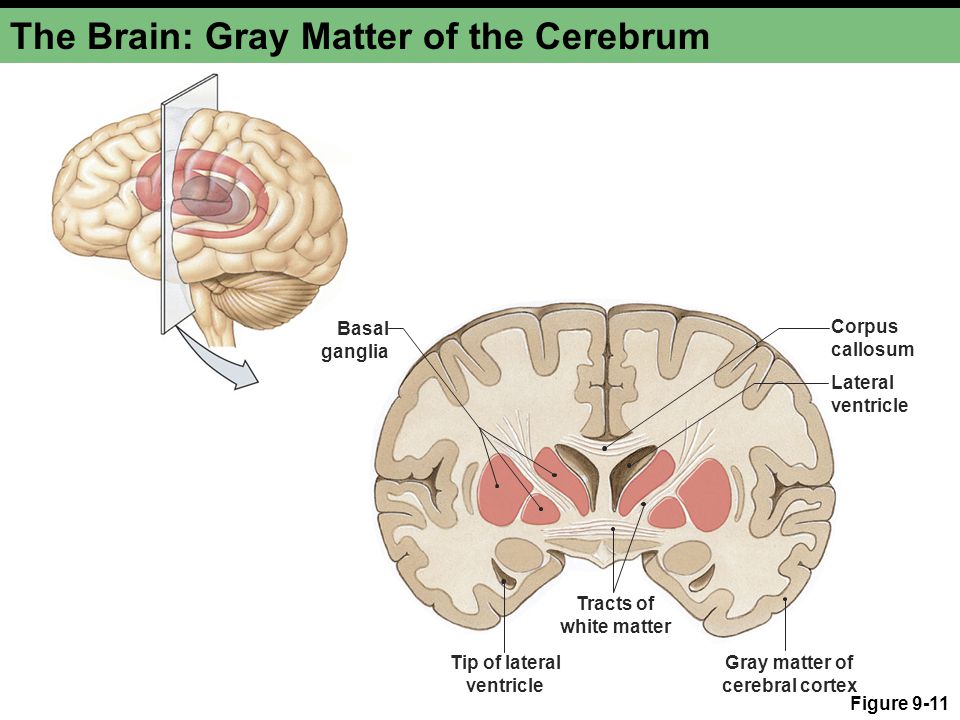
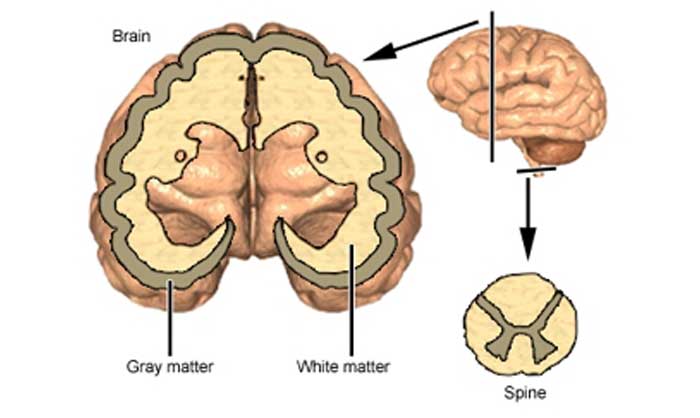
Increase grey matter in the brain
How Can You Improve Grey Matter in Your Brain? – Harmoni® Desk EU
Do you suffer from a loss of memory, otherwise known as memory fog? Over lockdown especially, more people than ever have been experiencing symptoms of memory loss whether on a small scale from forgetting what day it is, to more serious symptoms that could be a sign of an underlying health problem which needs to be addressed with a Doctor.
Feeling stressed out every once in a while is completely normal, but if you’ve been feeling tired and lethargic for two consecutive weeks and are finding it hard to think clearly, you might need some additional support.
We’ve put together some top tips on how you can boost your mood naturally to reduce stress
and at the same time increase the grey matter in your brain.
1) Meditation improves grey matter
Proven to help people relax and feel calmer, the benefits of meditation are far reaching. A study from Harvard University even found that long term meditators have increased the amounts of grey matter in the brain (within the insula and sensory regions and the auditory and sensory cortex).
Meditation is also a valuable tool which is not only free, but is also proven to alter your brainwave patterns. By restoring energy and helping you relax, you will be able to sleep better and feel more physically and mentally rested.
Try meditating twice a week for twenty minutes. Find somewhere which is quiet and calm, surround yourself with aromatic essentials oils, and watch as your breathing steadies and you feel a sense of relaxation and peace.
You can even upgrade your experience with a meditative exercise like Yoga! Check out these yoga essentials.
2) Physical activity increases grey matter in the brain
Along with the health benefits associated with physical exercise, working out is scientifically proven to increase the amount of grey matter in the brain. According to a study found in the Journal of Gerontology, ‘aerobic exercise training increases brain volume in aging humans’.
According to a study found in the Journal of Gerontology, ‘aerobic exercise training increases brain volume in aging humans’.
In fact, cardiovascular fitness is associated with enhancing the central nervous system. Particularly in older adults, exercise can help to improve the cognitive functioning of the human body and is a simple way to increase the amount of grey and white matter in the brain.
Try going up and down the stairs at home a few times today, or even a walk around the block during lunch. Additionally, you can try these free online exercise classes which you can do at home!
Also, be sure to use our Harmoni desk to increase the grey matter in your brain. By opting to stand to work, you will be more active and therefore improve your brain’s cognitive function.
Whilst most people would assume that playing video games is bad for your health, there is actually scientific evidence which suggests that video game users have an increase in
grey matter in the brain.
According to a study produced by the University of Electronic Science and Technology of China and the Marcquarie University of Sydney, video games alter the structure of the brain; heightening the connectivity between certain subregions in the insular cortex, and causing a larger surface area and volume of grey matter.
Enjoy playing guilt free! It’s supposedly healthy after all!
We’ve all been told that a balanced, healthy diet is important for our nutrition and health, but have you considered fasting before?
Proven to increase the production of the brain neurotrophic growth factor, a protein that promotes neuron growth and makes us more resilient to neurological stress; fasting reduces neurodegenerative disease and is proven to increase grey matter in the brain.
Additionally, fasting also helps existing neurons to survive while encouraging the growth of new neurons and the development of synapses. This helps to prevent conditions which are linked to Alzheimer’s and cognitive impairment.
This helps to prevent conditions which are linked to Alzheimer’s and cognitive impairment.
It’s no secret that sleep is incredibly important for the human body! In fact, lack of sleep, also known as sleep deprivation, is a well known cause of cognitive impairment. Sleep deprivation causes disruptions in the neuronal networks of the brain which in turn makes it harder to think and also remember.
In order to optimise the amount of grey matter in your brain, aim for eight to nine hours of high quality sleep each night. Also, try wearing blue light blocking sunglasses before bed or even removing any screens an hour prior. Just like any habit, building a healthy night routine into your day will take some time, but the benefit is that you will feel more relaxed and refreshed the next day. Who wouldn’t want that?
Conclusion
Improve the amount of grey matter in your brain today with these five simple steps. By taking a holistic approach to your wellness and incorporating some new habits into your dya, you will be sure to not only improve your memory, but also feel more energised and positive as a result.
By taking a holistic approach to your wellness and incorporating some new habits into your dya, you will be sure to not only improve your memory, but also feel more energised and positive as a result.
We hope you have enjoyed reading this article! If you have any questions, please do not hesitate to get in touch with us via email - [email protected]
Sources
<https://brainflow.co/index.php/2018/01/06/how-to-increase-iq-by-increasing-grey-brain-matter/>
<https://academic.oup.com/biomedgerontology/article/61/11/1166/630432>
‘What Causes Brain Fog?’ <https://www.medicinenet.com/brain_fog/article.htm>
‘Why Fast? Part 4 - Brain Health’ by Mark Sisson <https://www.marksdailyapple.com/fasting-brain-function/>
‘What is the effect of fasting on the lifespan of neurons?’ by Dilraj S. Kalsi <https://www.sciencedirect.com/science/article/pii/S156816371530012X>
‘How to Optimise Your Brain for Better Cognitive Performance’ by Chris Kresser <https://chriskresser. com/how-to-optimize-your-brain-for-better-cognitive-performance/>
com/how-to-optimize-your-brain-for-better-cognitive-performance/>
<https://www.yourbackyard.org.uk/free-exercise-classes-on-zoom/>
Katie Bodha
Older Post
Newer Post
Brief Mindfulness Meditation Induces Gray Matter Changes in a Brain Hub
1. Tang Y. Y., Holzel B. K., Posner M. I. The neuroscience of mindfulness meditation. Nature Reviews Neuroscience. 2015;16(4):213–225. doi: 10.1038/nrn3916. [PubMed] [CrossRef] [Google Scholar]
2. Goyal M., Singh S., Sibinga E. M. S., et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Hölzel B. K., Lazar S. W., Gard T., Schuman-Olivier Z., Vago D. R., Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [PubMed] [CrossRef] [Google Scholar]
Hölzel B. K., Lazar S. W., Gard T., Schuman-Olivier Z., Vago D. R., Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [PubMed] [CrossRef] [Google Scholar]
4. Tang Y. Y. The Neuroscience of Mindfulness Meditation: How the Body and Mind Work Together to Change our Behavior? London: Springer Nature; 2017. [CrossRef] [Google Scholar]
5. Fox K. C. R., Nijeboer S., Dixon M. L., et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience and Biobehavioral Reviews. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [PubMed] [CrossRef] [Google Scholar]
6. Hölzel B. K., Carmody J., Vangel M., et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. MacCoon D. G., Imel Z. E., Rosenkranz M. A., et al. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behaviour Research and Therapy. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Rosenkranz M. A., Davidson R. J., MacCoon D. G., Sheridan J. F., Kalin N. H., Lutz A. A comparison of mindfulness based stress reduction and an active control in modulation of neurogenic inflammation. Brain, Behavior, and Immunity. 2013;27(1):174–184. doi: 10.1016/j.bbi.2012.10.013. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. MacCoon D. G., MacLean K. A., Davidson R. J., Saron C. D., Lutz A. No sustained attention differences in a longitudinal randomized trial comparing mindfulness based stress reduction versus active control. PLoS One. 2014;9(6, article e97551) doi: 10.1371/journal.pone.0097551. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2014;9(6, article e97551) doi: 10.1371/journal.pone.0097551. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
10. Doll A., Hölzel B. K., Boucard C. C., Wohlschläger A. M., Sorg C. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Frontiers in Human Neuroscience. 2015;9:p. 461. doi: 10.3389/fnhum.2015.00461. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
11. Brewer J. A., Worhunsky P. D., Gray J. R., Tang Y. Y., Weber J., Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20254–20259. doi: 10.1073/pnas.1112029108. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
12. Creswell J. D., Taren A. A., Lindsay E. K., et al. Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: a randomized controlled trial. Biological Psychiatry. 2016;80(1):53–61. doi: 10.1016/j.biopsych.2016.01.008. [PubMed] [CrossRef] [Google Scholar]
Biological Psychiatry. 2016;80(1):53–61. doi: 10.1016/j.biopsych.2016.01.008. [PubMed] [CrossRef] [Google Scholar]
13. Wells R. E., Yeh G. Y., Kerr C. E., et al. Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neuroscience Letters. 2013;556:15–19. doi: 10.1016/j.neulet.2013.10.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
14. Leech R., Sharp D. J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12–32. doi: 10.1093/brain/awt162. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
15. Tang Y. Y., Ma Y., Wang J., et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Tang Y. Y., Ma Y., Fan Y., et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):8865–8870. doi: 10.1073/pnas.0904031106. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):8865–8870. doi: 10.1073/pnas.0904031106. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Tang Y. Y., Lu Q., Geng X., Stein E. A., Yang Y., Posner M. I. Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15649–15652. doi: 10.1073/pnas.1011043107. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
18. Tang Y. Y., Lu Q., Fan M., Yang Y., Posner M. I. Mechanisms of white matter changes induced by meditation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(26):10570–10574. doi: 10.1073/pnas.1207817109. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Mori S., Wakana S., van Zijl P. C. , et al. MRI Atlas of Human White Matter. New York: Elsevier Science; 2005. [Google Scholar]
, et al. MRI Atlas of Human White Matter. New York: Elsevier Science; 2005. [Google Scholar]
20. Wakana S., Jiang H., Nagae-Poetscher L. M., van Zijl P. C. M., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [PubMed] [CrossRef] [Google Scholar]
21. Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
22. Hagmann P., Cammoun L., Gigandet X., et al. Mapping the structural core of human cerebral cortex. PLoS Biology. 2008;6(7, article e159) doi: 10.1371/journal.pbio.0060159. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
23. Lu H., Song Y., Xu M., Wang X., Li X., Liu J. The brain structure correlates of individual differences in trait mindfulness: a voxel-based morphometry study. Neuroscience. 2014;272:21–28. doi: 10.1016/j.neuroscience.2014.04.051. [PubMed] [CrossRef] [Google Scholar]
Neuroscience. 2014;272:21–28. doi: 10.1016/j.neuroscience.2014.04.051. [PubMed] [CrossRef] [Google Scholar]
24. Chételat G., Mézenge F., Tomadesso C., et al. Reduced age-associated brain changes in expert meditators: a multimodal neuroimaging pilot study. Scientific Reports. 2017;7(1, article 10160) doi: 10.1038/s41598-017-07764-x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
25. Tang Y. Y., Hölzel B. K., Posner M. I. Traits and states in mindfulness meditation. Nature Reviews Neuroscience. 2016;17(1):p. 59. doi: 10.1038/nrn.2015.7. [PubMed] [CrossRef] [Google Scholar]
26. Rothbart M. K., Ahadi S. A., Evans D. E. Temperament and personality: origins and outcomes. Journal of Personality and Social Psychology. 2000;78(1):122–135. doi: 10.1037/0022-3514.78.1.122. [PubMed] [CrossRef] [Google Scholar]
27. Evans D. E., Rothbart M. K. Developing a model for adult temperament. Journal of Research in Personality. 2007;41(4):868–888. doi: 10.1016/j.jrp.2006.11.002. [CrossRef] [Google Scholar]
doi: 10.1016/j.jrp.2006.11.002. [CrossRef] [Google Scholar]
28. Tang Y.-Y., Tang R., Jiang C., Posner M. I. Short-term meditation intervention improves self-regulation and academic performance. Journal of Child and Adolescent Behaviour. 2014;2(4):p. 154. doi: 10.4172/2375-4494.1000154. [CrossRef] [Google Scholar]
29. Ding X., Tang Y. Y., Tang R., Posner M. I. Improving creativity performance by short-term meditation. Behavioral and Brain Functions. 2014;10(1):p. 9. doi: 10.1186/1744-9081-10-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
30. Fan Y., Tang Y. Y., Tang R., Posner M. I. Time course of conflict processing modulated by brief meditation training. Frontiers in Psychology. 2015;6:p. 911. doi: 10.3389/fpsyg.2015.00911. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
31. Reuter M., Schmansky N. J., Rosas H. D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage.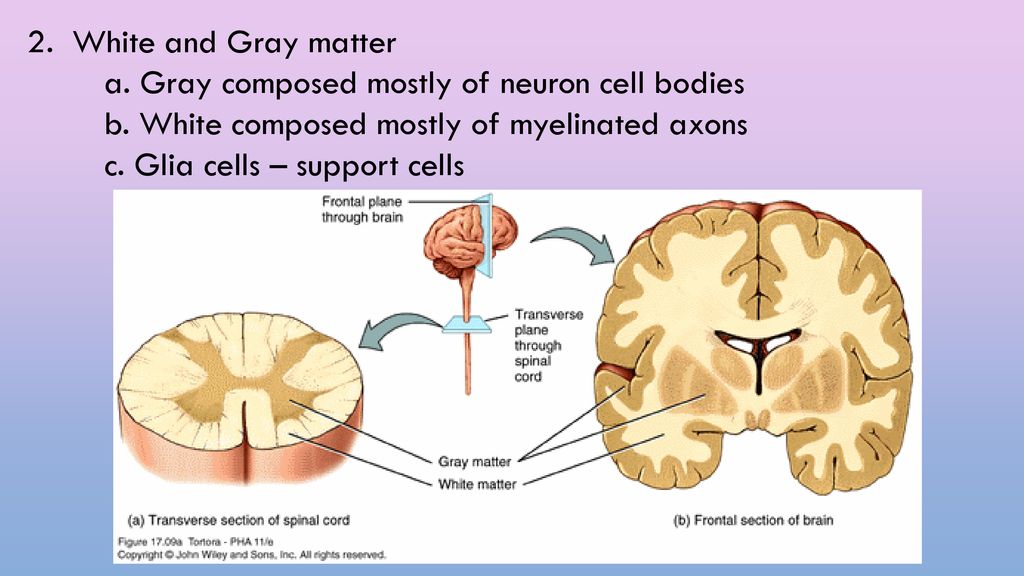 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
32. Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
33. Reuter M., Rosas H. D., Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
34. Lim H. K., Jung W. S., Ahn K. J., et al. Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology. 2012;37(3):838–849. doi: 10.1038/npp.2011.264. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Hervais-Adelman A., Moser-Mercer B., Murray M. M.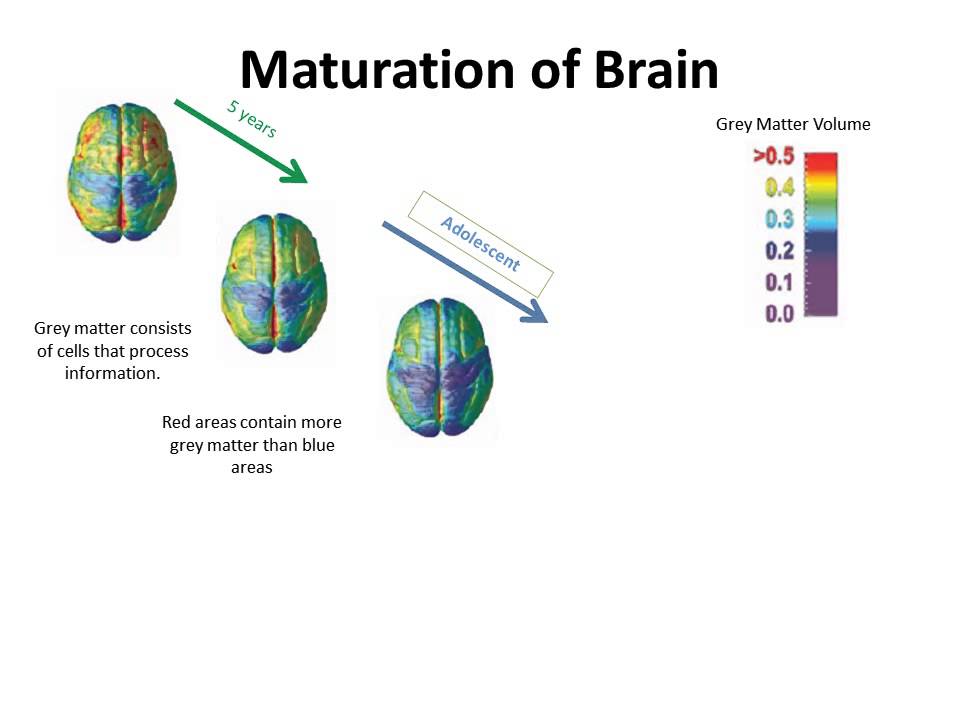 , Golestani N. Cortical thickness increases after simultaneous interpretation training. Neuropsychologia. 2017;98:212–219. doi: 10.1016/j.neuropsychologia.2017.01.008. [PubMed] [CrossRef] [Google Scholar]
, Golestani N. Cortical thickness increases after simultaneous interpretation training. Neuropsychologia. 2017;98:212–219. doi: 10.1016/j.neuropsychologia.2017.01.008. [PubMed] [CrossRef] [Google Scholar]
36. Pegueroles J., Vilaplana E., Montal V., et al. Longitudinal brain structural changes in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2017;13(5):499–509. doi: 10.1016/j.jalz.2016.08.010. [PubMed] [CrossRef] [Google Scholar]
37. Bercaw E. L., Hanks R. A., Millis S. R., Gola T. J. Changes in neuropsychological performance after traumatic brain injury from inpatient rehabilitation to 1-year follow-up in predicting 2-year functional outcomes. The Clinical Neuropsychologist. 2010;25(1):72–89. doi: 10.1080/13854046.2010.532813. [PubMed] [CrossRef] [Google Scholar]
38. Nesvåg R., Bergmann Ø., Rimol L. M., et al. A 5-year follow-up study of brain cortical and subcortical abnormalities in a schizophrenia cohort. Schizophrenia Research. 2012;142(1-3):209–216. doi: 10.1016/j.schres.2012.10.004. [PubMed] [CrossRef] [Google Scholar]
2012;142(1-3):209–216. doi: 10.1016/j.schres.2012.10.004. [PubMed] [CrossRef] [Google Scholar]
39. Storsve A. B., Fjell A. M., Tamnes C. K., et al. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. The Journal of Neuroscience. 2014;34(25):8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
40. Greenberg J., Romero V. L., Elkin-Frankston S., Bezdek M. A., Schumacher E. H., Lazar S. W. Reduced interference in working memory following mindfulness training is associated with increases in hippocampal volume. Brain Imaging and Behavior. 2019;13(2):366–376. doi: 10.1007/s11682-018-9858-4. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
41. Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
42. Tang Y. Y., Tang R., Gross J. J. Promoting psychological well-being through an evidence-based mindfulness training program. Frontiers in Human Neuroscience. 2019;13:p. 237. doi: 10.3389/fnhum.2019.00237. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
43. Lutz A., Slagter H. A., Dunne J. D., Davidson R. J. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
44. Hölzel B. K., Carmody J., Evans K. C., et al. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5(1):11–17. doi: 10.1093/scan/nsp034. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Takeuchi H., Taki Y., Sassa Y., et al. Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions.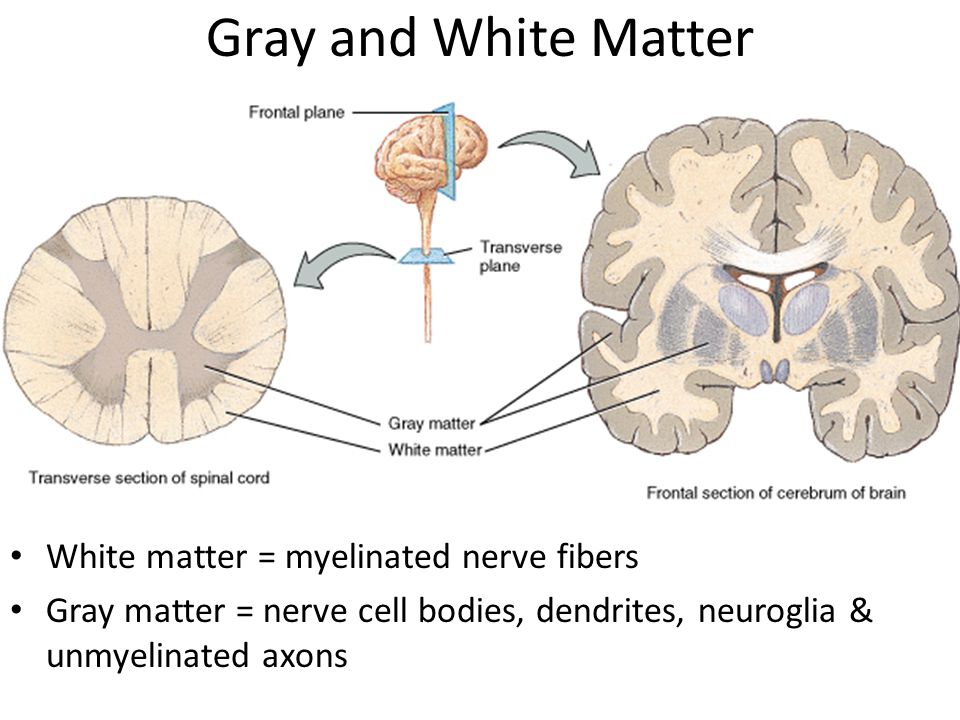 PLoS One. 2011;6(8, article e23175) doi: 10.1371/journal.pone.0023175. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
PLoS One. 2011;6(8, article e23175) doi: 10.1371/journal.pone.0023175. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
46. Gotink R. A., Vernooij M. W., Ikram M. A., et al. Meditation and yoga practice are associated with smaller right amygdala volume: the Rotterdam study. Brain Imaging and Behavior. 2018;12(6):1631–1639. doi: 10.1007/s11682-018-9826-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
47. Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde inihren Prinzipien dargestellt auf Grund des Zellenbaues. Berlin: Barth; 1909. [Google Scholar]
48. Vann S. D., Aggleton J. P., Maguire E. A. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10(11):792–802. doi: 10.1038/nrn2733. [PubMed] [CrossRef] [Google Scholar]
49. https://en.wikipedia.org/wiki/Isthmus_of_cingulate_gyrus.
50. Chrastil E. R. Heterogeneity in human retrosplenial cortex: a review of function and connectivity. Behavioral Neuroscience. 2018;132(5):317–338. doi: 10.1037/bne0000261. [PubMed] [CrossRef] [Google Scholar]
Behavioral Neuroscience. 2018;132(5):317–338. doi: 10.1037/bne0000261. [PubMed] [CrossRef] [Google Scholar]
51. Corcoran K. A., Yamawaki N., Leaderbrand K., Radulovic J. Role of retrosplenial cortex in processing stress-related context memories. Behavioral Neuroscience. 2018;132(5):388–395. doi: 10.1037/bne0000223. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
52. Huang Y., Hullfish J., de Ridder D., Vanneste S. Meta-analysis of functional subdivisions within human posteromedial cortex. Brain Structure & Function. 2019;224(1):435–452. doi: 10.1007/s00429-018-1781-3. [PubMed] [CrossRef] [Google Scholar]
53. McLaren M. E., Szymkowicz S. M., O’Shea A., Woods A. J., Anton S. D., Dotson V. M. Dimensions of depressive symptoms and cingulate volumes in older adults. Translational Psychiatry. 2016;6(4, article e788) doi: 10.1038/tp.2016.49. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
54. Caetano S. C., Kaur S.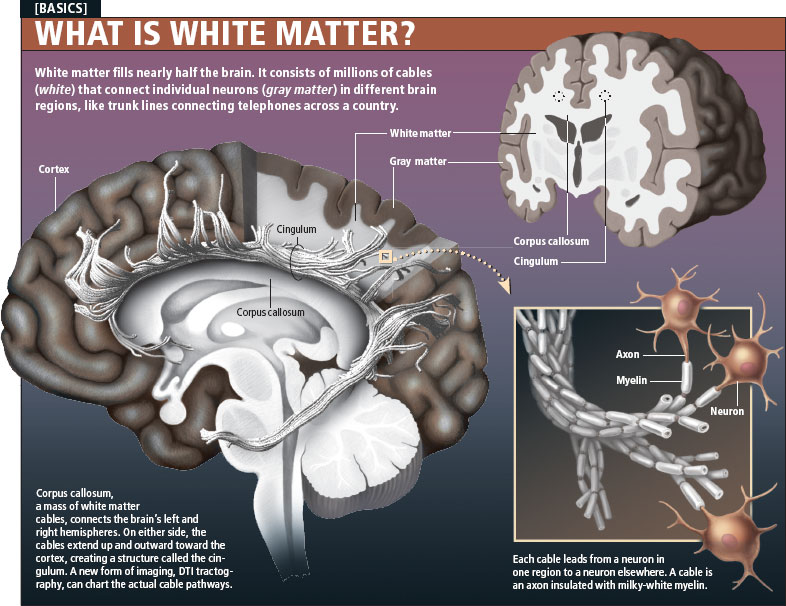 , Brambilla P., et al. Smaller cingulate volumes in unipolar depressed patients. Biological Psychiatry. 2006;59(8):702–706. doi: 10.1016/j.biopsych.2005.10.011. [PubMed] [CrossRef] [Google Scholar]
, Brambilla P., et al. Smaller cingulate volumes in unipolar depressed patients. Biological Psychiatry. 2006;59(8):702–706. doi: 10.1016/j.biopsych.2005.10.011. [PubMed] [CrossRef] [Google Scholar]
55. Yeh P. H., Zhu H., Nicoletti M. A., Hatch J. P., Brambilla P., Soares J. C. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: differences in latent volumetric structure. Psychiatry Research. 2010;184(3):177–185. doi: 10.1016/j.pscychresns.2010.07.007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
56. Pan P., Zhu L., Yu T. T., et al. Aberrant spontaneous low-frequency brain activity in amnestic mild cognitive impairment: a meta-analysis of resting-state fMRI studies. Ageing Research Reviews. 2017;35:12–21. doi: 10.1016/j.arr.2016.12.001. [PubMed] [CrossRef] [Google Scholar]
57. Wang H., Tan L., Wang H. F., et al. Magnetic resonance spectroscopy in Alzheimer’s disease: systematic review and meta-analysis. Journal of Alzheimer’s Disease. 2015;46(4):1049–1070. doi: 10.3233/JAD-143225. [PubMed] [CrossRef] [Google Scholar]
Journal of Alzheimer’s Disease. 2015;46(4):1049–1070. doi: 10.3233/JAD-143225. [PubMed] [CrossRef] [Google Scholar]
58. Tumati S., Martens S., Aleman A. Magnetic resonance spectroscopy in mild cognitive impairment: systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2013;37(10):2571–2586. doi: 10.1016/j.neubiorev.2013.08.004. [PubMed] [CrossRef] [Google Scholar]
59. Reiman E. M., Caselli R. J., Chen K., Alexander G. E., Bandy D., Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
60. Reiman E. M., Chen K., Alexander G. E., et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
61. Maarouf C. L., Kokjohn T. A., Walker D. G., et al. Biochemical assessment of precuneus and posterior cingulate gyrus in the context of brain aging and Alzheimer’s disease. PLoS One. 2014;9(8, article e105784) doi: 10.1371/journal.pone.0105784. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
62. He Y., Wang L., Zang Y., et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. NeuroImage. 2007;35(2):488–500. doi: 10.1016/j.neuroimage.2006.11.042. [PubMed] [CrossRef] [Google Scholar]
63. Sperling R. A., LaViolette P. S., O’Keefe K., et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
doi: 10.1016/j.neuron.2009.07.003. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
64. Ries M. L., Schmitz T. W., Kawahara T. N., Torgerson B. M., Trivedi M. A., Johnson S. C. Task-dependent posterior cingulate activation in mild cognitive impairment. NeuroImage. 2006;29(2):485–492. doi: 10.1016/j.neuroimage.2005.07.030. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
65. Fjell A. M., Walhovd K. B., Reinvang I., et al. Selective increase of cortical thickness in high-performing elderly--structural indices of optimal cognitive aging. NeuroImage. 2006;29(3):984–994. doi: 10.1016/j.neuroimage.2005.08.007. [PubMed] [CrossRef] [Google Scholar]
66. Gard T., Hölzel B. K., Lazar S. W. The potential effects of meditation on age-related cognitive decline: a systematic review. Annals of the New York Academy of Sciences. 2014;1307(1):89–103. doi: 10.1111/nyas.12348. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
67. Koenigsberg H.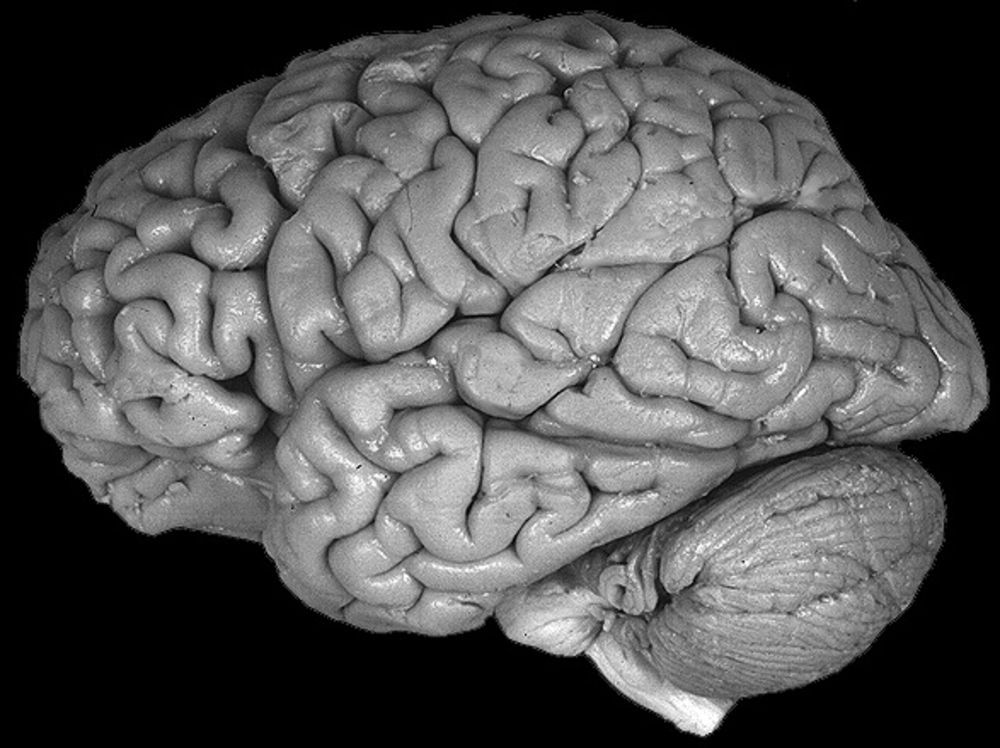 W., Fan J., Ochsner K. N., et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48(6):1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
W., Fan J., Ochsner K. N., et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48(6):1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
68. Morawetz C., Bode S., Derntl B., Heekeren H. R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews. 2017;72:111–128. doi: 10.1016/j.neubiorev.2016.11.014. [PubMed] [CrossRef] [Google Scholar]
69. Powers J. P., LaBar K. S. Regulating emotion through distancing: a taxonomy, neurocognitive model, and supporting meta-analysis. Neuroscience and Biobehavioral Reviews. 2019;96:155–173. doi: 10.1016/j.neubiorev.2018.04.023. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
70. Adapa R. M., Davis M. H., Stamatakis E. A., Absalom A. R., Menon D. K. Neural correlates of successful semantic processing during propofol sedation.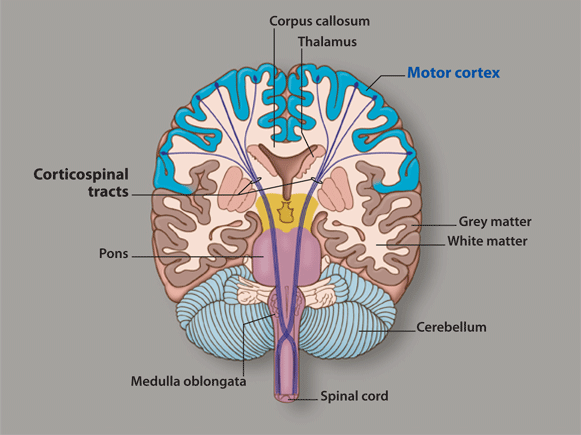 Human Brain Mapping. 2014;35(7):2935–2949. doi: 10.1002/hbm.22375. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Human Brain Mapping. 2014;35(7):2935–2949. doi: 10.1002/hbm.22375. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
71. Koch C., Massimini M., Boly M., Tononi G. Neural correlates of consciousness: progress and problems. Nature Reviews Neuroscience. 2016;17(5):307–321. doi: 10.1038/nrn.2016.22. [PubMed] [CrossRef] [Google Scholar]
72. Koch C., Massimini M., Boly M., Tononi G. Posterior and anterior cortex – where is the difference that makes the difference? Nature Reviews Neuroscience. 2016;17(10):p. 666. doi: 10.1038/nrn.2016.105. [PubMed] [CrossRef] [Google Scholar]
73. Vasey M. W., Harbaugh C. N., Lonigan C. J., et al. Dimensions of temperament and depressive symptoms: replicating a three-way interaction. Journal of Research in Personality. 2013;47(6):908–921. doi: 10.1016/j.jrp.2013.09.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
What do we know about gray matter pathology in multiple sclerosis
What do we know about gray matter pathology in multiple sclerosis Khachanova N. V.
V.
Federal State Budgetary Scientific Institution "Research Institute of Neurosurgery named after A.I. acad. N.N. Burdenko, Moscow
What do we know about gray matter pathology in multiple sclerosis
Journal: Journal of Neurology and Psychiatry. S.S. Korsakov. Special issues. 2018;118(8‑2): 18‑22
DOI 10.17116/jnevro201811808218
How to quote
Khachanova N.V. What do we know about gray matter pathology in multiple sclerosis. Journal of Neurology and Psychiatry. S.S. Korsakov. Special issues. 2018;118(8‑2):18‑22.
Khachanova NV. What do we know about the pathology of gray matter in multiple sclerosis. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova. 2018;118(8‑2):18‑22. (In Russ.).
https://doi.org/10.17116/jnevro201811808218
Authors:
Khachanova N.V.
Federal State Budgetary Scientific Institution "Research Institute of Neurosurgery named after A.I. acad. N.N. Burdenko, Moscow
All authors (1)
Read metadata
The advent of modern methods of immunohistochemistry and the development of MRI capabilities have led to a deeper understanding of the pathology of gray matter (GM) in multiple sclerosis (MS). SW involvement can be extensive, including both demyelination (cortical lesions) and neuroaxonal damage. Mechanisms of SV damage in MS remain poorly understood. There are two concepts: the lesion of SW is primary and proceeds simultaneously with changes in the white matter (WM) or it is secondary, i.e. is a consequence of a pathological process in BV. A more detailed study of SW pathology using the latest MRI techniques will contribute to understanding the pathogenesis of pathological changes in both cortical and deep SW.
SW involvement can be extensive, including both demyelination (cortical lesions) and neuroaxonal damage. Mechanisms of SV damage in MS remain poorly understood. There are two concepts: the lesion of SW is primary and proceeds simultaneously with changes in the white matter (WM) or it is secondary, i.e. is a consequence of a pathological process in BV. A more detailed study of SW pathology using the latest MRI techniques will contribute to understanding the pathogenesis of pathological changes in both cortical and deep SW.
Keywords:
multiple sclerosis
gray matter
demyelination
Authors:
Khachanova N.V.
Federal State Budgetary Scientific Institution "Research Institute of Neurosurgery named after A.I. acad. N.N. Burdenko, Moscow
Close metadata
An in-depth study of the mechanisms of damage to the nervous tissue in multiple sclerosis (MS) has expanded our understanding of the disease and made it possible to consider MS not only as a demyelinating but also as a neurodegenerative disease.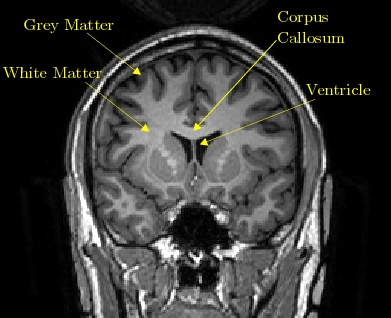 Traditionally R.S. was considered a disease of the white matter (WM) of the CNS, however, in the last century, several pathomorphological studies [1, 2] showed the presence of a focal lesion involving the gray matter (SW). These preliminary data on the potential pathology of SV were initially ignored due to the shortcomings of histochemical staining methods, which limited the study of cortical SV. In the second half of the 20th century, it became obvious that such clinical manifestations of the disease as cognitive dysfunction [3-5] or epileptic seizures [6-9], cannot be explained solely by the pathology of B.V. The advent of modern methods of immunohistochemical studies and the development of MRI capabilities have led to a deeper understanding of the pathology of SW in MS. It turned out that SW involvement can be extensive, including both demyelination (cortical lesions) and neuroaxonal damage.
Traditionally R.S. was considered a disease of the white matter (WM) of the CNS, however, in the last century, several pathomorphological studies [1, 2] showed the presence of a focal lesion involving the gray matter (SW). These preliminary data on the potential pathology of SV were initially ignored due to the shortcomings of histochemical staining methods, which limited the study of cortical SV. In the second half of the 20th century, it became obvious that such clinical manifestations of the disease as cognitive dysfunction [3-5] or epileptic seizures [6-9], cannot be explained solely by the pathology of B.V. The advent of modern methods of immunohistochemical studies and the development of MRI capabilities have led to a deeper understanding of the pathology of SW in MS. It turned out that SW involvement can be extensive, including both demyelination (cortical lesions) and neuroaxonal damage.
The introduction of immunohistochemical staining techniques has improved the visualization of SW pathology and allowed a more detailed description of cortical demyelination in MS.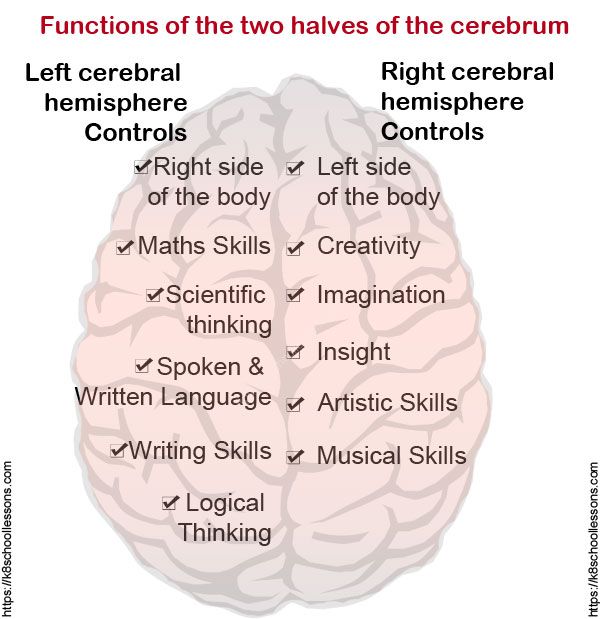 According to C. Gilmore et al. [10], the foci in the cerebral cortex are very small, but the volume of SW demyelination may exceed that of B.V. Cortical foci of demyelination occupy approximately 15–30% of the cortical CO [10–12] and are detected in 60–70% of patients [13, 14].
According to C. Gilmore et al. [10], the foci in the cerebral cortex are very small, but the volume of SW demyelination may exceed that of B.V. Cortical foci of demyelination occupy approximately 15–30% of the cortical CO [10–12] and are detected in 60–70% of patients [13, 14].
The original classification distinguished seven types of cortical lesions and was based on the relationship between lesions and cortical venous blood supply [15]. J. Peterson et al. [16] developed a less complex classification based on the location of lesions in different layers of the cortex for practical purposes and identified 3 subtypes: leukocortical, intracortical, and subpial. The most common are subpial plaques (type 3), which account for up to 60% of the total number of cortical lesions and 67% of the total area of focal lesions, leukocortical (type 1) and intracortical (type 2) demyelination foci less common [11, 17, 18]. In some cases of secondary progressive MS, cortical foci of demyelination can affect up to 68% of the total cortical surface [19]. The intensity of neuronal damage in type 1 lesions in SW is 3–4 times lower than in BV [20]. In the work of M. Vercellino et al. [21] showed that in cortical foci, a decrease in the density of neurons can reach 18–23%. Other signs of pathological processes occurring in the SW in MS, the loss of glial cells (36%) and synaptic contacts (47%), were noted in the work of S. Wegner et al. [eighteen]. Maps of the probability of damage to the SW and regional analysis of its atrophy made it possible to identify the specific topographic distribution of focal and diffuse damage [22]. Most lesions in the SW were found in the frontotemporal lobes, with a particular prevalence in the motor areas. A high probability of focal SW demyelination is observed in the hippocampus, deep SW, and insula [23, 24], while a significant increase in the distribution of diffuse subpial demyelination, assessed by MRI with a magnetic field strength of 7 T, is observed in the anterior cingulate gyrus [25]. Demyelination S.
The intensity of neuronal damage in type 1 lesions in SW is 3–4 times lower than in BV [20]. In the work of M. Vercellino et al. [21] showed that in cortical foci, a decrease in the density of neurons can reach 18–23%. Other signs of pathological processes occurring in the SW in MS, the loss of glial cells (36%) and synaptic contacts (47%), were noted in the work of S. Wegner et al. [eighteen]. Maps of the probability of damage to the SW and regional analysis of its atrophy made it possible to identify the specific topographic distribution of focal and diffuse damage [22]. Most lesions in the SW were found in the frontotemporal lobes, with a particular prevalence in the motor areas. A high probability of focal SW demyelination is observed in the hippocampus, deep SW, and insula [23, 24], while a significant increase in the distribution of diffuse subpial demyelination, assessed by MRI with a magnetic field strength of 7 T, is observed in the anterior cingulate gyrus [25]. Demyelination S. V. also observed in the limbic system and in the mesial temporal lobe [26].
V. also observed in the limbic system and in the mesial temporal lobe [26].
Immunohistochemical analysis revealed not only extensive demyelination of the neocortex, but also of archicortical structures (hippocampus) and subcortical structures (thalamus, putamen, globus pallidus, hypothalamus, substantia nigra, and cerebellum) [17, 19, 24, 27, 28]. In the hippocampal SW, the average area of demyelination can be 30%, and the decrease in neuron density correlates with a decrease in the average cross-sectional area of the hippocampus [29]. When studying the pathology of SW in the hippocampus, several types of intrahippocampal and mixed intrahippocampal-perihippocampal foci were found. In addition, the largest mixed lesions correlated with the severity of cognitive deficits. Moderate microglial activation was often found in the hippocampus, mainly along the edges of the demyelination focus [23]. The results of the study by R. Dutta et al. [30] found that, with similar axon densities in the normal-looking SW and demyelinated areas, the density of synaptic contacts is reduced in the demyelinated hippocampus. Other signs of neurodegeneration in the hippocampus, including impaired axonal transport, glutamate homeostasis, and neuronal survival, were also studied in this work [30].
Other signs of neurodegeneration in the hippocampus, including impaired axonal transport, glutamate homeostasis, and neuronal survival, were also studied in this work [30].
While the volume of BV remains fairly stable during the course of the disease, the volume of SV gradually decreases [31]. A large number of works in recent years have been devoted to the study of atrophy of the thalamus and hypothalamus and its relationship with increasing disability and progression of cognitive dysfunction [32, 33]. M. Houtchens et al. [34] found a decrease in the volume of the thalamus by 16.8% in patients with MS when compared with a group of healthy controls (control). Atrophy of the thalamus and putamen has already been described in the early stages of MS [35]. In secondary progressive MS (SPMS), the rate of CO volume loss can be 14 times higher than that in healthy people [36]. Another area of the brain with extensive cortical demyelination is the cerebellum [28]. A. Kutzelnigg et al. [28] described demyelination in approximately 38.8% of the cerebellar cortex in 19patients with SPMS and 36.9% in 10 patients with primary progressive MS (PPMS), while axonal spheroids and a decrease in Purkinje cells were also found in the cerebellum samples, which indicated ongoing neurodegeneration.
[28] described demyelination in approximately 38.8% of the cerebellar cortex in 19patients with SPMS and 36.9% in 10 patients with primary progressive MS (PPMS), while axonal spheroids and a decrease in Purkinje cells were also found in the cerebellum samples, which indicated ongoing neurodegeneration.
Microchip studies performed on cortical tissue revealed changes associated with mitochondrial function in demyelinated areas of the motor cortex [37], axonal transport and synaptic function [38], regulation of oligodendrocyte differentiation [39] and intrathecal synthesis of immunoglobulin [40]. Defeat S.V. usually characterized by the absence of lymphocytic infiltration, less deposition of antibodies and complement proteins, and the absence of disruption of the integrity of the blood-brain barrier compared to BV [16, 41, 42]. In the brain tissue of patients in the late stages of MS, a large number of immune cells were found only in type 1 (leukocortical) CV lesions: all other CV lesions were classified as relatively “non-inflammatory” [15–17]. C. Lucchinetti et al. [43] showed that the degree of SW lymphocytic infiltration depends on the stage of the disease: foamy macrophages involved in ongoing demyelination were found during biopsy in active SW lesions in 66% of patients with early MS, but were rarely detected in SW in patients with SPMS [44 ]. Despite the evidence of the role of CD4+ T-lymphocytes in the pathogenesis of the disease, according to autopsy studies, CD8+ T-cells are more often detected in the CB and in the brain parenchyma than CD4+ T-lymphocytes [44, 45]. The presence of perivascular CD8+ T cells was observed in 77% of intracortical biopsy lesions with varying frequency depending on the area of study (with a decrease in the number from leukocortical to intracortical lesions and the least in subpial plaques) [16, 44]. Perivascular T- and B-cell infiltrates have also been described in intracortical and subpial lesions in progressive MS, especially in the presence of signs of inflammation activation [46]. The reasons for the heterogeneous distribution of CD8+ T cells in the cerebral cortex, their relatively higher frequency in leukocortical and intracortical plaques compared to subpial lesions, and their localization in perivascular sleeves are currently unclear [44].
C. Lucchinetti et al. [43] showed that the degree of SW lymphocytic infiltration depends on the stage of the disease: foamy macrophages involved in ongoing demyelination were found during biopsy in active SW lesions in 66% of patients with early MS, but were rarely detected in SW in patients with SPMS [44 ]. Despite the evidence of the role of CD4+ T-lymphocytes in the pathogenesis of the disease, according to autopsy studies, CD8+ T-cells are more often detected in the CB and in the brain parenchyma than CD4+ T-lymphocytes [44, 45]. The presence of perivascular CD8+ T cells was observed in 77% of intracortical biopsy lesions with varying frequency depending on the area of study (with a decrease in the number from leukocortical to intracortical lesions and the least in subpial plaques) [16, 44]. Perivascular T- and B-cell infiltrates have also been described in intracortical and subpial lesions in progressive MS, especially in the presence of signs of inflammation activation [46]. The reasons for the heterogeneous distribution of CD8+ T cells in the cerebral cortex, their relatively higher frequency in leukocortical and intracortical plaques compared to subpial lesions, and their localization in perivascular sleeves are currently unclear [44]. In addition, a close topographic relationship has been demonstrated between subpial foci and meningeal inflammatory infiltrates, which, according to some researchers [19, 43, 47, 48], contribute to both cortical demyelination and progression of disability in R.S. Subpial demyelination and cortical atrophy are more pronounced in deep cortical invaginations [18, 28, 49]. Probably, regional differences in the movement of cerebrospinal fluid (CSF) and/or its stagnation can lead to the formation of a shielded niche (or microenvironment) for the persistence of lymphoid structures within the cerebral sulci. There is speculation that this situation may support a local immune response, especially enriched in CD20+ B-lymphocytes and plasmablasts, but also including CD4+ and CD8+ T cells and macrophages that chronically generate inflammatory, cytotoxic, and possibly myelinotoxic mediators circulating in the CSF. and freely distributed throughout the subarachnoid space. These mediators can cross the pial membrane in relation to the adjacent SW and mediate diffuse and focal subpial SW damage in MS [50].
In addition, a close topographic relationship has been demonstrated between subpial foci and meningeal inflammatory infiltrates, which, according to some researchers [19, 43, 47, 48], contribute to both cortical demyelination and progression of disability in R.S. Subpial demyelination and cortical atrophy are more pronounced in deep cortical invaginations [18, 28, 49]. Probably, regional differences in the movement of cerebrospinal fluid (CSF) and/or its stagnation can lead to the formation of a shielded niche (or microenvironment) for the persistence of lymphoid structures within the cerebral sulci. There is speculation that this situation may support a local immune response, especially enriched in CD20+ B-lymphocytes and plasmablasts, but also including CD4+ and CD8+ T cells and macrophages that chronically generate inflammatory, cytotoxic, and possibly myelinotoxic mediators circulating in the CSF. and freely distributed throughout the subarachnoid space. These mediators can cross the pial membrane in relation to the adjacent SW and mediate diffuse and focal subpial SW damage in MS [50].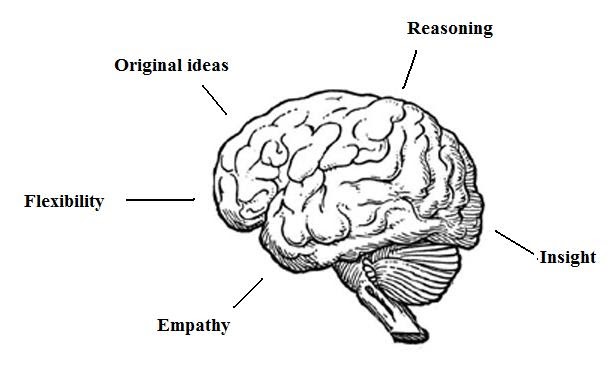 The release and circulation of specific but as yet unknown factors in the CSF, located in the cerebral cortex, may explain the fact that subpial cortical demyelination is one of the most specific features of pathological changes in MS [51]. Patients with extensive meningeal inflammation and ectopic B-cell follicles showed a more pronounced demyelination of the CB and a gradual loss of neurons from layers I to IV of the cortex [49]. At the same time, it should be noted that patients with ectopic B-cell follicles have a more aggressive course of the disease, an earlier onset of the disease, and early mortality [48]. Ectopic B-cell follicle-like structures described in deep sulci of the temporal, lumbar, insular, and frontal cortex in patients with early relapsing-remitting and progressive MS [43, 44, 46] are immunoreactive for Epstein–Barr virus (EBV) [48]. Based on several epidemiological studies, EBV has been hypothesized to be involved in the pathogenesis of MS [52–54], and EBV and RNA proteins have been found in B cells in the meninges and perivascular spaces of MS patients with extensive meningeal infiltrates and cortical demyelination [55–57 ].
The release and circulation of specific but as yet unknown factors in the CSF, located in the cerebral cortex, may explain the fact that subpial cortical demyelination is one of the most specific features of pathological changes in MS [51]. Patients with extensive meningeal inflammation and ectopic B-cell follicles showed a more pronounced demyelination of the CB and a gradual loss of neurons from layers I to IV of the cortex [49]. At the same time, it should be noted that patients with ectopic B-cell follicles have a more aggressive course of the disease, an earlier onset of the disease, and early mortality [48]. Ectopic B-cell follicle-like structures described in deep sulci of the temporal, lumbar, insular, and frontal cortex in patients with early relapsing-remitting and progressive MS [43, 44, 46] are immunoreactive for Epstein–Barr virus (EBV) [48]. Based on several epidemiological studies, EBV has been hypothesized to be involved in the pathogenesis of MS [52–54], and EBV and RNA proteins have been found in B cells in the meninges and perivascular spaces of MS patients with extensive meningeal infiltrates and cortical demyelination [55–57 ]. It has been suggested that failure to control latent EBV infection in an immune-privileged site such as the subarachnoid space may lead to recurrent intrathecal EBV reactivation and tissue damage in the nearby SW [58,59]. However, a number of other studies [60, 61] failed to detect EBV in the brain or plaques of patients with MS.
It has been suggested that failure to control latent EBV infection in an immune-privileged site such as the subarachnoid space may lead to recurrent intrathecal EBV reactivation and tissue damage in the nearby SW [58,59]. However, a number of other studies [60, 61] failed to detect EBV in the brain or plaques of patients with MS.
The behavior of activated microglia and its phenotype in MS cortical lesions also remains to be determined. It has been shown that the number of activated microglial cells and the degree of their activation correlate with the density of fragmented neurons in focal SW lesions [16], which indicates the vulnerability of dendrites and axons to microglial activation [62]. In post-mortem homogenates of demyelinated and non-demyelinated areas of the cerebral cortex obtained from patients with MS, it was found that CB demyelination is associated with increased activity of myeloperoxidase, which is expressed by the CD68-positive subtype of activated microglia, which was detected in active areas of CB demyelination along the plaque margin, but not microglia present in the adjacent non-demyelinated cortex [63].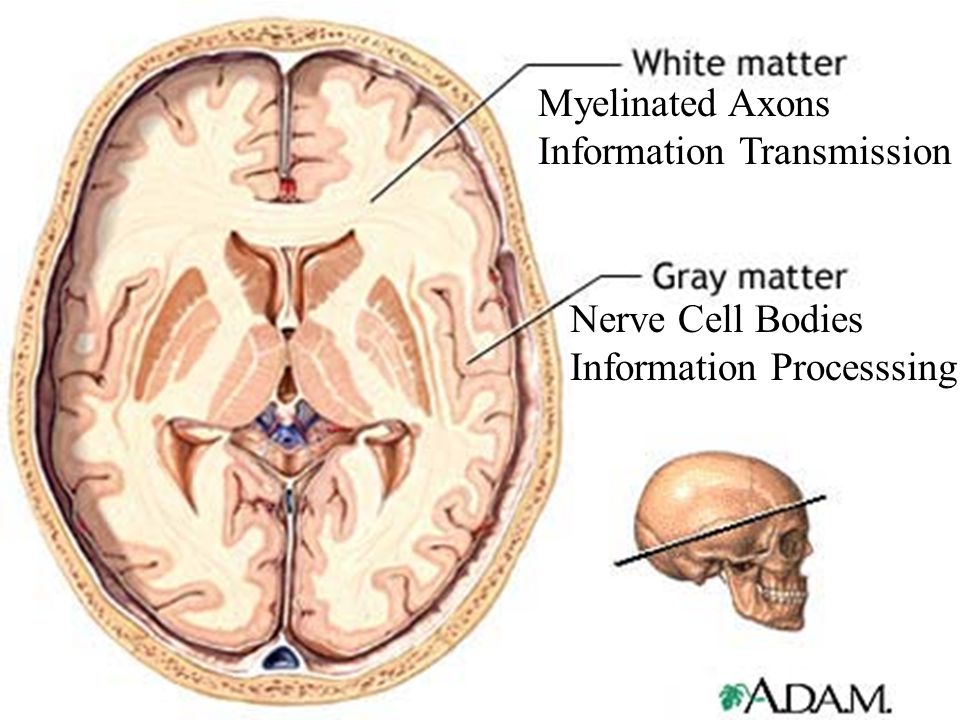 In addition, the presence of activated microglia in CV lesions in MS patients correlates with focal loss of glutamate transporters [64]. Mechanisms of SV damage in MS remain poorly understood.
In addition, the presence of activated microglia in CV lesions in MS patients correlates with focal loss of glutamate transporters [64]. Mechanisms of SV damage in MS remain poorly understood.
There are two concepts: damage to SW is primary and goes in parallel with changes in BV, or secondary, i.e., is a consequence of a pathological process in BV [20]. A more detailed study of SW pathology using the latest MRI techniques is likely to make a greater contribution to understanding the pathogenesis of pathological changes in both cortical and deep SW.
The authors declare no conflict of interest.
e-mail: [email protected]
Learning complex skills forever changes the structure of the brain - scientists , 12.10.2009
Learning complex skills permanently changes the structure of the brain - scientists
Learning complex skills, such as juggling, increases the amount of gray matter in the brain and improves communication between individual neurons with the help of axons - white matter. The discovery, described in an article in the journal Nature Neuroscience, could lead to the development of treatments for people with brain damage.
The discovery, described in an article in the journal Nature Neuroscience, could lead to the development of treatments for people with brain damage.
2009-10-12T11:19
2009-10-12T11:19
2009-10-12T11:19
/html/head/meta[@name='og:title']/@content
2 /html/head/meta[@name='og:description']/@content
https://cdnn21.img.ria.ru/images/sharing/article/188485620.jpg?1806327131255331993
RIA Novosti
1
5
4.7
96
7 495 645-6601
Rossiya Segodnya
https: //xn---c1acbl2abdlkab1og.xn--p1ai/Awards/
2009
RIA Novosti
1
5
4.7
9000 90004 -6601
FSUE MIA Rossiya Segodnya
https://xn--c1acbl2abdlkab1og.xn--p1ai/awards/
News
ru-RU
https://ria.ru/docs/about/ copyright.html
https://xn--c1acbl2abdlkab1og.xn--p1ai/
RIA Novosti
1
5
4. 7
7
96 9000
7 495 645-6603
FSUE MIA today
https: // XN-C1ACBL2ABDLKab1G. XN-P1AI/ Awards/
RIA Novosti
1
5
4.7
9000
7 495 645-6601
FSUE MIA Russia Today
HTTPS ://xn--c1acbl2abdlkab1og.xn--p1ai/awards/
RIA Novosti
1,000 .xn--p1ai/awards/
MOSCOW, Oct 12 - RIA Novosti. Learning complex skills, such as juggling, leads to an increase in the amount of gray matter in the brain and improves communication between individual neurons using axons - white matter. The discovery, described in an article in the journal Nature Neuroscience, could lead to the development of treatments for people with brain damage.
The gray matter of the brain consists mainly of nerve cells of neurons, while the white matter, axons, are their processes, their main task is the connection between neurons.
Despite the fact that scientists have already been able to show that teaching a person new complex skills leads to a change in the structure and increase in gray matter in certain areas of the brain, Jan Scholz of the University of Oxford and his colleagues were able to demonstrate for the first time that these changes in the brain, in fact, are more complex, irreversible, and an increase in gray matter is accompanied by an independent change in the structure and an increase in white matter.
In their experiment, a group of scientists using the method of diffusion magnetic resonance imaging scanned the brains of 24 volunteers - both men and women - each of whom learned to juggle according to instructions for two weeks. At the same time, for comparison, the researchers studied the brains of 24 people who were not trained in juggling.
After two weeks of training, it turned out that constant loads on the parts of the brain responsible for the coordination of movements, in particular, on the parietal lobe, and the connection of loads with the processing of visual information, led to an increase in the number of connections between neurons (white matter) in this area in all participants in the experiment. Moreover, Scholz, like his predecessors, also managed to detect an increase in gray matter in the same parts of the brain where changes in the structure of white occurred, but the degree of these changes in each case was different. This, according to scientists, suggests that the processes of changing white and gray matter as a result of learning a new skill occur independently.