatum, tuberous, interlimbic and mesocortical regions), where significant number of dopamine receptors.
Influence on central dopamine receptors leads to some endocrine disorders caused by antipsychotics. By blocking the dopamine receptors of the pituitary gland, they increase the secretion of prolactin and stimulate lactation, and acting on the hypothalamus, they inhibit the secretion of corticotropin and growth hormone.
An antipsychotic with pronounced antipsychotic activity, but practically no extrapyramidal side effects, is clozapine, a piperazino-dibenzodiazepine derivative. This feature of the drug is associated with its anticholinergic properties.
Most antipsychotics are well absorbed by different routes of administration (orally, intramuscularly), penetrate the BBB, but accumulate in the brain in much smaller amounts than in the internal organs (liver, lungs), metabolize in the liver and excreted in the urine, partly in the intestines . They have a relatively short half-life and after a single application, they act for a short time. Long-acting drugs (haloperidol decanoate, fluphenazine, etc. ) have been created that have a long-term effect when administered parenterally or orally.
) have been created that have a long-term effect when administered parenterally or orally.
Comparable modulators and divergents - two new base drug classes | Akhapkina V.I., Akhapkin R.V.
Discoveries in molecular biology in recent years have changed the concept of information transmission in the nervous system, which is not limited to monotonous or direct neurotransmission. This applies to neurotransmitters with their ability to exhibit both stimulating and inhibitory effects (depending on place, time, age, concentration), hormones, dynamic genes, immunity factors, co- and transcription factors. According to the concept of signaling biochemical networks, the nature of interactions between receptors, G-proteins and their effectors is stochastic, and cross-effects affect the regulation of cellular functions on the membrane surface, in the cytosol, in many cell compartments, including the nucleus [9-eleven]. As a result, even seemingly insignificant changes in the structure of receptors, caused, for example, by polymorphism of the genes encoding them, can cause serious consequences for signaling pathways, leading to profound pleiotropic changes in cell functioning [8]. Cell membranes are built of asymmetric material. Asymmetry and stereospecificity, biorhythmicity (relatively short, circadian and age-related rhythms), recessiveness and dominance play a huge role in the organization of the topology of the norm type and in the development of a disorder or pathology. At the same time, the cellular genome predetermines the individual characteristics and characteristics of a particular organism during its life cycle, starting from the papillary pattern and ending with the type of the nervous system.
Cell membranes are built of asymmetric material. Asymmetry and stereospecificity, biorhythmicity (relatively short, circadian and age-related rhythms), recessiveness and dominance play a huge role in the organization of the topology of the norm type and in the development of a disorder or pathology. At the same time, the cellular genome predetermines the individual characteristics and characteristics of a particular organism during its life cycle, starting from the papillary pattern and ending with the type of the nervous system.
Modulation and divergence are phenomena predetermined by nature in the structural and functional organization, fundamental and operational management in any living organism. They are rhythmic, interconnected and interdependent. Substances synthesized in the body play the role of modulators in some cases (for example, due to the conformation of molecules, conjugated-asymmetric bipolarity and stereospecificity of the action potential, providing reversibility of processes, etc.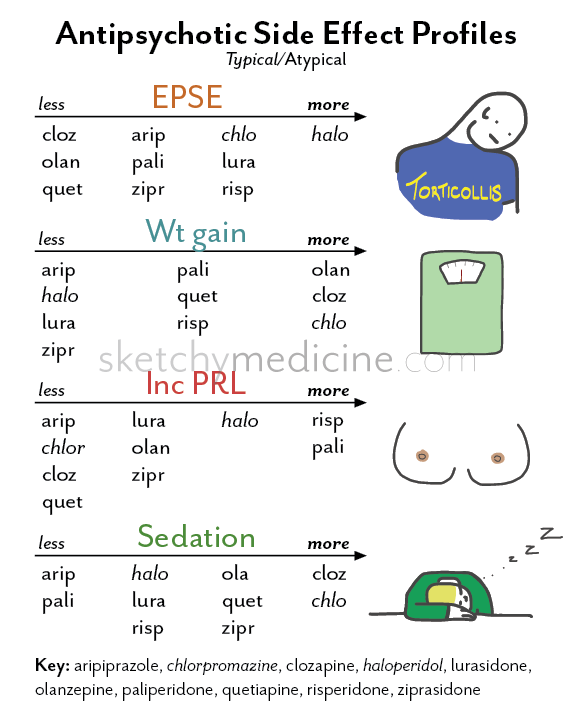 ), and in others - divergents with a clear effect of paradigm shift for due to a pronounced change in concentration, which is especially pronounced when the rhythm of sleep-wakefulness changes, and vice versa. Normally, for the body there are no separate concepts at all - stimulation, suppression, modulation, divergence. They are reciprocal with a constant change in their balance ratios.
), and in others - divergents with a clear effect of paradigm shift for due to a pronounced change in concentration, which is especially pronounced when the rhythm of sleep-wakefulness changes, and vice versa. Normally, for the body there are no separate concepts at all - stimulation, suppression, modulation, divergence. They are reciprocal with a constant change in their balance ratios.
The introduction of two new classes of drugs - modulators (from Latin modulatio - dimension, dimension) with a proportional (Latin - commensuratur, English - commensurate) influence and divergents (from Latin divergerens - discrepancy) was first proposed by V.I. Akhapkina in the report "Classification of drugs, its fundamental and applied foundations and problems" at the XIII Russian National Congress "Man and Medicine" in 2006. The modulator concept eliminated the distortion in understanding modulation, which has existed for many years in biology and medicine with the synonym modulating (t i.e. either summed modular synergy, or indirect one-type and unambiguous potentiation) influence. The divergent concept defines a place for drugs whose stimulatory and inhibitory effects diverge in a dose-dependent manner.
The divergent concept defines a place for drugs whose stimulatory and inhibitory effects diverge in a dose-dependent manner.
Modulatory activity is a commensurate effect of one or a complex of substances on the processes of stimulation and suppression, their commensurately consolidated conjugation and commensurate reversibility. The stimulating and suppressing effect of a proportional influence can manifest itself differentially predominantly depending on the type of the state of the organism in the norm and the type of disorder of the norm. Proportionate effect, including trigger transmission and reversibility, is the main distinguishing feature of modulators from other basic classes of drugs (stimulants, depressants, divergents). The substantiation and systematization of this class, the mechanisms of action are described in sufficient detail in the articles: “Fundamental foundations of the modulatory concept and classification of modulatory drugs”, “Modulatory concept as an innovative direction in medicine” and other materials [2,3,5,6].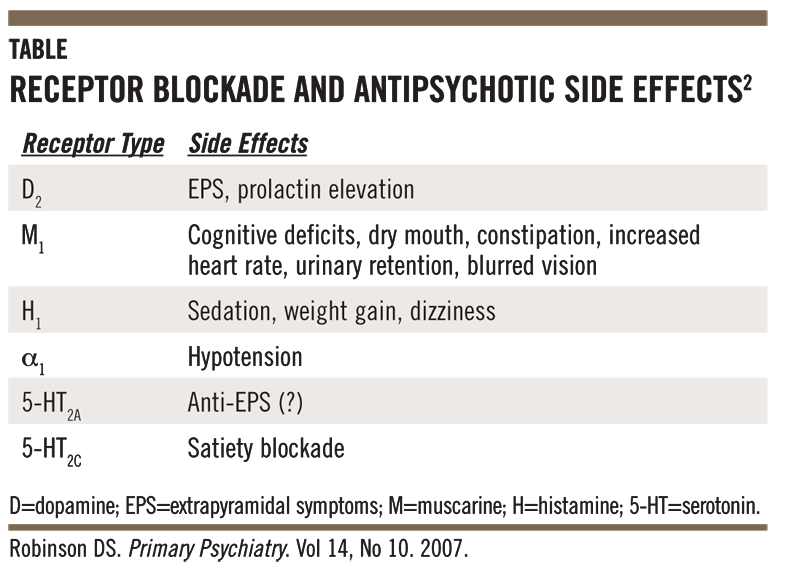
Divergent activity is a change (divergence) of the basic activity (stimulation and suppression) of one or a complex of substances depending on the dose. Signs of divergence in pharmacology, in contrast to divergence in mathematics and definitions regarding the origin of species by Charles Darwin, diverge not only from one point or from one species.
Modulators and divergents have their own systematization and their own obligate criteria (signs) for identifying and evaluating their pharmacological activity, concomitant action components, including exfoliative ones (from Latin exfoliatio - stratification). The introduction of two new base classes makes it possible to more objectively evaluate the effectiveness of drugs, their mechanisms of action and place in the systematization, to determine new approaches to the treatment, correction and prevention of various disorders and diseases with the most rational dosing and predicting the effect of a particular drug. At the same time, exfoliative and other concomitant and mediated components of action or secondary signs of modulators and divergents, as well as stimulants and inhibitors, are not decisive in their classification.
Secondary features are not named for their significance, but because they are not decisive for a particular class. Such signs appear depending on the basic activity and its mechanisms of action and are usually considered in groups and subgroups when systematizing a particular class. Often they have significant independent application in medical practice. These features include, in particular, the most common, for example, adaptogenic and nootropic effects. It must be recognized that disturbances in adaptive regulation, cognitive processes, metabolism and their plasticity occur to varying degrees both against the background of any disorder and disease without exception (especially in the stages of exacerbation, complications, chronic course), and in the postfertile period. Moreover, they occur not only against the background of repression, but also against the background of extensive expression and sensitization. Sensitization is especially facilitated by a discrete transmission (when one is turned on and the other is turned off).
Attempts to link the activity of many drugs with the nootropic concept did not give the desired result for objective reasons. Changing the fundamental position in the nootropic concept with a bell-shaped graphical dependence of the electrophysiological influence, including the components of the evoked transcallosal potential, on the stimulation of neurometabolism in the identification and evaluation of basic pharmacological activity, essentially does not change anything for the clinic in terms of stimulation. There is no reason to state that the stimulation of neurometabolism is exclusively the prerogative of nootropic activity, and the disruption of neurometabolism processes occurs exclusively in violation or insufficiency of cognitive functions. For example, any inflammatory process, intoxication will cause a violation of cognitive functions and cellular metabolism, and a nootropic drug will not eliminate them without eliminating the cognitive deficit that has arisen in such cases. In essence, the nootropic effect of known drugs is not a basic one, but one of the components of action and is very dose-dependent, exfoliating in most drugs with other components of action against the background of one or another basic activity and disappearing with increasing dose [4,6,7].
So, for example, in aminophenylbutyric and hopantenic acids, nootropic and adaptogenic activities are manifested in low therapeutic doses, and with increasing doses, these effects disappear, and a tranquilizing effect or even a hypnotic effect appears. These drugs are classical divergent agents, their stimulatory and inhibitory activity diverging depending on the dose. In piracetam, against the background of stimulating activity, antihypoxic with nootropic and adaptogenic components of action are clearly stratified. The stimulatory activity of piracetam increases with increasing dose, and at the same time, the nootropic effect disappears, but some antihypoxic effect appears in a very limited number of hypoxia models. At the same time, it is quite obvious that if a violation of intellectual activity is associated with hypoxia, then it is the antihypoxic effect that will mitigate or even completely eliminate this violation, eliminating hypoxia. An increase in the dose of piracetam is in clear conflict with the nootropic concept, causing impaired concentration, confusion and hallucinations, which is typical for psychostimulants in high doses. Known neurometabolic stimulants - neuropeptides - do not have antihypoxic activity, but have a pronounced antiamnestic effect. Phenamine and sydnoimine stimulants increase metabolism in nerve cells. Tranquilizers of the benzodiazepine series, having a pronounced antihypoxic effect, themselves cause amnesia, and in low - threshold doses they can show stimulating activity.
At the same time, it is quite obvious that if a violation of intellectual activity is associated with hypoxia, then it is the antihypoxic effect that will mitigate or even completely eliminate this violation, eliminating hypoxia. An increase in the dose of piracetam is in clear conflict with the nootropic concept, causing impaired concentration, confusion and hallucinations, which is typical for psychostimulants in high doses. Known neurometabolic stimulants - neuropeptides - do not have antihypoxic activity, but have a pronounced antiamnestic effect. Phenamine and sydnoimine stimulants increase metabolism in nerve cells. Tranquilizers of the benzodiazepine series, having a pronounced antihypoxic effect, themselves cause amnesia, and in low - threshold doses they can show stimulating activity.
Secondary signs soften to a certain extent the extensive and repressive activity of new generation drugs, especially if they are palliative, which depends on the mechanisms of action of a particular drug in certain doses. Considering that secondary signs depend on the basic activity and mechanisms of action of the drug, they can also be characterized differently, and this, in turn, determines their place in the systematization of the anatomical-therapeutic-chemical (ATC) classification of drugs. The inclusion of "true nootropics" in the class of psychostimulants (N06B - N06BX) is fully justified in terms of basic activity. However, it must be taken into account that the role and significance of cognitive functions, as well as adaptive regulation, is much broader and more diverse [2,3]. The study of the mechanisms of action in normal and pathological conditions, the preventive and therapeutic effects of drugs requires separate consideration and changes in approaches to them. What was acceptable for drugs with uniquely directed activity can be misleading in relation to true modulators and divergents. The vast majority of drugs from the group of nootropics in terms of basic activity are either stimulants (piracetam, cerebrolysin, pyriditol, etc.
Considering that secondary signs depend on the basic activity and mechanisms of action of the drug, they can also be characterized differently, and this, in turn, determines their place in the systematization of the anatomical-therapeutic-chemical (ATC) classification of drugs. The inclusion of "true nootropics" in the class of psychostimulants (N06B - N06BX) is fully justified in terms of basic activity. However, it must be taken into account that the role and significance of cognitive functions, as well as adaptive regulation, is much broader and more diverse [2,3]. The study of the mechanisms of action in normal and pathological conditions, the preventive and therapeutic effects of drugs requires separate consideration and changes in approaches to them. What was acceptable for drugs with uniquely directed activity can be misleading in relation to true modulators and divergents. The vast majority of drugs from the group of nootropics in terms of basic activity are either stimulants (piracetam, cerebrolysin, pyriditol, etc. ) or divergents (aminophenylbutyric and hopantenic acids).
) or divergents (aminophenylbutyric and hopantenic acids).
Identification and evaluation of modulatory and divergent activities do not require new research models, but require new approaches to them in comparison with just stimulants or inhibitory substances, including studies of the mechanisms of action. And if the divergent action has been studied quite well and only requires an objective independent place for itself in the classification of drugs, then modulators with a commensurate effect require not only an objective place for themselves, but also a more detailed consideration and study of their obligate criteria.
The underlying specific criterion of modulation is proportionality, therefore, first of all, it is necessary to establish the activity of the drug in the same doses when it is studied for one or another indicator, both against the background of its initially high and initially low levels in different types of normal and normal disorders. Class systematization is subdivided into: psychomodulators and psioperandmodulators, neuromodulators of the central and peripheral or autonomous level, endocrine modulators, immunomodulators, cytomodulators, tissue-specific and organ-specific modulators with their respective research models. The identification of promoter-modulatory activity should be carried out on a variety of models, and the unimodulatory action should not be limited not only by functional systems, but also by the method of application, the specifics of cells, organs and tissues. You should also consider the following:
The identification of promoter-modulatory activity should be carried out on a variety of models, and the unimodulatory action should not be limited not only by functional systems, but also by the method of application, the specifics of cells, organs and tissues. You should also consider the following:
- the severity of modulatory activity depending on the dose cannot violate the obligate criteria for identifying and evaluating one or another commensurate effect;
- divergence of effects depending on the dose (when only a stimulating effect is manifested in one dose, and only an inhibitory effect in another) is not an indication of the presence of a commensurate effect;
- the divergence of effects beyond single therapeutic doses cannot detract from the merits of modulators;
- cannot be a sign of the presence of a proportionate influence, the number of identified effects, if they do not meet its obligatory criteria;
- the exfoliative nature of various concomitant components of action depending on the dose cannot detract from the merits of one or another modulator, because in each case they can be not only individual, but also can manifest themselves to one degree or another depending on the dose.
For modulation and modulators with a specific commensurate effect, such general concepts as: modeling (model), modulating (module) and regulatory influence are not synonymous. Modeling and modulating influence are present in the identification and evaluation of any leading activity of the drug (whether it be stimulants, inhibitors, modulators, divergents), any concomitant effects and adverse reactions. Regulatory (regulatory) influence is inherent in the very philosophy of the concept of a drug. The organism is a functional system, and any single cell is a collection of sets as a whole, and none of these sets is isolated from others either in a particular module (for example, in a certain area of the brain), or from / from a particular module to other modules, nor in the body as a whole.
Modulation with a commensurate effect compared to suppression, stimulation and divergence is universal, but its universality can be limited by the evolutionary specifics of the cells and tissues of the body, and then it cannot have a unimodulatory (from lat. universalis - universal) status even in the presence of promotermodulatory (from lat. .promovere - I promote and ut - how) of the type of action. In such cases, modulatory activity cannot be characterized without a prefix or prefix indicating the level, and from the level of functional, pathogenetic, organotropic, etc. type of proportionate influence. It has its own ranges and boundaries of influence within the framework of ontogenesis and homeostasis, within the framework of the phenotype and age characteristics in the norm and in disorders of the norm. Therefore, formative (from Latin formans, formantis - generative) and fermatic (from Italian fermata - indefinite duration of a pause or action) types of plasticity, the scenario for the development of an organization, disorganization, degradation of the functional state are non-equivalent and changeable.
universalis - universal) status even in the presence of promotermodulatory (from lat. .promovere - I promote and ut - how) of the type of action. In such cases, modulatory activity cannot be characterized without a prefix or prefix indicating the level, and from the level of functional, pathogenetic, organotropic, etc. type of proportionate influence. It has its own ranges and boundaries of influence within the framework of ontogenesis and homeostasis, within the framework of the phenotype and age characteristics in the norm and in disorders of the norm. Therefore, formative (from Latin formans, formantis - generative) and fermatic (from Italian fermata - indefinite duration of a pause or action) types of plasticity, the scenario for the development of an organization, disorganization, degradation of the functional state are non-equivalent and changeable.
It is also necessary to take into account the fact that from postnatal to fertile age, the fermentative type of organization is normally aimed at achieving formative in the fertile period. In the post-fertile period, the fermentative type of organization has an out-direction. The rhythmicity of stimulation and suppression, their reciprocal-asymmetric relations and confrontation are never in an equilibrium state and in a state of absolute rest neither on one of the levels, nor between levels. Consequently, the principle of true (proportional) modulation is related not only to how many mechanisms of action are included in a particular state, but also what is the balance result of these relationships in a certain rhythm range at a certain state or transition to another state.
In the post-fertile period, the fermentative type of organization has an out-direction. The rhythmicity of stimulation and suppression, their reciprocal-asymmetric relations and confrontation are never in an equilibrium state and in a state of absolute rest neither on one of the levels, nor between levels. Consequently, the principle of true (proportional) modulation is related not only to how many mechanisms of action are included in a particular state, but also what is the balance result of these relationships in a certain rhythm range at a certain state or transition to another state.
The ancestor of the class of modulators with a commensurate effect is the innovative drug Phenotropil®TM or (RS)-2-(2-oxo-4-phenylpyrrolidin-1-yl)acetamide. The innovativeness of Phenotropil lies in the fact that the achieved not only laboratory, but industrial level of obtaining an almost chemically pure and stable composition of the substance allows you to completely change the idea of the properties and characteristics of this compound, previously known. It has been established that everything previously known about him concerned neither himself as such, but compositions burdened by the presence of other substances. It turned out that their presence, even at pharmacopoeially normalized quantitative content, can in some cases mask the biological activity of the compound, as in the previously known Phenotropil, and in others (carphedon, phenylpiracetam, phenylpiracetam, etc.) - completely distort the composition itself and, accordingly, its biological activity.
It has been established that everything previously known about him concerned neither himself as such, but compositions burdened by the presence of other substances. It turned out that their presence, even at pharmacopoeially normalized quantitative content, can in some cases mask the biological activity of the compound, as in the previously known Phenotropil, and in others (carphedon, phenylpiracetam, phenylpiracetam, etc.) - completely distort the composition itself and, accordingly, its biological activity.
Phenotropil does not and cannot originate from piracetam, as is sometimes erroneously believed. It does not and cannot belong to the family of racetams (piracetam, aniracetam, pramiracetam, oxiracetam, levetiracetam, etc.), since historically this family includes monomers and pyrrolidine-pyrrolidone derivatives that do not have substitutions of hydrogen for radicals at carbon atoms in the core of the pyrrolidone molecule. Phenotropil (diaphenyloxopyrrolidinylacetamide) is a complex preparation or dimer derived from the racemic mixture of fepirone / diaphenyloxopyrrolidone, which in turn is obtained from a cyclized linear racemic precursor (phenylamide butaric acid), and not from piracetam or pyrrolidone. Of the derivatives of diaphenylpyrrolidone, diaphenylpyrrolidinylacetyl is also known, which has not received medical use due to a pronounced damaging effect on internal organs, tissues and the nervous system. In Phenotropil, unlike phenylpiracetam and phenylpiracetam, both enantiomeric substances are biologically active. They have the ability to conform and transform into each other, and they do not worsen, as stated in studies WO 2007/104780 A2 of 20.09.2007, but improve its physicochemical, organoleptic and, most importantly, pharmacological properties and characteristics. The ability of Phenotropil molecules to conformations [3,6] and reversibility has been discovered. In experimental studies, Phenotropil has no restrictions on the use of age and sex. Currently undergoing clinical trials in pediatrics.
Of the derivatives of diaphenylpyrrolidone, diaphenylpyrrolidinylacetyl is also known, which has not received medical use due to a pronounced damaging effect on internal organs, tissues and the nervous system. In Phenotropil, unlike phenylpiracetam and phenylpiracetam, both enantiomeric substances are biologically active. They have the ability to conform and transform into each other, and they do not worsen, as stated in studies WO 2007/104780 A2 of 20.09.2007, but improve its physicochemical, organoleptic and, most importantly, pharmacological properties and characteristics. The ability of Phenotropil molecules to conformations [3,6] and reversibility has been discovered. In experimental studies, Phenotropil has no restrictions on the use of age and sex. Currently undergoing clinical trials in pediatrics.
It is likely that it is the ability to conformations and reversibility of optically active enantiomeric Phenotropil molecules that provides modulatory activity with a commensurate effect and its unusually wide range of pharmacological effects, depending on the state of the environment in which it is located during the pharmacokinetic distribution, allows it to overcome the specialization of cells, both when introduced into body, and when applied externally, and also does not allow extensive and repressive influence of the drug when the effect is achieved.
Innovative Phenotropil has neuromodulatory (at the levels and from the levels of the central and autonomic nervous system), psychomodulatory and psychoperandmodulatory, operandmodulatory (without limiting the specifics of cells), endocrine modulatory, immunomodulatory, cytomodulatory at the membrane and intracellular levels, training-stress-factor and adaptogenic activity in acute , subchronic and chronic negative stress. Phenotropil exhibits a pronounced neuroleptic (antipsychotic), antiparkinsonian, psychostimulant, anticonvulsant (for all models of seizures), antidepressant, anxiolytic, mnemotropic (with a genetically predetermined developmental delay), nootropic (with the manifestation of a bipolar trigger and reverse effect, which differs significantly from stimulants -nootropics), antiviral, anti-creving (with various forms of dependence), antihypoxic, anti-asthenic, antioxidant and pro-oxidant, anti-ischemic and anti-infarction (without limiting the specifics of organs and tissues), normotonic, anti-inflammatory, analgesic (including migraine with and without aura), anti-sickness , antitoxic, diuretic, decongestant, regenerative, reparative, slender (weight loss in obesity without manifestation of anorexigenic activity), rejuvenative (rejuvenating), antiulcerogenic, anticarcinogenic, antiapoptotic and thermoregulatory (especially with hypothermia and heat shock) action. It restores lost or reduced sensitivity of receptors. In animal and human studies, the drug restores fertility. Increases the average and maximum lifespan of animals. When applied externally, Phenotropil has a positive effect on various ENT, eye, dental, joint and skin diseases, helps to eliminate inflammation, heal damaged areas of various tissues without degenerative and destructive manifestations, restore joint mobility, eliminate vascular pattern and bundles, pigmentation, swelling and pain. sensations, reduces the aging process, regulates skin turgor, fat balance, the work of sebaceous channels in dry and oily seborrhea, increases the effectiveness of biologically active substances. The mechanisms of action of Phenotropil are diverse and unequal in different types of norms, disorders and various diseases [1-6].
It restores lost or reduced sensitivity of receptors. In animal and human studies, the drug restores fertility. Increases the average and maximum lifespan of animals. When applied externally, Phenotropil has a positive effect on various ENT, eye, dental, joint and skin diseases, helps to eliminate inflammation, heal damaged areas of various tissues without degenerative and destructive manifestations, restore joint mobility, eliminate vascular pattern and bundles, pigmentation, swelling and pain. sensations, reduces the aging process, regulates skin turgor, fat balance, the work of sebaceous channels in dry and oily seborrhea, increases the effectiveness of biologically active substances. The mechanisms of action of Phenotropil are diverse and unequal in different types of norms, disorders and various diseases [1-6].
The stable chemical composition of Phenotropil without any negative and masking effect of accompanying impurities allows us to fully confirm all the fundamentally designated areas of the modulatory concept, expand the effectiveness and safety of the drug with a significant change in the therapeutic index, increase its therapeutic breadth, expand the scope in medical practice.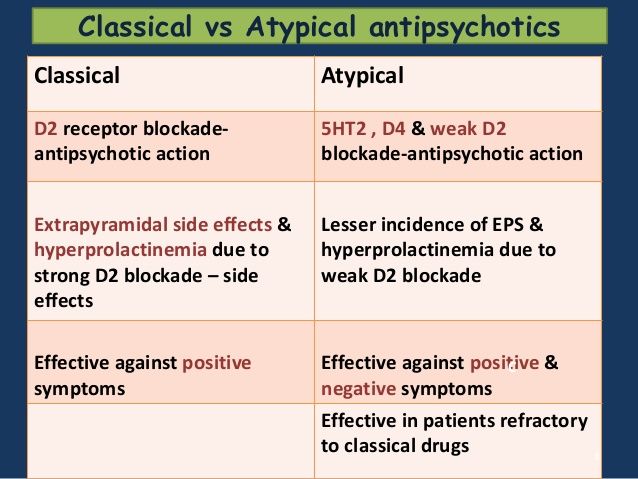 Phenotropil's unprecedentedly wide range of properties opens up new prospects for it in various medical fields, parapharmaceutics, including special biologically active additives (BAS), hygiene and cosmetic products. s
Phenotropil's unprecedentedly wide range of properties opens up new prospects for it in various medical fields, parapharmaceutics, including special biologically active additives (BAS), hygiene and cosmetic products. s
Literature
1. Akhapkin R.V., Akhapkina V.I. Properties and characteristics of innovatively original phenotropil // Abstracts of the report in Sat. Materials of the XIX Russian National Congress "Man and Medicine". - M., 2012. - S. 350-351.
2. Akhapkin R.V., Akhapkina V.I. Modulatory concept as an innovative direction in medicine // RMJ. - 2012. - No. 19. - P. 952–958.
3. Akhapkina V.I., Akhapkin R.V. Application for invention No. 2011138840 "Composition with modulatory activity with a commensurate effect, pharmaceutical substance (options), use of pharmaceutical substance, pharmaceutical and parapharmaceutical composition (options), method for producing pharmaceutical compositions" with priority dated September 22, 2011
4. Akhapkina V. I. Nootropic and mnemotropic activity as components of the action of drugs of various classes // Abstracts of the XIХ Russian National Congress "Man and Medicine". - M., 2012. - S. 351-352.
I. Nootropic and mnemotropic activity as components of the action of drugs of various classes // Abstracts of the XIХ Russian National Congress "Man and Medicine". - M., 2012. - S. 351-352.
5. Akhapkina V.I., Akhapkin R.V. Classification of modulatory drugs // Abstracts of the report in Sat. Materials of the XIX Russian National Congress "Man and Medicine". - M., 2012. - S. 465-466.
6. Akhapkina V.I., Akhapkin R.V. Fundamentals of the modulatory concept and classification of modulatory drugs // RMJ. - 2012. - No. 19. – S. 933– 951.
7. Voronina T.A. Experimental psychopharmacology of nootropics // Pharmacology of nootropics (experimental and clinical study). - M., 1989. - S. 8–19.
8. Clapham D.E. Mutations in G protein–linked receptors: Novel insights on disease // Cell. 1993 Vol. 75. P. 1237–1239.
9. Neves S.R., Ram P.T., Iyengar R. G protein pathways // Science. 2002 Vol. 296. P. 1636-1639.
10. Paul S.M., Purdy R.H. Neuroactive steroids // FASEBJ.